Abstract
Silver-haired bat rabies virus (SHBRV) infection induces a strong virus-specific immune response in the periphery of the host, but death is common due to the failure to open the blood-brain barrier (BBB) and deliver immune effectors to central nervous system (CNS) tissues. Mice with an SJL background are less susceptible to lethal infection with rabies viruses. In addition, these animals are known to have reduced hypothalamus-pituitary-adrenal (HPA) axis activity and an elevated capacity to mediate CNS inflammatory responses. We show here that approximately one-half of PLSJL mice survive an SHBRV infection that is invariably lethal for 129/SvEv mice. This difference is associated with the elevated capacity of PLSJL mice to mediate BBB permeability changes in response to the infection. The induction of more extensive BBB permeability and CNS inflammation in these animals results in greater virus clearance and improved survival. On the other hand, treatment of SHBRV-infected PLSJL mice with the steroid hormone dehydroepiandrosterone reduced BBB permeability changes and caused greater mortality. We conclude that the infiltration of immune effectors across the BBB is critical to surviving a rabies virus infection and that HPA axis activity may influence this process.
Fluid exchange and the entry of circulating cells and macromolecules into central nervous system (CNS) tissues are regulated by the blood-brain barrier (BBB), a specialization of the neurovasculature (11). While the loss of BBB integrity is often associated with pathological changes in the CNS tissues (1, 12, 25), transiently increased BBB permeability can be therapeutic. An example is when immune effectors are delivered to CNS tissues to clear an infection with the attenuated rabies virus (RV) strain CVS-F3 (21). The clearance of this virus from the CNS of 129/SvEv mice occurs without signs of disease and is associated with enhanced BBB permeability which is largely restricted to the cerebellum (21). The absence of this beneficial enhancement of BBB permeability can lead to a CNS infection becoming lethal, as is evidently the case for infection with the highly pathogenic silver-haired bat-associated RV (SHBRV) (23), the virus that is currently responsible for the majority of human deaths from rabies in the United States and Canada (18). Despite the normal development of RV-specific immunity in peripheral lymphoid organs, increased BBB permeability and immune cell invasion do not occur in the CNS tissues of SHBRV-infected 129/SvEv mice (23). Consequently, the virus is not cleared, and the animals die (23).
While the antiviral immune response cannot clear SHBRV from the CNS tissues of 129/SvEv mice, immune cells adoptively transferred from these animals are capable of causing elevated BBB permeability as well as CNS inflammation and clearance of CVS-F3 from the CNS of rag2−/− mice (23). We therefore speculated that the BBB in the cerebellum of SHBRV-infected animals becomes refractory to the signals that trigger enhanced permeability, and the virus-clearing CNS inflammatory response does not occur (23). In this case, the outcome of RV infection is dictated by the capacity to mediate CNS inflammation. However, by comparing infections of various mouse strains, a prior study has demonstrated that survival from RV infection is correlated with the level of virus-neutralizing antibody produced (17). In these studies, SJL mice developed the highest serum virus-neutralizing antibody titers, but resistance to lethal infection proved to be major histocompatibility complex independent (16), suggesting that the extent of antigen-specific immunity may not be the primary determinant of survival.
The SJL background has been associated with blunted hypothalamus-pituitary-adrenal (HPA) axis activity (2). The HPA axis regulates the production of corticosteroids and other steroid hormones, such as dehydroepiandrosterone (DHEA), that have a variety of functions in different tissues (5, 9, 30). With respect to immunity, corticosteroids are generally anti-inflammatory (3, 20); therefore, it may be for good reason that mice with an SJL background are extensively used in experimental allergic encephalomyelitis (EAE) studies (2). In addition, DHEA treatment has been demonstrated to inhibit CNS inflammation in an SJL model of EAE (10). This raises the possibility that, rather than elevated antiviral immunity, the deregulation of CNS inflammation due to reduced HPA activity may be responsible for the resistance of SJL-derived mice to lethal infection with RV. In this investigation, we first tested this hypothesis by comparing antiviral immunity, BBB permeability changes, CNS inflammation, and virus clearance in PLSJL and 129/SvEv mice infected with SHBRV. To further examine the association between BBB integrity, CNS inflammation, and virus clearance, we triggered an exaggerated CNS inflammatory response in SHBRV-infected PLSJL mice by the induction of EAE. Finally, to probe the possibility that the reduced production of DHEA impacts the antiviral response, the capacity of PLSJL mice treated with this hormone to clear SHBRV was assessed.
MATERIALS AND METHODS
Animals and virus infection.
129/SvEv and PLSJLF1/J (PLSJL) mice were purchased from Taconic (Germantown, NY) and Jackson Laboratories (Bar Harbor, ME), respectively, and used at 8 to 10 weeks of age. Mice were infected with SHBRV-17 (SHBRV), a pathogenic strain originally isolated from human brain tissue and expanded via multiple passages in neonatal mouse brain (8) via the intradermal route in both ears with 104 focus-forming units of virus as has been previously described (23). All procedures were carried out according to the protocols approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University.
Measurement of serum antibody titers.
Levels of RV-specific total immunoglobulin G (IgG) in sera from control and experimental mice were assessed by enzyme-linked immunosorbent assay (ELISA) as described previously (7). Briefly, 96-well plates (Nalge Nunc International, Rochester, NY) were coated with UV-inactivated RV (5 μg/ml), and antibodies captured from serially diluted samples of sera were detected using peroxidase-conjugated anti-mouse IgG (Sigma). 3,3′,5,5′-Tetramethylbenzidine dihydrochloride substrate (Sigma) supplemented with hydrogen peroxide was used for color development. The reaction was terminated by the addition of 2 M H2SO4 to the wells, and the absorbance was measured at 450 nm in a microplate spectrophotometer (Biotek, Winooski, VT).
QRT-PCR.
Levels of different gene-specific mRNAs were measured by quantitative real-time PCR (QRT-PCR) as previously described (21). Briefly, total RNA was isolated from the CNS tissues of RV-infected and uninfected control mice using a QIAGEN RNeasy kit (Valencia, CA). cDNAs were synthesized from mRNA by reverse transcription using oligo(dT) as primer. QRT-PCR was then performed using TaqMan PCR reagents (Applied Biosystems, Foster City, CA), gene-specific primers and probes, synthetic gene standards, and a Bio-Rad iCycler iQ real-time detection system (Hercules, CA). The mRNA copy numbers of a particular gene in each sample were normalized to the mRNA copy number of a housekeeping gene L13 in that sample. To estimate virus replication, virus-specific nucleoprotein mRNA expression was measured by QRT-PCR and expressed as the number of mRNA copies per mg of tissue. This has been demonstrated to be a reliable measure of RV replication (13). Sequences of the primers and probes used for quantitative PCR have been previously detailed (21, 23).
Assessment of BBB permeability.
The extent of BBB permeability was assessed by measuring the amount of leakage of a low-molecular-weight fluorescent marker (Na-fluorescein) from the circulation into CNS tissues as previously described (21). Briefly, 100 μl of 10% solution of Na-fluorescein was injected intraperitoneally, and after 10 min, mice were anesthetized and cardiac blood was collected, followed by transcardial perfusion with phosphate-buffered saline. CNS tissues were collected and homogenized in phosphate-buffered saline and centrifuged. Proteins were precipitated with 15% trichloroacetic acid from the supernatants of the tissue homogenate as well as from serum samples. Fluorescence in the clarified supernatant was measured using a CytoFluorII fluorimeter (PerSeptive Biosystems, Framingham, MA). The amount of Na-fluorescein in the CNS tissue of each animal was normalized to the level of Na-fluorescein detected in the serum from the corresponding animal.
MBP immunization.
Mice were immunized with myelin basic protein (MBP) as described previously (15). Briefly, mice were subcutaneously injected at three sites along the back with a total volume of 200 μl of a 1:1 emulsion of 100 μg MBP in complete Freund's adjuvant (Difco Labs, MI) supplemented with 4 mg/ml Mycobacterium tuberculosis H37RA (Difco). Mice also received two injections of 400 ng pertussis toxin (List Biologicals, CA) intraperitoneally, one on the day of immunization and the other 2 days after immunization. Mice were monitored daily for any signs of the disease.
DHEA treatment.
Mice were treated via subcutaneous injection with a daily dose of 2 mg DHEA (Sigma-Aldrich, St. Louis, MO) per animal dissolved in 20 μl of vehicle (dimethyl sulfoxide; Sigma). Treatment was started from the day of infection with the virus. Mice in control groups received a similar volume of vehicle without DHEA.
Statistical analyses.
Results are expressed as the mean ± the standard error of the mean. Statistical significance of the differences in gene expression between control and infected groups was tested using the Mann-Whitney test, while differences between serum antibody levels were assessed by the paired t test. Differences in survival rate between the groups were determined by Fisher's exact test for contingency.
RESULTS
PLSJL mice are less susceptible to lethal SHBRV infection than 129/SvEv mice.
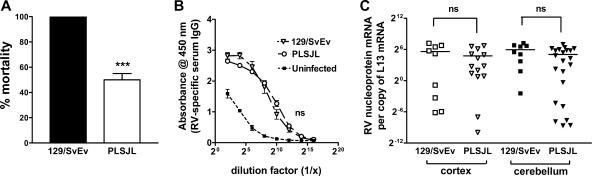
The genetic makeup of an animal is a major determinant of susceptibility to a variety of diseases. For example, SJL mice often survive infection with pathogenic strains of RV that are lethal in other strains, possibly because mice with an SJL background are prone to the development of stronger inflammatory responses (17). To examine this possibility with the highly pathogenic SHBRV, we compared the outcome of infection in 129/SvEv (H-2b) mice with that in PLSJL (H-2u/s) mice. 129/SvEv mice infected intradermally in the ear with 104 focus-forming units of SHBRV invariably die, while approximately one-half of similarly infected PLSJL mice survive (Fig. 1A). Regardless of whether or not they succumb to the infection, all infected mice develop comparably high levels of RV-specific antibodies in the circulation (Fig. 1B), as well as SHBRV nucleoprotein mRNA in CNS tissues (Fig. 1C).
FIG. 1.
Comparison of SHBRV replication levels in the CNS tissues of 129/SvEv and PLSJL mice. Groups of 129/SvEv and PLSJL mice (n = 30) were infected with SHBRV and monitored for morbidity and mortality (A). Similarly infected groups of 129/SvEv (n = 10) and PLSJL (n = 23) mice were euthanized on day 8 following infection to assess RV antigen-specific IgG titers in the sera using ELISA (B) and to quantify SHBRV nucleoprotein mRNA levels in the cortex (open symbols) as well as in the cerebellum (filled symbols) as a measure of virus replication (C). Infection, antibody titer determination by ELISA, and QRT-PCR analysis of mRNA levels were performed as described in Materials and Methods. Nucleoprotein mRNA levels are expressed as the number of copies per copy of the housekeeping gene L13 in a particular sample. A statistically significant difference in survival rate between the groups was determined by Fisher's exact test for contingency and is denoted by the symbol *** (P < 0.001). The absence of statistical differences in antibody and virus nucleoprotein levels between infected mice of the two mouse strains is denoted by ns (not significant).
The death of SHBRV-infected PLSJL mice is associated with high-level virus replication in the CNS without a concomitant deficit in immunity.
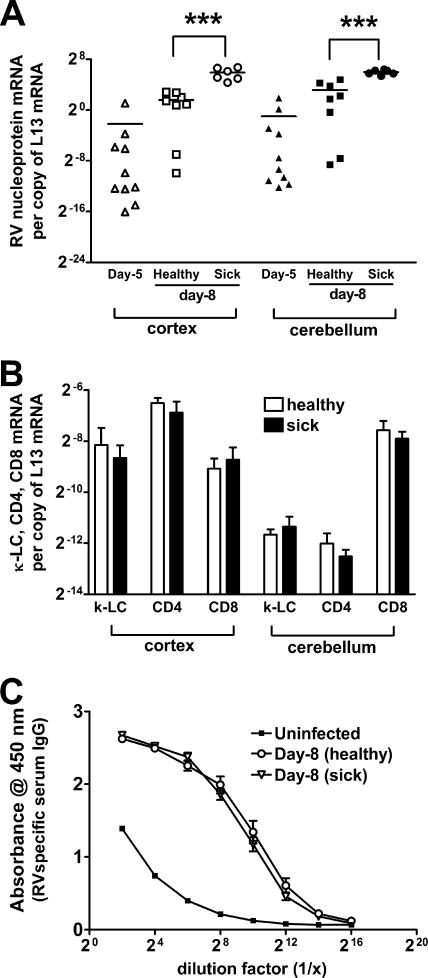
SHBRV infection is lethal in 129/SvEv mice as a consequence of the failure to mount a CNS inflammatory response and clear the virus from CNS tissues (23). This led us to question whether or not the difference in survival between individual PLSJL mice is the result of the inability of certain mice to mediate CNS inflammation. Between 8 and 12 days postinfection (p.i.), PLSJL mice that will die from SHBRV infection exhibit neurological signs of disease, such as agitation, convulsion, and muscle rigidity, while survivors remain healthy throughout. This enables us to predict the outcome and compare virus replication levels and immune functions between mice that will survive and those that will not survive the infection. At day 5 p.i., before mice show clinical signs of infection, nucleoprotein mRNA levels in both cortex and cerebellum are variable. At day 8 p.i., virus nucleoprotein-specific mRNA levels in these regions are significantly higher in mice exhibiting neurological signs of rabies than in healthy mice, despite the fact that several of the latter are expected to eventually develop clinical signs of infection and die (Fig. 2A). However, neither the accumulation in the CNS tissues of mRNAs specific for immune cells, CD4 and CD8 T cells, and B cells (Fig. 2B) nor serum RV-specific IgG antibodies in the circulation (Fig. 2C) differ between healthy and sick mice on day 8 p.i.
FIG. 2.
Comparison of the virus titer and the immune response between SHBRV-infected PLSJL mice with or without clinical signs of rabies. Groups of PLSJL mice (n = 20) were infected with SHBRV and monitored for morbidity and mortality. Five and eight days after infection, groups of mice were euthanized to assess the SHBRV nucleoprotein-specific mRNA levels in the cortex and the cerebellum. Amount of virus was compared between mice with (sick) or without (healthy) clinical signs of rabies at these time points (A). Levels of mRNAs specific for CD4, CD8, and κ-light chain (κ-LC) in the cortex and the cerebellum (B) and RV antigen-specific serum IgG titer of healthy and sick mice on day 8 p.i. (C) were measured by QRT-PCR and ELISA, respectively. Infection, mRNA analysis by QRT-PCR, and ELISA were performed as described in Materials and Methods. Statistically significant differences in the amount of virus-specific mRNA levels between groups of healthy and sick mice were determined by the Mann-Whitney test and are denoted by the symbol *** (P < 0.001). No statistical differences were found in immune cell markers and in antibody levels between healthy and sick mice.
The survival of SHBRV-infected PLSJL mice is improved by enhancing the CNS inflammatory response and the delivery of immune effectors to the CNS tissues.
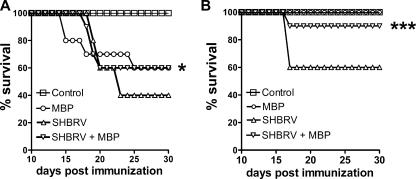
The above-mentioned results suggest that immune effector cells reach the CNS tissues of SHBRV-infected PLSJL mice and that death results in animals where virus levels in the CNS cannot be contained. If this is the case, increased immune cell infiltration into the CNS tissues may support the clearance of higher levels of virus, thereby allowing more animals to survive the infection. To test this hypothesis, we assessed whether or not the induction of extensive CNS inflammation would promote the survival of SHBRV-infected PLSJL mice. PLSJL mice were immunized with MBP using a protocol that induces elevated BBB permeability and, often, the autoimmune CNS inflammatory disease EAE within 16 to 22 days (15). Ten days after MBP immunization, mice were infected with SHBRV, the timing being such that extensive CNS inflammation would occur, concomitant with the development of an antiviral response. Significantly higher numbers of SHBRV-infected PLSJL mice survive the infection when they have been immunized with MBP to induce a stronger CNS inflammatory response (Fig. 3A and B). The incidence and severity of EAE in PLSJL mice following MBP immunization are variable (15, 26). Thus, in the experiment shown in Fig. 3A, where 40% of MBP-immunized but uninfected mice died of EAE, we cannot be certain as to whether the MBP-immunized, SHBRV-infected mice died of EAE or rabies. However, when MBP immunization did not cause lethal EAE, most of the immunized/infected mice also survived (Fig. 3B).
FIG. 3.
Effect of MBP immunization on survival of SHBRV-infected PLSJL mice. Groups of PLSJL mice (n = 20) were immunized with MBP as described in Materials and Methods. Ten days following immunization, half of the immunized mice (n = 10) as well as 10 nonimmunized mice were infected with SHBRV and monitored for mortality. The results expressed are from the two out of four independent experiments that exhibited the maximum (A) and minimum (B) mortality in groups of nonimmunized mice infected with SHBRV. Statistically significant differences in survival between groups of SHBRV-infected mice with or without MBP immunization were determined by the Mann-Whitney test and are denoted by the symbols * (P < 0.05) and *** (P < 0.001).
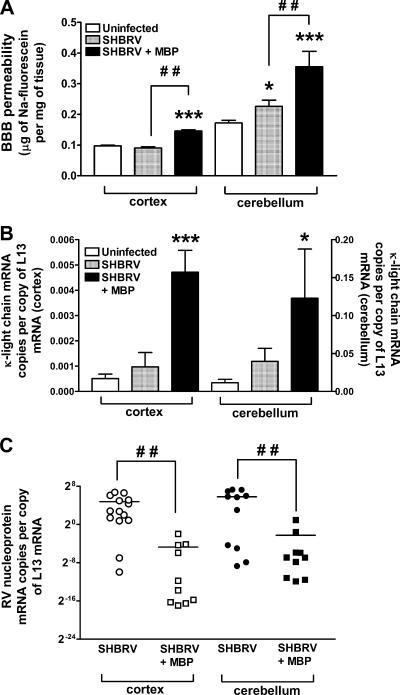
To provide further insight into why MBP immunization is therapeutic in SHBRV-infected mice, we next determined whether the increased survival of MBP-immunized, SHRBV-infected animals is a consequence of the enhanced delivery of immune effectors to CNS tissues and an elevated capacity to clear the virus. As shown in Fig. 4A, SHBRV-infected mice immunized with MBP develop significantly enhanced BBB permeability to Na-fluorescein compared to similarly infected mice that were not immunized. The production of antibodies by invading B cells, as evidenced by κ-light chain-specific mRNA levels, is also elevated in the CNS tissues of the immunized/infected mice (Fig. 4B), and levels of SHBRV nucleoprotein-specific mRNA are reduced (Fig. 4C).
FIG. 4.
BBB permeability and immune cell accumulation in the CNS tissues of SHBRV-infected PLSJL mice. (A) BBB permeability in the cortex and the cerebellum was assessed by measuring Na-fluorescein leakage from the circulation into CNS tissues in groups of PLSJL mice that were either uninfected (Uninfected), infected with SHBRV 8 days previously (SHBRV), or infected with SHBRV 8 days previously and immunized with MBP 10 days before infection (SHBRV + MBP). Infection and assessment of BBB permeability were performed as described in Materials and Methods. Levels of mRNA specific for κ-light chain (κ-LC) (B) and RV nucleoprotein (C) in the cortex and the cerebellum of these mice were measured by QRT-PCR as detailed in Materials and Methods. Statistically significant differences determined by the Mann-Whitney test between infected and uninfected control groups are denoted by the symbols * (P < 0.05) and *** (P < 0.001) and between infected mice with or without immunization are denoted by the symbol # # (P < 0.01). (Ten mice were used for each group.)
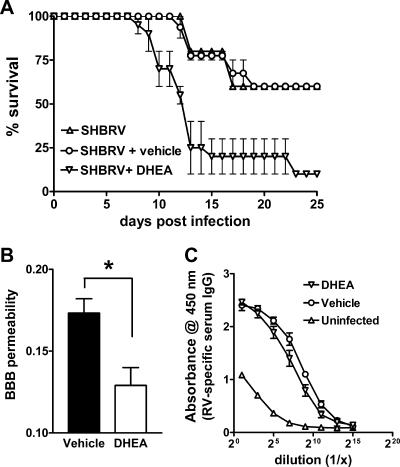
Inhibition of BBB permeability changes leads to death of SHBRV-infected PLSJL mice.
In our studies of SHBRV-infected 129/SvEv mice, we concluded that the inability to open the BBB is the critical defect leading to the death of the animals, despite the development of a strong antiviral immune response (23). We hypothesize that PLSJL mice survive the infection because of an elevated capacity to enhance BBB permeability and deliver immune effectors to the CNS due to the reduced production of regulatory steroid hormones. To confirm whether this may be the case, SHBRV-infected PLSJL mice were treated with the steroid hormone DHEA. DHEA treatment caused an increase in the death rate of SHBRV-infected PLSJL mice from 40 to 90 percent (Fig. 5A). Elevations in BBB permeability were also significantly reduced by DHEA (Fig. 5B). However, no major difference was observed in serum RV-specific IgG levels between DHEA- and vehicle-treated mice (Fig. 5C).
FIG. 5.
Effect of DHEA on BBB permeability and survival of SHBRV-infected PLSJL mice. Groups of PLSJL mice (n = 30) were infected with SHBRV and either left untreated or treated with DHEA or its vehicle dimethyl sulfoxide by daily subcutaneous injection, as described in Materials and Methods. Mice were monitored for mortality (A) or euthanized 8 days following infection to assess BBB permeability in the cerebellum (B) and RV-specific IgG level in serum (C), as described in Materials and Methods. Statistically significant differences determined by the Mann-Whitney test between DHEA- and vehicle-treated groups are denoted by the symbol * (P < 0.05).
DISCUSSION
The observation that individuals dying of rabies often have virus-neutralizing antibodies in their circulation suggests that the mere presence of antiviral immunity may not be enough to protect an individual from the disease (14). This is consistent with the fact that rabies postexposure prophylaxis, which is a combination of active and passive immunization, fails to protect individuals from a lethal outcome when administered after clinical signs of rabies appear (6, 32). We have previously provided evidence that the failure to clear SHBRV from the CNS tissues of 129/SvEv mice may be a consequence of the maintenance of BBB integrity, which prevents circulating RV-specific immune effectors from reaching infected CNS tissues (23). In the current study, we confirm this hypothesis by showing that increasing BBB permeability facilitates the infiltration of immune effectors into the infected CNS, thereby reducing the lethality of SHBRV infection.
The development of enhanced BBB permeability and a CNS inflammatory response is required for the clearance of attenuated RV from the infected CNS (21). Mice with an SJL background are known to develop more extensive CNS inflammation than mice of other backgrounds when subjected to similar stimuli (22, 28). Notably, with respect to the current investigation, mice with an SJL background more often survive infection with pathogenic RV variants than other mouse strains (16, 17). We found that a high percentage of PLSJL mice indeed survive infection with a dose of SHRBV that is uniformly lethal for 129/SvEv mice. More importantly, the ability of PLSJL mice to survive the infection is associated with their greater capacity to mediate BBB permeability changes and deliver immune effectors to the CNS. Enhancement in BBB permeability and invasion of immune effectors in 129/SvEv mice infected with attenuated RV occurs primarily in the cerebellum (21). Consistent with this observation, in the current study, PLSJL mice only developed significantly enhanced BBB permeability in the cerebellum following SHBRV infection (Fig. 4A).
Despite their natural resistance to lethal SHBRV infection, a significant proportion of SHBRV-infected PLSJL mice die of rabies. At day 8 p.i., mice with clinical signs of rabies that are destined to die invariably had higher virus titers in CNS tissues than their similarly infected but healthy counterparts that often survive. Differences in the extent of the CNS adaptive immune response are unlikely to be responsible for the variation in the levels of virus in the CNS of dying and healthy mice for several reasons: (i) the accumulation of mRNAs specific for immune cell subsets does not significantly differ between these two groups, (ii) similar variability is seen in the absence of immune cell infiltration in SHBRV-infected 129/SvEv mice at day 8 p.i., and (iii) variable levels of virus replication are seen in the CNS tissues of PLSJL mice at day 5 p.i., which is prior to the onset of BBB permeability changes (A. Roy and D. C. Hooper, unpublished data). Thus, for reasons that are not fully understood, the extent of virus replication in the CNS tissues of the mice varies with high levels predictive of death. Consequently, we speculate that at day 8 p.i., the levels of virus in the CNS tissues of some of the SHBRV-infected PLSJL mice are too high for the infiltrating immune effectors to clear.
If a proportion of SHBRV-infected PLSJL mice die because virus replication overwhelms the immune effectors reaching the CNS, enhancing immune effector infiltration into the CNS tissue may be expected to increase the capacity of the animal to clear the virus and improve survival. To test this hypothesis, we used an autoimmune CNS inflammatory response to increase BBB permeability and immune cell infiltration into the CNS tissues of SHBRV-infected PLSJL mice. This resulted in a higher percentage of the mice surviving despite the likelihood that some died of autoimmune encephalomyelitis. This confirms that the clearance of SHBRV from the CNS is primarily limited by the delivery of immune effectors to the CNS tissues rather than that the extent of the antiviral response that develops in the periphery and promoting immune effector infiltration into the CNS tissues of the SHBRV-infected host is therapeutic.
Virus replication in the CNS can stimulate the production of steroid hormones through effects on the HPA axis (4, 27). It is known that pathogenic strains of RV infect the hypothalamus and alter HPA activity (24, 29), resulting in the elevated production of steroid hormones and the reduced production of growth hormones (29). These changes may be responsible for the lymphoid organ atrophy and altered immune function (29, 31) seen during the late stages of rabies. Compared to other strains, mice with an SJL background (i) have reduced HPA axis activity (2), (ii) develop more extensive CNS inflammation in response to various stimuli (2, 22), and (iii) are resistant to lethal infection with RV (16, 17). These facts led us to speculate that the survival of PLSJL mice from a normally lethal dose of SHBRV may be due, at least in part, to the reduced production of steroid hormones as a result of a blunted HPA axis activity. DHEA is particularly interesting in this regard, as it is one of the most abundant hormones produced under HPA axis control (19) and is known to inhibit CNS inflammation (10). Administration of DHEA to PLSJL mice, while having no effect on the ability to produce RV-specific IgG, limited BBB permeability elevation and caused increased mortality following SHBRV infection. These findings suggest that the higher levels of DHEA expected in mice with normal HPA axis activity may contribute to increased SHBRV lethality by reducing BBB permeability changes.
Our findings indicate that the delivery of immune effectors to the CNS can reverse the outcome of an otherwise lethal RV infection even after the virus has reached the CNS. Certainly the induction of autoimmune CNS inflammation to open the BBB and deliver RV-specific immune effectors to CNS tissues is not an appropriate therapy for humans. However, the utilization of a mechanism to enhance BBB permeability that is unaffected by SHBRV infection may be crucial to the rescue of an individual who has been diagnosed with rabies based on the appearance of clinical signs. We expect that this may also apply to other CNS diseases where the delivery of therapeutic agents across the BBB is necessary.
Acknowledgments
This work was supported by National Institutes of Health grants AI09706 and AI060005.
We thank Timothy W. Phares and Rhonda B. Kean for helpful discussion and contribution to this work.
Footnotes
Published ahead of print on 16 May 2007.
REFERENCES
- 1.Andersen, I. H., O. Marker, and A. R. Thomsen. 1991. Breakdown of blood-brain barrier function in the murine lymphocytic choriomeningitis virus infection mediated by virus-specific CD8+ T cells. J. Neuroimmunol. 31:155-163. [DOI] [PubMed] [Google Scholar]
- 2.Andreini, I., C. Getuli, V. Pacelli, R. Manno, E. Ragazzoni, A. Nunziata, and P. Navarra. 2002. Function of the hypothalamus pituitary adrenal axis and humoral immune mechanisms during experimental allergic encephalomyelitis in SJL/J mice. Neuroimmunomodulation 10:9-16. [DOI] [PubMed] [Google Scholar]
- 3.Auphan, N., J. A. DiDonato, C. Rosette, A. Helmberg, and M. Karin. 1995. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science 270:286-290. [DOI] [PubMed] [Google Scholar]
- 4.Bailey, M., H. Engler, J. Hunzeker, and J. F. Sheridan. 2003. The hypothalamic-pituitary-adrenal axis and viral infection. Viral Immunol. 16:141-157. [DOI] [PubMed] [Google Scholar]
- 5.Baulieu, E. E., and P. Robel. 1998. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEA-S) as neuroactive neurosteroids. Proc. Natl. Acad. Sci. USA 95:4089-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1999. Human rabies prevention-United States, 1999. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. Recommend. Rep. 48:1-21. [PubMed] [Google Scholar]
- 7.Champion, J. M., R. B. Kean, C. E. Rupprecht, A. L. Notkins, H. Koprowski, B. Dietzschold, and D. C. Hooper. 2000. The development of monoclonal human rabies virus neutralizing antibodies as a substitute for pooled human immune globulin in the prophylactic treatment of rabies virus exposure. J. Immunol. Methods 235:81-90. [DOI] [PubMed] [Google Scholar]
- 8.Dietzschold, B., K. Morimoto, D. C. Hooper, J. S. Smith, C. E. Rupprecht, and H. Koprowski. 2000. Genotypic and phenotypic diversity of rabies virus variants involved in human rabies: implications for postexposure prophylaxis. J. Hum. Virol. 3:50-57. [PubMed] [Google Scholar]
- 9.Dillon, J. S. 2005. Dehydroepiandrosterone, dehydroepiandrosterone sulfate and related steroids: their role in inflammatory, allergic and immunological disorders. Curr. Drug Targets Inflamm. Allergy 4:377-385. [DOI] [PubMed] [Google Scholar]
- 10.Du, C., M. W. Khalil, and S. Sriram. 2001. Administration of dehydroepiandrosterone suppresses experimental allergic encephalomyelitis. J. Immunol. 167:7094-7101. [DOI] [PubMed] [Google Scholar]
- 11.Gloor, S. M., M. Wachtel, M. F. Bolliger, H. Ishihara, R. Landmann, and K. Frei. 2001. Molecular and cellular permeability control at the blood-brain barrier. Brain Res. Rev. 36:258-264. [DOI] [PubMed] [Google Scholar]
- 12.Hooper, D. C., R. B. Kean, G. Scott, S. Spitsin, T. Mikheeva, K. Morimoto, M. Bette, A. Rohrenbeck, B. Dietzschold, and E. Weihe. 2001. The central nervous system inflammatory response to neurotropic virus infection is peroxynitrite dependent. J. Immunol. 167:3470-3477. [DOI] [PubMed] [Google Scholar]
- 13.Hughes, G. J., J. S. Smith, C. A. Hanlon, and C. E. Rupprecht. 2004. Evaluation of a TaqMan PCR assay to detect rabies virus RNA: influence of sequence variation and application to quantification of viral loads. J. Clin. Microbiol. 42:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasempimolporn, S., T. Hemachudha, P. Khawplod, and S. Manatsathit. 1991. Human immune response to rabies nucleocapsid and glycoprotein antigens. Clin. Exp. Immunol. 84:195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kean, R. B., S. Spitsin, T. Mikheeva, G. Scott, and D. C. Hooper. 2000. The peroxynitrite scavenger uric acid prevents inflammatory cell invasion into the CNS in experimental allergic encephalomyelitis through maintenance of blood-CNS barrier integrity. J. Immunol. 165:6511-6518. [DOI] [PubMed] [Google Scholar]
- 16.Lodmell, D. L. 1983. Genetic control of resistance to street rabies virus in mice. J. Exp. Med. 157:451-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lodmell, D. L., and L. C. Ewalt. 1985. Pathogenesis of street rabies virus infections in resistant and susceptible strains of mice. J. Virol. 55:788-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messenger, S. L., J. S. Smith, L. A. Orciari, P. A. Yager, and C. E. Rupprecht. 2003. Emerging pattern of rabies deaths and increased viral infectivity. Emerg. Infect. Dis. 9:151-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orentreich, N., J. L. Brind, J. H. Vogelman, R. Andres, and H. Baldwin. 1992. Long-term longitudinal measurement of plasma dehydroepiandrosterone sulfate in normal men. J. Clin. Endocrinol. Metab. 75:1002-1004. [DOI] [PubMed] [Google Scholar]
- 20.Padgett, D. A., R. M. Loria, and J. F. Sheridan. 2000. Steroid hormone regulation of antiviral immunity. Ann. N. Y. Acad. Sci. 917:935-943. [DOI] [PubMed] [Google Scholar]
- 21.Phares, T. W., R. B. Kean, T. Mikheeva, and D. C. Hooper. 2006. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J. Immunol. 176:7666-7675. [DOI] [PubMed] [Google Scholar]
- 22.Rowell, J. F., and D. E. Griffin. 1999. The inflammatory response to nonfatal Sindbis virus infection of the nervous system is more severe in SJL than in BALB/c mice and is associated with low level of IL-4 mRNA and high level of IL-10-producing CD4+ T cells. J. Immunol. 162:1624-1632. [PubMed] [Google Scholar]
- 23.Roy, A., T. W. Phares, H. Koprowski, and D. C. Hooper. 2007. Failure to open the blood-brain barrier and deliver immune effectors to the CNS tissues leads to the lethal outcome of silver-haired bat rabies virus infection. J. Virol. 81:1110-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savino, W., E. Artz, and M. Dardenne. 1999. Immunoneuroendocrine connectivity: the paradigm of the thymus-hypothalamus/pituitary axis. Neuroimmunomodulation 6:126-136. [DOI] [PubMed] [Google Scholar]
- 25.Scott, G. S., R. Kean, M. Fabis, T. Mikheeva, C. Brimer, T. Phares, S. Spitsin, and D. C. Hooper. 2004. ICAM-1 upregulation in the spinal cords of PLSJL mice with experimental allergic encephalomyelitis is dependent upon TNF-α production triggered by the loss of blood-brain barrier integrity. J. Neuroimmunol. 155:32-42. [DOI] [PubMed] [Google Scholar]
- 26.Scott, G. S., S. V. Spitsin, R. B. Kean, T. Mikheeva, H. Koprowski, and D. C. Hooper. 2002. Therapeutic intervention in experimental allergic encephalomyelitis by administration of uric acid precursors. Proc. Natl. Acad. Sci. USA 99:16303-16308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silverman, M. N., B. D. Pearce, C. A. Biron, and A. H. Miller. 2005. Immune modulation of the hypothalamus-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol. 18:41-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh, A. K., J. Q. Yang, V. V. Parekh, J. Wei, C. R. Wang, S. Joyce, R. R. Singh, and L. Van Kaer. 2005. The natural killer cell ligand alpha-galactosylceramide prevents or promotes pristine-induced lupus in mice. Eur. J. Immunol. 35:1143-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torres-Anjel, M. J., D. Volz, M. J. R. Torres, M. Turk, and J. G. Tshikuka. 1988. Failure to thrive, wasting syndrome, and immunodeficiency in rabies: a hypophysical/hypothalamic/thymic axis effect of rabies virus. Rev. Infect. Dis. 10(s4):710-725. [DOI] [PubMed] [Google Scholar]
- 30.Valtysdottir, S. T., L. Wide, and R. Hallgren. 2001. Low serum dehydroepiandrosterone sulfate in women with primary Sjogren's syndrome as an isolated sign of impaired HPA axis function. J. Rheumatol. 28:1259-1265. [PubMed] [Google Scholar]
- 31.Wiktor, T. J., P. C. Doherty, and H. Koprowski. 1977. Suppression of cell-mediated immunity by street rabies virus. J. Exp. Med. 145:1617-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization Expert Committee on Rabies. 1992. Eighth report. WHO Tech. Rep. Ser. 824:1-84. [PubMed] [Google Scholar]