Abstract
Human metapneumovirus (hMPV) is a recently discovered paramyxovirus that is a major cause of lower-respiratory-tract disease. hMPV is associated with more severe disease in infants and persons with underlying medical conditions. Animal studies have shown that the hMPV fusion (F) protein alone is capable of inducing protective immunity. Here, we report the use of phage display technology to generate a fully human monoclonal antibody fragment (Fab) with biological activity against hMPV. Phage antibody libraries prepared from human donor tissues were selected against recombinant hMPV F protein with multiple rounds of panning. Recombinant Fabs then were expressed in bacteria, and supernatants were screened by enzyme-linked immunosorbent assay and immunofluorescent assays. A number of Fabs that bound to hMPV F were isolated, and several of these exhibited neutralizing activity in vitro. Fab DS7 neutralized the parent strain of hMPV with a 60% plaque reduction activity of 1.1 μg/ml and bound to hMPV F with an affinity of 9.8 ×10−10 M, as measured by surface plasmon resonance. To test the in vivo activity of Fab DS7, groups of cotton rats were infected with hMPV and given Fab intranasally 3 days after infection. Nasal turbinates and lungs were harvested on day 4 postinfection and virus titers determined. Animals treated with Fab DS7 exhibited a >1,500-fold reduction in viral titer in the lungs, with a modest 4-fold reduction in the nasal tissues. There was a dose-response relationship between the dose of DS7 and virus titer. Human Fab DS7 may have prophylactic or therapeutic potential against severe hMPV infection.
Human metapneumovirus (hMPV) is a recently described respiratory pathogen that is a major cause of upper- and lower-respiratory-tract infection in children and adults worldwide (5, 25, 26, 72, 81, 83). hMPV is related genetically to respiratory syncytial virus (RSV), which is the most significant viral respiratory pathogen of infancy and early childhood. Epidemiologic studies showed that hMPV is associated with significant morbidity in young infants and other high-risk populations, such as immunocompromised cancer and transplant patients and those with underlying conditions, including prematurity, asthma, and cardiopulmonary disease (4, 6, 10, 26-28, 30, 36, 47, 50, 52, 57, 71, 76, 80, 82). Hospitalization rates due to hMPV infection in previously healthy infants and in these high-risk groups are comparable to those caused by other common respiratory viruses, such as RSV, parainfluenzavirus (PIV), and influenza virus (4, 6, 20, 25, 26, 28, 29, 51, 74, 81). There is currently no licensed vaccine for hMPV. Several groups have published preclinical studies of candidate live attenuated hMPV vaccines generated using reverse genetics (8, 63, 64, 66, 67). However, live attenuated vaccines for use in infants face many obstacles to successful implementation, including safety concerns, difficulties achieving the appropriate balance between attenuation and immunogenicity, and poor immune response due to immunological immaturity of the neonate. Longstanding efforts to develop live attenuated vaccines against RSV and PIV attest to these obstacles (11, 13, 16, 21, 43, 53).
The hMPV fusion (F) protein is likely the most important target of protective immunity. Sequence analysis of the hMPV F protein shows that it is related to other paramyxovirus fusion proteins and appears to have homologous regions that likely have similar functions. Paramyxovirus fusion proteins are synthesized as inactive precursors (F0) that are cleaved by host cell proteases into the biologically fusion-active F1 and F2 domains. hMPV F contains one putative cleavage site that is highly conserved, as well as fusion peptide and heptad repeat domains. Recent data suggest that hMPV F alone expressed from transfected cDNA is capable of mediating cell-cell fusion (61). Fusion proteins are major antigenic determinants for all known paramyxoviruses and for other viruses that possess similar fusion proteins, such as human immunodeficiency virus, influenza virus, and Ebola virus. Two groups have shown that hMPV F expressed in a chimeric, live attenuated PIV vaccine is immunogenic and protective in rodents (64, 67). We previously generated recombinant hMPV F protein that was immunogenic and protective in cotton rats (17).
In the absence of a licensed vaccine, another option for prophylaxis or treatment of severe respiratory viral infections is to provide passive immunity in the form of neutralizing antibodies. Animal studies have shown the feasibility of this approach against RSV and PIV using both polyclonal and monoclonal antibodies (MAbs) (32, 33, 37, 55, 56, 62, 68, 89). Subsequent human trials of human polyclonal and humanized mouse MAbs against RSV showed protective efficacy in the prevention of severe lower-respiratory-tract disease and hospitalization (35, 54, 77, 79). Passive antibodies also have shown efficacy in the treatment of severe disease, especially in high-risk and immunocompromised persons (19, 24, 32, 34, 38, 44, 75). Most currently licensed MAbs are chimeric or humanized mouse immunoglobulin molecules. Although severe reactions such as anaphylaxis are less common with chimeric or humanized MAbs than with murine MAbs, human anti-chimeric-antibody and anti-humanized-antibody responses still occur, causing adverse reactions and limiting therapeutic efficacy (1, 41, 65). Thus, human MAbs are a preferred therapeutic choice, and the development of fully human MAbs remains a key goal of antibody research.
The preparation of combinatorial phage display libraries from variable heavy- and light-chain antibody genes provides an efficient method for the isolation of human antibody Fabs. The construction of antibody libraries on the surface of M13 phage and their application for the generation of human MAbs against numerous viruses have been described in several reports (2, 3, 9, 14, 15, 18, 60, 86). Many of these studies reported the isolation of antibodies that neutralize virus in vitro and in vivo. In the present study, we describe the development of fully human MAbs against hMPV F protein, using phage display technology. Several of these Fabs exhibited in vitro neutralizing activity, and the clone with the highest in vitro efficacy was effective therapeutically in the cotton rat model.
MATERIALS AND METHODS
HMPV F ectodomain expression in mammalian cells.
We previously generated a soluble, expression construct of the hMPV F gene truncated so as to remove the transmembrane (TM) domain. We used RT-PCR to amplify a full-length F sequence from a pathogenic clinical isolate designated TN/92-4, a prototype genogroup A2 strain according to the proposed nomenclature (73). The full TN/92-4 F sequence was sequence optimized by a commercial source (Aptagen) to alter suboptimal codon usage for mammalian tRNA bias, improve secondary mRNA structure, and remove AT-rich regions, increasing mRNA stability. We then generated an expression vector encoding the hMPV F ectodomain construct lacking the TM domain (pcDNA3.1-FΔTM). The optimized full-length cDNA of the F gene was PCR amplified with the primers 5′-GGAGGTACCATGAGCTGGAAG-3′ and 5′-GAAGCGGCCGCTGCCCTTCTC-3′, and the PCR product was digested and ligated into the KpnI/NotI sites (restriction sites are underlined) of the vector pcDNA3.1/myc-His B (Invitrogen). The pcDNA3.1-FΔTM recombinant plasmid was transfected into a suspension of 293-F cells (Freestyle 293 expression system; Invitrogen). At 96 h posttransfection, cells were centrifuged for 5 min at 100 × g at room temperature and the supernatant harvested. Supernatant was filtered through 0.2-μm filters before purification.
Purification of His6-tagged F ectodomain.
Protein purification was performed on an ÁKTA fast protein liquid chromatography system controlled by UNICORN 4.12 software (GE Healthcare). The His-tagged F ectodomain FΔTM was purified by immobilized metal ion-affinity chromatography using prepacked HisTrap Ni-Sepharose columns (GE Healthcare). Sample was diluted with concentrated binding buffer stock to adjust pH, salt, and imidazole concentrations before purification. Protein was loaded on a 5-ml HisTrap column with a loading flow rate of 5.0 ml/min, and the binding buffer contained 20 mM sodium phosphate, 0.5 M NaCl, and 30 mM imidazole (pH 7.4). Unrelated proteins were eluted in elution step 1 using 4 column volumes of 8% elution buffer, and the His6-tagged F protein was eluted in elution step 2 with 4 column volumes of 25% elution buffer. The elution buffer contained 20 mM sodium phosphate, 0.5 M NaCl, and 500 mM imidazole (pH 7.4). Purified protein was concentrated and dialyzed against phosphate-buffered saline (PBS) (Invitrogen) through Amicon Ultra centrifugal filters with molecular weight cutoffs of 30,000 and 100,000 (Millipore).
Construction and selection of antibody phage display libraries.
Antibody Fab immunoglobulin G1 (IgG1) (κ and/or λ chain) phage display libraries were cloned from the bone marrow tissue of 12 donors as described elsewhere (2, 86). Libraries ranged in size from 3 × 106 to 5 × 107 members. Libraries were selected individually against recombinant hMPV F protein bound to enzyme-linked immunosorbent assay (ELISA) wells using a biopanning procedure described in reference 2. Selected phage recovered from the fourth or fifth round of panning were converted to a soluble Fab expression system (2), and clones were tested individually for reactivity with the recombinant hMPV F protein-selecting antigen. Selected hMPV F protein-reactive Fab clones were purified by immunoaffinity chromatography (86).
Immunofluorescent assays.
LLC-MK2 cell culture monolayers were infected with hMPV at a multiplicity of infection of 1. At 24 h after infection, cells were fixed with 10% buffered formalin, washed with PBS-Tween (PBS-T), and then incubated with either Fabs or anti-hMPV serum (diluted 1:500) in PBS-T-milk for 1 h at 37°C. After washing with PBS-T, cells were stained with AlexaFluor568-conjugated goat anti-guinea pig Ig or AlexaFluor568-conjugated mouse anti-Fab antibody diluted 1:1,000 (Molecular Probes) in PBS-T-milk for 1 h at 37°C. Cell monolayers were examined on an inverted Nikon Diaphot microscope and images captured with a Nikon D100 digital camera. Images were cropped and figures constructed using Adobe Photoshop and Illustrator without digital adjusting or reprocessing of images.
In vitro neutralization assays.
hMPV-neutralizing titers were determined by a plaque reduction assay as described elsewhere (84), with the following modifications. Fab suspensions in serial fourfold dilutions, starting with no dilution, were incubated with a working stock of hMPV diluted to yield 50 plaques per well in a 24-well plate. The Fab and virus mixture was incubated for 1 h at 37°C with rotation. The Fab-virus mixtures then were plated in triplicate on LLC-MK2 monolayers in 24-well culture plates and allowed to adsorb at room temperature for 1 h. Wells were then overlaid with 0.75% methylcellulose in OptiMEM supplemented with trypsin and incubated at 37°C in a CO2 incubator for 4 days. Monolayers were rinsed, formalin-fixed, and stained with guinea pig anti-hMPV serum and peroxidase-labeled goat anti-guinea pig Ig as previously described (84). Plaques were counted, and 60% plaque reduction titers were calculated. hMPV-positive human serum was used as a positive control in all assays.
Genetic analysis of Fab clones.
The light-chain and heavy-chain variable-region sequences of hMPV-reactive antibody Fab clones were determined as described elsewhere (86). We analyzed VH or VL region sequences with the international ImMunoGeneTics database (http://imgt.cines.fr/) using the junction analysis program, reporting results with an updated nomenclature of the human Ig genes as recently summarized (31, 58). All VH and VL segment assignments were reviewed and confirmed by manual inspection. Mutations in the junction region were manually confirmed, and mutations in the remaining regions were manually scored and tabulated.
Competition capture ELISA for epitope mapping.
Fabs ACN044, AC59, LL01, DS1, DS6, and DS7 were biotinylated with a biotin labeling kit (Roche, Mannheim, Germany) according to the manufacturer's instructions. Different Fabs against hMPV FΔTM recombinant protein were bound to ELISA plates overnight at 4°C (0.5 μg/well in 50 μl PBS). Fab B12, recognizing human immunodeficiency virus, was used as a negative control. The wells were blocked at 37°C for 1 h with 1% bovine serum albumin (BSA) in PBS. Then diluted hMPV FΔTM protein was added to each well (0.2 μg/well in 50 μl 1% BSA in PBS), and plates were incubated at 37°C for 1 h. After washes with PBS-T, different biotin-conjugated Fabs (0.5 μg/well in 50 μl 1% BSA in PBS) were added to each well, and then the plates were incubated at 37°C for 1 h. The plates were again washed with PBS-T and incubated with 1:1,000-diluted alkaline phosphatase-conjugated streptavidin (50 μl/well in 1% BSA; Pierce) for 1 h at 37°C. After washing, phosphatase activity was tested with p-nitrophenyl phosphate (0.1%, wt/vol) in 0.1 M NaHCO3 buffer (pH 9.8), 50 μl/well. Optical density values were read at 405 nm.
Surface plasmon resonance (SPR).
Kinetic analyses of hMPV F-specific-MAb binding to hMPV F protein was performed on a Biacore 2000 (Biacore AB, Uppsala, Sweden). Purified recombinant hMPV F or RSV F protein was diluted to 30 μg/ml in 10 mM sodium acetate, pH 4.5, and covalently immobilized at 5 μl/min by amine coupling to the dextran matrix of a CM5 sensor chip (Biacore AB) with a target density of 1,200 response units (RU). Since amine coupling results in random orientations of the coupled ligand to the surface of the chip, a surface density of 1,200 RU was needed to yield a maximum binding signal (Rmax) of ∼100 RU during the binding experiments. Unreacted active ester groups were blocked with 1 M ethanolamine. For use as a reference, a blank surface, containing no protein, was prepared under identical immobilization conditions. Purified hMPV F antibodies and an RSV-specific MAb, palivizumab (Synagis; MedImmune, Inc., Gaithersburg, MD), at different concentrations ranging from 5 to 500 nM in HBS/Tween-20 buffer (Biacore AB), were injected over the immobilized hMPV F protein, RSV F protein, or reference cell surfaces. Antibody binding was measured at a flow rate of 30 μl/min for 180 s, and dissociation was monitored for an additional 360 s. Residual bound antibody was removed from the sensor chip by pulsing 50 mM HCl at 100 μl/min for 30 s. Association rates (Kon), dissociation rates (Koff), and equilibrium dissociation constants (KD) were calculated by aligning the binding curves globally to fit a 1:1 Langmuir binding model using BIAevaluation 4.1 software (Biacore AB). The goodness of each fit was based on the agreement between experimental data and the calculated fits, where the χ2 values were below 1.0.
In vivo infection and Fab treatment.
Cotton rats were purchased at 5 to 6 weeks of age from a commercial breeder (Harlan, Indianapolis, IN), fed a standard diet and water ad libitum, and kept in microisolator cages. Animals were anesthetized by isoflurane inhalation prior to virus or Fab inoculation. The virus strain used was a pathogenic clinical isolate of hMPV designated TN/94-49, a genotype group A2 virus, according the proposed nomenclature (73). This virus stock was determined to have a titer of 3.5 × 106 PFU/ml by plaque titration in LLC-MK2 cell monolayer cultures. Cotton rats in groups of five to seven were inoculated intranasally on day 0 with 3.5 × 105 PFU in a volume of 100 μl. On day 3 postinfection, solutions of Fab were instilled intranasally. An irrelevant, similarly prepared Fab designated B12 was used at 1 or 4 mg/kg body weight. The hMPV F-specific Fab DS7 was used at 0.06, 0.25, 1, or 4 mg/kg body weight. All Fab concentrations were adjusted to a uniform volume of 100 μl, except for the B12 4-mg/kg dose, which was given in a 225-μl volume due to the lower concentration. On day 4 postinfection (24 h after Fab administration), the animals were sacrificed by CO2 asphyxiation and exsanguinated. Nasal and lung tissues were harvested separately, weighed individually for each animal, and homogenized immediately. The lungs were pulverized in ice-cold glass homogenizers, and nasal turbinates were ground with sterile sand in a cold porcelain mortar and pestle in 3 ml of ice-cold Hanks' balanced salt solution. Tissue homogenates were centrifuged at 4°C for 10 min at 300 × g, and the supernatants were collected, aliquoted into cryovials, and snap-frozen in liquid nitrogen. Virus yields were measured by plaque titration as previously described (84). The Vanderbilt Institutional Animal Care and Use Committee approved the study.
Statistical analysis.
Viral titers between control groups were compared with the Kruskal-Wallis test. Viral titers in each of the hMPV F-specific Fab DS7-treated groups were compared with the viral loads in the combined control groups using a Wilcoxon rank sum test. Linear regression was used to examine a dose-response relationship between Fab DS7 and viral titer. Controls were not included in the dose-response analysis. The doses were log2 transformed, since the doses were 2−4, 2−2, 20, and 22 mg/kg, and tissue virus titers were log10 transformed to minimize the effect of a non-Gaussian distribution. Viral assays in which plaques were not detected were assigned a titer at the detection limit of 5 PFU/g before log10 transformation. In this model, a line was fitted to the data, since we reasoned that with only four distinct dose levels, models that fit flexible curves to the data could be overfitting the data. Titers of experimental groups were expressed as geometric mean titers.
RESULTS
Recovery of hMPV F-specific monoclonal Fab fragments by phage library panning.
Phage antibody Fab display libraries prepared from bone marrow tissues of 12 donors were selected individually against recombinant hMPV F protein bound to ELISA wells. Twenty or thirty antibody Fab clones present after the fourth or fifth round of phage panning were evaluated in an ELISA for reactivity against the selecting antigen. Antigen-specific clones were isolated from 5 of the 12 donor libraries. Analysis of the Fab light-chain and heavy-chain DNA sequences of the specific Fabs identified 14 different clones with distinct sequences (see Table S1 in the supplemental material).
Immunofluorescent detection of hMPV-infected cells.
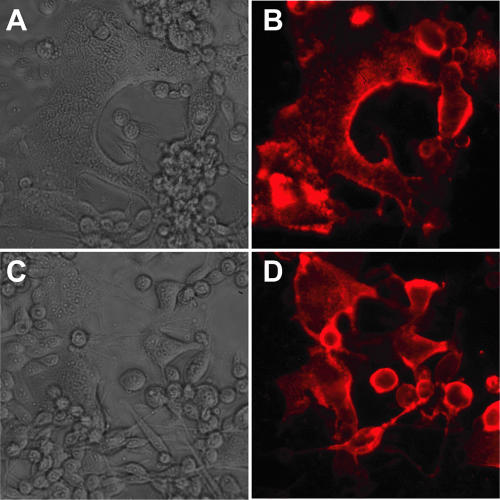
Bacterial supernatants from 14 Fab clones that specifically bound hMPV FΔTM by ELISA screening were tested further by immunofluorescent assays for the ability to bind specifically to hMPV-infected cell monolayers. Of the 14 Fab antibodies tested, 12 exhibited specific binding to hMPV-infected cells, and 1 of these 12 clones is shown (Fig. 1A and B). Several Fabs exhibited neutralizing activity in vitro (see below) and were purified from bacterial supernatants. These purified Fabs also bound to hMPV-infected cells, and one is shown (Fig. 1C and D). The F-specific Fabs detected both syncytia and single infected cells in a membrane-distributed pattern consistent with the expected localization of F protein. The pattern of fluorescence was similar to that seen previously with staining of hMPV-infected cells with polyclonal serum and cells transfected with cDNA encoding hMPV F alone (17). Fab clones that detected hMPV by immunofluorescence were tested further for in vitro neutralizing ability.
FIG. 1.
Light-microscopic and immunofluorescent images of hMPV-infected LLC-MK2 cell monolayers stained with human anti-hMPV F Fab and AlexaFluor568-conjugated goat anti-human IgG. (A and B) Fab ACN044; (C and D) Fab DS7. Magnification, ×20.
In vitro neutralization.
The majority of the Fab clones tested as bacterial supernatants did not exhibit in vitro neutralizing activity even at a 1:20 dilution. However, several clones showed activity at dilutions ranging from 1:55 to 1:65 (data not shown). These clones were expressed, purified, and then retested for neutralizing activity as purified Fabs (Table 1). The neutralizing titers of these Fabs ranged from 1:55 to 1:1,114. Adjustment for Fab concentration and calculation of specific neutralizing activity (i.e., the minimal concentration needed to accomplish 60% plaque reduction) showed that the specific neutralizing activities ranged from 1.1 μg/ml to 3.2 μg/ml (Table 1). Nonspecific Fabs generated against irrelevant antigens did not show neutralizing activity. We further tested the Fabs for their ability to neutralize viruses from each of the four major hMPV genetic lineages in a separate set of experiments. All Fabs had similar activity against the A2 strain as in previous testing (Table 2). However, DS1, DS6, and ACN044 did not exhibit activity against viruses from the other three lineages. DS7 neutralized A1 and B1 viruses at concentrations of 2.4 μg/ml and 9.8 μg/ml, respectively, similar to the specific activity against A2 virus. However, DS7 failed to neutralize B2 virus even at a concentration of 59 μg/ml.
TABLE 1.
In vitro neutralizing specific activity of selected Fabs against hMPV A2 strain TN/94-49a
| Fab | Concn (μg/ml) | Neutralizing dilution | Neutralizing concn (μg/ml) |
|---|---|---|---|
| DS1 | 160 | 1:65 | 2.5 |
| DS6 | 178 | 1:55 | 3.2 |
| DS7 | 1,180 | 1:1,114 | 1.1 |
| Han09 | 66 | <1:20 | NAb |
| ACN044 | 191 | 1:144 | 1.3 |
| B12c | 1,500 | <1:20 | NA |
Neutralizating activity is defined as the minimal concentration needed to accomplish 60% plaque reduction.
B12 is a human Fab directed against an irrelevant antigen.
NA, not applicable.
TABLE 2.
In vitro neutralizing specific activity of selected Fabs against hMPV strains from each genetic lineagea
| Fab | Concn (μg/ml) | Neutralizing activity against lineage
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| A1
|
A2
|
B1
|
B2
|
||||||
| Dilution | Concn (μg/ml) | Dilution | Concn (μg/ml) | Dilution | Concn (μg/ml) | Dilution | Concn (μg/ml) | ||
| DS1 | 160 | <1:20 | NA | 1:39 | 4.1 | <1:20 | NA | <1:20 | NA |
| DS6 | 178 | <1:20 | NA | 1:84 | 2.1 | <1:20 | NA | <1:20 | NA |
| DS7 | 1,180 | 1:120 | 9.8 | 1:1,042 | 1.1 | 1:488 | 2.4 | <1:20 | NA |
| ACN044 | 191 | <1:20 | NA | 1:86 | 2.2 | <1:20 | NA | <1:20 | NA |
In vitro neutralization assays were performed as described in the text. Strains used: A1, TN/96-12; A2, TN/94-49; B1, TN/982/42; B2, TN/994/19. NA, not applicable.
Variable gene segment usage and mutations.
The hMPV FΔTM-specific Fabs utilized a number of VH gene segments (see Table S1 in the supplemental material). VH3-23 was present in only one clone, despite being the most commonly used VH segment in the adult random circulating B-cell repertoire (7, 12, 78). VH1-03, which is utilized by fewer than 5% of random circulating B cells, was used by four clones. VH4-59 was utilized in three clones, two that were likely clonally related from one donor, and one from a separate donor. Of the four Fab clones with virus-neutralizing activity, two from a single donor (DS1 and DS6) had very similar VH segments and identical HCDR3 regions (see Table S1 in the supplemental material) but distinct light chains. The two clones with the highest neutralizing ability (ACN044 and DS7) comprised VH, JH, and light chains that were distinct at the nucleotide and amino acid levels but had very similar HCDR3 loops (see Table S2 in the supplemental material). Analysis of somatic mutations revealed that most of the Fab clones were highly mutated, with framework mutations predominant (see Table S1 in the supplemental material). However, there was no apparent correlation between the number of mutations and neutralizing activity.
Competition ELISA analysis of hMPV-binding Fabs.
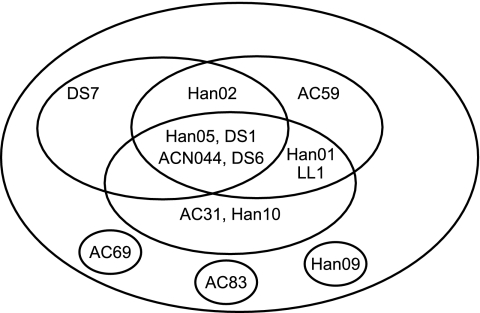
In order to determine the number of antigenic sites that these human Fabs recognized, competition ELISA experiments were carried out. The results of these experiments are shown in Table 3. The data show that there are several complex patterns of competition. The four Fabs with in vitro neutralizing activity (ACN044, DS1, DS6, and DS7) were all in the same competition group, along with several nonneutralizing Fabs. There were several partially overlapping competition groups that included both neutralizing and nonneutralizing Fabs. Three nonneutralizing Fabs (AC69, AC83, and Han09) competed only with the matched antibody. These data are represented pictorially by the epitope map in Fig. 2. The figure is not meant to indicate actual regions on the F protein but rather to illustrate the overlapping nature of the epitopes.
TABLE 3.
Competition ELISA using biotinylated Fabs
| Fab | Competition with biotinylated Faba
|
|||||
|---|---|---|---|---|---|---|
| ACN044 | AC59 | LL01 | DS1 | DS6 | DS7 | |
| AC31 | + | − | − | + | + | − |
| ACN044 | + | + | + | + | + | + |
| AC59 | − | + | − | − | − | − |
| AC69 | − | − | − | − | − | − |
| AC83 | − | − | − | − | − | − |
| DS1 | + | + | + | + | + | + |
| DS6 | + | + | + | + | + | + |
| DS7 | − | − | − | − | − | + |
| Han01 | + | + | + | + | + | − |
| Han02 | − | + | + | + | + | + |
| Han05 | + | + | + | + | + | + |
| Han09 | − | − | − | − | − | − |
| Han10 | + | − | − | − | − | − |
| LL01 | + | − | + | − | − | − |
+, competition; −, no competition.
FIG. 2.
Schematic map of epitopes recognized by hMPV A2 F protein-specific MAbs. Each circle represents an individual epitope on hMPV A2 F protein, with the Fab binding to that epitope shown inside the circle. Fab names inside the intersections of circles are those that have recognition sites composed of a portion of two or three epitopes.
SPR.
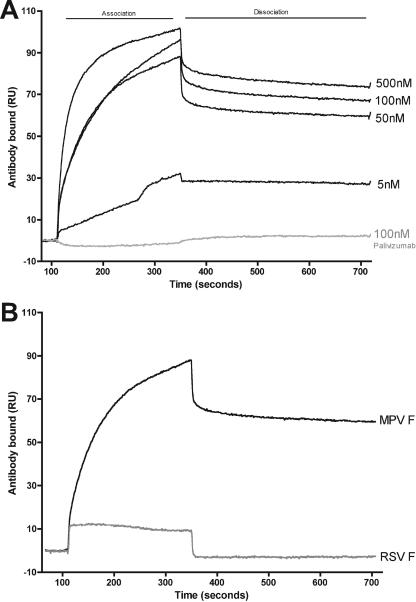
SPR studies indicated that hMPV-specific Fab bound hMPV FΔTM with high affinity, while as expected, the RSV F-specific MAb palivizumab did not. The binding curves of anti-hMPV Fab DS7 at concentrations ranging from 500 nM to 5 nM showed a pattern of specific binding to hMPV FΔTM (Fig. 3A). In contrast, Fig. 3A shows that palivizumab did not bind to hMPV FΔTM even at 100 nM concentration. We tested the binding ability of palivizumab to RSV-FΔTM, and it exhibited strong, specific binding (data not shown), showing that the lack of binding to hMPV FΔTM was the result of specificity and not related to the quality of the antibody. The Kon and Koff of Fab DS7 with hMPV FΔTM were measured at 3.54 × 105 M−1 s−1 and 3.48 × 10−4 M−1, respectively. The confidence in these kinetic values was strong based on small χ2 (<1.0) values for all of the 1:1 Langmuir fitted binding sensograms. The affinity of Fab DS7 for hMPV FΔTM was high, 9.84 × 10−10 M. These values suggest a strong, specific antibody-antigen binding. The human Fab DS7 showed specific binding to hMPV FΔTM, but it did not have a detectable affinity for RSV FΔTM protein (Fig. 3B).
FIG. 3.
SPR analysis of DS7 Fab. (A) Association-dissociation curves of decreasing concentrations of DS7 against immobilized hMPV FΔTM protein. Palivizumab (RSV F-specific MAb) was used as an irrelevant control. (B) Association-dissociation curves of DS7 at 100 nM against immobilized hMPV FΔTM protein and RSV FΔTM protein.
In vivo activity of DS7.
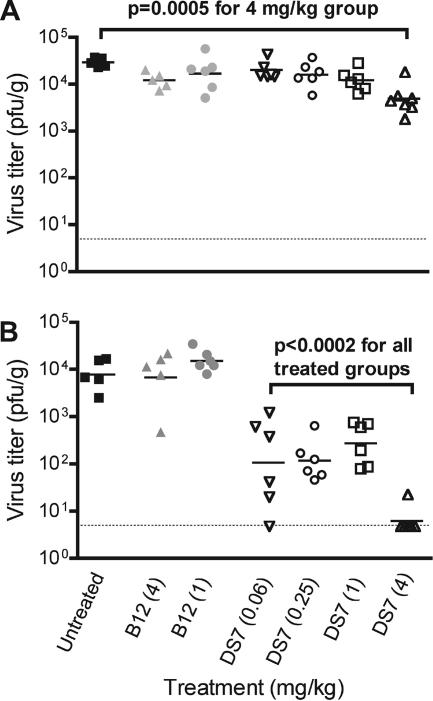
Cotton rats were infected intranasally with hMPV and given Fab intranasally on day 3, 1 day prior to the peak of hMPV replication (84). Animals were sacrificed and tissues harvested on day 4, and nasal and lung virus titers were determined. The virus titer in nasal turbinates was reduced only modestly by Fab DS7 treatment (Fig. 4A). There was a significant difference in virus titers between the control groups (P = 0.025), with those in the untreated control having a slightly higher geometric mean nasal virus titer than the two Fab B12-treated control groups (hMPV, 2.9 × 104 PFU/g; B12 at 4 mg/kg, 1.2 × 104 PFU/g; B12 at 1 mg/kg, 1.7 × 104 PFU/g). We compared the DS7-treated groups to the combined control groups, and only the 4-mg/kg DS7 dose was associated with a significant reduction in nasal virus titer (4.9 × 103 PFU/g versus 1.8 × 104 PFU/g, P = 0.0005) (Fig. 4A). There was a statistically significant relationship between dose and response (P = 0.0002): for every quadrupling of the dose, the expected viral load decreased by approximately −0.20 log10 PFU/g (95% confidence interval [CI] of −0.29, −0.11) (Fig. 5A).
FIG. 4.
Nasal (A) and lung (B) titers of hMPV. Groups are defined in the text. Tissue virus titers were log10 transformed for statistical analysis. Comparisons between groups were made using a Wilcoxon rank sum test. Horizontal bars represent geometric means; the dotted line indicates the limit of detection (5 PFU/g).
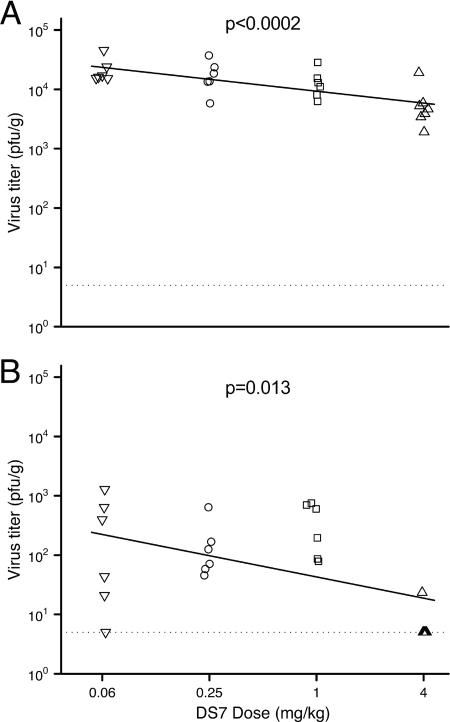
FIG. 5.
Dose-response relationship between DS7 and nasal (A) and lung (B) titers of hMPV. Groups are defined in the text. Linear regression was used to examine the relationship between Fab DS7 dose and log10-transformed viral titer, as described in text. The dotted line indicates the limit of detection (5 PFU/g).
DS7 was highly effective at reducing viral titers in the lungs (Fig. 4B). The control groups (either untreated or treated with Fab B12) had a mean lung virus titer of 9.6 × 103 PFU/g. The lung virus titers did not differ between the three control groups (P = 0.38). Each of the DS7-treated groups had a lower lung virus titer than the controls (P < 0.0002 for each group compared to controls). The mean virus titer in the lungs of DS7-treated animals ranged from 1.1 × 102 (0.06-mg/kg dose) to 6.2 × 100 PFU/g (4-mg/kg dose). Only one of seven animals in the 4-mg/kg DS7 group had detectable virus in the lungs. This represented a >1,500-fold reduction in the 4-mg/kg-treated cotton rats compared to controls. There was evidence that higher doses resulted in lower lung virus titers (P = 0.013; slope, −0.36 log10 PFU/g per dose quadrupling [95% CI of −0.62, −0.10]) (Fig. 5B). However, this result was driven by the 4-mg/kg dose; the data suggest that there may be a threshold dose between 1 and 4 mg/kg, above which neutralization occurs more strongly. There was no evidence to suggest that the lung virus titer differed between the 0.06-, 0.25-, and 1.0-mg/kg doses (P = 0.37). We also performed linear regression without log2 transforming the doses, and the reduction in virus titer for both nasal and lung tissues was still significant (P < 0.0001 for both; −0.62 log10 PFU/g [95% CI of −0.82, −0.43] for lung and −0.14 log10 PFU/g [95% CI of −0.20, −0.09] for nasal turbinates).
We evaluated the possibility that the observed reduction in titer of hMPV in the lungs was due to neutralization in vitro when the lung homogenates were prepared and assayed for virus titer by plaque titration. Individual lung homogenates of six hMPV-infected animals were prepared on day 4 postinfection, and each was mixed separately with an equal volume of lung homogenate derived from a different animal that had received 4 mg/kg of Fab DS7 24 h previously. Plaque assay of the six mixed homogenates yielded a virus titer that was slightly lower than that in the unmixed lung suspensions from the infected animals. The difference in geometric mean titer of the two groups was only 100.5. This indicated that the majority of the therapeutic effect observed in Fab recipients was due to an action of the Fab in vivo rather than neutralization of virus in vitro during homogenization of lung tissue.
DISCUSSION
We used a novel method of mammalian protein expression to generate a soluble form of the hMPV F protein (FΔTM) that was highly immunogenic and induced neutralizing antibodies in cotton rats. This construct was used to select fully human MAbs from combinatorial phage display libraries. This approach proved effective in isolating numerous human Fabs that bound to hMPV-infected cells. This work also shows that the FΔTM protein retains important neutralizing epitopes present on native F protein. Previous animal studies suggest that hMPV F is the major determinant of protection, and thus, an improved understanding of the antigenic characteristics of F is critical for the development of vaccines and prophylactic antibodies (63, 64, 66, 67). The Fabs that we describe here and additional Fab clones that we are isolating in ongoing work in our laboratories will be useful for mapping antigenic and neutralizing epitopes on the hMPV F protein.
Several of these Fabs exhibited neutralizing activity in vitro, but the majority of F-binding Fabs that were isolated did not neutralize virus, consistent with previous reports using phage display technology as a means of sampling specific antiviral human antibody repertoires (2, 14, 18, 86). However, of the 14 F-specific Fabs we identified by ELISA screening, 12 bound hMPV-infected cells, and 4 of these exhibited in vitro neutralizing activity. This finding suggests a high efficiency of the panning and screening method and supports the hypothesis that the recombinant construct FΔTM retains elements of mature F conformation as expressed on infected cells. Previous studies of human and mouse MAbs against RSV F have suggested that RSV F that was immunoaffinity purified from infected cell lysates presented an immature conformation and induced primarily nonneutralizing MAbs that did not recognize mature F protein (59). Our results suggest that hMPV FΔTM may be an antigen with greater conformational integrity.
Sequence analysis of multiple strains of hMPV isolated from diverse locations and over many years shows that there are four distinct genotypes of hMPV, provisionally designated A1, A2, B1, and B2, and that the proportion of genotypes represented in circulating strains varies from year to year (48, 49, 73, 85). The F protein is 94 to 96% conserved at the amino acid sequence level between groups. Reinfection with hMPV with homologous and heterologous genogroup viruses has been described (22, 52, 81, 85). It is not yet clear whether this represents incomplete immunity or immune escape resulting from antigenic variation, but preventive or therapeutic strategies against hMPV need to address this phenomenon. We tested the Fabs with neutralizing activity against fully sequenced and plaque-purified prototype strains from each lineage. Only DS7 neutralized strains from the A1 and B1 lineages in addition to the A2 lineage. DS7 failed to neutralize the B2 strain tested. It is not yet clear whether one virus lineage predominates in circulating strains, although studies conducted over a 20-year period found A2 and B2 viruses to be the most common (81, 85). We have sequenced the complete F open reading frame from 78 isolates collected over a 20-year period, and we observed only 23 conserved amino acid differences between A2 and B2 strains (J. V. Williams, C.-F. Yang, C. K. Wang, and J. E. Crowe, Jr., unpublished data). Thus, it seems likely that there are neutralizing epitopes shared by strains of differing genotypes that will induce broadly neutralizing activity. One group recently reported the isolation of mouse MAbs against hMPV F that had various degrees of neutralizing activity against all four genotypes, with 50% plaque reduction concentrations ranging from 0.03 μg/ml to 23.6 μg/ml (70). The specific in vitro neutralizing activity of DS7 against different subgroups of hMPV (1.1 to 9.8 μg/ml) is similar to that of these mouse MAbs and is also comparable to the specific activity of palivizumab (27.46 μg/ml as Fab) (87, 88).
The hMPV F-specific Fabs isolated represented diverse VH and VL gene segment usage, similar to other reports of phage display-derived Fabs directed at viral glycoproteins (45, 69, 86). The variable antibody gene segments present were segments that are not common in the repertoire of adult randomly selected B cells, but the natural repertoire of antibodies induced by hMPV infection is not known. It is important to note that phage display technology allows promiscuous heavy- and light-chain pairings that might not be present in the normally expressed repertoire. Two neutralizing clones from the same donor (DS1 and DS6) utilized distinct light chains but virtually identical heavy chains, suggesting that for these clones the heavy chain mediated the principal determinants of FΔTM binding. In contrast, the two clones with the highest in vitro neutralizing activity (ACN044 and DS7), which were derived from separate human donor libraries, utilized VH and JH segments that were quite distinct at the nucleotide and amino acid levels. These two clones had similar HCDR3 sequences, suggesting that for these two clones, HCDR3 mediates binding. For most antigens, it is thought that the HCDR3 loop is the most critical determinant of antigen binding because it is usually located in the center of the antigen binding surface of the combining site (90).
We compared the ability of the Fabs to neutralize different subgroups of hMPV and to compete for binding sites on the F protein, leading to the identification of nine epitopes. Three of these were distinct but recognized by nonneutralizing Fabs (AC69, AC83, and Han09). The remaining six epitopes have antibodies that recognize one of two or three independent epitopes or recognize the overlap between these epitopes (Fig. 2). Interestingly, three of the four neutralizing Fabs (ACN044, DS1, and DS6) recognized a single epitope, while Fab with the most potent neutralizing activity (DS7) recognized an overlapping epitope. A previous study of hMPV F-specific mouse MAbs identified several distinct neutralizing epitopes (70). We are generating monoclonal antibody-resistant mutants of hMPV that will allow the determination of the precise location on the F protein sequence to which the Fabs bind.
Fab DS7 exhibited high affinity for hMPV F, with a Kon of 3.54 × 105 s−1 M−1, Koff of 3.48 × 10−4 s−1, and KD of 0.98 nM. This is slightly higher than the affinity described for two mouse MAbs against hMPV F, which ranged from 1.42 to 4.49 nM (70). The values we obtained for DS7 are similar to the Kon, Koff, and KD of the RSV-neutralizing Fab palivizumab (1.26 s−1 M−1, 6.62 s−1, and 5.25 nM, respectively) (14, 87, 88). A comparison of two anti-RSV mouse MAbs suggested that affinity was the determining factor in their level of neutralizing activity (42). Recent reports of affinity-matured, ultrapotent MAbs derived from palivizumab showed that decreases in the dissociation constant (Koff) had the greatest effect on overall affinity and neutralizing potency (87, 88). We are conducting further studies to determine the epitope recognized by DS7 and the relationship between affinity, avidity (of full-length IgG), and neutralizing activity.
Fab DS7 provided a substantial degree of therapeutic effect against lung hMPV replication, reducing lung virus titers >1,500-fold at a dose of 4 mg/kg (380 μg for a 95-g cotton rat). This effect is similar to the degree of reduction in lung virus we observed previously with FΔTM vaccination of cotton rats (17). A significant though more modest reduction in lung virus was observed even with the dose of 0.06 mg/kg. These results are similar to studies showing that Fabs against the related virus RSV generated using phage display are therapeutically effective in the mouse model (14, 15). RSV also can cause fatal infections in immunocompromised hosts, and these infections are frequently treated with anti-RSV MAbs (19). These findings suggest that neutralizing Fabs could be used therapeutically to treat hMPV infection in high-risk hosts, such as immunocompromised patients, in whom hMPV infection can be severe and fatal (10, 23, 40, 46, 52, 82). Fabs offer promise as small-molecule aerosols for respiratory viruses that are limited to the respiratory epithelium. Also, Fabs generally are not immunomodulatory, since they lack the Fc domain and are cleared rapidly (39).
In summary, we used a novel F protein expression strategy combined with phage display to isolate human Fabs specific for hMPV F. Several of these Fabs exhibited in vitro neutralizing activity. Fab DS7 neutralized viruses from different genogroups, with a specific virus-neutralizing activity against the A2 strain of 1.1 μg/ml. DS7 bound to purified F protein with nanomolar affinity and effected a >1,500-fold reduction in lung virus titer in treated cotton rats. DS7 and other anti-hMPV Fabs may have therapeutic or prophylactic potential against hMPV, an important emerging pathogen.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (K08 AI-56170 to J.V.W.), and a Vanderbilt Department of Pediatrics Hazinski-Turner Award (J.V.W.). J.E.C. holds a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund.
We thank Hoyin (Sunny) Mok for assistance with animal studies and Gil Abalos III and Justin Cruite for technical assistance.
Footnotes
Published ahead of print on 23 May 2007.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Baert, F., M. Noman, S. Vermeire, G. Van Assche, G. D'Haens, A. Carbonez, and P. Rutgeerts. 2003. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N. Engl. J. Med. 348:601-608. [DOI] [PubMed] [Google Scholar]
- 2.Barbas, C. F., III, J. E. Crowe, Jr., D. Cababa, T. M. Jones, S. L. Zebedee, B. R. Murphy, R. M. Chanock, and D. R. Burton. 1992. Human monoclonal Fab fragments derived from a combinatorial library bind to respiratory syncytial virus F glycoprotein and neutralize infectivity. Proc. Natl. Acad. Sci. USA 89:10164-10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbas, C. F., III, A. S. Kang, R. A. Lerner, and S. J. Benkovic. 1991. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc. Natl. Acad. Sci. USA 88:7978-7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeckh, M., V. Erard, D. Zerr, and J. Englund. 2005. Emerging viral infections after hematopoietic cell transplantation. Pediatr. Transplant. 9(Suppl. 7):48-54. [DOI] [PubMed] [Google Scholar]
- 5.Boivin, G., Y. Abed, G. Pelletier, L. Ruel, D. Moisan, S. Cote, T. C. Peret, D. D. Erdman, and L. J. Anderson. 2002. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J. Infect. Dis. 186:1330-1334. [DOI] [PubMed] [Google Scholar]
- 6.Boivin, G., G. De Serres, S. Cote, R. Gilca, Y. Abed, L. Rochette, M. G. Bergeron, and P. Dery. 2003. Human metapneumovirus infections in hospitalized children. Emerg. Infect. Dis. 9:634-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brezinschek, H. P., R. I. Brezinschek, and P. E. Lipsky. 1995. Analysis of the heavy chain repertoire of human peripheral B cells using single-cell polymerase chain reaction. J. Immunol. 155:190-202. [PubMed] [Google Scholar]
- 8.Buchholz, U. J., S. Biacchesi, Q. N. Pham, K. C. Tran, L. Yang, C. L. Luongo, M. H. Skiadopoulos, B. R. Murphy, and P. L. Collins. 2005. Deletion of M2 gene open reading frames 1 and 2 of human metapneumovirus: effects on RNA synthesis, attenuation, and immunogenicity. J. Virol. 79:6588-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burioni, R., R. A. Williamson, P. P. Sanna, F. E. Bloom, and D. R. Burton. 1994. Recombinant human Fab to glycoprotein D neutralizes infectivity and prevents cell-to-cell transmission of herpes simplex viruses 1 and 2 in vitro. Proc. Natl. Acad. Sci. USA 91:355-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cane, P. A., B. G. van den Hoogen, S. Chakrabarti, C. D. Fegan, and A. D. Osterhaus. 2003. Human metapneumovirus in a haematopoietic stem cell transplant recipient with fatal lower respiratory tract disease. Bone Marrow Transplant. 31:309-310. [DOI] [PubMed] [Google Scholar]
- 11.Collins, P. L., and B. R. Murphy. 2005. New generation live vaccines against human respiratory syncytial virus designed by reverse genetics. Proc. Am. Thorac. Soc. 2:166-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbett, S. J., I. M. Tomlinson, E. L. Sonnhammer, D. Buck, and G. Winter. 1997. Sequence of the human immunoglobulin diversity (D) segment locus: a systematic analysis provides no evidence for the use of DIR segments, inverted D segments, “minor” D segments or D-D recombination. J. Mol. Biol. 270:587-597. [DOI] [PubMed] [Google Scholar]
- 13.Crowe, J. E., Jr. 2001. Respiratory syncytial virus vaccine development. Vaccine 20(Suppl. 1):S32-S37. [DOI] [PubMed] [Google Scholar]
- 14.Crowe, J. E., Jr., P. S. Gilmour, B. R. Murphy, R. M. Chanock, L. Duan, R. J. Pomerantz, and G. R. Pilkington. 1998. Isolation of a second recombinant human respiratory syncytial virus monoclonal antibody fragment (Fab RSVF2-5) that exhibits therapeutic efficacy in vivo. J. Infect. Dis. 177:1073-1076. [DOI] [PubMed] [Google Scholar]
- 15.Crowe, J. E., Jr., B. R. Murphy, R. M. Chanock, R. A. Williamson, C. F. Barbas III, and D. R. Burton. 1994. Recombinant human respiratory syncytial virus (RSV) monoclonal antibody Fab is effective therapeutically when introduced directly into the lungs of RSV-infected mice. Proc. Natl. Acad. Sci. USA 91:1386-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowe, J. E., Jr., and J. V. Williams. 2003. Immunology of viral respiratory tract infection in infancy. Paediatr. Respir. Rev. 4:112-119. [DOI] [PubMed] [Google Scholar]
- 17.Cseke, G., D. W. Wright, S. J. Tollefson, J. E. Johnson, J. E. Crowe, Jr., and J. V. Williams. 2006. Human metapneumovirus fusion protein vaccines that are immunogenic and protective in cotton rats. J. Virol. 81:698-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Carvalho Nicacio, C., R. A. Williamson, P. W. Parren, A. Lundkvist, D. R. Burton, and E. Bjorling. 2002. Neutralizing human Fab fragments against measles virus recovered by phage display. J. Virol. 76:251-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeVincenzo, J. P., R. L. Hirsch, R. J. Fuentes, and F. H. Top, Jr. 2000. Respiratory syncytial virus immune globulin treatment of lower respiratory tract infection in pediatric patients undergoing bone marrow transplantation—a compassionate use experience. Bone Marrow Transplant. 25:161-165. [DOI] [PubMed] [Google Scholar]
- 20.Dollner, H., K. Risnes, A. Radtke, and S. A. Nordbo. 2004. Outbreak of human metapneumovirus infection in Norwegian children. Pediatr. Infect. Dis. J. 23:436-440. [DOI] [PubMed] [Google Scholar]
- 21.Durbin, A. P., and R. A. Karron. 2003. Progress in the development of respiratory syncytial virus and parainfluenza virus vaccines. Clin. Infect. Dis. 37:1668-1677. [DOI] [PubMed] [Google Scholar]
- 22.Ebihara, T., R. Endo, N. Ishiguro, T. Nakayama, H. Sawada, and H. Kikuta. 2004. Early reinfection with human metapneumovirus in an infant. J. Clin. Microbiol. 42:5944-5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Englund, J. A., M. Boeckh, J. Kuypers, W. G. Nichols, R. C. Hackman, R. A. Morrow, D. N. Fredricks, and L. Corey. 2006. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann. Intern. Med. 144:344-349. [DOI] [PubMed] [Google Scholar]
- 24.Englund, J. A., P. A. Piedra, and E. Whimbey. 1997. Prevention and treatment of respiratory syncytial virus and parainfluenza viruses in immunocompromised patients. Am. J. Med. 102:61-70, 75-76. [DOI] [PubMed] [Google Scholar]
- 25.Esper, F., R. A. Martinello, D. Boucher, C. Weibel, D. Ferguson, M. L. Landry, and J. S. Kahn. 2004. A 1-year experience with human metapneumovirus in children aged <5 years. J. Infect. Dis. 189:1388-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falsey, A. R., D. Erdman, L. J. Anderson, and E. E. Walsh. 2003. Human metapneumovirus infections in young and elderly adults. J. Infect. Dis. 187:785-790. [DOI] [PubMed] [Google Scholar]
- 27.Falsey, A. R., and E. E. Walsh. 2006. Viral pneumonia in older adults. Clin. Infect. Dis. 42:518-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franquet, T., S. Rodriguez, R. Martino, T. Salinas, A. Gimenez, and A. Hidalgo. 2005. Human metapneumovirus infection in hematopoietic stem cell transplant recipients: high-resolution computed tomography findings. J. Comput. Assist. Tomogr. 29:223-227. [DOI] [PubMed] [Google Scholar]
- 29.Galiano, M., C. Videla, S. S. Puch, A. Martinez, M. Echavarria, and G. Carballal. 2004. Evidence of human metapneumovirus in children in Argentina. J. Med. Virol. 72:299-303. [DOI] [PubMed] [Google Scholar]
- 30.Gerna, G., P. Vitulo, F. Rovida, D. Lilleri, C. Pellegrini, T. Oggionni, G. Campanini, F. Baldanti, and M. G. Revello. 2006. Impact of human metapneumovirus and human cytomegalovirus versus other respiratory viruses on the lower respiratory tract infections of lung transplant recipients. J. Med. Virol. 78:408-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giudicelli, V., P. Duroux, C. Ginestoux, G. Folch, J. Jabado-Michaloud, D. Chaume, and M. P. Lefranc. 2006. IMGT/LIGM-DB, the IMGT comprehensive database of immunoglobulin and T cell receptor nucleotide sequences. Nucleic Acids Res. 34:D781-D784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham, B. S., T. H. Davis, Y. W. Tang, and W. C. Gruber. 1993. Immunoprophylaxis and immunotherapy of respiratory syncytial virus-infected mice with respiratory syncytial virus-specific immune serum. Pediatr. Res. 34:167-172. [DOI] [PubMed] [Google Scholar]
- 33.Graham, B. S., Y. W. Tang, and W. C. Gruber. 1995. Topical immunoprophylaxis of respiratory syncytial virus (RSV)-challenged mice with RSV-specific immune globulin. J. Infect. Dis. 171:1468-1474. [DOI] [PubMed] [Google Scholar]
- 34.Groothuis, J. R. 1994. The role of RSV neutralizing antibodies in the treatment and prevention of respiratory syncytial virus infection in high-risk children. Antiviral Res. 23:1-10. [DOI] [PubMed] [Google Scholar]
- 35.Groothuis, J. R., E. A. Simoes, V. G. Hemming, et al. 1995. Respiratory syncytial virus (RSV) infection in preterm infants and the protective effects of RSV immune globulin (RSVIG). 95:463-467. [PubMed] [Google Scholar]
- 36.Hamelin, M. E., S. Cote, J. Laforge, N. Lampron, J. Bourbeau, K. Weiss, R. Gilca, G. DeSerres, and G. Boivin. 2005. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin. Infect. Dis. 41:498-502. [DOI] [PubMed] [Google Scholar]
- 37.Hemming, V. G., and G. A. Prince. 1990. Immunoprophylaxis of infections with respiratory syncytial virus: observations and hypothesis. Rev. Infect. Dis. 12(Suppl. 4):S470-S475. [DOI] [PubMed] [Google Scholar]
- 38.Hemming, V. G., G. A. Prince, J. R. Groothuis, and G. R. Siber. 1995. Hyperimmune globulins in prevention and treatment of respiratory syncytial virus infections. Clin. Microbiol. Rev. 8:22-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holliger, P., and P. J. Hudson. 2005. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 23:1126-1136. [DOI] [PubMed] [Google Scholar]
- 40.Huck, B., M. Egger, H. Bertz, G. Peyerl-Hoffman, W. V. Kern, D. Neumann-Haefelin, and V. Falcone. 2006. Human metapneumovirus infection in a hematopoietic stem cell transplant recipient with relapsed multiple myeloma and rapidly progressing lung cancer. J. Clin. Microbiol. 44:2300-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang, W. Y., and J. Foote. 2005. Immunogenicity of engineered antibodies. Methods 36:3-10. [DOI] [PubMed] [Google Scholar]
- 42.Johnson, S., S. D. Griego, D. S. Pfarr, M. L. Doyle, R. Woods, D. Carlin, G. A. Prince, S. Koenig, J. F. Young, and S. B. Dillon. 1999. A direct comparison of the activities of two humanized respiratory syncytial virus monoclonal antibodies: MEDI-493 and RSHZl9. J. Infect. Dis. 180:35-40. [DOI] [PubMed] [Google Scholar]
- 43.Karron, R. A., R. B. Belshe, P. F. Wright, B. Thumar, B. Burns, F. Newman, J. C. Cannon, J. Thompson, T. Tsai, M. Paschalis, S. L. Wu, Y. Mitcho, J. Hackell, B. R. Murphy, and J. M. Tatem. 2003. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in young infants. Pediatr. Infect. Dis. J. 22:394-405. [DOI] [PubMed] [Google Scholar]
- 44.Kneyber, M. C., H. A. Moll, and R. de Groot. 2000. Treatment and prevention of respiratory syncytial virus infection. Eur. J. Pediatr. 159:399-411. [DOI] [PubMed] [Google Scholar]
- 45.Kramer, R. A., W. E. Marissen, J. Goudsmit, T. J. Visser, M. Clijsters-Van der Horst, A. Q. Bakker, M. de Jong, M. Jongeneelen, S. Thijsse, H. H. Backus, A. B. Rice, W. C. Weldon, C. E. Rupprecht, B. Dietzschold, A. B. Bakker, and J. de Kruif. 2005. The human antibody repertoire specific for rabies virus glycoprotein as selected from immune libraries. Eur. J. Immunol. 35:2131-2145. [DOI] [PubMed] [Google Scholar]
- 46.Larcher, C., C. Geltner, H. Fischer, D. Nachbaur, L. C. Muller, and H. P. Huemer. 2005. Human metapneumovirus infection in lung transplant recipients: clinical presentation and epidemiology. J. Heart Lung Transplant. 24:1891-1901. [DOI] [PubMed] [Google Scholar]
- 47.Levin, M. D., and G. J. van Doornum. 2004. An immunocompromised host with bilateral pulmonary infiltrates. Neth. J. Med. 62:197:210. [PubMed] [Google Scholar]
- 48.Ludewick, H. P., Y. Abed, N. van Niekerk, G. Boivin, K. P. Klugman, and S. A. Madhi. 2005. Human metapneumovirus genetic variability, South Africa. Emerg. Infect. Dis. 11:1074-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mackay, I. M., S. Bialasiewicz, Z. Waliuzzaman, G. R. Chidlow, D. C. Fegredo, S. Laingam, P. Adamson, G. B. Harnett, W. Rawlinson, M. D. Nissen, and T. P. Sloots. 2004. Use of the P gene to genotype human metapneumovirus identifies 4 viral subtypes. J. Infect. Dis. 190:1913-1918. [DOI] [PubMed] [Google Scholar]
- 50.Martinello, R. A., F. Esper, C. Weibel, D. Ferguson, M. L. Landry, and J. S. Kahn. 2006. Human metapneumovirus and exacerbations of chronic obstructive pulmonary disease. J. Infect. 53:248-254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mullins, J. A., D. D. Erdman, G. A. Weinberg, K. Edwards, C. B. Hall, F. J. Walker, M. Iwane, and L. J. Anderson. 2004. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg. Infect. Dis. 10:700-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pelletier, G., P. Dery, Y. Abed, and G. Boivin. 2002. Respiratory tract reinfections by the new human metapneumovirus in an immunocompromised child. Emerg. Infect. Dis. 8:976-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polack, F. P., and R. A. Karron. 2004. The future of respiratory syncytial virus vaccine development. Pediatr. Infect. Dis. J. 23:S65-S73. [DOI] [PubMed] [Google Scholar]
- 54.Pollack, P., and J. R. Groothuis. 2002. Development and use of palivizumab (Synagis): a passive immunoprophylactic agent for RSV. J. Infect. Chemother. 8:201-206. [DOI] [PubMed] [Google Scholar]
- 55.Prince, G. A., V. G. Hemming, R. L. Horswood, and R. M. Chanock. 1985. Immunoprophylaxis and immunotherapy of respiratory syncytial virus infection in the cotton rat. Virus Res. 3:193-206. [DOI] [PubMed] [Google Scholar]
- 56.Prince, G. A., R. L. Horswood, and R. M. Chanock. 1985. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J. Virol. 55:517-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richards, A., J. N. Chuen, C. Taylor, R. Jackson, G. Toms, and D. Kavanagh. 2005. Acute respiratory infection in a renal transplant recipient. Nephrol. Dialysis Transplant. 20:2848-2850. [DOI] [PubMed] [Google Scholar]
- 58.Ruiz, M., V. Giudicelli, C. Ginestoux, P. Stoehr, J. Robinson, J. Bodmer, S. G. Marsh, R. Bontrop, M. Lemaitre, G. Lefranc, D. Chaume, and M. P. Lefranc. 2000. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 28:219-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakurai, H., R. A. Williamson, J. E. Crowe, J. A. Beeler, P. Poignard, R. B. Bastidas, R. M. Chanock, and D. R. Burton. 1999. Human antibody responses to mature and immature forms of viral envelope in respiratory syncytial virus infection: significance for subunit vaccines. J. Virol. 73:2956-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanna, P. P., R. A. Williamson, A. De Logu, F. E. Bloom, and D. R. Burton. 1995. Directed selection of recombinant human monoclonal antibodies to herpes simplex virus glycoproteins from phage display libraries. Proc. Natl. Acad. Sci. USA 92:6439-6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schowalter, R. M., S. E. Smith, and R. E. Dutch. 2006. Characterization of human metapneumovirus F protein-promoted membrane fusion: critical roles for proteolytic processing and low pH. J. Virol. 80:10931-10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siber, G. R., J. Leszcynski, V. Pena-Cruz, C. Ferren-Gardner, R. Anderson, V. G. Hemming, E. E. Walsh, J. Burns, K. McIntosh, R. Gonin, et al. 1992. Protective activity of a human respiratory syncytial virus immune globulin prepared from donors screened by microneutralization assay. J. Infect. Dis. 165:456-463. [DOI] [PubMed] [Google Scholar]
- 63.Skiadopoulos, M. H., S. Biacchesi, U. J. Buchholz, E. Amaro-Carambot, S. R. Surman, P. L. Collins, and B. R. Murphy. 2006. Individual contributions of the human metapneumovirus F, G, and SH surface glycoproteins to the induction of neutralizing antibodies and protective immunity. Virology 345:492-501. [DOI] [PubMed] [Google Scholar]
- 64.Skiadopoulos, M. H., S. Biacchesi, U. J. Buchholz, J. M. Riggs, S. R. Surman, E. Amaro-Carambot, J. M. McAuliffe, W. R. Elkins, M. St. Claire, P. L. Collins, and B. R. Murphy. 2004. The two major human metapneumovirus genetic lineages are highly related antigenically, and the fusion (F) protein is a major contributor to this antigenic relatedness. J. Virol. 78:6927-6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stallmach, A., T. Giese, C. Schmidt, S. C. Meuer, and S. S. Zeuzem. 2004. Severe anaphylactic reaction to infliximab: successful treatment with adalimumab—report of a case. Eur. J. Gastroenterol. Hepatol. 16:627-630. [DOI] [PubMed] [Google Scholar]
- 66.Tang, R. S., K. Mahmood, M. Macphail, J. M. Guzzetta, A. A. Haller, H. Liu, J. Kaur, H. A. Lawlor, E. A. Stillman, J. H. Schickli, R. A. Fouchier, A. D. Osterhaus, and R. R. Spaete. 2005. A host-range restricted parainfluenza virus type 3 (PIV3) expressing the human metapneumovirus (hMPV) fusion protein elicits protective immunity in African green monkeys. Vaccine 23:1657-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang, R. S., J. H. Schickli, M. MacPhail, F. Fernandes, L. Bicha, J. Spaete, R. A. Fouchier, A. D. Osterhaus, R. Spaete, and A. A. Haller. 2003. Effects of human metapneumovirus and respiratory syncytial virus antigen insertion in two 3′ proximal genome positions of bovine/human parainfluenza virus type 3 on virus replication and immunogenicity. J. Virol. 77:10819-10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor, G., E. J. Stott, M. Bew, B. F. Fernie, P. J. Cote, A. P. Collins, M. Hughes, and J. Jebbett. 1984. Monoclonal antibodies protect against respiratory syncytial virus infection in mice. Immunology 52:137-142. [PMC free article] [PubMed] [Google Scholar]
- 69.Throsby, M., C. Geuijen, J. Goudsmit, A. Q. Bakker, J. Korimbocus, R. A. Kramer, M. Clijsters-van der Horst, M. de Jong, M. Jongeneelen, S. Thijsse, R. Smit, T. J. Visser, N. Bijl, W. E. Marissen, M. Loeb, D. J. Kelvin, W. Preiser, J. ter Meulen, and J. de Kruif. 2006. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile virus. J. Virol. 80:6982-6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ulbrandt, N. D., H. Ji, N. K. Patel, J. M. Riggs, Y. A. Brewah, S. Ready, N. E. Donacki, K. Folliot, A. S. Barnes, K. Senthil, S. Wilson, M. Chen, L. Clarke, M. MacPhail, J. Li, R. M. Woods, K. Coelingh, J. L. Reed, M. P. McCarthy, D. S. Pfarr, A. D. Osterhaus, R. A. Fouchier, P. A. Kiener, and J. A. Suzich. 2006. Isolation and characterization of monoclonal antibodies which neutralize human metapneumovirus in vitro and in vivo. J. Virol. 80:7799-7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ulloa-Gutierrez, R., P. Skippen, A. Synnes, M. Seear, N. Bastien, Y. Li, and J. C. Forbes. 2004. Life-threatening human metapneumovirus pneumonia requiring extracorporeal membrane oxygenation in a preterm infant. Pediatrics 114:e517-e519. [DOI] [PubMed] [Google Scholar]
- 72.van den Hoogen, B. G., J. C. de Jong, J. Groen, T. Kuiken, R. de Groot, R. A. Fouchier, and A. D. Osterhaus. 2001. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat. Med. 7:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van den Hoogen, B. G., S. Herfst, L. Sprong, P. A. Cane, E. Forleo-Neto, R. L. de Swart, A. D. Osterhaus, and R. A. Fouchier. 2004. Antigenic and genetic variability of human metapneumoviruses. Emerg. Infect. Dis. 10:658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van den Hoogen, B. G., G. J. van Doornum, J. C. Fockens, J. J. Cornelissen, W. E. Beyer, R. de Groot, A. D. Osterhaus, and R. A. Fouchier. 2003. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J. Infect. Dis. 188:1571-1577. [DOI] [PubMed] [Google Scholar]
- 75.van Woensel, J., and J. Kimpen. 2000. Therapy for respiratory tract infections caused by respiratory syncytial virus. Eur. J. Pediatr. 159:391-398. [DOI] [PubMed] [Google Scholar]
- 76.Vicente, D., M. Montes, G. Cilla, and E. Perez-Trallero. 2004. Human metapneumovirus and chronic obstructive pulmonary disease. Emerg. Infect. Dis. 10:1338-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang, E. E., and N. K. Tang. 2000. Immunoglobulin for preventing respiratory syncytial virus infection. Cochrane Database Syst. Rev. 2000:CD001725. [DOI] [PubMed] [Google Scholar]
- 78.Weitkamp, J. H., N. Kallewaard, K. Kusuhara, E. Bures, J. V. Williams, B. LaFleur, H. B. Greenberg, and J. E. Crowe, Jr. 2003. Infant and adult human B cell responses to rotavirus share common immunodominant variable gene repertoires. J. Immunol. 171:4680-4688. [DOI] [PubMed] [Google Scholar]
- 79.Welliver, R. C. 1998. Respiratory syncytial virus immunoglobulin and monoclonal antibodies in the prevention and treatment of respiratory syncytial virus infection. Semin. Perinatol. 22:87-95. [DOI] [PubMed] [Google Scholar]
- 80.Williams, J. V., J. E. Crowe, Jr., R. Enriquez, P. Minton, R. S. Peebles, Jr., R. G. Hamilton, S. Higgins, M. Griffin, and T. V. Hartert. 2005. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J. Infect. Dis. 192:1149-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williams, J. V., P. A. Harris, S. J. Tollefson, L. L. Halburnt-Rush, J. M. Pingsterhaus, K. M. Edwards, P. F. Wright, and J. E. Crowe, Jr. 2004. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N. Engl. J. Med. 350:443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams, J. V., R. Martino, N. Rabella, M. Otegui, R. Parody, J. M. Heck, and J. E. Crowe, Jr. 2005. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J. Infect. Dis. 192:1061-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williams, J. V., S. J. Tollefson, P. W. Heymann, H. T. Carper, J. Patrie, and J. E. Crowe. 2005. Human metapneumovirus infection in children hospitalized for wheezing. J. Allergy Clin. Immunol. 115:1311-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams, J. V., S. J. Tollefson, J. E. Johnson, and J. E. Crowe, Jr. 2005. The cotton rat (Sigmodon hispidus) is a permissive small animal model of human metapneumovirus infection, pathogenesis, and protective immunity. J. Virol. 79:10944-10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williams, J. V., C. K. Wang, C. F. Yang, S. J. Tollefson, F. S. House, J. M. Heck, M. Chu, J. B. Brown, L. D. Lintao, J. D. Quinto, D. Chu, R. R. Spaete, K. M. Edwards, P. F. Wright, and J. E. Crowe, Jr. 2006. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J. Infect. Dis. 193:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williamson, R. A., R. Burioni, P. P. Sanna, L. J. Partridge, C. F. Barbas III, and D. R. Burton. 1993. Human monoclonal antibodies against a plethora of viral pathogens from single combinatorial libraries. Proc. Natl. Acad. Sci. USA 90:4141-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu, H., D. S. Pfarr, S. Johnson, Y. A. Brewah, R. M. Woods, N. K. Patel, W. I. White, J. F. Young, and P. A. Kiener. 2007. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J. Mol. Biol. 368:652-665. [DOI] [PubMed] [Google Scholar]
- 88.Wu, H., D. S. Pfarr, Y. Tang, L. L. An, N. K. Patel, J. D. Watkins, W. D. Huse, P. A. Kiener, and J. F. Young. 2005. Ultra-potent antibodies against respiratory syncytial virus: effects of binding kinetics and binding valence on viral neutralization. J. Mol. Biol. 350:126-144. [DOI] [PubMed] [Google Scholar]
- 89.Wyde, P. R., D. K. Moore, T. Hepburn, C. L. Silverman, T. G. Porter, M. Gross, G. Taylor, S. G. Demuth, and S. B. Dillon. 1995. Evaluation of the protective efficacy of reshaped human monoclonal antibody RSHZ19 against respiratory syncytial virus in cotton rats. Pediatr. Res. 38:543-550. [DOI] [PubMed] [Google Scholar]
- 90.Xu, J. L., and M. M. Davis. 2000. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity 13:37-45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.