Abstract
Two models describing how alphaherpesviruses exit neurons differ with respect to whether nucleocapsids and envelope glycoproteins travel toward axon termini separately or as assembled enveloped virions. Recently, a pseudorabies virus glycoprotein D (gD)-green fluorescent protein fusion was found to colocalize with viral capsids, supporting anterograde transport of enveloped virions. Previous antibody staining experiments demonstrated that herpes simplex virus (HSV) glycoproteins and capsids are separately transported in axons. Here, we generated an HSV expressing a gD-yellow fluorescent protein (YFP) fusion and found that gD-YFP and capsids were transported separately in neuronal axons. Anti-gD antibodies colocalized with gD-YFP, indicating that gD-YFP behaves like wild-type HSV gD.
Herpes simplex virus (HSV), pseudorabies virus (PRV), and varicella-zoster virus establish life-long latency in neurons and periodically initiate reinfections in epithelial tissues. The ability of alphaherpesviruses to be rapidly transported in neuronal axons in a highly directed fashion is fundamental to successful virus spread and an ability to “outrun” robust host immunity. Specialized fast axonal microtubule transport mechanisms allow viral particles to travel in a bidirectional fashion in sensory neurons (8, 16, 17). In epithelial tissues, alphaherpesviruses enter neuronal axons, releasing capsids that travel on microtubules toward neuronal cell bodies (retrograde direction). Latency can be established in ganglia, and following reactivation, viruses return to epithelial tissues (anterograde direction), again using microtubules.
There is debate over how alphaherpesviruses are transported in the anterograde direction in neuronal axons, with two different models proposed (1, 2-5, 7, 9-12, 14, 15, 18). In the first model, denoted the separate model (14), unenveloped capsids are transported on axonal microtubules separately from vesicles containing viral glycoproteins. In this model, assembly of enveloped virions occurs at axon termini, by budding of capsids into membrane vesicles containing viral glycoproteins. Alternatively, the married model (1, 3, 4) suggests that capsids acquire an envelope containing glycoproteins in neuronal cell bodies and travel in axons as enveloped virions.
Several different imaging techniques have been used to track capsids and glycoprotein vesicles in the axons of cultured neurons, producing support for both models. Electron microscopy (EM) studies, using two- or three-chamber systems to separate axons from cell bodies, have described unenveloped HSV capsids moving in axons (7, 12, 14, 15). Immunogold labeling showed separate staining of glycoproteins and capsids (7, 13). By contrast, recent EM studies of PRV described enveloped particles moving in the anterograde direction (4). However, EM studies suffer from three important problems that make the results more difficult to interpret. First, capsids can be exceedingly rare in thin sections of axons (15). Second, without immuno-EM (which obscures the quality of the image), it is difficult to determine whether viral glycoproteins are present in a lipid envelope (4, 7). There is a real possibility that capsids are contained in vesicles lacking glycoproteins, with assembly following transport. This is really a third model apart from the previously defined separate and married models. Third, the situation is confused by observations that capsids can become enveloped at discrete structures along the way, including branch points, bifurcations, varicosities, and new-growth cones, as well as at axonal termini (15, 19).
Light microscopy studies suffer less from these three problems. Longer sections of axons can be examined, avoiding branch points and varicosities, with discrete puncta, representing capsids and membrane vesicles containing glycoproteins, observed (6, 12, 15, 16). Three sets of light microscopy studies have been reported, coming to two different conclusions about how alphaherpesvirus anterograde transport occurs. Smith and Enquist described PRV V26 green fluorescent protein (GFP)-labeled capsids that were separate from gB stained with antibodies in rat neurons (16). We performed deconvolution immunofluorescence microscopy (IF) and live-cell microscopy of HSV-infected rat and human neurons stained with a panel of glycoprotein- and capsid-specific antibodies and involving VP26 GFP-labeled capsids (18). Our observations were all consistent with the separate model for HSV. However, recently, Antinone and Smith (1) described experiments with a PRV recombinant expressing both gD-GFP and VP26-red fluorescent protein in chicken neurons, demonstrating colocalization of gD and capsids during anterograde transport. On the basis of these observations with this single PRV glycoprotein-GFP fusion, they concluded that the married model was correct. It is not clear how these observations fit together with the previous PRV and HSV immunostaining results (6, 12, 16, 18). We wondered whether the PRV gD-GFP accurately reflects the transport of other alphaherpesvirus glycoproteins. Related to this, several other PRV glycoprotein-GFP fusions were found to be detrimental to virus replication or were nonfluorescent (1) and there are similar unpublished observations with HSV glycoprotein-GFP fusions.
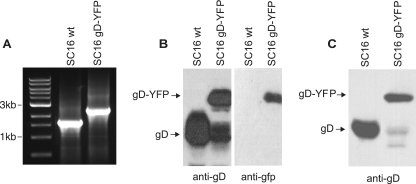
In terms of these IF microscopic studies, one missing piece of the puzzle was whether an HSV gD-GFP fusion would colocalize with capsids during anterograde transport. We constructed an HSV recombinant expressing gD-yellow fluorescent protein (YFP) by inserting YFP sequences into an engineered BglII site at the stop codon of gD (20). A single PCR product of 1,609 bp was detected in DNA produced from wild-type SC16-infected cells (Fig. 1A). By contrast, a 2,324-bp PCR product, corresponding in size to the gD-YFP gene fusion, was observed in DNA from SC16 gD-YFP-infected cells. An immunoblot with anti-gD monoclonal antibody (MAb) LP2 detected two proteins in SC16 gD-YFP-infected cells, a larger form corresponding to the gD-YFP fusion and a smaller form corresponding to wild-type gD (Fig. 1B) which was not detected with anti-YFP antibody (Fig. 1B). This smaller band likely results from proteolytic cleavage of YFP from gD-YFP. It is well known that GFP or YFP fusions are frequently partially cleaved between protein domains. This smaller gD protein provides an important control in our experiments. To examine whether gD-YFP was incorporated into the virion envelope, Vero cell culture supernatants (harvested before any obvious cell breakage) were subjected to high-speed centrifugation (100,000 × g) and pellets were immunoblotted with anti-gD MAb LP14. Both gD-YFP and the smaller form of gD were detected in these preparations (Fig. 1C), supporting the incorporation of both proteins into extracellular virions.
FIG. 1.
(A) Analysis of the gD-encoding gene in HSV SC16 gD-YFP. DNAs from SC16 gD-YFP and wild-type (wt) SC16 were analyzed by PCR with primers within the coding sequence of gD and near the start of the gene for gI. Products were separated on a 0.8% agarose gel. Lanes: 1, 1-kb molecular size marker; 2, HSV-1 SC16; 3, HSV-1 SC16 gD-YFP. (B) Western blot analysis of cell lysates infected with wild-type SC16 or SC16 gD-YFP separated on a 12% polyacrylamide gel, transferred to nitrocellulose membranes, and probed with either anti-gD LP2 or an anti-GFP MAb which reacts with YFP. (C) Western blot analysis of virions pelleted (100,000 × g) from wild-type SC16- or SC16 gD-YFP-infected cell supernatants separated on a 12% polyacrylamide gel, transferred to a nitrocellulose membrane, and probed with anti-gD MAb LP14.
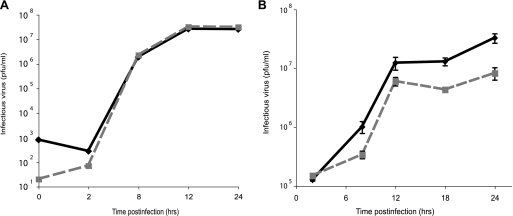
We examined the growth kinetics of SC16 gD-YFP on both Vero cells and SK-N-SH cells, a human neuroblastoma cell line differentiated with retinoic acid to produce axons (18). Growth of SC16 gD-YFP did not differ from that of wild-type SC16 on Vero cells (Fig. 2A), but it produced threefold less infectious virus at 12 h and sixfold less infectious virus at 18 and 24 h compared with wild-type HSV on SK-N-SH neurons (Fig. 2B).
FIG. 2.
Kinetics of HSV SC16 gD-YFP growth on different cell types. Vero cells (A) or differentiated SK-N-SH neurons (B) were infected at 1 PFU/cell with gD-YFP (dashed line) or wild-type HSV SC16 (solid line), pooled cells and cell culture supernatants were harvested at various times, and infectious virus titers were determined on Vero cells.
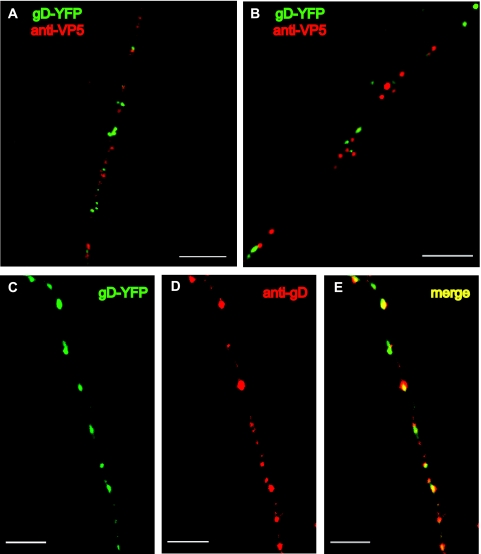
To determine whether gD-YFP colocalized with capsids, SK-N-SH cells were infected with SC16 gD-YFP (1 PFU/cell) for 26 h and neurons were fixed, permeabilized, and stained with a combination of MAbs specific for capsid protein VP5 as previously described (18). Although we previously observed that wild-type HSV type 1 (HSV-1) strain F capsids enter axons at 18 to 20 h, capsids did not enter axons until 24 to 26 h postinfection in both wild-type SC16- and SC16 gD-YFP-infected neurons. Most of the capsids (red puncta) localized separately from gD-YFP (green puncta) (Fig. 3A). Of the 60 capsids and 60 gD-YFP puncta quantitated in eight axons, only 6.6% exhibited signal overlap (defined as merging of >25% of the red and green puncta), with most of the overlap being nonconcentric and in regions of dense puncta. Similar results were observed in primary rat dorsal root ganglion neurons (Fig. 3B) prepared as previously described (18). Therefore, HSV gD-YFP does not colocalize with viral capsids during transport in axons. In other live-cell analyses, gD-YFP was transported at speeds similar to what we previously observed with VP26-GFP and consistent with fast axonal transport (not shown).
FIG. 3.
Localization of capsids and glycoproteins in human and rat neurons infected with HSV SC16 gD-YFP. SK-N-SH neurons (A) or rat dorsal root ganglion neurons (B) were infected with SC16 gD-YFP (1 PFU/cell) for 26 h, fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and stained with mouse anti-VP5 MAbs, followed by Texas Red-conjugated donkey anti-mouse immunoglobulin G as previously described (18). (C to E) SK-N-SH neurons were infected with SC16 gD-YFP, fixed, permeabilized, and stained with rabbit anti-gD polyclonal antibodies, followed by Texas Red-conjugated donkey anti-rabbit immunoglobulin G. Panels: C, gD-YFP fusion; D, rabbit anti-gD polyclonal antibody staining; E, merged fluorescence. Scale bars = 4 μm.
To determine whether the gD-YFP fusion protein colocalized with wild-type gD, SC16 gD-YFP-infected SK-N-SH neurons were stained with gD-specific polyclonal antibodies. The majority of gD-YFP puncta (green) overlapped with puncta observed with gD-specific antibodies (red) (Fig. 3C to E). Puncta varied significantly in intensity, perhaps related to variations in the number of gD and gD-YFP present in individual membrane vesicles or in the availability of gD epitopes. However, when each punctum was examined in separate channels, >95% contained green and red fluorescence. The extensive overlap of gD antibody staining and gD-YFP signal is consistent with the conclusion that gD-YFP accurately predicts the axonal localization of wild-type gD. In other experiments, gD-YFP puncta also overlapped extensively with puncta stained with anti-gB antibodies (not shown).
Previous antibody staining experiments involving PRV (16) and HSV (12, 14, 15, 18) supported the separate model of anterograde transport. By contrast, Antinone and Smith (1) described a dually labeled gD-GFP/VP26-YFP PRV that supported the married model. They further suggested that there are limitations to the use of antibodies and fixed cells to measure the distribution of alphaherpesvirus capsids and glycoproteins because these structures are dynamic in axons. It seems highly unlikely to us that antibody staining would provide an inaccurate picture of the subcellular localization of capsids and glycoproteins at steady state, especially when a panel of antibodies was used (18). Their conclusion that capsids are transported as enveloped virions (including glycoproteins) relied heavily on a single glycoprotein fusion protein, gD-GFP, that was separate from VP26 red fluorescent protein-labeled capsids. Other PRV glycoprotein-GFP fusions reduced virus replication or were not fluorescent (1).
Our HSV gD-YFP was transported separately from capsids that were stained with two anti-VP5 MAbs. Both VP26 GFP-labeled capsids (18) and gD-YFP-labeled vesicles moved in axons with the expected kinetics (data not shown). We can offer two possible explanations for the dramatic difference from what was found by Antinone and Smith with PRV gD-GFP. First, it is possible that HSV and PRV are different in the anterograde transport of capsids. Our studies and those of Cunningham and colleagues (12, 14, 15) clearly support the separate model for HSV. EM studies (4) and analysis with PRV gD-GFP support the married model for PRV. However, it is very difficult to believe that this fundamentally important process would occur by distinct mechanisms for different alphaherpesviruses. Second, it is conceivable that PRV gD-GFP inaccurately reflects the transport of gD and other PRV envelope glycoproteins or compromises some aspect of virus replication or transport. We showed that HSV gD-YFP behaved in a fashion similar to that of a smaller form of gD lacking YFP sequences, suggesting that gD-YFP was transported in axons normally. However, there was a 3- to 6-fold reduction in virus replication in neurons with SC16 gD-YFP, and we previously noted that an HSV expressing VP26-GFP is reduced 10-fold in neurons. Moreover, VP26-GFP capsids enter axons at much later times of infection compared with wild-type HSV capsids (18). It is not clear whether the PRV gD-GFP recombinant has defects in replication or transport in neurons or whether the fusion protein was transported normally. Unfortunately, HSV and PRV recombinants expressing other glycoprotein-GFP fusions are also defective in replication or are not fluorescent, preventing resolution of this incongruity by that approach. We believe this controversy must ultimately be resolved by the use of virus mutants, as with the controversy over herpesvirus egress (20).
Acknowledgments
We are grateful to Aurelie Snyder for assistance with deconvolution microscopy and to Tony Minson for helpful discussion.
This work was supported by the National Institute of Allergy and Infectious Diseases (grant AI73996 to D.J.C. and training grant T32-AI07472 to A.S.), the National Eye Institute (grant EY11245 to D.J.C.), and the Wellcome Trust, UK (grant WT068075 to H.B. and B.B.).
Footnotes
Published ahead of print on 23 May 2007.
REFERENCES
- 1.Antinone, S. E., and G. A. Smith. 2006. Two modes of herpesvirus trafficking in neurons: membrane acquisition directs motion. J. Virol. 80:11235-11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Card, J. P., L. Rinaman, R. B. Lynn, B. H. Lee, R. P. Meade, R. R. Miselis, and L. W. Enquist. 1993. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J. Neurosci. 13:2515-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ch'ng, T. H., and L. W. Enquist. 2005. Efficient axonal localization of alphaherpesvirus structural proteins in cultured sympathetic neurons requires viral glycoprotein E. J. Virol. 79:8835-8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ch'ng, T. H., and L. W. Enquist. 2005. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J. Virol. 79:10875-10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Rio, T., T. H. Ch'ng, E. A. Flood, S. P. Gross, and L. W. Enquist. 2005. Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies. J. Virol. 79:3903-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enquist, L. W., M. J. Tomishima, S. Gross, and G. A. Smith. 2002. Directional spread of an alpha-herpesvirus in the nervous system. Vet. Microbiol. 86:5-16. [DOI] [PubMed] [Google Scholar]
- 7.Holland, D. J., M. Miranda-Saksena, R. A. Boadle, P. Armati, and A. L. Cunningham. 1999. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J. Virol. 73:8503-8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristensson, K., E. Lycke, M. Röyttä, B. Svennerholm, and A. Vahlne. 1986. Neuritic transport of herpes simplex virus in rat sensory neurons in vitro. Effects of substances interacting with microtubular function and axonal flow [nocodazole, taxol and erythro-9-3-(2-hydroxynonyl)adenine]. J. Gen. Virol. 67(Pt. 9):2023-2028. [DOI] [PubMed] [Google Scholar]
- 9.Luxton, G. W., S. Haverlock, K. E. Coller, S. E. Antinone, A. Pincetic, and G. A. Smith. 2005. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc. Natl. Acad. Sci. USA 102:5832-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lycke, E., B. Hamark, M. Johansson, A. Krotochwil, J. Lycke, and B. Svennerholm. 1988. Herpes simplex virus infection of the human sensory neuron. An electron microscopy study. Arch. Virol. 101:87-104. [DOI] [PubMed] [Google Scholar]
- 11.Marchand, C. F., and M. E. Schwab. 1986. Binding, uptake and retrograde axonal transport of herpes virus suis in sympathetic neurons. Brain Res. 383:262-270. [DOI] [PubMed] [Google Scholar]
- 12.Miranda-Saksena, M., P. Armati, R. A. Boadle, D. J. Holland, and A. L. Cunningham. 2000. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 74:1827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miranda-Saksena, M., R. A. Boadle, P. Armati, and A. L. Cunningham. 2002. In rat dorsal root ganglion neurons, herpes simplex virus type 1 tegument forms in the cytoplasm of the cell body. J. Virol. 76:9934-9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saksena, M. M., H. Wakisaka, B. Tijono, R. A. Boadle, F. Rixon, H. Takahashi, and A. L. Cunningham. 2006. Herpes simplex virus type 1 accumulation, envelopment, and exit in growth cones and varicosities in mid-distal regions of axons. J. Virol. 80:3592-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith, G. A., S. P. Gross, and L. W. Enquist. 2001. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc. Natl. Acad. Sci. USA 98:3466-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith, G. A., L. Pomeranz, S. P. Gross, and L. W. Enquist. 2004. Local modulation of plus-end transport targets herpesvirus entry and egress in sensory axons. Proc. Natl. Acad. Sci. USA 101:16034-16039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder, A., T. W. Wisner, and D. C. Johnson. 2006. Herpes simplex virus capsids are transported in neuronal axons without an envelope containing the viral glycoproteins. J. Virol. 80:11165-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomishima, M. J., and L. W. Enquist. 2002. In vivo egress of an alphaherpesvirus from axons. J. Virol. 76:8310-8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whiteley, A., B. Bruun, T. Minson, and H. Browne. 1999. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J. Virol. 73:9515-9520. [DOI] [PMC free article] [PubMed] [Google Scholar]