Abstract
Herpes simplex viruses (HSV) reactivate at rates proportional to the viral loads in latently infected ganglia. However, these rates vary substantially among infected animals. We assessed whether the numbers of HSV-specific CD8+ T cells infiltrating latently infected ganglia also affect reactivation rates and contribute to their variability. Following corneal infection of mice with HSV type 2 (HSV-2), we quantified the latent viral loads in dissociated trigeminal ganglia by real-time PCR, the numbers of infiltrating CD8+ T cells by flow cytometry, and the rates of reactivation by the detection of cell-free virus released from ganglion cells cultured in 96-well plates. The reactivation rates correlated directly with the latent viral loads (P = 0.001) but did so more strongly (P = 10−7) when cultures were depleted of CD8+ T cells. Reactivation rates were reduced in a dose-dependent fashion by adding back ganglion CD8+ T cells to the cultures (P = 0.003). We related the latent viral loads, numbers of CD8+ T cells, and reactivation rates by mathematical equations. The rates of reactivation predicted from latent viral loads and numbers of infiltrating CD8+ T cells in dissociated ganglia correlated with the observed rates of reactivation (P = 0.04). The reactivation of HSV-2 from ganglia ex vivo is determined both by the latent viral load and the number of infiltrating CD8+ T cells.
The establishment of neuronal latency and periodic reactivation are definitive features of herpes simplex virus (HSV) infections in animals and humans. While the determinants of reactivation rates in humans are not well defined, the competence of the host cellular immune response is thought to be important in controlling reactivation (22, 43). In animals, the quantity of latent HSV type 1 (HSV-1) DNA in ganglia (the latent viral load) as estimated by levels of latency-associated transcripts (13, 16) and more precisely by quantitative PCR of viral DNA (29, 33-35) is an important determinant of recurrence rates ex vivo. We confirmed this association in vivo, as well, using infected guinea pigs in which genital disease due to HSV-1 or HSV-2 recurs spontaneously (17, 25).
Within individual studies, recurrence rates of HSV infection vary up to 10-fold, even among animals with comparable latent viral loads (17). Presumably, in these animals, other factors in addition to latent viral loads contribute to reactivation rates. An important clue to the nature of such factors was provided in recent seminal reports by Khanna et al. and Liu et al., who showed that HSV-specific, gamma interferon (IFN-γ)-positive, CD8+ T cells infiltrate latently infected mouse trigeminal ganglia (TG) and block the reactivation of HSV-1 from latency ex vivo in a dose-dependent manner (19, 20, 27). Neither HSV-1 nor HSV-2 undergoes spontaneous reactivation as defined by the production of infectious virus in mice; however, more sensitive molecular techniques have demonstrated low levels of spontaneous viral antigens and productive cycle transcripts in ganglia of mice latently infected with HSV-1 (14). HSV-1 and HSV-2 also do not reactivate very efficiently ex vivo in the absence of inductive stimuli, such as the treatment of mice with UV light (42), hyperthermia (36), or the addition of demethylating agents like hexamethylene bisacetamide to explanted ganglion cultures (1, 23).
We explored the hypothesis that infiltrating HSV-specific CD8+ T cells contribute to the inherently low rates and the variability of the rates of reactivation of virus from mouse TG. We tested whether the removal of CD8+ T cells from cultured, HSV-2-infected ganglia permitted virus to reactivate at higher rates that would correlate even more strongly with the latent viral loads and reduce the variability of reactivation rates compared to those for non-T-cell-depleted cultures. Further, we hypothesized and sought to confirm that infiltrating HSV-specific CD8+ T cells block HSV-2 reactivation from ganglia in a dose-dependent manner.
MATERIALS AND METHODS
Viruses and animal studies.
HSV-2 strain 333 was propagated in Vero cells and the titers of virus in Vero cells were determined as previously described (17). After the administration of an anesthetic comprising 0.12 mg of xylazine and 1.2 mg of ketamine per mouse, groups of 9- to 10-week-old C57BL/6 or DBA2 mice were challenged with HSV-2 strain 333 on both of their scarified (C57BL/6) or unscarified (DBA2) corneas with a range of inocula from 1.6 × 102 to 1.6 × 105 PFU/mouse. Because of the virulence of HSV-2 strain 333 in this ocular infection model, 0.5 ml of human immunoglobulin G (a 1:8 dilution in phosphate-buffered saline; Abbott Labs, Chicago, IL) was injected intraperitoneally into each mouse 24 h after infection so that most of the mice would survive the acute infection and attain substantive levels of latency (10). Twenty-seven days after immunoglobulin G treatment, 7 to 10 mice per group were sacrificed and their TG were harvested. Ganglia from each group of mice were pooled, and the pools were dispersed into single cell suspensions by using collagenase type I (3 mg/ml for 1.5 h; Sigma, St. Louis, MO). In pooled tissues, there were 4,000 to 10,000 neurons per TG as determined by morphology observed by microscopy. From the TG suspensions for each group, numbers of cells equivalent to those obtained from 3 to 4.5 ganglia were used for nondepleted cultures; numbers equivalent to those obtained from 8 to 10 TG were used for studies of CD8+-T-cell depletion in culture. Aliquots equivalent to one TG each were studied by real-time PCR to measure the latent viral load or subjected to flow cytometry to quantify infiltrating HSV-2-specific IFN-γ-expressing CD8+ T cells.
Quantitative real-time PCR.
Aliquots of suspension cultures to be used for viral load determinations were divided into two tubes each and stored at −80°C. DNA was isolated independently from each sample by using the Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN). The numbers of copies of DNA genomes of latent HSV-2 in each sample were determined by real-time PCR using the Taqman system (ABI 7700 sequence detector; PE Applied Biosystems, Foster City, CA) with primers and probes specific for HSV-2 gD, as described previously (17). Each reaction mixture included 200 ng of ganglion DNA. A standard curve for the assay was generated using known numbers of copies of a plasmid containing the HSV-2 gD coding region diluted in salmon sperm DNA. The detection limit of this PCR assay proved to be ∼4 copies/reaction mixture, and the assay results showed excellent linearity (R > 0.92) over 4 logs of DNA content. The latent viral load in infected ganglia was calculated from the means of results from at least two independent reactions performed in duplicate with two independently extracted pools of dissociated TG cells from each group of mice. Thus, determinations of latent viral load for each ganglion arose from the results of at least eight PCR assays.
Flow cytometry.
Flow cytometry analyses were done as previously reported (17). First, the CD8+ T cells infiltrating each TG were counted. Each aliquot of a TG suspension culture, equivalent to one entire latently infected TG, was passed through a cell strainer and stained with fluorescein isothiocyanate-conjugated anti-CD8 and peridinin chlorophyll-conjugated anti-CD45 (a panleukocyte marker) monoclonal antibodies (both from BD Pharmingen, San Diego, CA). The cells were examined promptly on a FACSCaliber cytometer (Becton Dickinson, Franklin Lakes, NJ), and data were analyzed using FlowJo software (version 4.5.9; Tree Star Inc., Ashland, OR). The numbers of CD8+ T cells among pooled TG varied somewhat, as expected given the use of two independent strains of mice and the wide range of virus inocula employed, but the numbers for each group of mice were normalized according to the numbers of CD45-negative cells, as these proved to be far less variable per ganglion.
Aliquots of TG suspensions equivalent to one TG each were used for quantifying HSV-2-specific IFN-γ-expressing CD8+ T cells as previously described (17). Briefly, P815 cells (kindly supplied by John Yewdell) were infected with HSV-2 strain 333 at a level of 3 PFU/cell for 5 h. Dissociated TG cells were resuspended in Iscove's Dulbecco modified medium supplemented with 10% fetal bovine serum, 20 U of interleukin-2 (IL-2)/ml, glutamine, and antibiotics. TG lymphocytes were stimulated by coincubation with P815 cells infected with HSV-2 in the presence of 10 μg of brefeldin A (Sigma)/ml for 5 more hours. The cells were washed and stained with fluorescein isothiocyanate-conjugated anti-CD8 and peridinin chlorophyll-conjugated anti-CD45 monoclonal antibodies (BD Pharmingen). After another washing, the cells were fixed and permeabilized with Cytofix/Cytoperm buffer (BD Pharmingen) and washed with Permwash buffer (BD Pharmingen) according to the manufacturer's protocol. Cells were stained with phycoerythrin-conjugated anti-IFN-γ monoclonal antibody (BD Pharmingen). After the final wash, the cells were analyzed by flow cytometry, as described above.
Depletion of virus-specific CD8+ T-cells in TG single-cell suspensions.
Aliquots of TG suspensions equivalent to eight to nine TG each were depleted of infiltrating HSV-2-specific CD8+ T cells by using CD8-immunomagnetic beads (Dynal Biotech, Norway) according to the manufacturer's instructions (five cycles of depletions with 6 to 10 beads/cell). Analyses of cells by flow cytometry before and after the cycles of depletion with immunobeads showed that more than 90% of CD8+ cells were removed from every immunobead-depleted aliquot of dissociated TG cells. During each individual cycle of depletion, about 10% of the infiltrating CD8+ T cells were lost nonspecifically, but the numbers of neurons in the starting and final cell suspensions were determined microscopically and adjusted relative to the numbers found in non-cell-depleted control culture wells.
CD8+-T-cell add-back studies.
Fifty DBA2 mice were infected ocularly with a total of 4 × 104 PFU per mouse. Twenty-nine days later, the mice were sacrificed and their 100 TG were harvested and dissociated. CD8+ T cells were recovered from these ganglia by using releasable beads (CELLection biotin kit; Dynal) with biotin-conjugated CD8b monoclonal antibody (BD Pharmingen) according to the manufacturer's protocol (10 beads/cell and four cycles each). After release from the beads, the recovered CD8+ T cells were counted and added back to dissociated TG cell culture wells at a range of cell numbers per well. The reactivation rates for each set of wells to which the same number of CD8+ T cells were added were calculated.
HSV-2 reactivation ex vivo.
The reactivation of latent HSV-2 from infected TG cell suspension cultures in 96-well plates was demonstrated by the release of infectious virus into the medium of each well as detected by the transfer of the medium to Vero cell monolayer indicator cultures and the serial examination of the cultures for cytopathic effects typical of HSV replication. In the present experiments, we determined and quantified the rates of reactivation ex vivo of HSV-2 from latently infected TG cell suspensions that had or had not been depleted specifically of CD8+ T cells by using immunomagnetic beads. For these assays, all TG cell suspensions were cultured in Iscove's Dulbecco modified medium supplemented with 10% fetal bovine serum, 10 U of IL-2/ml, glutamine, and antibiotics in 96-well flat-bottom culture plates at a cell density equivalent to 0.25 TG/well. Daily, or every other day, one-fourth of the volume of the supernatants from each well was transferred onto Vero cell monolayers and an equal volume of fresh medium was added to each well. All Vero cell cultures were examined microscopically for cytopathic effects after 2 days by using crystal violet staining.
In an initial control experiment, the effect of the T-cell depletion procedure upon rates of virus reactivation was tested by comparing reactivation rates for nondepleted TG cultures with those for mock-depleted cultures in which anti-biotin-conjugated beads were used without any biotin-conjugated antibodies for five cycles of incubation with ganglion cells. We observed no substantial effects of mock depletion on virus reactivation. Specifically, virus in one well among 16 wells of nondepleted cultures reactivated, whereas virus in two wells among 16 wells of mock-depleted cultures reactivated. Based on this result, nondepleted cultures were used in all subsequent experiments.
Calculation of rates of reactivation of HSV from latency.
To quantify the rates of reactivation of HSV ex vivo from depleted or nondepleted TG cell cultures, we assumed that the reactivation of HSV-2 from latency in any given TG culture is an entirely random event that takes place with the frequency r (the reactivation rate per TG per day). Thus, the probability of not reactivating is expressed as follows: 1 − r. Therefore, the probability that HSV will not reactivate in TG cell cultures during d consecutive days (PnonR) is as follows: PnonR = (1 − r)d. Taking the natural logarithm of both sides of the equation gives the following: ln(PnonR) = d·ln(1 − r). Since the reactivation rate r is reasonably assumed to be a small value, ln(1 − r) can be approximated by −r, and therefore, we have the following expression: ln(PnonR) ≈ −r·d. Thus, we can denote all cumulative reactivation events in ex vivo TG cultures (r·d) by the expression −ln(PnonR), which should be linear with respect to the number of days in culture. Therefore, when the cumulative reactivation events [−ln(PnonR)] for pooled TG cultures are plotted on the y axis with the number of days in culture plotted on the x axis, the slope of the linear regression line provides an estimation of r.
Statistical analysis.
All statistical analysis was done using JMP 5.01 software (SAS Institute Inc., Cary, NC).
RESULTS
HSV-specific CD8+ T cells infiltrate latently infected mouse ganglia.
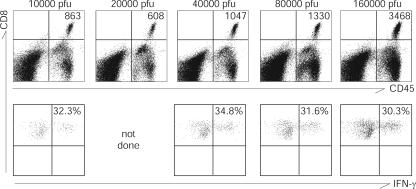
To address the potential effects of CD8+ T cells on HSV-2 reactivation ex vivo, we first needed to quantify these cells and characterize their phenotypes in infected ganglia. In serial, independent experiments, the eyes from two strains (CD57BL/6 and DBA2) of mice with different susceptibilities to HSV infection were infected with different titers of HSV-2. Twenty-eight days later, the TG from these mice were harvested and the numbers of infiltrating CD8+ T cells were determined using flow cytometry. Pooled TG from both strains of mice contained CD8+ CD45+ lymphocytes (Fig. 1, upper panels). Approximately 30 to 35% of these infiltrating CD8+ T cells were HSV-2 specific, as shown by the production of IFN-γ in response to HSV-2-infected antigen-presenting cells (Fig. 1, lower panels). In preliminary experiments, most of these infiltrating CD8+ cells were CD3+ and CD62L−, indicating that they had the effector memory T-cell phenotype; a small minority (approximately 3 to 5%, ranging from 1 to 10%) were central memory T cells (CD3+, CD62L+) (data not shown). The numbers of infiltrating CD8+ T cells were generally greater following infection with higher titers of HSV-2; however, the frequencies of HSV-specific IFN-γ-producing CD8+ T cells remained similar across the wide range of virus inocula used.
FIG. 1.
HSV-2-specific CD8+ T cells infiltrate the TG of latently infected mice. Unscarified corneas of DBA2 mice were infected with various amounts of virus, as indicated above each dot plot. Twenty-eight days after inoculation, six to seven mice from each group were sacrificed and TG from the same group were pooled. In the upper panels, the x axis indicates CD45 expression, and in the lower panels, the x axis indicates IFN-γ expression after incubation with HSV-2-infected P815 cells. The y axis indicates CD8 expression in both the upper and lower panels. For all panels, cells were gated on lymphocyte populations from forward and side scatter as determined by back gating using the CD45+ population. The numbers of CD8+ T cells per TG and the percentages of HSV-2-specific IFN-γ-producing CD8+ T cells are shown in the upper right quadrants of the upper and lower panels, respectively.
The latent viral load correlates with reactivation rates more strongly when cultures are depleted of CD8+ T cells.
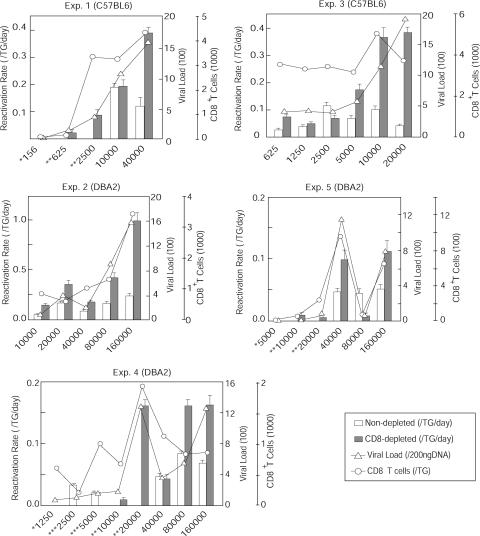
We performed five independent experiments using two strains of HSV-2-infected mice and calculated the reactivation rates. When the results of these experiments were pooled, the virus reactivation rates for CD8 cell-depleted cultures were significantly higher than those for nondepleted cultures (Fig. 2) (P, 0.002 by Wilcoxon test). Reactivation rates for both CD8 cell-depleted and nondepleted cultures correlated significantly with the titers of the inocula and the latent viral loads. However, the reactivation rates for both CD8 cell-depleted and nondepleted cultures correlated more strongly with the latent viral loads than with the titers of the inocula (P = 2 × 10−7 and 2 × 10−4 for the latent viral loads and the titers of the inocula, respectively, for CD8 cell-depleted cultures; P = 2 × 10−3 and 3 × 10−3 for the latent viral loads and the titers of the inocula, respectively, for nondepleted cultures; Spearman's rho test). Thus, the latent viral load is a better predictor of reactivation than the titer of the inoculum.
FIG. 2.
Latent viral loads, numbers of CD8+ T cells, and ex vivo reactivation rates for TG cultures from C57BL/6 and DBA2 mice. Reactivation rates for CD8 cell-depleted (gray bars) and nondepleted (white bars) cultures were determined. Error bars indicate standard errors. Latent viral loads (triangles) and numbers of CD8+ T cells (circles) were also determined. The y axis on the left of each panel shows the reactivation rate, and the y axis on the right of each panel shows the latent viral load and CD8+ T cell number. The strain of mice is shown at the top of each panel, and the amounts of virus (PFU) in the inocula are indicated at the bottom. * indicates that neither CD8 cell-depleted nor nondepleted cultures showed reactivation, ** indicates that nondepleted cultures did not show reactivation, and *** indicates that CD8 cell-depleted cultures did not show reactivation. Exp., experiment.
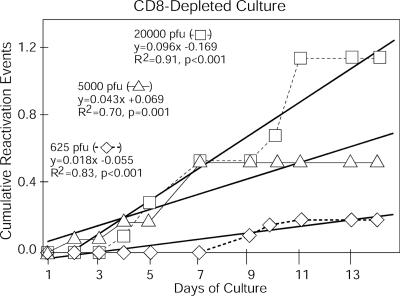
Using the stochastic model of in vitro reactivation of HSV from latency (see Materials and Methods), we determined the number of cumulative reactivation events. The number of these events increased linearly with increasing days in culture (data for three different titers of inocula from experiment 1 in Fig. 2 are plotted in Fig. 3). The mean correlation coefficient for 46 regression lines for all five experiments was 0.83 ± 0.03 (mean ± standard error), indicating that the stochastic model appropriately describes the occurrences of ex vivo reactivation events in cultured TG cells.
FIG. 3.
Linear increase in cumulative reactivation events with increasing time in culture. The numbers of cumulative reactivation events are indicated on the y axis, and days after culture ex vivo are indicated on the x axis. The input inoculum (PFU) used to infect each group of mice is indicated on the figure.
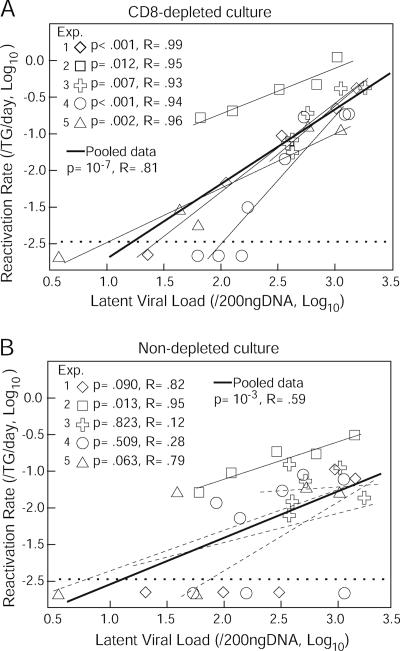
An analysis of pooled data from all five experiments showed that the correlation between latent viral loads and HSV-2 reactivation rates for CD8 cell-depleted cultures was highly significant and linear when plotted on a log scale (P = 10−7 by linear regression analysis) (Fig. 4A). Each independent experiment had a correlation coefficient R of ≥0.93. In contrast, the correlation between latent viral loads and HSV-2 reactivation rates for non-CD8 cell-depleted cultures was significant for only one of the five experiments (Fig. 4B). When the results of these five experiments were pooled, the correlation between reactivation rates for non-CD8-cell-depleted cultures and latent viral loads was significant (P = 10−3); however, the correlation coefficient for latent viral loads was much higher for CD8 cell-depleted cultures (R = 0.81) than for nondepleted cultures (R = 0.59). Thus, reactivation rates are higher and correlate more strongly with latent viral loads when CD8+ T cells are removed from ex vivo cultures.
FIG. 4.
Correlation between latent viral loads and reactivation rates. The log10 reactivation rates for the CD8 cell-depleted cultures (A) and the nondepleted cultures (B) are indicated on the y axis, and the log10 latent viral loads are shown on the x axis. Each type of symbol corresponds to one experiment (Exp.). Solid thin lines indicate statistically significant linear regression, while dashed thin lines indicate nonsignificant linear regression. The P and R values for the linear regression analysis for each experiment are shown. Thick lines indicate linear regression for pooled data. The horizontal dotted lines indicate the lower limit of detection. Experiments 1 and 3 were done with C57BL/6 mice, and experiments 2, 4, and 5 were done with DBA2 mice (see Fig. 2 for details).
HSV-2 reactivation rates for CD8-depleted cultures and latent viral load data from DBA2 mice and C57BL/6 mice showed no notable differences between the two strains of mice (P = 0.96 by analysis of covariance) (Fig. 4A). The equation of the linear regression line for pooled data from five experiments with CD8 cell-depleted cultures using the log10 scale was as follows: y = 0.998x − 3.711. For this equation, y and x are defined as follows: y = log10(reactivation rate/TG/day), and x = log10(latent viral load/200 ng of DNA). Therefore, the equation becomes as follows: log10(reactivation rate/TG/day) = 0.998 × log10(latent viral load/200 ng of DNA) − 3.711. Taking the antilog of both sides of the equation gives the following: 10log10(reactivation rate/TG/day)= 100.998 × log10(latent viral load/200 ng of DNA) − 3.711. This equation converts as follows: (reactivation rate/TG/day) = 10log10(latent viral load/200 ng of DNA) × 0.998× 10−3.711. This expression is the same as the following: (reactivation rate/TG/day) = (latent viral load/200 ng of DNA)0.998 × 10−3.711. Since 10−3.711 is 1.95 × 10−4, and if v is the latent viral load (genome copies per 200 ng of DNA), then the following equation applies to the CD8 cell-depleted cultures: (reactivation rate/TG/day) = 1.95 × 10−4·v0.998.
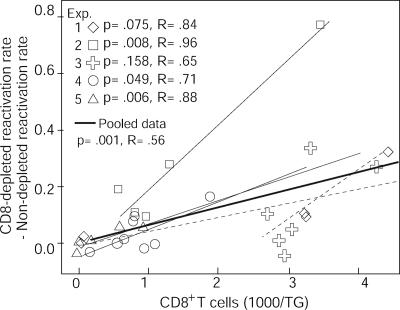
Dose-dependent reduction of HSV-2 reactivation rate by CD8+ T cells.
We postulated that the numbers of infiltrating CD8+ T cells should correlate with the reduction in virus reactivation rates in a dose-dependent manner. Since the rates of reactivation for the CD8 cell-depleted cultures were higher (Fig. 2 and 4), we calculated the extent to which the CD8+ T cells suppressed reactivation by subtracting the reactivation rates for the nondepleted cultures from the reactivation rates for the CD8 cell-depleted cultures. If the effect of CD8+ T cells on blocking reactivation rates is dose dependent, then there should be a positive correlation between the numbers of CD8+ T cells and the difference in reactivation rates for CD8 cell-depleted and nondepleted cultures. In three experiments (Fig. 5), the correlation was significant, while in two experiments (Fig. 5), the correlation did not reach significance. When data from the five experiments were pooled (Fig. 5), the correlation between numbers of CD8+ T cells and the difference in reactivation rates for CD8 cell-depleted and nondepleted cultures was significant (P = 0.001). The equation of the linear regression line is as follows: y = (6.61 × 10−5)t − 1.68 × 10−3, where t is the number of CD8+ T cells per TG and y is the reduction in the reactivation rate due to CD8+ T cells. Therefore, we estimate the amount of reduction in the reactivation rate due to CD8+ T cells to be as follows: 6.61 × 10−5(t − 25)/day for t CD8+ T cells.
FIG. 5.
Correlation between the numbers of infiltrating CD8+ T cells and the differences in reactivation rates for CD8 cell-depleted and nondepleted cultures. The differences in reactivation rates for CD8 cell-depleted and nondepleted cultures are indicated on the y axis, and the numbers of infiltrating CD8+ T cells per TG are indicated on the x axis. Each type of symbol represents one experiment (Exp.) and corresponds to the same animal as the same type of symbol in Fig. 4, as samples from the same animals were used for both experiments. Solid thin lines indicate statistically significant linear regression, dashed thin lines indicate nonsignificant linear regression, and thick lines indicate linear regression for pooled data. The P and R values for the linear regression analysis for each experiment are shown.
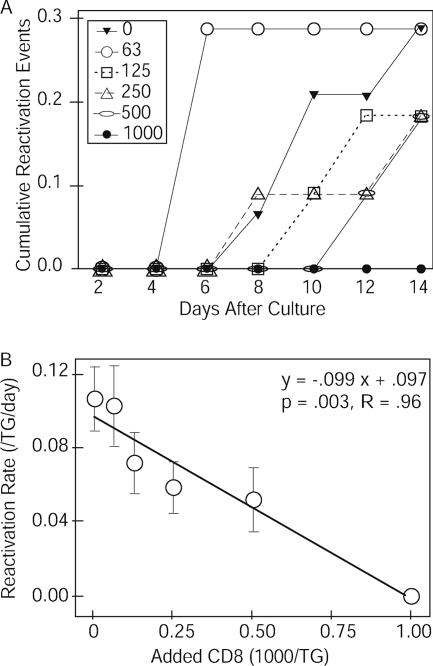
Next, we used a different technique to confirm the dose-dependent reduction in the reactivation rate by primary CD8+ T cells. Primary CD8+ T cells were recovered from latently infected mouse TG and added back to CD8 cell-depleted cultures in various numbers. The reactivation of HSV occurred more slowly when higher numbers of CD8+ T cells were added to the culture; when a large number (1,000) of CD8+ T cells was added, reactivation was completely blocked (Fig. 6A). The magnitude of reduction in the reactivation rate correlated significantly (P = 0.003) and linearly (R = 0.96) with the number of CD8+ T cells added to the culture (Fig. 6B).
FIG. 6.
Dose-dependent reduction in reactivation rates by the addition of infiltrating CD8+ T cells. Panel A shows the numbers of cumulative reactivation events (y axis) and days after culture (x axis). The numbers in the box are the numbers of CD8+ T cells added back to the cultures. Panel B shows the numbers of CD8+ T cells added to the culture wells (x axis) and the reactivation rates (y axis), with error bars indicating standard errors. The linear regression line in panel B is derived from the data in panel A.
Mathematical model for ex vivo reactivation rate.
Finally, we postulated that the reactivation rate should be able to be derived solely from the latent viral load and the number of infiltrating CD8+ T cells. By using the two equations given above, the predicted reactivation rate for a CD8 cell-depleted TG culture is as follows: (1.95 × 10−4)v0.998/day, where v is the latent viral load (genome copies per 200 ng of DNA). The reduction in the reactivation rate due to CD8+ T cells is as follows: 6.61 × 10−5(t − 25)/day, where t is the number of CD8+ T cells. Combining those two equations, we predicted the reactivation rate for nondepleted cultures (i.e., the reactivation rate for CD8 cell-depleted cultures minus the reduction in the reactivation rate due to CD8+ T cells), based on the latent viral load and the number of CD8+ T cells, to be as follows: (1.95 × 10−4)v0.998 − (6.61 × 10−5)(t − 25)/day.
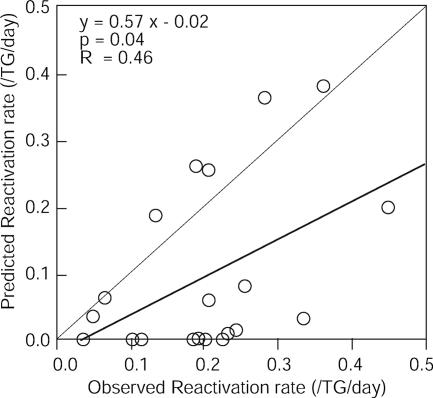
We performed an experiment to see if the equation would predict the reactivation rate for nondepleted cultures. Forty C57BL/6 mice were infected with three different doses of HSV-2 by the scarification of both corneas. Mice were sacrificed 28 days after infection. Twenty individual pools of TG, each containing four TG from two mice, were dispersed into single cell suspensions. The latent viral loads and the numbers of CD8+ T cells in a portion of the TG cell suspension (equivalent to one TG) were measured, and the reactivation rates for non-CD8-cell-depleted cultures were measured using the rest of the TG suspension (equivalent to three TG). From the latent viral loads and numbers of CD8+ T cells, we predicted the reactivation rates for nondepleted cultures. The predicted reactivation rates for 20 pooled TG correlated significantly with the observed reactivation rate (P = 0.04) (Fig. 7).
FIG. 7.
Mathematically predicted reactivation rates correlate significantly with observed reactivation rates. Forty mice were infected with HSV-2. The reactivation rates predicted based on the latent viral loads and the numbers of CD8+ T cells are plotted on the y axis, and the observed reactivation rates are plotted on the x axis. The thick line indicates the linear regression between predicted and observed reactivation rates, and the thin line represents the expression y = x, which is the reactivation rate predicted from the model. The equation, P value, and R value for the linear regression analysis are shown.
DISCUSSION
We have studied mice latently infected with HSV-2 to understand the interaction between HSV-2 and virus-specific cellular immune responses during latency. We found that (i) the ex vivo HSV-2 reactivation rate correlates directly with the latent viral load in the absence of CD8+ T cells, (ii) the number of infiltrating CD8+ T cells influences the ex vivo reactivation rate, but less directly than the latent viral load, and (iii) a mathematical model derived from the measurement of the latent viral load and the number of CD8+ T cells can predict the ex vivo reactivation rate. Our results were derived from ex vivo reactivation experiments with mice, and the interactions between HSV-2 and CD8+ T cells may be different during the spontaneous reactivation of HSV-2 in humans.
We have shown that the total numbers of CD8+ T cells in latently infected mouse ganglia correlate with the titers of the inocula used to infect the animals; however, the frequencies of HSV-2-specific CD8+ T cells in the ganglia are independent of the titers of the inocula. These results imply that assessing both virus-specific and nonspecific CD8+ T cells, rather than solely HSV-specific CD8+ T cells, gives a more accurate measure of the immune response induced by the latent infection of neurons with HSV. Most (more than 90%) of the CD8+ T cells in the ganglia were effector memory cells (CD62L−), indicating that the CD8+ T cells in latently infected ganglia are persistently stimulated rather than simply remaining in the ganglia after acute HSV infection. Our results agree with previous observations that activated memory CD8+ T cells accumulate in mouse TG latently infected with HSV (19) and that latent HSV infection provides antigenic stimulation to CD8+ T cells (40).
We found that 30 to 35% of the total CD8+ T cells in the ganglia of infected mice were HSV-2 specific and produced IFN-γ in response to virus. Khanna et al. showed that more than 60% of infiltrating CD8+ T cells in mouse ganglia recognize a single glycoprotein B peptide incorporated into a tetramer and that most of these CD8+ T cells are activated and express CD69 and CD44 at 1 month after infection (19). In addition to CD8+ T cells, other types of cells such as γδT cells, NK cells, CD4+ T cells, macrophages, and microglial cells may also affect the rate of reactivation. Some of these cells have been shown to control acute HSV infection (2, 5, 9, 18, 30, 31, 37), but the function of individual cell types other than CD8+ T cells during HSV reactivation from latently infected neurons has not been studied.
Infiltrating T cells in latently infected mouse TG secrete IFN-γ, tumor necrosis factor alpha, and other cytokines (e.g., IL-2, IL-10, IL-4, and RANTES) (4, 7, 15, 28). After the induction of reactivation by various stimuli, cytokine-producing lymphocytes accumulate around HSV antigen-positive neurons. Changes in levels of transcripts of immune response genes in latently infected ganglia have also been detected previously. A comparison of mRNAs in mouse TG latently infected with HSV with those in mock-infected TG showed that multiple immune response genes are significantly upregulated during latency, including genes for IFN-γ, tumor necrosis factor alpha, macrophage inflammatory protein 1α, macrophage inflammatory protein 1β, RANTES, CCR6, and CXCR3 (6, 8). The importance of the innate immune response in latency and the control of reactivation is evident from the results of experiments showing that latent HSV-1 reactivates at higher frequencies in IFN-γ or IFN-γ receptor knockout mice than in wild-type mice and that IFN-γ can block the in vitro reactivation of HSV-1 even after the expression of late viral gene products (3, 12, 24, 26).
We found that primary HSV-specific CD8+ T cells, obtained from the ganglia of HSV-2-infected mice, can block the reactivation of HSV from latency ex vivo in a dose-dependent manner. We demonstrated this both (i) by comparing reactivation rates for cultures depleted of CD8+ T cells with those for cultures not depleted of these cells (Fig. 5) and (ii) by adding increasing numbers of CD8+ T cells to HSV-2-infected TG cell cultures (Fig. 6). The results of our experiments using primary infiltrating CD8+ T cells are consistent with those of studies by Khanna et al. in which an HSV-specific CD8+-T-cell clone, specific for glycoprotein B, inhibited the reactivation of HSV-1 from latently infected mouse TG cultures ex vivo in a dose-dependent manner (19, 21) and with those of studies by Noisakran and Carr using lymphocytes from immune mice (32). One important difference between the findings of our study and those of Khanna et al. is the number of CD8+ T cells needed to block reactivation. Khanna et al. found that 106 HSV-specific CD8+ T cells derived from the T-cell clone did not block reactivation completely when added to TG cell cultures; however, we found that 103 HSV-specific primary CD8+ T cells completely blocked reactivation (Fig. 6). The difference in our findings may be due to differences in culture systems or strains of animals or viruses but also may indicate that the primary infiltrating CD8+ T cells are more effective in blocking reactivation than an HSV-specific CD8+-T-cell clone.
We developed a mathematical model, based on the latent viral load and the number of infiltrating CD8+ T cells in ganglia, to predict the ex vivo reactivation rate and validated the model prospectively. The difference in the predicted reactivation rate and the observed rate may be due to biological variation, other types of cells that contribute to reactivation (e.g., CD4+ T cells and NK cells), or local concentrations of cytokines or chemokines. The numbers of infiltrating CD8+ T cells and the latent viral loads correlated significantly (P = 10−6) when data from five experiments were pooled (data not shown). An increased latent viral load may increase the probability of virus reactivation from neurons; however, a higher viral load may also increase the number of CD8+ T cells infiltrating the ganglia. Both of these effects likely contribute to the observed rate of reactivation. We speculate that low-level, subclinical reactivation of latent HSV in mouse TG, as proposed by Feldman et al. (14), maintains CD8+ T cells in TG long after acute infection is over. In the present study, we used TG infected with HSV-2. While most HSV-2 infections result in genital disease, HSV-2 does cause ocular and oral disease. We have found the ocular model of HSV-2 infection to be useful for studies of immunity, latency, and the reactivation of HSV-2 (17, 42). Nonetheless, the rates of reactivation of HSV-2 from the TG differ from those from the lumbosacral ganglia.
Our findings have implications for the development of both therapeutic and prophylactic HSV-2 vaccines. CD8+ T cells in human TG from HSV-seropositive subjects have been detected previously (39), a finding similar to our observations with mice. An immunotherapeutic vaccine which seeks to reduce HSV-2 reactivation rates should aim to increase the number of infiltrating virus-specific CD8+ T cells in the ganglia. Therapeutic vaccines have shown efficacy in guinea pig models of HSV infection when given shortly after primary infection (17, 41); however, a therapeutic vaccine for humans would need to be effective in persons who were infected several months or years prior to vaccination. Large-scale clinical trials of therapeutic vaccines using recombinant glycoprotein D or virus with the deletion of glycoprotein H have not been successful in reducing recurrence rates in HSV-2-seropositive persons with recurrent genital herpes (11, 38). Our studies suggest that the quantification of virus-specific CD8+ T cells in the ganglia of animals, especially over time, may provide a useful surrogate marker to predict the ability of a vaccine to reduce the rate of reactivation.
A prophylactic vaccine should similarly increase the level of virus-specific cells in the ganglia; if the vaccine contains live virus that can reactivate, it should yield a low or absent latent viral load in the ganglia. Our data indicate that a low latent viral load is the most critical factor to prevent reactivation. While a number of vaccines have been effective in animal studies, a vaccine that effectively prevents HSV-2 disease in humans does not yet exist. Our studies provide a mathematical model for the relationship of the latent viral load, the HSV-specific CD8+-T-cell response, and the rate of reactivation that may be useful in the development of future vaccines and the testing of these vaccines in animals.
Acknowledgments
This study was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases. Part of Yo Hoshino's work was supported by the Japan Herpes Virus Infection Forum (JHIF).
We thank Kennichi Dowdell, Kening Wang, Anthony Nicola, and Kozaburo Hayashi for suggestions and assistance and Jonathan Yewdell for the P815 cell line.
Footnotes
Published ahead of print on 23 May 2007.
This paper is dedicated to the memory of Stephen E. Straus, who was our mentor and inspiration for this paper.
REFERENCES
- 1.Bernstein, D. I., and J. C. Kappes. 1988. Enhanced in vitro reactivation of latent herpes simplex virus from neural and peripheral tissues with hexamethylenebisacetamide. Arch. Virol. 99:57-65. [DOI] [PubMed] [Google Scholar]
- 2.Bonneau, R. H., and S. R. Jennings. 1989. Modulation of acute and latent herpes simplex virus infection in C57BL/6 mice by adoptive transfer of immune lymphocytes with cytolytic activity. J. Virol. 63:1480-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantin, E., B. Tanamachi, H. Openshaw, J. Mann, and K. Clarke. 1999. Gamma interferon (IFN-gamma) receptor null-mutant mice are more susceptible to herpes simplex virus type 1 infection than IFN-gamma ligand null-mutant mice. J. Virol. 73:5196-5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantin, E. M., D. R. Hinton, J. Chen, and H. Openshaw. 1995. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J. Virol. 69:4898-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr, D. J., and S. Noisakran. 2002. The antiviral efficacy of the murine alpha-1 interferon transgene against ocular herpes simplex virus type 1 requires the presence of CD4+, α/β T-cell receptor-positive T lymphocytes with the capacity to produce gamma interferon. J. Virol. 76:9398-9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr, D. J., S. Noisakran, W. P. Halford, N. Lukacs, V. Asensio, and I. L. Campbell. 1998. Cytokine and chemokine production in HSV-1 latently infected trigeminal ganglion cell cultures: effects of hyperthermic stress. J. Neuroimmunol. 85:111-121. [DOI] [PubMed] [Google Scholar]
- 7.Chen, S. H., D. A. Garber, P. A. Schaffer, D. M. Knipe, and D. M. Coen. 2000. Persistent elevated expression of cytokine transcripts in ganglia latently infected with herpes simplex virus in the absence of ganglionic replication or reactivation. Virology 278:207-216. [DOI] [PubMed] [Google Scholar]
- 8.Cook, W. J., M. F. Kramer, R. M. Walker, T. J. Burwell, H. A. Holman, D. M. Coen, and D. M. Knipe. 2004. Persistent expression of chemokine and chemokine receptor RNAs at primary and latent sites of herpes simplex virus 1 infection. Virol. J. 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham, A. L., and T. C. Merigan. 1983. gamma interferon production appears to predict time of recurrence of herpes labialis. J. Immunol. 130:2397-2400. [PubMed] [Google Scholar]
- 10.Dalai, S. K., L. Pesnicak, G. F. Miller, and S. E. Straus. 2002. Prophylactic and therapeutic effects of human immunoglobulin on the pathobiology of HSV-1 infection, latency, and reactivation in mice. J. Neurovirol. 8:35-44. [DOI] [PubMed] [Google Scholar]
- 11.de Bruyn, G., M. Vargas-Cortez, T. Warren, S. K. Tyring, K. H. Fife, J. Lalezari, R. C. Brady, M. Shahmanesh, G. Kinghorn, K. R. Beutner, R. Patel, M. A. Drehobl, P. Horner, T. O. Kurtz, S. McDermott, A. Wald, and L. Corey. 2006. A randomized controlled trial of a replication defective (gH deletion) herpes simplex virus vaccine for the treatment of recurrent genital herpes among immunocompetent subjects. Vaccine 24:914-920. [DOI] [PubMed] [Google Scholar]
- 12.Decman, V., P. R. Kinchington, S. A. Harvey, and R. L. Hendricks. 2005. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J. Virol. 79:10339-10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ecob-Prince, M. S., F. J. Rixon, C. M. Preston, K. Hassan, and P. G. Kennedy. 1993. Reactivation in vivo and in vitro of herpes simplex virus from mouse dorsal root ganglia which contain different levels of latency-associated transcripts. J. Gen. Virol. 74:995-1002. [DOI] [PubMed] [Google Scholar]
- 14.Feldman, L. T., A. R. Ellison, C. C. Voytek, L. Yang, P. Krause, and T. P. Margolis. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc. Natl. Acad. Sci. USA 99:978-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halford, W. P., B. M. Gebhardt, and D. J. Carr. 1996. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J. Immunol. 157:3542-3549. [PubMed] [Google Scholar]
- 16.Hill, J. M., B. M. Gebhardt, R. Wen, A. M. Bouterie, H. W. Thompson, R. J. O'Callaghan, W. P. Halford, and H. E. Kaufman. 1996. Quantitation of herpes simplex virus type 1 DNA and latency-associated transcripts in rabbit trigeminal ganglia demonstrates a stable reservoir of viral nucleic acids during latency. J. Virol. 70:3137-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoshino, Y., S. K. Dalai, K. Wang, L. Pesnicak, T. Y. Lau, D. M. Knipe, J. I. Cohen, and S. E. Straus. 2005. Comparative efficacy and immunogenicity of replication-defective, recombinant glycoprotein, and DNA vaccines for herpes simplex virus 2 infections in mice and guinea pigs. J. Virol. 79:410-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kassim, S. H., N. K. Rajasagi, X. Zhao, R. Chervenak, and S. R. Jennings. 2006. In vivo ablation of CD11c-positive dendritic cells increases susceptibility to herpes simplex virus type 1 infection and diminishes NK and T-cell responses. J. Virol. 80:3985-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khanna, K. M., R. H. Bonneau, P. R. Kinchington, and R. L. Hendricks. 2003. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 18:593-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khanna, K. M., A. J. Lepisto, V. Decman, and R. L. Hendricks. 2004. Immune control of herpes simplex virus during latency. Curr. Opin. Immunol. 16:463-469. [DOI] [PubMed] [Google Scholar]
- 21.Khanna, K. M., A. J. Lepisto, and R. L. Hendricks. 2004. Immunity to latent viral infection: many skirmishes but few fatalities. Trends Immunol. 25:230-234. [DOI] [PubMed] [Google Scholar]
- 22.Koelle, D. M., and L. Corey. 2003. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin. Microbiol. Rev. 16:96-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeBlanc, R. A., L. Pesnicak, M. Godleski, and S. E. Straus. 1999. Treatment of HSV-1 infection with immunoglobulin or acyclovir: comparison of their effects on viral spread, latency, and reactivation. Virology 262:230-236. [DOI] [PubMed] [Google Scholar]
- 24.Lekstrom-Himes, J. A., R. A. LeBlanc, L. Pesnicak, M. Godleski, and S. E. Straus. 2000. Gamma interferon impedes the establishment of herpes simplex virus type 1 latent infection but has no impact on its maintenance or reactivation in mice. J. Virol. 74:6680-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lekstrom-Himes, J. A., L. Pesnicak, and S. E. Straus. 1998. The quantity of latent viral DNA correlates with the relative rates at which herpes simplex virus types 1 and 2 cause recurrent genital herpes outbreaks. J. Virol. 72:2760-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, T., K. M. Khanna, B. N. Carriere, and R. L. Hendricks. 2001. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 75:11178-11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, T., K. M. Khanna, X. Chen, D. J. Fink, and R. L. Hendricks. 2000. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 191:1459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, T., Q. Tang, and R. L. Hendricks. 1996. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J. Virol. 70:264-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maggioncalda, J., A. Mehta, Y. H. Su, N. W. Fraser, and T. M. Block. 1996. Correlation between herpes simplex virus type 1 rate of reactivation from latent infection and the number of infected neurons in trigeminal ganglia. Virology 225:72-81. [DOI] [PubMed] [Google Scholar]
- 30.Nash, A. A., A. Jayasuriya, J. Phelan, S. P. Cobbold, H. Waldmann, and T. Prospero. 1987. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J. Gen. Virol. 68:825-833. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura, H., T. Yajima, Y. Kagimoto, M. Ohata, T. Watase, K. Kishihara, F. Goshima, Y. Nishiyama, and Y. Yoshikai. 2004. Intraepithelial γδ T cells may bridge a gap between innate immunity and acquired immunity to herpes simplex virus type 2. J. Virol. 78:4927-4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noisakran, S., and D. J. Carr. 1999. Lymphocytes delay kinetics of HSV-1 reactivation from in vitro explants of latent infected trigeminal ganglia. J. Neuroimmunol. 95:126-135. [DOI] [PubMed] [Google Scholar]
- 33.Sawtell, N. M. 1997. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J. Virol. 71:5423-5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawtell, N. M. 1998. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J. Virol. 72:6888-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sawtell, N. M., D. K. Poon, C. S. Tansky, and R. L. Thompson. 1998. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J. Virol. 72:5343-5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawtell, N. M., and R. L. Thompson. 1992. Rapid in vivo reactivation of herpes simplex virus in latently infected murine ganglionic neurons after transient hyperthermia. J. Virol. 66:2150-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheridan, J. F., A. D. Donnenberg, L. Aurelian, and D. J. Elpern. 1982. Immunity to herpes simplex virus type 2. IV. Impaired lymphokine production during recrudescence correlates with an imbalance in T lymphocyte subsets. J. Immunol. 129:326-331. [PubMed] [Google Scholar]
- 38.Straus, S. E., A. Wald, R. G. Kost, R. McKenzie, A. G. Langenberg, P. Hohman, J. Lekstrom, E. Cox, M. Nakamura, R. Sekulovich, A. Izu, C. Dekker, and L. Corey. 1997. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoproteins D and B: results of a placebo-controlled vaccine trial. J. Infect. Dis. 176:1129-1134. [DOI] [PubMed] [Google Scholar]
- 39.Theil, D., T. Derfuss, I. Paripovic, S. Herberger, E. Meinl, O. Schueler, M. Strupp, V. Arbusow, and T. Brandt. 2003. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am. J. Pathol. 163:2179-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Lint, A. L., L. Kleinert, S. R. Clarke, A. Stock, W. R. Heath, and F. R. Carbone. 2005. Latent infection with herpes simplex virus is associated with ongoing CD8+ T-cell stimulation by parenchymal cells within sensory ganglia. J. Virol. 79:14843-14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wachsman, M., M. Kulka, C. C. Smith, and L. Aurelian. 2001. A growth and latency compromised herpes simplex virus type 2 mutant (ICP10DeltaPK) has prophylactic and therapeutic protective activity in guinea pigs. Vaccine 19:1879-1890. [DOI] [PubMed] [Google Scholar]
- 42.Wang, K., L. Pesnicak, E. Guancial, P. R. Krause, and S. E. Straus. 2001. The 2.2-kilobase latency-associated transcript of herpes simplex virus type 2 does not modulate viral replication, reactivation, or establishment of latency in transgenic mice. J. Virol. 75:8166-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitley, R. J. 2002. Herpes simplex virus infection. Semin. Pediatr. Infect. Dis. 13:6-11. [DOI] [PubMed] [Google Scholar]