Abstract
Entry of vaccinia virus into cells occurs by an endosomal route as well as through the plasma membrane. Evidence for an endosomal pathway was based on findings that treatment at a pH of <6 of mature virions attached to the plasma membrane enhances entry, whereas inhibitors of endosomal acidification reduce entry. Inactivation of infectivity by low-pH treatment of virions prior to membrane attachment is characteristic of many viruses that use the endosomal route. Nevertheless, we show here that the exposure of unattached vaccinia virus virions to low pH at 37°C did not alter their infectivity. Instead, such treatment stably activated virions as indicated by their accelerated entry upon subsequent addition to cells, as measured by reporter gene expression. Moreover, the rate of entry was not further enhanced by a second low-pH treatment following adsorption to the plasma membrane. However, the entry of virions activated prior to adsorption remained sensitive to inhibitors of endosomal acidification, whereas virions treated with low pH after adsorption were resistant. Activation of virions by low pH was closely mimicked by proteinase digestion, suggesting that the two treatments operate through a related mechanism. Although proteinase cleavage of the virion surface proteins D8 and A27 correlated with activation, mutant viruses constructed by individually deleting these genes did not exhibit an activated phenotype. We propose a two-step model of vaccinia virus entry through endosomes, in which activating or unmasking the fusion complex by low pH or by proteinase is rate limiting but does not eliminate a second low-pH step mediating membrane fusion.
Poxviruses are large DNA viruses that replicate in the cytoplasm of vertebrate or invertebrate cells (22). The mechanism by which these viruses enter cells is being actively investigated, with most of the information coming from studies with vaccinia virus (VACV), the prototype member of the family (23). The most abundant infectious form of VACV, called the mature virion (MV), consists of an outer lipoprotein membrane, lateral bodies of an undefined nature, and a core containing the genome and early transcription system. Although MVs can be released by cell lysis, a subset are wrapped by modified trans-Golgi or endosomal membranes and transported to the periphery of the cell where exocytosis from intact cells occurs (32). This extracellular form of VACV is essentially an MV with one additional membrane that is discarded prior to entry and consequently is not directly involved in the merging of viral and cellular membranes (19).
The entry of enveloped viruses into cells can be divided into three stages: attachment, activation, and membrane fusion (11). In the case of VACV, three membrane proteins contribute to the binding of MVs to glycosaminoglycans at the cell surface (7, 12, 20), and a complex composed of at least nine other proteins is required for membrane fusion and entry as well as for syncytium formation (4, 16, 26, 29-31, 34, 35, 37). Importantly, the same fusion complex mediates neutral pH entry of VACV through the plasma membrane and low-pH entry through endocytic vesicles (36).
The conclusion that VACV enters cells by fusion with the plasma membrane at neutral pH came primarily from electron microscopy images (2, 5, 6). However, recent studies indicate that VACV also uses a low-pH endosomal entry pathway (36). The latter indication was based on the following evidence: (i) a brief treatment at a pH of <6 of MVs attached to the plasma membrane enhanced entry 10-fold, as determined by firefly luciferase (Luc) reporter gene expression and corroborated by electron microscopy; (ii) inhibitors of endosomal acidification reduced entry by 70 to 80%; and (iii) low-pH treatment of bound MVs triggered fusion with the plasma membrane and alleviated the effect of inhibitors of endosomal acidification (36).
Inactivation of the fusion apparatus by low-pH exposure prior to association with the target membrane is a characteristic of many viruses that use the endosomal pathway (11), including influenza virus (18, 25, 33), Semliki Forest virus (3), tick-borne encephalitis virus (8), and lymphocytic choriomeningitis virus (9). Nevertheless, we show here that low pH stably activates VACV MVs, which then exhibit accelerated entry as measured by Luc expression, when they are subsequently adsorbed to cells. Nevertheless the entry of activated MVs remains sensitive to inhibitors of endosomal acidification, suggesting two distinct low-pH steps. Furthermore, activation of MVs by low pH can be mimicked by proteinase digestion, suggesting that the two treatments operate through a related mechanism.
MATERIALS AND METHODS
Viruses.
Experiments were performed with the Western Reserve (WR) strain of VACV (ATCC VR-1354) and a previously characterized recombinant VACV WR virus that expresses Luc via a synthetic early-late promoter (WRvFire) (36). In addition, two new derivatives of WRvFIRE, consisting of one in which the A27L gene was replaced by a cassette that expresses green fluorescent protein and xanthine-guanine phosphoribosyltransferase to form ΔA27vFire and the other in which the D8L gene was replaced with a cassette that expresses red fluorescent protein to form ΔD8vFire, were used. Genes were denoted both by the old nomenclature (comprising the letter designation of the HindIII fragment, the number of the open reading frame within that fragment, and L or R to denote direction of transcription) and by the reading frame number derived from annotation of the complete WR genome (NC 006998). VACV gene products were denoted by the old nomenclature, except that the direction of transcription was omitted.
Purification of VACV.
HeLa-S3 cells were infected with VACV WR or WRvFire or recombinants derived from these strains, at a multiplicity of 5 PFU. After 48 h, progeny MV were released from infected cells by Dounce homogenization and purified by sedimentation twice through a 36% sucrose cushion and banding on a 25% to 40% sucrose gradient as described previously (10). Purified virus stocks were stored at −80°C and sonicated on ice three times for 1 min each prior to infection or treatment.
Activation of virions by low-pH or papain treatment.
For low-pH treatment, purified MVs were incubated with phosphate-buffered saline (PBS) that had been adjusted to pH 5 with HCl and 1 mM 2-morpholinoethanesulfonic acid or, as a control, PBS at pH 7.4, in a final volume of 50 to 100 μl for 3 min at 37°C. The pH was then neutralized with an excess of Earle's minimal essential medium (EMEM; Quality Biologicals, Gaithersburg, MD) containing 2.5% fetal bovine serum (FBS) and 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and the suspension was placed on ice.
For papain (Sigma-Aldrich, St. Louis, MO) treatment, purified virions were resuspended in 50 μl of PBS (pH 7.4) containing 5 mM l-cysteine. Virion suspensions were then treated with the desired concentration of papain or were mock treated for 30 min at 37°C. Papain digestion was quenched by diluting the reaction mixtures to 1:50 or 1:100 with EMEM containing 2.5% FBS, and samples were placed on ice.
Luc activity assays.
BS-C-1 cells in 12-well plates were cooled to 4°C, and virus adsorption (0.2 PFU/cell) was allowed to proceed for 1 h at 4°C. In some experiments, the cells were pretreated or mock treated with inhibitors of endosomal acidification (bafilomycin A1 or monensin; Sigma-Aldrich) in EMEM containing 2.5% FBS for 1 h at 37°C. Cells were subsequently washed with cold EMEM containing 2.5% FBS and overlaid with prewarmed (37°C) PBS (pH 7.4) or PBS that had been adjusted to pH 5 with HCl and 1 mM 2-morpholinoethanesulfonic acid. Following two washes with EMEM at 37°C, cells were incubated in 1 ml of EMEM containing 2.5% FBS at 37°C with or without inhibitors. At the desired time, cells were washed with PBS (pH 7.4) and lysed in 300 μl of cell culture lysis reagent (Promega, Madison, WI) for 30 min at room temperature with gentle agitation. Samples (20 μl) of the resulting cell lysate were added to 100 μl of Luc activity assay substrate (Promega) and vortexed, and chemiluminescence was quantified by using a Berthold Sirius luminometer (Bad Wildbad, Germany).
Analysis of papain-treated virions.
Virions were incubated with various concentrations of papain for 30 min at 37°C, as described above. Following this step, papain was inactivated by the addition of 40 mM N-ethyl maleimide (1), virions were pelleted, and the pellet and supernatant fractions were solubilized in lithium dodecyl sulfate sample buffer containing NuPage reducing agent (Invitrogen, Carlsbad, CA). Proteins from disrupted virions were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 4 to 12% bis-Tris NuPage gels (Invitrogen) and transferred to nitrocellulose membranes for Western blotting analysis. Membranes were blocked with Tris-buffered saline supplemented with 5% nonfat dried milk and 0.05% Tween 20 for 1 h at room temperature. Subsequently, membranes were incubated with rabbit polyclonal antibodies, washed, and incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Pierce Biotechnology, Rockford, IL). Western blots were analyzed using chemiluminescence reagents (Pierce Biotechnology).
Syncytium assay.
Purified virions, mock treated or treated with papain or low pH, at a multiplicity of infection of 300 PFU (calculated prior to treatments) were adsorbed to BS-C-1 cells for 1 h at 4°C. The cell monolayers were washed with ice-cold EMEM containing 2.5% FBS and then incubated with a pH 5 buffer or a pH 7.4 buffer for 3 min at 37°C. Cells were washed twice and then incubated in EMEM containing 2.5% FBS and 300 μg/ml cycloheximide (Sigma-Aldrich) for 3 h at 37°C. Cells were fixed with 3% paraformaldehyde in PBS for 20 min at room temperature, washed three times with PBS, and quenched with 2% glycine. To stain filamentous actin, cells were incubated with either Alexa Fluor 568- or Alexa Fluor 488-conjugated phalloidin (Invitrogen) diluted in 10% complement-inactivated FBS in PBS for 1 h at 37°C. Cells were washed three times with PBS prior to incubation with diamidino-2-phenylindole dihydrochloride (DAPI; Invitrogen) to visualize DNA. Images were obtained using a Leica TCS-NT/SP2 inverted confocal microscope with an attached argon laser (Coherent Inc., Santa Clara, CA).
RESULTS
Activation of MVs by a brief low-pH treatment in the absence of target membranes.
We previously studied entry using a recombinant VACV (strain WR) called WRvFire that expresses Luc under the control of an early-late promoter (36). Although this is a postfusion assay, Luc synthesis begins almost immediately after entry into the cytoplasm because the early transcription system is carried within the virus core. Purified WRvFire MVs were allowed to attach to cells at 4°C and then were briefly incubated with pH 5 buffer at 37°C. The latter step accelerated the rate of Luc expression, and this was correlated with the rapid fusion of virus at the plasma membrane and the deposition of cores in the cytoplasm, as determined by transmission electron microscopy (36).
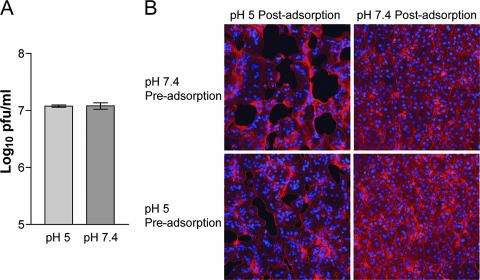
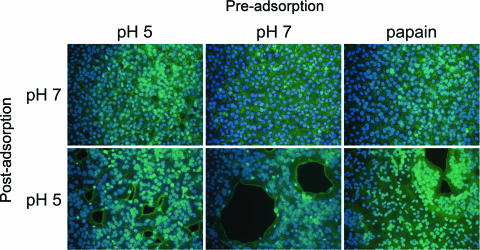
In the present study, we wanted to see the effect of low pH on virions that were unattached to cells, since such treatment frequently inactivates viruses that use the endosomal pathway (11). After MVs were incubated in pH 5.0 or 7.4 buffer at 37°C for 3 min, infectivity was determined by plaque assay with BS-C-1 cells. Low-pH treatment had no effect on the titer of infectious particles (Fig. 1A), and SDS-PAGE did not reveal changes in the pattern of silver-stained protein bands (not shown). In addition, low-pH-treated MVs still induced syncytium formation by a process called “fusion from without” when the pH was lowered following adsorption (Fig. 1B). These results indicated that low-pH treatment of MVs that were unattached to membranes did not result in irreversible inactivation of the fusion apparatus or infectivity or affect the aggregation state of the virus.
FIG. 1.
Effect of a brief low-pH treatment in the absence of target membranes on MV infectivity and syncytium formation. (A) Purified VACV WR MVs were exposed to pH 5 or pH 7.4 buffer for 3 min at 37°C, and the infectivity titer was determined by plaque assay. (B) Purified VACV WR MVs were exposed to pH 5 or pH 7.4 buffer for 3 min at 37°C (Pre-adsorption) and adsorbed to BS-C-1 monolayers for 1 h at 4°C. The cells were then immersed in either pH 5 or pH 7.4 buffer at 37°C (Post-adsorption). After 3 min, the buffer was replaced with EMEM containing cycloheximide and incubated for 3 h at 37°C. The cells were fixed and stained with Alexa Fluor 568-phalloidin and DAPI to display actin filaments and DNA, respectively.
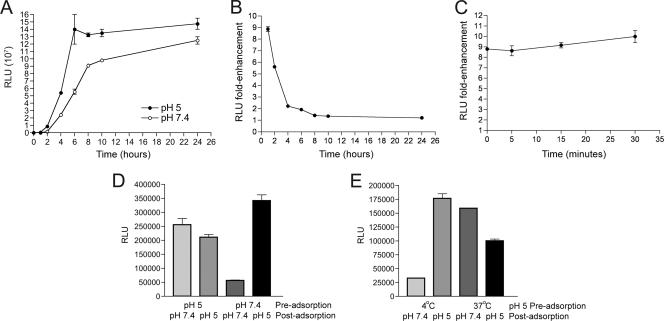
Next, we determined the effect of low-pH treatment on the rate of virus entry, using the Luc activity assay. 1-β-d-Arabinofuranosylcytosine (araC) was present in order to prevent postreplicative expression of Luc. WRvFire MVs that had been pretreated with pH 5 buffer at 37°C for 3 min were allowed to adsorb to cells at 4°C, a temperature too low for entry. After removing unbound virus, the temperature was raised to 37°C, and Luc activity was measured at intervals between 1 and 24 h to measure entry of adsorbed virus over time. Luc activity increased for 4 to 6 h and then reached a plateau (Fig. 2A). In cells infected with control virus, which had been exposed to pH 7.4 buffer, Luc activity increased more slowly but approached values similar to that of low-pH-treated virus by 24 h (Fig. 2A). At the earliest time point, the difference in Luc activity between low- and neutral-pH-treated virus was 9-fold (Fig. 2B). Over time, this difference decreased, and by 8 to 10 h, the ratio was similar for MVs treated prior to adsorption with pH 5 or 7.4 buffer (Fig. 2B); thus, the major enhancing effect was on kinetics. Furthermore, the activated state of MVs persisted for at least 30 min after neutralization of the pH (Fig. 2C).
FIG. 2.
Activation of MVs by low-pH treatment in the absence of target membranes. (A) BS-C-1 cells were treated with araC for 1 h at 37°C and then incubated with WRvFire MVs that had been subjected to a 3-min incubation with either pH 5 or pH 7.4 buffer at 37°C. After 1 h at 4°C, unadsorbed virus was removed by washing, and the cells were incubated at 37°C. At the indicated times after the temperature was raised, cells were lysed, and Luc activity was determined. Luc activity was expressed as relative light units (RLU). Experiments were performed in duplicate, and data points represent the means ± standard errors of the means. (B) The n-fold enhancement represents the ratio of Luc activities of cells infected with pH 5-treated versus pH 7.4-treated MVs from panel A. (C) Purified WRvFire MVs were incubated in pH 5 or pH 7.4 buffer, as shown in panel A, and then incubated at pH 7.4 at 37°C for up to 30 min. After the times indicated on the horizontal axis, the virus suspensions were adsorbed to cells, which were then incubated for 1 h at 37°C, following which Luc activity was determined as described in the legend to panel A. (D) Purified WRvFire MVs were exposed to pH 5 or pH 7.4 buffer for 3 min at 37°C and adsorbed to cells as described in the legend to panel A. The cells were then washed and incubated with either pH 5 or pH 7.4 buffer for 3 min at 37°C. Following this step, cells were incubated at 37°C for 1 h, lysed, and then assayed for Luc activity as described for panel A. (E) Purified WRvFire MVs were exposed to pH 5 or pH 7.4 buffer for 3 min at 4°C or 37°C and subsequently neutralized in an excess of EMEM with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid chilled to 4°C. Virus was adsorbed to BS-C-1 monolayers at 4°C for 1 h. After this, cell monolayers were washed and treated with either pH 5 or pH 7.4 buffer for 3 min at 37°C. Cells were then incubated at 37°C for 1 h, lysed, and assayed for Luc activity.
The next experiment was designed to determine whether the activation resulting from treatment of MVs with low pH prior to adsorption and the activation occurring with low-pH treatment after adsorption were additive. MVs were (i) exposed to pH 5 or 7.4 at 37°C for 3 min prior to adsorption (preadsorption), (ii) neutralized and allowed to adsorb to cells at 4°C for 1 h, (iii) exposed to pH 5 or 7.4 postadsorption for 3 min at 37°C, and (iv) neutralized and incubated at 37°C for 1 h to allow Luc expression. Luc activity levels were similar, whether pH 5-treated MVs were incubated at pH 7.4 or 5 after adsorption (Fig. 2D), indicating that low-pH activation was not additive. In contrast, Luc activity after 1 h was much higher when MVs that were incubated at pH 7.4 prior to adsorption were incubated at pH 5 after adsorption than at pH 7.4 after adsorption (Fig. 2D). Thus, as measured by the Luc activity assay, MVs pretreated with low pH did not show further activation when treated with low pH again after adsorption to membranes.
To determine the temperature requirement for preadsorption activation, MVs were (i) incubated in pH 5 buffer at 4°C or 37°C for 3 min, (ii) neutralized and adsorbed to cells at 4°C for 1 h, (iii) incubated at pH 7.4 or 5 postadsorption at 37°C for 3 min, and (iv) neutralized and incubated for 1 h at 37°C for 1 h to allow Luc expression. Activation only occurred if the preadsorption low-pH exposure was carried out at 37°C (Fig. 2E).
Entry of low-pH-activated MVs is dependent on endosomal acidification.
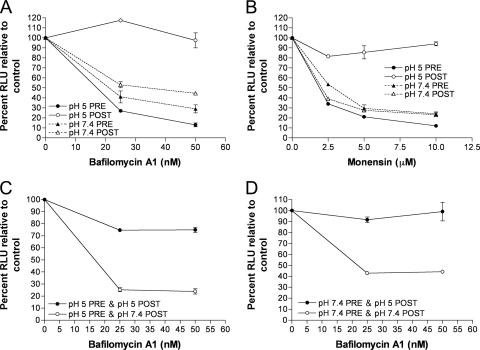
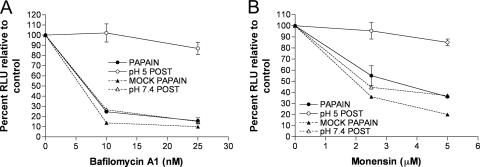
As described above (Fig. 1B), we showed that fusion from without only occurred when low-pH treatment was applied after adsorption of MVs, regardless of whether the MVs had been activated by exposure to low pH prior to adsorption. Since syncytium formation is dependent on the deposition of the viral membrane proteins in the plasma membrane, we suspected that the MVs, which were activated prior to adsorption, were still entering through an endosomal route. Bafilomycin A1 and monensin, inhibitors of endosomal acidification, were used to determine whether this was true. We confirmed previous observations (36) that bafilomycin A1 and monensin reduced the entry of control MVs by 70 to 80% as judged by Luc activity (Fig. 3A and B), consistent with endosomal entry. We also confirmed the observation (36) that these inhibitors have no effect if the pH of the medium is briefly lowered after virus adsorption (Fig. 3A and B), so that the predominant route of entry is through the plasma membrane. The latter control demonstrated that the drugs did not inhibit Luc activity per se. Importantly, we found that entry of the MVs that were activated by low pH prior to adsorption was still inhibited by bafilomycin A1 and monensin (Fig. 3A and B). However, when the pH 5-activated MVs were allowed to attach to cells and were then treated with low pH, they became resistant to bafilomycin A1 (Fig. 3C and D). Thus, MVs activated by low pH prior to adsorption to cells, unlike MVs that are activated after absorption, still enter through a low-pH-dependent endosomal pathway.
FIG. 3.
MVs activated by low pH in the absence of target membranes still enter cells through a low-pH-dependent pathway. (A and B) Purified WRvFire MVs were exposed to pH 5 (pH 5 PRE) or pH 7.4 buffer (pH 7.4 PRE) for 3 min at 37°C, neutralized, and overlaid onto BS-C-1 monolayers that had been pretreated for 1 h at 37°C with the indicated amounts of bafilomycin A1 or monensin. Virus adsorption was allowed to continue for 1 h at 4°C before the cell monolayers were washed and incubated in the presence of the appropriate inhibitor for an additional 1 h at 37°C. The cells were then lysed and assayed for Luc activity. As controls, untreated WRvFire MVs were adsorbed to BS-C-1 monolayers pretreated with bafilomycin A1 or monensin and induced to fuse at the plasma membrane by 3 min of incubation with pH 5 buffer (pH 5 POST) or were allowed to enter via a low-pH endocytic pathway by mock treatment with pH 7.4 buffer (pH 7.4 POST). Experiments were performed in duplicate, and data points represent the means ± standard errors of the means. RLU, relative light units. (C and D) Purified WRvFire MVs were treated with pH 5 (pH 5 PRE) or pH 7.4 buffer (pH 7.4 PRE) for 3 min at 37°C and overlaid onto BS-C-1 monolayers that had been pretreated with bafilomycin A1 for 1 h at 37°C. Virus adsorption was allowed to continue for 1 h at 4°C before the cells were washed and incubated with either pH 5 (pH 5 POST) or pH 7.4 (pH 7.4 POST) buffer for 3 min at 37°C. The cells were then incubated in the presence of bafilomycin A1 for a further 1 h at 37°C, lysed, and assayed for Luc activity.
Taken together, these data indicate the existence of two separable low-pH processes. By exposing MVs to low pH prior to adsorption, the MVs are activated so that entry is accelerated, but the MVs still use an endosomal route and therefore require a second low-pH step.
Activation of MVs by proteinase treatment.
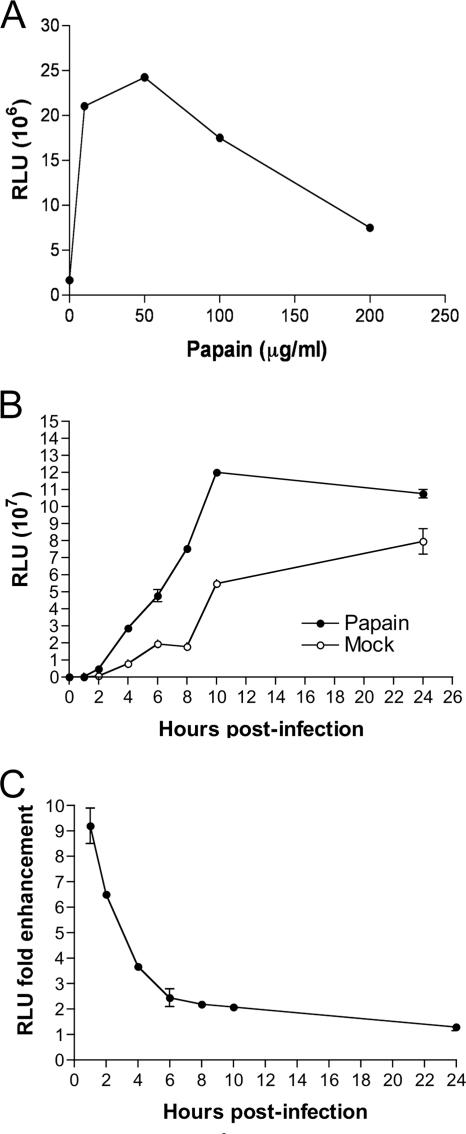
The finding that MVs were stably activated by low pH could be explained by a conformational or covalent change in proteins of the fusion apparatus itself or by unmasking or activating the entry apparatus by modification of other proteins. In this respect, we recalled that a number of previous workers had either used proteinases during VACV purification (17, 27) or reported that proteolysis enhanced infectivity or virus penetration (13, 15). In the following experiments, MVs were treated with a proteinase for 1 h, the proteinase was inactivated or highly diluted with medium containing serum, and the digested MVs were allowed to bind to cells for 1 h at 4°C. The temperature was then raised to 37°C, and Luc activity was measured 1 h later. Trypsin at a concentration of 250 μg per ml produced a severalfold enhancement of Luc activity (not shown). However, the effect was more dramatic with papain; the enhancement was greater than 10-fold following treatment with a concentration of 5 to 50 μg of papain per ml (Fig. 4A). With higher papain concentrations, the Luc activity was diminished, either because of inactivation of virus or to an effect of residual diluted papain on the cells. Evidence supporting the former was determined by infectivity assays. Even at 50 μg per ml, infectivity was reduced by 60 to 70% compared to mock-treated virus (data not shown), and presumably greater infectivity losses occurred with higher concentrations of papain due to digestion of essential virion proteins. The loss of infectivity at 50 μg/ml of papain also rules out MV disaggregation as a mechanism of activation.
FIG. 4.
Activation of MVs by papain digestion. (A) Purified WRvFire MVs were incubated with the indicated amounts of papain or mock treated for 30 min at 37°C and then quenched by dilution into cold EMEM containing 2.5% FBS. The MVs were then added to BS-C-1 monolayers, and virus adsorption was allowed to continue for 1 h at 4°C. Unadsorbed virus was removed by washing, and the cells were incubated at 37°C for 1 h, lysed, and then assayed for Luc activity. (B) BS-C-1 cell monolayers were treated with araC for 1 h at 37°C prior to inoculation with WRvFire MVs that had been subjected to digestion with 50 μg/ml papain or mock treated for 30 min at 37°C. Unadsorbed virus was removed by washing, and the cells were incubated at 37°C. At the indicated times, cells were lysed, and Luc activity was measured as relative light units (RLU). Experiments were performed in duplicate, and data points represent the means ± standard errors of the means. (C) The n-fold enhancement represents the ratio of Luc activities of cells infected with papain-treated versus those of mock-treated MVs from panel B.
A time course experiment showed a more rapid increase in Luc activity following infection with proteinase-treated MVs than with mock-treated MVs (Fig. 4B and C), similar to the difference seen with MVs treated with low pH versus neutral pH (Fig. 2A and B). Thus, both pH 5 treatment and papain treatment appeared to accelerate virus entry. When papain-treated MVs were adsorbed to cells and then briefly treated with low pH, there was only a low and variable enhancement of Luc activity (data not shown).
The activation produced by papain digestion was similar in magnitude and kinetics to that produced by low-pH treatment of unbound MVs. This similarity extended to the inhibition of entry of proteinase-activated MVs by bafilomycin A1 and monensin (compare Fig. 5A and B with Fig. 3). We also found that low-pH treatment was still necessary to trigger cell-cell fusion following adsorption of MVs activated by low pH or papain (Fig. 6).
FIG. 5.
MVs activated by papain digestion enter cells through a low-pH-dependent pathway. Purified WRvFire MVs were incubated with 50 μg/ml papain for 30 min at 37°C (PAPAIN), or mock treated (MOCK PAPAIN), quenched in an excess of EMEM plus 2.5% FBS, and then overlaid onto BS-C-1 monolayers that had been pretreated with bafilomycin A1 (A) or monensin (B) for 1 h at 37°C. RLU, relative light units. Virus adsorption was allowed to continue for 1 h at 4°C before the cell monolayers were washed and incubated in the presence of inhibitor for an additional 1 h at 37°C. The cells were then lysed and assayed for Luc activity. As controls, untreated WRvFire MVs were adsorbed to BS-C-1 monolayers pretreated with bafilomycin A1 or monensin and induced to fuse at the plasma membrane by transient incubation with pH 5 buffer (pH 5 POST) or allowed to enter via a low-pH endocytic pathway by mock treatment with pH 7.4 buffer (pH 7.4 POST).
FIG. 6.
Activated MVs induce cell-cell fusion only after a postadsorption low-pH treatment. Purified WR MVs were treated before adsorption (Pre-adsorption) with either pH 5 or pH 7.4 buffer for 3 min at 37°C or were digested with 50 μg/ml of papain for 30 min at 37°C and then adsorbed to BS-C-1 cells at a multiplicity of 300 PFU for 1 h at 4°C. The cells were then immersed in either pH 5 or pH 7.4 buffer at 37°C (Post-adsorption). After 3 min, the buffers were replaced with EMEM containing 300 μg of cycloheximide per ml and incubated for 3 h at 37°C. The cells were fixed and stained with Alexa Fluor 568-phalloidin and DAPI to display actin filaments and DNA, respectively.
Ichihashi and coworkers (15) had shown that when VACV was incubated with 10 to 100 μg of papain, surface proteins of 88, 32, and 14 kDa were digested. We used Western blotting to identify potential papain-sensitive proteins A21 (VACVWR140) and L5 (VACVWR092), two proteins representative of the entry/fusion complex; three proteins, A27 (VACVWR150), D8 (VACVWR113), and H3 (VACVWR101), involved in attachment; and L1 (VACVWR088), a membrane protein required for assembly. Each protein was digested to some degree with increasing concentrations of papain (Fig. 7A). However, A27 and D8 were the most susceptible and were almost completely cleaved with 10 μg/ml of papain. The cleavage of D8 was quite specific, as a large protein fragment remained associated with virus particles even after digestion with high concentrations of papain. A27 (12.6 kDa) and D8 (35.3 kDa) correspond to the 14- and 32-kDA proteins, respectively, previously described as papain sensitive (15).
FIG. 7.
Digestion of MV surface proteins by papain and effects of deletions on activation. (A) Purified WRvFire MVs were incubated with the indicated concentrations of papain for 30 min at 37°C. After this time, papain was inactivated by the addition of 40 mM N-ethylmaleimide, and particles were collected by centrifugation. The proteins were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and subjected to Western blotting with rabbit polyclonal antibodies to the MV membrane-associated proteins A21, L5, A27, H3, D8, and L1. Masses of marker proteins in kilodaltons are indicated on the right. (B) WRvFire recombinant viruses, deleted for either the A27L (ΔA27vFire) gene or the D8L (ΔD8vFire) gene, were constructed by homologous recombination. Purified MVs of ΔA27vFire, ΔD8vFire, and WRvFire were adsorbed to BS-C-1 monolayers at 4°C for 1 h. After this time, cell monolayers were washed and exposed to pH 5 or pH 7.4 buffer for 3 min at 37°C. Cells were then incubated at 37°C for 1 h, lysed, and then assayed for Luc activity. The n-fold enhancement represents the ratios of Luc activities for pH 5 and 7.4 treatments.
A27 and D8 are nonessential proteins, and their sensitivity to papain correlated with activation of entry. Therefore, one hypothesis was that degradation of A27 or D8 unmasked the fusion complex. To investigate this idea, we constructed A27 and D8 deletion mutants expressing Luc, referred to as ΔA27vFire and ΔD8vFire, respectively. The idea was that MVs lacking one of these proteins might be “preactivated” so that no further activation would occur after low-pH treatment of adsorbed virions. The MVs were purified, adsorbed to cells, and then exposed to pH 5 or 7.4 buffer. After 1 h, Luc activity was measured, and the effect of low-pH treatment was assessed by comparing the enhancement levels (n-fold). Luc activity of the ΔA27vFire, ΔD8vFire, and control WRvFire MVs showed similar levels of enhancement by low-pH treatment (Fig. 7B), indicating that they did not exhibit a “preactivated” phenotype.
DISCUSSION
Enveloped viruses enter cells by a variety of membrane fusion mechanisms. Some require a low-pH activation step that occurs in endosomes, whereas others are activated at neutral pH by receptor binding at the plasma membrane (11). Herpes simplex virus has been reported to enter through the plasma membrane as well as by low-pH and neutral-pH endocytosis, depending on the cell type (21, 24). VACV entry resembles that of herpes simplex virus in that it can occur at the plasma membrane as well as by low-pH-mediated endocytosis, though with VACV this can occur simultaneously in the same cell type (36). VACV seems unique in that a very large number of proteins, at least eight, are required for fusion to occur (4, 30), though the roles of the individual proteins are unknown. Here we showed that the activation of VACV is unique in other respects as well.
It is generally thought that fusion proteins on the virion surface exist in a metastable state in which a hydrophobic peptide sequence is hidden or shielded and that exposure to low pH in the endosome or a specific interaction at neutral pH with a receptor on the cell surface triggers conformational changes that lead to merging of membranes and entry of internal components (11). Although there are exceptions (28), the low-pH activation step is usually irreversible, and premature activation results in loss of fusion capability, as shown for Semliki Forest virus (3), tick-borne encephalitis virus (8), lymphocytic choriomeningitis virus (9) and influenza virus (18, 25, 33). In the present study, we showed that low-pH treatment of unattached VACV virions stably activated them, as measured by Luc activity, following cell adsorption and incubation at 37°C. The effect was attributed to entry rate, as the difference between treated and untreated virions diminished with time and there was no increase in infectious units, as measured by plaque titration. Nevertheless, because of the assay, we cannot rule out a postentry mechanism. However, a second low-pH treatment following adsorption to cells did not further accelerate entry. The activation of MVs by low pH could be explained by a conformational or covalent change in proteins of the fusion apparatus itself or by unmasking or activating the entry apparatus by modification of other proteins.
Consistent with an unmasking mechanism, we found that papain treatment also activated VACV virions as measured by the accelerated rate of virus entry. Potentially, proteinase treatment can have multiple effects depending on the physical state of the purified virus and the type and amounts of enzyme used (13-15) as well as the strain of VACV (A. C. Townsley and B. Moss, unpublished data). Activation did not represent dispersal of aggregates in our experiments as the infectivity titer was decreased at the optimal concentration of papain. Proteinase activation may occur naturally during infection, since the addition of phenylmethylsulfonyl fluoride during the period of virus penetration decreases infection (14). Two surface proteins, A27 and D8, which are involved in cell attachment, were extremely sensitive to papain, and their cleavage correlated with activation. This was unexpected because A27 D8 double deletion mutants have low infectivity presumably because of poor attachment to cells (9). The papain-treated virus, however, was not equivalent to a double deletion mutant, since a large fragment of D8 remained associated with the activated virions. We found that deletion of the gene encoding A27 or D8 individually, which has little effect on attachment (9), did not accelerate entry, and both mutants were still activated by low pH. This result ruled out a simple scenario in which activation occurs when either of these proteins is removed.
Previously, we demonstrated that a brief low-pH treatment of virions that were adsorbed to cells induced fusion with the plasma membrane and abolished sensitivity to inhibitors of endosomal acidification. Nevertheless, entry of virions activated in the unattached state by either low pH or proteinase was still sensitive to such inhibitors. To explain this difference, we invoke a model in which low-pH or proteinase treatment of virions eliminates an initial rate-limiting step but not a second low-pH step mediating membrane fusion. We are seeking ways to confirm or refute this hypothesis.
Acknowledgments
We thank Norman Cooper for supplying cells, Andrea Weisberg for electron microscopy, Linda Wyatt for help revising figures, and Zain Bengali for discussions.
The work was supported by the Division of Intramural Research, NIAID.
Footnotes
Published ahead of print on 6 June 2007.
REFERENCES
- 1.Anderson, B. M., and E. C. Vasini. 1970. Nonpolar effects in reactions of the sulfhydryl group of papain. Biochemistry 9:3348-3352. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, J. A., D. H. Metz, and M. R. Young. 1973. The mode of entry of vaccinia virus into L cells. J. Gen. Virol. 21:533-537. [DOI] [PubMed] [Google Scholar]
- 3.Bron, R., J. M. Wahlberg, H. Garoff, and J. Wilschut. 1993. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J. 12:693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, E., T. G. Senkevich, and B. Moss. 2006. Vaccinia virus F9 virion membrane protein is required for entry but not virus assembly, in contrast to the related l1 protein. J. Virol. 80:9455-9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter, G. C., M. Law, M. Hollinshead, and G. L. Smith. 2005. Entry of the vaccinia virus intracellular mature virion and its interactions with glycosaminoglycans. J. Gen. Virol. 86:1279-1290. [DOI] [PubMed] [Google Scholar]
- 6.Chang, A., and D. H. Metz. 1976. Further investigations on the mode of entry of vaccinia virus into cells. J. Gen. Virol. 32:275-282. [DOI] [PubMed] [Google Scholar]
- 7.Chung, C.-S., J.-C. Hsiao, Y.-S. Chang, and W. Chang. 1998. A27L protein mediates vaccinia virus interaction with cell surface heparin sulfate. J. Virol. 72:1577-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corver, J., A. Ortiz, S. L. Allison, J. Schalich, F. X. Heinz, and J. Wilschut. 2000. Membrane fusion activity of tick-borne encephalitis virus and recombinant subviral particles in a liposomal model system. Virology 269:37-46. [DOI] [PubMed] [Google Scholar]
- 9.Di Simone, C., and M. J. Buchmeier. 1995. Kinetics and pH dependence of acid-induced structural changes in the lymphocytic choriomeningitis virus glycoprotein complex. Virology 209:3-9. [DOI] [PubMed] [Google Scholar]
- 10.Earl, P. L., N. Cooper, S. Wyatt, B. Moss, and M. W. Carroll. 1998. Preparation of cell cultures and vaccinia virus stocks, p. 16.16.1-16.16.3. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. John Wiley and Sons, New York, NY. [Google Scholar]
- 11.Earp, L. J., S. E. Delos, H. E. Park, and J. M. White. 2005. The many mechanisms of viral membrane fusion proteins. Curr. Top. Microbiol. Immunol. 285:25-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsiao, J.-C., C.-S. Chung, and W. Chang. 1999. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J. Virol. 73:8750-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichihashi, Y., and M. Oie. 1980. Adsorption and penetration of the trypsinized vaccinia virion. Virology 101:50-60. [DOI] [PubMed] [Google Scholar]
- 14.Ichihashi, Y., and M. Oie. 1982. Proteolytic activation of vaccinia virus for penetration phase of infection. Virology 116:297-305. [DOI] [PubMed] [Google Scholar]
- 15.Ichihashi, Y., T. Tsuruhara, and M. Oie. 1982. The effect of proteolytic enzymes on the infectivity of vaccinia virus. Virology 122:279-289. [DOI] [PubMed] [Google Scholar]
- 16.Izmailyan, R. A., C.-Y. Huang, S. Mohammad, S. N. Isaacs, and W. Chang. 2006. The envelope G3L protein is essential for entry of vaccinia virus into host cells. J. Virol. 80:8402-8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joklik, W. K. 1962. The purification of four strains of poxvirus. Virology 18:9-18. [DOI] [PubMed] [Google Scholar]
- 18.Korte, T., K. Ludwig, F. P. Booy, R. Blumenthal, and A. Herrmann. 1999. Conformational intermediates and fusion activity of influenza virus hemagglutinin. J. Virol. 73:4567-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Law, M., G. C. Carter, K. L. Roberts, M. Hollinshead, and G. L. Smith. 2006. Ligand-induced and non-fusogenic dissolution of a viral membrane. Proc. Natl. Acad. Sci. USA 103:5989-5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, C.-L., C.-S. Chung, H. G. Heine, and W. Chang. 2000. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 74:3353-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milne, R. S., A. V. Nicola, J. C. Whitbeck, R. J. Eisenberg, and G. H. Cohen. 2005. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J. Virol. 79:6655-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moss, B. 2007. Poxviridae: the viruses and their replication, p. 2905-2946. In D. M. Knipe (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 23.Moss, B. 2006. Poxvirus entry and membrane fusion. Virology 344:48-54. [DOI] [PubMed] [Google Scholar]
- 24.Nicola, A. V., A. M. McEvoy, and S. E. Straus. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 77:5324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nir, S., N. Duzgunes, M. C. de Lima, and D. Hoekstra. 1990. Fusion of enveloped viruses with cells and liposomes. Activity and inactivation. Cell Biophys. 17:181-201. [DOI] [PubMed] [Google Scholar]
- 26.Ojeda, S., T. G. Senkevich, and B. Moss. 2006. Entry of vaccinia virus and cell-cell fusion require a highly conserved cysteine-rich membrane protein encoded by the A16L gene. J. Virol. 80:51-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Planterose, N. D., C. Nishimura, and N. P. Salzman. 1962. The purification of vaccinia virus from cell cultures. Virology 18:294-301. [DOI] [PubMed] [Google Scholar]
- 28.Puri, A., J. Winick, R. J. Lowy, D. Covell, O. Eidelman, A. Walter, and R. Blumenthal. 1988. Activation of vesicular stomatitis virus fusion with cells by pretreatment at low pH. J. Biol. Chem. 263:4749-4753. [PubMed] [Google Scholar]
- 29.Senkevich, T. G., and B. Moss. 2005. Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell-cell fusion. J. Virol. 79:4744-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senkevich, T. G., S. Ojeda, A. Townsley, G. E. Nelson, and B. Moss. 2005. Poxvirus multiprotein entry-fusion complex. Proc. Natl. Acad. Sci. USA 102:18572-18577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Senkevich, T. G., B. M. Ward, and B. Moss. 2004. Vaccinia virus entry into cells is dependent on a virion surface protein encoded by the A28L gene. J. Virol. 78:2357-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, G. L., and M. Law. 2004. The exit of vaccinia virus from infected cells. Virus Res. 106:189-197. [DOI] [PubMed] [Google Scholar]
- 33.Stegmann, T., I. Bartoldus, and J. Zumbrunn. 1995. Influenza hemagglutinin-mediated membrane fusion: influence of receptor binding on the lag phase preceding fusion. Biochemistry 34:1825-1832. [DOI] [PubMed] [Google Scholar]
- 34.Townsley, A., T. G. Senkevich, and B. Moss. 2005. The product of the vaccinia virus L5R gene is a fourth membrane protein encoded by all poxviruses that is required for cell entry and cell-cell fusion. J. Virol. 79:10988-10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Townsley, A., T. G. Senkevich, and B. Moss. 2005. Vaccinia virus A21 virion membrane protein is required for cell entry and fusion. J. Virol. 79:9458-9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Townsley, A. C., A. S. Weisberg, T. R. Wagenaar, and B. Moss. 2006. Vaccinia virus entry into cells via a low-pH-dependent endosomal pathway. J. Virol. 80:8899-8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagenaar, T. R., and B. Moss. 2007. Association of vaccinia virus fusion regulatory proteins with the multicomponent entry/fusion complex. J. Virol. 81:6286-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]