Abstract
Although recombinants based on the attenuated poxvirus vectors MVA and NYVAC are currently in clinical trials, the nature of the genes triggered by these vectors in antigen-presenting cells is poorly characterized. Using microarray technology and various analysis conditions, we compared specific changes in gene expression profiling following MVA and NYVAC infection of immature human monocyte-derived dendritic cells (MDDC). Microarray analysis was performed at 6 h postinfection, since these viruses induced extensive cytopathic effects, rRNA breakdown, and apoptosis at late times postinfection. MVA- and NYVAC-infected MDDC shared upregulation of 195 genes compared to uninfected cells: MVA specifically upregulated 359 genes, and NYVAC upregulated 165 genes. Microarray comparison of NYVAC and MVA infection revealed 544 genes with distinct expression patterns after poxvirus infection and 283 genes specifically upregulated after MVA infection. Both vectors upregulated genes for cytokines, cytokine receptors, chemokines, chemokine receptors, and molecules involved in antigen uptake and processing, including major histocompatibility complex genes. mRNA levels for interleukin 12β (IL-12β), beta interferon, and tumor necrosis factor alpha were higher after MVA infection than after NYVAC infection. The expression profiles of transcription factors such as NF-κB/Rel and STAT were regulated similarly by both viruses; in contrast, OASL, MDA5, and IRIG-I expression increased only during MVA infection. Type I interferon, IL-6, and Toll-like receptor pathways were specifically induced after MVA infection. Following MVA or NYVAC infection in MDDC, we found similarities as well as differences between these virus strains in the expression of cellular genes with immunological function, which should have an impact when these vectors are used as recombinant vaccines.
Attenuated strains of vaccinia virus (VACV), MVA and NYVAC, are currently being tested as vaccine vectors (8, 35, 49, 77). NYVAC is a derivative of VACV strain Copenhagen, from which 18 open reading frames were specifically deleted from the parental viral genome; genes involved in host range, virulence, and pathogenesis were thus lost (75). NYVAC-derived vectors are able to express antigens from a broad range of species (75). A number of examples using NYVAC as a delivery system for recombinant vaccines to pathogens and tumors have been reported (22, 39, 52, 71). Phase I/II clinical trials using NYVAC against human immunodeficiency virus (HIV) type 1 and pathogens are currently under way and showed immunogenicity and a good safety profile (55). MVA was generated after more than 500 passages in chicken embryo fibroblasts and has lost approximately 15% of the parental viral genome (5, 48); the structural genes remained unaltered, but genes involved in immune evasion factors and host range (5, 48, 78) have been deleted or fragmented. In mammals, MVA recombinants induce protective immunity against a wide spectrum of pathogens (49, 66). Differences in the degree and magnitude of the immune response to HIV proteins have been observed between MVA and NYVAC vectors (24). Phase I/II clinical trials with MVA-based recombinants have been performed or are under way for HIV type 1, malaria, and tumors (13, 18, 23, 33). MVA is also a potentially safe candidate for a vaccine against smallpox should this virus reemerge as a bioterrorism weapon (10).
While there is major interest in the use of MVA and NYVAC as vectors for antigen delivery and as vaccines for pathogens and tumors, little is known of the impact of these vectors on host genome expression by antigen-presenting cells (APC). Whereas other viruses productively infect dendritic cells (DC), including cytomegalovirus, varicella zoster virus, and measles virus (1, 26, 59), several studies indicate that poxviruses do not produce an infectious cycle in DC (20, 21, 38, 40), while Langerhans cells allow VACV replication (19). Recent findings show that MVA infection of human DC interrupts cell maturation and leads to apoptosis associated with a decrease in Bcl-2 and Bcl-XL levels late in infection in a virus multiplicity-dependent manner (15). Both MVA and NYVAC are potent in vivo activators of T-cell-specific immune responses to recombinant antigens, indicating efficient antigen delivery in APC and the activation of immune T cells, possibly due to virus infection of activated DC (80).
DC, the best-known group of APC, are bone marrow-derived leukocytes. They act as sentinels of the immune system and are present in an immature state in almost all peripheral tissues, where they can induce specific T-cell-mediated immune responses (6). Maturation is induced by the contact of immature DC with various products of infectious agents (4, 14). During maturation, DC lose their ability to take up antigens and migrate from the sites of antigen accumulation to the areas of antigen presentation, primarily the T-cell zones of secondary lymphoid organs (6, 58). Due to the essential role of DC in immune response development, we characterized the impact of two vaccine poxvirus vectors, MVA and NYVAC, on the gene expression profile of human monocyte-derived dendritic cells (MDDC) infected for a relatively brief period (6 h postinfection [hpi]). This infection time was chosen to identify upregulated genes, since rRNA breakdown effects at this time are minimal compared to those at late times postinfection, when rRNA breakdown and apoptosis are found in infected MDDC (15). DNA microarray technology allows monitoring of the expression of several thousand individual genes (32) and has been used to identify the genomic expression profiles of human HeLa cells in response to infection by both virulent VACV (WR strain) as well as attenuated MVA and NYVAC (28-30) and other VACV strains (43, 46).
In this investigation, we have defined the characteristics of MVA and NYVAC infection of MDDC in culture and show that MVA infection upregulates a larger number of genes encoding immunomodulatory molecules than NYVAC, with higher expression levels. We demonstrated a distinct regulation of host genes by the poxvirus vectors in infected MDDC under three microarray conditions, comparing MVA- or NYVAC-infected with uninfected cells, MVA-infected with NYVAC-infected cells, and MVA/NYVAC-infected HeLa cells with MVA/NYVAC-infected MDDC. Levels of alpha interferon (IFN-α), tumor necrosis factor alpha (TNF-α), and proinflammatory cytokines such as interleukin 6 (IL-6) were higher in MVA-infected MDDC than in NYVAC-infected MDDC. Genes involved in the antiviral response such as retinoic acid-inducible protein I (RIG-I), melanoma differentiation-associated gene 5 (MDA5), and 2′-5′-oligoadenylate (5-OA) synthetase-like (OASL) were upregulated exclusively in MVA-infected MDDC. Our findings show similarities and differences in the genes induced by MVA and NYVAC in human MDDC. These genes are important for the innate immune response and could influence the extent of the host response and protective efficacy when these two poxvirus vectors are used as vaccines.
MATERIALS AND METHODS
Cells, viruses, and infection conditions.
HeLa cells (ATCC) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% newborn bovine serum and antibiotics. Human MDDC were generated as previously reported, with minor modifications (51, 56). Peripheral blood mononuclear cells were obtained by using a standard Ficoll gradient for heparinized blood extracted from healthy individuals. To obtain human monocytes, peripheral blood mononuclear cells (3 × 106 to 4 × 106 cells/ml) were incubated (2 h at 37°C) in a humidified atmosphere with 5% CO2 in MDDC medium (serum-free XVIVO-15 medium; BioWhittaker, Walkersville, MD) with 1% human blood group AB serum, 50 μg/ml gentamicin (Braun, Melsungen, Germany), and 2.5 μg/ml amphotericin B (Bristol-Myers Squibb, Rueil-Malmaison, France). Adherent cells were washed four times with prewarmed serum-free XVIVO-10 medium and cultured in MDDC medium as described above. To obtain immature MDDC, cells were stimulated for 5 days by the addition of 1,000 U/ml each of IL-4 and granulocyte-macrophage colony-stimulating factor (both from Prospec-Tany Technogene, Rehovot, Israel) at days 0 and 2. MDDC immunophenotyping was confirmed by flow cytometry using the following monoclonal antibodies to cell surface markers: fluorescein isothiocyanate (FITC)-conjugated anti-HLA-DR, anti-CD14, and anti-CD19 and an immunoglobulin Gγ1 (IgGγ1) isotype-matched control; phycoerythrin (PE)-conjugated anti-HLA-DR, anti-CD11c, anti-CD14, anti-CD40, anti-CD45, and anti-CD56 and an IgG-γ1 control; and peridinin-chlorophyll-protein complex-anti-CD3 and -anti-CD14 and an IgG-γ1 control (all from BD Biosciences, San Diego, CA). PE-anti-CD80, -CD83, and -CD86 were from Coulter, and PE-anti-CD209 was from eBioscience (San Diego, CA). Cells were washed with phosphate-buffered saline (PBS), resuspended at 2 × 106 cells/ml (50 μl/tube), and incubated with FITC-, PE-, and/or PerCP-conjugated monoclonal antibody (30 min at 4°C). Cells were washed with PBS, fixed with 1% formaldehyde in PBS, and analyzed by flow cytometry in an EPICS Profile cytometer (Coulter, Hialeah, FL). Cell populations were selected by forward- and side-light-scatter parameters. This analysis showed that the purity of MDDC was ≥95%, and the phenotype observed was characteristic of immature MDDC: CD3− CD8− CD14− CD19− CD56− HLA-DR+ CD80− CD83− CD86+ CD11c+ CD40+ CD45+ CD209+.
These immature MDDC were used for virus infection. NYVAC (28a, 75) and MVA (24, 29) strains were cultured in chicken embryo fibroblast cells, purified by two sucrose cushions, and titrated on BHK-21 cells by immunostaining of fixed infected cultures with a polyclonal anti-VACV antibody (62). MDDC were infected at 5 PFU/cell, virus inoculum was removed after 1 h, fresh medium was added, and infection continued for another 5 h. Cells were collected and centrifuged, supernatants were saved for an enzyme-linked immunosorbent assay (ELISA), and cells were washed twice with PBS and processed for RNA extraction or Western blot analyses.
Metabolic labeling of proteins.
MDDC were infected with 5 PFU/cell, and at the indicated times (106 cells/time postinfection), cells were washed with methionine-free medium and incubated in methionine-free medium containing [35S]methionine (50 μCi/well for 30 min at 37°C). Proteins from cell extracts prepared in lysis buffer were fractionated by 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and developed by autoradiography.
Microarray labeling.
Ultraspect_II RNA (Biotecx, Houston, TX) was used to isolate total RNA from purified human MDDC infected with NYVAC or MVA (3 × 106 cells/time postinfection; 5 PFU/cell), or mock infected. RNA was then purified with Megaclear (Ambion, Foster City, CA), and the integrity was confirmed by using an Agilent (Santa Clara, CA) 2100 Bioanalyzer. Total RNA (1.5 μg) was amplified with an Amino Allyl MessageAmp aRNA kit (Ambion); 54 to 88 μg of amplified RNA (aRNA) was obtained. The mean RNA size was 1,500 nucleotides, as observed using the Agilent 2100 Bioanalyzer. For each sample, 6 μg aRNA was labeled with one aliquot of Cy3 or Cy5 Mono NHS Ester (CyDye postlabeling reactive dye pack; GE Healthcare) and purified using Megaclear. Incorporation of Cy5 and Cy3 was measured using 1 μl of probe in a Nanodrop spectrophotometer (Nanodrop Technologies). For each hybridization, Cy5 and Cy3 probes (150 mol each) were mixed and dried by speed vacuum and resuspended in 9 μl RNase-free water. Labeled aRNA was fragmented by adding 1 μl 10× fragmentation buffer (Ambion), followed by incubation (70°C for 15 min). The reaction was terminated with the addition of 1 μl stop solution (Ambion) to the mixture. Two dye-swapped hybridizations were performed for each comparison; in one, the mock-infected sample was Cy3 labeled, and the MVA-infected sample was Cy5 labeled; in the second, labeling was reversed. Double labeling was used to abolish dye-specific labeling and hybridization differences.
Slide treatment and hybridization.
Slides containing 22,264 spots (19,256 different oligonucleotides) corresponding to Human Genome Oligo set version 2.2 (QIAGEN, Hilden, Germany) were obtained from the Genomic and Microarrays Laboratory (Cincinnati University, Cincinnati, OH). Information about printing and the oligonucleotide set can be found on their website (http://microarray.uc.edu). Slides were prehybridized and hybridized as described previously (28-30). Images from Cy3 and Cy5 channels were equilibrated and captured with an Axon 4000B scanner, and spots were quantified using GenePix 5.1 software. Data for replicates were analyzed using Almazen software (Bioalma, Spain). Basically, Lowess normalization was applied to each replicate, and the log ratios were merged with the corresponding standard deviations and z scores.
Gene expression analysis.
The original data set contained 19,256 oligonucleotides per slide. In each analysis, genes with an interreplicate mean signal of <100 or an interreplicate standard deviation of >1 were filtered out. Genes were considered to be differently expressed if the expression change (n-fold) was <−2 (downregulated) or >2 (upregulated). Functional analyses of regulated genes were generated by Ingenuity Pathways Analysis (Ingenuity Systems [www.ingenuity.com]). Hierarchical clustering was carried out using SpotFire Decision Site for Functional Genomics software. Ward's method with an average value-ordering function and a half-square Euclidean distance function was used.
Quantitative real-time RT-PCR.
RNA (1 μg) was reverse transcribed using the superscript first-strand synthesis system for reverse transcription (RT)-PCR (Invitrogen, Carlsbad, CA). A 1:40 dilution of the RT reaction mixture was used for quantitative PCR. The primers and probe set used to amplify TNF, IFN-β, IFN-stimulated gene 15 (ISG15), NF-κB-2, IL-12, IL-7, IL-6, IFN-γ, OASL, ATF-3, ADORA, and H2AFY were purchased from Applied Biosystems. RT-PCRs were performed according to Assay-on-Demand, optimized for TaqMan Universal PCR MasterMix, No AmpErase UNG (28-30). All samples were assayed in duplicate. Threshold cycle values were used to plot a standard curve in which the threshold cycle decreased in linear proportion to the log of the template copy number. Correlation values of standard curves were always >99%.
Immunofluorescence.
MDDC cultured on coverslips were infected with MVA or NYVAC (5 × 105 cells/time postinfection; 5 PFU/cell). At 6 and 16 hpi, cells were washed with PBS, fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton X-100 in PBS (room temperature for 10 min). Cells were incubated with primary anti-WR (anti-VV), anti-A36R or anti-B5R (both obtained from R. Blasco, INIA, Spain), or anti-E3L (obtained from B. L. Jacobs, University of Arizona) antibodies, followed by fluorescein- or Texas red-conjugated isotype-specific secondary antibodies. F-actin was stained with fluorescein-conjugated phalloidin (Molecular Probes, Carlsbad, CA); DNA was stained with ToPro (Molecular Probes). Images were obtained using a Bio-Rad Radiance 2100 confocal laser microscope.
Western blot.
HeLa and MDDC were infected (106 cells/time postinfection; 5 PFU/cell) with MVA or NYVAC and collected, and cell extracts were prepared at 2, 6, and 16 hpi by lysis in buffer (50 mM Tris-HCl [pH 8.0], 0.5 M NaCl, 10% NP-40, 1% SDS) for 5 min on ice. Protein lysates (100 μg) were fractionated by 14% or 8% SDS-PAGE, transferred onto nitrocellulose membranes, and incubated with anti-poly(ADP-ribose) polymerase (PARP) (Cell Signaling, Boston, MA), anti-actin (Santa Cruz, Santa Cruz, CA), anti-E3L, anti-A14L (64), anti-A4L (60), anti-A27L (63), anti-Al7L (61), anti-phosphorylated interferon-responsive factor 3 (IRF-3) (Upstate, Chicago, IL), anti-IRF-3 (Cell Signaling), anti-IRF-7 (Santa Cruz), anti-B5R, anti-phosphorylated IF-2α (Biosource, Camarillo, CA), or anti-alpha subunit of eukaryotic initiation factor 2 (eIF-2α) (Santa Cruz) antibodies, followed by secondary antibodies (mouse and rabbit peroxidase conjugates). Protein expression was detected using ECL reagents (Amersham, Uppsala, Sweden).
Cytokine determination.
IL-2, IL-4, IL-6, IL-10, TNF-α, IFN-γ, IL-1β, IL-8, and IL-12 levels in 30 μl of supernatants were determined using Cytometric Bead Array, human Th1/Th2 cytokine, and Cytometric Bead Array human inflammation kits (BD Bioscience) according to the manufacturer's protocol.
RESULTS
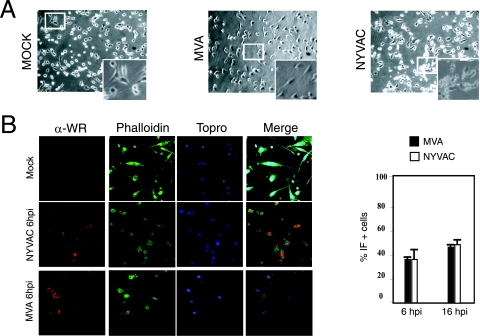
Infection with attenuated poxvirus vectors MVA and NYVAC causes extensive cell damage in human MDDC. To characterize the impact of MVA and NYVAC infection on immature human MDDC, we first defined the induced cytopathic effects by phase-contrast and immunofluorescence (IF) microscopy. At 6 hpi, MVA or NYVAC infection at 5 PFU/cell resulted in alterations in cell morphology characterized by cell rounding and cytoplasmic contraction; the cytopathic effect was more pronounced in NYVAC than after MVA infection (Fig. 1A). The effects on cell morphology were severe by 16 hpi in MDDC infected with either virus strain (not shown). The number of MVA- or NYVAC-infected MDDC was monitored by IF after staining with a polyclonal antibody to VACV proteins. At 6 hpi, approximately 35% of MVA- or NYVAC-infected MDDC stained for viral proteins, which increased to 45% by 16 hpi (Fig. 1B). Since the use of higher virus multiplicities caused extensive MDDC damage, we used 5 PFU/cell in these studies.
FIG. 1.
Cellular and biochemical changes in human immature MDDC following infection with MVA or NYVAC poxvirus vectors. (A) Morphological changes in immature MDDC mock infected or infected with MVA or NYVAC (5 PFU/cell) in six-well plates; changes were examined by phase-contrast microscopy at 6 hpi. The upper panels show representative fields (magnification, ×4); the lower panels show the indicated areas at a higher magnification. (B) IF analysis. MDDC cultured on coverslips were infected with MVA or NYVAC (5 PFU/cell), and cells were fixed at 6 hpi and treated with a rabbit polyclonal antibody to VACV proteins (α-WR), followed by a Texas Red-labeled secondary antibody, phalloidin-FITC (for actin staining), and ToPro (for DNA staining) (left panels). Cells expressing viral antigens were quantified by IF in four independent experiments (right panel).
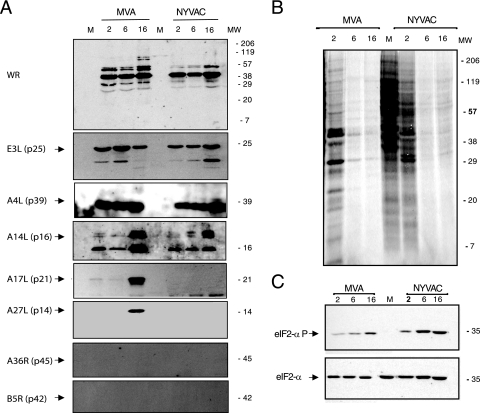
To examine viral protein synthesis in MDDC during MVA or NYVAC infection, we performed Western blotting using antibodies specific for early p25 (E3L) and late viral proteins p14 (A27L), p21 (A17L), p16 (A14L), p39 (A4L), and p42 (B5R). Proteins encoded by the E3L, A4L, and A14L genes were detected efficiently in lysates of cells infected with both viruses (Fig. 2A). The late proteins encoded by the A17L and A27L genes were detected only in lysates of MVA-infected cells, concurring with previous reports for HeLa cells (53). The protein encoded by the B5R gene was not detected in lysates of cells infected with either virus, as determined by Western blot (Fig. 2A) and IF (not shown) analyses. MVA-infected MDDC gave a similar result for A36R gene expression, as previously described (15).
FIG. 2.
Protein synthesis evaluation in MDDC after MVA or NYVAC infection. (A) Viral protein expression during NYVAC and MVA infection. MDDC were mock infected (M) or infected with MVA or NYVAC (5 PFU/cell). At the indicated times postinfection, equal amounts of proteins from cell extracts were fractionated by SDS-PAGE, transferred onto nitrocellulose, and treated with antibodies to VACV proteins (WR) or specific virus early (p25) and late proteins (p21, p14, p39, p16, and p42). Molecular weight (MW) (in thousands) is indicated based on protein standards. (B) Metabolic labeling of proteins during NYVAC and MVA infection. MDDC were mock infected (M) or infected with MVA or NYVAC (5 PFU/cell). At the times indicated, cells were labeled (30 min) with [35S]Met-Cys Promix (50 μCi/ml), and equal amounts of proteins were analyzed by SDS-PAGE (10%) and autoradiography. (C) The gel in B was transferred onto nitrocellulose and incubated with antibodies to total eIF-2α or eIF-2α phosphorylated (P) at Ser51. Molecular weight (MW) (in thousands) is indicated based on protein standards.
To document the overall protein expression pattern and the shutoff effect, MDDC were metabolically labeled with [35S]methionine at 2, 6, and 16 h after MVA or NYVAC infection and analyzed by SDS-PAGE and autoradiography. We observed a severe translational block in protein synthesis by 6 hpi in cells infected with either virus (Fig. 2B). This blockade coincided with an increase in phosphorylation of the small subunit of the initiation factor eIF-2α (Fig. 2C), as described previously for NYVAC in HeLa cells (28, 53). These findings show that MVA or NYVAC infection of MDDC caused shutoff by 6 hpi, whereas the synthesis of early and some late viral proteins continued, indicating functional cell translational machinery. It has been previously reported that the viral C7L gene is able to prevent eIF-2α phosphorylation in NYVAC-infected HeLa cells (53). The presence of this gene in the MVA genome might explain the different levels in eIF-2α phosphorylation between MVA- and NYVAC-infected MDDC, although we cannot rule out the possibility of other mechanisms.
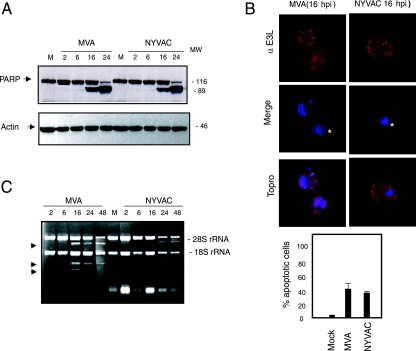
Since NYVAC infection of HeLa cells induces apoptosis (28a, 53), we used an antibody that recognizes both full-length and cleaved PARP-1 (73) to analyze whether apoptosis occurs in infected MDDC. In both MVA and NYVAC infection, cleavage of 89-kDa PARP-1 was evident by 16 hpi, with no apoptosis at 6 hpi (Fig. 3A). After IF staining with the E3 antibody for an early viral protein and ToPro for the appearance of apoptotic bodies, we found similar numbers of apoptotic cells for both viruses at 16 hpi (nearly 40% of total infected cells) (Fig. 3B). This result contrasts with the low apoptotic effect induced by MVA in human HeLa cells (28a, 53).
FIG. 3.
Apoptosis induction and rRNA breakdown during MVA or NYVAC infection of MDDC. (A) Time course of PARP-1 cleavage during MVA and NYVAC infection. MDDC were mock infected (M) or infected with MVA or NYVAC (5 PFU/cell); at the times indicated, total proteins (100 μg) were fractionated by SDS-PAGE, transferred onto nitrocellulose, and immunoblotted with anti-PARP-1. An 89-kDa PARP-1 cleavage product was observed at 16 hpi. Molecular weight standards (MW) (in thousands) are indicated (right). Equivalence of protein loading was confirmed using anti-actin controls. (B) Quantification of cells in apoptosis after MVA or NYVAC infection. MDDC were mock infected (M) or infected with MVA or NYVAC (5 PFU/cell); at 16 hpi, cells were fixed and processed to visualize apoptosis by IF using antibodies to the viral E3 protein (red) and ToPro for DNA staining (blue). The percentage of cells in apoptosis was determined by counting ∼2,000 cells in two independent experiments (bottom). (C) MVA or NYVAC infection of MDDC causes rRNA breakdown. Total rRNA was isolated from uninfected MDDC (M) or MDDC infected with MVA or NYVAC at the indicated times postinfection; 2 μg of each sample was applied for electrophoresis, and the gel was stained with ethidium bromide. Arrows indicate bands corresponding to characteristic rRNA degradation products.
We previously showed that NYVAC but not MVA infection of HeLa cells triggers rRNA degradation late in infection, with the same cleavage pattern observed during activation of the interferon-induced 2-5A oligonucleotide synthetase/RNase L system (53). We therefore examined rRNA integrity in MVA- and NYVAC-infected MDDC. Total RNA was isolated from infected and mock-infected cells and fractionated by formaldehyde-agarose gel electrophoresis. There was no rRNA degradation in uninfected cells or in infected cells at 6 hpi; in contrast, we observed a breakdown of 28S and 18S rRNA at later times in cells infected with both viruses (Fig. 3C). These results revealed that MVA and NYVAC infection of MDDC does not induce apoptosis or rRNA degradation at 6 hpi, but these effects appeared later in infection.
Differential gene regulation following MVA or NYVAC infection of human MDDC compared to mock-infected cells.
Since MDDC are the most potent APC and the only cells able to activate naive T cells (47), we studied the impact of both viruses on MDDC gene expression at a time when host rRNA had not been degraded by virus infection. We used chips carrying oligonucleotides from 19,256 human genes to profile MDDC gene expression and hybridized cDNA samples from infected and uninfected (mock-infected) cells at 6 hpi. The gene expression data were selected as described in Materials and Methods. Compared to uninfected cells, we identified 1,215 genes differentially expressed in MDDC after MVA infection (21.6% of the genes selected), 554 of which were upregulated and 661 of which were downregulated (see examples in Table S1 in the supplemental material). A similar experiment comparing NYVAC-infected with uninfected MDDC showed variance in the regulation of 728 genes after NYVAC infection (13.5% of the genes selected), 360 of which were upregulated and 368 of which were downregulated (see examples in Table S2 in the supplemental material).
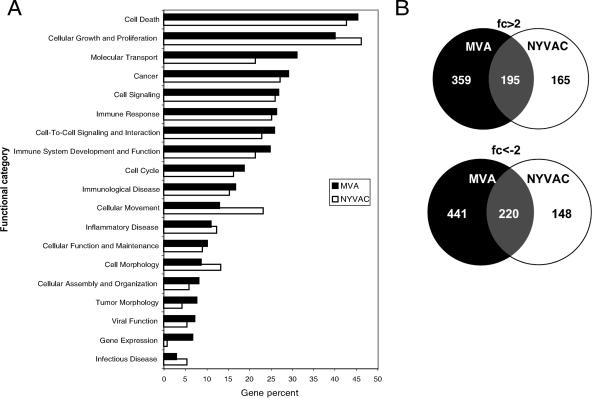
Host genes with altered expression in MVA- or NYVAC-infected MDDC belong to a number of functional categories (Fig. 4A). Both poxvirus vectors regulated similar numbers of genes involved in cell death, cancer, gene expression, cell development, and organism survival. NYVAC nonetheless selectively regulated more genes involved in cell growth, proliferation, and morphology than MVA. In contrast, MVA regulated a larger number of genes involved in the immune response and immune system development than NYVAC (Fig. 4A). Examples of the differentially regulated genes are presented in Table 1. Genes differentially expressed after poxvirus infection were represented using Venn diagrams to display differently and similarly regulated genes for MVA and NYVAC (Fig. 4B). MVA and NYVAC shared 195 genes that were upregulated and 220 that were downregulated, whereas 359 genes were upregulated only by MVA and 165 genes were upregulated only by NYVAC. In infected MDDC, MVA specifically downregulated more genes than NYVAC (441 versus 148 genes). Both viruses produced an increase in specific immune molecules such as CXCL2, TNF-α, and several interferon-induced proteins (IFIT1, IFIT4, ISG15, and ISG20), with higher expression levels in MVA-infected MDDC than in NYVAC-infected MDDC. An alternative visualization using hierarchical cluster analysis was also developed (not shown).
FIG. 4.
Functional classification of genes with altered expression in MVA- or NYVAC-infected MDDC versus uninfected cells after microarray analysis. Genes with altered expression in MVA- or NYVAC-infected MDDC were compared with uninfected cells. (A) The y axis shows the most representative high-level functions associated with genes regulated in MVA- or NYVAC-infected MDDC, according to ingenuity pathway analysis. The x axis represents the percentage of the total number of regulated genes associated with a given function. (B) Venn diagrams represent common or specific genes upregulated (>2-fold) or downregulated (<−2-fold) in MVA- or NYVAC-infected MDDC compared to mock-infected cells.
TABLE 1.
Representative genes regulated by MVA or NYVAC in infected MDDC according to predicted biological function
| Description | GenBank accession no. | Gene | Fold change in transcription
|
|
|---|---|---|---|---|
| MVA | NYVAC | |||
| IFN and IFN-induced genes | ||||
| IFN-induced protein with tetratricopeptide repeats 4 | NM_001549 | IFIT4 | 52.45 | 3.37 |
| IFN-induced protein with tetratricopeptide repeats 1 | NM_001548 | IFIT1 | 47.8 | 4.65 |
| IFN-induced, hepatitis C virus-associated microtubular aggregate protein (44 kDa) | NM_006417 | MTAP44 | 20.95 | 2.63 |
| IRF-2 | NM_002199 | IRF2 | 6.54 | 3.48 |
| IRF-7 | NM_004031 | IRF7 | 2.52 | 1.3 |
| IFN-α1 | NM_024013 | IFNA1 | 2.51 | 1.92 |
| Myxovirus (influenza virus) resistance 1, IFN-inducible protein p78 (mouse) | NM_002462 | MX1 | 2.26 | 1.44 |
| IRF-5 | NM_002200 | IRF5 | −2.31 | 1.06 |
| IFN-α-inducible protein 27 | NM_005532 | IFI27 | −2.46 | −1.62 |
| IFN-γ-inducible protein 30 | NM_006332 | IFI30 | −2.79 | −2.79 |
| Interleukins | ||||
| IL-6 (IFN, beta 2) | NM_000600 | IL6 | 4.05 | 1 |
| IL-1α | NM_000575 | IL1A | −1.43 | 2.01 |
| IL-8 | NM_000584 | IL8 | −2.9 | −1.39 |
| IL-1β | NM_000576 | IL1B | −2.25 | 1.39 |
| TNF (TNF superfamily, member 2) | NM_000594 | TNF | 52.21 | 6.79 |
| Other cytokines | ||||
| GRO2 oncogene (SCYB2), CXCL2 | NM_002089 | GRO2 | 6.58 | 2.22 |
| Small inducible cytokine A3 | NM_002983 | SCYA3 | 4.22 | 1.12 |
| Small inducible cytokine A5 (RANTES) | NM_002985 | SCYA5 | 4.17 | −1.17 |
| Small inducible cytokine A4 | NM_002984 | SCYA4 | 3.74 | −1.14 |
| Colony-stimulating factor 2 (granulocyte-macrophage) | NM_000758 | CSF2 | 3.13 | −1.08 |
| Apoptosis | ||||
| Nuclear factor of kappa light polypeptide gene enhancer in B cells inhibitor α | NM_020529 | NFKBIA | 9.59 | 6.02 |
| MAIL, NFKBIZ | NM_031419 | 12.99 | 4.91 | |
| BCL2-associated athanogene 3 | NM_004281 | BAG3 | 8.42 | 1.28 |
| Calpain 1, (mu/I) large subunit | NM_005186 | CAPN1 | 4.1 | 1.78 |
| Antiviral immune response | ||||
| Melanoma differentiation-associated protein 5 | NM_022168 | MDA5 | 7.77 | 1.67 |
Comparison of MVA and NYVAC infection of human MDDC shows specific differences in host gene expression levels.
To further document specific differences between the two vectors, we performed microarrays with cDNAs prepared from MDDC infected with MVA and NYVAC for 6 h, but we now compared NYVAC-infected samples with MVA-infected samples. We evaluated specific transcriptional differences between the two vectors by removing those genes regulated equally by both viruses from the processed data. We identified 11,800 genes with similar expression levels for both viruses; 544 genes showed expression pattern differences after poxvirus infection (4.4% of the genes selected), 283 of which had at least twofold-higher transcriptional levels after MVA than after NYVAC infection. Human genes differentially expressed in MVA versus NYVAC infection of MDDC are shown in Table 2 and in Table S3 in the supplemental material. Genes including TNF-α, IFN-β, and IL-12β were increased by fivefold or higher after MVA infection compared to NYVAC infection. MVA and NYVAC infections produced 12- and 4-fold upregulation, respectively, of a recently described member of the NF-κB family, such as MAIL (molecule possessing ankyrin repeats induced by lipopolysaccharide) (Tables 1 and 2).
TABLE 2.
Differential gene expression profiling of MVA-infected versus NYVAC-infected human DCa
| Description | GenBank accession no. | Gene | Fold change of MVA/NYVAC |
|---|---|---|---|
| IFNs and IFN-induced genes | |||
| IFN-induced protein with tetratricopeptide repeats 4 | NM_001549 | IFIT4 | 11.45 |
| IFN-induced protein with tetratricopeptide repeats 1 | NM_001548 | IFIT1 | 7.38 |
| IFN-induced, hepatitis C virus-associated microtubular aggregate protein (44 kDa) | NM_006417 | MTAP44 | 5.52 |
| Guanylate binding protein 5 | NM_052942 | GBP5 | 5.37 |
| IFN-β1, fibroblast | NM_002176 | IFNB1 | 5.06 |
| IFN-stimulated protein, 15 kDa | NM_005101 | ISG15 | 2.56 |
| IFN-stimulated gene (20 kDa) | BC016341 | ISG20 | 2.51 |
| IRF-7 | NM_004031 | IRF7 | 2.31 |
| IFN-α1 | NM_024013 | IFNA1 | 1.18 |
| Interleukins | |||
| IL-12β (NK cell-stimulatory factor 2) | NM_002187 | IL12B | 5.44 |
| IL-6 (IFN-β2) | NM_000600 | IL6 | 4.05 |
| IL-1α | NM_000575 | IL1A | −2.42 |
| IL-1β | NM_000576 | IL1B | −3.14 |
| TNF and related genes | |||
| TNF (TNF superfamily, member 2) | NM_000594 | TNF | 10.38 |
| TNF receptor-associated factor 6 | NM_004620 | TRAF6 | 1.7 |
| Tumor necrosis factor receptor superfamily, member 10b | AF016266 | TNFRSF10B | −1.07 |
| Other cytokines | |||
| Small inducible cytokine A4 | NM_002984 | SCYA4 | 5.61 |
| Small inducible cytokine A5 (RANTES) | NM_002985 | SCYA5 | 3.06 |
| GRO2 oncogene (SCYB2), CXCL2 | NM_002089 | GRO2 | 2.78 |
| Small inducible cytokine subfamily B member 10, CXCL10, IP-10 | NM_001565 | SCYB10 | 2.35 |
| Colony-stimulating factor 2 (granulocyte-macrophage) | NM_000758 | CSF2 | 1.94 |
| Apoptosis | |||
| BCL2-associated athanogene 3 | NM_004281 | BAG3 | 4.55 |
| MAIL, NFKBIZ | NM_031419 | 2.85 | |
| Calpain 1 (mu/I) large subunit | NM_005186 | CAPN1 | 1.72 |
| Antiviral immune response | |||
| RNA helicase | NM_014314 | RIG-I | 5.72 |
| Melanoma differentiation-associated protein 5 | NM_022168 | MDA5 | 5.05 |
| OASL | NM_003733 | OASL | 4.53 |
Shown is a comparison of gene expression profiling between MVA- and NYVAC-infected MDDC. Representative human genes specifically regulated by each vector according to predicted biological function are shown.
The microarray findings indicate that both poxvirus vectors triggered similar but also distinct regulatory pathways in MDDC, with MVA upregulating more host genes than NYVAC.
Validation of microarray data by quantitative real-time RT-PCR.
To confirm the microarray results, we validated the changes in transcript levels using real-time RT-PCR to verify the transcriptional changes in 12 selected genes (TNF, IFN-β, ISG15, NF-κB-2, IL12, IL7, IL6, IFN-γ, OASL, ATF-3, ADORA, and H2AFY); HPRT was used as an internal control. The assay was performed with the same RNAs as those used in the microarray experiment (Table 2). The expression pattern and relative mRNA abundance of the selected genes concurred with microarray data in all cases, with only slight variations, validating the microarray findings (see Table S4 in the supplemental material). Indeed, clear differences were observed in the expression levels of selected genes for both viruses.
To further validate these results (see Table S4 in the supplemental material), we used cDNAs from MDDC obtained from two other healthy volunteers. MDDC were infected with MVA or NYVAC for 6 h, cDNA was prepared, and RT-PCR was performed as described above (Table 3). The results for three donors revealed a similar pattern of cytokine gene expression induced by the two poxvirus vectors (Table 3), validating the microarray data.
TABLE 3.
Expression levels of selected genes by real-time RT-PCRa
| Gene product | Fold change by real-time RT-PCR
|
|||||
|---|---|---|---|---|---|---|
| MVA vs mock
|
NYVAC vs mock
|
|||||
| DC 1 | DC 2 | DC 3 | DC 1 | DC 2 | DC 3 | |
| TNF | 36.63 | 25.45 | 44.32 | 2.23 | 4.95 | 4.48 |
| IFN-β | 43.86 | 28.87 | 27.76 | 2.15 | 4.98 | 1.85 |
| ISG15 | 3.82 | 4.62 | 3.81 | 2.15 | 2.27 | 2.26 |
| IL-12 | 7.91 | 5.89 | 7.98 | 2.08 | 1.98 | 1.79 |
| IL-7 | 1.78 | 0.90 | 2.07 | 1.27 | 1.55 | 0.81 |
| IL-6 | 2.01 | 2.36 | 3.03 | 1.16 | 1.41 | 2.54 |
| IFN-γ | 1.45 | 1.82 | 1.4 | 1.19 | 0.98 | 1 |
| NFκ2 | 2.02 | 3.05 | 3.64 | 1.68 | 2.06 | 2 |
| OASL | 27.66 | 37.66 | 26.63 | 2.13 | 1.43 | 0.519 |
| ATF-3 | 1.96 | 2.19 | 1.98 | 3.51 | 3.75 | 3.91 |
| H2AFY | 1.44 | 1.89 | 1.98 | 3.73 | 0.75 | 0.91 |
| ADORAA2 | 0.714 | 1.18 | 3.6 | 1.28 | 1.18 | 0.63 |
MDDC from three donors (DC1, DC2, and DC3) were mock, MVA, or NYVAC infected and processed for RT-PCR.
Differences in host gene expression levels in MVA- and NYVAC-infected MDDC compared to infected HeLa cells.
To provide further evidence for distinct gene regulation by NYVAC and MVA, we compared gene expression levels between HeLa cells infected with MVA or NYVAC with MDDC infected with the same poxvirus vectors. Genes expressed in 6-h NYVAC- or MVA-infected HeLa cells were subtracted from the genes regulated by both viruses in infected MDDC. Thus, we selected those genes showing at least twofold-higher expression levels in one cell type than in the other. We identified 1,245 genes that were differentially expressed after MVA infection of MDDC compared to HeLa cells (34.5% of the genes selected); of these genes, 463 were upregulated and 782 were downregulated in infected MDDC compared to infected HeLa cells. In the case of NYVAC infection, 1,970 genes were differentially expressed in MDDC versus HeLa cells (51.1% of the selected genes); of these genes, 1,009 were upregulated and 961 were downregulated in infected MDDC compared to HeLa cells. MVA- and NYVAC-infected MDDC shared 268 upregulated and 380 downregulated genes (data not shown). Functional analysis of genes with at least twofold-higher expression levels in MVA- or NYVAC-infected MDDC showed similar percentages for the main functional categories including immune response, cell death, or cell signaling (data not shown). Examples of these genes are shown in Table 4 and in Tables S4 and S5 in the supplemental material.
TABLE 4.
Differential gene expression in MVA/NYVAC-infected DC compared to that in MVA/NYVAC-infected HeLa cellsa
| Description | GenBank accession | Gene | Fold change
|
|
|---|---|---|---|---|
| MVA | NYVAC | |||
| Immune response, inflammatory response | ||||
| Small inducible cytokine A3 | NM_002983 | SCYA3 | 66.81 | 10.63 |
| IFN-induced protein with tetratricopeptide repeats 4 | NM_001549 | IFIT4 | 32.52 | 10.87 |
| TNF (TNF superfamily, member 2) | NM_000594 | TNF | 31.40 | 5.60 |
| IL-8 | NM_000584 | IL8 | 20.53 | 31.45 |
| V-fos FBJ murine osteosarcoma viral oncogene homolog | NM_005252 | FOS | 20.49 | 1.75 |
| Guanylate binding protein 5 | NM_052942 | GBP5 | 15.21 | |
| CD83 antigen (activated B lymphocytes, immunoglobulin superfamily) | NM_004233 | CD83 | 14.53 | 12.41 |
| IFN-β1, fibroblast | NM_002176 | IFNB1 | 13.70 | 4.52 |
| GRO2 oncogene | NM_002089 | GRO2 | 13.21 | 1.64 |
| Pre-B-cell colony-enhancing factor | NM_005746 | PBEF | 8.01 | 13.38 |
| MDA5 | NM_022168 | MDA5 | 6.66 | |
| Nuclear factor of kappa light polypeptide gene enhancer in B cells 1 (p105) | NM_003998 | NFKB1 | 5.70 | 12.59 |
| RNA helicase | NM_014314 | RIG-I | 5.42 | |
| IFN-α-inducible protein (clone IFI-6-16) | NM_022873 | G1P3 | 4.69 | 2.77 |
| IRF-7 | NM_004031 | IRF7 | 4.24 | 3.37 |
| IFN-induced protein with tetratricopeptide repeats 1 | NM_001548 | IFIT1 | 3.88 | 8.63 |
| IFN-γ-inducible protein 30 | NM_006332 | IFI30 | 3.51 | 5.85 |
| Myxovirus (influenza virus) resistance 1, IFN-inducible protein p78 (mouse) | NM_002462 | MX1 | 3.45 | |
| Small inducible cytokine A4 | NM_002984 | SCYA4 | 14.94 | |
| Apoptosis | ||||
| TNF-α-induced protein 3 | NM_006290 | TNFAIP3 | 18.24 | 20.04 |
| Apolipoprotein E | NM_000041 | APOE | 15.45 | 4.58 |
| TNF receptor-associated factor 1 | NM_005658 | TRAF1 | 2.43 | 4.92 |
| Death-associated protein 6 | NM_001350 | DAXX | −1.22 | 6.17 |
| IL-1β | NM_000576 | IL1B | 5.49 | |
| Cathepsin B | NM_001908 | CTSB | 11.43 | |
| Cell cycle, signaling | ||||
| Carcinoembryonic antigen-related cell adhesion molecule 6 | M18216 | CEACAM6 | 78.47 | 10.14 |
| Endothelial PAS domain protein 1 | NM_001430 | EPAS1 | 11.21 | 7.29 |
| Leupaxin | NM_004811 | LPXN | 9.59 | 7.45 |
| Fibrinogen, gamma polypeptide | NM_021870 | FGG | 7.79 | 13.30 |
| Prostaglandin E receptor 1 (subtype EP1), 42kD | NM_000955 | PTGER1 | 5.76 | 10.54 |
| Mitogen-activated protein kinase 6 | NM_002748 | MAPK6 | 5.33 | 8.48 |
| Wingless-type MMTV integration site family, member 10B | NM_003394 | WNT10B | 4.25 | 6.44 |
| Pleckstrin | NM_002664 | PLEK | 11.08 | |
| CD53 antigen | NM_000560 | CD53 | 9.48 | |
| RAP1B, member of RAS oncogene family | NM_015646 | RAP1B | 15.26 | |
Both cell types were infected with MVA or NYVAC (5 PFU/cell), and total RNA was extracted at 6 hpi and processed for microarray and data analysis (see Materials and Methods and Results). FBJ, Finkel-Biskis-Junkins; PAS, Per-Arnt-Sim.
Several genes that were upregulated in MVA-infected MDDC also showed higher expression levels than their counterparts in MVA-infected HeLa cells or in NYVAC-infected MDDC. These include TNF-α, IFN-β, interferon-induced IFIT1 and IFIT4 genes, cytokines such as GRO2 (CXCL2), and genes involved in the antiviral immune response (RIG-I, MDA5, and GBP5).
The comparative profiling of infected MDDC versus HeLa cells provides additional evidence that both poxvirus vectors affect host gene expression differently.
Induction of immunomodulatory molecules and activation of IFN pathways in MVA- and NYVAC-infected MDDC.
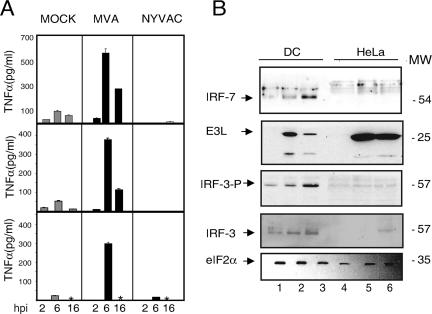
While the above-described experiments indicate that both MVA and NYVAC produced an increase in gene expression of certain cytokines (see Tables S1 and S2 in the supplemental material), MVA elicited higher expression levels of specific immunomodulatory molecules such as TNF-α, IFN-β, CCL5, and IL-12. IL-1α and IL-1β expression levels were nonetheless slightly enhanced after NYVAC infection. Genes involved in the antiviral response, such as OASL, RIG-I, and MDA5, were upregulated after MVA infection. We therefore analyzed the correlation between transcription and translational levels of specific immunomodulatory molecules after poxvirus infection of MDDC. Since we observed high transcriptional levels of TNF-α after MVA infection of MDDC in the microarrays, we used ELISA to evaluate TNF-α levels in supernatants of infected MDDC from three healthy volunteers. High TNF-α levels were induced in MDDC from all volunteers after MVA infection compared to NYVAC infection, with higher levels at 6 than at 16 hpi (Fig. 5A).
FIG. 5.
Validation of microarray data at the protein level. (A) TNF-α levels in cell supernatants after MVA or NYVAC infection. MDDC from three different donors were mock infected or infected with MVA or NYVAC (5 PFU/cell) for 2, 6, and 16 h, and TNF levels in supernatants were measured by ELISA. Values indicate duplicate samples in two independent experiments. An asterisk indicates a lack of data. (B) IRF-3 and IRF-7 levels in virus-infected MDDC. Mock (lanes 1 and 4)-, MVA (lanes 2 and 5)-, or NYVAC (lanes 3 and 6)-infected (5 PFU/cell) MDDC or HeLa cell extracts were fractionated by SDS-PAGE, transferred onto nitrocellulose, and incubated with antibodies to IRF-7, IRF-3, phosphorylated IRF-3 (IRF-3-P), eIF-2α (as a protein loading control), and the viral protein E3L (as a virus infection control). The molecular weight of protein standards (MW) (in thousands) is indicated (right).
Since high levels of type I IFN gene expression (Table 2) were produced after poxvirus infection of MDDC, and IFN expression is regulated by IRFs (IRF-3 and IRF-7), we analyzed the levels of these two factors involved in the IFN-responsive pathway. Western blot analysis showed increased IRF-7 levels after MVA and NYVAC infection of MDDC but not after infection of HeLa cells (Fig. 5B). To induce IFN gene expression, IRF phosphorylation is needed (72). We thus used Western blotting to determine levels of IRF-3, both unphosphorylated and phosphorylated, in MVA- or NYVAC-infected MDDC. The results showed an increase in IRF-3 phosphorylation in MVA- and NYVAC-infected compared to uninfected cells (Fig. 5B). Phosphorylation levels were higher in NYVAC- than in MVA-infected MDDC, although this increase was not observed in HeLa cells. Levels of the VACV protein E3, an inhibitor of IRF-3 phosphorylation (72), were considerably higher after MVA infection than after NYVAC infection.
These findings indicate that the enhanced transcriptional levels of genes encoding immunomodulatory molecules observed in microarray after MVA or NYVAC infection correlate with protein levels, at least for TNF, and that the induction of type I IFN gene expression may be mediated by poxvirus-induced expression of RIG-I/MDA5. This in turn would trigger the phosphorylation of IRF-3, translocation to the nucleus, and the activation of IFN gene expression.
DISCUSSION
Efficient antiviral immunity involves both innate and adaptive immune responses. Adaptive immunity leads to viral clearance and generates long-term immunological memory through the generation of specific T and B cells. DC found in an immature state in all tissues orchestrate the development of adaptive immunity (6). Following pathogen recognition, there are changes in the expression of DC proinflammatory genes, including those coding for cytokines, chemokines, and costimulatory molecules, in a process known as DC maturation (6, 58). These functional changes are necessary for initiating adaptive immunity. Immediately after viral infection, host innate immune defenses are induced. These immune responses are critical for the activation of adaptive immunity. There is nonetheless a 4- to 7-day delay before the adaptive immune response is initiated, during which time the innate immune response has an important role in early viral clearance (37). VACV attempts to subvert the innate response by secreting proteins that inactivate complement (41), inhibit the IFN response (9, 74), protect it from inflammatory responses, and prevent natural killer (NK) cell activation (2).
Poxvirus vectors are efficient activators of host immune responses to virally expressed antigens of distinct origin and are being used as potential vaccines for several pathogens and tumors (49, 50, 55). It is therefore important to define the transcriptional changes in human APC, particularly in MDDC, after VACV vector infection. Here, we showed that MDDC infected with MVA or NYVAC at 5 PFU/cell caused extensive morphological damage to the cells at late times postinfection. This is characterized by the severe inhibition of protein synthesis by 6 hpi and apoptosis induction together with rRNA cleavage at 16 hpi. In spite of a general translational block induced by the two vectors, some of the early (E3) and late (A4, A14, and A27) viral proteins examined were produced in the infected cells, although differences were observed between these vectors. In contrast to MVA infection, NYVAC-infected MDDC do not produce A17 or A27 proteins, and both viruses do not synthesize A36R or B5R proteins. The difference in viral gene expression between the two vectors might be related to the extent of eIF-2α phosphorylation and RNA degradation induced during infection as well as the presence or absence of certain viral genes in the poxvirus vectors. In agreement with other investigators, MVA induced apoptosis late in the infection and inhibition of some of the late proteins. In view of the severity of the effect triggered by the two poxvirus vectors in MDDC late in the infection, we used 6 hpi and a multiplicity of 5 PFU/cell for gene expression profiling. We applied genomic studies using microarrays and various data analysis conditions to define the impact of infection of immature human MDDC with the two attenuated VACV strains MVA and NYVAC. We identified genes regulated by MVA and NYVAC infection of MDDC at a postinfection time before rRNA breakdown and defined that these two vectors trigger some similar and some distinct host gene expression profiles. For this study, we used three approaches. First, we identified genes that were selectively expressed in MVA- or NYVAC-infected MDDC compared to mock-infected cells. We subsequently defined genes that were specifically regulated by MVA in comparison with NYVAC infection, and finally, we described genes regulated in MVA- or NYVAC-infected HeLa cells compared to MDDC infected by these viruses. Our analysis showed that in infected human MDDC, MVA upregulates more genes than NYVAC. Since the expression of genes involved in host defense is markedly enhanced after MVA infection, we discuss the contribution of some of these genes to MDDC responses, taking the viral genes that counteract host immune responses into consideration. It should be noted that the effect of the poxvirus vectors on host genome profiling is due to the contribution of the infected and noninfected cells (Fig. 1) together with the immunomodulators released during infection acting in an autocrine and paracrine manner.
TLR.
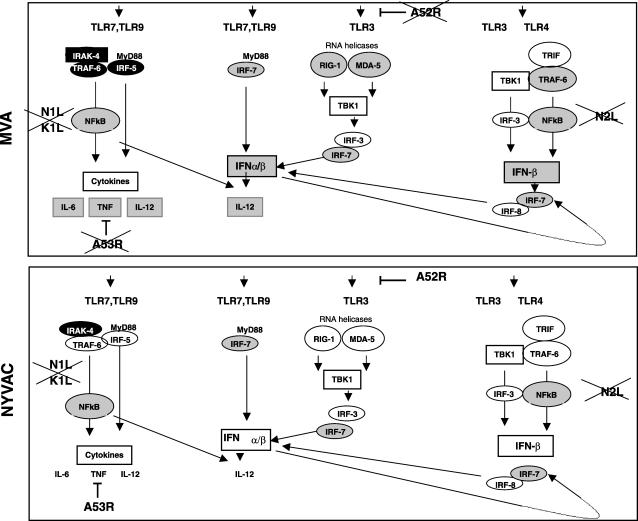
An efficient innate immune response is a prerequisite for the activation of adaptive immune responses. Toll-like receptors (TLR) are expressed predominantly in APC such as macrophages and MDDC. TLR3 is a double-stranded RNA receptor with a key role in antiviral and inflammatory responses through cross-priming of several physiological pathways including the activation of IFN responses, NF-κB, mitogen-activated protein kinase pathways, and the caspase cascade (54). We found that TLR3 expression was clearly upregulated after MVA infection of MDDC, implying a possible role for TLR3 in double-stranded RNA-initiated antiviral and inflammatory responses. Altered TLR expression has been linked to enhanced responsiveness to viral infection, as reported previously for viruses such as respiratory syncytial virus (27), VACV (34), and hepatitis C virus (45). In VACV, two viral genes have been implicated in TLR-dependent signaling, the A46R and A52R genes. While the A46R gene is present in both MVA and NYVAC genomes, the A52R gene is found only in NYVAC. Since this viral gene is involved in the blockade of NF-κB by several TLR, including TLR3, we propose that the upregulation of the TLR3 pathway after MVA infection (Fig. 6) could be due to the absence of the A52R gene in its genome. This activation was not observed after NYVAC infection. The A52R gene has been described to associate with both IL-1 receptor-associated kinase 2 (IRAK2) and TNF receptor-associated factor 6 (TRAF6), two key proteins in TLR signal transduction (34). We observed the upregulation of TRAF6 and the downregulation of IL-1α and IL-1β only in MVA-infected MDDC.
FIG. 6.
Signaling pathways regulated by MVA or NYVAC infection in MDDC. Genes whose expression levels are enhanced in MDDC by the poxvirus vector infection are shown in gray, and downregulated genes are shown in black. Viral genes known to interfere with selected pathways are indicated. MVA lacks genes that interfere TLR signaling (A52R and A53R), which are present in NYVAC.
Comparison of a TLR signaling pathway in MVA- and NYVAC-infected MDDC showed similar transcription levels in genes encoding receptor/adaptors, with some genes slightly repressed (phosphatidylinositol 3-kinase and IRAK1) and some genes slightly enhanced (TRAF6 and TBK1). Transcription levels were similar in genes encoding transcription factors and inflammatory cytokines, with some enhanced genes in MVA-infected (IFN-α, IFN-β, TNF-α, IL-12β, CCL3, CCL4, CCL5, and CXCL10) and in NYVAC-infected (IFN-β and TNF-α) cells. Neither MVA nor NYVAC had any effect on costimulatory molecule expression (CD40, CD80, and CD86). Transcription of genes involved in the antiviral immune response and cytokine production was clearly more enhanced in MVA-infected MDDC than in NYVAC-infected MDDC.
Type I IFN production and antiviral response.
The most consistent changes in MDDC infection by the attenuated poxvirus vectors involved genes implicated in the type I IFN-α/β response (Tables 1 to 3). By generating an intracellular environment that restricts viral replication, type I IFN represent a first line of defense against virus infection (25). The IFN signaling system produces a broadly effective innate response by creating an antiviral state in both an autocrine and a paracrine manner (81). IFN gene expression is regulated by IRF-3 phosphorylation, homodimerization, and nuclear translocation (67). Once in the nucleus, IRF-3 interacts with IRF-3-responsive promoters and the transcriptional coactivator histone acetyltransferase CBP/p300, leading to the transcription of IRF-responsive genes; together with NF-κB and AP-1, IRF-3 also promotes IFN-β transcription. The microarray experiments showed a clear upregulation of RIG-I and MDA5 levels only after MVA infection (Tables 1 to 3). As RIG-I and MDA-5 mRNA expression levels are strongly enhanced by type I IFN (70), we propose that the elevated IFN levels produced after MVA infection might be involved in the upregulation of RIG-I and MDA5, which can also activate the expression of type I IFN in a feedback mechanism (Fig. 6). This is supported by the enhanced IRF-3 phosphorylation observed in MVA-infected MDDC cells (Fig. 5B).
RIG-I and MDA5 upregulation was not observed in previous studies using mRNA microarrays from MVA-infected HeLa cells (29). The specificity of RIG-I and MDA5 expression in MVA-infected MDDC was also confirmed in a microarray experiment comparing the expression profiles of MVA-infected HeLa cells and MDDC at 6 hpi (Table 4; also see Table S4 in the supplemental material). These observations indicate a cell type-specific involvement of RIG-I in the antiviral response to poxvirus infection. This concurs with the observations made previously by Ichikawa et al., where type I IFN induction in fibroblasts and myeloid DC was RIG-I dependent, while type I IFN induction in periferical DC was independent of RIG-I (36). RNA virus recognition by RIG-I and MDA5 is reported to involve distinct mechanisms (36), but little is known of the mechanisms used by their helicases in infection with DNA viruses.
Type I IFN levels were notably higher during MVA infection than during NYVAC infection. There was no apparent RIG-I or MDA-5 upregulation during NYVAC infection, possibly due to low IFN levels or to specific differences between MVA and NYVAC genomes. VACV genes involved in inhibiting the IFN response include the E3L (16), B18R (17), K3L (12), and N1L (31) genes. Although MVA and NYVAC share common deleted genes, including serpins (the B13R and B14R genes), the M2L and N1L genes, which are involved in signaling, and host range genes (the K1L gene), most other deleted genes differ between the two strains. The differences in deleted genes between MVA and NYVAC must play a role in the host genome expression pattern induced after MDDC infection with these vectors (Fig. 6).
Proinflammatory cytokine production (TNF-α, IL-12, and IL-6).
Another important difference between MVA and NYVAC infection was the activation of TNF-α, IL-12, and IL-6, which were upregulated more than 10-, 5-, and 4-fold, respectively, in MVA versus NYVAC infection. Since NF-κB plays an important role in the expression of inflammatory cytokines, including TNF-α and IL-12, our results suggest an activation of this pathways by MVA, as previously described for HeLa cells (28, 29). Upregulation of these immunomodulators is likely to have an important role in MDDC function. TNF-α would be released by MDDC while in peripheral tissues to further recruit MDDC precursors and sustain antigen capture and presentation. On the contrary, IL-12 would be released by MDDC in lymph nodes to polarize Th cells toward a Th1 phenotype (42). The fact that viruses encode proteins that act to subvert nearly all aspects of TNF-α signaling (11) emphasizes the importance of the TNF-α/TNF receptor axis in antiviral immunity and virus-host interactions. Poxviruses have evolved various strategies to prevent apoptosis, including the ability to inhibit secreted TNF-α (57). In microarrays and Western blots, we found an increase in TNF-α mRNA (Tables 1 to 4) and protein levels (Fig. 5A) in MVA-infected but not in NYVAC-infected MDDC. As the A53R gene is deleted in MVA and is intact in NYVAC, we propose that by its high-affinity binding to human TNF (3), the A53R gene product may be responsible for the decreased TNF levels observed in NYVAC-infected MDDC supernatants.
Apoptosis signaling.
During our transcriptional profiling analysis of MVA- and NYVAC-infected MDDC, we observed a clear rRNA breakdown associated with infection by MVA and NYVAC (Fig. 3). MVA infection produced high levels of the 5-OA synthetase-like messenger (Table 2) at 6 hpi, and this is probably the reason for the apparent increase in RNA degradation by MVA compared to NYVAC infection. Activation of this enzyme mediates an antiviral and antitumor function by cleaving cellular and viral RNAs, promoting a general inhibition of protein synthesis and apoptosis (53, 69). The rRNA breakdown products are identical to those produced in cells infected with a VACV recombinant expressing RNase L (not shown), suggesting that MVA or NYVAC infection in MDDC triggers the activation of the 5-OA synthetase/RNase L pathway at late times postinfection (Fig. 3C). Both MVA and NYVAC induced apoptosis in infected MDDC, indicating that a poxvirus-infected MDDC will eventually die. Taking into consideration that the viral E3 protein, a PKR inhibitor (7, 9, 44, 65, 68, 79), is produced at high levels after infection of MDDC with both viruses (Fig. 2), which should block PKR activation, in view of the levels eIF-2α phosphorylation triggered by the poxvirus vectors, we cannot rule out the possibility of another kinase being responsible for eIF-2α phosphorylation and apoptosis induction after MDDC infection. The role of apoptosis in the immune response is still unclear, but antigens produced by apoptotic cells have been reported to increase antigen immunogenicity, which is likely to be more effective in cross-priming (76).
In summary, we have identified important differences in host genome expression profiling of human immature MDDC after infection with the attenuated poxvirus vectors MVA and NYVAC. This has been achieved using microarray technology and direct comparison of cDNAs from three different systems: MVA- or NYVAC-infected versus mock-infected MDDC, MVA- versus NYVAC-infected MDDC, and MVA- or NYVAC- infected MDDC versus infected HeLa cells. We identified a number of genes that were expressed similarly as well as others that showed differential expression profiling by these viral vectors. In general, MVA infection upregulated more genes than NYVAC infection. Of note are the differences in TNF-α and IFN-β levels, which were higher after MVA infection of MDDC than after NYVAC infection. These differences in vector behavior might be related to the number of genes in the genome of each vector, with MVA lacking more immunomodulatory genes than NYVAC. These poxvirus vectors induced apoptosis in MDDC, suggesting that a cell infected in vivo will not survive. Furthermore, the differences in host gene expression and levels of immunomodulatory molecules produced in MDDC might affect the quality of the immune response induced by each of these vectors when used as vaccines against pathogens and tumors.
Supplementary Material
Acknowledgments
We are indebted to R. Bablanian for critically reviewing the manuscript, V. Jiménez for expert technical assistance, and C. Mark for excellent editorial help. We thank Alberto Pascual-Montano and Integromics, SL, for help in clustering. We thank J. Tartaglia (Sanofi-Pasteur) for the generous gift of NYVAC, G. Sutter for MVA, and B. L. Jacobs for anti-E3 antibody.
This work was supported by grants from the Spanish Ministry of Education and Science (BIO2004-03954 and SAF2005-05566), the Spanish Ministry of Health (FIS2006-1259), the Spanish Foundation for AIDS Research (FIPSE 36344/02 and FIPSE 36536-05), Fundación Botín, and the European Union (EuroVac QLRT-PL-1999-01321 and QLK2-CT-2002-01867). The Department of Immunology and Oncology was founded and is supported by the Spanish National Research Council (CSIC) and by Pfizer.
Footnotes
Published ahead of print on 30 May 2007.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Abendroth, A., G. Morrow, A. L. Cunningham, and B. Slobedman. 2001. Varicella-zoster virus infection of human dendritic cells and transmission to T cells: implications for virus dissemination in the host. J. Virol. 75:6183-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcami, A. 2003. Viral mimicry of cytokines, chemokines and their receptors. Nat. Rev. Immunol. 3:36-50. [DOI] [PubMed] [Google Scholar]
- 3.Alcami, A., A. Khanna, N. L. Paul, and G. L. Smith. 1999. Vaccinia virus strains Lister, USSR and Evans express soluble and cell-surface tumour necrosis factor receptors. J. Gen. Virol. 80:949-959. [DOI] [PubMed] [Google Scholar]
- 4.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 5.Antoine, G., F. Scheiflinger, F. Dorner, and F. G. Falkner. 1998. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology 244:365-396. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 7.Beattie, E., K. L. Denzler, J. Tartaglia, M. E. Perkus, E. Paoletti, and B. L. Jacobs. 1995. Reversal of the interferon-sensitive phenotype of a vaccinia virus lacking E3L by expression of the reovirus S4 gene. J. Virol. 69:499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beattie, E., E. B. Kauffman, H. Martinez, M. E. Perkus, B. L. Jacobs, E. Paoletti, and J. Tartaglia. 1996. Host-range restriction of vaccinia virus E3L-specific deletion mutants. Virus Genes 12:89-94. [DOI] [PubMed] [Google Scholar]
- 9.Beattie, E., E. Paoletti, and J. Tartaglia. 1995. Distinct patterns of IFN sensitivity observed in cells infected with vaccinia K3L− and E3L− mutant viruses. Virology 210:254-263. [DOI] [PubMed] [Google Scholar]
- 10.Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 100:9458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benedict, C. A., P. S. Norris, and C. F. Ware. 2002. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 3:1013-1018. [DOI] [PubMed] [Google Scholar]
- 12.Carroll, K., O. Elroy-Stein, B. Moss, and R. Jagus. 1993. Recombinant vaccinia virus K3L gene product prevents activation of double-stranded RNA-dependent, initiation factor 2 alpha-specific protein kinase. J. Biol. Chem. 268:12837-12842. [PubMed] [Google Scholar]
- 13.Cebere, I., L. Dorrell, H. McShane, A. Simmons, S. McCormack, C. Schmidt, C. Smith, M. Brooks, J. E. Roberts, S. C. Darwin, P. E. Fast, C. Conlon, S. Rowland-Jones, A. J. McMichael, and T. Hanke. 2006. Phase I clinical trial safety of DNA- and modified virus Ankara-vectored human immunodeficiency virus type 1 (HIV-1) vaccines administered alone and in a prime-boost regime to healthy HIV-1-uninfected volunteers. Vaccine 24:417-425. [DOI] [PubMed] [Google Scholar]
- 14.Cella, M., M. Salio, Y. Sakakibara, H. Langen, I. Julkunen, and A. Lanzavecchia. 1999. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 189:821-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chahroudi, A., D. A. Garber, P. Reeves, L. Liu, D. Kalman, and M. B. Feinberg. 2006. Differences and similarities in viral life cycle progression and host cell physiology after infection of human dendritic cells with modified vaccinia virus Ankara and vaccinia virus. J. Virol. 80:8469-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang, H. W., J. C. Watson, and B. L. Jacobs. 1992. The E3L gene of vaccinia virus encodes an inhibitor of the interferon-induced, double-stranded RNA-dependent protein kinase. Proc. Natl. Acad. Sci. USA 89:4825-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colamonici, O. R., B. Porterfield, P. Domanski, R. K. Handa, S. Flex, C. E. Samuel, R. Pine, and M. O. Diaz. 1994. Ligand-independent anti-oncogenic activity of the alpha subunit of the type I interferon receptor. J. Biol. Chem. 269:27275-27279. [PubMed] [Google Scholar]
- 18.Corona Gutierrez, C. M., A. Tinoco, T. Navarro, M. L. Contreras, R. R. Cortes, P. Calzado, L. Reyes, R. Posternak, G. Morosoli, M. L. Verde, and R. Rosales. 2004. Therapeutic vaccination with MVA E2 can eliminate precancerous lesions (CIN 1, CIN 2, and CIN 3) associated with infection by oncogenic human papillomavirus. Hum. Gene Ther. 15:421-431. [DOI] [PubMed] [Google Scholar]
- 19.Deng, L., P. Dai, W. Ding, R. D. Granstein, and S. Shuman. 2006. Vaccinia virus infection attenuates innate immune responses and antigen presentation by epidermal dendritic cells. J. Virol. 80:9977-9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drillien, R., D. Spehner, A. Bohbot, and D. Hanau. 2000. Vaccinia virus-related events and phenotypic changes after infection of dendritic cells derived from human monocytes. Virology 268:471-481. [DOI] [PubMed] [Google Scholar]
- 21.Engelmayer, J., M. Larsson, M. Subklewe, A. Chahroudi, W. I. Cox, R. M. Steinman, and N. Bhardwaj. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762-6768. [PubMed] [Google Scholar]
- 22.Franchini, G., S. Gurunathan, L. Baglyos, S. Plotkin, and J. Tartaglia. 2004. Poxvirus-based vaccine candidates for HIV: two decades of experience with special emphasis on canarypox vectors. Expert Rev. Vaccines 3:S75-S88. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert, S. C., V. S. Moorthy, L. Andrews, A. A. Pathan, S. J. McConkey, J. M. Vuola, S. M. Keating, T. Berthoud, D. Webster, H. McShane, and A. V. Hill. 2006. Synergistic DNA-MVA prime-boost vaccination regimes for malaria and tuberculosis. Vaccine 24:4554-4561. [DOI] [PubMed] [Google Scholar]
- 24.Gomez, C. E., J. L. Najera, V. Jimenez, K. Bieler, J. Wild, L. Kostic, S. Heidari, M. Chen, M. J. Frachette, G. Pantaleo, H. Wolf, P. Liljestrom, R. Wagner, and M. Esteban. 2007. Generation and immunogenicity of novel HIV/AIDS vaccine candidates targeting HIV-1 Env/Gag-Pol-Nef antigens of clade C. Vaccine 25:1969-1992. [DOI] [PubMed] [Google Scholar]
- 25.Grandvaux, N., B. R. tenOever, M. J. Servant, and J. Hiscott. 2002. The interferon antiviral response: from viral invasion to evasion. Curr. Opin. Infect. Dis. 15:259-267. [DOI] [PubMed] [Google Scholar]
- 26.Grosjean, I., C. Caux, C. Bella, I. Berger, F. Wild, J. Banchereau, and D. Kaiserlian. 1997. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J. Exp. Med. 186:801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groskreutz, D. J., M. M. Monick, L. S. Powers, T. O. Yarovinsky, D. C. Look, and G. W. Hunninghake. 2006. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J. Immunol. 176:1733-1740. [DOI] [PubMed] [Google Scholar]
- 28.Guerra, S., L. A. Lopez-Fernandez, M. Angel Garcia, A. Zaballos, and M. Esteban. 2006. Human gene profiling in response to the active protein kinase, interferon-induced serine/threonine protein kinase (PKR), in infected cells. Involvement of the transcription factor ATF-3 in PKR-induced apoptosis. J. Biol. Chem. 2 81:18734-18745. [DOI] [PubMed] [Google Scholar]
- 28a.Guerra, S., L. A. López-Fernández, A. Pascual-Montano, J. L. Nájera, A. Zaballos, and M. Estaban. 2006. Host response to the attenuated poxvirus vector NYVAC: upregulation of apoptotic genes and NF-κB-responsive genes in infected HeLa cells. J. Virol. 80:985-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerra, S., L. A. Lopez-Fernandez, R. Conde, A. Pascual-Montano, K. Harshman, and M. Esteban. 2004. Microarray analysis reveals characteristic changes of host cell gene expression in response to attenuated modified vaccinia virus Ankara infection of human HeLa cells. J. Virol. 78:5820-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerra, S., L. A. Lopez-Fernandez, A. Pascual-Montano, M. Munoz, K. Harshman, and M. Esteban. 2003. Cellular gene expression survey of vaccinia virus infection of human HeLa cells. J. Virol. 77:6493-6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haga, I. R., and A. G. Bowie. 2005. Evasion of innate immunity by vaccinia virus. Parasitology 130:S11-S25. [DOI] [PubMed] [Google Scholar]
- 32.Harper, N., M. A. Hughes, S. N. Farrow, G. M. Cohen, and M. MacFarlane. 2003. Protein kinase C modulates tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by targeting the apical events of death receptor signaling. J. Biol. Chem. 278:44338-44347. [DOI] [PubMed] [Google Scholar]
- 33.Harrop, R., N. Connolly, I. Redchenko, J. Valle, M. Saunders, M. G. Ryan, K. A. Myers, N. Drury, S. M. Kingsman, R. E. Hawkins, and M. W. Carroll. 2006. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin. Cancer Res. 12:3416-3424. [DOI] [PubMed] [Google Scholar]
- 34.Harte, M. T., I. R. Haga, G. Maloney, P. Gray, P. C. Reading, N. W. Bartlett, G. L. Smith, A. Bowie, and L. A. O'Neill. 2003. The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J. Exp. Med. 197:343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hornemann, S., O. Harlin, C. Staib, S. Kisling, V. Erfle, B. Kaspers, G. Hacker, and G. Sutter. 2003. Replication of modified vaccinia virus Ankara in primary chicken embryo fibroblasts requires expression of the interferon resistance gene E3L. J. Virol. 77:8394-8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ichikawa, T., K. Nakao, K. Nakata, K. Hamasaki, Y. Takeda, Y. Kajiya, S. Higashi, K. Ohkubo, Y. Kato, N. Ishii, and K. Eguchi. 2001. Geranylgeranylacetone induces antiviral gene expression in human hepatoma cells. Biochem. Biophys. Res. Commun. 280:933-939. [DOI] [PubMed] [Google Scholar]
- 37.Janeway, C., Jr., and R. Medzhitov. 2000. Viral interference with IL-1 and Toll signaling. Proc. Natl. Acad. Sci. USA 97:10682-10683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenne, L., C. Hauser, J. F. Arrighi, J. H. Saurat, and A. W. Hugin. 2000. Poxvirus as a vector to transduce human dendritic cells for immunotherapy: abortive infection but reduced APC function. Gene Ther. 7:1575-1583. [DOI] [PubMed] [Google Scholar]
- 39.Kanesa-thasan, N., J. J. Smucny, C. H. Hoke, D. H. Marks, E. Konishi, I. Kurane, D. B. Tang, D. W. Vaughn, P. W. Mason, and R. E. Shope. 2000. Safety and immunogenicity of NYVAC-JEV and ALVAC-JEV attenuated recombinant Japanese encephalitis virus-poxvirus vaccines in vaccinia-nonimmune and vaccinia-immune humans. Vaccine 19:483-491. [DOI] [PubMed] [Google Scholar]
- 40.Kastenmuller, W., I. Drexler, H. Ludwig, V. Erfle, C. Peschel, H. Bernhard, and G. Sutter. 2006. Infection of human dendritic cells with recombinant vaccinia virus MVA reveals general persistence of viral early transcription but distinct maturation-dependent cytopathogenicity. Virology 350:276-288. [DOI] [PubMed] [Google Scholar]
- 41.Kotwal, G. J., S. N. Isaacs, R. McKenzie, M. M. Frank, and B. Moss. 1990. Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science 250:827-830. [DOI] [PubMed] [Google Scholar]
- 42.Langenkamp, A., M. Messi, A. Lanzavecchia, and F. Sallusto. 2000. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat. Immunol. 1:311-316. [DOI] [PubMed] [Google Scholar]
- 43.Langland, J. O., J. C. Kash, V. Carter, M. J. Thomas, M. G. Katze, and B. L. Jacobs. 2006. Suppression of proinflammatory signal transduction and gene expression by the dual nucleic acid binding domains of the vaccinia virus E3L proteins. J. Virol. 80:10083-10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee, S. B., and M. Esteban. 1994. The interferon-induced double-stranded RNA-activated protein kinase induces apoptosis. Virology 199:491-496. [DOI] [PubMed] [Google Scholar]
- 45.Li, K., E. Foy, J. C. Ferreon, M. Nakamura, A. C. Ferreon, M. Ikeda, S. C. Ray, M. Gale, Jr., and S. M. Lemon. 2005. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl. Acad. Sci. USA 102:2992-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ludwig, H., J. Mages, C. Staib, M. H. Lehmann, R. Lang, and G. Sutter. 2005. Role of viral factor E3L in modified vaccinia virus Ankara infection of human HeLa Cells: regulation of the virus life cycle and identification of differentially expressed host genes. J. Virol. 79:2584-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mellman, I., and R. M. Steinman. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell 106:255-258. [DOI] [PubMed] [Google Scholar]
- 48.Meyer, H., G. Sutter, and A. Mayr. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen. Virol. 72:1031-1038. [DOI] [PubMed] [Google Scholar]
- 49.Moss, B. 1996. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc. Natl. Acad. Sci. USA 93:11341-11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moss, B., M. W. Carroll, L. S. Wyatt, J. R. Bennink, V. M. Hirsch, S. Goldstein, W. R. Elkins, T. R. Fuerst, J. D. Lifson, M. Piatak, N. P. Restifo, W. Overwijk, R. Chamberlain, S. A. Rosenberg, and G. Sutter. 1996. Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv. Exp. Med. Biol. 397:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munoz-Fontela, C., M. Collado, E. Rodriguez, M. A. Garcia, A. Alvarez-Barrientos, J. Arroyo, C. Nombela, and C. Rivas. 2005. Identification of a nuclear export signal in the KSHV latent protein LANA2 mediating its export from the nucleus. Exp. Cell Res. 311:96-105. [DOI] [PubMed] [Google Scholar]
- 52.Myagkikh, M., S. Alipanah, P. D. Markham, J. Tartaglia, E. Paoletti, R. C. Gallo, G. Franchini, and M. Robert-Guroff. 1996. Multiple immunizations with attenuated poxvirus HIV type 2 recombinants and subunit boosts required for protection of rhesus macaques. AIDS Res. Hum. Retrovir. 12:985-992. [DOI] [PubMed] [Google Scholar]
- 53.Najera, J. L., C. E. Gomez, E. Domingo-Gil, M. M. Gherardi, and M. Esteban. 2006. Cellular and biochemical differences between two attenuated poxvirus vaccine candidates (MVA and NYVAC) and role of the C7L gene. J. Virol. 80:6033-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishiya, T., E. Kajita, S. Miwa, and A. L. Defranco. 2005. TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. J. Biol. Chem. 280:37107-37117. [DOI] [PubMed] [Google Scholar]
- 55.Ockenhouse, C. F., P. F. Sun, D. E. Lanar, B. T. Wellde, B. T. Hall, K. Kester, J. A. Stoute, A. Magill, U. Krzych, L. Farley, R. A. Wirtz, J. C. Sadoff, D. C. Kaslow, S. Kumar, L. W. Church, J. M. Crutcher, B. Wizel, S. Hoffman, A. Lalvani, A. V. Hill, J. A. Tine, K. P. Guito, C. de Taisne, R. Anders, W. R. Ballou, et al. 1998. Phase I/IIa safety, immunogenicity, and efficacy trial of NYVAC-Pf7, a pox-vectored, multiantigen, multistage vaccine candidate for Plasmodium falciparum malaria. J. Infect. Dis. 177:1664-1673. [DOI] [PubMed] [Google Scholar]
- 56.Pacheco, R., J. M. Martinez-Navio, M. Lejeune, N. Climent, H. Oliva, J. M. Gatell, T. Gallart, J. Mallol, C. Lluis, and R. Franco. 2005. CD26, adenosine deaminase, and adenosine receptors mediate costimulatory signals in the immunological synapse. Proc. Natl. Acad. Sci. USA 102:9583-9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pomerantz, J., N. Schreiber-Agus, N. J. Liegeois, A. Silverman, L. Alland, L. Chin, J. Potes, K. Chen, I. Orlow, H. W. Lee, C. Cordon-Cardo, and R. A. DePinho. 1998. The Ink4a tumor suppressor gene product, p19Arf, interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell 92:713-723. [DOI] [PubMed] [Google Scholar]
- 58.Ridge, J. P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393:474-478. [DOI] [PubMed] [Google Scholar]
- 59.Riegler, S., H. Hebart, H. Einsele, P. Brossart, G. Jahn, and C. Sinzger. 2000. Monocyte-derived dendritic cells are permissive to the complete replicative cycle of human cytomegalovirus. J. Gen. Virol. 81:393-399. [DOI] [PubMed] [Google Scholar]
- 60.Risco, C., J. R. Rodriguez, W. Demkowicz, R. Heljasvaara, J. L. Carrascosa, M. Esteban, and D. Rodriguez. 1999. The vaccinia virus 39-kDa protein forms a stable complex with the p4a/4a major core protein early in morphogenesis. Virology 265:375-386. [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez, D., M. Esteban, and J. R. Rodriguez. 1995. Vaccinia virus A17L gene product is essential for an early step in virion morphogenesis. J. Virol. 69:4640-4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodriguez, D., J. R. Rodriguez, J. F. Rodriguez, D. Trauber, and M. Esteban. 1989. Highly attenuated vaccinia virus mutants for the generation of safe recombinant viruses. Proc. Natl. Acad. Sci. USA 86:1287-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodriguez, J. F., R. Janeczko, and M. Esteban. 1985. Isolation and characterization of neutralizing monoclonal antibodies to vaccinia virus. J. Virol. 56:482-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodriguez, J. R., C. Risco, J. L. Carrascosa, M. Esteban, and D. Rodriguez. 1998. Vaccinia virus 15-kilodalton (A14L) protein is essential for assembly and attachment of viral crescents to virosomes. J. Virol. 72:1287-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romano, P. R., F. Zhang, S.-L. Tan, M. T. Garcia-Barrio, M. G. Katze, T. E. Dever, and A. G. Hinnebusch. 1998. Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: role of complex formation and the E3 N-terminal domain. Mol. Cell. Biol. 18:7304-7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 67.Servant, M. J., B. ten Oever, C. LePage, L. Conti, S. Gessani, I. Julkunen, R. Lin, and J. Hiscott. 2001. Identification of distinct signaling pathways leading to the phosphorylation of interferon regulatory factor 3. J. Biol. Chem. 276:355-363. [DOI] [PubMed] [Google Scholar]
- 68.Sharp, T. V., F. Moonan, A. Romashko, B. Joshi, G. N. Barber, and R. Jagus. 1998. The vaccinia virus E3L gene product interacts with both the regulatory and the substrate binding regions of PKR: implications for PKR autoregulation. Virology 250:302-315. [DOI] [PubMed] [Google Scholar]
- 69.Silverman, R. H. 2003. Implications for RNase L in prostate cancer biology. Biochemistry 42:1805-1812. [DOI] [PubMed] [Google Scholar]
- 70.Siren, J., T. Imaizumi, D. Sarkar, T. Pietila, D. L. Noah, R. Lin, J. Hiscott, R. M. Krug, P. B. Fisher, I. Julkunen, and S. Matikainen. 2006. Retinoic acid inducible gene-I and mda-5 are involved in influenza A virus-induced expression of antiviral cytokines. Microbes Infect. 8:2013-2020. [DOI] [PubMed] [Google Scholar]
- 71.Sivanandham, M., P. Shaw, S. F. Bernik, E. Paoletti, and M. K. Wallack. 1998. Colon cancer cell vaccine prepared with replication-deficient vaccinia viruses encoding B7.1 and interleukin-2 induce antitumor response in syngeneic mice. Cancer Immunol. Immunother. 46:261-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith, E. J., I. Marie, A. Prakash, A. Garcia-Sastre, and D. E. Levy. 2001. IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA-dependent protein kinase R or Ikappa B kinase but is blocked by vaccinia virus E3L protein. J. Biol. Chem. 276:8951-8957. [DOI] [PubMed] [Google Scholar]
- 73.Soldani, C., and A. I. Scovassi. 2002. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis 7:321-328. [DOI] [PubMed] [Google Scholar]
- 74.Symons, J. A., A. Alcami, and G. L. Smith. 1995. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell 81:551-560. [DOI] [PubMed] [Google Scholar]
- 75.Tartaglia, J., M. E. Perkus, J. Taylor, E. K. Norton, J. C. Audonnet, W. I. Cox, S. W. Davis, J. van der Hoeven, B. Meignier, M. Riviere, et al. 1992. NYVAC: a highly attenuated strain of vaccinia virus. Virology 188:217-232. [DOI] [PubMed] [Google Scholar]
- 76.Terenzi, F., M. J. deVeer, H. Ying, N. P. Restifo, B. R. Williams, and R. H. Silverman. 1999. The antiviral enzymes PKR and RNase L suppress gene expression from viral and non-viral based vectors. Nucleic Acids Res. 27:4369-4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vanderplasschen, A., and P. P. Pastoret. 2003. The uses of poxviruses as vectors. Curr. Gene Ther. 3:583-595. [DOI] [PubMed] [Google Scholar]
- 78.Wyatt, L. S., M. W. Carroll, C. P. Czerny, M. Merchlinsky, J. R. Sisler, and B. Moss. 1998. Marker rescue of the host range restriction defects of modified vaccinia virus Ankara. Virology 251:334-342. [DOI] [PubMed] [Google Scholar]
- 79.Xiang, Y., R. C. Condit, S. Vijaysri, B. Jacobs, B. R. Williams, and R. H. Silverman. 2002. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus. J. Virol. 76:5251-5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yates, N. L., and M. A. Alexander-Miller. 2006. Vaccinia virus infection of mature dendritic cells results in activation of virus-specific naïve CD8+ T cells: a potential mechanism for direct presentation. Virology 359:349-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.