Abstract
As the diversity of potential immunogens increases within certain classes of vectors, the possibility has arisen of employing heterologous prime/boost immunizations using diverse members of the same family of vectors. The present study was initiated to explore the use of divergent pox vectors in a prime/boost regimen to elicit high-frequency cellular immune responses to human immunodeficiency virus type 1 envelope and simian immunodeficiency virus gag in rhesus monkeys. We demonstrated that monkeys vaccinated with a recombinant modified vaccinia virus Ankara (rMVA) prime/recombinant fowlpox virus (rFPV) boost regimen and monkeys vaccinated with a recombinant vaccinia virus prime/rFPV boost regimen developed comparable cellular immune responses that were greater in magnitude than those elicited by a homologous prime/boost with rMVA. Nevertheless, comparable magnitude recall cellular immune responses were observed in monkeys vaccinated with heterologous and homologous recombinant poxvirus following challenge with the CXCR4-tropic SHIV-89.6P. Consistent with this finding, comparable levels of containment of viral replication and CD4+ T-lymphocyte preservation were seen in these groups of recombinant poxvirus-vaccinated monkeys. This study supports further exploration of combining recombinant vectors of the same family in prime/boost immunization strategies to optimize vaccine-elicited cellular immune responses.
There is accruing evidence that the magnitude of a vaccine-elicited cellular immune response determines the extent to which primate immunodeficiency virus replication is controlled in nonhuman primates following challenge (10, 11, 14). This has been well documented in CXCR4-tropic simian human immunodeficiency virus (SHIV)/macaque studies (1, 2, 22, 25) and has recently been shown as well in CCR5-tropic simian immunodeficiency virus/macaque studies (14, 27). These findings have provided a rationale for developing AIDS vaccine strategies based upon the induction of anti-HIV-1 cellular immune responses.
The most robust cellular immune responses induced to date have been generated with prime/boost vaccination regimens employing live, recombinant vaccine vectors. The responses elicited through homologous prime/boost immunizations have, however, proven disappointing because the anti-vector immunity induced following priming blunts the immunogenicity of the boosting inoculum (3, 4, 24). Heterologous prime/boost immunizations employing plasmid DNA immunogens for priming and live recombinant vectors for boosting have generated promising results (1, 23, 25). Nevertheless, concern remains that plasmid DNA immunogens are not a clinically viable vaccine modality because of the requirement for multiple inoculations and the cost associated with their production.
As the diversity of potential immunogens increases within certain classes of vectors, the possibility has arisen of employing heterologous prime/boost immunizations using diverse members of the same family of vectors. For example, with the ongoing development of alternative serotype, chimpanzee-origin (6, 7), and even chimeric adenovirus vectors (19), it is now possible to consider performing prime/boost immunizations with divergent adenovirus immunogens. Further, a variety of poxviruses have been developed as potential vaccine vectors (18, 21), including vaccinia virus, modified vaccinia virus Ankara (MVA), NYVac, canarypox, and fowlpox (FPV). It may prove possible to combine disparate pox vectors to elicit robust cellular immune responses.
The present study was initiated to explore the use of divergent pox vectors in a prime/boost regimen to elicit high-frequency cellular immune responses in nonhuman primates. We demonstrate that a heterologous immunization with recombinant MVA (rMVA) or recombinant vaccinia virus (rVac), followed by recombinant FPV (rFPV), generates robust cellular immune responses that confer substantial clinical protection against an SHIV-89.6P challenge.
MATERIALS AND METHODS
Generation of poxvirus recombinants expressing SHIV-89.6P env and SIVmac239 gag.
rVac strains expressing SHIV-89.6P env and SIVmac239 gag were constructed by inserting these genes in the HindIII M region of TBC-Wy, Therion strain of vaccinia virus as previously described (16). rFPVs expressing these same genes were constructed by inserting the genes in the Bam J HI region of the POXVAC-TC (Schering-Plough, Kenilworth, NJ) strain of FPV as described previously (13). rMVAs were generated by inserting these genes in the deletion III region of a plaque-purified isolate of the replication-defective strain of vaccinia virus designated MVA (15, 17). The env and gag genes of the recombinant viruses were under the control of the vaccinia virus 40K (H5R) promoter (9). All of the viruses also contained the Escherichia coli lacZ gene under the control of the fowlpox C1 promoter (13) to facilitate their use in a colorimetric screen for recombinant viruses. The genomic structures of these recombinant viruses were determined by PCR amplification and sequencing. The expression of Gag p55 was demonstrated by Western blot assay with an anti-p27 antibody (Advanced Biotechnologies, Inc.), and the expression of Env gp160/gp41 was demonstrated by Western blot assay with an anti-gp41 antibody. The purity of the recombinant viruses was assessed by in situ immunostaining using the same antibodies. Nonrecombinant wild-type vaccinia virus (VV-WT) (Wyeth strain), FPV-WT, and MVA-WT were used as control vector immunogens.
Immunization and challenge of rhesus monkeys.
Twenty-four rhesus monkeys were housed at Advanced Bioscience Laboratories (Kensington, MD). The animals were maintained in accordance with the guidelines of the National Institutes of Health and Harvard Medical School. Monkeys were divided into four groups, each consisting of six animals. Immunizations were carried out as described below. At week 64, 21 weeks following the last boost, all monkeys were challenged with 50 50% monkey infectious doses of SHIV-89.6P by the intravenous route.
IFN-γ ELISPOT assays.
Multiscreen 96-well plates were coated overnight with 100 μl per well of 5 μg/ml anti-human gamma interferon antibody (IFN-γ) (B27; BD Pharmingen) in endotoxin-free Dulbecco's phosphate-buffered saline (D-PBS). The plates were then washed three times with D-PBS containing 0.25% Tween-20, blocked for 2 h with D-PBS containing 5% fetal bovine serum to remove the Tween 20, and incubated with peptide pools and 2 × 105 peripheral blood mononuclear cells (PBMCs) in triplicate in 100-μl reaction mixture volumes. The peptide pool used in this study spanning the SIVmac239 Gag protein was comprised of 15 amino acid peptides overlapping by 11 amino acids; that spanning the HIV-1 89.6P (KB9) Env protein was comprised of 20 amino acid peptides overlapping by 10 amino acids. Each peptide in a pool was present at a 1-μg/ml concentration. Following an 18-h incubation at 37°C, the plates were washed nine times with D-PBS containing 0.25% Tween-20 and once with distilled water. The plates were then incubated with 2 μg/ml biotinylated rabbit anti-human IFN-γ (Biosource) for 2 h at room temperature, washed six times with Coulter Wash (Beckman Coulter), and incubated for 2.5 h with a 1:500 dilution of streptavidin-alkaline phosphatase (Southern Biotechnology). After five washes with Coulter Wash and one with D-PBS, the plates were developed with NBT/BCIP chromogen (Pierce), the process was stopped by washing with tap water, and the plates were air dried and read with an enzyme-linked immunospot (ELISPOT) reader (Hitech Instruments) using Image-Pro Plus image-processing software (version 4.1) (Media Cybernetics, Des Moines, IA).
PBMC stimulation and intracellular cytokine staining.
PBMCs were incubated at 37°C in a 5% CO2 environment for 6 h in the presence of RPMI 1640-10% fetal calf serum medium alone (unstimulated), a pool of 20-mer HIV-1 Env peptides (5 μg/ml each peptide), or staphylococcal enterotoxin B (5 μg/ml, Sigma-Aldrich) as a positive control. All cultures contained monensin (GolgiStop; BD Biosciences) as well as 1 μg/ml anti-CD49d (BD Biosciences). The cultured cells were stained with monoclonal antibodies specific for cell surface molecules (CD3, CD4, and CD8). After fixing with Cytofix/Cytoperm solution (BD Biosciences), cells were permeabilized and stained with antibodies specific for IFN-γ. Labeled cells were fixed in 1.5% formaldehyde-PBS. Samples were collected on an LSR II instrument (BD Biosciences) and analyzed using FlowJo software (Tree Star). Approximately 200,000 to 1,000,000 events were collected per sample. The background level of cytokine staining varied from sample to sample, but was typically less than 0.01% of the CD4+ T cells and less than 0.05% of the CD8+ T cells. The only samples considered positive were those in which the percentage of cytokine-staining cells was at least twice that of the background or in which there was a distinct population of cytokine brightly positive cells.
Neutralizing antibody assays.
Neutralizing antibodies were measured in TZM-bl cells (NIH AIDS Research and Reference Reagent Program) as described previously (28), except that trypsinized cells were added (10,000 cells/well) to the virus serum dilutions and indinavir was added to inhibit progeny virions. Titers of neutralizing antibodies are the reciprocal plasma dilution at which relative luminescence units were reduced 50% compared to virus control wells (no sample). The assay stock of SHIV-89.6P was grown in human PBMCs.
CD4+ T-lymphocyte counts and viral RNA levels.
CD4+ T-lymphocyte counts were determined by multiplying the total lymphocyte count by the percentage of CD3+ CD4+ T cells determined by monoclonal antibody staining and flow cytometric analysis. Plasma viral RNA levels were measured by an ultrasensitive branched DNA amplification assay with a detection limit of 125 copies/ml (Bayer Diagnostics).
RESULTS
Vaccine trial design.
Twenty-four Indian-origin rhesus monkeys were distributed into three experimental and one control group, each consisting of six animals. Twelve monkeys in the first two experimental groups received priming immunizations with 109 PFU of rMVA expressing HIV-1 89.6P Env gp140 (KB9) and 109 PFU of rMVA expressing SIVmac239 Gag, administered both intramuscularly and intradermally at weeks 0 and 8. Six monkeys in the third experimental group were primed by the same routes with 109 PFU of rVac expressing HIV-1 89.6P Env gp140 (KB9) and 109 PFU of rVac expressing SIVmac239 Gag. Monkeys in the control groups received inoculations with 2 × 109 PFU of empty MVA vector. At weeks 26 and 43, monkeys in the first experimental group were boosted with 109 PFU of rMVA-HIV-1 89.6P Env and 109 PFU of rMVA-SIVmac239 Gag, administered by both intramuscular and intradermal routes. Monkeys in the second and third experimental groups were boosted with 109 PFU of rFPV-HIV-1 89.6P Env and 109 PFU of rFPV-SIVmac239 Gag. Monkeys in the control group were inoculated with 2 × 109 PFU of empty fowlpox vector.
Vaccine-elicited cellular immune responses.
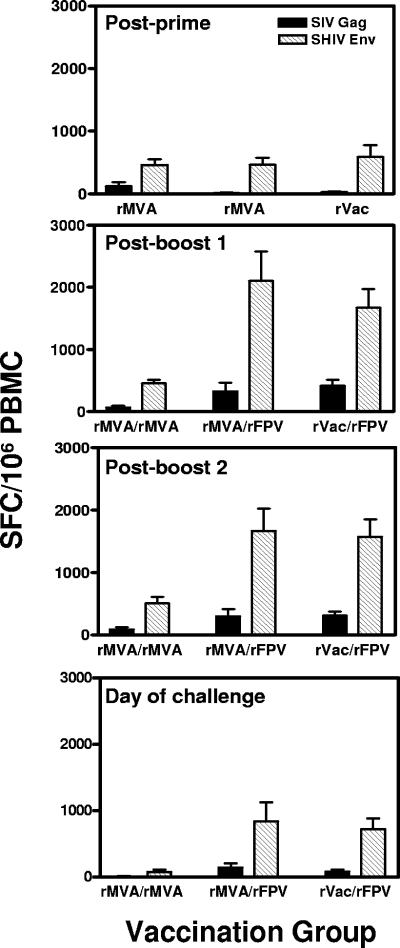
The cellular immune responses elicited by these vaccine constructs were assessed using pooled peptide IFN-γ ELISPOT assays. Vaccine-elicited ELISPOT responses to HIV-1 Env and simian immunodeficiency virus (SIV) Gag peptides were detected in the PBMCs of all experimentally vaccinated monkeys 2 to 4 weeks after the first inoculation (data not shown). Two weeks after the second inoculations, PBMCs of all three groups of vaccinated monkeys had IFN-γ responses to HIV-1 89.6P Env antigen of comparable magnitudes, 460 to 590 spot-forming cells (SFCs)/106 PBMCs. SIV Gag-stimulated IFN-γ responses were very low in the PBMCs of these monkeys (Fig. 1) (postprime), consistent with the low expression of Gag by the recombinant pox vectors seen in Western blots (data not shown).
FIG. 1.
Vaccine-elicited cellular immune responses to SIVmac251 Gag and HIV-1 89.6P Env. PBMCs were isolated at weeks 10 (panel 1, postprime), 28 (panel 2, postboost 1), 45 (panel 3, postboost 2), and 64 (panel 4, day of challenge) postimmunization and assessed in IFN-γ ELISPOT assays following in vitro exposure to peptide pools spanning SIV Gag and HIV-1 89.6P Env proteins. The bars represent the means ± standard error of the means (error bars) of SFC responses to individual viral proteins for six monkeys in each group. PBMCs of the control-vaccinated monkeys demonstrated SFC responses of less than 20/106 PBMC.
At week 26, the monkeys were boosted. One group that was primed with rMVA also received a boosting inoculation with rMVA (rMVA/rMVA). The other group of rMVA-primed monkeys and the group of monkeys primed with rVac were boosted with rFPV (rMVA/rFPV and rVac/rFPV, respectively). PBMC IFN-γ ELISPOT responses to SIV Gag and HIV-1 Env peptides were assessed in all three groups of animals at week 28, 2 weeks following this boost. Boosting with rMVA did not augment the Env- or Gag-specific T-lymphocyte IFN-γ ELISPOT responses in the monkeys primed with rMVA immunogens. In fact, the magnitude of the T-lymphocyte response to SIV Gag protein was less at week 28 than at week 10 in this experimental group (postboost 1) (Fig. 1). Monkeys that received rMVA/rFPV had fivefold increases in their IFN-γ ELISPOT responses to both SIV Gag and HIV-1 Env proteins, reaching 2,429 ± 475 total SFCs. The increase in these responses to HIV-1 Env but not to SIV Gag protein was statistically significant (P = 0.008, unpaired t test) in this group of monkeys. Similarly, monkeys primed with rVac and boosted with rFPV had increases in their IFN-γ ELISPOT responses to both SIV Gag and HIV-1 Env proteins of more than threefold, reaching 2,085 ± 301 total SFCs. This increase in responses to both SIV Gag as well as HIV-1 Env proteins achieved statistical significance (P = 0.008 for SIV Gag, P = 0.004 for HIV-1 Env, unpaired t tests). IFN-γ ELISPOT responses were comparable in magnitude in the two groups of monkeys receiving a heterologous prime/boost immunization.
Monkeys received a second boost with either rMVA (rMVA/rMVA) or rFPV (rMVA/rFPV and rVac/rFPV) at week 43. As shown in the third panel of Fig. 1, the ELISPOT responses in all three groups of vaccinated monkeys either were of the same magnitude or were lower than those seen following the first boost. These responses declined slowly in the rMVA/rFPV- and rVac/rFPV-immunized monkeys, and by week 64, 21 weeks following the last boost, there was a twofold decrease from the peak responses in the total SFC responses. In contrast to this finding, monkeys receiving rMVA/rMVA had almost sevenfold contractions in their total SFC responses by week 64 (Fig. 1, bottom panel).
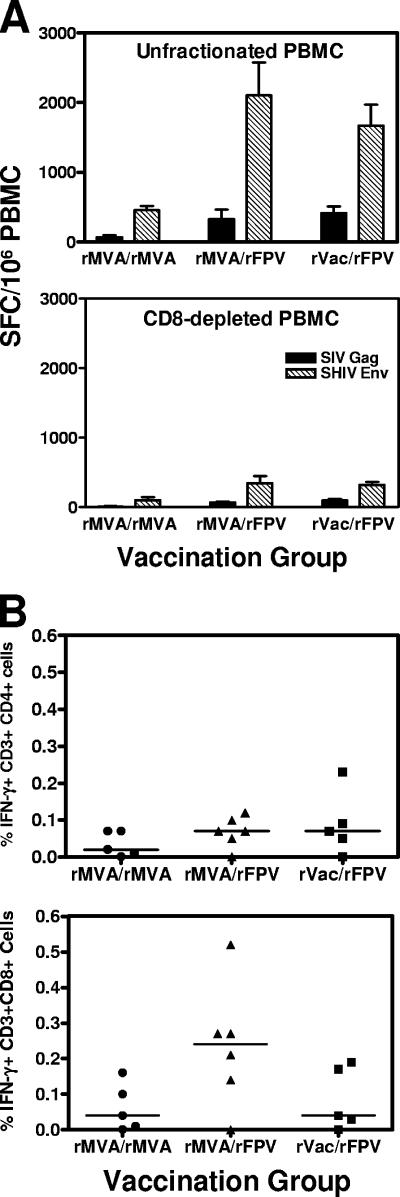
Studies were performed 2 weeks following the first boost immunizations to determine whether these vaccinations elicited CD4+ or CD8+ lymphocyte-biased immune responses. Freshly isolated PBMCs from the immunized monkeys were incubated with phycoerythrin-labeled anti-CD8 antibody and then with anti-phycoerythrin-labeled magnetic beads. These labeled PBMCs were sorted using a Miltenyi AutoMACS cell sorter to deplete CD8+ T lymphocytes, and IFN-γ ELISPOT responses were measured in both the unfractionated and CD8+ T-lymphocyte-depleted PBMCs (Fig. 2A). The CD8+ lymphocyte depletion eliminated approximately 80% of the ELISPOT responses. Therefore, the vaccinations elicited a CD8+ T-lymphocyte-biased response, but did generate both virus-specific CD4+ and CD8+ T-lymphocyte populations.
FIG. 2.
(A) Cellular immunity elicited by recombinant pox vector prime-recombinant pox vector boost immunizations is mediated by both CD4+ and CD8+ T lymphocytes. IFN-γ ELISPOT responses were measured in freshly isolated unfractionated (top panel) as well as CD8+ T-lymphocyte-depleted (bottom panel) PBMCs of the monkeys 2 weeks following the first recombinant pox vector boost immunizations. (B) IFN-γ secretion by CD4+ and CD8+ T lymphocytes was measured by intracellular cytokine staining following stimulation of PBMCs with peptide pools spanning the HIV-1 Env protein 1 week following the second recombinant pox vector boost. Error bars indicate standard errors of the means.
One week following the second boost, peripheral blood lymphocytes from the monkeys were exposed to overlapping peptides spanning the HIV-1 Env protein, and the fractions of CD4+ or CD8+ T cells producing IFN-γ were determined by intracellular cytokine staining. No statistically significant differences were noted in the frequencies of IFN-γ-producing CD4+ T lymphocytes from rMVA/rMVA-, rMVA/rFPV-, or rVac/rFPV-vaccinated monkeys (Kruskal-Wallis test). However, a trend toward higher frequencies of IFN-γ-producing CD8+ lymphocytes was seen in monkeys that received rMVA prime and rFPV boost (Fig. 2B).
Vaccine-elicited humoral immune responses.
To assess the vaccine-elicited humoral immune responses, serum samples from the vaccinated and control monkeys were analyzed for HIV-1 89.6P neutralizing antibodies. No HIV-1 89.6P-specific neutralization above background was detected in sera of most of the vaccinated monkeys in the rMVA/rMVA and rMVA/rFPV groups at the time of peak vaccine-elicited immunity. However, three of the six monkeys in the rVac/rFPV group had very low-titer neutralizing antibody responses detectable at week 46 (Table 1).
TABLE 1.
Neutralizing antibody titers against HIV-1 89.6P in sera of monkeys
| Group | Titer for:
|
|
|---|---|---|
| Wk 46a | Wk 4 PCb | |
| rMVA/rMVA | <20 | 499 |
| 27 | 137 | |
| <20 | 258 | |
| <20 | 129 | |
| <20 | 239 | |
| 20 | 188 | |
| rMVA/rFPV | <20 | 236 |
| <20 | 69 | |
| <20 | 150 | |
| <20 | 521 | |
| <20 | 1,868 | |
| <20 | 603 | |
| rVac/rFPV | <20 | 484 |
| 95 | 118 | |
| 50 | 43,650 | |
| <20 | 32,010 | |
| <20 | 575 | |
| 117 | >43,740 | |
| Control | 21 | 26 |
| <20 | 67 | |
| <20 | <20 | |
| <20 | <20 | |
| <20 | 149 | |
| <20 | 28 | |
Neutralizing antibody titers were measured in the sera of the monkeys 2 weeks following the final boost immunization.
Neutralizing antibody titers in the sera of the monkeys 4 weeks following SHIV-89.6P challenge. PC, postchallenge.
Cellular and humoral immune responses following viral challenge.
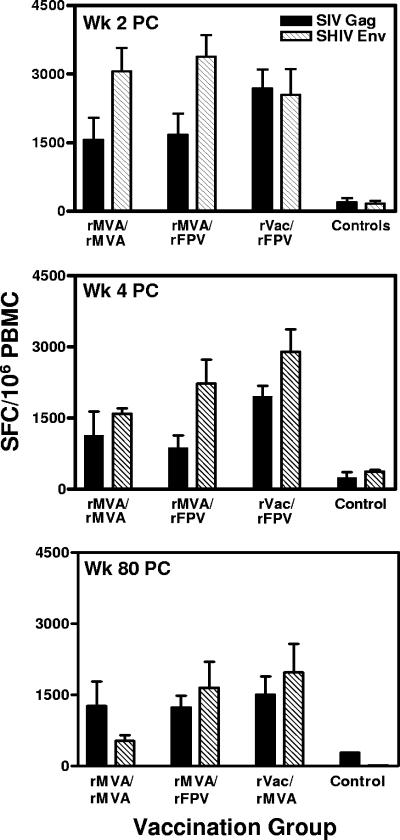
Twenty-one weeks after the final immunization, all monkeys were challenged with 50 50% monkey infectious doses of cell-free SHIV-89.6P by the intravenous route. Since the virus-specific cellular immune responses postchallenge should contribute to the early control of the virus, we were interested in determining the relative magnitudes of the SHIV-89.6P-specific cellular immune responses in these monkeys. Interestingly, the magnitudes of the SHIV-89.6P-specific IFN-γ ELISPOT responses were comparable in all three groups of experimentally vaccinated monkeys 2 weeks postchallenge (Fig. 3, top panel). The control animals had much lower ELISPOT responses. Therefore, although the vaccine-elicited prechallenge peak and plateau immune responses were greater in the groups of monkeys primed and then boosted with heterologous recombinant pox vectors than in the group receiving a homologous rMVA prime/boost immunization, the postchallenge peak secondary responses were equivalent in magnitude in all three groups of experimentally vaccinated animals.
FIG. 3.
PBMC IFN-γ ELISPOT responses in the vaccinated and control monkeys following viral challenge. Freshly isolated PBMCs were assessed for IFN-γ ELISPOT responses after in vitro exposure to peptide pools spanning the SIVmac251 Gag and HIV-1 89.6P Env proteins at weeks 2 (Wk 2 PC), 4 (Wk 4 PC), and 80 (Wk 80 PC) following intravenous SHIV-89.6P challenge. The bars represent the means ± standard errors of the means (error bars) of values of SFC responses to individual viral proteins for six monkeys in each group of experimentally vaccinated as well as control-vaccinated animals. PC, postchallenge.
IFN-γ ELISPOT responses were again measured at the end of the acute phase of infection, at week 4 following viral challenge. As shown in Fig. 3 (middle panel), monkeys in the rMVA/rMVA and rMVA/rFPV groups had substantial contractions in their ELISPOT responses compared with those measured at 2 weeks following infection. In contrast, monkeys in the rVac/rFPV group had only small contractions in their ELISPOT responses measured at 2 weeks postchallenge. At week 4 following viral challenge, this group had 1.5-fold higher total SFC responses than did the other two experimental groups. Seventy-six weeks later, at week 80 following challenge, there was very little diminution of the ELISPOT responses in these monkeys. The animals in the rVac/rFPV group had a 1.2-fold-higher total SFC response than did the other two experimental groups of monkeys.
The monkeys were also assessed for the degree to which vaccination primed for a secondary neutralizing antibody response that emerged after virus infection. At week 4 following SHIV-89.6P challenge, comparable peak neutralizing antibody titers could be measured in the rMVA/rMVA- and rMVA/rFPV-immunized groups of monkeys. Interestingly, very high-titer HIV-1 89.6P neutralizing antibody responses were measured in three of the six monkeys in the rVac/rFPV group of animals. One of the six control monkeys developed a neutralizing antibody response following viral challenge, and three other control animals developed barely measurable responses (Table 1). Thus, vaccination primed for a recall neutralizing antibody response, and this priming was most marked in the rVac/rFPV-immunized monkeys. Four weeks later, at week 8 following challenge, a decline was observed in the titer of these neutralizing antibody responses (data not shown).
Plasma viral RNA levels and CD4 T-cell counts.
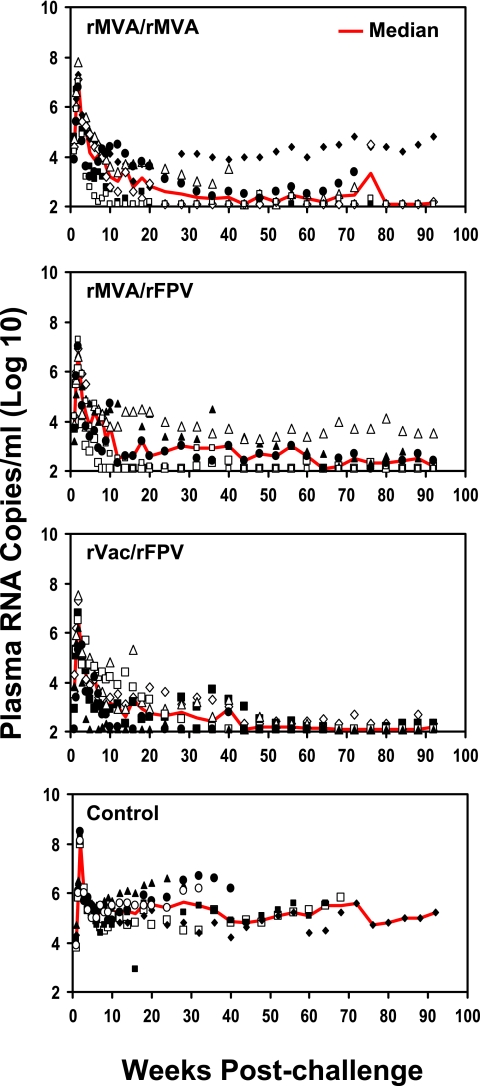
Viral replication in the SHIV-89.6P-challenged monkeys was assessed by quantitating plasma viral RNA levels. The median values for each group of animals at each sampling time are shown in Fig. 4. The median values of the peak plasma viral RNA levels in each of the three groups of experimentally vaccinated monkeys were 7.05 (rMVA/rMVA), 6.8 (rMVA/rFPV), and 6.65 (rVac/rFPV) log copies of viral RNA/ml of plasma, significantly lower than the median 8.1 log copies of the control vaccinees (P = 0.002, Wilcoxon rank sum test). Comparisons between the three experimental groups did not reveal significant differences in their peak viral loads.
FIG. 4.
Plasma viral RNA levels following intravenous challenge with SHIV-89.6P. These values were determined by an ultrasensitive branched DNA amplification assay with a detection limit of 125 copies/ml. Viral RNA levels are shown as the log copies of plasma viral RNA/ml of plasma for individual monkeys for each time point. Red lines indicate the median values for each experimental group at each sampling time.
The plasma viral RNA level in each of the experimentally vaccinated and control monkeys was also assessed following challenge during the postacute period, defined for each monkey as the median value of six determinations performed on specimens obtained between days 35 and 70 following challenge. During this postacute period, the median values of the peak plasma viral RNA levels in the three groups of experimentally vaccinated monkeys were 4.0 (rMVA/rMVA), 3.9 (rMVA/rFPV), and 3.4 (rVac/rFPV) log copies of viral RNA/ml of plasma, significantly lower than the median 5.25 log copies for the control vaccinees (P = 0.002 [rMVA/rMVA], P = 0.004 [rMVA/rFPV], and P = 0.002 [rVac/rFPV], Wilcoxon rank sum test). No significant differences in plasma viral RNA levels were observed between the groups of vaccinated monkeys during the postacute infection period.
The groups of experimentally vaccinated monkeys had lower long-term set point plasma viral RNA levels than did the control monkeys. The set point plasma viral RNA value used for the evaluation of each monkey was the median of eight data points obtained between days 84 and 224 postchallenge. Using these set point values, the six control monkeys had a median value of 5.35 log copies/ml. The median plasma viral RNA levels at set point in the experimentally vaccinated monkeys were 2.55 (rMVA/rMVA), 2.6 (rMVA/rFPV), and 2.8 (rVac/rFPV) log copies/ml. Therefore, at set point the vaccinated animals had plasma viral RNA levels almost 2 logs lower than those of the control animals (P = 0.002 [rMVA/rMVA], P = 0.004 [rMVA/rFPV], and P = 0.002 [rVac/rFPV], Wilcoxon rank sum test). The three groups of experimentally vaccinated monkeys did not have statistically significant differences in their long-term set point plasma viral RNA levels.
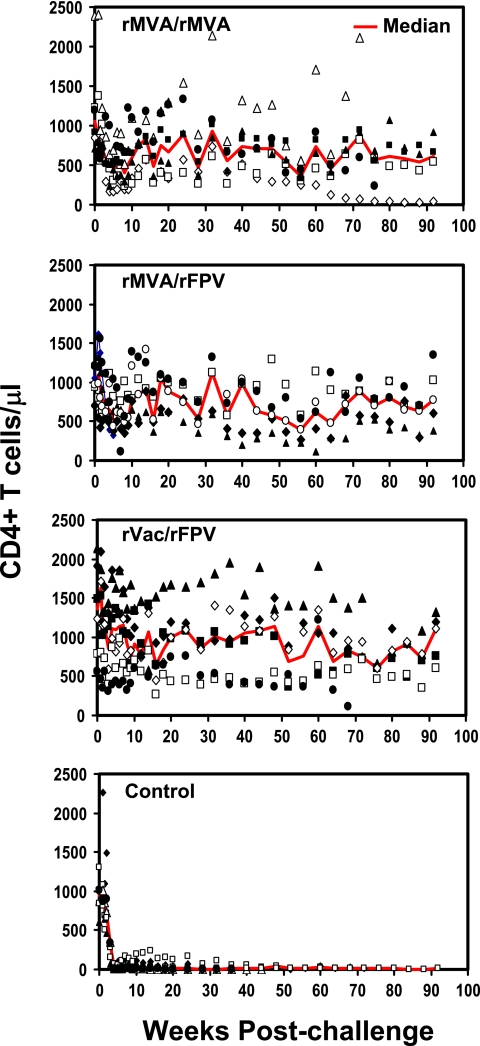
Peripheral blood CD4+ T-lymphocyte counts were also measured for all the monkeys to assess the vaccine-mediated clinical protection against the pathogenic SHIV-89.6P challenge. All six control monkeys had greater losses of peripheral blood CD4+ T lymphocytes than did the experimentally vaccinated animals by day 14 after challenge (Fig. 5, bottom panel). The median CD4+ T-lymphocyte counts of each group of monkeys at each sampling time are shown in Fig. 5. During the postacute period (days 35 through 70 following challenge), all six control monkeys developed profound losses of peripheral blood CD4+ T lymphocytes. In contrast, the three groups of experimentally vaccinated animals had a transient decline in peripheral blood CD4+ T lymphocytes detected by day 50 following challenge, but this cell loss was substantially reversed in 17 of the 18 monkeys.
FIG. 5.
Peripheral blood CD4+ T lymphocytes following SHIV-89.6P challenge. The values were determined by multiplying percentage of CD3+ CD4+ T lymphocytes by the total lymphocyte counts. Red lines indicate the median CD4+ T-cell count for each experimental group at each sampling time.
To quantitate the CD4+ T-lymphocyte counts of the monkeys at the time of long-term set point plasma viremia, the median peripheral blood CD4+ T-lymphocyte count was determined between days 84 and 224 postchallenge for each animal. Using these values, the control monkeys had a median CD4+ T-lymphocyte count of 13 and the experimentally vaccinated animals had median CD4+ T-lymphocyte counts of 689 (rMVA/rMVA), 861 (rMVA/rFPV), and 1,007 (rVac/rFPV) (P = 0.002 [rMVA/rMVA], P = 0.004 [rMVA/rFPV] and P = 0.002 [rVac/rFPV], Wilcoxon rank sum test). The three groups of experimentally vaccinated monkeys did not have statistically significant differences in their peripheral blood CD4+ T-lymphocyte counts. Therefore, the CD4+ T-lymphocyte counts in these monkeys reflected their plasma viral RNA levels postchallenge.
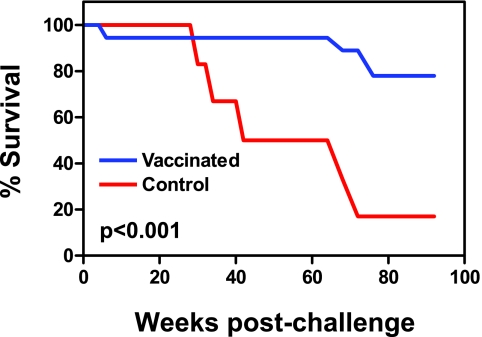
Consistent with their plasma viral RNA levels and CD4+ T-lymphocyte counts, five of the six control monkeys had to be euthanized because of advanced disease by week 72 postchallenge (Fig. 6). In contrast, one animal in the rMVA/rFPV group had to be euthanized at week 4 postchallenge and one of the monkeys vaccinated with rVac/rFPV had to be euthanized at week 68 postchallenge because of advanced disease. At week 76 postchallenge, two monkeys from the rMVA/rMVA group were also euthanized.
FIG. 6.
Mortality of the SHIV-89.6P challenged monkeys that received experimental or sham vaccines. The P value calculated by the Wilcoxon rank sum test was <0.001.
DISCUSSION
The present study demonstrates that potent and effective cellular immune responses can be generated in nonhuman primates using a prime/boost vaccination regimen with heterologous recombinant pox vectors. Importantly, the study shows that two vectors that are well tolerated in humans, MVA and FPV, can be used in such a regimen to elicit vigorous cell-mediated immune responses that provide durable clinical protection against a pathogenic SHIV challenge.
While poxviruses have been given to humans as vaccines since the late 18th century, very little data exist on the relative immunogenicity of these vaccine strain viruses when administered via different routes of inoculation or according to different temporal schedules. Certainly no systematic studies have been performed to compare the safety and immunogenicity of rVac, rMVA, and rFPV administered by different routes of inoculation. To guarantee the optimal immunogenicity of each of the vaccine constructs in this study, monkeys received the recombinant poxvirus vectors simultaneously through two routes of inoculation, intramuscular and intradermal. Moreover, each priming immunogen was administered twice and each boosting immunogen was also administered twice. This assured that the true potential immunogenicity of these constructs would be realized in the present study.
Although the immunogenicity of all of the recombinant pox-HIV-1 Env constructs was impressive, the immunogenicity of the recombinant pox-SIV Gag constructs was disappointing. In fact, both the rVac-SIV gag and the rMVA-SIV gag constructs expressed the transgene product at lower levels than did the rVac-SIV env and rMVA-SIV env constructs, as demonstrated by the Western blot studies. The less impressive immunogenicity of the recombinant SIV gag constructs was likely explained, therefore, by transgene expression levels. However, the impressive immunogenicity of the recombinant pox envelope constructs attests to the potential utility of these vectors as HIV-1 immunogens.
While SHIVs should be excellent challenge viruses for assessing HIV-1 vaccine candidates, no ideal SHIV has been constructed to date for use in this setting. Both the R5-tropic and clade C SHIVs that have been developed cause infections in macaques with peak and set point plasma viral RNA levels that are too variable to allow a useful comparison of different vaccine strategies (12, 26). The clade B SHIV-89.6P acts in vivo like a CXCR4-tropic virus and preferentially eliminates naïve CD4+ T lymphocytes, while HIV-1 usually infects CCR5-expressing CD4+ T lymphocytes and, therefore, preferentially eliminates memory CD4+ T lymphocytes (20). Nevertheless, the clinical protection against this virus afforded by vaccination is comparable to that seen in vaccinated monkeys challenged with a CXCR4-tropic primate lentivirus (5, 8). Therefore, on the basis of the present study, there is reason to assume that the vaccination of humans with recombinant poxviruses should afford some degree of clinical protection against HIV-1 infection.
While the different vaccination regimens employed in the present study generated immune responses of different magnitudes, the immunologic and virologic findings postchallenge in the three cohorts of experimentally immunized monkeys were, in general, comparable. We previously showed that different magnitudes of cellular immune responses were generated in plasmid DNA-primed monkeys that were boosted with DNA alone or different recombinant pox immunogens. Yet, postchallenge, these groups of monkeys developed comparable recall cellular immune responses and demonstrated comparable levels of lentiviral control. The results of the present study are consistent with these earlier observations. These findings may simply reflect the fact that this lentiviral challenge system cannot discriminate sensitively between the clinical protection afforded by these various vaccine regimens. It also possible, however, that even modest vaccine-elicited immunity will translate into robust clinical protection.
The finding of durable recall cellular immune responses as well as detectable neutralizing antibody responses postchallenge in the monkeys primed with rVac and boosted with rFPV constructs suggests that this regimen may be superior to the other vaccination regimens. This possibility is lent further credence by the finding of modestly higher peripheral blood CD4+ T-lymphocyte counts in this group of monkeys than in the other groups of vaccinated animals. Nevertheless, the use of vaccinia virus-based immunogens will likely not be acceptable in humans because of the possibility of vaccinia virus dissemination in the setting of clinical immunosuppression. Thus, if an individual is vaccinated who has an undiagnosed illness with associated immune suppression, exposure to vaccinia virus may result in systemic spread of the virus, with a resulting encephalitis and even death. Therefore, the present study suggests that the use of a combination of rMVA and rFPV immunogens is the most promising of these regimens.
This study therefore supports further exploration of combining recombinant vectors of the same family in prime/boost immunization strategies to optimize vaccine-elicited cellular immune responses. In particular, these results suggest that combining rMVA with rFPV may prove to be a useful regimen for generating HIV-1-specific cellular immunity.
Acknowledgments
We acknowledge Nancy Miller, Keith Reimann, Sharon Orndorff, Jim Treece, Deborah Weiss, Niem Nguyen, and Jim Monroe for generous advice, assistance, and reagents.
This work was supported by NIAID Simian Vaccine Evaluation Unit contract N01-AI60005, NIH contract N01-AI30033, and Harvard University Center for AIDS Research (CFAR) program grant NIH AI-060354.
Footnotes
Published ahead of print on 6 June 2007.
REFERENCES
- 1.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. I. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 2.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T.-M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 3.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290-6297. [DOI] [PubMed] [Google Scholar]
- 4.Casimiro, D. R., L. Chen, T. M. Fu, R. K. Evans, M. J. Caulfield, M. E. Davies, A. Tang, M. Chen, L. Huang, V. Harris, D. C. Freed, K. A. Wilson, S. Dubey, D. M. Zhu, D. Nawrocki, H. Mach, R. Troutman, L. Isopi, D. Williams, W. Hurni, Z. Xu, J. G. Smith, S. Wang, X. Liu, L. Guan, R. Long, W. Trigona, G. J. Heidecker, H. C. Perry, N. Persaud, T. J. Toner, Q. Su, X. Liang, R. Youil, M. Chastain, A. J. Bett, D. B. Volkin, E. A. Emini, and J. W. Shiver. 2003. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J. Virol. 77:6305-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casimiro, D. R., F. Wang, W. A. Schleif, X. Liang, Z.-Q. Zhang, T. W. Tobery, M.-E. Davies, A. B. McDermott, D. H. O'Connor, A. Fridman, A. Bagchi, L. G. Tussey, A. J. Bett, A. C. Finnefrock, T. Fu, A. Tang, K. A. Wilson, M. Chen, H. C. Perry, G. J. Heidecker, D. C. Freed, A. Carella, K. S. Punt, K. J. Sykes, L. Huang, V. I. Ausensi, M. Bachinsky, U. Sadasivan-Nair, D. I. Watkins, E. A. Emini, and J. W. Shiver. 2005. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 79:15547-15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farina, S. F., G.-P. Gao, Z. Q. Xiang, J. J. Rux, R. M. Burnett, M. R. Alvira, J. Marsh, H. C. Ertl, and J. M. Wilson. 2001. Replication-defective vector based on a chimpanzee adenovirus. J. Virol. 75:11603-11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald, J. C., G. P. Gao, A. Reyes-Sandoval, G. N. Pavlakis, Z. Q. Xiang, A. P. Wlazlo, W. Giles-Davis, J. M. Wilson, and H. C. Ertl. 2003. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J. Immunol. 170:1416. [DOI] [PubMed] [Google Scholar]
- 8.Friedrich, T. C., L. E. Valentine, L. J. Yant, E. G. Rakasz, S. M. Piaskowski, J. R. Furlott, K. L. Weisgrau, B. Burwitz, G. E. May, E. J. León, T. Soma, G. Napoe, S. V. Capuano III, N. A. Wilson, and D. I. Watkins. 2007. Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J. Virol. 81:3465-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gritz, L., A. Destree, N. Cormier, E. Day, V. Stallard, T. Caiazzo, G. Mazzara, and D. Panicali. 1990. Generation of hybrid genes and proteins by vaccinia virus-mediated recombination: application to human immunodeficiency virus type 1 env. J. Virol. 64:5948-5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hel, Z., J. Nacsa, E. Tryniszewska, W. P. Tsai, R. W. Parks, D. C. Montefiori, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2002. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J. Immunol. 169:4778-4787. [DOI] [PubMed] [Google Scholar]
- 11.Hirsch, V. M., T. R. Fuerst, G. Sutter, M. W. Carroll, L. C. Yang, S. Goldstein, M. Piatak, Jr., W. R. Elkins, W. G. Alvord, D. C. Montefiori, B. Moss, and J. D. Lifson. 1996. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 70:3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu, M., S. H. Ho, P. Balfe, A. Gettie, J. Harouse, J. Blanchard, and C. Cheng-Mayer. 2005. A CCR5-tropic simian-HIV molecular clone capable of inducing AIDS in rhesus macaques. J. Acquir. Immune Defic. Syndr. 40:383. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins, S., L. Gritz, C. H. Fedor, E. M. O'Neill, L. K. Cohen, and D. L. Panicali. 1991. Formation of lentivirus particles by mammalian cells infected with recombinant fowlpox virus. AIDS Res. Hum. Retrovir. 7:991-998. [DOI] [PubMed] [Google Scholar]
- 14.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayr, A., V. Hochstein-Mintzel, and H. Stickl. 1975. Abstammung, Eigenschaften und Verwendung des attenuierten vaccinia-Stammes MVA. Infection 3:6-14. [Google Scholar]
- 16.Mazzara, G. P., A. Destree, and A. Mahr. 1993. Generation and analysis of vaccinia virus recombinants. Methods Enzymol. 217:557-581. [DOI] [PubMed] [Google Scholar]
- 17.Meyer, H., G. Sutter, and A. Mayr. 1991. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J. Gen. Virol. 72:1031-1038. [DOI] [PubMed] [Google Scholar]
- 18.Moss, B., M. W. Carroll, L. S. Wyatt, J. R. Bennink, V. M. Hirsch, S. Goldstein, W. R. Elkins, J. D. Lifson, M. Piatak, N. P. Restifo, W. Owerwijk, R. Chamberlain, S. A. Rosenberg, and G. Sutter. 1996. Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv. Exp. Med. Biol. 397:7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nanda, A., D. M. Lynch, J. Goudsmit, A. A. Lemckert, B. A. Ewald, S. M. Sumida, D. M. Truitt, P. Abbink, M. G. Kishko, D. A. Gorgone, M. A. Lifton, L. Shen, A. Carville, K. G. Mansfield, M. J. Havenga, and D. H. Barouch. 2005. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J. Virol. 79:14161-14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishimura, Y., T. Igarashi, O. K. Donau, A. Buckler-White, C. Buckler, B. A. P. Lafont, R. M. Goeken, S. Goldstein, V. M. Hirsch, and M. A. Martin. 2004. Highly pathogenic SHIVs and SIVs target different CD4+ T cell subsets in rhesus monkeys, explaining their divergent clinical courses. Proc. Natl. Acad. Sci. USA 101:12324-12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paoletti, E. 1996. Applications of pox virus vectors to vaccination: an update. Proc. Natl. Acad. Sci. USA 93:11349-11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 23.Santra, S., D. H. Barouch, B. K. Schmitz, C. I. Lord, G. R. Krivulka, F. Yu, M. H. Beddall, D. A. Gorgone, M. A. Lifton, A. Miura, V. Philippon, K. Manson, P. D. Markham, J. Parrish, M. J. Kuroda, J. E. Schmitz, R. S. Gelman, J. W. Shiver, D. C. Montefiori, D. Panicali, and N. L. Letvin. 2004. Recombinant poxvirus boosting of DNA-primed rhesus monkeys augments peak but not memory T lymphocyte responses. Proc. Natl. Acad. Sci. USA 101:11088-11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiver, J. W., and E. A. Emini. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55:355-372. [DOI] [PubMed] [Google Scholar]
- 25.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 26.Song, R. J., A.-L. Chenine, R. A. Rasmussen, C. R. Ruprecht, S. Mirshahidi, R. D. Grisson, W. Xu, J. B. Whitney, L. M. Goins, H. Ong, P.-L. Li, E. Shai-Kobiler, T. Wang, C. M. McCann, H. Zhang, C. Wood, C. Kankasa, W. E. Secor, H. M. McClure, E. Strobert, J. G. Else, and R. M. Ruprecht. 2006. Molecularly cloned SHIV-1157ipd3N4: a highly replication-competent, mucosally transmissible R5 simian-human immunodeficiency virus encoding HIV clade C env. J. Virol. 80:8729-8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun, Y., J. E. Schmitz, A. P. Buzby, B. R. Barker, S. S. Rao, L. Xu, Z. Y. Yang, J. R. Mascola, G. J. Nabel, and N. L. Letvin. 2006. Virus-specific cellular immune correlates of survival in vaccinated monkeys after simian immunodeficiency virus challenge. J. Virol. 80:10950-10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]