Abstract
Viral particles preferentially incorporate extra- and intracellular constituents of host cell lipid rafts, a phenomenon central to pseudotyping. Based on this mechanism, we have developed a system for the predictable decoration of enveloped viruses with functionally active cytokines that circumvents the need to modify viral proteins themselves. Human interleukin-2 (hIL-2), hIL-4, human granulocyte-macrophage colony-stimulating factor (hGM-CSF), and murine IL-2 (mIL-2) were used as model cytokines and fused at their C terminus to the glycosylphosphatidylinositol (GPI) acceptor sequence of human Fcγ receptor III (CD16b). We show here that genetically modified cytokines are all well expressed on 293 producer cells. However, only molecules equipped with GPI anchors but not those linked to transmembrane/intracellular regions of type I membrane proteins are efficiently targeted to lipid rafts and consequently to virus-like particles (VLP) induced by Moloney murine leukemia virus Gag-Pol. hIL-4::GPI and hGM-CSF::GPI coexpressed on VLP were found to differentiate monocytes towards dendritic cells. Apart from myeloid-committed cell types, VLP-bound cytokines also act efficiently on lymphocytes. hIL-2::GPI strongly costimulated T-cell receptor (TCR)/CD3 dependent T-cell activation in vitro and mIL-2::GPI-coactivated antigen-specific T cells in vivo. On a molar basis, the functional activity of VLP-bound hIL-2::GPI was found to be comparable to that of soluble hIL-2. VLP decorated with hIL-2::GPI and coexpressing a TCR/CD3 ligand have an IL-2-specific activity of 5 × 104 units/mg protein. Virus particles decorated with lipid-modified cytokines might help to improve viral strains for vaccination purposes, the propagation of factor-dependent cell types, as well as gene transfer by viral systems in the future.
The augmentation of immune responses against antigens by the coadministration of appropriate adjuvants became general practice following the demonstration by Ramon more than 80 years ago that increased antitoxin responses in the presence of “helper substances” can be achieved (reviewed in references 3 and 21). Adjuvants can be separated into agents causing depot formation, vaccine delivery systems, and immunostimulatory agents (66) according to their modes of action, although several adjuvants consist of complex formulations and belong to more than one category. The delivery systems are mostly particulate formulations and function mainly by targeting associated antigens to antigen-presenting cells (52). In contrast, immunostimulatory adjuvants such as lipopolysaccharide, monophosphoryl lipid A, or CpG DNA primarily activate cells of the innate immune system (3).
Meuer and others have described a more direct approach to promote immune system function during vaccination. In their studies, soluble cytokines coadministered with antigen were found to overcome nonresponsiveness to, e.g., hepatitis B virus vaccination in immunodeficient individuals (42, 43, 51). Rabies virus and herpes simplex virus vaccines supplemented with interleukin-2 (IL-2) were found to be successful in animals at about the same time (50, 76). Subsequently, other cytokines have been evaluated as adjuvants in a variety of experimental vaccines (22, 23, 25, 26). In a related approach, naked DNA or recombinant vaccine strains have been engineered to express soluble cytokines upon administration to the host (6, 7, 24, 27, 35, 36). Similar methods have been applied to enhance the immunogenicity of tumor cells for therapeutic oncolysis (38, 54) or to substitute essential hematopoietic growth factors (9, 34). Frequently, however, the introduction of soluble cytokines leads to undesired systemic adverse reactions (2, 18, 37, 71). In order to restrict the action of cytokines to the site of administration, several groups have resorted to the membrane attachment of cytokines to, e.g., tumor cells modified for vaccination purposes (12, 17, 69, 80).
In this paper, we address the feasibility of designing a simple and versatile system for the decoration of viral particles with biologically active cytokines. For that purpose, we have built upon and explored the recently elucidated mechanism responsible for viral pseudotyping (39, 49, 56, 63). Pseudotyping is the consequence of a mixed infection of a host cell with two different enveloped viruses, which results in the production of virus progeny with chimeric envelope constituents, i.e., in which the core of one virus is circumscribed by a lipid envelope bearing both its own envelope protein and the envelope protein of the second virus (13, 29, 30, 82). We and others have shown that detergent-insoluble lipid rafts of the plasma membrane function as a natural meeting point for the transmembrane and core components of a phylogenetically diverse collection of enveloped viruses (39, 49, 56, 63) and hence represent the biological basis for the pseudotyping phenomenon. This phenomenon is driven at least in part by posttranslational lipid acylation of viral envelope and core proteins, leading to their enrichment in the lipid-ordered domains, also called lipid rafts, of the plasma membrane (48, 61, 64, 78, 79).
As a proof of principle, we have evaluated the ability of membrane-anchored human and murine cytokines to “pseudotype” Moloney murine leukemia virus (MoMLV) particles. For this purpose, we have fused the cytokine gene products at their C termini to the glycosylphosphatidylinositol (GPI) anchor attachment sequence of CD16b, a well-defined GPI-anchored molecule of human granulocytes (65). Here, we show that this leads to the efficient surface expression of biologically active cytokines on virus-like particles (VLP) as demonstrated in vitro and in vivo. Targeting different varieties of cytokines to viral envelopes might help to raise the immunogenicity of virus preparations. In addition, viruses or VLP decorated with cytokines might become useful experimental tools for the ex vivo expansion of diverse hematopoietic and nonhematopoietic precursor/stem cell populations and might lead to the development of improved viral gene therapy vehicles in the future.
MATERIALS AND METHODS
Cell lines and primary cells.
A laboratory isolate of the 293 cell line (human embryonic kidney epithelial cells) was maintained in IMDM (Sigma Chemicals, St. Louis, MO) plus 10% fetal calf serum (Invitrogen, Carlsbad, CA) supplemented with 2 mM l-glutamine, 50 μM 2-mercaptoethanol, and 15 μg/ml gentamicin sulfate. A stable transfectant of the 293 cell line expressing a single-chain variable fragment (scFv) of the CD3ɛ-specific hybridoma OKT3 attached to the CD14 molecule (OKT3scFv::GPI) was established and grown in the presence of 1 μg/ml of puromycin (Sigma Chemicals, St. Louis, MO) as described elsewhere (16). Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood of healthy adult donors upon informed consent by standard density gradient centrifugation with Lymphoprep (Technoclone, Vienna, Austria). Purified CD14+ monocytes (>95%) were obtained by positive selection with CD14 monoclonal antibodies (mAbs) (Miltenyi Biotech, Bergisch Gladbach, Germany) as described previously (55).
Plasmid constructs and transfections.
The human IL-2 (hIL-2)-specific cDNA sequence was amplified with primers IL-2 for (5′-CGCGGGAAGCTTGCCACCATGTACAGGATGCAACTCCTG) and IL-2 rev (5′-CCCGCGGCTAGCGCCGCCGCCAGTCAGTGTTGAGATGATGC) from clone pTCGF-11 (ATCC 39673; American Type Culture Collection, Manassas, VA), which is derived from phytohemagglutinin (PHA)-activated peripheral blood cells. The murine IL-2 (mIL-2)-specific cDNA sequence was amplified with primers mIL-2 for (5′-CCCGCGAAGCTTGCCACCATGTACAGCATGCAGCTCGC) and mIL-2 rev (5′-CGCGGGGCTAGCGCCGCCGCCTTGAGGGCTTGTTGAGATGATG) from clone pcDmouseIL-2[MT-1] (ATCC 39892; American Type Culture Collection, Manassas, VA), which is derived from concanavalin A-induced T-cell line LB2-1. The hIL-4-specific cDNA sequence was amplified with primers IL-4 for (5′-CGCGGGAAGCTTGCCACCATGGGTCTCACCTCCCAACTG) and IL-4 rev (5′-CCCGCGTCTAGAGCCGCCGCCGCTCGAACACTTTGAATATTTCTC), from clone pcD-IL-4 (ATCC 57592,), which is derived from the concanavalin A-activated T-helper cell line 2F1. The human granulocyte-macrophage colony-stimulating factor (hGM-CSF)-specific cDNA sequence was amplified with primers GM-CSF for (5′-CCCGCGAAGCTTGCCACCATGTGGCTGCAGAGCCTG) and GM-CSF rev (5′-CGCGGGGCTAGCGCCGCCGCCCTCCTGGACTGGCTCCCA) from clone pCSF-1 (ATCC 39754), which is derived from the hairy cell leukemia line Mo. At the 5′ ends of the cytokine cDNAs, optimized translational initiation sites preceded by a HindIII restriction site were inserted. The 3′ stop codons of the cytokine genes were replaced with an NheI restriction site to allow fusion with the CD16 GPI anchor acceptor sequence. After amplification, digestion with restriction enzymes, and gel purification, the DNA fragments were ligated into the pEAK12-CD80::CD16 plasmid from which the CD80 ectodomain had been released (16). For the construction of IL-2 fusions with transmembrane/intracellular (TM) regions of type I transmembrane molecules, the CD16 GPI anchor acceptor sequence was replaced by CD80 or CD99 transmembrane/intracellular regions. For that purpose, we amplified CD80 with CD80 for (5′-CGCGGGGCTAGCTGGGCCATTACCTTAATCTCAGTA) and CD80 rev (5′-CGCGGGCCGGCCGCTTTATACAGGGCGTACACTTTCCCTTC) from pEAK12-CD80 (16) and CD99 with CD99 for (5′-CGCGGGGCTAGCGTGATCCCCGGGATTGTGG) and CD99 rev (5′-CCCGCGGCGGCCGCTTTATTTCTCTAAAAGAGTACG) from a human endothelial cell library (Edge Biosystems, Gaithersburg, ME). Correctness of the constructs was determined by DNA sequencing. Original MoMLV gag-pol (OGP) was expressed using the mammalian expression vector pMD.gagpol (53), kindly provided by R. Mulligan (Children's Hospital, Boston, MA). The expression constructs harbored in pEAK12 and coding for human CD54::GPI, CD80::GPI, and OKT3scFv::GPI and mouse H-2Db::GPI and β2-microglobulin (mβ2m) and the peptide minigene coding for amino acids 33 to 41 of the lymphocytic choriomeningitis virus glycoprotein (LCMV-GP33-41) were designed and constructed as described previously (16).
Immunofluorescence analyses.
For membrane staining, 5 × 105 293 cells were incubated at 4°C with the fluorochrome-conjugated IL-2-specific mAb MQ1-17H12, the IL-4-specific mAb MP4-25D2, and the GM-CSF-specific mAb BVD2-21C11 conjugated with phycoerythrin (PE) (Caltag, Burlingham, CA); the CD59-specific mAb MEM-43 (kindly provided by Horejsi, Academy of Sciences, Praha, Czech Republic); the CD99-specific mAb 3B2/TA8 (Caltag); or an irrelevant control mAb (VIAP, recognizing calf intestinal alkaline phosphatase, kindly provided by Majdic, Institute of Immunology, Vienna, Austria) used at a concentration of 20 μg/ml for 30 min. After washing the cells twice with phosphate-buffered saline (PBS)-1% bovine serum albumin, the resulting membrane fluorescence was analyzed on a FACSCalibur flow cytometer supported by CellQuest software (Becton Dickinson, San Jose, CA). Phosphatidylinositol-specific phospholipase C (PI-PLC) treatment of 293 cells with PI-PLC from Bacillus thuringiensis (American Radiolabeled Chemicals, St. Louis, MO) was performed according to the manufacturer's recommendations. Briefly, 293 cells were washed in PBS without Ca2+ and Mg2+ and resuspended at a concentration of 1 × 107 cells/ml in PBS. 100 mU of PI-PLC was added to 1 × 107 cells and incubated at 37°C for 2 h. Subsequently, cells were washed in PBS-1% bovine serum albumin and subjected to membrane staining.
Generation of VLP.
For the generation of VLP, 293 cells were transiently transfected using the modified calcium phosphate precipitation method (32) as described previously (16). Briefly, 1 day prior to transfection, wild-type 293 cells or 293 cells stably expressing OKT3scFv::GPI were seeded onto 100-mm culture dishes at a concentration of 3 × 106 cells and were transfected the day after with 2 ml transfection mix. Total DNA amounts of 30 μg per dish included 7.5 μg of the MoMLV original gag-pol (OGP) encoding plasmid pMD.gagpol and, as indicated, expression plasmids in pEAK12 encoding membrane-bound hIL-2::GPI, mIL-2::GPI, hIL-2::TM(CD80), hIL-2::TM(CD99), hIL-4::GPI, hGM-CSF::GPI, hCD80::GPI, hCD54::GPI, H-2Db::GPI, mβ2m, and the LCMV-GP33-41 minigene. If necessary, DNA amounts were adjusted with a control vector (empty pEAK12 vector or, where indicated, full-length CD16b in pEAK12). Eighteen hours to 24 h after transfection, medium was changed. Supernatants (10 ml) were harvested 3 days after transfection and cleared of cellular debris by filtration through 0.45-μm syringe filters (Millipore, Billerica, MA), concentrated by ultracentrifugation in a Beckman-Optima LE-80K centrifuge (Beckman Instruments, Palo Alto, CA) using an SW41 Ti rotor at 100,000 × g for 1 h, and washed once in a large volume of PBS. Amounts of VLP were determined by standard protein assay (Micro BCA; Pierce) and adjusted to protein concentrations as described above for individual experiments. If required, they were sterile filtered through 0.22-μm syringe filters (Millipore) and used directly or, alternatively, stored at −80°C, which did not alter the functional activities of VLP (not shown).
Biochemical analyses of producer cells and VLP.
Whole-cell lysates were obtained by incubating 1 × 106 cells in 10 μl lysis buffer containing 1% NP-40, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 20 μg/ml aprotinin, and 20 μg/ml leupeptin in MBS (25 mM MES [morpholineethanesulfonic acid], 150 mM NaCl) for 1 h on ice. Subsequently, lysates were centrifuged at 80 × g in an Eppendorf microcentrifuge to remove insoluble material at 4°C for 10 min and mixed with 4× Laemmli sample buffer. VLP were concentrated and washed, and the protein concentration was determined as described above, followed by resuspension in 1× Laemmli sample buffer. Lipid rafts were prepared as previously described (56). Cellular and VLP lysates were resolved by 4 to 20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Anamed, Darmstadt, Germany). Subsequently, proteins were transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA) and subjected to Western blotting. hIL-2-specific mAb IL-2.1E7 (kindly provided by O. Majdic, Institute of Immunology, Vienna, Austria); hIL4-, hGM-CSF-, and mIL-2-specific polyclonal antisera (biotinylated) (Peprotech, London, United Kingdom); MoMLV p30 Gag mAb R187 (ATCC); and CD14 antiserum AB383 (R&D, Minneapolis, MN) were used at 1 μg/ml. Horseradish peroxidase (HRP)-conjugated secondary reagents (DAKO, Glostrup, Denmark, and Amersham Pharmacia, Little Chalfont, United Kingdom) were used at a dilution of 1:104. Blots were developed with a luminol-based indicator system (Western lightning; Perkin-Elmer, Boston, MA) and exposed to X-ray films (Eastman Kodak, Rochester, NY).
Electron microscopy.
VLP of gag-pol (OGP)-transfected 293 cells that were cotransfected with hIL-2::GPI or control vector were pelleted and washed by ultracentrifugation as described above. VLP were loaded onto Formvar-carbon-coated electron microscopy grids for 20 min and fixed in 2% paraformaldehyde for 30 min. After quenching with PBS-50 mM glycine, samples were immunogold labeled by incubation with mouse anti-human IL-2 antibody IL-2.1E7 mAb or the isotype-matched control mAb VIAP (immunoglobulin G1 [IgG1]), followed by rabbit anti-mouse Ig (Dakopatts, Denmark) and protein A coupled to 10-nm gold particles (Cell Microscopy Center, AZU, Utrecht, The Netherlands). After washing with PBS, samples were postfixed in 1% glutaraldehyde and contrasted and embedded with a mixture of 2% methylcellulose-.4% uranyl acetate (pH 4). Observations were made with a CM120 Twin Phillips electron microscope (FEI Company, Eindoven, The Netherlands) equipped with a KeenView digital camera (Soft Imaging System, Germany).
Differentiation of CD14+ monocytes towards dendritic cells (DC).
Highly purified CD14+ peripheral blood monocytes were cultured at a cell density of 2 × 106 cells/ml in 24-well culture plates (Becton Dickinson, Franklin Lakes, NY) in RPMI 1640-10% fetal calf serum medium alone, in medium containing undecorated VLP (5 μg/ml), or in medium containing VLP bearing hIL-4::GPI plus hGM-CSF::GPI (5 μg/ml) at 37°C in a humidified CO2-containing atmosphere for 5 to 8 days. As a positive control, a combination of soluble recombinant hGM-CSF (500U/ml) and hIL-4 (1000 U/ml) was used (Peprotech, London, United Kingdom). Based on the biochemical data, amounts of hIL-4::GPI and hGM-CSF::GPI added to the cultures were estimated to range between 100 and 1,000 U/ml assuming similar activities for soluble and membrane-bound cytokines.
Determination of cellular morphology of differentiated monocytes.
Aliquots (50 μl) of CD14+ monocytes cultured for 5 to 8 days with the indicated soluble or membrane-bound cytokine combinations, undecorated VLP, or medium alone were centrifuged at 100 × g for 5 min onto microscope slides using a Cytospin-2 centrifuge (Shandon Southern Products, Astmoor, United Kingdom), followed by staining with May-Grünwald-Giemsa solution and analysis by light microscopy on a Nikon Eclipse E600 microscope (Nikon, Tokyo, Japan).
T-cell stimulation assays.
PBMC (105/well) were incubated with the indicated VLP preparations (2 μg/ml) or controls (5 μg/ml PHA or medium) in 96-well plates in triplicates (final volume, 200 μl). CD3 mAb OKT3 (eBioscience, San Diego, CA), CD28 mAb 28.2 (BD Pharmingen, San Jose, CA), and/or control mAb VIAP was coated onto 4.5-μm goat anti-mouse IgG magnetic beads (Dynabeads, Dynal Biotech) at 150 fg per bead and was used at a bead-to-cell ratio of 1:1. Recombinant, soluble hIL-2 was purchased from Peprotech (London, United Kingdom) and used at the indicated concentrations. After spin inoculation by centrifugation (62) in a Sigma 4K15 centrifuge (Sigma, Osterode, Germany) using rotor 11150 at 900 × g for 2 h at 30°C, plates were incubated for 3 days in a humidified atmosphere at 37°C and then pulsed with [methyl-3H]thymidine (1 μCi/well) for 18 h, and their DNA was collected onto filter plates (Packard, Meriden, CT). Incorporated radioactivity was quantified with a microplate scintillation counter (Packard).
Mice.
P14 T-cell receptor (TCR) transgenic mice expressing TRVA2/TRVB8 TCR-specific LCMV-GP33-41 in association with the H-2Db molecule have been described previously (57) and were kindly provided by R. M. Zinkernagel (Institute of Experimental Immunology, University Hospital, Zurich, Switzerland). Animals were kept under conventional conditions and were used for experiments at 8 to 16 weeks of age.
In vivo activation of LCMV-GP33-41-specific CD8+ T cells with VLP coexpressing mIL-2::GPI.
P14 TCR transgenic mice, subline 327, with 80 to 90% of CD8+ T cells expressing TRVA2, were intravenously (i.v.) challenged with 400 μg (in 200 μl) VLP expressing H-2Db::GPI along with mβ2m and a peptide minigene coding for the immunodominant CD8+ T-cell epitope of LCMV-GP33-41 essentially as described previously by Derdak et al. (16). Where indicated, such particles coexpressed mIL-2::GPI or were supplemented with 2 × 104 units soluble mIL-2 (Peprotech) or PBS only. PBS and PBS supplemented with 2 × 104 units soluble mIL-2 served as additional controls. Eighteen hours later, single-cell suspensions were prepared from spleens and were incubated at 4°C with anti-mouse CD69-fluorescein isothiocyanate (FITC) (clone H1.2F3; Caltag Laboratories, Burlingame, CA), anti-mouse TRAV2-PE (clone B20.1; Pharmingen-BD, San Jose, CA), and anti-mouse CD8a-allophycocyanin (clone 5H10; Caltag Laboratories) for 30 min. Control stainings were performed by replacing the CD69 mAb with either VIAP-FITC (negative control mAb) or anti-mouse CD3-FITC (positive control mAb, clone 500A2; Caltag Laboratories). Viable cells (3 × 104) were acquired with a FACSCalibur flow cytometer and analyzed by using CellQuest software (Becton Dickinson, Mountain View, CA).
RESULTS
Construction of membrane-anchored forms of human cytokines by replacement of intrinsic stop codons with GPI anchor acceptor sequences.
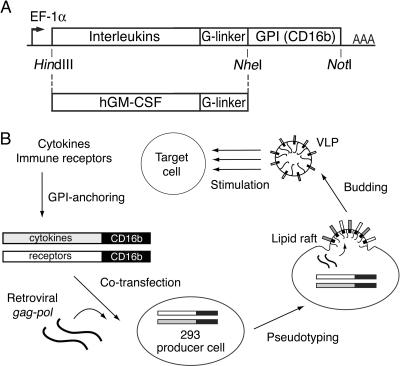
The coding sequences of hIL-2, hIL-4, and hGM-CSF as well as of mIL-2 were PCR amplified from cDNA clones with specific oligonucleotides, as detailed in Materials and Methods. The naturally occurring stop codons of the cytokines were replaced by a polyglycine spacer (8, 31), followed by the GPI anchor acceptor sequence (5, 73) of the human Fcγ receptor III, CD16b (65) (Fig. 1A). For this purpose, we took advantage of a CD80::GPI expression cassette based on CD16b, which was originally designed to target costimulatory transmembrane molecules to lipid rafts (16). hIL-2 fused to the type I TM region of the type I transmembrane molecule CD80 or CD99 served as a control. The constructs were cloned into the pEAK12 expression vector under the control of the EF-1α promoter.
FIG. 1.
Diagram of GPI-modified cytokine constructs and scheme for production of cytokine-decorated VLP. (A) The cytokine coding sequences were PCR amplified and tailed with HindIII and NheI restriction sites, respectively. For optimal expression levels, the consensus transcriptional initiation site GCCACC was introduced upstream of the ATG codons. At the 3′ end of the cytokine cDNAs, the intrinsic stop codons were replaced by a polyglycine linker sequence. After digestion with the indicated restriction enzymes, the cytokine genes were fused to the CD16b GPI anchor acceptor sequence located in the pEAK12 expression vector, directing gene expression by the EF-1α promoter. (B) Cytokines or immune receptors of interest are modified by GPI anchor acceptor sequences, which, upon transfection into 293 cells, target them to lipid rafts of the plasma membrane. Coexpression of original MoMLV gag-pol (OGP) induces the secretion of plasma membrane-derived VLP by the producer cell line. VLP harvested from the supernatant can be used directly or purified by ultracentrifugation in order to stimulate target cells.
Expression levels of GPI-anchored cytokines on 293 cells upon transfection.
In order to check for proper surface expression of the modified cytokines, 293 cells were transiently transfected using the calcium phosphate precipitation method. Flow cytometric analyses of transfectants stained with mAbs specific for hIL-2, hIL-4, and hGM-CSF demonstrated that both the cytokines fused to GPI anchor acceptor sequences, i.e., hIL-2::GPI, hIL-4::GPI, and hGM-CSF::GPI, and the cytokine fused to a type I transmembrane/intracellular region, i.e., hIL-2::TM(CD99), are clearly expressed on the majority of viable 293 cells (Fig. 2). mIL-2::GPI, hIL-7::GPI, hIL-15::GPI, and hIL-2::TM(CD80) revealed similar expression levels (not shown). In contrast, 293 cells transfected with empty vector DNA as a control did not express detectable levels of the indicated cytokines. Substantial proportions of the GPI-modified interleukins and growth factors are indeed attached to the cell membrane via GPI anchors as shown by their release upon coincubation with PI-PLC from B. thuringiensis (Fig. 2). These experiments revealed that 59.5% ± 5.5% of the hIL-2::GPI, 85.4% ± 3.6% of the hIL-4::GPI, and 96.0% ± 3.5% of the hGM-CSF::GPI were released from the cell surface of transfected 293 cells upon PI-PLC treatment (Table 1 and Fig. 2). A clear-cut release was also observed for the constitutively expressed GPI-anchored molecule CD59 (94.2% ± 1.8%), which served as a positive control. In marked contrast, both the transmembrane hIL-2::TM(CD99) fusion protein (3.5% ± 1.5%) as well as the constitutively expressed type I transmembrane molecule CD99 (2.5% ± 1.5%) remained largely unaffected by PI-PLC treatment (Table 1 and Fig. 2).
FIG. 2.
Expression levels of GPI-anchored and type I transmembrane-linked cytokines on 293 cells. 293 cells were transfected by the calcium phosphate precipitation method with empty vector (control), hIL-2::GPI, hIL-2::TM(CD99), hIL-4::GPI, or hGM-CSF::GPI. Seventy-two hours later, transfectants were harvested, and aliquots of the transfectants (1 × 107 cells) were treated with 100 mU/ml of PI-PLC at 37°C for 2 h. Subsequently, both PI-PLC-treated and nontreated cells (5 × 105) were incubated with the hIL-2-specific mAb MQ1-17H12, the hIL-4-specific mAb MP4-25D2, and the hGM-CSF-specific mAb BVD2-21C11 conjugated with PE (Caltag, Burlingham, CA). In addition, the constitutively expressed molecules CD59 (GPI anchored) and CD99 (type I transmembrane molecule) were probed with mAbs MEM-43 and 3B2/TA8, respectively. The resulting cellular fluorescence was analyzed by flow cytometry on a FACSCalibur cytometer (Becton Dickinson). Dead cells were excluded from the analyses by means of propidium iodide staining. In the overlay histograms, the solid lines correspond to the PI-PLC-treated cells, the dashed lines represent the untreated cells, and the percent values indicate the degree of release upon PI-PLC treatment. The data shown are representative of three independently performed experiments.
TABLE 1.
GPI-modified cytokines are released upon PI-PLC treatmenta
| Molecule on HEK-293 cells | % Release upon PI-PLC treatment ± SD |
|---|---|
| hIL-2::GPI | 59.5 ± 5.5 |
| hIL-2::TM(CD99) | 3.5 ± 1.5 |
| hIL-4::GPI | 85.4 ± 3.6 |
| hGM-CSF::GPI | 96.0 ± 3.5 |
| CD59 | 94.2 ± 1.8 |
| CD99 | 2.5 ± 1.5 |
Percent release of transfected or constitutively expressed molecules from 293 cells upon PI-PLC treatment. Mean fluorescence intensities of PI-PLC-treated cells were compared to mean fluorescence intensities of mock-treated cells and are expressed as relative values calculated according to the following formula: 100 − [(MFIPI-PLC/MFImock) × 100]. Data show mean values ± standard deviations from three independently performed experiments.
Human GPI-modified IL-2 is concentrated in lipid rafts and becomes targeted to VLP.
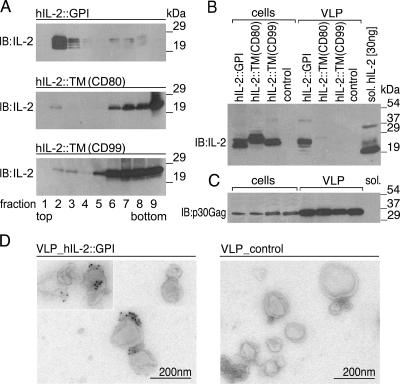
As a proof of principle, we set out to decorate MoMLV core particles with GPI-modified cytokines (production scheme in Fig. 1B). For that purpose, 293 cells were cotransfected with original MoMLV gag-pol (OGP) and hIL-2::GPI. As control molecules, hIL-2 fused to the type I membrane anchors of CD80 [hIL-2::TM(CD80)] and CD99 [hIL-2::TM(CD99)] were used. After 72 h, membrane fractionation experiments on producer cell lysates revealed that hIL-2::GPI localized exclusively to the lipid raft containing fractions 2 and 3 (Fig. 3A). Comparable targeting was observed for the constitutively GPI-anchored molecule CD59 (not shown). In contrast, hIL-2::TM(CD80) and hIL-2::TM(CD99) were targeted mainly to fractions 6 to 9 of gradients containing detergent-soluble molecules. In parallel, VLP were harvested from cell culture supernatants, cleared of residual producer cells by filtration, and concentrated by ultracentrifugation. Two micrograms of VLP, equivalent to the amount of VLP in 1 ml of supernatant, was resolved by SDS-PAGE under reducing conditions and analyzed by immunoblotting. The VLP preparations were compared to whole-cell lysates derived from 1 × 106 293 producer cells. Figure 3B shows the results obtained with the various hIL-2 fusion proteins, compared to 30 ng of soluble recombinant hIL-2, which served as a positive control. Significantly, only hIL-2::GPI was clearly targeted to VLP, although all three molecules were expressed at comparable levels in producer cells. That similar amounts of secreted particles and cell lysates were applied is shown by the loading control (Fig. 3C). The hIL-2 fusion proteins had a distinctly higher molecular mass than the soluble hIL-2, which is likely caused by the additional mass brought about by the different membrane anchors. Similar shifts were obtained with other cytokines such as hIL-4::GPI and hGM-CSF::GPI (Fig. 4A) and mIL-2::GPI (see Fig. 6C). Neither cells nor VLP preparations of control vector-transfected cells showed a specific signal in the IL-2 immunoblot. The immunoblot analyses revealed that under these conditions, approximately 20 to 30 ng of IL-2 is expressed on 2 μg of VLP. In order to directly test whether GPI-modified cytokines are able to decorate VLP, we performed immunoelectron microscopy analyses on various VLP preparations. Consistent with previous observations (16), electron microscopy showed spherical plasma membrane-like structures with an average diameter of ∼100 nm in supernatants of producer cells. In agreement with the biochemical experiments described above, the transfection of producer cells with MoMLV gag-pol plus hIL-2::GPI led to the production of hIL-2-decorated VLP, which were clearly recognized by the hIL-2-specific mAb IL-2.1E7 (Fig. 3D, left). In contrast, transfection of producer cells with MoMLV gag-pol plus control vector revealed undecorated VLP, which remained unrecognized by the hIL-2-specific mAb (Fig. 3D, right). No staining of hIL-2-decorated and undecorated VLP was observed with the isotype control mAb VIAP (not shown).
FIG. 3.
Expression of hIL-2::GPI on 293 producer cells and VLP. (A) GPI anchor attachment targets hIL-2 to lipid rafts. 293 cells transfected with hIL-2::GPI, hIL-2:TM(CD80), or hIL-2::TM(CD99) were lysed at 4°C with 1% Triton X-100 and fractionated on 5 to 40% sucrose into nine fractions (top to bottom). Equal amounts of each fraction were resolved by SDS-PAGE, blotted, and probed (immunoblotting) with the hIL-2-specific mAb IL-2.1E7. Bound antibody was visualized by goat anti-mouse Ig conjugated to HRP. (B) Preferential targeting of hIL-2::GPI to VLP. 293 cells were transfected with the indicated cDNAs or control vector and cell lysates (cells), corresponding VLP preparations, as well as 30 ng of soluble (sol.) recombinant hIL-2 and were analyzed by SDS-PAGE under reducing conditions followed by immunoblotting (IB) with the hIL-2-specific mAb IL-2.1E7. One out of several typical experiments is shown. (C) As a control for proper vesicle induction and loading in B, viral core protein (p30 Gag) expression was determined in cell lysates (cells) and VLP. (D) VLP isolated from the cell culture supernatant of producer cells cotransfected with MoMLV gag-pol and hIL-2::GPI (left micrograph) or MoMLV gag-pol and control vector (right micrograph) were put onto Formvar-carbon-coated electron microscopy grids, fixed, and immunogold labeled by incubation with the hIL-2-specific mAb IL-2.1E7 followed by rabbit-anti-mouse Ig and protein A coupled to 10-nm gold particles and contrasted as described in Materials and Methods.
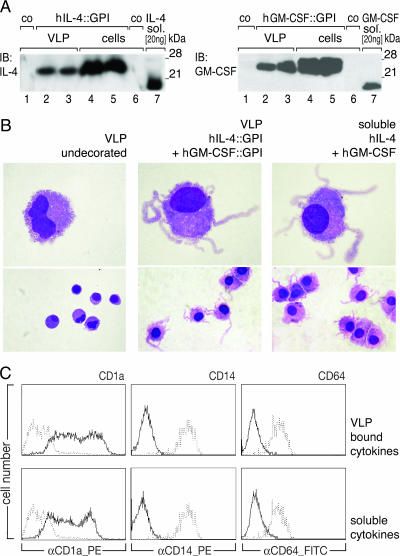
FIG. 4.
Morphology and marker profiles of monocyte-derived DC generated by VLP decorated with hIL-4::GPI plus hGM-CSF::GPI. (A) VLP were isolated from supernatants by ultracentrifugation and washed once in a large volume of PBS. Two micrograms of VLP was subjected to SDS-PAGE (4 to 20% acrylamide) under nonreducing (lane 2) and reducing (lane 3) conditions. Lanes 4 and 5 correspond to the respective cell lysates of producer cells. Soluble (sol.) hIL-4 (20 ng ≈ 100 U) and soluble hGM-CSF (20 ng ≈ 250 U) were used as positive controls (lane 7). co refers to VLP (lane 1) and cell lysates (lane 6) from control vector-transfected 293 cells. Proteins transferred onto nitrocellulose membranes were probed with biotinylated rabbit anti-human IL-4 or GM-CSF antibodies (Peprotech Ltd., London, United Kingdom), followed by streptavidin-conjugated HRP and a luminol-based detection reaction (Perkin-Elmer, Boston, MA). Molecular mass is indicated in kilodaltons. One out of several typical experiments is shown. IB, immunoblot. (B) Representative photographs of Wright-Giemsa-stained cytospin preparations of highly purified CD14+ monocytes stimulated for 7 days with undecorated VLP, with VLP expressing membrane-bound hIL-4::GPI plus hGM-CSF::GPI, or with soluble hIL-4 (1,000 U/ml) plus hGM-CSF (500 U/ml), used as a positive control, are shown. Pictures show typical cell morphologies (high and low magnifications) observed in four independently performed experiments. (C) Marker profiles of monocyte-derived cells obtained upon culture with VLP expressing hIL-4::GPI plus hGM-CSF::GPI. After 7 days of culture, cells were harvested and incubated with fluorochrome-conjugated mAbs directed against CD1a (VIT6b), CD14 (VIM13), and CD64 (10.1). Cellular fluorescence was determined by flow cytometry on a FACSCalibur cytometer (Becton Dickinson). Panels show overlay histograms of monocytes stimulated with VLP expressing hIL-4::GPI plus hGM-CSF::GPI or soluble hIL-4 plus hGM-CSF (solid lines) compared to cells stimulated with undecorated VLP (dotted lines). The abscissa represents fluorescence intensity (log10 scale), and the ordinate represents the respective cell numbers. Data are representative of three independently performed experiments.
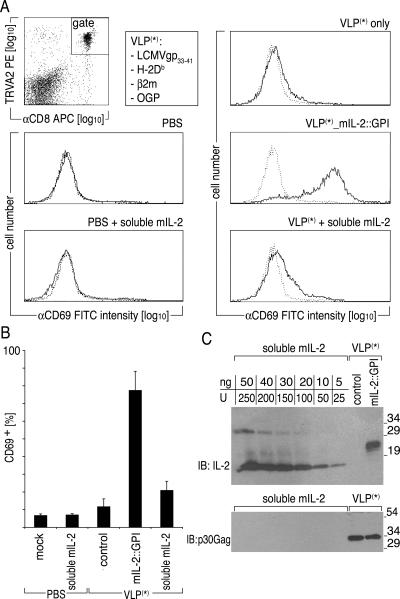
FIG. 6.
mIL-2::GPI on antigen-specific VLP induces up-regulation of the activation marker CD69 on CD8+ LCMV-GP33-41-specific T cells. 327 TCR transgenic mice were i.v. challenged with PBS alone, PBS containing 2 × 104 U soluble mIL-2, 400 μg LCMV-GP33-41-specific VLP only, 400 μg LCMV-GP33-41-specific VLP coexpressing mIL-2::GPI, or 400 μg LCMV-GP33-41-specific VLP supplemented with 2 × 104 U soluble mIL-2. Eighteen hours after challenge, splenocytes were stained with CD69-FITC or VIAP-FITC in combination with TRVA2-PE and CD8-allophycocyanin (APC) mAbs and analyzed by flow cytometry. (A) Data are gated on TRVA2+/CD8+ lymphocytes in two-parameter dot plots, and their CD69 FITC fluorescence intensity is displayed in single histograms (solid line) overlaid by the fluorescence of the FITC-conjugated control mAb (dotted line). (B) Percentage of CD69+/TRVA2+/CD8+ lymphocytes from three different mice within each group ± standard deviation is shown. (C) Determination of mIL-2::GPI-specific protein concentration in VLP preparations. Graded amounts of soluble mIL-2 were resolved along with 2 μg of the indicated VLP preparations followed by immunoblotting (IB) using either a rabbit anti-mouse IL-2-specific antiserum (Peprotech) or a rat mAb specific for p30 Gag (R187). Binding of antibodies was visualized by HRP-conjugated secondary reagents, followed by a luminol-based detection reaction (Perkin-Elmer, Boston, MA). Molecular mass is indicated in kilodaltons.
VLP simultaneously decorated with hIL-4::GPI plus hGM-CSF::GPI are biologically active and induce differentiation of monocytes to DC.
Highly purified CD14+ monocytes can be differentiated towards DC in the presence of soluble hIL-4 plus hGM-CSF (55, 83). To clarify whether VLP can be decorated with two cytokines simultaneously, expression levels of hIL-4::GPI and hGM-CSF::GPI were determined on VLP biochemically. For that purpose, 2 μg of VLP preparations (equivalent to 1 ml of supernatant) was resolved together with 20 ng of the respective soluble cytokines by SDS-PAGE under nonreducing and reducing conditions and analyzed by immunoblotting. The VLP preparations were compared to whole-cell lysates derived from 1 × 106 293 producer cells. Figure 4A shows that approximately 1 to 10 ng of hIL-4::GPI and approximately 20 ng of hGM-CSF::GPI are expressed on 2 μg of VLP, corresponding to 5 to 50 units of soluble hIL-4 and 250 units of soluble hGM-CSF, respectively, assuming equivalent biological activities of membrane-bound and soluble growth factors. To test whether VLP bearing these molecules would have similar functionality, we exposed purified monocytes to VLP produced from cells coexpressing the two cytokines as GPI fusion proteins. As a positive control, soluble hIL-4 plus hGM-CSF were applied, and as a negative control, VLP without membrane-bound cytokines (“undecorated VLP”) or medium without further additions was used. After 7 days of stimulation, cell morphology was evaluated, and the expression of typical monocyte/DC marker molecules was determined (55). Cultivation of monocytes with medium alone (not shown) or in the presence of undecorated VLP did not lead to differentiation as revealed by cellular morphology (Fig. 4B). In marked contrast, the addition of VLP coexpressing hIL-4::GPI plus hGM-CSF::GPI caused the monocytes to differentiate to typical DC with large cell membrane projections (Fig. 4B). The dramatic changes in cell morphology induced by the VLP-bound cytokines were also mirrored by characteristic changes in the marker profiles of the treated cells (Fig. 4C). The signature DC marker molecule CD1a was induced, whereas the lipopolysaccharide coreceptor CD14 as well as the high-affinity Fcγ receptor CD64 were repressed (Fig. 4C). The resulting cells were indistinguishable in both morphology and marker profiles from monocytes differentiated with the classical cytokine cocktail consisting of soluble hIL-4 plus hGM-CSF (Fig. 4B and C). Undecorated VLP or medium alone did not induce changes in cell morphology and marker profiles (not shown).
VLP decorated with hIL-2::GPI costimulate human T lymphocytes in vitro.
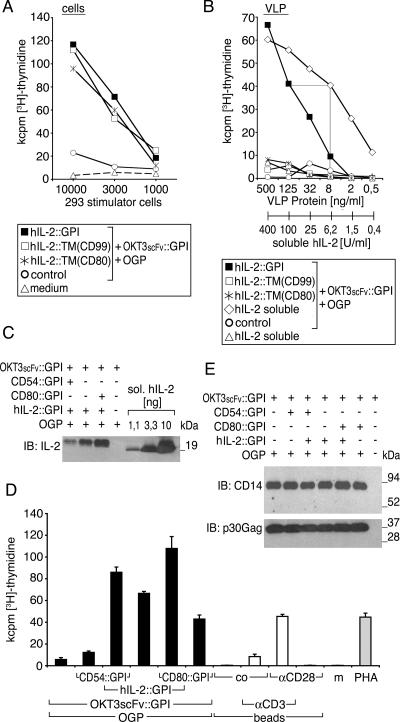
To investigate the ability of membrane-bound cytokines to also modulate T-lymphocyte function, we prepared VLP, which we refer to as immunosomes, equipped with a surrogate TCR/CD3 complex ligand consisting of an anti-CD3ɛ single-chain variable fragment attached to the GPI-anchored CD14 molecule (OKT3scFv::CD14) (16). Producer cells were in addition transfected with the indicated hIL-2 fusion proteins or control vector. Figure 5A shows that graded numbers of producer cells expressing either GPI-anchored or transmembrane-anchored versions of hIL-2 in combination with the TCR/CD3 ligand induced comparable levels of T-cell proliferation as measured by [3H]thymidine uptake. No stimulation was detectable with control vector-transfected producer cells. This indicates that the GPI-anchored hIL-2::GPI as well as the transmembrane versions hIL-2::TM(CD80) and hIL-2::TM(CD99) are functionally intact. In contrast, Fig. 5B shows that only VLP from hIL-2::GPI- and OKT3scFv::GPI-cotransfected producer cells were able to stimulate T-lymphocyte proliferation in a dose-dependent manner. No T-cell proliferation was observed with VLP from hIL-2::TM(CD80)- or hIL-2:TM(CD99)-transfected producer cells coexpressing the surrogate TCR/CD3 ligand (Fig. 5B). These findings are consistent with the biochemical experiments shown in Fig. 3B and indicate that although both the GPI and the transmembrane versions of hIL-2 are functionally expressed on producer cells, only the GPI-anchored version is efficiently decorating VLP.
FIG. 5.
VLP expressing GPI-anchored hIL-2 modulate human T-lymphocyte activation. The biological activity of IL-2 fusion proteins on producer cells (A) and resulting VLP (B) was determined upon cotransfection with OKT3scFv::GPI. As controls, control vector-transfected producer cells and particles thereof, OKT3scFv::GPI-decorated particles supplemented with soluble hIL-2, soluble hIL-2 alone, or medium alone was used. PBMC (105) were incubated with titrated amounts of the indicated 293 cells starting with a cell number of 1 × 104 in A or VLP or VLP/soluble hIL-2 mixtures starting with 500 μg/ml of particles in B. Cell proliferation was determined after 4 days by [3H]thymidine uptake. Data show mean values of triplicate cultures representative for two independently performed experiments. (C) A total of 360 ng of VLP coexpressing hIL-2::GPI (same particles as in B, D, and E) was compared to a titration curve of soluble hIL-2 (1.1, 3.3, and 10 ng) by immunoblotting (IB) using the hIL-2-specific mAb IL-2.1F10. Binding of antibodies in C was visualized by HRP-conjugated secondary reagents, followed by a luminol-based detection reaction (Perkin-Elmer, Boston, MA). Molecular mass is indicated in kilodaltons. One out of several typical experiments is shown. (D) Human PBMC (105) were cocultured with supernatants of stably OKT3scFv::GPI-expressing 293 cells that were cotransfected with original MoMLV gag-pol (OGP), CD80::GPI, CD54::GPI, and hIL-2::GPI or control vector combined as indicated (black bars). Microbeads (105/well) substituted with CD3 mAb, CD28 mAb, control mAb (co), or combinations thereof were used for comparison (white bars). PHA or culture medium (m) served as additional controls (gray bars). Cell proliferation was determined after 4 days by [3H]thymidine uptake. Data show mean values ± standard deviations of triplicate cultures representative of several independently performed experiments. (E) The amounts of VLP in the various supernatants used for proliferation assays in A were determined by immunoblotting for OKT3scFv::GPI using a CD14 mAb and for viral core proteins using a p30 Gag mAb. Binding of antibodies was visualized by HRP-conjugated secondary reagents, followed by a luminol-based detection reaction (Perkin-Elmer). Molecular mass is indicated in kilodaltons.
In the next step, we determined the biological activity of VLP-bound hIL-2::GPI with that of defined amounts of soluble hIL-2 supplementing the same amount of VLP. According to the linear parts of our titration curves, 125 ng/ml hIL-2::GPI plus OKT3scFv::GPI decorated VLP and showed a biological activity comparable to 6.2 units/ml of soluble hIL-2 supplementing OKT3scFv::GPI-decorated particles (Fig. 5B). Thus, hIL-2::GPI-decorated VLP have a specific activity of 5 × 104 units/mg VLP protein. In parallel, we determined the protein amounts of hIL-2::GPI on VLP by immunoblotting (Fig. 5C). For reasons which remain to be solved in future experiments, CD54::GPI competed to some extent with hIL-2::GPI expression levels on VLP. Immunoblotting experiments (Fig. 5C) revealed that 360 ng of hIL-2::GPI-decorated VLP plus OKT3scFv::GPI-decorated VLP expressed approximately 2 ng of hIL-2::GPI. Considering a specific activity of 1 unit per 100 pg for hIL-2::GPI also, this would correspond to a specific activity of 6 × 104 units/mg of VLP. Thus, on a molar basis, the functional activity of particle-bound hIL-2::GPI seems to be similar to that of soluble hIL-2.
In a next step, we wanted to clarify whether hIL-2::GPI functionally synergizes with classical costimulatory or adhesion molecules on OKT3scFv::GPI-decorated VLP. Figure 5D shows that signal 1 alone (OKT3scFv::GPI) led to only a very moderate proliferative response, which was augmented more than 11-fold by the coexpression of hIL-2::GPI. Under similar conditions, additional coexpression of CD54::GPI or CD80::GPI led to an even further increase in T-cell activation (15-fold and 19-fold, respectively). In the absence of signal 1, hIL-2::GPI did not activate human T lymphocytes (not shown). Control experiments revealed that the T-lymphocyte-activating potency of OKT3scFv::GPI- plus hIL-2::GPI- or of OKT3scFv::GPI- plus CD80::GPI- plus hIL-2::GPI-decorated VLP was clearly superior to microbeads substituted with CD3 and/or CD28 mAbs or to the polyclonal T-cell-activating lectin PHA (Fig. 5D). Importantly, immunoblotting analyses of OKT3scFv::GPI (CD14) and viral core protein p30 Gag showed that the amount of secreted particles were comparable in the various VLP preparations used to stimulate T lymphocytes (Fig. 5E). No significant export of GPI fusion proteins, as shown by CD14 immunoblotting recognizing the OKT3scFv::GPI molecule, was detectable in the absence of MoMLV gag-pol (OGP) expression (Fig. 5E, lane 7).
mIL-2::GPI-decorating antigen-specific VLP coactivate T lymphocytes in vivo.
In a final step, we sought to determine the biological activity of VLP-bound cytokines in vivo. For that purpose, P14 TCR transgenic mice expressing a TCR specific for LCMV-GP33-41 were i.v. challenged with VLP (400 μg) expressing H-2Db::GPI along with murine β2m and LCMV-GP33-41 in the form of a minigene (16). Such particles were also decorated with mIL-2::GPI. Amounts of mIL-2::GPI on VLP preparations were determined by immunoblotting and revealed that 2 μg of VLP expresses approximately 20 ng of mIL-2::GPI (Fig. 6C). Controls consisted of antigen-specific VLP (400 μg) lacking mIL-2::GPI, which were supplemented with 2 × 104 units soluble mIL-2 or with PBS alone. Additional controls consisted of PBS or PBS supplemented with 2 × 104 units soluble mIL-2. T-cell activation in the spleen was assessed 18 h later by analyzing the expression of the surface activation marker CD69 on antigen-specific TRVA2+/CD8+ cells. The challenge with antigen-specific VLP decorated with mIL-2::GPI elicited clear-cut CD69 expression on the majority of antigen-specific TRVA2+/CD8+ cells (Fig. 6A) but not on TRVA2− bystander cells (not shown). CD69 expression upon challenge with antigen-specific VLP expressing signal 1 alone (VLP only) or of such particles supplemented with soluble mIL-2 (2 × 104 units), PBS plus soluble mIL-2, or PBS alone was negligible, indicating that colocalized secondary signals, e.g., those provided by membrane-bound cytokines, are clearly required to induce sustained T-cell activation with our antigen-specific VLP. Significantly, the majority of the spleen-resident, transgenic LCMV-GP33-41-specific CD8+ T cells were activated within 18 h after challenge (78.0% ± 10.4% of TRVA2+/CD8+ cells were expressing CD69) (Fig. 6B). Figure 6C shows by immunoblotting for p30 Gag that similar amounts of antigen-specific VLP were produced irrespective of whether or not mIL-2::GPI was coexpressed.
DISCUSSION
Cytokines are secreted or membrane-bound proteins that regulate the growth, differentiation, and activation of immune cells (45). Although most cytokines are expressed in a secreted form, many of them exert their functions in a locally restricted manner. However, when they are administered systemically, adverse effects are frequently apparent (2, 18, 37, 71). To control the pleiotropic effects of therapeutically delivered cytokines, it is desirable to target them to the preferred site of action, e.g., by attaching them to the plasma membrane of target cells (12, 17, 69, 80). Once this is achieved, cytokines that are otherwise not well tolerated can be applied even at high concentrations (11).
Here, we have demonstrated a procedure for targeting functionally active cytokines to the membrane surface of enveloped viruses or VLP without the need to modify viral proteins themselves. In this approach, human cytokines are targeted to lipid rafts of producer cells with the help of posttranslationally attached GPI anchors (5, 46, 73). Since many enveloped viruses bud from the lipid rafts of infected cells and since distinct lipid-modified molecules (i.e., those with covalently linked saturated acyl chains) have the inherent tendency to accumulate in lipid rafts (39, 49, 56, 63), whole viruses or VLP can be conveniently equipped with molecules of choice through the genetic introduction of such lipid acceptor sites. In contrast to previous attempts in which cytokines have been fused to Env molecules genetically (40, 41, 75), we have tried to lay the ground for a more universal strategy that would be suitable for the large and phylogenetically diverse collection of enveloped viruses independent of genus-specific (envelope) proteins.
Basic research might benefit from the findings made in this paper since cytokines can be tested from now on in a relevant membrane context together with selected immune receptors, all on a reductionist platform brought about by lipid-raft-enriched viral membrane envelopes. Such platforms may open novel opportunities for the maintenance, differentiation, and activation of progenitor or immune effector cells. We have shown recently that VLP equipped with TCR/CD3 complex ligands and costimulatory molecules, so-called immunosomes, induce massive, accessory-cell-independent T-cell proliferation, which can be enhanced by coexpressed adhesion molecules (16). Such particles are stable enough to become purified by ultracentrifugation on sucrose gradients and stored for prolonged periods of time at −80°C. The introduction of membrane-bound cytokines as signal 3 (14, 15) into such immunostimulatory VLP shall help to further shape lymphocyte responses. As a proof of principle, this concept was tested by us with a small collection of well-defined T-lymphocyte growth factors (1, 19, 20, 67, 70, 77, 81) in cellular activation assays. In fact, our experiments revealed that VLP-bound hIL-2::GPI costimulates T-lymphocyte activation considerably. Other cytokines, such as hIL-7::GPI and hIL-15::GPI, demonstrated a similar although somewhat weaker tendency to costimulate T cells (not shown). Moreover, cellular proliferation induced by TCR/CD3 triggering plus CD54 (ICAM-1) and/or CD80 (B7.1) was further augmented by membrane-bound hIL-2::GPI. Of significance, dose-response relationship analyses revealed that the specific functional activity of VLP-bound hIL-2::GPI is similar to that of soluble hIL-2. Parallel biochemical experiments revealed that lipid raft targeting as well as enrichment of hIL-2 on VLP were observed only when the membrane attachment of hIL-2 was brought about by a bona fide GPI anchor but not when hIL-2 was linked to transmembrane/intracellular regions of type I membrane proteins such as CD80 or CD99, although expression levels of molecules on producer cells were similar. Furthermore, immunoelectron microscopy allowed the direct assessment of targeting of hIL-2::GPI to VLP. The decoration of VLP with hIL-2::GPI was observed only when VLP were derived from producer cells that had been cotransfected with hIL-2::GPI but not with control vector.
In order to clarify whether two cytokines would also synergize with each other when coexpressed on VLP, we studied membrane-bound hIL-4::GPI and hGM-CSF::GPI on VLP and contrasted the behavior of these structures with that of their soluble counterparts. IL-4 was initially identified as a B-cell growth factor (28) but is also one of the principal T-helper-2-inducing cytokines (58, 59) and has a major impact on antibody production and class switching (4). GM-CSF belongs to the family of myeloid colony-stimulating factors and has been shown to induce a wide range of effector functions in neutrophils and monocytes (45). The combination of IL-4 with GM-CSF has revolutionized the field of DC biology, since it opened the opportunity to generate DC from highly purified peripheral blood CD14+ monocytes (55, 83) and from CD34+ progenitor cells (60). Here, we have shown that hIL-4 and hGM-CSF, when bound to the membrane of VLP, have a similar potential to generate DC compared to their soluble counterparts. This indicates that the colocalization of more than one cytokine on the VLP surface does not necessarily compromise their individual function.
GPI-anchored cytokines may allow us to choose among the immune effector mechanisms to be enhanced in the future, e.g., during vaccination, in order to foster protective immune responses and at the same time to minimize unfavorable ones. With this approach, the antigenic stimulus and the immunomodulatory influence of the “adjuvant” are colocalized and hence should maximally synergize. To directly test the immunomodulatory capacity of membrane-bound cytokines in vivo, we i.v. challenged P14 TCR transgenic mice with antigen-specific VLP expressing LCMV-GP33-41 in the context of H-2Db::GPI in the presence or absence of mIL-2::GPI. Antigen-specific VLP lacking mIL-2::GPI but supplemented with a comparable concentration of soluble mIL-2 (2 × 104 units) or with PBS alone or PBS supplemented with soluble mIL-2 were used as controls. In fact, clear-cut activation of TRVA2+/CD8+ T lymphocytes, as measured by CD69 expression after 18 h, was observed only in splenocytes of mice that had been challenged with antigen-specific VLP coexpressing mIL-2::GPI. In contrast, only negligible CD69 expression on a small fraction of TRVA2+/CD8+ lymphocytes was observed in splenocytes of mice that had been challenged with antigen-specific VLP supplemented with soluble mIL-2. The in vivo results show two important findings: (i) VLP-bound cytokines are also functionally active in vivo, and (ii) colocalization of the cytokine with the antigen of interest enhances antigen-specific immune responses. Future experiments using a collection of whole viral envelope proteins as model antigens will have to characterize the potential of VLP-bound cytokines as adjuvants in further detail.
In addition, GPI-anchored immunomodulators might also serve as novel components for the improvement of viral gene transfer (10, 44, 47, 72). In the past, soluble growth factors have been shown to increase gene transfer rates of retroviruses for hematopoietic stem cells (33). Furthermore, antibody fragments and cytokines fused to N-terminal sequences of viral envelope proteins were demonstrated to improve the targeting efficiency of retroviruses/lentiviruses (41, 68, 75). Those strategies required the direct manipulation of the respective viral envelope proteins in order to target viruses to their host cells. In contrast, immunomodulators bound to the viral lipid bilayer envelope via GPI anchors could theoretically be introduced into any enveloped virus particle (40, 41, 75) without the need to directly manipulate viral sequences. In fact, the coexpression of a GPI-anchored anti-CD3 single-chain variable fragment (scFv) on MoMLV- or human immunodeficiency virus-based viruses was found to significantly increase their transduction efficiency (V. M. Leb et al., unpublished data). Consequently, cytokines fused to GPI anchors might also be combinable with envelope proteins, including those not native to the viral strain used for transduction. This might be especially attractive for gene delivery into cell types, such as primary T lymphocytes, which ideally should not become activated by the transfection procedure (74).
Acknowledgments
We are indebted to Eva Schmitt for animal care and for expert technical assistance with in vivo experiments. We thank Peter Steinberger for critically reading the manuscript and Otto Majdic for providing hIL-2-specific mAb IL-2.1E7.
This work was supported by grants SFB F1816-B13 and P-15634 of the Austrian Science foundation (to W.F.P., H.-J.K., V.M.L., K.G.S., and A.N.), by the Österreichsche Forschungsförderungsgesellschaft (grant 812079), by Biomay, Austria (D.H.), and by a grant from the Austrian Academy of Sciences (CeMM 20030 to S.V.D.). C.S. was supported by grant P-16412 from the Austrian Science Foundation. B.S. was supported by grants from the U.S. National Institutes of Health.
Footnotes
Published ahead of print on 30 May 2007.
REFERENCES
- 1.Armitage, R. J., A. E. Namen, H. M. Sassenfeld, and K. H. Grabstein. 1990. Regulation of human T cell proliferation by IL-7. J. Immunol. 144:938-941. [PubMed] [Google Scholar]
- 2.Atkins, M. B., M. J. Robertson, M. Gordon, M. T. Lotze, M. DeCoste, J. S. DuBois, J. Ritz, A. B. Sandler, H. D. Edington, P. D. Garzone, J. W. Mier, C. M. Canning, L. Battiato, H. Tahara, and M. L. Sherman. 1997. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin. Cancer Res. 3:409-417. [PubMed] [Google Scholar]
- 3.Audibert, F. M., and L. D. Lise. 1993. Adjuvants: current status, clinical perspectives and future prospects. Immunol. Today 14:281-284. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., F. Briere, Y. J. Liu, and F. Rousset. 1994. Molecular control of B lymphocyte growth and differentiation. Stem Cells 12:278-288. [DOI] [PubMed] [Google Scholar]
- 5.Berger, J., A. D. Howard, L. Brink, L. Gerber, J. Hauber, B. R. Cullen, and S. Udenfriend. 1988. COOH-terminal requirements for the correct processing of a phosphatidylinositol-glycan anchored membrane protein. J. Biol. Chem. 263:10016-10021. [PubMed] [Google Scholar]
- 6.Berzofsky, J. A. 2001. Design of engineered vaccines for systemic and mucosal immunity to HIV. Pathol. Biol. (Paris) 49:466-467. [DOI] [PubMed] [Google Scholar]
- 7.Berzofsky, J. A., J. D. Ahlers, and I. M. Belyakov. 2001. Strategies for designing and optimizing new generation vaccines. Nat. Rev. Immunol. 1:209-219. [DOI] [PubMed] [Google Scholar]
- 8.Bird, R. E., K. D. Hardman, J. W. Jacobson, S. Johnson, B. M. Kaufman, S. M. Lee, T. Lee, S. H. Pope, G. S. Riordan, and M. Whitlow. 1988. Single-chain antigen-binding proteins. Science 242:423-426. [DOI] [PubMed] [Google Scholar]
- 9.Bonham, L., T. Palmer, and A. D. Miller. 1996. Prolonged expression of therapeutic levels of human granulocyte colony-stimulating factor in rats following gene transfer to skeletal muscle. Hum. Gene Ther. 7:1423-1429. [DOI] [PubMed] [Google Scholar]
- 10.Buchschacher, G. L., Jr., and F. Wong-Staal. 2000. Development of lentiviral vectors for gene therapy for human diseases. Blood 95:2499-2504. [PubMed] [Google Scholar]
- 11.Cavallo, F., P. Signorelli, M. Giovarelli, P. Musiani, A. Modesti, M. J. Brunda, M. P. Colombo, and G. Forni. 1997. Antitumor efficacy of adenocarcinoma cells engineered to produce interleukin 12 (IL-12) or other cytokines compared with exogenous IL-12. J. Natl. Cancer Inst. 89:1049-1058. [DOI] [PubMed] [Google Scholar]
- 12.Chakrabarti, R., Y. Chang, K. Song, and G. J. Prud'homme. 2004. Plasmids encoding membrane-bound IL-4 or IL-12 strongly costimulate DNA vaccination against carcinoembryonic antigen (CEA). Vaccine 22:1199-1205. [DOI] [PubMed] [Google Scholar]
- 13.Choppin, P. W., and R. W. Compans. 1970. Phenotypic mixing of envelope proteins of the parainfluenza virus SV5 and vesicular stomatitis virus. J. Virol. 5:609-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curtsinger, J. M., C. M. Johnson, and M. F. Mescher. 2003. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 171:5165-5171. [DOI] [PubMed] [Google Scholar]
- 15.Curtsinger, J. M., C. S. Schmidt, A. Mondino, D. C. Lins, R. M. Kedl, M. K. Jenkins, and M. F. Mescher. 1999. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J. Immunol. 162:3256-3262. [PubMed] [Google Scholar]
- 16.Derdak, S. V., H. J. Kueng, V. M. Leb, A. Neunkirchner, K. G. Schmetterer, E. Bielek, O. Majdic, W. Knapp, B. Seed, and W. F. Pickl. 2006. Direct stimulation of T lymphocytes by immunosomes: virus-like particles decorated with T cell receptor/CD3 ligands plus costimulatory molecules. Proc. Natl. Acad. Sci. USA 103:13144-13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dranoff, G. 2004. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 4:11-22. [DOI] [PubMed] [Google Scholar]
- 18.Dranoff, G., E. Jaffee, A. Lazenby, P. Golumbek, H. Levitsky, K. Brose, V. Jackson, H. Hamada, D. Pardoll, and R. C. Mulligan. 1993. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl. Acad. Sci. USA 90:3539-3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrar, J. J., W. R. Benjamin, M. L. Hilfiker, M. Howard, W. L. Farrar, and J. Fuller-Farrar. 1982. The biochemistry, biology, and role of interleukin 2 in the induction of cytotoxic T cell and antibody-forming B cell responses. Immunol. Rev. 63:129-166. [DOI] [PubMed] [Google Scholar]
- 20.Grabstein, K. H., J. Eisenman, K. Shanebeck, C. Rauch, S. Srinivasan, V. Fung, C. Beers, J. Richardson, M. A. Schoenborn, M. Ahdieh, et al. 1994. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 264:965-968. [DOI] [PubMed] [Google Scholar]
- 21.Gupta, R. K., and G. R. Siber. 1995. Adjuvants for human vaccines—current status, problems and future prospects. Vaccine 13:1263-1276. [DOI] [PubMed] [Google Scholar]
- 22.Hadden, J. W. 1994. T-cell adjuvants. Int. J. Immunopharmacol. 16:703-710. [DOI] [PubMed] [Google Scholar]
- 23.Hancock, G. E., J. D. Smith, and K. M. Heers. 2000. The immunogenicity of subunit vaccines for respiratory syncytial virus after co-formulation with aluminum hydroxide adjuvant and recombinant interleukin-12. Viral Immunol. 13:57-72. [DOI] [PubMed] [Google Scholar]
- 24.Hanlon, L., D. Argyle, D. Bain, L. Nicolson, S. Dunham, M. C. Golder, M. McDonald, C. McGillivray, O. Jarrett, J. C. Neil, and D. E. Onions. 2001. Feline leukemia virus DNA vaccine efficacy is enhanced by coadministration with interleukin-12 (IL-12) and IL-18 expression vectors. J. Virol. 75:8424-8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heath, A. W., and J. H. Playfair. 1993. Cytokine-antigen vaccines. Nature 364:493. [DOI] [PubMed] [Google Scholar]
- 26.Heath, A. W., and J. H. Playfair. 1992. Cytokines as immunological adjuvants. Vaccine 10:427-434. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman, S. J., F. P. Polack, D. A. Hauer, M. Singh, M. A. Billeter, R. J. Adams, and D. E. Griffin. 2003. Vaccination of rhesus macaques with a recombinant measles virus expressing interleukin-12 alters humoral and cellular immune responses. J. Infect. Dis. 188:1553-1561. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 28.Howard, M., J. Farrar, M. Hilfiker, B. Johnson, K. Takatsu, T. Hamaoka, and W. E. Paul. 1982. Identification of a T cell-derived B cell growth factor distinct from interleukin 2. J. Exp. Med. 155:914-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang, A. S., P. Besmer, L. Chu, and D. Baltimore. 1973. Growth of pseudotypes of vesicular stomatitis virus with N-tropic murine leukemia virus coats in cells resistant to N-tropic viruses. J. Virol. 12:659-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang, A. S., E. L. Palma, N. Hewlett, and B. Roizman. 1974. Pseudotype formation between enveloped RNA and DNA viruses. Nature 252:743-745. [DOI] [PubMed] [Google Scholar]
- 31.Huston, J. S., D. Levinson, M. Mudgett-Hunter, M. S. Tai, J. Novotny, M. N. Margolies, R. J. Ridge, R. E. Bruccoleri, E. Haber, and R. Crea. 1988. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc. Natl. Acad. Sci. USA 85:5879-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan, M., A. Schallhorn, and F. M. Wurm. 1996. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 24:596-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiem, H. P., R. G. Andrews, J. Morris, L. Peterson, S. Heyward, J. M. Allen, J. E. Rasko, J. Potter, and A. D. Miller. 1998. Improved gene transfer into baboon marrow repopulating cells using recombinant human fibronectin fragment CH-296 in combination with interleukin-6, stem cell factor, FLT-3 ligand, and megakaryocyte growth and development factor. Blood 92:1878-1886. [PubMed] [Google Scholar]
- 34.Koeberl, D. D., L. Bonham, C. L. Halbert, J. M. Allen, T. Birkebak, and A. D. Miller. 1999. Persistent, therapeutically relevant levels of human granulocyte colony-stimulating factor in mice after systemic delivery of adeno-associated virus vectors. Hum. Gene Ther. 10:2133-2140. [DOI] [PubMed] [Google Scholar]
- 35.Kuwata, T., T. Miura, T. Haga, I. Kozyrev, and M. Hayami. 2000. Construction of chimeric simian and human immunodeficiency viruses that produce interleukin 12. AIDS Res. Hum. Retrovir. 16:465-470. [DOI] [PubMed] [Google Scholar]
- 36.Leutenegger, C. M., F. S. Boretti, C. N. Mislin, J. N. Flynn, M. Schroff, A. Habel, C. Junghans, S. A. Koenig-Merediz, B. Sigrist, A. Aubert, N. C. Pedersen, B. Wittig, and H. Lutz. 2000. Immunization of cats against feline immunodeficiency virus (FIV) infection by using minimalistic immunogenic defined gene expression vector vaccines expressing FIV gp140 alone or with feline interleukin-12 (IL-12), IL-16, or a CpG motif. J. Virol. 74:10447-10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lollini, P. L., A. D'Errico, C. De Giovanni, L. Landuzzi, F. Frabetti, G. Nicoletti, F. Cavallo, M. Giovarelli, W. F. Grigioni, and P. Nanni. 1995. Systemic effects of cytokines released by gene-transduced tumor cells: marked hyperplasia induced in small bowel by gamma-interferon transfectants through host lymphocytes. Int. J. Cancer 61:425-430. [DOI] [PubMed] [Google Scholar]
- 38.Mach, N., and G. Dranoff. 2000. Cytokine-secreting tumor cell vaccines. Curr. Opin. Immunol. 12:571-575. [DOI] [PubMed] [Google Scholar]
- 39.Manie, S. N., S. Debreyne, S. Vincent, and D. Gerlier. 2000. Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly. J. Virol. 74:305-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maurice, M., S. Mazur, F. J. Bullough, A. Salvetti, M. K. Collins, S. J. Russell, and F. L. Cosset. 1999. Efficient gene delivery to quiescent interleukin-2 (IL-2)-dependent cells by murine leukemia virus-derived vectors harboring IL-2 chimeric envelope glycoproteins. Blood 94:401-410. [PubMed] [Google Scholar]
- 41.Maurice, M., E. Verhoeyen, P. Salmon, D. Trono, S. J. Russell, and F. L. Cosset. 2002. Efficient gene transfer into human primary blood lymphocytes by surface-engineered lentiviral vectors that display a T cell-activating polypeptide. Blood 99:2342-2350. [DOI] [PubMed] [Google Scholar]
- 42.Meuer, S. C., H. Dumann, K. H. Meyer zum Buschenfelde, and H. Kohler. 1989. Low-dose interleukin-2 induces systemic immune responses against HBsAg in immunodeficient non-responders to hepatitis B vaccination. Lancet i:15-18. [DOI] [PubMed] [Google Scholar]
- 43.Meuer, S. C., M. Hauer, P. Kurz, K. H. Meyer zum Buschenfelde, and H. Kohler. 1987. Selective blockade of the antigen-receptor-mediated pathway of T cell activation in patients with impaired primary immune responses. J. Clin. Investig. 80:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller, A. D. 1996. Cell-surface receptors for retroviruses and implications for gene transfer. Proc. Natl. Acad. Sci. USA 93:11407-11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mire-Sluis, A. R., and R. Thorpe. 1998. Cytokines. Academic Press, San Diego, CA.
- 46.Moran, P., and I. W. Caras. 1991. Fusion of sequence elements from non-anchored proteins to generate a fully functional signal for glycophosphatidylinositol membrane anchor attachment. J. Cell Biol. 115:1595-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulligan, R. C. 1993. The basic science of gene therapy. Science 260:926-932. [DOI] [PubMed] [Google Scholar]
- 48.Naeve, C. W., and D. Williams. 1990. Fatty acids on the A/Japan/305/57 influenza virus hemagglutinin have a role in membrane fusion. EMBO J. 9:3857-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nunberg, J. H., M. V. Doyle, S. M. York, and C. J. York. 1989. Interleukin 2 acts as an adjuvant to increase the potency of inactivated rabies virus vaccine. Proc. Natl. Acad. Sci. USA 86:4240-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh, Y. K., T. Sohn, J. S. Park, M. J. Kang, H. G. Choi, J. A. Kim, W. K. Kim, J. J. Ko, and C. K. Kim. 2004. Enhanced mucosal and systemic immunogenicity of human papillomavirus-like particles encapsidating interleukin-2 gene adjuvant. Virology 328:266-273. [DOI] [PubMed] [Google Scholar]
- 52.O'Hagan, D. T. 2001. Recent developments in vaccine delivery systems. Curr. Drug Targets Infect. Disord. 1:273-286. [DOI] [PubMed] [Google Scholar]
- 53.Ory, D. S., B. A. Neugeboren, and R. C. Mulligan. 1996. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc. Natl. Acad. Sci. USA 93:11400-11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pardoll, D. M. 1995. Paracrine cytokine adjuvants in cancer immunotherapy. Annu. Rev. Immunol. 13:399-415. [DOI] [PubMed] [Google Scholar]
- 55.Pickl, W. F., O. Majdic, P. Kohl, J. Stockl, E. Riedl, C. Scheinecker, C. Bello-Fernandez, and W. Knapp. 1996. Molecular and functional characteristics of dendritic cells generated from highly purified CD14+ peripheral blood monocytes. J. Immunol. 157:3850-3859. [PubMed] [Google Scholar]
- 56.Pickl, W. F., F. X. Pimentel-Muinos, and B. Seed. 2001. Lipid rafts and pseudotyping. J. Virol. 75:7175-7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pircher, H., K. Burki, R. Lang, H. Hengartner, and R. M. Zinkernagel. 1989. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature 342:559-561. [DOI] [PubMed] [Google Scholar]
- 58.Powrie, F., and R. L. Coffman. 1993. Cytokine regulation of T-cell function: potential for therapeutic intervention. Immunol. Today 14:270-274. [DOI] [PubMed] [Google Scholar]
- 59.Romagnani, S. 1992. Induction of TH1 and TH2 responses: a key role for the ‘natural’ immune response? Immunol. Today 13:379-381. [DOI] [PubMed] [Google Scholar]
- 60.Romani, N., D. Reider, M. Heuer, S. Ebner, E. Kampgen, B. Eibl, D. Niederwieser, and G. Schuler. 1996. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J. Immunol. Methods 196:137-151. [DOI] [PubMed] [Google Scholar]
- 61.Rose, J. K., G. A. Adams, and C. J. Gallione. 1984. The presence of cysteine in the cytoplasmic domain of the vesicular stomatitis virus glycoprotein is required for palmitate addition. Proc. Natl. Acad. Sci. USA 81:2050-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanyal, A., and F. G. Schuening. 1999. Increased gene transfer into human cord blood cells by centrifugation-enhanced transduction in fibronectin fragment-coated tubes. Hum. Gene Ther. 10:2859-2868. [DOI] [PubMed] [Google Scholar]
- 63.Scheiffele, P., A. Rietveld, T. Wilk, and K. Simons. 1999. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 274:2038-2044. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt, M. F., and B. Lambrecht. 1985. On the structure of the acyl linkage and the function of fatty acyl chains in the influenza virus haemagglutinin and the glycoproteins of Semliki Forest virus. J. Gen. Virol. 66:2635-2647. [DOI] [PubMed] [Google Scholar]
- 65.Simmons, D., and B. Seed. 1988. The Fc gamma receptor of natural killer cells is a phospholipid-linked membrane protein. Nature 333:568-570. [DOI] [PubMed] [Google Scholar]
- 66.Singh, M., and I. Srivastava. 2003. Advances in vaccine adjuvants for infectious diseases. Curr. HIV Res. 1:309-320. [DOI] [PubMed] [Google Scholar]
- 67.Smith, K. A. 1980. T-cell growth factor. Immunol. Rev. 51:337-357. [DOI] [PubMed] [Google Scholar]
- 68.Somia, N. V., M. Zoppe, and I. M. Verma. 1995. Generation of targeted retroviral vectors by using single-chain variable fragment: an approach to in vivo gene delivery. Proc. Natl. Acad. Sci. USA 92:7570-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soo Hoo, W., K. A. Lundeen, J. R. Kohrumel, N. L. Pham, S. W. Brostoff, R. M. Bartholomew, and D. J. Carlo. 1999. Tumor cell surface expression of granulocyte-macrophage colony-stimulating factor elicits antitumor immunity and protects from tumor challenge in the P815 mouse mastocytoma tumor model. J. Immunol. 162:7343-7349. [PubMed] [Google Scholar]
- 70.Spits, H., H. Yssel, Y. Takebe, N. Arai, T. Yokota, F. Lee, K. Arai, J. Banchereau, and J. E. de Vries. 1987. Recombinant interleukin 4 promotes the growth of human T cells. J. Immunol. 139:1142-1147. [PubMed] [Google Scholar]
- 71.Tjuvajev, J., B. Gansbacher, R. Desai, B. Beattie, M. Kaplitt, C. Matei, J. Koutcher, E. Gilboa, and R. Blasberg. 1995. RG-2 glioma growth attenuation and severe brain edema caused by local production of interleukin-2 and interferon-gamma. Cancer Res. 55:1902-1910. [PubMed] [Google Scholar]
- 72.Trono, D. 2000. Lentiviral vectors: turning a deadly foe into a therapeutic agent. Gene Ther. 7:20-23. [DOI] [PubMed] [Google Scholar]
- 73.Udenfriend, S., and K. Kodukula. 1995. How glycosylphosphatidylinositol-anchored membrane proteins are made. Annu. Rev. Biochem. 64:563-591. [DOI] [PubMed] [Google Scholar]
- 74.Unutmaz, D., V. N. KewalRamani, S. Marmon, and D. R. Littman. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 189:1735-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verhoeyen, E., V. Dardalhon, O. Ducrey-Rundquist, D. Trono, N. Taylor, and F. L. Cosset. 2003. IL-7 surface-engineered lentiviral vectors promote survival and efficient gene transfer in resting primary T lymphocytes. Blood 101:2167-2174. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 76.Weinberg, A., and T. C. Merigan. 1988. Recombinant interleukin 2 as an adjuvant for vaccine-induced protection. Immunization of guinea pigs with herpes simplex virus subunit vaccines. J. Immunol. 140:294-299. [PubMed] [Google Scholar]
- 77.Welch, P. A., A. E. Namen, R. G. Goodwin, R. Armitage, and M. D. Cooper. 1989. Human IL-7: a novel T cell growth factor. J. Immunol. 143:3562-3567. [PubMed] [Google Scholar]
- 78.Yang, C., and R. W. Compans. 1996. Palmitoylation of the murine leukemia virus envelope glycoprotein transmembrane subunits. Virology 221:87-97. [DOI] [PubMed] [Google Scholar]
- 79.Yang, C., C. P. Spies, and R. W. Compans. 1995. The human and simian immunodeficiency virus envelope glycoprotein transmembrane subunits are palmitoylated. Proc. Natl. Acad. Sci. USA 92:9871-9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yei, S., R. M. Bartholomew, P. Pezzoli, A. Gutierrez, E. Gouveia, D. Bassett, W. Soo Hoo, and D. J. Carlo. 2002. Novel membrane-bound GM-CSF vaccines for the treatment of cancer: generation and evaluation of mbGM-CSF mouse B16F10 melanoma cell vaccine. Gene Ther. 9:1302-1311. [DOI] [PubMed] [Google Scholar]
- 81.Yokota, T., T. Otsuka, T. Mosmann, J. Banchereau, T. DeFrance, D. Blanchard, J. E. De Vries, F. Lee, and K. Arai. 1986. Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities. Proc. Natl. Acad. Sci. USA 83:5894-5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zavada, J. 1972. Pseudotypes of vesicular stomatitis virus with the coat of murine leukaemia and of avian myeloblastosis viruses. J. Gen. Virol. 15:183-191. [DOI] [PubMed] [Google Scholar]
- 83.Zhou, L. J., and T. F. Tedder. 1996. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc. Natl. Acad. Sci. USA 93:2588-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]