Abstract
In contrast to most negative-stranded RNA viruses, hantaviruses and other viruses in the family Bunyaviridae mature intracellularly, deriving the virion envelope from the endoplasmic reticulum (ER) or Golgi compartment. While it is generally accepted that Old World hantaviruses assemble and bud into the Golgi compartment, some studies with New World hantaviruses have raised the possibility of maturation at the plasma membrane as well. Overall, the steps leading to virion assembly remain largely undetermined for hantaviruses. Because hantaviruses do not have matrix proteins, the nucleocapsid protein (N) has been proposed to play a key role in assembly. Herein, we examine the intracellular trafficking and morphogenesis of the prototype Old World hantavirus, Hantaan virus (HTNV). Using confocal microscopy, we show that N colocalized with the ER-Golgi intermediate compartment (ERGIC) in HTNV-infected Vero E6 cells, not with the ER, Golgi compartment, or early endosomes. Brefeldin A, which effectively disperses the ER, the ERGIC, and Golgi membranes, redistributed N with the ERGIC, implicating membrane association; however, subcellular fractionation experiments showed the majority of N in particulate fractions. Confocal microscopy revealed that N was juxtaposed to and distributed along microtubules and, over time, became surrounded by vimentin cages. To probe cytoskeletal association further, we probed trafficking of N in cells treated with nocodazole and cytochalasin D, which depolymerize microtubules and actin, respectively. We show that nocodazole, but not cytochalasin D, affected the distribution of N and reduced levels of intracellular viral RNA. These results suggested the involvement of microtubules in trafficking of N, whose movement could occur via molecular motors such as dynein. Overexpression of dynamitin, which is associated with dynein-mediated transport, creates a dominant-negative phenotype blocking transport on microtubules. Overexpression of dynamitin reduced N accumulation in the perinuclear region, which further supports microtubule components in N trafficking. The combined results of these experiments support targeting of N to the ERGIC prior to its movement to the Golgi compartment and the requirement of an intact ERGIC for viral replication and, thus, the possibility of virus factories in this region.
Hantaviruses are present throughout the world, yet hantaviral illnesses in humans occur predominantly in geographically localized, mostly sporadic and unpredictable outbreaks (65). Presumably, this reflects the ecology of rodents in which hantaviruses maintain a persistent infection without illness. In Europe and Asia, the Old World hantaviruses cause hemorrhagic fever with renal syndrome, with 1 to 15% mortality. Hantaan virus (HTNV), a prototype Old World hantavirus, is the major etiological agent for hemorrhagic fever with renal syndrome, with as many as 50,000 to 100,000 cases per year (26, 41). In the Americas, New World hantaviruses cause hantavirus pulmonary syndrome, with up to 40% mortality (52). Unfortunately, there are no FDA-approved therapeutics available for treatment of either disease, hence, care is supportive. Basic mechanistic questions regarding components of the life cycle of hantaviruses such as trafficking, replication, and assembly remain largely unanswered.
Hantaviral particles contain a tripartite, single-stranded RNA genome (viral RNA [vRNA]) of negative polarity (64, 66). The S, M, and L segments encode the nucleocapsid protein (N), glycoproteins (Gn and Gc), and L protein (an RNA-dependent RNA polymerase), respectively. Studies of the infection of tracheal endothelial cells with Andes virus suggest that hantaviruses can enter and replicate in the respiratory epithelium following inhalation (59). Entry of most hantaviruses into host epithelial cells begins with the interaction of Gn with β-1 and β-3 integrins (19, 20), which is followed by receptor-mediated endocytosis through clathrin-coated pits (25). Jin et al. suggested that HTNV particles remain in the endosomal compartments until moving to late endosomes or lysosomes (25). Numerous studies have shown that the glycoprotein is cotranslationally processed into Gn and Gc, which traffic together from the endoplasmic reticulum (ER) to the Golgi compartment; virions form by budding into the Golgi compartment (64, 66, 75). One unanswered question is whether the N and L proteins, after translation in the cytoplasm, target to the Golgi compartment directly to mediate replication, transcription, and assembly. Alternatively, replication and transcription could occur at a different site within the cell. Difficulty in working with the large 240-kDa L protein has hampered experimental progress. However, we and others have made some progress in characterizing N. The hantaviral N is the most abundant protein in the virion and in virus-infected cells (66). This multifunctional protein presumably interacts with other hantaviral proteins, and possibly with host cell components, to mediate virus replication and assembly. There have been, however, relatively few studies that demonstrate its functions or show at what site(s) within the cell it performs its functions. At present, we know that N interacts with viral RNA (68, 82), itself (1, 2, 30, 31, 33, 47, 48), and perhaps the L protein, Gn (8, 17, 75), and cellular factors (32, 34, 43). One study has shown it to be required for replication and/or transcription (17). Although the mechanistic details concerning the switch from primary transcription to replication are currently lacking, the concentration of N may drive this switch (28). Clearly, these interactions and functions require trafficking of N within the cell, and as with other viruses, it is possible that the replication and assembly occur at discrete sites within the cell.
The studies reported herein were designed to address how N traffics in the cell prior to viral assembly. Studies of N have been limited to localization in both Old World (25, 29, 31, 34, 54) and New World viruses (54, 59, 76), which have primarily shown trafficking of N to the perinuclear region. In cells transiently expressing N from Black Creek Canal virus (BCCV), N colocalizes with the cis-medial Golgi marker α-mannosidase II (Mann II) (54). In contrast, studies performed with Seoul virus show no colocalization between N and the Golgi marker, although N accumulated in the perinuclear area (29). A signal in the C-terminal region of the Tula virus and BCCV N promotes perinuclear targeting (31, 54). Herein, we demonstrate that HTNV N colocalized with the ER-Golgi intermediate compartment (ERGIC) and microtubules but not with the ER, Golgi compartment, early endosomal, or actin markers. We show that the movement of N depended on the microtubule network and, further, that disruption of this network reduced levels of vRNA. Our analyses suggest that N traffics to the ERGIC prior to its movement to the Golgi compartment, that an intact ERGIC is essential for viral replication, and that there might be virus factories in the ERGIC region.
MATERIALS AND METHODS
Cell culture, antibodies, and inhibitors.
Vero E6 cells (ATCC CRL 1586) were maintained in complete DMEM (Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 1% l-glutamine). Primary antibodies included mouse monoclonal antibodies (MAbs) against β-tubulin (Upstate), α-tubulin, vimentin, and filamentous actin (F-actin; Sigma); ERGIC-53 (Alexis); protein disulfide isomerase (PDI; Abcam); early endosomal antigen 1 (EEA1; BD Biosciences); a rabbit polyclonal antibody against Mann II (Abcam); phalloidin conjugated to tetramethylrhodamine (TRITC; Sigma); and wheat germ agglutinin (WGA) conjugated to Alexa 594 (Molecular Probes). Secondary antibodies used were goat anti-mouse antibody conjugated to Alexa 488 or 594 and goat anti-rabbit antibody conjugated to Alexa 488 or 594 (Molecular Probes) and goat anti-mouse antibody and goat anti-rabbit antibody conjugated to horseradish peroxidase from KPL. We purchased brefeldin A (BFA), nocodazole (NOC), and cytochalasin D (CytD) from Sigma, and we purchased ribavirin (RBV) from ICN Pharmaceuticals.
Production of HTNV N-specific antibodies in rabbits and mice.
N was expressed with the T7 polymerase expression system and purified as hexahistidine-tagged fusion proteins from the soluble fraction, as previously described (27). Rabbit polyclonal antibody (no. 143) was raised to the HTNV N antigen in New Zealand White rabbits by Southern Biotechnology Associates (Birmingham, AL). A murine MAb (E-314) was raised to the same HTNV N antigen by Cell Essentials, Inc. (Boston, MA). Five mice were immunized per antigen, and enzyme-linked immunosorbent assay (ELISA)-positive animals with the best antibody response to the antigen were euthanized. Spleens were removed for collection of cells and subsequent fusion by standard methods. Cell culture supernatants of fusion clones were screened by ELISA for the presence of virus-specific antibodies. For those clones that showed a positive signal by ELISA, we expanded the supernatant to produce 50 ml of antibody-rich supernatant, from which we purified this MAb. Cells were frozen in liquid nitrogen to safeguard the stability of the cell line. Hybridoma cells were cultured in HyClone medium (HYQSFM4Mab) supplemented with 10% fetal clone III serum and 0.01% penicillin-streptomycin. Hybridoma clones were grown to 1 × 106/ml for each T-150 sterile flask and incubated at 37°C, 5% CO2 until >90% confluence. At >90% confluence, cells were stressed in the same medium until 30 to 40% were dead (3 to 5 days). Cells were checked for the percentage dead with trypan blue. Supernatant from stressed hybridoma clones was removed to a sterile conical tube and centrifuged at 150 × g for 5 min at room temperature. The supernatant containing the MAb was recovered from the cell pellet and purified with a MabTrap kit (Amersham Biosciences) by following the manufacturer's protocol.
Virus strain, RNA isolation, and construction of recombinant plasmids.
HTNV (strain 76-118) was used for all experiments. The open reading frame encoding N was amplified by reverse transcription-PCR (RT-PCR) from total RNA extracted from HTNV-infected cells with TRIzol (Invitrogen). RT-PCRs used SuperScript III reverse transcriptase (Invitrogen), Pfu DNA polymerase (Stratagene), a gene-specific forward primer (5′ TAGTAGTAGACTCCCTAAAGAGCT 3′), and a reverse primer (5′ GGCCCTCTAGAGTTTCAAAGGCTCTTGGTTGGAG 3′). PCR products were digested with XbaI and were phosphorylated and blunt-end cloned into pcDNA3.1 (Invitrogen) to produce pcHTNVN. The nucleotide sequence of pcHTNVN was confirmed by bidirectional sequencing with universal cytomegalovirus forward and bovine growth hormone reverse primers using an ABI 3130xl genetic analyzer (Applied Biosystems). The plasmid p50-green fluorescent protein (p50-GFP), a gift from William Britt, was described previously (9).
Confocal and immunofluorescence microscopies.
For all microscopy studies, Vero E6 cells were seeded in Lab-Tek II 2-well chamber slides (Nalge Nunc International). For infection studies, Vero E6 cells at 60% confluence were infected with HTNV at a multiplicity of infection (MOI) of 0.1 or as noted for 1 h at 37°C with 5% CO2 as previously described (69), or they were transfected with 1 μg of plasmid DNA using Lipofectamine 2000 in OptiMEM (Invitrogen) according to manufacturer's instructions. After 1 h of incubation, complete DMEM was added to the wells, and the mixtures were incubated at 37°C in a 5% CO2 chamber. At different time points, Vero E6 cells were fixed either in acetone for 15 min or with 3.5% paraformaldehyde for 30 min at room temperature, followed by permeabilization with 0.1% Triton X-100 for 5 min. Slides were washed three times with phosphate-buffered saline (PBS).
The HTNV N was detected by incubating the cells with either HTNV N MAb E-314 at a 1:200 dilution or rabbit polyclonal antibody no. 143 at a dilution of 1:10,000 for 1 h at room temperature. Golgi compartments were stained with TRITC-conjugated Alexa 594 or with polyclonal anti-Mann II antibody at a dilution of 1:75. PDI, EEA1, ERGIC-53, actin, β-tubulin, and vimentin were detected with the respective MAbs listed above at a dilution of 1:100 for 1 h at room temperature. Slides were washed three times with PBS and were incubated with secondary goat anti-mouse or anti-rabbit antibodies conjugated to Alexa 488 or Alexa 594 for 30 min at room temperature at a dilution of 1:400. F-actin was visualized by being stained with phalloidin conjugated to Alexa 594 for 30 min at room temperature. Slides were mounted with Fluoromount-G (Southern Biotechnology Associates), and confocal imaging was performed with a Leica DMIRBE inverted epifluorescence microscope outfitted with Leica TCS NT SP1 laser confocal optics at the High Resolution Imaging Facility at the University of Alabama—Birmingham. Epifluorescence imaging was also performed with a Zeiss Axiovert 200 microscope outfitted with an ApoTome for deconvolution purposes. The ApoTome uses the grid projection or structured illumination principle to obtain images with an improved signal-to-noise ratio, and it approximately doubles the resolution in the axial (z) direction. Final images were obtained by averaging four independent scans of the fields using ×40, ×63, or ×100 magnification, corrected for oil immersion. Quantification of N in slides was calculated by measuring the area of the cell occupied by indirect labeling of N versus the total area of the cell using Image J image analysis software (National Institutes of Health).
Determination of the effect of drug treatment of hantaviral replication and release of infectious virus.
Vero E6 cells grown to 100% confluence in 6-well plates were infected with HTNV at an MOI of 0.1, as described previously (69). Eight hours postinfection (p.i.), 15.0 μg/ml of BFA, CytD, NOC, or RBV was added, and cells were incubated at 37°C for an additional 36 h in a 5% CO2 incubator, with replenishment of drug in complete DMEM every 12 h. Levels of infectious virus released into the supernatant were measured using an infectious virus center assay, and cellular vRNA levels were quantified by a quantitative real-time RT-PCR assay, as described previously (42, 78). For the infectious virus center assay, virus was allowed to adsorb to the cells for 1 h at 37°C, 5% CO2. Cells were rinsed twice with PBS and replenished with 100 μl of 0.7% methyl cellulose containing complete DMEM. After 5 days, cells were fixed and treated with a 1:3 solution of H2O2-methanol. Cells were washed and incubated with a 1:500 dilution of HTNV N MAb E-314, followed by a secondary goat anti-mouse antibody conjugated with horseradish peroxidase using a TrueBlue peroxidase staining kit (KPL). Infectious centers were photographed with a Camedia C-5060 5.1-megapixel camera (Olympus) and counted after enlargement of the images.
Membrane floatation.
Vero E6 cells were cultured in T-75 flasks, grown to 80% confluence, and transfected with 10 μg of pcHTNVN. At 18 h posttransfection (p.t.), the cells were washed and resuspended in 5.0 ml of 10 mM Tris, pH 7.4, containing 0.25 M sucrose and complete protease inhibitor cocktail (Roche), and were subjected to membrane floatation as described by others (6). Briefly, the extracts were brought to 1.4 M sucrose and layered onto a discontinuous sucrose gradient (0.8, 1.2, 1.4, and 1.6 M) and centrifuged at 110,000 × g overnight at 4°C in an SW 50.1 rotor (Beckman). Fractions were collected and acetone precipitated and then resolved by sodium dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis, followed by immunoblotting using rabbit anti-N antibody no. 143, as described previously (82), with enhanced chemiluminescent technology (Pierce).
Microsomal membrane fractionation.
Vero E6 cells were grown to 80% confluence in T-75 flasks and were transfected with 10 μg of pcHTNVN plasmid. At 18 h p.t., cells were washed twice with ice-cold PBS and resuspended in 200 μl of 5 mM Tris, pH 7.4, 0.5 mM MgCl2, and complete protease inhibitor cocktail. Cells were homogenized by being passed 15 to 18 times through a 26-gauge needle and then were brought to a final concentration of 0.25 M sucrose. Cell debris and nuclei were removed by low-speed centrifugation at 1,000 × g for 10 min. Postnuclear supernatant (PNS) was centrifuged at 100,000 × g for 1 h using a TLA 100.2 rotor (Beckman) to separate the particulate pellet and soluble cytosolic fractions. Pellets were resuspended in 10 mM Tris-HCl, pH 6.8, 1% SDS, and complete protease inhibitor cocktail, brought to a final concentration of 1 M NaCl, 50 mM sodium bicarbonate, 1.0% Triton X-100, or 1.0% NP-40, and incubated for 1 h on ice. Samples were centrifuged for 1 h at 120,000 × g. Proteins from the pellet and supernatant fractions were acetone precipitated and subjected to SDS-polyacrylamide gel electrophoresis followed by immunoblotting, as described previously (82).
RESULTS
Temporal distribution of HTNV N in Vero E6 cells.
The multifunctional roles of N require an understanding of its temporal movement in the cell. To assess the temporal distribution of N during infection, Vero E6 cells were infected with HTNV, and the distribution of N was monitored by confocal microscopy at several time points p.i. The expression of N was detected as early as 4 h p.i., and by 12 h most of N was detected predominantly as small granular structures dispersed throughout the cell cytoplasm (Fig. 1A; the upper panels show phase contrast/immunofluorescence, and the lower panels show immunofluorescence only). A significant change in the distribution of N was observed at 24 h p.i., at which time punctate structures containing N accumulated in the perinuclear region. The presence of N as granular cytoplasmic particles continued, albeit at a lower rate, and by 72 h p.i. most of N was clustered in the perinuclear region. At day 5 p.i., cells contained highly condensed structures of N, some of which appeared tubular in nature (data not shown).
FIG. 1.
Temporal distribution of N in Vero E6 cells infected with HTNV or transfected with plasmid expressing N. (A) To ensure maximal infection, Vero E6 cells were infected with HTNV at an MOI of 5.0 and were examined for the presence of N at 4, 12, 24, and 72 h with (upper row) or without (lower row) phase contrast. At each of these times, cells were acetone fixed and stained by indirect immunofluorescence for N (red) with rabbit polyclonal anti-N antibody no. 143 as described in Materials and Methods. Scale bar, 20 μm, with 63× objectives (Axiovert 200 microscope). The nucleus was stained with 4′,6′-diamidino-2-phenylindole. (B) Vero E6 cells were transfected with pcHTNVN and examined for the presence of N at 4, 12, 24, and 48 h. At each of these times, cells were acetone fixed and stained by indirect immunofluorescence for N (red) with rabbit polyclonal anti-N antibody no. 143 as described in Materials and Methods. Scale bar, 20 μm, with 63× objectives (Axiovert 200 microscope).
To investigate the ability of N to accumulate in the perinuclear region and its temporal distribution in the absence of other viral components, we transfected Vero E6 cells with a plasmid that constitutively expressed N (pcHTNVN). N accumulated in the perinuclear region as early as 12 h p.t., and by 24 h p.t. most cells showed perinuclear localization of N (Fig. 1B). At 48 h p.t., N was highly compacted around the perinuclear region; hence, we chose 24 h for subsequent studies. The 24-h pattern of N alone was similar to the pattern observed with HTNV-infected cells at 72 h p.i. (Fig. 1). As reported for the BCCV and Tula virus N proteins (31, 54), our studies suggest that the HTNV N contains the necessary intrinsic sequences to target itself to the perinuclear compartment.
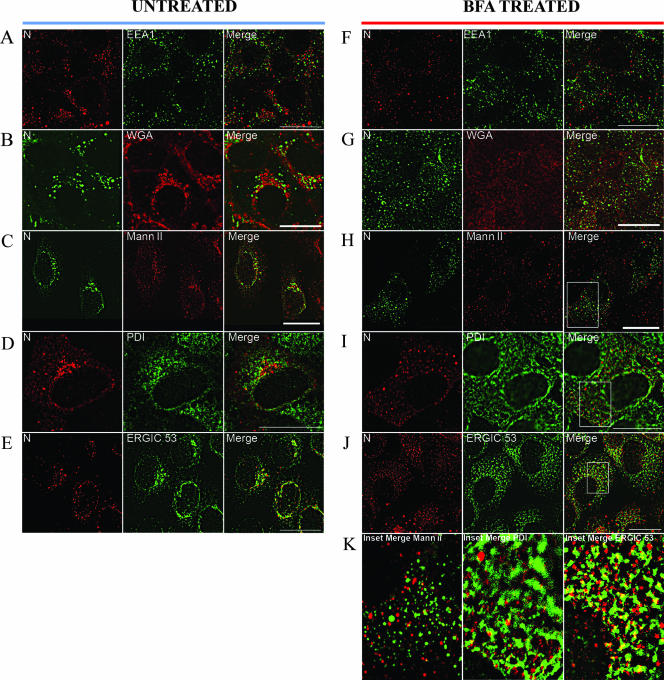
N colocalizes with ERGIC-53 and redistributes with the ERGIC upon BFA treatment.
It is well established that the hantaviral Gn and Gc glycoproteins traffic from the ER to the Golgi compartment and that virions form by budding into the Golgi compartment (75). One unanswered question is whether N targets the Golgi compartment directly to facilitate replication and assembly. To identify the compartment targeted following HTNV N synthesis, we performed dual-immunofluorescence labeling experiments with confocal laser-scanning microscopy of HTNV-infected Vero E6 cells using antibodies against HTNV N and various subcellular organelles. Specifically, we examined the localization of N relative to markers of the early endosome (EE), Golgi compartment, ER, and ERGIC.
We used EEA1 as a marker for the EE and looked at the relative distribution of N and EEA1 in infected cells. At 72 h p.i., N accumulated in the perinuclear region but did not colocalize with EEA1 (Fig. 2A). We chose WGA to label the trans-Golgi compartment (Fig. 2B), Mann II to label the cis- and medial-Golgi compartments (Fig. 2C), and PDI to label the ER (Fig. 2D). Confocal imaging revealed that none of the markers had colocalized with N, although a small amount of spectral overlap was noted between PDI and N (Fig. 2D). However, our dual-labeling analysis with antibodies against N and ERGIC-53 showed colocalization, as indicated by the yellow signal in the merged image (Fig. 2E).
FIG. 2.
Colocalization of HTNV N with ERGIC-53 and redistribution of N with BFA. Vero E6 cells were infected with HTNV at an MOI of 0.1, and after 3 days slides were acetone fixed (except for Mann II staining, in which case paraformaldehyde was used). Prior to fixation, slides F to K were treated with BFA for 1 h as described in Materials and Methods. Slides were costained with WGA (B and G) or antibodies (anti-N monoclonal E-314 [green] or polyclonal no. 143 antibody [red]) against EEA1 (A and F), Mann II (C and H), PDI (D and I), or ERGIC-53 (E and J) as described in Materials and Methods. Enlarged merged images of the insets in panels H, I, and J are presented in panel K. Scale bars, 20 μm, using 100× objectives (Leica confocal microscope).
We asked whether N was associated with ERGIC membranes by using BFA, which rapidly and reversibly redistributes membranes of the ERGIC and the Golgi compartment (44, 49). HTNV-infected Vero E6 cells were treated with BFA at 72 h p.i. for 1 h and were analyzed for the pattern of N localization. Our data showed that BFA treatment redistributed N in HTNV-infected cells from a perinuclearly accumulated pattern to a more granularly distributed pattern (for example, compare N in Fig. 2A to N in Fig. 2F). The redistributed N did not colocalize with EEA1 (Fig. 2F), WGA (Fig. 2G), or Mann II (Fig. 2H and K, left panels). As can be seen in Fig. 2D, a very small amount of overlap was noted with PDI (Fig. 2I and K, middle panels). As can be seen Fig. 2E, we observed colocalization of N and ERGIC-53 in cells treated with BFA (Fig. 2J and K, right panels). This suggested that N may associate with ERGIC membranes.
Since N colocalized with ERGIC in HTNV-infected cells, we asked whether transiently expressed N could target the ERGIC independent of other viral proteins. Dual-immunofluorescence labeling of Vero E6 cells transfected with pcHTNVN showed very little colocalization of N and the ERGIC compared to that of the infected cells (Fig. 3D). Furthermore, we did not detect any colocalization between N and ER or Golgi markers in these transfection studies in the presence or absence of BFA (Fig. 3A to H). The data suggest that transfected N alone is deficient in proper trafficking to the ERGIC and requires one or more viral components. In infection studies, a small amount of spectral overlap was noted with N and the ER marker PDI (Fig. 2K), but none was noted in transfection studies (Fig. 3G). The difference noted between transfection and infection studies of N protein at the ER could be due to the rapidly recycling of N via the early secretory pathway from the Golgi compartment to the ER, promoted by the presence of Gn/Gc. Thus, N directly targeted the ERGIC in virus-infected cells.
FIG. 3.
Colocalization studies of HTNV N in Vero E6 cells expressing N alone, as well as redistribution of N with BFA, with various subcellular markers. Vero E6 cells were transfected with pcHTNVN, which expresses N alone, and after 18 h, the cells were fixed with acetone (except for Mann II staining, in which case paraformaldehyde was used). Prior to fixation, the slides shown in panels E to H were treated with BFA for 1 h. Slides were costained with anti-N monoclonal E-314 (red) or polyclonal no. 143 antibody (green) as well as WGA (A and E) and antibodies against Mann II (B and F), PDI (C and G), or ERGIC-53 (D and H). Scale bars, 20 μm, using 100× objectives (Leica confocal microscope).
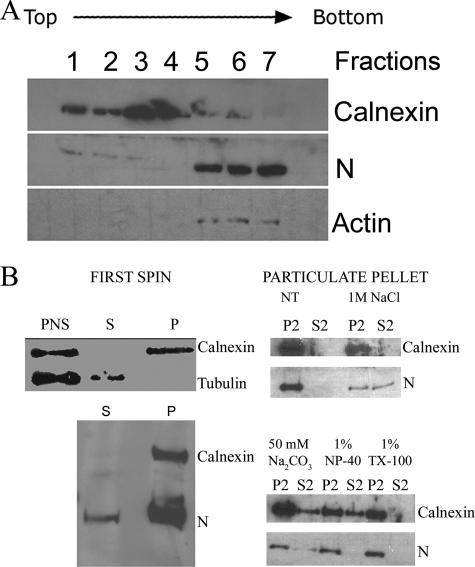
N association with membranes.
To further address membrane association, we conducted membrane subcellular fractionation experiments. Vero E6 cells were transfected with pcHTNVN, which transcribes the mRNA for N from a cytomegalovirus promoter, and were allowed to produce N for 16 h. Extracts from Vero E6 cells transiently expressing N were subjected to floatation analysis in a discontinuous sucrose gradient (Fig. 4A). We chose calnexin (CNX) and actin as markers for membrane-containing fractions and cytosolic fractions, respectively. Most N was observed in the cytosolic fractions of the gradient (Fig. 4A, fractions 5 to 7). However, a very tiny fraction of N was also noted in membrane-containing fractions 1 to 3 (Fig. 4A). We repeated this analysis using HTNV-infected Vero E6 cells, and we noted similar results (data not shown).
FIG. 4.
Association of HTNV N with membrane fractions. Vero E6 cells were transfected with pcHTNVN, and after 18 h they were subjected to membrane floatation (A) or subcellular fractionation (B). Fractions were subjected to Western blotting and were probed with antibody to CNX, N, or actin. (A) Vero E6 cell extracts containing transiently expressed N were brought to 1.4 M sucrose and centrifuged as described in Materials and Methods. Fractions were collected from the top, and proteins were precipitated. (B) Immunoblot analyses of PNS, soluble supernatant (S), and particulate pellet (P) fractions by following the treatment or no treatment (NT) and centrifugation regimen described in Materials and Methods. TX-100, Triton X-100.
Subcellular fractionation and detergent and salt treatments were used to analyze the nature of the particulate fraction of N. Cells were lysed at 18 h after transfection and subjected to high-speed centrifugation to separate the soluble cytosolic fraction (S) and the particulate fraction containing the microsomal membranes (P). As shown in Fig. 4B (top left panel), PNS contained both CNX and β-tubulin bands, which mark cytosolic and membrane compartments, respectively. Following centrifugation, the cytosolic fractions contained tubulin and the membrane fractions contained CNX (Fig. 4B, top left panel). Interestingly, the majority of N banded in the pellet with the microsomal membranes as expected, but a small amount was detected in the soluble fraction (Fig. 4B, lower left panel). The particulate fraction was further treated with various chemicals and separated into soluble and particulate fractions with high-speed centrifugation. Interestingly, treatment of the particulate fraction with 1 M NaCl resulted in partitioning of N in roughly equal amounts between the particulate and soluble fractions. However, treatments with 50 mM sodium bicarbonate, 1.0% Triton X-100, or NP-40 did not release the N from the particulate fraction. These results suggest that the majority of N is not primarily membrane associated.
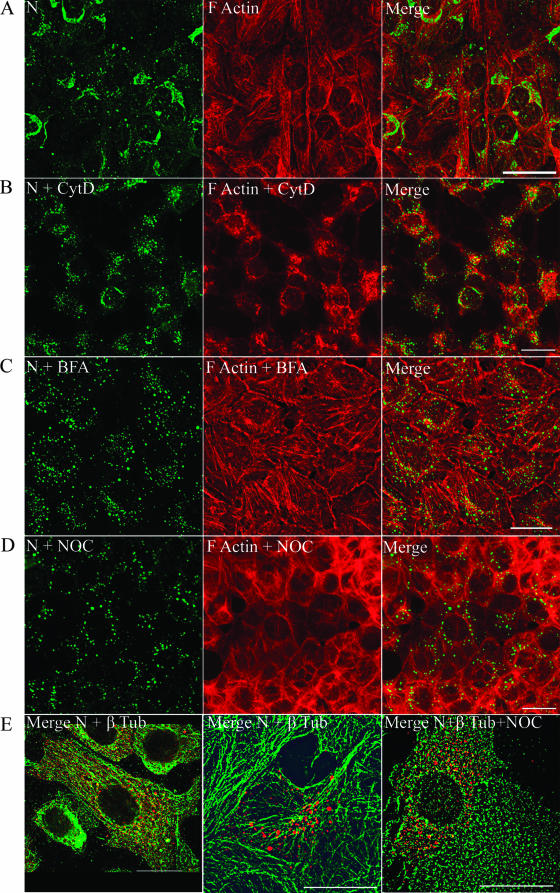
N associates with microtubules and not actin.
Several viruses employ cellular cytoskeletal machinery, such as actin, microtubules, and their associated molecular motors, to traffic to the site of replication, assembly, and egress (14, 15, 39, 53, 70, 72, 74). Ravkov et al. have suggested that actin filaments may play a role in the biogenesis of BCCV, a New World hantavirus (55). To test the association of actin with N, we performed dual-immunofluorescence analysis of N and actin in HTNV-infected cells. F-actin was stained with TRITC-conjugated phalloidin. Our confocal microscopy studies showed a pattern for N that neither colocalized nor juxtaposed with actin (Fig. 5A). As an alternative approach to probe for its interaction with actin, HTNV-infected Vero E6 cells were treated with 10.0 μg/ml of CytD, an actin-depolymerizing drug, for 1 h (Fig. 5B). At 24 h p.i., CytD treatment disrupted most of the actin; however, the distribution of N was not affected, as shown by the unchanged, perinuclear accumulation of N (compare the distribution of N in Fig. 5A with that of N in Fig. 5B). To explore the association with the microtubular cytoskeleton, NOC, a reversible microtubule-depolymerizing agent, was used to probe HTNV-infected cells. In contrast to CytD, NOC treatment of HTNV-infected cells resulted in extensive redistribution of N from perinuclear to peripheral sites (Fig. 5D). BFA is shown for comparison in Fig. 5C.
FIG. 5.
Redistribution of N in HTNV-infected Vero E6 cells and redistribution upon treatment with CytD, BFA, or NOC. Vero E6 cells were infected with HTNV at an MOI of 0.1, and after 3 days the cells were treated for 1 h with a mock vector (A), CytD (B), BFA (C), or NOC (D and E). Slides were acetone fixed and processed for indirect immunofluorescence. Cells were costained either with anti-N E-314 antibody to detect N (green) and TRITC-conjugated phalloidin to detect filamentous actin (red) (A to D) or with rabbit polyclonal anti-N no. 143 antibody to detect N (red) and anti-β-tubulin to detect the microtubules (green) (E). Scale bars: panels A to D, 20 μm, using 63× objectives; panel E, 20 μm, with 100× objectives (Leica confocal microscope). β-tub, β-tubulin.
The redistribution of N upon treatment of NOC in virus-infected cells and the lack of colocalization with actin led us to examine the possible association of N with microtubules. Antibodies against β-tubulin and N were used to analyze the relative distribution of these proteins in HTNV-infected cells (Fig. 5E). At 24 h p.i., we observed a juxtaposition of N with the microtubules that resembled a classic bead-on-a-string pattern (Fig. 5E, left panel and middle panel). Further, treatment of HTNV-infected cells with NOC for 1 h redistributed N into a pattern that resembled depolymerized microtubules (Fig. 5E, right panel).
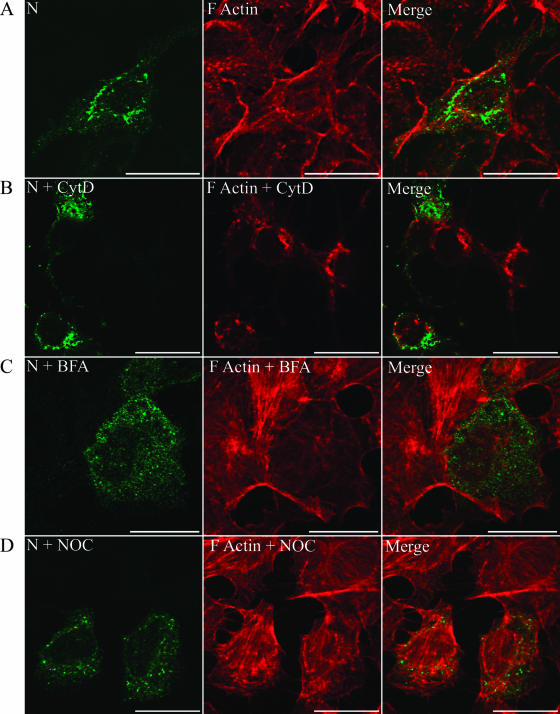
Microtubules promote transport of N in the absence of other viral proteins.
Since N was redistributed from the perinuclear region upon NOC treatment, and not upon CytD treatment, in HTNV-infected cells, we repeated the drug treatment studies to analyze the distribution of the HTNV N protein in transiently expressed cells in the absence of other viral proteins. Dual-immunofluorescence labeling of Vero E6 cells transfected with pcHTNVN showed perinuclear accumulation of N that did not coincide with the F-actin staining (Fig. 6A). Furthermore, we did not see any redistribution of N upon treatment of cells with CytD, although the drug affected the actin distribution (Fig. 6B). In contrast, treatment of Vero E6 cells with BFA or NOC for 1 h resulted in redistribution of N proteins without affecting the distribution of the actin filaments (Fig. 6C and D). Overall, our data support the transport of N via microtubules expressed alone or in the context of other viral components.
FIG. 6.
Distribution of N in Vero E6 cells and redistribution upon treatment with CytD, BFA, or NOC. Vero E6 cells were transfected with pcHTNVN, which expresses N, and after 18 h the cells were treated with a mock vector (A), CytD (B), BFA (C), or NOC (D). Slides were acetone fixed, stained, and processed for indirect immunofluorescence as mentioned for infected cells in the legend to Fig. 5. Scale bars, 20 μm, with 100× objectives (Leica confocal microscope).
Involvement of dynein in microtubule-mediated HTNV N transport.
The molecular motors kinesin and dynein play important roles in cellular trafficking (63, 79), including trafficking of viruses (14, 21). Cytoplasmic dynein, together with its activator dynactin, is a multisubunit macromolecular complex necessary for cargo transport (21, 63, 80, 81). Overexpression of dynamitin (p50), a component of the dynein complex, results in disruption of dynein-dependent transport by a dominant-negative effect (9, 16, 35, 77). The accumulation of N within a perinuclear region suggested the involvement of dynein motor activity for transport of N. Hence, we overexpressed dynamitin in the presence of N to explore the involvement of dynein in trafficking of N to the perinuclear region.
Vero E6 cells were transfected with a plasmid expressing a GFP-tagged dynamitin, and after 4 h they were infected with HTNV. Two days after viral infection, cells were treated and processed for immunofluorescence. Representative images of three treatments are shown in Fig. 7A (N alone [left], N plus NOC [central], and N plus dynamitin [right]). We quantified the area of the cell occupied by N in untreated cells and in cells treated with NOC or expressing dynamitin (Fig. 6B). Ten to 20 cells were randomly chosen, and the intracellular distribution of N was quantified by measuring the ratio of the area occupied by N in the cells (Fig. 7A, inner circle) to the total cell area (Fig. 7A, outer circle), as depicted. The area of the HTNV-infected cells occupied by N was 14.7% of the total cell area (Fig. 7B, column 1). In HTNV-infected cells treated with NOC, the area of the cell occupied by N was 42% (Fig. 7B, column 2). In cells expressing N and dynamitin (p50), the area of the cell occupied by N was 44.3% (Fig. 7B, column 4). NOC washout (recovery) after a 1-h treatment of infected cells with NOC resulted in a decrease in the area of the cell occupied by N to 24.9% (Fig. 7B, column 3). Such a decrease was absent or was negligible in HTNV-infected cells expressing dynamitin (Fig. 7B, column 5). Finally, in experiments that coexpressed N alone or with p50-GFP, we observed the absence of perinuclear N distribution in transfected Vero E6 cells (data not shown). This suggests that motor-mediated transport via microtubules facilitates N delivery to the perinuclear region of the cell. Cells expressing dynamitin limited accumulation of N in the perinuclear region.
FIG. 7.
Overexpression of dynamitin abrogated accumulation of N in the perinuclear region. Vero E6 cells were cotransfected with p50-GFP (green) and pcHTNVN (red). (A) Vero E6 cells infected with HTNV are shown, either mock treated (left panel), treated for 1 h with NOC (center panel), or cotransfected with p50 (right panel). Scale bars, 20 μm, using 63× objectives (Axiovert 200 microscope). (B) Examples of measurements that were used to obtain values for various treatments. The following equation was used: % HTNV N area/cell = (total area of N occupied in the cell/total area of the cell) × 100. The percentage of N found in the perinuclear region and/or p50-GFP in the presence or absence of NOC is indicated. Error bars for each treatment condition were calculated as the means ± standard deviations of measurements of 10 to 20 cells picked randomly from different fields within the respective treatment groups.
BFA and NOC affect viral replication.
Our experiments showed that N in HTNV-infected cells was redistributed upon BFA or NOC treatment. We hypothesized that redistribution of N and associated structures may inhibit virus replication. We also expected that BFA and NOC would inhibit virus production, since an intact Golgi compartment is required for HTNV maturation. To determine the requirement for ERGIC and associated perinuclear structures in virus replication and virus production, we compared the effects of BFA, NOC, CytD, and RBV on vRNA levels and virus produced at 1 h prior to HTNV infection (pretreatment) and 8 h p.i. (posttreatment). RBV inhibits HTNV replication and served as a positive control of vRNA inhibition (69). The concentrations of the drugs used showed no cytotoxicity (data not shown). Relative HTNV vRNA levels were measured by quantitative RT-PCR from total RNA extractions of Vero E6 cells, and infectious virus production was measured by infectious virus center assay.
As expected, all of the drug pretreatments inhibited the level of infectious virus released to nearly 100% (data not shown). Posttreatment with BFA or NOC inhibited infectious virus production; however, CytD had a reduced effect (Table 1). These results were not unexpected, since the main inhibitory target of CytD is virus entry and BFA would be expected to affect assembly and/or budding, which requires an intact Golgi compartment. Interestingly, both NOC and BFA reduced levels of vRNA in posttreatment studies (Table 1). CytD had no effect on replication. This suggests a requirement for microtubules and an intact ERGIC in the production of vRNA.
TABLE 1.
Relative percentages of inhibition of HTNV vRNA S-segment levels and levels of infectious virus in the absence and presence of drugs
| Treatment | % Inhibition of vRNA at 8 h p.i.a | Level of infectious virusb (PFU/ml) |
|---|---|---|
| None | 0 | 0 |
| BFA | 52 | 100 |
| CytD | 0 | 71 |
| NOC | 56 | 92 |
| RBV | 73 | 91 |
Relative HTNV vRNA levels were quantified using the 2−ΔΔCT method and 18S rRNA as an internal control.
Wild-type infection levels corresponded to 2.5 × 105 PFU/ml.
Infection with HTNV causes vimentin rearrangement.
Vimentin is a major component of type III intermediate filaments (5, 18), and it has been implicated as a structural component in the replication processes of several viruses (10-12, 40, 50, 51, 77). We noted in our studies that N did not completely colocalize with ERGIC, although the majority of N did. Therefore, it was worth asking whether vimentin was associated with N in HTNV-infected cells.
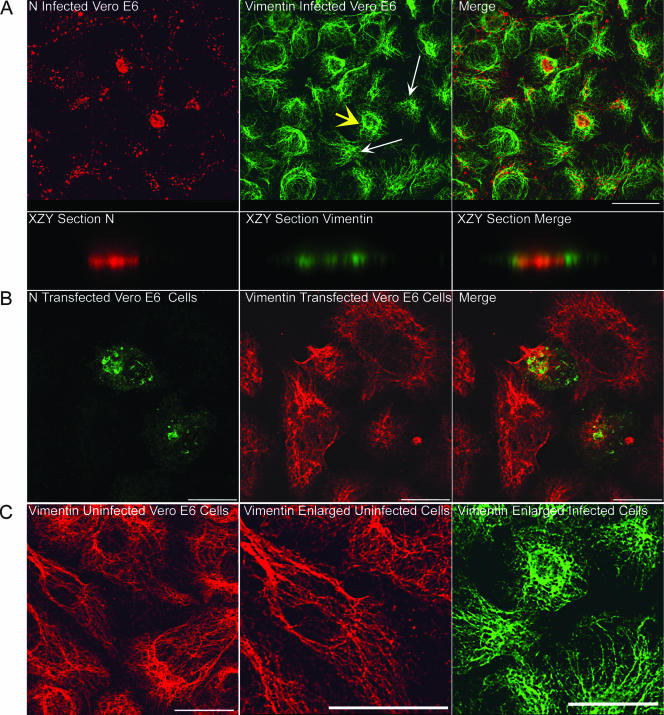
We employed confocal microscopy and costained HTNV-infected Vero E6 cells with antibodies against vimentin and N. We analyzed the relative distribution of these proteins on day 5 p.i. (Fig. 8A). Cross-sections of vimentin with N in the z axis showed localization in the same plane (Fig. 8A, bottom panels). Interestingly, vimentin filaments appeared to form cages around highly condensed N that had accumulated in the perinuclear region (see the merged image in Fig. 8A). Additionally, vimentin remodeling was observed in cells transiently expressing N (Fig. 8B). In the absence of HTNV infection, vimentin filaments were distributed throughout the cell (Fig. 8C, left and central panels), unlike what was seen with HTNV-infected cells (Fig. 8C, right panel). This suggests that N alone can induce the formation of these vimentin structures.
FIG. 8.
Relationship of N and vimentin in HTNV-infected and pcHTNVN-transfected Vero E6 cells. Vero E6 cells were infected with HTNV at an MOI of 0.1 (A) or were transfected with pcHTNVN (B), and after 5 days or 24 h, respectively, slides were acetone fixed and processed for indirect immunofluorescence. (A) Infected cells were costained for N (red) and vimentin (green); (B) transfected cells were costained for N (green) and vimentin (red). (C) Enlargements of uninfected (left and middle panels) and infected (right panel) Vero E6 cells. Scale bars, 20 μm, using 63× objectives (Leica confocal microscope). (A) The yellow arrow points to vimentin cages. The cell in the upper middle panel (marked by the yellow arrow) was enlarged, and a z section was performed on the xzy axes to demonstrate the plane of cage formation around N. Note the distinct small circles formed upon cage formation. The white arrows point to vimentin redistribution and aster formation.
DISCUSSION
Herein we address where and how hantaviral N traffics within the cell and how this may be important for virus replication. Using antibodies to various subcellular compartments and the HTNV structural N, we show that N colocalized with ERGIC but not with the Golgi compartment, ER, F-actin, or EE. These data suggest that N directly targeted the ERGIC prior to movement to the Golgi compartment and imply that once HTNV N traffics to the Golgi compartment, it rapidly assembles into the virion. The ERGIC constitutes an independent structure that is not continuous with the ER or the cis-Golgi compartment (7, 37, 67). ERGIC is maintained by a continuous flow of membranes mediated by molecular motor proteins that include the microtubule-minus-end-directed protein dynein, the microtubule-plus-end-directed motor kinesin, microtubules, actin, and various Rab GTPases. Together, they ferry cargo bidirectionally to the ER and to the cis-Golgi body from ERGIC (4, 58). ERGIC participates in the biogenesis of many viruses (36, 45, 56, 57, 60-62, 73). Within the Bunyaviridae family, Uukuniemi virus (UUKV) particles localize to peripherally and centrally localized elements within the ERGIC. Further, budding of UUKV has been reported to begin in the ERGIC and continue in the Golgi compartment (24). Additional immunocytochemical and electron microscopic studies of other genera in the Bunyaviridae are warranted to determine the use of this compartment in morphogenesis in general.
The redistribution of N from the perinuclear region upon treatment with NOC and BFA suggested a possible association of N with membranes. There is no evidence so far for any kind of membrane-associated, posttranslational modifications in N for any of the hantaviruses (66). However, the N proteins expressed from plasmids from UUKV (24) and BCCV (54) as well as the N and L proteins of the Tula virus (38) have been shown to associate with microsomal membranes. Our membrane floatation and subcellular fractionation of N, expressed from a plasmid or from HTNV-infected cells, showed that only a tiny fraction of HTNV N associated with membranes. Because of the very small amount, it will be difficult to determine experimentally the composition of the membrane in the fraction that floated with N; however, it is highly likely that this N fraction is associated with the Golgi compartment.
BCCV N colocalizes with actin microfilaments, and actin has been proposed to be involved in BCCV biogenesis (55). Similarly, actin colocalizes with N of Crimean-Congo hemorrhagic fever virus (CCHFV), a member of the Nairovirus genus within the Bunyaviridae. Treatment of CCHFV-infected cells with 1 μg/ml CytD resulted in the redistribution of CCHFV N from the perinuclear region (3). In our studies, NOC, but not CytD, caused a rapid redistribution of N in virus-infected cells and transfected cells, suggesting that microtubules, but not actin, are involved in N trafficking and/or retention at the ERGIC. Further, coexpression of dynamitin with N resulted in the abrogation of perinuclear N transport, thus providing evidence for dynein-mediated microtubule transport of N.
We asked if microtubules and the ERGIC were necessary for HTNV replication. We examined vRNA levels in the absence or presence of BFA, NOC, and CytD. BFA and NOC inhibited HTNV replication at the level of RNA synthesis when added at 8 h p.i., while CytD had no effect on vRNA synthesis. BFA is known to inhibit replication of poliovirus, a positive-stranded RNA virus (13, 23, 46), and vesicular stomatitis virus, a negative-stranded RNA virus (22). Microtubule-depolymerizing drugs such as NOC interfere with the delivery of many unrelated viral proteins to the intracellular sites of replication (reviewed in reference 71). BFA- and NOC-mediated inhibition of hantaviral RNA synthesis suggests that the ERGIC is important for viral replication. Further studies are required to understand the mechanism by which BFA and NOC inhibit HTNV vRNA replication.
Several studies have shown targeting of N to the perinuclear region, and based on early electron microscopy work, it has been assumed that hantaviral N targets the Golgi body (29, 31, 38, 54, 76). Our studies show the involvement of microtubules in HTNV N transport and that HTNV N initially targets the ERGIC region and not the Golgi compartment. These findings, and the decrease in viral replication with BFA or NOC treatment, suggest a function for this region in the virus life cycle. Further, we show that N accumulation coincided with the remodeling of the vimentin structure and formation of cage-like structures that surround N. Vimentin remodeling has been reported to play important roles in many aspects of virus replication (10-12, 40, 50, 51, 77). It is possible that during HTNV infection, vimentin generates a unique scaffold to enhance replication of the virus or to create an environment inside or outside of these structures that facilitates virus replication and transcription. Clearly, additional studies with cellular and viral markers at the ultrastructural level could yield valuable insight into these structures. In summary, our studies support a central role for the ERGIC in the hantaviral life cycle prior to assembly. Future studies will address the interplay of the ERGIC and the Golgi compartment and how these interactions lead to the assembly of this fascinating emerging virus.
Acknowledgments
We thank William Britt for providing the p50-GFP construct.
H.N.R. was supported by an internship made available through the Southern Research Institute.
Footnotes
Published ahead of print on 30 May 2007.
REFERENCES
- 1.Alfadhli, A., Z. Love, B. Arvidson, J. Seeds, J. Willey, and E. Barklis. 2001. Hantavirus nucleocapsid protein oligomerization. J. Virol. 75:2019-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfadhli, A., E. Steel, L. Finlay, H. P. Bachinger, and E. Barklis. 2002. Hantavirus nucleocapsid protein coiled-coil domains. J. Biol. Chem. 277:27103-27108. [DOI] [PubMed] [Google Scholar]
- 3.Andersson, I., M. Simon, A. Lundkvist, M. Nilsson, A. Holmstrom, F. Elgh, and A. Mirazimi. 2004. Role of actin filaments in targeting of Crimean Congo hemorrhagic fever virus nucleocapsid protein to perinuclear regions of mammalian cells. J. Med. Virol. 72:83-93. [DOI] [PubMed] [Google Scholar]
- 4.Appenzeller-Herzog, C., and H. P. Hauri. 2006. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J. Cell Sci. 119:2173-2183. [DOI] [PubMed] [Google Scholar]
- 5.Azumi, N., and H. Battifora. 1987. The distribution of vimentin and keratin in epithelial and nonepithelial neoplasms. A comprehensive immunohistochemical study on formalin- and alcohol-fixed tumors. Am. J. Clin. Pathol. 88:286-296. [DOI] [PubMed] [Google Scholar]
- 6.Balch, W. E., W. G. Dunphy, W. A. Braell, and J. E. Rothman. 1984. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell 39:405-416. [DOI] [PubMed] [Google Scholar]
- 7.Bannykh, S. I., T. Rowe, and W. E. Balch. 1996. The organization of endoplasmic reticulum export complexes. J. Cell Biol. 135:19-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop, D. H. L. 1996. Biology and molecular biology of bunyaviruses, p. 19-61. In R. M. Elliot (ed.), The Bunyaviridae. Plenum Press, New York, NY.
- 9.Burkhardt, J. K., C. J. Echeverri, T. Nilsson, and R. B. Vallee. 1997. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139:469-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvalho, Z. G., A. P. De Matos, and C. Rodrigues-Pousada. 1988. Association of African swine fever virus with the cytoskeleton. Virus Res. 11:175-192. [DOI] [PubMed] [Google Scholar]
- 11.Chen, M., R. Goorha, and K. G. Murti. 1986. Interaction of frog virus 3 with the cytomatrix. IV. Phosphorylation of vimentin precedes the reorganization of intermediate filaments around the virus assembly sites. J. Gen. Virol. 67:915-922. [DOI] [PubMed] [Google Scholar]
- 12.Cordo, S. M., and N. A. Candurra. 2003. Intermediate filament integrity is required for Junin virus replication. Virus Res. 97:47-55. [DOI] [PubMed] [Google Scholar]
- 13.Doedens, J., L. A. Maynell, M. W. Klymkowsky, and K. Kirkegaard. 1994. Secretory pathway function, but not cytoskeletal integrity, is required in poliovirus infection. Arch. Virol. Suppl. 9:159-172. [DOI] [PubMed] [Google Scholar]
- 14.Dohner, K., C. H. Nagel, and B. Sodeik. 2005. Viral stop-and-go along microtubules: taking a ride with dynein and kinesins. Trends Microbiol. 13:320-327. [DOI] [PubMed] [Google Scholar]
- 15.Dohner, K., and B. Sodeik. 2005. The role of the cytoskeleton during viral infection. Curr. Top. Microbiol. Immunol. 285:67-108. [DOI] [PubMed] [Google Scholar]
- 16.Dohner, K., A. Wolfstein, U. Prank, C. Echeverri, D. Dujardin, R. Vallee, and B. Sodeik. 2002. Function of dynein and dynactin in herpes simplex virus capsid transport. Mol. Biol. Cell 13:2795-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flick, K., J. W. Hooper, C. S. Schmaljohn, R. F. Pettersson, H. Feldmann, and R. Flick. 2003. Rescue of Hantaan virus minigenomes. Virology 306:219-224. [DOI] [PubMed] [Google Scholar]
- 18.Franke, W. W., E. Schmid, S. Winter, M. Osborn, and K. Weber. 1979. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp. Cell. Res. 123:25-46. [DOI] [PubMed] [Google Scholar]
- 19.Gavrilovskaya, I. N., E. J. Brown, M. H. Ginsberg, and E. R. Mackow. 1999. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by β3 integrins. J. Virol. 73:3951-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavrilovskaya, I. N., M. Shepley, R. Shaw, M. H. Ginsberg, and E. R. Mackow. 1998. β3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. USA 95:7074-7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greber, U. F., and M. Way. 2006. A superhighway to virus infection. Cell 124:741-754. [DOI] [PubMed] [Google Scholar]
- 22.Irurzun, A., L. Perez, and L. Carrasco. 1993. Brefeldin A blocks protein glycosylation and RNA replication of vesicular stomatitis virus. FEBS Lett. 336:496-500. [DOI] [PubMed] [Google Scholar]
- 23.Irurzun, A., L. Perez, and L. Carrasco. 1992. Involvement of membrane traffic in the replication of poliovirus genomes: effects of brefeldin A. Virology 191:166-175. [DOI] [PubMed] [Google Scholar]
- 24.Jantti, J., P. Hilden, H. Ronka, V. Makiranta, S. Keranen, and E. Kuismanen. 1997. Immunocytochemical analysis of Uukuniemi virus budding compartments: role of the intermediate compartment and the Golgi stack in virus maturation. J. Virol. 71:1162-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin, M., J. Park, S. Lee, B. Park, J. Shin, K. J. Song, T. I. Ahn, S. Y. Hwang, B. Y. Ahn, and K. Ahn. 2002. Hantaan virus enters cells by clathrin-dependent receptor-mediated endocytosis. Virology 294:60-69. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, K. 1999. Introduction, p. 1-6. In C. C. Ho Wang Lee and Connie Schmaljohn (ed.), Manual of hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. WHO Collaborating Center for Virus Reference and Research (Hantaviruses), Asian Institute for Life Sciences, Seoul, Korea.
- 27.Jonsson, C. B., J. Gallegos, P. Ferro, W. Severson, X. Xu, and C. S. Schmaljohn. 2001. Purification and characterization of the Sin Nombre virus nucleocapsid protein expressed in Escherichia coli. Protein Expr. Purif. 23:134-141. [DOI] [PubMed] [Google Scholar]
- 28.Jonsson, C. B., and C. S. Schmaljohn. 2001. Replication of hantaviruses. Curr. Top. Microbiol. Immunol. 256:15-32. [DOI] [PubMed] [Google Scholar]
- 29.Kariwa, H., H. Tanabe, T. Mizutani, Y. Kon, K. Lokugamage, N. Lokugamage, M. A. Iwasa, T. Hagiya, K. Araki, K. Yoshimatsu, J. Arikawa, and I. Takashima. 2003. Synthesis of Seoul virus RNA and structural proteins in cultured cells. Arch. Virol. 148:1671-1685. [DOI] [PubMed] [Google Scholar]
- 30.Kaukinen, P., V. Koistinen, O. Vapalahti, A. Vaheri, and A. Plyusnin. 2001. Interaction between molecules of hantavirus nucleocapsid protein. J. Gen. Virol. 82:1845-1853. [DOI] [PubMed] [Google Scholar]
- 31.Kaukinen, P., V. Kumar, K. Tulimaki, P. Engelhardt, A. Vaheri, and A. Plyusnin. 2004. Oligomerization of hantavirus N protein: C-terminal α-helices interact to form a shared hydrophobic space. J. Virol. 78:13669-13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaukinen, P., A. Vaheri, and A. Plyusnin. 2005. Hantavirus nucleocapsid protein: a multifunctional molecule with both housekeeping and ambassadorial duties. Arch. Virol. 150:1693-1713. [DOI] [PubMed] [Google Scholar]
- 33.Kaukinen, P., A. Vaheri, and A. Plyusnin. 2003. Mapping of the regions involved in homotypic interactions of Tula hantavirus N protein. J. Virol. 77:10910-10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaukinen, P., A. Vaheri, and A. Plyusnin. 2003. Non-covalent interaction between nucleocapsid protein of Tula hantavirus and small ubiquitin-related modifier-1, SUMO-1. Virus Res. 92:37-45. [DOI] [PubMed] [Google Scholar]
- 35.Kim, S., H.-Y. Kim, S. Lee, S. W. Kim, S. Sohn, K. Kim, and H. Cho. 2007. Hepatitis B virus X protein induces perinuclear mitochondrial clustering in microtubule- and dynein-dependent manners. J. Virol. 81:1714-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klumperman, J., J. K. Locker, A. Meijer, M. C. Horzinek, H. J. Geuze, and P. J. Rottier. 1994. Coronavirus M proteins accumulate in the Golgi complex beyond the site of virion budding. J. Virol. 68:6523-6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klumperman, J., A. Schweizer, H. Clausen, B. L. Tang, W. Hong, V. Oorschot, and H. P. Hauri. 1998. The recycling pathway of protein ERGIC-53 and dynamics of the ER-Golgi intermediate compartment. J. Cell Sci. 111:3411-3425. [DOI] [PubMed] [Google Scholar]
- 38.Kukkonen, S. K., A. Vaheri, and A. Plyusnin. 2004. Tula hantavirus L protein is a 250 kDa perinuclear membrane-associated protein. J. Gen. Virol. 85:1181-1189. [DOI] [PubMed] [Google Scholar]
- 39.Lakadamyali, M., M. J. Rust, and X. Zhuang. 2006. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell 124:997-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lake, J. A., J. Carr, F. Feng, L. Mundy, C. Burrell, and P. Li. 2003. The role of Vif during HIV-1 infection: interaction with novel host cellular factors. J. Clin. Virol. 26:143-152. [DOI] [PubMed] [Google Scholar]
- 41.Lee, H. W., and G. van der Groen. 1989. Hemorrhagic fever with renal syndrome. Prog. Med. Virol. 36:62-102. [PubMed] [Google Scholar]
- 42.Lee, P. W., C. J. Gibbs, Jr., D. C. Gajdusek, and R. Yanagihara. 1985. Serotypic classification of hantaviruses by indirect immunofluorescent antibody and plaque reduction neutralization tests. J. Clin. Microbiol. 22:940-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, X. D., T. P. Makela, D. Guo, R. Soliymani, V. Koistinen, O. Vapalahti, A. Vaheri, and H. Lankinen. 2002. Hantavirus nucleocapsid protein interacts with the Fas-mediated apoptosis enhancer Daxx. J. Gen. Virol. 83:759-766. [DOI] [PubMed] [Google Scholar]
- 44.Lippincott-Schwartz, J., L. C. Yuan, J. S. Bonifacino, and R. D. Klausner. 1989. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56:801-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackenzie, J. M., M. K. Jones, and E. G. Westaway. 1999. Markers for trans-Golgi membranes and the intermediate compartment localize to induced membranes with distinct replication functions in flavivirus-infected cells. J. Virol. 73:9555-9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maynell, L. A., K. Kirkegaard, and M. W. Klymkowsky. 1992. Inhibition of poliovirus RNA synthesis by brefeldin A. J. Virol. 66:1985-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mir, M. A., and A. T. Panganiban. 2005. The hantavirus nucleocapsid protein recognizes specific features of the viral RNA panhandle and is altered in conformation upon RNA binding. J. Virol. 79:1824-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mir, M. A., and A. T. Panganiban. 2004. Trimeric hantavirus nucleocapsid protein binds specifically to the viral RNA panhandle. J. Virol. 78:8281-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misumi, Y., Y. Misumi, K. Miki, A. Takatsuki, G. Tamura, and Y. Ikehara. 1986. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J. Biol. Chem. 261:11398-11403. [PubMed] [Google Scholar]
- 50.Murti, K. G., R. Goorha, and M. W. Klymkowsky. 1988. A functional role for intermediate filaments in the formation of frog virus 3 assembly sites. Virology 162:264-269. [DOI] [PubMed] [Google Scholar]
- 51.Nedellec, P., P. Vicart, C. Laurent-Winter, C. Martinat, M. C. Prevost, and M. Brahic. 1998. Interaction of Theiler's virus with intermediate filaments of infected cells. J. Virol. 72:9553-9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peters, C. J., and A. S. Khan. 2002. Hantavirus pulmonary syndrome: the new American hemorrhagic fever. Clin. Infect. Dis. 34:1224-1231. [DOI] [PubMed] [Google Scholar]
- 53.Radtke, K., K. Dohner, and B. Sodeik. 2006. Viral interactions with the cytoskeleton: a hitchhiker's guide to the cell. Cell. Microbiol. 8:387-400. [DOI] [PubMed] [Google Scholar]
- 54.Ravkov, E. V., and R. W. Compans. 2001. Hantavirus nucleocapsid protein is expressed as a membrane-associated protein in the perinuclear region. J. Virol. 75:1808-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ravkov, E. V., S. T. Nichol, C. J. Peters, and R. W. Compans. 1998. Role of actin microfilaments in Black Creek Canal virus morphogenesis. J. Virol. 72:2865-2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Risco, C., J. R. Rodriguez, C. Lopez-Iglesias, J. L. Carrascosa, M. Esteban, and D. Rodriguez. 2002. Endoplasmic reticulum-Golgi intermediate compartment membranes and vimentin filaments participate in vaccinia virus assembly. J. Virol. 76:1839-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez, J. R., C. Risco, J. L. Carrascosa, M. Esteban, and D. Rodriguez. 1997. Characterization of early stages in vaccinia virus membrane biogenesis: implications of the 21-kilodalton protein and a newly identified 15-kilodalton envelope protein. J. Virol. 71:1821-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roghi, C., and V. J. Allan. 1999. Dynamic association of cytoplasmic dynein heavy chain 1a with the Golgi apparatus and intermediate compartment. J. Cell Sci. 112:4673-4685. [DOI] [PubMed] [Google Scholar]
- 59.Rowe, R. K., and A. Pekosz. 2006. Bidirectional virus secretion and nonciliated cell tropism following Andes virus infection of primary airway epithelial cell cultures. J. Virol. 80:1087-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salanueva, I. J., J. L. Carrascosa, and C. Risco. 1999. Structural maturation of the transmissible gastroenteritis coronavirus. J. Virol. 73:7952-7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salmons, T., A. Kuhn, F. Wylie, S. Schleich, J. R. Rodriguez, D. Rodriguez, M. Esteban, G. Griffiths, and J. K. Locker. 1997. Vaccinia virus membrane proteins p8 and p16 are cotranslationally inserted into the rough endoplasmic reticulum and retained in the intermediate compartment. J. Virol. 71:7404-7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sanchez, V., E. Sztul, and W. J. Britt. 2000. Human cytomegalovirus pp28 (UL99) localizes to a cytoplasmic compartment which overlaps the endoplasmic reticulum-Golgi-intermediate compartment. J. Virol. 74:3842-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schliwa, M., and G. Woehlke. 2003. Molecular motors. Nature 422:759-765. [DOI] [PubMed] [Google Scholar]
- 64.Schmaljohn, C. 1996. Molecular biology of hantaviruses, p. 63-90. In R. Elliot (ed.), The Bunyaviridae. Plenum Press, New York, NY.
- 65.Schmaljohn, C., and B. Hjelle. 1997. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 3:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schmaljohn, C. S., and J. W. Hooper. 2001. Bunyaviridae: the viruses and their replication, p. 1581-1633. In B. N. Fields and D. M. Knipe (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 67.Sesso, A., F. P. de Faria, E. S. Iwamura, and H. Correa. 1994. A three-dimensional reconstruction study of the rough ER-Golgi interface in serial thin sections of the pancreatic acinar cell of the rat. J. Cell Sci. 107:517-528. [PubMed] [Google Scholar]
- 68.Severson, W., X. Xu, M. Kuhn, N. Senutovitch, M. Thokala, F. Ferron, S. Longhi, B. Canard, and C. B. Jonsson. 2005. Essential amino acids of the Hantaan virus N protein in its interaction with RNA. J. Virol. 79:10032-10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Severson, W. E., C. S. Schmaljohn, A. Javadian, and C. B. Jonsson. 2003. Ribavirin causes error catastrophe during Hantaan virus replication. J. Virol. 77:481-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith, A. E., and A. Helenius. 2004. How viruses enter animal cells. Science 304:237-242. [DOI] [PubMed] [Google Scholar]
- 71.Smith, G. A., and L. W. Enquist. 2002. Break ins and break outs: viral interactions with the cytoskeleton of mammalian cells. Annu. Rev. Cell Dev. Biol. 18:135-161. [DOI] [PubMed] [Google Scholar]
- 72.Sodeik, B. 2000. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 8:465-472. [DOI] [PubMed] [Google Scholar]
- 73.Sodeik, B., R. W. Doms, M. Ericsson, G. Hiller, C. E. Machamer, W. van't Hof, G. van Meer, B. Moss, and G. Griffiths. 1993. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J. Cell Biol. 121:521-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136:1007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spiropoulou, C. F. 2001. Hantavirus maturation. Curr. Top. Microbiol. Immunol. 256:33-46. [DOI] [PubMed] [Google Scholar]
- 76.Spiropoulou, C. F., C. S. Goldsmith, T. R. Shoemaker, C. J. Peters, and R. W. Compans. 2003. Sin Nombre virus glycoprotein trafficking. Virology 308:48-63. [DOI] [PubMed] [Google Scholar]
- 77.Stefanovic, S., M. Windsor, K. I. Nagata, M. Inagaki, and T. Wileman. 2005. Vimentin rearrangement during African swine fever virus infection involves retrograde transport along microtubules and phosphorylation of vimentin by calcium calmodulin kinase II. J. Virol. 79:11766-11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun, Y., D. H. Chung, Y. K. Chu, C. B. Jonsson, and W. B. Parker. 2007. Activity of ribavirin against Hantaan virus correlates with production of ribavirin-5′-triphosphate, not with inhibition of IMP dehydrogenase. Antimicrob. Agents Chemother. 51:84-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vallee, R. B., J. C. Williams, D. Varma, and L. E. Barnhart. 2004. Dynein: an ancient motor protein involved in multiple modes of transport. J. Neurobiol. 58:189-200. [DOI] [PubMed] [Google Scholar]
- 80.Vaughan, K. T., and R. B. Vallee. 1995. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J. Cell Biol. 131:1507-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vaughan, P. S., J. D. Leszyk, and K. T. Vaughan. 2001. Cytoplasmic dynein intermediate chain phosphorylation regulates binding to dynactin. J. Biol. Chem. 276:26171-26179. [DOI] [PubMed] [Google Scholar]
- 82.Xu, X., W. Severson, N. Villegas, C. S. Schmaljohn, and C. B. Jonsson. 2002. The RNA binding domain of the Hantaan virus N protein maps to a central, conserved region. J. Virol. 76:3301-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]