Abstract
Opioids, via the mu opioid receptor (MOR), can exacerbate bacterial infections and the immunopathogenesis of human immunodeficiency virus type 1 (HIV-1) infection. Recently, an HIV-1 transgenic (HIV-1Tg) rat model containing circulating HIV-1 gp120 was created. Using real-time reverse transcription-PCR, we found that MOR mRNA levels were significantly higher in the peritoneal macrophages of the HIV-1Tg rat than those in control animals. Lipopolysaccharide, a bacterial endotoxin, induced secretion of the inflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-β (IL-β), and IL-10 in the HIV-1Tg rat and further increased MOR expression. Ex vivo studies showed that MOR expression was up-regulated in the peritoneal macrophages of F344 control rats by exposure to serum from HIV-1Tg rats and that MOR up-regulation was abolished by addition of gp120 antibody to the serum. In human TPA-differentiated HL-60 cells, which are macrophage-like cells, LPS-induced MOR mRNA up-regulation was greater in gp120-pretreated cells than in vehicle-pretreated cells. Our data suggest that in individuals infected with HIV-1, the MOR is up-regulated, possibly by circulating HIV-1 proteins such as gp120, and HIV-1 proteins may play a significant role in modulating the response to bacterial infection in opioid-using HIV-infected individuals. Furthermore, our results demonstrate that the new HIV-1Tg rat model can be a valuable tool with which to study MOR gene expression and its effects in the continuous presence of HIV viral proteins.
The mu opioid receptor (MOR) mediates the actions of opioids such as morphine and is expressed in the central nervous system as well as on various cells of the immune system, including lymphocytes and macrophages (6, 28, 29, 37, 42, 48). In the immune system, activation of the MOR suppresses lymphocyte proliferation, decreases antibody production (9), and decreases macrophage-mediated immunity (18, 28, 38). Previous reports by Suzuki et al. have even shown that morphine changes the progression of human immunodeficiency virus (HIV) infection to AIDS in monkey models (14, 44).
Expression of the MOR can be modulated by various cytokines, including interleukin-1β (IL-1β) (46), tumor necrosis factor alpha (TNF-α) (26), IL-4 (27), and IL-6 (7). In response to microbial infections, both bacterial and viral, these proinflammatory cytokines, along with anti-inflammatory cytokines such as IL-10, are produced by the host's cells (15, 24). The balance between proinflammatory cytokines and anti-inflammatory cytokines may be directly related to the progression of HIV infection to AIDS (8).
The bacterial endotoxin lipopolysaccharide (LPS) is a well-characterized active complex glycolipid isolated from the outer membrane of gram-negative bacteria (19, 33). LPS is routinely used as a surrogate to study gram-negative bacterial infection, since it can induce an inflammatory response, such as production of cytokines (19, 31). We and others have previously shown that systemic administration of LPS induces secretion of TNF-α, IL-1β, and IL-6 into the circulation (23, 30, 45) and increases the expression of these cytokines in the brain (11, 45).
Macrophages play an essential role in modulating the initiation and perpetuation of the inflammatory response to bacterial infection. Rat peritoneal macrophages have been shown to respond to LPS by secreting proinflammatory cytokines, including TNF-α (45). In turn, these cytokines have also been demonstrated to mediate the actions of LPS (18). HIV-1 infection results from the actions of HIV-1 viral proteins, including the envelope protein, glycoprotein 120 (gp120), on the host's T cells and macrophages (12). gp120 mediates the binding of HIV-1 to CD4 receptors and chemokine coreceptors to initiate the entry of the virus into the immune cells (13). Unlike T cells, macrophages are relatively resistant to the cytopathic effects of the HIV-1 virus (39).
HL-60 promyelocytic leukemia cells are well-characterized, immortal cells capable of differentiating into macrophage-like cells by treatment with 12-O-tetradecanoyl-phorbol-13-acetate (TPA) (4, 5). We previously reported that MOR is expressed in TPA-differentiated HL-60 (TPA-HL-60) cells, and recently we showed that HIV gp120 induces the secretion of TNF-α from these cells, which then up-regulates the expression of the MOR in an autocrine/paracrine manner (3).
A novel, noninfectious HIV transgenic (HIV-1Tg) rat model was recently created by Reid et al. (36). The HIV-1Tg rat model carries an HIV-1 genome with gag and pol deleted under the control of the HIV-1 viral promoter and expresses seven of the nine HIV genes, including gp120 (36). Thus, there is no viral replication in the HIV-1Tg rat model. Even though the macrophages do not act as a viral reservoir in this animal model, the viral proteins are expressed in various organs and are present in the circulating blood (36). In addition, the HIV-1Tg rat develops many characteristics similar to those of humans infected with HIV-1, including expression of viral genes, immune response alterations, pathologies with advancing age (36), T-cell abnormalities (35), and kidney failure (34). There are also extensive and progressive neurobehavioral and neuropathologic changes within the brain parenchyma of these animals (36). Studies using the peritoneal macrophages isolated from the HIV-1Tg rat may lead to a better understanding of the functional roles of these immune cells in the course of HIV infection other than as viral reservoirs. Thus, these two models, HIV-1Tg rat peritoneal macrophages and TPA-HL-60 cells, are unique tools for studying the effects of an HIV viral protein such as gp120 on the immune system.
MATERIALS AND METHODS
Materials.
LPS and TPA were obtained from Sigma-Aldrich (St. Louis, MO). All cell culture materials were obtained from Gibco BRL, Gaithersburg, MD. A full-length recombinant HIV-1 IIIB gp120 glycoprotein (50 μg/500 μl in phosphate-buffered saline) was obtained from Immunodiagnostic, Inc. (Woburn, MA). HIV-1 IIIB anti-gp120 envelope glycoprotein was purchased from Advanced Biotechnologies, Inc. (Columbia, MD). TRIzol reagent for RNA extraction was obtained from Invitrogen (Grand Island, NY). Oligonucleotides for real-time reverse transcription-PCR (RT-PCR) of the MOR and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were synthesized and purified by Integrated DNA Technologies, Inc. (Coralville, IA). Enzyme-linked immunosorbent assay (ELISA) kits for rat TNF-α, IL-1β, and IL-10 were obtained from R&D Systems (Minneapolis, MN). Protein determination was assayed using Bio-Rad Bradford reagents (Hercules, CA).
Animals.
Male HIV-1 transgenic (HIV-1Tg) rats and parental wild-type inbred F344/NHsd control background strain rats, 5 to 12 months of age, were purchased from Harlan, Inc. (Indianapolis, IN). The animals were singly housed in standard plastic rodent cages in a temperature-controlled environment with standard rat diet and water available ad libitum in a 12-h light/dark cycle. The experimental procedures were approved by the Institutional Animal Care and Use Committee of Seton Hall University.
Treatment of animals.
To obtain serum for the measurement of cytokine levels, six HIV-1Tg rats and six F344 rats were randomly treated with a nonpyrogenic dosage of LPS (intraperitoneal [i.p.], 250 μg/kg of body weight) or saline. The dosage and duration of treatment were adapted from our previous studies reported by Ocasio et al. (30). At 2 h after injection, tail vein blood was collected from each animal for serum extraction. To obtain peritoneal macrophages for determination of MOR mRNA levels, six HIV-1Tg rats and six F344 rats were randomly treated with either LPS (i.p., 1.25 mg/kg) or saline. At 24 h after injection, the animals were sacrificed and the peritoneal macrophages harvested as previously described (41).
Preparation of peritoneal macrophages.
HIV-1Tg and F344 rats were sacrificed by asphyxiation using halothane (Halothane Laboratories) in an asphyxiation chamber. The peritoneal macrophages were isolated from the rats as previously described (41). Briefly, the peritoneal fluid was extracted and centrifuged at 200 × g for 10 min. The supernatant was discarded, and the cells were washed in Hanks' balanced salt solution, followed by centrifugation. The cells were then stained with trypan blue and counted on a hemacytometer. The cell density of the suspension was adjusted to 2 × 106/ml, and the cells were plated at 1 ml/well in a six-well plate in RPMI 1640 supplemented with 20% fetal bovine serum and 1% penicillin-streptomycin. The plates were incubated at 37°C in a 5% CO2 atmosphere. After 4 h of incubation, the cells were washed twice with phosphate-buffered saline to remove any nonadherent cells. The peritoneal macrophages isolated from the HIV-1Tg and F344 rats were then collected into cell pellets for RNA isolation.
Treatment of peritoneal macrophages.
The serum collected from the HIV-1Tg rats was heated for 30 min at 56°C to inactivate the complement system. The peritoneal macrophages isolated from the F344 rats were treated with either heated serum (prepared as above), heated serum plus gp120 monoclonal antibody, recombinant gp120 protein (1 nM), LPS (10 μg/ml), or control vehicle (culture medium) for 18 h. At the end of the treatment, the cells were harvested into cell pellets for RNA isolation and real-time RT-PCR.
ELISA for cytokines.
Two hours following the injection with LPS (i.p., 250 μg/kg), tail vein blood was collected from each animal. The blood samples were centrifuged at 4°C (1,000 × g) for 30 min to extract the serum. The sera were stored in aliquots at −80°C until use. ELISAs were performed according to the manufacturer's protocol (R&D Systems, Minneapolis, MN).
Cell culture.
HL-60 human promyelocytic leukemia cells were obtained from ATCC (Rockville, MD). HL-60 cells were cultured in RPMI 1640 medium containing 20% fetal bovine serum and 1% penicillin-streptomycin. The HL-60 cells were treated with 16 nM TPA in a control vehicle of 0.1% ethanol for 4 days. The cell culture medium containing TPA was changed every 48 h until the cells were differentiated into macrophage/monocytic-like cells (5), which were designated as TPA-HL-60 cells (3, 4).
Treatment of TPA-HL-60 cells.
The TPA-HL-60 cells were first pretreated with recombinant gp120 protein (1 nM) or control vehicle (culture medium) for 24 h. The cells were then treated with either 10 ng/ml or 100 ng/ml LPS or control vehicle (culture medium) for an additional 12 h. At the end of the subsequent treatment, the cells were harvested for RNA isolation.
RNA isolation and real-time RT-PCR.
Total RNA from peritoneal macrophage cells or TPA-HL-60 cells was isolated with TRIzol reagent according to the manufacturer's protocol (Invitrogen, Grand Island, NY). One microgram of total RNA was reverse transcribed into cDNA in a GeneAmp 2400 Thermocycler (Perkin-Elmer, Foster City, CA) for 1 h at 37°C and then for 10 min at 67°C. Real-time RT-PCR assays were performed to quantify the MOR mRNA expression in the TPA-HL-60 cells and rat peritoneal macrophages. A 2-μl aliquot of the cDNA was amplified by real-time RT-PCR in 50 μl of PCR master mix (25 μl of 1× PCR Mastermix, 400 nM of each primer, Taq-Man probe, in 24.7 μl of diethyl pyrocarbonate-H2O) using an ABI Prism 7000 (Applied Biosystems, Foster City, CA). The cDNA was amplified by real-time RT-PCR using the following human MOR sense primer, antisense primers, and TaqMan probe primers, respectively: 5′-TCATCATTACCGTGTGCTATGGA-3′, 5′-TCCTTTTCTTTGGAGCCAGAGAA-3′, and 5′-CTTGCGCCTCAAGAGTGTCCGCA-3. For human GAPDH, the sense, antisense, and TaqMan probe primers were 5′-GGAAGCTCACTGGCATGG-C-3′, 5′-CCCCACTGCCAACGTGTCAGTG-3′, and 5′-CCCCACTGCCAACGTGTCAGTG-3′. The rat MOR sense, antisense, and TaqMan probe primers were 5′-CAGCCCTTCCATGGTCACAG-3′, 5′-TACTGGTCGCTAAGGGGTCTG-3′, and 5′-CATTTTGGTGTATCTTACAATCACAT-3. The rat GAPDH sense, antisense, and TaqMan probe primers were 5′-GAACATCATCCCTGCATCCA-3′, 5′-CCAGTGAGCTTCCCGTTCA-3′, and 5′-CTTGCCCACACGCTTGGCAGC-3′. All TaqMan probes were labeled with the fluorophore 6-carboxyfluorescein at the 5′ end and a quencher, 4-(4′-dimethylaminophenylaso) benzoic acid (DABCYL), at the 3′ end (3, 4, 10). Thermal cycling conditions included an initial denaturation step for 10 min at 95°C and then 45 cycles for 18 s at 95°C for TPA-HL-60 cells and 60 cycles for 18 s at 95°C for rat peritoneal macrophages, followed by 1 min at 60°C. At the end of each PCR run, the data were automatically analyzed by the system and amplification plots were obtained. A MOR RNA standard curve was generated from a known concentration of RNA, and the quantity of MOR mRNA copies in the samples was calculated from the standard curve. For normalization of MOR mRNA levels, a GAPDH mRNA fragment in these cells was amplified and used in the calculations for the MOR mRNA copy number per microgram of total RNA. All amplification reactions were done in triplicate.
Statistical analysis.
All data are presented as the means ± standard error. All data were analyzed by a one-way analysis of variance followed by a Tukey's post hoc test. Statistical significance was accepted at P < 0.05.
RESULTS
MOR expression in peritoneal macrophages of HIV-1Tg rats.
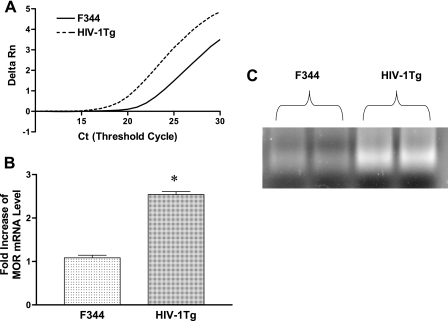
Using real-time RT-PCR, we first determined the basal level of MOR expression in the peritoneal macrophages of the HIV-1Tg rats compared to those of the F344 control rats. Figure 1A shows a representative amplification curve for rat MOR from HIV-1Tg rats (solid curve) and F344 animals (broken curve). The shift to the left of the amplification curve reflects the higher level of MOR mRNA in the HIV-1Tg rat peritoneal macrophages. The level of MOR mRNA was significantly higher (P < 0.05) in the peritoneal macrophages of the HIV-1Tg rats than those in the F344 animals (Fig. 1B). Agarose gel electrophoresis (Fig. 1C) confirmed the specificity of the single bands of the real-time RT-PCR products.
FIG. 1.
MOR mRNA levels in peritoneal macrophages of HIV-1Tg rats. MOR mRNA levels in the peritoneal macrophages from HIV-1Tg and F344 rats were compared using real-time RT-PCR (A and B). Delta Rn refers to the delta reaction that is the normalized intensity of fluorescence emission from the reporter dye for MOR while MOR is amplified as a function of the reaction cycle (Ct). Real-time RT-PCR of the cDNA from the peritoneal macrophages was run an additional 7 cycles after crossing the amplification threshold, and the PCR products were electrophoresed on a 3% agarose gel (C). n = 3; *, P < 0.05 compared to F344 control rats.
LPS-induced serum levels of TNF-α, IL-1β, and IL-10 in HIV-1Tg rats.
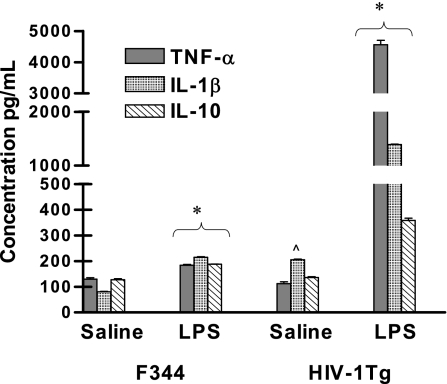
LPS, a bacterial endotoxin, induces the production of cytokines such as TNF-α, IL-1β, and IL-10, and TNF-α has been shown to regulate MOR expression in macrophages (3). Hence, we compared the LPS-induced serum levels of TNF-α, IL-1β, and IL-10 in HIV-1Tg and F344 rats (Fig. 2). Of the three cytokines examined, only the basal level of IL-1β was slightly increased in the HIV-1Tg rats given saline compared to that in the saline-treated F344 animals. Treatment with LPS increased the serum level of TNF-α and further increased the level of IL-1β about 1- and 1.5-fold, respectively, in the F344 rats compared to basal levels. The LPS-induced increase of both TNF-α and IL-1β in the F344 control rats was consistent with our previous report using healthy rats (30). In the HIV-1Tg rats, the LPS-induced increases in IL-1β and TNF-α were 7- and 38-fold higher, respectively, than those in the control F344 animals (P < 0.05). There was a 0.8-fold increase in IL-10 in response to LPS in the F344 rats (Fig. 2) and a 1.5-fold increase in IL-10 in the HIV-1Tg rats (P < 0.05) compared to basal levels.
FIG. 2.
LPS-induced serum levels of TNF-α, IL-1β, and IL-10 in HIV-1Tg rats. Serum levels of TNF-α, IL-1β, and IL-10 from HIV-1Tg and F344 rats were measured by ELISA 2 h following an i.p. injection with either saline or LPS (250 μg/kg). n = 3; *, P < 0.05 compared to saline treatment; ^, P < 0.05 compared to saline-treated F344 rats.
LPS-induced MOR expression in the peritoneal macrophages of the HIV-1Tg rats.
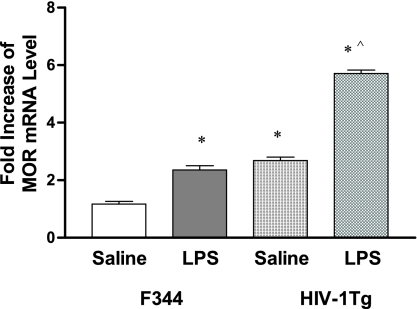
We then examined MOR expression in response to LPS treatment in the peritoneal macrophages of the HIV-1Tg rats compared to F344 control animals using real-time RT-PCR. LPS increased MOR mRNA levels in both the HIV-1Tg rats and F344 rats (Fig. 3) compared to saline (P < 0.05); however, the up-regulation of MOR expression in response to LPS in the HIV-1Tg rats (Fig. 3) was significantly higher than that in the F344 rats (P < 0.05).
FIG. 3.
LPS-induced MOR expression in peritoneal macrophages of HIV-1Tg rats. HIV-1Tg and F344 control rats were injected (i.p.) with either saline or LPS (1.25 mg/kg). Twenty-four hours after treatment, the animals were sacrificed and the peritoneal macrophages were isolated for analysis of MOR mRNA by real-time RT-PCR. n = 3; *, P < 0.05 compared to F344 rats given saline; ^, P < 0.05 compared to F344 rats given LPS.
MOR expression in the peritoneal macrophages of F344 rats treated with HIV-1Tg rat serum.
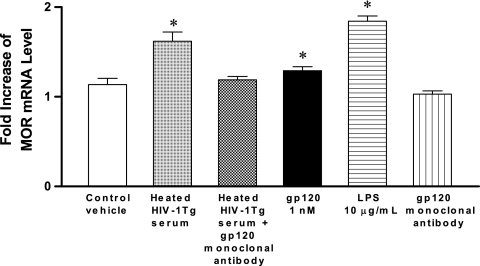
In order to examine whether circulating HIV-1 viral proteins such as gp120 are involved in regulating MOR expression in HIV-1Tg rats, peritoneal macrophages from F344 control rats were treated with either heated HIV-1Tg rat serum (20%), HIV recombinant gp120 protein (1 nM), LPS (10 μg/ml), gp120 monoclonal antibody, HIV-1Tg rat serum (20%) plus gp120 monoclonal antibody, or control vehicle (culture medium) for 18 h at 37°C. MOR mRNA expression was up-regulated in the F344 peritoneal macrophages treated with heated HIV-1Tg rat serum, recombinant gp120 protein alone, and LPS alone (Fig. 4) compared to that in cells treated with control vehicle (P < 0.05). gp120 monoclonal antibody alone had no effect on MOR expression (Fig. 4), and the addition of gp120 monoclonal antibody to the HIV-1Tg rat serum abolished the up-regulation of the MOR (Fig. 4) that occurred with HIV-1Tg serum alone.
FIG. 4.
Effects of HIV-1Tg serum on MOR expression in F344 rats. Peritoneal macrophages were isolated from six F344 control rats, and treated with either heated HIV-1Tg rat serum (20%), recombinant gp120 protein (1 nM), LPS (10 μg/ml), gp120 monoclonal antibody alone, heated HIV-1Tg rat serum (20%) plus gp120 monoclonal antibody, or control vehicle (culture medium) for 18 h at 37°C. The cells were harvested for total RNA isolation, and MOR mRNA levels were determined by real-time RT-PCR analysis. n = 3; *, P < 0.05 compared to control vehicle.
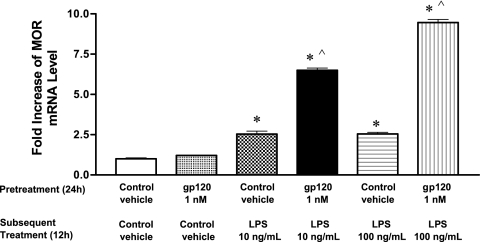
The effect of pretreatment with gp120 on LPS-induced up-regulation of MOR expression in TPA-HL-60 cells.
Our data in the HIV-1Tg rat model indicated that gp120 could augment the effects of LPS on MOR expression. To determine if this observation would translate into similar results in human immune cells, we pretreated human TPA-differentiated HL-60 cells with either 1 nM recombinant gp120 protein or control vehicle (culture medium) for 24 h. We then treated the cells with different concentrations of LPS (10 ng/ml or 100 ng/ml) for an additional 12 h and examined MOR mRNA expression using real-time RT-PCR (Fig. 5). The level of MOR mRNA was greater in the cells pretreated with gp120 followed by LPS (P < 0.05) than in the cells pretreated with control vehicle followed by LPS (P < 0.05). When the TPA-HL-60 cells were pretreated with gp120 for 24 h and then treated again with gp120 (1 nM), no difference in MOR expression was observed compared to treatment with control vehicle or no treatment at all (data not shown).
FIG. 5.
Effects of pretreatment with gp120 protein on LPS-induced up-regulation of MOR mRNA expression in TPA-HL-60 cells. TPA-HL-60 cells were pretreated with either vehicle medium or 1 nM recombinant gp120 protein for 24 h and then treated for an additional 12 h with control vehicle (culture medium) or different concentrations of LPS (10 ng/ml or 100 ng/ml). The effects of gp120 pretreatment on LPS-induced up-regulation of MOR expression were then examined by real-time RT-PCR. n = 3; *, P < 0.05 compared to control vehicle; ^, P < 0.05 compared to treatment with vehicle followed by LPS at 10 ng/ml and 100 ng/ml, respectively.
DISCUSSION
The MOR was originally discovered in the central nervous system. However, during the last two decades, the expression of the MOR in the immune system has been well characterized (6, 28, 29, 37, 42, 48). Activation of the MOR by agonists such as morphine can result in immunosuppression (18, 28, 38). Many individuals infected with the HIV-1 virus are opioid abusers (32), and even HIV-1 patients who are not drug abusers sometimes receive treatment with a MOR agonist, including morphine, for pain management (32). In addition, in vitro studies by Guo et al. demonstrated that morphine increases HIV-1 infection of human monocyte-derived macrophages (20).
HIV-1 infection is marked by an array of pathologies and a variety of secondary infections (25), and HIV-positive patients often contract bacterial infections (25). Few studies, thus far, have examined the interactive effects of bacterial endotoxins and viral proteins in the course of HIV infection using either animal models or human subjects. In this study, we used both the HIV-1Tg rat model and human cell cultures to investigate the interaction between the bacterial endotoxin LPS and the HIV-1 glycoprotein gp120 on MOR expression.
Highly active antiretroviral therapy (HAART) consists of inhibitors that target viral entry, reverse transcriptase, and viral protease to control viral replication, restore immunity, and delay disease progression, but it cannot eliminate the infection (1, 25, 47). The clinical challenge in this post-HAART era is, therefore, the persistent infection that results from the presence of HIV-1 viral proteins in the host (25, 47). The development of various manifestations of human HIV-1 infection in the HIV-1Tg rat, without viral replication, indicates that the presence of viral proteins in the host is sufficient to affect the target cells, including the immune cells such as T cells and macrophages, and cause the clinical progression to AIDS. Thus, the HIV-1Tg rat appears to mimic the condition of patients given HAART, who have limited (controlled) viral replication but persistent HIV infection that eventually advances to AIDS.
In this study, we were particularly interested in observing the effects of viral proteins such as gp120 on immune cells in vivo in the absence of viral replication. Macrophages have long been known to act as reservoirs for the HIV-1 virus, where the virus can replicate and remain latent (39). These cells have been shown to harbor the HIV-1 virus in both the peripheral blood and bone marrow as well as in target organs such as the brain, lungs, lymph nodes, and skin (22, 38, 40). In contrast, the macrophages in the HIV-1Tg rat model do not act as viral reservoirs; however, viral proteins are still being expressed in blood and tissues. Thus, the peritoneal macrophages in the HIV-1Tg rat can be used to help delineate the effects of viral proteins such as gp120 on immune function in the absence of viral replication.
Our data show that the basal level of MOR mRNA in the peritoneal macrophages of the HIV-1Tg rats is significantly higher than that in the F344 control animals, indicating that the HIV-1 transgene with the gag-pol defect may itself increase MOR expression. As gag and pol are mainly responsible for HIV viral replication, our results suggest that one or more of the other seven remaining viral proteins may be involved in the up-regulation of MOR expression. In addition, since the macrophages do not act as the viral reservoir as in the patients infected with HIV-1, the information obtained using peritoneal macrophages can apply to any cells on which the HIV viral proteins exert their actions.
Immunocompromised HIV-positive patients often contract secondary bacterial infections (25). LPS, a bacterial endotoxin, is commonly used to study a host's response to a bacterial infection, including the production of inflammatory cytokines. We have previously shown that systemic treatment with a nonpyrogenic dose of LPS increases the serum levels of proinflammatory cytokines, including TNF-α, IL-1β, and IL-6, in healthy Sprague-Dawley rats (30). In this study, we found that in F344 control rats, there were comparable increases in the serum levels of IL-1β and TNF-α (1- to 1.5-fold increases, respectively) following treatment with the same nonpyrogenic dose of LPS (30). However, in the HIV-1Tg rats, the same dose of LPS induced a much greater increase in serum IL-1β and TNF-α levels (7- and 38-fold increases, respectively). In contrast to the robust increase in serum IL-1β and TNF-α levels induced by LPS in HIV-1Tg rats, the LPS-induced increases in the serum level of IL-10, an anti-inflammatory cytokine, in the HIV-1Tg rats and F344 control animals were more comparable (0.8- versus 1.5-fold increases, respectively). Taken together, our data suggest that there may be an imbalance between the levels of production of pro- and anti-inflammatory cytokines in response to bacterial endotoxin in HIV-1-infected individuals. Such an imbalance has been reported to be related to the progression of HIV-1 infection to AIDS (8), and, thus, this imbalance can have detrimental consequences.
We and others have demonstrated that the MOR is constitutively expressed in peritoneal macrophages isolated from rats and mice (37; unpublished data). In this study, although MOR expression in the peritoneal macrophages of both HIV-1Tg rats and F344 control animals was increased following systemic treatment with LPS, the LPS-induced up-regulation of MOR expression was significantly greater in the HIV-1Tg rats than in the F344 control animals. This is the first in vivo study to demonstrate that not only does LPS up-regulate the MOR but the LPS-induced up-regulation of the MOR is greater in the presence of HIV proteins.
In this study, we used an ex vivo approach to show that serum prepared from the HIV-1Tg rats up-regulated MOR mRNA in the peritoneal macrophages isolated from the F344 control animals. Furthermore, preincubation of HIV-1Tg serum with a monoclonal antibody to human gp120 abolished the up-regulation of MOR mRNA in the F344 peritoneal macrophages. These data not only confirm our observation that MOR mRNA levels are significantly higher in the HIV-1Tg rats than in the F344 animals but also suggest that circulating viral proteins in the HIV-1Tg rat, specifically gp120, may be involved in the up-regulation of the MOR.
Many laboratories, including our own, routinely use HL-60 promyelocytic leukemia cells as an in vitro macrophage cell model because of their ability to differentiate into macrophage-like cells in response to treatment with TPA (4, 5). We have previously shown that TPA-HL-60 cells respond to gp120 stimulation by secreting TNF-α and up-regulating MOR expression (3). To demonstrate that our observations in the HIV-1Tg rat model could have clinical implications, we showed that pretreatment with gp120 potentiates LPS-induced up-regulation of MOR expression in human TPA-HL-60 cells. Taken together, both our in vitro and ex vivo studies suggest that there may be some underlying molecular and cellular mechanisms that would explain why HIV-1-infected individuals who use opioids are significantly more susceptible to opportunistic bacterial infections. There have been a number of studies showing that the use or abuse of morphine can have adverse effects on immune function (16, 17, 30). Several previous studies have shown that exposure to opioids induces septic shock, which leads to a compromised immune response to bacterial infection (16, 17, 23, 30). Morphine use can result in immunosuppression, which can further aggravate the progression of HIV infection to full-blown AIDS (2, 21, 32, 43). Our results show that the MOR is up-regulated in the presence of HIV proteins such as gp120. Furthermore, these results indicate that the HIV-infected individual could be placed in an immune deficit situation via the actions of HIV proteins on MOR expression. Since HIV-infected patients often contract bacterial infections and, in addition, may also be given a MOR agonist for pain management, our data provide important information with significant clinical implications.
Acknowledgments
This work was partially supported by National Institutes of Health PHS grants R01 (DA007058), K02 (DA016149), and R21 (DA019836) to S.L.C.
Footnotes
Published ahead of print on 6 June 2007.
REFERENCES
- 1.Barbaro, G., A. Scozzafava, A. Mastrolorenzo, and C. T. Supuran. 2005. Highly active antiretroviral therapy: current state of the art, new agents and their pharmacological interactions useful for improving therapeutic outcome. Curr. Pharm. Des. 11:1805-1843. [DOI] [PubMed] [Google Scholar]
- 2.Beck, M., A. Mirmohammadsadegh, B. Franz, J. Blanke, and U. R. Hengge. 2002. Opioid receptors on white blood cells: effect of HIV infection and methadone treatment. Pain 98:187-194. [DOI] [PubMed] [Google Scholar]
- 3.Beltran, J. A., A. Pallur, and S. L. Chang. 2006. HIV-1 gp120 up-regulation of the mu opioid receptor in TPA-differentiated HL-60 cells. Int. Immunopharmacol. 6:1459-1467. [DOI] [PubMed] [Google Scholar]
- 4.Beltran, J. A., J. Peek, and S. L. Chang. 2006. Expression and regulation of the mu opioid peptide receptor in TPA-differentiated HL-60 promyelocytic leukemia cells. Int. Immunopharmacol. 6:1331-1340. [DOI] [PubMed] [Google Scholar]
- 5.Bestilny, L. J., and K. T. Riabowol. 2000. A role for serine proteases in mediating phorbol ester-induced differentiation of HL-60 cells. Exp. Cell Res. 256:264-271. [DOI] [PubMed] [Google Scholar]
- 6.Bidlack, J. M. 2000. Detection and function of opioid receptors on cells from the immune system. Clin. Diagn. Lab. Immunol. 7:719-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Börner, C., J. Kraus, H. Schroder, H. Ammer, and V. Höllt. 2004. Transcriptional regulation of the human mu-opioid receptor gene by interleukin-6. Mol. Pharmacol. 66:1719-1726. [DOI] [PubMed] [Google Scholar]
- 8.Breen, E. C. 2002. Pro- and anti-inflammatory cytokines in human immunodeficiency virus infection and acquired immunodeficiency syndrome. Pharmacol. Ther. 95:295-304. [DOI] [PubMed] [Google Scholar]
- 9.Bussiere, J. L., M. W. Adler, T. J. Rogers, and T. K. Eisenstein. 1993. Cytokine reversal of morphine-induced suppression of the antibody response. J. Pharmacol. Exp. Ther. 264:591-597. [PubMed] [Google Scholar]
- 10.Chang, S. L., and M. Vigorito. 2006. Role of HIV-1 infection in addictive behavior: a study of a HIV-1 transgenic rat model. Am. J. Infect. Dis. 2:98-106. [Google Scholar]
- 11.Chen, R., H. Zhou, J. Beltran, L. Malellari, and S. L. Chang. 2005. Differential expression of cytokines in the brain and serum during endotoxin tolerance. J. Neuroimmunol. 163:53-72. [DOI] [PubMed] [Google Scholar]
- 12.Cicala, C., J. Arthos, S. M. Selig, G. Dennis, Jr., D. A. Hosack, D. VanRyk, M. L. Spangler, T. D. Steenbeke, P. Khazanie, N. Gupta, J. Yang, M. Daucher, R. A. Lempicki, and A. S. Fauci. 2002. HIV envelope induces a cascade of cell signals in non-proliferating target cells that favor virus replication. Proc. Natl. Acad. Sci. USA 99:9380-9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conti, L., L. Fantuzzi, M. Del Corno, F. Belardelli, and S. Gessani. 2004. Immunomodulatory effects of the HIV-1 gp120 protein on antigen presenting cells: implications for AIDS pathogenesis. Immunobiology 209:99-115. [DOI] [PubMed] [Google Scholar]
- 14.Donahoe, R. M. 2004. Multiple ways that drug abuse might influence AIDS progression: clues from a monkey model. J. Neuroimmunol. 147:28-32. [DOI] [PubMed] [Google Scholar]
- 15.Eskandari, F., J. I. Webster, and E. M. Sternberg. 2003. Neural immune pathways and their connection to inflammatory diseases. Arthritis Res. Ther. 5:251-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng, P., J. J. Meissler, Jr., M. W. Adler, and T. K. Eisenstein. 2005. Morphine withdrawal sensitizes mice to lipopolysaccharide: elevated TNF-alpha and nitric oxide with decreased IL-12. J. Neuroimmunol. 164:57-65. [DOI] [PubMed] [Google Scholar]
- 17.Feng, P., A. L. Truant, J. J. Meissler, Jr., J. P. Gaughan, M. W. Adler, and T. K. Eisenstein. 2006. Morphine withdrawal lowers host defense to enteric bacteria: spontaneous sepsis and increased sensitivity to oral Salmonella enterica serovar Typhimurium infection. Infect. Immun. 74:5221-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman, H., C. Newton, and T. W. Klein. 2003. Microbial infections, immunomodulation, and drugs of abuse. Clin. Microbiol. Rev. 16:209-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujihara, M., M. Muroi, K. I. Tanamoto, T. Suzuki, H. Azuma, and H. Ikeda. 2003. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol. Ther. 100:171-194. [DOI] [PubMed] [Google Scholar]
- 20.Guo, C. J., Y. Li, S. Ting, X. Wang, S. D. Douglas, and W. Z. Ho. 2002. Morphine enhances HIV infection of human blood mononuclear phagocytes through modulation of beta-chemokines and CCR5 receptor. J. Investig. Med. 50:435-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauser, K. F., N. El-Hage, S. Buch, J. R. Berger, W. R. Tyor, A. Nath, A. J. Bruce-Keller, and P. E. Knapp. 2005. Molecular targets of opiate drug abuse in neuroAIDS. Neurotoxicol. Res. 8:63-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herbein, G., S. Keshav, M. Collin, L. J. Montaner, and S. Gordon. 1994. HIV-1 induces tumour necrosis factor and IL-1 gene expression in primary human macrophages independent of productive infection. Clin. Exp. Immunol. 95:442-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hilburger, M. E., M. W. Adler, A. L. Truant, J. J. Meissler, Jr., V. Satishchandran, T. J. Rogers, and T. K. Eisenstein. 1997. Morphine induces sepsis in mice. J. Infect. Dis. 176:183-188. [DOI] [PubMed] [Google Scholar]
- 24.Holguin, A., K. A. O'Connor, J. Biedenkapp, J. Campisi, J. Wieseler-Frank, E. D. Milligan, M. K. Hansen, L. Spataro, E. Maksimova, C. Bravmann, D. Martin, M. Fletcher, S. F. Maier, and L. R. Watkin. 2004. HIV-1 gp120 stimulates proinflammatory cytokine-mediated pain facilitation via activation of nitric oxide synthase-I (nNOS). Pain 110:517-530. [DOI] [PubMed] [Google Scholar]
- 25.Jones, L. E., and A. S. Perelson. 2005. Opportunistic infection as a cause of transient viremia in chronically infected HIV patients under treatment with HAART. Bull. Math. Biol. 67:1227-1251. [DOI] [PubMed] [Google Scholar]
- 26.Kraus, J., C. Börner, E. Giannini, and V. Höllt. 2003. The role of nuclear factor kappaB in tumor necrosis factor-regulated transcription of the human mu-opioid receptor gene. Mol. Pharmacol. 64:876-884. [DOI] [PubMed] [Google Scholar]
- 27.Kraus, J., C. Börner, E. Giannini, K. Hickfang, H. Braun, P. Mayer, M. R. Hoehe, A. Ambrosch, W. König, and V. Höllt. 2001. Regulation of mu-opioid receptor gene transcription by interleukin-4 and influence of an allelic variation within a STAT6 transcription factor binding site. J. Biol. Chem. 276:43901-43908. [DOI] [PubMed] [Google Scholar]
- 28.McCarthy, L., I. Szabo, J. F. Nitsche, J. E. Pintar, and T. J. Rogers. 2001. Expression of functional mu-opioid receptors during T cell development. J. Neuroimmunol. 114:173-180. [DOI] [PubMed] [Google Scholar]
- 29.Miyagi, T., L. F. Chuang, K. M. Lam, H. Kung, J. M. Wang, B. I. Osbum, and R. Y. Chuang. 2000. Opioids suppress chemokine-mediated migration of monkey neutrophils and monocytes—an instant response. Immunopharmacology 47:53-62. [DOI] [PubMed] [Google Scholar]
- 30.Ocasio, F. M., Y. Jiang, S. D. House, and S. L. Chang. 2004. Chronic morphine accelerates the progression of lipopolysaccharide-induced sepsis to septic shock. J. Neuroimmunol. 149:90-100. [DOI] [PubMed] [Google Scholar]
- 31.Prabhakar, U., T. M. Conway, P. Murdock, J. L. Mooney, S. Clark, P. Hedge, B. C. Bond, E. C. Jazwinska, M. R. Barnes, F. Tobin, V. Damien-Iordachi, L. Greller, M. Hurle, A. P. Stubbs, Z. Li, E. I. Valoret, C. Erickson-Miller, L. Cass, B. Levitt, H. M. Davis, D. K. Jorkasky, and W. V. Williams. 2005. Correlation of protein and gene expression profiles of inflammatory proteins after endotoxin challenge in human subjects. DNA Cell Biol. 24:410-431. [DOI] [PubMed] [Google Scholar]
- 32.Quang-Cantagrel, N. D., M. S. Wallace, N. Ashar, and C. Mathews. 2001. Long-term methadone treatment: effect on CD4+ lymphocyte counts and HIV-1 plasma RNA level in patients with HIV infection. Eur. J. Pain 5:415-420. [DOI] [PubMed] [Google Scholar]
- 33.Raetz, C. R., and C. Whitfield. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71:635-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray, P. E., X. H. Liu, L. R. Robinson, W. Reid, L. Xu, J. W. Owens, O. D. Jones, F. Denaro, H. G. Davis, and J. L. Bryant. 2003. A novel HIV-1 transgenic rat model of childhood HIV-1-associated nephropathy. Kidney Int. 63:2242-2253. [DOI] [PubMed] [Google Scholar]
- 35.Reid, W., S. Abdelwahab, M. Sadowska, D. Huso, A. Neal, A. Aheam, J. Bryant, R. C. Gallo, G. K. Lewis, and M. Reitz. 2004. HIV-1 transgenic rats develop T cell abnormalities. Virology 321:111-119. [DOI] [PubMed] [Google Scholar]
- 36.Reid, W., M. Sadowska, F. Denaro, S. Rao, F. Foulke, Jr., N. Hayes, O. Jones, D. Doodnauth, H. Davis, A. Sill, P. O'Driscoll, D. Husso, T. Fouts, G. Lewis, M. Hill, R. Kamin-Lewis, C. Wei, P. Ray, R. C. Gallo, M. Reitz, and J. Bryant. 2001. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc. Natl. Acad. Sci. USA 98:9271-9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy, S., J. H. Wang, S. Balasubramanian, S. Sumandeep, R. Charboneau, R. Barke, and H. H. Loh. 2001. Role of hypothalamic-pituitary axis in morphine-induced alteration in thymic cell distribution using mu-opioid receptor knockout mice. J. Neuroimmunol. 116:147-155. [DOI] [PubMed] [Google Scholar]
- 38.Roy, S., J. Wang, J. Kelshenbach, L. Koodie, and J. Martin. 2006. Modulation of immune function by morphine: implication for susceptibility to infection. J. Neuroimmune Pharmacol. 1:77-89. [DOI] [PubMed] [Google Scholar]
- 39.Roy, S., and M. A. Wainberg. 1988. Role of the mononuclear phagocyte system in the development of acquired immunodeficiency syndrome (AIDS). J. Leukoc. Biol. 43:91-97. [DOI] [PubMed] [Google Scholar]
- 40.Sanduzzi, A., M. Fraziano, and F. Mariani. 2001. Monocytes/macrophages in HIV infection and tuberculosis. J. Biol. Regul. Homeost. Agents 15:294-298. [PubMed] [Google Scholar]
- 41.Sedqi, M., S. Roy, S. Ramakrishnan, R. Elde, and H. H. Loh. 1995. Complementary DNA cloning of a mu-opioid receptor from rat peritoneal macrophages. Biochem. Biophys. Res. Commun. 209:563-574. [DOI] [PubMed] [Google Scholar]
- 42.Sharp, B. M. 2004. Opioid receptor expression and function. J. Neuroimmunol. 147:3-5. [DOI] [PubMed] [Google Scholar]
- 43.Steele, A. D., E. E. Henderson, and T. J. Rogers. 2003. Mu-opioid modulation of HIV-1 coreceptor expression and HIV-1 replication. Virology 309:99-107. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki, S., A. J. Chuang, L. F. Chung, R. H. Doi, and R. Y. Chung. 2002. Morphine promotes simian acquired immunodeficiency syndrome virus replication in monkey peripheral mononuclear cells: induction of CC chemokine receptor 5 expression for virus entry. J. Infect. Dis. 185:1826-1829. [DOI] [PubMed] [Google Scholar]
- 45.Turrin, N. P., D. Gayle, S. E. Ilyin, M. C. Flynn, W. Langhans, G. J. Schwartz, and C. R. Plata-Salaman. 2001. Pro-inflammatory and anti-inflammatory cytokine mRNA induction in the periphery and brain following intraperitoneal administration of bacterial lipopolysaccharide. Brain Res. Bull. 54:443-453. [DOI] [PubMed] [Google Scholar]
- 46.Vidal, E. L., N. A. Patel, G. Wu, M. Fiala, and S. L. Chang. 1998. Interleukin-1 induces the expression of mu opioid receptors in endothelial cells. Immunopharmacology 38:261-266. [DOI] [PubMed] [Google Scholar]
- 47.Vigano, A., D. Trabattoni, L. Schneider, F. Ottaviani, A. Aliffi, E. Longhi, S. Rusconi, and M. Clerici. 2006. Failure to eradicate HIV despite fully successful HAART initiated in the first days of life. J. Pediatr. 148:389-391. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, N., and J. J. Oppenheim. 2005. Crosstalk between chemokines and neuronal receptors bridges immune and nervous systems. J. Leukoc. Biol. 78:1210-1214. [DOI] [PubMed] [Google Scholar]