Abstract
Virus-specific CD4 T cells are endowed with multiple functions, such as cytokine production, CD40 ligand (CD40L) expression (associated with the costimulation of CD8 and B cells), and degranulation (associated with cytotoxic potential). Here, we used antiviral CD4 T cells present in human blood to evaluate the relationship between cytokine production and other functions of CD4 T cells. Antiviral CD4 T cells specific for a virus causing persistent infection, cytomegalovirus (CMV), and two viruses causing nonpersistent infections, influenza virus and the smallpox vaccine virus (vaccinia virus), were studied. CD4 T cells specific for each of the viruses produced all seven possible combinations of the cytokines gamma interferon (IFN-γ), interleukin-2, and tumor necrosis factor alpha. Cells producing three or two cytokines (triple producers and double producers) represented nearly 50% of the total response to each of the viruses. Triple producers expressed the highest levels of cytokines per cell, and single producers expressed the lowest. Following stimulation, higher frequencies of triple producers than single producers expressed CD40L. Only CMV-specific CD4 T cells underwent degranulation. However, higher frequencies of CMV-specific triple producers than single producers showed this functional characteristic. In contrast to the functional phenotypes, the memory phenotypes of triple producers and IFN-γ single producers did not differ. These results demonstrate a strong positive association between the cytokine coproduction capacity of a virus-specific CD4 T cell and its other functional characteristics and suggest that vaccines should aim to elicit T cells that coproduce more than one cytokine.
Antiviral CD4 T cells play a vital role in the control of many viral infections. CD4 T cells are critical for the generation of functional CD8 T cells and neutralizing antibody. CD8 T-cell priming in the absence of CD4 help results in the generation of CD8 T cells that are defective in their ability to expand following a secondary antigen exposure (7, 15, 22, 24). CD4 T-cell help is also critical for the maintenance of functional CD8 responses (25). Similarly, CD4 T cells are crucial for the entry of B cells into germinal center reaction sites for affinity maturation, a process that facilitates the generation of neutralizing antibody and the formation of memory B cells (10, 17).
Characteristics that are important for T-cell function in the control of viral infections include the production of cytokines, such as gamma interferon (IFN-γ), interleukin-2 (IL-2), and tumor necrosis factor alpha (TNF-α) (12, 23, 32), and proliferative capacity. Functional CD8 T-cell exhaustion is associated with a progressive loss of cytokine production and proliferative capacity, with the loss of IL-2 production being the first indicator of T-cell exhaustion, followed by the loss of TNF-α production (31). Apart from cytokine secretion, other indicators of CD4 T-cell function include costimulation via the expression of the CD40 ligand (CD40L) and cytotoxicity. Cytolytic CD4 T cells have been shown to help control chronic viral infections (16, 18, 27). The degranulation potential, measured as the surface mobilization of CD107a and CD107b, of CD8 (3) and CD4 (8) T cells has been shown to be associated with the cytolytic potential of these cells. Thus, CD4 T cells are endowed with a multitude of functions, and the coexpression of more than one function may be associated with enhanced viral control, as has been shown previously for human immunodeficiency virus-specific CD8 T cells (4).
There are very limited data available on the cytokine coexpression profiles of virus-specific CD4 T cells (6, 8, 9, 11, 13, 14), and a comprehensive analysis of the relationship between the ability of virus-specific CD4 T cells to coexpress multiple cytokines and other functions has not been performed. With the availability of multicolor flow cytometry, it is now possible to simultaneously measure multiple functions of virus-specific CD4 T cells (19). Here, we evaluated the cytokine coexpression profiles of CD4 T cells specific for antigens of different viruses causing human infections and then further evaluated the levels of cytokine expression, CD40L expression (costimulatory potential), and degranulation (cytotoxic potential). Our results demonstrate that triple producers are functionally superior to single producers not only in the numbers but also in the amounts of cytokines produced per cell and in costimulatory and degranulation potential. The results also demonstrate a strong positive association between the multiple-cytokine production capacity of virus-specific CD4 T cells and their other functions.
MATERIALS AND METHODS
Study participants.
Normal healthy subjects with measurable CD4 T-cell responses to vaccinia virus (n = 5)-, cytomegalovirus (CMV; n = 10)-, or influenza (flu) virus (n = 12)-specific antigens were recruited into the study. These individuals were identified based on the reactivity of their blood samples to the respective viral antigens as assessed by an intracellular cytokine staining assay. All healthy volunteers were employees of Emory University, Atlanta, GA. The Emory University Institutional Review Board approved this study. Signed forms indicating informed consent were obtained from all individuals before enrollment in the study.
Antigens and peptides.
Virus-specific cytokine-producing CD4 T cells were assayed by stimulating peripheral blood mononuclear cells (PBMC) with viral antigens. CMV, flu virus, and control lysates were obtained from Institute Virion Ltd., Ruschlikon, Switzerland. Vaccinia virus strain WR was used to study vaccinia virus-specific immune responses as previously described (1). Staphylococcal enterotoxin B (SEB; Toxin Technology Inc., Sarasota, FL) was used as a positive control.
Intracellular cytokine staining analysis using polychromatic flow cytometry.
Intracellular cytokine production was assessed as previously described (2) with a few modifications. Briefly, 2 million PBMC were resuspended in 100 μl of RPMI plus 10% fetal bovine serum (FBS) in a 5-ml polypropylene tube and stimulated in a total volume of 200 μl. The CMV lysate and the control lysate for CMV were used at 1:20 final dilution, and the flu virus lysate and the corresponding control lysate were used at a 1:100 final dilution. SEB was used at a concentration of 1 μg/ml. Stimulations were conducted in the presence of anti-CD28 antibody and anti-CD49d antibody (1 μg/ml; BD Pharmingen). Cells were incubated at 37°C in the presence of 5% CO2. Brefeldin A (10 μg per ml) was added after 2 h of incubation. Tube contents were mixed briefly, and tubes were then incubated for an additional 4 h. For vaccinia virus-specific responses, approximately 5 × 106 PFU of vaccinia virus strain WR (multiplicity of infection of ∼2) in a volume of 100 μl was added. After 6 h of incubation at 37°C, brefeldin A was added and cells were cultured for an additional 4 h. Cells were washed once with cold phosphate-buffered saline (PBS) containing 2% FBS; surface stained with anti-human CD4-peridinin chlorophyll protein (clone L200; BD Pharmingen), anti-human CD8-AmCyan (clone SK1; BD Biosciences), anti-human CCR7-fluorescein isothiocyanate (CCR7-FITC; clone 150503 [R&D Systems]), and anti-human CD45RA-phycoerythrin (CD45RA-PE; clone ALB11 [Beckman Coulter]); fixed with Cytofix/Cytoperm (BD Pharmingen); and permeabilized with 1× PermWash (BD Pharmingen). Cells were then incubated for 30 min at 4°C with a cocktail of three anti-human monoclonal antibodies conjugated to different fluorochromes for the detection of cytokines IFN-γ, IL-2, and TNF-α. Cells were washed twice with 1× PermWash and once with 2% FBS in PBS and resuspended in 1% formalin in PBS. Approximately 500,000 lymphocytes were acquired on the LSRII system (BD Immunocytometry Systems) and analyzed using FlowJo software (Treestar, Inc., San Carlos, CA). Lymphocytes were identified based on their scatter patterns, and CD3+, CD8−, CD4+ cells were considered to be CD4 T cells. These CD4 T cells were then gated for cells positive for the respective cytokines. Boolean combination gating was then performed (see http://www.flowjo.com/v8/html/boolcomb.html for an example) to calculate the frequencies of expression profiles corresponding to the seven different combinations of cytokines by using the FlowJo software. After subtracting the background values, the proportions of the seven different subsets were expressed as percentages of total cytokine-positive cells and the results for each antigen per donor were plotted. Responses corresponding to more than 0.07% of total CD4 T cells were considered for analysis. This criterion was defined based on the facts that we were dividing the total response into seven subsets and that our detection limit was 0.01%.
To assess the specificity of the assay, we stimulated PBMC obtained from seven Dryvax-naïve and nine CMV-seronegative individuals with the respective viral lysates and analyzed the PBMC for the frequency of IFN-γ-, IL-2-, or TNF-α-producing CD4 T cells by using the same conditions described above. The magnitude of responses to any of the three viruses corresponded to less than 0.02% of the total CD4 T cells (data not shown).
For the analysis of degranulation and costimulatory potential, cells were prestained with anti-human CD107a-FITC (clone H4A3; BD Pharmingen) and anti-human CD107b-FITC (clone H4B4; BD Pharmingen) and stimulated in the presence of these antibodies. At the end of stimulation, cells were stained as described above except that anti-human CD154-PE (clone TRAP1; BD Pharmingen) was used in place of the CD45RA antibody and the CCR7-FITC antibody was excluded (Table 1).
TABLE 1.
Staining panel for multicolor flow cytometry
| Antibody | Fluorochrome(s)a | Clone | Company |
|---|---|---|---|
| CD107a | FITC | H4A3 | BD Pharmingen |
| CD107b | FITC | H4B4 | BD Pharmingen |
| CD154 | PE | TRAP1 | BD Pharmingen |
| CD4 | PerCP | L200 | BD Pharmingen |
| IL-2 | APC | MQ1-17H12 | BD Pharmingen |
| IFN-γ | Alexa Fluor 700 | B27 | BD Pharmingen |
| TNF-α | PE-Cy7 | MAb11 | eBiosciences |
| CD3 | Pacific blue | SP34.2 | BD Pharmingen |
| CD8 | AmCyan | SK1 | BD Biosciences |
PerCP, peridinin chlorophyll protein; APC, allophycocyanin.
Statistical analysis.
A paired t test was used for comparing cytokine mean fluorescence intensities for triple producers and single producers. The Wilcoxon signed-rank test was used for comparing the magnitudes of cytokine-positive cells. A two-sided P value of <0.05 was considered statistically significant. The Bonferroni method was used to adjust P values for multiple comparisons. Statistical analyses were performed using the software program S-PLUS 7.0.
RESULTS
To study cytokine coexpression profiles, we measured the coexpression of IFN-γ, IL-2, and TNF-α by vaccinia virus-, flu virus-, and CMV-specific CD4 T cells in human blood. Normal healthy subjects with measurable CD4 T-cell responses to vaccinia virus-, CMV-, or flu virus-specific antigens were recruited into the study. CD4 T cells specific for vaccinia and flu viruses were used to represent nonpersistent viral infections, and CD4 T cells specific for CMV represented a persistent viral infection. The cytokine coexpression subsets were further characterized by factors relating to other functions, such as the level of cytokine production per cell, the upregulation of CD40L (costimulation function), and degranulation (cytotoxic potential), to determine whether multiple-cytokine-producing cells are functionally superior to single-cytokine-producing cells. Below, we first present data on the frequency of total cytokine-positive cells expressing IFN-γ, IL-2, or TNF-α (magnitude) and then coexpression profiles for these cytokines (quality) and their relationship to the expression of other functions.
Frequencies of cytokine-positive cells specific for the studied viruses.
CD4 T cells specific for the three viruses differed in their frequencies and patterns of cytokine production (Fig. 1). The frequency of CMV-specific IFN-γ-producing CD4 T cells was higher than the frequency of vaccinia or flu virus-specific IFN-producing cells. The CMV-specific CD4 T cells produced predominantly IFN-γ, followed by TNF-α and then IL-2, with CMV-specific cells positive for these cytokines constituting arithmetic means of 0.55, 0.38, and 0.11%, respectively, of the total CD4 T cells. The frequencies of vaccinia and flu virus-specific CD4 T cells were each about 0.1%. Vaccinia virus-specific CD4 T cells produced predominantly TNF-α, followed by IL-2 and IFN-γ, with vaccinia virus-specific cells positive for these cytokines accounting for arithmetic means of 0.14, 0.11, and 0.09%, respectively, of total CD4 T cells. Flu virus-specific cells produced predominantly IFN-γ, followed by IL-2 and TNF-α at similar levels, and the arithmetic mean percentages of flu virus-specific cells producing each type of cytokine among the total CD4 T cells were 0.16, 0.09, and 0.09%, respectively.
FIG. 1.
Magnitude of virus-specific CD4 T cells expressing IFN-γ, IL-2, or TNF-α. PBMC were stimulated with viral lysates, and the virus-specific CD4 T-cell responses were measured using an intracellular cytokine staining assay. The percentage of total cytokine-positive (+ve) CD4 T cells specific for the indicated virus and positive for each cytokine is plotted.
Cytokine coexpression profiles of vaccinia virus-, flu virus-, and CMV-specific CD4 T cells.
To learn more about the cytokine coexpression profiles of CD4 T cells specific for vaccinia virus, flu virus, and CMV, we measured the coexpression of cytokines IFN-γ, IL-2, and TNF-α and categorized the cytokine-positive cells into seven different subsets consisting of triple producers, double producers, and single producers (Fig. 2). About 50% of the CD4 T cells specific for vaccinia virus, flu virus, and CMV produced more than one cytokine (Fig. 3). However, the patterns of expression and the relative proportions of triple, double, and single producers were different for the three different viruses. The proportion of triple producers among vaccinia virus-specific cells (34%) was highest, followed by that among flu virus-specific cells (26%) and then that among CMV-specific cells (9%). The proportions of double producers among vaccinia virus (38%)- and CMV (42%)-specific cells were higher than that among flu virus-specific cells (26%). Among the double producers, the vaccinia virus-specific response consisted predominantly of IL-2- and TNF-α-coproducing cells and the CMV-specific response consisted predominantly of IFN-γ- and TNF-α-coproducing cells, whereas the response to the flu virus was equally represented by the three subsets of double producers. Among the single producers, the vaccinia virus-specific response consisted predominantly of TNF-α-producing cells, whereas the flu virus- and CMV-specific responses consisted predominantly of IFN-γ-producing cells.
FIG. 2.
Schematic for the analysis of cytokine coexpression profiles of CD4 T cells following stimulation with SEB. Lymphocytes were gated based on forward scattering (FSC) and side scattering (SSC), and CD4 T cells (CD3+, CD8−, and CD4+) were then analyzed for IFN-γ, IL-2, and TNF-α expression. Cytokine coexpression profiles were determined using the Boolean gating function of FlowJo software. I, IFN-γ; L, IL-2; and T, TNF-α.
FIG. 3.
Cytokine coexpression profiles of vaccinia virus-, flu virus-, and CMV-specific CD4 T cells. PBMC were stimulated with viral lysates, and cytokine coexpression profiles were determined using the Boolean gating function of FlowJo software. (A) The proportions of subsets of virus-specific CD4 T cells positive for specific cytokines were expressed as percentages of total cytokine-positive (+ve) virus-specific CD4 T cells and plotted using GraphPad Prism. I, IFN-γ; L, IL-2; and T, TNF-α. (B) The pie charts present the mean frequencies of the T-cell subsets positive for the indicated cytokines in each group. TP, triple producers; DP, double producers; and SP, single producers.
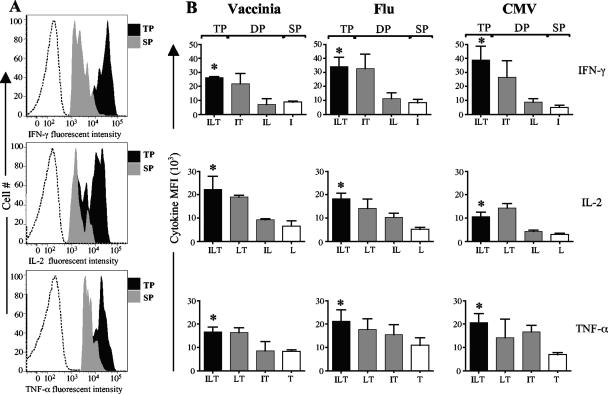
Triple producers produce more cytokine per cell than single producers.
We next investigated whether the cytokine coexpression subsets differed in the amount of cytokine expression per cell (Fig. 4). This was determined based on the mean fluorescence intensity for each cytokine per subset. The triple producers produced higher levels of cytokine per cell than the corresponding single producers (P < 0.05). This was true for all three cytokines. However, the double producers produced levels of cytokine per cell similar to those produced by triple producers, with the exception of the IFN-γ- and IL-2-coproducing cells, which produced lower levels of IFN-γ and IL-2. These findings held for all the studied virus-specific CD4 T cells. These results show that triple producers are functionally more active in cytokine production than single producers. The CMV-specific cells expressed the lowest levels of IL-2 per cell among the IL-2-expressing subsets of all three groups of virus-specific cells (P < 0.01), suggesting that antigen persistence may diminish IL-2 production by the respective virus-specific CD4 T cells.
FIG. 4.
Triple producers produce higher levels of cytokine per cell than single producers. (A) Representative plots showing the fluorescence intensities for IFN-γ, IL-2, and TNF-α from triple producers (TP) and single producers (SP). Dotted lines represent data for the isotype control. (B) Summary of mean fluorescence intensities (MFI) for vaccinia virus-, flu virus-, and CMV-specific triple producers, double producers (DP), and single producers. I, IFN-γ; L, IL-2; and T, TNF-α. Note that for double and single producers, only the dominant subsets in each virus-specific group are shown. Asterisks, significantly higher than single producers.
Higher proportions of triple producers than single producers express CD40L.
Higher proportions of triple-cytokine-producing than single-cytokine-producing CD4 cells expressed CD40L following stimulation (Fig. 5). CD4 T cells upregulate the expression of CD40L for the costimulation of CD8 T cells and B cells. We investigated whether the various cytokine coexpression subsets differed in their abilities to express CD40L. For double and single producers, we restricted our analysis to the respective dominant subsets among each virus-specific group. As can be seen in Fig. 5B, more than 90% of the vaccinia virus-, flu virus-, and CMV-specific triple producers expressed CD40L, whereas lower proportions (10 to 60%) of the single producers expressed CD40L. Similarly, the triple producers expressed higher levels of CD40L per cell than the single producers as measured by the mean fluorescence intensities (Fig. 5C). Interestingly, the flu virus-specific cells expressed higher levels of CD40L per cell than vaccinia virus- or CMV-specific cells (P < 0.01). The double producers were fairly similar to the triple producers in their ability to express CD40L following stimulation. These results suggest that following antigen stimulation, triple- and double-cytokine-producing CD4 T cells provide better costimulation to CD8 T cells and B cells than single-cytokine-producing CD4 T cells.
FIG. 5.
Higher proportions of triple producers than single producers express CD40L. (A) Representative plots showing the levels of surface expression of CD40L (CD154) by triple and single producers. Bars indicate CD154-positive cells, and numbers indicate CD154-positive cells as a percentage of total cytokine-positive cells. (B) Summary of the proportions of CD40L-positive cells as percentages of the respective cytokine coexpression subsets. Bars indicate median value for the group. TP, triple producers; DP, double producers; SP, single producers; I, IFN-γ; L, IL-2; and T, TNF-α. (C) Summary of mean fluorescence intensity (MFI) data. Note that for double and single producers, only the dominant subsets in each virus-specific group are shown.
Higher proportions of CMV-specific triple producers than single producers degranulate.
Higher proportions of CMV-specific triple-cytokine- and double-cytokine-producing CD4 cells than single-cytokine-producing cells degranulated following stimulation (Fig. 6). We investigated whether the various cytokine coexpression subsets differed in their abilities to degranulate following stimulation and thereby might differ in their cytolytic potentials. The CMV- but not the vaccinia or flu virus-specific CD4 T cells degranulated following stimulation (Fig. 6A). Among the CMV-specific CD4 T cells, the cytokine coexpression subsets that did not express IFN-γ also failed to degranulate, suggesting a strong association between IFN-γ production and degranulation (data not shown). However, the proportions of IFN-γ-positive cells that degranulated differed based on the coexpression of other cytokines by these cells (Fig. 6B and C). About 50% of the CMV-specific triple producers and IFN-γ and TNF-α double producers degranulated following stimulation, whereas less than 15% of the IFN-γ single producers degranulated. Among the double producers, IFN-γ-positive cells coexpressing TNF-α degranulated more frequently than IFN-γ-positive cells coexpressing IL-2 (Fig. 6C). The degranulation-positive cells were also positive for granzyme B expression (Fig. 6D). These results suggest that following antigen stimulation, triple and double producers preferentially degranulate over single producers and thus may possess better cytolytic potential.
FIG. 6.
Higher proportions of CMV-specific triple producers than single producers degranulate. (A) Representative flow charts showing the degranulation potential of vaccinia virus-, flu virus-, and CMV-specific IFN-γ-producing CD4 T cells. Percentage of total CD4 cells is indicated. (B) Representative flow charts showing the overlay of total CD4 T cells (gray) with CMV-specific triple producers, IFN-γ and TNF-α double producers, and IFN-γ single producers (black). Numbers on the graphs indicate the frequencies of virus-specific CD4 T cells as percentages of the respective cytokine coexpression subsets. (C) Summary of CD107a/b and CD154 expression in different cytokine coexpression subsets. TP, triple producers; DP, double producers; SP, single producers; I, IFN-γ; L, IL-2; T, TNF-α; and +ve, positive. (D) Coexpression of CD107a/b and granzyme B by CMV-specific CD4 T cells.
Triple producers do not differ from IFN-γ single producers in their memory phenotypes.
CD4 T cells specific for different viral antigens exhibited different patterns of memory phenotypes. We evaluated the memory phenotypes of cytokine coexpression subsets to determine the association between coexpression profiles and memory phenotypes (Fig. 7). The cells of the cytokine coexpression subsets were characterized as having an effector (CCR7− and CD45RA−) or central memory (CCR7+ and CD45RA−) phenotype based on the expression of CD45RA and CCR7 as described previously (14, 21). A clear association between any of the cytokine coexpression subsets and memory phenotypes was not evident except for IL-2 single producers and IL-2 and TNF-α double producers, which predominantly showed a central memory phenotype in all the studied virus-specific groups. Among the flu virus-specific cells, the majority of cells (>60%) in any cytokine subset had a central memory phenotype; in contrast, among vaccinia virus- and CMV-specific CD4 T cells, the majority of cells in any cytokine subset had an effector memory phenotype. These differences were striking considering that in flu and vaccinia virus infections the antigen is cleared after the initial infection or immunization. The memory phenotype of the triple producers of a particular virus-specific group was similar to that of the IFN-γ single producers of the same group. These data demonstrate that triple producers cannot be distinguished from single producers based on their memory phenotypes.
FIG. 7.
Memory phenotypes of cytokine coexpression subsets. (A) Representative data showing the overlay of symbols for total CD4 T cells (light gray) with symbols for cytokine coexpression subsets (black) for different virus-specific cells. The numbers on the graphs represent the frequencies of different subsets as percentages of the respective total cytokine coexpression subsets. I, IFN-γ; L, IL-2; and T, TNF-α. (B) Summary of levels of CCR7 expression (shown as percentages of CCR7-positive cells among the respective cytokine coexpression subsets) by different cytokine coexpression subsets specific for vaccinia virus (V), flu virus (F), and CMV (C). Black bars represent triple producers, gray bars represent double producers, and white bars represent single producers. +ve, positive.
DISCUSSION
The advent of intracellular cytokine staining has allowed the examination of antiviral CD4 and CD8 T cells in highly quantitative functional ex vivo assays (29). Numerous studies in the past have evaluated the frequencies of antiviral CD4 T cells producing different cytokines to assess the magnitudes of specific responses. Both the magnitude and the quality of T-cell responses are critical for the control of viral infections. Here, we evaluated the cytokine coexpression profiles of CD4 T cells specific for viruses representing one persistent and two nonpersistent infections to better understand the quality of virus-specific CD4 T-cell responses. We then studied the association between cytokine coexpression profiles and other functions of virus-specific CD4 T cells. Our results demonstrate that about 50% of the total cytokine-positive cells specific for each of the viruses produced more than one cytokine and that multiple-cytokine-producing cells were functionally superior to single-cytokine-producing cells (Table 2).
TABLE 2.
Functional profiles of triple producers, double producers, and single producers among CD4 T cells specific for different viral antigensa
| Expression subset | Levelb of total cytokine response by cells specific for:
|
Level of cytokine production/cell specific for:
|
Levelc of CD40L upregulation by cells specific for:
|
Leveld of degranulation of cells specific for:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinia virus | Flu virus | CMV | Vaccinia virus | Flu virus | CMV | Vaccinia virus | Flu virus | CMV | Vaccinia virus | Flu virus | CMV | |
| Triple producers | ++ | ++ | ± | High | High | High | ++++ | ++++ | ++++ | − | − | ++ |
| Double producers | ++ | ++ | ++ | High to intermediate | High to intermediate | High to intermediate | ++++ | ++++ | ++++ | − | − | ++ |
| Single producers | ++ | ++ | ++ | Low | Low | Low | ++ | +++ | ++ | − | − | + |
−, <1%; ±, 1 to 10%; +, 11 to 25%; ++, 25 to 50%; +++, 51 to 75%; ++++, 76 to 100%. Note that for double and single producers, only the dominant subsets are considered for determinations.
Expressed as the percentage of cells of the indicated expression subset among total cytokine-positive CD4 T cells of the virus-specific group.
Expressed as the percentage of cells of the indicated expression subset exhibiting CD40L upregulation.
Expressed as the percentage of cells of the indicated expression subset exhibiting degranulation.
Multiple-cytokine producers expressed more cytokine per cell than single producers of the same cytokine (Table 2). This was true for IFN-γ, IL-2, and TNF-α and for CD4 T cells specific for all three studied viruses. In addition to the difference in the level of cytokine production, the proportion of triple producers expressing CD40L was higher than that of single producers, suggesting that the former may provide better costimulation to CD8 T cells and B cells than the latter (Table 2). The degranulation function of triple producers among CMV-specific CD4 T cells was similarly enhanced over that of single producers. CMV-specific CD4 T cells can degranulate following antigen exposure (8) and exhibit a cytolytic function (26, 28). Interestingly, higher proportions of triple producers and double producers than single producers degranulated, suggesting that cells that are capable of expressing more than one cytokine possess enhanced cytolytic potential (Table 2). Collectively, these results indicate that triple producers are functionally superior to single producers.
Interestingly, the triple producers and the IFN-γ single producers did not differ in their memory phenotypes (Table 2). Furthermore, the memory phenotypes of triple producers differed among CD4 T cells specific for the different viral antigens. Among flu virus-specific triple producers, the central memory phenotype (CD45RA− CCR7+) was predominant, whereas among the CMV- and vaccinia virus-specific triple producers, the effector memory phenotype (CD45RA− CCR7−) was predominant. A similar phenomenon for other cytokine subsets was observed. These results demonstrate that cytokine coexpression subsets cannot be distinguished based on the memory phenotypes of the cells, as has been shown previously for human immunodeficiency virus-specific CD8 T cells (4). This was true for memory cells defined based on CD28 and CD95 or CD28 and CD27 expression (data not shown).
Interestingly, each group of virus-specific cells exhibited a unique cytokine coexpression profile. The patterns of dominance of expressed cytokines differed even between the two groups corresponding to nonpersistent infections (vaccinia and flu). The vaccinia virus-specific double producers expressed predominantly IL-2 and TNF-α, and the CMV-specific double producers expressed predominantly IFN-γ and TNF-α. Interestingly, the differences in cytokine coproduction by double producers did not influence the relative ability to perform other functions. At this point, it is not clear what contributes to these observed differences. However, we speculate that antigen persistence (30) and unique interactions between different viruses and the innate immune response (20) determine differences in cytokine coexpression profiles.
Proliferative capacity, a key characteristic of virus-specific T cells, has a critical role in viral control. In the present study, we did not investigate the association between cytokine coexpression and proliferative capacity because of our inability to isolate live T cells producing more than two cytokines. However, previous studies have associated IL-2 production with proliferative capacity (5, 33). This possibility suggests that multiple-cytokine-producing CD4 T cells that produce high levels of IL-2 may support better proliferation of T cells than IL-2 single producers, which produce relatively low levels of IL-2.
The quality of CD4 T cells specific for the virus representing persistent infection (CMV) differed from the quality of CD4 T cells specific for the two viruses representing nonpersistent infections (vaccinia and flu viruses). Higher proportions of the total cytokine responses to vaccinia and flu viruses than of the response to CMV consisted of triple producers. The CMV-specific CD4 cells expressed smaller amounts of IL-2 (a marker for proliferative potential) and CD40L (a marker for costimulation potential) per cell than the vaccinia or flu virus-specific CD4 T cells. In contrast, the CMV-specific CD4 T cells degranulated, whereas the vaccinia and flu virus-specific cells failed to degranulate. This result was true for triple as well as single producers. Collectively, these data suggest that the CMV-specific cells perform a cytolytic function and have weak costimulatory and proliferative potentials and that the vaccinia and flu virus-specific cells have strong costimulatory and proliferative potentials and no cytolytic function.
In conclusion, our results from the study of cytokine coexpression profiles demonstrate that triple producers are functionally superior to single producers not only in the numbers but also in the levels of cytokines produced per cell and in costimulatory potential and degranulation. Our results demonstrate a strong positive association between the multiple-cytokine production capacity of virus-specific CD4 T cells and their other functions. This strong association suggests that vaccines targeting viral infections should elicit CD4 T cells capable of producing more than one cytokine.
Acknowledgments
We thank James Herndon for help with statistical analysis and Helen Drake-Perrow for outstanding administrative support. We are thankful to the Yerkes Division of Research Resources for the consistent excellence of pathology support. Most of all, we are very grateful to all of the volunteers who selflessly participated in this study.
This work was supported by National Institutes of Health National Institute of Allergy and Infectious Diseases grants R01 AI57029 to R.R.A. and P01 AI49364 to H.L.R., Emory/Atlanta Center for AIDS Research grant P30 AI050409, and Yerkes National Primate Research Center base grant P51 RR00165.
Footnotes
Published ahead of print on 6 June 2007.
REFERENCES
- 1.Amara, R. R., P. Nigam, S. Sharma, J. Liu, and V. Bostik. 2004. Long-lived poxvirus immunity, robust CD4 help, and better persistence of CD4 than CD8 T cells. J. Virol. 78:3811-3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amara, R. R., J. M. Smith, S. Staprans, D. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 76:6138-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betts, M. R., J. M. Brenchley, D. A. Price, S. C. De Rosa, D. C. Douek, M. Roederer, and R. A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65-78. [DOI] [PubMed] [Google Scholar]
- 4.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blattman, J. N., J. M. Grayson, E. J. Wherry, S. M. Kaech, K. A. Smith, and R. Ahmed. 2003. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 9:540-547. [DOI] [PubMed] [Google Scholar]
- 6.Boaz, M. J., A. Waters, S. Murad, P. J. Easterbrook, and A. Vyakarnam. 2002. Presence of HIV-1 Gag-specific IFN-γ+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. J. Immunol. 169:6376-6385. [DOI] [PubMed] [Google Scholar]
- 7.Bourgeois, C., H. Veiga-Fernandes, A. M. Joret, B. Rocha, and C. Tanchot. 2002. CD8 lethargy in the absence of CD4 help. Eur. J. Immunol. 32:2199-2207. [DOI] [PubMed] [Google Scholar]
- 8.Casazza, J. P., M. R. Betts, D. A. Price, M. L. Precopio, L. E. Ruff, J. M. Brenchley, B. J. Hill, M. Roederer, D. C. Douek, and R. A. Koup. 2006. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med. 203:2865-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Correa, R., A. Harari, F. Vallelian, S. Resino, M. A. Munoz-Fernandez, and G. Pantaleo. 2007. Functional patterns of HIV-1-specific CD4 T-cell responses in children are influenced by the extent of virus suppression and exposure. AIDS 21:23-30. [DOI] [PubMed] [Google Scholar]
- 10.Crotty, S., E. N. Kersh, J. Cannons, P. L. Schwartzberg, and R. Ahmed. 2003. SAP is required for generating long-term humoral immunity. Nature 421:282-287. [DOI] [PubMed] [Google Scholar]
- 11.Duvall, M. G., A. Jaye, T. Dong, J. M. Brenchley, A. S. Alabi, D. J. Jeffries, M. van der Sande, T. O. Togun, S. J. McConkey, D. C. Douek, A. J. McMichael, H. C. Whittle, R. A. Koup, and S. L. Rowland-Jones. 2006. Maintenance of HIV-specific CD4+ T cell help distinguishes HIV-2 from HIV-1 infection. J. Immunol. 176:6973-6981. [DOI] [PubMed] [Google Scholar]
- 12.Guidotti, L. G., and F. V. Chisari. 2000. Cytokine-mediated control of viral infections. Virology 273:221-227. [DOI] [PubMed] [Google Scholar]
- 13.Harari, A., F. Vallelian, P. R. Meylan, and G. Pantaleo. 2005. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J. Immunol. 174:1037-1045. [DOI] [PubMed] [Google Scholar]
- 14.Harari, A., F. Vallelian, and G. Pantaleo. 2004. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur. J. Immunol. 34:3525-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852-856. [DOI] [PubMed] [Google Scholar]
- 16.Landais, E., X. Saulquin, E. Scotet, L. Trautmann, M. A. Peyrat, J. L. Yates, W. W. Kwok, M. Bonneville, and E. Houssaint. 2004. Direct killing of Epstein-Barr virus (EBV)-infected B cells by CD4 T cells directed against the EBV lytic protein BHRF1. Blood 103:1408-1416. [DOI] [PubMed] [Google Scholar]
- 17.McHeyzer-Williams, M. G., and R. Ahmed. 1999. B cell memory and the long-lived plasma cell. Curr. Opin. Immunol. 11:172-179. [DOI] [PubMed] [Google Scholar]
- 18.Norris, P. J., H. F. Moffett, O. O. Yang, D. E. Kaufmann, M. J. Clark, M. M. Addo, and E. S. Rosenberg. 2004. Beyond help: direct effector functions of human immunodeficiency virus type 1-specific CD4+ T cells. J. Virol. 78:8844-8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perfetto, S. P., P. K. Chattopadhyay, and M. Roederer. 2004. Seventeen-colour flow cytometry: unravelling the immune system. Nat. Rev. Immunol. 4:648-655. [DOI] [PubMed] [Google Scholar]
- 20.Pulendran, B., K. Palucka, and J. Banchereau. 2001. Sensing pathogens and tuning immune responses. Science 293:253-256. [DOI] [PubMed] [Google Scholar]
- 21.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 22.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337-339. [DOI] [PubMed] [Google Scholar]
- 23.Slifka, M. K., and J. L. Whitton. 2000. Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J. Immunol. 164:208-216. [DOI] [PubMed] [Google Scholar]
- 24.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun, J. C., M. A. Williams, and M. J. Bevan. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5:927-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suni, M. A., S. A. Ghanekar, D. W. Houck, H. T. Maecker, S. B. Wormsley, L. J. Picker, R. B. Moss, and V. C. Maino. 2001. CD4+ CD8dim T lymphocytes exhibit enhanced cytokine expression, proliferation and cytotoxic activity in response to HCMV and HIV-1 antigens. Eur. J. Immunol. 31:2512-2520. [DOI] [PubMed] [Google Scholar]
- 27.van Leeuwen, E. M., E. B. Remmerswaal, M. T. Vossen, A. T. Rowshani, P. M. Wertheim-van Dillen, R. A. van Lier, and I. J. ten Berge. 2004. Emergence of a CD4+CD28− granzyme B+, cytomegalovirus-specific T cell subset after recovery of primary cytomegalovirus infection. J. Immunol. 173:1834-1841. [DOI] [PubMed] [Google Scholar]
- 28.van Leeuwen, E. M. M., E. B. M. Remmerswaal, M. H. M. Heemskerk, I. J. M. ten Berge, and R. A. W. van Lier. 2006. Strong selection of virus-specific cytotoxic CD4+ T-cell clones during primary human cytomegalovirus infection. Blood 108:3121-3127. [DOI] [PubMed] [Google Scholar]
- 29.Waldrop, S. L., C. J. Pitcher, D. M. Peterson, V. C. Maino, and L. J. Picker. 1997. Determination of antigen-specific memory/effector CD4+ T cell frequencies by flow cytometry: evidence for a novel, antigen-specific homeostatic mechanism in HIV-associated immunodeficiency. J. Clin. Investig. 99:1739-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wherry, E. J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78:5535-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong, G. H., J. F. Krowka, D. P. Stites, and D. V. Goeddel. 1988. In vitro anti-human immunodeficiency virus activities of tumor necrosis factor-alpha and interferon-gamma. J. Immunol. 140:120-124. [PubMed] [Google Scholar]
- 33.Zimmerli, S. C., A. Harari, C. Cellerai, F. Vallelian, P. A. Bart, and G. Pantaleo. 2005. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc. Natl. Acad. Sci. USA 102:7239-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]