Abstract
Previous studies have suggested that monoclonal antibodies (MAbs) to flavivirus nonstructural protein-1 (NS-1) protect against infection in mice through an Fc-γ receptor-dependent pathway. To identify a specific mechanism, we evaluated the protective activity of anti-NS1 MAbs to WNV using mice and cells with deficiencies of specific Fc-γ receptors. Our results suggest that only MAbs that recognize cell surface-associated NS1 trigger Fc-γ receptor I- and/or IV-mediated phagocytosis and clearance of WNV-infected cells. These findings may be relevant for generating novel therapeutic MAbs or vaccines against flaviviruses that target the NS1 protein.
West Nile virus (WNV) is a positive polarity single-stranded RNA mosquito-borne virus of the Flaviviridae family and is related to other viruses that cause human disease including dengue virus (DENV), yellow fever virus (YFV), Japanese virus, St. Louis virus, and tick-borne encephalitis virus. WNV has become endemic in North America and other parts of the world, with annual outbreaks of encephalitis occurring mostly in immunocompromised or elderly individuals. At present, treatment is supportive, and no vaccine exists for human use.
Innate and adaptive immune responses are essential for the control of WNV infection (reviewed in reference 23). The humoral response limits flavivirus infection in vivo, and this protection has been mapped to antibodies that recognize the envelope (E) and nonstructural-1 (NS-1) proteins (11, 22). Studies have shown that some anti-WNV and anti-YFV NS1 monoclonal antibodies (MAbs) protect through Fc-γ receptor-dependent pathways (6, 24-26). We evaluated here the Fc-γ receptor-dependent mechanism for protective anti-NS1 MAbs against WNV.
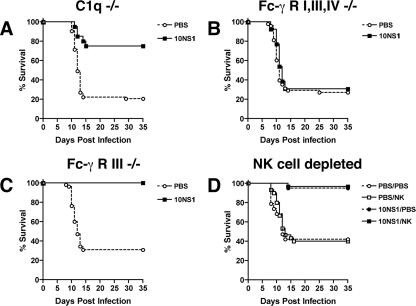
A previous study showed that passive transfer of five different MAbs (10NS1, 14NS1, 16NS1, 17NS1, or 22NS1) against WNV NS1 protein protected mice against lethal challenge (6). To gain further insight into their mechanism of control, we evaluated in detail how one of the MAbs, 10NS1, limited WNV infection. Similar to studies with other anti-NS1 and E MAbs against WNV and YFV (6, 19, 26), we tested whether the effector functions of 10NS1 MAb were linked to its protective activity. Passive antibody transfer studies were initially performed in wild-type, C1q−/−, or Fc-γ receptor I−/−, III−/−, and IV−/− congenic C57BL/6 mice. The Fc-γ receptor-deficient animals lack the common accessory γ-chain that carries an immunoreceptor tyrosine-based activation motif required for activation and efficient expression of all activating Fc-γ receptors in mice, including the newly described Fc-γ receptor IV (17, 18). In C1q−/− mice, which cannot activate complement by the antibody-dependent classical pathway, 10NS1 maintained virtually all of its protective effect (Fig. 1A, P < 0.0001) with a ∼75% survival rate. Consistent with this, passive transfer of protective anti-NS1 MAbs also significantly prevented lethal WNV infection in C3−/− mice (data not shown). In contrast, in Fc-γ receptor I−/−, III−/−, and IV−/− mice, which lack the common signaling γ-chain and are impaired in antibody-dependent effector responses (28), the beneficial effect of 10NS1 was lost (Fig. 1B, P = 0.6). These results suggested that 10NS1, as had been previously observed with two other anti-WNV NS1 MAbs, 16NS1 and 17NS1 (6), required interaction with activating Fc-γ receptors for its protective effect.
FIG. 1.
Efficacy of 10NS1 MAb in C1q−/−, Fc-γ receptor I−/−, III−/−, and IV−/−, Fc-γ receptor III−/−, and NK cell-depleted mice. C1q−/− (A), Fc-γ receptor I−/−, III−/−, and IV−/− (B), or Fc-γ receptor III−/− (C) C57BL/6 mice were inoculated via footpad with 102 PFU of WNV on day 0. On the same day, mice were administered PBS or a single dose of 10NS1 MAb (500 μg) by an intraperitoneal route. The difference in survival curves between antibody and PBS treatments was statistically significant for the C1q−/− (n = 20, P < 0.0001) and Fc-γ receptor III−/− mice (n = 15, P < 0.0001) but not for Fc-γ receptor I−/−, III−/−, and IV−/− mice (n = 13, P = 0.6). (D) NK cells were depleted from wild-type mice after treatment with 150 μg of anti-NK1.1 MAb 2 days before and after infection. Depletion of NK cells was confirmed as >99% by flow cytometry of peripheral blood lymphocytes. Mice were infected with WNV and treated with 10NS1 or PBS as described above. There was no statistically significant difference in mortality between 10NS1-treated, NK-depleted, and nondepleted mice (n = 30, P = 0.8). The survival curves were constructed from two to three independent experiments.
NS1 is a secreted nonstructural glycoprotein that is absent from the virion, accumulates in cell supernatants, and becomes plasma membrane-associated through as-yet-undetermined mechanisms (32, 33). Because activating Fc-γ receptors were essential for 10NS1 protection, we speculated that natural killer (NK) cells might control infection by detecting and lysing NS1-expressing WNV-infected cells through antibody-dependent cellular cytotoxicity (ADCC). To test this, passive protection experiments were repeated with 10NS1 in congenic Fc-γ receptor III−/− mice: NK cells express only Fc-γ receptor III, and thus NK-mediated ADCC is abolished in these mice (12). Notably, 10NS1 completely maintained its beneficial effect in Fc-γ receptor III−/− mice (Fig. 1C, P < 0.0001). Although these data suggested that NK cell did not contribute to 10NS1-mediated protection against WNV, we further investigated this using cell depletion experiments. NK cells were depleted from wild-type C57BL/6 mice by administering an MAb (150 μg) against the NK cell-restricted surface antigen NK 1.1 (27). Two days later, depletion was confirmed, with <0.1% of NK cells detected in peripheral blood by flow cytometric analysis (data not shown). Subsequently, mice treated with NK1.1, an isotype control MAb (anti-SARS coronavirus ORF7a), or phosphate-buffered saline (PBS) were administered 10NS1, infected with WNV, and evaluated for survival (Fig. 1D and data not shown). Depletion of NK cells, which was sustained by an additional dose of NK1.1 at day 2 after infection, did not affect WNV pathogenesis or 10NS1-mediated protection. Based on these studies, we conclude that 10NS1-mediated protection does not depend on NK cells, NK cell-mediated ADCC, or other Fc-γ receptor III-triggered effector events in other cells, including granulocytes and macrophages. Thus, 10NS1 MAb protects mice against WNV infection in vivo through Fc-γ receptor I- and/or IV-dependent mechanisms.
All of the anti-NS1 MAbs (10NS1, 16NS1, and 17NS1) that protected against WNV through a Fc-γ receptor-dependent mechanism share a common feature: they were of the mouse IgG2a subclass (Table 1). Of the three activating Fc-γ receptors (I, III, and IV) in mice, Fc-γ receptor I is unique and is the only high-affinity receptor for monomeric mouse IgG2a (21, 29). Based on this, we speculate that Fc-γ receptor I mediates protection through interaction with anti-NS1 MAbs of the IgG2a subclass. However, the unavailability of Fc-γ receptor I−/− C57BL/6 mice precludes direct testing of this hypothesis at this time.
TABLE 1.
Relationship between protective activity and binding of anti-NS1 MAbs
| Antibody | % Surviving micea | Antibody isotypea | NS1 MAb domain localizationa | % Positiveb
|
||
|---|---|---|---|---|---|---|
| Yeast binding | Raji-WNV intracellular NS1 | Raji-WNV surface-associated NS1 | ||||
| PBS | 17 | |||||
| 1NS1 | 25 | IgG1 | 0.2 | NDc | ND | |
| 2NS1 | 40 | IgG1 | III | 0.1 | 63 | 5 |
| 3NS1 | 50* | IgG2b | I | 54 | 97 | 30 |
| 4NS1 | 50* | IgG2b | III | 54 | 99 | 84 |
| 5NS1 | 40 | IgG1 | I | 54 | 93 | 0.6 |
| 6NS1 | 20 | IgG1 | III | 24 | 98 | 78 |
| 7NS1 | 0 | IgG1 | I | 54 | 91 | 0.6 |
| 8NS1 | 20 | IgG2a | I | 52 | 97 | 1.3 |
| 9NS1 | 40 | IgG1 | III | 52 | 98 | 48 |
| 10NS1 | 80* | IgG2a | I, II-III | 52 | 98 | 77 |
| 11NS1 | 50* | IgG2b | I | 55 | 94 | 65 |
| 12NS1 | 10 | IgG2a | I | 51 | 95 | 5.1 |
| 13NS1 | 30 | IgG1 | III | 48 | 98 | 79 |
| 14NS1 | 97* | IgG2a | III | 44 | 99 | 71 |
| 15NS1 | 0 | IgG2a | I | 50 | 94 | 0.9 |
| 16NS1 | 90* | IgG2a | I | 55 | 98 | 82 |
| 17NS1 | 75* | IgG2a | I, III | 52 | 93 | 81 |
| 18NS1 | 10 | IgG2b | III | 18 | 93 | 82 |
| 19NS1 | 20 | IgG1 | III | 39 | 97 | 66 |
| 21NS1 | 40 | IgG1 | I | 25 | 96 | 0.9 |
| 22NS1 | 70* | IgG2a | II-III | 41 | 99 | 52 |
| 23NS1 | 0 | IgG1 | I | 52 | 93 | 0.9 |
Data were adapted from a table in a previous publication (6).
Intracellular and cell surface-associated NS1 levels on Raji-WNV-Rep and yeast cells were measured in permeabilized or nonpermeabilized cells by flow cytometry. The values are expressed as the percent positive cells compared to a negative control antibody. The results are representative of at least three independent experiments. *, The percentage of surviving mice after transfer of the indicated NS1 MAb was statistically different (P < 0.05) from saline controls.
ND, not determined.
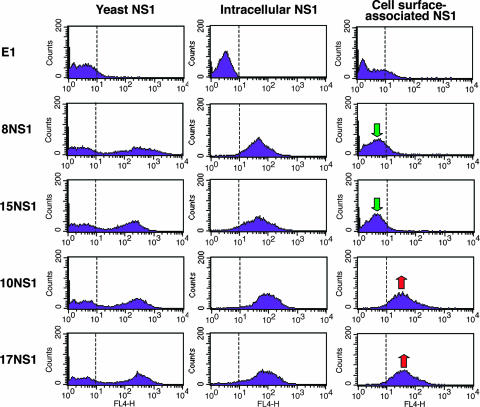
As some but not all anti-WNV NS1 MAbs controlled infection through a Fc-γ receptor-dependent mechanism, we sought to define the basis for differential MAb protection. Because biochemical studies with DENV NS1 suggested that soluble and cell surface NS1 were arranged as distinct oligomers (hexamer versus dimer, respectively) (9, 20, 33), we speculated that the disparate protective phenotypes could be due to differential recognition of soluble and cell-associated forms of NS1. Virtually all of our anti-NS1 MAbs equivalently recognized a full-length NS1 protein in solution, as a fusion protein expressed on the surface of yeast cells and as an intracellular protein (Fig. 2, Table 1, and data not shown). In the yeast expression system, however, NS1 attaches to the cell surface via a unique mechanism: the Aga2 N-terminal part of the fusion protein binds to Aga1, which is constitutively expressed on the yeast surface (5). The surface orientation of NS1 on infected cells, which binds as a dimer (20), may be distinct and recognized differentially by antibodies. To define the reactivity of MAbs against cell surface-associated NS1, we used a cell line, Raji-WNV-Rep, that propagates a WNV subgenomic replicon (10, 31) and expresses the viral proteins NS1 through NS5 of the New York 1999 strain of WNV. Each antibody was tested for surface and intracellular staining of NS1 on this cell. All NS1 MAbs that protected mice through an Fc-γ receptor-dependent mechanism (10NS1, 16NS1, and 17NS1) were of the IgG2a subclass and exhibited strong immunoreactivity against both cell surface and intracellular forms of NS1. However, other IgG2a MAbs (8NS1, 12NS1, and 15NS1) that failed to protect mice did not recognize cell surface-associated NS1. Finally, other MAbs (6NS1, 13NS1, and 19NS1) of the IgG1 subclass recognized cell surface NS1 and yet did not protect in vivo. Similar results were obtained with BHK cells infected with live (New York 2000 strain) of WNV (data not shown). When these results were analyzed in the context of previous mapping data (6), we observed that anti-NS1 IgG2a that failed to recognize cell surface NS1 or protect against infection in mice all mapped to the N-terminal fragment (FR-I) of NS1 (Table 1). Thus, MAbs differentially recognize cell surface forms of NS1, and this pattern, in combination with the IgG subclass, determines whether an anti-NS1 MAb is protective in vivo through an Fc-γ-receptor I- and/or IV-dependent mechanism.
FIG. 2.
Flow cytometry histograms showing immunoreactivity of NS1 on the surface of yeast, within permeabilized Raji-WNV-Rep cells, and on the surface of Raji-WNV-Rep cells with individual anti-NS1 MAbs. Representative histograms are shown for four NS1 MAbs (8NS1, 15NS1, 10NS1, and 17NS1). The E1 MAb against WNV E protein (19) was used as a negative control. WNV NS1 immunostaining was performed as described previously (6). Intracellular and cell surface-associated NS1 levels on Raji-WNV-Rep were measured in permeabilized or nonpermeabilized cells, respectively. Green and red arrows indicate the absence or presence, respectively, of binding of MAbs to cell surface-associated NS1. The data shown are representative of several independent experiments.
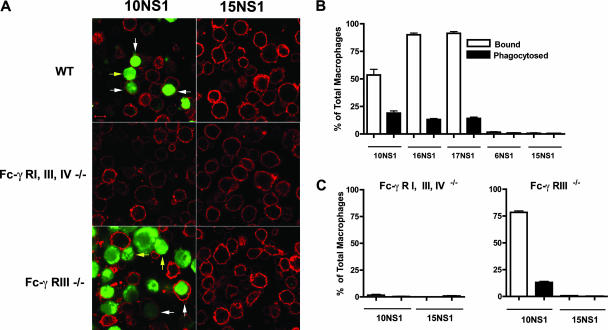
Although our studies suggested that protection by anti-NS1 IgG2a was Fc-γ receptor dependent and NK cell independent, no specific mechanism was identified. We hypothesized that anti-NS1 MAbs might target infected cells that display high levels of cell surface NS1 for phagocytosis by tissue macrophages that express high levels of Fc-γ receptor I (4). To test this, we compared the ability of peritoneal macrophages from wild-type, Fc-γ receptor III−/−, or Fc-γ receptor I−/−, III−/−, and IV−/− mice to phagocytose carboxy-fluorescein succinimidyl ester (CFSE)-labeled Raji-WNV-Rep cells in the presence or absence of anti-NS1 IgG2a MAbs that bind (10NS1) or fail to bind (15NS1) cell surface NS1. Phagocytosis by macrophages was assessed and quantitated by confocal microscopy. Opsonization with 10NS1 MAb resulted in efficient binding and internalization of Raji-WNV-Rep cells by wild-type and Fc-γ receptor III−/− but not Fc-γ receptor I−/−, III−/−, and IV−/− peritoneal macrophages (Fig. 3). Similar results were observed with the two other anti-NS1 IgG2a MAbs (16NS1 and 17NS1) that protect in vivo via an Fc-γ receptor-dependent mechanism (Fig. 3B) and with cells infected with live WNV (data not shown). Importantly, 10NS1 MAb did not facilitate any binding and/or phagocytosis of the parent Raji cell line that lacked expression of the WNV replicon (data not shown). Surface staining of cells with cholera toxin and confocal microscopic analysis confirmed that 10NS1-opsonized Raji-WNV-Rep cells were phagocytosed by wild-type macrophages (data not shown). In contrast, internalization of Raji-WNV-Rep cells was not observed after the addition of 15NS1 in wild-type or deficient macrophages (Fig. 3). These experiments suggest that anti-NS1 MAbs of a given IgG subclass that bind to cell surface-associated NS1 facilitate phagocytosis and clearance of WNV-infected cells through Fc-γ receptors I and/or IV. Consistent with this, some but not all human IgG switch variants of 17NS1 promoted phagocytosis of Raji-WNV-Rep cells (data not shown). These studies, however, do not exclude a possible independent protective mechanism by ADCC in macrophages (16).
FIG. 3.
Anti-NS1 MAb-dependent phagocytosis by peritoneal macrophages. (A) Thioglycolate-elicited peritoneal macrophages were isolated after peritoneal lavage from wild-type, Fc-γ receptor I−/−, III−/−, and IV−/−, or Fc-γ receptor III−/− mice and adhered (5 × 105 cells) on poly-d-lysine and laminin-coated coverslips. NS1-expressing Raji-WNV-Rep target cells (2 × 106) labeled with CFSE were opsonized with 10NS1 or 15NS1 and incubated with wild-type or Fc-γ receptor-deficient peritoneal macrophages for 1 h at 37°C. After being washed, cells were fixed with paraformaldehyde, stained with Alexa-555-conjugated cholera toxin subunit B, and imaged by confocal microscopy. The yellow and white arrows indicate CFSE-labeled Raji-WNV-Rep target cells bound to and engulfed by macrophages, respectively. (B) Quantitation of the percentage of bound or phagocytosed target cells by wild-type peritoneal macrophages with different anti-NS1 MAbs. Ten random fields were counted (×63 magnification) per antibody treatment, resulting in more than 600 total cells counted. The difference in number between 10NS1, 16NS1, 17NS1, and 6NS1 or 15NS1 treatment was statistically significant for bound (P ≤ 0.0001) and phagocytosed (P ≤ 0.0001) target cells. (C) Quantitation of the number of bound or engulfed target cells by Fc-γ receptor I−/−, III−/−, or IV−/− (left) or Fc-γ receptor III−/− (right) peritoneal macrophages with 10NS1 or 15NS1 MAbs. The difference in the percentage of bound or phagocytosed Raji-WNV-Rep target cells between 10NS1 and 15NS1 was not different for Fc-γ receptor I−/−, III−/−, and IV−/− macrophages but was for Fc-γ receptor III−/− macrophages (P < 0.0001).
In summary, our investigations suggest that vaccines or antibody-based therapeutics that target the NS1 protein could enhance protection and/or viral clearance against flaviviruses. In support of this, recent preclinical subunit vaccines that contain NS1 showed promising protection against WNV and DENV in rodents (7, 14, 15, 30, 34). Given our findings on IgG subclass and cell surface recognition requirements, further genetic and immunologic manipulation could enhance NS1 antibody-mediated protection through Fc-γ-receptor-dependent phagocytic mechanisms. Nonetheless, since NS1 can also bind to the surface of uninfected cells (32), in flavivirus infection models where high levels of soluble NS1 accumulate (1, 13, 35) it is possible that some anti-NS1 MAbs could have pathogenic consequences (2, 3, 8) due to targeting and phagocytosis of uninfected cells.
Acknowledgments
We thank A. Blasius, M. Cella, and M. Noll for technical suggestions and help with some of the experiments.
This study was supported by grants from the National Institutes of Health (AI061373 [M.S.D.] and U54 AI057160 [Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research]) and the Pediatric Dengue Vaccine Initiative (D.H.F and M.S.D.).
Footnotes
Published ahead of print on 20 June 2007.
REFERENCES
- 1.Alcon, S., A. Talarmin, M. Debruyne, A. Falconar, V. Deubel, and M. Flamand. 2002. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40:376-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcon-LePoder, S., P. Sivard, M. T. Drouet, A. Talarmin, C. Rice, and M. Flamand. 2006. Secretion of flaviviral nonstructural protein NS1: from diagnosis to pathogenesis. Novartis Found. Symp. 277:233-253. [DOI] [PubMed] [Google Scholar]
- 3.Avirutnan, P., N. Punyadee, S. Noisakran, C. Komoltri, S. Thiemmeca, K. Auethavornanan, A. Jairungsri, R. Kanlaya, N. Tangthawornchaikul, C. Puttikhunt, S. N. Pattanakitsakul, P. T. Yenchitsomanus, J. Mongkolsapaya, W. Kasinrerk, N. Sittisombut, M. Husmann, M. Blettner, S. Vasanawathana, S. Bhakdi, and P. Malasit. 2006. Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J. Infect. Dis. 193:1078-1088. [DOI] [PubMed] [Google Scholar]
- 4.Barnes, N., A. L. Gavin, P. S. Tan, P. Mottram, F. Koentgen, and P. M. Hogarth. 2002. FcγRI-deficient mice show multiple alterations to inflammatory and immune responses. Immunity 16:379-389. [DOI] [PubMed] [Google Scholar]
- 5.Boder, E. T., and K. D. Wittrup. 1997. Yeast surface display for screening combinatorial polypeptide libraries. Nat. Biotechnol. 15:553-557. [DOI] [PubMed] [Google Scholar]
- 6.Chung, K. M., G. E. Nybakken, B. S. Thompson, M. J. Engle, A. Marri, D. H. Fremont, and M. S. Diamond. 2006. Antibodies against West Nile virus nonstructural (NS)-1 protein prevent lethal infection through Fc gamma receptor-dependent and independent mechanisms. J. Virol. 80:1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa, S. M., A. S. Azevedo, M. V. Paes, F. S. Sarges, M. S. Freire, and A. M. Alves. 2007. DNA vaccines against dengue virus based on the ns1 gene: the influence of different signal sequences on the protein expression and its correlation to the immune response elicited in mice. Virology 358:413-423. [DOI] [PubMed] [Google Scholar]
- 8.Falconar, A. K. 1997. The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis. Arch. Virol. 142:897-916. [DOI] [PubMed] [Google Scholar]
- 9.Flamand, M., F. Megret, M. Mathieu, J. Lepault, F. A. Rey, and V. Deubel. 1999. Dengue virus type 1 nonstructural glycoprotein NS1 is secreted from mammalian cells as a soluble hexamer in a glycosylation-dependent fashion. J. Virol. 73:6104-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geiss, B. J., T. C. Pierson, and M. S. Diamond. 2005. Actively replicating West Nile virus is resistant to cytoplasmic delivery of siRNA. Virol. J. 53:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibson, C. A., J. J. Schlesinger, and A. D. Barrett. 1988. Prospects for a virus nonstructural protein as a subunit vaccine. Vaccine 6:7-9. [DOI] [PubMed] [Google Scholar]
- 12.Hazenbos, W. L., J. E. Gessner, F. M. Hofhuis, H. Kuipers, D. Meyer, I. A. Heijnen, R. E. Schmidt, M. Sandor, P. J. Capel, M. Daeron, J. G. van de Winkel, and J. S. Verbeek. 1996. Impaired IgG-dependent anaphylaxis and Arthus reaction in Fc gamma RIII (CD16) deficient mice. Immunity 5:181-188. [DOI] [PubMed] [Google Scholar]
- 13.Libraty, D. H., P. R. Young, D. Pickering, T. P. Endy, S. Kalayanarooj, S. Green, D. W. Vaughn, A. Nisalak, F. A. Ennis, and A. L. Rothman. 2002. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 186:1165-1168. [DOI] [PubMed] [Google Scholar]
- 14.Lieberman, M. M., D. E. Clements, S. Ogata, G. Wang, G. Corpuz, T. Wong, T. Martyak, L. Gilson, B. A. Coller, J. Leung, D. M. Watts, R. B. Tesh, M. Siirin, A. Travassos da Rosa, T. Humphreys, and C. Weeks-Levy. 2007. Preparation and immunogenic properties of a recombinant West Nile subunit vaccine. Vaccine 25:414-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellado-Sanchez, G., J. Garcia-Cordero, R. Luria-Perez, L. Lazaro-Olan, L. Santos-Argumedo, B. Gutierrez-Castaneda, I. Estrada-Garcia, and L. Cedillo-Barron. 2005. DNA priming E and NS1 constructs-homologous proteins boosting immunization strategy to improve immune response against dengue in mice. Viral Immunol. 18:709-721. [DOI] [PubMed] [Google Scholar]
- 16.Moore, T., G. A. Ananaba, J. Bolier, S. Bowers, T. Belay, F. O. Eko, and J. U. Igietseme. 2002. Fc receptor regulation of protective immunity against Chlamydia trachomatis. Immunology 105:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nimmerjahn, F., P. Bruhns, K. Horiuchi, and J. V. Ravetch. 2005. FcγRIV: a novel FcR with distinct IgG subclass specificity. Immunity 23:41-51. [DOI] [PubMed] [Google Scholar]
- 18.Nimmerjahn, F., and J. V. Ravetch. 2006. Fcgamma receptors: old friends and new family members. Immunity 24:19-28. [DOI] [PubMed] [Google Scholar]
- 19.Oliphant, T., M. Engle, G. Nybakken, C. Doane, S. Johnson, L. Huang, S. Gorlatov, E. Mehlhop, A. Marri, K. M. Chung, G. D. Ebel, L. D. Kramer, D. H. Fremont, and M. S. Diamond. 2005. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat. Med. 11:522-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pryor, M. J., and P. J. Wright. 1993. The effects of site-directed mutagenesis on the dimerization and secretion of the NS1 protein specified by dengue virus. Virology 194:769-780. [DOI] [PubMed] [Google Scholar]
- 21.Ravetch, J. V., and S. Bolland. 2001. IgG Fc receptors. Annu. Rev. Immunol. 19:275-290. [DOI] [PubMed] [Google Scholar]
- 22.Roehrig, J. T., L. A. Staudinger, A. R. Hunt, J. H. Mathews, and C. D. Blair. 2001. Antibody prophylaxis and therapy for flaviviral encephalitis infections. Ann. N. Y. Acad. Sci. 951:286-297. [DOI] [PubMed] [Google Scholar]
- 23.Samuel, M. A., and M. S. Diamond. 2006. Pathogenesis of West Nile virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J. Virol. 80:9349-9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlesinger, J. J., M. W. Brandriss, J. R. Putnak, and E. E. Walsh. 1990. Cell surface expression of yellow fever virus nonstructural glycoprotein NS1: consequences of interaction with antibody. J. Gen. Virol. 71(Pt. 3):593-599. [DOI] [PubMed] [Google Scholar]
- 25.Schlesinger, J. J., M. W. Brandriss, and E. E. Walsh. 1985. Protection against 17D yellow fever encephalitis in mice by passive transfer of monoclonal antibodies to the nonstructural glycoprotein gp48 and by active immunization with gp48. J. Immunol. 135:2805-2809. [PubMed] [Google Scholar]
- 26.Schlesinger, J. J., M. Foltzer, and S. Chapman. 1993. The Fc portion of antibody to yellow fever virus NS1 is a determinant of protection against YF encephalitis in mice. Virology 192:132-141. [DOI] [PubMed] [Google Scholar]
- 27.Shrestha, B., M. A. Samuel, and M. S. Diamond. 2006. CD8+ T cells require perforin to clear West Nile virus from infected neurons. J. Virol. 80:119-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takai, T., M. Li, D. Sylvestre, R. Clynes, and J. V. Ravetch. 1994. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell 76:519-529. [DOI] [PubMed] [Google Scholar]
- 29.Unkeless, J. C., E. Scigliano, and V. H. Freedman. 1988. Structure and function of human and murine receptors for IgG. Annu. Rev. Immunol. 6:251-281. [DOI] [PubMed] [Google Scholar]
- 30.Watts, D. M., R. B. Tesh, M. Siirin, A. T. Rosa, P. C. Newman, D. E. Clements, S. Ogata, B. A. Coller, C. Weeks-Levy, and M. M. Lieberman. 2007. Efficacy and durability of a recombinant subunit West Nile vaccine candidate in protecting hamsters from West Nile encephalitis. Vaccine 25:2913-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitby, K., T. C. Pierson, B. Geiss, K. Lane, M. Engle, Y. Zhou, R. W. Doms, and M. S. Diamond. 2005. Castanospermine, a potent inhibitor of dengue virus infection in vitro and in vivo. J. Virol. 79:8698-8706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winkler, G., S. E. Maxwell, C. Ruemmler, and V. Stollar. 1989. Newly synthesized dengue-2 virus nonstructural protein NS1 is a soluble protein but becomes partially hydrophobic and membrane-associated after dimerization. Virology 171:302-305. [DOI] [PubMed] [Google Scholar]
- 33.Winkler, G., V. B. Randolph, G. R. Cleaves, T. E. Ryan, and V. Stollar. 1988. Evidence that the mature form of the flavivirus nonstructural protein NS1 is a dimer. Virology 162:187-196. [DOI] [PubMed] [Google Scholar]
- 34.Wu, S. F., C. L. Liao, Y. L. Lin, C. T. Yeh, L. K. Chen, Y. F. Huang, H. Y. Chou, J. L. Huang, M. F. Shaio, and H. K. Sytwu. 2003. Evaluation of protective efficacy and immune mechanisms of using a nonstructural protein NS1 in DNA vaccine against dengue 2 virus in mice. Vaccine 21:3919-3929. [DOI] [PubMed] [Google Scholar]
- 35.Young, P. R., P. A. Hilditch, C. Bletchly, and W. Halloran. 2000. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 38:1053-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]