Abstract
The potential of neural stem and progenitor cell (NSPC) transplantation in neurodegenerative disease raises a concern about immunosuppressive agents and opportunistic neurotropic pathogens that may interfere with engraftment. Cytomegalovirus (CMV) is an important opportunistic pathogen infecting the central nervous system, where it may remain latent for life, following transplacental transmission. Cyclosporine (Cs), an immunosuppressive drug used in organ transplantation, where its use is associated with CMV reactivation, suppressed murine CMV (MCMV) infection in cultured NSPCs but not in fibroblasts. This activity of Cs appears to be mediated via cyclophilin (CyP) rather than via calcineurin. First, the calcineurin-specific inhibitor FK506 failed to suppress replication. Second, the CyP-specific inhibitor NIM811 strongly suppressed replication in NSPC. NSPCs maintained in the presence of NIM811 retained viral genomes for several weeks without detectable viral gene expression or obvious deleterious effects. The withdrawal of NIM811 reactivated viral replication, suggesting that the inhibitory mechanism was reversible. Finally, inhibition of endogenous CyP A (CyPA) by small interfering RNA also inhibited replication in NSPCs. These results show that MCMV replication depends upon cellular CyPA pathways in NSPCs (in a specific cell type-dependent fashion), that CyPA plays an important role in viral infection in this cell type, and that inhibition of viral replication via CyP leads to persistence of the viral genome without cell damage. Further, the calcineurin-signaling pathway conferring immunosuppression in T cells does not influence viral replication in a detectable fashion.
Neural stem cells (NSC) have self-renewal capacity and an ability to differentiate into neurons, glia, and oligodendrocytes (40). Transplantation of neural stem and progenitor cells (NSPC) is being tested as a treatment for neurodegenerative disease (31), although a number of challenges remain (17). Because allogeneic or xenogeneic rejection poses an obstacle in NPSC transplantation (4), immunosuppression with cyclosporine (Cs) has been commonly incorporated in experimental models using this approach (4). One consequence of such therapies is reactivation and amplification of latent opportunistic infectious agents such as cytomegalovirus (CMV), a common problem associated with allogeneic transplantation. Little is know about the direct effect of immunosuppressive agents on pathogens such as CMV that are likely to reactivate during NSPC transplantation.
Human CMV (HCMV) is an important opportunistic pathogen in immunocompromised patients (8) and is an important neurotropic pathogen when transmitted to the fetus during pregnancy. Infection accompanies ca. 1% of live births (60), with a 5 to 10% incidence of central nervous system (CNS) disease at birth (11) and an additional 10% who do not show symptoms at birth but develop progressive CNS-related disorders such as hearing loss in the first few years of life (13, 38). There is a possibility that CMV may remain latent in the CNSs of newborns who survive congenital infection to become a problem during NSPC transplantation.
The mechanisms of CNS damage and reasons underlying differential cellular susceptibility to CMV infection and disease remain unknown. Since all CMVs exhibit strict species specificity, HCMV cannot be studied directly in any laboratory animal. Murine CMV (MCMV) (27, 56) and guinea pig CMV (45) have both provided insights into general aspects of viral pathogenesis, as well as specific evidence for the involvement of the CNS analogous to HCMV. Importantly, NSPC appear to be susceptible to MCMV infection (22, 26). This evidence supports a role for NSPC in viral pathogenesis and raises the possibility that NSC may be a reservoir of viral latency (55) and further suggests that NSC may contribute to CMV-associated disease.
The potential presence of CMV in the brain of donor and recipient brain cells prompted further investigation of the biology of this virus in mouse NSPC and NSC. We have investigated the direct impact of the widely used immunological modulators, Cs and FK506 on MCMV replication in NSPC. Cs and FK506 have a common target, calcineurin, that mediates immunosuppression in T cells (44). A Cs/cyclophilin (CyP) and FK506/FKBP (FK506 binding protein) complex are essential for the immunosuppression of lymphocyte activation (35) binding to and inhibiting phosphatase activity necessary for calcineurin signaling, thereby silencing nuclear factor of activated T cells (NF-AT) activity (see Fig. 10). Cs and FK506 are known as calcineurin inhibitors, with variable sensitivity in different cell types (28, 29). CyP was originally discovered as a cellular factor with high affinity for the immunosuppressant Cs (46). CyP is a peptidyl prolyl cis/trans isomerase and catalyzes the cis-trans interconversion of peptide bonds amino terminal to proline residues to facilitate protein folding (52). We show here that Cs and NIM811 (a CyP inhibitor lacking calcineurin activity) suppressed replication, whereas FK506 failed to suppress replication in NPSC, implicating CyP-dependent pathways in CMV infection, especially in neural progenitor lineage cells.
FIG. 10.
Model for inhibition mechanism of Cs and NIM811 to MCMV infection. Forming of complex of Cs and CyP or complex of NIM811 and CyP inhibits CyP functions to lead the suppression of MCMV ie1/ie3 transcripts and MCMV proliferation upstream of the MCMV IE-promoter (pretranscription level) (red). This complex does not inhibit AP-1, NF-κB, or MCMV IE promoter activity in NSPC (black line). In T cells, the Cs/CyP or FK506/FKBP complex directly binds to calcineurin and inhibits the phosphatase activity of calcineurin, resulting in the silencing of transcription factor NF-AT (blue line). The inhibition of MCMV infection by Cs and NIM811 uses CyP-mediated pathway independent of calcineurin/NF-AT pathway.
MATERIALS AND METHODS
Cell culture and differentiation of NSPC.
Murine epidermal growth factor (EGF) (purified from mouse submaxillary glands; Becton Dickinson, Bedford, MA) and basic fibroblast growth factor (bFGF) (recombinant basic human FGF purified from Escherichia coli; Sigma, St. Louis, MO)-responsive NSPC were prepared from the subventricular zone of BALB/c mouse embryos (gestational day 15) or from 7- or 21-day-old BALB/c mice (SLC, Tokyo, Japan) as described previously (26). To test the ability of cells that formed neurospheres to regenerate, cells from disrupted neurospheres were plated into 96-well-plates. After 7 days in culture, the wells were inspected for the presence or absence of neurospheres as described previously (26). These secondary neurospheres were confirmed as NSPC by immunostaining with a rabbit antibody to a neuroepithelial cell marker anti-nestin, a rat monoclonal antibody (19) specific to mouse Musashi1, and a rat monoclonal antibody (MAb) specific to CD133 (Chemicon International). After 20 passages, we used the regenerated a neurosphere called “secondary neurosphere” for all experiments.
The induction of differentiation of the NSPC was performed as described by Reynolds and Weiss (41) and analyzed by immunocytochemistry by using rabbit polyclonal antibody against bovine glial fibrillary acidic protein (Dako), mouse MAb specific to β-tubulin III (a neuronal marker) (Sigma), and affinity-purified anti-human Olig2 rabbit immunoglobulin G (IBL). The proportion of each cell type for glia, neurons, and oligodendrocytes was determined after counting in three high-power fields under phase-contrast microscopy. Three independent counts were performed for each marker. Mouse embryonal fibroblasts (MEF) were prepared by a standard method (26). To check the viability, NSPC and MEF were stained with 5 μg of propidium iodide (PI)/ml (28) with or without drugs, and flow cytometry was performed. Cells that displayed a low permeability to PI after 7 days in culture were considered viable.
Cs, FK506, NIM811, and PSC833.
Cs (Sigma) was initially dissolved in ethanol, and stock solution was stored at −30°C. FK506 (tacrolimus) was kindly provided by Fujisawa Comp Japan. The Cs derivatives NIM811 and PSC833 were generously provided by Novartis Pharma AG, Basel, Switzerland, and were dissolved in ethanol. Immediately before addition these derivatives were diluted to the desired concentrations by using Dulbecco modified Eagle medium-F-12 medium.
Virus preparation and infection.
Recombinant viruses (RM4503) derived from the K181+ strain of MCMV capable of expressing enhanced green fluorescence protein (GFP) were used in these studies (57). The dissociated NSPC in suspension or MEF were infected with MCMV at the indicated multiplicities of infection (MOI), and after removal of the inoculum the cells were cultured in fresh medium. Cs, FK506, NIM811, or PSC833 were added at this time to medium using the concentrations indicated in Results. Virus titers were measured in aliquots of the cells by a plaque assay on MEF monolayers. Other aliquots of cells were processed for detection of MCMV antigen by immunofluorescence and flow cytometry.
Flow cytometry.
For flow cytometry, neurospheres of the CNS stem/progenitor cells were determined as previously described (26). The cells were reacted in suspension with MAb N2, specific to the MCMV immediate-early (IE)-89K antigen (48) and with rabbit antibody specific to CyPA (Upstate Cell Signaling, Lake Placid, NY). For the secondary labeling, phycoerythrin- or fluorescein isothiocyanate-conjugated rabbit anti-rat immunoglobulins (Dako) was used for the detection of the MCMV antigen. Fluorescein isothiocyanate-conjugated swine anti-rabbit immunoglobulins (Dako) or phycoerythrin-conjugated affinity-purified goat anti-rabbit immunoglobulin G (Rockland Immunochemicals, Inc., Gilbertsville, PA) were used for the CyPA antigen. The stained cells were analyzed by flow cytometry using an EPICS profile analyzer (Coulter, Miami, FL).
Transfection and reporter assay.
Transfection into mouse NSPC was performed by using a Nucleofector electroporator (Amaxa Biosystems, Germany) according to the manufacturer's recommended protocol. The reporter assay of transcriptional factors (NF-AT, NF-κB, and AP-1) and MCMV IE promoter activity were performed by using the dual-luciferase reporter assay system (Promega Madison, WI) and the β-galactosidase enzyme assay system (Promega).
RNA interference technique.
Small interfering RNA (siRNA) duplexes (si-CyPA containing 5′-CACCAUUUCCGACUGUGGA-3′ overhanging sequences, and si-cyclophilin B (CyPB) containing 5′-GGAAAGACUGUUCCAAAAA-3′ overhanging sequences) and siCONTROL were purchased from Dharmacon (Lafayette, CO). The siRNAs were transfected into mouse NSPC and a fibroblast cell line (NIH 3T3) by using a Nucleofector electroporator (Amaxa Biosystems) according to the manufacturer's recommended protocol. At 48 h after transfection, MCMV (RM4503) was infected at an MOI of 1. The percentage of IE1-positive cells was checked for NSPC at 7 days postinfection (dpi) and for NIH 3T3 at 3 dpi by flow cytometry and compared between CyPA siRNA transfected cells and control cells. At 72 h posttransfection, the CyPA and CyPB expression levels were examined by Western blotting.
Western blotting.
CyPA protein and CyPB protein were detected by using rabbit antibody specific to CyPA (Upstate Cell Signaling) and CyPB (Affinity BioReagents). Sample loads were standardized by detecting β-actin with mouse MAb specific to β-actin (Sigma). YY1 is detected using mouse monoclonal antibody specific to YY1 (Santa Cruz Biotechnology). The blots were incubated with biotin-conjugated secondary antibody (Nichirei, Tokyo, Japan) followed by horseradish peroxidase-conjugated avidin-biotin reaction. Immunoreactive bands were visualized by using enhanced chemiluminescence substrate (Amersham Pharmacia Biotech).
Reverse transcription-PCR.
NSPC were infected with an MOI of 1 PFU/cell. The cultures were incubated for 30 min prior to infection in the presence or absence of NIM811 (1 μM). Total RNA was isolated at 3 and 6 h after infection by using the RNeasy minikit (QIAGEN, Hilden, Germany) according to the manufacturer's protocol. RNA samples were treated with RNase-free DNase I for 15 min at room temperature, and the DNase was inactivated at 65°C for 15 min. The RNA was reverse transcribed by using oligo(dT) primers at 50°C for 50 min, and reactions were terminated by heating at 70°C for 15 min. The reverse-transcribed products were treated with RNase H for 20 min at 37°C and amplified by using specific primers. The primers ie1-R (5′-TAC AGG ACA ACA GAA CGC TC-3′) and ie1/ie3-F (5′-CCT CGA GTC TGG AAC CGA AA-3′) were used to amplify a 188-bp product within the ie1 gene, primers ie3-R (5′-TGT GAG GCA GTA GTT ATA CC-3′) and ie1/ie3-F were used to amplify a 299-bp fragment within the ie3 gene, and primers HPRT-R (5′-AGA TTC AAC TTG CGC TCA TCT TAG GC-3′) and HPRT-F (5′-TTG GAT AAC AGG CCA GAA CTT TGT TGG-3′) were used to amplify a 163-bp product within the hypoxanthine phosphoribosyltransferase (HPRT) cellular gene (1). PCRs were performed under the following conditions with using Phusion kit (FINNZYMES, Esopoo, Finland): 1 cycle at 94°C for 30 sec; 30 cycles of 10 s at 94°C, 10 s at the corresponding annealing temperature, and 20 s at 72°C; and 1 cycle at 72°C for 5 min. Annealing temperatures were as follows: 60°C for ie1, ie3, and HPRT primers. The presence of introns in the viral ie1 and ie3 genes and the cellular HPRT gene made it possible to distinguish the correct amplified RNA from contaminant viral or cellular DNA by its size. Control reactions carried out in the absence of reverse transcriptase were used to assess the specific detection of RNA. Amplified products were separated on a 2% agarose gel and visualized by ethidium bromide staining.
Reversal experiments with recombinant CyPA.
NSPC infected with recombinant MCMV were cultured in the presence of Cs (0.5 μM) or NIM811 (0.5 μM), and then 1 to 100 ng of human recombinant CyPA (BioMol, Plymouth Meeting, PA)/ml was added to the medium. The percentage of IE1-positive NSPC was examined at 10 dpi by flow cytometry.
RESULTS
Cs inhibition of MCMV replication in NSPC.
NSPC were isolated from the subventricular zone of 21-day-old BALB/c mice and separated to single cells (Fig. 1A) capable of generating primary neurospheres (Fig. 1B). Single cells from neurospheres formed secondary neurospheres in a clonal growth assay. NSPC were positive for nestin (Fig. 1C), Musashi-1 (Fig. 1D), and CD133 (Fig. 1E) but were negative for β-III tubulin (data not shown) under conditions previously described (41). A phase-contrast image of NSPC is shown for comparison (Fig. 1F to H). Neurospheres retained the potential to form neurons (Fig. 1I), astrocytes (Fig. 1J), and oligodendrocytes (Fig. 1K) when induced to differentiate (42). NSPC differentiated into neurons (Fig. 1L), astrocytes (Fig. 1M), and oligodendrocytes (Fig. 1N) in the presence of Cs (0.5 μM), indicating that the drug had little effect on differentiation and did not impact the proportion of each cell type observed in cultures (data not shown).
FIG. 1.
Isolation and assay of NSPC. (A) Phase-contrast micrograph of a single neural stem cell. (B) Phase-contrast micrograph of a neurosphere after 7 days of proliferation in response to EGF and bFGF. (C to H) Secondary neurospheres were immunostained with antibodies specific to the neural stem cell markers such as nestin (C), Musashi-1 (D), and CD133 (E). Phase-contrast micrograph of each marker's neurosphere (nestin [F], Musashi-1 [G], and CD133 [H]). (I to K) NSPC from neurospheres were dissociated and plated onto glass coverslips that had been coated with poly-d-lysine and were differentiated into neurons (β-III tubulin) (I), astrocytes (glial fibrillary acidic protein [GFAP]) (J), and oligodendrocytes (Olig-2) (K). (L to N) NSPC were dissociated and plated onto glass coverslips that had been coated with poly-d-lysine and treated with Cs (0.5 μM) and were differentiated into neurons (L), astrocytes (M), and oligodendrocytes (N). Bar, 20 μm.
Immediately after isolation and dissociation, NSPC were infected with MCMV (RM4503) at an of MOI of 1 (determined on MEF). The infected cells first showed GFP fluorescence starting at 3 dpi, which increased by 7 dpi (Fig. 2A). Infected cells treated with Cs showed colony formation similar to untreated cells, but fewer GFP-positive cells were observed by day 7 (Fig. 2B). Immunocytostaining with MAb N2 (19) specific to IE1 antigen (48) revealed that ca. 10% of cells were IE1 positive at day 5, 25% of cells were positive by day 7, and 75% were positive by day 10 postinfection (Fig. 2C), suggesting a considerable delay in the replication cycle in NSPC that was not studied further. Infection was specifically blocked by Cs treatment, as evidenced by the reduced levels of IE1+ cells (Fig. 2C) and the production of infectious virus (Fig. 2D) during the period from 5 to 10 dpi. The immunosuppressant significantly (>100-fold) reduced virus titers (Fig. 2D). Untreated and Cs-treated NSPC cultures showed the same level of infectivity 1 day after infection, suggesting that Cs did not affect virus binding but reduced some subsequent step in replication. To investigate the effect of input dose on Cs-mediated inhibition, the percentage of IE1-positive cells was assessed at 7 dpi at an MOI of 0.1, 1, or 10 in the presence or absence of 0.5 μM Cs. Cs treatment suppressed MCMV infection at all doses of virus but was most effective at an MOI of 1 (Fig. 2E). Although NSPC from 21-day-old BALB/c mice were susceptible, embryonic NSPC isolated at 15 days of gestation or from 7-day-old BALB/c mice showed a threefold higher susceptibility to infection (Fig. 2F). Replication in at any of these developmental stages showed a similar level of inhibition with Cs.
FIG. 2.
Suppression of MCMV replication by Cs in NSPC cultures. (A and B) Phase-contrast micrograph (top) and immunofluorescence image for GFP (bottom) of NSPC cultures at 7 dpi with MCMV (RM4503) at an MOI of 1 and left untreated (A) or treated with 0.5 μM Cs (B). (C) Flow cytometric analysis of the percentage of MCMV IE1 antigen-positive cells in NSPC cultures infected at an MOI of 1 and left untreated (control) or treated with 0.5 μM Cs (**, P < 0.01). (D) Virus titers determined by plaque assay in NSPC cultures infected at an MOI of 1 and left untreated (control) or treated with 0.5 μM Cs (**, P < 0.01). (E) Percentage of IE1-positive cells (7 dpi) in infected NSPC cultures after different input doses of virus (MOIs of 0.1, 1, or 10) and left untreated (control) or treated with 0.5 μM Cs and assayed (**, P < 0.01; *, P < 0.05). (F) Percentage of IE1-positive cells (7 dpi) in NSPC cultures infected at an MOI of 1 and left untreated (control) or treated with 0.5 μM Cs from mice at embryonic day 15 (E15), postnatal day 7 (P7), and postnatal day 21 (P21) (**, P < 0.01).
We next evaluated the effect of Cs on MCMV replication in a commonly used permissive cell type, MEF. MEF showed the expected >60% GFP+ or IE1+ cells by 3 dpi (data not shown), in contrast to >1 week time in NSPC (Fig. 2C). In contrast to NSPC, MCMV infection of MEF was insensitive to Cs (0.5 μM), with nearly normal levels of GFP-positive cells, cytopathic effect (Fig. 3A), IE1-antigen-positive cells (Fig. 3B), or release of progeny virus (Fig. 3C). Cs inhibited MCMV replication in MEF at higher concentrations (>5 μM), but these doses also reduced cell viability, although MEF growth was not inhibited by treatment with 0.5 μM Cs (data not shown).
FIG. 3.
(A) MEF were infected with MCMV (RM4503) at an MOI of 1 and left untreated (control) or treated with Cs at 0.5 or 5 μM. Photomicrographs showed fluorescent image (green, GFP) and phase-contrast images. (B) Comparison of time course of IE1 antigen-positive cells in MEF left untreated (control) or treated with Cs at 0.5 or 5 μM. (C) Virus titers in infected MEF cultures left untreated (control) or treated with Cs at 0.5 μM, analyzed by plaque assay.
Inhibition of MCMV replication independent of calcineurin-mediated immunosuppressive activity but dependent on CyP inhibition.
Cs and FK506 have a common target, calcineurin, that mediates immunosuppression in T cells (44). A Cs/CyP and FK506/FKBP (FK506 binding protein) complex is essential for immunosuppression of lymphocyte activation (35), silencing NF-AT activity through calcineurin inhibition (28, 29). It was interesting that FK506 did not inhibit MCMV replication in NSPC when assayed by GFP detection (Fig. 4A). We investigated the dose dependence of Cs and FK506 inhibition in NSPC (MOI of 1) and observed that Cs inhibited MCMV in a dose-dependent manner, whereas FK506 had little effect on IE1 detection (Fig. 4B), even when at 0.5 μM, a dose in excess of that necessary to suppress lymphocyte activation (44). Thus, the inhibitory effect of Cs on MCMV replication seemed to be independent of the calcineurin pathway. Because Cs binds CyP and inactivates peptidyl prolyl cis/trans isomerase function (35), we sought out CyP-specific inhibitors. NIM811 is one such CyP inhibitor that does not inhibit calcineurin-dependent pathways (5) and, as a result, is not immunosuppressive. NIM811 strongly suppressed MCMV replication (MOI = 1) at 0.5 μM (Fig. 4A, C, and D). NIM811 treatment reduced the percentage of IE1-positive NSPC in a dose-dependent manner (Fig. 4C). Plaque assay showed that this concentration of NIM811 suppressed MCMV more dramatically than Cs, reducing virus titers between 2 and 3 orders of magnitude (Fig. 4D). NIM811 showed ca. 90% inhibition of MCMV replication at 0.5 μM even when NSPC were infected at an MOI 10 (data not shown). NIM811 has been known as an inhibitor of p-glycoprotein, as well as CyPs (59); however, PSC833, a Cs derivative that is a specific p-glycoprotein inhibitor and targets neither CyP- or calcineurin-dependent pathways (24), did not affect the level of MCMV IE1 expression (Fig. 4C) or replication (Fig. 4D). These results indicate that CyP is involved in MCMV replication. NSPC viability (PI uptake) and cell growth were not affected by treatment with doses as high as 1 μM Cs (Fig. 4E), FK506 (Fig. 4F), NIM811 (Fig. 4G), or PSC833 (Fig. 4H).
FIG. 4.
The inhibitory effect of Cs on MCMV replication is independent of calcineurin function and dependent on CyP. (A) Phase-contrast micrographs (top) and GFP fluorescence (bottom) analyses of MCMV infection at 7 dpi in control cells and in the presence of 0.5 μM FK506, NIM811, and PSC833, as indicated. (B) Comparison of the percentage of MCMV IE1-antigen positive cells (7 dpi) in untreated NSPC cultures and in cultures maintained in Cs or FK506, with inhibitors at 0.1, 0.5, or 1.0 μM as determined by flow cytometry. (C) Comparison of the percentage of MCMV IE1-antigen positive cells at 7 dpi in untreated NSPC cultures and in cultures maintained in NIM811 or PSC833, with inhibitors at 0.1, 0.5, or 1.0 μM as determined by flow cytometry. (D) Comparison of virus titers in control and infected NSPC cultures treated with FK506, PSC833, or NIM811 (0.5 μM) until 7 dpi (**, P < 0.01). (E to H) Viability of NSPC treated with increasing doses of Cs (E), FK506 (F), NIM811 (G), or PSC833 (H) for 7 days, as determined by cell count and PI uptake (104 cells/ml).
We confirmed that Cs (Fig. 5A) and FK506 (Fig. 5B) had a small effect on NF-AT activity in NSPC at concentrations used to assess an impact on MCMV. As expected, NF-AT was suppressed by Cs at concentrations as low as 0.1 μM in the T-lymphocyte cell line EL-4 (Fig. 5C). FK506 also inhibited NF-AT activity at a concentration as low as 0.01 μM in T lymphocytes (Fig. 5C). Thus, NF-AT is not critical to MCMV replication in NSPC.
FIG. 5.
Reporter cell assay analysis. (A to E) NSPC and EL-4 cells were transfected with pNF-AT-luc (A, B, and C) or pAP-1-luc (D), NF-κB-luc (E), or pRL-TK reporter plasmid as a control, followed by treatment with different doses of Cs (A, C, D, and E) and FK506 (B and C). At 24 h after transfection, the luciferase activities of whole lysates were measured. (F and G) NSPC were transfected with MCMV IE-promoter-β-Gal, followed by treatment with different doses of Cs (F) and NIM811 (G). At 24 h after transfection, the β-Gal activities of whole lysates were measured. All of the data represent the means of the relative luciferase and β-galactosidase activities in three independent experiments.
Cs may affect the activities of AP-1 and NF-κB, known targets outside the calcineurin/NF-AT pathway (36, 43). Reporter assays showed that Cs did not influence AP-1 (Fig. 5D) or NF-κB (Fig. 5E) signaling pathways in NSPC and that the activity of the MCMV-IE promoter/enhancer was not suppressed by Cs (Fig. 5F) or NIM811 (Fig. 5G) in NSPC despite the presence of AP-1 and NF-κB binding sites. Thus, the inhibition of viral replication by Cs or NIM811 appears to be independent of viral IE gene expression.
NIM811 inhibits ie1/ie3 mRNA expression in NSPC.
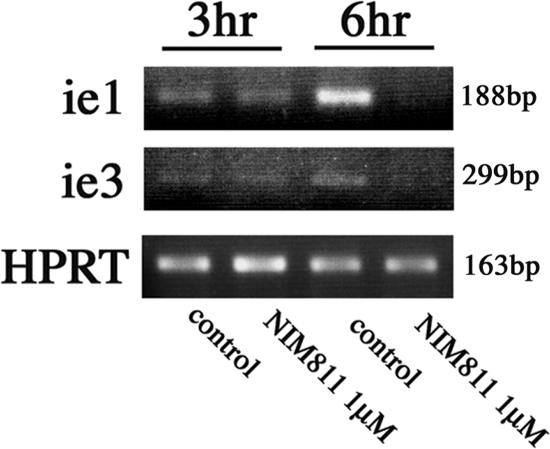
We found that NIM811 inhibited ie1/ie3 mRNA expression at 3 h postinfection (hpi) and 6 hpi, a time point during the IE/early phase of the infection cycle before the onset of viral DNA replication (23). The NSPC were incubated for 30 min prior to infection in the presence or absence of NIM811 (1 μM) and then were infected with MCMV at an MOI of 1. Total RNA was isolated at 3 and 6 hpi from each culture. The RNA was reverse transcribed, and semiquantitative reverse transcription-PCR was performed with primers for ie1 and ie3 transcripts. HPRT was used as a standard control. We could not detect the significant difference between NIM811 treated and untreated cultures at 3 hpi, but at 6 hpi the ie1/ie3 transcripts levels were significantly inhibited in NIM811-treated NSPC (Fig. 6).
FIG. 6.
Detection of viral transcripts after infection with NIM811. NSPC were infected at an MOI of 1 PFU per cell with MCMV in the presence or absence of NIM811 (1 μM). Whole-cell RNA was harvested at 3 hpi, treated with DNase at 6 hpi, and reverse transcribed with oligo(dT). PCRs were performed with primer sets specific for ie1, ie3, and HPRT as described in Materials and Methods. Amplified products were separated on 2% agarose gels and visualized by ethidium bromide staining. NIM811 (1 μM) inhibited ie1/ie3 mRNA expression at 6 hpi in NSPC.
Viral persistence in NIM811-treated NSPC cultures.
NSPC cultures were infected with MCMV at an MOI of 1 in the presence of Cs (0.5 μM), and cells were washed and transferred to Cs-free medium at 7 dpi. The numbers of MCMV IE1-positive cells significantly increased after the removal of Cs compared to cells kept in Cs-containing medium (Fig. 7A), demonstrating that the inhibition of viral replication by Cs was reversible. To determine whether the persistence of the viral genome in the presence of Cs was CyP dependent and calcineurin independent, NSPC were infected with MCMV at an MOI of 0.1 in the presence of NIM811 (1 μM). At 2 weeks postinfection, virus-derived GFP expression was rarely observed with NIM811 (Fig. 7B), and the percentage of IE1-positive cells remained low (Fig. 7D). NSPC infected with MCMV and maintained in the presence of NIM811 were divided into an NIM811-treated culture and a NIM811-free culture at 2-week intervals. GFP-positive cells were rarely observed in NIM811-treated cultures monitored for 6 weeks (Fig. 7B). GFP-positive and IE-1 positive cells appeared in most cells within 2 weeks when NIM811 was removed from the culture. Levels rose from 59% at 2 weeks to 70% at 4 weeks and dropped to 38% at 6 weeks and 26% at 8 weeks (Fig. 7C and D to G). Evidence of infection was not detectable at 10 weeks. Cells were able to yield infectious virus up to 8 weeks in culture, after which time infected NSPC cultures failed to proliferate virus.
FIG. 7.
MCMV persistent infection in the presence of Cs or NIM811 and reactivation after the removal. (A) NSPC were cultured in the presence of Cs (0.5 μM) for 7 days, when cultures were washed, split, and cultured in the presence or absence of Cs for analysis of MCMV IE1 antigen-positive cells. The arrow indicates the day when Cs was removed from cultures. (B and C) GFP fluorescence micrographs and phase-contrast micrographs of NSPC. NSPC were infected with MCMV (RM4503) at an MOI of 0.1 and cultured for 2, 4, or 6 weeks, when the cells were divided into cultures without or with NIM811 (1.0 μM) and cultured for an additional 2 weeks as indicated below the panels. (B and C) Continual NIM811-treated NSPC (B) and NIM811-released NSPC (C). Phase-contrast micrographs (top) and GFP fluorescence micrographs (bottom) are shown. (D to G) Comparison of the percentage of IE1-positive cells in cultures infected with MCMV (RM4503) at an MOI of 0.1 and treated with NIM811 (1 μM). Cultures were released from suppression by NIM811 every 2 weeks up to 8 weeks postinfection (2 weeks of culture [D], 4 weeks of culture [E], 6 weeks of culture [F], and 8 weeks of culture [G]). The data are expressed as ± the standard deviation of at least three independent experiments (**, P < 0.01).
Role of CyPA in MCMV infection in NSPC.
Cyclophilin A (CypA) and CyPB are the most abundant subtypes of CyP (58). We assessed the importance of these in CMV replication by using siRNA to inhibit endogenous CyPA and CyPB compared to control β-actin (Fig. 8A). Knockdown of CyPA in NSPC was associated with a 50% inhibition of MCMV yields, whereas knockdown of CyPB had no effect (Fig. 8B), even though this siRNA blocked CyPB expression effectively. As expected from the use of inhibitors, knockdown of endogenous CyPA (Fig. 8C) had no effect on MCMV replication in fibroblasts (Fig. 8D).
FIG. 8.
Function of CyPA in MCMV infection in NSPC. (A) The expression and knockdown of CyPA and CyPB by each specific siRNA and si-control were confirmed by immunoblot analysis in total cell lysate of NSPC. β-Actin was detected as an internal control by immunoblot analysis. (B) Inhibition of endogenous CyPA inhibits MCMV infection in NSPC significantly at 7 dpi (MOI = 1) (n = 9; **; P < 0.001). (C) Expression and knockdown of CyPA by each specific siRNA and si-control were confirmed by immunoblot analysis in total cell lysates of NIH 3T3 cells. β-Actin was detected as an internal control by immunoblot analysis. (D) Inhibition of endogenous CyPA did not inhibit MCMV IE1 expression in NIH 3T3 cells at 3 dpi (MOI = 1) (n = 3).
Five days after infection (MOI = 1), staining of MCMV-IE1 antigen and CyPA was investigated by flow cytometry, where nearly 39% of the cells were CyPA positive, 21% of the cells were IE1 positive, and nearly 15% of the cells double stained for both (Fig. 9A). Thus, 70% of IE1-positive cells also exhibited elevated CyPA, suggesting that these cells provided better support for MCMV gene expression.
FIG. 9.
(A) Two-color flow cytometry of MCMV IE1 antigen and CyPA (5 dpi). (B) Upregulation of CyPA by CMV infection in NSPC (3 dpi). The expression of CyPA and IE1 of MCMV was confirmed by immunoblot analysis. β-Actin was detected as an internal control. (C to F) Reversal of MCMV inhibition with Cs and NIM811 by adding recombinant CyPA in medium. (C) NSPC infected with MCMV (RM4503) at an MOI of 1 were cultured in the presence of Cs alone or together with human recombinant CyPA. Comparison of the percentage of IE1-positive cells in control untreated (left) and Cs-treated (0.5 μM) cultures in the absence (middle) or presence of CyPA (100 ng/ml) (right), as assessed by flow cytometry. (E) Comparison of the percentage of IE1-positive cells in control, untreated (left) and NIM811-treated (0.5 μM) cultures in the absence (middle) or presence of CyPA (100 ng/ml) (right), as assessed by flow cytometry. (D) CyPA (1, 10, or 100 ng/ml) reversed the suppression of MCMV infection by Cs (0.5 μM) examined by flow cytometry. (F) CyPA (1, 10, or 100 ng/ml) reversed the suppression of MCMV infection by NIM811 (0.5 μM) examined by flow cytometry. (G) Comparison of CyPA and YY1 expression between MEF and NSPC. Each total cell lysate of a similar number of cells (104) from MEF and NSPC was compared and detected by blotting with antibody to CyPA and YY1.
CyPA is known as a proinflammatory cytokine that is upregulated and secreted into plasma after inflammatory stimulation (20, 47). We investigated whether MCMV infection would upregulate endogenous CyPA in NSPC. Western blotting analysis showed that CyPA was upregulated in NSPC infected with high MOI at 3 dpi (Fig. 9B). We also investigated the ability of exogenous CyPA to reverse the inhibition of MCMV by Cs and NIM811. Human recombinant CyPA was added to infected NSPC (MOI = 1) in the presence of Cs or NIM811 (0.5 μM). Given that IE1 expression correlated with productive infection (Fig. 2C and D), the proportion of IE1-positive cells were measured by flow cytometry. CyPA (at 100 ng/ml) was found to reverse the inhibition of MCMV by Cs (Fig. 9C) or NIM811 (Fig. 9E). Furthermore, the addition of CyPA to infected NSPC cultures reversed Cs-mediated inhibition at starting concentrations as low as 1 ng/ml (Fig. 9D). CyPA reversed NIM811-mediated inhibition of MCMV in a dose-dependent manner (Fig. 9F).
To examine the differential inhibitory effect of Cs on infected MEF and NSPC, we compared the expression levels of CyPA by Western blotting. Because of the difference in cell size and the nuclear/cytoplasmic ratio between NSPC and MEF, as well as differences in host proteins such as β-actin that might be used as a control, we compared the expression level of CyPA in similar numbers of cells. The blot showed that the level of CyPA was significantly higher in MEF than in NSPC when similar numbers of cells were analyzed (Fig. 9G), suggesting that one reason that inhibitors were inactive in MEF was the level of expression. In contrast to CyPA, the level of YY1 (an inhibitor of CMV replication) is higher in NSPC than in MEF (Fig. 9G), a finding consistent with evidence that YY1 is higher in less differentiated cells and is downregulated during differentiation as cells become more permissive to virus (32). Thus, the induction of CyPA, as well as other differences, may underlie the sensitivity of infected cells to CyPA inhibitors.
DISCUSSION
Cs and the CyP-specific inhibitor NIM811 reduce MCMV replication by reducing the level of viral gene expression in NSPC. Although Cs is a potent immunosuppressor acting through the calcineurin pathway (15), the anti-MCMV effects described here are dependent on inhibition of CyP by this drug. This was confirmed showing that FK506 (another calcineurin inhibitor) did not inhibit MCMV replication in NSPC as well as by the ability of NIM811 (CyP inhibitor and p-glycoprotein inhibitor) or siRNA targeted at CyPA to inhibit viral replication, as well as by showing that inhibition was reversed by supplementing CyP and that PSC833 (p-glycoprotein inhibitor) failed have any effect. Infection of NSPC with MCMV could be suppressed and was reversed upon removal of drug up to 8 weeks in the presence of 1 μM NIM811. CyP, in particular appears to play a crucial role in the process of MCMV replication in NSPC, and this may contribute to the behavior of this and related viruses in the host. Any of the many cellular processes that rely on CyP could be involved. CyP has peptidyl-prolyl cis-trans isomerase activity that catalyzes conformational changes, assisting primary protein folding, and accelerating the rate of polypeptide refolding (14, 52) associated with secretion (12), mitochondrial physiology and cell death suppression (37), RNA splicing (19), and histone deacetylation (2).
We compared the role of CyPA and CyPB, two of many family members, to show that Cs or NIM811 was inhibiting replication via CyPA but not CyPB. Infected cells have higher levels of CyPA, suggesting an important role in CMV replication in fibroblasts, as well as NSPC. CyP family members control the replication efficiency of human immunodeficiency virus (HIV) (34), vaccinia virus (10), vesicular stomatitis virus (6), and hepatitis C virus (59). CyPA was reported to bind HIV-1 Gag polyprotein in a Cs-sensitive complex (34). CyPB interacts with the HCV RNA polymerase NS5B to directly stimulate its RNA-binding activity (59). Our report is the first to implicate CyPA in herpesvirus replication, but it has not identified any unique target of this protein.
Although the precise mechanism by which Cs and NIM811 inhibit MCMV replication is not yet clear, our results suggest a postentry block that reduces or blocks viral gene expression, although whether this is focused at the IE, early, or late phases is not known. Inhibition of major immediate-early promoter (MIEP), which controls the transcription of the critical ie1/ie3 transcription unit, would reduce or prevent MCMV productive infection (1). Cs did not block MIEP transcription in transient assays (49) that were sensitive to the activation of transcription factors AP-1 or NF-κB. In addition, ie1/ie3 mRNA expression was significantly inhibited by NIM811 when added at 6 hpi, which is a time when the histone deacetylase (HDAC) effect is maximal (54). These results suggest an impact on CyP-dependent events as broad as virus trafficking, chromatin remodeling, and the regulatory activity of virus proteins at the pretranscription level (Fig. 10). If viral trafficking is the central mechanism of anti-MCMV effect of NIM811, the ie1/ie3 transcripts level must have had a great difference between control culture and NIM811-treated culture at 3 hpi. In our experiment, the levels of ie1/ie3 transcripts were almost the same at 3 hpi. At this time, transcription is influenced by chromatin modification even though there is no evidence that the viral genome itself has a chromatin structure. Nevertheless, viral genomes may be silenced by HDAC under control of IE3 (53). It has been demonstrated that CyPA specifically interacts with SIN3-Rpd3 HDAC in vitro, suggesting that CyPA affects gene expression by physically interacting with HDAC (2). Lu et al. reported that CyPA has a function of protecting the paternal allele of Peg3 from DNA methylation and inactive histone modifications. Stable knockdown of CyPA led to the silencing of Peg3 expression in p19 embryonic carcinoma cells through interacting with HDAC and methyltransferase (33). CyPA may play an important role in regulation of MIEP chromatin modification through interacting with HDACs and methyltransferases during CMV infection. Investigation of protein(s) that may interact with CyPA is now in progress.
This study is the first to report the inhibitory effect of nontoxic doses of Cs on CMV. The immunosuppressive consequences of Cs on CMV in the host have been intensively studied. Although Cs might inhibit CMV proliferation in vivo, immunosuppression caused by Cs may outweigh the anti-CMV effect of Cs. Our work confirmed a previous report (18) that MCMV-infected MEF are resistant to Cs, which may result from higher expression of CyPA in MEF than in NSPC. This mechanism is similar to that reported by Gatanaga et al. (16). showing that the amount of CyPA in different cell types is a determinant of the HIV-1 replication level. The anti-CMV effect of Cs or NIM811 may also be dependent on other NSPC characteristics. Although these are stem/progenitor cells, their susceptibility to CMV appears to be greater than hematopoietic stem cells. In terms of susceptibility of CMV, NSPC may be classified as an intermediate cell type between nonpermissive cells (e.g., embryonic stem cells or hematopoietic stem cells) and permissive cells (e.g., MEF). The levels of YY1 (Fig. 9G) and HDAC2 (data not shown) may contribute to the pattern of susceptibility by many mechanisms, including silencing (2), as well as chromatin modification.
Transplantation of the NSPC has been proposed as a treatment for neurodegenerative disorders (31). This approach will likely encounter the allogeneic and xenogeneic rejection response (4), which is highly inflammatory (7, 30). Although the brain is an immunologically privileged site, inflammation upregulates CyPA (20) and may drive CMV reactivation from latency and replication (51). Neural stem transplantation may upregulate CyPA and lead to CMV-related disease. The fact that CMV replication enhances endogenous CyPA expression further reinforces the likelihood of increased inflammation (61).
In addition to being an immunosuppressant, Cs provides a neuroprotection (50) that may be shared with nonimmunosuppressive immunophilin ligands such as NIM811 (21). Castilho et al. reported that Cs enhances survival of grafted rat embryonic dopamine neurons (9). The administration of Cs or CyP inhibitors may facilitate NSPC survival, as well as block the replication of opportunistic viruses such as HCMV. Thus, the immunosuppressive characteristics of Cs may be expected to predispose to CMV disease such encephalitis (39), while CyP inhibitor-mediated effects of this drug may provide some level of antiviral activity in a tissue-specific fashion, especially in the CNS. Although most CMV encephalitis is related to HIV infection and AIDS, a small proportion is related to solid organ transplantation, where Cs is commonly used (3, 25). Our work suggests that Cs inhibition of viral replication and the control of the CyP level may help prevent CMV reactivation and replication in the future neural stem cell transplantation.
Acknowledgments
This study has been supported in part by grant 17790259 from the Ministry of Education, Science, and Culture of Japan.
We thank Shimotohno, Laboratory of Human Tumor Viruses, Department of Viral Oncology, Institute for Virus Research, Kyoto University, for fruitful discussions. The Cs derivatives NIM811 and PSC833 were kindly provided by Novartis (Basel, Switzerland).
Footnotes
Published ahead of print on 6 June 2007.
REFERENCES
- 1.Angulo, A., P. Ghazal, and M. Messerle. 2000. The major immediate-early gene ie3 of mouse cytomegalovirus is essential for viral growth. J. Virol. 74:11129-11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arevalo-Rodriguez, M., M. E. Cardenas, X. Wu, S. D. Hanes, and J. Heitman. 2000. Cyclophilin A and Ess1 interact with and regulate silencing by the Sin3-Rpd3 histone deacetylase. EMBO J. 19:3739-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arribas, J. R., G. A. Storch, D. B. Clifford, and A. C. Tselis. 1996. Cytomegalovirus encephalitis. Ann. Intern. Med. 125:577-587. [DOI] [PubMed] [Google Scholar]
- 4.Barker, R. A., and H. Widner. 2004. Immune problems in central nervous system cell therapy. Neurorx 1:472-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billich, A., F. Hammerschmid, P. Peichl, R. Wenger, G. Zenke, V. Quesniaux, and B. Rosenwirth. 1995. Mode of action of SDZ NIM 811, a nonimmunosuppressive cyclosporin A analog with activity against human immunodeficiency virus (HIV) type 1: interference with HIV protein-cyclophilin A interactions. J. Virol. 69:2451-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bose, S., M. Mathur, P. Bates, N. Joshi, and A. K. Banerjee. 2003. Requirement for cyclophilin A for the replication of vesicular stomatitis virus New Jersey serotype. J. Gen. Virol. 84:1687-1699. [DOI] [PubMed] [Google Scholar]
- 7.Briscoe, D. M., S. I. Alexander, and A. H. Lichtman. 1998. Interactions between T lymphocytes and endothelial cells in allograft rejection. Curr. Opin. Immunol. 10:525-531. [DOI] [PubMed] [Google Scholar]
- 8.Britt, W. J., and C. A. Alford. 1996. Cytomegalovirus, 3rd ed. Lippincott-Raven, Philadelphia, PA.
- 9.Castilho, R. F., O. Hansson, and P. Brundin. 2000. Improving the survival of grafted embryonic dopamine neurons in rodent models of Parkinson's disease. Prog. Brain Res. 127:203-231. [DOI] [PubMed] [Google Scholar]
- 10.Castro, A. P., T. M. Carvalho, N. Moussatche, and C. R. Damaso. 2003. Redistribution of cyclophilin A to viral factories during vaccinia virus infection and its incorporation into mature particles. J. Virol. 77:9052-9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cinque, P., R. Marenzi, and D. Ceresa. 1997. Cytomegalovirus infections of the nervous system. Intervirology 40:85-97. [DOI] [PubMed] [Google Scholar]
- 12.Colley, N. J., E. K. Baker, M. A. Stamnes, and C. S. Zuker. 1991. The cyclophilin homolog ninaA is required in the secretory pathway. Cell 67:255-263. [DOI] [PubMed] [Google Scholar]
- 13.Conboy, T. J., R. F. Pass, S. Stagno, W. J. Britt, C. A. Alford, C. E. McFarland, and T. J. Boll. 1986. Intellectual development in school-aged children with asymptomatic congenital cytomegalovirus infection. Pediatrics 77:801-806. [PubMed] [Google Scholar]
- 14.Freskgard, P. O., N. Bergenhem, B. H. Jonsson, M. Svensson, and U. Carlsson. 1992. Isomerase and chaperone activity of prolyl isomerase in the folding of carbonic anhydrase. Science 258:466-468. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi, M. K., and R. Khanna. 2004. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect. Dis. 4:725-738. [DOI] [PubMed] [Google Scholar]
- 16.Gatanaga, H., D. Das, Y. Suzuki, D. D. Yeh, K. A. Hussain, A. K. Ghosh, and H. Mitsuya. 2006. Altered HIV-1 Gag protein interactions with cyclophilin A (CypA) on the acquisition of H219Q and H219P substitutions in the CypA binding loop. J. Biol. Chem. 281:1241-1250. [DOI] [PubMed] [Google Scholar]
- 17.Ginis, I., and M. S. Rao. 2003. Toward cell replacement therapy: promises and caveats. Exp. Neurol. 184:61-77. [DOI] [PubMed] [Google Scholar]
- 18.Gui, X., M. Ho, and P. E. Camp. 1982. Effect of cyclosporin A on murine natural killer cells. Infect. Immun. 36:1123-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horowitz, D. S., E. J. Lee, S. A. Mabon, and T. Misteli. 2002. A cyclophilin functions in pre-mRNA splicing. EMBO J. 21:470-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin, Z. G., A. O. Lungu, L. Xie, M. Wang, C. Wong, and B. C. Berk. 2004. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler. Thromb. Vasc. Biol. 24:1186-1191. [DOI] [PubMed] [Google Scholar]
- 21.Kaminska, B., K. Gaweda-Walerych, and M. Zawadzka. 2004. Molecular mechanisms of neuroprotective action of immunosuppressants—facts and hypotheses. J. Cell Mol. Med. 8:45-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawasaki, H., I. Kosugi, Y. Arai, and Y. Tsutsui. 2002. The amount of immature glial cells in organotypic brain slices determines the susceptibility to murine cytomegalovirus infection. Lab. Investig. 82:1347-1358. [DOI] [PubMed] [Google Scholar]
- 23.Keil, G. M., A. Ebeling-Keil, and U. H. Koszinowski. 1984. Temporal regulation of murine cytomegalovirus transcription and mapping of viral RNA synthesized at immediate-early times after infection. J. Virol. 50:784-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller, R. P., H. J. Altermatt, K. Nooter, G. Poschmann, J. A. Laissue, P. Bollinger, and P. C. Hiestand. 1992. SDZ PSC 833, a non-immunosuppressive cyclosporine: its potency in overcoming P-glycoprotein-mediated multidrug resistance of murine leukemia. Int. J. Cancer 50:593-597. [DOI] [PubMed] [Google Scholar]
- 25.Kleinschmidt-DeMasters, B. K., and D. H. Gilden. 2001. The expanding spectrum of herpesvirus infections of the nervous system. Brain Pathol. 11:440-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosugi, I., Y. Shinmura, H. Kawasaki, Y. Arai, R. Y. Li, S. Baba, and Y. Tsutsui. 2000. Cytomegalovirus infection of the central nervous system stem cells from mouse embryo: a model for developmental brain disorders induced by cytomegalovirus. Lab. Investig. 80:1373-1383. [DOI] [PubMed] [Google Scholar]
- 27.Krmpotic, A., I. Bubic, B. Polic, P. Lucin, and S. Jonjic. 2003. Pathogenesis of murine cytomegalovirus infection. Microbes Infect. 5:1263-1277. [DOI] [PubMed] [Google Scholar]
- 28.Kung, L., T. D. Batiuk, S. Palomo-Pinon, J. Noujaim, L. M. Helms, and P. F. Halloran. 2001. Tissue distribution of calcineurin and its sensitivity to inhibition by cyclosporine. Am. J. Transplant. 1:325-333. [DOI] [PubMed] [Google Scholar]
- 29.Kung, L., and P. F. Halloran. 2000. Immunophilins may limit calcineurin inhibition by cyclosporine and tacrolimus at high drug concentrations. Transplantation 70:327-335. [DOI] [PubMed] [Google Scholar]
- 30.Lalor, P. F., and D. H. Adams. 2000. Lymphocyte homing to allografts. Transplantation 70:1131-1139. [DOI] [PubMed] [Google Scholar]
- 31.Lindvall, O., Z. Kokaia, and A. Martinez-Serrano. 2004. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat. Med. 10(Suppl.):S42-S50. [DOI] [PubMed] [Google Scholar]
- 32.Liu, R., J. Baillie, J. G. Sissons, and J. H. Sinclair. 1994. The transcription factor YY1 binds to negative regulatory elements in the human cytomegalovirus major immediate-early enhancer/promoter and mediates repression in non-permissive cells. Nucleic Acids Res. 22:2453-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu, Y. C., J. Song, H. Y. Cho, G. Fan, K. K. Yokoyama, and R. Chiu. 2006. Cyclophilin a protects Peg3 from hypermethylation and inactive histone modification. J. Biol. Chem. 281:39081-39087. [DOI] [PubMed] [Google Scholar]
- 34.Luban, J., K. L. Bossolt, E. K. Franke, G. V. Kalpana, and S. P. Goff. 1993. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell 73:1067-1078. [DOI] [PubMed] [Google Scholar]
- 35.Matsuda, S., and S. Koyasu. 2000. Mechanisms of action of cyclosporine. Immunopharmacology 47:119-125. [DOI] [PubMed] [Google Scholar]
- 36.Mattila, P. S., K. S. Ullman, S. Fiering, E. A. Emmel, M. McCutcheon, G. R. Crabtree, and L. A. Herzenberg. 1990. The actions of cyclosporin A and FK506 suggest a novel step in the activation of T lymphocytes. EMBO J. 9:4425-4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagawa, T., S. Shimizu, T. Watanabe, O. Yamaguchi, K. Otsu, H. Yamagata, H. Inohara, T. Kubo, and Y. Tsujimoto. 2005. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434:652-658. [DOI] [PubMed] [Google Scholar]
- 38.Pass, R. F., S. Stagno, G. J. Myers, and C. A. Alford. 1980. Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics 66:758-762. [PubMed] [Google Scholar]
- 39.Reuter, J. D., D. L. Gomez, J. H. Wilson, and A. N. Van Den Pol. 2004. Systemic immune deficiency necessary for cytomegalovirus invasion of the mature brain. J. Virol. 78:1473-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynolds, B. A., W. Tetzlaff, and S. Weiss. 1992. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 12:4565-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynolds, B. A., and S. Weiss. 1996. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev. Biol. 175:1-13. [DOI] [PubMed] [Google Scholar]
- 42.Rietze, R. L., H. Valcanis, G. F. Brooker, T. Thomas, A. K. Voss, and P. F. Bartlett. 2001. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature 412:736-739. [DOI] [PubMed] [Google Scholar]
- 43.Rincon, M., and R. A. Flavell. 1994. AP-1 transcriptional activity requires both T-cell receptor-mediated and co-stimulatory signals in primary T lymphocytes. EMBO J. 13:4370-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawada, S., G. Suzuki, Y. Kawase, and F. Takaku. 1987. Novel immunosuppressive agent, FK506. In vitro effects on the cloned T-cell activation. J. Immunol. 139:1797-1803. [PubMed] [Google Scholar]
- 45.Schleiss, M. R. 2002. Animal models of congenital cytomegalovirus infection: an overview of progress in the characterization of guinea pig cytomegalovirus (GPCMV). J. Clin. Virol. 25(Suppl. 2):S37-S49. [DOI] [PubMed] [Google Scholar]
- 46.Schreiber, S. L. 1991. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science 251:283-287. [DOI] [PubMed] [Google Scholar]
- 47.Sherry, B., N. Yarlett, A. Strupp, and A. Cerami. 1992. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc. Natl. Acad. Sci. USA 89:3511-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shinmura, Y., S. Aiba-Masago, I. Kosugi, R. Y. Li, S. Baba, and Y. Tsutsui. 1997. Differential expression of the immediate-early and early antigens in neuronal and glial cells of developing mouse brains infected with murine cytomegalovirus. Am. J. Pathol. 151:1331-1340. [PMC free article] [PubMed] [Google Scholar]
- 49.Smith, C. L., and G. L. Hager. 1997. Transcriptional regulation of mammalian genes in vivo: a tale of two templates. J. Biol. Chem. 272:27493-27496. [DOI] [PubMed] [Google Scholar]
- 50.Snyder, S. H., M. M. Lai, and P. E. Burnett. 1998. Immunophilins in the nervous system. Neuron 21:283-294. [DOI] [PubMed] [Google Scholar]
- 51.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1997. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91:119-126. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi, N., T. Hayano, and M. Suzuki. 1989. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature 337:473-475. [DOI] [PubMed] [Google Scholar]
- 53.Tang, Q., and G. G. Maul. 2006. Immediate-early interactions and epigenetic defense mechanisms. Caister Academic Press, Norfork, United Kingdom.
- 54.Tang, Q., and G. G. Maul. 2003. Mouse cytomegalovirus immediate-early protein 1 binds with host cell repressors to relieve suppressive effects on viral transcription and replication during lytic infection. J. Virol. 77:1357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsutsui, Y., H. Kawasaki, and I. Kosugi. 2002. Reactivation of latent cytomegalovirus infection in mouse brain cells detected after transfer to brain slice cultures. J. Virol. 76:7247-7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsutsui, Y., I. Kosugi, and H. Kawasaki. 2005. Neuropathogenesis in cytomegalovirus infection: indication of the mechanisms using mouse models. Rev. Med. Virol. 15:327-345. [DOI] [PubMed] [Google Scholar]
- 57.van Den Pol, A. N., E. Mocarski, N. Saederup, J. Vieira, and T. J. Meier. 1999. Cytomegalovirus cell tropism, replication, and gene transfer in brain. J. Neurosci. 19:10948-10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Waldmeier, P. C., K. Zimmermann, T. Qian, M. Tintelnot-Blomley, and J. J. Lemasters. 2003. Cyclophilin D as a drug target. Curr. Med. Chem. 10:1485-1506. [DOI] [PubMed] [Google Scholar]
- 59.Watashi, K., N. Ishii, M. Hijikata, D. Inoue, T. Murata, Y. Miyanari, and K. Shimotohno. 2005. Cyclophilin B is a functional regulator of hepatitis C virus RNA polymerase. Mol. Cell 19:111-122. [DOI] [PubMed] [Google Scholar]
- 60.Weller, T. H. 1971. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. I. N. Engl. J. Med. 285:203-214. [DOI] [PubMed] [Google Scholar]
- 61.Yurchenko, V., G. Zybarth, M. O'Connor, W. W. Dai, G. Franchin, T. Hao, H. Guo, H. C. Hung, B. Toole, P. Gallay, B. Sherry, and M. Bukrinsky. 2002. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J. Biol. Chem. 277:22959-22965. [DOI] [PubMed] [Google Scholar]