Abstract
A subset of simian immunodeficiency virus (SIV)-infected macaques progresses rapidly to disease with transient SIV-specific immune responses and high viral loads. Unique SIV variants with convergent Env mutations evolve in these rapid progressor (RP) macaques. To address the pathogenic significance of RP-specific variants, we generated infectious molecular clones from the terminal-phase plasma of an RP macaque. Inoculation of macaques with a representative clone, SIVsmH635FC, resulted in a persistent viremia, comparable to that produced by pathogenic SIVsmE543-3, and a chronic disease with progressive loss of CD4+ T cells. However, SIVsmH635FC did not reproduce the rapid-disease phenomenon. Molecular analyses of viruses from these macaques revealed rapid reversion to the wild-type SIVsmE543-3 sequence at two RP-specific sites and slower reversion at another three sites. SIVsmH635FC infection was not sufficient to cause rapid progression even following coinoculation with SIVsmE543-3, despite acute depletion of memory CD4+ T cells. SIVsmH635FC competed efficiently during primary infection in the coinoculated macaques, but SIVsmE543-3 predominated after the development of SIV-specific immune responses. These data suggest that the replication fitness of the RP variant was similar to that of SIVsmE543-3 in a naïve host; however, SIVsmH635FC was at a disadvantage following the development of SIV-specific immune responses. Consistent with these findings, neutralization assays revealed that SIVsmH635FC was highly sensitive to neutralization but that the parental SIVsmE543-3 strain was highly resistant. This study suggests that the evolution of RP-specific variants is the result of replication in a severely immunocompromised host, rather than the direct cause of rapid progression.
Rapid progression to disease is observed in patients infected with human immunodeficiency virus (HIV) (9, 13, 30, 38, 40, 60) and macaques experimentally infected with simian immunodeficiency virus (SIV) (15, 18, 21, 22, 24, 31, 44, 47, 52, 64). In both cases, the level of plasma viremia is significantly higher than in conventional progressors, and the cellular and humoral immune responses are very weak and only transiently observed. In this study, as well as prior studies, we defined rapid progressors (RPs) as SIV-inoculated macaques with persistent antigenemia that failed to develop or maintain antibody responses and showed clinical deterioration that necessitated euthanasia before 6 months of inoculation. Recent studies have shown that SIV-infected RP macaques suffer an early massive loss of memory CD4+ T cells, presumably due to virus-induced destruction (24, 31, 34, 37, 44, 45, 52, 61). The loss of memory CD4+ T cells is profound and irreversible in RP macaques, suggesting a strong correlation between the maintenance of CD4+ memory T cells and rapid progression to disease (31, 44, 45, 52). Although these studies of RPs using the SIV model are useful for understanding the mechanism of rapid progression in HIV infection, it is not clear why the depletion of memory CD4+ T cells is so profound in RP macaques and how they develop disease rapidly after the depletion.
Recent studies have shown that RP macaques can be distinguished from conventional progressors in terms of pathological manifestations (2, 18, 21, 31). RP macaques uniformly exhibit SIV encephalitis and pneumonia, the presence of multinucleated giant cells, and a predominance of SIV-expressing macrophages in nonlymphoid tissues, such as lung and brain. In contrast, conventional progressor macaques show the pathological features more analogous to AIDS in humans, i.e., the predominance of opportunistic infections. However, the definitive hallmark of rapid progression is the failure to maintain SIV-specific immune responses (18, 21, 64). RP macaques mount an initial humoral and cellular immune response at the appropriate time following infection, but these responses wane rapidly within the first 3 to 4 weeks of infection (21). This immune defect is observed not only in immunity to SIV, but also in immune responses against unrelated antigens, including recall antigens (tetanus toxoid) or new antigen (hepatitis A virus). This profound and global immune failure is consistent with the early loss in T-helper function caused by a massive loss of memory CD4+ T cells (24, 31, 34, 37, 44, 45, 52, 61) and may be critical for the subsequent rapid progression of disease.
Despite the lack of immune pressure, the envelope of SIV undergoes unique molecular evolution in RP macaques (8, 31). Sequence analysis of viruses in three SIVsmE543-3-infected RP macaques (8) revealed a unique convergent pattern of substitutions in env, including the loss of a highly conserved potential N-linked glycosylation (PNG) site in the V1-V2 region (N158D/S or S160N/G), substitutions in the V3 analog (P337T/S/H/L and R348W), and substitutions in the highly conserved GDPE motif (G386R and D388N/V). These RP-specific mutations are associated with the acquisition of CD4-independent usage of CCR5 in cell fusion assays (8, 11, 57). In addition, mutational analyses have shown that, like in HIV type 1 (HIV-1) (33, 46), the GDPE motif and/or V3 loop analog of SIV are important for cell tropism and interactions with CD4 and coreceptors (11, 20, 49, 57). These data suggested that RP-specific mutations were selected in RP macaques to adapt to a specific microenvironment in RP macaques through altered cell tropism and receptor usage.
In order to study the role of these variants with RP-specific mutations in rapid progression, seven infectious molecular clones were generated from the terminal-phase plasma of an RP macaque (31). We initially predicted that these clones would show an advantage over parental SIV in terms of replication efficacy or cell tropism that would explain the high viral loads in the plasma and tissues of RP macaques. However, clones with RP-specific mutations replicated less efficiently than their parent, SIVsmE543-3, in primary rhesus peripheral blood mononuclear cells and macrophages (31). From these in vitro analyses, it was not clear why RP variants predominated in RP macaques or whether RP variants contributed to the development of rapid disease progression.
The goal of the present study was to analyze the biological properties of RP variants in vivo using infectious molecular clones. For this purpose, we chose SIVsmH635FC, since it was closest in terms of sequence to the consensus sequence of plasma virus in the donor animal. Macaques were inoculated with SIVsmH635FC alone or in combination with parental SIVsmE543-3, and virus replication, changes in CD4+ T-cell subsets in blood and mucosal sites, antibody responses, and the stability of RP-specific mutations were evaluated. The coinfected animals allowed us to evaluate the in vivo fitness of SIVsmH635FC relative to that of SIVsmE543-3.
MATERIALS AND METHODS
Viruses and macaques.
SIVsmE543-3 is a pathogenic molecular clone which was derived from a rhesus macaque (E543) with SIV-induced encephalitis and AIDS (8, 14, 15, 18). The molecular clone SIVsmH635FC was generated from the plasma of an RP rhesus macaque (H635), which was inoculated with virus isolated from an SIVsmE543-3-infected RP macaque (H445), as previously described (31). Virus stocks were prepared from the supernatant of 293 cells (17) that were transfected by plasmid DNA using FuGENE 6 transfection reagent (Roche Diagnostics, Indianapolis, IN). The p27 titer of virus stocks was measured by SIV core antigen assay (Coulter, Miami, FL), and the stocks were adjusted to 50 ng/ml p27 for inoculation into macaques. Four rhesus macaques (H704, H709, H714, and H723) were intravenously inoculated with 1 ml of SIVsmH635FC, and three other macaques (H711, H712, and H713) were coinoculated with 0.5 ml of SIVsmE543-3 and 0.5 ml of SIVsmH635FC. Blood and cerebrospinal fluid (CSF) samples were collected sequentially and evaluated for viral RNA load. Blood, bronchoalveolar lavage (BAL), peripheral lymph node (LN), and rectosigmoid colon (ReCo) samples were collected to analyze lymphocyte subsets. Western blotting was performed using SIVsmE543-3 as an antigen to examine the antibody response, as previously described (18). Macaques were euthanized when they developed intractable diarrhea and weight loss of greater than 15% of body weight.
Quantification of viruses in plasma and CSF.
The viral RNA loads in plasma and CSF were determined by real-time reverse transcriptase-PCR (RT-PCR) using a Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA) as described previously (19), a new set of primers, and a probe to amplify an 85-bp fragment. The primers and probe were as follows: forward primer, 5′-CATCTAGTGGTGGAAACAGGAACA-3′ (nucleotides [nt] 1383 to 1406); reverse primer, 5′-AATTTCCTCCTCTGCCACTAGGT-3′ (nt 1445 to 1467); and probe, 5′-ATGCCAGCAACAAGCAGACCAACAGC-3′ (nt 1416 to 1441). The sequences of these primers and probe are 100% identical to those of SIVsmE543-3 and SIVsmH635FC.
Lymphocyte isolation from tissues.
BAL samples were obtained as previously described (25, 31). Briefly, the lavage fluid was filtered through a 70-μm cell strainer and centrifuged. The cell pellet was then washed twice with phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml gentamicin. Lymphocytes were isolated from peripheral LN by Ficoll density gradient and washed twice with Hanks' balanced salt solution (HBSS). Lymphocytes were isolated from ReCo biopsy samples using the modified method which was previously described (39, 63). Briefly, small biopsy specimens were incubated at 37°C for 30 min with vigorous shaking in HBSS containing 1 mM EDTA, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml gentamicin. The supernatants were filtered and centrifuged, and the cells were resuspended in HBSS containing 5% fetal bovine serum (FBS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml gentamicin (HBSS-5% FBS). The residual tissue fragments were incubated at 37°C for 1 h with vigorous shaking in RPMI 1640 containing 5% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamicin, and 20 U/ml type II collagenase (Sigma, St. Louis, MO). The cells were filtered, centrifuged, and resuspended in HBSS-5% FBS. The cells obtained by both EDTA and collagenase treatment were pooled and pelleted by centrifugation. The pellets were resuspended in 44% Percoll (GE Healthcare, Piscataway, NJ), layered on 70% Percoll, and centrifuged at 1,800 rpm for 20 min. Cells at the interface between the 44% and 70% Percoll layers were collected. All cells isolated from BAL, LN, and ReCo samples were resuspended in stain buffer (BD Biosciences, San Jose, CA) and used for staining with monoclonal antibodies.
Flow cytometric analysis.
Blood, BAL, LN, and ReCo samples were stained with combinations of the following fluorochrome-conjugated monoclonal antibodies: CD3 (fluorescein isothiocyanate [FITC] or peridinin chlorophyll protein-Cy5.5), CD4 (phycoerythrin [PE], peridinin chlorophyll protein-Cy5.5, or allophycocyanin [APC]), CD28 (FITC), CD95 (APC), CCR5 (PE), CD8 (FITC or APC), CD8 beta (PE), CD20 (FITC or PE), and mouse immunoglobulin G1 and G2 isotype-matched controls. All antibodies were obtained from BD Biosciences except CD8 beta (Beckman Coulter, Fullerton, CA) and analyzed by four-color flow cytometry (FACSCalibur; BD Biosciences). Percentages of memory/naïve cells in CD4+ T cells were determined using CD28 and CD95 as makers, as described before (44, 45). Data analysis was performed using CellQuest (BD Biosciences) and Flowjo (TreeStar, San Carlos, CA).
Sequencing analysis of viruses in the infected macaques.
Sequence analysis of the env V1-V5 region was performed as previously described (31). Briefly, viral RNA samples were isolated with a QIAamp viral RNA kit (QIAGEN, Hilden, Germany) and were used for reverse transcription by SuperScript III (Invitrogen, Carlsbad, CA) and the primer 3U3-R. The region of gp120 from V1 to V5 was amplified using primers SME1 and SME2 (31) and was cloned using the TOPO TA cloning kit (Invitrogen). Resultant clones from plasma and CSF samples, as well as PCR products, were sequenced. Sequences were aligned by MacVector (Accelrys, San Diego, CA) and compared with the sequences of SIVsmE543-3 (GenBank accession number U72748) and SIVsmH635FC (DQ201174). Numbering of nucleotides and amino acids used in this study are based on the sequence of SIVsmE543-3. Numbers of nonsynonymous and synonymous substitutions per site were calculated using SNAP (synonymous/nonsynonymous analysis program, available at http://www.hiv.lanl.gov), which utilizes the method of Nei and Gojobori (43; see also references 29 and 48). Pairwise comparisons with SIVsmH635FC were performed using sequence data of clones from SIVsmH635FC-infected macaques. The average number of substitutions at each codon was plotted to detect locations of positive selection. The rates of synonymous substitution per synonymous site (dS) and nonsynonymous substitution per nonsynonymous site (dN) were calculated. The unpaired t test was used to compare the group means of dS and dN values for individual animals.
Assay for virus-specific neutralizing antibody.
GHOST (3) Hi-5 cells (AIDS Research and Reference Reagent Program, DAIDS, NIAID, NIH, from V. N. KewalRamani and D. R. Littman) (41) were used for the virus-neutralizing assay. GHOST (3) Hi-5 cells were selected as target cells, because they are highly susceptible to both SIVsmE543-3 and SIVsmH635FC. Cells were maintained in complete medium, which is Dulbecco's modified Eagle's medium containing 10% heat-inactivated FBS, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin in the presence of antibiotics (500 μg/ml G418, 100 μg/ml hygromycin, and 1 μg/ml puromycin). The virus stocks of SIVsmE543-3 and SIVsmH635FC were prepared from the supernatant of 293 cells that had been transfected by plasmid DNA using FuGENE 6 transfection reagent. The 50% tissue culture infectious dose (TCID50) was determined using GHOST (3) Hi-5 cells. Virus stocks were adjusted to 2 × 104 TCID50/ml, which generally resulted in 5 to 10% green fluorescent protein-positive (GFP+) cells in a positive control without plasma. Sequential plasma samples from SIVsmH635-infected macaques in this study were used for the neutralization assay. To assess homologous- and heterologous-antibody neutralization, plasma samples from two SIVsmE543-infected macaques (H461 and H596) at 16 wpi and SIVsmE660-infected macaques (D4 and D5) at 20 wpi (50) were also used for the assay. Plasma samples were inactivated by heating them at 58°C for 30 min.
The neutralizing assay was performed by a modified method based on previous studies (5, 10, 32). GHOST (3) Hi-5 cells were seeded in a 48-well plate at 2 × 104 cells/well the day before infection. Fourfold serial dilutions of plasma were mixed with equal volumes of virus stock. The mixture was incubated for 1 h at 37°C. One hundred microliters of the virus-plasma mixture was added to duplicate wells containing GHOST (3) Hi-5 cells in 100 μl complete medium supplemented with 40 μg/ml Polybrene (Millipore, Billerica, MA). After overnight incubation, the virus-plasma mixture was removed and the cells were washed once with PBS. Then, 500 μl of complete medium per well was added, and the mixture was incubated for one more day. The cells were washed once with PBS, resuspended in PBS containing 1 mM EDTA, and fixed in 2% formaldehyde. GFP expression was measured by flow cytometry using a FACSCalibur. The percent neutralization was calculated using the number of GFP+ cells observed in the absence of plasma as a positive control. The highest dilution of plasma that resulted in a reduction in the number of GFP+ cells by more than 90% is shown as the neutralization titer.
RESULTS
Replication of the RP clone SIVsmH635FC in rhesus macaques.
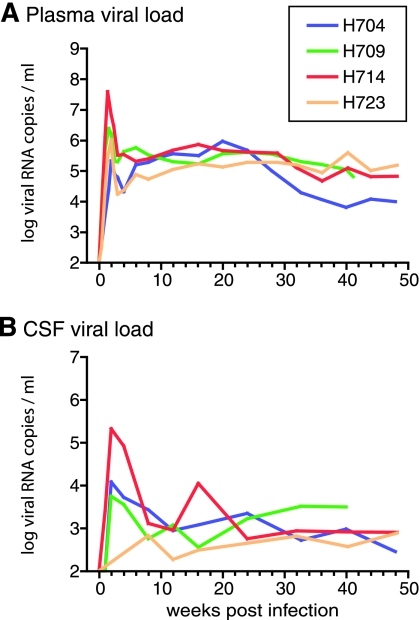
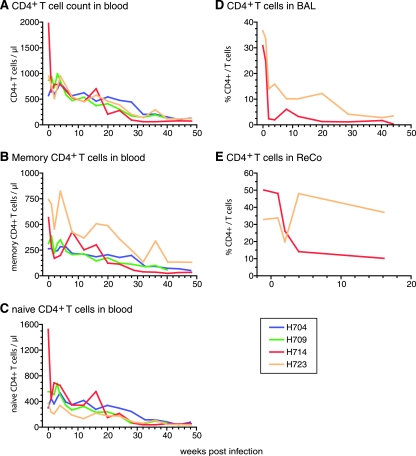
Infectious molecular clones were previously generated from RP macaque H635 (31). Six of the seven clones had specific substitutions in the env gene that we have observed previously in RP macaques (8, 31) and appeared to replicate inefficiently in vitro (31). To determine the in vivo pathogenicity of RP variants, four rhesus macaques were inoculated with one of these clones, SIVsmH635FC. This virus was closest in terms of sequence to the consensus sequence of the virus in the plasma of the donor macaque, H635. Infection was established in all four macaques as shown by the presence of viral RNA in plasma (Fig. 1A) and CSF (Fig. 1B), and seroconversion as assessed by Western blot analyses (data not shown). However, none of the macaques developed rapid progression. Peak plasma viremia varied from 2 × 105 to 4 × 107 copies/ml among the inoculated macaques, with approximately 105 copies/ml being indicative of stable persistent viremia (Fig. 1A). These peak and postacute-phase viral loads were equivalent to those observed previously in macaques inoculated with other pathogenic SIV clones, such as the parental clone SIVsmE543-3 (21, 31, 44). CD4+ T cells in the blood progressively declined in SIVsmH635FC-inoculated macaques (Fig. 2A), due to a decline in both the memory (CD95highCD28high or CD95highCD28low) and naïve (CD95lowCD28high) subsets of CD4 T cells (Fig. 2B and C). Acute depletion of CD4+ T cells at mucosal sites, such as BAL fluid (Fig. 2D) and the ReCo (Fig. 2E), was observed in the two animals examined. However, partial replenishment was observed in one animal, H723 (Fig. 2D and E). These kinetics of CD4+ T cells are similar to those of SIVsmE543-3-infected conventional progressors (31, 44, 45, 52). Three of these macaques remained healthy for over 1 year of infection while showing progressive depletion of both memory and naïve CD4+ T cells (Fig. 2B and C). One macaque, H709, died unexpectedly due to ventricular thromboembolism at 41 wpi. Although no opportunistic infections were observed, this animal exhibited lymphadenopathy, enterocolitis, and mild SIV encephalitis, all evidence of SIV-related disease. Therefore, SIVsmH635FC appeared to be pathogenic in rhesus macaques. However, infection was not associated with rapid disease progression.
FIG. 1.
Viral RNA loads in the plasma (A) and CSF (B) of four SIVsmH635FC-inoculated rhesus macaques (H704, H709, H714, and H723). CSF viral loads of H723 at 1 and 2 wpi were not determined because CSF samples were not available.
FIG. 2.
CD4+ T-cell depletion in four SIVsmH635FC-inoculated macaques. The progressive declines in numbers of CD4+ T cells (A), memory (CD95highCD28high or CD95highCD28low) CD4 T cells (B), and naive (CD95lowCD28high) CD4 T cells (C) in blood are shown by absolute numbers. The depletion of CD4+ T cells in mucosal tissues is shown by percentages of CD4+ cells in total T cells from BAL (D) and ReCo (E) samples. BAL and ReCo samples were collected only from H714 and H723.
Reversion to the parental SIVsmE543-3 sequence in SIVsmH635FC-infected macaques.
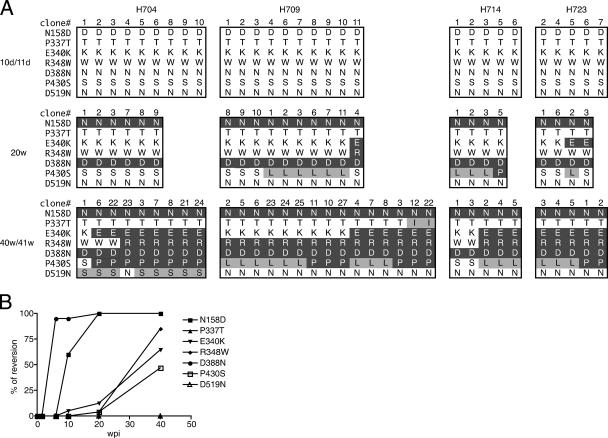
The env V1-V5 region cloned from plasma samples was sequenced to determine the stability of RP-specific mutations in vivo. SIVsmH635FC has seven substitutions in this region relative to its parent, SIVsmE543-3 (31). These differences are the loss of a highly conserved PNG site in the V1-V2 region (N158D), substitutions in or near the V3 loop analog (P337T, E340K, and R348W), a substitution in the GDPE motif (D388N), P430S in the V4 region, and D519N in the C terminus of gp120. Most of these substitutions, such as N158D, three substitutions in the V3 analog, and substitutions in the GDPE motif (D388N), are specifically observed in RP macaques (8). As shown in Fig. 3A, each of these unique substitutions was present during primary viremia (day 10 or 11). However, reversion to SIVsmE543-3 residues was subsequently observed in two sites (N158D and D388N) by week 20 and occurred more slowly at three other sites (E340K, R348W, and P430S) by week 40/41. Only two of the sites, P337T in the V3 loop and D519N at the C terminus of gp120, did not revert. This tendency was common to all the macaques, suggesting that the same selection pressure(s) was driving the reversion. To examine whether these changes were due to positive selection, synonymous and nonsynonymous substitutions at these seven codons were analyzed in 58 clones derived from week 20 and 40/41 samples (data not shown). Despite many nonsynonymous substitutions that are apparent from Fig. 3A, there was only one synonymous substitution found at codon 430 in H714 at week 40. All 58 clones had nonsynonymous substitutions at the two rapid reversion sites (N158D and D388N), with no synonymous substitutions. This extremely high ratio of nonsynonymous to synonymous substitutions strongly suggests positive selection of these reversions. Analysis of additional time points showed that reversion of the D388N mutation was almost complete at 6 wpi (Fig. 3B), suggesting a very strong selection pressure against this residue. Reversion of the N158D mutation was slower than that of the D388N mutation, though greater than 50% of the clones had reverted by 10 wpi. These two rapid-reversion sites are highly conserved in clones from conventional SIV infections and have been shown to be important for the tropism of the virus and its ability to bind CD4 or CCR5 (8, 11, 20, 42, 49, 57). In addition, changes in PNG sites in the V1-V2 region greatly affect sensitivity to antibody neutralization (6, 36). Thus, the phenotypic changes which result from the reversions may impart a significant advantage for replication in macaques.
FIG. 3.
Reversion to the parental SIVsmE543-3 sequence in SIVsmH635FC-infected macaques. (A) The env V1-V5 region was amplified by RT-PCR from plasma samples from day 10 or 11 (10d/11d), week 20 (20w), and week 40 or 41 (40w/41w). RT-PCR products were cloned into plasmids and sequenced. Reversion to the SIVsmE543-3 sequence at the seven locations which differ between SIVsmE543-3 and SIVsmH635FC in the env V1-V5 region is shown by amino acids. The seven locations of the amino acid substitutions, namely, N158D, P337T, E340K, R348W, D388N, P430S, and D519N, are shown on the left side. Amino acid residues at these locations of the obtained clones are boxed to represent the sampling date and macaques. Clone numbers are shown at the tops of the boxes. Amino acids that reverted to SIVsmE543-3 amino acids are shown by white letters in a black background. Amino acid changes other than the changes that revert to the SIVsmE543-3 sequence are shaded in gray. (B) The kinetics of reversion to the SIVsmE543-3 sequence are shown. The percentage of reversion was calculated using all the clones from the four SIVsmH635FC-infected macaques at day 10 or 11, week 20, and week 40 or 41 shown in panel A. The percentages of reversion at 6 and 10 weeks were calculated using clones from two macaques, H704 and H709.
Wide variation in the V1-V2 and V4 regions of Env gp120 in viruses from SIVsmH635FC-infected macaques.
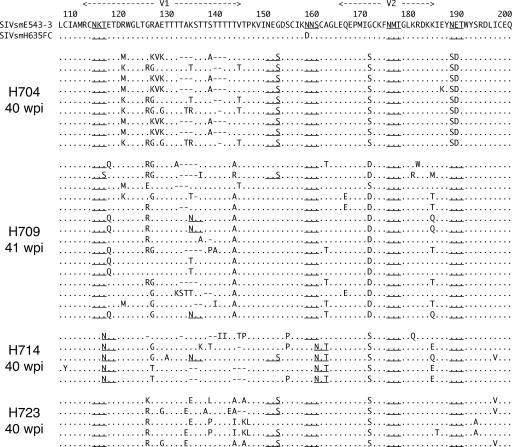
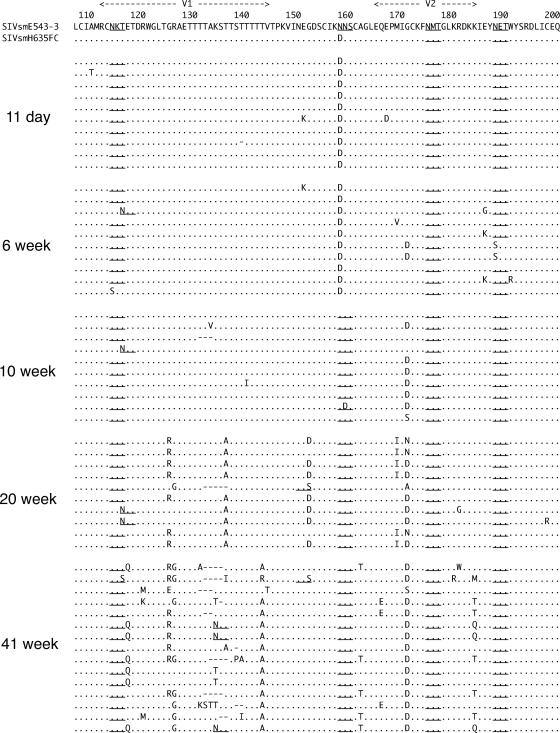
In addition to reversions, many other substitutions were observed in the V1 toV5 region of envelope cloned from SIVsmH635FC-infected macaques. Most of these substitutions were concentrated in the V1-V2 and V4 regions, in which insertion/deletion polymorphisms and changes in PNG sites were observed. The wide variation in these variable regions, as well as high conservation of other regions, suggested that these substitutions might be selected to escape from immune recognition, as previously reported (3, 6, 7, 26, 28, 36, 51, 55, 56). Figure 4 shows the extent of variation in the V1-V2 regions of Env proteins cloned from the plasma of all four macaques at 40/41 wpi. Despite many mutations, the reversion of the N158D mutation and one spontaneous substitution of G171S/D were the only dominant substitutions that were commonly observed in all the macaques. Thus, the viruses appeared to evolve independently, with the selection of different dominant substitutions in each macaque. For example, the loss of a glycosylation site by N188S was observed in nine of the nine clones in macaque H704 but was not observed at all in the other macaques. The highly dominant T143A/R/I substitution in H709 and H723 was not observed at all in H704 and H714.
FIG. 4.
Amino acid alignment of the Env V1-V2 region of clones amplified from plasma samples of the four SIVsmH635FC-infected macaques, H704, H709, H714, and H723, at 40 or 41 wpi. The amino acid sequence of SIVsmE543-3 is shown at the top in the single-letter code, with dots below indicating identical amino acids in aligned sequences. Amino acid substitutions are indicated, gaps are shown by dashes, and PNG sites are underlined.
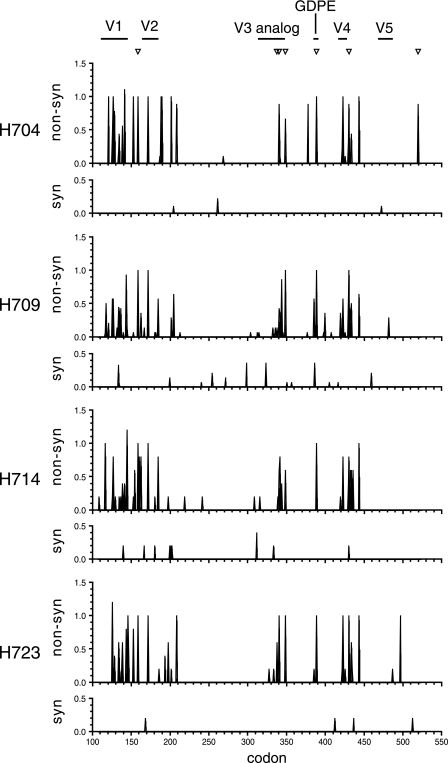
To clarify whether the envelope proteins were under positive selection at these later time points, nonsynonymous and synonymous mutations at each codon were analyzed using sequence data from 40/41 wpi (Fig. 5). Synonymous mutations were sporadically observed throughout the region analyzed in all macaques and represented a minor population (average of <0.4 mutation/codon). In contrast, nonsynonymous substitutions were heavily concentrated in the V1-V2 and V4 regions, as well as the regions containing the reversion mutations, which include the V3 analog and GDPE motif. The average number of nonsynonymous substitutions was greater than 0.5 mutation/codon and frequently in the range of 1.0 mutation/codon, indicating that these substitutions were highly dominant in the viral population. The calculation of the rate of nonsynonymous and synonymous substitutions (dN and dS) suggested positive selection in the entire env V1-V5 region. The mean dN (0.0193 to 0.0222) was significantly greater than the mean dS (0.0018 to 0.0103) in all macaques at 40/41 wpi, resulting in low dS/dN ratios (0.0797 to 0.5311). The results indicated that the variation in the V1-V2 and V4 regions, as well as the reversion of RP-specific sites, was strongly selected for in the SIVsmH635FC-infected macaques.
FIG. 5.
Average nonsynonymous and synonymous substitutions of each codon in the env V1-V5 region. Pairwise comparisons with SIVsmH635FC were performed using nucleic acid sequence data of clones amplified from plasma samples of SIVsmH635FC-infected macaques at 40/41 wpi. Numbers of nonsynonymous (non-syn) and synonymous (syn) substitutions per site were calculated with SNAP. The locations of seven nucleotide changes between SIVsmE543-3 and SIVsmH635FC are indicated by inverted triangles at the top. The V1 to V5 regions and GDPE motif are also indicated at the top. The codon numbers of env are shown at the bottom.
Temporal changes in the Env V1-V2 region are shown by an alignment of clones obtained from sequential plasma samples of a representative macaque, H709 (Fig. 6). The extent of variability in the V1-V2 region increased over the course of infection. Most of the mutations were observed to be dominant changes, such as G171D at 10 wpi; G125R, T136A, G152D, and M169I at 20 wpi; and T143A/R and K184M/T/Q at 41 wpi. Of these, T136A, G152D, and M169I at 20 wpi reverted to the original residues at 41 wpi. Variants with changes in PNG sites, such as T116N, N114S, N188S, G152S, and K134N, transiently emerged as minor populations. The deletions in V1 varied in length and location among clones at different time points, as well as at the same time point. This suggested that new variants continuously emerged and were selected for after inoculation with SIVsmH635FC. This is consistent with the hypothesis that these mutations in variable regions are due to escape from antibody recognition (3, 6, 7, 26, 28, 36, 51, 55, 56).
FIG. 6.
Amino acid alignment of the Env V1-V2 regions of clones amplified from sequential plasma samples of SIVsmH635FC-infected macaque H709. The amino acid sequence of SIVsmE543-3 is shown at the top in the single-letter code, with dots below indicating identical amino acids in aligned sequences. Amino acid substitutions are indicated, gaps are shown by dashes, and PNG sites are shown by underlines.
Coinfection with SIVsmH635FC and SIVsmE543-3.
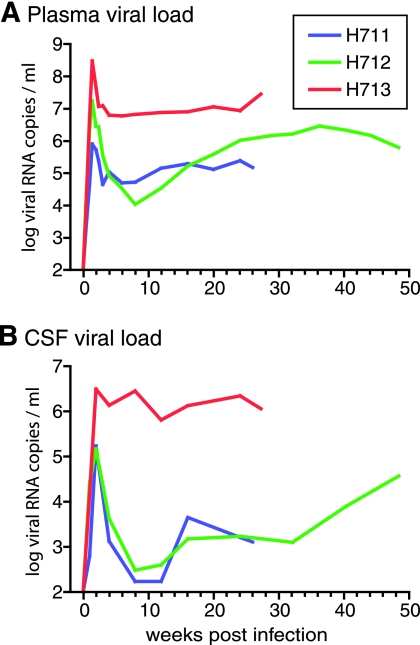
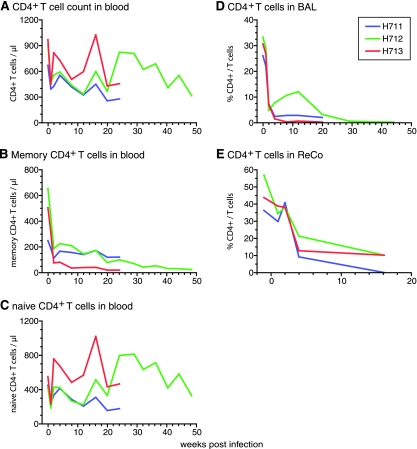
To study the in vivo fitness of SIVsmH635FC relative to that of SIVsmE543-3, three additional rhesus macaques (H711, H712, and H713) were coinoculated with equivalent amounts of SIVsmH635FC and SIVsmE543-3. As shown by the robust viremia noted in Fig. 7, each of the animals became infected. The peak plasma viremia varied from 8 × 105 to 3 × 108 copies/ml (Fig. 7A), and all animals had viral RNA in their CSF (1.5 × 105 to 3 × 106 copies/ml) (Fig. 7B). The level of peak viremia was not significantly different from that of SIVsmH635FC-infected macaques (P was 0.297 for plasma and P was 0.340 for CSF). However, one coinfected macaque, H713, exhibited higher peak plasma and CSF viral loads, with a much higher set point viremia in its plasma (107 copies/ml) and CSF (106 copies/ml), than the other coinfected macaques. The SIV-specific antibody response in H713 was extremely weak and was directed primarily towards Env gp120 and gp41, whereas the other two macaques showed robust antibody responses (data not shown). A transient decrease in blood CD4+ T cells was observed at 1 week in all three macaques (Fig. 8A), with significant acute memory CD4+ T-cell depletion. Memory CD4+ T-cell depletion was most pronounced in the animal with the highest viremia, H713 (Fig. 8B). Acute depletion of CD4+ T cells in mucosal tissues, such as BAL and ReCo tissues, was also observed (Fig. 8D and E). CD4+ T-cell depletion was generally severe in BAL fluid but was most profound in H713 (Fig. 8D). Less-consistent CD4+ T-cell depletion was observed in ReCo biopsy samples, with the most severe decline observed in H711 (Fig. 8E). Mucosal CD4+ T-cell depletion was more severe in coinoculated macaques than in SIVsmH635FC-infected macaques (Fig. 2D and E). These kinetics of CD4+ T-cell depletion, the acute depletion of the memory subset, and the preservation of the naïve subset are similar to what occurs in RP macaques (31, 44) but are different from what occurs in SIVsmH635FC-infected macaques (Fig. 2B and C).
FIG. 7.
Viral RNA loads in the plasma (A) and CSF (B) of three rhesus macaques (H711, H712, and H713) that were coinoculated with SIVsmE543-3 and SIVsmH635FC.
FIG. 8.
Kinetics of CD4+ T cells in three rhesus macaques (H711, H712, and H713) that were coinoculated with SIVsmE543-3 and SIVsmH635FC. Decreases in numbers of CD4+ T cells (A), memory (CD95highCD28high or CD95highCD28low) CD4+ T cells (B), and naive (CD95lowCD28high) CD4+ T cells (C) in blood are shown with absolute numbers. The depletion of CD4+ T cells in mucosal tissues is shown by percentages of CD4+ cells in total T cells from BAL (D) and ReCo (E) samples.
Disease progression was more rapid in the coinoculated macaques, with survival times of 26, 27, and 60 weeks. The macaque with the highest viremia, H713, was euthanized at 27 wpi due to chronic diarrhea and weight loss; pathology revealed moderate SIV encephalitis, SIV pneumonia, and protozoal enterocolitis. The continuous high plasma and CSF viral loads, weak antibody responses, and pathological findings for this animal were characteristic of RP macaques. Consistent with a previous study (52), proliferation of memory CD4+ T cells, as assessed by Ki-67 expression, was significantly lower in an RP macaque, H713, at 8 wpi (22.8%) than in other macaques (40.1 to 63.9%). Macaque H711 died at 26 wpi as a result of a mesenteric root torsion resulting in intestinal necrosis and peritonitis, most likely unrelated to SIV infection. Other pathological findings included severe lymphoid depletion and a mild SIV meningoencephalitis. H712 was euthanized at 60 wpi due to cytomegalovirus pneumonia; this animal also had SIV encephalitis with a granuloma formation in the choroid plexus. Although the rate of disease progression was faster in the coinfected macaques than in macaques inoculated only with SIVsmH635FC, survival times were not significantly different from those of macaques inoculated with SIVsmE543-3 (15, 18, 21, 44, 45). Rapid disease progression was observed in only one of the three coinfected macaques, suggesting that coinfection with SIVsmH635FC and SIVsmE543-3 was not sufficient to induce rapid progression consistently.
SIVsmH635FC efficiently replicated in coinfected macaques during acute infection.
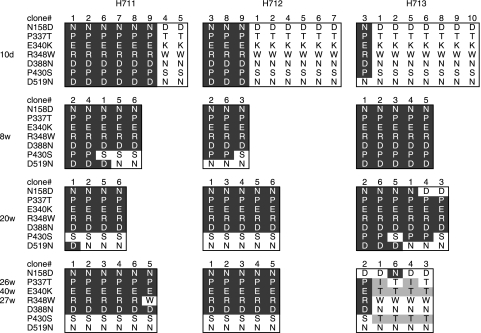
In order to examine the relative proportions of each of the viruses in the coinfected macaques, sequence analysis of the V1-V5 region of Env was performed using viral RNA isolated from plasma samples. SIVsmE543-3 and SIVsmH635FC were differentiated from one another by seven amino acids substitutions in the Env V1-V5 region. Both viruses were detected in the plasma of all three coinfected macaques during primary infection (10 days) (Fig. 9), although their relative proportions differed between the three animals. SIVsmH635FC predominated in H712 and H713, whereas SIVsmE543-3 predominated in H711 (six of eight clones). Direct sequencing of PCR products revealed that SIVsmH635FC was dominant in the CSF of all the macaques at 2 wpi (data not shown). These data suggested that SIVsmH635FC replicated efficiently during acute infection. Following primary infection, the SIVsmH635FC genotype was not observed at 8 wpi and 20 wpi in any of the macaques, except with two SIVsmH635-specific mutations, P430S and D519N. SIVsmE543-3 remained the dominant genotype in H711 and H712. In contrast, SIVsmH635FC became dominant again in RP macaque H713 at necropsy and persisted in its CSF throughout infection (data not shown). Viruses in H713 at the end stage included new variants with additional mutations in three sites, P337I/T, E340T, and P430T, which have been observed previously in other RP macaques (8). Although it is unclear why these new variants emerged, the genotype of these variants is apparently specific to RP macaques. The reemergence of RP-specific mutations at the end stage in H713 is consistent with the association between these genotypes and rapid disease (8, 31).
FIG. 9.
Differentiation of viruses in three macaques coinfected with SIVsmE543-3 and SIVsmH635FC. The env V1-V5 region was amplified by RT-PCR from viral RNA isolated from plasma samples of these macaques at 10 days (10d), 8 weeks (8w), 20 weeks, and 26 weeks (H711), 40 weeks (H712), or 27 weeks (H713) postinoculation. PCR products were cloned into plasmids, and the sequences were analyzed. Amino acids at the seven locations which differ between SIVsmE543-3 and SIVsmH635FC in the Env V1-V5 region are shown as described for Fig. 3A. Amino acids that are identical to those of SIVsmE543-3 are shown by white letters in a black background. Amino acids that are identical to those of SIVsmH635FC are shown by black letters in a white background. Amino acid changes other than changes from SIVsmE543-3 and SIVsmH635FC are shaded.
The coinfection studies demonstrated that SIVsmH635FC replicated as efficiently as SIVsmE543-3 in macaques during acute infection. However, following primary infection, SIVsmH635FC was observed only in an RP macaque. In other words, SIVsmH635FC replicated efficiently prior to the development of SIV-specific immune responses and in an RP macaque without active immune responses.
Neutralizing antibody responses occur in concert with reversion.
Since sequence analysis suggested that SIVsmH635FC replicated efficiently only in the absence of immune responses, we examined the ability of sequential plasma samples from SIVsmH635FC-infected macaques to neutralize SIVsmH635FC. As shown in Table 1, neutralizing antibodies against SIVsmH635FC were detected by 4 wpi and increased rapidly to high titers. The appearance of reversion mutations (Fig. 3A) overlapped the timing of neutralizing antibody responses, consistent with the hypothesis that the antibody response is a potent selection pressure for reversion mutations. In spite of vigorous neutralization of SIVsmH635FC, these plasma samples did not neutralize the closely related virus SIVsmE543-3 until 40 wpi.
TABLE 1.
Titers of antibodiesa in sequential plasma samples from SIVsmH635-infected macaques that neutralized SIVsmE543-3 and SIVsmH635FC
| Virus | Animal | Neutralization titer
|
|||||
|---|---|---|---|---|---|---|---|
| Preinoculation | 1.5 wpi | 4 wpi | 8 wpi | 16 wpi | 40 wpi | ||
| SIVsmE543-3 | H704 | 1:<20 | 1:<20 | 1:<20 | 1:<20 | 1:<20 | 1:20 |
| H709 | 1:<20 | 1:<20 | 1:<20 | 1:<20 | 1:<20 | 1:20 | |
| H714 | 1:<20 | 1:<20 | 1:<20 | 1:<20 | 1:<20 | 1:80 | |
| H723 | 1:<20 | 1:<20 | 1:<20 | 1:<20 | 1:<20 | 1:<20 | |
| SIVsmH635FC | H704 | 1:<20 | 1:<20 | 1:320 | 1:1,280 | 1:5,120 | 1:20,480 |
| H709 | 1:<20 | 1:<20 | 1:320 | 1:1,280 | 1:5,120 | 1:20,480 | |
| H714 | 1:<20 | 1:<20 | 1:20 | 1:1,280 | 1:5,120 | 1:20,480 | |
| H723 | 1:<20 | 1:<20 | 1:320 | 1:1,280 | 1:5,120 | 1:20,480 | |
GHOST (3) Hi-5 cells were infected with a mixture of virus and diluted plasma, and the highest dilutions of plasma which resulted in a >90% reduction in the number of infected cells were determined.
Neutralizations of homologous and heterologous antibodies were evaluated using plasma samples from the macaques infected in the present study or from SIVsmE543-3- or SIVsmE660-infected macaques to examine whether the RP mutations conferred a greater sensitivity to antibody neutralization. As shown in Table 2, SIVsmE543-3, which previous studies have shown to be resistant to neutralization (18, 21), was not neutralized by any of the plasma samples, including those from macaques infected with autologous virus. In contrast, SIVsmH635FC was neutralized by all the plasma samples. The high neutralization titers of SIVsmH635FC by plasma samples from SIVsm635FC-infected or -coinfected macaques (1:5,120) compared to those from SIVsmE543-3-infected macaques (1:320 or 1:1,280) suggested that antibodies were efficiently induced against the autologous strain in a type-specific manner. However, the neutralization of SIVsmH635FC by plasma samples from macaques infected with SIVsmE660, which is genetically divergent from SIVsmH635FC, demonstrated the high sensitivity of SIVsmH635FC to heterologous antibodies. These data clearly demonstrated that SIVsmH635FC was much more sensitive to antibody neutralization than its parent, SIVsmE543-3.
TABLE 2.
Titers of plasma that neutralized homologous and heterologous SIVsmE543-3 and SIVsmH635FCa
| Virus | Neutralization titer of plasma from indicated macaque infected with:
|
||||||
|---|---|---|---|---|---|---|---|
| SIVsmE543-3 and SIVsmH635FC
|
SIVsmE543-3
|
SIVsmE660
|
|||||
| H711 | H712 | H713 | H461 | H596 | D4 | D5 | |
| SIVsmE543-3 | 1:<20 | 1:<20 | 1:<20 | 1:<20 | 1:<20 | 1:<20 | 1:<20 |
| SIVsmH635FC | 1:5,120 | 1:5,120 | 1:20 | 1:320 | 1:1,280 | 1:1,280 | 1:80 |
GHOST (3) Hi-5 cells were infected with a mixture of virus and diluted plasma, and the highest dilutions of plasma which resulted in a >90% reduction in the number of infected cells were determined. Plasma samples from macaques coinfected with SIVsmE543-3 and SIVsmH635FC and macaques infected with SIVsmE543-3 were obtained at 16 wpi. Plasma samples from the SIVsmE660-infected macaques were obtained at 20 wpi.
DISCUSSION
In the present study, we evaluated the in vivo replication and evolution of an RP variant clone in naïve rhesus macaques either alone or when coinoculated with SIVsmE543-3. Our prior studies of virus evolution in RP macaques (8, 31) had suggested either of two scenarios with respect to the significance of RP-specific variants in disease pathogenesis. These variants were either the direct cause of rapid disease progression or the result of virus replicating after the immune failure. In the present study, the RP variant clone SIVsmH635FC was capable of initiating infection in an SIV-naïve host but did not induce rapid disease. SIVsmH635FC replicated as efficiently as its closely related parent virus, SIVsmE543-3, in vivo and induced a progressive loss of CD4+ T cells, consistent with pathogenicity. However, the RP-specific substitutions rapidly reverted to wild-type sequences after the establishment of infection. Similarly, SIVsmH635FC was dominant only during acute infection in the coinfection with SIVsmE543-3. These studies suggest that the SIV variants commonly selected in RP macaques are not the direct cause of rapid disease de novo in naïve macaques. The evolution of RP-specific variants appears to be the result of replication in a severely immunocompromised host.
The cause of rapid progression is therefore more complex than the phenotype of the virus inoculum. The early predictors of rapid progression in SIV-infected macaques are high primary viremia, severe early generalized loss of memory CD4+ T cells, and transient immune responses against SIV (15, 18, 21, 22, 24, 31, 44, 45, 47, 52, 64). We speculate that there is a threshold in terms of the amount of viremia and the early loss of memory CD4+ T cells that is essential for precipitating the failure to maintain immune responses. Therefore, this phenomenon is likely a complex early interplay between the virus and the host. Clearly, the virulence of the virus inoculum is important; attenuated or minimally pathogenic SIVs are never associated with this phenomenon. However, the host response is also equally important. For example, depletion of CD8+ T cells during primary SIVmac infection results in more-rapid disease progression (35, 59). The length of CD8+ T-cell depletion is also critical, since disease is most rapid in animals with long-term depletion (59). The rate of disease progression in HIV/SIV infection is also influenced by major histocompatibility complex alleles (4, 12, 27, 58). Additionally, the intrinsic susceptibility of CD4+ target cells appears to influence early viremia in macaques (15). Peripheral blood mononuclear cells from the macaques used in this study were highly susceptible to SIVsmE543-3 infection (104 TCID50/ml or more as determined by in vitro susceptiblity assay). However, the in vitro susceptibility to SIVsmE543-3 infection was not correlated with the level of viremia after infection with SIVsmH635FC. Other host factors, such as innate and humoral immune responses, are also likely to play a critical role.
Although SIVsmH635FC did not induce rapid disease progression, it was not attenuated, as is evident by high primary and persistent viremia. It retained much of the pathogenicity of its parent but had several features that may distinguish it from SIVsmE543-3. First, virus load was more reproducible between macaques infected with SIVsmH635FC than those infected with SIVsmE543-3 (15, 21, 44). All SIVsmH635FC-infected macaques showed set point viremia of around 105 copies/ml, whereas the set point viremia in SIVsmE543-3-infected macaques can range over 4 logs (15, 44). Second, previous studies have shown that memory CD4+ T cells in the blood are acutely depleted in most SIVsmE543-3-infected macaques (44). In contrast, CD4+ memory T cells were relatively preserved during primary SIVsmH635FC infection, with loss restricted mainly to mucosal compartments. Finally, SIVsmH635FC-infected macaques all showed a progressive decline of both memory and naive CD4+ T cells during chronic infection. Although the numbers of animals studied were not sufficient to make definitive conclusions, this pattern of CD4+ T-cell decline suggests that SIVsmH635FC has minimal cytopathic effects during acute infection but retains the ability to cause subsequent CD4+ T-cell loss and disease.
In SIVsmH635FC-infected macaques, vigorous viral mutations, including reversion to SIVsmE543-3 residues and extensive variation in the V1-V2 and V4 regions, were observed. Since SIVsmH635FC replicates well during primary infection, the reversion cannot be explained by reduced overall replication fitness relative to its parent, SIVsmE543-3. We speculated that immune selection would exert the most potent pressure on this antibody-sensitive virus. The reversion may be the readaptation to replicate in the presence of immune pressure. Indeed, one of the RP mutations (N158D) that was significantly correlated with neutralizing sensitivity appeared to revert rapidly in the infected macaques. The N158D mutation, which causes a loss of the PNG site in the V1-V2 region, is associated with the exposure of the CCR5 binding domain, which is known to be a target for neutralizing antibodies (53, 54). The carbohydrate at this site is suggested to be indispensable for efficient replication under immune pressure. Compared with this rapid reversion, changes in the V1-V2 and V4 regions of Env that are commonly observed in a conventional escape from neutralizing antibodies (3, 6, 7, 26, 28, 36, 51, 55, 56) occurred by later time points. The observation of positive selection of amino acid changes, insertion/deletion polymorphisms, and changes of PNG sites in the V1-V2 and V4 regions are characteristic of escape from neutralizing antibodies (3, 6, 7, 26, 28, 36, 51, 55, 56). The early and strong neutralizing antibody response in SIVsmH635FC-infected macaques and the high sensitivity of SIVsmH635FC against antibody neutralization are consistent with intense immune pressure. Although cytotoxic T lymphocytes were not examined in this study, cellular immune responses (1, 16) may also have caused escape mutations in other regions of the viral genome. The dominance of the highly neutralization-resistant SIVsmE543-3 clone in coinoculated macaques after the development of immune responses is also entirely consistent with potent selection by immune pressure, which is effective against neutralization-sensitive SIVsmH635FC.
In summary, although SIVsmH635FC virus retained much of the pathogenicity of its parent strain, it did not reproduce rapid disease in naïve macaques. The substitutions that make it unique were observed only during primary viremia and late in the disease course in the single RP macaque in the present study. The rapid reversion of RP variants in immunocompetent macaques, as well as the high sensitivity of SIVsmH635FC to neutralization, suggests that RP variants have evolved specifically to occupy a unique niche in severely immunosuppressed macaques. The necessity to seek alternative cell targets for infection is presumably the major driving force behind the evolution in RP macaques. However, since this evolution occurs in the absence of immune selection, these viruses also accumulate substitutions that increase their sensitivity to neutralization. Clearly, the end-stage variants are not responsible for inducing acute immune failure, the first step in rapid disease progression. Rather, we speculate that a unique interplay of the virus and host allows robust virus replication, resulting in the early destruction of memory CD4+ T cells and immune failure. The subsequent disease is accompanied by an expansion of RP variants that may play a role in the development of encephalitis, gastrointestinal disorders, and pneumonia through their effects on tissue macrophages.
Acknowledgments
We thank Yoshiaki Nishimura and Tatsuhiko Igarashi for helpful discussions and Que Dang for critical reading of the manuscript.
This work was supported by the Intramural Program of the NIAID, NIH.
Footnotes
Published ahead of print on 27 June 2007.
REFERENCES
- 1.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 2.Brown, C. R., M. Czapiga, J. Kabat, Q. Dang, I. Ourmanov, Y. Nishimura, M. A. Martin, and V. M. Hirsch. 2007. Unique pathology in simian immunodeficiency virus-infected rapid-progressor macaques is consistent with a pathogenesis distinct from that of classical AIDS. J. Virol. 81:5594-5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns, D. P., C. Collignon, and R. C. Desrosiers. 1993. Simian immunodeficiency virus mutants resistant to serum neutralization arise during persistent infection of rhesus monkeys. J. Virol. 67:4104-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrington, M., G. W. Nelson, M. P. Martin, T. Kissner, D. Vlahov, J. J. Goedert, R. Kaslow, S. Buchbinder, K. Hoots, and S. J. O'Brien. 1999. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science 283:1748-1752. [DOI] [PubMed] [Google Scholar]
- 5.Cecilia, D., V. N. KewalRamani, J. O'Leary, B. Volsky, P. Nyambi, S. Burda, S. Xu, D. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 72:6988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chackerian, B., L. M. Rudensey, and J. Overbaugh. 1997. Specific N-linked and O-linked glycosylation modifications in the envelope V1 domain of simian immunodeficiency virus variants that evolve in the host alter recognition by neutralizing antibodies. J. Virol. 71:7719-7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole, K. S., M. Alvarez, D. H. Elliott, H. Lam, E. Martin, T. Chau, K. Micken, J. L. Rowles, J. E. Clements, M. Murphey-Corb, R. C. Montelaro, and J. E. Robinson. 2001. Characterization of neutralization epitopes of simian immunodeficiency virus (SIV) recognized by rhesus monoclonal antibodies derived from monkeys infected with an attenuated SIV strain. Virology 290:59-73. [DOI] [PubMed] [Google Scholar]
- 8.Dehghani, H., B. A. Puffer, R. W. Doms, and V. M. Hirsch. 2003. Unique pattern of convergent envelope evolution in simian immunodeficiency virus-infected rapid progressor macaques: association with CD4-independent usage of CCR5. J. Virol. 77:6405-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demarest, J. F., N. Jack, F. R. Cleghorn, M. L. Greenberg, T. L. Hoffman, J. S. Ottinger, L. Fantry, J. Edwards, T. R. O'Brien, K. Cao, B. Mahabir, W. A. Blattner, C. Bartholomew, and K. J. Weinhold. 2001. Immunologic and virologic analyses of an acutely HIV type 1-infected patient with extremely rapid disease progression. AIDS Res. Hum. Retrovir. 17:1333-1344. [DOI] [PubMed] [Google Scholar]
- 10.Dreyer, K., E. G. Kallas, V. Planelles, D. Montefiori, M. P. McDermott, M. S. Hasan, and T. G. Evans. 1999. Primary isolate neutralization by HIV type 1-infected patient sera in the era of highly active antiretroviral therapy. AIDS Res. Hum. Retrovir. 15:1563-1571. [DOI] [PubMed] [Google Scholar]
- 11.Edinger, A. L., J. L. Mankowski, B. J. Doranz, B. J. Margulies, B. Lee, J. Rucker, M. Sharron, T. L. Hoffman, J. F. Berson, M. C. Zink, V. M. Hirsch, J. E. Clements, and R. W. Doms. 1997. CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc. Natl. Acad. Sci. USA 94:14742-14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans, D. T., L. A. Knapp, P. Jing, J. L. Mitchen, M. Dykhuizen, D. C. Montefiori, C. D. Pauza, and D. I. Watkins. 1999. Rapid and slow progressors differ by a single MHC class I haplotype in a family of MHC-defined rhesus macaques infected with SIV. Immunol. Lett. 66:53-59. [DOI] [PubMed] [Google Scholar]
- 13.Garland, F. C., C. F. Garland, E. D. Gorham, S. K. Brodine, et al. 1996. Western blot banding patterns of HIV rapid progressors in the U.S. Navy Seropositive Cohort: implications for vaccine development. Ann. Epidemiol. 6:341-347. [DOI] [PubMed] [Google Scholar]
- 14.Glamann, J., and V. M. Hirsch. 2000. Characterization of a macaque recombinant monoclonal antibody that binds to a CD4-induced epitope and neutralizes simian immunodeficiency virus. J. Virol. 74:7158-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein, S., C. R. Brown, H. Dehghani, J. D. Lifson, and V. M. Hirsch. 2000. Intrinsic susceptibility of rhesus macaque peripheral CD4+ T cells to simian immunodeficiency virus in vitro is predictive of in vivo viral replication. J. Virol. 74:9388-9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goulder, P. J., C. Brander, Y. Tang, C. Tremblay, R. A. Colbert, M. M. Addo, E. S. Rosenberg, T. Nguyen, R. Allen, A. Trocha, M. Altfeld, S. He, M. Bunce, R. Funkhouser, S. I. Pelton, S. K. Burchett, K. McIntosh, B. T. Korber, and B. D. Walker. 2001. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature 412:334-338. [DOI] [PubMed] [Google Scholar]
- 17.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch, V., D. Adger-Johnson, B. Campbell, S. Goldstein, C. Brown, W. R. Elkins, and D. C. Montefiori. 1997. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J. Virol. 71:1608-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch, V. M., T. R. Fuerst, G. Sutter, M. W. Carroll, L. C. Yang, S. Goldstein, M. Piatak, Jr., W. R. Elkins, W. G. Alvord, D. C. Montefiori, B. Moss, and J. D. Lifson. 1996. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 70:3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirsch, V. M., J. E. Martin, G. Dapolito, W. R. Elkins, W. T. London, S. Goldstein, and P. R. Johnson. 1994. Spontaneous substitutions in the vicinity of the V3 analog affect cell tropism and pathogenicity of simian immunodeficiency virus. J. Virol. 68:2649-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirsch, V. M., S. Santra, S. Goldstein, R. Plishka, A. Buckler-White, A. Seth, I. Ourmanov, C. R. Brown, R. Engle, D. Montefiori, J. Glowczwskie, K. Kunstman, S. Wolinsky, and N. L. Letvin. 2004. Immune failure in the absence of profound CD4+ T-lymphocyte depletion in simian immunodeficiency virus-infected rapid progressor macaques. J. Virol. 78:275-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirsch, V. M., P. M. Zack, A. P. Vogel, and P. R. Johnson. 1991. Simian immunodeficiency virus infection of macaques: end-stage disease is characterized by widespread distribution of proviral DNA in tissues. J. Infect. Dis. 163:976-988. [DOI] [PubMed] [Google Scholar]
- 23.Reference deleted.
- 24.Holterman, L., H. Niphuis, P. J. ten Haaft, J. Goudsmit, G. Baskin, and J. L. Heeney. 1999. Specific passage of simian immunodeficiency virus from end-stage disease results in accelerated progression to AIDS in rhesus macaques. J. Gen. Virol. 80:3089-3097. [DOI] [PubMed] [Google Scholar]
- 25.Igarashi, T., O. K. Donau, H. Imamichi, M. J. Dumaurier, R. Sadjadpour, R. J. Plishka, A. Buckler-White, C. Buckler, A. F. Suffredini, H. C. Lane, J. P. Moore, and M. A. Martin. 2003. Macrophage-tropic simian/human immunodeficiency virus chimeras use CXCR4, not CCR5, for infections of rhesus macaque peripheral blood mononuclear cells and alveolar macrophages. J. Virol. 77:13042-13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jurkiewicz, E., G. Hunsmann, J. Schaffner, T. Nisslein, W. Luke, and H. Petry. 1997. Identification of the V1 region as a linear neutralizing epitope of the simian immunodeficiency virus SIVmac envelope glycoprotein. J. Virol. 71:9475-9481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaslow, R. A., M. Carrington, R. Apple, L. Park, A. Munoz, A. J. Saah, J. J. Goedert, C. Winkler, S. J. O'Brien, C. Rinaldo, R. Detels, W. Blattner, J. Phair, H. Erlich, and D. L. Mann. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2:405-411. [DOI] [PubMed] [Google Scholar]
- 28.Kinsey, N. E., M. G. Anderson, T. J. Unangst, S. V. Joag, O. Narayan, M. C. Zink, and J. E. Clements. 1996. Antigenic variation of SIV: mutations in V4 alter the neutralization profile. Virology 221:14-21. [DOI] [PubMed] [Google Scholar]
- 29.Korber, B. 2000. HIV signature and sequence variation analysis, p. 55-72. In A. G. Rodrigo and G. H. Learn, (ed.), Computational analysis of HIV molecular sequences. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 30.Kourtis, A. P., C. Ibegbu, A. J. Nahmias, F. K. Lee, W. S. Clark, M. K. Sawyer, and S. Nesheim. 1996. Early progression of disease in HIV-infected infants with thymus dysfunction. N. Engl. J. Med. 335:1431-1436. [DOI] [PubMed] [Google Scholar]
- 31.Kuwata, T., H. Dehghani, C. R. Brown, R. Plishka, A. Buckler-White, T. Igarashi, J. Mattapallil, M. Roederer, and V. M. Hirsch. 2006. Infectious molecular clones from a simian immunodeficiency virus-infected rapid-progressor (RP) macaque: evidence of differential selection of RP-specific envelope mutations in vitro and in vivo. J. Virol. 80:1463-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuwata, T., T. Shioda, T. Igarashi, E. Ido, K. Ibuki, Y. Enose, C. Stahl-Hennig, G. Hunsmann, T. Miura, and M. Hayami. 1996. Chimeric viruses between SIVmac and various HIV-1 isolates have biological properties that are similar to those of the parental HIV-1. AIDS 10:1331-1337. [DOI] [PubMed] [Google Scholar]
- 33.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 35.Madden, L. J., M. A. Zandonatti, C. T. Flynn, M. A. Taffe, M. C. Marcondes, J. E. Schmitz, K. A. Reimann, S. J. Henriksen, and H. S. Fox. 2004. CD8+ cell depletion amplifies the acute retroviral syndrome. J. Neurovirol. 10(Suppl. 1):58-66. [DOI] [PubMed] [Google Scholar]
- 36.Mahalanabis, M., V. M. Hirsch, and N. L. Haigwood. 2005. Infection with a molecularly cloned SIVsm virus elicits high titer homologous neutralizing antibodies with heterologous neutralizing activity. J. Med. Primatol. 34:253-261. [DOI] [PubMed] [Google Scholar]
- 37.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 38.Michael, N. L., A. E. Brown, R. F. Voigt, S. S. Frankel, J. R. Mascola, K. S. Brothers, M. Louder, D. L. Birx, and S. A. Cassol. 1997. Rapid disease progression without seroconversion following primary human immunodeficiency virus type 1 infection—evidence for highly susceptible human hosts. J. Infect. Dis. 175:1352-1359. [DOI] [PubMed] [Google Scholar]
- 39.Miyake, A., K. Ibuki, Y. Enose, H. Suzuki, R. Horiuchi, M. Motohara, N. Saito, T. Nakasone, M. Honda, T. Watanabe, T. Miura, and M. Hayami. 2006. Rapid dissemination of a pathogenic simian/human immunodeficiency virus to systemic organs and active replication in lymphoid tissues following intrarectal infection. J. Gen. Virol. 87:1311-1320. [DOI] [PubMed] [Google Scholar]
- 40.Montagnier, L., C. Brenner, S. Chamaret, D. Guetard, A. Blanchard, J. de Saint Martin, J. D. Poveda, G. Pialoux, and M. L. Gougeon. 1997. Human immunodeficiency virus infection and AIDS in a person with negative serology. J. Infect. Dis. 175:955-959. [DOI] [PubMed] [Google Scholar]
- 41.Morner, A., A. Bjorndal, J. Albert, V. N. KewalRamani, D. R. Littman, R. Inoue, R. Thorstensson, E. M. Fenyo, and E. Bjorling. 1999. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J. Virol. 73:2343-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrison, H. G., F. Kirchhoff, and R. C. Desrosiers. 1995. Effects of mutations in constant regions 3 and 4 of envelope of simian immunodeficiency virus. Virology 210:448-455. [DOI] [PubMed] [Google Scholar]
- 43.Nei, M., and T. Gojobori. 1986. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 3:418-426. [DOI] [PubMed] [Google Scholar]
- 44.Nishimura, Y., T. Igarashi, A. Buckler-White, C. Buckler, H. Imamichi, R. M. Goeken, W. R. Lee, B. A. Lafont, R. Byrum, H. C. Lane, V. M. Hirsch, and M. A. Martin. 2007. Loss of naive cells accompanies memory CD4+ T-cell depletion during long-term progression to AIDS in simian immunodeficiency virus-infected macaques. J. Virol. 81:893-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishimura, Y., T. Igarashi, O. K. Donau, A. Buckler-White, C. Buckler, B. A. Lafont, R. M. Goeken, S. Goldstein, V. M. Hirsch, and M. A. Martin. 2004. Highly pathogenic SHIVs and SIVs target different CD4+ T cell subsets in rhesus monkeys, explaining their divergent clinical courses. Proc. Natl. Acad. Sci. USA 101:12324-12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olshevsky, U., E. Helseth, C. Furman, J. Li, W. Haseltine, and J. Sodroski. 1990. Identification of individual human immunodeficiency virus type 1 gp120 amino acids important for CD4 receptor binding. J. Virol. 64:5701-5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Orandle, M. S., K. C. Williams, A. G. MacLean, S. V. Westmoreland, and A. A. Lackner. 2001. Macaques with rapid disease progression and simian immunodeficiency virus encephalitis have a unique cytokine profile in peripheral lymphoid tissues. J. Virol. 75:4448-4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ota, T., and M. Nei. 1994. Variance and covariances of the numbers of synonymous and nonsynonymous substitutions per site. Mol. Biol. Evol. 11:613-619. [DOI] [PubMed] [Google Scholar]
- 49.Otto, C., B. A. Puffer, S. Pohlmann, R. W. Doms, and F. Kirchhoff. 2003. Mutations in the C3 region of human and simian immunodeficiency virus envelope have differential effects on viral infectivity, replication, and CD4-dependency. Virology 315:292-302. [DOI] [PubMed] [Google Scholar]
- 50.Ourmanov, I., M. Bilska, V. M. Hirsch, and D. C. Montefiori. 2000. Recombinant modified vaccinia virus Ankara expressing the surface gp120 of simian immunodeficiency virus (SIV) primes for a rapid neutralizing antibody response to SIV infection in macaques. J. Virol. 74:2960-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petry, H., K. Pekrun, G. Hunsmann, E. Jurkiewicz, and W. Luke. 2000. Naturally occurring V1-env region variants mediate simian immunodeficiency virus SIVmac escape from high-titer neutralizing antibodies induced by a protective subunit vaccine. J. Virol. 74:11145-11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Picker, L. J., S. I. Hagen, R. Lum, E. F. Reed-Inderbitzin, L. M. Daly, A. W. Sylwester, J. M. Walker, D. C. Siess, M. Piatak, Jr., C. Wang, D. B. Allison, V. C. Maino, J. D. Lifson, T. Kodama, and M. K. Axthelm. 2004. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 200:1299-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puffer, B. A., S. Pohlmann, A. L. Edinger, D. Carlin, M. D. Sanchez, J. Reitter, D. D. Watry, H. S. Fox, R. C. Desrosiers, and R. W. Doms. 2002. CD4 independence of simian immunodeficiency virus Envs is associated with macrophage tropism, neutralization sensitivity, and attenuated pathogenicity. J. Virol. 76:2595-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reitter, J. N., R. E. Means, and R. C. Desrosiers. 1998. A role for carbohydrates in immune evasion in AIDS. Nat. Med. 4:679-684. [DOI] [PubMed] [Google Scholar]
- 55.Rudensey, L. M., J. T. Kimata, E. M. Long, B. Chackerian, and J. Overbaugh. 1998. Changes in the extracellular envelope glycoprotein of variants that evolve during the course of simian immunodeficiency virus SIVMne infection affect neutralizing antibody recognition, syncytium formation, and macrophage tropism but not replication, cytopathicity, or CCR-5 coreceptor recognition. J. Virol. 72:209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rybarczyk, B. J., D. Montefiori, P. R. Johnson, A. West, R. E. Johnston, and R. Swanstrom. 2004. Correlation between env V1/V2 region diversification and neutralizing antibodies during primary infection by simian immunodeficiency virus sm in rhesus macaques. J. Virol. 78:3561-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryzhova, E., J. C. Whitbeck, G. Canziani, S. V. Westmoreland, G. H. Cohen, R. J. Eisenberg, A. Lackner, and F. Gonzalez-Scarano. 2002. Rapid progression to simian AIDS can be accompanied by selection of CD4-independent gp120 variants with impaired ability to bind CD4. J. Virol. 76:7903-7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sauermann, U., A. Arents, and G. Hunsmann. 1996. PCR-RFLP-based Mamu-DQB1 typing of rhesus monkeys: characterization of two novel alleles. Tissue Antigens 47:319-328. [DOI] [PubMed] [Google Scholar]
- 59.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 60.Shearer, W. T., T. C. Quinn, P. LaRussa, J. F. Lew, L. Mofenson, S. Almy, K. Rich, E. Handelsman, C. Diaz, M. Pagano, V. Smeriglio, L. A. Kalish, et al. 1997. Viral load and disease progression in infants infected with human immunodeficiency virus type 1. N. Engl. J. Med. 336:1337-1342. [DOI] [PubMed] [Google Scholar]
- 61.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 62.Reference deleted.
- 63.Veazey, R. S., M. Rosenzweig, D. E. Shvetz, D. R. Pauley, M. DeMaria, L. V. Chalifoux, R. P. Johnson, and A. A. Lackner. 1997. Characterization of gut-associated lymphoid tissue (GALT) of normal rhesus macaques. Clin. Immunol. Immunopathol. 82:230-242. [DOI] [PubMed] [Google Scholar]
- 64.Zhang, J. Y., L. N. Martin, E. A. Watson, R. C. Montelaro, M. West, L. Epstein, and M. Murphey-Corb. 1988. Simian immunodeficiency virus/delta-induced immunodeficiency disease in rhesus monkeys: relation of antibody response and antigenemia. J. Infect. Dis. 158:1277-1286. [DOI] [PubMed] [Google Scholar]