Abstract
Heterosubtypic immunity (HSI) is defined as cross-protection to infection with an influenza A virus serotype other than the one used for primary infection. Although HSI has been thought to be mediated by serotype cross-reactive cytotoxic T lymphocytes (CTL) that recognize conserved epitopes of structural proteins, recent studies suggest that antibodies (Abs) may make a significant contribution. In this study, we provide further evidence for the role of Abs in HSI using transgenic mice lacking terminal deoxyribonucleotidyltransferase (TdT), which adds N nucleotides to V-D and D-J junctions of the complementary determining region 3 (CDR3) (TdT−/−) and mice with altered Ab repertoires due to replacement of the complete locus of heavy chain diversity segments (DH) with an altered DH segment (namely, ΔD-iD). Both types of mice failed to generate complete HSI, although they were able to mount protective immunity to a homologous challenge. Lower levels of virus-specific antibodies along with more severely impaired HSI were observed in TdT−/− mice compared to those in ΔD-iD mice, while CTL activity remained unchanged in both types of mice. These findings indicate that a properly diversified antibody repertoire is required for HSI and that N addition by TdT is a more effective mechanism in the induction of a properly diversified antibody repertoire and, therefore, complete HSI. The results suggest that the diversity of the antibody repertoire as determined by the composition of the D region of HCDR3 and by N addition are among the mechanisms selected for in evolution to create a favorable environment to resolve infections with mutated viruses.
Infection with influenza A virus usually affords complete protection against reinfection with homologous virus. To a lesser, but still significant, extent, infection also provides a level of immunity against virus of a different subtype. The latter, termed heterosubtypic immunity (HSI), occurs in the absence of virus-neutralizing (VN) antibodies that recognize the outer membrane proteins (35). It has been proposed that HSI is mediated, in part, by subtype cross-reactive cytotoxic T lymphocytes (CTL) that recognize conserved epitopes of structural proteins shared by influenza A virus subtypes (5, 18, 28, 33, 34, 38, 40, 43, 44, 46). However, B cells and antibodies (Abs) have been shown to contribute significantly to HSI (29, 41). There are indications that subtype cross-reactive Abs (10, 22, 23, 29, 37, 41) specific for internal viral proteins expressed on the surface of infected cells may reduce the production of progeny virus and inhibit the spread of infection (25). For example, induction of Abs to the conserved transmembrane matrix protein 2 (M2) by immunization is associated with HSI (8, 27, 36). In addition, CD4+ T helper cells and T-cell-dependent virus-specific Ab responses are important for induction of complete HSI (29), since CD4+ T cells provide help for B cells and antigen-specific Ab responses by secretion of cytokines (for review, see reference 7).
The ability to generate diversified lymphocyte antigen receptor repertoires is one of the evolutionally advanced mechanisms used by the adaptive immune system to deal with a wide array of pathogens and toxins (1, 6, 13, 30, 39). The diversity of the Ab and T-cell receptor (TCR) repertoires is created both by the combinatorial rearrangement of germ line-encoded V(D)J gene segments and by junctional somatic diversity created during the process of V(D)J rearrangement. The addition of N nucleotides by deoxyribonucleotidyltransferase (TdT) to V-D and D-J junctions occurs in an untemplated fashion. This diversity is focused in complementary determining region 3 (CDR-3), which lies at the center of the antigen-binding site and often dictates antigen specificity (15, 32, 45). Deletion of TdT activity by targeted gene inactivation leads to an almost total absence of N nucleotides in adult B- and T-cell V-D-J junctions and results in Ab and TCR repertoires of limited, primarily germ line, diversity (12, 17). Although TdT-deficient (TdT−/− or TdT knockout) mice are healthy and respond to most complex antigens (11, 12, 17), the T-cell repertoire in these mice is more promiscuous with regards to peptide recognition (9). In contrast, the B-cell repertoire from these TdT−/− mice is less polyreactive than that of the N region-containing repertoire in wild-type mice (42).
Combinatorial diversification of immunoglobulin (Ig) heavy chain CDR-3 (CDR-H3) is created de novo by the rearrangement and juxtaposition of individual V, D, and J gene segments (1, 6, 39). Mammalian IgH loci typically contain multiple DH gene segments (15, 19, 32, 45) that are highly similar. Recently, we generated mice with a globally altered antibody repertoire due to replacement of the complete locus of 13 DH gene segments with an altered DFL16.1, the most JH-distal DH segment (ΔD-iD mice) (14). The central codons for reading frame 1 (RF1)-encoded tyrosine and glycine in DFL16.1 were replaced by codons for arginine, histidine, and asparagine. Combinatorial diversification of Ig CDR-H3 in these mice is limited to a single DH gene segment encoding positively charged amino acids in Ig CDR-H3. Such ΔD-iD mice have impaired B-cell development and Ab production (14).
In an attempt to better define the role of Abs in HSI, transgenic mice with limited Ab repertoires were used in this study. Unlike the immune responses to primary infection or homotypic immunity against reinfection with the same subtype, HSI to challenge with different subtypes seems to require more complex Ab responses, including properly diversified Abs as well as a TCR repertoire of T helper cells. The results obtained from this study provide further evidence for the role of Ab responses in effector mechanisms of HSI to influenza A viruses. This has implications for the development of universal vaccines against infections with different influenza A virus subtypes.
MATERIALS AND METHODS
Viruses.
Influenza virus strains A/Udorn/307/72 (H3N2) (A/Udorn; a gift from Brian Murphy, National Institutes of Health, Bethesda, MD), A/PR/8/34 (H1N1) (a gift from Thomas M. Moran, Mount Sinai School of Medicine, New York, NY), A/Philippines/2/82/X-79 (H3N2) (A/Philippines; a gift from Ker-Sang Chen, U.S. Food and Drug Administration, Bethesda, MD), and A/Panama/2007/99 (A/Panama; obtained from the Centers for Disease Control and Prevention, Atlanta, GA) were prepared as previously reported (28). Mouse-adapted viruses A/PR/8/34 and A/Philippines prepared as lung homogenates of intranasally infected mice were used for challenge.
Mice.
Wild-type (WT) BALB/cJ (H-2d) mice were purchased at 6 to 8 weeks of age from The Jackson Laboratory (Bar Harbor, ME). TdT−/− mice (12) on a C57BL/6 background (kind gift of Diane Mathis) were backcrossed for 8 to 10 generations onto a BALB/cJ mice background. ΔD-iD mice were generated from a gene-targeted BALB/c embryonic stem cell line as described elsewhere (14). All of these mice were maintained in a specific-pathogen-free barrier facility in microisolator caging (Lab Products, Inc., Maywood, NJ). All experiments and animal procedures conformed to protocols approved by the University of Alabama Institutional Animal Care and Use Committee.
Immunization and challenge of mice.
For immunization via the total respiratory tract (TRT), ketamine-anesthetized mice (1 μg/100 μl/mouse) were inoculated intranasally with a sublethal dose of 2.5 × 105 PFU of A/Udorn (H3N2) virus in 50 μl of phosphate-buffered saline (PBS) per mouse. For heterologous or homologous virus challenge, ketamine-anesthetized mice were infected throughout the TRT with 250 PFU (5× the 50% lethal dose [LD50]) of A/PR/8/34 (H1N1) virus or 1,000 PFU (5× LD50) of A/Philippines (H3N2) resuspended in 50 μl PBS per animal, respectively.
Cell lines.
Murine P815 (H-2d) mastocytoma cells, EL-4 (H-2b) T-cell lymphoma, and Madin-Darby canine kidney cells (ATCC, Manassas, VA) were maintained in standard complete medium (RPMI 1640; Gibco, Grand Island, NY) containing 10% fetal bovine serum (FBS) and antibiotics.
ELISA.
The enzyme-linked immunosorbent assay (ELISA) was performed as previously described (28). Briefly, 96-well MaxiSorp Nunc-Immuno plates (Nalgene Nunc International, Naperville, IL) were coated with whole influenza virus type A/Udorn, purified by sucrose gradient centrifugation, at a concentration of 0.5 μg/ml. Dilutions of serum were incubated overnight on coated and blocked ELISA plates, and the bound immunoglobulins were detected with horseradish peroxidase-labeled F(ab′)2 of goat IgG against mouse Ig (Southern Biotechnology Associates, Birmingham, AL). At the end of the incubation time (3 h at 37°C), the peroxidase substrate 2,2′-azino-bis-(3-ethylbenzthiazoline) sulfonic acid (Sigma, St. Louis, MO) in citrate buffer (pH 4.2) containing 0.0075% H2O2 was added. The color that developed was measured in a Vmax photometer (Molecular Devices, Palo Alto, CA) at 414 nm. The reproducibility of the assay was ascertained by applying on each plate a control hyperimmune mouse serum. The results were expressed as end point titration values.
Generation of antigen-specific CTL effector cells.
Spleens and mediastinal lymph nodes (MLN) were harvested from five mice per group, and pooled single-cell suspensions were used for further analysis. A portion of the spleen cell suspension (stimulator cells) was infected with influenza virus A/Udorn at a multiplicity of infection (MOI) of 2 to 4 or with A/PR/8/34 at an MOI of 4 to 6 in a small volume (0.2 ml) of PBS or RPMI 1640 medium without FBS. After incubation for 30 min at 37°C, 5% CO2, RPMI 1640 complete medium was added, and the cell suspension was incubated for at least 3 h, irradiated (3,000 R), washed, and mixed with the remaining splenocytes (responder cells) at a ratio of 2:1 (3 × 106 to 4 × 106 responder cells/ml) in CTL complete medium containing 10% FBS, 10 mM HEPES, 100 U/ml of penicillin, 100 μg/ml of streptomycin, 0.03% glutamine, and 3 × 10−5 M 2-mercaptoethanol (Sigma, St. Louis, MO). Murine recombinant interleukin-2 (R&D Systems, Inc., Minneapolis, MN) was added to cultures at day 3 (20 U/ml), followed by an additional 3-day incubation at 37°C, 5% CO2, after which CTL effector cells were washed and tested with virus-infected major histocompatibility complex (MHC)-matched target cells in a 51Cr release assay.
Preparation of target cells.
P815 (H-2d) and EL-4 (H-2b) cells (MHC-mismatched control) were infected at an MOI of 5 with A/Udorn or A/PR/8/34 influenza virus in 100 μl of incomplete medium (without serum) for 20 min at 37°C. The cells were washed to remove unbound virus and were cultured for 2 h in 500 μl of complete medium containing 100 μCi of 51Cr per 106 cells. Prior to assessing cytotoxic activity, 51Cr-labeled cells were washed three times. The cells were counted and used for target cells in the cytotoxicity assay, as described below.
Cytotoxicity assay.
The 51Cr-labeled P815 and EL-4 target cells were washed three times and resuspended in complete medium at 105 cells/ml; 100-μl aliquots of the cell suspension were added to 96-well, round-bottom microtiter plates containing triplicate 100-μl samples of serially diluted effector cells. The microtiter plates were centrifuged at 400 × g for 5 min and then incubated for 4 h at 37°C, 5% CO2. The level of released radioactivity in 100 μl of supernatant from each well was measured in a gamma counter (Cobra II Auto-Gamma; Packard Instrument Co., Downers Grove, IL). Specific lysis was calculated from the 51Cr released in counts per minute (cpm) using the following formula: percent specific lysis = (experimental cpm − spontaneous cpm)/(maximal release cpm − spontaneous cpm) × 100. The cpm values for spontaneous and maximal release were determined by incubating target cells with either 100 μl of medium or 100 μl of 5% Triton X-100, respectively. Spontaneous release of 51Cr in the absence of effector cells was usually less than 15% and did not exceed 20%. Standard errors of the means (SEM) were always less than 5% of the mean value and are not included.
Lymphocyte proliferation assay.
Single-cell suspensions from five mice per group were pooled and cultured with viral antigens (whole virus particles) or irradiated virus-infected splenocytes (as described above for stimulator cells used for generation of antigen-specific CTL effector cells) for 3 days at 37°C, 5% CO2. Cell cultures with irradiated uninfected splenocytes, concanavalin A (5 μg/ml), or without antigen were included as controls. About 16 h before cell culture harvest, 0.5 μCi of [3H]thymidine was added to each well. Tritium uptake was determined by using a scintillation counter, and the results are presented as the stimulation index, calculated as follows: (cpm of stimulated culture)/(cpm of nonstimulated culture, in the absence of antigen).
Statistics.
The data are expressed as the mean ± 1 SEM and were compared using a two-tailed Student's t test or an unpaired Mann-Whitney U test. The results were analyzed using the InStat 2.00 statistical program (GraphPad Software, San Diego, CA) for Macintosh computers and were considered to be statistically significant if P values were less than 0.05.
RESULTS
Susceptibility of TdT−/− and ΔD-iD mice to influenza A virus infection.
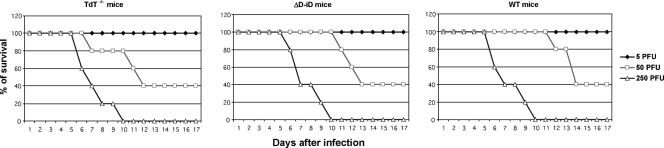
We first determined whether the absence of terminal TdT or replacement of the complete heavy chain diversity segments (DH) with an altered DH-segment (ΔD-iD) affected the susceptibility to infection with mouse-adapted influenza virus. We used the LD50 (50 PFU) of mouse-adapted A/PR/8/34 as determined in our previous studies (28, 29) for infection of TdT−/−, ΔD-iD, and WT mice. As shown in Fig. 1, the LD50s of mouse-adapted A/PR/8/34 were comparable in all examined mice, including WT mice, suggesting that the absence of TdT or replacement of the complete heavy chain diversity segments with an altered DH segment did not alter significantly the susceptibility to infection with mouse-adapted influenza virus.
FIG. 1.
Susceptibility of TdT−/− and ΔD-iD mice to infection with mouse-adapted influenza virus A/PR/8/34 (H1N1). Naïve TdT−/− and ΔD-iD mice were infected with mouse-adapted A/PR/8/34 (H1N1) at different doses. Mortality was monitored daily for 3 weeks and is expressed as the percentage in each group of 8 to 10 mice that survived the infection.
Protection against challenge with lethal doses of homologous and heterologous influenza viruses.
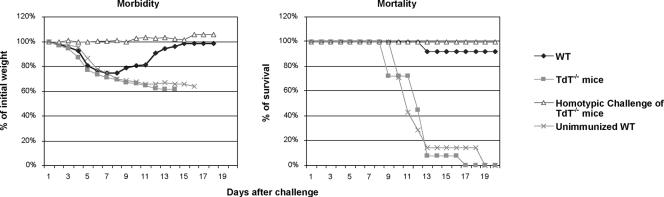
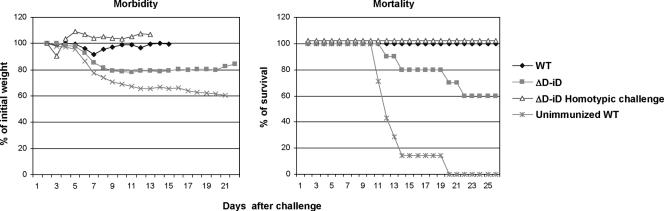
We next examined the effect of altering or limiting the lymphocyte antigen receptor repertoire on the outcome of reinfection with either a homologous or a heterologous virus subtype. TdT−/−, ΔD-iD, and WT mice were immunized with a sublethal dose of A/Udorn (H3N2). Four weeks later, they were challenged with a lethal dose (5× LD50) of either homologous A/Philippines (H3N2) or heterologous A/PR/8/34 (H1N1) subtype virus. Both TdT−/− (Fig. 2) and ΔD-iD (Fig. 3) mice survived reinfection with homologous A/Philippines (H3N2) subtype virus. No morbidity (weight loss) was observed after the homotypic challenge, indicating that the immunized mice were completely protected from reinfection with a homologous subtype. However, when challenged with heterologous subtype A/PR/8/34 (H1N1), none of the TdT−/− mice and only 60% of ΔD-iD mice survived the challenge. As a control, all WT mice completely recovered from challenge after transient weight loss. The TdT−/− mice displayed more severe weight loss starting at day 2 following challenge compared to ΔD-iD and WT mice. These results demonstrate that TdT−/− mice with a limited lymphocyte antigen receptor repertoire and ΔD-iD mice with an altered antibody repertoire failed to develop complete HSI against challenge with a heterologous influenza virus subtype. Of these two types of mice, TdT−/− mice exhibited greater weight loss and a higher mortality, suggesting that the impairment of HSI was greater in TdT−/− mice than ΔD-iD mice.
FIG. 2.
Morbidity and mortality in TdT−/− mice after challenge with mouse-adapted heterologous influenza virus. TdT−/− and WT littermates were immunized with live influenza virus A/Udorn (H3N2) via the TRT. Four weeks later, the mice were challenged with the heterologous mouse-adapted A/PR/8/34 (H1N1) or homologous mouse-adapted A/Philippines (H3N2) strain by the same route. Morbidity and mortality were monitored daily for at least 3 weeks. The body weights of individual mice were measured until all animals regained their initial weight. The values are the means of 12 to 16 mice in each group. Mortality is expressed as the percentage of mice that survived the challenge.
FIG. 3.
Morbidity and mortality in ΔD-iD mice after challenge with mouse-adapted heterologous influenza virus. ΔD-iD and WT littermates were immunized with the live influenza virus A/Udorn (H3N2) via the TRT. Four weeks later they were challenged with the heterologous mouse-adapted A/PR/8/34 (H1N1) or homologous mouse-adapted A/Philippines (H3N2) strain by the same route. Morbidity and mortality were monitored daily for at least 3 weeks. Body weights of individual mice were measured until all animals regained their initial weight. The values are the means of 10 to 14 mice in each group. Mortality is expressed as the percentage of mice that survived the challenge.
Virus-specific Ab levels induced after primary infection.
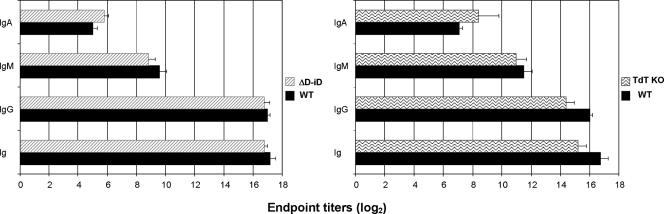
Since the outcome of HSI depends significantly on the virus-specific Ab responses induced after the immunization (29), we asked whether the impairment of HSI in TdT−/− and ΔD-iD mice is indeed related to reduction of virus-specific Ab responses in these mice. One month after primary infection with A/Udorn and a day before the challenge, we examined the levels of serum virus-specific Abs by A/Udorn-coated ELISA in immunized ΔD-iD and TdT−/− mice. As shown in Fig. 4, virus-specific Ab responses were impaired in TdT−/− mice. The levels of virus-specific Abs of major isotypes induced in TdT−/− mice after primary infection were significantly lower than those in infected WT mice (P < 0.01). Of note, a slightly increased level of virus-specific IgA was observed in the sera of immunized TdT−/− compared to that of immunized WT mice (Fig. 4). However, the titer was relatively low compared to that of other isotypes. The levels of virus-specific Abs of all isotypes were also lower in ΔD-iD mice compared to WT, but the difference in titer did not achieve statistical significance (P > 0.05). Thus, in terms of the magnitude, virus-specific Ab responses induced by primary infection in ΔD-iD mice were relatively similar to those observed in WT.
FIG. 4.
Virus-specific serum antibody titers in TdT−/−, ΔD-iD, and WT mice following immunization with live influenza A virus strain A/Udorn. Four weeks later, virus-specific antibody titers in serum were determined by influenza virus A/Udorn-coated ELISA plates before challenge with the heterologous mouse-adapted A/PR/8/34 (H1N1) strain via the TRT. The values represent the means and SEM of the end point ELISA antibody titers for five mice per group.
Cell-mediated immune responses.
Since the deletion of TdT affects the diversity of the TCR as well as the antibody repertoire, we asked whether or not the TdT deficiency affects recognition and lysis of influenza virus-infected cells by T cells and CTL responses, respectively. We analyzed the induction of virus-specific cell-mediated immune responses in TdT−/− mice after primary exposure to live virus. Four weeks after primary infection, mononuclear cells from spleens and MLN of immunized mice were isolated and cultured with viruses or irradiated virus-infected cells as antigen-presenting cells for virus-specific proliferation responses and CTL effector expansion. As shown in Fig. 5, splenic CTL responses induced in TdT−/− mice upon primary infection were slightly greater and mediastinal lymph node CTL responses in both TdT−/− and ΔD-iD mice were slightly lower than those induced in WT mice. However, neither of these findings reached statistical significance (P > 0.05). Proliferation of mononuclear cells derived from MLN and spleens of TdT−/− and ΔD-iD mice in response to viral antigen as whole or presented by syngeneic splenocytes was comparable with that in WT mice (Fig. 6). Slightly higher responses were seen in the cell cultures derived from TdT−/− mice; however, the difference was not statistically significant (P > 0.05). The results suggest that antigen recognition by T cells and/or cytolytic activity of CTL was relatively unaffected by the deletion of TdT or the replacement of the complete heavy chain diversity segments (DH) with an altered DH segment.
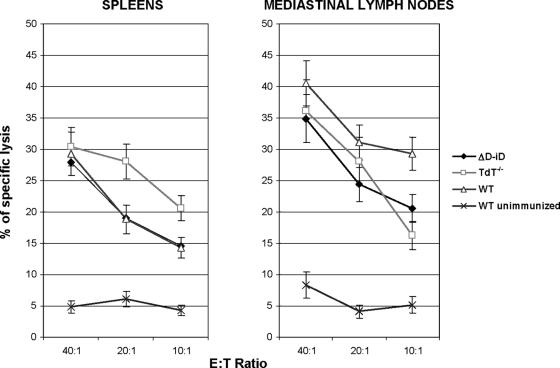
FIG. 5.
Heterosubtypic CTL responses. TdT−/−, ΔD-iD, and WT mice were exposed throughout the TRT to live A/Udorn (H3N2) influenza virus. Four weeks later, the mice were challenged with a lethal dose of mouse-adapted A/PR/8/34 (H1N1) virus. On day 3 after the challenge, five mice from each subgroup were sacrificed. Mononuclear cells isolated from MLN and spleens were stimulated in vitro and assayed for CTL activity against H1N1 influenza virus-infected P815 (H-2d) target cells. Specific CTL activities were determined by subtracting nonspecific cytotoxic activity against mock-infected and MHC-mismatched virus-infected EL-4 (H-2b) target cells from the cytotoxic activity against virus-infected MHC-matched P815 (H-2d) target cells. The data represent results from two independent experiments of five mice per group.
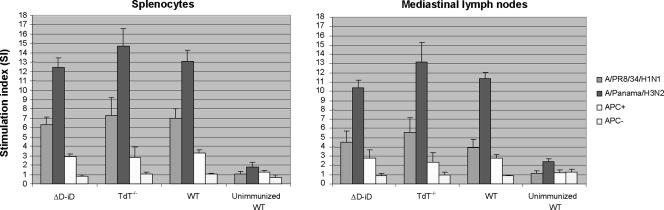
FIG. 6.
Proliferation of mononuclear cells derived from MLN and spleens of TdT−/− and ΔD-iD mice in response to viruses and virus-infected cells. TdT−/−, ΔD-iD, and WT mice were exposed throughout the TRT to the live A/Udorn (H3N2) influenza virus. Four weeks later the mice were challenged with a lethal dose of mouse-adapted A/PR/8/34 (H1N1) virus. On day 3 after the challenge, five mice from each subgroup were sacrificed. Mononuclear cells isolated from MLN and spleens were cultured in the presence of live viruses or irradiated syngeneic splenocytes infected with homologous [A/Panama/2007/99 (H3N2)] or heterologous [A/PR/8/34 (H1N1)] viruses. The data are presented as the stimulation index, calculated as follows: (cpm of stimulated culture)/(cpm of nonstimulated culture, in the absence of antigen). The data represent results from two independent experiments of five mice per group. APC, antigen-presenting cells.
DISCUSSION
In this study, we have tested the role of antibody and TCR diversity in the induction of HSI to influenza A virus infection. We used genetically altered mice limited to germ line antibody and TCR diversity in the case of the TdT−/− mice as well as mice with wild-type TCR loci, but where replacement of the DH locus with a single, altered DH led to a globally altered antibody repertoire in the case of ΔD-iD. Neither a limitation to germ line diversity in the Ab and TCR repertoire nor global alteration of the Ab repertoire impaired the ability of the gene-targeted mice to respond appropriately to the initial infection with influenza A virus. Immune responses to primary viral infection involve a variety of components of the immune system, including natural Abs (mainly IgM) secreted by B-1 cells (2, 3) and Th-independent B cells (26). Since TdT−/− mice generate predominantly B-1 cells (11) and unchanged levels of secreted IgM (Fig. 4), our data on susceptibility of these transgenic mice to mouse-adapted influenza virus infection indicate that limitations to the diversity of the Ab repertoire in TdT−/− and ΔD-iD mice could be either compensated by more effective innate immunity or that an antibody repertoire of normal wild-type diversity is not necessary for the induction of immune responses to a primary infection with virus. In fact, TdT−/− mice have been found to be healthy and to respond to most complex antigens (11, 12, 17). Although the ability of ΔD-iD mice to generate normal levels of IgG in the serum appears to be impaired, they are able to generate normal levels of total IgM and IgA (13). In the present study, virus-specific IgM reached wild-type levels upon infection (Fig. 4).
When subsequently challenged with a homologous subtype, both types of mice with a limited antigen receptor repertoire or a globally altered Ab repertoire were protected from the reinfection, suggesting that they were able to generate normal protective, presumably VN, Abs, since VN Abs are responsible for prevention of reinfection with the same subtype (29, 35). Our findings support the notion that a single Ab isotype specific for a single antigenic epitope of hemagglutinin could be sufficient for VN (24). Even with a limited or altered Ab repertoire, it would appear that there is sufficient diversity to generate Abs specific for hemagglutinin, the most abundant viral surface glycoprotein (48). Such Abs with VN activity could be protective when present at low levels (25).
Interestingly, when challenged with a virus of a heterologous subtype, both types of mice developed impaired HSI. In TdT−/− mice, morbidity and mortality correlated with impaired induction of the virus-specific Ab response. Indeed, virus-specific Ab responses were down-regulated in TdT−/− mice compared to those in WT littermates. In contrast to homotypic immunity, complete HSI seems to require a set of different Ab isotypes specific to a set of antigen epitopes. The observations support the findings that treatment with a single Ab isotype specific for the conserved transmembrane M2 proteins, the Ab that mediates HSI (16, 47), could provide partial protection (25), while immunization with a protein complex containing M2 (8, 23, 27) or DNA plasmid containing the influenza virus M gene (31) could provide complete HSI. Since the B-cell repertoire from TdT−/− mice is presumed to be less flexible than that of the N-region-containing repertoire in WT mice (42), our results suggest that an Ab repertoire with high polyreactivity could play an important role in HSI. Interestingly, virus-specific IgA was found to be increased in TdT−/− but not in WT mice (Fig. 4). Such IgA could derive from B-1 cells through an extrasplenic pathway of Ig rearrangement (21). Although the role for IgA in HSI is controversial (4, 20), an increased level of cross-reactive IgA in TdT−/− mice that failed to develop HSI suggests that IgA produced by B-1 cells may not be necessary for HSI and that B-2-cell-derived IgA may possibly make a greater contribution to HSI.
Since depletion of TdT also resulted in a limited TCR repertoire (12, 17) and could affect the antigen recognition by T cells, including cytotoxic T cells, we examined virus-specific CD8+ CTL responses. Four weeks after immunization, the mice were challenged with a lethal dose of A/PR/8/34 (H1N1) virus. On day 3 after the challenge, mononuclear cells isolated from spleens and MLN were subjected to 7-day in vitro culture for stimulation of antigen-specific CTL effector cells. Pronounced subtype-specific CTL systemic (spleen and MLN) responses were observed in TdT−/− mice (Fig. 5). When CD8+ T cells were depleted from the effector cells by treating the cells with monoclonal antibody specific for the CD8 molecule before performing the cytotoxicity assay, specific lysis was almost completely eliminated. This result indicates that subtype-specific CTL activity is mediated predominantly by CD8+ T cells. Thus, the ability to mount virus-specific CD8+ CTL responses was intact in TdT−/− mice. As expected, ΔD-iD mice mounted normal virus-specific CD8+ CTL responses (Fig. 5), since the replacement of DH segments resulted in alteration of the antibody, but not the TCR, repertoire. Proliferation activities of spleen- or MLN-derived mononuclear cells to the virus and the virus-infected cells were relatively unaffected in TdT−/− and ΔD-iD mice compared to WT littermates (Fig. 6). The results indicate that antigen recognition by CD4+ and CD8+ T cells was not functionally altered by genetic deletion of TdT or replacement of the DH segments. Although the role for CD4+ T cells in providing help for B cells and antigen-specific Ab responses by secretion of cytokines was not examined in this study, the increased morbidity and mortality observed in TdT−/− mice after the challenge compared to those seen in ΔD-iD mice (with an intact TCR repertoire) suggest a role for a properly diversified TCR repertoire for the outcome of HSI. The results support our previous finding that CD4+ T cells are required for induction of cross-reactive Abs and, subsequently, complete HSI (29). Thus, impaired induction of virus-specific Abs along with lack of HSI, but intact virus-specific CTL responses, in TdT−/− mice suggests a greater role for B cells and Abs in HSI, as previously described (22, 29, 37, 41).
The death of 40% of the ΔD-iD mice after the heterosubtypic challenge in spite of the presence of comparable (to WT) virus-specific Ab levels after immunization (Fig. 4) that offered complete protection against challenge with homotypic virus indicates that the virus-specific Abs produced by the ΔD-iD mice were functionally not equivalent to the Abs from WT mice. This raises questions regarding whether the mutant surviving mice had responded to different viral epitopes than the mice that died and/or whether they responded to the same epitopes but differed from their brethren in the ability of their antibodies to protect them against infection. These hypotheses are not mutually exclusive, and their examination remains a focus of our laboratories.
Normal immune responses to primary infection or reinfection with the same subtype of influenza A viruses but impaired heterosubtypic immunity in TdT−/− and ΔD-iD mice suggest that a normally diversified adult antibody repertoire provides optimal heterosubtypic immunity for influenza virus. The ΔD-iD mouse is a laboratory construct, but the TdT−/− mouse mimics the restriction in diversity observed in neonates (11). The perinatal period is one that is known to be associated with increased susceptibility to influenza virus. Our findings would thus support the view that limitations in the diversity of lymphocyte antigen receptors may underlie the physiologic immune deficiency exhibited by neonates.
Acknowledgments
This work was supported in part by U.S. PHS grants AI 28147, K12HD 01402-02, AI 42732, AI 48115, and HD 043327 and by Humboldt-Foundation FLF 1071857 and DFG SFB/TR22 TPA17.
Animal experiments in this study were performed according to the animal experimentation guidelines of the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Footnotes
Published ahead of print on 13 June 2007.
REFERENCES
- 1.Alt, F. W., and D. Baltimore. 1982. Joining of immunoglobulin heavy chain gene segments: implications from a chromosome with evidence of three D-JH fusions. Proc. Natl. Acad. Sci. USA 79:4118-4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgarth, N., O. C. Herman, G. C. Jager, L. Brown, and L. A. Herzenberg. 1999. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc. Natl. Acad. Sci. USA 96:2250-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgarth, N., O. C. Herman, G. C. Jager, L. E. Brown, L. A. Herzenberg, and J. Chen. 2000. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J. Exp. Med. 192:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benton, K. A., J. A. Misplon, C. Y. Lo, R. R. Brutkiewicz, S. A. Prasad, and S. L. Epstein. 2001. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or γδ T cells. J. Immunol. 166:7437-7445. [DOI] [PubMed] [Google Scholar]
- 5.Boon, A. C., G. de Mutsert, D. van Baarle, D. J. Smith, A. S. Lapedes, R. A. Fouchier, K. Sintnicolaas, A. D. Osterhaus, and G. F. Rimmelzwaan. 2004. Recognition of homo- and heterosubtypic variants of influenza A viruses by human CD8+ T lymphocytes. J. Immunol. 172:2453-2460. [DOI] [PubMed] [Google Scholar]
- 6.Brundler, M. A., P. Aichele, M. Bachmann, D. Kitamura, K. Rajewsky, and R. M. Zinkernagel. 1996. Immunity to viruses in B cell-deficient mice: influence of antibodies on virus persistence and on T cell memory. Eur. J. Immunol. 26:2257-2262. [DOI] [PubMed] [Google Scholar]
- 7.Doherty, P. C., D. J. Topham, R. A. Tripp, R. D. Cardin, J. W. Brooks, and P. G. Stevenson. 1997. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 159:105-117. [DOI] [PubMed] [Google Scholar]
- 8.Frace, A. M., A. I. Klimov, T. Rowe, R. A. Black, and J. M. Katz. 1999. Modified M2 proteins produce heterotypic immunity against influenza A virus. Vaccine 17:2237-2244. [DOI] [PubMed] [Google Scholar]
- 9.Gavin, M. A., and M. J. Bevan. 1995. Increased peptide promiscuity provides a rationale for the lack of N regions in the neonatal T cell repertoire. Immunity 3:793-800. [DOI] [PubMed] [Google Scholar]
- 10.Gerhard, W., K. Mozdzanowska, and M. Furchner. 1996. The nature of heterosubtypic immunity, p. 235-243. In L. E. Brown, A. W. Hampson, and R. G. Webster (ed.), Options for the control of influenza III. Elsevier, Amsterdam, The Netherlands.
- 11.Gilfillan, S., C. Benoist, and D. Mathis. 1995. Mice lacking terminal deoxynucleotidyl transferase: adult mice with a fetal antigen receptor repertoire. Immunol. Rev. 148:201-219. [DOI] [PubMed] [Google Scholar]
- 12.Gilfillan, S., A. Dierich, M. Lemeur, C. Benoist, and D. Mathis. 1993. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science 261:1175-1178. [DOI] [PubMed] [Google Scholar]
- 13.Hood, L., and D. Galas. 2003. The digital code of DNA. Nature 421:444-448. [DOI] [PubMed] [Google Scholar]
- 14.Ippolito, G. C., R. L. Schelonka, M. Zemlin, I. I. Ivanov, R. Kobayashi, C. Zemlin, G. L. Gartland, L. Nitschke, J. Pelkonen, K. Fujihashi, K. Rajewsky, and H. W. Schroeder, Jr. 2006. Forced usage of positively charged amino acids in immunoglobulin CDR-H3 impairs B cell development and antibody production. J. Exp. Med. 203:1567-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabat, E. A., T. T. Wu, H. C. Perry, K. S. Gottesman, and C. Foeller. 1991. Sequences of proteins of immunological interest. U.S. Department of Health and Human Services, Bethesda, MD.
- 16.Kaya, Z., M. Afanasyeva, Y. Wang, K. M. Dohmen, J. Schlichting, T. Tretter, D. Fairweather, V. M. Holers, and N. R. Rose. 2001. Contribution of the innate immune system to autoimmune myocarditis: a role for complement. Nat. Immunol. 2:739-745. [DOI] [PubMed] [Google Scholar]
- 17.Komori, T., A. Okada, V. Stewart, and F. W. Alt. 1993. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science 261:1171-1175. [DOI] [PubMed] [Google Scholar]
- 18.Kreijtz, J. H., R. Bodewes, G. van Amerongen, T. Kuiken, R. A. Fouchier, A. D. Osterhaus, and G. F. Rimmelzwaan. 2007. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine 25:612-620. [DOI] [PubMed] [Google Scholar]
- 19.Lefranc, M. P. 2003. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 31:307-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liew, F. Y., S. M. Russell, G. Appleyard, C. M. Brand, and J. Beale. 1984. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur. J. Immunol. 14:350-356. [DOI] [PubMed] [Google Scholar]
- 21.Macpherson, A. J., D. Gatto, E. Sainsbury, G. R. Harriman, H. Hengartner, and R. M. Zinkernagel. 2000. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 288:2222-2226. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell, J. A., T. D. Green, R. A. Bright, and T. M. Ross. 2003. Induction of heterosubtypic immunity to influenza A virus using a DNA vaccine expressing hemagglutinin-C3d fusion proteins. Vaccine 21:902-914. [DOI] [PubMed] [Google Scholar]
- 23.Mozdzanowska, K., J. Feng, M. Eid, G. Kragol, M. Cudic, L. Otvos, Jr., and W. Gerhard. 2003. Induction of influenza type A virus-specific resistance by immunization of mice with a synthetic multiple antigenic peptide vaccine that contains ectodomains of matrix protein 2. Vaccine 21:2616-2626. [DOI] [PubMed] [Google Scholar]
- 24.Mozdzanowska, K., M. Furchner, G. Washko, J. Mozdzanowski, and W. Gerhard. 1997. A pulmonary influenza virus infection in SCID mice can be cured by treatment with hemagglutinin-specific antibodies that display very low virus-neutralizing activity in vitro. J. Virol. 71:4347-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mozdzanowska, K., K. Maiese, M. Furchner, and W. Gerhard. 1999. Treatment of influenza virus-infected SCID mice with nonneutralizing antibodies specific for the transmembrane proteins matrix 2 and neuraminidase reduces the pulmonary virus titer but fails to clear the infection. Virology 254:138-146. [DOI] [PubMed] [Google Scholar]
- 26.Mozdzanowska, K., K. Maiese, and W. Gerhard. 2000. Th cell-deficient mice control influenza virus infection more effectively than Th- and B cell-deficient mice: evidence for a Th-independent contribution by B cells to virus clearance. J. Immunol. 164:2635-2643. [DOI] [PubMed] [Google Scholar]
- 27.Neirynck, S., T. Deroo, X. Saelens, P. Vanlandschoot, W. M. Jou, and W. Fiers. 1999. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat. Med. 5:1157-1163. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen, H. H., Z. Moldoveanu, M. J. Novak, F. W. van Ginkel, E. Ban, H. Kiyono, J. R. McGhee, and J. Mestecky. 1999. Heterosubtypic immunity to lethal influenza A virus infection is associated with virus-specific CD8+ cytotoxic T lymphocyte responses induced in mucosa-associated tissues. Virology 254:50-60. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen, H. H., F. W. van Ginkel, H. L. Vu, J. R. McGhee, and J. Mestecky. 2001. Heterosubtypic immunity to influenza A virus infection requires B cells but not CD8+ cytotoxic T lymphocytes. J. Infect. Dis. 183:368-376. [DOI] [PubMed] [Google Scholar]
- 30.Nossal, G. J. 2003. The double helix and immunology. Nature 421:440-444. [DOI] [PubMed] [Google Scholar]
- 31.Okuda, K., A. Ihata, S. Watabe, E. Okada, T. Yamakawa, K. Hamajima, J. Yang, N. Ishii, M. Nakazawa, K. Okuda, K. Ohnari, K. Nakajima, and K. Q. Xin. 2001. Protective immunity against influenza A virus induced by immunization with DNA plasmid containing influenza M gene. Vaccine 19:3681-3691. [DOI] [PubMed] [Google Scholar]
- 32.Padlan, E. A. 1994. Anatomy of the antibody molecule. Mol. Immunol. 31:169-217. [DOI] [PubMed] [Google Scholar]
- 33.Powell, T. J., T. Strutt, J. Reome, J. A. Hollenbaugh, A. D. Roberts, D. L. Woodland, S. L. Swain, and R. W. Dutton. 2007. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J. Immunol. 178:1030-1038. [DOI] [PubMed] [Google Scholar]
- 34.Sambhara, S., A. Kurichh, R. Miranda, T. Tumpey, T. Rowe, M. Renshaw, R. Arpino, A. Tamane, A. Kandil, O. James, B. Underdown, M. Klein, J. Katz, and D. Burt. 2001. Heterosubtypic immunity against human influenza A viruses, including recently emerged avian H5 and H9 viruses, induced by FLU-ISCOM vaccine in mice requires both cytotoxic T-lymphocyte and macrophage function. Cell. Immunol. 211:143-153. [DOI] [PubMed] [Google Scholar]
- 35.Schulman, J. L., and E. D. Kilbourne. 1965. Induction of partial specific heterotypic immunity in mice by a single infection with influenza virus. J. Bacteriol. 89:170-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slepushkin, V. A., J. M. Katz, R. A. Black, W. C. Gamble, P. A. Rota, and N. J. Cox. 1995. Protection of mice against influenza A virus challenge by vaccination with baculovirus-expressed M2 protein. Vaccine 13:1399-1402. [DOI] [PubMed] [Google Scholar]
- 37.Takada, A., S. Matsushita, A. Ninomiya, Y. Kawaoka, and H. Kida. 2003. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine 21:3212-3218. [DOI] [PubMed] [Google Scholar]
- 38.Taylor, P. M., J. Davey, K. Howland, J. B. Rothbard, and B. A. Askonas. 1987. Class I MHC molecules rather than other mouse genes dictate influenza epitope recognition by cytotoxic T cells. Immunogenetics 26:267-272. [DOI] [PubMed] [Google Scholar]
- 39.Tonegawa, S. 1983. Somatic generation of antibody diversity. Nature 302:575-581. [DOI] [PubMed] [Google Scholar]
- 40.Townsend, A. R. M., A. J. McMichael, N. P. Carter, J. A. Huddleston, and G. G. Brownlee. 1984. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell 9:13-25. [DOI] [PubMed] [Google Scholar]
- 41.Tumpey, T. M., M. Renshaw, J. D. Clements, and J. M. Katz. 2001. Mucosal delivery of inactivated influenza vaccine induces B-cell-dependent heterosubtypic cross-protection against lethal influenza A H5N1 virus infection. J. Virol. 75:5141-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weller, S., C. Conde, A. M. Knapp, H. Levallois, S. Gilfillan, J. L. Pasquali, and T. Martin. 1997. Autoantibodies in mice lacking terminal deoxynucleotidyl transferase: evidence for a role of N region addition in the polyreactivity and in the affinities of anti-DNA antibodies. J. Immunol. 159:3890-3898. [PubMed] [Google Scholar]
- 43.Wiley, J. A., R. J. Hogan, D. L. Woodland, and A. G. Harmsen. 2001. Antigen-specific CD8+ T cells persist in the upper respiratory tract following influenza virus infection. J. Immunol. 167:3293-3299. [DOI] [PubMed] [Google Scholar]
- 44.Wraith, D. C., A. E. Vessey, and B. A. Askonas. 1987. Purified influenza virus nucleoprotein protects mice from lethal infection. J. Gen. Virol. 68:433-440. [DOI] [PubMed] [Google Scholar]
- 45.Xu, J. L., and M. M. Davis. 2000. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity 13:37-45. [DOI] [PubMed] [Google Scholar]
- 46.Yewdell, J. W., J. R. Bennink, G. L. Smith, and B. Moss. 1985. Influenza virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 82:1785-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Youngner, J. S., J. J. Treanor, R. F. Betts, and P. Whitaker-Dowling. 1994. Effect of simultaneous administration of cold-adapted and wild-type influenza A viruses on experimental wild-type influenza infection in humans. J. Clin. Microbiol. 32:750-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zebedee, S. L., and R. A. Lamb. 1988. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J. Virol. 62:2762-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]