Abstract
Most children in Africa receive their vaccine against tuberculosis at birth. Those infants born to human immunodeficiency virus type 1 (HIV-1)-positive mothers are at high risk of acquiring HIV-1 infection through breastfeeding in the first weeks of their lives. Thus, the development of a vaccine which would protect newborns against both of these major global killers is a logical yet highly scientifically, ethically, and practically challenging aim. Here, a recombinant lysine auxotroph of Mycobacterium bovis bacillus Calmette-Guérin (BCG), a BCG strain that is safer than those currently used and expresses an African HIV-1 clade-derived immunogen, was generated and shown to be stable and to induce durable, high-quality HIV-1-specific CD4+- and CD8+-T-cell responses. Furthermore, when the recombinant BCG vaccine was used in a priming-boosting regimen with heterologous components, the HIV-1-specific responses provided protection against surrogate virus challenge, and the recombinant BCG vaccine alone protected against aerosol challenge with M. tuberculosis. Thus, inserting an HIV-1-derived immunogen into the scheduled BCG vaccine delivered at or soon after birth may prime HIV-1-specific responses, which can be boosted by natural exposure to HIV-1 in the breast milk and/or by a heterologous vaccine such as recombinant modified vaccinia virus Ankara delivering the same immunogen, and decrease mother-to-child transmission of HIV-1 during breastfeeding.
Since the first report of AIDS in 1981, an estimated more than 60 million people have become infected with human immunodeficiency virus type 1 (HIV-1), of whom some 25 million have died. Over 60% of the global HIV-1-infected population lives in Africa, and about a half of the infected adults are women of childbearing age. Up to 40% of pregnant women attending large urban-center clinics in Kenya are HIV-1 seropositive. Despite the fact that approximately half of mother-to-child transmissions (MTCT) are due to prolonged breastfeeding (43), for many HIV-1-positive mothers, bottle feeding is not an option for social, practical, and health reasons. Bottle-fed babies of infected mothers have higher morbidity and mortality due to increased exposure and susceptibility to other infections (32). Although antiretroviral therapy can significantly reduce the risk of MTCT at parturition, it is less clear whether it is practical to use antiretroviral drugs to prevent HIV-1 transmission through breast milk. The drugs are very expensive and would have to be administered at birth and throughout the whole period of breastfeeding; in addition, their effectiveness may be compromised by the emergence of resistant mutants. Thus, the best hope for protecting newborns against MTCT of HIV-1 in developing countries is the development of safe, effective, accessible prophylactic vaccines, which would both reduce the adult burden of infection and protect neonates against vertical HIV-1 transmission.
The development of an HIV-1 vaccine remains on the horizon. Although in principle it is possible, the induction of broadly neutralizing antibodies effective across HIV-1 clades has been extremely difficult to achieve by active immunization (6). While various technologies are becoming more successful at inducing HIV-1-specific T-cell responses (33), a single correlate of protection against HIV-1 infection and AIDS remains elusive and may be nonexistent, as protection is most likely to be multifactorial. Therefore, before further progress in this area is made, the interim aim of HIV-1 vaccination is to elicit responses comprising T cells that are high in frequency, multispecific, multifunctional, capable of rapid proliferation, long-lived, and of the central memory phenotype. The in vivo functionality of vaccine-induced T cells can be assessed using surrogate models of viral challenge. The ultimate goal, vaccine efficacy, can be confirmed only by adequately powered clinical trials with humans.
For a number of years, we have been developing an HIV-1 vaccine focusing on the induction of protective cell-mediated responses. Our starting platform was based on a heterologous regimen consisting of a DNA priming vaccine and a modified vaccinia virus Ankara (MVA) boosting vaccine (44) delivering a common immunogen called HIVA, which is derived from consensus HIV-1 clade A Gag protein, i.e., an immunogen derived from an HIV-1 strain prevalent in central and eastern Africa, and a string of CD8+-T-cell epitopes (17). Extensive studies with mice, nonhuman primates, and more than 400 healthy and HIV-1-infected humans have shown that the vaccines are safe and immunogenic (18). In humans, DNA vaccine pTHr.HIVA weakly but consistently primes HIV-1-specific responses involving mostly CD4+ T cells and MVA expressing HIVA (MVA.HIVA) delivers a strong and consistent boost, expanding populations of both CD4+ and CD8+ HIV-1-specific T cells, particularly if they are well primed, e.g., by HIV-1 infection (12, 15, 37).
Infection with Mycobacterium tuberculosis kills about 2 million people each year (42). M. bovis bacillus Calmette-Guérin (BCG) is the only licensed vaccine and protects significantly against childhood and miliary tuberculosis (52). Globally, 80% percent of children are vaccinated with BCG (50), the majority of them at birth. Thus, the development of a combined vaccine that would protect neonates against tuberculosis and MTCT of HIV-1 through breastfeeding is a logical effort in the fight against these two major global killers. One such obvious vaccine or vaccine component is a recombinant BCG (rBCG) strain expressing an HIV-1-derived immunogen.
BCG as a vaccine vector has a number of attractive features (10). BCG has a proven record of safety as a vaccine against tuberculosis from its use in more than 2 billion individuals, including neonates (26). BCG infects and colonizes macrophages and dendritic cells, where it can survive and replicate for a long period of time. By its persistence and potent adjuvancy through its cell wall components, it can induce long-lasting humoral and cellular immune responses. Immunity against tuberculosis following neonatal BCG vaccination lasts 10 to 15 years (20) and, therefore, fails to protect adults from pulmonary disease (9). Thus, it should easily cover the danger period of breastfeeding, and a later booster vaccine may offer children further protection in adolescence. BCG can be given at or any time after birth and is not affected by maternal antibodies. The manufacturing of BCG-based vaccines is cheap as live bacteria are easy to purify. Finally, BCG is one of the most heat-stable vaccines in present use (14).
It is not known how early in life T cells can be educated to launch a protective response against intracellular microorganisms, and this factor most likely differs from pathogen to pathogen. Qualitative and quantitative differences between responses to a number of infections in human newborns and adults have been observed previously (1, 16, 31, 45). Weaker CD8+ T-cell responses in HIV-1-infected infants than in adults may play an important role in fast disease progression (7, 27, 41); children account for 4% of HIV-1 infections, yet they represent 20% of AIDS deaths (16). At the same time, mature responses to certain infections and vaccines during the postnatal and even fetal periods have been demonstrated previously (28). This observation is particularly true for BCG vaccine-induced responses, which promote adult-like Th1 responses in newborns (21, 29, 38, 49). Therefore, there is some evidence suggesting that protective T-cell-mediated responses could be elicited by vaccines early in life and that BCG as a vaccine vector may be very well suited to prime them (30).
In this study, we have constructed a recombinant lysine auxotroph of BCG expressing the HIVA immunogen from both replicative and integrative vectors. After confirmation of the HIVA gene sequence, plasmid stability, and protein expression, the BCG strain expressing HIVA from an episomal plasmid (BCG.HIVA) alone and BCG.HIVA in a heterologous priming-boosting combination were studied for the induction of HIV-1- and M. tuberculosis-specific immune responses in a murine model. Protection against both surrogate virus expressing HIVA and aerosol M. tuberculosis challenge was achieved.
MATERIALS AND METHODS
Construction of Escherichia coli-mycobacterium shuttle vectors expressing HIVA.
Parental plasmids pJH222 and pJH223 were kindly provided by W. R. Jacobs, Jr., B. R. Bloom, and T. Hsu. The coding sequence of the HIVA gene was fused to the M. tuberculosis nucleotides coding for the 19-kDa lipoprotein signal sequence in a PCR, and the chimeric gene was cloned into the pJH222 and pJH223 plasmids as a HindIII-HindIII fragment under the control of the M. tuberculosis α-antigen promoter by using standard recombinant-DNA techniques.
Mycobacterial culture.
A lysine auxotroph of BCG, kindly provided by W. R. Jacobs, Jr., B. R. Bloom, and T. Hsu, was transformed by electroporation. Mycobacterial cultures were grown in Middlebrook 7H9 broth medium or on Middlebrook agar 7H10 medium supplemented with albumin-dextrose complex (ADC; Difco) and containing 0.05% Tween 80 and 25 μg of kanamycin/ml. The l-lysine monohydrochloride (Sigma) was dissolved in distilled water and used at a concentration of 40 μg/ml. For transformation, BCG cultures were grown to an optical density at 600 nm of 0.9, transformed using a Bio-Rad gene pulser electroporator at 2.5 kV, 25 mF, and 1,000 Ω, and plated onto ADC-supplemented Middlebrook agar 7H10 medium containing 0.05% Tween 80 and 25 μg of kanamycin/ml.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis.
Cell lysates of mid-logarithmic-phase BCG transformants were prepared, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and electroblotted. HIVA protein was detected using anti-Pk antibodies with an ECL kit (Amersham International).
Mice.
For all animal experiments, groups of four to five 5- to 8-week-old female BALB/c mice were used unless otherwise specified. All animal procedures and care were approved by local ethical committees and strictly conformed to United Kingdom Home Office guidelines.
Immunizations and isolation of splenocytes.
Under general anesthesia, mice were immunized intramuscularly with 100 μg of endotoxin-free pTHr.HIVA DNA (Cobra Therapeutics, United Kingdom) or 106 PFU of MVA.HIVA intramuscularly or intraperitoneally (i.p.) with 106 CFU of parental BCG (BCG.p) or BCG.HIVA. For dose titration, mice received 103 to 107 CFU of BCG.HIVA i.p. On the day of sacrifice, spleens were removed and pressed individually through a cell strainer (Falcon) with a 5-ml syringe rubber plunger. Following the removal of red blood cells with red blood cell lysing buffer (Sigma), splenocytes were washed and resuspended in lymphocyte medium (R10 [RPMI 1640 supplemented with 10% fetal calf serum {FCS} and penicillin-streptomycin], 20 mM HEPES, and 15 mM 2-mercaptoethanol) at a concentration of 2 ×107 cells/ml.
In vivo stability of plasmid pJH222.HIVA.
The growth of rBCG and the stability of the extrachromosomal plasmid pJH222.HIVA were established by the recovery of BCG.HIVA colonies from the spleens of mice 15 weeks after immunization. Spleens were homogenized and plated onto Middlebrook 7H10 medium supplemented with ADC (Difco) and containing 0.05% Tween 80 and 25 μg of kanamycin/ml. With specific primers, the HIVA gene was detected among well-separated mycobacterial colonies by PCR.
Peptides.
For assessing the immunogenicity of HIVA in the BALB/c mice, the following peptides were used: H-2Dd-restricted epitope P18-I10 (RGPGRAFVTI) (48), herein designated epitope H, and the subdominant H-2Kd-restricted P epitope (IFQSSMTKI) (22). Three epitope peptides derived from p24 were pooled to investigate the major histocompatibility complex (MHC) class II-restricted response: MHQALSPRTLNAQVKVIEEK, NPPIPVGDIYKRWIILGLNK, and FRDYVDRFFKTLRAEQATQE.
In vivo killing assay.
Naïve syngeneic mice were sacrificed, the splenocytes were prepared as described above, and the isolated splenocytes were incubated without or with 2 μg of peptides/ml in R10 at 37°C in 5% CO2 for 90 min and washed three times. Target cells not pulsed with peptides were labeled with 5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethyl rhodamine (CMTMR; Molecular Probes) only, while peptide-pulsed target cells were labeled with carboxyfluorescein diacetate succinimidyl ester either without (P peptide) or combined with (H peptide) CMTMR. Briefly, H or P peptide-pulsed splenoctyes resuspended in phosphate-buffered saline (PBS) at 2 × 107 cells/ml were incubated with 80 nM carboxyfluorescein diacetate succinimidyl ester (Molecular Probes) in the dark at room temperature for 10 min, followed by the quenching of the reaction with an equal volume of FCS and three washing steps. H peptide-pulsed cells were then resuspended in R10 at 2 × 107 cells/ml and incubated with 10 μM CMTMR at 37°C for 15 min and in fresh R10 only for a further 15 min. Three differentially labeled cell cultures were washed, resuspended in PBS, and combined for intravenous adoptive transfer, with each animal receiving approximately 2 × 106 cells of each population. After 12 h, animals were sacrificed, and their splenocytes were isolated and analyzed using flow cytometry. Cytotoxicity was calculated using the following formula: adjusted percentage of surviving cells = 100 × (percentage of surviving peptide-pulsed cells/mean percentage of surviving unpulsed cells). Next, the percentage of specific lysis was calculated as follows: percentage of specific lysis = 100 − adjusted percentage of surviving cells (19).
Intracellular cytokine staining.
Two million splenocytes were added to each well of a 96-well round-bottomed plate (Falcon) and pulsed with peptides at 2 μg/ml (CD8 epitopes) to 5 μg/ml (CD4 epitopes) or a 5-μg/ml concentration of purified protein derivative (PPD) tuberculin (Statens Serum Institut, Copenhagen, Denmark), together with antibodies against lysosome-associated membrane proteins anti-CD107a-fluorescein isothiocyanate (FITC) and anti-CD107b-FITC (BD Biosciences) (2), and kept at 37°C and 5% CO2 for 90 min, followed by the addition of GolgiStop (BD Biosciences) containing monensin. After a further 5-h incubation, the reaction was terminated and the cells were washed with FACS wash buffer (PBS, 2% FCS, 0.01% azide) and blocked with anti-CD16/32 (BD Biosciences) at 4°C for 30 min. All subsequent antibody staining procedures were performed using the same conditions. Cells were then washed and stained with anti-CD8-peridinin chlorophyll protein or anti-CD4-peridinin chlorophyll protein (BD Biosciences), washed again, and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences). Perm/Wash buffer (BD Biosciences) was used to wash cells before staining with anti-interleukin-2 (anti-IL-2)-FITC, anti-tumor necrosis factor alpha (anti-TNF-α)-phycoerythrin, and anti-gamma interferon (anti-IFN-γ)-allophycocyanin (BD Biosciences). Cells were fixed with CellFIX (BD Biosciences) and stored at 4°C until analysis.
Vaccinia virus challenge and protection assay.
Groups of four to five naïve or immunized BALB/c female mice were challenged i.p. with 4 × 106 PFU of recombinant replication-competent vaccinia virus strain Western Reserve expressing the HIVA immunogen (WR.HIVA). Four days later, ovaries were collected and sonicated. Confluent 143B cells negative for human thymidine kinase in 6-well plates were infected with 10-fold serial dilutions of the homogenated ovaries. Cells were stained with 0.1% crystal violet in 20% ethanol, and the plaques were counted.
Fluorescence-activated cell sorter analysis.
All chromogen-labeled cells were analyzed by flow cytometry using the CellQuest software (BD Biosciences).
M. tuberculosis challenge and protection assay.
Specific-pathogen-free 6- to 8-week-old BALB/c mice were maintained under barrier conditions in a category III safety facility. Groups of seven to nine mice were vaccinated subcutaneously in the left hind foot with 3 × 105 CFU of either BCG.HIVA, BCG.p, or BCG wild-type 1173 p2 12 weeks prior to challenge. M. tuberculosis Erdman was obtained from M. Brennan (World Health Organization), kept at −80°C, and sonicated before use. Mice were challenged with aerosol spray containing M. tuberculosis by using a modified Henderson apparatus (40) and exposing only the nose during challenge with a system from ADG Developments (United Kingdom). Bacterial loads in lungs and spleens were determined 4 weeks after challenge by plating 10-fold serial dilutions of organ homogenates onto Middlebrook plates and counting the CFU following a 3-week incubation at 37°C. Deposition in the lungs 24 h after challenge was estimated and determined to be around 25 CFU/lung.
RESULTS
Construction of recombinant M. bovis BCG expressing HIV-1 clade A immunogen.
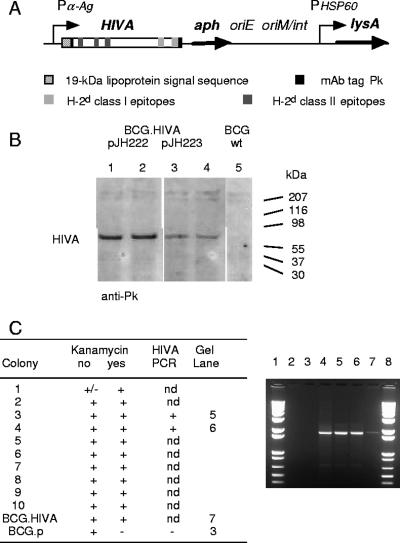
To construct a candidate HIV-1 vaccine with M. bovis BCG as the vector, we used an immunologically well-characterized protein, HIVA, containing the monoclonal antibody (MAb) tag Pk (17). The HIVA gene was synthesized utilizing humanized GC-rich codons (5), which are similar to those used by mycobacteria (4). The HIVA open reading frame was fused at its 5′ end to nucleotides coding for the 19-kDa lipoprotein signal sequence, which facilitates the expression of foreign proteins in the mycobacterial membrane and was shown to increase the foreign protein immunogenicity (47). To facilitate the preclinical development of candidate vaccines, the HIVA immunogen contains an immunodominant H-2Dd-restricted epitope, P18-I10 (48), herein designated epitope H. In addition, it also contains at least three other subdominant H-2d epitopes recognized by CD8+ T cells, including epitope P, lipoprotein, and three CD4+-T-helper epitopes (24). The chimeric 19-kDa signal sequence-HIVA gene was expressed from E. coli-mycobacterium shuttle plasmids pJH222 and pJH223 under the control of the M. tuberculosis α-antigen promoter (Fig. 1A). pJH222 is a low-copy-number replicative episomal vector and contains a mycobacterial origin of replication (oriM). pJH223 is an integrative vector that carries an attachment site (attP) and the integrase gene (int) from the mycobacteriophage L5 and integrates as a single copy into the mycobacterial chromosome. Both vectors also contained the kanamycin resistance gene (aph) as a selectable marker, an E. coli origin of replication (oriE), and a wild-type lysine A-complementing gene for vector maintenance in the BCG auxotroph. The lysine auxotroph of M. bovis BCG host strain Pasteur (39) was transformed with recombinant pJH222.HIVA and pJH223.HIVA. The expression of the full-size chimeric 19-kDa lipoprotein signal sequence-HIVA protein with an Mr of 65 kDa was confirmed on a Western blot of transformed mycobacterial whole-cell lysates using anti-Pk MAb (Fig. 1B). This analysis also revealed that rBCG carrying episomal pJH222.HIVA expressed moderately higher levels of HIVA than rBCG with the integrated pJH223.HIVA plasmid. The growth of the transformed mycobacteria and the in vivo stability of the episomal plasmid were established by the recovery of HIVA-expressing rBCG from the spleens of BALB/c mice 15 weeks after immunization (Fig. 1C). For further experiments, rBCG harboring episomal vector pJH222.HIVA was used and referred to as BCG.HIVA.
FIG. 1.
Construction of BCG.HIVA. (A) A synthetic GC-rich HIVA gene was fused to the region encoding the 19-kDa lipoprotein signal sequence and inserted into the episomal pJH222 or integrative pJH223 E. coli-mycobacterium shuttle plasmid. These plasmids both contained kanamycin resistance (aph) and complementing lysA genes and an E. coli origin of replication (oriE). In addition, pJH222 contained the mycobacterial origin of replication (oriM) and pJH223 carried the attachment site (attP) and the integrase gene (int) of mycobacteriophage L5. The BALB/c mouse T-cell and MAb Pk epitopes used in this work are depicted. Pα-Ag, M. tuberculosis α-antigen promoter; PHSP60, heat shock protein 60 gene promoter. (B) Western blot of lysates of rBCG containing pJH222.HIVA (lanes 1 and 2) and pJH223.HIVA (lanes 3 and 4) and of BCG.p (lane 5) are shown. HIVA was detected using the anti-Pk MAb followed by horseradish peroxidase-protein A and enhanced chemiluminescence. wt, wild type. (C) Stability of rBCG harboring pJH222.HIVA. Mice were injected i.p. with 107 CFU of BCG.HIVA, and rBCG was recovered from homogenized spleens 15 weeks later and plated (without kanamycin). Ten randomly picked mycobacterial colonies were tested for kanamycin resistance, and two of these colonies were tested for the presence of the HIVA gene by HIVA gene-specific PCR. Lanes 1 and 8, molecular markers; lane 2, BCG.p; lane 3, no template; lane 4, plasmid pJH222.HIVA; lanes 5 and 6, kanamycin-resistant colonies; and lane 7, BCG.HIVA vaccine stock. nd, not done.
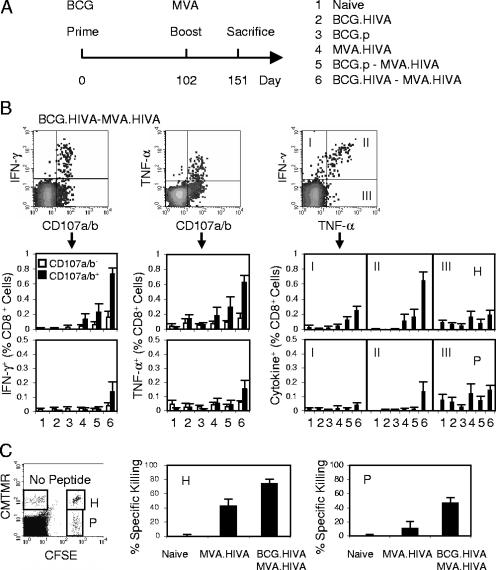
BCG.HIVA primes and enhances MVA.HIVA-elicited HIV-1-specific CD8+-T-cell responses.
The ability of the candidate BCG.HIVA vaccine to induce HIV-1-specific immune responses in BALB/c mice was determined (Fig. 2A). On day 0, mice were immunized using either rBCG with the episomal plasmid or BCG.p or left unimmunized, and on day 102, half of the animals received a booster dose of MVA.HIVA. Because of the activation induced by BCG, detectable HIVA-specific responses peaked after 12 weeks (data not shown). On day 151, the mice were sacrificed and the functional quality of the elicited T cells in terms of their ability to produce IFN-γ and TNF-α and to degranulate (exhibit surface expression of CD107a/b) in response to peptide stimulation was investigated in a multicolor flow cytometric analysis (Fig. 2B). A number of observations were made. First, BCG.HIVA alone induced undetectable, HIV-1-specific CD8+ T-cell responses with the possible exception of degranulating (CD107a/b+) cells producing TNF-α (Fig. 2B, middle dot blot). Nevertheless, the same BCG.HIVA priming increased the MVA.HIVA-elicited frequencies of the H- and subdominant P-specific CD8+ splenocytes producing IFN-γ and degranulating by 5 (P ≤ 0.0004)- and 14 (P ≤ 0.02)-fold, respectively. Although some increase in the H responses after the BCG.p priming was also observed, this increase was not statistically significant. Thus, the BCG.HIVA enhancement of MVA.HIVA responses was not a nonspecific stimulation of bystander T cells by innate anti-BCG responses but rather a HIVA-specific response. Second, of all the regimens tested, the BCG.HIVA priming-MVA.HIVA boosting regimen elicited the highest proportion of HIV-1-specific bifunctional cells in all three analyses presented (Fig. 2B). Finally, no HIVA-induced CD4+-T-cell responses were detected (data not shown). HIV-1-specific cellular responses in MVA.HIVA- and BCG.HIVA-MVA.HIVA-vaccinated mice were also assessed using an in vivo killing assay (Fig. 2C). This assay clearly demonstrated strong in vivo lytic activities against both the H and P epitopes primed by BCG.HIVA, which approximately doubled and tripled, respectively, responses elicited by MVA.HIVA alone.
FIG. 2.
Induction of multifunctional HIV-1-specific CD8+ T cells by the BCG.HIVA priming-MVA.HIVA boosting regimen. (A) Mice were either left unimmunized or immunized with 106 CFU of p.BCG or BCG.HIVA and subsequently given a booster dose of 106 PFU of MVA.HIVA as indicated. (B) Analysis of bifunctional vaccine-elicited CD8+ T cells. The top panels provide examples of dot blots as generated for group 6 and epitope H, and the bottom panels summarize the data obtained for each vaccination group by using the H (top) and P (bottom) epitopes. For the IFN-γ/CD107a/b and TNF-α/CD107a/b analyses, the frequencies of nondegranulating (empty bars) and degranulating (full bars) cells producing cytokine are shown. For the IFN-γ/TNF-α analysis, group average frequencies corresponding to dot blot quadrants I, II, and III are plotted. Data are presented as means ± standard deviations (SD; n, 4 to 5 mice). (C) Results of a 12-h procedure of in vivo killing of syngeneic peptide-pulsed cells from naïve and vaccinated animals. The left panel is an example dot blot showing data for splenocytes reisolated from naïve mice. Right panels show H- and P-specific killing as means ± SD (n = 5).
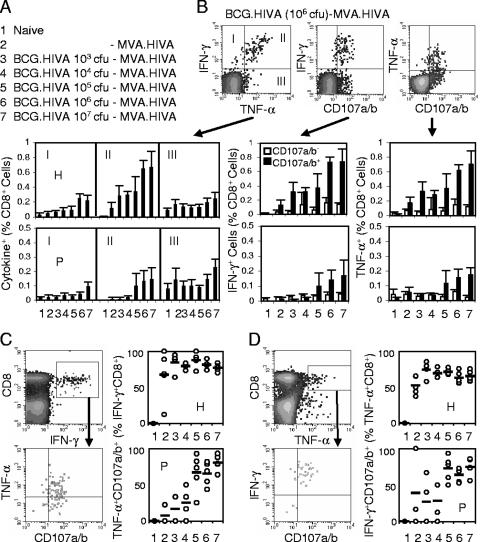
Priming BCG.HIVA dose affects vigor and quality of CD8+-T-cell response to a subdominant epitope.
Next, the effect of the BCG.HIVA dose on the vigor and quality of CD8+-T-cell responses induced by the heterologous BCG.HIVA-MVA.HIVA regimen was investigated. According to a schedule similar to that described above, BALB/c mice were either left unvaccinated or primed with increasing doses of BCG.HIVA ranging from 103 to 107 CFU per animal and given a constant booster dose of MVA.HIVA (Fig. 3A). First, the frequencies of bifunctional IFN-γ+ CD107a/b+, TNF-α+ CD107a/b+, and IFN-γ+ TNF-α+ T cells specific for the H and P epitopes were assessed. We found that for immunodominant epitope H, bifunctional responses steadily increased in magnitude along with the dose and reached a maximum of approximately 0.7% CD8+ splenocytes at 106 CFU of BCG.HIVA. In contrast, for subdominant epitope P, 105 CFU of BCG.HIVA appeared to be the threshold for response induction and the P response increased with doses of up to 107 CFU, reaching approximately 0.2% CD8+ splenocytes (Fig. 3B). Analyzing trifunctional cells revealed that for the immunodominant H epitope, the BCG.HIVA dose did not significantly affect the relatively high fraction of CD8+ IFN-γ+ splenocytes that were also capable of TNF-α production and degranulation. The highest group average proportion of trifunctional cells was 88% CD8+ IFN-γ+ splenocytes, which was detected at 105 CFU of BCG.HIVA (Fig. 3C). In contrast, responses to subdominant epitope P peaked at 107 CFU of BCG.HIVA and reached 80% CD8+ IFN-γ+ splenocytes. Similar results were obtained by the reciprocal analysis, i.e., identifying the fractions of CD8+ TNF-α+ cells that could both produce IFN-γ and degranulate (Fig. 3D). Thus, the priming dose of BCG.HIVA affected both the strength and quality of CD8+-T-cell responses induced by a heterologous rBCG priming-recombinant MVA (rMVA) boosting regimen. A high priming BCG.HIVA dose was particularly important for improving the quality of responses to the subdominant P epitope, i.e., for the breadth of the vaccine-induced T-cell responses.
FIG. 3.
Effect of the BCG.HIVA priming on the induction of HIV-1-specific CD8+ T cells. (A) Immunization groups. Mice were either left unimmunized or primed with increasing doses of BCG.HIVA and given a booster dose of 106 PFU of MVA.HIVA on a schedule similar to that shown in Fig. 2A. (B) The top right panels provide examples of dot blots for the analysis of bifunctional vaccine-elicited CD8+ T cells as generated for group 6 and epitope H. The bottom panels summarize the data obtained for each vaccination group by using the H (top) and P (bottom) epitopes. For the IFN-γ/CD107a/b and TNF-α/CD107a/b analyses, the frequencies of nondegranulating (empty bars) and degranulating (full bars) cells producing cytokine are shown. For the IFN-γ/TNF-α analysis, average frequencies corresponding to dot blot quadrants I, II, and III are plotted. Data are presented as means ± SD (n, 4 to 5 mice). (C and D) Analyses of trifunctional vaccine-elicited T cells. The two left panels indicate the gating. The right panels give the frequencies of trifunctional cells corresponding to the upper right quadrants for individual mice (circles) and groups (bars; values are means for the groups) as obtained with the H (top) and P (bottom) epitopes. Frequencies are expressed as percentages of CD8+ IFN-γ+ (C) and CD8+ TNF-α+ (D) cells.
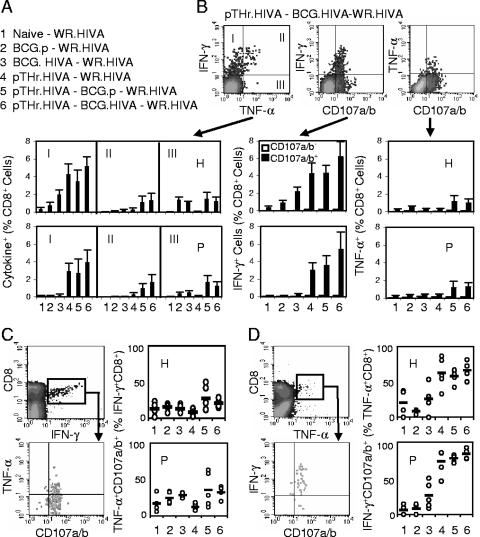
pTHr.HIVA DNA priming-BCG.HIVA boosting protects against a surrogate virus challenge.
The relevant functionality of vaccine-induced responses against viral infection is best tested by an in vivo virus challenge. However, HIV-1 does not replicate in mice, and there is not a challenge relevant for the HIV-1-derived immunogen HIVA in nonhuman primate models other than the infection of chimpanzees with HIV-1, which is prohibitively costly. Thus, a surrogate challenge with WR.HIVA was used as described previously (25). To avoid the induction of antipoxvirus immune responses by MVA.HIVA, we utilized a priming-boosting regimen with heterologous vaccines consisting of a pTHr.HIVA DNA priming dose on day 0 and a BCG.HIVA booster dose on day 33, together with several control groups (Fig. 4A). Mice were challenged on day 150 with WR.HIVA and sacrificed 4 days later, and both the HIVA-specific immune responses and the WR.HIVA loads in the ovaries were determined. While a 4-day infection with WR.HIVA did not elicit any HIVA-specific responses in naïve and BCG.p-vaccinated mice, H- and P-specific responses in mice that had received BCG.HIVA, pTHr.HIVA-BCG.p, and pTHr.HIVA-BCG.HIVA prior to the WR.HIVA challenge were readily detected (Fig. 4B). The highest frequencies of T cells were detected in the pTHr.HIVA-BCG.HIVA-vaccinated WR.HIVA-challenged group, in which the T cells produced mainly IFN-γ and degranulated. The TNF-α-producing cells were lower in frequency than the IFN-γ+ cells, but the majority of both the H- and P-specific TNF-α+ T cells were trifunctional, again suggesting the generation of high-quality T cells (Fig. 4C and D).
FIG. 4.
Immunogenicity of the pTHr.HIVA DNA priming-BCG.HIVA boosting regimen for CD8+ T cells. (A) Mice were left unimmunized or primed with 106 CFU of BCG.HIVA or BCG.p or 100 μg of pTHr.HIVA DNA, groups 5 and 6 were given booster doses of 106 CFU of BCG.p or BCG.HIVA, and then all groups were challenged with 4 × 106 PFU of WR.HIVA. (B) The top panels provide examples of dot blots for the analysis of bifunctional CD8+ T cells as generated for group 6 and epitope H. The bottom panels summarize the data obtained for each vaccination group by using the H (top) and P (bottom) epitopes. For the IFN-γ/CD107a/b and TNF-α/CD107a/b analyses, the frequencies of nondegranulating (empty bars) and degranulating (full bars) cells producing cytokine are shown. For the IFN-γ/TNF-α analysis, average frequencies corresponding to dot blot quadrants I, II, and III are plotted. Data are presented as means ± SD (n, 4 to 5 mice). (C and D) Analyses of trifunctional vaccine-elicited T cells. The two left panels indicate the gating. The right panels give the frequencies of trifunctional cells corresponding to the upper right quadrants for individual mice (circles) and groups (bars; values are means for the groups) as obtained with the H (top) and P (bottom) epitopes. Frequencies are expressed as percentages of CD8+ IFN-γ+ (C) and CD8+ TNF-α+ (D) cells.
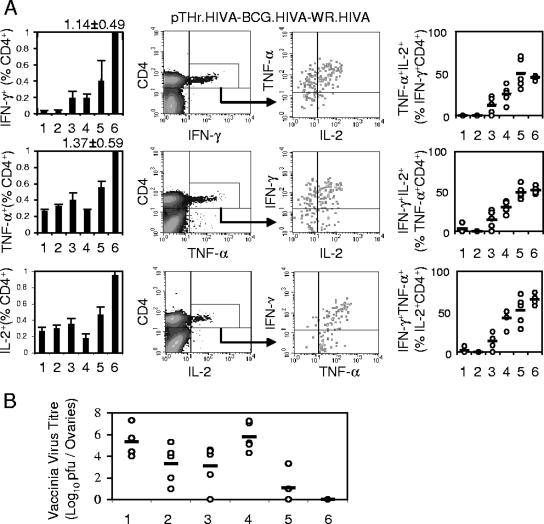
In this series of experiments, CD4+ responses to three known HIVA epitopes in all groups primed with the HIVA immunogen were also detected. Significantly higher frequencies of bifunctional splenocytes were elicited by the pTHr.HIVA DNA-BCG.HIVA immunization and the WR.HIVA challenge than by the other immunization-challenge regimens (Fig. 5A, left panels). Interestingly, for CD4+ cells, both BCG.HIVA and BCG.p boosting of pTHr.HIVA-primed responses achieved the highest quality of CD4+ IFN-γ+ TNF-α+ IL-2+ splenocytes observed among all treatment groups (Fig. 5A, right panels), suggesting, at least for the CD4+-T-cell response, an augmenting role for the BCG-stimulated innate response.
FIG. 5.
Induction of high-quality HIV-1-specific CD4+ T cells and complete protection against surrogate virus challenge. The mice and the treatment groups (1 through 6) were the same as those described in the legend to Fig. 4A. (A) The leftmost panels summarize the data obtained for each cytokine and vaccination group. Data are presented as means ± SD (n, 4 to 5 mice). The middle panels demonstrate the gating for IFN-γ-, TNF-α-, and IL-2-producing CD4+ T cells as generated by group 6 for a cocktail of three MHC class II epitopes. The rightmost panels give the upper-right-quadrant data for trifunctional HIV-1-specific CD4+ T cells from individual mice (circles) and groups (bars). Data are presented as means ± SD (n, 4 to 5 mice). (B) Mice were either left naïve (1) or vaccinated with BCG.p (2), BCG.HIVA (3), pTHr.HIVA DNA (4), pTHr.HIVA DNA and BCG.p (5), or pTHr.HIVA and BCG.HIVA (6) and challenged with WR.HIVA. The WR.HIVA loads in ovaries were determined 4 days later. Data for individual mice (circles) and group means (bars; n, 4 to 5 mice) are shown.
Indeed, a similar synergism between the HIVA-specific and nonspecific responses in the control of WR.HIVA replication in the ovaries was observed (Fig. 5B). Compared to no immunization, immunization with BCG.HIVA alone, but also that with BCG.p alone, decreased the WR.HIVA titer in ovaries by 2 orders of magnitude, thus strongly implicating HIVA-nonspecific protective mechanisms. While pTHr.HIVA DNA on its own provided no protection, pTHr.HIVA priming improved the BCG.p-generated control of WR.HIVA replication by a further 2 orders of magnitude compared to that by BCG.p alone. Finally, a complete WR.HIVA clearance from ovaries of five out of five mice was achieved by the combination of HIVA-specific and nonspecific responses following the pTHr.HIVA priming-BCG.HIVA boosting regimen. Although we used a surrogate virus challenge, the quality of the BCG-elicited responses and the complete protection are encouraging for further development of the BCG.HIVA vaccine.
Lysine auxotroph BCG.HIVA gives a level of protection against M. tuberculosis challenge similar to that given by the presently used BCG vaccine.
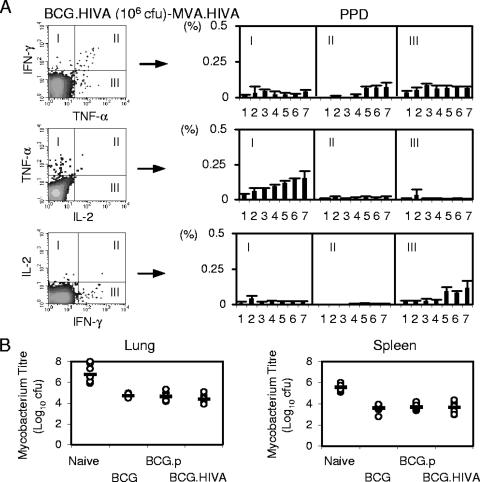
We also assessed the immunological response to PPD following the vaccine regimen consisting of BCG.HIVA dose response priming and a constant MVA.HIVA booster dose as described in the legend to Fig. 3A and found PPD-specific cells producing mainly IFN-γ and TNF-α at the higher BCG.HIVA doses (Fig. 6A). Upon an M. tuberculosis aerosol challenge, the present BCG vaccine (BCG wild-type 1173 p2), BCG.p, and BCG.HIVA provided equivalent levels of protection as demonstrated by a 2-order-of-magnitude decrease in M. tuberculosis loads in both lungs and spleens (Fig. 6B). Thus, at least this murine experiment suggests that the safer lysine auxotroph BCG.HIVA can replace the neonatal BCG vaccine against M. tuberculosis without losing the benefits of the BCG vaccination and that, therefore, its use will not compromise or interfere with any other scheduled pediatric vaccination.
FIG. 6.
Immune responses and protection against M. tuberculosis challenge by BCG.HIVA. (A) The mice and the treatment groups were the same as those described in the legend to Fig. 3A (immunized mice were primed with increasing doses of BCG.HIVA). Left panels show examples of bifunctional analyses using PPD as an antigenic stimulus. On the right, data corresponding to dot blot quadrants I, II, and III for each immunization group are shown as means ± SD (n, 4 to 5 mice). (B) Mice were left naïve or immunized subcutaneously in their left hind legs with the presently used BCG vaccine, a parental BCG, or lysine auxotrophic BCG.HIVA and challenged with inhaled M. tuberculosis. M. tuberculosis loads in lungs (left) and spleens (right) were determined 4 weeks later. Data for individual mice (circles) and geometric means for each group (n, 7 to 9 mice) are shown.
DISCUSSION
Here, we have engineered a novel candidate vaccine for both HIV-1 and M. tuberculosis infection that is vectored by a lysine auxotroph of M. bovis BCG (the Pasteur ΔlysA5::res strain), which expresses the HIV-1 clade A-derived immunogen HIVA. We demonstrated with BALB/c mice that BCG.HIVA can both prime novel and boost preexisting HIV-1-specific cellular immune responses, which are mediated by CD4+ and CD8+ T cells of high quality as judged by their long-term persistence and capacity to proliferate and produce multiple cytokines upon antigenic reexposure. Furthermore, sequential heterologous immunization involving pTHr.HIVA DNA and BCG.HIVA conferred complete protection of mice against a surrogate challenge with vaccinia virus expressing the HIVA immunogen, while BCG.HIVA alone provided a level of protection against M. tuberculosis challenge comparable to that achieved by a presently used BCG vaccine strain.
Here, we constructed rBCG stably expressing the immunogen HIVA from both the episomal pJH222.HIVA and the integrative pJH223.HIVA plasmids. To express the HIVA protein, both plasmids used the α-antigen promoter and a 19-kDa lipoprotein signal sequence, which were found to be optimal for antigen secretion and immunogenicity (10), perhaps through the acylation of the signal sequence, which was important for entering into the MHC class I presentation and interaction with Toll-like receptor 2 (36). Based on higher levels of HIVA expression by pJH222.HIVA, we chose to further characterize the episomal construct. The same plasmid backbones in a strain of M. smegmatis, a rapidly replicating mycobacterium expressing the HIV-1 gp120 envelope glycoprotein, were recently compared (8, 53). Despite higher levels of gp120 expression from the episomal plasmid than from the integrated plasmid, the levels of immunogenicity in BALB/c mice were identical.
To date, BCG-vectored vaccines against a number of infectious agents, such as Leishmania species, Borrelia burgdorferi, Streptococcus pneumoniae, Bordetella pertussis, malaria parasites, cottontail rabbit papillomavirus, measles virus, and indeed, HIV and simian immunodeficiency virus (SIV), have been successfully constructed (23). Many of these vaccines show immunogenicity and protection in murine models, while some are also immunogenic in nonhuman primates (3, 51, 53). The only volunteer study conducted to date suggested that rBCG is only moderately efficient in eliciting immune responses to passenger proteins in humans, but this study did not use a heterologous priming-boosting regimen (13). In fact, only a small fraction of the above-mentioned animal experiments used rBCG strains in heterologous priming-boosting regimens. However, when BCG-SIVGag-induced responses were boosted by defective poxvirus-SIVGag, partial protection against a pathogenic simian-human immunodeficiency virus challenge was obtained (3). Here, the inclusion of BCG.HIVA in heterologous-vaccine protocols consistently improved the frequency, quality, and durability of the generated HIV-1-specific responses. This improvement was reflected by the detection of the highest fractions of multifunctional HIVA-specific T cells in the mice that received BCG.HIVA in a heterologous-vaccine regimen versus the other regimens tested; these responses were present over 14 weeks after rBCG administration. Also, the frequency and functionality of CD8+ T cells specific for the subdominant epitope P were significantly elevated by the inclusion of rBCG, suggesting that BCG may be a useful vaccine vector for the induction of specificity, which may be overshadowed by immunodominance under other circumstances, although this possibility will need to be confirmed for other epitopes and immunogens. Detectable HIV-1-specific CD4+ T-cell responses were found only in the WR.HIVA challenge experiment but not following immunization with BCG.HIVA alone or BCG.HIVA-MVA.HIVA. These results may simply reflect the generally lower frequencies of elicited CD4+ T cells than of their CD8+ counterparts or the fact that in the former experiment, the CD4+ T-cell responses were also boosted by the vigorously replicating WR.HIVA. Nevertheless, the ability to elicit strong, multispecific, and multifunctional CD8+ T-cell responses is likely to be a critical feature for the success of a candidate HIV-1 vaccine.
The BCG.HIVA vaccine candidate in this study was vectored by a lysine auxotroph of BCG that carried an E. coli-mycobacterial shuttle plasmid with a lysine A-complementing gene. This inclusion theoretically increases the plasmid stability in vivo, prevents genetic rearrangements of the heterologous genes, and adds an additional safety feature to the vaccine. Here, we demonstrated that BCG.HIVA was as efficient in protecting against M. tuberculosis as the presently used BCG wild-type 1173 p2 vaccine in a mouse model. Also, this is the first report of an HIV-1 vaccine vectored by an auxotroph of BCG and the first study using an rBCG priming-rMVA boosting protocol as a potential anti-HIV-1 regimen, which combines the benefits of both of these promising vaccine vectors.
Protection against M. tuberculosis challenge offers the possibility to use BCG.HIVA in neonates as a substitute for the BCG vaccine in the first month of their lives (35) and, thus, for BCG.HIVA to serve as a dual vaccine against HIV-1 and tuberculosis. The relevance of the “artificial” consensus clade A HIVA immunogen to the viruses presently circulating in Nairobi has been demonstrated with HIV-1-infected and HIV-1-exposed uninfected infants, of whom 38 to 52% made T-cell responses that recognized the Gag and polyepitope domains of HIVA (46). The high frequency of exposed uninfected infants whose T cells recognize HIVA (52%) is encouraging, as it suggests that exposure to virus through breast milk does not inevitably lead to infection and can prime or boost T-cell immunity. Thus, the T-cell responses in these vaccinated infants may exceed those to the same vaccines in HIV-1-unexposed adults. HIVA's usefulness was also shown by boosting HIV-1-specific responses in patients on highly active antiretroviral therapy who were infected with diverse HIV-1 clades (11, 12, 37). Therefore, we propose to further develop this rBCG strain as a pediatric vaccine to protect children born to HIV-1-positive mothers against becoming infected with HIV-1 through breastfeeding. The regimen would consist of BCG.HIVA priming at birth, followed by boosting with MVA.HIVA at 1 or 2 months of age along with the babies' scheduled Extended Programme of Immunization. This booster vaccine could be combined with MVA.85A to strengthen the tuberculosis protection (34). In addition, the BCG.HIVA-primed responses can be also expanded by natural exposure to HIV-1 through breast milk. The evaluation of the safety and immunogenicity of this regimen in newborn nonhuman primates is the next aim. However, for a more relevant challenge with SIV, an equivalent immunogen derived from SIV inner proteins will have to be constructed.
Acknowledgments
This work was supported by MRC UK, HIVACAT, the Foundation for Research and Prevention of AIDS in Spain (FIPSE 36338/02), Fundación Mutua Madrileña de Automóviles (second call for proposals), and Fundacio BCN SIDA 2002. H.M. is a Wellcome Trust senior clinical fellow, C.S. is funded by AFTBVAC (the European Commission, the Fifth Framework Programme), and D.T. is supported by the A*Star Graduate Academy of Singapore. T.H. and H.S. are The Jenner Institute Investigators.
Footnotes
Published ahead of print on 27 June 2007.
REFERENCES
- 1.Adkins, B., C. Leclerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553-564. [DOI] [PubMed] [Google Scholar]
- 2.Alter, G., J. M. Malenfant, and M. Altfeld. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 294:15-22. [DOI] [PubMed] [Google Scholar]
- 3.Ami, Y., Y. Izumi, K. Matsuo, K. Someya, M. Kanekiyo, S. Horibata, N. Yoshino, K. Sakai, K. Shinohara, S. Matsumoto, T. Yamada, S. Yamazaki, N. Yamamoto, and M. Honda. 2005. Priming-boosting vaccination with recombinant Mycobacterium bovis bacillus Calmette-Guerin and a nonreplicating vaccinia virus recombinant leads to long-lasting and effective immunity. J. Virol. 79:12871-12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson, G. E., and P. M. Sharp. 1996. Codon usage in the Mycobacterium tuberculosis complex. Microbiology 142:915-925. [DOI] [PubMed] [Google Scholar]
- 5.Andre, S., B. Seed, J. Eberle, W. Schraut, A. Bultmann, and J. Haas. 1998. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J. Virol. 72:1497-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. [DOI] [PubMed] [Google Scholar]
- 7.Buseyne, F., M. Burgard, J. P. Teglas, E. Bui, C. Rouzioux, M. J. Mayaux, S. Blanche, and Y. Riviere. 1998. Early HIV-specific cytotoxic T lymphocytes and disease progression in children born to HIV-infected mothers. AIDS Res. Hum. Retrovir. 14:1435-1444. [DOI] [PubMed] [Google Scholar]
- 8.Cayabyab, M. J., A. H. Hovav, T. Hsu, G. R. Krivulka, M. A. Lifton, D. A. Gorgone, G. J. Fennelly, B. F. Haynes, W. R. Jacobs, Jr., and N. L. Letvin. 2006. Generation of CD8+ T-cell responses by a recombinant nonpathogenic Mycobacterium smegmatis vaccine vector expressing human immunodeficiency virus type 1 Env. J. Virol. 80:1645-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 10.Dennehy, M., and A. L. Williamson. 2005. Factors influencing the immune response to foreign antigen expressed in recombinant BCG vaccines. Vaccine 23:1209-1224. [DOI] [PubMed] [Google Scholar]
- 11.Dorrell, L., H. Yang, A. K. Iversen, C. Conlon, A. Suttill, M. Lancaster, T. Dong, I. Cebere, A. Edwards, S. Rowland-Jones, T. Hanke, and A. J. McMichael. 2005. Therapeutic immunization of highly active antiretroviral therapy-treated HIV-1-infected patients: safety and immunogenicity of an HIV-1 gag/poly-epitope DNA vaccine. AIDS 19:1321-1323. [DOI] [PubMed] [Google Scholar]
- 12.Dorrell, L., H. Yang, B. Ondondo, T. Dong, K. di Gleria, A. Suttill, C. Conlon, D. Brown, P. Williams, P. Bowness, N. Goonetilleke, T. Rostron, S. Rowland-Jones, T. Hanke, and A. J. McMichael. 2006. Expansion and diversification of virus-specific T cells following immunization of human immunodeficiency virus type 1 (HIV-1)-infected individuals with a recombinant modified vaccinia virus Ankara/HIV-1 Gag vaccine. J. Virol. 80:4705-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edelman, R., K. Palmer, K. G. Russ, H. P. Secrest, J. A. Becker, S. A. Bodison, J. G. Perry, A. R. Sills, A. G. Barbour, C. J. Luke, M. S. Hanson, C. K. Stover, J. E. Burlein, G. P. Bansal, E. M. Connor, and S. Koenig. 1999. Safety and immunogenicity of recombinant bacille Calmette-Guerin (rBCG) expressing Borrelia burgdorferi outer-surface protein A (OspA) lipoprotein in adult volunteers: a candidate Lyme disease vaccine. Vaccine 17:904-919. [DOI] [PubMed] [Google Scholar]
- 14.Gheorghiu, M., P. H. Lagrange, and C. Fillastre. 1988. The stability and immunogenicity of a dispersed-grown freeze-dried Pasteur BCG vaccine. J. Biol. Stand. 16:15-26. [DOI] [PubMed] [Google Scholar]
- 15.Goonetilleke, N., S. Moore, L. Dally, N. Winstone, N. Mahmoud, I. Cebere, S. Pinheiro, G. Gillespie, D. Brown, V. Loach, J. Roberts, A. Guimaraes-Walker, P. Hayes, K. Loughran, C. Smith, P. Fast, L. Dorrell, T. Hanke, and A. McMichael. 2006. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J. Virol. 80:4717-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goulder, P. J., P. Jeena, G. Tudor-Williams, and S. Burchett. 2001. Paediatric HIV infection: correlates of protective immunity and global perspectives in prevention and management. Br. Med. Bull. 58:89-108. [DOI] [PubMed] [Google Scholar]
- 17.Hanke, T., and A. J. McMichael. 2000. Design and construction of an experimental HIV-1 vaccine for a year-2000 clinical trial in Kenya. Nat. Med. 6:951-955. [DOI] [PubMed] [Google Scholar]
- 18.Hanke, T., A. J. McMichael, and L. Dorrell. 2007. Clinical experience with plasmid DNA- and modified vaccinia vaccine Ankara (MVA)-vectored HIV-1 clade A vaccine inducing T cells. J. Gen. Virol. 88:1-12. [DOI] [PubMed] [Google Scholar]
- 19.Hermans, I. F., J. D. Silk, J. Yang, M. J. Palmowski, U. Gileadi, C. McCarthy, M. Salio, F. Ronchese, and V. Cerundolo. 2004. The VITAL assay: a versatile fluorometric technique for assessing CTL- and NKT-mediated cytotoxicity against multiple targets in vitro and in vivo. J. Immunol. Methods 285:25-40. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz, M. A., and G. Harth. 2003. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect. Immun. 71:1672-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussey, G. D., M. L. Watkins, E. A. Goddard, S. Gottschalk, E. J. Hughes, K. Iloni, M. A. Kibel, and S. R. Ress. 2002. Neonatal mycobacterial specific cytotoxic T-lymphocyte and cytokine profiles in response to distinct BCG vaccination strategies. Immunology 105:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Im, E.-J., and T. Hanke. 2007. Pre-clinical evaluation of candidate HIV-1 vaccines in inbred strains and an outbred stock of mice. AIDS Res. Hum. Retrovir. 33:857-862. [DOI] [PubMed] [Google Scholar]
- 23.Joseph, J., N. Saubi, E. Pezzat, and J. M. Gatell. 2006. Progress towards an HIV vaccine based on recombinant bacillus Calmette-Guerin: failures and challenges. Exp. Rev. Vaccines 5:827-838. [DOI] [PubMed] [Google Scholar]
- 24.Larke, N., E.-J. Im, R. Wagner, C. Williamson, A.-L. Williamson, A. J. McMichael, and T. Hanke. 2007. Combined single-clade candidate HIV-1 vaccines induce T cell responses limited by multiple forms of in vivo immune interference. Eur. J. Immunol. 37:566-577. [DOI] [PubMed] [Google Scholar]
- 25.Larke, N., A. Murphy, C. Wirblich, D. Teoh, M. J. Estcourt, A. J. McMichael, P. Roy, and T. Hanke. 2005. Induction of human immunodeficiency virus type 1-specific T cells by a bluetongue virus tubule-vectored vaccine prime-recombinant modified virus Ankara boost regimen. J. Virol. 79:14822-14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotte, A., O. Wasz-Hockert, N. Poisson, N. Dumitrescu, M. Verron, and E. Couvet. 1984. BCG complications. Estimates of the risks among vaccinated subjects and statistical analysis of their main characteristics. Adv. Tuberc. Res. 21:107-193. [PubMed] [Google Scholar]
- 27.Luzuriaga, K., D. Holmes, A. Hereema, J. Wong, D. L. Panicali, and J. L. Sullivan. 1995. HIV-1-specific cytotoxic T lymphocyte responses in the first year of life. J. Immunol. 154:433-443. [PubMed] [Google Scholar]
- 28.Marchant, A., V. Appay, M. Van Der Sande, N. Dulphy, C. Liesnard, M. Kidd, S. Kaye, O. Ojuola, G. M. Gillespie, A. L. Vargas Cuero, V. Cerundolo, M. Callan, K. P. McAdam, S. L. Rowland-Jones, C. Donner, A. J. McMichael, and H. Whittle. 2003. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J. Clin. Investig. 111:1747-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchant, A., T. Goetghebuer, M. O. Ota, I. Wolfe, S. J. Ceesay, D. De Groote, T. Corrah, S. Bennett, J. Wheeler, K. Huygen, P. Aaby, K. P. McAdam, and M. J. Newport. 1999. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J. Immunol. 163:2249-2255. [PubMed] [Google Scholar]
- 30.Marchant, A., and M. Goldman. 2005. T cell-mediated immune responses in human newborns: ready to learn? Clin. Exp. Immunol. 141:10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marchant, A., and M. Newport. 2000. Prevention of infectious diseases by neonatal and early infantile immunization: prospects for the new millennium. Curr. Opin. Infect. Dis. 13:241-246. [DOI] [PubMed] [Google Scholar]
- 32.Mbori-Ngacha, D., R. Nduati, G. John, M. Reilly, B. Richardson, A. Mwatha, J. Ndinya-Achola, J. Bwayo, and J. Kreiss. 2001. Morbidity and mortality in breastfed and formula-fed infants of HIV-1-infected women: a randomized clinical trial. JAMA 286:2413-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMichael, A. J. 2006. HIV vaccines. Annu. Rev. Immunol. 24:227-255. [DOI] [PubMed] [Google Scholar]
- 34.McShane, H., A. A. Pathan, C. R. Sander, S. M. Keating, S. C. Gilbert, K. Huygen, H. A. Fletcher, and A. V. Hill. 2004. Recombinant modified vaccinia virus Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 10:1240-1244. [DOI] [PubMed] [Google Scholar]
- 35.Moss, W. J., C. J. Clements, and N. A. Halsey. 2003. Immunization of children at risk of infection with human immunodeficiency virus. Bull. W. H. O. 81:61-70. [PMC free article] [PubMed] [Google Scholar]
- 36.Neyrolles, O., K. Gould, M. P. Gares, S. Brett, R. Janssen, P. O'Gaora, J. L. Herrmann, M. C. Prevost, E. Perret, J. E. Thole, and D. Young. 2001. Lipoprotein access to MHC class I presentation during infection of murine macrophages with live mycobacteria. J. Immunol. 166:447-457. [DOI] [PubMed] [Google Scholar]
- 37.Ondondo, B., H. Yang, T. Dong, K. di Gleria, A. Suttill, C. Conlon, D. Brown, P. Williams, P. Bowness, S. L. Rowland-Jones, T. Hanke, A. J. McMichael, and L. Dorrell. 2006. Immunisation with recombinant modified vaccinia virus Ankara expressing HIV-1 gag in HIV-1-infected subjects stimulates broad functional CD4+ T cell responses. Eur. J. Immunol. 36:2585-2594. [DOI] [PubMed] [Google Scholar]
- 38.Ota, M. O., J. Vekemans, S. E. Schlegel-Haueter, K. Fielding, M. Sanneh, M. Kidd, M. J. Newport, P. Aaby, H. Whittle, P. H. Lambert, K. P. McAdam, C. A. Siegrist, and A. Marchant. 2002. Influence of Mycobacterium bovis bacillus Calmette-Guerin on antibody and cytokine responses to human neonatal vaccination. J. Immunol. 168:919-925. [DOI] [PubMed] [Google Scholar]
- 39.Pavelka, M. S., Jr., and W. R. Jacobs, Jr. 1999. Comparison of the construction of unmarked deletion mutations in Mycobacterium smegmatis, Mycobacterium bovis bacillus Calmette-Guerin, and Mycobacterium tuberculosis H37Rv by allelic exchange. J. Bacteriol. 181:4780-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillpotts, R. J., T. J. Brooks, and C. S. Cox. 1997. A simple device for the exposure of animals to infectious microorganisms by the airborne route. Epidemiol. Infect. 118:71-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pikora, C. A., J. L. Sullivan, D. Panicali, and K. Luzuriaga. 1997. Early HIV-1 envelope-specific cytotoxic T lymphocyte responses in vertically infected infants. J. Exp. Med. 185:1153-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raviglione, M. C., D. E. Snider, Jr., and A. Kochi. 1995. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 273:220-226. [PubMed] [Google Scholar]
- 43.Rousseau, C. M., R. W. Nduati, B. A. Richardson, M. S. Steele, G. C. John-Stewart, D. A. Mbori-Ngacha, J. K. Kreiss, and J. Overbaugh. 2003. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J. Infect. Dis. 187:741-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. S. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 45.Siegrist, C. A. 2001. Neonatal and early life vaccinology. Vaccine 19:3331-3346. [DOI] [PubMed] [Google Scholar]
- 46.Slyker, J. A., B. L. Lohman, D. A. Mbori-Ngacha, M. Reilly, E. G. Wee, T. Dong, A. J. McMichael, S. L. Rowland-Jones, T. Hanke, and G. John-Stewart. 2005. Modified vaccinia Ankara expressing HIVA antigen stimulates HIV-1-specific CD8 T cells in ELISpot assays of HIV-1 exposed infants. Vaccine 23:4711-4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stover, C. K., G. P. Bansal, M. S. Hanson, J. E. Burlein, S. R. Palaszynski, J. F. Young, S. Koenig, D. B. Young, A. Sadziene, and A. G. Barbour. 1993. Protective immunity elicited by recombinant bacille Calmette-Guerin (BCG) expressing outer surface protein A (OspA) lipoprotein: a candidate Lyme disease vaccine. J. Exp. Med. 178:197-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takahashi, H., J. Cohen, A. Hosmalin, K. B. Cease, R. Houghten, J. L. Cornette, C. DeLisi, B. Moss, R. N. Germain, and J. A. Berzofsky. 1988. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility molecule-restricted murine cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 85:3105-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vekemans, J., A. Amedei, M. O. Ota, M. M. D'Elios, T. Goetghebuer, J. Ismaili, M. J. Newport, G. Del Prete, M. Goldman, K. P. McAdam, and A. Marchant. 2001. Neonatal bacillus Calmette-Guerin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur. J. Immunol. 31:1531-1535. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization. 2001. Outbreak news. Wkly. Epidemiol. Rec. 76:33-40.11218337 [Google Scholar]
- 51.Yasutomi, Y., S. Koenig, S. S. Haun, C. K. Stover, R. K. Jackson, P. Conard, A. J. Conley, E. A. Emini, T. R. Fuerst, and N. L. Letvin. 1993. Immunization with recombinant BCG-SIV elicits SIV-specific cytotoxic T lymphocytes in rhesus monkeys. J. Immunol. 150:3101-3107. [PubMed] [Google Scholar]
- 52.Young, D., and C. Dye. 2006. The development and impact of tuberculosis vaccines. Cell 124:683-687. [DOI] [PubMed] [Google Scholar]
- 53.Yu, J.-S., J. W. Peacock, S. Vanleeuwen, T. Hsu, W. R. Jacobs, Jr., M. J. Cayabyab, N. L. Letvin, R. Fronthingham, H. F. Staats, H. X. Liao, and B. F. Haynes. 2006. Generation of mucosal anti-human immunodeficiency virus type 1 T-cell responses by recombinant Mycobacterium smegmatis. Clin. Vaccine Immunol. 13:1204-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]