Abstract
Dendritic cells (DCs) potently stimulate the transmission of human immunodeficiency virus type 1 (HIV-1) to CD4+ T cells. Immature DCs (iDCs) located in submucosal tissues can capture HIV-1 and migrate to lymphoid tissues, where they become mature DCs (mDCs) for effective antigen presentation. DC maturation promotes HIV-1 transmission; however, the underlying mechanisms remain unclear. Here we have compared monocyte-derived iDCs and mDCs for their efficiencies and mechanisms of HIV-1 transmission. We have found that mDCs significantly facilitate HIV-1 endocytosis and efficiently concentrate HIV-1 at virological synapses, which contributes to mDC-enhanced viral transmission, at least in part. mDCs were more efficient than iDCs in transferring HIV-1 to various types of target cells independently of C-type lectins, which partially accounted for iDC-mediated HIV-1 transmission. Efficient HIV-1 trans-infection mediated by iDCs and mDCs required contact between DCs and target cells. Moreover, rapid HIV-1 degradation occurred in both iDCs and mDCs, which correlated with the lack of HIV-1 retention-mediated long-term viral transmission. Our results provide new insights into the mechanisms underlying DC-mediated HIV-1 transmission, suggesting that HIV-1 exploits mDCs to facilitate its dissemination within lymphoid tissues.
Dendritic cells (DCs) perform an essential role in the induction and regulation of the adaptive immune response (4). In opposition to the immune function of DCs presenting processed antigens, human immunodeficiency virus type 1 (HIV-1 [referred to subsequently as HIV]) hijacks DCs to promote viral spread. DCs are proposed to be among the first cells that encounter HIV at the mucosa and to play an important role in HIV infection and dissemination (54). After capture or uptake of HIV, immature DCs (iDCs) located in submucosal tissues migrate to lymphoid tissues and become mature DCs (mDCs) to potently present antigens to T cells. Interestingly, the transmission efficiency of HIV is enhanced by DC maturation (11, 23, 27, 34, 42, 50), suggesting that mDCs efficiently facilitate HIV transfer to activated CD4+ T cells in lymphoid tissues. Increased mDC-T-cell interactions may augment HIV transfer to CD4+ T cells (34, 42); however, the mechanisms underlying mDC-enhanced HIV transmission remain elusive.
DCs can transmit HIV to CD4+ T cells through trans-infection and cis-infection (reviewed in reference 54). Trans-infection mediated by DCs can occur by two pathways: HIV transmission across the synaptic junctions or infectious/virological synapses (2, 34, 48) and HIV transmission by immature monocyte-derived DCs via an exocytic pathway that involves HIV-associated exosomes (52). HIV cis-infection of DCs results in de novo viral production and long-term transmission of HIV, although viral replication in DCs is less efficient than that in CD4+ T cells (8, 23, 31, 38, 48). These mechanisms of HIV transmission may coexist in vivo and contribute to viral dissemination; however, whether mDC-enhanced HIV transmission involves these pathways is unclear.
Initial observations suggested that a C-type lectin, DC-SIGN (for “DC-specific intercellular adhesion molecule 3 [ICAM-3]-grabbing nonintegrin”), facilitates DC-mediated HIV trans-infection (22). Subsequent studies have indicated that DCs also have DC-SIGN-independent mechanisms of HIV trans-infection of CD4+ T cells (5, 6, 24-26, 46, 49, 53, 57). Other C-type lectin molecules may be involved in DC-SIGN-independent HIV transmission mediated by DCs (54). However, whether mDC-enhanced HIV transmission is dependent on C-type lectins remains to be examined.
Previous studies indicated that DC contact with CD4+ T cells is required for efficient HIV trans-infection (34, 42, 47). HIV trafficking has been suggested to be important for DC-mediated viral transmission (21, 29, 34, 52). Immature DCs can internalize HIV to late endosomal compartments or multivesicular bodies (52), while mDCs sequester internalized HIV into an endocytic compartment that is distinct from the conventional multivesicular bodies (21). In contrast, a recent study suggests that DC-mediated HIV trans-infection of CD4+ T cells primarily originates from virions bound on DC surfaces (11). The discordance of these studies may result from different approaches to DC generation, various stimuli of DC maturation, and different HIV reporter systems. Nevertheless, the role of HIV trafficking in iDC- and mDC-mediated viral transmission remains to be defined.
Here we report functionally distinct HIV trans-infection of CD4+ T cells mediated by iDCs and mDCs. mDCs were more efficient than iDCs in transmitting HIV to various types of target cells independently of C-type lectins, which partially accounts for iDC-mediated HIV transmission. Compared with iDCs, mDCs significantly enhanced HIV endocytosis and efficiently concentrated HIV at virological synapses, which likely play a role in promoting viral transmission to CD4+ T cells. Our results suggest that HIV exploits mDCs to facilitate its dissemination within lymphoid tissues.
MATERIALS AND METHODS
Cell culture.
Peripheral blood lymphocytes (PBLs) and CD14+ monocytes were isolated from buffy coat units of healthy donors (provided by the Blood Center of Wisconsin, Milwaukee, WI) as previously described (49, 53). iDCs were generated from purified monocytes treated with granulocyte-macrophage colony-stimulating factor and interleukin 4 (IL-4) for 6 days, as described previously (57). mDCs were generated by adding 10 ng/ml of lipopolysaccharide (LPS) (Escherichia coli strain O55:B5; Sigma-Aldrich) to iDCs and cultured for an additional 2 days. The monocyte-differentiated iDCs were more than 98.5% pure by DC-SIGN, HLA-DR, CD11b, and CD11c staining but were negative for CD3 and CD14. PBLs were activated with 5 μg/ml of phytohemagglutinin (Sigma-Aldrich) and cultured in the presence of 20 IU/ml of IL-2 (NIH AIDS Research and Reference Reagent Program), as described previously (49). The human embryonic kidney cell line HEK293T, the CD4+ T-cell line Hut/CCR5, the human B-cell line Raji/DC-SIGN, and the HIV indicator cell line GHOST/R5 (kind gifts from Vineet KewalRamani, National Cancer Institute, Frederick, MD) have been described previously (49, 57).
Flow cytometry.
DCs (1 × 105) were stained with specific monoclonal antibodies (MAbs) or isotype-matched immunoglobulin G (IgG) controls, as previously described (55). Phycoerythrin-conjugated mouse anti-human MAbs (BD Biosciences [unless specified]) against the following molecules (clone numbers are given in parentheses) were used for staining: DC-SIGN (120507; R&D Systems), CD3 (HIT3a), CD11b (VIM12), CD11c (BU15), CD14 (TÜK4), HLA-DR (TÜ 36), CD80 (L307.4), CD86 (2331), and IgG isotype control MAbs. When indicated, DCs were treated with 0.25 mg/ml of trypsin (without EDTA) (Invitrogen) at room temperature for 4 min or with 0.25 mg/ml of pronase E (P6911; Sigma-Aldrich) on ice for 10 min. DCs were subsequently neutralized with culture medium and washed before staining for DC-SIGN. Stained cells were analyzed with a FACSCalibur flow cytometer (Becton Dickinson).
HIV stocks.
Single-cycle infectious HIV stocks were generated by calcium phosphate cotransfections of HEK293T cells with pLai3ΔenvLuc2 (58) (a kind gift from Michael Emerman, Fred Hutchinson Cancer Research Center) and expression plasmids for HIV envelope protein (Env) of JRFL (R5-tropic) or HXB2 (X4-tropic), as described previously (57). The infectivity of the virus stocks was evaluated by limiting dilution on GHOST/R5 cells (57). Aldrithiol-2 (AT-2)-inactivated R5-tropic HIV (Bal/Supt1-CCR5 cl30) was a kind gift from Jeffery Lifson (AIDS Vaccine Program, SAIC, Frederick, MD).
HIV binding and internalization assays.
iDCs and mDCs (7.5 × 104) were incubated separately with infectious HIV-Luc/JRFL or AT-2-inactivated HIV (30 ng of p24) for 2 h at 37°C or 4°C. Cells were then washed intensively and lysed for HIV Gag p24 quantification with an enzyme-linked immunosorbent assay kit (PerkinElmer), as previously described (49). When indicated, HIV-pulsed DCs were treated with 0.25 mg/ml of trypsin (without EDTA) at room temperature for 4 min; cells were subsequently neutralized with culture medium and washed before lysis for HIV p24 quantification.
HIV transmission and infection assays.
HIV transmission and direct infection assays using luciferase viruses were performed as previously described (57). Cell lysates were analyzed for luciferase activity with a commercially available kit (Promega). When indicated, iDCs and mDCs were preincubated with either 10 μg/ml of anti-DC-SIGN (cocktail of clones 120518, 120526, and 120612 [R&D Systems]) at room temperature or 20 μg/ml of mannan (Sigma-Aldrich) at 37°C for 30 min prior to HIV incubation, as described previously (57). Transwell culture plates (Costar) with inserts of polycarbonate membranes (pore size, 3 μm) were used to separate donor cells from target cells as described previously (56). When indicated, HIV-pulsed DCs were treated either with 0.25 mg/ml of trypsin (without EDTA) at room temperature for 4 min or with pronase E at various concentrations on ice for 10 min. Cells were subsequently neutralized with culture medium and washed before coculturing with Hut/CCR5 cells.
For HIV transmission assays using trafficking inhibitors, DCs (3 × 105) were incubated separately with 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid acetoxymethyl ester (BAPTA-AM) (25 μM), ammonium chloride (NH4Cl) (10 mM), monensin sodium (30 μM), brefeldin A (3.6 μM), and epoxomicin (0.2 μM) (all inhibitors were purchased from Sigma-Aldrich) at 37°C for 30 min and then pulsed with HIV at 37°C for 2 h in the presence of the inhibitors before coculture with Hut/CCR5 cells. DC viability after the inhibitor treatment was examined with 7-amino-actinomycin D staining (Annexin V-PE apoptosis detection kit; BD Pharmingen) and flow cytometry.
Electron microscopy.
For visualization of HIV trafficking, iDCs and mDCs (6 × 105) were incubated separately with AT-2-inactivated HIV (2 μg of p24) at 37°C for 1.5 h. After a wash, DCs were cultured for 1 h and fixed and processed for conventional transmission electron microscopy as previously described (49). For HIV transmission from DCs to CD4+ T cells, after incubation with AT-2-inactivated HIV as described above, DCs were washed extensively and cocultured with Hut/CCR5 cells (6 × 105) for 1 h prior to fixation and sample preparation. Cells were washed and processed for electron microscopy as previously described (49). For ruthenium red (RR) labeling of plasma membranes, cells were washed with 0.1 M ice-cold sodium-cacodylate buffer and then fixed with 1% glutaraldehyde in 0.1 M sodium-cacodylate buffer containing 0.05% RR for 1 h on ice. After a wash, cells were postfixed in 1% osmium tetroxide containing 0.05% RR for 1 h on ice. Thin sections were examined with a transmission electron microscope (Hitachi H-600 or JEOL 2100 LaB6).
Statistical analyses.
Statistical analyses were performed with the Wilcoxon paired t test with Prism software or Dunnett's multiple comparison test with the SAS program.
RESULTS
mDCs enhance HIV transmission to different types of target cells independently of C-type lectins.
To better understand the cell-cell interactions underlying mDC-enhanced HIV transmission, the efficiencies of HIV trans-infection mediated by iDCs and mDCs were compared and the role of C-type lectins in viral transmission was examined. Purified CD14+ monocytes were used to generate iDCs, and the maturation of iDCs was achieved by LPS treatment (34). The phenotypes of iDCs and mDCs were confirmed by immunostaining of cell surface markers. As expected, iDCs and mDCs uniformly expressed high levels of CD11c, and they were nearly negative for CD14 at day 7 of differentiation (Fig. 1A). HLA-DR and CD86 expression levels were significantly increased in mDCs relative to iDCs, indicating efficient DC maturation, while the surface expression of DC-SIGN was decreased in mDCs (Fig. 1A).
FIG. 1.
Mature DCs enhance HIV transmission to different types of target cells independently of C-type lectins. (A) Surface markers of iDCs and mDCs. Monocyte-derived iDCs were cultured with LPS to generate mDCs. Cell surface markers were stained with specific MAbs or isotype-matched IgG controls and analyzed by flow cytometry. The histogram peaks of CD11c staining on iDCs and mDCs were overlapped. (B) Enhanced HIV transmission by mDCs is independent of C-type lectins. DCs and Raji/DC-SIGN cells were preincubated separately with medium, anti-DC-SIGN cocktails, and mannan prior to HIV incubation, as described previously (57). Raji/DC-SIGN cells, iDCs, and mDCs were pulsed separately with single-cycle, luciferase reporter R5-tropic HIV-Luc/JRFL (multiplicity of infection [MOI], 0.2), washed, and cocultured separately with autologous PBLs, the CD4+ T-cell line Hut/CCR5, and the HIV indicator cells GHOST/R5. HIV infection was determined after 2 days by measuring the luciferase activity. (C) No detectable HIV cis-infection in DCs and Raji/DC-SIGN cells. Cells were infected with HIV-Luc/JRFL (MOI, 0.2), and viral infection was determined at 2 dpi. (D) mDCs enhance transmission of HIV pseudotyped with X4-tropic Env (HXB2). Transmission of HIV-Luc/HXB2 with DCs as donors and Hut/CCR5 cells as targets was performed as described for panel B. The asterisk indicates a significant difference (P < 0.05) compared with iDCs. The data show the means ± standard deviations of triplicate samples. One representative experiment out of four is shown. cps, counts per second.
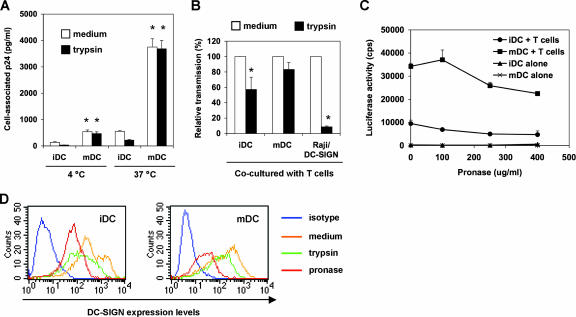
To quantify HIV transmission efficiency mediated by iDCs and mDCs, a single-cycle luciferase reporter HIV was used. The virus was pseudotyped separately with R5- or X4-tropic HIV Env. Various types of target cells were used in HIV transmission assays, including activated autologous PBLs, the human CD4+ T-cell line Hut/CCR5 (57), and the human osteosarcoma cell line GHOST/R5, and were engineered to express HIV receptors (12). When DCs were pulsed with small amounts of R5-tropic HIV and cocultured separately with different types of target cells, mDCs were 3-fold (P < 0.05), 18-fold (P < 0.001), and 8-fold (P < 0.01) more effective than iDCs in transmitting HIV infection to activated PBLs, Hut/CCR5 cells, and GHOST/R5 cells, respectively (Fig. 1B). Similar results were observed in independent experiments using autologous DCs and PBLs derived from four different donors and using HIV pseudotyped with different R5-tropic Env proteins (data not shown).
Preincubation of iDCs with cocktails of DC-SIGN MAbs reduced HIV transmission to various types of target cells by 33% to 54% (P < 0.05) (Fig. 1B), a finding consistent with our previous results (49, 57). Similarly, blockade of iDCs with mannan, an inhibitor of mannose-binding C-type lectins, decreased HIV transmission by 22% to 56% (P < 0.05). However, DC-SIGN MAbs and mannan had no effect on HIV transmission mediated by mDCs (Fig. 1B). These results suggest that mDC-enhanced HIV transmission is independent of C-type lectins, which partly contribute to iDC-mediated HIV transmission. To confirm the effective function of DC-SIGN MAbs and mannan for neutralizing HIV transmission, Raji/DC-SIGN cells (55), a human B-cell line engineered high levels of DC-SIGN expression, were used as a positive control. Consistent with our previous results (53, 56, 57), HIV transmission to Hut/CCR5 cells by Raji/DC-SIGN cells was abolished by DC-SIGN MAbs and mannan (Fig. 1B).
The HIV infection observed in cocultures was a direct result of DC-mediated trans-infection of target cells, as no viral infection was detected at 2 days postinfection (dpi) in DCs and Raji/DC-SIGN cells that were pulsed with small amounts of HIV (Fig. 1C). HIV infection in GHOST/R5 cells was significantly higher (149-fold and 6-fold) than that in PBLs and Hut/CCR5 cells (Fig. 1C), consistent with the increased HIV infection observed in coculture assays (Fig. 1B). In addition, mDCs were threefold (P < 0.05) more potent than iDCs in stimulating X4-tropic HIV trans-infection of CD4+ T cells (Fig. 1D). Together, these data suggest that unique mDC-HIV interactions may account for enhanced HIV transmission to target cells. HIV pseudotyped with R5-tropic-Env was used in the following assays, given that the infection and transmission rate of X4-tropic HIV in DCs is significantly lower than that of R5-tropic HIV (20, 23, 38, 49).
DC-target cell contact is required for efficient HIV transmission mediated by iDCs and mDCs.
To evaluate whether mDC-enhanced HIV transmission requires cell-cell contact, HIV transmissions to different types of target cells by iDCs and mDCs were compared with transwell culture plates. HIV-pulsed DCs were separated from Hut/CCR5 or GHOST/R5 target cells by the use of transwell culture plates with permeable membranes (56). The transwell membranes (pore size, 3 μm) are permeable for HIV but not for DCs or target cells (data not shown). Compared with DC-alone controls, HIV infection was enhanced 32-fold or 168-fold when iDCs or mDCs, respectively, were in cocultures with Hut/CCR5 target cells (Fig. 2A). Similarly, compared with DC-alone controls, HIV infection was enhanced 66-fold or 400-fold when iDCs or mDCs, respectively, were in cocultures with GHOST/R5 target cells (Fig. 2B). When iDCs and mDCs were separated from target cells by the permeable membranes, HIV transmission decreased to background levels (Fig. 2). Similar results were obtained when transwell plates with membrane pore sizes of 0.4 μm were used (data not shown). Thus, DC-target cell contact is required for efficient DC-mediated HIV transmission.
FIG. 2.
DC-target cell contact is required for efficient HIV transmission mediated by iDCs and mDCs. Transmission of HIV-Luc/JRFL with iDCs and mDCs as donors and (A) Hut/CCR5 cells or (B) GHOST/R5 cells as targets was performed as described in the legend to Fig. 1B. Transwell culture plates with membrane pore sizes of 3 μm were used to separate DCs and target cells (Transwell). HIV infection was determined after 2 dpi by measuring the luciferase activity. The data show the means ± standard deviations of triplicate samples. One representative experiment out of three is shown. cps, counts per second.
Enhanced HIV endocytosis and distinct viral trafficking in mDCs relative to iDCs.
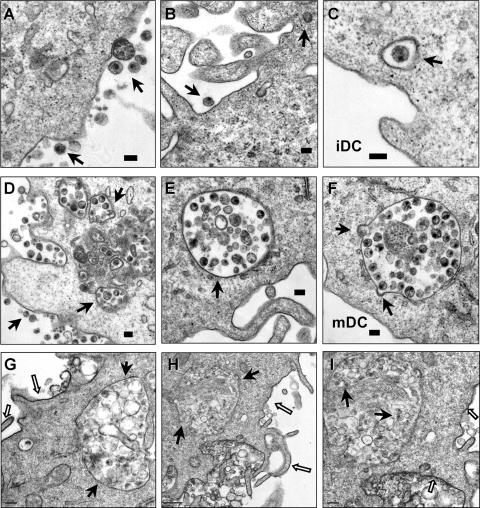
To visualize HIV trafficking and interactions with iDCs and mDCs, AT-2-inactivated HIV was used for electron microscopy assays. AT-2-inactivated HIV is conformationally authentic and interacts with DCs similarly to infectious HIV (19, 48). Using electron microscopy, previous studies have investigated interactions of AT-2-inactivated simian immunodeficiency virus and human DCs or macaque DCs (19, 48). After a 1.5-h HIV exposure, cell surface-associated HIV and a few internalized viral particles in clathrin-coated vesicles were observed in iDCs (Fig. 3A, B, and C). By contrast, in addition to the surface-associated HIV, numerous intact HIV particles were observed within intracellular endocytic compartments in mDCs (Fig. 3D, E, and F). No HIV particles were observed in controls without HIV incubation (not shown).
FIG. 3.
Enhanced HIV endocytosis and distinct viral trafficking in mDCs relative to iDCs. DCs were exposed to AT-2-inactivated R5 HIV for 1.5 h, washed thoroughly, fixed, and prepared for electron microscopy. (A, B, and C) Cell surface-bound HIV and internalized viral particles in iDCs. (D, E, and F) HIV internalization is significantly enhanced in mDCs. Arrows indicate DC surface-associated HIV particles or intracellular compartments that trapped intact HIV particles. (G, H, and I) RR labeling of mDC plasma membranes. (I) Higher-magnification image of panel H (a partial area). The open arrows indicate RR-labeled mDC plasma membranes, and the black arrows point to HIV-containing compartments and HIV particles that were not labeled with RR. Scale bars, 0.1 μm (A to F), 0.2 μm (G, I), and 0.5 μm (H).
Given the complexity of DC membranes, to confirm that the HIV-containing compartments in mDCs were truly endocytosed structures rather than cell surface invaginations, RR was used during fixation as a membrane-impermeable dye. RR binds to carbohydrate moieties on the cell surface (32) and readily penetrates membrane invaginations due to its small size (14). Fixation of DCs at 4°C prevents the internalization of RR. Upon postfixation, RR forms an electron-dense precipitate that can be visualized by electron microscopy. This method has been used to study HIV entry and assembly in macrophages (15, 33, 51). Results showed that mDC surfaces and the invaginations were strongly labeled with RR, as expected, while the membranes of the HIV-containing vesicles were largely resistant to RR staining (Fig. 3G, H, and I). These results confirm that significant amounts of HIV were internalized in the intracellular compartments in mDCs.
mDCs are more potent than iDCs in protecting HIV from proteolysis.
The above viral trafficking studies indicated that mDCs facilitated HIV internalization and altered HIV localization compared with iDCs. To determine whether viral trafficking contributes to DC-mediated HIV transmission, iDCs and mDCs were compared for binding and internalization of HIV and viral protection from proteolysis.
To measure HIV binding and internalization, iDCs and mDCs were pulsed separately with small amounts of infectious HIV for 2 h, and DC-associated HIV p24 was quantified. To test the proteolysis sensitivity of DC-associated HIV, DCs were treated with trypsin after the HIV incubation. At 4°C, HIV binding to mDCs was nearly fourfold (P < 0.01) higher than that to iDCs (Fig. 4A). Trypsin treatment reduced iDC-associated HIV to background levels, indicating that virus mainly remained on the iDC surface upon binding at 4°C, while the trypsin treatment only slightly decreased mDC-bound HIV, by 13%. HIV internalization of mDCs at 37°C was sevenfold greater (P < 0.01) than that of iDCs and was sevenfold greater (P < 0.01) than HIV binding to mDCs at 4°C. Nearly 50% iDC-associated HIV at 37°C was sensitive to proteolysis, whereas the mDC-associated HIV was completely resistant to trypsin treatment (Fig. 4A). These results suggest that increased HIV internalization and protection by mDCs may contribute to enhanced efficiency of viral transmission.
FIG. 4.
mDCs are more potent than iDCs in protecting HIV from proteolysis. (A) mDCs enhance HIV binding and internalization. DCs were incubated with HIV-Luc/JRFL (30 ng of p24) at 4°C or 37°C for 2 h, washed and treated with trypsin or medium, and then lysed for HIV p24 quantification. Asterisks indicate significant differences (P < 0.01) compared with iDCs at the same temperature. (B) DCs protect captured HIV from trypsin cleavage. HIV-pulsed iDCs, mDCs, and Raji/DC-SIGN cells were separately treated with trypsin before coculture with Hut/CCR5 target cells. Transmission of HIV-Luc/JRFL to Hut/CCR5 target cells was performed as described in the legend to Fig. 1B. The average results of four independent experiments are shown. Values for medium controls were set at 100%. Asterisks indicate significant differences (P < 0.05) between trypsin-treated samples and medium controls. (C) DCs protect captured HIV from pronase cleavage. HIV-pulsed iDCs and mDCs were treated with increasing concentrations of pronase before coculture with Hut/CCR5 target cells. The data show the means ± standard deviations of triplicate samples. One representative experiment out of two is shown. cps, counts per second. (D) Decreased surface DC-SIGN levels on DCs after protease treatment. DCs were stained for surface DC-SIGN after separate treatments with trypsin or pronase and analyzed by flow cytometry. Medium treatment was used as a control.
To detect whether DCs protect HIV from protease treatment and further transfer HIV to T cells, HIV-pulsed iDCs and mDCs were treated with trypsin (250 μg/ml) before coculture with Hut/CCR5 cells. Compared with medium controls, the average results of four independent experiments revealed that trypsin treatment reduced iDC-mediated HIV transmission by 43% ± 16% (P < 0.05), while a 17% ± 9% decrease in viral transmission was observed in trypsin-treated mDCs (Fig. 4B). These data suggest that mDCs are more potent at protecting internalized and surface-bound HIV from protease digestion. As a control, trypsin treatment of HIV-pulsed Raji/DC-SIGN cells under the same conditions significantly reduced viral transmission, by 91% (P < 0.001) (Fig. 4B). We have carefully optimized the trypsin treatment conditions to ensure cell viability and sufficient HIV cleavage on cell surfaces (49); however, the trypsin treatment might not completely cleave DC surface-bound HIV given the complexity of DC membranes.
A recent study proposed that trypsin might be less potent than pronase at removing DC surface-bound HIV, although no comparison data were shown (11). To further demonstrate that DC-mediated HIV transmission is partially resistant to proteolysis, HIV-pulsed DCs were treated with pronase and then cocultured with Hut/CCR5 cells. HIV transmission gradually decreased when DCs were treated with increasing concentrations of pronase (Fig. 4C). Interestingly, pronase treatment (250 μg/ml) reduced iDC- and mDC-mediated HIV transmission by 48% (P < 0.05) and 24%, respectively. These data were comparable to those of trypsin treatment at the same concentration, suggesting that both trypsin and pronase may efficiently strip surface HIV from DCs. Furthermore, when DCs were treated with 400 μg/ml of pronase, HIV transmission by iDCs and mDCs was decreased by 51% (P < 0.05) and 34%, respectively (Fig. 4C), indicating that mDC-associated HIV is more resistant to proteolysis. Together, these data suggest that DC-mediated HIV transmission may involve recycling of the internalized viruses in addition to DC surface-bound HIV. To confirm the effective proteolysis function, DC-SIGN on DC surfaces was stained after separate treatments with trypsin or pronase. Results showed that both trypsin and pronase treatments efficiently reduced surface DC-SIGN levels on iDCs and mDCs (Fig. 4D), which may also partially contribute to the decreased efficiency of iDC-mediated HIV transmission after protease treatment.
Effects of trafficking inhibitors on DC-mediated viral transmission.
To quantify the effect of HIV trafficking on DC-mediated viral transmission, iDCs and mDCs were examined for their efficiencies in supporting HIV trans-infection after treatment with various trafficking inhibitors. DCs were incubated separately with various inhibitors for 30 min and pulsed with small amounts of HIV in the presence of the inhibitors for 2 h. To avoid the effect of inhibitors on HIV infection of target cells, HIV-pulsed DCs were washed and cocultured with Hut/CCR5 cells for 2 days in the absence of inhibitors. Various trafficking inhibitors included an intracellular Ca2+ chelator, BAPTA-AM, which can eliminate exosome secretion (43); NH4Cl, a weak base that neutralizes acidic endomembrane compartments (1); monensin, a polyether antibiotic that disrupts the structure of the Golgi apparatus and inhibits vesicular transport in eukaryotic cells (18); brefeldin A, a macrocyclic lactone that inhibits small GTP-binding proteins and induces the rapid redistribution of the Golgi apparatus into the endoplasmic reticulum (28); and epoxomicin, a potent and selective proteasome inhibitor (35). Medium that contained dissolvent was used as a control.
The average results of four independent experiments revealed that monensin significantly reduced iDC- and mDC-mediated HIV transmission, by 68% and 72% (P < 0.01 [Dunnett's multiple comparison test]), respectively (Fig. 5A and B). These data suggest potential involvement of the Golgi apparatus or vesicular transport of HIV in DC-mediated HIV transmission. BAPTA-AM blocked 42% of iDC-mediated HIV transmission (P < 0.05) (Fig. 5A), indicating that HIV transmission by iDCs is partially dependent on C-type lectins. As a control, monensin treatment did not significantly change DC-associated HIV p24 (91% to 119%, relative to medium controls). While BAPTA-AM treatment reduced iDC-associated HIV p24 by 43%, it had no effect on mDC-associated HIV p24. Some inhibitor-dependent effects (such as NH4Cl) on cellular trafficking events are reversible after removal of the inhibitors. Thus, potential effects of other inhibitors on HIV trans-infection could not be ruled out. No significant decrease in DC viability was observed after inhibitor treatment and 3 days in culture. The viability of inhibitor-treated DCs remained 76% to 94%, compared with the 88% to 94% viability of the medium controls (Fig. 5C). Together, these data suggest that intracellular trafficking inhibitors may disrupt DC-mediated viral transmission.
FIG. 5.
Effects of trafficking inhibitors on DC-mediated viral transmission. iDCs (A) and mDCs (B) were incubated separately with various inhibitors for 0.5 h and pulsed with HIV-Luc/JRFL in the presence of the inhibitors for 2 h at 37°C. DCs were washed and cocultured with Hut/CCR5 cells for 2.5 days. Medium that contained dissolvent was used as a control. The average relative transmission of four independent experiments using DCs from different donors is shown (medium controls were set at 100%). Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01) compared with medium controls. (C) Viability of inhibitor-treated DCs after 3 days in culture. DCs were incubated separately with various inhibitors at 37°C for 2.5 h, washed, and cultured 3 days before staining with 7-amino-actinomycin D. Stained DCs were analyzed by flow cytometry. The average DC viability of three independent experiments is shown (medium controls were set at 100%).
mDCs efficiently concentrate HIV at virological synapses.
To visualize the formation of virological synapses between DCs and CD4+ T cells, an electron microscopy-based assay was developed. After a 1.5-h exposure of AT-2-inactivated HIV to iDCs and mDCs, DCs were washed thoroughly and cocultured with Hut/CCR5 cells for 1 h to allow DC-T-cell interactions and viral transfer. Upon contact with CD4+ T cells, a large amount of intact HIV particles were concentrated and polarized at mDC-T-cell synapses (Fig. 6A to D). Interestingly, membrane continuity between an HIV-containing compartment and the plasma membrane of an mDC was observed at an mDC-T-cell synapse (Fig. 6C and D). Although it is difficult to conclude that HIV was redistributed from the intracellular compartments following T-cell contact, the membrane continuity with concentrated HIV particles could not be observed in HIV-pulsed mDCs without T-cell coculture (Fig. 3D to I). Large amounts of intact HIV particles were easily observed at numerous mDC-T-cell synapses; by contrast, the virological synapses formed between iDCs and T cells were not readily found (Fig. 6E and F and data not shown). A few intact HIV-like particles were observed at iDC-T-cell junctions (Fig. 6E and F). These results suggest that mDCs are more efficient than iDCs in concentrating captured HIV at virological synapses.
FIG. 6.
mDCs efficiently concentrate HIV at virological synapses. After a 1.5-h exposure to AT-2-inactivated R5 HIV, iDCs and mDCs were washed and cocultured separately with Hut/CCR5 cells for 1 h, fixed, and prepared for electron microscopy. Hut/CCR5 cells exhibit more condensed chromatins; DCs show typical surface dendrites, less-condensed chromatin, and electron-dense lysosome-like granules. (A) Large amount of intact HIV particles concentrated at the mDC-T-cell junction. (B) Higher-magnification images of the boxed areas from panel A. Black arrows indicate HIV particles that were concentrated at the synapses. (C) HIV particles concentrated at the mDC-T-cell junction. Membrane continuity was observed between an HIV-containing compartment and the plasma membrane of an mDC. (D) Higher-magnification images of the boxed areas from panel C. White arrows indicate HIV particles that were concentrated at the mDC-T-cell synapses. (E) Fewer HIV-like particles were observed at the iDC-T-cell junction. (F) A number of intact HIV-like particles were observed at the iDC-T-cell junction. Black arrows indicate HIV-like particles at the synapses. TC, Hut/CCR5 cells; scale bars, 1 μm (A to E) and 0.5 μm (F).
Intracellular HIV degradation and time course viral transmission mediated by DCs.
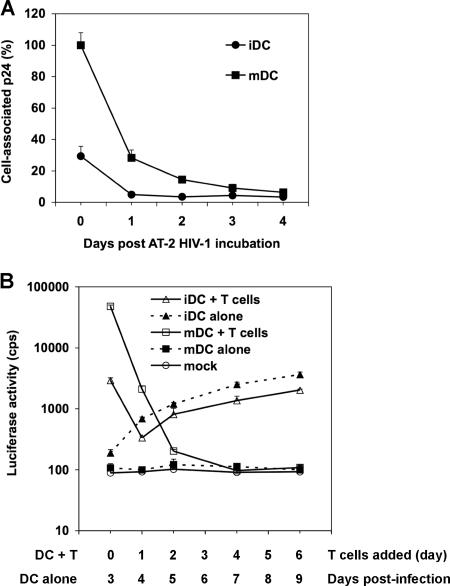
To compare intracellular HIV degradation in iDCs and mDCs, DCs were exposed to small amounts of AT-2-inactivated HIV and trypsinized, and DC-associated HIV p24 from aliquots was measured daily for 4 days. The mDC-associated p24 was 3.4-fold higher than that of iDCs at day 0 (Fig. 7A). After 1 day in culture, iDC- and mDC-associated HIV was rapidly degraded, by 83% and 72%, respectively. After 3 days, almost no HIV p24 was detectable in iDCs and mDCs (Fig. 7A), suggesting that there is no long-term retention of HIV in DCs. These data are in agreement with previous studies showing that most incoming HIV is degraded in DCs within 24 h (36, 37, 48).
FIG. 7.
Intracellular HIV degradation and time course viral transmission mediated by DCs. (A) HIV degradation in DCs. DCs (7.5 × 105) were incubated with AT-2-inactivated R5-tropic HIV (50 ng of p24), washed, and treated with trypsin. Aliquots of DCs were cultured, and DC-associated HIV p24 was measured daily. The p24 result (3,897 pg/ml) for mDCs at day 0 was set at 100%, and relative results are shown. (B) Time course HIV transmission by DCs. Transmission of HIV-Luc/JRFL (multiplicity of infection, 0.2) with iDCs and mDCs as donors and Hut/CCR5 cells as targets was performed as described in the legend to Fig. 1B. At 0, 1, 2, 4, and 6 dpi, aliquots of HIV-pulsed iDCs and mDCs were cocultured separately with Hut/CCR5 cells for an additional 3 days. In parallel, HIV infection of DC-alone controls was determined by measuring the luciferase activity at 3, 4, 5, 7, and 9 dpi. Mock controls of iDCs and mDCs without HIV infection were identical. All data are the means ± standard deviations of triplicate samples. One representative experiment out of three is shown. cps, counts per second.
To determine whether the small proportions of HIV retained in DCs can mediate long-term trans-infection of CD4+ T cells, time course viral transmission by iDCs and mDCs was examined. Use of single-cycle HIV in this assay had the advantage of avoiding viral transmission of progeny viruses that replicate in cis-infected DCs. At 0, 1, 2, 4, and 6 dpi, aliquots of HIV-pulsed iDCs and mDCs were separately cocultured with Hut/CCR5 cells for an additional 3 days to quantify viral transmission. In parallel, HIV-infected DC-alone controls were harvested at 3, 4, 5, 7, and 9 dpi to measure cis-infection.
HIV trans-infection mediated by mDCs was 16-fold (P < 0.001) higher than that by iDCs when DCs were cocultured with Hut/CCR5 cells at 0 dpi (Fig. 7B). After 1 dpi, viral transmission mediated by iDCs and mDCs decreased by 9- and 23-fold (P < 0.001), respectively, while mDCs were still 6-fold more potent than iDCs at enhancing viral transmission. At 2 dpi, mDC-mediated HIV transmission further decreased 10-fold, to almost basal level. Of note, in the above-described transmission assays (Fig. 1, 2, 4, and 5), HIV-pulsed DCs alone did not become detectably infected at 2 to 2.5 dpi, whereas an increasing HIV infection in iDCs became detectable after 3 to 4 dpi during the time course. The increased HIV infection in iDCs correlated with iDC-mediated viral transmission. Consistently, no HIV infection was detected in mDCs (Fig. 7B). HIV infection in iDC-alone samples was around twofold higher than that in iDC-T-cell cocultures at 4 dpi to 9 dpi, a result that was likely due to T-cell-induced DC maturation (Fig. 7B). Together, these results suggest that most incoming HIV is rapidly degraded by iDCs and mDCs and that there is no viral retention-mediated long-term transmission.
DISCUSSION
Understanding HIV-host cell interactions and defining the mechanisms of DC-mediated virus transmission are essential for developing effective strategies to combat HIV infection (54). Here we have compared the efficiency and mechanisms of HIV transmission mediated by iDCs and mDCs by using single-cycle HIV quantification assays. We have found that mDCs significantly facilitate HIV endocytosis and efficiently concentrate HIV at virological synapses, which is likely to contribute to mDC-enhanced HIV transmission, at least in part. mDCs were more efficient than iDCs in transmitting HIV to various types of target cells independently of C-type lectins. Moreover, DC-target cell contact was required for efficient HIV-1 transmission mediated by iDCs and mDCs. These results suggest that HIV may exploit mDCs to efficiently spread viral infection in lymphoid tissues, which are the major resources for HIV replication (17).
The enhanced efficiency of HIV transmission by LPS-induced mDCs has potential clinical implications for HIV pathogenesis. A recent finding indicated that significantly increased plasma LPS levels in HIV-infected humans correlate with AIDS progression and systemic immune activation. The increased plasma LPS levels may result from microbial translocation through a breach in the integrity of the mucosal barrier in the gut (7). Indeed, LPS can induce mouse DC maturation in vivo (16). Although the LPS concentration that we used (10 ng/ml) for in vitro DC maturation is about 50-fold higher than that found in the plasma of HIV-infected patients (7), it is conceivable that increased LPS in HIV-infected individuals may induce DC maturation and potently stimulate HIV dissemination in vivo. In addition, HIV coinfection with other sexually transmitted pathogens can increase inflammatory stimulations at the mucosae (44), which may directly activate DCs in vivo and promote HIV spread. Further studies using myeloid DCs, plasmacytoid DCs, or Langerhans cells from HIV-infected individuals may be required to test this hypothesis.
Our data suggest that both cell surface-bound and internalized HIV contributes to DC-mediated viral transmission. In contrast, a recent study indicates that DC-mediated HIV trans-infection mainly derives from DC surface-bound virions (11). Although it is difficult to directly compare these results owing to the different approaches used in the studies, the dynamic recycling of internalized HIV to DC surfaces may also mediate HIV trans-infection, which should be an important consideration. It has been shown that HIV trafficking to the infectious synapse between LPS-induced mDCs and CD4+ T cells occurs via a tetraspanin-sorting pathway (21). HIV is internalized into endocytic compartments in LPS-induced mDCs, which are nonconventional, nonlysosomal vesicles (21). Upon contact with T cells, internalized HIV in mDCs redistributes to form infectious synapses (21, 48). Together, these results support a model in which intracellular HIV trafficking contributes to HIV transmission mediated by DCs, particularly to mDC-enhanced viral transmission. Although endocytosis of HIV by DCs may not occur at 4°C, we have observed that the invaginations of mDC plasma membranes were strongly labeled with RR at 4°C. We observed that trypsin treatment only slightly decreased mDC-bound HIV at 4°C, by 13%, which might be due to viral protection by the invaginations of mDC plasma membranes.
We found that monensin, an intracellular trafficking inhibitor, significantly blocked iDC- and mDC-mediated HIV transmission to CD4+ T cells. In addition to inhibiting vesicular transport in eukaryotic cells, monensin can also disrupt the structure of the Golgi apparatus and glycoprotein synthesis (18, 39). In our experiments, monensin was washed away after the 2.5-h incubation with DCs, and no significant cytotoxic effects on DCs were observed after 3 days in culture. Therefore, it is unlikely that the reduced HIV transmission by monensin was mainly due to disrupted protein synthesis, although the possibility cannot be ruled out. Monensin is used as an antiprotozoal, antibacterial, or antifungal agent and as a growth promoter in veterinary medicine (9). It might be interesting to further explore whether monensin can be used as an antiviral agent against HIV transmission in vivo.
Previous results (34, 42) and the present study indicate that DC-T-cell contact is required for efficient HIV trans-infection mediated by iDCs and mDCs. The exocytosis of HIV-associated exosomes also can play a role in iDC-mediated HIV trans-infection (52), but it may not be an efficient pathway in mDC-enhanced HIV transmission given that iDCs produce more exosomes than do mDCs (45). Although cell-free supernatants from single-cycle HIV-pulsed mDCs were positive for HIV Gag p24, they failed to initiate HIV infection in GHOST/R5 cells or Hut/CCR5 cells (data not shown). Nevertheless, the efficiency of exosome-mediated trans-infection by mDCs remains to be confirmed with replication-competent HIV.
Increased ICAM-1 expression on mDCs has been shown to correlate with mDC-enhanced HIV transmission (42). This is possibly due to stronger DC-T-cell interactions through ICAM-1 binding to T-cell-expressed LFA-1 (for “leukocyte function-associated molecule 1”) (25, 42). Despite the lack of expression of any identified ICAM ligands, such as LFA-1, CD11b/CD18, and CD11c, GHOST/R5 cells efficiently supported mDC-enhanced HIV transmission (Fig. 2B and data not shown). Moreover, ICAM-1 MAb blockade of DCs, GHOST/R5 cells, or both did not significantly affect HIV transmission mediated by iDCs or mDCs (data not shown). Therefore, ICAM-1 may not be the only cellular factor that contributes to mDC-enhanced efficiency of HIV trans-infection. Cell-type-dependent HIV trafficking may play a role in mDC-enhanced viral transmission, at least in part.
Our results indicate that HIV capture by iDCs is less efficient than that by mDCs; thus, the differences in viral transmission efficiencies and virological synapses between iDCs and mDCs may only reflect the low levels of viral capture by iDCs. HIV entry in DCs can occur through endocytosis and viral receptor-mediated fusion, while productive HIV replication requires viral fusion (8, 23, 38). To visualize viral interaction with DCs, high concentrations of AT-2-inactivated HIV were used in a previous study (2 to 3 μg of p24/106 DCs) (48) and in our electron microscopy assays (2 μg of p24/6 × 105 DCs). Given that AT-2-inactivated HIV can mediate viral fusion with cell membranes (41), the majority of iDC-associated HIV particles may undergo fusion, uncoating, or degradation processes in iDCs or in cocultured T cells. Therefore, intact HIV particles could not be easily observed in iDC-T-cell cocultures by electron microscopy (Fig. 6E and F and data not shown).
It has been shown that HIV fusion to DCs decreases as cells mature (10). The entry of HIV into LPS-induced mDCs seemed to be primarily through endocytosis. The large intracellular compartments that confined numerous HIV particles in mDCs (Fig. 3D to I) appeared morphologically similar to macropinocytosis-mediated HIV entry in macrophages and brain microvascular endothelia (30, 33). Activation of DCs can trigger extensive and prolonged macropinocytic activity, enabling DCs to sample large volumes of the extracellular milieu for immune surveillance (13). Although mDC-associated HIV was rapidly degraded, by 72%, after 1 day, about 14% and 9% of HIV p24 remained at day 2 and 3 in mDCs, respectively (Fig. 7A). Due to the high capacity in enhancing HIV trans-infection by mDCs, these intracellularly retained viruses could represent an important HIV reservoir in vivo.
Cellular restriction factors that block productive HIV infection in DCs may reflect the intrinsic antiviral immunity of the antigen-presenting cells. It has been suggested that reduced viral replication in mDCs is due to a block in reverse transcription (23), postintegration blocks at the transcriptional level (3), and decreased viral fusion (10). It has been recently reported that APOBEC3G and APOBEC3F (for “apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G and 3F”) mediate the postentry block to HIV replication in DCs (40). However, when the efficiency and mechanisms of HIV infection and transmission between different subsets of DCs are compared, it is extremely important to consider different approaches to DC generation and different stimuli for DC maturation (54). Using replication-competent and single-cycle HIV, we have found that HIV infection and transmission are functionally distinct from different subsets of mDCs induced by various stimuli (Dong et al. and L. Wu, unpublished results). Further understanding of the regulation of antiretroviral immunity in DCs may provide new insights into more effective interventions against HIV infection and dissemination mediated by DCs.
Acknowledgments
We thank T. Zahrt and J. Barbieri for critical reading of the manuscript. We thank M. Emerman, V. KewalRamani, and J. Lifson for the kind gift of reagents and C. Wells for expert assistance with electron microscopy. IL-2 was obtained from M. Gately (Hoffmann-La Roche Inc.) through the AIDS Research and Reference Reagent Program, NIAID, NIH.
This work was supported by grants to L.W. from the NIH (R01-AI068493) and the Research Affairs Committee of the Medical College of Wisconsin.
Footnotes
Published ahead of print on 13 June 2007.
REFERENCES
- 1.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrighi, J. F., M. Pion, E. Garcia, J. M. Escola, Y. van Kooyk, T. B. Geijtenbeek, and V. Piguet. 2004. DC-SIGN-mediated infectious synapse formation enhances X4 HIV-1 transmission from dendritic cells to T cells. J. Exp. Med. 200:1279-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakri, Y., C. Schiffer, V. Zennou, P. Charneau, E. Kahn, A. Benjouad, J. C. Gluckman, and B. Canque. 2001. The maturation of dendritic cells results in postintegration inhibition of HIV-1 replication. J. Immunol. 166:3780-3788. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Baribaud, F., S. Pohlmann, G. Leslie, F. Mortari, and R. W. Doms. 2002. Quantitative expression and virus transmission analysis of DC-SIGN on monocyte-derived dendritic cells. J. Virol. 76:9135-9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boggiano, C., N. Manel, and D. R. Littman. 2007. Dendritic cell-mediated trans-enhancement of human immunodeficiency virus type 1 infectivity is independent of DC-SIGN. J. Virol. 81:2519-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenchley, J. M., D. A. Price, T. W. Schacker, T. E. Asher, G. Silvestri, S. Rao, Z. Kazzaz, E. Bornstein, O. Lambotte, D. Altmann, B. R. Blazar, B. Rodriguez, L. Teixeira-Johnson, A. Landay, J. N. Martin, F. M. Hecht, L. J. Picker, M. M. Lederman, S. G. Deeks, and D. C. Douek. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365-1371. [DOI] [PubMed] [Google Scholar]
- 8.Burleigh, L., P.-Y. Lozach, C. Schiffer, I. Staropoli, V. Pezo, F. Porrot, B. Canque, J.-L. Virelizier, F. Arenzana-Seisdedos, and A. Amara. 2006. Infection of dendritic cells (DCs), not DC-SIGN-mediated internalization of human immunodeficiency virus, is required for long-term transfer of virus to T cells. J. Virol. 80:2949-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butaye, P., L. A. Devriese, and F. Haesebrouck. 2003. Antimicrobial growth promoters used in animal feed: effects of less well known antibiotics on gram-positive bacteria. Clin. Microbiol. Rev. 16:175-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavrois, M., J. Neidleman, J. F. Kreisberg, D. Fenard, C. Callebaut, and W. C. Greene. 2006. Human immunodeficiency virus fusion to dendritic cells declines as cells mature. J. Virol. 80:1992-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavrois, M., J. Neidleman, J. F. Kreisberg, and W. C. Greene. 2007. In vitro derived dendritic cells trans-infect CD4 T cells primarily with surface-bound HIV-1 virions. PLoS Pathog. 3:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cecilia, D., V. N. KewalRamani, J. O'Leary, B. Volsky, P. Nyambi, S. Burda, S. Xu, D. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 72:6988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conner, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the cell. Nature 422:37-44. [DOI] [PubMed] [Google Scholar]
- 14.Damke, H., T. Baba, D. E. Warnock, and S. L. Schmid. 1994. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127:915-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deneka, M., A. Pelchen-Matthews, R. Byland, E. Ruiz-Mateos, and M. Marsh. 2007. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9, and CD53. J. Cell Biol. 177:329-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Smedt, T., B. Pajak, E. Muraille, L. Lespagnard, E. Heinen, P. De Baetselier, J. Urbain, O. Leo, and M. Moser. 1996. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. J. Exp. Med. 184:1413-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Douek, D. C., L. J. Picker, and R. A. Koup. 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21:265-304. [DOI] [PubMed] [Google Scholar]
- 18.Fliesler, S. J., and S. F. Basinger. 1987. Monensin stimulates glycerolipid incorporation into rod outer segment membranes. J. Biol. Chem. 262:17516-17523. [PubMed] [Google Scholar]
- 19.Frank, I., M. Piatak, Jr., H. Stoessel, N. Romani, D. Bonnyay, J. D. Lifson, and M. Pope. 2002. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs. J. Virol. 76:2936-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganesh, L., K. Leung, K. Lore, R. Levin, A. Panet, O. Schwartz, R. A. Koup, and G. J. Nabel. 2004. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J. Virol. 78:11980-11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia, E., M. Pion, A. Pelchen-Matthews, L. Collinson, J. F. Arrighi, G. Blot, F. Leuba, J. M. Escola, N. Demaurex, M. Marsh, and V. Piguet. 2005. HIV-1 trafficking to the dendritic cell-T-cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic 6:488-501. [DOI] [PubMed] [Google Scholar]
- 22.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 23.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granelli-Piperno, A., A. Pritsker, M. Pack, I. Shimeliovich, J. F. Arrighi, C. G. Park, C. Trumpfheller, V. Piguet, T. M. Moran, and R. M. Steinman. 2005. Dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J. Immunol. 175:4265-4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gummuluru, S., V. N. KewalRamani, and M. Emerman. 2002. Dendritic cell-mediated viral transfer to T cells is required for human immunodeficiency virus type 1 persistence in the face of rapid cell turnover. J. Virol. 76:10692-10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gummuluru, S., M. Rogel, L. Stamatatos, and M. Emerman. 2003. Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC-SIGN and mannose binding C-type lectin receptors via a cholesterol-dependent pathway. J. Virol. 77:12865-12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Izquierdo-Useros, N., J. Blanco, I. Erkizia, M. T. Fernández-Figueras, F. E. Borràs, M. Naranjo-Gómez, M. Bofill, L. Ruiz, B. Clotet, and J. Martinez-Picado. 2007. Maturation of blood-derived dendritic cells enhances human immunodeficiency virus type 1 capture and transmission. J. Virol. 81:7559-7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klausner, R. D., J. G. Donaldson, and J. Lippincott-Schwartz. 1992. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116:1071-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon, D. S., G. Gregorio, N. Bitton, W. A. Hendrickson, and D. R. Littman. 2002. DC-SIGN-mediated internalization of HIV is required for trans-enhancement of T cell infection. Immunity 16:135-144. [DOI] [PubMed] [Google Scholar]
- 30.Liu, N. Q., A. S. Lossinsky, W. Popik, X. Li, C. Gujuluva, B. Kriederman, J. Roberts, T. Pushkarsky, M. Bukrinsky, M. Witte, M. Weinand, and M. Fiala. 2002. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J. Virol. 76:6689-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lore, K., A. Smed-Sorensen, J. Vasudevan, J. R. Mascola, and R. A. Koup. 2005. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J. Exp. Med. 201:2023-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luft, J. H. 1971. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat. Rec. 171:347-368. [DOI] [PubMed] [Google Scholar]
- 33.Marechal, V., M. C. Prevost, C. Petit, E. Perret, J. M. Heard, and O. Schwartz. 2001. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J. Virol. 75:11166-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDonald, D., L. Wu, S. M. Bohks, V. N. KewalRamani, D. Unutmaz, and T. J. Hope. 2003. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300:1295-1297. [DOI] [PubMed] [Google Scholar]
- 35.Meng, L., R. Mohan, B. H. Kwok, M. Elofsson, N. Sin, and C. M. Crews. 1999. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc. Natl. Acad. Sci. USA 96:10403-10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moris, A., C. Nobile, F. Buseyne, F. Porrot, J. P. Abastado, and O. Schwartz. 2004. DC-SIGN promotes exogenous MHC-I-restricted HIV-1 antigen presentation. Blood 103:2648-2654. [DOI] [PubMed] [Google Scholar]
- 37.Moris, A., A. Pajot, F. Blanchet, F. Guivel-Benhassine, M. Salcedo, and O. Schwartz. 2006. Dendritic cells and HIV-specific CD4+ T cells: HIV antigen presentation, T-cell activation, and viral transfer. Blood 108:1643-1651. [DOI] [PubMed] [Google Scholar]
- 38.Nobile, C., C. Petit, A. Moris, K. Skrabal, J. P. Abastado, F. Mammano, and O. Schwartz. 2005. Covert human immunodeficiency virus replication in dendritic cells and in DC-SIGN-expressing cells promotes long-term transmission to lymphocytes. J. Virol. 79:5386-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pal, R., R. C. Gallo, and M. G. Sarngadharan. 1988. Processing of the structural proteins of human immunodeficiency virus type 1 in the presence of monensin and cerulenin. Proc. Natl. Acad. Sci. USA 85:9283-9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pion, M., A. Granelli-Piperno, B. Mangeat, R. Stalder, R. Correa, R. M. Steinman, and V. Piguet. 2006. APOBEC3G/3F mediates intrinsic resistance of monocyte-derived dendritic cells to HIV-1 infection. J. Exp. Med. 203:2887-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossio, J. L., M. T. Esser, K. Suryanarayana, D. K. Schneider, J. W. Bess, Jr., G. M. Vasquez, T. A. Wiltrout, E. Chertova, M. K. Grimes, Q. Sattentau, L. O. Arthur, L. E. Henderson, and J. D. Lifson. 1998. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J. Virol. 72:7992-8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanders, R. W., E. C. de Jong, C. E. Baldwin, J. H. Schuitemaker, M. L. Kapsenberg, and B. Berkhout. 2002. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J. Virol. 76:7812-7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Savina, A., M. Furlan, M. Vidal, and M. I. Colombo. 2003. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 278:20083-20090. [DOI] [PubMed] [Google Scholar]
- 44.Shattock, R. J., and J. P. Moore. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 1:25-34. [DOI] [PubMed] [Google Scholar]
- 45.Thery, C., A. Regnault, J. Garin, J. Wolfers, L. Zitvogel, P. Ricciardi-Castagnoli, G. Raposo, and S. Amigorena. 1999. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 147:599-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trumpfheller, C., C. G. Park, J. Finke, R. M. Steinman, and A. Granelli-Piperno. 2003. Cell type-dependent retention and transmission of HIV-1 by DC-SIGN. Int. Immunol. 15:289-298. [DOI] [PubMed] [Google Scholar]
- 47.Tsunetsugu-Yokota, Y., S. Yasuda, A. Sugimoto, T. Yagi, M. Azuma, H. Yagita, K. Akagawa, and T. Takemori. 1997. Efficient virus transmission from dendritic cells to CD4+ T cells in response to antigen depends on close contact through adhesion molecules. Virology 239:259-268. [DOI] [PubMed] [Google Scholar]
- 48.Turville, S. G., J. J. Santos, I. Frank, P. U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak, Jr., J. D. Lifson, M. Pope, and A. L. Cunningham. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103:2170-2179. [DOI] [PubMed] [Google Scholar]
- 49.Wang, J. H., A. M. Janas, W. J. Olson, V. N. Kewalramani, and L. Wu. 2007. CD4 coexpression regulates DC-SIGN-mediated transmission of human immunodeficiency virus type 1. J. Virol. 81:2497-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weissman, D., Y. Li, J. M. Orenstein, and A. S. Fauci. 1995. Both a precursor and a mature population of dendritic cells can bind HIV. However, only the mature population that expresses CD80 can pass infection to unstimulated CD4+ T cells. J. Immunol. 155:4111-4117. [PubMed] [Google Scholar]
- 51.Welsch, S., O. T. Keppler, A. Habermann, I. Allespach, J. Krijnse-Locker, and H. G. Krausslich. 2007. HIV-1 buds predominantly at the plasma membrane of primary human macrophages. PLoS Pathog. 3:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiley, R. D., and S. Gummuluru. 2006. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc. Natl. Acad. Sci. USA 103:738-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu, L., A. A. Bashirova, T. D. Martin, L. Villamide, E. Mehlhop, A. O. Chertov, D. Unutmaz, M. Pope, M. Carrington, and V. N. KewalRamani. 2002. Rhesus macaque dendritic cells efficiently transmit primate lentiviruses independently of DC-SIGN. Proc. Natl. Acad. Sci. USA 99:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu, L., and V. N. KewalRamani. 2006. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat. Rev. Immunol. 6:859-868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu, L., T. D. Martin, M. Carrington, and V. N. KewalRamani. 2004. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology 318:17-23. [DOI] [PubMed] [Google Scholar]
- 56.Wu, L., T. D. Martin, Y. C. Han, S. K. Breun, and V. N. KewalRamani. 2004. Trans-dominant cellular inhibition of DC-SIGN-mediated HIV-1 transmission. Retrovirology 1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu, L., T. D. Martin, R. Vazeux, D. Unutmaz, and V. N. KewalRamani. 2002. Functional evaluation of DC-SIGN monoclonal antibodies reveals DC-SIGN interactions with ICAM-3 do not promote human immunodeficiency virus type 1 transmission. J. Virol. 76:5905-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamashita, M., and M. Emerman. 2004. Capsid is a dominant determinant of retrovirus infectivity in nondividing cells. J. Virol. 78:5670-5678. [DOI] [PMC free article] [PubMed] [Google Scholar]