Abstract
Recombination between two strains is a known phenomenon for enteroviruses replicating within a single cell. We describe a recombinant strain recovered from human stools, typed as coxsackievirus B4 (CV-B4) and CV-B3 after partial sequencing of the VP1 and VP2 coding regions, respectively. The strain was neutralized by a polyclonal CV-B3-specific antiserum but not by a CV-B4-specific antiserum. The nucleotide sequence analysis of the whole structural genomic region showed the occurrence of a recombination event at position 1950 within the VP3 capsid gene, in a region coding for the 2b antigenic site previously described for CV-B3. This observation evidences for the first time the occurrence of an interserotypic recombination within the VP2-VP3-VP1 capsid region between two nonpoliovirus enterovirus strains. The neutralization pattern suggests that the major antigenic site is located within the VP2 protein.
Enteroviruses are small, nonenveloped, positive single-stranded RNA viruses belonging to the family Picornaviridae. Human enteroviruses (HEV) comprise 68 serotypes, subdivided into five species on the basis of genetic properties: HEV-A to HEV-D and poliovirus (PV) (38). These viruses are responsible for a wide range of acute and chronic clinical manifestations (32).
The RNA genome, 7.5 kb long, is constituted by a single open reading frame flanked by 5′ and 3′ untranslated regions. The coding region is translated into a single polyprotein of 2,200 amino acids and is then processed to generate four structural proteins (VP1 to VP4) and seven nonstructural proteins (2A to 2C and 3A to 3D). The four structural proteins constitute the icosahedral capsid that contains the major antigenic determinants (22) and the principal attachment sites to the cellular receptors (for a review, see reference 10).
Recombination between two strains is a known phenomenon for enteroviruses replicating within a single cell. Although recombination has long been recognized as an important property of PV (9, 17), several recent publications have demonstrated that it is also extremely frequent in non-PV enteroviruses (2, 6-8, 15, 17-20, 24, 27, 28, 31, 35). Despite this high level of genetic instability, the occurrence of intra- and interserotypic recombination events in the VP2-VP3-VP1 structural coding region has been shown to be a rare phenomenon and up to now restricted to PV strains (5, 14, 21, 40). Structural requirements of the virion shell or of receptor binding were thought to be involved in the restriction of recombination within this region (17, 30, 36).
In this paper, we describe an interserotypic recombination event occurring in the VP3 coding region of a clinical strain of HEV-B and leading to a chimeric coxsackievirus B3 (CV-B3)/CV-B4 type.
MATERIALS AND METHODS
Virus isolation and identification.
The analyzed virus strain (SE-03-78616) was isolated from the KB cell line in 2003 from stools of a patient admitted for meningitis in the pediatric unit of Toulon Hospital (France). The strain was purified by the limiting dilution method in cell culture. Three different viral clones were used in neutralization and typing experiments.
Identification of strain SE-03-78616 was performed by neutralization of the cytopathic effect using A to H and J to P Lim-Benyesh Melnick intersecting pools of specific monovalent antisera (Statens Serum Institute, Copenhagen, Denmark) (23). Further neutralization experiments were conducted with specific polyclonal antisera directed toward the six CV-B serotypes (Statens Serum Institute, Copenhagen, Denmark). Clinical isolates of CV-B3, CV-B4, and CV-B5 were used as positive controls.
Molecular typing.
The molecular typing of strain SE-03-78616 was performed by partial sequencing of the VP1 gene, as described previously (29), and of the VP2 gene using original primers (AM12 and AM32) (Table 1). Viral RNA was extracted from 140 μl of tissue culture supernatant and then reverse transcribed using SuperScriptIII reverse transcriptase (Invitrogen, Cergy Pontoise, France). Amplification reactions were done with a proofreading Taq DNA polymerase (Platinum Pfx; Invitrogen, Cergy Pontoise, France). The amplified products were purified with the QIAquick gel extraction kit (QIAGEN, Courtaboeuf, France) and sequenced with the Genome Lab Dye Terminator Cycle Sequencing Quick Start kit (Beckman Coulter, Villepinte, France) according to the manufacturer's recommendations. Sequence alignment was performed with BLAST software (1).
TABLE 1.
Primers used for amplification of the P1 region
| Primera | Positionb | Polarity | Sequence (5′-3′) | Genomic region |
|---|---|---|---|---|
| RC1 | 614-634 | Sense | CCA TAT AGY TAT TGG ATT GGC | 5′ noncoding |
| RC2 | 1199-1180 | Antisense | GGR AAY TTC CAC CAC CAI CC | VP2 |
| AM12* | 961-980 | Sense | GAR GAR TGY GGI TAY AGY GA | VP2 |
| AM32* | 1531-1515 | Antisense | TTD ATC CAY TGR TGI GG | VP2 |
| RC3 | 1460-1479 | Sense | GAG AGT TGT ATA TAA CGC AG | VP2 |
| RC4 | 2609-2590 | Antisense | GCA TGG TGT CAC TTG GAT CC | VP1 |
| 292† | 2559-2574 | Sense | MIG CIG YIG ARA CNG G | VP1 |
| 222† | 2908-2890 | Antisense | CIC CIG GIG GIA YRW ACA T | VP1 |
| RC5 | 2814-2833 | Sense | GAC TTT TGT CAT AAC CAG CC | VP1 |
| RC6 | 3702-3694 | Antisense | ACI ACR CCY TCI CCI CCC AT | 2A |
Symbols: *, designed for molecular typing of enterovirus in the VP2 region; †, published in 2003 by Oberste et al. (29).
Relative to the genome sequence of CV-B3 reference strain Woodruff.
Sequencing of the full-length genome.
In an initial set of experiments, the entire genomic region that encodes the four structural proteins (VP1, VP2, VP3, and VP4) was sequenced (2,562 nucleotides) with five pairs of primers (Table 1). Additional reverse transcription-PCR assays were conducted to sequence the complete genome of the strain by using 17 pairs of primers targeting the whole genome of HEV-B (data not shown). The complete nucleotide sequence is available in the GenBank sequence database under the accession number EF371880.
Sequence, recombination, and phylogenetic analyses.
The P1 region (VP4 to VP1) sequence of the strain was compared to that of reference and clinical CV-B strains (http://www.picornaviridae.com/enterovirus/hev-b/hev-b_seqs.htm) with Clustal W (version 1.81) (39). Similar alignments were performed for the P2 (2A to 2C) and P3 (3A to 3D) regions by comparison to reference and clinical HEV-B strains.
The location of recombination events was determined by similarity plotting of the resulting alignment and bootscanning analysis (34) using the SimPlot program, version 2.5 (http://sray.med.som.jhmi.edu/SCRoftware/SimPlot) (16), with a sliding window of 200 nucleotides moving in 20-nucleotide steps.
Phylogenetic trees were designed by imputing the aligned sequences into the MEGA program (version 3.1) (13) and constructed with the neighbor-joining algorithm (33). Genetic distances were calculated with the Kimura-2 parameter model (12) with a transition/transversion ratio of 2.0, and the reliability of the trees was determined by bootstrap analysis with 1,000 pseudoreplicate data sets (11).
Nucleotide sequence accession numbers.
The GenBank accession numbers of the sequences used in the molecular analyses are provided in Fig. 1, 2, 3, and 5.
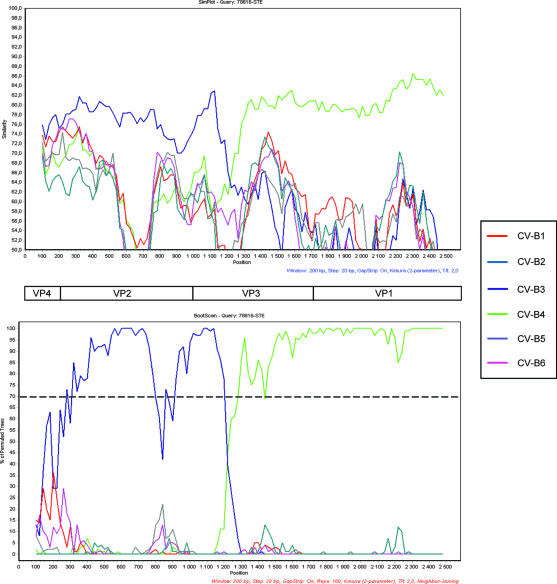
FIG. 1.
Similarity plot and bootscanning analysis of the P1 region comparing strain SE-03-78616 and CV-B strains with complete genomes available in databases. (Top) Similarity plot of strain SE-03-78616 versus CV-B strains constructed by the SimPlot program, version 2.5, with a sliding window of 200 nucleotides moving in 20-nucleotide steps. (Bottom) Bootscan graph of strain SE-03-78616 versus CV-B strains constructed by using the neighbor-joining tree algorithm, the Kimura-2 distance model, and 100 pseudoreplicates. The dotted line shows an arbitrary 70% reliability threshold. The following sequences (with GenBank accession numbers given in parentheses) were used: CV-B1 (AY186745 to AY186748, M16560); CV-B2 (AF081485, EF174468, EF174469); CVB-3 (AF231763 to AF231765, AY752944 to AY752946, M16572, M33854, M88483), CV-B4 (AF311939, DQ480420, X05690), CV-B5 (AF114383, AY875692, X67706), CV-B6 (AF039205).
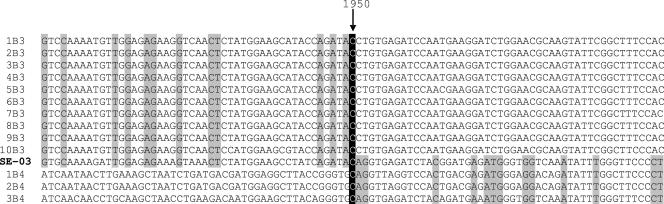
FIG. 2.
Location of the breakpoint site of strain SE-03-78616 by nucleotide sequence alignment with the CV-B3 and CV-B4 strains listed in Fig. 1 (1B3, AY752949; 2B3, AF231765; 3B3, AF231762; 4B3, M88483; 5B3, M33854; 6B3, AY752945; 7B3, AY752944; 8B3, AF231763; 9B3, M16572; 10B3, U57056; 1B4, DQ480420; 2B4, X05690; 3B4, AF311939). Regarding positions exhibiting base homology for all tested strains of a single serotype but divergence between the two serotypes, the nucleotide shared with strain SE-03-78616 is shown against a gray background. The breakpoint site is represented by a black background.
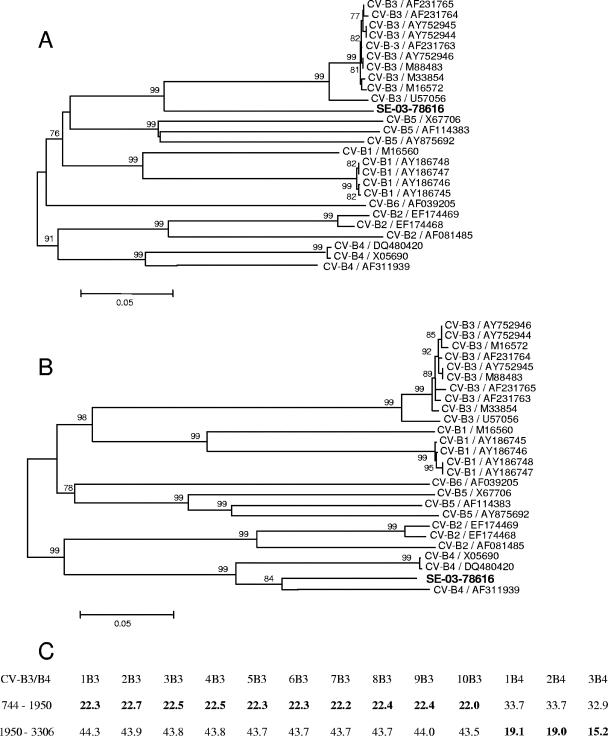
FIG. 3.
Phylogenetic relationships in the P1 region between strain SE-03-78616 and the CV-B strains listed in Fig. 1. (A) Phylogenetic tree of the genomic region situated upstream of the breakpoint site (1,206 nucleotides, position 745 to 1950). (B) Phylogenetic tree of the genomic region situated downstream of the breakpoint site (1,357 nucleotides, position 1950 to 3306). Only bootstrap values over 75% are shown at tree nodes. (C) Nucleotide divergence (derived from the MEGA program [13] and expressed as a percentage) between strain SE-03-78616 and CV-B3/CV-B4 strains (abbreviated according to the legend to Fig. 2), upstream and downstream of the breakpoint site.
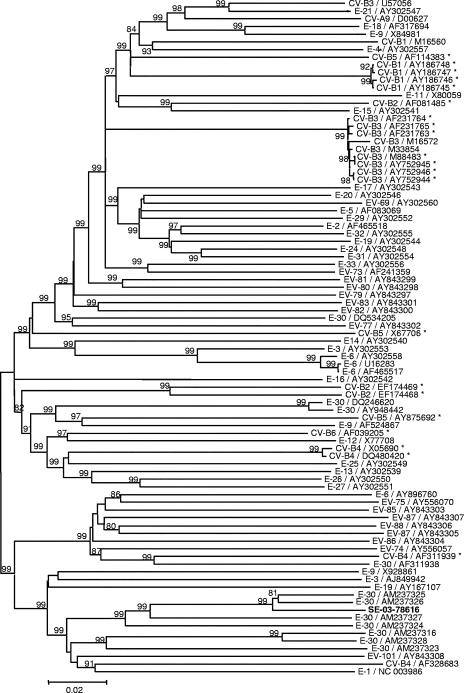
FIG. 5.
Phylogenetic tree showing relationships between strain SE-03-78616 and HEV-B strains in the P2-P3 region. Only bootstrap values over 75% are shown at tree nodes. The HEV-B strains are indicated by serotype and accession number in the GenBank database. Asterisks identify the strains used in Fig. 3.
RESULTS
Identification of strain SE-03-78616.
Strain SE-03-78616 was not neutralized by Lim-Benyesh Melnick intersecting pools of specific monovalent antisera. By molecular typing, it was identified as CV-B3 in the VP2 region and CV-B4 in the VP1 region. These results were confirmed by successive typing of three different clones.
Molecular characterization of strain SE-03-78616.
To further explore the discrepant results obtained by two different molecular typing strategies, the entire P1 region of the viral genome was sequenced. As depicted in Fig. 1, the obtained sequence (2,562 nucleotides) was shown to be close to that of CV-B3 in the 5′ part of the genome until the first third of VP3 and to that of CV-B4 after this position, suggesting the occurrence of an interserotypic recombination event in the capsid region. To locate precisely the breakpoint, the sequence was compared by alignment with other CV-B3 and CV-B4 strains. Figure 2 shows that the recombination site corresponds to nucleotide 1207 of the coding region (nucleotide 1950 with reference to the entire genome). For all positions shared by strains of the same serotype but diverging between the two serotypes, strain SE-03-78616 clustered with CV-B3 and CV-B4 strains upstream and downstream of the breakpoint, respectively (Fig. 2). One of the primer pairs used to sequence the structural coding region (RC3 and RC4 [Table 1]) covered unambiguously the recombinant region.
Phylogenetic analyses (Fig. 3) confirmed that strain SE-03-78616 is the result of a recombination event in the VP3 coding region between two CV-B types. Despite the fact that, in the region located upstream of the breakpoint, this strain is relatively distant from the other strains of CV-B3 used to construct the tree depicted in Fig. 3A, the nucleotide divergence is less than 25% (Fig. 3C), confirming that the strain belongs to the CV-B3 serotype in this region. By contrast, downstream of the breakpoint, the phylogenetic profile and nucleotide divergence data identify the strain as CV-B4 (Fig. 3B and 3C, respectively).
Functional analysis of the breakpoint site.
One hundred 50% tissue culture infective doses of three clones of strain SE-03-78616 were completely neutralized by a horse polyclonal anti-CV-B3 monovalent antiserum diluted 1:50 to 1:200 for at least 72 h; in contrast, no neutralization was observed under the same experimental conditions by antisera directed toward CV-B1, CV-B2, CV-B4, CV-B5, or CV-B6.
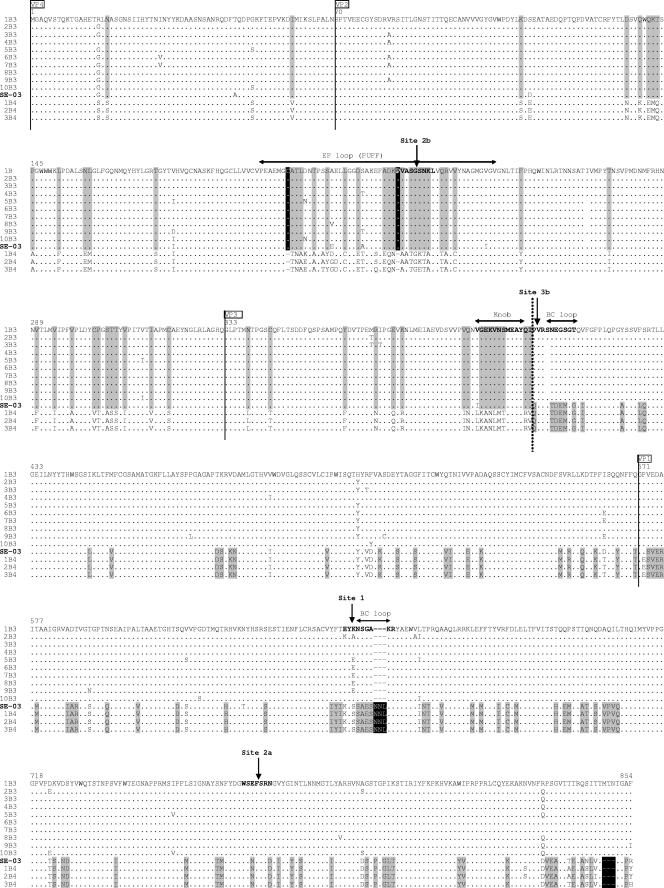
In order to further explore the putative relationship between the antigenic sites and the location of the breakpoint, an amino acid alignment was performed in the whole capsid region with reference to the available sequences of CV-B3 and CV-B4 strains (Fig. 4). The topography of the antigenic sites was represented according to the mapping described by Auvinen et al. (3). As shown in Fig. 4, the recombination site was found to be located in the central part of the 3b antigenic site in the VP3 protein. In addition, this alignment illustrates the analogy of amino acid sequences with CV-B3 in the 5′ part of the recombinant strain and CV-B4 in the 3′ part, confirming that this strain is the result of a recombinant event between these two CV-B serotypes. This finding is reinforced by the identification of significant differences in the amino acid sequence (deletions or insertions) between the two serotypes, notably in antigenic site 2b of VP2, in antigenic site 1 of VP1, and in the 3′-terminal part of the latter protein (Fig. 4).
FIG. 4.
Amino acid sequence alignment of the structural proteins of strain SE-03-78616 with the CV-B3 and CV-B4 strains listed in Fig. 1 and abbreviated according to the legend to Fig. 2. Regarding positions exhibiting amino acid homology for all tested strains of a single serotype but divergence between the two serotypes, the amino acid shared with strain SE-03-78616 is shown against a gray background. Deletions or insertions having the same characteristics are shown against a black background. The solid vertical lines delineate the four structural viral proteins (VP). The dotted vertical line locates the CV-B3/CV-B4 switch on the amino acid sequence of the recombinant strain. The antigenic sites described for CV-B3 by Auvinen et al. (3) are depicted in bold type and designated according to the nomenclature of those authors. The amino acids are numbered according to the sequence of the reference strain of CV-B3 (accession number M16572). The positions of the main tertiary structures described for CV-B3 (25) are indicated by double-headed horizontal arrows above the sequences.
Localization of other recombinant events in the nonstructural coding region of strain SE-03-78616.
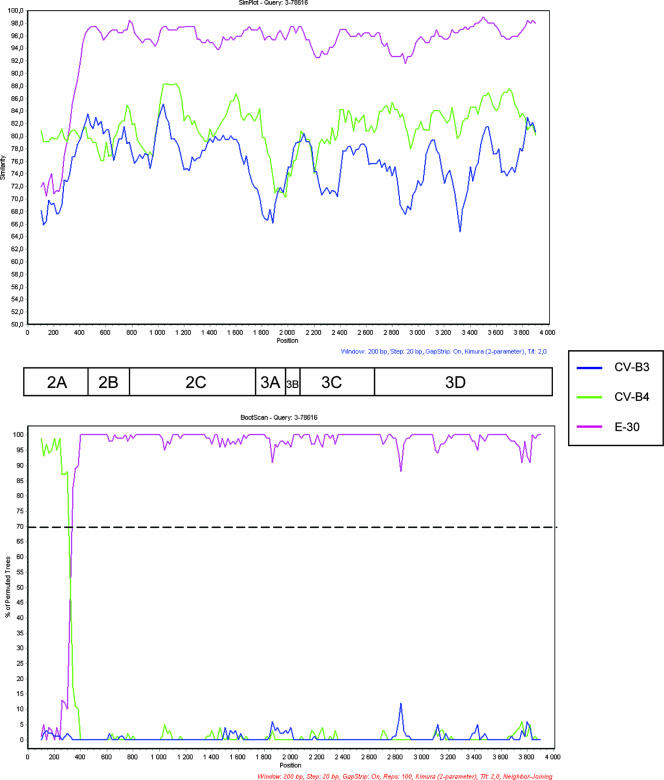
Figure 5 shows the phylogenic clustering of strain SE-03-78616 by comparison with different HEV-B strains in the nonstructural coding region. In the latter region, the strain was shown to be close to strains identified as echovirus 30 (E-30) in the P1 region. The similarity plots and the bootscanning analysis of the alignment of the sequences coding for the nonstructural proteins of SE-03-78616, E-30, CV-B3, and CV-B4 strains showed the presence of an additional breakpoint in the 2A region (Fig. 6). Taken together, these results indicate that SE-03-78616 is a chimeric virus constituted by sequences from three different HEV-B strains.
FIG. 6.
Similarity plot (Top) and bootscanning analysis (Bottom) of the P2-P3 region comparing strain SE-03-78616 with CV-B3 (U57056), CV-B4 (X05690), and E-30 (AM237325) strains. The graphs were constructed as described in the legend to Fig. 1.
DISCUSSION
Despite the fact that the enterovirus genome can be considered a “stable symbiosis of genes” (18), different studies have suggested that interserotypic recombination events within the capsid region are relatively rare (36, 37), whereas similar events are more common between members of the same serotype (CV-B1 [30], CV-A9 [35]). Recently, Lukashev et al. showed that the VP4 protein coding region could be a recombination hot spot within serotypes of HEV-B species and that, consequently, it is the VP2-VP3-VP1 block that must be considered relatively free of interserotypic recombination within the P1 region (19). By using phylogenetic compatibility matrices, Simmonds and Welch showed that this block was protected from recombination events within HEV-A, HEV-B, and HEV-C (37).
In this study, we analyzed a strain of HEV-B that represents an exception to the above rule, as it was clearly found to exhibit a recombination event within the VP3 gene, with a CV-B3 genome at the 5′ end and a CV-B4 genome at the 3′ end, as evidenced by bootscanning, alignment, nucleotide divergence, and phylogenetic analyses. A technical artifact of the polymerases was ruled out, since the recombination event was confirmed on three different clones. Except for PV serotypes that have been shown to recombine within the VP1 gene (5, 21, 40) and the VP2 genes (14), this is the first report of an intertypic recombination event within the capsid region of HEV.
The rarity of this naturally occurring phenomenon could be due to the fact that the recombinant strain would not be able to recognize its cellular receptor; however, in the present case, the two serotypes participating in the recombination have the same receptor (10). Another explanation could be linked to capsid constraints within the VP2-VP3-VP1 block that prevent the occurrence of recombination events. For strain SE-03-78616, the precise location of the recombination site is position 1950 of the VP3 gene. As shown in Fig. 4, the breakpoint is located in the βB barrel at the junction between the knob and the BC loop previously described in the tertiary structure of CV-B3 (25), a region known to harbor the 3b antigenic site of this serotype (3).
With regard to neutralization epitopes, it is striking to note that the recombinant strain was neutralized by a CV-B3 monovalent polyclonal antiserum but not by the Lim-Benyesh-Melnick pools of antisera. This finding could be explained by the fact that the two anti-CV-B3 neutralizing antisera are not directed toward the same epitope; alternatively, the dilution inherent to the constitution of pooled antisera could account for the lower sensitivity of this reagent. More interestingly, although strain SE-03-78616 exhibited a CV-B4-like VP1 region, it was not recognized by the CV-B4 monovalent antiserum. This finding suggests that the major neutralization antigenic site(s) is located in the proximal part of the VP2-VP3-VP1 block. In fact, Beatrice et al. reported that the VP2 peptide was the sole immunogen capable of inducing neutralizing antibodies against CV-B3 (4). These findings support the fact that typing methods based only on the VP1 region may underestimate the weight of the VP2/VP3 region in the antigenic variability of enteroviruses.
In complement to the description of a recombination event within the capsid region of strain SE-03-78616, the occurrence of another breakpoint was documented in its nonstructural coding region. This observation illustrates that recombination in the latter part of the enterovirus genome, which is very common in HEV-B circulating strains (36), is also possible in an isolate exhibiting a recombined capsid region.
Finally, from the taxonomic point of view, this finding suggests the putative existence of chimeric viruses within the HEV-B species which could be considered equivalents to the recombinant forms between different clades of human immunodeficiency virus type 1 strains belonging to subtype M (26), adding to the complexity of type identification within the HEV-B species. The extensive use of typing methods targeting two different parts of the capsid region, VP1 and VP2, will contribute to an evaluation of the impact of interserotypic recombination in terms of enterovirus identification and functional activity.
Acknowledgments
D.N. is supported by funds from the “Comité Mixte de Coopération Universitaire (CMCU) Franco-Tunisien” (program no. 03S0804) and the Doctoral School of Monastir.
Footnotes
Published ahead of print on 30 May 2007.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, P., K. Edman, and A. M. Lindberg. 2002. Molecular analysis of the echovirus 18 prototype. Evidence of interserotypic recombination with echovirus 9. Virus Res. 85:71-83. [DOI] [PubMed] [Google Scholar]
- 3.Auvinen, P., M. J. Makela, M. Roivainen, M. Kallajoki, R. Vainionpaa, and T. Hyypia. 1993. Mapping of antigenic sites of coxsackievirus B3 by synthetic peptides. APMIS 101:517-528. [DOI] [PubMed] [Google Scholar]
- 4.Beatrice, S. T., M. G. Katze, B. A. Zajac, and R. L. Crowell. 1980. Induction of neutralizing antibodies by the coxsackievirus B3 virion polypeptide, VP2. Virology 104:426-438. [DOI] [PubMed] [Google Scholar]
- 5.Blomqvist, S., A. L. Bruu, M. Stenvik, and T. Hovi. 2003. Characterization of a recombinant type 3/type 2 poliovirus isolated from a healthy vaccinee and containing a chimeric capsid protein VP1. J. Gen. Virol. 84:573-580. [DOI] [PubMed] [Google Scholar]
- 6.Bolanaki, E., C. Kottaridi, P. Markoulatos, L. Margaritis, and T. Katsorchis. 2006. Evolution of 2B and 2C genomic parts of species B coxsackie viruses. Phylogenetic study and comparison with other regions. Virus Genes 32:249-259. [DOI] [PubMed] [Google Scholar]
- 7.Chan, Y. F., and A. Sazaly. 2006. Phylogenetic evidence for inter-typic recombination in the emergence of human enterovirus 71 subgenotypes. BMC Microbiol. 6:74-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevaliez, S., A. Szendroï, V. Caro, J. Balanant, S. Guillot, G. Berencsi, and F. Delpeyroux. 2004. Molecular comparison of echovirus 11 strains circulating in Europe during an epidemic of multisystem hemorrhagic disease of infants indicates that evolution generally occurs by recombination. Virology 325:56-70. [DOI] [PubMed] [Google Scholar]
- 9.Cuervo, N. S., S. Guillot, N. Romanenkova, M. Combiescu, A. Aubert-Combiescu, M. Seghier, V. Caro, R. Crainic, and F. Delpeyroux. 2001. Genomic features of intertypic recombinant Sabin poliovirus strains excreted by primary vaccinees. J. Virol. 75:5740-5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans, D. J., and J. W. Almond. 1998. Cell receptors for picornaviruses as determinants of cell tropism and pathogenesis. Trends Microbiol. 6:198-202. [DOI] [PubMed] [Google Scholar]
- 11.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 12.Kimura, M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol 16:111-120. [DOI] [PubMed] [Google Scholar]
- 13.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 14.Kyriakopoulou, Z., C. Kottaridi, E. Dedepsidis, E. Bolanaki, S. Levidiotou-Stefanou, and P. Markoulatos. 2006. Molecular characterization of wild-type polioviruses isolated in Greece during the 1996 outbreak in Albania. J. Clin. Microbiol. 44:1150-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindberg, A. M., P. Andersson, C. Savolainen, M. N. Mulders, and T. Hovi. 2003. Evolution of the genome of human enterovirus B: incongruence between phylogenies of the VP1 and 3CD regions indicates frequent recombination within the species. J. Gen. Virol. 84:1223-1235. [DOI] [PubMed] [Google Scholar]
- 16.Lole, K. S., R. C. Bollinger, R. S. Paranjape, D. Gadkari, S. S. Kulkarni, N. G. Novak, R. Ingersoll, H. W. Sheppard, and S. C. Ray. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73:152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukashev, A. N. 2005. Role of recombination in evolution of enteroviruses. Rev. Med. Virol. 15:157-167. [DOI] [PubMed] [Google Scholar]
- 18.Lukashev, A. N., V. A. Lashkevich, O. E. Ivanova, G. A. Koroleva, A. E. Hinkkanen, and J. Ilonen. 2003. Recombination in circulating enteroviruses. J. Virol. 77:10423-10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lukashev, A. N., V. A. Lashkevich, O. E. Ivanova, G. A. Koroleva, A. E. Hinkkanen, and J. Ilonen. 2005. Recombination in circulating Human enterovirus B: independent evolution of structural and non-structural genome regions. J. Gen. Virol. 86:3281-3290. [DOI] [PubMed] [Google Scholar]
- 20.Lukashev, A. N., V. A. Lashkevich, G. A. Koroleva, J. Ilonen, and A. E. Hinkkanen. 2004. Recombination in uveitis-causing enterovirus strains. J. Gen. Virol. 85:463-470. [DOI] [PubMed] [Google Scholar]
- 21.Martin, J., E. Samoilovich, G. Dunn, A. Lackenby, E. Feldman, A. Heath, E. Svirchenvskaya, G. Cooper, M. Yermalovich, and P. D. Minor. 2002. Isolation of an intertypic poliovirus capsid recombinant from a child with vaccine-associated paralytic poliomyelitis. J. Virol. 76:10921-10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mateu, M. G. 1995. Antibody recognition of picornaviruses and escape from neutralization: a structural view. Virus Res. 38:1-24. [DOI] [PubMed] [Google Scholar]
- 23.Melnick, J. L., and I. L. Wimberly. 1985. Lyophilized combination pools of enterovirus equine antisera: new LBM pools prepared from reserves of antisera stored frozen for two decades. Bull. WHO 63:543-550. [PMC free article] [PubMed] [Google Scholar]
- 24.Mirand, A., C. Henquell, C. Archimbaud, H. Peigue-Lafeuille, and J. L. Bailly. 2007. Emergence of recent echovirus 30 lineages is marked by serial genetic recombination events. J. Gen. Virol. 88:166-176. [DOI] [PubMed] [Google Scholar]
- 25.Muckelbauer, J. K., M. Kremer, P. Minor, G. Diana, F. J. Dutko, J. Groarke, D. C. Pevear, and M. G. Rossmann. 1995. The structure of coxsackievirus B3 at 3.5 Å resolution. Structure 3:653-667. [DOI] [PubMed] [Google Scholar]
- 26.Najera, R., E. Delgado, L. Perez-Alvarez, and M. M. Thomson. 2002. Genetic recombination and its role in the development of the HIV-1 pandemic. AIDS 16:S3-S16. [DOI] [PubMed] [Google Scholar]
- 27.Norder, H., L. Bjerregaard, and L. O. Magnius. 2002. Open reading frame sequence of an Asian enterovirus 73 strain reveals that the prototype from California is recombinant. J. Gen. Virol. 83:1721-1728. [DOI] [PubMed] [Google Scholar]
- 28.Oberste, M. S., K. Maher, and M. A. Pallansch. 2004. Evidence for frequent recombination within species Human enterovirus B based on complete genomic sequences of all thirty-seven serotypes. J. Virol. 78:855-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberste, M. S., W. A. Nix, K. Maher, and M. A. Pallansch. 2003. Improved molecular identification of enteroviruses by RT-PCR and amplicon sequencing. J. Clin. Virol. 26:375-377. [DOI] [PubMed] [Google Scholar]
- 30.Oberste, M. S., S. Penaranda, and M. A. Pallansch. 2004. RNA recombination plays a major role in genomic change during circulation of coxsackie B viruses. J. Virol. 78:2948-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oprisan, G., M. Combiescu, S. Guillot, V. Caro, A. Combiescu, F. Delpeyroux, and R. Crainic. 2002. Natural genetic recombination between co-circulating heterotypic enteroviruses. J. Gen. Virol. 83:2193-2200. [DOI] [PubMed] [Google Scholar]
- 32.Pallansch, M. A., and R. Roos. 2007. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses, p. 839-893. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 5th ed., vol. 1. Lippincott Williams and Wilkins, Philadelphia, PA. [Google Scholar]
- 33.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 34.Salminen, M. O., J. K. Carr, D. S. Burke, and F. E. McCutchan. 1995. Identification of breakpoints in intergenotypic recombinants of HIV type 1 by bootscanning. AIDS Res. Hum. Retroviruses 11:1423-1425. [DOI] [PubMed] [Google Scholar]
- 35.Santti, J., T. Hyypia, L. Kinnunen, and M. Salminen. 1999. Evidence of recombination among enteroviruses. J. Virol. 73:8741-8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simmonds, P. 2006. Recombination and selection in the evolution of picornaviruses and other mammalian positive-stranded RNA viruses. J. Virol. 80:11124-11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simmonds, P., and J. Welch. 2006. Frequency and dynamics of recombination within different species of human enteroviruses. J. Virol. 80:483-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanway, G., F. Brown, and P. Christian. 2005. Picornaviridae, p. 757-778. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy—classification and nomenclature of viruses. Eighth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, Amsterdam, The Netherlands.
- 39.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang, C. F., H. Y. Chen, J. Jorba, H. C. Sun, S. J. Yang, H. C. Lee, Y. C. Huang, T. Y. Lin, P. J. Chen, H. Shimizu, Y. Nishimura, A. Utama, M. A. Pallansch, T. Miyamura, O. Kew, and J. Y. Yang. 2005. Intratypic recombination among lineages of type 1 vaccine-derived poliovirus emerging during chronic infection of an immunodeficient patient. J. Virol. 79:12623-12634. [DOI] [PMC free article] [PubMed] [Google Scholar]