Abstract
Three distinct chimpanzee Fabs against the A33 envelope glycoprotein of vaccinia virus were isolated and converted into complete monoclonal antibodies (MAbs) with human γ1 heavy-chain constant regions. The three MAbs (6C, 12C, and 12F) displayed high binding affinities to A33 (Kd of 0.14 nM to 20 nM) and may recognize the same epitope, which was determined to be conformational and located within amino acid residues 99 to 185 at the C terminus of A33. One or more of the MAbs were shown to reduce the spread of vaccinia virus as well as variola virus (the causative agent of smallpox) in vitro and to more effectively protect mice when administered before or 2 days after intranasal challenge with virulent vaccinia virus than a previously isolated mouse anti-A33 MAb (1G10) or vaccinia virus immunoglobulin. The protective efficacy afforded by anti-A33 MAb was comparable to that of a previously isolated chimpanzee/human anti-B5 MAb. The combination of anti-A33 MAb and anti-B5 MAb did not synergize the protective efficacy. These chimpanzee/human anti-A33 MAbs may be useful in the prevention and treatment of vaccinia virus-induced complications of vaccination against smallpox and may also be effective in the immunoprophylaxis and immunotherapy of smallpox and other orthopoxvirus diseases.
The recent outbreaks of human cases of monkeypox (35) and concerns that variola (smallpox) virus might be used as a biological weapon (18) have led to renewed interest in the prevention and therapy of pox diseases. While vaccination is generally safe and effective for prevention of smallpox, it is well documented that various adverse reactions in individuals have been caused by vaccination with existing licensed vaccines (16). Furthermore, although vaccination can provide long-term protection, time is required for development of the immune response. Given the unpredictable nature of emerging and bioterrorist-related infections, it is important to have available a rapid intervention that does not depend on active immunization. Passive administration of neutralizing monoclonal antibodies (MAbs) is such an intervention. Studies have shown that antibodies play the most important role in vaccine-mediated protection against orthopoxviruses (4, 12, 32, 47). The importance of antibodies in biodefense has been discussed in detail by Casadevall (7).
There are two major forms of infectious vaccinia virus (VACV): intracellular mature virus (MV) and extracellular enveloped virus (EV). Most of the MV remains within the cell until lysis, at which time it is disseminated as free virus, but some virus particles are wrapped in additional membranes and exocytosed as EV. Most EV remains attached to the outside of the plasma membrane and is responsible for direct cell-to-cell spread; however, in some strains appreciable amounts are released and these can infect distant cells in vivo and can cause comet-like satellite plaques in vitro (5, 6). The EV membrane is fragile and is disrupted prior to fusion of the inner MV membrane with the cell (24). It has been speculated that the MV is responsible for host-to-host spread, whereas EV is important for virus dissemination within the host as well as in cultured cells (34, 43).
Viral proteins A27, L1, H3, D8, and A17 are known targets for MV-neutralizing antibodies, and immunization with A27 (21, 36), L1 (14, 19), H3 (10, 36), or D8 (36) protected against challenge with virulent virus in mice or macaques. In contrast, viral glycoproteins B5 and A33 are targets for antibodies that protect against EV, and immunization with these two proteins also can protect against VACV in animal models (3, 14, 17, 19, 27). In general, the best protection has been achieved with a combination of MV- and EV-specific target proteins (14, 15, 20, 48). Consistent with the results from protein immunization, the passive administration of MAbs against B5, A33, L1, and A27 (8, 17, 28, 38) also conferred protection in animal models.
Although some human anti-VACV neutralizing MAbs have been reported (40), with one exception, all of the neutralizing MAbs used to date in passive transfer studies are of rodent origin and thus require humanization to be useful. The exception, a chimpanzee/human MAb against the B5 glycoprotein (8) was derived from the bone marrow of a chimpanzee that had been vaccinated with VACV. Because of the near identity of chimpanzee and human immunoglobulin G (IgG) (13, 41), this antibody should not require humanization, thus increasing its therapeutic value.
In order to expand the repertoire of useful reagents against poxviruses, we panned the same phage library against recombinant A33 glycoprotein. Three anti-A33 antibodies were isolated and characterized extensively.
MATERIALS AND METHODS
Reagents.
Recombinant truncated A33 protein consisting of amino acids 89 to 185 was produced in a baculovirus expression system and was used as a panning antigen for selection of A33-reactive phage. Restriction enzymes and other enzymes used in molecular cloning were purchased from New England BioLabs (Beverly, MA). Oligonucleotides were synthesized by Invitrogen (Carlsbad, CA). Anti-His-horseradish peroxidase (HRP) conjugate, anti-human Fab-HRP conjugate, and anti-human Fab-agarose beads were purchased from Sigma (St. Louis, MO). Nickel-agarose beads were from Invitrogen. VACV WR (ATCC VR-1354), IHD-J (from S. Dales, Rockefeller University), and VV-NP-siinfekl-EGFP (expressing enhanced green fluorescent protein) were grown in HeLa S3 cells (ATCC CCL-2.2), purified, and the titer determined in BS-C-1 cells as described previously (11). A mouse anti-A33 MAb from hybridoma 1G10 (19) was purified from ascitic fluid (Taconic Biotechnology, Germantown, NY). VACV Ig (VIG) (Cangene) was obtained from BEI Resource and the CDC (C. Allen, Drug Service, Atlanta, GA).
Animals.
Chimpanzees 3863 and 3915 were immunized twice approximately 19 years apart (initially at Bioqual, Inc., Rockville, MD and subsequently at the University of Texas M. D. Anderson Cancer Center, Bastrop, TX) with VACV WR (31). Bone marrow was aspirated from the iliac crests of these animals 11 weeks after the second immunization. Mice were purchased from Taconic Biotechnology (Germantown, NY). All animal experiments were conducted at facilities that are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International and performed under protocols approved by the respective institutions as well as the NIAID Animal Care and Use Committee and the NIH Interagency Animal Model Committee.
Library construction and selection.
The library was constructed and selected for phages specific to A33 by the method described previously (8).
Sequence analysis.
The genes encoding the variable regions of the heavy (VH) and light (VL) chains of A33-specific clones were sequenced, and their corresponding amino acid sequences were aligned. The presumed family usage and germ line origin were established for each VH and VL gene by search of V-Base (9).
Expression and purification of Fab and IgG.
Fab and IgG were expressed and purified by the procedure described previously (8).
ELISA.
A33 and nonrelated proteins (bovine serum albumin [BSA], cytochrome c, thyroglobulin, lysozyme, and phosphorylase b) were tested for binding specificity in an enzyme-linked immunosorbent assay (ELISA) as described before (8).
Affinity measurement.
Since A33 dimerizes, the procedure described previously (8) was modified slightly. Briefly, A33 was immobilized to the surface and the kinetics of binding and dissociation of anti-A33 Fab were recorded for 40 to 50 min and 3 to 5 h, respectively, at anti-A33 Fab concentrations between 1 and 100 nM. Kd and koff were determined by globally modeling the kinetic data as a continuous distribution of affinity and rate constants and integration of the resulting peaks (44). For the anti-A33 Fabs that exhibited very slow kinetics, additional solution competition experiments were conducted as a control, in which different concentrations of A33 were preincubated with anti-A33 Fab for 24 h and the concentration of unbound anti-A33 Fab was determined from the initial slope of the surface binding when passing the mixture over the A33-functionalized surface.
Epitope mapping.
Pilot experiments showed that A33 peptides produced either through in vitro translation or in the cytoplasm of Escherichia coli were not reactive to anti-A33 MAbs in ELISA, whereas A33 peptides that were secreted into the periplasmic space of E. coli reacted well. Therefore, an ELISA based on soluble A33 peptides of different lengths was performed to map the epitopes recognized by anti-A33 MAbs. Since the panning antigen was a truncated A33 protein containing residues 89 to 185, DNA fragments encoding A33 peptides covering amino acids 89 to 185, 99 to 185, 101 to 185, 110 to 185, and 89 to 179 were cloned into plasmid vector pAK400 (23) and transformed into E. coli TG1. Inserts and the presence of a His tag were confirmed by DNA sequencing. The production of A33 peptides in E. coli was performed as described for expression of soluble Fabs and their purification from the cell lysate on nickel columns (8). The amounts of the A33 peptides were estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (NuPAGE MOP; Invitrogen), and the identity of the peptides was confirmed by Western blotting with anti-His-HRP.
For the binding assays, the wells of a 96-well plate were coated with serial dilutions of each peptide and the peptides were tested for binding to anti-His as well as to anti-A33. The term “positive binding” meant that peptide bound to anti-His and anti-A33 equally well; “negative binding” meant the peptide bound to anti-His, but not to anti-A33; and “partial binding” meant the peptide bound to both anti-His and anti-A33, but preferentially to anti-His.
Comet reduction assay for VACV and variola virus.
The comet reduction analysis was performed according to the procedure described previously (8).
Passive immunization and challenge with VACV strain WR.
Groups of 7-week old female BALB/c mice (Taconic Biotechnology, Germantown, NY) were inoculated intraperitoneally with antibody diluted in phosphate-buffered saline (PBS). Nonimmunized controls were injected with the same volume of PBS. Either 24 h after or 48 h before immunization, mice were challenged intranasally (i.n.) with 104 to 106 PFU of VACV WR as described previously (28). Mice were weighed daily for 16 days and sacrificed if their weight diminished to 70% of the initial weight, in accordance with NIAID Animal Care and Use protocols.
Statistical analysis.
Statistical differences in weight loss between groups of mice were assessed by analysis of variance using StatView software (SAS Institute, Inc., Cary, NC).
RESULTS
Isolation and characterization of VACV A33-specific Fabs.
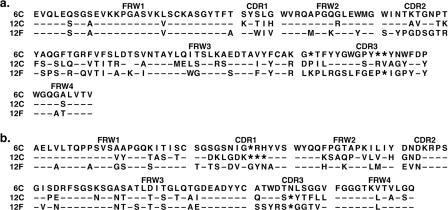
The chimpanzee Fab-displaying phage library was panned against recombinant VACV A33 protein, and 96 clones were randomly picked and screened for binding to A33 by phage ELISA with BSA as a negative control. Ninety percent of the phage clones preferentially bound to A33. DNA sequencing of the variable regions of heavy (VH) and light (VL) chains from 16 positive clones identified three distinct clones, designated 6C, 12C, and 12F. The sequences of VH and VL genes and the closest human germ line gene for each are shown in Fig. 1a and b and Table 1.
FIG. 1.
Deduced amino acid sequences of variable domains of heavy (a) and light (b) chains of chimpanzee/human anti-A33 MAbs. Complementarity-determining regions (CDR1, CDR2, and CDR3) and framework regions (FWR1, FWR2, and FWR3) are indicated above the sequence alignment. Substitutions relative to 6C are expressed as single letters denoting amino acids. Dashes indicate identical residues, and asterisks denote the absence of corresponding residues relative to the longest sequence.
TABLE 1.
Human Ig germ line genes most closely related to chimpanzee heavy and light chains of anti-A33 MAbs
| MAb | Germ line gene in:a
|
||||||
|---|---|---|---|---|---|---|---|
| VH family | Segment
|
Vλ family | Segment
|
||||
| VH | D | JH | Vλ | Jλ | |||
| 6C | VH7 | VI-4.1B | D3-10 | J5b | VλI | 1b.366F5 | Jλ 3b |
| 12C | VH1 | DP-25 | D3-10 | J4b | VλIII | 3r.9C5 | Jλ 2/3a |
| 12F | VH5 | DP-73 | D3-3 | J5b | VλII | 2a2.272A12 | Jλ 2/3a |
The closest human VH and Vλ germ lines were identified by the V-Base database.
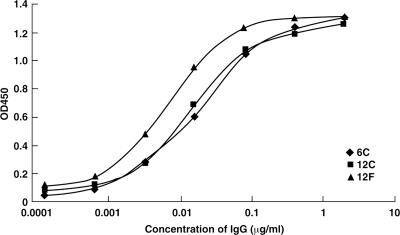
The Fab sequences were converted into full-length IgG with human γ1 constant regions, and the IgGs were examined for their binding specificity by ELISA. Anti-A33 IgGs did not bind to the unrelated proteins (BSA, thyroglobulin, phosphorylase b, lysozyme, and cytochrome c) (data not shown) but bound strongly to A33 protein with the IgG concentration for 50% maximum binding ranging from 0.05 nM to 0.2 nM (7.5 ng/ml to 30 ng/ml) (Fig. 2).
FIG. 2.
ELISA titration of anti-A33 MAbs. The wells of ELISA plates were coated with recombinant A33 or unrelated proteins (BSA, thyroglobulin, lysozyme, and phosphorylase b) and then incubated with serial dilutions of 6C, 12C, or 12F IgG. Bound IgG was detected by the addition of peroxidase-conjugated anti-human Fc followed by tetramethylbenzidine (TMB) substrate. Anti-A33 MAbs did not bind to the unrelated proteins (data not shown). OD450, optical density at 450 nm.
Epitope recognized by the anti-A33 MAb.
Competition ELISA indicated that the three MAbs recognized the same or closely related epitopes since they competed with each other for binding to A33 protein (data not shown). Therefore, 6C was initially chosen for epitope mapping as it had been used extensively in neutralization assays. Two His-tagged soluble A33 peptides generated by N-terminal deletions (89-185 and 99-185) were produced in bacteria and affinity purified through a nickel column. Both expressed peptides reacted strongly with MAbs 6C, 12C, and 12F in the ELISA. In Western blots performed under reducing conditions, the two peptides reacted with anti-His but not with anti-A33 MAb 6C, which suggested that the epitope recognized by the anti-A33 MAb was conformational (data not shown). Further deletions from either the N or C terminus of A33 (peptide 89-179, 101-185, or 110-185) abrogated expression in E. coli and prevented finer mapping. Therefore, the epitope for anti-A33 MAbs may be conformational and is located within amino acid residues 99 to 185 at the C terminus of A33.
Binding affinity and in vitro comet reduction activity.
The affinity of the three chimpanzee/human MAbs for A33 protein was measured by surface plasmon resonance (SPR) biosensor. A Kd range of 0.14 nM to 20 nM and a dissociation rate constant of ∼10−4/s to ∼ 10−5/s were observed for the three MAbs (Table 2). MAb 12F had the highest affinity, as determined by ELISA or SPR.
TABLE 2.
Binding affinities of anti-A33 MAbsa
| MAb | kon (M−1 s−1) | koff (s−1) | Kd (nM) |
|---|---|---|---|
| 6C | 6.8 × 103 | 1.4 × 10−4 | 20.0 |
| 12C | 4.6 × 104 | 2.2 × 10−5 | 0.46 |
| 12F | 1.9 × 105 | 2.6 × 10−5 | 0.14 |
Recombinant A33 proteins were immobilized on the SPR sensor surfaces. The antibody (Fab) binding responses to A33 protein were collected at a range of concentrations between 1 and 100 nM of Fab. The kinetic and equilibrium constants were determined by modeling the surface binding kinetics as a distribution of rate and affinity constants (44).
In vitro neutralization activity of anti-A33 MAbs was measured by the comet reduction assay, an established method that measures the inhibition of comet-like plaque formation by the released EV form of the virus (2, 25). The IHD-J strain of VACV is usually used for this assay because of the release of large amounts of EV. Comet-shaped plaques formed in the absence of antibodies, but the formation of comets was completely blocked by the addition of an excess of rabbit hyperimmune serum to VACV (Fig. 3a). The monoclonal anti-A33 clones 6C, 12C, and 12F reduced the formation of comet-like plaques of VACV EV in a dose-dependent manner (Fig. 3a). Similarly, the formation of comet-shaped plaques of the Solaimen strain of variola virus EV was inhibited by 6C in a dose-dependent manner (Fig. 3b), indicating that the 6C MAb possessed neutralizing activity against EV of both viruses.
FIG. 3.
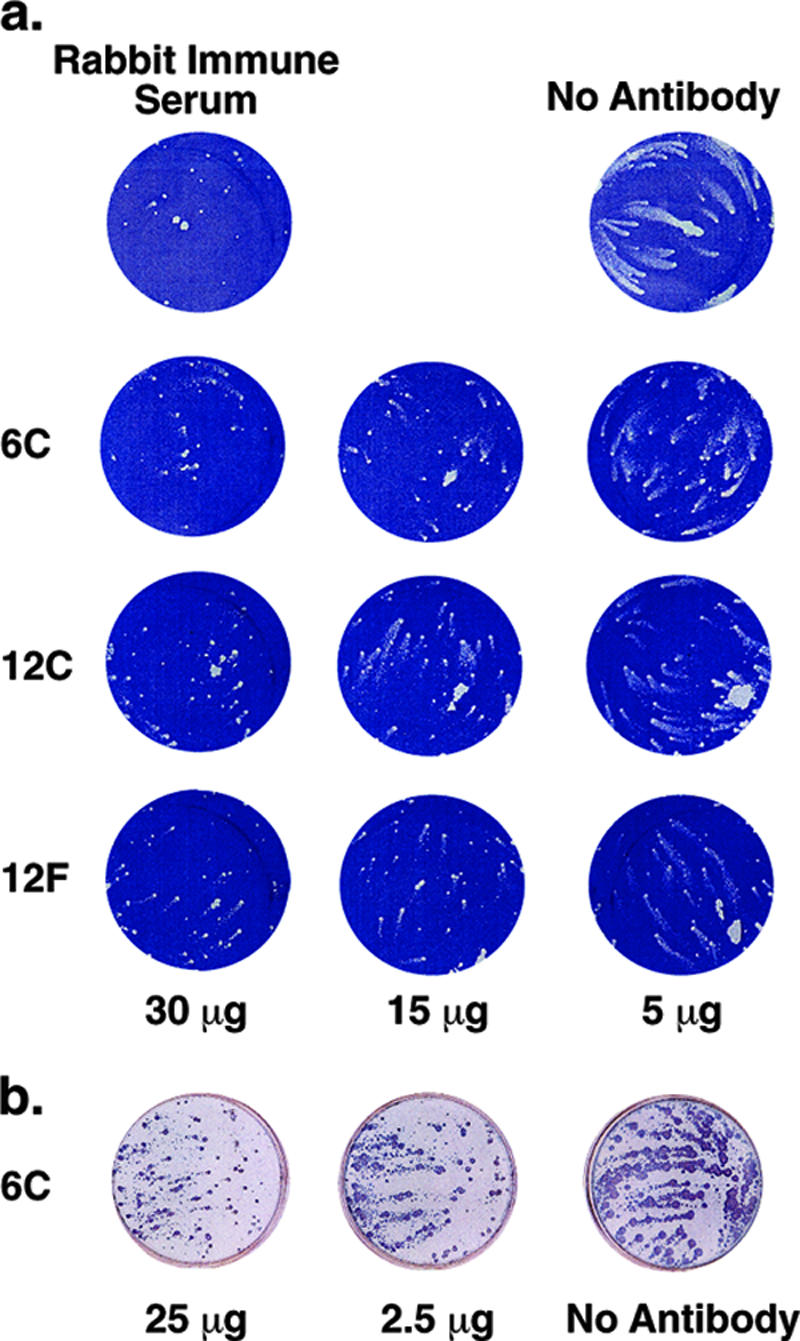
In vitro neutralizing activity of anti-A33 MAbs measured by a comet reduction assay. (a) In vitro neutralization of VACV by 6C, 12C, and 12F MAbs. BS-C-1 cells were infected with approximately 50 PFU of VACV, strain IHD-J. After 2 h at 37°C, the monolayer was washed and fresh medium containing indicated amounts of chimpanzee anti-A33 6C, 12C, or 12F was added. PBS and rabbit hyperimmune serum served as negative and positive controls, respectively. After 48 h, the monolayers were stained with crystal violet. (b) In vitro neutralization of variola virus by 6C MAb. Monolayers of BS-C-40 cells were infected with the Solaimen strain of variola virus. After 1 h, the medium was aspirated; cells were washed twice and overlaid with RPMI medium containing 25 μg, 2.5 μg, or 0 μg of 6C IgG. The plates were then incubated in a CO2 incubator for 4 days at 35.5°C. Cells were fixed and reacted with polyclonal rabbit anti-variola virus antibody. Following incubation with goat anti-rabbit-HRP conjugate, comets were visualized by addition of TruBlue peroxidase substrate.
Protection of mice against challenge with virulent VACV.
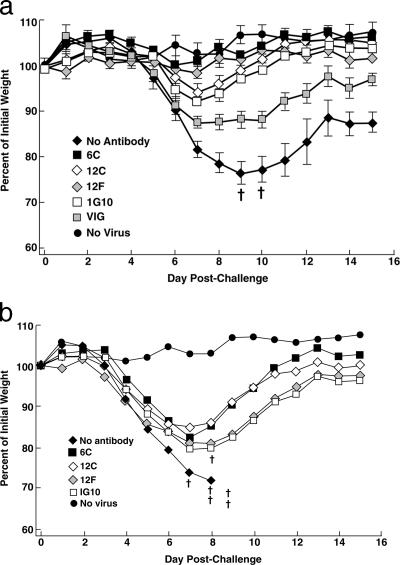
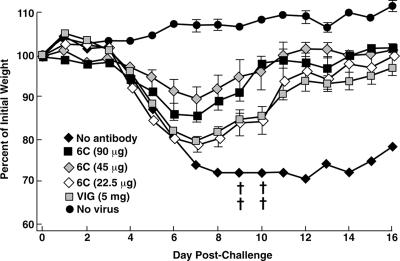
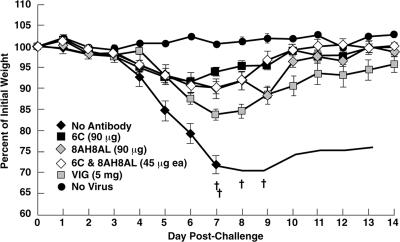
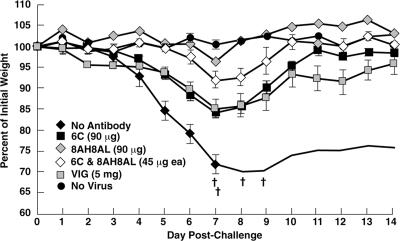
The BALB/c mouse pneumonia model of VACV disease (42, 46) was used for the following reasons: the i.n. route is believed to be the major avenue for transmission of variola virus; weight loss and death are correlated with viral replication in the lungs, allowing the onset and progress of disease to be monitored by a noninvasive method that reduces the number of animals needed for significance (26); and the model has been used for active immunization studies with live VACV as well as with individual VACV proteins (14) and for passive immunization studies with antisera prepared against VACV and VACV proteins (26, 29, 48). To compare the protective efficacies of different antibodies, groups of five BALB/c mice were inoculated intraperitoneally with 90 μg of purified chimpanzee/human MAbs 6C, 12C, and 12F; mouse MAb 1G10 (19); or 5 mg of human VACV Ig (VIG). After 24 h, the mice were inoculated i.n. with 105 PFU of VACV WR. As shown in Fig. 4a, the control inoculated mice, which did not receive antibody, lost weight continuously, starting on day 5 following challenge, and two of the five mice were sacrificed because their weights declined to 70% of starting weight. In contrast, the mice that were injected with MAb 6C or 12F did not lose weight after the identical virus challenge, suggesting that full protection was achieved. The mice receiving 12C experienced only slight weight loss, followed by rapid recovery. The protection achieved by the chimpanzee/human MAbs was statistically significant compared to the no-antibody control (P < 0.0001). The mice receiving the mouse MAb 1G10 or human VIG were protected (Fig. 4a), but the protection was inferior to that afforded by the chimpanzee/human MAbs. The differences were statistically significant (P < 0.0001 and P = 0.0037 for MAbs 6C and 12F versus MAb 1G10 and P < 0.0001 for MAbs 6C and 12F versus VIG). When the challenge dose was increased to 106 PFU, all of the mice not given antibodies died and all of the mice receiving antibodies suffered heavy weight losses. However, mice receiving chimpanzee/human MAbs lost less weight and all of the mice survived, whereas mice receiving mouse MAb lost more weight and one of the mice died (Fig. 4b). These data confirmed that the chimpanzee/human anti-A33 MAbs were superior to mouse anti-A33 MAb in protecting against VACV challenge in the mouse model.
FIG. 4.
Comparison of protective efficacy between different MABs and VIG. Groups of five BALB/c mice were inoculated intraperitoneally with 90 μg of purified IgG or 5 mg of VIG. Twenty-four hours later, mice were challenged i.n. with 105 (a) or 106 (b) PFU of the WR strain of VACV. Mice were weighed individually, and mean percentages of starting weight ± standard error were plotted. Controls were unimmunized (no antibody) or unchallenged (no virus). †, died naturally or killed because of 30% weight loss.
To determine the minimum effective dose of anti-A33 6C, groups of mice (five mice per group) were given decreasing amounts of 6C (90, 45, and 22.5 μg per mouse) or a single 5-mg dose of human VIG (2.5× the recommended human dose on a weight basis). Twenty-four hours later, the animals were challenged i.n. with 5 × 104 PFU of VACV WR. As shown in Fig. 5, mice receiving 45 μg or 90 μg of 6C lost less weight than mice receiving 5 mg of human VIG and comparable levels of protection were achieved with 22.5 μg of 6C and 5 mg of VIG. Two anti-A33 MAbs, 6C and 12F, had an approximately 140-fold difference in affinities (Table 2), yet had very similar protective efficacies at a dose of 90 μg (Fig. 4). To confirm this functional similarity, we used a lower dose, 22.5 μg. The two antibodies again showed similar protective efficacies; this was also comparable to that achieved by 5 mg of VIG (data not shown). This indicated that 6C and 12F had very similar neutralizing potencies despite their difference in affinities.
FIG. 5.
Minimal effective dose of anti-A33 6C for protection in mice. Groups of five BALB/c mice were inoculated intraperitoneally with 90, 45, and 22.5 μg of purified IgG or 5 mg of VIG. Twenty-four hours later, mice were challenged i.n. with 5 × 104 PFU of the WR strain of VACV. Mice were weighed individually, and mean percentages of starting weight ± standard error were plotted. Controls were unimmunized (no antibody) or unchallenged (no virus). †, died naturally or killed because of 30% weight loss.
To assess the therapeutic value of MAb 6C and to determine if there was a synergistic effect between anti-B5 MAb 8AH8AL (8) and anti-A33 MAb 6C, we compared the protective efficacies of the individual MAbs and VIG with that of a combination of the two MAbs in mice challenged with WR before or after administration of antibody (Fig. 6 and 7). The protective efficacy of the two MAbs together was not greater than that of the individual MAbs. MAb 6C appeared to protect slightly better than MAb 8AH8AL when administered before challenge, whereas 8AH8AL provided much stronger protection than 6C when administered after challenge (P < 0.0001 on day 7).
FIG. 6.
Prophylactic protection in mice by anti-B5 and by anti-A33 MAbs and investigation of synergism with a combination of the two MAbs. Groups of five BALB/c mice were inoculated intraperitoneally with 90 μg of purified IgG of each MAb, with a mixture of 45 μg of each of the two MAbs, or with 5 mg of human VIG. Twenty-four hours later, mice were challenged i.n. with 5 × 105 PFU of the WR strain of vaccinia virus. Mice were weighed individually, and mean percentages of starting weight ± standard error were plotted. Controls were unimmunized (no antibody) or unchallenged (no virus). †, died naturally or killed because of 30% weight loss.
FIG. 7.
Therapeutic protection in mice by anti-B5 MAbs and anti-A33 MAbs and investigation of synergism with the combination of two MAbs. Groups of five BALB/c mice were inoculated i.n. with 5 × 105 PFU of VACV WR. After 48 h, the mice were injected intraperitoneally with 90 μg of purified IgG of each MAb, a mixture of 45 μg of each of the two MAbs, or 5 mg of human VIG. Mice were weighed individually, and mean percentages of starting weight ± standard error were plotted. Controls were unimmunized (no antibody) or unchallenged (no virus). †, died naturally or killed because of 30% weight loss.
DISCUSSION
Our previous study demonstrated that chimpanzee MAbs against VACV B5 protein (an EV-specific protein) alone were sufficient not only to protect mice from lethal challenge with virulent VACV but also to confer therapeutic protection of mice when administered 2 days after infection (8). Here, we show that similar protection is achieved by chimpanzee/human MAbs against VACV A33 protein (another EV-specific protein). These results reinforce the notion that antibodies against EV play a critical role in protective immunity (1, 45).
Two types of in vitro EV neutralization assays have been developed. In the first assay, EVs isolated from the medium of cells are incubated with antibody and then allowed to adsorb to cells and plaque formation is measured under a solid or semisolid overlay. In the second, the antibody is added to a liquid overlay following adsorption of virus to cells and the formation of satellite plaques known as comets is measured. In both cases, the IHD-J strain of VACV is usually used because of the large amount of EV released due to a point mutation in the A34 open reading frame (6). For unknown reasons, antibody to the B5 protein is effective in both assays, whereas antibody to A33 only works in the comet reduction assay. Consequently anti-B5 contributes the most to direct EV-neutralizing activity of immune sera (3, 37). Even though anti-A33 antibody cannot directly neutralize EV, it has been shown to be protective in animal models (17, 29). In this study, the direct comparison of chimpanzee/human anti-B5 and anti-A33 MAbs in protective efficacy in the mouse model suggested that anti-B5 and anti-A33 may play similar roles in protective immunity in vivo since roughly comparable levels of protection were observed for the two antibodies. The absence of synergism with the combination of anti-B5 and anti-A33 is consistent with a previous finding (28) and supports the notion that better protection may require a combination of EV-specific and MV-specific neutralizing MAbs.
In general, antibody affinity correlates well with protective efficacy (8, 30). However, this was not the case for anti-A33 MAbs. We found that although 6C had about 140-fold-lower affinity than 12F, the protective efficacies were similar for the two antibodies. One possible explanation for this unexpected result is that because A33 forms dimers in nature (33, 39), the binding strength between A33 and bivalent IgG is determined by avidity rather than affinity, which is measured by monovalent binding. Such avidity resulting from multivalent binding could be substantially higher than intrinsic affinity. For example, the measured enhancement between monovalent and multivalent binding for anti-DNP IgM is in the range of 106 to 107 (22). This hypothesis is supported by the observation that in an ELISA binding assay using IgG, where binding strength is measured by avidity rather than affinity, the IgG concentration for 50% maximum binding for 6C was 0.2 nM and that for 12F was 0.05 nM, only a fourfold difference (Fig. 2). This difference is much smaller than the affinity measured by Fab (Table 2).
We showed that the anti-A33 MAbs recognized a conformational epitope at the C terminus of A33, but because of difficulty in expressing smaller truncated peptides in bacteria, we could not determine the exact epitope. There are four cysteine residues in A33 protein, which are located at residues 100, 109, 126, and 180. We suspect that these cysteine residues are important in maintaining correct folding of the peptide since the deletion of any one of the cysteines, which occurred in peptides 89-179, 101-185, and 110-185, prevented expression. This hypothesis was supported by the observation that in the Western blots, denatured A33 protein was reactive with 6C under nonreducing conditions, but it was not reactive under reducing conditions (data not shown).
Our anti-A33 MAbs exhibited higher protective efficacy than did a mouse anti-A33 MAb or human VIG. Competition ELISA showed that chimpanzee/human and mouse MAbs did not compete with each other for binding to A33 (data not shown), suggesting that they recognize different epitopes, which may contribute to their different protective efficacies. The likely reason that human VIG is inferior to the chimpanzee/human MAbs in animal studies is that the concentration of protective antibodies in VIG is low.
The use of anti-A33 MAbs in treatment of smallpox vaccine-associated complications would overcome the limitations posed by VIG, such as low titer of neutralizing activity, variability, and risk of transmission of infectious agents. It is especially important that anti-VACV A33 MAbs cross-reacted with variola virus A33 and reduced variola virus comet formation in vitro. The latter result indicates that the epitope is readily accessible on the surface of the variola virus EV. Unfortunately, there is no good animal model of smallpox that can be used to test protection. Amino acid sequence comparison of A33 at residues 99 to 185 (containing the neutralization epitope recognized by the anti-A33 MAb) from vaccinia, variola and monkeypox viruses revealed that variola and monkeypox viruses have the same five amino acid differences from VACV and all three viruses have four cysteines at positions 100, 109, 126, and 180. Therefore, it is likely that anti-A33 MAb would recognize monkeypox virus in addition to variola virus. It is conceivable that an anti-A33 MAb alone or in conjunction with other MAbs could be used directly in treatment of bioterrorist-associated smallpox or in cases of naturally acquired monkeypox (35).
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, NIAID, and was partly supported by NIH contracts NO2-OR-04021 and NO1-AO-62713 for providing research resources. G.C. and R.J.E. were funded by National Institutes of Health grants, NIAID Mid-Atlantic Regional Center of Excellence grant U54 AI057168, and a University of Pennsylvania Health Research Faculty Development block grant.
We thank Yi-Hua Zhou for initial work and helpful discussions in epitope mapping and Victoria A. Olson and Kevin Karem for help with comet reduction assays of variola virus. We are grateful to J. C. Whitbeck, L. Aldaz-Carroll, and Huan Lou for providing us with the vaccinia virus protein A33.
Footnotes
Published ahead of print on 20 June 2007.
REFERENCES
- 1.Appleyard, G., and C. Andrews. 1974. Neutralizing activities of antisera to poxvirus soluble antigens. J. Gen. Virol. 23:197-200. [DOI] [PubMed] [Google Scholar]
- 2.Appleyard, G., A. J. Hapel, and E. A. Boulter. 1971. An antigenic difference between intracellular and extracellular rabbitpox virus. J. Gen. Virol. 13:9-17. [DOI] [PubMed] [Google Scholar]
- 3.Bell, E., M. Shamim, J. C. Whitbeck, G. Sfyroera, J. D. Lambris, and S. N. Isaacs. 2004. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology 325:425-431. [DOI] [PubMed] [Google Scholar]
- 4.Belyakov, I. M., P. Earl, A. Dzutsev, V. A. Kuznetsov, M. Lemon, L. S. Wyatt, J. T. Snyder, J. D. Ahlers, G. Franchini, B. Moss, and J. A. Berzofsky. 2003. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc. Natl. Acad. Sci. USA 100:9458-9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blasco, R., and B. Moss. 1992. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J. Virol. 66:4170-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blasco, R., J. R. Sisler, and B. Moss. 1993. Dissociation of progeny vaccinia virus from the cell membrane is regulated by a viral envelope glycoprotein: effect of a point mutation in the lectin homology domain of the A34R gene. J. Virol. 67:3319-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casadevall, A. 2002. Antibodies for defense against biological attack. Nat. Biotechnol. 20:114. [DOI] [PubMed] [Google Scholar]
- 8.Chen, Z., P. Earl, J. Americo, I. Damon, S. K. Smith, Y. H. Zhou, F. Yu, A. Sebrell, S. Emerson, G. Cohen, R. J. Eisenberg, J. Svitel, P. Schuck, W. Satterfield, B. Moss, and R. Purcell. 2006. Chimpanzee/human mAbs to vaccinia virus B5 protein neutralize vaccinia and smallpox viruses and protect mice against vaccinia virus. Proc. Natl. Acad. Sci. USA 103:1882-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook, G. P., and I. M. Tomlinson. 1995. The human immunoglobulin VH repertoire. Immunol. Today 16:237-242. [DOI] [PubMed] [Google Scholar]
- 10.Davies, D. H., M. M. McCausland, C. Valdez, D. Huynh, J. E. Hernandez, Y. Mu, S. Hirst, L. Villarreal, P. L. Felgner, and S. Crotty. 2005. Vaccinia virus H3L envelope protein is a major target of neutralizing antibodies in humans and elicits protection against lethal challenge in mice. J. Virol. 79:11724-11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Earl, P. L., B. Moss, L. S. Wyatt, and M. W. Carroll. 1998. Current protocols in molecular biology, vol. 2. Greene & Wiley, New York, NY.
- 12.Edghill-Smith, Y., H. Golding, J. Manischewitz, L. R. King, D. Scott, M. Bray, A. Nalca, J. W. Hooper, C. A. Whitehouse, J. E. Schmitz, K. A. Reimann, and G. Franchini. 2005. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 11:740-747. [DOI] [PubMed] [Google Scholar]
- 13.Ehrlich, P. H., Z. A. Moustafa, K. E. Harfeldt, C. Isaacson, and L. Ostberg. 1990. Potential of primate monoclonal antibodies to substitute for human antibodies: nucleotide sequence of chimpanzee Fab fragments. Hum. Antib. Hybrid. 1:23-26. [PubMed] [Google Scholar]
- 14.Fogg, C., S. Lustig, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2004. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J. Virol. 78:10230-10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogg, C. N., J. L. Americo, S. Lustig, J. W. Huggins, S. K. Smith, I. Damon, W. Resch, P. L. Earl, D. M. Klinman, and B. Moss. 2007. Adjuvant-enhanced antibody responses to recombinant proteins correlates with protection of mice and monkeys to orthopoxvirus challenges. Vaccine 25:2787-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fulginiti, V. A., A. Papier, J. M. Lane, J. M. Neff, and D. A. Henderson. 2003. Smallpox vaccination: a review, part II. Adverse events. Clin. Infect. Dis. 37:251-271. [DOI] [PubMed] [Google Scholar]
- 17.Galmiche, M. C., J. Goenaga, R. Wittek, and L. Rindisbacher. 1999. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology 254:71-80. [DOI] [PubMed] [Google Scholar]
- 18.Henderson, D. A. 1999. The looming threat of bioterrorism. Science 283:1279-1282. [DOI] [PubMed] [Google Scholar]
- 19.Hooper, J. W., D. M. Custer, C. S. Schmaljohn, and A. L. Schmaljohn. 2000. DNA vaccination with vaccinia virus L1R and A33R genes protects mice against a lethal poxvirus challenge. Virology 266:329-339. [DOI] [PubMed] [Google Scholar]
- 20.Hooper, J. W., D. M. Custer, and E. Thompson. 2003. Four-gene-combination DNA vaccine protects mice against a lethal vaccinia virus challenge and elicits appropriate antibody responses in nonhuman primates. Virology 306:181-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooper, J. W., E. Thompson, C. Wilhelmsen, M. Zimmerman, M. A. Ichou, S. E. Steffen, C. S. Schmaljohn, A. L. Schmaljohn, and P. B. Jahrling. 2004. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J. Virol. 78:4433-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hornick, C. L., and F. Karuch. 1972. Antibody affinity. 3. The role of multivalance. Immunochemistry 9:325-340. [DOI] [PubMed] [Google Scholar]
- 23.Krebber, A., S. Bornhauser, J. Burmester, A. Honegger, J. Willuda, H. R. Bosshard, and A. Pluckthun. 1997. Reliable cloning of functional antibody variable domains from hybridomas and spleen cell repertoires employing a reengineered phage display system. J. Immunol. Methods 201:35-55. [DOI] [PubMed] [Google Scholar]
- 24.Law, M., G. C. Carter, K. L. Roberts, M. Hollinshead, and G. L. Smith. 2006. Ligand-induced and nonfusogenic dissolution of a viral membrane. Proc. Natl. Acad. Sci. USA 103:5989-5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Law, M., R. Hollinshead, and G. L. Smith. 2002. Antibody-sensitive and antibody-resistant cell-to-cell spread by vaccinia virus: role of the A33R protein in antibody-resistant spread. J. Gen. Virol. 83:209-222. [DOI] [PubMed] [Google Scholar]
- 26.Law, M., M. M. Putz, and G. L. Smith. 2005. An investigation of the therapeutic value of vaccinia-immune IgG in a mouse pneumonia model. J. Gen. Virol. 86:991-1000. [DOI] [PubMed] [Google Scholar]
- 27.Law, M., and G. L. Smith. 2001. Antibody neutralization of the extracellular enveloped form of vaccinia virus. Virology 280:132-142. [DOI] [PubMed] [Google Scholar]
- 28.Lustig, S., C. Fogg, J. C. Whitbeck, R. J. Eisenberg, G. H. Cohen, and B. Moss. 2005. Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J. Virol. 79:13454-13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lustig, S., C. Fogg, J. C. Whitbeck, and B. Moss. 2004. Synergistic neutralizing activities of antibodies to outer membrane proteins of the two infectious forms of vaccinia virus in the presence of complement. Virology 328:30-35. [DOI] [PubMed] [Google Scholar]
- 30.Maynard, J. A., C. B. Maassen, S. H. Leppla, K. Brasky, J. L. Patterson, B. L. Iverson, and G. Georgiou. 2002. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat. Biotechnol. 20:597-601. [DOI] [PubMed] [Google Scholar]
- 31.Moss, B., G. L. Smith, J. L. Gerin, and R. H. Purcell. 1984. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature 311:67-69. [DOI] [PubMed] [Google Scholar]
- 32.Panchanathan, V., G. Chaudhri, and G. Karupiah. 2006. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J. Virol. 80:6333-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Payne, L. G. 1992. Characterization of vaccinia virus glycoproteins by monoclonal antibody precipitation. Virology 187:251-260. [DOI] [PubMed] [Google Scholar]
- 34.Payne, L. G. 1980. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J. Gen. Virol. 50:89-100. [DOI] [PubMed] [Google Scholar]
- 35.Perkins, S. 2003. Monkeypox in the United States. Contemp. Top. Lab. Anim. Sci. 42:70, 72. [PubMed] [Google Scholar]
- 36.Pulford, D. J., A. Gates, S. H. Bridge, J. H. Robinson, and D. Ulaeto. 2004. Differential efficacy of vaccinia virus envelope proteins administered by DNA immunization in protection of BALB/c mice from a lethal intranasal poxvirus challenge. Vaccine 22:3358-3366. [DOI] [PubMed] [Google Scholar]
- 37.Putz, M. M., C. M. Midgley, M. Law, and G. L. Smith. 2006. Quantification of antibody responses against multiple antigens of the two infectious forms of Vaccinia virus provides a benchmark for smallpox vaccination. Nat. Med. 12:1310-1315. [DOI] [PubMed] [Google Scholar]
- 38.Ramirez, J. C., E. Tapia, and M. Esteban. 2002. Administration to mice of a monoclonal antibody that neutralizes the intracellular mature virus form of vaccinia virus limits virus replication efficiently under prophylactic and therapeutic conditions. J. Gen. Virol. 83:1059-1067. [DOI] [PubMed] [Google Scholar]
- 39.Roper, R. L., L. G. Payne, and B. Moss. 1996. Extracellular vaccinia virus envelope glycoprotein encoded by the A33R gene. J. Virol. 70:3753-3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmaljohn, C., Y. Cui, S. Kerby, D. Pennock, and K. Spik. 1999. Production and characterization of human monoclonal antibody Fab fragments to vaccinia virus from a phage-display combinatorial library. Virology 258:189-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schofield, D. J., W. Satterfield, S. U. Emerson, and R. H. Purcell. 2002. Four chimpanzee monoclonal antibodies isolated by phage display neutralize hepatitis A virus. Virology 292:127-136. [DOI] [PubMed] [Google Scholar]
- 42.Smee, D. F., K. W. Bailey, M. H. Wong, and R. W. Sidwell. 2001. Effects of cidofovir on the pathogenesis of a lethal vaccinia virus respiratory infection in mice. Antivir. Res. 52:55-62. [DOI] [PubMed] [Google Scholar]
- 43.Smith, G. L., and A. Vanderplasschen. 1998. Extracellular enveloped vaccinia virus. Entry, egress, and evasion. Adv. Exp. Med. Biol. 440:395-414. [PubMed] [Google Scholar]
- 44.Svitel, J., A. Balbo, R. A. Mariuzza, N. R. Gonzales, and P. Schuck. 2003. Combined affinity and rate constant distributions of ligand populations from experimental surface binding kinetics and equilibria. Biophys. J. 84:4062-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turner, G. S., and E. J. Squires. 1971. Inactivated smallpox vaccine: immunogenicity of inactivated intracellular and extracellular vaccinia virus. J. Gen. Virol. 13:19-25. [DOI] [PubMed] [Google Scholar]
- 46.Williamson, J. D., R. W. Reith, L. J. Jeffrey, J. R. Arrand, and M. Mackett. 1990. Biological characterization of recombinant vaccinia viruses in mice infected by the respiratory route. J. Gen. Virol. 71:2761-2767. [DOI] [PubMed] [Google Scholar]
- 47.Wyatt, L. S., P. L. Earl, L. A. Eller, and B. Moss. 2004. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc. Natl. Acad. Sci. USA 101:4590-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao, Y., L. Aldaz-Carroll, A. M. Ortiz, J. C. Whitbeck, E. Alexander, H. Lou, H. L. Davis, T. J. Braciale, R. J. Eisenberg, G. H. Cohen, and S. N. Isaacs. 2007. A protein-based smallpox vaccine protects mice from vaccinia and ectromelia virus challenges when given as a prime and single boost. Vaccine 25:1214-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]