Abstract
Enterovirus 71 (EV71) is a causative agent of hand, foot, and mouth disease and is also sometimes associated with serious neurological disorders. In this study, we characterized the antigenicity and tissue specificity of an attenuated strain of EV71 [EV71(S1-3′)], which belongs to genotype A, in a monkey infection model. Three cynomolgus monkeys were inoculated with EV71(S1-3′), followed by lethal challenge with the parental virulent strain EV71(BrCr-TR) via an intravenous route on day 45 postinoculation of EV71(S1-3′). Monkeys inoculated with EV71(S1-3′) showed a mild neurological symptom (tremor) but survived lethal challenge by virulent EV71(BrCr-TR) without exacerbation of the symptom. The immunized monkey sera showed a broad spectrum of neutralizing activity against different genotypes of EV71, including genotypes A, B1, B4, C2, and C4. For the strains examined, the sera showed the highest neutralization activity against the homotype (genotype A) and the lowest neutralization activity against genotype C2. The order of decreasing neutralization activity of sera was as follows: A > B1 > C4 > B4 > C2. To examine the tissue specificity of EV71(S1-3′), two monkeys were intravenously inoculated with EV71(S1-3′), followed by examination of virus distribution in the central nervous system (CNS) and extraneural tissues. In the CNS, EV71(S1-3′) was isolated only from the spinal cord. These results indicate that EV71(S1-3′) acts as an effective antigen, although this attenuated strain was still neurotropic when inoculated via the intravenous route.
Enterovirus 71 (EV71) is a small nonenveloped virus with a genome of single-strand positive RNA of about 7,500 nucleotides and belongs to the genus Enterovirus of the family Picornaviridae (8, 48). EV71 is classified as Human enterovirus species A along with some coxsackie A (CA) viruses, such as CA10 and CA16 (8, 44). CA10, CA16, and EV71 cause hand, foot, and mouth disease (HFMD) and herpangina. EV71 infection is also sometimes associated with severe neurological diseases, such as brain stem encephalitis and poliomyelitis-like paralysis, mainly in infants and young children (11, 34, 56). The neuropathogenic features of EV71 were first emphasized during an outbreak in Bulgaria in 1975 in which poliomyelitis-like paralysis was the major symptom (21.1% of patients), with a high fatality rate (29.5%) among the paralytic cases (51). In recent large-scale outbreaks of HFMD in Malaysia (1997) (1, 50) and Taiwan (1998 and 2000) (20, 28, 29, 55), several fatal encephalitis cases were reported. In the EV71 outbreak in Taiwan in 1998, out of 129,106 cases of HFMD or herpangina, there were 405 severe cases, including 78 fatal cases (20); thus, the severity rate of this outbreak was <0.3%. These findings underscore the high neuropathogenicity of EV71 as well as poliovirus (PV), which causes poliomyelitis in 0.1 to 1.0% of infected individuals (reviewed in reference 36).
Most of the fatal EV71 cases in Taiwan were in young children (age, ≤5 years) and involved pulmonary edema and/or pulmonary hemorrhage (20). These symptoms were of neurogenic origin, and brain stem involvement (direct destruction of the vasomotor and respiratory centers) was critical (10, 21, 25, 31, 57). Disseminated infection of EV71 in the central nervous system (CNS) might in part explain the EV71-specific neuropathogenesis (40).
Molecular epidemiological studies indicate that EV71 consists of at least 10 genotypes (A, B1 to -5, and C1 to -4) (7, 26, 33, 37, 49). The prototype BrCr strain is the sole member of genotype A (8). In the HFMD outbreak in Malaysia in 1997, the predominant genotype was B3, but in the outbreak in Taiwan in 1998, the predominant genotype was C2. Isolates from the outbreak in Bulgaria in 1975 and the outbreak in Hungary in 1978 belong to genotype B1. In more recent EV71 outbreaks in Asia, genotype C4 was predominant (26, 27, 37). These findings indicate that, in general, the genotype is not the sole determinant of the observed pathogenesis of EV71 (14, 50). The relationship between EV71 genotypes and cross-neutralizing reactivity remains unclear.
Recently, we generated an attenuated strain of EV71 [EV71(S1-3′)] derived from the prototype BrCr strain by defined genetic manipulation (5). The manipulation was based on the temperature-sensitive determinants of the type 1 PV vaccine strain (Sabin 1) (45), some of which are located in the conserved regions of the enterovirus genome, i.e., the 5′ nontranslated region (NTR), the 3Dpol, and the 3′ NTR (24, 43, 53). Monkeys inoculated with EV71(S1-3′) exhibited a mild, nonlethal neurological disorder with limited spread in the CNS on day 10 postinoculation (p.i.).
In the present study, we characterized the antigenicity and tissue specificity of EV71(S1-3′) in cynomolgus monkeys. We examined the tissue specificity of EV71(S1-3′) in the CNS and extraneural tissues. We examined the humoral immune response of monkeys inoculated with EV71(S1-3′) and the antigenicity of EV71(S1-3′) against EV71 strains belonging to genotypes A, B1, B4, C2, and C4.
MATERIALS AND METHODS
Cells and viruses.
Vero cells (derived from African green monkey kidney cells) and RD cells (derived from human rhabdomyosarcoma) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). Vero cells were used for preparation of stocks of the viruses EV71(S1-3′) and EV71(BrCr-TR), titration of those viruses, and isolation of viruses from monkey tissues. RD cells were used for preparation of stocks of other virus strains, titration of those viruses, and neutralization assays. The following EV71 strains were used: EV71(BrCr-TR), a genotype A temperature-resistant variant of the prototype BrCr strain; EV71(S1-3′), a genotype A temperature-sensitive mutant of the prototype BrCr strain; Nagoya (genotype B1) (16); C7-Osaka (genotype B4); 1095 (genotype C2) (49); 75-Yamagata-2003 (genotype C4); 2399-Yamagata-2003 (genotype C4); and 1530-Yamagata-2003 (genotype C4) (26, 27, 37). In cynomolgus monkeys, EV71(BrCr-TR) has the neurovirulent phenotype of the BrCr strain and EV71(S1-3′) has an attenuated phenotype (5, 17, 39, 40). The virus stocks of EV71(BrCr-TR) and EV71(S1-3′) were prepared in Vero cells by RNA transfection of the transcripts derived from corresponding infectious clones, as described elsewhere (5). The virus stocks of other strains were prepared in RD cells by RNA transfection of the transcripts derived from corresponding infectious clones [EV71(Nagoya) and EV71(Nagoya-HIS)] or by direct amplification from the original virus stocks. For the enzyme-linked immunosorbent assay (ELISA), EV71(Nagoya-HIS) was propagated from the virus stock in RD cells cultured in serum-free medium (VP-SFM; Gibco).
General methods of molecular cloning.
Escherichia coli strain XL10gold (Stratagene) was used for the preparation of plasmids. Ligation of DNA fragments was performed using a Quick Ligation kit (New England BioLabs). Site-directed mutagenesis (SDM) was performed using KOD plus DNA polymerase (Toyobo) (47).
RNA extraction, RT-PCR, and sequencing.
Viral genomic RNA was extracted from the culture fluid of infected cells using a High-pure viral RNA purification kit (Roche). Reverse transcription-PCR (RT-PCR) was performed using a ReverTra-Plus kit (TOYOBO). PCR products were purified using a PCR purification kit (QIAGEN). Direct sequence analysis of full-length genomic sequences of EV71(Nagoya) was performed using DNA fragments amplified by RT-PCR as the templates of the sequencing. The 5′ end of the viral genome was sequenced using the 5′RACE system, version 2.0 (Invitrogen), according to the manufacturer's instructions. The 3′ end of viral genomes was sequenced using an RT-PCR product obtained using the primers 7200F+ and EcoRI-3END (Table 1). DNA sequencing was performed using a BigDye Terminator v3.0 cycle sequencing ready reaction kit (Applied Biosystems), and the results were analyzed using an ABI PRIZM 3130 genetic analyzer (Applied Biosystems).
TABLE 1.
Primers used for construction of infectious clones of EV71(Nagoya) and EV71 (Nagoya-HIS)
| Primer | Sequencea |
|---|---|
| 7200F+ | AACACTCAAGATCACGTGCGCTCCC |
| EcoRI-3END- | ACTGGAATTCTTTTTTTTTTTTTTTTTTTTTTTTTV |
| SnaBI-T7-Nagoya+ | TTAATACGTATTAATACGACTCACTATAGGTTAAAACAGCCTGTGGGTTGTTCC |
| Nagoya2200+ | CTACGTGGTTCCAATTGGGGCGCC |
| Nagoya3800- | CTAGCTTCCACATATATGAGGCTGG |
| A2MluI- | AAAAACGCGTTTTTTTTTTTTTTTTTTTTTTTTTTGCTATTCTGG |
| NagoyaVP1-HIS+ | CACCATCATCACCATCACACTAACCCAAATGGTTATGCTAACTG |
| NagoyaVP1-HIS- | GTGTGATGGTGATGATGGTGAGTACCCTCAAGAGGGAGGTCTATCTCC |
The variable sequence position in the primer is expressed according to the IUPAC system. Sequences read from the 5′ position on the left.
RNA transfection.
RNA transcripts were obtained using a RiboMAX large-scale RNA production system T7 kit (Promega), using AvrII-linearized DNA of the infectious clones of EV71(Nagoya) or EV71(Nagoya-HIS) as the template. The in vitro-synthesized RNA transcripts were transfected onto monolayers of RD cells in six-well plates (Falcon), using the DEAE-dextran method, followed by incubation at 37°C in 10% FCS-DMEM (2 ml per well) (30). The cells were harvested when all the cells exhibited the cytopathic effect (CPE) and were then stored at −70°C. The titers of recovered viruses were about 107 50% cell culture infectious doses (CCID50) per ml.
Virus titration.
The virus titer was determined by measuring the CCID50 in a microtitration assay using RD cells, as described elsewhere (40). Briefly, inoculated RD cells were cultured at 37°C for 7 days and were then observed for CPE. The CCID50 was calculated using the Behrens-Kärber method (23).
Titration of neutralization activity of monkey serum.
The neutralization titer of monkey serum was determined using two different methods: (i) observation of CPE in infected cells (CPE method), and (ii) counting the number of infected cells by indirect immunofluorescence (IF method) (2).
For the CPE method, twofold dilutions of each monkey serum were prepared using 10% FCS-DMEM. Then, in 96-well plates (Falcon; two wells per dilution), 50 μl of each dilution was mixed with 50 μl of 10% FCS-DMEM containing 20 CCID50 of an EV71 strain, followed by incubation at 37°C for 3 h. Then, 100 μl of RD cell suspension (containing 2.0 × 105 cells) was added to each well, followed by culturing at 37°C for 7 days for observation of CPE. The reciprocal of the highest dilution of serum that protected cells from infection in at least one of the two inoculated wells (per dilution) was recorded as the neutralization titer.
For the IF method, we used the following infectious doses of EV71 in 50 μl of 10% FCS-DMEM, which each yielded about 100 infected cells, as detected by indirect immunofluorescence: EV71(BrCr-TR), 3.8 × 102 CCID50; EV71(Nagoya), 1.9 × 102 CCID50; EV71(C7-Osaka), 3.3 × 102 CCID50; EV71(1095), 1.1 × 102 CCID50; EV71(75-Yamagata-2003), 2.1 × 103 CCID50; EV71(2399-Yamagata-2003), 5.6 × 104 CCID50; EV71(1530-Yamagata-2003), 2.3 × 102 CCID50. These doses were incubated with 10-fold dilutions of monkey sera in 50 μl of 10% FCS-DMEM, or were incubated with 50 μl of 10% FCS-DMEM without monkey sera (control), in 96-well plates (Falcon) at 37°C for 3 h. After incubation, 100 μl of RD cell suspension (2.0 × 105cells) was added, followed by culturing at 37°C for 16 h, except for cells inoculated with 75-Yamagata-2003, which were cultured at 37°C for 8 h because 75-Yamagata-2003 spreads faster than other strains in RD cells. Cells were fixed with 3% paraformaldehyde in phosphate-buffered saline [PBS(-); 10 mM phosphate buffer, pH 7.0, 137 mM NaCl, and 2.6 mM KCl] at room temperature for 10 min and were then incubated with rabbit anti-EV71(C7-Osaka) hyperimmune serum [1:100 dilution in 0.1% Triton X-100-PBS(-)] at 37°C for 1 h. After washing three times with PBS(-), goat anti-rabbit immunoglobulin G (IgG; H+L) conjugated with fluorescein isothiocyanate [1:300 dilution in 0.1% Triton X-100/PBS(-); Zymed] was added to the cells, followed by incubation at 37°C for 20 min. The cells were observed with a Biozero fluorescence microscopy system (KEYENCE). The number of infected cells obtained in the control wells (without serum) was designated as 100%, and the percentage of neutralization was calculated for each dilution. The 50% neutralization units (NU50) for 50 μl of monkey serum was calculated according to the Behrens-Kärber method (23).
Construction of infectious cDNA clones of EV71(Nagoya) and EV71(Nagoya-HIS).
A DNA fragment, containing 4 kb of the 5′ region of the viral genome of EV71(Nagoya), was amplified by RT-PCR using the primers SnaBI-T7-Nagoya+ and Nagoya3800- (Table 1). The resultant cDNA fragment was cloned into plasmid pEV71(BrCr-TR), which contains the infectious cDNA of strain BrCr-TR (5), after digestion by SnaBI and AgeI. Next, the 3′-end sequence of EV71(Nagoya) was amplified by RT-PCR using the primers Nagoya2200+ and A2MluI- and was then cloned into the above construct after digestion by AgeI and MluI. The resultant construct was sequenced and then differences in nucleotides between this construct and the parental Nagoya strain were corrected by SDM using appropriate primers. This infectious clone of EV71(Nagoya) was designated as pEV71(Nagoya).
We constructed an infectious clone of EV71(Nagoya-HIS), which is a mutant of EV71(Nagoya) that contains a histidine tag in the BC loop of VP1 protein between amino acid residues 100 and 101 on the VP1 protein. The clone was constructed from pEV71(Nagoya) by SDM using the primers NagoyaVP1-HIS+ and NagoyaVP1-HIS-. This infectious clone of EV71(Nagoya-HIS) was designated as pEV71(Nagoya-HIS).
Detection of anti-EV71 IgM and IgG in monkey serum.
A virus solution of EV71(Nagoya-HIS) (1.0 × 108 CCID50/ml) was diluted with PBS(-) (1:10 dilution) and then added to HisGrab nickel-coated plates (Pierce) (100 μl per well). The plates were incubated at 4°C overnight for the adsorption of EV71(Nagoya-HIS) virions to the plates. After adsorption, 1% skim milk-PBS(-) was added to the plates (100 μl per well), which were then incubated at room temperature for 3 h. The plates were washed three times with 0.05% Tween 20-PBS(-) [T-PBS(-)], followed by addition of 100 μl of diluted monkey serum [1:10,000 or 1:20,000 dilution with 0.5% skim milk-0.5% Tween 20-PBS(-), for detection of IgM or IgG, respectively] and incubation at room temperature for 1 h. The plates were washed three times with T-PBS(-), followed by addition of 100 μl of horseradish peroxidase (HRP)-conjugated goat anti-monkey IgM or IgG antibody per well [1:10,000 dilution with 0.5% skim milk-0.5% Tween 20-PBS(-); Nordic Immunology] for the detection of IgM or IgG, respectively. The plates were incubated at room temperature for 1 h and were then washed three times with T-PBS(-). Finally, 100 μl of substrate solution (0.01% 3,3′,5,5′-tetramethylbenzidine) per well was added to the plates. After sufficient incubation at room temperature, the reaction was stopped by adding 100 μl of 2 N H2SO4 per well. Then, the optical density at 450 nm was measured for each well using a Benchmark Plus microplate spectrophotometer (Bio-Rad).
Monkey neurovirulence test.
Five female cynomolgus monkeys (Macaca fascicularis; age, 7 to 23 years) were inoculated with EV71(S1-3′), followed by lethal challenge with EV71(BrCr-TR). All animal procedures were approved by the Committee for Biosafety and Animal Handling and the Committee for Ethical Regulation of the National Institute of Infectious Diseases, Japan. Animal care, breeding, virus inoculation, and observation were performed in accordance with the guidelines of these committees.
Under light anesthesia with ketaral and xylazine, 1 ml of EV71(S1-3′) virus solution (containing 107 CCID50 of virus) was intravenously inoculated into the right tibial vein. Monkeys were examined daily for neurological manifestations for the first 10 days and were examined weekly from day 10 p.i. to day 45 p.i. On day 45 p.i., three monkeys were challenged with a lethal dose (107 CCID50) of EV71(BrCr-TR) (5) by intravenous inoculation as described above. Those three monkeys were examined daily for neurological manifestations for the first 10 days after the inoculation of EV71(BrCr-TR) and were examined weekly from 10 to 21 days after the inoculation of EV71(BrCr-TR) (55 to 66 days p.i.) (see Fig. 2. below). Autopsy was performed on two monkeys 4 days after the inoculation of EV71(S1-3′). At autopsy, various parts of the CNS, nonneural tissues, and blood were collected for histopathological and virological analysis. Histological changes in the CNS (lesion score) were evaluated using the method recommended by the WHO (40). Lesions were scored as follows: 0, no lesion; 1, cellular infiltration; 2, cellular infiltration with minimal neuronal damage; 3, cellular infiltration with extensive neuronal damage; 4, massive neuronal damage with or without cellular infiltration. For virus isolation, a portion of each excised tissue was stored at −80°C. We also isolated viruses from nonneural tissues of monkeys previously inoculated with EV71(BrCr-TR) and autopsied on day 6 p.i. (5). After freezing and thawing, tissue homogenates (10% [wt/vol]) in minimal essential medium containing 2% FCS were centrifuged at 10,000 × g for 10 min to remove cell debris. Supernatants were used for virus isolation in Vero cells. The cells were observed for CPE for 1 week, and then blind passage was performed for CPE-negative samples after freezing and thawing of the first-round passage. If no CPE was observed in the first- or second-round cultures, the result of the virus isolation was recorded as negative.
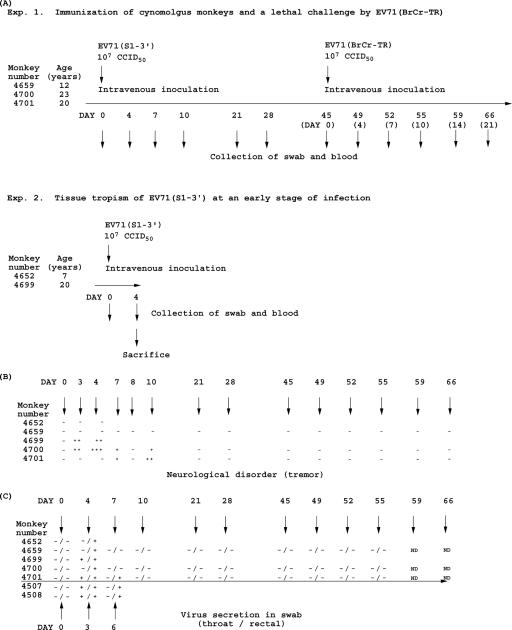
FIG. 2.
Experimental schedule, clinical symptoms, and virus excretion of cynomolgus monkeys inoculated with EV71(S1-3′). (A) Experimental schedule. In experiment 1 (upper panel), the antigenicity of EV71(S1-3′) in cynomolgus monkeys was examined. Three monkeys were intravenously inoculated with EV71(S1-3′) on day 0 and were then challenged by lethal inoculation with EV71(BrCr-TR) on day 45 p.i. In experiment 2 (lower panel), the tissue specificity of EV71(S1-3′) in the early phase of infection was examined. Two monkeys were sacrificed on day 4 p.i., and virus distribution and histopathology in those two monkeys were examined. The assigned numbers and ages of individual monkeys are shown. Numbers in parentheses represent days after the lethal challenge with EV71(BrCr-TR). The swabs and blood were collected at the times indicated. (B) Clinical symptoms of the monkeys. Severity of tremor is represented by +, ++, and +++. (C) Virus excretion in the throat and from rectal swabs of the monkeys inoculated with EV71(S1-3′). The swabs from which EV71(S1-3′) or EV71(BrCr-TR) was isolated are shown as positive, and swabs from which EV71(S1-3′) or EV71(BrCr-TR) was not isolated are shown as negative. Swab samples from monkeys inoculated with EV71(BrCr-TR) (numbers 4507 and 4508) were collected in our previous study (5). ND, not determined.
RESULTS
Clinical symptoms of cynomolgus monkeys inoculated with EV71(S1-3′).
To characterize the antigenicity and tissue specificity of EV71(S1-3′), we inoculated five cynomolgus monkeys with 107 CCID50 of EV71(S1-3′) via an intravenous route (Fig. 1). Three monkeys were used to examine antigenicity (Fig. 2A, Exp. 1), and two monkeys were used to examine tissue specificity in the early phase of infection as indicated by clinical symptoms (day 4 p.i.) (Fig. 2A, Exp. 2).
FIG. 1.
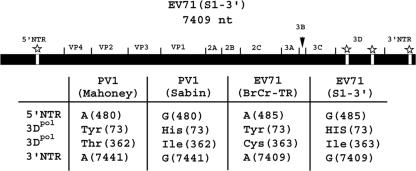
Schematic diagram of the EV71(S1-3′) genome. The sequences derived from the parental EV71(BrCr-TR) genome are represented by the black regions, and the mutations derived from PV1(Sabin) are represented by the white regions with stars above them. Corresponding mutations of PV1(Mahoney), PV1(Sabin), and EV71(BrCr-TR) are also shown. The numbers in parentheses are the nucleotide positions in the genome or the amino acid positions in the 3Dpol protein. The figure was adapted from a previously published figure with the permission of the Society for General Microbiology (5).
EV71(S1-3′) infection caused tremor in three monkeys (age, 20 to 23 years), but no clinical symptoms were observed in two other monkeys (age, 7 and 12 years) (Fig. 2B). Tremor appeared as early as day 3 p.i., disappeared on day 8 p.i., reappeared on day 10 p.i., and then disappeared again before day 21 p.i. The legs of the three monkeys that exhibited tremor remained weak throughout the period of observation. The virus was isolated from both throat and rectal swabs until day 7 p.i., predominantly from rectal swabs (Fig. 2C), suggesting transient infection of EV71(S1-3′) in the inoculated monkeys.
Humoral immune response of cynomolgus monkeys inoculated with EV71(S1-3′).
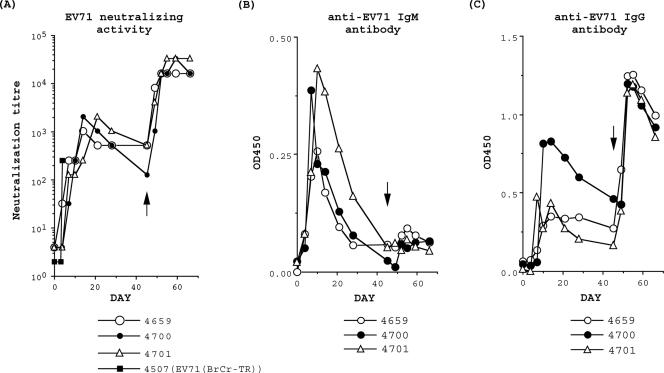
To examine the humoral immune response of monkeys inoculated with EV71(S1-3′), we measured the neutralizing and binding activities of anti-EV71 IgG and IgM antibodies in sera from the five monkeys inoculated with EV71(S1-3′) via the intravenous route. Induction of anti-EV71 antibodies was evaluated using EV71(Nagoya) (genotype B1), which is a prime strain of EV71 (16). We also used a genetically engineered EV71(Nagoya) mutant carrying a histidine tag in the BC loop of the VP1 protein [EV71(Nagoya-His)] (Fig. 3A and C). The neutralizing activity of the monkey sera was comparable to that of a monkey (number 4507) inoculated with virulent parental EV71(BrCr-TR) in a previous study (Fig. 3A, 0 to 6 days p.i.) (5) and peaked (neutralization titer, 1,024 to 2,048) at 14 to 21 days p.i., followed by a gradual decline in activity (primary immune response). After the lethal challenge with EV71(BrCr-TR) on day 45 p.i., the neutralizing activity of the monkey sera markedly increased as a consequence of the secondary immune response. In the secondary immune response, the neutralizing activity peaked (neutralization titer, 16,384 to 32,768) at 7 to 14 days after the lethal challenge (52 to 59 days p.i.) (Fig. 3A).
FIG. 3.
Anti-EV71 antibody titers of monkeys inoculated with EV71(S1-3′). The time point of lethal challenge with EV71(BrCr-TR) is indicated by an arrow. (A) Anti-EV71 neutralization activity of monkey serum. The neutralization titer of 50 μl of the serum, determined by the CPE method, is shown over the course of the infection. The serum of a monkey (number 4507) inoculated with virulent EV71(BrCr-TR) was collected in a previous study (5). (B and C) Measurement of anti-EV71 IgM (B) or anti-EV71 IgG (C) in monkey serum. Histidine-tagged EV71 virions were adsorbed on a nickel-coated plate and then monkey serum was added to the plate. Monkey anti-EV71 antibodies bound to the histidine-tagged virions were detected by HRP-conjugated goat anti-monkey IgM or anti-monkey IgG antibodies. OD450, optical density at 450 nm.
The binding activity and/or the amounts of anti-EV71 IgG and anti-EV71 IgM antibodies were measured by ELISA. Previously, we developed an IgM capture ELISA for the detection of anti-EV71 IgM in human serum (52). However, that system is not suitable for monkey serum because of the strong cross-reactivity between the antibodies, which results in high background levels (data not shown). To minimize cross-reactivity between the antibodies, we developed a new ELISA system using histidine-tagged EV71(Nagoya-His) virus as the antigen (Fig. 3B and C). In this ELISA system, histidine-tagged EV71 virions are adsorbed on a nickel-coated plate and then monkey serum is added to the plate. The anti-EV71 IgG or IgM antibodies bound to the histidine-tagged virions are detected using goat anti-monkey IgG or anti-monkey IgM antibodies conjugated with HRP (see Materials and Methods). Using this ELISA system, we detected anti-EV71 IgM and IgG antibodies in the monkey sera (Fig. 3B and C). IgM was the predominant type of anti-EV71 antibody in the primary immune response (peak at 7 to 10 days p.i.), but apparently no anti-EV71 IgM antibody was induced in the secondary immune response after the lethal challenge with EV71(BrCr-TR). In contrast, the amount or the binding capacity of anti-EV71 IgG antibody was low in the primary immune response (peak at 10 to 14 days p.i.) and markedly increased in the secondary immune response (peak at 7 days after the lethal challenge [52 days p.i.]). These results indicate that the infection with EV71(S1-3′) induced an efficient humoral immune response in cynomolgus monkeys.
Antigenicity of EV71(S1-3′) in cynomolgus monkeys.
Next, we examined the genotype specificity of the neutralizing activity of the monkey sera. We examined neutralizing activity against EV71 strains belonging to genotypes A, B1, B4, C2, and C4 (Tables 2 and 3). First, we determined the neutralization titer by observing the CPE caused by virus that escaped neutralization (CPE method; see Materials and Methods). The highest neutralization titer was against homotypic strain EV71(BrCr-TR) [the parental strain of EV71(S1-3′)]. The order of decreasing neutralization activity in the CPE assay was as follows: BrCr-TR (genotype A) > Nagoya (genotype B1) > 75-Yamagata-2003 (genotype C4) and 2399-Yamagata-2003 (genotype C4) > C7-Osaka (genotype B4) and 1095 (genotype C2). However, we were unable to determine the neutralization titer against strain 1530-Yamagata-2003 (genotype C4) using this method because of the late appearance of CPE, attributable to the nonneutralizable aggregated form of virus (54). Therefore, we determined the neutralization titer against strain 1530-Yamagata-2003 by indirect immunofluorescence with rabbit anti-EV71 antiserum (IF method; see Materials and Methods). The IF method is useful for strains of enterovirus that contain a small amount of nonneutralizable aggregated form of the virus, which can account for 0.005% to 30% of total virions (54). The nonneutralizable aggregated form of virus makes it difficult to determine the end point, due to incomplete neutralization by the antibody. The monkey sera had substantial neutralizing activity against strain 1530-Yamagata-2003 (Table 3). The order of decreasing neutralization activity in the IF assay was as follows: BrCr-TR (genotype A) > Nagoya (genotype B1) and 75-Yamagata-2003 (genotype C4) > 1530-Yamagata-2003 (genotype C4) > C7-Osaka (genotype B4) > 1095 (genotype C2) and 2399-Yamagata-2003 (genotype C4).
TABLE 2.
Neutralization titers of monkey sera against EV71 strains as determined by CPE assay
| Monkey | Day p.i.a | Neutralization titer (% relative to BrCr-TR)b with EV71 strain (genotype)c:
|
|||||
|---|---|---|---|---|---|---|---|
| BrCr-TR (A) | Nagoya (B1) | C7-Osaka (B4) | 1095 (C2) | 75-Yamagata-2003 (C4) | 2399-Yamagata-2003 (C4) | ||
| 4659 | 14 | 3.6 (100) | 3.3 (50) | 2.1 (3.1) | 2.4 (5.8) | 3.6 (100) | 3.3 (50) |
| 59 (14) | 4.5 (100) | 3.9 (25) | 2.7 (1.6) | 2.7 (1.6) | 3.6 (13) | 3.6 (13) | |
| 4700 | 14 | 3.3 (100) | 3.0 (50) | 2.4 (13) | 2.4 (13) | 3.0 (50) | 2.1 (6.3) |
| 59 (14) | 4.8 (100) | 3.9 (13) | 3.3 (3.1) | 3.3 (3.1) | 3.9 (13) | 3.9 (13) | |
| 4701 | 21 | 3.0 (100) | 2.7 (50) | 2.1 (13) | 2.1 (13) | 2.4 (25) | 1.8 (6.3) |
| 59 (14) | 4.8 (100) | 4.2 (25) | 3.3 (3.1) | 3.3 (3.1) | 3.9 (13) | 3.9 (13) | |
Numbers in parentheses represent the day postinfection at which a lethal challenge with EV71(BrCr-TR) was administered to the monkeys.
The log10 of the neutralization titer in 50 μl of monkey serum. Numbers in parentheses represent the percentage of the neutralization titer relative to the titer against the BrCr-TR strain, which was taken as 100%.
The genotype of each EV71 strain is shown in parentheses. For strain 1530-Yamagata-2003, the neutralization titer could not be determined in this assay.
TABLE 3.
Neutralization titers of monkey sera against EV71 strains as determined by IF assay
| Monkey | Day p.i.a | Neutralization titer (% relative to BrCr-TR)b with EV71 strain (genotype):
|
||||||
|---|---|---|---|---|---|---|---|---|
| BrCr-TR (A)c | Nagoya (B1) | C7-Osaka (B4) | 1095 (C2) | 75-Yamagata-2003 (C4) | 2399-Yamagata-2003 (C4) | 1530-Yamagata-2003 (C4) | ||
| 4659 | 14 | 4.1 (100) | 3.9 (57) | 3.6 (32) | 3.1 (8.3) | 3.9 (57) | 3.6 (35) | 4.0 (87) |
| 59 (14) | 5.5 (100) | 5.3 (69) | 4.7 (15) | 4.1 (4.0) | 5.4 (73) | 4.4 (7.1) | 5.2 (50) | |
| 4700 | 14 | 4.6 (100) | 4.2 (43) | 4.2 (40) | 3.9 (25) | 4.4 (60) | 3.7 (13) | 4.6 (92) |
| 59 (14) | 5.5 (100) | 5.0 (37) | 4.6 (16) | 4.4 (8.1) | 5.1 (43) | 5.1 (38) | 5.0 (33) | |
| 4701 | 21 | 3.9 (100) | 4.3 (230) | 3.5 (36) | 3.6 (41) | 3.7 (50) | 3.5 (36) | 3.9 (88) |
| 59 (14) | 5.8 (100) | 5.6 (63) | 5.1 (18) | 5.2 (21) | 5.1 (20) | 4.7 (7.6) | 4.9 (12) | |
Numbers in parentheses represent the day postinfection at which a lethal challenge with EV71(BrCr-TR) was administered to the monkeys.
The neutralization titer was determined by indirect immunofluorescence and is the log10 of the NU50 in 50 μl of monkey serum (see Materials and Methods). Numbers in parentheses represent the percentage of the neutralization titer relative to the titer against the BrCr-TR strain, which was taken as 100%.
Thus, the monkey sera exhibited a broad spectrum of neutralization activity against EV71 strains belonging to different genotypes, with the highest activity against the homotypic genotype A and the lowest activity against genotype C2 (1.6% of neutralization titer against homotypic strain).
Protection of cynomolgus monkeys from lethal challenge by virulent EV71(BrCr-TR).
Three monkeys inoculated with EV71(S1-3′) were challenged with a lethal dose (107 CCID50) of EV71(BrCr-TR) via an intravenous route (Fig. 2) (5, 39). After the challenge, no clinical symptoms were observed in any of the three monkeys. Virus was not isolated from swab samples obtained after the challenge (Fig. 2B). These results indicate that EV71(S1-3′) acted as an effective antigen that protected the monkeys from lethal infection with the homotypic virulent strain.
Tissue specificity of EV71(S1-3′) in cynomolgus monkeys.
We examined the tissue specificity of EV71(S1-3′) in the cynomolgus monkeys on day 4 p.i., in the early phase of the infection as indicated by clinical symptoms (Tables 4 and 5). In our previous study (5), in the late phase of the infection (day 10 p.i.), virus was isolated only from the CNS of the monkeys inoculated with EV71(S1-3′), although inflammation was observed in a broad area of the CNS.
TABLE 4.
Tissue specificity of EV71(S1-3′) in the CNS of cynomolgus monkeys
| Tissue | Virus isolation (inflammation) for monkey no. [treatment]a:
|
|||||
|---|---|---|---|---|---|---|
| 4652 [EV71(S1-3′), day 4 p.i.] | 4699 [EV71(S1-3′), day 4 p.i.] | 4513b [EV71(S1-3′), day 10 p.i.] | 4514b [EV71(S1-3′), day 10 p.i.] | 4507c [EV71(BrCr-TR), day 6 p.i.] | 4508c [EV71(BrCr-TR), day 6 p.i.] | |
| Cerebrum | − (−) | − (−) | − (−) | − (+) | + (+) | + (+) |
| Midbrain | − (−) | − (−) | − (−) | − (+) | + (+) | + (+) |
| Pons | − (+) | − (−) | − (−) | − (+) | + (+) | + (+) |
| Cerebellum | − (−) | − (−) | − (−) | − (+) | + (+) | + (+) |
| Medulla | − (+) | − (−) | − (−) | − (+) | + (+) | + (+) |
| Cervical cord | + (+) (101.75) | − (−) | − (−) | − (+) | + (+) | + (+) |
| Lumbar cord [lesion score]d | + (+) (103.5) [0.9] | + (+) (103.0) [0.3] | − (−) [0.0] | + (+) [1.3] | + (+) [2.9] | + (+) [3.2] |
The virus isolation results are shown as positive or negative. The presence of inflammation in the tissues is shown in parentheses as positive or negative. The inoculated viruses and the day sacrificed are also indicated. The virus titer (CCID50) in 100 μl of the homogenate is shown, in parentheses following the isolation and inflammation results, for the samples collected from two monkeys, numbers 4652 and 4699.
Samples from monkeys numbers of 4513 and 4514 were collected in a previous study (5).
Data on monkeys inoculated with virulent EV71(BrCr-TR) (numbers 4507 and 4508) were adapted from a previous publication with the permission of the Society for General Microbiology (5).
The lesion score of the lumbar cord was determined for each monkey and is shown in brackets.
TABLE 5.
Tissue specificity of EV71(S1-3′) and EV71(BrCr-TR) in extraneural tissues of cynomolgus monkeys
| Tissue | Virus isolation (inflammation) for monkey no. [treatment]a:
|
|||
|---|---|---|---|---|
| 4652 [EV71(S1-3′), day 4 p.i.] | 4699 [EV71(S1-3′), day 4 p.i.] | 4507b [EV71(BrCR-TR), day 6 p.i.] | 4508b [EV71(BrCR-TR), day 6 p.i.] | |
| Tonsil | − (−) | − (−) | − (−) | − (−) |
| Heart | − (−) | − (−) | − (−) | − (−) |
| Lung | − (−) | − (−) | − (−) | − (−) |
| Liver | − (−) | − (−) | − (−) | − (−) |
| Spleen | − (−) | − (−) | + (−) | − (−) |
| Kidney | − (−) | + (+) (105.75) | + (−) | + (+) |
| Muscle | − (−) | − (−) | ND | ND |
| Deep cervical lymph node | + (−) (<100.5) | + (−) (102.5) | + (−) | + (−) |
| Dorsal root ganglion | + (−) (101.25) | − (−) (<100.5) | + (+) | + (+) |
The virus isolation results are shown as positive or negative. The presence of inflammation in the tissues is shown in parentheses as positive or negative. The inoculated viruses and the day sacrificed are also indicated. The virus titer (CCID50) in 100 μl of the homogenate is shown, in parentheses following the isolation and inflammation results, for the samples collected from two monkeys, numbers 4652 and 4699.
Samples of monkeys numbers 4507 and 4508 were collected in our previous study (5). ND, not determined.
In the CNS of the inoculated monkeys, inflammation (perivascular cuffing with mononuclear cells) was observed in a limited area, mainly in gray matter, on day 4 p.i. (Table 4). In one monkey (number 4652), inflammation was observed in the pons, the medulla, and the cervical and lumbar spinal cord. In another monkey (number 4699), inflammation was observed only in the lumbar spinal cord. Virus was isolated from the lumbar spinal cords of both monkeys. In one monkey (number 4513) on day 10 p.i., no virus was isolated and no inflammation was observed. In another monkey (number 4514), virus was isolated only from the lumbar spinal cord, but inflammation was observed in all CNS tissues. These results suggest that EV71(S1-3′) infection can cause inflammation in a broad range of CNS tissues with inefficient replication. Efficient replication of EV71(S1-3′) was limited to the spinal cord, predominantly the lumbar spinal cord.
Next, we isolated virus from extraneural tissues of the monkeys inoculated with EV71(S1-3′) or with the virulent parental EV71(BrCr-TR) (Table 5). Virus was isolated from the spleen, kidney, deep cervical lymph node, and dorsal root ganglion. There was no clear difference in extraneural tissue specificity between EV71(S1-3′) and EV71(BrCr-TR). In the kidney and dorsal root ganglion, inflammation was observed, suggesting that viral replication occurred in these tissues. However, on day 10 p.i. after inoculation of EV71(S1-3′), virus was not isolated from extraneural tissues (data not shown). Thus, EV71(S1-3′) infected extraneural tissues of cynomolgus monkeys in the early phase of the infection, but the infection of extraneural tissues was transient.
DISCUSSION
To evaluate the antigenicity of EV71(S1-3′) in cynomolgus monkeys, we used an intravenous inoculation route instead of the oral route. This is because in a previous study EV71 infection via the oral route did not efficiently cause neurological disorders in the inoculated monkeys (1 out of 10 inoculated monkeys) (18).
In the present study, the monkeys inoculated with EV71(S1-3′) via the intravenous route exhibited tremor followed by a slight weakness of the legs (Fig. 2C and data not shown). One monkey (number 4699) clearly exhibited tremor on day 3 and day 4 p.i. However, in the CNS of that monkey, the only lesions that were observed were in the lumbar spinal cord, which had a low lesion score (0.3, perivascular cuffing with mononuclear cells) (Table 4). Also in that monkey, no extrapyramidal lesions were detected in the CNS (40). Extrapyramidal lesions directly cause tremor, suggesting that extrapyramidal lesions present at undetectable levels can cause tremor in EV71-infected monkeys and that detectable pyramidal lesions would exacerbate such tremors. This suggests that the tremors observed in the present monkeys inoculated with EV71(S1-3′) have a different origin than those observed in monkeys inoculated with the virulent parental strain EV71(BrCr-TR) in previous studies, in which extrapyramidal lesions were detected (39).
The spinal cord is a niche of enterovirus infection (4, 13). Actually, a PV replicon caused severe poliomyelitis-like paralysis in inoculated mice with limited lesions of the lumbar spinal cord (<1.4% of motor neurons) (3). However, EV71 replication in the spinal cord of cynomolgus monkeys was not sufficient to cause tremor. In the young monkeys (ages, 7 and 12 years), EV71 replication was detected in the lumbar spinal cord, but the animals did not exhibit tremor, in contrast to the older monkeys (ages, 20 to 23 years). Interestingly, the lumbar spinal cord of monkey 4699 had a lower lesion score (0.3) than that of monkey 4652 (lesion score, 0.9), which exhibited no clinical symptoms. In poliomyelitis, adults are much more likely than children to be severely affected by infection in the spinal cord (42). Thus, detectable lesions and viral replication are not the only determinants of the occurrence of tremor in monkeys inoculated with EV71(S1-3′); other factors are critical.
Consistent with the mild neurological symptoms in the present cynomolgus monkeys inoculated with EV71(S1-3′), the distribution of EV71(S1-3′) in the CNS of the monkeys was limited to a small region on day 4 p.i. in the early phase of the infection (Table 4). Inflammation (perivascular cuffing with mononuclear cells) was observed in a broad area of the CNS without a detectable level of viral antigen. The virus was isolated from only the lumbar spinal cord on day 10 p.i. (5). Therefore, the inflammation observed in a broad area on day 10 p.i. was caused by inefficient infection of EV71(S1-3′) and was not caused by the vigorous infection in the early phase. Previously, we isolated an EV71 mutant [EV71(3′)], which contains temperature-sensitive mutations in the 3Dpol and 3′ NTR, from both the lumbar spinal cord and brain stem of inoculated monkeys (5). Thus, it appears that suppressed infection of EV71(S1-3′) in the brain stem is promoted by mutation in the 5′ NTR or by a combination of mutations in the 5′ NTR, 3Dpol, and 3′ NTR. Interestingly, studies indicate that PV1 and EV71 viruses with a mutation in the 5′ NTR [PV1(Sabin) and EV71(S1-3′)] are fairly stable in the lumbar spinal cord of inoculated monkeys (5, 24). The lesions of the brain stem are critical causes of fatality from EV71 infection (10, 21, 25, 31, 57), suggesting that the introduced attenuation determinant of PV1(Sabin) contributes to EV71-specific pathogenesis.
In the present study, we observed transient infection of EV71(S1-3′) in extraneural tissues, including the kidney, deep cervical lymph node, and dorsal root ganglion (Table 5). The infection in the dorsal root ganglion may simply reflect EV71 infection in sensory neurons (40). The deep cervical lymph node is thought to play a central role in establishment of the viremic phase of PV infection, according to a model proposed by Bodian (6; reviewed in reference 35). The kidney may also play an important role in the establishment of viremia as a transiently susceptible extraneural tissue in EV71 infection, as Sabin has proposed for PV infection, although the identity of the extraneural tissue involved in PV infection remains unknown (46).
The monkeys inoculated with EV71(S1-3′) were protected from a lethal challenge with EV71(BrCr-TR) (Fig. 2). We immunized monkeys with EV71(S1-3′) via an intravenous route with a high dose (107 CCID50) intended to cause experimentally transient high-level viremia (2.9 × 104 to 5.1 × 104 CCID50 per ml). Even the viremogenic PV1(Mahoney) has been shown to cause viremia on the order of 105 CCID50 per ml in cynomolgus monkeys (6). Therefore, if EV71(S1-3′) were inoculated via the oral route (i.e., the natural route of EV71 infection), a much weaker humoral immune reaction would be expected in terms of levels of anti-EV71 IgG and IgM in the serum. The effectiveness of EV71(S1-3′) inoculation via the oral route, which depends on the induction of anti-EV71 secretory IgA for protection, remains to be further evaluated.
The neutralizing titers of the sera of monkeys immunized with EV71(S1-3′) were highest against the homotypic strain EV71 (genotype A) and were lowest against strains belonging to genotype C2, with a maximum difference of 60-fold between neutralization titers for different strains (Tables 2 and 3). It should be noted that genotype specificity was not eliminated after a single booster with the homotypic strain. Antigenic heterogeneity within a single serotype is a typical feature of enterovirus; for example, for echovirus 30, differences in the neutralization titer of >100-fold of the sera of rhesus monkeys have been observed (58). There have also been reports of antigenic heterogeneity among EV71 isolates, including a maximum difference in neutralization titers of 32-fold between different rabbit sera after EV71 inoculation (19). Frequent changes in the genotype of circulating EV71 may in part reflect antigenic variation in cross-neutralization between genotypes that may be critical for the perpetuation of EV71 (27, 37, 41, 55).
EV71(S1-3′) is a promising vaccine candidate, although it is still neurovirulent, at least when inoculated via the intravenous route. Several strategies have been proposed for attenuation and stabilization of the phenotype of PV (4, 9, 12, 15, 22, 32, 38). However, in general, attenuated PV strains exhibit impaired virus growth, even in cells cultured in vitro. Therefore, evaluation of the fitness of EV71(S1-3′) required for effective antigenicity is necessary to ensure that further attenuation produces effective vaccine strains.
In summary, we found that the attenuated strain EV71(S1-3′), which belongs to genotype A, acts as an effective antigen against several genotypes of EV71 and has attenuated neurovirulence in cynomolgus monkeys.
Acknowledgments
We are grateful to Ayako Harashima, Yuko Sato, and Junko Wada for their excellent assistance.
This work was supported by grants-in-aid from the Japan Society for Promotion of Science and for Research on Emerging and Re-emerging Infectious Diseases from the Ministry of Health, Labor and Welfare.
Footnotes
Published ahead of print on 13 June 2007.
REFERENCES
- 1.Abubakar, S., H. Y. Chee, N. Shafee, K. B. Chua, and S. K. Lam. 1999. Molecular detection of enteroviruses from an outbreak of hand, foot and mouth disease in Malaysia in 1997. Scand. J. Infect. Dis. 31:331-335. [DOI] [PubMed] [Google Scholar]
- 2.Arita, M., H. Horie, M. Arita, and A. Nomoto. 1999. Interaction of poliovirus with its receptor affords a high level of infectivity to the virion in poliovirus infections mediated by the Fc receptor. J. Virol. 73:1066-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arita, M., N. Nagata, T. Sata, T. Miyamura, and H. Shimizu. 2006. Quantitative analysis of poliomyelitis-like paralysis in mice induced by a poliovirus replicon. J. Gen. Virol. 87:3317-3327. [DOI] [PubMed] [Google Scholar]
- 4.Arita, M., H. Shimizu, and T. Miyamura. 2004. Characterization of in vitro and in vivo phenotypes of poliovirus type 1 mutants with reduced viral protein synthesis activity. J. Gen. Virol. 85:1933-1944. [DOI] [PubMed] [Google Scholar]
- 5.Arita, M., H. Shimizu, N. Nagata, Y. Ami, Y. Suzaki, T. Sata, T. Iwasaki, and T. Miyamura. 2005. Temperature-sensitive mutants of enterovirus 71 show attenuation in cynomolgus monkeys. J. Gen. Virol. 86:1391-1401. [DOI] [PubMed] [Google Scholar]
- 6.Bodian, D. 1954. Viremia in experimental poliomyelitis. I. General aspects of infection after intravascular inoculation with strains of high and of low invasiveness. Am. J. Hyg. 60:339-357. [PubMed] [Google Scholar]
- 7.Brown, B. A., M. S. Oberste, J. P. Alexander, Jr., M. L. Kennett, and M. A. Pallansch. 1999. Molecular epidemiology and evolution of enterovirus 71 strains isolated from 1970 to 1998. J. Virol. 73:9969-9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown, B. A., and M. A. Pallansch. 1995. Complete nucleotide sequence of enterovirus 71 is distinct from poliovirus. Virus Res. 39:195-205. [DOI] [PubMed] [Google Scholar]
- 9.Burns, C. C., J. Shaw, R. Campagnoli, J. Jorba, A. Vincent, J. Quay, and O. Kew. 2006. Modulation of poliovirus replicative fitness in HeLa cells by deoptimization of synonymous codon usage in the capsid region. J. Virol. 80:3259-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, L. Y., T. Y. Lin, K. H. Hsu, Y. C. Huang, K. L. Lin, C. Hsueh, S. R. Shih, H. C. Ning, M. S. Hwang, H. S. Wang, and C. Y. Lee. 1999. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet 354:1682-1686. [DOI] [PubMed] [Google Scholar]
- 11.Chumakov, M., M. Voroshilova, L. Shindarov, I. Lavrova, L. Gracheva, G. Koroleva, S. Vasilenko, I. Brodvarova, M. Nikolova, S. Gyurova, M. Gacheva, G. Mitov, N. Ninov, E. Tsylka, I. Robinson, M. Frolova, V. Bashkirtsev, L. Martiyanova, and V. Rodin. 1979. Enterovirus 71 isolated from cases of epidemic poliomyelitis-like disease in Bulgaria. Arch. Virol. 60:329-340. [DOI] [PubMed] [Google Scholar]
- 12.De Jesus, N., D. Franco, A. Paul, E. Wimmer, and J. Cello. 2005. Mutation of a single conserved nucleotide between the cloverleaf and internal ribosome entry site attenuates poliovirus neurovirulence. J. Virol. 79:14235-14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dufresne, A. T., and M. Gromeier. 2004. A nonpolio enterovirus with respiratory tropism causes poliomyelitis in intercellular adhesion molecule 1 transgenic mice. Proc. Natl. Acad. Sci. USA 101:13636-13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujimoto, T., M. Chikahira, S. Yoshida, H. Ebira, A. Hasegawa, A. Totsuka, and O. Nishio. 2002. Outbreak of central nervous system disease associated with hand, foot, and mouth disease in Japan during the summer of 2000: detection and molecular epidemiology of enterovirus 71. Microbiol. Immunol. 46:621-627. [DOI] [PubMed] [Google Scholar]
- 15.Gromeier, M., B. Bossert, M. Arita, A. Nomoto, and E. Wimmer. 1999. Dual stem loops within the poliovirus internal ribosomal entry site control neurovirulence. J. Virol. 73:958-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagiwara, A., I. Tagaya, and T. Yoneyama. 1978. Epidemic of hand, foot and mouth disease associated with enterovirus 71 infection. Intervirology 9:60-63. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto, I., and A. Hagiwara. 1983. Comparative studies on the neurovirulence of temperature-sensitive and temperature-resistant viruses of enterovirus 71 in monkeys. Acta Neuropathol. (Berlin) 60:266-270. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto, I., and A. Hagiwara. 1982. Pathogenicity of a poliomyelitis-like disease in monkeys infected orally with enterovirus 71: a model for human infection. Neuropathol. Appl. Neurobiol. 8:149-156. [DOI] [PubMed] [Google Scholar]
- 19.Hattori, M., M. Oseto, Y. Yamashita, M. Mori, H. Inouye, Y. Ishimaru, and S. Nakano. 1994. An epidemic of hand, foot and mouth disease associated with enterovirus 71 infection in Ehime in 1993. Annu. Rep. Ehime Prefectural Inst. Public Health Environ. Sci. 55:11-14. [Google Scholar]
- 20.Ho, M., E. R. Chen, K. H. Hsu, S. J. Twu, K. T. Chen, S. F. Tsai, J. R. Wang, and S. R. Shih. 1999. An epidemic of enterovirus 71 infection in Taiwan. N. Engl. J. Med. 341:929-935. [DOI] [PubMed] [Google Scholar]
- 21.Huang, C. C., C. C. Liu, Y. C. Chang, C. Y. Chen, S. T. Wang, and T. F. Yeh. 1999. Neurologic complications in children with enterovirus 71 infection. N. Engl. J. Med. 341:936-942. [DOI] [PubMed] [Google Scholar]
- 22.Iizuka, N., M. Kohara, K. Hagino-Yamagishi, S. Abe, T. Komatsu, K. Tago, M. Arita, and A. Nomoto. 1989. Construction of less neurovirulent polioviruses by introducing deletions into the 5′ noncoding sequence of the genome. J. Virol. 63:5354-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karber, G. 1931. Beitrag zur kollektiven Behandlung pharmakologischer Reihenversuche. Arch. Exp. Path. Pharm. 162:480. [Google Scholar]
- 24.Kawamura, N., M. Kohara, S. Abe, T. Komatsu, K. Tago, M. Arita, and A. Nomoto. 1989. Determinants in the 5′ noncoding region of poliovirus Sabin 1 RNA that influence the attenuation phenotype. J. Virol. 63:1302-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komatsu, H., Y. Shimizu, Y. Takeuchi, H. Ishiko, and H. Takada. 1999. Outbreak of severe neurologic involvement associated with enterovirus 71 infection. Pediatr. Neurol. 20:17-23. [DOI] [PubMed] [Google Scholar]
- 26.Li, L., Y. He, H. Yang, J. Zhu, X. Xu, J. Dong, Y. Zhu, and Q. Jin. 2005. Genetic characteristics of human enterovirus 71 and coxsackievirus A16 circulating from 1999 to 2004 in Shenzhen, People's Republic of China. J. Clin. Microbiol. 43:3835-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin, K. H., K. P. Hwang, G. M. Ke, C. F. Wang, L. Y. Ke, Y. T. Hsu, Y. C. Tung, P. Y. Chu, B. H. Chen, H. L. Chen, C. L. Kao, J. R. Wang, H. L. Eng, S. Y. Wang, L. C. Hsu, and H. Y. Chen. 2005. Evolution of EV71 genogroup in Taiwan from 1998 to 2005: an emerging of subgenogroup C4 of EV71. J. Med. Virol. 78:254-262. [DOI] [PubMed] [Google Scholar]
- 28.Lin, T. Y., S. J. Twu, M. S. Ho, L. Y. Chang, and C. Y. Lee. 2003. Enterovirus 71 outbreaks, Taiwan: occurrence and recognition. Emerg. Infect. Dis. 9:291-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu, C. Y., C. Y. Lee, C. L. Kao, W. Y. Shao, P. I. Lee, S. J. Twu, C. C. Yeh, S. C. Lin, W. Y. Shih, S. I. Wu, and L. M. Huang. 2002. Incidence and case-fatality rates resulting from the 1998 enterovirus 71 outbreak in Taiwan. J. Med. Virol. 67:217-223. [DOI] [PubMed] [Google Scholar]
- 30.Lu, H. H., L. Alexander, and E. Wimmer. 1995. Construction and genetic analysis of dicistronic polioviruses containing open reading frames for epitopes of human immunodeficiency virus type 1 gp120. J. Virol. 69:4797-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lum, L. C., K. T. Wong, S. K. Lam, K. B. Chua, A. Y. Goh, W. L. Lim, B. B. Ong, G. Paul, S. AbuBakar, and M. Lambert. 1998. Fatal enterovirus 71 encephalomyelitis. J. Pediatr. 133:795-798. [DOI] [PubMed] [Google Scholar]
- 32.Macadam, A. J., G. Ferguson, D. M. Stone, J. Meredith, S. Knowlson, G. Auda, J. W. Almond, and P. D. Minor. 2006. Rational design of genetically stable, live-attenuated poliovirus vaccines of all three serotypes: relevance to poliomyelitis eradication. J. Virol. 80:8653-8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMinn, P., K. Lindsay, D. Perera, H. M. Chan, K. P. Chan, and M. J. Cardosa. 2001. Phylogenetic analysis of enterovirus 71 strains isolated during linked epidemics in Malaysia, Singapore, and western Australia. J. Virol. 75:7732-7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMinn, P. C. 2002. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol. Rev. 26:91-107. [DOI] [PubMed] [Google Scholar]
- 35.Minor, P. 1997. Poliovirus, p. 555-574. In N. Nathanson et al. (ed.), Viral pathogenesis. Lippincott-Raven Publishers, Philadelphia, PA.
- 36.Minor, P. D. 1992. The molecular biology of polio vaccines. J. Gen. Virol. 73:3065-3077. [DOI] [PubMed] [Google Scholar]
- 37.Mizuta, K., C. Abiko, T. Murata, Y. Matsuzaki, T. Itagaki, K. Sanjoh, M. Sakamoto, S. Hongo, S. Murayama, and K. Hayasaka. 2005. Frequent Importation of enterovirus 71 from surrounding countries into the local community of Yamagata, Japan, between 1998 and 2003. J. Clin. Microbiol. 43:6171-6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller, S., D. Papamichail, J. R. Coleman, S. Skiena, and E. Wimmer. 2006. Reduction of the rate of poliovirus protein synthesis through large-scale codon deoptimization causes attenuation of viral virulence by lowering specific infectivity. J. Virol. 80:9687-9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagata, N., T. Iwasaki, Y. Ami, Y. Tano, A. Harashima, Y. Suzaki, Y. Sato, H. Hasegawa, T. Sata, T. Miyamura, and H. Shimizu. 2004. Differential localization of neurons susceptible to enterovirus 71 and poliovirus type 1 in the central nervous system of cynomolgus monkeys after intravenous inoculation. J. Gen. Virol. 85:2981-2989. [DOI] [PubMed] [Google Scholar]
- 40.Nagata, N., H. Shimizu, Y. Ami, Y. Tano, A. Harashima, Y. Suzaki, Y. Sato, T. Miyamura, T. Sata, and T. Iwasaki. 2002. Pyramidal and extrapyramidal involvement in experimental infection of cynomolgus monkeys with enterovirus 71. J. Med. Virol. 67:207-216. [DOI] [PubMed] [Google Scholar]
- 41.Nathanson, N. 2005. Virus perpetuation in populations: biological variables that determine persistence or eradication. Arch. Virol. Suppl. 19:3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nathanson, N., and J. R. Martin. 1979. The epidemiology of poliomyelitis: enigmas surrounding its appearance, epidemicity, and disappearance. Am. J. Epidemiol. 110:672-692. [DOI] [PubMed] [Google Scholar]
- 43.Omata, T., M. Kohara, S. Kuge, T. Komatsu, S. Abe, B. L. Semler, A. Kameda, H. Itoh, M. Arita, E. Wimmer, and A. Nomoto. 1986. Genetic analysis of the attenuation phenotype of poliovirus type 1. J. Virol. 58:348-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pulli, T., P. Koskimies, and T. Hyypia. 1995. Molecular comparison of coxsackie A virus serotypes. Virology 212:30-38. [DOI] [PubMed] [Google Scholar]
- 45.Sabin, A. B. 1965. Oral poliovirus vaccine. History of its development and prospects for eradication of poliomyelitis. JAMA 194:872-876. [DOI] [PubMed] [Google Scholar]
- 46.Sabin, A. B. 1956. Pathogenesis of poliomyelitis: reappraisal in the light of new data. Science 123:1151-1157. [DOI] [PubMed] [Google Scholar]
- 47.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed., p, 13.19-13.25. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 48.Schmidt, N. J., E. H. Lennette, and H. H. Ho. 1974. An apparently new enterovirus isolated from patients with disease of the central nervous system. J. Infect. Dis. 129:304-309. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu, H., A. Utama, N. Onnimala, C. Li, Z. Li-Bi, M. Yu-Jie, Y. Pongsuwanna, and T. Miyamura. 2004. Molecular epidemiology of enterovirus 71 infection in the western Pacific region. Pediatr. Int. 46:231-235. [DOI] [PubMed] [Google Scholar]
- 50.Shimizu, H., A. Utama, K. Yoshii, H. Yoshida, T. Yoneyama, M. Sinniah, M. A. Yusof, Y. Okuno, N. Okabe, S. R. Shih, H. Y. Chen, G. R. Wang, C. L. Kao, K. S. Chang, T. Miyamura, and A. Hagiwara. 1999. Enterovirus 71 from fatal and nonfatal cases of hand, foot and mouth disease epidemics in Malaysia, Japan and Taiwan in 1997-1998. Jpn. J. Infect. Dis. 52:12-15. [PubMed] [Google Scholar]
- 51.Shindarov, L. M., M. P. Chumakov, M. K. Voroshilova, S. Bojinov, S. M. Vasilenko, I. Iordanov, I. D. Kirov, E. Kamenov, E. V. Leshchinskaya, G. Mitov, I. A. Robinson, S. Sivchev, and S. Staikov. 1979. Epidemiological, clinical, and pathomorphological characteristics of epidemic poliomyelitis-like disease caused by enterovirus 71. J. Hyg. Epidemiol. Microbiol. Immunol. 23:284-295. [PubMed] [Google Scholar]
- 52.Tano, Y., H. Shimizu, M. Shiomi, T. Nakano, and T. Miyamura. 2002. Rapid serological diagnosis of enterovirus 71 infection by IgM ELISA. Jpn. J. Infect. Dis. 55:133-135. [PubMed] [Google Scholar]
- 53.Tardy-Panit, M., B. Blondel, A. Martin, F. Tekaia, F. Horaud, and F. Delpeyroux. 1993. A mutation in the RNA polymerase of poliovirus type 1 contributes to attenuation in mice. J. Virol. 67:4630-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallis, C., and J. L. Melnick. 1967. Virus aggregation as the cause of the non-neutralizable persistent fraction. J. Virol. 1:478-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, J. R., Y. C. Tuan, H. P. Tsai, J. J. Yan, C. C. Liu, and I. J. Su. 2002. Change of major genotype of enterovirus 71 in outbreaks of hand-foot-and-mouth disease in Taiwan between 1998 and 2000. J. Clin. Microbiol. 40:10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, S. M., H. Y. Lei, K. J. Huang, J. M. Wu, J. R. Wang, C. K. Yu, I. J. Su, and C. C. Liu. 2003. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: roles of cytokines and cellular immune activation in patients with pulmonary edema. J. Infect. Dis. 188:564-570. [DOI] [PubMed] [Google Scholar]
- 57.Wang, S. M., C. C. Liu, H. W. Tseng, J. R. Wang, C. C. Huang, Y. J. Chen, Y. J. Yang, S. J. Lin, and T. F. Yeh. 1999. Clinical spectrum of enterovirus 71 infection in children in southern Taiwan, with an emphasis on neurological complications. Clin. Infect. Dis. 29:184-190. [DOI] [PubMed] [Google Scholar]
- 58.Wenner, H. A., P. Harmon, A. M. Behbehani, H. Rouhandeh, and P. S. Kamitsuka. 1967. The antigenic heterogeneity of type 30 echoviruses. Am. J. Epidemiol. 85:240-249. [DOI] [PubMed] [Google Scholar]