Abstract
The sialic acid-binding lectin sialoadhesin (Sn) is a macrophage-restricted receptor for porcine reproductive and respiratory syndrome virus (PRRSV). To investigate the importance of pSn sialic acid-binding activity for PRRSV infection, an R116-to-E mutation was introduced in the predicted sialic acid-binding domain of pSn, resulting in a mutant, pSnRE, that could not bind sialic acids. PSn, but not pSnRE, allowed PRRSV binding and internalization. These data show that the sialic acid-binding activity of pSn is essential for PRRSV attachment to pSn and thus identifies the variable, N-terminal domain of Sn as a PRRSV binding domain.
Porcine reproductive and respiratory syndrome virus (PRRSV) is a member of the family Arteriviridae, which belongs, together with the Coronaviridae and Roniviridae, to the order Nidovirales (12, 14, 19, 23). The virus causes reproductive disorders in pregnant sows and boars and respiratory problems in pigs of all ages. In vivo, the virus has a tropism for a subpopulation of macrophages (10, 11), and only primary pig macrophages and continuous cell lines derived from African green monkey kidney cells, such as Marc-145 cells, allow efficient virus replication in vitro (1, 16, 28, 29). Heparan sulfate is a PRRSV receptor on macrophages that mediates virus attachment, and the viral matrix protein has been shown to be a heparin-binding protein, suggesting its potential role as a viral ligand for heparan sulfate (8, 25). Sialoadhesin (Sn) was identified as an essential PRRSV receptor that mediates both attachment and internalization on macrophages (5-7, 9, 26). Although Sn was shown to be essential for infection of macrophages, other, unidentified, factors are essential for productive infection, since expression of Sn in PRRSV nonpermissive cells, such as PK-15 and CHO K1 cells, allows virus internalization but no virus uncoating, genome release, or production of infectious virus (5, 26). Other putative PRRSV receptors have been described. Macrophage-specific monoclonal antibodies (MAbs) that block or reduce infection of macrophages were shown to be directed, respectively, against a protein of approximately 220 kDa and a 150-kDa protein doublet, but these proteins have not been identified and their exact role in PRRSV infection is not established (31). Recently, another MAb that blocks PRRSV infection of Marc-145 cells was shown to recognize a complex of cytoskeletal proteins (17), and an intact cytoskeleton was shown to be important for efficient infection of Marc-145 cells (2).
The PRRSV receptor pSn contains, like other sialoadhesins, an N-terminal variable immunoglobulin (Ig)-like domain, followed by 16 constant Ig domains. The conservation of the critical amino acids of the sialic acid-binding variable Ig-like domain in pSn and the observation that sialic acid-carrying sheep red blood cells agglutinate to porcine Sn-expressing macrophages suggest that porcine Sn is also a sialic acid-binding lectin, but this has not been conclusively demonstrated (7, 26). The domain of pSn that is involved in the interaction with PRRSV has not been identified. The fact that neuraminidase treatment of the porcine arterivirus blocks infection of macrophages may indicate that sialic acids present on viral glycoproteins are important for virus interaction with Sn (7). Therefore, it was investigated in this study whether the sialic acid-binding activity of porcine Sn can be eliminated by site-directed mutagenesis and whether the absence of sialic acid-binding activity has an effect on the interaction of the porcine arterivirus with Sn.
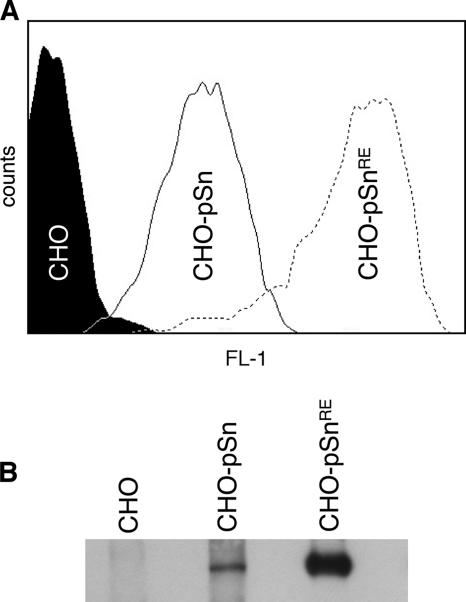
The amino acid residues critical for sialic acid-binding activity have been identified for mouse Sn by a combination of site-directed mutagenesis, nuclear magnetic resonance, and crystallography (4, 18, 20, 27). To generate a sialic acid-binding mutant of pSn, the amino acid R116, which is by analogy to mouse Sn critical for the sialic acid-binding activity of pSn, was changed to an E residue by site-directed mutagenesis (QuikChange; Stratagene) of the pcDNA3.1/pSn vector (26) with the primers pSnRE_F (TCGGGCTCCTATAACTTCGAATTTGAGATCAGCGAGGGC) and pSnRE_R (GCCCTCGCTGATCTCAAATTCGAAGTTATAGGAGCCCGA). Sequencing confirmed the presence of the R116-to-E mutation and the absence of other mutations in pSnRE. Continuous cell lines expressing pSn (CHO-pSn) or pSnRE (CHO-pSnRE) were selected from transfected CHO K1 cells by growth in medium containing G418 sulfate (Invitrogen), followed by cloning. Surface expression and total expression of both wild-type and mutant Sn in transfected CHO K1 cells were detected, respectively, by fluorescence-activated cell sorting analysis and by Western blotting using the pSn-specific MAb 41D3. Surprisingly, both surface-expressed and total expression levels of Sn with a mutation in the sialic acid-binding domain were at levels 10- to 20-fold higher than wild-type Sn (Fig. 1). Similar findings were described for mouse Sn, but how this is caused is not exactly known (22). One explanation might be that the sialic acid-binding capacity of Sn predisposes the protein for rapid turnover or degradation, but clearly this needs further investigation.
FIG. 1.
Analysis of porcine Sn expression in CHO cells expressing pSn (CHO-pSn) and CHO cells expressing a pSn with a mutation in the sialic acid-binding domain (CHO-pSnRE) by flow cytometric analysis (surface expression) (A) and Western blotting (total expression levels) (B). Histograms and Western blots are representative of three independent experiments.
To confirm that pSnRE lacks sialic acid-binding capacity, CHO K1, CHO-pSn, and CHO-pSnRE cells were used for red blood cell binding assays (3, 7). Briefly, CHO cells were pretreated for 30 min at 37°C with different concentrations of Vibrio cholerae neuraminidase (Roche) to remove potential cis-acting sialic acids that could interfere with the sialic acid-binding capacity of pSn and were then washed and incubated with sialic acid-containing sheep red blood cells for 30 min at room temperature. Qualitative analysis of red blood cell binding was done with light microscopy. Quantitative analysis of red blood cell binding was based on the presence of pseudoperoxidase activity of hemoglobin in red blood cells. Plates were fixed with methanol; a peroxidase substrate (Substrate Reagent Pack; R&D Systems) was added and processed, according to the manufacturer's recommendations; and the optical density at 450 nm (OD450) was measured. When transfected cells were not pretreated with neuraminidase, no red blood cell binding was detected (Fig. 2), suggesting the presence of inhibitory cis-acting sialic acids (3, 13). Strong red blood cell binding was observed for CHO-pSn cells after pretreatment with 10 mU/ml neuraminidase. Pretreatment of the cells with higher concentrations of neuraminidase resulted in small increases in red blood cell binding (Fig. 2). Binding of red blood cells was strongly reduced by treatment of the red blood cells with 50 mU/ml V. cholerae neuraminidase (Roche) or by incubation of the CHO cells with 100 μg/ml purified pSn-specific MAb 41D3, confirming the sialic acid and Sn dependence of red blood cell binding (data not shown). No red blood cell binding was observed for the CHO K1 and CHO-pSnRE cells (Fig. 2). These data clearly show that pSn is indeed a sialic acid-binding lectin and demonstrate the loss of the sialic acid-binding capacity of pSnRE.
FIG. 2.
Analysis of the sialic acid-binding capacity of different CHO cell lines by a sheep red blood cell binding assay. (A) Parental CHO cells (black bars), CHO cells expressing pSn (CHO-pSn) (gray bars), or CHO cells expressing a pSn with a mutation in the sialic acid-binding domain (CHO-pSnRE) (white bars) were treated with different concentrations of neuraminidase and incubated with sheep red blood cells. Data are means ± standard deviations from three experiments. (B) Microscopic images of the cells described above.
Previously, it was shown that CHO K1 and PK-15 cells allow PRRSV attachment and internalization upon expression of pSn (5, 26). To investigate the importance of the sialic acid-binding capacity of pSn for porcine arterivirus interaction with pSn, we used CHO-pSn, CHO-pSnRE, or parental CHO K1 cells. Alternatively, PK-15 cells were used after transient transfection with pSn and pSnRE by use of Lipofectamine (Invitrogen). Cells were inoculated with the European prototype PRRSV strain Lelystad virus (LV) (29), the American prototype PRRSV strain VR-2332 (1), or the Belgian isolate 94V360 (9) at a multiplicity of infection of 5. After 1 h of incubation at 37°C, cells were washed and fixed with methanol. LV and 94V360 virus particles were stained with the PRRSV nucleocapsid-specific MAb P3/27 (30), and the VR-2332 virus particles were stained with the American-type PRRSV nucleocapsid-specific MAb SDOW17 (21), followed by fluorescein isothiocyanate-labeled goat anti-mouse IgG. Cortical actin, which forms a thin layer just beneath the plasma membrane, was stained with Texas Red-labeled Phalloidin (Invitrogen) to allow discrimination between attached and internalized virus particles. Image acquisition and analysis of virus attachment and internalization were done by confocal microscopy with a Leica TCS SP2 laser-scanning spectral confocal system. For the CHO K1 cells that were mock transfected, few virus particles were stained for the three PRRSV isolates tested (Fig. 3). As previously shown, this low level of virus attachment most likely results from the interaction of PRRSV with heparan sulfate (5). Very abundant PRRSV staining was observed for CHO-pSn cells that expressed recombinant pSn (Fig. 3). For CHO-pSnRE cells, few virus particles were stained, suggesting that the sialic acid-binding capacity of pSn is essential for efficient interaction of PRRSV with pSn. Similarly, only PK-15 cells with expression of pSn showed a clear staining of internalized PRRSV particles, while no internalization was observed in wild-type PK-15 cells or PK-15 cells expressing the sialic acid-binding mutant pSnRE. To quantify virus internalization, the number of virus particles present inside the ring of cortical actin was counted (15 cells for each experimental condition). Virus particles were clearly internalized in cells expressing pSn but not in the parental cells or the cells expressing pSnRE, which lack the sialic acid-binding capacity (Fig. 4).
FIG. 3.
Confocal microscopic analysis of PRRSV attachment and internalization in CHO cells. PRRSV is stained green, cortical actin is stained red, and the overlay shows a superposition of the two images. Staining of the cortical actin, which lies just beneath the plasma membrane, allows discrimination of bound and internalized virus particles. The number of internalized PRRSV particles was analyzed with parental CHO cells, with CHO-pSn cells, which express recombinant porcine Sn, and with CHO-pSnRE cells, which express a mutant porcine Sn that lacks sialic acid-binding activity. Each image represents one confocal z-section through the middle of the cell.
FIG. 4.
Quantification of PRRSV internalization in CHO and PK-15 cells. The number of internalized PRRSV particles was analyzed by confocal microscopy of parental cell lines (black bars), cells expressing pSn (gray bars), and cells expressing pSnRE (white bars). Data are means ± standard deviations from three experiments.
In conclusion, the results of the present study show that the sialic acid-binding domain of pSn is essential for efficient Sn-dependent PRRSV binding and internalization. Furthermore, these data support the importance of sialic acids on PRRSV glycoproteins for interaction with pSn (7). Nevertheless, the results exclude neither the involvement of other Ig domains of pSn nor the involvement of nonsialylated PRRSV proteins in the interaction of PRRSV with pSn. Construction of soluble forms of pSn containing various amounts of pSn Ig domains, as was done previously (4, 15, 20, 24), and identification of the PRRSV protein that binds to pSn using these soluble receptors will certainly provide further insights into the interaction of PRRSV with pSn.
Acknowledgments
We thank Chantal Vanmaercke and Carine Boone for highly appreciated technical assistance.
P.L.D. was supported by an EMBO fellowship (ASTF 132-05) and a fellowship (B/06524) from the Special Research Fund of Ghent University. W.V.B. was supported by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen).
Footnotes
Published ahead of print on 13 June 2007.
REFERENCES
- 1.Benfield, D. A., E. Nelson, J. E. Collins, L. Harris, S. M. Goyal, D. Robison, W. T. Christianson, R. B. Morrison, D. Gorcyca, and D. Chladek. 1992. Characterization of swine infertility and respiratory syndrome (SIRS) virus (isolate ATCC VR-2332). J. Vet. Diagn. Investig. 4:127-133. [DOI] [PubMed] [Google Scholar]
- 2.Cafruny, W. A., R. G. Duman, G. H. Wong, S. Said, P. Ward-Demo, R. R. Rowland, and E. A. Nelson. 2006. Porcine reproductive and respiratory syndrome virus (PRRSV) infection spreads by cell-to-cell transfer in cultured MARC-145 cells, is dependent on an intact cytoskeleton, and is suppressed by drug-targeting of cell permissiveness to virus infection. Virol. J. 3:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crocker, P. R., S. Mucklow, V. Bouckson, A. McWilliam, A. C. Willis, S. Gordon, G. Milon, S. Kelm, and P. Bradfield. 1994. Sialoadhesin, a macrophage sialic acid binding receptor for haemopoietic cells with 17 immunoglobulin-like domains. EMBO J. 13:4490-4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crocker, P. R., M. Vinson, S. Kelm, and K. Drickamer. 1999. Molecular analysis of sialoside binding to sialoadhesin by NMR and site-directed mutagenesis. Biochem. J. 341:355-361. [PMC free article] [PubMed] [Google Scholar]
- 5.Delputte, P. L., S. Costers, and H. J. Nauwynck. 2005. Analysis of porcine reproductive and respiratory syndrome virus attachment and internalization: distinctive roles for heparan sulphate and sialoadhesin. J. Gen. Virol. 86:1441-1445. [DOI] [PubMed] [Google Scholar]
- 6.Delputte, P. L., and H. J. Nauwynck. 2006. Porcine arterivirus entry in macrophages: heparan sulfate-mediated attachment, sialoadhesin-mediated internalization, and a cell-specific factor mediating virus disassembly and genome release. Adv. Exp. Med. Biol. 581:247-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delputte, P. L., and H. J. Nauwynck. 2004. Porcine arterivirus infection of alveolar macrophages is mediated by sialic acid on the virus. J. Virol. 78:8094-8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delputte, P. L., N. Vanderheijden, H. J. Nauwynck, and M. B. Pensaert. 2002. Involvement of the matrix protein in attachment of porcine reproductive and respiratory syndrome virus to a heparinlike receptor on porcine alveolar macrophages. J. Virol. 76:4312-4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duan, X., H. J. Nauwynck, H. W. Favoreel, and M. B. Pensaert. 1998. Identification of a putative receptor for porcine reproductive and respiratory syndrome virus on porcine alveolar macrophages. J. Virol. 72:4520-4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan, X., H. J. Nauwynck, and M. B. Pensaert. 1997. Effects of origin and state of differentiation and activation of monocytes/macrophages on their susceptibility to porcine reproductive and respiratory syndrome virus (PRRSV). Arch. Virol. 142:2483-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan, X., H. J. Nauwynck, and M. B. Pensaert. 1997. Virus quantification and identification of cellular targets in the lungs and lymphoid tissues of pigs at different time intervals after inoculation with porcine reproductive and respiratory syndrome virus (PRRSV). Vet. Microbiol. 56:9-19. [DOI] [PubMed] [Google Scholar]
- 12.Enjuanes, L., W. J. Spaan, E. Snijder, and D. Cavanagh. 2000. Nidovirales, p. 827-834. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carsten, M. K. Estes, S. M. Lemon, D. J. McGeoch, J. Maniloff, M. A. Mayo, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. Classification and nomenclature of viruses. Academic Press, San Diego, CA.
- 13.Freeman, S. D., S. Kelm, E. K. Barber, and P. R. Crocker. 1995. Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood 85:2005-2012. [PubMed] [Google Scholar]
- 14.Gorbalenya, A. E., L. Enjuanes, J. Ziebuhr, and E. J. Snijder. 2006. Nidovirales: evolving the largest RNA virus genome. Virus Res. 117:17-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartnell, A., J. Steel, H. Turley, M. Jones, D. G. Jackson, and P. R. Crocker. 2001. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood 97:288-296. [DOI] [PubMed] [Google Scholar]
- 16.Kim, H. S., J. Kwang, I. J. Yoon, H. S. Joo, and M. L. Frey. 1993. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogeneous subpopulation of MA-104 cell line. Arch. Virol. 133:477-483. [DOI] [PubMed] [Google Scholar]
- 17.Kim, J. K., A. M. Fahad, K. Shanmukhappa, and S. Kapil. 2006. Defining the cellular target(s) of porcine reproductive and respiratory syndrome virus blocking monoclonal antibody 7G10. J. Virol. 80:689-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.May, A. P., R. C. Robinson, R. T. Aplin, P. Bradfield, P. R. Crocker, and E. Y. Jones. 1997. Expression, crystallization, and preliminary X-ray analysis of a sialic acid-binding fragment of sialoadhesin in the presence and absence of ligand. Protein Sci. 6:717-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayo, M. A. 2002. A summary of taxonomic changes recently approved by ICTV. Arch. Virol. 147:1655-1663. [DOI] [PubMed] [Google Scholar]
- 20.Nath, D., P. A. van der Merwe, S. Kelm, P. Bradfield, and P. R. Crocker. 1995. The amino-terminal immunoglobulin-like domain of sialoadhesin contains the sialic acid binding site. Comparison with CD22. J. Biol. Chem. 270:26184-26191. [DOI] [PubMed] [Google Scholar]
- 21.Nelson, E. A., J. Christopher-Hennings, T. Drew, G. Wensvoort, J. E. Collins, and D. A. Benfield. 1993. Differentiation of U.S. and European isolates of porcine reproductive and respiratory syndrome virus by monoclonal antibodies. J. Clin. Microbiol. 31:3184-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oetke, C., G. Kraal, and P. R. Crocker. 2006. The antigen recognized by MOMA-I is sialoadhesin. Immunol. Lett. 106:96-98. [DOI] [PubMed] [Google Scholar]
- 23.Snijder, E. J., and J. J. Meulenberg. 1998. The molecular biology of arteriviruses. J. Gen. Virol. 79:961-979. [DOI] [PubMed] [Google Scholar]
- 24.van den Berg, T. K., D. Nath, H. J. Ziltener, D. Vestweber, M. Fukuda, I. van Die, and P. R. Crocker. 2001. Cutting edge: CD43 functions as a T cell counterreceptor for the macrophage adhesion receptor sialoadhesin (Siglec-1). J. Immunol. 166:3637-3640. [DOI] [PubMed] [Google Scholar]
- 25.Vanderheijden, N., P. Delputte, H. Nauwynck, and M. Pensaert. 2001. Effects of heparin on the entry of porcine reproductive and respiratory syndrome virus into alveolar macrophages. Adv. Exp. Med. Biol. 494:683-689. [DOI] [PubMed] [Google Scholar]
- 26.Vanderheijden, N., P. L. Delputte, H. W. Favoreel, J. Vandekerckhove, J. Van Damme, P. A. van Woensel, and H. J. Nauwynck. 2003. Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages. J. Virol. 77:8207-8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinson, M., P. A. van der Merwe, S. Kelm, A. May, E. Y. Jones, and P. R. Crocker. 1996. Characterization of the sialic acid-binding site in sialoadhesin by site-directed mutagenesis. J. Biol. Chem. 271:9267-9272. [DOI] [PubMed] [Google Scholar]
- 28.Wensvoort, G., E. P. de Kluyver, J. M. Pol, F. Wagenaar, R. J. Moormann, M. M. Hulst, R. Bloemraad, A. den Besten, T. Zetstra, and C. Terpstra. 1992. Lelystad virus, the cause of porcine epidemic abortion and respiratory syndrome: a review of mystery swine disease research at Lelystad. Vet. Microbiol. 33:185-193. [DOI] [PubMed] [Google Scholar]
- 29.Wensvoort, G., C. Terpstra, J. M. Pol, E. A. ter Laak, M. Bloemraad, E. P. de Kluyver, C. Kragten, L. van Buiten, A. den Besten, F. Wagenaar, et al. 1991. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet. Q. 13:121-130. [DOI] [PubMed] [Google Scholar]
- 30.Wieczorek-Krohmer, M., F. Weiland, K. Conzelmann, D. Kohl, N. Visser, P. van Woensel, H. J. Thiel, and E. Weiland. 1996. Porcine reproductive and respiratory syndrome virus (PRRSV): monoclonal antibodies detect common epitopes on two viral proteins of European and U.S. isolates. Vet. Microbiol. 51:257-266. [DOI] [PubMed] [Google Scholar]
- 31.Wissink, E. H., H. A. van Wijk, J. M. Pol, G. J. Godeke, P. A. van Rijn, P. J. Rottier, and J. J. Meulenberg. 2003. Identification of porcine alveolar macrophage glycoproteins involved in infection of porcine respiratory and reproductive syndrome virus. Arch. Virol. 148:177-187. [DOI] [PMC free article] [PubMed] [Google Scholar]