Abstract
Vitamin K-dependent coagulation factors can promote adenoviral cell transduction in vitro. In vivo, warfarin pretreatment ablates liver targeting of an adenovirus serotype 5 (Ad5) vector deleted of CAR binding capability. Here, we assess in vivo transduction and biodistribution of Ad5 vectors with nonmodified fibers (Ad5) and a serotype 47 fiber-pseudotyped Ad5 (Ad5/47; subgroup D) virus following intravascular injection. Warfarin reduced liver transduction by both viruses. However, no impact on early liver virus accumulation was observed, suggesting no effect on Kupffer cell interactions. Hence, coagulation factors play a pivotal role in selectively mediating liver hepatocyte transduction of Ad5 and Ad5/47 vectors.
Adenovirus (Ad) vectors are commonly used biological tools for in vitro and in vivo gene delivery. The route of Ad delivery principally defines both the infectivity profile and the subsequent toxicity of the virus. The delivery of Ad via the intravascular route is broadly appealing both for liver-directed gene therapy (7, 13) and for targeting of nonhepatic tissues via alternate receptors (5, 19). In the paradigm of intravascular gene delivery, there is a complex yet poorly characterized pattern of virus-host interactions, including those with blood cells and plasma proteins. A fuller understanding of these interactions is necessary for the development of safe and efficient targeting to individual organs. Prior studies have demonstrated Ad interactions with red blood cells (2, 12) and white blood cells (9), as well as plasma proteins such as coagulation zymogens and complement pathway components (15, 18, 23). Recent evidence has shown a substantial role for coagulation zymogens in the delivery of Ad to the liver following intravascular injection (15, 18). This contrasts sharply with the accepted mechanism of Ad serotype 5 (Ad5) vector transduction in vitro with tethering to the cell surface through the fiber knob domain, which binds CAR (1, 21), and internalization through the penton base, which engages αv integrins (22). Potential redundancy of CAR in Ad5-mediated transduction of the liver is supported by studies showing little or no difference in liver sequestration for CAR binding and non-CAR binding Ad (reviewed in reference 11). Anatomically, the distribution of CAR expression is restricted to tight junctions (3); hence, the considerable levels of hepatocyte transduction observed with systemically administered Ad vectors would be difficult to reconcile with such restricted expression of CAR.
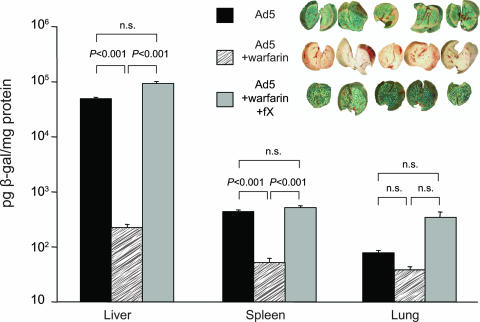
We recently documented a definitive role for plasma coagulation zymogens with the Gla-EGF-EGF-SP domain structure (factor VII [FVII], FIX, FX, and protein C) in enhancing hepatocyte transduction in vitro of both Ad5 and non-CAR binding Ad5 (15) and for Ad5 capsids pseudotyped with fibers from subgroup D Ad (14). Using a model system involving in vivo warfarin pretreatment of mice to reduce circulating levels of functional Gla domain-containing zymogens, we showed that warfarin blocked the ability of a non-CAR binding Ad5 (AdKO1) to target the liver (15). Liver targeting was reconstituted by injection of physiological concentrations of FX 30 min prior to virus injection, thereby confirming a particularly important role for FX. Because many studies have suggested that CAR is redundant in Ad-mediated liver targeting, we sought to assess the effect of coagulation zymogen depletion on liver targeting mediated by Ad vectors with a nonmodified capsid (Ad5). We injected Ad5 vectors into MF-1 mice in the presence or absence of warfarin pretreatment (Fig. 1) and first assessed transgene expression levels and then virion levels in organs postinjection. In an assessment of transduction at 48 h postinfection, a substantial reduction of liver targeting by warfarin was observed (Fig. 2). Hence, taken with data on CAR binding-deleted Ad vectors (15), this supports a redundancy of the CAR pathway in hepatocyte transduction in vivo, previously a controversial issue (4; reviewed in reference 11). Liver transduction was fully restored by FX injection (Fig. 2). FX injection resulted in physiological levels of circulating FX, confirmed by enzyme-linked immunosorbent assay (ELISA) (not shown). Hence, warfarin-sensitive coagulation factors are fundamentally important in Ad5 liver transduction. Although, as expected, Ad5 levels of transduction in the spleen were far lower than in the liver, transduction was again substantially reduced by the presence of warfarin and rescued by FX infusion (Fig. 2). This suggests that binding of Ad5 to coagulation factors also dictates Ad5 targeting to permissive cells in the spleen. Levels in the lung were lower than in the liver and spleen but were not significantly altered by warfarin or FX infusion (Fig. 2).
FIG. 1.
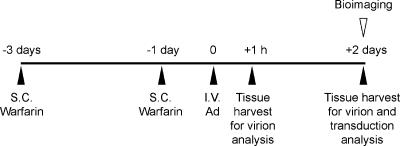
Schematic of the experimental design. MF1 outbred mice were injected subcutaneously (S.C.) with 133 μg of warfarin/mouse at 3 and 1 day prior to intravenous (I.V.) Ad injection (time zero). At 1 or 48 h postinjection, mice were perfused with phosphate-buffered saline and livers were taken for assay of virion accumulation by quantitative PCR. Transgene levels were also evaluated at 48 h by β-galactosidase ELISA or by whole-body in vivo bioimaging for luciferase.
FIG. 2.
Assessment of liver targeting by CAR binding Ad5 and the influence of FX modulation. Mice were pretreated with warfarin or carrier and injected with 4 × 1011 VP of Ad5/mouse with or without preinjection of FX at 30 min prior to virus injection. β-Galactosidase levels were quantitated by ELISA of the left lobe of the liver, spleen, and lungs. The median lobe of the liver was fixed in 70% ethanol before treatment with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside for visualization of β-galactosidase activity (inset). *, P < 0.05 (as indicated) (Student's t test); n, 5/group; n.s., not significant.
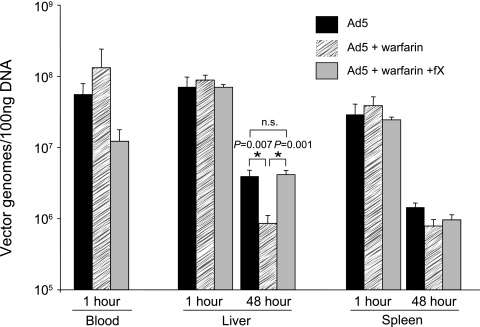
We next sought to define early virion accumulation in liver and spleen postinjection to ascertain whether warfarin could block virion sequestration in addition to cell transduction. Early Ad accumulation in liver and spleen is strongly associated with substantial targeting and clearance of Ad through Kupffer cells, an effect that is acute (8, 10, 20). We therefore assessed Ad virion levels at 1 h and 48 h postinfusion in the warfarin pretreatment and FX rescue model using quantitative Taqman PCR. Ad virion levels at 1 h postinjection were not significantly different with warfarin treatment or FX reconstitution from those in control mice (Fig. 3). This contrasted sharply with the levels quantified at 48 h postinjection, which, for the liver, paralleled transduction profiles (Fig. 3). This observation suggests that coagulation zymogens do not affect the substantial Kupffer cell uptake and supports the concept that coagulation zymogens play a pivotal role selectively in transduction of cells, both in the liver and in the spleen.
FIG. 3.
Effects of warfarin and FX on Ad5 virion accumulation. Ad5 vector (4 × 1011 VP/mouse) was injected into MF1 mice, and virion accumulation was assessed by quantitative real-time PCR. Mice were sacrificed at 1 h or 48 h postinfection and perfused to exsanguination; recovered DNA was subjected to real-time PCR (Applied Biosystems Prism 7900HT) and compared to standard curves generated from known concentrations of virus. *, P < 0.05 (versus controls) (Student's t test); n, 5/group; ns, not significant.
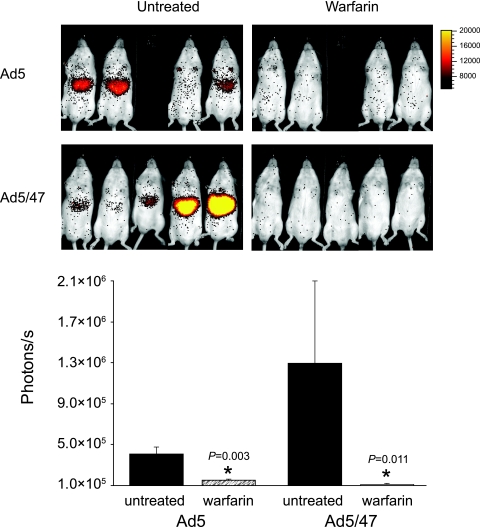
To confirm that the in vivo effects of warfarin are not limited to Ad5, we assessed the effect on liver targeting of a fiber-pseudotyped virus containing fibers from subgroup D (Ad5/47). Previously, we showed in vitro that FX at physiological concentrations promotes cell attachment and transduction of HepG2 hepatocytes mediated by a number of fiber-pseudotyped vectors with fibers from subgroup D (14). We therefore injected luciferase-expressing A5/47 into mice at 2 × 1010 virus particles (VP) per mouse and assessed transgene expression by bioluminescence at 48 h (Fig. 4). As shown, and in similarity to injection of the control Ad5 vector expressing luciferase (6), the liver infectivity of Ad5/47 was substantially reduced by warfarin pretreatment (Fig. 4). Hence, the effect of coagulation factors in vivo is relevant to Ad vectors with fibers derived from subgroup D as well as from subgroup C (Ad5).
FIG. 4.
In vivo imaging and quantitation of the effects of warfarin on liver transduction by Ad5/5 and Ad5/47. Mice received warfarin or carrier prior to intravenous Ad5/5 or Ad5/47. At 48 h, intraperitoneal injection of luciferin was followed by in vivo bioimaging with a Xenogen IVIS-50 cooled charge-coupled-device camera. Luciferase expression was quantitated as photon flux from the upper abdomen. *, P < 0.05 (as indicated) (Student's t test).
Combined with our previous studies (14, 15), we now show that both CAR binding and non-CAR binding Ad vectors predominantly utilize coagulation factors for liver and spleen transduction in vivo, since warfarin substantially reduces transduction of both viruses, an effect fully restored by FX infusion at physiological levels. For Ad5, this suggests that CAR binding plays no role in liver targeting when delivered via the bloodstream. This explains why many studies have shown no effect of CAR binding mutants on in vivo Ad liver transduction. For vector retargeting strategies, however, it remains unknown whether blocking of Ad-coagulation factor binding will allow efficient retargeting of virus, since early virus accumulation in the liver is not affected, at least for Ad5 (Fig. 3). Hence, Kupffer cell-depleting or avoidance strategies may also be required. Observations from this and previous studies, however, do provide evidence to suggest that blockade of coagulation factor-mediated liver transduction by Ad may be a useful approach for achieving transductional targeting. In the presence of a high-affinity retargeting strategy using bispecific antibodies to retarget Ad to the angiotensin converting enzyme, detectable transduction was achieved in target lung endothelium; however, the majority of Ad still transduced the liver (17). Transcriptional control was required to completely eliminate liver transgene expression (16). Clearly, in this paradigm, assessing the effect of warfarin depletion of coagulation factors on angiotensin converting enzyme-targeted Ad biodistribution is warranted. Our experiments with Ad5/47 also highlight the breadth of the effect that coagulation zymogens have on the biology of different human Ad serotypes upon contact with the bloodstream. In sum, our study defines the importance of vitamin K-dependent coagulation zymogens on Ad5- and Ad5/47-mediated liver transduction. It is likely that such mechanisms are relevant in defining the in vivo infectivity of many human Ad vectors being developed for gene-based therapeutics.
Acknowledgments
This work was supported by the European Commission and the Biotechnology and Biophysical Research Council. Hemostasis and Thrombosis are supported by the Medical Research Council. S.N.W. is a Philip Gray Memorial Fellow, Katharine Dormandy Trust.
Footnotes
Published ahead of print on 6 June 2007.
REFERENCES
- 1.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 2.Cichon, G., S. Boeckh-Herwig, D. Kuemin, C. Hoffmann, H. H. Schmidt, E. Wehnes, W. Haensch, U. Schneider, U. Eckhardt, R. Burger, and P. Pring-Akerblom. 2003. Titer determination of Ad5 in blood: a cautionary note. Gene Ther. 10:1012-1017. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, C. J., J. T. C. Shieh, R. J. Pickles, T. Okegawa, J.-T. Hsieh, and J. M. Bergelson. 2001. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 98:15191-15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Einfeld, D. A., R. Schroeder, P. W. Roelvink, A. Lizonova, C. R. King, I. Kovesdi, and T. J. Wickham. 2001. Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J. Virol. 75:11284-11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 6.Havenga, M. J. E., A. A. C. Lemckert, O. J. A. E. Ophorst, M. V. Meijer, W. T. V. Germeraad, J. Grimbergen, M. A. V. D. Doel, R. Vogels, J. V. Duetekom, A. A. M. Janson, J. D. D. Bruijn, F. Uytdehaag, P. H. A. Quax, T. Logtenberg, M. Mehtali, and A. Bout. 2002. Exploiting the natural diversity in adenovirus tropism for therapy and prevention of disease. J. Virol. 76:4612-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huard, J., H. Lochmuller, G. Ascadi, A. Jani, B. Massie, and G. Karpati. 1995. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther. 107:107-115. [PubMed] [Google Scholar]
- 8.Lieber, A., C. Y. He, L. Meuse, D. Schowalter, I. Kirillova, B. Winther, and M. A. Kay. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 71:8798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyons, M., D. Onion, N. K. Green, K. Aslan, R. Rajaratnam, M. Bazan-Peregrino, S. Phipps, S. Hale, V. Mautner, L. W. Seymour, and K. D. Fisher. 2006. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol. Ther. 14:118-128. [DOI] [PubMed] [Google Scholar]
- 10.Manickan, E., J. S. Smith, J. Tian, T. L. Eggerman, J. N. Lozier, J. Muller, and A. P. Byrnes. 2005. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol. Ther. 13:108-117. [DOI] [PubMed] [Google Scholar]
- 11.Nicklin, S., E. Wu, G. Nemerow, and A. Baker. 2005. The influence of adenovirus fiber structure and function on vector development for gene therapy. Mol. Ther. 12:384-393. [DOI] [PubMed] [Google Scholar]
- 12.Nicol, C., D. Graham, W. Miller, S. White, T. Smith, S. Nicklin, S. Stevenson, and A. Baker. 2004. Effect of adenovirus serotype 5 fiber and penton modifications on in vivo tropism in rats. Mol. Ther. 10:343-353. [DOI] [PubMed] [Google Scholar]
- 13.Oka, K., L. Pastore, I. Kim, A. Merched, S. Nomura, H. Lee, M. Merched-Sauvage, C. Arden-Riley, B. Lee, M. Finegold, A. Beaudet, and L. Chan. 2001. Long term stable correction of low-density lipoprotein receptor-deficient mice with a helper-dependent adenoviral vector expressing the very low-density lipoprotein receptor. Circulation 103:1274-1281. [DOI] [PubMed] [Google Scholar]
- 14.Parker, A. L., J. H. McVey, J. H. Doctor, O. Lopez-Franco, S. N. Waddington, M. J. E. Havenga, S. A. Nicklin, and A. H. Baker. 2007. Influence of coagulation factor zymogens on the infectivity of adenoviruses pseudotyped with fibers from subgroup D. J. Virol. 81:3627-3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker, A. L., S. N. Waddington, C. G. Nicol, D. M. Shayakhmetov, S. M. Buckley, L. Denby, G. Kemball-Cook, S. Ni, A. Lieber, J. H. McVey, S. A. Nicklin, and A. H. Baker. 2006. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes Blood. 108:2554-2561. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds, P. N., S. A. Nicklin, L. Kaliberova, B. G. Boatman, W. E. Grizzle, I. V. Balyasnikova, A. H. Baker, S. M. Danilov, and D. T. Curiel. 2001. Combined transductional and transcriptional targeting improves the specificty of transgene expression in vivo. Nat. Biotechnol. 19:838-842. [DOI] [PubMed] [Google Scholar]
- 17.Reynolds, P. N., K. R. Zinn, V. D. Gavrilyuk, I. V. Balyasnikova, B. E. Rogers, D. J. Buchsbaum, M. H. Wang, D. J. Miletich, W. E. Grizzle, J. T. Douglas, S. M. Danilov, and D. T. Curiel. 2000. A targetable, injectable adenoviral vector for selective gene delivery to pulmonary endothelium in vivo. Mol. Ther. 2:562-578. [DOI] [PubMed] [Google Scholar]
- 18.Shayakhmetov, D., A. Gaggar, S. Ni, Z.-Y. Li, and A. Lieber. 2005. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 79:7478-7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shayakhmetov, D., Z. Li, S. Ni, and A. Lieber. 2002. Targeting of adenovirus vectors to tumor cells does not enable efficient transduction of breast cancer metastases. Cancer Res. 62:1063-1068. [PubMed] [Google Scholar]
- 20.Tao, N., G. P. Gao, M. Parr, J. Johnston, T. Baradet, J. M. Wilson, J. Barsoum, and S. E. Fawell. 2001. Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol. Ther. 3:28-35. [DOI] [PubMed] [Google Scholar]
- 21.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 23.Zinn, K. R., A. Szalai, A. Stargel, V. Krasnykh, and T. R. Chaudhuri. 2004. Bioluminescence imaging reveals a significant role for complement in liver transduction following intravenous delivery of adenovirus. Gene Ther. 11:1482-1486. [DOI] [PubMed] [Google Scholar]