Abstract
The Sydney Blood Bank Cohort (SBBC) consists of eight blood transfusion recipients infected with nef-attenuated human immunodeficiency virus type 1 (HIV-1) acquired from a single donor. Here, we show that viral phenotypes and antibody responses differ considerably between individual cohort members, despite the single source of infection. Replication of isolated virus varied from barely detectable to similar to that of the wild-type virus, and virus isolated from five SBBC members showed coreceptor usage signatures unique to each individual. Higher viral loads and stronger neutralizing antibody responses were associated with better-replicating viral strains, and detectable viral replication was essential for the development of strong and sustained humoral immune responses. Despite the presence of strong neutralizing antibodies in a number of SBBC members, disease progression was not prevented, and each cohort member studied displayed a unique outcome of infection with nef-attenuated HIV-1.
Attenuation of human immunodeficiency virus type 1 (HIV-1) or simian immunodeficiency virus, via deletions in the nef gene, has been shown to result in lower plasma viral loads and delayed disease progression in infected humans and macaques (5, 13, 23, 25, 26, 30, 35, 46, 47). Such viruses have been proposed for use as vaccines to protect against HIV-1 infection (10, 58). However, long-term studies revealed that infection with nef-attenuated viruses resulted in disease progression in some infected individuals, albeit delayed (1, 6, 8, 13, 33).
The Sydney Blood Bank Cohort (SBBC) provides an important model of attenuated HIV-1 infection. It consists of eight individuals infected with HIV-1 (clade B) containing deletions in the nef and long terminal repeat (LTR) regions obtained from a single blood donor, D36, between 1981 and 1984 (3, 8, 9, 13, 32, 33). All cohort members maintained stable CD4+ T-cell counts and undetectable or low plasma viral loads (less than 16,000 HIV-1 RNA copies/ml) (Table 1) without therapeutic intervention for at least 17 to 18 years postinfection (3, 8, 9, 33).
TABLE 1.
Background data for study subjectsa
| Subject | Sample | Date | No. of yr | CD4 | VL | Isolation |
|---|---|---|---|---|---|---|
| nef attenuated | ||||||
| D36 | D36II | 6/5/95 | 14.4 | ND | 1,400 | − |
| D36III | 8/2/96 | 15.2 | 609 | 1,100 | + | |
| D36IV | 10/4/96 | 15.3 | 504 | 7,700 | + | |
| D36V | 9/7/96 | 15.6 | 414 | 2,600 | + | |
| D36VI | 23/10/96 | 15.8 | 432 | 1,100 | + | |
| D36VII | 30/1/97 | 16.1 | 361 | 3,200 | + | |
| D36VIII | 20/5/97 | 16.4 | 540 | 4,000 | + | |
| D36IX | 23/12/97 | 17.0 | 390 | 7,500 | + | |
| D36X | 15/7/98 | 17.6 | 325 | ND | + | |
| D36XI | 27/1/99 | 18.1 | 214 | ND | + | |
| D36XII | 2/3/99 | 18.3 | 323 | BD | − | |
| D36XIII | 26/4/00 | 19.3 | 284 | <400 | − | |
| D36XIV | 27/9/00 | 19.8 | ND | ND | − | |
| D36XVI | 8/11/00 | 19.9 | ND | <400 | + | |
| C18 | C18(2) | 26/7/93 | 9.8 | ND | ND | + |
| C18(3) | 14/10/93 | 10.1 | ND | ND | + | |
| C18(4) | 7/3/94 | 10.5 | ND | 2,804 | + | |
| C49 | C49I | 28/2/94 | 9.8 | 1,045 | ND | − |
| C49II | 18/10/94 | 10.3 | 918 | BD | − | |
| C49III | 31/1/95 | 10.6 | ND | ND | − | |
| C49IV | 13/4/95 | 10.8 | ND | ND | − | |
| C49V | 9/10/95 | 11.3 | 1,008 | BD | − | |
| C49VI | 25/6/96 | 12.0 | 1,248 | BD | − | |
| C49VII | 17/10/96 | 12.3 | ND | ND | − | |
| C49VIII | 10/12/97 | 13.5 | 918 | BD | − | |
| C49IX | 23/6/99 | 15.0 | 605 | BD | − | |
| C49X | 25/10/00 | 16.3 | 752 | BD | − | |
| C49XI | 31/10/01 | 17.3 | 624 | ND | ND | |
| C54 | C54III | 7/11/94 | 10.3 | 2,006 | 8,200 | + |
| C54IV | 21/6/95 | 10.9 | 1,504 | 3,000 | + | |
| C54V | 20/12/95 | 11.4 | 1,054 | 400 | + | |
| C54VI | 4/3/96 | 11.7 | 1,188 | 1,500 | + | |
| C54VII | 19/6/96 | 11.9 | 972 | 3,600 | + | |
| C54VIII | 16/9/96 | 12.2 | 1,120 | 1,800 | + | |
| C54IX | 18/12/96 | 12.4 | 1,073 | 3,400 | − | |
| C54X | 3/3/97 | 12.7 | 882 | 3,400 | + | |
| C54XI | 14/5/97 | 12.8 | 1,286 | 5,500 | + | |
| C54XII | 11/8/97 | 13.1 | 1,419 | 1,700 | + | |
| C54XIII | 17/11/97 | 13.3 | 1,054 | 1,600 | + | |
| C54XIV | 5/5/99 | 14.8 | 1,288 | 1,200 | + | |
| C54XV | 6/3/00 | 15.7 | 840 | 1,600 | + | |
| C54XVI | 16/10/00 | 16.3 | 1,225 | ND | − | |
| C64 | C64III | 23/11/95 | 12.5 | 850 | BD | − |
| C64IV | 28/2/96 | 12.8 | 875 | BD | + | |
| C64V | 6/5/96 | 13.0 | 851 | BD | − | |
| C64VII | 6/11/96 | 13.5 | 910 | BD | − | |
| C64VIII | 10/2/97 | 13.8 | 851 | ND | − | |
| C64IX | 12/5/97 | 14.0 | 1,050 | BD | − | |
| C64XI | 24/11/97 | 14.5 | 936 | BD | − | |
| C64XII | 20/4/99 | 15.9 | 1,026 | ND | − | |
| C64XIII | 15/11/99 | 16.5 | 1,332 | ND | − | |
| C64XIV | 12/1/00 | 16.7 | 1,156 | ND | − | |
| C64XV | 24/10/00 | 17.4 | ND | BD | − | |
| C64XVII | 27/3/01 | 17.8 | 1,414 | BD | − | |
| C98 | C98II | 7/12/94 | 12.9 | 462 | 1,000 | + |
| C98III | 9/10/95 | 13.8 | 576 | 670 | + | |
| C98IV | 7/2/96 | 14.1 | 435 | 200 | + | |
| C98V | 22/5/96 | 14.3 | 693 | 290 | + | |
| C98VI | 7/8/96 | 14.6 | 512 | 330 | + | |
| C98VII | 4/11/96 | 14.8 | 646 | 690 | + | |
| C98VIII | 31/1/97 | 15.0 | 629 | 770 | + | |
| C98IX | 7/5/97 | 15.3 | 529 | 760 | + | |
| C98X | 27/8/97 | 15.6 | 612 | 170 | + | |
| C98XI | 26/11/97 | 15.8 | 400 | ND | + | |
| C98XII | 25/9/98 | 16.7 | 387 | BD | + | |
| C98XIII | 3/3/99 | 17.2 | 476 | 800 | + | |
| C98XIV | 9/11/99 | 17.8 | 585 | BD | + | |
| C98XV | 8/3/00 | 18.2 | 468 | BD | ND | |
| C98XVI | 8/11/00 | 18.8 | 230 | BD | ND | |
| C124 | C124I | 8/3/93 | 11.9 | 720 | ND | − |
| C135 | C135I | 12/3/96 | 15.0 | 646 | BD | − |
| C135IV | 14/7/97 | 16.4 | 392 | BD | − | |
| C135V | 15/10/97 | 16.7 | 420 | BD | − | |
| C135VI | 21/6/99 | 18.3 | ND | ND | − | |
| C135VIII | 28/2/00 | 19.0 | ND | ND | − | |
| C135IX | 7/9/00 | 19.6 | 510 | BD | − | |
| C135X | 16/10/00 | 19.7 | 594 | ND | − | |
| Nef1 | Nef1(1) | 19/6/03 | 18.5 | 390 | 70,000 | + |
| Nef1(2) | 2/7/03 | 18.6 | ND | ND | ND | |
| Control | ||||||
| S1 | 26/5/94 | 8.2 | 1,200 | ND | ||
| 28/11/94 | 8.7 | 1,200 | 8,000 | |||
| 13/8/96 | 10.4 | 874 | 16,464 | |||
| 8/4/97 | 10.9 | 1,078 | 11,000 | |||
| 20/10/99 | 13.4 | 540 | 23,100 | |||
| S2 | 20/6/94 | 10.9 | ND | ND | ||
| 21/1/95 | 11.6 | ND | 11,000 | |||
| 16/7/96 | 13.0 | 864 | 3,400 | |||
| 15/5/97 | 13.9 | 899 | 35,000 | |||
| S4 | 12/10/94 | 9.9 | ND | ND | ||
| 14/6/96 | 11.6 | 546 | 79,000 | |||
| S12 | 24/10/95 | 10.1 | 805 | ND | ||
| 17/4/96 | 10.6 | 560 | ND | |||
| 29/11/00 | 15.2 | 680 | 3,500 | |||
| S13 | 21/12/95 | 5.0 | 483 | 3,700 | ||
| 4/7/96 | 5.6 | 418 | 8,962 | |||
| 3/4/97 | 6.3 | 260 | ND | |||
| 14/12/99 | 9.0 | ND | ND |
For each of the studied members of the nef-attenuated and control LTNP/LTS groups, the sample name, date (day/month/year), number of years postinfection (SBBC) or after identification of infection (control group), viral load (VL) (RNA copies/ml), CD4+ T-cell count (CD4) (cells/μl), and success of virus isolation (Isolation) (+, successful; −, unsuccessful) are shown. The italicized samples were used in this study. Samples in boldface were obtained while the subject was undergoing antiretroviral therapy. For the control group, viral load and CD4+ cell count data are not always coincident and were derived from the closest time point available. BD, below detection; ND, not done.
Since monitoring began in 1993, a slow decline in CD4+ T cells was observed for subjects D36, C98, and, to a lesser extent, C54 (3, 33). These subjects are considered long-term survivors (LTS). For D36, this was associated with a high viral load in the cerebrospinal fluid (greater than 750,000 copies/ml compared with 9,900 copies/ml in plasma), and a switch in coreceptor usage from dual-tropic CCR5/CXCR4 to CCR5 occurred after the commencement of antiretroviral therapy (8). In contrast, for C98, the viral load in the cerebrospinal fluid just prior to the commencement of therapy (900 copies/ml) was low by comparison (unpublished data). Subjects D36 and C98 were placed on antiretroviral therapy in 1999 (9). Subjects C49, C64, and C135 are long-term nonprogressors (LTNP), as they have remained asymptomatic with undetectable plasma viral loads and stable CD4+ T-cell counts (3, 9, 33). Although five cohort members have died—C18 (1995, aged 88), C54 (2001, aged 74), C83 (1987, aged 23), C98 (2001, aged 64), and C124 (1994, aged 77)—no deaths were considered directly attributable to HIV-1 infection, with the possible exception of C83 (9, 33).
A number of studies have suggested an increased frequency of LTNP and LTS possessing strong, cross-reactive neutralizing antibody responses (7, 43, 57). However, very few studies have investigated antibody responses in LTNP/LTS infected with nef-attenuated HIV-1, despite a number of studies showing strong neutralizing antibody responses in macaques infected with nef-attenuated simian immunodeficiency virus (12, 17, 24, 31, 38, 51). Greenough et al. (22) showed measurable neutralizing antibody responses concurrent with a detectable viral load for a single individual infected with nef-attenuated HIV-1. Dyer et al. (16) observed strong cytotoxic T-lymphocyte responses for SBBC members D36, C18, C49, and C98, which, except for C49, were associated with low but detectable levels of viremia.
This study follows the antibody responses in nef-attenuated HIV-1 infection and details the replication phenotype and unique coreceptor usage of virus isolated from different cohort members. Analysis of humoral immune responses revealed a strong association between neutralizing antibody responses, viral load, isolation of infectious virus, and viral replication phenotype in individuals infected with nef-attenuated HIV-1. The study emphasizes the requirement for viral replication for infected individuals to mount robust humoral immune responses. For nef-attenuated virus infections, a spectrum of responses was observed that developed much more slowly overall than those of a control group of LTNP/LTS. This may contribute to more potent neutralizing antibody responses.
MATERIALS AND METHODS
Subject samples.
Plasma was obtained from eight SBBC members between 1993 and 2001 (Table 1). An additional individual (Nef1) was shown to be infected with HIV-1 containing deletions in the nef and LTR regions via PCR fragment length analysis. Nef1 remained asymptomatic and antiretroviral therapy naïve until 2004, 19 years after infection, when the subject commenced antiretroviral therapy due to a high viral load and a declining CD4+ T-cell count. Plasma was also obtained from a control group of five LTNP/LTS infected with HIV-1 containing wild-type nef, selected from two LTNP/LTS cohorts—a sexually acquired-HIV-1-infected cohort (Australian Red Cross Blood Service [ARCBS], Sydney, Australia) and the Australian Long Term Non Progressor Study Group (National Centre in HIV Epidemiology and Clinical Research, Australia) (3, 46, 50). Two of the five members of this group (S4 and S12) were heterozygous for the CCR5-Δ32 mutation (34). All control group members remained asymptomatic and antiretroviral therapy naïve for at least 11 years after the identification of infection, except S13, who began antiretroviral therapy 9 years after the identification of infection when he showed signs of disease progression. Clinical details of study subjects corresponding to plasma samples are shown in Table 1. The plasma was heat inactivated at 56°C for 30 min and stored at −20°C until immediately before use.
Cells.
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll gradient centrifugation from buffy coats obtained from the ARCBS (Melbourne, Victoria, Australia), taken from healthy HIV-1-seronegative individuals, as described previously (53). PBMC were maintained in RF10 medium (RPMI 1640 medium supplemented with 10% [vol/vol] fetal bovine serum, 25 μg/ml l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin) and were activated using 10 μg/ml phytohemagglutinin 72 h prior to use. Donor PBMC were selected for the ability to support the replication of primary and nef-attenuated isolates prior to use.
Viruses.
The HIV-1 isolates HIV-1NL43 (X4, subtype B) and HIV-1AD8 (R5, subtype B) were obtained from Malcolm Martin (National Institute of Allergy and Infectious Disease [NIAID], NIH, Bethesda, MD). HIV-1BCB93 (subtype D) and HIV-192TH024 (R5, subtype CRF01_AE) were obtained from the NIH AIDS Reference and Reagent Program (NIAID, Bethesda, MD). HIV-1ROK39 (R5, subtype A) and HIV-1SE364 (R5, subtype C) were obtained from Paul Cameron (University of Melbourne, Parkville, Victoria, Australia). The Australian isolates HIV-1MBC200 (X4, subtype B) and HIV-1MBC925 (R5, subtype B) were isolated from patient PBMC and characterized as described previously (42). HIV-1ADA was obtained from H. Gendelman (18). HIV-189.6 was generated by transfection of 293T cells with HIV-189.6 proviral DNA obtained from R. Collman (11). Virus stocks were produced by infection and culture of 3-day phytohemagglutinin-activated PBMC in interleukin 2 (IL-2) medium (RF10 containing 12 mM HEPES, 10 U/ml IL-2, 0.2% Polybrene, and 0.1% hydrocortisone).
Virus was isolated from SBBC PBMC using standard PBMC coculture techniques (41). Subject PBMC were isolated from fresh whole blood as described previously (53) and were cocultured with selected activated donor PBMC for up to 28 days. The culture supernatant was tested for cell-free reverse transcriptase (RT) activity (49) and/or extracellular p24 antigen by enzyme immunoassay (EIA) (Biomérieux, Baulkham Hills, NSW, Australia). For some isolations prior to 1996, subject PBMC were irradiated with UV light prior to coculture to promote virus production from latently infected cells (52).
Virus was isolated from plasma collected from subject Nef1 by centrifugation over a 20% (wt/vol) sucrose cushion at 45,000 × g using a Heraeus Biofuge Stratos centrifuge. The pelleted virus was resuspended in IL-2 medium containing 5 × 106 selected activated donor PBMC and cultured for 14 days. The culture supernatant was tested for cell-free RT activity.
Coreceptor usage assay.
Virus coreceptor usage was analyzed as described previously (19-21). Briefly, Cf2-Luc cells were transfected with CD4 alone or cotransfected with CCR2b, CCR3, CCR5, CCR8, CXCR4, CX3CR1, Gpr1, Gpr15, Strl33, or Apj before infection with each HIV-1 isolate. Cells were harvested 48 h postinfection and assayed for luciferase activity.
Western blotting.
HIV-1 viral-lysate-based Western blots were performed using total immunoglobulin G (IgG) or IgG3 secondary antibodies as described previously (56).
Neutralization assay.
Plasma (25 μl, to give final twofold dilutions from 1:100 to 1:3,200, except where otherwise stated) was added to 50 μl of HIV-1 at 10,000 50% tissue culture infective doses/ml, or 50 μl of neat culture supernatant if less than 10,000 50% tissue culture infective doses/ml, in quadruplicate wells of a 96-well tissue culture plate. This mixture was incubated for 1 h at 37°C and 5% CO2 before the addition of 2 × 105 selected activated PBMC pooled from two different HIV-1-seronegative donors. After incubation for 2 h, 100 μl of IL-2 medium was added to each well. The PBMC were washed on day 1 postinfection by performing three half-medium changes using fresh growth medium. Neutralization was measured during the logarithmic growth phase of virus replication by analysis of cell-free RT activity and/or p24 production and was calculated as the percent decrease in replication compared to a control with no antibody present.
RESULTS
Virus isolation.
To facilitate autologous antibody testing for SBBC members, virus isolation was attempted for the eight members studied via coculture of subject PBMC with selected seronegative donor PBMC. The success of isolation correlated with the coincident plasma viral load for the majority of samples tested (Table 1). Virus was consistently isolated from subjects D36, C18, C54, and C98. For subjects with undetectable viral loads (C49, C64, and C135), virus was isolated for C64 on only a single occasion. No virus was isolated from the one time point tested for C124. A single attempt at virus isolation from plasma collected from Nef1 was successful (Table 1).
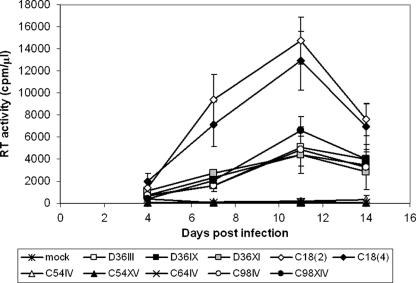
Replication of nef-attenuated isolates in selected activated HIV-1-seronegative donor PBMC yielded three distinct replication phenotypes (Fig. 1). For each infected individual, viruses isolated over time behaved with similar replication kinetics. Two separate isolates from C18 [C18(2) and C18(4)] replicated with rapid kinetics, peaking at a high level of RT activity (12,925 and 14,730 cpm/μl, respectively). Viruses derived from D36 (D36III, D36IX, and D36XI) and C98 (C98IV and C98XIV) replicated more slowly, but still to detectable levels of RT activity (4,380 to 6,605cpm/μl). Replication of viruses derived from C54 (C54IV and C54XV) and C64 (C64IV) was barely detectable by analysis of RT activity (<125 cpm/μl) but was confirmed by extracellular p24 antigen expression (data not shown). Virus isolated from Nef1 plasma replicated with kinetics similar to that of the C18 isolates (data not shown).
FIG. 1.
Replication kinetics of SBBC isolates. HIV-1 isolated from members of the SBBC was used to infect selected activated PBMC derived from healthy seronegative donors. Virus replication was measured by cell-free RT activity, and replication of HIV-1C54IV, HIV-1C54XV, and HIV-1C64IV was confirmed by quantitation of extracellular p24 antigen (not shown). The error bars indicate standard deviations.
Unique pattern of coreceptor usage for SBBC isolates.
The coreceptor usage of selected SBBC isolates was analyzed by infection of transfected Cf2-Luc cells, alongside the control viruses HIV-1NL4-3 (X4), HIV-1ADA (R5), and HIV-189.6 (R5X4). All SBBC isolates studied used CCR5 as the principal coreceptor for virus entry (Table 2), with the exception of all D36 isolates, which displayed a dual-tropic phenotype (R5X4), as reported previously (8). Some isolates were able to utilize additional coreceptors, such as CCR2b (D36 and C54 isolates), CCR3 (C18, C54, and C98 isolates), and Gpr15 (C18 isolates). No change in isolate coreceptor usage over time was observed for any SBBC member studied, except that previously reported for D36 (8). The signatures of multiple coreceptor usage remained unique for all viruses isolated from each subject. Virus isolated from Nef1 was found to use CCR5, but not CXCR4, as a primary coreceptor (data not shown).
TABLE 2.
Coreceptor usage of SBBC isolatesa
| Virus | Coreceptor usage
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CCR2b | CCR3 | CCR5 | CCR8 | CXCR4 | CX3CR1 | Gpr1 | Gpr15 | Strl33 | Apj | |
| NL4-3 | − | − | − | − | +++ | − | − | − | − | + |
| ADA | − | ++ | +++ | + | − | ± | − | + | + | + |
| 89.6 | + | ++ | +++ | − | +++ | − | ± | − | − | + |
| D36II | ± | − | ++ | − | +++ | − | − | − | − | − |
| D36VIII | ± | − | +++ | − | +++ | − | − | − | − | − |
| D36IX | ± | − | +++ | − | +++ | − | − | − | − | − |
| D36XI | ± | − | +++ | − | +++ | − | − | − | − | − |
| C18(2) | − | + | +++ | − | − | − | − | + | − | − |
| C18(4) | − | + | +++ | − | − | − | − | + | − | − |
| C54III | ± | + | ++ | − | − | − | − | − | − | − |
| C54VIII | ± | + | ++ | − | − | − | − | − | − | − |
| C54XIV | ± | + | +++ | − | − | − | − | − | − | − |
| C64IV | − | − | ++ | − | − | − | − | − | − | − |
| C98I | − | + | ++ | − | − | − | − | − | − | − |
| C98VI | − | + | +++ | − | − | − | − | − | − | − |
| C98XIV | − | + | +++ | − | − | − | − | − | − | − |
Cf2-Luc cells were transfected with CD4 alone or cotransfected with selected HIV-1 coreceptors before infection with each HIV-1 isolate. Cells were harvested 48 h postinfection and assayed for luciferase activity. Entry levels were scored as +++ (>50,000 luciferase activity units), ++ (between 30,000 and 50,000 luciferase activity units), + (between 10,000 and 30,000 luciferase activity units), ± (between 5,000 and 10,000 luciferase activity units), or − (<5,000 luciferase activity units) as described elsewhere (20).
Immune responses to nef-attenuated HIV-1 infection.
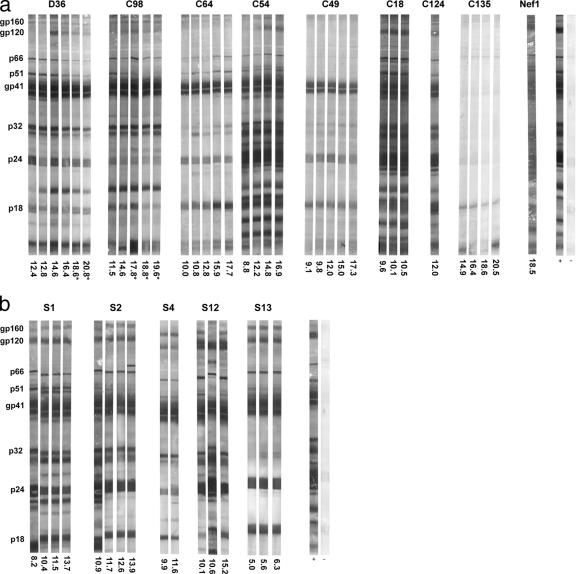
The total anti-HIV-1 IgG responses over time for members of the nef-attenuated cohort were initially analyzed by Western blotting (Fig. 2a). Total IgG responses for the nef-attenuated cohort showed concordance with the viral load, with the strongest overall responses shown by individuals with detectable viral loads (Nef1, D36, C18, C54, and C98), as well as C124 (viral load unknown). IgG3 isotype reactivity to particular HIV-1 antigens was also examined. Subjects C18 and Nef1 displayed IgG3 isotype responses to multiple viral antigens, compared with weaker responses for D36, C54, C64, and C98 (data not shown). Subjects C49 and C64, who maintained consistently undetectable viral loads, showed weaker total IgG responses. Western blot and EIA analyses detected no gp120-specific antibodies for these individuals (data not shown). Subject C135 had not fully seroconverted on Western blots after more than 20 years of infection, extending previous findings (45). Total IgG responses for D36 and C98 waned after the commencement of antiretroviral therapy.
FIG. 2.
Total IgG responses in individuals infected with nef-attenuated (a) or non-nef-attenuated (b) HIV-1. Total IgG reactivity to HIV-1 lysate in plasma derived from HIV-1-infected individuals was analyzed by Western blotting. Strips were probed with biotinylated anti-human IgG polyclonal antibody and streptavidin-alkaline phosphatase conjugate and visualized using 5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium phosphate substrate (56). Plasma samples are identified by the number of years postinfection (a) or after identification of infection (b) and do not correspond to those used for neutralization assays. Reactivities were compared with those of a recently seroconverted individual (+) and a seronegative individual (−).
In contrast, total IgG responses in the control group were more uniformly reactive to all HIV-1 proteins (Fig. 2b). No association with the viral load was observed, although the control group did not contain any antiretroviral therapy-naïve individuals with undetectable viral loads. Analysis of IgG3 isotype responses for the five controls revealed no association with the viral load. Plasmas from subjects S2 and S12 had strong reactivity to numerous HIV-1 proteins, whereas plasmas from S1, S4, and S13 showed weak reactivity to single proteins (data not shown).
Neutralization of nef-attenuated HIV-1 is associated with viral phenotype and viral load.
Plasma samples from selected members of the nef-attenuated and control cohorts were tested for neutralization of two SBBC isolates, HIV-1D36III and HIV-1C18(2), using PBMC (Table 3). The results shown are from representative experiments using the same donor PBMC, allowing reproducible cross-comparisons (15). Experiments in our laboratory showed that measurement of neutralization using the RT assay was reproducible and comparable to results gained using the p24 antigen assay (data not shown). For the nef-attenuated group, virus neutralization was associated with the viral load, replication of isolated virus, and the strength of antiviral IgG responses; C54 was the exception. The most potent neutralization was observed for plasmas derived from subjects D36, C18, and Nef1. Plasma from later time points in infection showed little change in neutralizing ability. No neutralization was observed for plasmas derived from cohort members with undetectable or low viral loads. Plasma samples derived from all members of the control group, except for the seronegative individual, were able to neutralize HIV-1C18(2) with titers generally higher than those of the nef-attenuated cohort. No association of neutralization with viral load or IgG responses was observed for the control cohort.
TABLE 3.
Neutralization of SBBC virus isolates by plasma samples derived from the nef-attenuated and control groupsa
| Virus | Plasma | Yr postinfection | ID50 | ID80 |
|---|---|---|---|---|
| HIV-1D36III | D36III/VIII | 14.5/16.4 | 2,510/1,723 | <100/<100 |
| C18(2)/(4) | 9.8/10.5 | 818/1,091 | <100/<100 | |
| C49I | 17.0 | NT | NT | |
| C54IV/VIII | 10.9/15.6 | 314/216 | <100/<100 | |
| C64IV/XII | 12.9/16.0 | <100/<100 | <100/<100 | |
| C98IV/XIV | 14.0/17.8 | <100/<100 | <100/<100 | |
| C135I | 19.6 | NT | NT | |
| Nef1(1) | 18.5 | NT | NT | |
| HIV-1C18(2) | D36III/VIII | 14.5/16.4 | 674/836 | 150/157 |
| C18(2)/(4) | 9.8/10.5 | 904/945 | <100/<100 | |
| C49I | 17.0 | <25 | <25 | |
| C54IV/VIII | 10.9/15.6 | <100/<100 | <100/<100 | |
| C64IV/XII | 12.9/16.0 | <100/<100 | <100/<100 | |
| C98IV/XIV | 14.0/17.8 | <100/<100 | <100/<100 | |
| C135I | 19.6 | <25 | <25 | |
| Nef1(1) | 18.5 | 1,266 | 315 | |
| HIV-1C18(2) | S1 | 8.2 | 2,299 | <100 |
| S2 | 10.9 | 2,710 | 353 | |
| S4 | 9.9 | 1,398 | <100 | |
| S12 | 10.6 | 709 | <100 | |
| S13 | 5.2 | 2,661 | 703 | |
| Seronegative | N/A | <25 | <25 |
Plasma samples derived from members of the nef-attenuated and control cohorts were tested for neutralization of two SBBC isolates, HIV-1D36III and HIV-1C18(2). The plasma samples were tested at twofold dilutions from 1:100 to 1:3,200, except for C49I, C135I, and the seronegative plasma (1:25 to 1:800). Fifty percent or 80% inhibitory dilutions (ID50 or ID80) are shown as the reciprocal of the plasma dilution calculated to cause 50% or 80% neutralization.
Presence of broadly neutralizing antibodies against HIV-1.
Plasma derived from members of the nef-attenuated and control groups were tested for neutralization of the clade B isolates HIV-1NL43, HIV-1AD8, HIV-1MBC200, and HIV-1MBC925 (Table 4). Potent neutralization was observed for plasmas derived from Nef1 and C18 and, to a lesser extent, for D36, C54, and C98. Little or no neutralization was observed for plasma samples derived from C49, C64, and C135. Plasma derived from subject C18 was able to strongly neutralize HIV-1ROK39 (clade A), HIV-1SE364 (clade C), HIV-1BCB93 (clade D), and HIV-192TH024 (CRF01_AE), while plasma from C98 was only able to weakly neutralize HIV-1ROK39 and HIV-1SE364 (Table 5). Neutralization of clade B isolates by plasma samples derived from members of the control group varied, with plasma from S13, and to a lesser extent S1, S2, and S4, strongly neutralizing and no neutralization observed for plasma from S12. HIV-1MBC925, a brain-derived CCR5-using strain isolated from a patient with AIDS, was the most difficult clade B isolate to neutralize, with only three plasma samples [C18(2), Nef1, and S13] showing moderate neutralization of this isolate. This is consistent with previous findings (37). Together, these isolates represent a diverse group of viruses to be neutralized, some which have been used in other neutralization studies, including both R5 and X4 phenotypes (2, 28, 37, 40).
TABLE 4.
Neutralization of heterologous virus isolates by plasma samples derived from the nef-attenuated and control groupsa
| Plasma | HIV-1NL4-3
|
HIV-1AD8
|
HIV-1MBC200
|
HIV-1MBC925
|
||||
|---|---|---|---|---|---|---|---|---|
| ID50 | ID80 | ID50 | ID80 | ID50 | ID80 | ID50 | ID80 | |
| D36III | 639 | 159 | 579 | 128 | 184 | <100 | <100 | <100 |
| C18(2) | 1979 | 158 | 2,969 | 352 | 1,211 | <100 | 390 | <100 |
| C49I | NT | NT | <25 | <25 | 39 | <25 | <25 | <25 |
| C54IV | 701 | 203 | 1,162 | 338 | 308 | <100 | <100 | <100 |
| C64IV | <100 | <100 | 236 | <100 | <100 | <100 | <100 | <100 |
| C98IV | 184 | <100 | 1,929 | <100 | <100 | <100 | <100 | <100 |
| C135I | NT | NT | <50 | <50 | <25 | <25 | <25 | <25 |
| Nef1(1) | NT | NT | 1,385 | 499 | >3,200 | 792 | 894 | 403 |
| S1 | NT | NT | >3,200 | <100 | 2,440 | 208 | <100 | <100 |
| S2 | NT | NT | 1,454 | <100 | 1,153 | 302 | <100 | <100 |
| S4 | NT | NT | >3,200 | <100 | 2,052 | <100 | <100 | <100 |
| S12 | NT | NT | <100 | <100 | <100 | <100 | <100 | <100 |
| S13 | NT | NT | >3,200 | 449 | >3,200 | 1196 | 1302 | 532 |
| Seronegative | NT | NT | <25 | <25 | <25 | <25 | <25 | <25 |
Selected plasma samples derived from members of the nef-attenuated and control cohorts were tested for neutralization of four subtype B isolates, HIV-1NL4-3 (X4), HIV-1AD8 (R5), HIV-1MBC200 (X4), and HIV-1MBC925 (R5). All plasma samples were tested at twofold dilutions from 1:100 to 1:3,200, except for C49I, C135I, and the seronegative plasma (1:25 to 1:800). Fifty percent and 80% inhibitory dilutions (ID50 and ID80) are shown as the reciprocal of the plasma dilution calculated to cause 50% or 80% neutralization. NT, not tested.
TABLE 5.
Neutralization of heterologous virus isolates by plasma samples derived from the nef-attenuated groupa
| Plasma | HIV-1ROK39
|
HIV-1SE364
|
HIV-1BCB93
|
HIV-192TH024
|
||||
|---|---|---|---|---|---|---|---|---|
| ID50 | ID80 | ID50 | ID80 | ID50 | ID80 | ID50 | ID80 | |
| C18(2) | >3,200 | <100 | >3,200 | 263 | 2,128 | 325 | >3,200 | <100 |
| C98XIV | 551 | <100 | 305 | <100 | <100 | <100 | <100 | <100 |
Plasma samples derived from subjects C98 and C18 were tested for neutralization of four isolates representing different subtypes, HIV-1ROK39 (subtype A, R5), HIV-1SE364 (subtype C, R5), HIV-1BCB93 (subtype D), and HIV-192TH024 (CRF01_AE, R5). All plasma samples were tested at twofold dilutions from 1:100 to 1:3,200. Fifty percent and 80% inhibitory dilutions (ID50 and ID80) are shown as the reciprocal of the plasma dilution calculated to cause 50% or 80% neutralization. HIV-1ROK39, HIV-1SE364, HIV-1BCB93, and HIV-192TH024 were tested for neutralization by C98XIV plasma; HIV-1BCB93 and HIV-192TH024 were tested for neutralization by C18(4) plasma.
Changes in neutralization over time.
Sequential plasma samples derived from the five SBBC members from whom virus had been isolated (D36, C18, C54, C64, and C98) were tested for the ability to neutralize virus isolated from the same individual at different time points (Table 6). For subject D36, virus isolated at 14.5 years postinfection (D36III), prior to the increasing viral load and decreasing CD4+ T-cell count, showed increased neutralization sensitivity compared with virus isolated at later time points. D36 plasma from early time points was able to neutralize autologous virus before neutralization decreased, accompanying signs of disease progression. Interestingly, neutralization titers increased following the commencement of antiretroviral therapy.
TABLE 6.
Longitudinal analysis of plasma-neutralizing responses and HIV-1 neutralization sensitivities for selected members of the SBBCa
| Virus | Plasma | Yr postinfection | ID50 | ID80 |
|---|---|---|---|---|
| HIV-1D36III | D36III | 14.5 | 668 | <100 |
| D36VIII | 16.4 | 298 | <100 | |
| D36IX | 17.0 | NT | NT | |
| D36XI | 18.1 | 132 | <100 | |
| D36XVI | 19.8 | 813 | <100 | |
| HIV-1D36IX | D36III | 14.5 | 319 | <100 |
| D36VIII | 16.4 | <100 | <100 | |
| D36IX | 17.0 | 597 | <100 | |
| D36XI | 18.1 | <100 | <100 | |
| D36XVI | 19.8 | 395 | <100 | |
| HIV-1D36XI | D36III | 14.5 | 158 | <100 |
| D36VIII | 16.4 | <100 | <100 | |
| D36IX | 17.0 | NT | NT | |
| D36XI | 18.1 | <100 | <100 | |
| D36XVI | 19.8 | 245 | <100 | |
| HIV-1C18(2) | C18(2) | 9.8 | >3200 | 531 |
| C18(4) | 10.5 | 2886 | 140 | |
| HIV-1C18(4) | C18(2) | 9.8 | >3200 | 262 |
| C18(4) | 10.5 | 2144 | <100 | |
| HIV-1C54IV | C54IV | 10.9 | >3200 | <100 |
| C54XV | 15.6 | 1173 | <100 | |
| HIV-1C54XV | C54IV | 10.9 | 640 | <100 |
| C54XV | 15.6 | 708 | <100 | |
| HIV-1C64IV | C64IV | 12.9 | <25 | <25 |
| C64XII | 16.0 | <25 | <25 | |
| C64XVII | 18.3 | <25 | <25 | |
| HIV-1C98IV | C98II | 12.8 | 255 | <100 |
| C98IV | 14.0 | <100 | <100 | |
| C98XII | 16.7 | 103 | <100 | |
| C98XIV | 17.8 | 1311 | <100 | |
| C98XVI | 18.8 | 556 | 151 | |
| HIV-1C98XIV | C98II | 12.8 | 699 | <100 |
| C98IV | 14.0 | 796 | <100 | |
| C98XII | 16.7 | <100 | <100 | |
| C98XIV | 17.8 | 1061 | <100 | |
| C98XVI | 18.8 | 1193 | 413 |
Sequential plasma samples from SBBC members from whom HIV-1 had been isolated (D36, C18, C54, C64, and C98) were tested for neutralization of sequential virus isolates from the same individual. Plasma samples were tested at twofold dilutions from 1:100 to 1:3,200, except for C64 plasma (1:25 to 1:800). Replication was measured by cell-free RT activity, except for HIV-1C54IV and HIV-1C54XV, which were analyzed for supernatant p24 antigen production. Fifty percent and 80% inhibitory dilutions (ID50 and ID80) are shown as the reciprocal of the plasma dilution calculated to cause 50% or 80% neutralization. Boldface, subject was undergoing antiretroviral therapy at the time of sampling.
Only two samples were available for subject C18 after monitoring began and prior to the death of the subject in 1995. Both C18(2) and C18(4) plasmas were strongly able to neutralize autologous virus, although neutralization titers declined marginally for C18(4) plasma.
Neutralization titers for plasma derived from subject C54 decreased from 10.9 to 15.4 years postinfection, as did the neutralization sensitivity of isolated virus. For this subject, isolated virus was neutralized more strongly by contemporaneous plasma than by plasma taken at earlier or later time points. This suggests an evolution of neutralizing antibody responses and virus neutralization sensitivity over time for this individual, despite the poor replication of isolated virus over time, as demonstrated in Fig. 1.
The single C64 virus isolate was not able to be neutralized by any autologous plasma. For subject C98, virus isolated at 14.0 and 17.8 years postinfection displayed similar sensitivities to neutralization, although the neutralizing ability of the plasma decreased over time from 12.8 to 16.7 years postinfection. As was observed for D36, neutralization increased after the commencement of antiretroviral therapy.
DISCUSSION
This study identified a relationship between the viral load, replication phenotype, and antibody responses for individuals infected with nef-attenuated HIV-1. This was not observed for LTNP/LTS infected with HIV-1 containing wild-type nef sequences. Due to the small sample population, statistical analysis of significance was not performed.
Members of the nef-attenuated cohort displayed lower viral loads than the control group, with the exceptions of D36 (cerebrospinal fluid) and Nef1 (plasma) (750,000 and 70,000 RNA copies/ml, respectively), for whom the relatively high viral loads were associated with signs of disease progression. Virus replication fell into three distinct phenotypes—robust (HIV-1C18 and HIV-1Nef1), intermediate (HIV-1D36 and HIV-1C98), and poor (HIV-1C54 and HIV-1C64). An exception was observed in subject C54, who maintained a detectable viral load but whose isolated virus replicated poorly, similar to virus isolated from C64, who maintained a consistently undetectable viral load. C54 did not display a decrease in the CD4+ cell count, suggesting reduced pathogenicity of the infecting strain. However, findings for this individual are complicated by coinfection with hepatitis C, which has been implicated in increased mortality for HIV-1-infected individuals (54). All viruses isolated from the SBBC members studied were found to use CCR5 as the primary coreceptor, with the exception of isolates from the donor, D36, which were able to utilize both CCR5 and CXCR4. The unique signature of coreceptor usage for viruses isolated from different cohort members suggests independent evolution of each virus after infection of each individual from the same or similar virus quasispecies. This supports recent longitudinal analyses of nef and LTR sequences for D36, C98, C54, C64, and C49 (9), which showed each individual to have evolved unique sequences. Minimal change of virus coreceptor usage was observed for each subject over the period of observation.
For the individuals with nef-attenuated HIV-1 infection, the strengths of total IgG responses as analyzed by Western blotting was associated with viral load and virus replication kinetics, highlighting the importance of adequate antigenic stimulation to drive antibody production. However, this did not extend to members of the control group, who showed a range of detectable viral loads (3,700 to 79,000 RNA copies/ml for S13 and S4, respectively). Members of the control group showed a much narrower range of IgG responses than the nef-attenuated cohort. Interestingly, plasma samples derived from Nef1, C18, and C54 showed p24 antigen-specific IgG3 isotype responses, previously identified in our laboratory as a marker of early HIV-1 infection, when tested with seroconversion panels by Western blotting, as did S4 and S13 by EIA (56), previously identified in our laboratory as a marker of early HIV-1 infection, when tested with seroconversion panels.
Detailed neutralization studies revealed concordance between the viral load and neutralizing antibody responses for studied members of the nef-attenuated cohort. The subjects could be ranked by the strengths of neutralizing responses against all viruses tested: Nef1 > C18 > C54 > D36 > C98 > C64. With the exception of C54, this was also consistent with the replication of isolated virus. Plasma samples from Nef1 and C18 showed 50% neutralization approaching or greater than a dilution of 1:3,200, which was exceptionally potent against the diverse isolates tested. Such high-titer antibody responses were observed previously for LTNP (57). Only small differences in neutralization were observed for sequential plasma samples from cohort members, suggesting minimal evolution had occurred over the time period tested. In contrast to published data, neutralization of contemporaneous virus was observed for those SBBC LTS with neutralizing antibody responses, further confirming minimal viral evolution (4). No evidence of complement-dependent neutralization was observed (data not shown). All members of the control group showed robust antibody responses (Fig. 2). Despite this, only plasma derived from S13 showed broad and potent neutralization similar to that of Nef1 and C18. This individual showed signs of disease progression by 9 years after initial identification as HIV-1 positive, earlier than other control group members. We cannot exclude the possibility that this individual may be a slow progressor. No correlation between IgG responses and neutralizing antibody responses was observed for the control group.
The SBBC isolate HIV-1C18(2) was neutralized more readily by plasma derived from the control group than the other clade B isolates tested, but not by plasma from the nef-attenuated group. Highly attenuated isolates, such as HIV-1C54IV and HIV-1C64IV, were readily neutralized by most plasma samples from the nef-attenuated cohort (data not shown). In contrast, the local primary isolate HIV-1MBC925 was poorly neutralized by most plasma samples tested. This may reflect the robustness of replication or viral fitness.
Longitudinal analysis of neutralizing antibody responses and neutralization sensitivities of isolated virus revealed only small changes over the time period tested for subjects D36, C18, C54, C64, and C98. A small decline in neutralizing responses over time was observed for most subjects, which accompanied signs of disease progression for D36 and C98. C54 was the only subject to show increased neutralization of contemporaneous virus. Neutralizing antibody responses for D36 and C98 were observed to increase over time after the commencement of antiretroviral therapy, a phenomenon that has been observed previously (27, 29, 48). While antiretrovirals present in test plasma may contribute to the observation of neutralization (14, 39), experiments in our laboratory showed minimal contributions by four antiretrovirals (the RT inhibitors stavudine and didanosine and the phosphatidylinositol inhibitors ritonavir and indinavir) added exogenously at expected circulating levels and testing dilutions of plasma up to 1:200 (O. H. Ho and D. A. McPhee, unpublished data). Antiretroviral drug therapy for D36 and C98 included abacavir, zidovudine, nevirapine, lamivudine, stavudine, and indinavir (9). Hence, while we suggest only a minimal input, we cannot exclude the possibility that antiretrovirals may have contributed to the observed neutralization while the individuals were on therapy. The neutralization sensitivity of isolated virus declined for a number of subjects; however, it remains unclear whether this resulted from changes to the envelope glycoproteins, decreasing recognition by neutralizing antibodies, or from phenotypic changes, increasing viral fitness. The inverse relationship between virus replication and neutralization sensitivity may suggest the latter.
For the members of the nef-attenuated cohort studied, the strongest antibody responses were observed for individuals with low but detectable viral-load set points, less than 10,000 copies/ml. This is consistent with the recent observation of an undetectable viral load and weak, delayed antibody responses in an unrelated individual infected with nef-attenuated HIV-1 (30). Although Nef1 appears to be an exception, the viral-load data for this individual were available for only the single time point tested. This study was limited by the window of time from which samples were available, well after infection was established. The results suggest that neutralizing antibody responses may have been declining during the window of observation for some subjects.
This study shows that infection with nef-attenuated HIV-1 can, in some individuals, induce antibody responses capable of potently neutralizing a broad range of isolates. The diverse group of viral strains offered a tier 1-type test of neutralizing antibody responses (36). It is possible that the breadth of antibody responses observed for the nef-attenuated cohort may be associated with unrestricted immunoglobulin class switching, which is inhibited by Nef (44). The strongest antibody responses in the nef-attenuated cohort were observed in individuals with detectable viral loads, supporting the model of a viral-load threshold that must be reached to provide adequate antigenic stimulation to drive antibody production (55). However, this higher level of virus replication places these individuals at the greatest risk of disease progression. Signs of progression were observed for three cohort members (D36, C98, and Nef1), demonstrating that the strong neutralizing antibody responses observed did not protect them from eventual disease progression. These results suggest continuing viral evolution, albeit very slow, in the face of a strong neutralizing antibody response associated with low but detectable levels of viral turnover. The marked variability of these responses within a well-defined cohort, where infection from a single source is known, shows the importance of the host in the evolution of the virus and of host responses. While the broadly protective antibodies may not offer long-term protection in individuals with established infection, they may be critical in preventing a new infection. In addition, it is possible that they may be important in the maintenance of low viral loads and delayed disease progression, observed for some members of the nef-attenuated cohort.
The viral loads and neutralizing antibody responses were highly variable for different cohort members, despite the fact that all SBBC members were infected with closely related viruses. Isolated SBBC viruses show common elements of the deletions in nef and the LTR, which alter over time (9). The results reported here highlight the importance of both the host and the infecting viral strain in determining the unique outcome of infection with nef-attenuated HIV-1 for each individual. This presents additional complications for the induction of consistent and robust immune responses for any future vaccine against HIV-1.
Acknowledgments
We are indebted to the Burnet Institute (Melbourne, Victoria, Australia) for ongoing laboratory support. We thank the ARCBS for the provision of donor cells, as well as Paul Cameron, Malcolm Martin, and the AIDS Research and Reference Reagent Program for the provision of viruses.
This study was supported by the Burnet Institute, the National Centre for HIV Virology Research (Melbourne, Victoria, Australia), and the National Serology Reference Laboratory (Melbourne, Victoria, Australia). E.E.V. was supported by an Australian Postgraduate Award (Monash University, Clayton, Victoria, Australia). P.R.G. was supported in part by grants from the Australian National Health and Medical Research Council (NHMRC) (251520) and NIH/NIAID (AI054207) and is the recipient of an NHMRC R. Douglas Wright Biomedical Career Development Award. P.R.G. and D.G. were supported by the NIH (NS37277).
Footnotes
Published ahead of print on 13 June 2007.
REFERENCES
- 1.Baba, T. W., Y. S. Jeong, D. Pennick, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820-1825. [DOI] [PubMed] [Google Scholar]
- 2.Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232-13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birch, M. R., J. C. Learmont, W. B. Dyer, N. J. Deacon, J. J. Zaunders, N. Saksena, A. L. Cunningham, J. Mills, and J. S. Sullivan. 2001. An examination of signs of disease progression in survivors of the Sydney Blood Bank Cohort (SBBC). J. Clin. Virol. 22:263-270. [DOI] [PubMed] [Google Scholar]
- 4.Bradney, A. P., S. Scheer, J. M. Crawford, S. P. Buchbinder, and D. C. Montefiori. 1999. Neutralization escape in human immunodeficiency virus type 1-infected long-term nonprogressors. J. Infect. Dis. 179:1264-1267. [DOI] [PubMed] [Google Scholar]
- 5.Brambilla, A., L. Turchetto, A. Gatti, C. Bovolenta, F. Veglia, E. Santagostino, A. Gringeri, M. Clementi, G. Poli, P. Bagnarelli, and E. Vicenzi. 1999. Defective nef alleles in a cohort of hemophiliacs with progressing and nonprogressing HIV-1 infection. Virology 259:349-368. [DOI] [PubMed] [Google Scholar]
- 6.Carl, S., R. Daniels, A. J. Iafrate, P. Easterbrook, T. C. Greenough, J. Skowronski, and F. Kirchhoff. 2000. Partial “repair” of defective NEF genes in a long-term nonprogressor with human immunodeficiency virus type 1 infection. J. Infect. Dis. 181:132-140. [DOI] [PubMed] [Google Scholar]
- 7.Carotenuto, P., D. Looij, L. Keldermans, F. de Wolf, and J. Goudsmit. 1998. Neutralizing antibodies are positively associated with CD4+ T-cell counts and T-cell function in long-term AIDS-free infection. AIDS 12:1591-1600. [DOI] [PubMed] [Google Scholar]
- 8.Churchill, M., J. Sterjovski, L. Gray, D. Cowley, C. Chatfield, J. Learmont, J. S. Sullivan, S. M. Crowe, J. Mills, B. J. Brew, S. L. Wesselingh, D. A. McPhee, and P. R. Gorry. 2004. Longitudinal analysis of nef/long terminal repeat-deleted HIV-1 in blood and cerebrospinal fluid of a long-term survivor who developed HIV-associated dementia. J. Infect. Dis. 190:2181-2186. [DOI] [PubMed] [Google Scholar]
- 9.Churchill, M. J., D. I. Rhodes, J. C. Learmont, J. S. Sullivan, S. L. Wesselingh, I. R. Cooke, N. J. Deacon, and P. R. Gorry. 2006. Longitudinal analysis of human immunodeficiency virus type 1 nef/long terminal repeat sequences in a cohort of long-term survivors infected from a single source. J. Virol. 80:1047-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen, J. 1997. Novel campaign to test live HIV vaccine. Science 277:1035. [DOI] [PubMed] [Google Scholar]
- 11.Collman, R., J. W. Balliet, S. A. Gregory, H. Friedman, D. L. Kolson, N. Nathanson, and A. Srinivasan. 1992. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J. Virol. 66:7517-7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cranage, M. P., A. M. Whatmore, S. A. Sharpe, N. Cook, N. Polyanskaya, S. Leech, J. D. Smith, E. W. Rud, M. J. Dennis, and G. A. Hall. 1997. Macaques infected with live attenuated SIVmac are protected against superinfection via the rectal mucosa. Virology 229:143-154. [DOI] [PubMed] [Google Scholar]
- 13.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, et al. 1995. Genomic structure of an attenuated quasispecies of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 14.Dreyer, K., E. G. Kallas, V. Planelles, D. Montefiori, M. P. McDermott, M. S. Hasan, and T. G. Evans. 1999. Primary isolate neutralization by HIV type 1-infected patient sera in the era of highly active antiretroviral therapy. AIDS Res. Hum. Retrovir. 15:1563-1571. [DOI] [PubMed] [Google Scholar]
- 15.D'Souza, M. P., G. Milman, J. A. Bradac, D. McPhee, C. V. Hanson, and R. M. Hendry. 1995. Neutralization of primary HIV-1 isolates by anti-envelope monoclonal antibodies. AIDS 9:867-874. [DOI] [PubMed] [Google Scholar]
- 16.Dyer, W. B., G. S. Ogg, M. A. Demoitie, X. Jin, A. F. Geczy, S. L. Rowland-Jones, A. J. McMichael, D. F. Nixon, and J. S. Sullivan. 1999. Strong human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte activity in Sydney Blood Bank Cohort patients infected with nef-defective HIV type 1. J. Virol. 73:436-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Enose, Y., M. Ui, A. Miyake, H. Suzuki, H. Uesaka, T. Kuwata, J. Kunisawa, H. Kiyono, H. Takahashi, T. Miura, and M. Hayami. 2002. Protection by intranasal immunization of a nef-deleted, nonpathogenic SHIV against intravaginal challenge with a heterologous pathogenic SHIV. Virology 298:306-316. [DOI] [PubMed] [Google Scholar]
- 18.Gendelman, H. E., J. M. Orenstein, M. A. Martin, C. Ferrua, R. Mitra, T. Phipps, L. A. Wahl, H. C. Lane, A. S. Fauci, D. S. Burke, et al. 1988. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J. Exp. Med. 167:1428-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorry, P. R., G. Bristol, J. A. Zack, K. Ritola, R. Swanstrom, C. J. Birch, J. E. Bell, N. Bannert, K. Crawford, H. Wang, D. Schols, E. De Clercq, K. Kunstman, S. M. Wolinsky, and D. Gabuzda. 2001. Macrophage tropism of human immunodeficiency virus type 1 isolates from brain and lymphoid tissues predicts neurotropism independent of coreceptor specificity. J. Virol. 75:10073-10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray, L., M. J. Churchill, N. Keane, J. Sterjovski, A. M. Ellett, D. F. Purcell, P. Poumbourios, C. Kol, B. Wang, N. K. Saksena, S. L. Wesselingh, P. Price, M. French, D. Gabuzda, and P. R. Gorry. 2006. Genetic and functional analysis of R5X4 human immunodeficiency virus type 1 envelope glycoproteins derived from two individuals homozygous for the CCR5δ32 allele. J. Virol. 80:3684-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray, L., J. Sterjovski, M. Churchill, P. Ellery, N. Nasr, S. R. Lewin, S. M. Crowe, S. L. Wesselingh, A. L. Cunningham, and P. R. Gorry. 2005. Uncoupling coreceptor usage of human immunodeficiency virus type 1 (HIV-1) from macrophage tropism reveals biological properties of CCR5-restricted HIV-1 isolates from patients with acquired immunodeficiency syndrome. Virology 337:384-398. [DOI] [PubMed] [Google Scholar]
- 22.Greenough, T. C., M. Somasundaran, D. B. Brettler, R. M. Hesselton, A. Alimenti, F. Kirchhoff, D. Panicali, and J. L. Sullivan. 1994. Normal immune function and inability to isolate virus in culture in an individual with long-term human immunodeficiency virus type 1 infection. AIDS Res. Hum. Retrovir. 10:395-403. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann-Lehmann, R., J. Vlasak, A. L. Williams, A. L. Chenine, H. M. McClure, D. C. Anderson, S. O'Neil, and R. M. Ruprecht. 2003. Live attenuated, nef-deleted SIV is pathogenic in most adult macaques after prolonged observation. AIDS 17:157-166. [DOI] [PubMed] [Google Scholar]
- 24.Igarashi, T., Y. Ami, H. Yamamoto, R. Shibata, T. Kuwata, R. Mukai, K. Shinohara, T. Komatsu, A. Adachi, and M. Hayami. 1997. Protection of monkeys vaccinated with vpr- and/or nef-defective simian immunodeficiency virus strain mac/human immunodeficiency virus type 1 chimeric viruses: a potential candidate live-attenuated human AIDS vaccine. J. Gen. Virol. 78:985-989. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, R. P., R. L. Glickman, J. Q. Yang, A. Kaur, J. T. Dion, M. J. Mulligan, and R. C. Desrosiers. 1997. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J. Virol. 71:7711-7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 27.Kim, J. H., J. R. Mascola, S. Ratto-Kim, T. C. VanCott, L. Loomis-Price, J. H. Cox, N. L. Michael, L. Jagodzinski, C. Hawkes, D. Mayers, B. L. Gilliam, D. C. Birx, and M. L. Robb. 2001. Selective increases in HIV-specific neutralizing antibody and partial reconstitution of cellular immune responses during prolonged, successful drug therapy of HIV infection. AIDS Res. Hum. Retrovir. 17:1021-1034. [DOI] [PubMed] [Google Scholar]
- 28.Kim, Y. B., M. K. Lee, D. P. Han, and M. W. Cho. 2001. Development of a safe and rapid neutralization assay using murine leukemia virus pseudotyped with HIV type 1 envelope glycoprotein lacking the cytoplasmic domain. AIDS Res. Hum. Retrovir. 17:1715-1724. [DOI] [PubMed] [Google Scholar]
- 29.Kimura, T., K. Yoshimura, K. Nishihara, Y. Maeda, S. Matsumi, A. Koito, and S. Matsushita. 2002. Reconstitution of spontaneous neutralizing antibody response against autologous human immunodeficiency virus during highly active antiretroviral therapy. J. Infect. Dis. 185:53-60. [DOI] [PubMed] [Google Scholar]
- 30.Kondo, M., T. Shima, M. Nishizawa, K. Sudo, S. Iwamuro, T. Okabe, Y. Takebe, and M. Imai. 2005. Identification of attenuated variants of HIV-1 circulating recombinant form 01_AE that are associated with slow disease progression due to gross genetic alterations in the nef/long terminal repeat sequences. J. Infect. Dis. 192:56-61. [DOI] [PubMed] [Google Scholar]
- 31.Kumar, A., S. Mukherjee, J. Shen, S. Buch, Z. Li, I. Adany, Z. Liu, W. Zhuge, M. Piatak, Jr., J. Lifson, H. McClure, and O. Narayan. 2002. Immunization of macaques with live simian human immunodeficiency virus (SHIV) vaccines conferred protection against AIDS induced by homologous and heterologous SHIVs and simian immunodeficiency virus. Virology 301:189-205. [DOI] [PubMed] [Google Scholar]
- 32.Learmont, J., B. Tindall, L. Evans, A. Cunningham, P. Cunningham, J. Wells, R. Penny, J. Kaldor, and D. A. Cooper. 1992. Long-term symptomless HIV-1 infection in recipients of blood products from a single donor. Lancet 340:863-867. [DOI] [PubMed] [Google Scholar]
- 33.Learmont, J. C., A. F. Geczy, J. Mills, L. J. Ashton, C. H. Raynes-Greenow, R. J. Garsia, W. B. Dyer, L. McIntyre, R. B. Oelrichs, D. I. Rhodes, N. J. Deacon, and J. S. Sullivan. 1999. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 340:1715-1722. [DOI] [PubMed] [Google Scholar]
- 34.Liu, R., W. A. Paxton, S. Choe, D. Ceradini, S. R. Martin, R. Horuk, M. E. MacDonald, H. Stuhlmann, R. A. Koup, and N. R. Landau. 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86:367-377. [DOI] [PubMed] [Google Scholar]
- 35.Mariani, R., F. Kirchhoff, T. C. Greenough, J. L. Sullivan, R. C. Desrosiers, and J. Skowronski. 1996. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 70:7752-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mascola, J. R., P. D'Souza, P. Gilbert, B. H. Hahn, N. L. Haigwood, L. Morris, C. J. Petropoulos, V. R. Polonis, M. Sarzotti, and D. C. Montefiori. 2005. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J. Virol. 79:10103-10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McPhee, D., A. Hines, and R. Kiernan. 1993. Inability of polyclonal and monoclonal antibodies to neutralize primary clinical HIV-1 isolates, p. 151-158. In R. A. Lerner, H. Ginsberg, R. M. Chanock and F. Brown (ed.), Vaccines 93. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 38.Montefiori, D. C., T. W. Baba, A. Li, M. Bilska, and R. M. Ruprecht. 1996. Neutralizing and infection-enhancing antibody responses do not correlate with the differential pathogenicity of SIVmac239delta3 in adult and infant rhesus monkeys. J. Immunol. 157:5528-5535. [PubMed] [Google Scholar]
- 39.Montefiori, D. C., T. S. Hill, H. T. Vo, B. D. Walker, and E. S. Rosenberg. 2001. Neutralizing antibodies associated with viremia control in a subset of individuals after treatment of acute human immunodeficiency virus type 1 infection. J. Virol. 75:10200-10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore, J. P., Y. Cao, J. Leu, L. Qin, B. Korber, and D. D. Ho. 1996. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J. Virol. 70:427-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neate, E. V., R. C. Pringle, J. B. Jowett, D. S. Healey, and I. D. Gust. 1987. Isolation of HIV from Australian patients with AIDS, AIDS related conditions and healthy antibody positive individuals. Aust. N. Z. J. Med. 17:461-466. [DOI] [PubMed] [Google Scholar]
- 42.Oelrichs, R. B., V. A. Lawson, K. M. Coates, C. Chatfield, N. J. Deacon, and D. A. McPhee. 2000. Rapid full-length genomic sequencing of two cytopathically heterogeneous Australian primary HIV-1 isolates. J. Biomed. Sci. 7:128-135. [DOI] [PubMed] [Google Scholar]
- 43.Pilgrim, A. K., G. Pantaleo, O. J. Cohen, L. M. Fink, J. Y. Zhou, J. T. Zhou, D. P. Bolognesi, A. S. Fauci, and D. C. Montefiori. 1997. Neutralizing antibody responses to human immunodeficiency virus type 1 in primary infection and long-term-nonprogressive infection. J. Infect. Dis. 176:924-932. [DOI] [PubMed] [Google Scholar]
- 44.Qiao, X., B. He, A. Chiu, D. M. Knowles, A. Chadburn, and A. Cerutti. 2006. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat. Immunol. 7:302-310. [DOI] [PubMed] [Google Scholar]
- 45.Rhodes, D., A. Solomon, W. Bolton, J. Wood, J. Sullivan, J. Learmont, and N. Deacon. 1999. Identification of a new recipient in the Sydney Blood Bank Cohort: a long-term HIV type 1-infected seroindeterminate individual. AIDS Res. Hum. Retrovir. 15:1433-1439. [DOI] [PubMed] [Google Scholar]
- 46.Rhodes, D. I., L. Ashton, A. Solomon, A. Carr, D. Cooper, J. Kaldor, N. Deacon, et al. 2000. Characterization of three nef-defective human immunodeficiency virus type 1 strains associated with long-term nonprogression. J. Virol. 74:10581-10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salvi, R., A. R. Garbuglia, A. Di Caro, S. Pulciani, F. Montella, and A. Benedetto. 1998. Grossly defective nef gene sequences in a human immunodeficiency virus type 1-seropositive long-term nonprogressor. J. Virol. 72:3646-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarmati, L., E. Nicastri, G. el-Sawaf, L. Ventura, A. Salanitro, L. Ercoli, S. Vella, and M. Andreoni. 1997. Increase in neutralizing antibody titer against sequential autologous HIV-1 isolates after 16 weeks saquinavir (Invirase) treatment. J. Med. Virol. 53:313-318. [PubMed] [Google Scholar]
- 49.Silburn, K. A., D. A. McPhee, A. L. Maerz, P. Poumbourios, R. G. Whittaker, A. Kirkpatrick, W. G. Reilly, M. K. Manthey, and C. C. Curtain. 1998. Efficacy of fusion peptide homologs in blocking cell lysis and HIV-induced fusion. AIDS Res. Hum. Retrovir. 14:385-392. [DOI] [PubMed] [Google Scholar]
- 50.Stewart, G. J., L. J. Ashton, R. A. Biti, R. A. Ffrench, B. H. Bennetts, N. R. Newcombe, E. M. Benson, A. Carr, D. A. Cooper, J. M. Kaldor, et al. 1997. Increased frequency of CCR-5 delta 32 heterozygotes among long-term non-progressors with HIV-1 infection. AIDS 11:1833-1838. [DOI] [PubMed] [Google Scholar]
- 51.Stipp, H. L., A. Kumar, and O. Narayan. 2000. Characterization of immune escape viruses from a macaque immunized with live-virus vaccine and challenged with pathogenic SHIVKU-1. AIDS Res. Hum. Retrovir. 16:1573-1580. [DOI] [PubMed] [Google Scholar]
- 52.Valerie, K., A. Delers, C. Bruck, C. Thiriart, H. Rosenberg, C. Debouck, and M. Rosenberg. 1988. Activation of human immunodeficiency virus type 1 by DNA damage in human cells. Nature 333:78-81. [DOI] [PubMed] [Google Scholar]
- 53.Verity, E. E., L. A. Williams, D. N. Haddad, V. Choy, C. O'Loughlin, C. Chatfield, N. K. Saksena, A. Cunningham, F. Gelder, and D. A. McPhee. 2006. Broad neutralization and complement-mediated lysis of HIV-1 by PEHRG214, a novel caprine anti-HIV-1 polyclonal antibody. AIDS 20:505-515. [DOI] [PubMed] [Google Scholar]
- 54.Weis, N., B. O. Lindhardt, G. Kronborg, A. B. Hansen, A. L. Laursen, P. B. Christensen, H. Nielsen, A. Moller, H. T. Sorensen, and N. Obel. 2006. Impact of hepatitis C virus coinfection on response to highly active antiretroviral therapy and outcome in HIV-infected individuals: a nationwide cohort study. Clin. Infect. Dis. 42:1481-1487. [DOI] [PubMed] [Google Scholar]
- 55.Whitney, J. B., and R. M. Ruprecht. 2004. Live attenuated HIV vaccines: pitfalls and prospects. Curr. Opin. Infect. Dis. 17:17-26. [DOI] [PubMed] [Google Scholar]
- 56.Wilson, K. M., E. I. Johnson, H. A. Croom, K. M. Richards, L. Doughty, P. H. Cunningham, B. E. Kemp, B. M. Branson, and E. M. Dax. 2004. Incidence immunoassay for distinguishing recent from established HIV-1 infection in therapy-naive populations. AIDS 18:2253-2259. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, Y. J., C. Fracasso, J. R. Fiore, A. Bjorndal, G. Angarano, A. Gringeri, and E. M. Fenyo. 1997. Augmented serum neutralizing activity against primary human immunodeficiency virus type 1 (HIV-1) isolates in two groups of HIV-1-infected long-term nonprogressors. J. Infect. Dis. 176:1180-1187. [DOI] [PubMed] [Google Scholar]
- 58.Zuniga, J., et al. 1997. Setting the record straight: IAPAC's HIV vaccine initiative. J. Int. Assoc. Physicians AIDS Care 3:38-39. [PubMed] [Google Scholar]