Abstract
Hepatitis C virus (HCV) nonstructural protein 4A (NS4A) is only 54 amino acids (aa) in length, yet it is a key regulator of the essential serine protease and RNA helicase activities of the NS3-4A complex, as well as a determinant of NS5A phosphorylation. Here we examine the structure and function of the C-terminal acidic region of NS4A through site-directed mutagenesis of a Con1 subgenomic replicon and through biophysical characterization of a synthetic peptide corresponding to this region. Our genetic studies revealed that in 8 of the 15 C-terminal residues of NS4A, individual Ala substitutions or charge reversal substitutions led to severe replication phenotypes, as well as decreased NS5A hyperphosphorylation. By selecting for replication-competent mutants, several second-site changes in NS3 were identified and shown to suppress these defects in replication and NS5A hyperphosphorylation. Circular-dichroism spectroscopy and nuclear magnetic resonance spectroscopy on a peptide corresponding to the C-terminal 19 aa of NS4A revealed that this region can adopt an alpha-helical conformation, but that this folding requires neutralization of a cluster of acidic residues. Taken together, these data suggest that the C terminus of NS4A acts as a dynamic regulator of NS3-4A interaction, NS5A hyperphosphorylation, and HCV replicase activity.
Hepatitis C virus (HCV) is an enveloped, positive-strand RNA virus in the family Flaviviridae. The viral genome is an uncapped, nonpolyadenylated, 9.6-kb RNA that encodes a long open reading frame of ∼3,011 codons (reviewed in references 2 and 43). This translation product is processed by viral and host proteases into at least 10 distinct products. The N-terminal one-third of the genome encodes the viral structural proteins (C, E1, and E2), followed by a small ion channel protein, p7, and the nonstructural (NS) proteins NS2, NS3, NS4A, NS4B, NS5A, and NS5B (Fig. 1A). In addition, a small protein of unknown function, F for frame shift, may be translated from an alternative reading frame within the C gene (reviewed in reference 9).
FIG. 1.
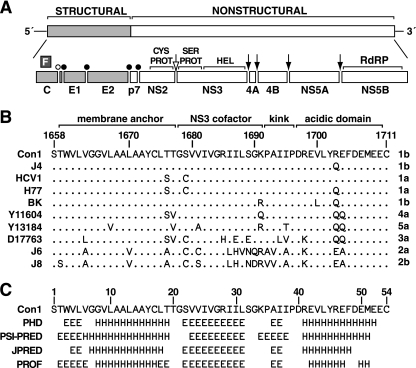
Overview of NS4A. (A) HCV genome and polyprotein-processing strategy. Structural genes are light gray, and NS proteins are white. Cleavage sites are indicated by an open bullet (signal peptide peptidase), closed bullets (signal peptidase), an open arrow (NS2 cysteine protease [CYS PROT]), and closed arrows (NS3-4A serine protease [SER PROT]). HEL, RNA helicase; RdRP, RNA-dependent RNA polymerase. (B) Sequence alignment of NS4A from several HCV isolates. The isolate name and genotype are indicated to the left and right of each sequence, respectively. Numbers above the alignment indicate positions within the polyprotein of the Con1 strain. Within the alignment, periods indicate positions with identity to Con1. (C) Secondary-structure predictions of NS4A are indicated as extended (E), helical (H), or undetermined (blank). Numbers above the predictions indicate positions within the NS4A protein. Predictions were made by using the web-based algorithms PHD (58), PSI-PRED (10), JPred (14), and PROF (59).
HCV encodes two essential proteases, i.e., the NS2 cysteine autoprotease, which cleaves at the NS2/3 junction, and the NS3-4A serine protease, which is responsible for cleavage at the NS3/4A, NS4A/4B, NS4B/NS5A, and NS5A/NS5B junctions (reviewed in references 2 and 43). In addition to its role in viral polyprotein processing, the NS3-4A serine protease can downregulate the host innate antiviral response (22) by cleaving the cellular proteins TRIF (37) and IPS-1 (also known as MAVS, VISA, or Cardif) (38, 41, 45, 47). Additional cellular substrates of the NS3-4A serine protease have not been described.
NS3-4A is a bifunctional enzyme; the N-terminal one-third of NS3 encodes the serine protease domain, while the C-terminal region of NS3 encodes an RNA helicase/nucleoside triphosphatase domain (reviewed in reference 54). Full activity of both enzymes requires interaction between the serine protease domain and the viral NS4A protein, which contributes one strand of the first β-barrel in the chymotrypsin-like fold (4, 19, 30, 39, 53, 70).
At 54 amino acids (aa), NS4A is the smallest NS protein, yet it has multiple functions in the viral life cycle. The hydrophobic N-terminal region of NS4A is responsible for anchoring the NS3-4A complex to the endoplasmic reticulum and mitochondrial outer membrane (50, 52, 64, 68). As mentioned, the central region of NS4A serves as the serine protease cofactor (4, 19, 39). NS4A also augments NS3 RNA helicase activity, perhaps via interactions between the helicase and serine protease domains (23, 35, 53). Although it is unclear whether NS4A is found outside of the NS3-4A complex in HCV-infected cells, overexpression of free NS4A inhibits translation (21, 29, 34) and can lead to mitochondrial damage (52). Furthermore, the hyperphosphorylation of NS5A is dependent on NS4A expression (3, 28, 64), and mutations in NS3, NS4A, or NS4B can alter NS5A hyperphosphorylation (31). The mechanisms of these additional NS4A activities are unknown but may involve interactions between NS4A and NS4B, NS5A, NS5B, and uncleaved NS4B-5A (15, 26, 40).
While the membrane-anchoring and serine protease cofactor activities are encoded within the first 34 aa of NS4A, very little is known about the downstream region of this protein. To further clarify the role of the C-terminal acidic region of NS4A, we examined its function through site-directed mutagenesis of an HCV genotype 1b subgenomic replicon and show that the C-terminal region of NS4A has an important role in HCV RNA replication. Further analysis of these mutants revealed additional genetic interactions between NS4A and NS3. To understand the structural basis of these effects, the C-terminal 19 aa of NS4A was examined through circular-dichroism (CD) spectroscopy and nuclear magnetic resonance (NMR) spectroscopy. These data indicated that the C terminus of NS4A can undergo pH-dependent folding into an alpha helix, suggesting that its folding may be induced by interaction with a positively charged surface in the context of the viral replicase.
MATERIALS AND METHODS
Abbreviations used in this report.
The following abbreviations are used in this report: Bsd, blasticidin S deaminase; DM, n-dodecyl-β-d-maltoside; DPC, dodecyl phosphocholine; HPLC, high-performance liquid chromatography; NOE, nuclear Overhauser enhancement; HSQC, heteronuclear single-quantum correlation; NOESY, NOE spectroscopy; SDS, sodium dodecyl sulfate; TFE, 2,2,2-trifluoroethanol; TOCSY, total correlation spectroscopy.
Replicon constructs.
Standard molecular biology methods were used throughout (60). Plasmid sequences were verified by dye-labeled DNA sequencing at The Rockefeller DNA Sequencing Facility. Con1b/SG-Bsd(I) and Con1b/SG-Bsd(T) are subgenomic replicons that express the blasticidin S resistance gene (Bsd) and contain the cell culture-adaptive mutations S2204I and A2199T, respectively (7). These replicons (and their derivatives) were transcribed from ScaI-linearized plasmids pBDL429P+S+/BBvii and pBDL429P+S+/BBix, respectively. Both plasmids were derived from the Bsd-expressing, S2204I-containing Con1 replicon plasmid previously described (17). However, to facilitate cloning, these constructs were engineered to contain useful restriction sites by silent mutagenesis. Specifically, a unique SacII site was introduced near the beginning of the NS3 gene by silent mutation of codon 1036 (numbered according to the Con1 genome, GenBank accession no. AJ238799) from ACG to ACC and codon 1037 from CGA to CGC. Similarly, a unique PmeI site was introduced into the middle of the NS3 gene by mutating codons 1411 to 1413 from GGA CTC AAT to GGT TTA AAC, and a unique SpeI site was introduced at the beginning of the NS4A gene in two steps. First, unique SpeI and XbaI sites in the vector backbone were sequentially destroyed by cleavage with each enzyme, filling in with the Klenow fragment of DNA polymerase I, and religation. Codon 1661 was then mutated from GTG to GTA, and codon 1662 was mutated from CTG to CTA. Finally, a unique AflII site was introduced into the variable region of the 3′ noncoding region by mutating nucleotide 9392 from C to T and nucleotide 9393 from C to A (G. Randall and C. M. Rice, unpublished data). These alterations had no discernible effects on HCV RNA replication or blasticidin S-resistant colony formation. Con1b/SG-Bsd(Pol−) was derived from pBDL429P+S+(I) by subcloning the polymerase active-site mutations GDD to AAG (7).
Mutations were introduced into NS4A as follows. First, an intermediate construct, pSLH1b3453-6534PX, was constructed by subcloning the 3,407-bp SstII/MluI fragment of pBSL429P+S+/BBvii into similarly digested pSL1180 (Pharmacia, Piscataway, NJ). pSLH1b3453-6534PX was then used as a template for site-directed mutagenesis of the corresponding NS4A residues in QuikChange reactions according to the manufacturer's suggestions (Stratagene, La Jolla, CA) with appropriate primer pairs (Table 1). After sequence confirmation, the mutations were subcloned back into pBSL429P+S+/BBvii or pBSL429P+S+/BBix by using SstII and MluI as described above.
TABLE 1.
Primers used for site-directed mutagenesis of NS4A
| Mutation | Orientationa | Primer | Sequenceb |
|---|---|---|---|
| D1697A | Fwd | RU-O-3389 | 5′-GCCATCATTCCCGCCAGGGAAGTCCTTTACCG-3′ |
| Rev | RU-O-3398 | 5′-CGGTAAAGGACTTCCCTGGCGGGAATGATGGC-3′ | |
| R1698A | Fwd | RU-O-3390 | 5′-GCCATCATTCCCGACGCCGAAGTCCTTTACCG-3′ |
| Rev | RU-O-3399 | 5′-CGGTAAAGGACTTCGGCGTCGGGAATGATGGC-3′ | |
| E1699A | Fwd | RU-O-3391 | 5′-GCCATCATTCCCGACAGGGCCGTCCTTTACCG-3′ |
| Rev | RU-O-3400 | 5′-CGGTAAAGGACGGCCCTGTCGGGAATGATGGC-3′ | |
| V1700A | Fwd | RU-O-4145 | 5′-TTCCCGACAGGGAAGCCCTTTACCGGGAGTTCGAT-3′ |
| Rev | RU-O-4159 | 5′-ATCGAACTCCCGGTAAAGGGCTTCCCTGTCGGGAA-3′ | |
| L1701A | Fwd | RU-O-4146 | 5′-TTCCCGACAGGGAAGTCGCCTACCGGGAGTTCGAT-3′ |
| Rev | RU-O-4160 | 5′-ATCGAACTCCCGGTAGGCGACTTCCCTGTCGGGAA-3′ | |
| Y1702A | Fwd | RU-O-4147 | 5′-CGACAGGGAAGTCCTTGCCCGGGAGTTCGAT-3′ |
| Rev | RU-O-4161 | 5′-ATCGAACTCCCGGGCAAGGACTTCCCTGTCG-3′ | |
| R1703A | Fwd | RU-O-3392 | 5′-AGGGAAGTCCTTTACGCCGAGTTCGATGAGATGG-3′ |
| Rev | RU-O-3401 | 5′-CCATCTCATCGAACTCGGCGTAAAGGACTTCCCT-3′ | |
| E1704A | Fwd | RU-O-3393 | 5′-AGGGAAGTCCTTTACCGGGCCTTCGATGAGATGG-3′ |
| Rev | RU-O-3402 | 5′-CCATCTCATCGAAGGCCCGGTAAAGGACTTCCCT-3′ | |
| F1705A | Fwd | RU-O-4148 | 5′-ACAGGGAAGTCCTTTACCGGGAGGCCGATGAGATGGAAGAGT-3′ |
| Rev | RU-O-4162 | 5′-ACTCTTCCATCTCATCGGCCTCCCGGTAAAGGACTTCCCTGT-3′ | |
| D1706A | Fwd | RU-O-3394 | 5′-CTTTACCGGGAGTTCGCCGAGATGGAAGAGTGCGCCTCACAC-3′ |
| Rev | RU-O-3403 | 5′-GTGTGAGGCGCACTCTTCCATCTCGGCGAACTCCCGGTAAAG-3′ | |
| E1707A | Fwd | RU-O-3395 | 5′-CTTTACCGGGAGTTCGATGCCATGGAAGAGTGCGCCTCACAC-3′ |
| Rev | RU-O-3404 | 5′-GTGTGAGGCGCACTCTTCCATGGCATCGAACTCCCGGTAAAG-3′ | |
| M1708A | Fwd | RU-O-4149 | 5′-CTTTACCGGGAGTTCGATGAGGCCGAAGAGTGCGCCTCACA-3′ |
| Rev | RU-O-4163 | 5′-TGTGAGGCGCACTCTTCGGCCTCATCGAACTCCCGGTAAAG-3′ | |
| E1709A | Fwd | RU-O-3396 | 5′-GGAGTTCGATGAGATGGCCGAGTGCGCCTCACACCTCCC-3′ |
| Rev | RU-O-3405 | 5′-GGGAGGTGTGAGGCGCACTCGGCCATCTCATCGAACTCC-3′ | |
| E1710A | Fwd | RU-O-3397 | 5′-GGAGTTCGATGAGATGGAAGCCTGCGCCTCACACCTCCC-3′ |
| Rev | RU-O-3406 | 5′-GGGAGGTGTGAGGCGCAGGCTTCCATCTCATCGAACTCC-3′ | |
| D1697R | Fwd | RU-O-4136 | 5′-GCCATCATTCCCCGCAGGGAAGTCCTTTACCG-3′ |
| Rev | RU-O-4150 | 5′-CGGTAAAGGACTTCCCTGCGGGGAATGATGGC-3′ | |
| R1698D | Fwd | RU-O-4137 | 5′-GCCATCATTCCCGACGACGAAGTCCTTTACCG-3′ |
| Rev | RU-O-4151 | 5′-CGGTAAAGGACTTCGTCGTCGGGAATGATGGC-3′ | |
| E1699R | Fwd | RU-O-4138 | 5′-GCCATCATTCCCGACAGGCGCGTCCTTTACCG-3′ |
| Rev | RU-O-4152 | 5′-CGGTAAAGGACGCGCCTGTCGGGAATGATGGC-3′ | |
| R1703D | Fwd | RU-O-4139 | 5′-AGGGAAGTCCTTTACGACGAGTTCGATGAGATGG-3′ |
| Rev | RU-O-4153 | 5′-CCATCTCATCGAACTCGTCGTAAAGGACTTCCCT-3′ | |
| E1704R | Fwd | RU-O-4140 | 5′-AGGGAAGTCCTTTACCGGCGCTTCGATGAGATGG-3′ |
| Rev | RU-O-4154 | 5′-CCATCTCATCGAAGCGCCGGTAAAGGACTTCCCT-3′ | |
| D1706R | Fwd | RU-O-4141 | 5′-CTTTACCGGGAGTTCCGCGAGATGGAAGAGTGCGCCTCACAC-3′ |
| Rev | RU-O-4155 | 5′-GTGTGAGGCGCACTCTTCCATCTCGCGGAACTCCCGGTAAAG-3′ | |
| E1707R | Fwd | RU-O-4142 | 5′-CTTTACCGGGAGTTCGATCGCATGGAAGAGTGCGCCTCACAC-3′ |
| Rev | RU-O-4156 | 5′-GTGTGAGGCGCACTCTTCCATGCGATCGAACTCCCGGTAAAG-3′ | |
| E1709R | Fwd | RU-O-4143 | 5′-GGAGTTCGATGAGATGCGCGAGTGCGCCTCACACCTCCC-3′ |
| Rev | RU-O-4157 | 5′-GGGAGGTGTGAGGCGCACTCGCGCATCTCATCGAACTCC-3′ | |
| E1710R | Fwd | RU-O-4144 | 5′-GGAGTTCGATGAGATGGAACGCTGCGCCTCACACCTCCC-3′ |
| Rev | RU-O-4158 | 5′-GGGAGGTGTGAGGCGCAGCGTTCCATCTCATCGAACTCC-3′ |
Fwd, forward; Rev, reverse.
The codon targeted for mutagenesis in each sequence is underlined.
Cell culture and RNA transfection.
Huh-7.5 cells (8) were maintained in Dulbecco's modified Eagle medium containing 10% fetal calf serum and 1 mM nonessential amino acids (all from Invitrogen [Carlsbad, CA]). Cells were transfected by electroporation as previously described (42). To monitor Bsd transduction efficiency, replicon-transfected cells were serially diluted into cells that had been transfected with the replication-defective control replicon Con1b/SG-Bsd(Pol−). Cells were then seeded at a density of 6 × 105/10-cm dish. Four days after transfection, the medium was changed to the above medium containing 3 μg/ml blasticidin S (Invivogen, San Diego, CA) and cells were maintained by changing the blasticidin S-containing medium every third or fourth day. Colony-forming activity was assessed after 3 weeks of selection by fixing the cells in formalin, staining them with crystal violet (1% [wt/vol] in 20% ethanol), and calculating the efficiency of colony formation.
Identification of suppressor mutations.
Blasticidin S-resistant colonies were isolated by trypsinization in cloning cylinders and expanded in blasticidin S-containing medium. Total RNA was extracted by using 0.75 ml TRIzol (Invitrogen, Carlsbad, CA) per 9.4-cm2 well on a six-well plate, according to the manufacturer's instructions. RNA was resuspended to 250 ng/μl in 2 mM sodium citrate, pH 6.5. Random-primed cDNAs were synthesized by using random hexamers and Superscript II (Invitrogen) at 50°C for 1 h, followed by a 15-min digestion at 37°C with RNase H. cDNAs were cleaned up by using QIAquick PCR spin columns and eluted into 50 μl elution buffer (QIAGEN, Valencia, CA). DNA was amplified with Klentaq LA (DNA Polymerase Technology, Inc., St. Louis, MO), 5 μl of purified cDNA, 400 nM each primer, and 200 nM each deoxynucleoside triphosphate, according to the manufacturer's instructions. The primers used for each PCR are listed in Table 2. PCR products were purified with QIAquick as described above and adjusted to 8.5 ng/μl. Population sequencing was performed on the PCR products with region-specific primers at The Rockefeller University DNA Sequencing Core Facility. To reconstruct replicons containing second-site mutations, PCR products were cloned into pCR2.1-TOPO (Invitrogen), their sequences were verified, and the corresponding regions were subcloned back into pBSL429P+S+/BBvii or pBSL429P+S+/BBix by using common restriction sites.
TABLE 2.
PCR primers used to amplify the HCV NS genes
| PCR no. | Primera | Sequence |
|---|---|---|
| 1 | 3841 (F) | CTCTCCTCAAGCGTATTCAAC |
| 3842 (R) | GTGGCGGCGACGGACGGGTTC | |
| 2 | 3843 (F) | TTCCAGGTGGCCCATCTACAC |
| 3844 (R) | GGTAAAGCCCGTCATTAGAGC | |
| 3 | 3845 (F) | GGCCTTGATGTATCCGTCATA |
| 3846 (R) | GGGAGGTGTGAGGCGCACTCT | |
| 4 | 3847 (F) | GACAACAGGCAGCGTGGTCAT |
| 3848 (R) | CGTGCTGCAGCGTCGCTCTCA | |
| 5 | 3849 (F) | GGATGAACCGGCTGATAGCGT |
| 3850 (R) | TTAGCCGTCTCCGCCGTAATG | |
| 6.2 | 3851 (F) | GTCGGGCTCAATCAATACCTG |
| 3854 (R) | TCTGCCCTTTAGAATTAGTCA | |
| 7 | 3853 (F) | GCTAGTGAGGACGTCGTCTGC |
| 3854 (R) | TCTGCCCTTTAGAATTAGTCA | |
| 8 | 3855 (F) | ACAGGCCATAAGGTCGCTCAC |
| 3856 (R) | ACTGGGACGCAGCCGGGATTG | |
| 9 | 3857 (F) | CTCCATGGCCTTAGCGCATTT |
| 3902 (R) | AAAAACAAGGATGGCCTATTGG |
F, forward; R, reverse.
Protein expression.
Huh-7.5 cells were seeded in six-well plates and infected for 1 h at a multiplicity of infection of 10 with the T7 RNA polymerase-expressing vaccinia virus vTF7-3 (24). The medium was changed to OptiMEM (Invitrogen), and cells were transfected with 1 μg of EcoRI-linearized, replicon-containing plasmids and 8 μl FuGENE 6 (Roche, Indianapolis, IN), according to the manufacturer's recommendations. Eight to 12 h later, the cells were lysed in 0.2 ml sample buffer (100 mM Tris [pH 6.8], 20 mM dithiothreitol, 4% [wt/vol] SDS, 20% [vol/vol] glycerol, 0.2% [wt/vol] bromophenol blue) and homogenized by five passes through a 22-gauge needle. Samples (10 μl each) were denatured by boiling, resolved on SDS-polyacrylamide gels, and transferred to Immobilon P membranes (Millipore, Bedford, MA). Membranes were blocked with TBS-T (20 mM Tris [pH 7.4], 150 mM NaCl, 0.1% [vol/vol] Tween 20 [polyoxyethylene sorbitan monolaurate]) containing 5% (wt/vol) dry milk and probed with this blocking buffer containing primary monoclonal antibodies against NS3 (7019 from Maine Biotech; now available as 1878 from ViroStat, Portland, ME), NS4B (4B-52) (25), NS5A (9E10) (42), NS5B (3B1.5.3) (49), or β-actin (AC-15; Sigma, St. Louis, MO). Following several washes with TBS-T, membranes were probed with horseradish peroxidase-conjugated secondary antibodies and washed repeatedly, and antigens were detected with SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL).
NS4A[36-54] peptide.
A peptide corresponding to NS4A residues 36 to 54 (Con1 strain residues 1693 to 1711), AIIPDREVLYREFDEMEEC, was synthesized at The Rockefeller University Protein Resource Center. This peptide contained an N-terminal biotin group and a C-terminal amide group, the latter to help stabilize the terminal Cys. The peptide was purified by HPLC with a gradient of 1 to 60% acetonitrile, and its mass was confirmed by mass spectrometry at the Protein Resource Center.
CD.
CD spectra were recorded on a Jobin Yvon CD6 spectrometer calibrated with 1S-(+)-10-camphorsulfonic acid. Measurements were carried out at room temperature in a 0.1-cm path length quartz cuvette (Hellma, Müllheim, Germany), with peptide concentrations ranging from 35 to 60 μM. Spectra were recorded in the 190- to 250-nm wavelength range with a 0.2-nm increment and a 2-s integration time. Spectra were processed with CD6 software, baseline corrected, and smoothed by using a third-order least-square polynomial fit. Spectral units were expressed as the molar ellipticity per residue by using peptide concentrations determined by measuring the UV light absorbance of tyrosine at 280 nm (molar extinction coefficient of 1,536 M−1 · cm−1). The alpha-helical content was estimated at 222 nm by using the empirical equation of Chen et al. (13) as detailed previously (48) with a calculated theoretical molar ellipticity of −34,160° · cm2 · dmol−1 for 100% helical conformation for the peptide in the various media.
NMR spectroscopy.
Three milligrams of the peptide was dissolved in 50% TFE-d2 (2,2,2-trifluoroethyl-1,1-d2 alcohol, >99% isotopic enrichment). The final peptide concentration was 1.3 mM at pH 3.8. Sodium 2,2-dimethyl-2-silapentane-5-sulfonate was added as an internal reference. Spectra were acquired under nonspinning conditions at temperatures of 288 and 298 K. Multidimensional NMR experiments were performed on a Varian Unity Plus 500 MHz equipped with a triple-resonance 5-mm probe with a self-shielded z gradient coil. NOESY (mixing times between 100 and 250 ms), clean-TOCSY (isotropic mixing time of 80 ms), and 1H-13C HSQC experiments were performed with conventional optimized pulse sequences as detailed previously (20, 48, 55, and references therein). Varian VNMR software was used to collect and process all data, and Sparky (kindly provided by T. D. Goddard and D. G. Kneller, University of California, San Francisco) was used for spectral analysis. Intraresidue backbone resonances and aliphatic side chains were identified from two-dimensional 1H TOCSY and 1H-13C HSQC. Sequential assignments were determined by correlating intraresidue assignments with interresidue cross peaks observed in two-dimensional 1H NOESY. NMR-derived 1Hα and 13Cα chemical shifts are reported relative to the random coil chemical shifts in TFE (46, 66).
NMR-derived distance constraints and structure calculations.
NOE distances used as input for structure calculations were obtained from the NOESY spectrum recorded at 288 K with a 150-ms mixing time. NOE intensities were partitioned into three categories that were converted into distances ranging from a common lower limit of 1.8 Å to upper limits of 2.8 Å, 3.9 Å, and 5.0 Å for strong, medium, and weak intensities, respectively. The cross-peak intensity of the vicinal methylene proton pair of the residue D49 side chain was used as a distance reference (1.7 Å). Because of the lack of resolution mainly due to overlap of glutamic acid residues, 23 canonical helical distance constraints in the region from R41 to D49 were added to the 111 distance constraints extracted from the NOESY spectrum. No hydrogen bond or dihedral-angle restraints were introduced. Protons without stereospecific assignments were treated as pseudoatoms, and correction factors were added to the upper and lower distance constraints (69). Three-dimensional structures were generated from NOE distances by the dynamical simulated annealing protocol with the Xplor-NIH 2.9.7 program (61) by using the standard force fields and default parameter sets. A set of 50 structures was initially calculated, from which 47 structures were selected on the basis of low energy and absence of NOE violations and used to calculate the average peptide structure.
RESULTS
Mutagenesis of the NS4A C-terminal acidic domain.
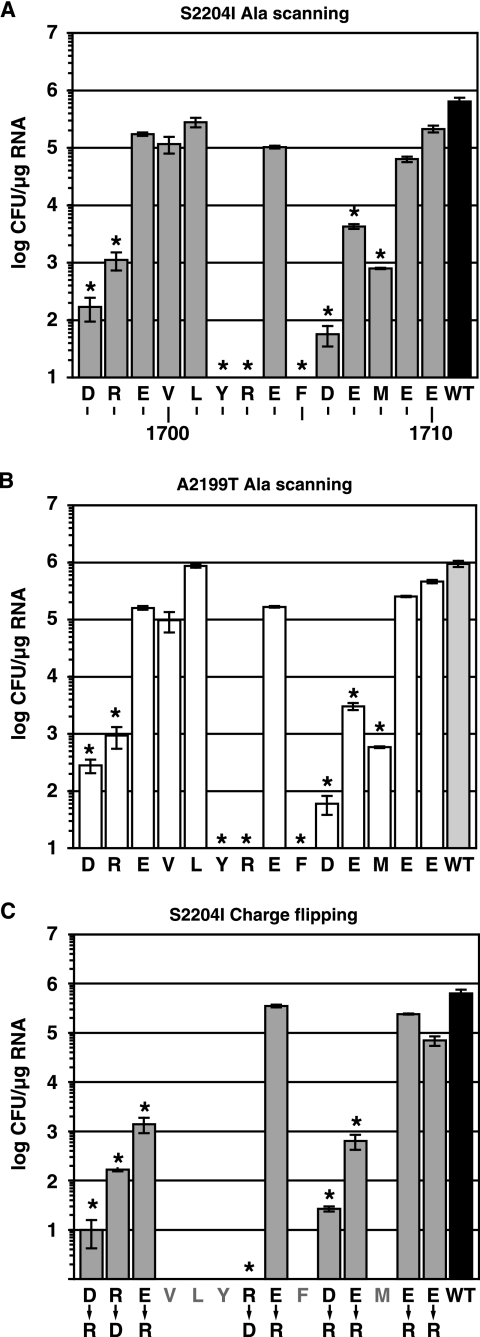
The C terminus of NS4A contains a short acidic region that is highly conserved among diverse HCV isolates (Fig. 1B) and is predicted to form an alpha helix (Fig. 1C). Similar acidic regions are also found at the NS4A C termini of pestiviruses and GB virus B (not shown). To examine whether this region is important in HCV RNA replication, residues 1697 to 1710 (numbered according to the Con1 genome) were individually mutated to Ala in the context of Con1b/SG-Bsd(I) or Con1b/SG-Bsd(T), Con1-derived subgenomic replicons that express the blasticidin S resistance gene (Bsd). These replicons also contained cell culture-adaptive mutations in NS5A (S2204I or A2199T, respectively) that permit efficient RNA replication in transfected Huh-7 and Huh-7.5 cells. The C-terminal Cys residue of NS4A was not targeted for mutagenesis because this position is necessary for NS4A/NS4B cleavage (5, 32, 33, 36, 63). Upon transfection into Huh-7.5 cells, several mutants demonstrated significant decreases in Bsd transduction efficiency (Fig. 2A and B) and HCV RNA accumulation (not shown). The most severe effects were seen with Y1702A, R1703A, and F1705A mutants, which were unable to form detectable colonies (detection limit, 10 CFU/μg). In contrast, conserved residues 1699 to 1701, 1709, and 1710 were tolerant of the Ala substitutions. Taken together, these data indicated that residues 1697, 1698, 1702, 1703, 1705, 1706, 1707, and 1708 are important determinants of HCV replication. In addition, a panel of charge reversal mutations, substituting Arg for acidic residues and Asp for basic residues, was constructed in Con1b/SG-Bsd(I). These mutants confirmed the importance of residues 1697, 1698, 1703 1706, and 1707 and revealed a preference for an acidic residue at position 1699 (Fig. 2C).
FIG. 2.
Replication phenotypes of NS4A mutants. (A) The efficiency of Bsd-resistant colony formation was determined for each Con1b/SG-Bsd(I) Ala scanning mutant. Plotted values represent the average of at least three experiments; error bars indicate the standard deviation of the mean. No colonies were detected for the Y1702A, R1703A, and F1705A mutants (limit of detection, 10 CFU/μg RNA). Asterisks indicate statistically significant differences (P ≤ 0.01 [Student's t test]) from the wild-type (WT) Con1b/SG-Bsd(I) parent. (B) The efficiency of Bsd-resistant colony formation was determined for each Con1b/SG-Bsd(T) Ala scanning mutant as in panel A and compared to the wild-type Con1b/SG-Bsd(T) parent. (C) The efficiency of Bsd-resistant colony formation was determined for each Con1b/SG-Bsd(I) charge-flipping mutant. The wild-type Con1b/SG-Bsd(T) parent is reproduced from panel A for comparison.
Identification of suppressor mutants.
We hypothesized that NS4A mutants with reduced replication would be under selective pressure to accumulate compensatory second-site changes. We therefore amplified HCV cDNA by reverse transcription-PCR from several Bsd-expressing colonies and directly sequenced these products (Table 3). Nine of the 10 selected D1697A clones reverted back to the wild-type residue at position 1697. However, a single D1697A clone was found to retain the engineered mutation and contained additional changes in NS3 (R1187L) and NS5A (Q2153P). Several R1698A, Y1702A, and M1708A clones also retained the original mutation in NS4A and contained additional mutations at positions Q1112 and P1115 of NS3, most commonly to basic residues. Furthermore, a single M1708A clone contained a change of S1369R in NS3. We also detected reversion to the original NS4A codons in nine of the D1706A-derived colonies. In two cases, M1708A had mutated to A1708V, suggesting a preference for large hydrophobic residues at this position.
TABLE 3.
Sequence analysis of replicons in selected blasticidin S-resistant cell clones
| Polyprotein position | Original codon | Mutant codon | No. of clones | Consensus codon | Additional mutation(s) detected (gene in which it occurs) |
|---|---|---|---|---|---|
| 1697 | Asp (GAC) | Ala (GCC) | 9 | Asp (GAC) | R1187L (NS3), Q2153P (NS5A) |
| 1 | Ala (GCC) | ||||
| 1698 | Arg (AGG) | Ala (GCC) | 2 | Ala (GCC) | Q1112R (NS3) |
| 1 | Ala (GCC) | Q1112K (NS3) | |||
| 1 | Ala (GCC) | P1115R (NS3) | |||
| 1702 | Tyr (UAC) | Ala (GCC) | 2 | Ala (GCC) | P1115R (NS3) |
| 1706 | Asp (GAU) | Ala (GCC) | 9 | Asp (GAC) | |
| 1708 | Met (AUG) | Ala (GCC) | 2 | Val (GUC) | |
| 1 | Ala (GCC) | Q1112R (NS3) | |||
| 1 | Ala (GCC) | P1115L (NS3) | |||
| 1 | Ala (GCC) | S1369R (NS3) |
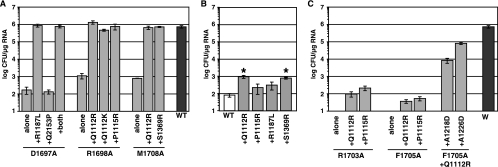
We tested whether the detected second-site changes could suppress NS4A-mediated defects in HCV replication by subcloning them back into replicons containing the original NS4A mutations. As shown in Fig. 3A, the R1187L mutation fully restored the replication of the D1697A mutant while Q2153P did not. As expected, the combination of these two mutations also fully restored replication. Furthermore, Q1112R, Q1112K, and P1115R all fully restored the replication of the R1698A mutant. Similarly, the Q1112R and S1369R mutations suppressed the M1708A defect. Although we did not reconstruct the P1115L mutation in the M1708A background, we did test the effect of P1115R on this mutant, and it also fully restored replication (not shown). To test whether these mutations in NS3 simply had a general positive effect on HCV replication, these changes were engineered into Con1b/SG-Bsd, which lacks cell culture-adaptive changes or mutations in NS4A. As shown in Fig. 3B, Q1112R and S1369R conferred a mild (10-fold) improvement in replication while P1115R and R1187L did not. Taken together, these data show that second-site mutations in NS3 suppress replication defects caused by mutations in the NS4A C-terminal acidic domain.
FIG. 3.
Second-site mutations in NS3 suppress defects caused by mutations in the NS4A acidic region. Data are presented as described in the legend to Fig. 2. (A) The indicated single, double, and triple mutations were tested in the context of Con1b/SG-Bsd(I). The replication phenotypes of D1697A, R1698A, M1708, and the wild type (WT) are reproduced from Fig. 1A for comparison. (B) The effects of each NS3 mutation were tested in the context of wild-type Con1b/SG-Bsd, which lacks cell culture-adaptive mutations. Asterisks indicate statistically significant differences (P ≤ 0.01 [Student's t test]) from the wild type. (C) The indicated single, double, and triple mutations were tested in the context of Con1b/SG-Bsd(I). The replication phenotypes of R1703A, F1705A, and the wild type are reproduced from Fig. 1A for comparison.
It was interesting that mutations at NS3 residues Q1112 and P1115 arose multiple times and could suppress replication defects caused by different NS4A mutations. The Q1112R and P1115R mutations were therefore tested for the ability to suppress defects caused by additional NS4A mutations. Interestingly, two of the most severe NS4A defects, R1703A and F1705A, were partially suppressed by these changes in NS3 (Fig. 3C). In contrast, the replication of the Y1702A and D1706A mutants was not improved by either Q1112R or P1115R (data not shown).
Since the R1703A and F1705A mutants containing Q1112R or P1115R showed a modest improvement in Bsd transduction efficiency, we expanded several of these colonies and sequenced the NS3-to-NS5A regions contained within them (Table 4). In all cases, the original mutations in NS4A and second-site mutations in NS3 were retained. These data also revealed several additional mutations in NS3, i.e., I1097T, A1218D, and A1226D. In a few cases, additional changes in NS4A were found near the original mutation. We therefore reconstructed triple mutants containing F1705A, Q1112R, and either A1218D or A1226D. As shown in Fig. 3C, both “third-site” mutations in NS3 strongly contributed to the suppression of the replication defect caused by the F1705A mutation in NS4A.
TABLE 4.
Sequence analysis of replicons in selected blasticidin S-resistant cell clones
| NS4A mutation | NS3 mutation | No. of clones | Additional mutation detected (gene in which it occurs) |
|---|---|---|---|
| R1703A | Q1112R | 1 | I1097T (NS3) |
| 3 | Y1702F (NS4A) | ||
| P1115R | 1 | F1705L (NS4A) | |
| F1705A | Q1112R | 3 | A1226D (NS3) |
| P1115R | 2 | A1226D (NS3) | |
| 2 | A1218D (NS3) |
Polyprotein processing and NS5A hyperphosphorylation of mutant NS4A proteins.
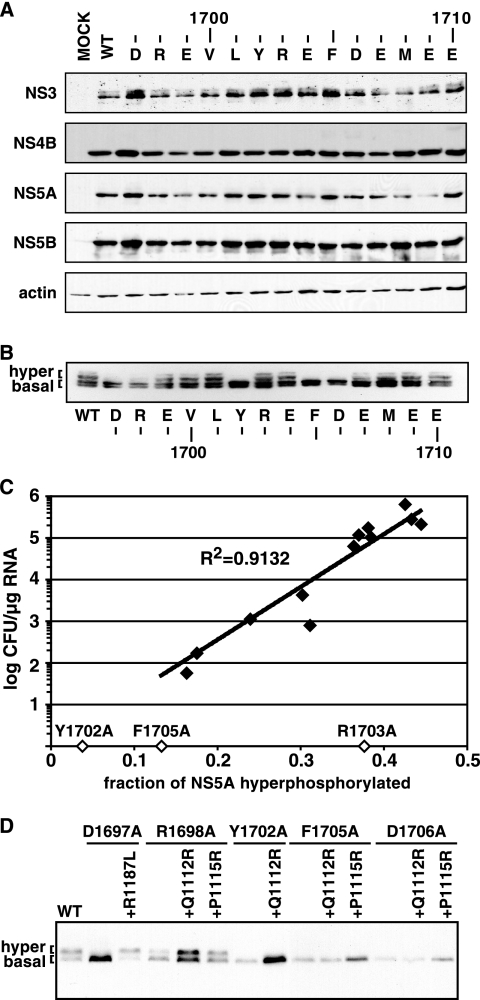
Since NS4A is an important cofactor for HCV serine protease activity, we wondered whether the replication defects of our mutant proteins could simply be due to defective polyprotein processing. In addition, mutations at positions 1706 and 1707 could have directly interfered with NS4A/4B cleavage since the NS3-4A serine protease prefers an acidic residue at substrate position P5 or P6 relative to the protease cleavage site (5, 32, 33, 36, 63). Processing of the wild-type and mutant polyproteins was therefore studied in transfected cells. To alleviate differences in protein expression arising from the inability of some mutants to replicate, Huh-7.5 cells were infected with vTF7-3, a vaccinia virus that expresses T7 RNA polymerase, and transfected with the T7-driven cDNA clone of each Con1b/SG-Bsd(I) mutant replicon. As shown by Western blotting, we did not detect major differences in the expression of NS3, NS4B, NS5A, or NS5B by Con1b/SG-Bsd(I) replicons containing Ala substitutions in the NS4A C-terminal acidic domain (Fig. 4A). We were unable to directly examine NS4A expression in our mutant panel because all of the available NS4A-specific antibodies recognize the region that we had mutated (12, 73, 74). Nevertheless, these data indicate that mutations in the NS4A C-terminal acidic domain do not strongly inhibit polyprotein processing and that mutations at positions P5 and P6 of the NS4A/4B cleavage site (residues 1706 and 1707) are individually tolerated.
FIG. 4.
Polyprotein processing and NS5A phosphorylation status of NS4A Ala scanning mutants. (A) Huh-7.5 cells were infected with vTF7-3 and transfected with T7-driven cDNAs of each Con1b/SG-Bsd(I) Ala scanning mutant, the wild-type (WT) replicon, or an irrelevant control plasmid (MOCK). The indicated proteins were detected by Western blotting as described in Materials and Methods. (B) NS5A expressed by each Con1b/SG-Bsd(T) Ala scanning mutant or wild-type replicon was analyzed as for panel A. (C) The replication phenotype of each mutant (closed diamonds) was plotted against the fraction of hyperphosphorylated NS5A, which was calculated from Western blot assays by digital scanning and image processing with NIH Image. The Y1702A, R1703A, and F1705A mutants, which did not produce detectable colonies, are indicated by open diamonds. (D) NS5A expressed by wild-type Con1b/SG-Bsd(T) or the indicated single or double mutant, as analyzed for panel B.
Mutations in the C-terminal region of NS4A were previously shown to affect NS5A hyperphosphorylation (31). We therefore examined the phosphorylation status of NS5A expressed by our panel of Ala substitutions through vTF7-3 infection and cDNA transfection. Preliminary studies confirmed that the basal (56-kDa) and hyperphosphorylated (58-kDa) forms of NS5A could be resolved by SDS-polyacrylamide gel electrophoresis and that these forms could be melded into an ∼55-kDa form by treatment with calf intestine alkaline phosphatase (not shown). Consistent with previous results (7), NS5A containing the S2204I adaptive mutation was not hyperphosphorylated, regardless of the mutations in NS4A (Fig. 4A). In contrast, several NS4A mutants containing the A2199T adaptive mutation exhibited decreased hyperphosphorylation of NS5A (Fig. 4B). For those replicons that were able to form colonies, replication fitness (as measured by colony-forming efficiency) correlated with the level of NS5A hyperphosphorylation (Fig. 4C). Among the three mutants that were unable to form colonies, two of them (Y1702A and F1705A) also exhibited greatly reduced NS5A hyperphosphorylation, while NS5A hyperphosphorylation was only slightly reduced in the R1703A mutant (Fig. 4C).
Given the correlation between replication and NS5A hyperphosphorylation, we went on to test whether second-site suppressor mutations found in NS3 had effects on the phosphorylation status of NS5A. The R1187L mutation, which restored the replication of the D1697A mutant, also restored NS5A hyperphosphorylation (Fig. 4D). Similarly, the Q1112R and P1115R mutations, which fully restored the replication of the D1698A mutant, also restored NS5A hyperphosphorylation. In contrast, Q1112R and P1115R conferred no improvement of Y1702A and D1706A replication and only modest improvement of F1705A replication and had no effect on NS5A phosphorylation in the Y1702A, F1705A, and D1706A mutants (Fig. 4D). Taken together, these data indicate that mutations in the C terminus of NS4A and compensatory changes in NS3 coordinately affect NS5A hyperphosphorylation and RNA replication.
Suppressing mutations map to surface residues of NS3.
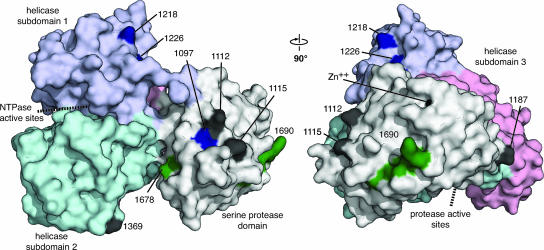
Suppressor mutations reside on discontinuous surfaces of NS3, distal from the serine protease and RNA helicase/nucleoside triphosphatase active sites (Fig. 5). Several of these sites could be involved in interactions between the serine protease and helicase domains; R1187 lies on the serine protease domain and is closely apposed to RNA helicase subdomain 3, and A1218 and A1226 map to the start of the RNA helicase domain, near the peptide that links the serine protease and helicase domains. S1369, which lies in RNA helicase subdomain 2, faces the serine protease domain at a distance of around 20 Å. Interestingly, suppressor mutations Q1112, P1115, and I1097 reside on a common surface near where the NS4A cofactor exits the serine protease fold. We therefore wondered whether NS4A might fold back and interact with one or more of these NS3 surface residues. Indeed, crystal structures of the dengue and West Nile virus NS2B-3 serine protease indicate that the C terminus of NS2B, which performs a cofactor function similar to that of NS4A, can fold back to interact with the substrate-bound serine protease domain (16). Nevertheless, structural data on the C terminus of HCV NS4A are lacking.
FIG. 5.
Suppressor mutations map to the surface of NS3. The surface structure of single-chain NS3-4A is shown (PDB accession code 1CU1 chain A). In this rendering, the serine protease domain of NS3 is white and NS3 helicase domains 1, 2, and 3 are purple, cyan, and pink, respectively. The cofactor peptide of NS4A (polyprotein residues 1678 to 1690) is green, and the synthetic linker between NS4A and NS3 has been removed. The locations of second- and third-site suppressor mutations are black or blue, respectively. The approximate locations of the serine protease and helicase/nucleoside triphosphatase active sites are indicated by dashed lines. This rendering was prepared with PyMOL (http://pymol.sourceforge.net/).
Conformation analysis of the NS4A C-terminal region by CD.
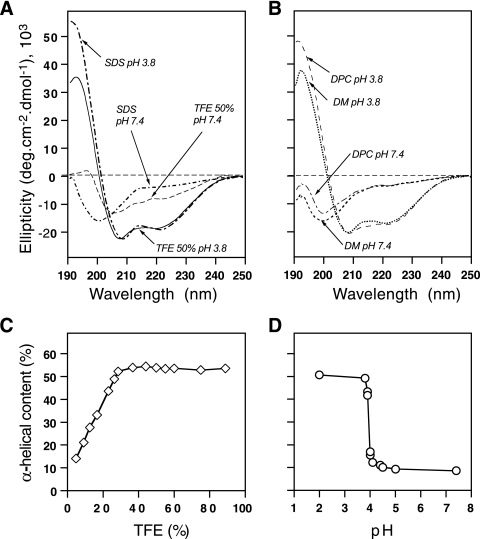
To gain insight into the structure of the NS4A C-terminal region, we synthesized a peptide corresponding to the C-terminal 19 residues of NS4A (polyprotein residues 1693 to 1711). To be consistent with standard protein chemistry nomenclature, this peptide will be referred to as NS4A[36-54] and residues within this peptide will be numbered from the start of the NS4A protein. The secondary structure of this peptide was analyzed by CD spectroscopy under various stabilizing conditions with cosolvents (TFE) or detergents (SDS, DM, DPC) that mimic the environment found in the hydrophobic core of globular proteins and membranes. TFE is well known to induce folding of peptides that have an intrinsic propensity to adopt an alpha-helical structure by stabilizing short-range hydrogen bonds (for an overview of TFE, see references 11 and 48). In the presence of 50% TFE at an acidic pH (3.8), the peptide exhibited a CD spectrum typical of alpha-helical folding, with minima at 208 and 222 nm and a maximum at 192 nm (Fig. 6A). Peptide folding was titrated with increasing concentrations of TFE, yielding spectra that were typical for alpha helices above 20% TFE (Fig. 6C). Maximal amplitude was reached at 35% TFE, and no change was found at higher TFE concentrations. These results were consistent with the suggestion that, for a peptide with a helical propensity, helicity is generally evident at 20 to 30% TFE and folding is complete by 50% TFE (27). In the presence of the various detergents tested under acidic conditions (SDS, DM, and DPC), CD spectra of the peptide again showed the typical spectrum of alpha-helical folding with an amplitude minimum at 222 nm, very similar to that observed in 50% TFE (Fig. 6A and B). Quantitatively, about 55% helical content was estimated at 222 nm by the method of Chen et al. (13) at pH 3.8 in 50% TFE or 100 mM SDS and dropped to about 50% helical content in 100 mM DM or 100 mM DPC. Interestingly, the typical spectral shape of helical folding disappeared at a neutral pH, both in TFE and in detergents (Fig. 6A and B). Indeed, the spectra recorded in the various detergents exhibit a large negative band at around 200 nm and a shoulder at about 220 nm, indicating a mixture of random coil configuration and some residual structure, respectively. In contrast, the CD spectrum observed in 50% TFE suggests the presence of some residual alpha-helical structure (about 22%, assuming that the ellipticity at 222 nm is only due to an alpha helix). The pH dependency of the helical fold was further analyzed by recording the CD spectra of the peptide dissolved in the nonionic detergent DM at various pHs. Figure 6D shows that the peptide is mainly alpha helical below pH 4.0 and essentially unstructured above this pH. The helix-to-random-coil transition occurs in a very narrow range of 0.1 to 0.2 pH unit. The apparent pK of this transition is about 4.0, a value that is slightly below the pKas of the acidic side chains of Glu and Asp (4.5 and 4.6, respectively). As the peptide contains five Glu and one Asp acid residues, it appears that protonation of these residues is required for the peptide to adopt an alpha-helical fold. In other words, the repulsive electrostatic interactions between the negatively charged groups of Glu and Asp prevent alpha-helical folding of the peptide. Furthermore, the very narrow pH range of the transition suggests a stringently cooperative process, whereby almost all of the acidic groups must be protonated to allow the helix to form.
FIG. 6.
pH-dependent folding of NS4A[36-54]. (A and B) CD spectra of the peptide in 10 mM sodium phosphate buffer (pH 3.8 or 7.4) containing 50% TFE or 100 mM SDS (A) or 100 mM DM or DPC (B). (C and D) The alpha-helical content of the peptide, as described in the text, is plotted as a function of percent TFE (C) or pH (D).
NMR structure of the NS4A C-terminal region.
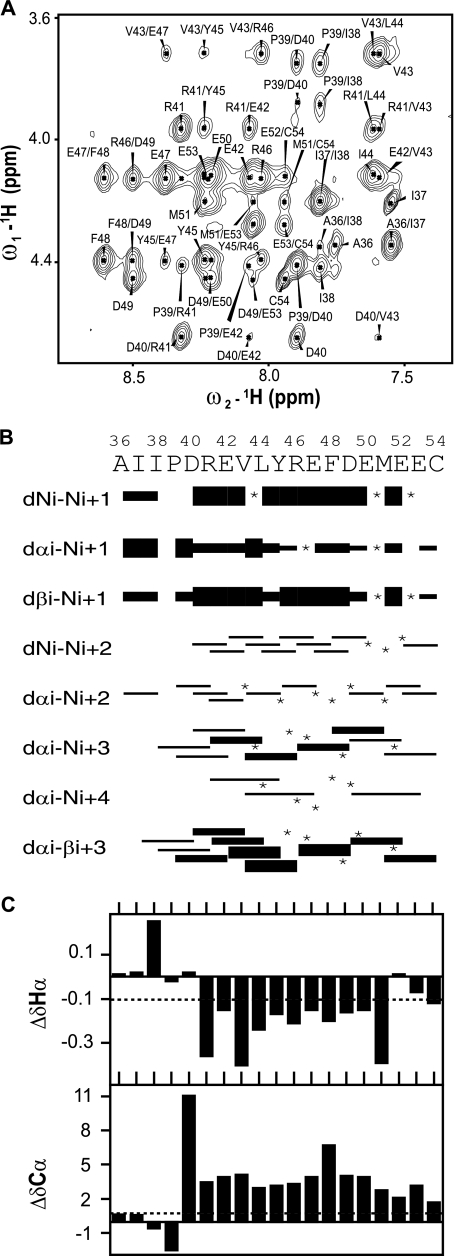
We used NMR spectroscopy to further investigate the conformation of the NS4A[36-54] peptide. Fifty percent TFE appeared to be an appropriate medium since a similar alpha-helical content was observed for the peptide in TFE or in detergents at an acidic pH. The two-dimensional homo- and heteronuclear NMR analyses of the peptide in 50% TFE-d2 yielded rather well-resolved spectra, as illustrated by the extract of the NOESY spectrum (Fig. 7A). Sequential attribution of all spin systems was complete despite the overlap of NMR signals, especially for the five Glu residues exhibiting close chemical shifts. An overview of the sequential and medium-range NOE connectivities is shown in Fig. 7B. Despite the lack of numerous connectivities caused by overlapping cross-peaks, the NOE connectivity patterns clearly show that the main body of the peptide (D40 to M51) displays typical characteristics of an alpha helix, including strong dNN(i,i+1) and medium dαN(i,i+1) sequential connectivities, weak dαN(i,i+2), medium dαN(i,i+3), medium or strong dαβ (i,i+3), and weak dαN(i,i+4) medium-range connectivities. C-terminal residues 52 to 54 also exhibit some NOEs characteristic of an alpha helix with fewer medium-range connectivities, suggestive of a fraying helix terminus. In contrast, the residues flanking the N-terminal end of the helix remain unstructured with almost no medium-range connectivities. Differences of 1Hα and 13Cα chemical shifts from those found in a random coil conformation are additional indicators of secondary structure (67). The long series of negative 1Hα (Δ1Hα ≤ −0.1 ppm) and positive 13Cα chemical shift differences (Δ13Cα ≥ 0.7 ppm) observed in Fig. 7C for residues 41 to 51 (R1698 to M1708) are typical of an alpha-helical conformation. These data are in close agreement with the NOE connectivities described above.
FIG. 7.
NMR analysis of NS4A[36-54]. (A) Extract of the amide-aliphatic region of the NOESY spectrum recorded with the peptide at pH 3.8 in 50% TFE. (B) Summary of sequential (i,i+1) and medium-range (i,i+2 to i+4) NOEs. Intensities of NOEs are indicated by bar thickness; asterisks indicate that the presence of a NOE is not confirmed because of resonance overlap. (C) Chemical shift differences for 1Hα and 13Cα at each position.
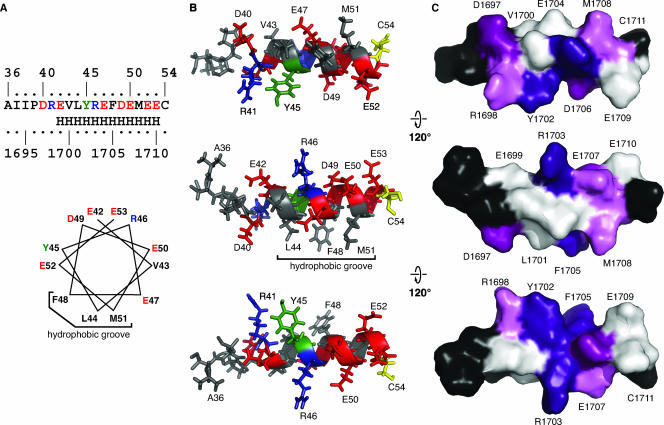
Because of the overlapping NMRs described above, the number of NOE-derived interproton distance constraints was insufficient for calculating the peptide structure by using only the experimentally identified distance constraints. As the NOE connectivities and chemical shift differences unambiguously showed that residues 41 to 51 (polyprotein residues R1698 to M1708) are folded as an alpha helix, we introduced 23 canonical helical distance constraints in the region from 41 to 49 (R1698 to D1706) in addition to the 111 distance constraints extracted from the NOESY spectrum to calculate a semiexperimental average NS4A[36-54] structure that fully satisfied the experimental NMR data. The structural model shown in Fig. 8 illustrates the amphipathic nature of the alpha-helix segment with the hydrophobic and aromatic residues forming a narrow hydrophobic groove while the charged and polar residues are exposed on the hydrophilic side. It should be noted that the structure of full-length NS4A might be somewhat different in the context of NS3-4A, where additional contacts are likely to occur.
FIG. 8.
Structural model of the NS4A C-terminal acidic region. (A) The experimentally determined helical region is shown as in Fig. 1C (top) or as a helical wheel (bottom). Residue colors are as follows on the basis of the chemical properties of their side chains: acidic, red; basic, blue; hydrophobic, black. Tyr and Cys, which have unique chemical properties, are green and yellow, respectively. (B) Semiexperimental model of the NS4A C-terminal acidic region deduced from the CD and NMR data. Residues are numbered from the start of NS4A and colored as in panel A. (C) Surface rendering of the NS4A C terminus acidic region. Residues are numbered according to their positions in the Con1 polyprotein and colored as follows on the basis of the phenotype of Ala substitutions: severe defects, deep purple; strong defects, purple; intermediate defects, pink; no phenotype, white; untested, black. These renderings were prepared with PyMOL (http://pymol.sourceforge.net/).
Despite the preliminary nature of this structural model, it provides a useful framework for understanding our prior results. Remarkably, peptide residues 47 (E1704), 49 (D1706), 50 (E1707), 52 (E1709), and 53 (E1710) form an acidic cluster at the alpha-helix surface (Fig. 8A and B). From an electrostatic point of view, such a cluster would not be stable at neutral pH because of repulsions between the acidic side groups. Conversely, the abolition of these electrostatic repulsions by protonation of these acidic groups at a low pH allows the alpha helix to fold. Alternatively, attractive electrostatic interactions between this acidic cluster and a positively charged target could also induce the alpha-helix folding by neutralizing the repulsive negative charges of the acidic residues. Furthermore, this structural model also suggests that several residues that had strong-to-severe replication phenotypes (Y1702, R1703, F1705, and D1707) may form a cluster on the surface of the NS4A[36-54] peptide (Fig. 8C). Future work will address whether this hypothetical surface is responsible for mediating critical interactions of NS4A in the context of the HCV replicase.
DISCUSSION
NS4A is known to mediate NS3-4A membrane association and enzyme activity, yet it also appears to control other aspects of the viral life cycle through unknown mechanisms. We therefore examined the structure and function of the NS4A C-terminal acidic region. An important aspect of our structural model of the NS4A C-terminal region is that several acidic residues must be neutralized in order for it to fold as an alpha helix. While this was conveniently performed in our CD analysis by altering the pH, interaction with a positively charged surface of another protein will likely induce a similar shift in the conformation of NS4A. The identity of this putative binding partner remains unknown, although it should be noted that NS5A contains several basic surfaces, including a suspected RNA binding groove (65), as well as a region previously proposed to be involved in mediating interaction with NS4A (3).
We found that several mutations in the NS4A C-terminal region reduced the replication fitness of HCV. The most severe phenotypes were exhibited by Y1702A, R1703A, R1703D, and F1705A, each of which abolished colony formation (Fig. 2). It is notable that two of these sites, Y1702 and F1705, are invariant among numerous HCV isolates. Since Gln or Glu residues frequently occur at position 1703 in other HCV isolates, the sequence requirements at this position are not yet clear and may covary with other positions within the HCV genome. On the other hand, several invariant residues were surprisingly tolerant of substitutions, including E1704A, E1704R, E1709A, E1709R, and E17010A, and E17010R. On the basis of our structural model, these mutations should affect the pH threshold for alpha-helix formation. However, the large number of acidic residues in this region may be able to compensate for changes at individual positions. In addition, sequence conservation at these positions may reflect their role in other aspects of the viral life cycle not addressed by our replication assays.
The replication defects of several NS4A mutants were suppressed by mutations in NS3 (Tables 1 and 2 and Fig. 3). These changes mapped to different regions on the surface of NS3, including a cluster of residues (I1097, Q1112, and P1115) near where the cofactor portion of NS4A exits the serine protease domain (Fig. 5). Given this location, we hypothesize that NS4A may fold back to directly interact with this surface. However, attempts to computationally dock the NS4A C-terminal alpha helix onto the surface of NS3 did not yield a convincing model and additional structural studies on NS3-4A are necessary to address this hypothesis. Additional suppressor mutations (R1187L, A1218D, A1226D, and S1369R) mapped to residues at or near the interface between the NS3 serine protease and helicase domains. Given that NS4A regulates both serine protease and RNA helicase activities of NS3 (53), perhaps these residues contribute to interdomain interactions within NS3. It should be noted that there is likely to be flexibility between the serine protease and helicase domains of NS3 that is not apparent from the crystal structure of full-length, single-chain NS3-4A, which captured the C terminus of NS3 bound to the serine protease substrate binding surface (71).
Previous work has shown that mutations in NS3 can lead to cell culture adaptation and increased HCV RNA replication (reviewed in reference 6). Indeed, mutations at Q1112, P1115, and A1226 were previously noted in other cell culture-adapted replicons, although their relative contributions to replication fitness were not specifically addressed (44, 62, 72, 75). We found that P1115R and R1187L did not enhance the replication of the wild-type Con1 replicon, while Q1112R and S1369R only moderately enhanced Con1 replication efficiency. Thus, the ability of these mutations to dramatically suppress the replication defects of NS4A mutants likely reflects their ability to compensate for a change in NS3-4A conformation and/or ability to interact with another partner, rather than as general enhancers of replication. This further suggests that these sites may be directly involved in interaction with NS4A or another replicase component.
Given the importance of NS4A in regulating NS3-4A serine protease activity, we tested whether our panel of Ala substitutions would exhibit decreased polyprotein processing but saw no effect. However, the vaccinia virus-T7 system drives high-level expression in Huh-7.5 cells and is likely to miss subtle differences in serine protease activity and NS5A hyperphosphorylation. Detailed enzymatic analysis of purified mutant NS3-4A complexes is necessary to discern the effects of these mutations on the serine protease and RNA helicase activities. For a few of our NS4A mutants, full-length NS3-4A has been purified and no significant differences in serine protease activity have been noted (R.K.F.B., B.D.L., and A.M.P., unpublished data). The helicase activity of these mutants is still under investigation.
Our panel of NS4A Ala substitutions exhibited a striking correlation between defective replication and decreased NS5A hyperphosphorylation. These results are consistent with previous observations that NS5A hyperphosphorylation is an important determinant of RNA replication. In addition, it appears that the R1703A mutation may inhibit replication by a process other than altered NS5A hyperphosphorylation. Although NS5A phosphorylation acceptor sites have not yet been fully defined, there is mounting evidence that hyperphosphorylation includes phosphorylation at position S2204 by casein kinase Iα and that the efficiency of this phosphorylation event depends on prior phosphorylation of S2201 by another kinase (56, 57).
The functional role of NS5A hyperphosphorylation is not yet fully understood. On the one hand, HCV RNA replication can be greatly enhanced by mutations in NS5A that block hyperphosphorylation (1, 7, 18) or by proprietary kinase inhibitors that block NS5A hyperphosphorylation (51). On the other hand, HCV replication is attenuated by RNAi-mediated silencing of casein kinase Iα expression, which also blocks hyperphosphorylation (56). One model to explain these seemingly disparate results is that the physical interaction between NS5A and casein kinase Iα may be required for HCV replication, but that the kinase activity of this binding partner may, in turn, downregulate replication efficiency. For instance, it is known that NS5A loses its ability to interact with human vesicle-associated membrane protein A (hVAP-A), a putative replicase assembly factor, upon hyperphosphorylation (18). Thus, the dynamics of NS5A hyperphosphorylation may lead to alternating cycles of RNA replication and silent infection, which may confer an advantage on persistent virus infections in vivo.
Given that NS4A mutations that block hyperphosphorylation also block RNA replication and that these effects are relieved by second-site suppressor mutations in NS3, we hypothesize that NS4A likely acts upstream of casein kinase Iα and that the NS4A-mediated conformation of NS3-4A may influence this NS5A-kinase interaction. This model is in line with previous studies showing that NS4A is required for NS5A hyperphosphorylation (3, 28) and that NS5A hyperphosphorylation is blocked by clustered Ala substitutions in the NS4A C-terminal acidic domain (31).
In conclusion, the present work shows that the C-terminal acidic region of NS4A encodes one or more activities that are critical for NS5A hyperphosphorylation and HCV replication, that second-site changes in NS3 can compensate for changes in this region, and that alpha-helical folding of this region depends on local electrostatic interactions and could behave as a molecular switch. Further work is necessary to examine the role of this region in the enzymatic activities of NS3-4A, to clarify the interaction with NS5A and/or other binding partners, and to define the structural basis for these activities.
Acknowledgments
We thank M. Evans for the Bsd replicon, B. Moss and T. Tellinghuisen for providing vTF7-3, A. Gauthier for sharing her NS4B Western blot assay conditions, and P. Holst for administrative and technical support.
This work was funded through United States PHS grants K01CA107092 (to B.D.L.), F32GM071120 (to R.K.F.B.), R01GM60620 (to A.M.P.), and R01CA057973 (to C.M.R.); the French Centre National de la Recherche Scientifique (to F.P.); and the Agence Nationale de Recherche sur le Sida et les Hepatites Virales (to F.P. and R.M.).
Footnotes
Published ahead of print on 20 June 2007.
REFERENCES
- 1.Appel, N., T. Pietschmann, and R. Bartenschlager. 2005. Mutational analysis of hepatitis C virus nonstructural protein 5A: potential role of differential phosphorylation in RNA replication and identification of a genetically flexible domain. J. Virol. 79:3187-3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appel, N., T. Schaller, F. Penin, and R. Bartenschlager. 2006. From structure to function: new insights into hepatitis C virus RNA replication. J. Biol. Chem. 281:9833-9836. [DOI] [PubMed] [Google Scholar]
- 3.Asabe, S. I., Y. Tanji, S. Satoh, T. Kaneko, K. Kimura, and K. Shimotohno. 1997. The N-terminal region of hepatitis C virus-encoded NS5A is important for NS4A-dependent phosphorylation. J. Virol. 71:790-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartenschlager, R., L. Ahlborn-Laake, J. Mous, and H. Jacobsen. 1994. Kinetic and structural analyses of hepatitis C virus polyprotein processing. J. Virol. 68:5045-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartenschlager, R., L. Ahlborn-Laake, K. Yasargil, J. Mous, and H. Jacobsen. 1995. Substrate determinants for cleavage in cis and in trans by the hepatitis C virus NS3 proteinase. J. Virol. 69:198-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartenschlager, R., M. Frese, and T. Pietschmann. 2004. Novel insights into hepatitis C virus replication and persistence. Adv. Virus Res. 63:71-180. [DOI] [PubMed] [Google Scholar]
- 7.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. [DOI] [PubMed] [Google Scholar]
- 8.Blight, K. J., J. A. McKeating, and C. M. Rice. 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 76:13001-13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branch, A. D., D. D. Stump, J. A. Gutierrez, F. Eng, and J. L. Walewski. 2005. The hepatitis C virus alternate reading frame (ARF) and its family of novel products: the alternate reading frame protein/F-protein, the double-frameshift protein, and others. Semin. Liver Dis. 25:105-117. [DOI] [PubMed] [Google Scholar]
- 10.Bryson, K., L. J. McGuffin, R. L. Marsden, J. J. Ward, J. S. Sodhi, and D. T. Jones. 2005. Protein structure prediction servers at University College London. Nucleic Acids Res. 33:W36-W38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buck, M. 1998. Trifluoroethanol and colleagues: cosolvents come of age. Recent studies with peptides and proteins. Q. Rev. Biophys. 31:297-355. [DOI] [PubMed] [Google Scholar]
- 12.Cerino, A., and M. U. Mondelli. 1991. Identification of an immunodominant B cell epitope on the hepatitis C virus nonstructural region defined by human monoclonal antibodies. J. Immunol. 147:2692-2696. [PubMed] [Google Scholar]
- 13.Chen, Y. H., J. T. Yang, and K. H. Chau. 1974. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry 13:3350-3359. [DOI] [PubMed] [Google Scholar]
- 14.Cuff, J. A., M. E. Clamp, A. S. Siddiqui, M. Finlay, and G. J. Barton. 1998. JPred: a consensus secondary structure prediction server. Bioinformatics 14:892-893. [DOI] [PubMed] [Google Scholar]
- 15.Dimitrova, M., I. Imbert, M. P. Kieny, and C. Schuster. 2003. Protein-protein interactions between hepatitis C virus nonstructural proteins. J. Virol. 77:5401-5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erbel, P., N. Schiering, A. D'Arcy, M. Renatus, M. Kroemer, S. P. Lim, Z. Yin, T. H. Keller, S. G. Vasudevan, and U. Hommel. 2006. Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat. Struct. Mol. Biol. 13:372-373. [DOI] [PubMed] [Google Scholar]
- 17.Evans, M. J., C. M. Rice, and S. P. Goff. 2004. Genetic interactions between hepatitis C virus replicons. J. Virol. 78:12085-12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans, M. J., C. M. Rice, and S. P. Goff. 2004. Phosphorylation of hepatitis C virus nonstructural protein 5A modulates its protein interactions and viral RNA replication. Proc. Natl. Acad. Sci. USA 101:13038-13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Failla, C., L. Tomei, and R. De Francesco. 1994. Both NS3 and NS4A are required for proteolytic processing of hepatitis C virus nonstructural proteins. J. Virol. 68:3753-3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favier, A., B. Brutscher, M. Blackledge, A. Galinier, J. Deutscher, F. Penin, and D. Marion. 2002. Solution structure and dynamics of Crh, the Bacillus subtilis catabolite repression HPr. J. Mol. Biol. 317:131-144. [DOI] [PubMed] [Google Scholar]
- 21.Florese, R. H., M. Nagano-Fujii, Y. Iwanaga, R. Hidajat, and H. Hotta. 2002. Inhibition of protein synthesis by the nonstructural proteins NS4A and NS4B of hepatitis C virus. Virus Res. 90:119-131. [DOI] [PubMed] [Google Scholar]
- 22.Foy, E., K. Li, C. Wang, R. Sumpter, Jr., M. Ikeda, S. M. Lemon, and M. Gale, Jr. 2003. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science 300:1145-1148. [DOI] [PubMed] [Google Scholar]
- 23.Frick, D. N., R. S. Rypma, A. M. Lam, and B. Gu. 2004. The nonstructural protein 3 protease/helicase requires an intact protease domain to unwind duplex RNA efficiently. J. Biol. Chem. 279:1269-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hügle, T., F. Fehrmann, E. Bieck, M. Kohara, H. G. Kräusslich, C. M. Rice, H. E. Blum, and D. Moradpour. 2001. The hepatitis C virus nonstructural protein 4B is an integral endoplasmic reticulum membrane protein. Virology 284:70-81. [DOI] [PubMed] [Google Scholar]
- 26.Ishido, S., T. Fujita, and H. Hotta. 1998. Complex formation of NS5B with NS3 and NS4A proteins of hepatitis C virus. Biochem. Biophys. Res. Commun. 244:35-40. [DOI] [PubMed] [Google Scholar]
- 27.Jasanoff, A., and A. R. Fersht. 1994. Quantitative determination of helical propensities from trifluoroethanol titration curves. Biochemistry 33:2129-2135. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko, T., Y. Tanji, S. Satoh, M. Hijikata, S. Asabe, K. Kimura, and K. Shimotohno. 1994. Production of two phosphoproteins from the NS5A region of the hepatitis C viral genome. Biochem. Biophys. Res. Commun. 205:320-326. [DOI] [PubMed] [Google Scholar]
- 29.Kato, J., N. Kato, H. Yoshida, S. K. Ono-Nita, Y. Shiratori, and M. Omata. 2002. Hepatitis C virus NS4A and NS4B proteins suppress translation in vivo. J. Med. Virol. 66:187-199. [DOI] [PubMed] [Google Scholar]
- 30.Kim, J. L., K. A. Morgenstern, C. Lin, T. Fox, M. D. Dwyer, J. A. Landro, S. P. Chambers, W. Markland, C. A. Lepre, E. T. O'Malley, S. L. Harbeson, C. M. Rice, M. A. Murcko, P. R. Caron, and J. A. Thomson. 1996. Crystal structure of the hepatitis C virus NS3 protease domain complexed with a synthetic NS4A cofactor peptide. Cell 87:343-355. [DOI] [PubMed] [Google Scholar]
- 31.Koch, J. O., and R. Bartenschlager. 1999. Modulation of hepatitis C virus NS5A hyperphosphorylation by nonstructural proteins NS3, NS4A, and NS4B. J. Virol. 73:7138-7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolykhalov, A. A., E. V. Agapov, and C. M. Rice. 1994. Specificity of the hepatitis C virus NS3 serine protease: effects of substitutions at the 3/4A, 4A/4B, 4B/5A, and 5A/5B cleavage sites on polyprotein processing. J. Virol. 68:7525-7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komoda, Y., M. Hijikata, S. Sato, S. Asabe, K. Kimura, and K. Shimotohno. 1994. Substrate requirements of hepatitis C virus serine proteinase for intermolecular polypeptide cleavage in Escherichia coli. J. Virol. 68:7351-7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kou, Y. H., S. M. Chou, Y. M. Wang, Y. T. Chang, S. Y. Huang, M. Y. Jung, Y. H. Huang, M. R. Chen, M. F. Chang, and S. C. Chang. 2006. Hepatitis C virus NS4A inhibits cap-dependent and the viral IRES-mediated translation through interacting with eukaryotic elongation factor 1A. J. Biomed. Sci. 13:861-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuang, W. F., Y. C. Lin, F. Jean, Y. W. Huang, C. L. Tai, D. S. Chen, P. J. Chen, and L. H. Hwang. 2004. Hepatitis C virus NS3 RNA helicase activity is modulated by the two domains of NS3 and NS4A. Biochem. Biophys. Res. Commun. 317:211-217. [DOI] [PubMed] [Google Scholar]
- 36.Leinbach, S. S., R. A. Bhat, S. M. Xia, W. T. Hum, B. Stauffer, A. R. Davis, P. P. Hung, and S. Mizutani. 1994. Substrate specificity of the NS3 serine proteinase of hepatitis C virus as determined by mutagenesis at the NS3/NS4A junction. Virology 204:163-169. [DOI] [PubMed] [Google Scholar]
- 37.Li, K., E. Foy, J. C. Ferreon, M. Nakamura, A. C. Ferreon, M. Ikeda, S. C. Ray, M. Gale, Jr., and S. M. Lemon. 2005. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl. Acad. Sci. USA 102:2992-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li, X. D., L. Sun, R. B. Seth, G. Pineda, and Z. J. Chen. 2005. Hepatitis C virus protease NS3/4A cleaves mitochondrial antiviral signaling protein off the mitochondria to evade innate immunity. Proc. Natl. Acad. Sci. USA 102:17717-17722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin, C., J. A. Thomson, and C. M. Rice. 1995. A central region in the hepatitis C virus NS4A protein allows formation of an active NS3-NS4A serine proteinase complex in vivo and in vitro. J. Virol. 69:4373-4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin, C., J. W. Wu, K. Hsiao, and M. S. Su. 1997. The hepatitis C virus NS4A protein: interactions with the NS4B and NS5A proteins. J. Virol. 71:6465-6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin, R., J. Lacoste, P. Nakhaei, Q. Sun, L. Yang, S. Paz, P. Wilkinson, I. Julkunen, D. Vitour, E. Meurs, and J. Hiscott. 2006. Dissociation of a MAVS/IPS-1/VISA/Cardif-IKKɛ molecular complex from the mitochondrial outer membrane by hepatitis C virus NS3-4A proteolytic cleavage. J. Virol. 80:6072-6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623-626. [DOI] [PubMed] [Google Scholar]
- 43.Lindenbach, B. D., H. J. Thiel, and C. M. Rice. 2007. Flaviviridae: the viruses and their replication, p. 1101-1152. In D. M. Knipe and P. M. Howley (ed.), Fields virology, fifth ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, PA. [Google Scholar]
- 44.Lohmann, V., S. Hoffmann, U. Herian, F. Penin, and R. Bartenschlager. 2003. Viral and cellular determinants of hepatitis C virus RNA replication in cell culture. J. Virol. 77:3007-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loo, Y. M., D. M. Owen, K. Li, A. K. Erickson, C. L. Johnson, P. M. Fish, D. S. Carney, T. Wang, H. Ishida, M. Yoneyama, T. Fujita, T. Saito, W. M. Lee, C. H. Hagedorn, D. T. Lau, S. A. Weinman, S. M. Lemon, and M. Gale, Jr. 2006. Viral and therapeutic control of IFN-β promoter stimulator 1 during hepatitis C virus infection. Proc. Natl. Acad. Sci. USA 103:6001-6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merutka, G., H. J. Dyson, and P. E. Wright. 1995. ‘Random coil’ 1H chemical shifts obtained as a function of temperature and trifluoroethanol concentration for the peptide series GGXGG. J. Biomol. NMR 5:14-24. [DOI] [PubMed] [Google Scholar]
- 47.Meylan, E., J. Curran, K. Hofmann, D. Moradpour, M. Binder, R. Bartenschlager, and J. Tschopp. 2005. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437:1167-1172. [DOI] [PubMed] [Google Scholar]
- 48.Montserret, R., M. J. McLeish, A. Bockmann, C. Geourjon, and F. Penin. 2000. Involvement of electrostatic interactions in the mechanism of peptide folding induced by sodium dodecyl sulfate binding. Biochemistry 39:8362-8373. [DOI] [PubMed] [Google Scholar]
- 49.Moradpour, D., E. Bieck, T. Hugle, W. Wels, J. Z. Wu, Z. Hong, H. E. Blum, and R. Bartenschlager. 2002. Functional properties of a monoclonal antibody inhibiting the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 277:593-601. [DOI] [PubMed] [Google Scholar]
- 50.Mottola, G., G. Cardinali, A. Ceccacci, C. Trozzi, L. Bartholomew, M. R. Torrisi, E. Pedrazzini, S. Bonatti, and G. Migliaccio. 2002. Hepatitis C virus nonstructural proteins are localized in a modified endoplasmic reticulum of cells expressing viral subgenomic replicons. Virology 293:31-43. [DOI] [PubMed] [Google Scholar]
- 51.Neddermann, P., M. Quintavalle, C. Di Pietro, A. Clementi, M. Cerretani, S. Altamura, L. Bartholomew, and R. De Francesco. 2004. Reduction of hepatitis C virus NS5A hyperphosphorylation by selective inhibition of cellular kinases activates viral RNA replication in cell culture. J. Virol. 78:13306-13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nomura-Takigawa, Y., M. Nagano-Fujii, L. Deng, S. Kitazawa, S. Ishido, K. Sada, and H. Hotta. 2006. Non-structural protein 4A of hepatitis C virus accumulates on mitochondria and renders the cells prone to undergoing mitochondria-mediated apoptosis. J. Gen. Virol. 87:1935-1945. [DOI] [PubMed] [Google Scholar]
- 53.Pang, P. S., E. Jankowsky, P. J. Planet, and A. M. Pyle. 2002. The hepatitis C viral NS3 protein is a processive DNA helicase with cofactor enhanced RNA unwinding. EMBO J. 21:1168-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Penin, F., J. Dubuisson, F. A. Rey, D. Moradpour, and J. M. Pawlotsky. 2004. Structural biology of hepatitis C virus. Hepatology 39:5-19. [DOI] [PubMed] [Google Scholar]
- 55.Penin, F., C. Geourjon, R. Montserret, A. Bockmann, A. Lesage, Y. S. Yang, C. Bonod-Bidaud, J. C. Cortay, D. Negre, A. J. Cozzone, and G. Deleage. 1997. Three-dimensional structure of the DNA-binding domain of the fructose repressor from Escherichia coli by 1H and 15N NMR. J. Mol. Biol. 270:496-510. [DOI] [PubMed] [Google Scholar]
- 56.Quintavalle, M., S. Sambucini, C. Di Pietro, R. De Francesco, and P. Neddermann. 2006. The α isoform of protein kinase CKI is responsible for hepatitis C virus NS5A hyperphosphorylation. J. Virol. 80:11305-11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quintavalle, M., S. Sambucini, V. Summa, L. Orsatti, F. Talamo, R. De Francesco, and P. Neddermann. 2007. Hepatitis C virus NS5A is a direct substrate of casein kinase I-α, a cellular kinase identified by inhibitor affinity chromatography using specific NS5A hyperphosphorylation inhibitors. J. Biol. Chem. 282:5536-5544. [DOI] [PubMed] [Google Scholar]
- 58.Rost, B. 1996. PHD: predicting one-dimensional protein structure by profile-based neural networks. Methods Enzymol. 266:525-539. [DOI] [PubMed] [Google Scholar]
- 59.Rost, B., G. Yachdav, and J. Liu. 2004. The PredictProtein server. Nucleic Acids Res. 32:W321-W326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, third ed., vol. 1. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 61.Schwieters, C. D., J. J. Kuszewski, N. Tjandra, and G. M. Clore. 2003. The Xplor-NIH NMR molecular structure determination package. J. Magn. Reson. 160:65-73. [DOI] [PubMed] [Google Scholar]
- 62.Sumpter, R., Jr., C. Wang, E. Foy, Y. M. Loo, and M. Gale, Jr. 2004. Viral evolution and interferon resistance of hepatitis C virus RNA replication in a cell culture model. J. Virol. 78:11591-11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanji, Y., M. Hijikata, Y. Hirowatari, and K. Shimotohno. 1994. Hepatitis C virus polyprotein processing: kinetics and mutagenic analysis of serine proteinase-dependent cleavage. J. Virol. 68:8418-8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanji, Y., M. Hijikata, S. Satoh, T. Kaneko, and K. Shimotohno. 1995. Hepatitis C virus-encoded nonstructural protein NS4A has versatile functions in viral protein processing. J. Virol. 69:1575-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tellinghuisen, T. L., J. Marcotrigiano, and C. M. Rice. 2005. Structure of the zinc-binding domain of an essential component of the hepatitis C virus replicase. Nature 435:374-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thanabal, V., D. O. Omecinsky, M. D. Reily, and W. L. Cody. 1994. The 13C chemical shifts of amino acids in aqueous solution containing organic solvents: application to the secondary structure characterization of peptides in aqueous trifluoroethanol solution. J. Biomol. NMR 4:47-59. [DOI] [PubMed] [Google Scholar]
- 67.Wishart, D. S., and B. D. Sykes. 1994. The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J. Biomol. NMR 4:171-180. [DOI] [PubMed] [Google Scholar]
- 68.Wölk, B., D. Sansonno, H. G. Kräusslich, F. Dammacco, C. M. Rice, H. E. Blum, and D. Moradpour. 2000. Subcellular localization, stability, and trans-cleavage competence of the hepatitis C virus NS3-NS4A complex expressed in tetracycline-regulated cell lines. J. Virol. 74:2293-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wüthrich, K. 1986. NMR of proteins and nucleic acids. John Wiley, New York, NY.
- 70.Yan, Y., Y. Li, S. Munshi, V. Sardana, J. L. Cole, M. Sardana, C. Steinkuehler, L. Tomei, R. De Francesco, L. C. Kuo, and Z. Chen. 1998. Complex of NS3 protease and NS4A peptide of BK strain hepatitis C virus: a 2.2 Å resolution structure in a hexagonal crystal form. Protein Sci. 7:837-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao, N., P. Reichert, S. S. Taremi, W. W. Prosise, and P. C. Weber. 1999. Molecular views of viral polyprotein processing revealed by the crystal structure of the hepatitis C virus bifunctional protease-helicase. Structure 7:1353-1363. [DOI] [PubMed] [Google Scholar]
- 72.Yi, M., and S. M. Lemon. 2004. Adaptive mutations producing efficient replication of genotype 1a hepatitis C virus RNA in normal Huh7 cells. J. Virol. 78:7904-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yusim, K., R. Richardson, N. Tao, A. Dalwani, A. Agrawal, J. Szinger, R. Funkhouser, B. Korber, and C. Kuiken. 2005. Los Alamos hepatitis C immunology database. Appl. Bioinformatics 4:217-225. [DOI] [PubMed] [Google Scholar]
- 74.Zhang, Z. X., M. Chen, C. Hultgren, A. Birkett, D. R. Milich, and M. Sällberg. 1997. Immune responses to the hepatitis C virus NS4A protein are profoundly influenced by the combination of the viral genotype and the host major histocompatibility complex. J. Gen. Virol. 78(Pt. 11):2735-2746. [DOI] [PubMed] [Google Scholar]
- 75.Zhu, Q., J. T. Guo, and C. Seeger. 2003. Replication of hepatitis C virus subgenomes in nonhepatic epithelial and mouse hepatoma cells. J. Virol. 77:9204-9210. [DOI] [PMC free article] [PubMed] [Google Scholar]