Abstract
This study describes a method for increasing the immunogenicity of influenza virus vaccines by exploiting the natural anti-Gal antibody to effectively target vaccines to antigen-presenting cells (APC). This method is based on enzymatic engineering of carbohydrate chains on virus envelope hemagglutinin to carry the α-Gal epitope (Galα1-3Galβ1-4GlcNAc-R). This epitope interacts with anti-Gal, the most abundant antibody in humans (1% of immunoglobulins). Influenza virus vaccine expressing α-Gal epitopes is opsonized in situ by anti-Gal immunoglobulin G. The Fc portion of opsonizing anti-Gal interacts with Fcγ receptors on APC and induces effective uptake of the vaccine virus by APC. APC internalizes the opsonized virus to transport it to draining lymph nodes for stimulation of influenza virus-specific T cells, thereby eliciting a protective immune response. The efficacy of such an influenza vaccine was demonstrated in α1,3galactosyltransferase (α1,3GT) knockout mice, which produce anti-Gal, using the influenza virus strain A/Puerto Rico/8/34-H1N1 (PR8). Synthesis of α-Gal epitopes on carbohydrate chains of PR8 virus (PR8αgal) was catalyzed by recombinant α1,3GT, the glycosylation enzyme that synthesizes α-Gal epitopes in cells of nonprimate mammals. Mice immunized with PR8αgal displayed much higher numbers of PR8-specific CD8+ and CD4+ T cells (determined by intracellular cytokine staining and enzyme-linked immunospot assay) and produced anti-PR8 antibodies with much higher titers than mice immunized with PR8 lacking α-Gal epitopes. Mice immunized with PR8αgal also displayed a much higher level of protection than PR8 immunized mice after being challenged with lethal doses of live PR8 virus. We suggest that a similar method for increasing immunogenicity may be applicable to avian influenza vaccines.
The increased risk of an influenza pandemic raises the need for the generation of effective vaccines that will elicit both humoral and cellular immune responses for prevention of infection in the respiratory tract and destruction of virus-infected cells in the body. A prerequisite for the efficacy of any influenza vaccine is its effective uptake at the vaccination site by antigen-presenting cells (APC) such as dendritic cells (DC) and macrophages (46). This applies to vaccines that are comprised of inactivated virus, subunit hemagglutinin (HA) vaccine, or a recombinant protein such as HA (7, 45). APC internalize the vaccine and transport it to draining lymph nodes, where they present the immunogenic viral peptides on cell surface major histocompatibility complex class I (MHC-I) and class II molecules for activation of virus-specific CD8+ and CD4+ T cells, respectively (46). Activated CD8+ T cells become cytotoxic T lymphocytes (CTL), which destroy infected cells, and activated CD4+ T cells, help virus-specific B cells to produce antiviral antibodies and CD8+ T cells to become CTL. Since the currently used influenza vaccines lack markers that identify them as molecules or particulate material that have to be internalized by APC, the uptake of these vaccines is likely to be suboptimal and to be mediated only by random endocytosis. The suboptimal efficacy of influenza vaccines is further suggested by the finding that in immunized populations, particularly the elderly, 25% to 50% of individuals may contract the disease during the flu season (7, 25, 45).
Targeting of vaccines to APC can be achieved by opsonizing them (i.e., forming immune complexes) with the corresponding immunoglobulin G (IgG) molecules. This targeting occurs because APC, including DC, Langerhans cells of the skin, and macrophages, all express Fcγ receptors (FcγR) for the Fc portion of the antigen-bound IgG antibodies (5, 31, 33, 35, 43). The interaction between the Fc portion of the opsonizing antibodies and FcγR on APC is considered the most effective mechanism by which APC identify and internalize antigens that should be targeted for an effective immune response (5). Accordingly, administration of antigens in the form of immune complexes with various antigens, including tetanus toxoid (9, 31), hepatitis B antigen (3), Eastern equine encephalomyelitis virus (23), and simian immunodeficiency virus (44), increases immunogenicity by 10- to 1,000-fold. We proposed to exploit the natural anti-Gal antibody, which is present in all humans as 1% of IgG, for such targeting of influenza virus vaccines to APC. We developed a method for in situ formation of immune complexes between influenza vaccines and the natural anti-Gal antibody, thereby achieving effective targeting of viral vaccines to APC.
Anti-Gal is the most abundant natural antibody in humans, constituting ∼1% of serum IgG (17). This antibody interacts specifically with the α-Gal epitope (Galα1-3Galβ1-4GlcNAc-R) on glycolipids and glycoproteins (10, 12, 15). Anti-Gal is produced throughout life as a result of antigenic stimulation by bacteria of the gastrointestinal flora (16). The α-Gal epitope is absent in humans but is synthesized by the glycosylation enzyme α1,3galactosyltransferase (α1,3GT) in very large amounts in cells of nonprimate mammals, prosimians, and New World monkeys (13, 19). The α1,3GT gene was inactivated in ancestral Old World primates (19, 20, 24, 27, 28, 29); thus, humans, apes, and Old World monkeys all lack α-Gal epitopes but produce the anti-Gal antibody in large amounts (11, 13, 19). If cells, viruses, or molecules expressing α-Gal epitopes are introduced into humans or Old World monkeys, anti-Gal binds avidly in vivo to these epitopes. This is indicated in xenotransplantation, in which in vivo binding of anti-Gal to α-Gal epitopes on transplanted pig hearts or kidneys is the main cause of the rapid rejection of such grafts in humans and Old World monkeys (6, 11, 21, 34). This in situ interaction of anti-Gal with α-Gal epitopes may be exploited for targeting viral vaccines expressing α-Gal epitopes to APC. In fact, anti-Gal is the only antibody in humans that can serve the purpose of targeting antigens to APC, because it is the only natural antibody known to be produced ubiquitously in large amounts in humans (10, 17). Therefore, any particulate or soluble antigen that has α-Gal epitopes will form immune complexes with anti-Gal and will be targeted for effective uptake by APC (1, 8, 18, 30).
In a recent study using a unique model of α1,3GT knockout mice, we showed that the recombinant gp120 protein of the human immunodeficiency virus envelope increases its immunogenicity by >100-fold if the vaccine protein is engineered to express α-Gal epitopes (1). This increased immunogenicity is achieved because of the in vivo formation of immune complexes between anti-Gal and the vaccinating gp120 expressing the α-Gal epitopes. We found that we could synthesize multiple α-Gal epitopes on the N-linked carbohydrate chains of gp120 by using recombinant α1,3GT that we produced in the Pichia pastoris yeast expression system (4). This observation raised the question of whether increasing immunogenicity by anti-Gal-mediated targeting to APC may also be achieved by using intact, inactivated influenza virus engineered to express multiple α-Gal epitopes in a vaccine.
The HA molecule on the envelope of influenza virus is a glycoprotein with seven carbohydrate chains capped with N-acetyllactosamines (Galβ1-4GlcNAc-R) (26, 32) on which α-Gal epitopes can be synthesized by using recombinant α1,3GT (22). We hypothesized that the synthesis of α-Gal epitopes on inactivated influenza virus and subsequent immunization with inactivated influenza virus expressing α-Gal epitopes would result in a significantly higher level of immune response than that measured following immunization with unprocessed inactivated influenza virus that lacks α-Gal epitopes. Such a difference in the immune response would be associated with anti-Gal-mediated targeting of the vaccine virus expressing α-Gal epitopes to APC and would result in differential protection against challenge with the live virus.
The only available nonprimate mammalian experimental model which produces anti-Gal and thus enables analysis of immunogenicity of anti-Gal opsonized vaccines is the α1,3GT gene knockout (KO) mouse, in which the α1,3GT gene was disrupted by targeted insertion of the neomycin resistance gene (41, 42). This mouse mimics the relevant human immune characteristics, as it lacks α-Gal epitopes and can produce anti-Gal as well as humans (1, 8, 30, 39, 40). In contrast, other nonprimate mammals (e.g., wild-type mice, rats, rabbits, ferrets, etc.) express α-Gal epitopes on their cells and thus are immunotolerant to this epitope and cannot produce anti-Gal (13, 19, 39). The characteristics of anti-Gal produced in KO mice are similar to those in humans (1). We studied the immune response to the A/Puerto Rico/8/34- H1N1 (PR8) influenza virus strain in these KO mice. As described below, we found that vaccine containing inactivated PR8 virus processed to express α-Gal epitopes (PR8αgal) is much more immunogenic than the unprocessed inactivated virus.
MATERIALS AND METHODS
Supplies.
The recombinant glycosylation enzyme α1,3galactosyltransferase (α1,3GT) was produced in the expression system of Pichia pastoris as previously described (4). The monoclonal anti-Gal antibody (designated M86) was obtained in tissue culture supernatants of hybridoma M86 cells, as previously described (14). Horseradish-peroxidase (HRP)-conjugated anti-mouse IgG and anti-mouse IgA antibodies were purchased from Accurate Laboratories (Westbury, NY). The glycoprotein comprised of synthetic α-Gal epitopes linked to bovine serum albumin (α-Gal BSA) was purchased from Dextra Laboratories (Reading, United Kingdom). Mouse anti-Gal was isolated from the sera of KO mice that were repeatedly immunized with pig kidney membrane homogenate in order to elicit production of anti-Gal, as previously described (1). The antibody was isolated from the sera by affinity columns of synthetic α-Gal epitopes linked to silica beads (Synsorb, Alberta, Canada) (12, 13). The Ribi adjuvant, trehalose dicorynomycolate, was purchased from Corixa (Hamilton, MT).
Virus preparation and inactivation.
Influenza virus strain A/PR/8/34 (H1N1) (PR8 virus) was propagated, as we previously described, in the allantoic cavity of 11-day-old embryonated chicken eggs (18, 22). The virus was precipitated from the allantoic fluid by polyethylene glycol and purified on a continuous sucrose gradient as previously described (37). The protein concentration was measured by color reaction, using a Pierce bicinchoninic acid protein assay (Pierce, Rockford, IL). Virus titers were measured by a hemagglutination assay with chicken red blood cells (ChRBC; Lampire Biological Laboratories, Inc., Pipersville, PA) and by a plaque-forming assay with MDCK cells.
Viruses were inactivated by incubation for 45 min at 65°C. Prior to immunization, the efficacy of the inactivation was confirmed by the complete loss of hemagglutinating activity by influenza viruses incubated with ChRBC, even at a concentration corresponding to 104 hemagglutinating units (HAU)/ml.
Mice and immunization procedures.
The mice used were α1,3GT KO mice on an H-2b genetic background (42), which were bred and maintained at the animal facility of the University of Massachusetts Medical Center. These mice lack α-Gal epitopes and thus are not immunotolerant to it and can produce the anti-Gal antibody (1, 8, 30, 39, 40). In contrast, the wild-type (WT) mouse counterpart (C57BL/6) expresses α-Gal epitopes on its cells and cannot produce anti-Gal (39). Experiments were performed with both males and females and found to yield similar results. All experiments with mice were performed according to AAALAC guidelines. The mice were immunized intraperitoneally 3 to 4 times with 50 mg pig kidney membrane homogenate to induce anti-Gal production at titers similar to those in humans (titers of 1:200 to 1:2,000, as measured by enzyme-linked immunosorbent assay [ELISA] with α-Gal BSA used as a solid-phase antigen) (1). Following demonstration of anti-Gal IgG production, the mice received a subcutaneous injection (1 μg in Ribi adjuvant) of inactivated PR8 or inactivated PR8 influenza virus that was enzymatically engineered to express α-Gal epitopes (PR8αgal virus). The injection was repeated after 2 weeks. Both antibody and cellular immune responses were evaluated 14 days after the second injection.
Enzymatic engineering of PR8 to express α-Gal epitopes.
The enzymatic reaction to synthesize α-Gal epitopes on PR8 viruses was performed in an enzyme buffer containing 0.1 M MES (methylethyl morpholino sulfonate; pH 6.0) and 25 mM MnCl2, as previously described (1, 22). α-Gal epitopes were synthesized on PR8 (100 μg/ml) by recombinant α1,3GT (30 μg/ml) and UDP-Gal (1 mM) for 2 h at 37°C. The control PR8 virus was incubated under similar conditions with inactivated α1,3GT and 1 mM UDP-Gal. Inactivation of α1,3GT was achieved by incubating the enzyme for 10 min in boiling water.
Western blotting and silver-staining analyses.
PR8 virus was run under denaturing and reducing conditions on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and tested for binding with purified mouse anti-Gal or monoclonal anti-Gal M86 antibodies. Viral proteins were separated on 10% precast polyacrylamide gel (NuSep, Australia) for 2 h at 100 V. The proteins were then transferred to polyvinylidene difluoride membranes by electroblotting and blocked overnight in a solution containing 1% fat-free milk powder. The membranes were then incubated in either mouse anti-Gal or monoclonal M86 antibodies in 1% BSA for 2 h at room temperature, followed by four washes for 15 min each in Tween-PBS. The anti-Gal blots were incubated with HRP-conjugated goat anti-mouse IgG (for mouse anti-Gal) or goat anti-mouse IgM (for M86) antibodies (Accurate Chemical & Scientific, NY) diluted 1:500 for 1 h at room temperature and washed as described above. Subsequently, the membranes were exposed to diamino benzidine (Sigma) until the staining developed. For silver staining, the gel was fixed with 100 ml of fixing solution (50% ethanol-10% acetic acid in ultra-pure water). After 40 min, the gel was washed with 30% ethanol, and then a ProteoSilver stain kit (Sigma) was used according to the manufacturer's protocol.
ELISA assays.
Anti-Gal titers in mice immunized with pig kidney membrane and the level of production of anti-PR8 antibodies were determined by ELISA as previously described (1). Briefly, ELISA wells were coated with α-Gal BSA (10 μg/ml) or PR8 virus (1 μg/ml) overnight at 4°C. The plates were washed once with PBS and blocked with 1% BSA in PBS. Serum samples at various dilutions (50-μl aliquots) were plated in the wells for 2 h at room temperature. After the plates were washed, HRP-coupled goat anti-mouse IgG or goat anti-mouse IgA was added for 1 h. Color reactions were developed with ortho-phenylene diamine, and absorbance was measured at 492 nm. In assays using the monoclonal anti-Gal antibody M86 (14), HRP-anti-mouse IgM was used as a secondary antibody. For antibody analysis within the sera, the first dilution was 1:10, whereas for analysis of the supernatants of lung homogenates, the first dilution was 1:1.
HI assay.
The ability of anti-HA antibodies to inhibit viral agglutination of ChRBC was measured as described previously (18). Briefly, 5 HAU/ml of PR8 virus was plated in 96-well V-bottom plates (Corning). Sera were diluted in PBS containing 0.08% sodium azide. Aliquots of sera at serial twofold dilutions (beginning with a serum dilution of 1:10) were added to the virus and incubated overnight at 4°C. Subsequently, 50 μl of 1% ChRBC suspension was added and incubated for 30 min at room temperature. HA inhibition (HI) titers were determined as the highest serum dilution at which the hemagglutination of ChRBC was inhibited.
APC for ELISPOT and intracellular-cytokine-staining assays.
The dendritic cell line DC2.4 (36) used in this study was a gift from K. Rock (University of Massachusetts Medical Center, Worcester, MA). These cells were pulsed with 10 μg (4 × 103 HAU) of heat-inactivated PR8 virus overnight to allow protein processing. The cells were then washed three times with PBS and used for enzyme-linked immunospot (ELISPOT) or intracellular-cytokine-staining (ICS) assays.
IFN-γ ELISPOT assay.
ELISPOT assays for gamma interferon (IFN-γ)-secreting cells were performed with a commercial kit (Mabtech, OH) according to the manufacturer's protocol, as we described previously (1). Briefly, 96-well ELISPOT plates were coated with 100 μl/well of the anti-IFN-γ monoclonal antibody AN18 overnight at 4°C. The plates were washed with PBS and blocked with PBS containing 10% fetal calf serum for 30 min at room temperature. Freshly isolated splenocytes (2 × 105 cells per well) were plated in triplicate together with 2 × 104 cells of the dendritic cell line DC2.4 that were prepulsed by incubation with inactivated PR8 virus as described above. After being incubated overnight at 37°C in 5% CO2, the cells were removed by washing with PBS, and aliquots of 100 μl of anti-IFN-γ-biotin (monoclonal antibody R4-6A2; Mabtech) were added to each well for 2 h at room temperature. The plates were then washed with PBS, 100 μl of streptavidin-alkaline phosphatase was added per well, and the plates were incubated for 1 h at room temperature. After a washing with PBS, 100 μl of chromogenic substrate (NBT-plus; Mabtech) was added to each well for 15 min to allow color development and the formation of spots. The color reaction was stopped by the addition of water. The wells were then air dried, and the spots were counted with an ELISPOT automated reader system (performed by Zellnet, Fort Lee, NJ). Calculated frequencies were based on the averages of the results for triplicate wells. The results were expressed as the numbers of PR8-specific IFN-γ-secreting T cells per 106 splenocytes.
ICS for determining T-cell activation.
For ICS, the spleens from six mice/group were harvested, and the splenocytes were adjusted to 2 × 106 cells per tube for each mouse. Spleen lymphocytes were incubated at 37°C in 5% CO2 with 2 × 104 nonpulsed cells of the dendritic cell line DC.2.4 or DC.2.4 cells pulsed by preincubation with heat-inactivated PR8 virus, as described above for the ELISPOT studies. Recombinant human interleukin-2 (10 unit/ml) was added to all samples, and after 1 h of culture, 1 μl/ml of brefeldin A (10 μg/ml; Sigma Chemical Co., St. Louis, MO) was added to impair secretion of cytokines. After another 5 h of incubation, the cells were washed once in 2% fetal calf serum-PBS and kept overnight at 4°C. The following day, these cells were stained for surface markers and intracellular cytokines as described by Bottrel et al. (2). Briefly, cultured cells were stained for surface markers by incubation for 30 min on ice, using fluorescein isothiocyanate-labeled anti-CD3 and peridinin-chlorophyll-protein-labeled anti-CD8 or phycoerythrin-labeled anti-CD4 monoclonal antibodies (BD Pharmingen, CA), followed by two washes and fixation, using solution A (Fix & Perm kit; Caltag Laboratories). These cells were washed and permeabilized, using solution B (Fix & Perm kit; Caltag Laboratories), and stained with APC-labeled anti-IFN-γ (BD Pharmingen, CA). After 20 min, the cells were washed and resuspended in 2% paraformaldehyde. A minimum of 50,000 splenocyte-gated events were acquired using a lymphocyte gate and were determined by size and granularity profiles. The data were analyzed by CELLQuest software (Becton Dickinson, San Jose, CA). Antigen-specific cells were defined as CD3+CD8+ or CD3+CD4+ T lymphocytes that coexpressed IFN-γ.
Challenging mice with live PR8 virus.
Mice immunized with PR8αgal or with PR8 were challenged intranasally with a dose of 2,000 PFU of PR8 virus in a 50-μl solution applied to the nostrils of anesthetized mice. The mice infected with PR8 were monitored for survival for 30 days postchallenge. In separate studies, mice immunized with PR8αgal or PR8 were euthanized 3 days postchallenge, and their lungs were harvested and homogenized in PBS to a total volume of 1 ml. The supernatants of the homogenates were subjected to analysis of virus titers by a standard assay to determine the tissue culture infectious dose (TCID) in wells with MDCK cells and analysis of the levels of cytopathic effect after 96 h and by hemagglutination of ChRBC. The supernatants were further assayed for anti-PR8 IgA antibodies by ELISA, as described above.
RESULTS
Synthesis of α-Gal epitopes on PR8 virus.
At a concentration of 100 μg/ml, the virus was incubated with 30 μg/ml of recombinant α1,3GT and 1 mM UDP-Gal (UDP-galactose) as a sugar donor. This enzyme transfers galactose from UDP-Gal and links it as Galα1-3 to the N-acetyllactosamines (Galβ1-4GlcNAc-R) of the multiple HA carbohydrate chains to generate α-Gal epitopes by a reaction that is identical to that naturally occurring within the Golgi apparatus of nonprimate mammalian cells (Fig. 1A). The presence of de novo synthesized α-Gal epitopes on PR8 could be demonstrated by Western blotting (Fig. 1B) with mouse serum anti-Gal and monoclonal anti-Gal (M86) (14) and by ELISA with this monoclonal anti-Gal (Fig. 1C).
FIG. 1.
Synthesis of α-Gal epitopes on influenza virus HA. (A) Illustration of enzymatic synthesis. α-Gal epitopes (Galα1-3Galβ1-4GlcNAc-R) were synthesized on the N (Asn)-linked carbohydrate chains on HA by the linking of galactosyls (Gal) from the sugar donor, UDP-GAL, to N-acetyllactosamine (Galβ1-4GlcNAc-R) residues as a result of the catalytic activity of recombinant α1,3GT. (B) Expression of α-Gal epitopes on PR8αgal, as shown by Western blots stained with serum anti-Gal purified from KO mouse serum and with monoclonal anti-Gal M86. Note that both anti-Gal antibodies bind only to HA1 from PR8αgal and not to the HA1 from unprocessed PR8. (C) α-Gal epitope expression levels on PR8αgal virus attached to ELISA wells and studied for binding of the monoclonal anti-Gal M86. PR8αgal virus treated with 0.2% formalin for 20 h had the same level of binding as PR8αgal virus generated by incubation of PR8 with active recombinant α1,3GT. The data shown are representative of three independent experiments with similar results. ▵, unprocessed PR8 virus; □, PR8 virus incubated with inactivated recombinant α1,3GT; ○, PR8αgal virus generated by incubation of PR8 with active recombinant α1,3GT.
Separation of PR8 proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions demonstrates the distinct band of HA1 (∼60 kDa) and other viral proteins in the range of 20 to 40 kDa. Blotting of the proteins, followed by staining with anti-Gal from mouse sera or with the monoclonal anti-Gal antibody M86, demonstrated the distinct staining of the HA1 band in PR8αgal but not in the same band of the unprocessed PR8 virus (Fig. 1B). The binding of these antibodies is highly specific and was not observed with other proteins of the PR8αgal virus or with any of the proteins of PR8 virus. It is not clear whether α-Gal epitopes were expressed on carbohydrate chains of HA2 and envelope neuraminidase, since anti-Gal binding was too weak to determine distinct expression. These findings suggest that the synthesis of α-Gal epitopes by recombinant α1,3GT occurs primarily on the carbohydrate chains of HA1.
Expression of α-Gal epitopes on PR8αgal could be demonstrated on the intact virus by ELISA with monoclonal anti-Gal. This antibody bound to PR8αgal as a solid-phase antigen in ELISA wells but not to unprocessed PR8 virus or to PR8 virus incubated with inactivated recombinant α1,3GT and UDP-Gal (Fig. 1C). Since some influenza virus vaccines include inactivation of the virus by formalin, it was of interest to determine whether formalin treatment affects α-Gal epitope expression on the virus. Thus, ELISA was performed in parallel with solid-phase PR8 virus that was incubated for 20 h with 0.2% formalin (i.e., a fourfold-higher concentration than that used for vaccine preparation). Monoclonal anti-Gal binding to formalin-treated PR8αgal was identical to binding to untreated PR8αgal, as shown in Fig. 1C. This was to be expected, since formalin does not interact with carbohydrate chains. These data imply that α-Gal epitope expression on the vaccine virus is not affected by formalin-mediated inactivation of the virus.
ELISPOT analyses of T-cell responses in mice immunized with PR8αgal or PR8.
We hypothesized that vaccination of α1,3GT KO mice with inactivated PR8αgal virus would result in opsonization of the vaccinating virus by anti-Gal and the subsequent targeting of this vaccine virus to APC by the antibody (see Fig. 7). This, in turn, would result in increased transport of the virus to lymph nodes draining the vaccination site and the processing and presentation of immunogenic viral peptides, ultimately resulting in a higher level of activation of virus-specific T cells than that following vaccination with the unprocessed PR8 virus, which lacks α-Gal epitopes. The KO mice used in our studies were immunized three to four times with 50 mg of pig kidney membrane homogenate in order to elicit anti-Gal production at titers similar to those found naturally in humans. The studies below (Fig. 2 to 5) describe data obtained from the same six PR8αgal-immunized mice and six PR8-immunized mice. The data on antibody production and ELISPOT assays are representative of 15 mice per group, with similar results for increased responses in ∼66% of mice immunized with PR8αgal. However, since analysis of ICS was performed for only six of the mice in each group, the data for these six mice are presented in Fig. 2 to 5 for comparison of the various types of immune responses.
FIG. 7.
Anti-Gal-mediated targeting of vaccinating PR8αgal virus to APC. Inactivated PR8αgal virus with α-Gal epitopes (red diamonds) was injected as a vaccine. Anti-Gal binds to the α-Gal epitopes on the virus and opsonizes it. The Fc portion of anti-Gal interacts with FcγR on APC and induces uptake of the vaccine by the APC. The internalized virus undergoes processing in the endocytic vesicles and the cytoplasm. The viral immunogenic peptides are presented on MHC class I molecules for activation of CD8+ CTL precursors (Tc cells) and on MHC class II molecules for activation of helper T cells (Th cells). Signal 2 provided by the APC also facilitates this activation. Activated Th cells provide help for the antibody response by B cells and for CTL activation. Activated Tc cells differentiate into CTL, which kill virus-infected cells. TCR, T-cell receptor.
FIG. 2.
Analysis by ELISPOT assay of IFN-γ secretion levels in mice immunized with PR8 or PR8αgal. Lymphocytes from six mice immunized with inactivated PR8αgal virus (#1 to #6) or inactivated PR8 (#7 to #12) were obtained 14 days after the second immunization and incubated with cells of the dendritic cell line DC2.4, prepulsed with inactivated PR8, and subjected to ELISPOT (hatched columns). Data for lymphocytes incubated with DC that were not prepulsed with PR8 are presented as empty columns. The data are presented as means ± standard deviations of the results for triplicate wells.
FIG. 5.
HI activity in KO mice immunized with PR8αgal or PR8. Results are presented as reciprocals of the serum dilutions that displayed HI activity. Note that the levels of HI activity in mice immunized twice with 1 μg PR8αgal is much higher than that in the sera of mice immunized with 1 μg PR8 (with the exception of mice no. 5 and 6).
KO mice, which were confirmed to produce anti-Gal by ELISA with synthetic α-Gal epitopes linked to BSA (1), were immunized subcutaneously twice at 2-week intervals with 1 μg of inactivated PR8αgal (corresponding to 400 HAU prior to inactivation) or with a similar amount of inactivated PR8 lacking α-Gal epitopes. The vaccines were delivered in Ribi adjuvant. The mice were studied for anti-PR8 cellular immune responses 14 days after the second immunization. PR8-specific T cells were identified in spleen lymphocyte populations of the immunized mice by ELISPOT assays. For these assays, the lymphocytes were coincubated with cells of the dendritic cell line DC2.4 that were prepulsed for 24 h with inactivated PR8 virus. The number of T cells that secreted IFN-γ in the absence of PR8 did not exceed 80 per 106 lymphocytes in any of the mice tested (Fig. 2). In mice immunized twice with the inactivated unprocessed PR8 virus (mice no. 7 to no. 12), the number of activated virus-specific T cells ranged between 400 and 700 per 106 lymphocytes, with an average ± standard deviation of 510 ± 103. The number of PR8-specific T cells in four of the six mice immunized with PR8αgal (mice no. 1 to no. 4) was severalfold higher and ranged between 1,650 and 2,510 per 106 lymphocytes. In the remaining two mice (mice no. 5 and no. 6), the number of these T cells was 750 and 1,200 per 106 lymphocytes (Fig. 2). As shown below, the humoral immune response in these two mice was also lower than in the four mice with higher ELISPOT values. The average ± standard deviations of the ELISPOT values for the mice immunized with PR8αgal were 1,805 ± 760.
Analysis of PR8-specific CD8+ and CD4+ T-cell responses by ICS.
In order to determine whether the virus-specific T cells of mice immunized with PR8 or PR8αgal included both CD8+ T cells (i.e., precursors of CTL) and CD4+ T cells (Th1 helper T cells) specific for PR8, we performed ICS assays. This staining assay aims to detect IFN-γ production in activated T cells that are also stained with CD8- or CD4-specific antibodies. The spleen lymphocytes were coincubated for 24 h with cells of the dendritic cell line DC2.4 prepulsed with inactivated PR8 or with control nonpulsed DC2.4 cells. Lymphocytes incubated with these control APC that were not pulsed, displayed no significant ICS (<0.1%; data not shown). As shown in Fig. 3A, 2.6 to 4.4% of CD8+ T cells from the six PR8-immunized mice (mice no. 7 to no. 12) were primed in vivo by PR8 peptides and thus were activated in vitro by the viral peptides presented on DC2.4 cells. In contrast, in the four mice immunized with PR8αgal (mice no. 1 to no. 4) as many as 19.5 to 23.3% of CD8+ T cells were activated by the process and presented PR8 peptides. However, the two mice (no. 5 and no. 6) for which ELISPOT values were lower than those for the other four mice (Fig. 2) also had low numbers of IFN-γ-producing CD8+ T cells, similar to mice immunized with PR8.
FIG. 3.
Intracellular staining of IFN-γ in CD8+ (A) and CD4+ (B) T cells in PR8αgal- or PR8-immunized mice. ICS analysis of levels of CD8+ (A, including two left panels) and CD4+ (B, including two right panels) T cells in PR8αgal (mice no. 1 to 6)- or PR8 (mice no. 7 to 12)-immunized mice. Lymphocytes were obtained as described in the legend to Fig. 2 and four-color stained for CD3+, CD8+, CD4+ membrane marker, and intracellular IFN-γ. Gated CD3+/CD8+- or CD3+/CD4+-positive events were analyzed for IFN-γ production. The percentage of CD8+ or CD4+ T cells with intracellular IFN-γ is indicated in the upper right quadrant for each mouse. Note that four of the six mice immunized with PR8αgal (#1 to #4) had much higher proportions of IFN-γ-producing CD8+ and CD4+ T cells than mice immunized with PR8.
The differential response of T cells to the PR8 peptides presented by DC was also observed among CD4+ T cells. For four of the mice immunized with PR8αgal (mice no. 1 to no. 4), 12 to 13.7% of CD4+ T cells were activated, whereas no significant activation of CD4+ T cells from the six PR8-immunized mice (mice no. 7 to no. 12) was observed (Fig. 3B). CD4+ cells activated to produce IFN-γ represent the PR8-specific Th1 population. The two PR8αgal-immunized mice (mice no. 5 and no. 6) with low levels of CD8+ activation also had very low levels of CD4+ activation, implying that in these mice there was no measurable increase in the antivirus cellular immune response, as determined by ICS and ELISPOT assays. Note that the very high number of activated T cells in both the CD8+ and CD4+ populations of PR8αgal-immunized mice are unusually high for ICS assays, for which the response to a defined immunogenic peptide is only one to a few percent. The >10% response of CD8+ and CD4+ T cells of mice no. 1 to no. 4 in Fig. 3 is likely to reflect the immune response to multiple immunogenic viral peptides of the various proteins comprising the immunizing PR8αgal influenza virus. Also note that the low immune response in two of the six PR8αgal-immunized mice is not associated with low levels of anti-Gal response, since all the mice were found to produce anti-Gal at titers comparable to those in humans (data not shown).
Production of anti-PR8 antibodies in immunized mice.
The sera from KO mice immunized with PR8 or PR8αgal were assayed for production of antibodies to the unprocessed PR8 virus used as a solid-phase antigen in ELISA (Fig. 4A). The levels of anti-PR8 IgG antibody activities in the six mice immunized with PR8αgal were much higher than those in PR8-immunized mice. Four mice immunized with PR8αgal had very high anti-PR8-antibody activity (mice no. 1 to no. 4), as indicated by an average of 50% maximum binding (i.e., 1.5 optical density) at a serum dilution of 1:102,400. Even mice no. 5 and no. 6 (mice displaying low levels of CD4+ and CD8+ activation) (Fig. 2 and 3) displayed 50% of the maximum anti-PR8 IgG activity at serum dilutions of 1:12,800 and 1:6,400, respectively. In contrast, for mice immunized with PR8 (mice no. 7 to no. 12) (Fig. 2 and 3), 50% of the maximum binding level was observed at a serum dilution of 1:400 (i.e., >200-fold lower than for PR8αgal-immunized mice no. 1 to no. 4).
FIG. 4.
Production of anti-PR8 antibodies in mice immunized twice with 1 μg inactivated PR8αgal (•) or with inactivated PR8 (○) in Ribi adjuvant and measured by ELISA with PR8 virus as a solid-phase antigen. (A) Anti-PR8 IgG response in KO mice. (B) Anti-PR8 IgG response in WT mice. (C) Anti-PR8 IgA response in KO mice (n = 6 per group). The two KO mice in panels A and C with the lowest levels of response (•) are mice no. 5 and 6 in Fig. 2 and 3 above.
The association between the high levels of anti-PR8 antibody response and anti-Gal-mediated targeting of the vaccinating virus can be further inferred from the data obtained with C57BL/6 WT mice. These mice express α-Gal epitopes on their cells and thus cannot produce anti-Gal, despite four immunizations with pig kidney membranes (1, 39). Repeating the immunization studies with PR8 or PR8αgal with these WT mice resulted in no significant differences in anti-PR8 antibody responses between the two groups (Fig. 4B). Thus, in the absence of anti-Gal, the expression of α-Gal epitopes on the immunizing virus has no effect on the immunogenicity of the virus.
The differential humoral immune response in KO mice immunized with PR8αgal versus those immunized with PR8 was also evident in analyses of anti-PR8 IgA antibodies. The significance of this immunoglobulin class is primarily in mucosal immunity, which prevents viral infection of respiratory tract cells. As shown in Fig. 4C, for mice no. 1 to no. 4 anti-PR8 IgA activity was 50- to 100-fold higher in PR8-immunized mice no. 7 to no. 12. This increased antibody response was indicated by the finding that anti-PR8 antibody activity in serum dilutions of 1:50 for mice no. 7 to no. 12 was similar to the activity in serum dilutions of 1:3,200 to 1:6,400 for mice no. 1 to no. 4.
Inhibition of hemagglutinating antibodies in immunized mice.
One of the distinct antibody activities that protects against infection by the influenza virus is that of antibodies binding to the HA of the infectious virus, indicated by the prevention of subsequent binding of this envelope glycoprotein to target cells for infection. The activity of these antibodies can be determined by the inhibition of hemagglutination of ChRBC by the PR8 virus, as determined by assays at virus concentrations of 5 HAU/ml. The virus was incubated with 50 μl of sera at twofold serial dilutions, starting at a dilution of 1:10. After 24 h at 4°C, 50 μl of 1% ChRBC was added, and HI was scored after 30 min. As shown in Fig. 5, the levels of HI activity in PR8αgal-immunized KO mice was many times higher than that in PR8-immunized mice. The average HI titer in the former group was 1:135, whereas in the latter group it was 1:15, i.e., ninefold lower. Two mice (no. 5 and no. 6) among the PR8αgal-immunized mice displayed a much lower level of HI activity, in agreement with the other parameter of low levels of anti-influenza virus cellular and humoral immune responses shown in Fig. 2 to 4. The average level of HI activity in the highly responsive mice in that group (no. 1 to no. 4) is 1:180, which is 12-fold higher than in the control group immunized with unmodified PR8 (Fig. 5).
Induction of a protective immune response against challenge with PR8.
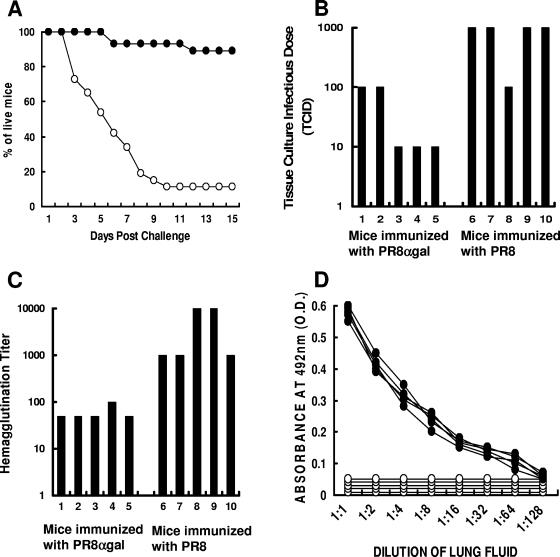
KO mice immunized with heat-inactivated PR8 or PR8αgal virus (25 mice/group) were studied for resistance to intranasal challenge with 2,000 PFU of live PR8 virus. The mice were subsequently monitored for survival for a period of 30 days. Immunization of the mice with inactivated PR8 virus did not confer significant protection against challenge with PR8 (Fig. 6A). By day 10 postchallenge, 89% of the mice had died of the infection. In contrast, mice immunized with inactivated PR8αgal virus were much more resistant to the challenge. Only 11% of the mice succumbed to the viral infection, and the remaining 89% survived. Survival data for day 30 were the same as for day 15, as shown in Fig. 6A. These results strongly suggest that the increased cellular and humoral immune responses in PR8αgal-immunized mice, shown in Fig. 2 to 5, correlate with increased resistance to infection by the PR8 virus.
FIG. 6.
Survival rates and analyses of lungs from mice immunized twice with inactivated PR8 or PR8αgal and receiving intranasal challenge with live PR8. (A) PR8 (○)- or PR8αgal (•)-immunized mice were challenged with 2,000 PFU of live PR8 in 50-μl solutions (n = 25/group). Survival data are presented as percentages of live mice at different time points postchallenge. The survival data for day 30 were similar to those for day 15 postchallenge. (B) Analysis of virus titers as the TCID in lungs of immunized mice 3 days postchallenge (n = 5/group). The supernatants of lung homogenates were incubated in serial 10-fold dilutions in 96-well plates with MDCK cell monolayers. The levels of cytopathic effects were scored after 96 h. (C) Analysis of virus titer by hemagglutination in lungs of immunized mice 3 days postchallenge (n = 5/group). (D) Levels of anti-PR8 IgA antibodies in lungs of immunized mice 3 days post challenge (n = 5/group). •, PR8αgal-immunized mice; ○, PR8-immunized mice (n = 5 mice/group).
In order to further study this protective immune response, the immunizations were repeated for five mice per group. The mice were euthanized 3 days after intranasal challenge, and their lungs were harvested and homogenized in PBS to a total volume of 1 ml. The virus titers in supernatants of the homogenate were determined by measuring the TCID in MDCK cell monolayers (i.e., the highest dilution resulting in a cytopathic effect on MDCK cells) and the hemagglutination of ChRBC. Incubation of the virus at serial 10-fold dilutions with MDCK cells for 96 h indicated that two of the PR8αgal-immunized mice had TCIDs of 100 and the remaining three mice had TCIDs of 10; i.e., the lung supernatant dilution of 1:10 was the highest dilution resulting in a cytopathic effect on MDCK cells (Fig. 6B). In contrast, four of the five PR8-immunized mice had TCIDs of 1,000, and only one had a TCID of 100. The average TCID among the PR8αgal-immunized mice was ∼18-fold lower than that in PR8-immunized mice. This difference in the presence of live PR8 in the lungs of the PR8-challenged mice was confirmed by analysis of the hemagglutination titers with ChRBC. As shown in Fig. 6C, incubation of lung homogenate supernatants from PR8αgal-immunized mice with ChRBC resulted in hemagglutination titers (reciprocal of end point dilution displaying agglutination) of 50 for four mice and 100 for one mouse. In contrast, the titer for three of the PR8-immunized mice was 1,000, and for the remaining two mice it was 10,000.
One of the factors preventing virus infection and subsequent virus production in the lung cells of the PR8αgal-immunized mice is likely the anti-PR8 IgA antibody activity in the lungs. The activity of these antibodies was determined by ELISA, using serial twofold dilutions of the lung homogenate supernatants (Fig. 4C). As shown in Fig. 6D, the lung homogenates from PR8-immunized mice contained no detectable anti-PR8 IgA antibodies, whereas those from all five PR8αgal-immunized mice had distinct anti-PR8 IgA activity. The lack of detectable anti-PR8 IgA antibodies in the lungs of PR8-immunized mice corresponds to the low level of such antibodies in the sera of PR8-immunized mice (Fig. 4C) and suggests that the level of these antibodies in the lungs is beneath the detection level for ELISA.
DISCUSSION
The present study demonstrates a method for increasing the immunogenicity of inactivated influenza virus vaccines by synthesizing α-Gal epitopes on the multiple N-linked carbohydrate chains on envelope glycoproteins of the virus. Injection of inactivated PR8 virus expressing α-Gal epitopes resulted in opsonization by anti-Gal, which in turn targeted the vaccinating virus to APC. This mechanism is illustrated in Fig. 7. The suboptimal uptake of vaccines that lack markers for recognition by APC at the vaccination site is a major limiting factor in their immunogenicity. The present study indicates that anti-Gal-mediated opsonization of the inactivated PR8αgal virus vaccine leads to a marked increase in the cellular immune response. This was shown for four of six PR8αgal-immunized mice, for which the numbers of PR8-specific CD8+ and CD4+ T cells were many times higher than those in PR8-immunized mice (Fig. 2 and 3). Accordingly, the level of anti-PR8 antibody response in the four high responder PR8αgal-immunized mice was >200-fold higher than that for PR8-immunized mice. The increased immune response in PR8αgal-immunized mice correlated with marked resistance to respiratory tract infection with live PR8 virus: ∼90% of PR8αgal-immunized mice versus ∼10% of PR8 immunized mice survived. The present study is the first to demonstrate protection against challenge with a live virus following immunization with an inactivated virus engineered to express α-Gal epitopes. The increased resistance of PR8αgal-immunized mice was also demonstrated by the lower numbers of infectious virus in the lungs of the challenged mice, as assayed 3 days postchallenge.
In previous studies, we demonstrated the increased in vitro uptake of anti-Gal-opsonized PR8 by APC (18). In those studies, expression of α-Gal epitopes on PR8 was achieved by propagating the virus in MDBK (Madin-Darby bovine kidney) and MDCK cells. The endogenous α1,3GT activity in these bovine and canine cells resulted in synthesis of α-Gal epitopes on a portion of the carbohydrate chains on viral envelope glycoproteins transported through the Golgi apparatus. In vitro opsonization of such inactivated PR8 virus by anti-Gal resulted in increased uptake by APC, as indicated by an ∼10-fold increase in the subsequent activation of an HA-specific T-cell clone by processed peptides presented on the APC, compared to the level of activation of these HA-specific T cells in the absence of anti-Gal (18). These in vitro data support the results of the present study, which indicate that this increased uptake by APC results in increased immunogenicity.
The in vitro synthesis of α-Gal epitopes by recombinant α1,3GT demonstrated in the present study is preferable to synthesis by propagation in MDBK or MDCK cells, because the intracellular synthesis of α-Gal epitopes on PR8 is suboptimal, as many of the carbohydrate chains are capped with sialic acid, due to the catalytic activity of sialyltransferase. This enzyme shares a compartment with α1,3GT within the trans-Golgi apparatus; thus, the two enzymes compete with each other for the capping of the carbohydrate chains on glycoconjugates passing through the Golgi apparatus (38). In order to maximize the number of α-Gal epitopes on the influenza virions, the virus can be incubated in vitro with α1,3GT and UDP-Gal. In previous studies on the synthesis of α-Gal epitopes on PR8 with radiolabeled UDP-Gal, we estimated that the de novo expression of α-Gal epitopes is >3,000 per virion (22). Thus, a high concentration of α-Gal epitopes on PR8αgal is likely to result in the binding of many anti-Gal IgG molecules that will effectively target the vaccine virions to APC at the vaccination site.
Our Western blot immunostaining studies (Fig. 1B) suggested that most of the α-Gal epitopes synthesized on PR8 incubated with α1,3GT are present on the carbohydrate chains of HA. These findings are particularly significant for formulating treatment plans to use inactivated virus or recombinant HA vaccines for future pandemics of highly virulent viruses such as H5N1 avian influenza virus converted into a form which is transmitted from human to human. Based on GenBank sequences, H5-HA, like H1-HA, carries carbohydrate chains at six to eight N-glycosylation sites. Our data suggest that influenza virus vaccines comprised of PR8 virus containing the HA-H5 gene of avian influenza virus and processed to express α-Gal epitopes are likely to be much more immunogenic than the same unprocessed vaccine lacking this epitope.
Expression of α-Gal epitopes on recombinant HA-H5 produced in CHO cells may also significantly increase the immunogenicity of such a recombinant vaccine that will be risk free for vaccinees. The immunogenicity of recombinant protein vaccines lacking α-Gal epitopes may be suboptimal because of limited uptake by APC at the vaccination site. Synthesis of α-Gal epitopes on the carbohydrate chains of recombinant H5-HA produced in CHO cells can be achieved by the reaction used in the present study. α-Gal epitope synthesis on the recombinant glycoprotein also requires the addition of neuraminidase to remove sialic acid, as we previously performed with gp120 (1). As a result of such an enzymatic reaction, all the complex carbohydrate chains on recombinant gp120 were capped with α-Gal epitopes to generate gp120αgal. Our in vivo studies with recombinant gp120αgal demonstrated a >100-fold increase in immunogenicity due to anti-Gal-mediated targeting of the vaccine recombinant protein to APC in KO mice (1). Similar studies with recombinant HA expressing α-Gal epitopes will enable us to determine the extent of increased immunogenicity of this recombinant protein and its ability to confer protection against challenge with live PR8 virus. If successful, such studies will indicate that expression of α-Gal epitopes on recombinant envelope proteins is likely to offer an effective alternative to inactivated virus vaccines.
Acknowledgments
The authors thank Kenneth Rock for providing the dendritic cell line DC2.4.
This work was supported in part by NIH grant CA34461 (R.M.W.) and NIH training grant AI07439 (H.M.G.).
Footnotes
Published ahead of print on 3 July 2007.
REFERENCES
- 1.Abdel-Motal, U., S. Wang, S. Lu, K. Wigglesworth, and U. Galili. 2006. Increased immunogenicity of human immunodeficiency virus gp120 engineered to express Galα1-3Galβ1-4GlcNAc-R epitopes. J. Virol. 80:6943-6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottrel, R. L. A., W. O. Dutra, F. A. Martins, B. Gontijo, E. Carvalho, M. Barral-Netto, A. Barral, R. P. Almeida, W. Mayrink, R. Locksley, and K. J. Gollob. 2001. Flow cytometric determination of cellular sources and frequencies of key cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis. Infect. Immun. 69:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Celis, E., and T. W. Chang. 1984. Antibodies to hepatitis B surface antigen potentiate the response of human T lymphocyte clones to the same antigen. Science 224:297-299. [DOI] [PubMed] [Google Scholar]
- 4.Chen, Z. C., M. Tanemura, and U. Galili. 2001. Synthesis of α-Gal epitopes (Galα1-3Galβ1-4GlcNAc-R) on human tumor cells by recombinant α1,3galactosyltransferase produced in Pichia pastoris. Glycobiology 11:577-586. [DOI] [PubMed] [Google Scholar]
- 5.Clynes, R., Y. Takechi, Y. Moroi, A. Houghton, and J. V. Ravetch. 1998. Fc receptors are required in passive and active immunity to melanoma. Proc. Natl. Acad. Sci. USA 95:652-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, B. H., A. H. Cotterell, K. R. McCurry, C. G. Alvarado, J. C. Magee, W. Parker, and J. L. Platt. 1995. Cardiac xenografts between primate species provide evidence for the importance of the α-Galactosyl determinant in hyperacute rejection. J. Immunol. 154:5500-5510. [PubMed] [Google Scholar]
- 7.Couch, R. B., W. A. Keitel, and T. R. Cate. 1997. Improvement of inactivated influenza virus vaccines. J Infect. Dis. 176(Suppl. 1):S38-S44. [DOI] [PubMed] [Google Scholar]
- 8.Deriy, L., H. Ogawa, G. P. Gao, and U. Galili. 2005. In vivo targeting of vaccinating tumor cells to antigen-presenting cells by a gene therapy method with adenovirus containing the α1,3galactosyltransferase gene. Cancer Gene Ther. 12:528-539. [DOI] [PubMed] [Google Scholar]
- 9.Fanger, N. A., K. Wardwell, L. Shen, T. F. Tedder, and P. M. Guyre. 1996. Type I (CD64) and type II (CD32) Fc gamma receptor-mediated phagocytosis by human blood dendritic cells. J. Immunol. 157:541-548. [PubMed] [Google Scholar]
- 10.Galili, U. 1993. Evolution and pathophysiology of the human natural anti-α-Galactosyl IgG (anti-Gal) antibody. Springer Semin. Immunopathol. 15:155-171. [DOI] [PubMed] [Google Scholar]
- 11.Galili, U. 1993. Interaction of the natural anti-Gal antibody with α-Galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol. Today 14:480-482. [DOI] [PubMed] [Google Scholar]
- 12.Galili, U., J. Buehler, S. B. Shohet, and B. A. Macher. 1987. The human natural anti-Gal IgG. III. The subtlety of immune tolerance in man as demonstrated by crossreactivity between natural anti-Gal and anti-B antibodies. J. Exp. Med. 165:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galili, U., M. R. Clark, S. B. Shohet, J. Buehler, and B. A. Macher. 1987. Evolutionary relationship between the natural anti-Gal antibody and the Galα1→3Gal epitope in primates. Proc. Natl. Acad. Sci. USA 84:1369-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galili, U., D. C. LaTemple, and M. Z. Radic. 1998. A sensitive assay for measuring α-Gal epitope expression on cells by a monoclonal anti-Gal antibody. Transplantation 65:1129-1132. [DOI] [PubMed] [Google Scholar]
- 15.Galili, U., B. A. Macher, J. Buehler, and S. B. Shohet. 1985. Human natural anti-α-Galactosyl IgG. II. The specific recognition of α(1-3)-linked galactose residues. J. Exp. Med. 162:573-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galili, U., R. E. Mandrell, R. M. Hamadeh, S. B. Shohet, and J. M. Griffiss. 1988. Interaction between human natural anti-α-Galactosyl immunoglobulin G and bacteria of the human flora. Infect. Immun. 56:1730-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galili, U., E. A. Rachmilewitz, A. Peleg, and I. Flechner. 1984. A unique natural human IgG antibody with anti-α-Galactosyl specificity. J. Exp. Med. 160:1519-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galili, U., P. M. Repik, F. Anaraki, K. Mozdzanowska, G. Washko, and W. Gerhard. 1996. Enhancement of antigen presentation of influenza virus hemagglutinin by the natural human anti-Gal antibody. Vaccine 14:321-328. [DOI] [PubMed] [Google Scholar]
- 19.Galili, U., S. B. Shohet, E. Kobrin, C. L. Stults, and B. A. Macher. 1988. Man, apes, and Old World monkeys differ from other mammals in the expression of α-Galactosyl epitopes on nucleated cells. J. Biol. Chem. 263:17755-17762. [PubMed] [Google Scholar]
- 20.Galili, U., and K. Swanson. 1991. Gene sequences suggest inactivation of α-1,3-Galactosyltransferase in catarrhines after the divergence of apes from monkeys. Proc. Natl. Acad. Sci. USA 88:7401-7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Good, A. H., D. K. Cooper, A. J. Malcolm, R. M. Ippolito, E. Koren, F. A. Neethling, Y. Ye, N. Zuhdi, and L. R. Lamontagne. 1992. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc. 24:559-562. [PubMed] [Google Scholar]
- 22.Henion, T. R., W. Gerhard, F. Anaraki, and U. Galili. 1997. Synthesis of α-Gal epitopes on influenza virus vaccines, by recombinant α1,3galactosyltransferase, enables the formation of immune complexes with the natural anti-Gal antibody. Vaccine 15:1174-1182. [DOI] [PubMed] [Google Scholar]
- 23.Houston, W. E., R. J. Kremer, C. L. Crabbs, and R. O. Spertzel. 1977. Inactivated Venezuelan equine encephalomyelitis virus vaccine complexed with specific antibody: enhanced primary immune response and altered pattern of antibody class elicited. J. Infect. Dis. 135:600-610. [DOI] [PubMed] [Google Scholar]
- 24.Joziasse, D. H., J. H. Shaper, D. H. Van den Eijnden, A. J. Van Tunen, and N. L. Shaper. 1989. Bovine α1-3-Galactosyltransferase: isolation and characterization of a cDNA clone. Identification of homologous sequences in human genomic DNA. J. Biol. Chem. 264:14290-14297. [PubMed] [Google Scholar]
- 25.Katz, J. M., J. Plowden, M. Renshaw-Hoelscher, X. Lu, T. M. Tumpey, and S. Sambhara. 2004. Immunity to influenza: the challenges of protecting an aging population. Immunol. Res. 29:113-124. [DOI] [PubMed] [Google Scholar]
- 26.Keil, W., R. Geyer, J. Dabrowski, U. Dabrowski, H. Niemann, S. Stirm, and H. D. Klenk. 1985. Carbohydrates of influenza virus. Structural elucidation of the individual glycans of the FPV hemagglutinin by two-dimensional 1H n.m.r. and methylation analysis. EMBO J. 4:2711-2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koike, C., J. J. Fung, D. A. Geller, R. Kannagi, T. Libert, P. Luppi, I. Nakashima, J. Profozich, W. Rudert, S. B. Sharma, T. E. Starzl, and M. Trucco. 2002. Molecular basis of evolutionary loss of the α1,3-Galactosyltransferase gene in higher primates. J. Biol. Chem. 277:10114-10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanteri, M., V. Giordanengo, F. Vidal, P. Gaudray, and J. C. Lefebvre. 2002. A complete α,3-Galactosyltransferase gene is present in the human genome and partially transcribed. Glycobiology 12:785-792. [DOI] [PubMed] [Google Scholar]
- 29.Larsen, R. D., C. A. Rivera-Marrero, L. K. Ernst, R. D. Cummings, and J. B. Lowe. 1990. Frameshift and nonsense mutations in a human genomic sequence homologous to a murine UDP-Gal:β-d-Gal(1,4)-d-GlcNAc α(1,3)-Galactosyltransferase cDNA. J. Biol. Chem. 265:7055-7061. [PubMed] [Google Scholar]
- 30.LaTemple, D. C., J. T. Abrams, S. Y. Zhang, and U. Galili. 1999. Increased immunogenicity of tumor vaccines complexed with anti-Gal: studies in knockout mice for α1,3galactosyltransferase. Cancer Res 59:3417-3423. [PubMed] [Google Scholar]
- 31.Manca, F., D. Fenoglio, G. Li Pira, A. Kunkl, and F. Celada. 1991. Effect of antigen/antibody ratio on macrophage uptake, processing, and presentation to T cells of antigen complexed with polyclonal antibodies. J. Exp. Med. 173:37-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto, A., H. Yoshima, and A. Kobata. 1983. Carbohydrates of influenza virus hemagglutinin: structures of the whole neutral sugar chains. Biochemistry 22:188-196. [DOI] [PubMed] [Google Scholar]
- 33.Regnault, A., D. Lankar, V. Lacabanne, A. Rodriguez, C. Thery, M. Rescigno, T. Saito, S. Verbeek, C. Bonnerot, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Fcγ receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization. J. Exp. Med. 189:371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandrin, M. S., H. A. Vaughan, P. L. Dabkowski, and I. F. McKenzie. 1993. Anti-pig IgM antibodies in human serum react predominantly with Gal(α1-3)Gal epitopes. Proc. Natl. Acad. Sci. USA 90:11391-11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuurhuis, D. H., A. Ioan-Facsinay, B. Nagelkerken, J. J. van Schip, C. Sedlik, C. J. M. Melief, J. S. Verbeek, and F. Ossendorp. 2002. Antigen-antibody immune complexes empower dendritic cells to efficiently prime specific CD8+ CTL responses in vivo. J. Immunol. 168:2240-2246. [DOI] [PubMed] [Google Scholar]
- 36.Shen, Z., G. Reznikoff, G. Dranoff, and K. L. Rock. 1997. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 158:2723-2730. [PubMed] [Google Scholar]
- 37.Skehel, J. J., and M. D. Waterfield. 1975. Studies on the primary structure of the influenza virus hemagglutinin. Proc. Natl. Acad. Sci. USA 72:93-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith, D. F., R. D. Larsen, S. Mattox, J. B. Lowe, and R. D. Cummings. 1990. Transfer and expression of a murine UDP-Gal:β-d-Gal-α1,3-Galactosyltransferase gene in transfected Chinese hamster ovary cells. Competition reactions between the α1,3-Galactosyltransferase and the endogenous α2,3-sialyltransferase. J. Biol. Chem. 265:6225-6234. [PubMed] [Google Scholar]
- 39.Tanemura, M., H. Ogawa, D. P. Yin, Z. C. Chen, V. J. DiSesa, and U. Galili. 2002. Elimination of anti-Gal B cells by α-Gal ricin. Transplantation 73:1859-1868. [DOI] [PubMed] [Google Scholar]
- 40.Tanemura, M., D. Yin, A. S. Chong, and U. Galili. 2000. Differential immune responses to α-Gal epitopes on xenografts and allografts: implications for accommodation in xenotransplantation. J. Clin. Investig. 105:301-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tearle, R. G., M. J. Tange, Z. L. Zannettino, M. Katerelos, T. A. Shinkel, B. J. Van Denderen, A. J. Lonie, I. Lyons, M. B. Nottle, T. Cox, C. Becker, A. M. Peura, P. L. Wigley, R. J. Crawford, A. J. Robins, M. J. Pearse, and A. J. d'Apice. 1996. The α-1,3-Galactosyltransferase knockout mouse. Implications for xenotransplantation. Transplantation 61:13-19. [DOI] [PubMed] [Google Scholar]
- 42.Thall, A. D., P. Maly, and J. B. Lowe. 1995. Oocyte Gal α1,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J. Biol. Chem. 270:21437-21440. [DOI] [PubMed] [Google Scholar]
- 43.Unkeless, J. C. 1989. Function and heterogeneity of human Fc receptors for immunoglobulin G. J. Clin. Investig. 83:355-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villinger, F., A. E. Mayne, P. Bostik, K. Mori, P. E. Jensen, R. Ahmed, and A. A. Ansari. 2003. Evidence for antibody-mediated enhancement of simian immunodeficiency virus (SIV) Gag antigen processing and cross presentation in SIV-infected rhesus macaques. J. Virol. 77:10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webster, R. G. 2000. Immunity to influenza in the elderly. Vaccine 18:1686-1689. [DOI] [PubMed] [Google Scholar]
- 46.Zinkernagel, R. M., S. Ehl, P. Aichele, S. Oehen, T. Kundig, and H. Hengartner. 1997. Antigen localisation regulates immune responses in a dose- and time-dependent fashion: a geographical view of immune reactivity. Immunol. Rev. 156:199-209. [DOI] [PubMed] [Google Scholar]