Abstract
The oncogenic human gammaherpesviruses, Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV), are latent in cultured lymphoma cells. We asked whether reactivation from latency of either virus requires de novo protein synthesis. Using Northern blotting and quantitative reverse transcriptase PCR, we measured the kinetics of expression of the lytic cycle activator genes and determined whether abundance of mRNAs encoding these genes from either virus was reduced by treatment with cycloheximide (CHX), an inhibitor of protein synthesis. CHX blocked expression of mRNAs of EBV BZLF1 and BRLF1, the two EBV lytic cycle activator genes, when HH514-16 Burkitt lymphoma cells were treated with histone deacetylase (HDAC) inhibitors, sodium butyrate or trichostatin A, or a DNA methyltransferase inhibitor, 5-Aza-2′-deoxycytidine. CHX also inhibited EBV lytic cycle activation in B95-8 marmoset lymphoblastoid cells by phorbol ester phorbol-12-myristate-13-acetate (TPA). EBV lytic cycle induction became resistant to CHX between 4 and 6 h after application of the inducing stimulus. KSHV lytic cycle activation, as assessed by ORF50 mRNA expression, was rapidly induced by the HDAC inhibitors, sodium butyrate and trichostatin A, in HH-B2 primary effusion lymphoma cells. In HH-B2 cells, CHX did not inhibit, but enhanced, expression of the KSHV lytic cycle activator gene, ORF50. In BC-1, a primary effusion lymphoma cell line that is dually infected with EBV and KSHV, CHX blocked EBV BRLF1 lytic gene expression induced by TPA and sodium butyrate; KSHV ORF50 mRNA induced simultaneously in the same cells by the same inducing stimuli was resistant to CHX. The experiments show, for the cell lines and inducing agents studied, that the EBV BZLF1 and BRLF1 genes do not behave with “immediate-early” kinetics upon reactivation from latency. KSHV ORF50 is a true “immediate-early” gene. Our results indicate that the mechanism by which HDAC inhibitors and TPA induce lytic cycle gene expression of the two viruses differs and suggest that EBV but not KSHV requires one or more proteins to be newly synthesized between 4 and 6 h after application of an inducing stimulus.
In this report we address the question of whether viral genes that regulate the lytic cycles of oncogenic human gammaherpesviruses behave with “immediate-early” kinetics upon reactivation from latency. Classical studies of the temporal pattern of expression of transcripts of bacteriophage T4 defined “early” genes, which were transcribed before DNA replication, and “late” genes, which were transcribed after viral DNA replication (41). Early genes were subdivided into “immediate-early” and “delayed-early” groups. Immediate-early transcripts appeared within 1 min after infection and were synthesized in the presence of chloramphenicol, an inhibitor of protein synthesis. Immediate-early, but not delayed-early, viral transcripts could be synthesized in vitro from DNA which was mechanically disrupted by shearing or sonication. This result implied that immediate-early genes were encoded by a limited region of the genome, whereas delayed-early genes were more diffusely distributed on the genome.
Three temporal groups of viral polypeptides, designated alpha, beta, and gamma, corresponding to immediate-early, delayed-early, and late gene products, were defined in cells infected with herpes simplex virus (24). The alpha group of proteins was synthesized at the highest rates 3 to 4 h after infection. Alpha polypeptides were made immediately following release of a blockade of protein synthesis by cycloheximide (CHX), an inhibitor of eukaryotic protein synthesis. Transcripts of the immediate-early genes, synthesized in the presence of CHX, hybridized to limited regions of the herpes simplex virus genome, again indicating that they were a subset of early genes (12).
For a viral gene to be transcribed in the presence of an inhibitor of protein synthesis and be classified as immediate-early, transcription factors that positively regulate viral gene expression must either preexist in uninfected cells or be packaged in virions that infect the cells. Herpes simplex virus packages a transactivator protein, variably called alpha trans inducing factor or viral protein 16, which interacts with preexisting cellular proteins, Oct 1 and host cell factor, to positively regulate genes of the immediate-early class (3, 9, 28, 31, 45).
Both Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV) encode lytic cycle activator genes which control the transition from latency to the lytic cycle. Two genes of EBV, BZLF1 and BRLF1, encode multifunctional proteins (ZEBRA and Rta) which together are responsible for activating viral delayed-early gene expression, lytic viral DNA replication, and late gene expression (13, 14, 23, 46, 61). The positional and functional homologue of EBV BRLF1 in KSHV, namely ORF50, regulates early gene transcription, and the product of KSHV ORFK8, the homologue of EBV BZLF1, functions primarily in DNA replication (22, 35, 39, 52). The lytic cycle activator genes of both viruses are repressed during latency.
The switch between latency and the lytic cycle of EBV and KSHV can be envisioned in two main phases: “upstream” events that lead to derepression and expression of the virally encoded lytic cycle activator genes and “downstream” events that result from a multitude of actions triggered by the products of the lytic cycle activator genes. Many chemical and biological stimuli can trigger upstream events. These include phorbol esters which are protein kinase C agonists, histone deacetylase (HDAC) inhibitors, DNA methyltransferase inhibitors, and anti-immunoglobulin (5, 15, 38, 54, 63).
Three unsolved mysteries about upstream events are suggested by this partial list of lytic cycle inducing agents. First, the agents work by different mechanisms, and it is unclear whether a final pathway is common to all lytic cycle inducing stimuli. Second, the virus latent in different cell backgrounds may be activated by a subset or by none of the inducing stimuli (21). Third, even in a responsive cell line, only a subpopulation of cells treated with an inducing agent responds (6). It remains unclear what determines susceptibility or refractoriness to lytic cycle induction.
The question of whether EBV BZLF1 and BRLF1 are immediate-early genes has been investigated under two different experimental conditions: infection with the virus and reactivation from latency. The results have been conflicting. When Raji cells were superinfected with EBV strain P3J-HR-1, the BZLF1 and BRLF1 mRNAs were classified as immediate-early since they were detected 12 h after superinfection of cells treated with anisomycin, an inhibitor of protein synthesis (7). However, the P3J-HR-1 virus stocks contained defective viral genomes (so-called “het DNA”) in which the EBV BZLF1 and BRLF1 genes were rearranged so that they were positioned next to constitutively active latency promoters (11, 14, 27). It was likely that the BZLF1 and BRLF1 mRNAs, classified as immediate-early, were encoded by these defective viral genomes. Therefore, this experiment did not assess the kinetic class of BZLF1 and BRLF1 in the configuration of a standard viral genome (57). However, in recently published experiments, the BZLF1 gene behaved with immediate-early kinetics when fluorescent marked virus harvested from Akata cells was used to infect primary B cells and EBV-negative B-cell lines (58).
Reactivation from latency has been studied with two cell lines derived from Burkitt lymphoma, Raji and Akata (1, 32, 56). When Raji cells were exposed to phorbol-12-myristate-13-acetate (TPA), expression of all viral lytic cycle genes that were induced by this treatment was inhibited by CHX when the mRNAs were analyzed 8 h after treatment. Thus, no viral genes could be classified as immediate-early upon reactivation from latency in Raji cells (32).
In Akata Burkitt lymphoma cells, the EBV lytic cycle is inducible by cross-linking surface immunoglobulin G (IgG) with anti-Ig. BZLF1 mRNA can be detected within 2 to 4 h after this treatment, which mimics activation of the B-cell receptor. Two studies concluded that BZLF1 and BRLF1 were immediate-early genes, while a third study showed that expression of these genes was blocked by inhibitors of protein synthesis. In the first study, BZLF1 mRNA was detected in Akata cells 6 h after treatment with anti-Ig and CHX (56). In the second study, BZLF1 and BRLF1 mRNAs were present, though decreased in abundance, 4 h and 20 h after anti-Ig-plus-anisomycin treatment of Akata cells (44). When examined in parallel, the mRNAs of the BMLF1 and BALF2 delayed-early genes were inhibited by anisomycin. The third similar study found that BZLF1 and BRLF1 mRNA induction was inhibited by a combination of anisomycin and CHX, an effect the authors attributed to inhibition of production of the ZEBRA and Rta proteins and a concomitant decrease in autostimulation and cross-stimulation of the BZLF1 and BRLF1 genes (19).
More recently the problem has been studied with stable lines of Akata cells containing extrachromosomal reporter plasmids in which Zp and Rp, the promoters of the BZLF1 and BRLF genes, were linked to luciferase (1, 8). Zp but not Rp could be activated by anti-Ig plus anisomycin, suggesting that BZLF1 but not BRLF1 was immediate-early. However, in the same group of experiments, BRLF1 mRNA from the EBV genome in Akata cells was synthesized in the presence of anisomycin.
These conflicting results could be explained by effects attributable to the cell backgrounds or to the specific lytic cycle-inducing stimulus, by the type and dose of the inhibitor of protein synthesis, by the time at which the effects on BZLF1 and BRLF1 mRNA abundance was assessed, and by the assays employed.
Lytic cycle activation of KSHV by chemical stimuli can be experimentally manipulated in cultured B-cell lines derived from primary effusion lymphoma (PEL) (10, 43, 48, 53). Nearly all PEL cell lines contain KSHV; some are dually infected with KSHV and EBV. Lytic cycle induction of KSHV has been examined in some detail with three PEL cell lines. These include BC-1, a dually infected cell line (10), and HH-B2 (20) and BCBL-1 (29, 48), which harbor only KSHV. The lytic cycles of both KSHV and EBV can be induced in BC-1 cells by TPA or sodium butyrate (NaB). TPA preferentially activates EBV, and NaB preferentially stimulates KSHV lytic gene expression (42). The 3.6-kb transcript of the KSHV ORF50 gene was originally defined as immediate-early in BC-1 cells. It was induced within 4 h after treatment with NaB, and its expression was resistant to CHX (52, 53). Transcripts of the KSHV K8 gene, the homologue of the EBV BZLF1 gene, appeared later, at 8 h, and were inhibited by CHX (53). Other KSHV immediate-early transcripts were identified in BC-1 cells treated with a combination of NaB and CHX by means of a cDNA subtraction strategy (62). These included a 2.0-kb mRNA containing ORFK4.2 and a 1.7-kb mRNA containing ORF45 (62).
The 3.6-kb ORF50 mRNA also behaved with immediate-early kinetics in HH-B2 cells. Eight hours after treatment of HH-B2 cells with NaB, the abundance of this mRNA was increased in the presence of CHX (20). By means of transient transfections of HH-B2 cells with reporter constructs, the butyrate-responsive region of the ORF50 promoter was mapped to an Sp1/Sp3 site, located 103 to 112 nucleotides upstream of the translational start site of the ORF50 protein (60). TPA activated the entire KSHV lytic cycle in BCBL-1 cells, leading to production of nucleocapsids and the release of encapsidated viral DNA (48). However, the effects of inhibiting protein synthesis with CHX on KSHV lytic cycle activation by TPA have not been described for any PEL cell line, possibly because of the toxicity of this combination of chemicals (62).
Studies with two PEL cell lines, BC-1 and HH-B2, showed that ORF50 expression behaved with immediate-early kinetics following KSHV reactivation induced by NaB. Since no comparable studies had been published examining the requirement for protein synthesis for EBV lytic cycle activation induced by HDAC inhibitors, we initially asked whether de novo protein synthesis was required for EBV lytic cycle induction following treatment with two HDAC inhibitors, NaB, a short chain fatty acid, and trichostatin A (TSA), an antifungal antibiotic. After we discovered that EBV reactivation induced by HDAC inhibitors required protein synthesis, we demonstrated a requirement for protein synthesis for EBV lytic induction by a DNA methyltransferase inhibitor and phorbol esters. We determined the interval of time after application of the inducing stimulus when new proteins needed to be made. We compared the requirements for protein synthesis for KSHV and EBV reactivation in cultured B-cell lines latently infected with one of the two viruses. We also directly compared the requirements for protein synthesis for KSHV and EBV reactivation in dually infected BC-1 cells. Since the experiments clearly indicated that EBV but not KSHV requires protein synthesis for lytic cycle gene expression, the results suggest that the pathways of reactivation from latency of the two viruses differ.
MATERIALS AND METHODS
Cell lines.
KSHV-infected PEL cell lines, HH-B2 and BC-1, were maintained in RPMI 1640 supplemented with 15% fetal calf serum. EBV-infected HH514-16 Burkitt lymphoma cells and B95-8 marmoset lymphoblastoid cells were cultured in RPMI 1640 supplemented with 8% fetal calf serum. All cultures were treated with penicillin (50 U/ml), streptomycin (50 U/ml), and amphotericin B (1 μg/ml). Cells were grown at 37°C under 5% CO2.
Reagents.
NaB, purchased from Sigma, was used at 3 mM. TSA, from Wako, was used at 5 μM. 5-Aza-2′-deoxycytidine (5Aza2′dC), from Sigma, was used at 5 μM. TPA, purchased from CalBiochem, was used at 20 ng/ml. CHX, purchased from CalBiochem, was used at 33 μg/ml. All chemical doses were determined to be in the optimal range for their effects.
Northern blots.
Cell pellets were collected at intervals up to 8 h after application of a lytic cycle-inducing stimulus. Total RNA was isolated from 1.5 × 107 HH514-16, B95-8, HH-B2, or BC-1 cells using the “RNeasy Mini” kit (QIAGEN no. 74104). RNA was quantified by UV spectrometry. Twenty micrograms of RNA per lane was fractionated on a 1% formaldehyde agarose gel and transferred to nylon membranes (HYBOND-N+; Amersham Biosciences). Transfer and hybridization were done as described previously (30) with one or more of the following probes: Z(301), a 301-bp PCR product of BZLF1 using primers 5′-GGCAGTTAGCTGGCCTTGTGGCAGAGGCTC-3′ and 5′-AACCATGACATCACAGAGGAGGCTGGTGCC-3′, encompassing EBV nucleotides (nt) 102927 to 103227; KSR(243), a 243-bp PCR product of KSHV ORF50 using primers 5′-ATCATCCCCTTTAACCCATCG-3′ and 5′-TGCAGGAGTGGACGCTGAC-3′, encompassing KSHV nt 73521 to 73763; KSR(339), a 339-bp PCR product of KSHV ORF50 using primers 5′-GAAAGCTTCGTCGGCCTCTCGGAC-3′ and 5′GTCAGAAATGCCTTTTCTGTGC3′, encompassing KSHV nt 72599 to 72937; RNaseP HI, a 350-bp NcoI-to-PstI fragment of cDNA from the HI RNA component of human RNaseP (2). The probes were radiolabeled with 32P by the random-prime method.
Real-time quantitative reverse transcription (RT)-PCR.
Each RNA sample was treated with 30 U DNase I, using the “RNase-Free DNase” set (QIAGEN). A 0.2-μg amount of total RNA was used as a template for real-time RT-PCR to amplify a 115-bp fragment from KSHV ORF50 mRNA or a 160-bp fragment from EBV BRLF1 mRNA using the “SuperScript One-step” RT-PCR system (Invitrogen). The ORF50 primer pair was as follows: forward, 5′-GCAGCCACAAAAATGGCGCAAGATG-3′; reverse, 5′-GGAGCACACACTGGTAGAGTTGG-3′. This primer pair spans the only intron of the ORF50 gene so that the PCR product could be differentiated from amplification of genomic DNA (∼1.1 kbp). The primer pair for EBV BRLF1 also spans the only intron: forward, 5′-CCATACAGGACACAACACCTCA-3′ (EBV nt 106144 to 106165); reverse, 5′-ACTCCCGGCTGTAAATTCCT-3′ (EBV nt 105061 to 105080). PCRs were quantitated on a Cepheid Smart Cycler II real-time PCR machine using the CYBRgreen incorporation detection method. Two standards were used to generate standard curves to quantitate the BRLF1 and ORF50 mRNAs: serial dilutions of total RNA from NaB-treated HH514-16 and HH-B2 cells and serial dilutions of gel-purified 115-bp ORF50 and 160-bp BRLF1 cDNA fragments.
Southern blots.
Twenty-four hours after chemical treatment, total cellular DNA was isolated as described previously (49). The DNA was digested with BamHI (for EBV) and SacI (for KSHV). Ten micrograms of DNA per lane was electrophoresed and transferred to nitrocellulose filters. The blots were hybridized in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M Na citrate), 0.1% Denhardt's solution, 0.5% sodium dodecyl sulfate (SDS), and 100 μg/ml of salmon sperm DNA at 65°C overnight using double-stranded random-primed 32P-labeled probes of KSR(339) or excised EBV BamHI W fragment. After hybridization, the blots were washed sequentially in 2× SSC, 0.5% SDS at 65° for 10 to 15 min, in 0.1× SSC, 0.5% SDS one to three times at 65° for 10 min each, and in 0.1× SSC for 5 min at room temperature. Radioactive bands were visualized by exposure of the blot to Kodak XAR film.
Immunoblots.
BC-1 cells were untreated or treated with NaB or TPA with or without addition of CHX. After 24 h of incubation at 37°C, cell extracts were prepared, electrophoresed in SDS-10% polyacrylamide gels, and transferred to nitrocellulose. The immunoblots were reacted with 1:100 dilutions in skim milk of monospecific rabbit antibodies to EBV Rta (amino acids 1 to 320) (46) and KSHV ORF50 (amino acids 351 to 691) (60), followed by 125I-labeled protein A. Mouse antibody to β-actin (A5316; Sigma) was used to control for protein loading.
RESULTS
Induction of EBV lytic gene expression in HH514-16 cells is inhibited by CHX.
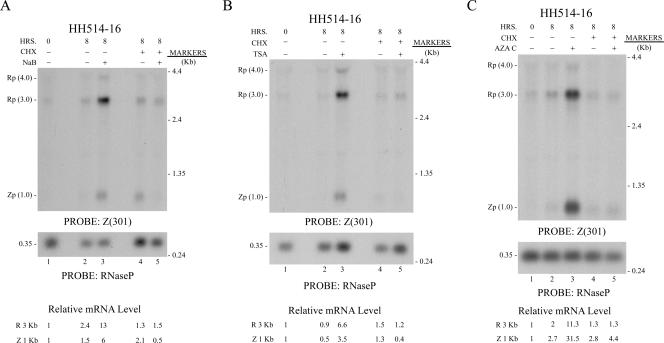
In HH514-16, a subclone of the P3J-HR1 Burkitt lymphoma cell line, there is minimal spontaneous EBV lytic gene expression and a robust response to a subset of lytic cycle activation stimuli (20, 46). The EBV lytic cycle can be induced in this cell line by HDAC inhibitors, such as NaB and TSA, as well as by the DNA methyltransferase inhibitor 5Aza2′dC (Fig. 1). EBV lytic cycle activation is evidenced by the appearance of transcripts encoding the EBV lytic cycle activator genes, BRLF1 and BZLF1. These transcripts are readily detectable by Northern blotting when cells are harvested 8 h after application of the inducing chemical (Fig. 1). All three inducing agents caused about a sixfold increase in BRLF1 mRNA compared to levels in untreated cells also examined at 8 h. The two HDAC inhibitors induced a four- to sevenfold increase in BZLF1 mRNA. 5Aza2′dC reproducibly activated expression of BZLF1 mRNA to a higher level (11.7-fold) than BRLF1 mRNA (5.6-fold) (Fig. 1C; also data not shown).
FIG. 1.
CHX inhibits induction of EBV BRLF1 and BZLF1 mRNAs by HDAC inhibitors and a DNA methyltransferase inhibitor in HH415-16 cells. Cells were untreated (−) or treated (+) for 8 h with chemicals to induce the EBV lytic cycle, NaB (A), TSA (B), or 5Aza2′dC (AZA C) (C). CHX was added to one set of untreated or chemically treated cultures. Total cellular RNA was prepared at 0 h or 8 h. A Northern blot was probed with Z(301), which detects both BRLF1 (Rp) and BZLF1 (Zp) mRNAs. The blots were reprobed for RNase P to control for RNA loading. The relative levels of the BRLF1 3.0-kb and BZLF1 1.0-kb mRNAs were determined by densitometry and corrected for RNase P expression.
Addition of CHX, together with the inducing chemical, markedly reduced the response to all three inducing stimuli. CHX inhibited the response by 85% to 90% and equally affected both BRLF1 and BZLF1 mRNA abundance. These observations suggested that de novo protein synthesis was required for appearance of stable BRLF1 and BZLF1 mRNAs in HH514-16 cells treated with two HDAC inhibitors and a DNA methyltransferase inhibitor.
Quantitation of inhibitory effect of CHX on EBV BRLF1 expression.
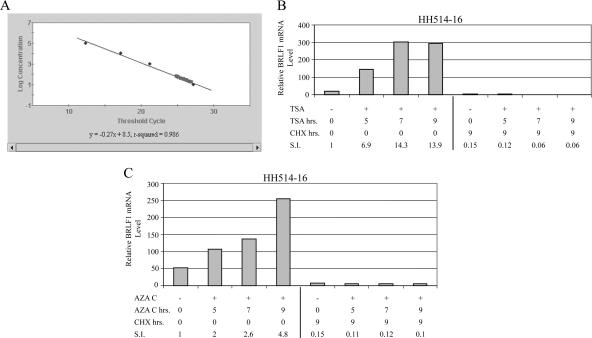
Quantitative RT-PCR (QRT-PCR) was used to measure the abundance of BRLF1 mRNA following exposure of HH514-16 cells to TSA (Fig. 2B) or 5Aza2′dC (Fig. 2C) for various durations. The response to TSA was more rapid and robust than the response to 5Aza2′dC. After 9 h of treatment, BRLF1 was induced 14-fold by TSA and approximately 5-fold by 5Aza2′dC. CHX abolished the response to both inducing chemicals. CHX also reduced the basal level of BRLF1 mRNA to 15% of that for the untreated control (Fig. 2B and C).
FIG. 2.
Quantitation of the inhibitory effect of CHX on EBV lytic cycle induction by real-time reverse transcriptase PCR(QRT-PCR). (A) Standard curve for QRT-PCR detection of BRLF1 mRNA. (B and C) HH514-16 cells were untreated (−) or treated (+) for various lengths of time with EBV lytic cycle inducing agents, either TSA (B) or 5Aza2′dC (AZA C) (panel C). One set of cultures exposed to the lytic cycle inducing agent for different lengths of time was also treated with CHX. RNA was harvested 9 h after the start of the experiment. The relative levels of BRLF1 mRNA were measured by QRT-PCR. S.I., stimulation index.
EBV lytic gene expression becomes resistant to CHX after an initial period of exposure to the inducing agent.
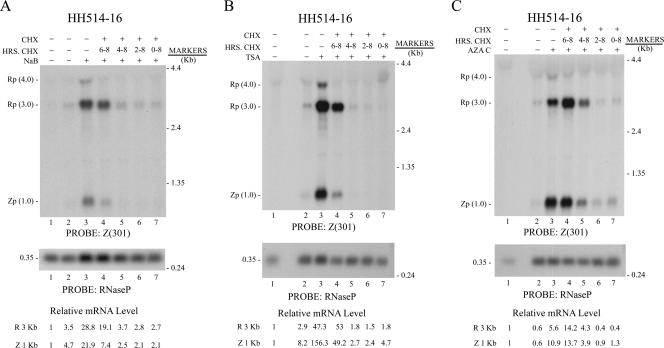
The experiments of Fig. 1 and 2 showed that EBV lytic activation was sensitive to CHX when the inhibitor was present for the entire duration of exposure to the inducing stimulus. The next experiments defined the time period after exposure of HH514-16 cells to the inducing chemical during which EBV BRLF1 and BZLF1 mRNA expression was sensitive to inhibition by CHX (Fig. 3). HH514-16 cells were exposed to NaB, TSA, or 5Aza2′dC; CHX was added together with the inducing agent or at 2-h intervals after exposure to the inducing chemicals (Fig. 3A, B, and C, respectively). The abundance of mRNAs of EBV lytic cycle activator genes was measured by Northern blotting of RNA harvested at 8 h. The expression of BRLF1 and BZLF1 mRNAs became independent of new protein synthesis when CHX was added in the last 2 h of treatment with the HDAC inhibitors (Fig. 3A and B, lane 4). Resistance to CHX appeared sooner, between 2 and 4 h after treatment with 5Aza2′dC (Fig. 3C, compare lanes 5 and 6). The BRLF1 and BZLF1 mRNAs in 5Aza2′dC-induced cells also treated for 2 h with CHX were more abundant than in 5Aza2′dC-treated cells which did not receive the inhibitor of protein synthesis (Fig. 3C, compare lanes 3 and 4). This result suggested that once the BRLF1 and BZLF1 mRNAs are expressed, CHX may stabilize them. The enhancing effect of CHX was reproducibly more pronounced with the 3.0-kb BRLF1 mRNA than with the 1.0-kb BZLF1 mRNA (Fig. 3C; also data not shown). These findings indicated that newly synthesized proteins were required to mediate lytic cycle induction in HH514-16 cells by the HDAC inhibitors and 5Aza2′dC. These newly made proteins are already present by 4 to 6 h after exposure to the inducing stimuli, since addition of CHX after this time no longer inhibits the appearance of the BRLF1 and BZLF1 mRNAs.
FIG. 3.
Determination of time after addition of the inducing agent when CHX inhibits EBV lytic cycle activation in HH514-16 cells. Cells were untreated (lanes 1 and 2) or treated (lanes 3 to 7) with NaB (A), TSA (B), or AZAC (C). CHX was added with the inducing agent or at 2-h intervals thereafter. RNA was prepared at 0 h (lane 1) or 8 h (lanes 2 to 7) after the start of the experiment. Northern blots were probed with Z(301); mRNA abundance was quantitated by densitometry. Rp, BRLF1; Zp, BZLF1.
Lytic cycle induction by phorbol esters in B95-8 cells is also sensitive to inhibition by CHX.
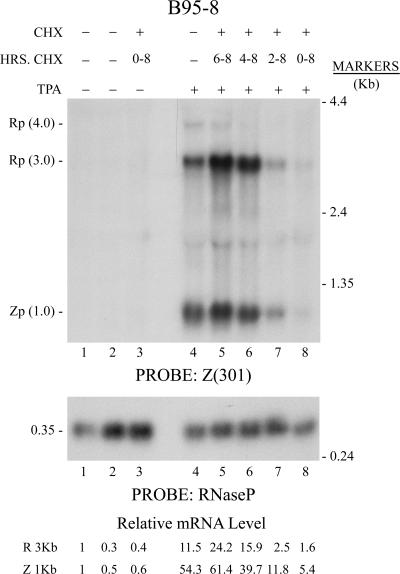
HDAC inhibitors and DNA methyltransferase inhibitors, such as 5Aza2′dC, may affect EBV lytic gene expression by promoting epigenetic alterations such as histone modifications and inhibition of DNA methylation on the promoters of BZLF1 and BRLF1 (17, 26). The EBV lytic cycle in B95-8 cells is not activated by either of these two classes of agents, but EBV can be induced into the lytic cycle by phorbol esters, such as TPA, which activate the protein kinase C signal transduction cascade (21). To determine whether lytic cycle activation by this signaling pathway was also dependent on new protein synthesis, an experiment, similar to that illustrated in Fig. 3, was conducted; CHX was added at intervals after TPA treatment of B95-8 cells (Fig. 4). Inclusion of CHX for the 8-h duration of the experiment inhibited the activation of BZLF1 and BRLF1 by TPA by 85% to 90%. In B95-8 cells, the BZLF1 and BRLF1 mRNAs became resistant to the inhibitory action of CHX between 2 and 4 h after exposure to TPA (Fig. 4, compare lanes 6 and 7). A 2-h exposure to CHX in the presence of TPA increased the abundance of the BRLF1 and BZLF1 mRNAs (Fig. 4, compare lanes 4 and 5). The time at which resistance to CHX appeared and the relative levels of BRLF1 and BZLF1 mRNAs were similar following treatment of B95-8 cells with TPA and following treatment of HH514-16 cells with 5Aza2′dC (compare Fig. 3C and Fig. 4).
FIG. 4.
CHX inhibits EBV early lytic cycle mRNA expression in B95-8 cells treated with phorbol ester. B95-8 cells received (+) (lanes 4 to 8) or did not receive (−) (lanes 1 to 3) TPA. CHX was added for the durations indicated (0 to 8 h). Total cellular RNA was harvested at 0 h (lane 1) or 8 h (lanes 2 to 8). Northern blots were probed with Z(301) and quantitated by densitometry. Rp, BRLF1; Zp, BZLF1.
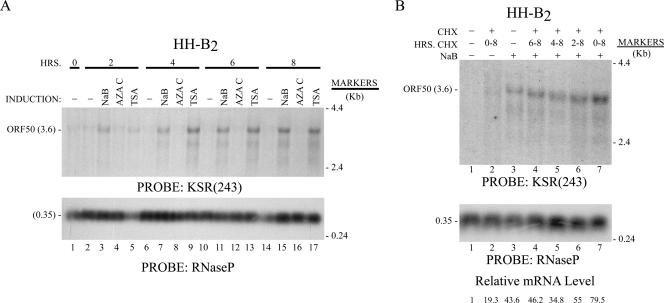
Induction of KSHV ORF50 mRNA in the HH-B2 PEL cell line by HDAC inhibitors is resistant to inhibition by CHX.
The results in Fig. 1 to 4 showed that induction of the mRNAs of EBV lytic gene activators, BRLF1 and BZLF1, by agents thought to work by different mechanisms, namely, HDAC inhibitors, DNA methyltransferase inhibitors, and protein kinase C agonists, was sensitive to CHX. This result was surprising, since our previous experiments showed that induction of the mRNA of the ORF50 gene by NaB was resistant and in some cells enhanced in the continuous presence of CHX (20, 53, 60). We reexamined the effects of inhibiting protein synthesis on lytic cycle activation of KSHV with the same reagents and in parallel with the studies of EBV reactivation (Fig. 5). We also studied the kinetics of ORF50 activation. In HH-B2 cells, ORF50 mRNA was activated by both HDAC inhibitors but not by 5Aza2′dC. The HDAC inhibitors worked rapidly. NaB stimulated the 3.6-kb ORF50 mRNA threefold, and TSA activated its expression eightfold, as assessed by Northern blotting of RNAs harvested 4 h after exposure of the cells to the inducing agents (Fig. 5A, lanes 7 and 9; also data not shown). In HH-B2 cells, CHX treatment increased the abundance of the ORF50 mRNA. When CHX was present continuously together with NaB, ORF50 mRNA was approximately twofold more abundant than when cells were treated with NaB but not with CHX. CHX also enhanced expression of ORF50 mRNA in HH-B2 cells that were not exposed to NaB (Fig. 5B, lane 2).
FIG. 5.
Activation of KSHV ORF50 mRNA in HH-B2 cells by NaB is resistant to CHX. (A) Kinetics of activation ORF50 mRNA in HH-B2 cells by two HDAC inhibitors. RNA prepared at the times indicated after exposure to NaB, AZAC, or TSA was analyzed by Northern blotting with the probe KSR(243), which detects the 3.6-kb ORF50 mRNA (52). (B) HH-B2 cells were untreated (lanes 1 and 2) or treated with NaB (lanes 3 to 7). CHX was added for the durations indicated. RNA harvested at 8 h was analyzed by Northern blotting with the KSR(243) probe.
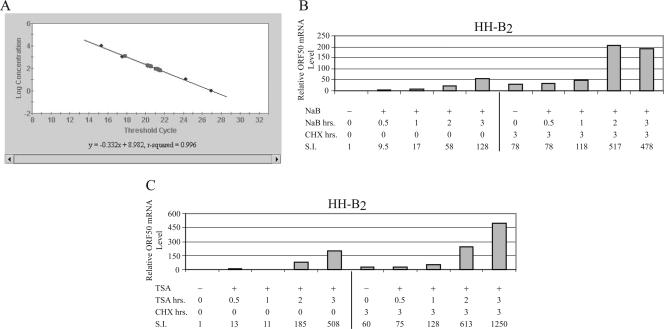
QRT-PCR was used to finely map the kinetics of the response of ORF50 mRNA to the HDAC inhibitors and to quantitate the effects of CHX (Fig. 6). Ten- to 13-fold increases in ORF50 mRNA abundance could be detected with as little as 0.5 h of exposure to NaB (Fig. 6B) or TSA (Fig. 6C). A 3-h exposure to the HDAC inhibitors caused marked amplification of ORF50 mRNA, 128-fold by NaB and 508-fold by TSA, with respect to untreated cells. With each duration of exposure to the HDAC inhibitors, there was significant enhancement of ORF50 mRNA levels in the presence of CHX. This enhancement by CHX varied between 2.4- and 11.6-fold.
FIG. 6.
CHX enhances expression of ORF50 mRNA in HH-B2 cells treated with HDAC inhibitors. (A) Standard curve for QRT-PCR detection of ORF50 mRNA. (B and C) HH-B2 cells were untreated (−) or treated (+) with NaB (B) or TSA (C) for different durations. One set of cultures received CHX for the duration of the experiment (3 h). RNA harvested at 3 h was analyzed by QRT-PCR.
Differential effects of CHX on EBV and KSHV early viral lytic gene expression induced by TPA in BC-1 PEL cells dually infected with both viruses.
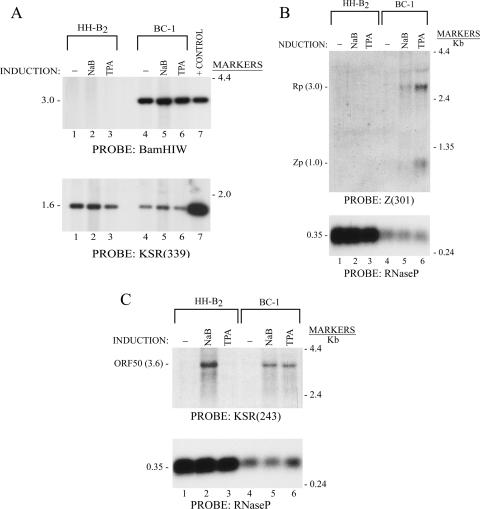
The previous experiments (Fig. 1 to 6) showed that CHX inhibited lytic cycle activation of EBV by HDAC inhibitors and TPA, while the protein synthesis inhibitor enhanced expression of the KSHV lytic cycle activator genes induced by HDAC inhibitors. A limitation of these experiments was that the effects of CHX on lytic cycle reactivation of the two viruses were studied with different cell backgrounds. PEL cell lines which are dually infected with EBV and KSHV offer an experimental system with which the lytic cycle reactivation of two viruses can be studied simultaneously (42). BC-1 is one such cell line (10). Some laboratory stocks of the BC-1 line contained both EBV and KSHV DNA, as detected by Southern blotting (Fig. 7A), although in other BC-1 cell stocks, EBV DNA could not be detected (data not shown). EBV BRLF1 and BZLF1 mRNAs were induced by NaB and by TPA; TPA had the greater effect (Fig. 7B). The same chemicals also induced expression of ORF50 mRNA (Fig. 7C). The HH-B2 cell line, studied in parallel, did not contain EBV DNA detectable by Southern blotting (Fig. 7A), nor did EBV lytic cycle transcripts appear following exposure to NaB or TPA (Fig. 7B). KSHV lytic gene expression in HH-B2 cells did not respond to TPA but was strongly activated by NaB (Fig. 7C, lane 2).
FIG. 7.
NaB and TPA activate EBV and KSHV lytic gene expression in dually infected BC-1 cells. (A) Southern blot of DNA from HH-B2 or BC-1 cells probed for EBV and KSHV DNA sequences. Probe for EBV DNA was BamHI W. Probe for KSHV DNA was KSR(339). (B) Northern blot for detection of EBV BRLF1 and BZLF1 mRNAs with the Z(301) probe. (C) Northern blot for detection of KSHV ORF50 mRNA with KSR(243) probe.
Figure 8 illustrates an experiment in which the effects of CHX on lytic cycle induction of EBV and KSHV in BC-1 cells were examined. The cells were untreated or treated with TPA, which activates both viruses (Fig. 7). One aliquot of cells that did not receive TPA was treated with CHX. CHX was also added to the TPA-treated cells for different durations. RNA harvested from the cells after 8 h was analyzed by Northern blotting, with probes for EBV BRLF1 and KSHV ORF50.
FIG. 8.
Differential effect of CHX on EBV BRLF1 and KSHV ORF50 mRNA levels in BC-1 cells treated with TPA. BC-1 cells were untreated (−) or treated (+) with TPA. CHX was added for the indicated durations, from 2 to 8 h before harvesting of RNA. RNA was prepared at 8 h. Northern blots were probed with Z(301) (A) or KSR(243) (B).
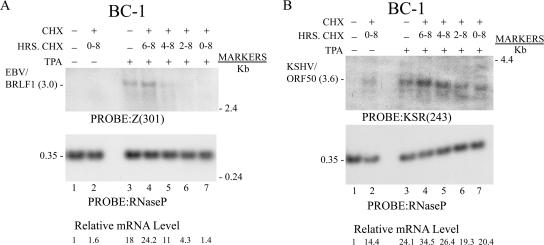
EBV BRLF1 expression was inhibited when CHX was added together with TPA or 2 and 4 h after addition of TPA (Fig. 8A, lanes 5, 6, and 7). Thereafter, BRLF1 mRNA was resistant to CHX or slightly enhanced in the presence of the inhibitor of protein synthesis. In this experiment, the BZLF1 mRNA could not be detected by Northern blotting, even after prolonged exposure of the autoradiograph. At no time in TPA-treated BC-1 cells did CHX decrease ORF50 mRNA abundance. In fact, KSHV ORF50 mRNA expression was increased by treating cells with CHX alone (Fig. 8B, lane 2). A short, 2-h exposure to CHX in the presence of TPA enhanced ORF50 expression about 1.5-fold relative to that for cells treated with TPA alone (Fig. 8B, compare lanes 3 and 4).
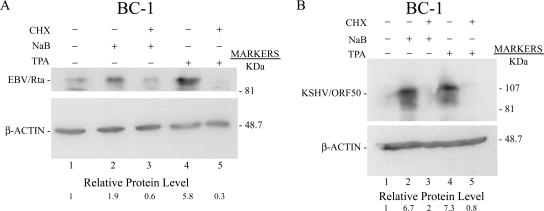
Other aliquots of the same BC-1 cells which were exposed to TPA and NaB in the absence or presence of CHX, a portion of which were used in the Northern blot experiment illustrated in Fig. 8, were analyzed for expression of the EBV Rta and KSHV ORF50 proteins by immunoblotting after 24 h (Fig. 9). The EBV Rta protein was spontaneously expressed at a low level, minimally (1.9×) induced by NaB, and more markedly (5.8×) induced by TPA (Fig. 9A). These results at the protein level were consistent with the greater stimulatory effect of TPA than of NaB on EBV BRLF1 mRNA levels in BC-1 cells. CHX reduced NaB induction of EBV Rta below background levels and abolished TPA induction of the Rta protein. TPA and NaB were equally active at inducing ORF50 protein expression (Fig. 9B). This result was consistent with results (Fig. 7C) showing that both agents were approximately equivalent in their capacity to induce ORF50 mRNA (Fig. 7C). CHX markedly reduced ORF50 protein expression, regardless of the inducing stimulus.
FIG. 9.
CHX inhibits expression of the EBV Rta and KSHV ORF50 proteins in BC-1 cells. Expression of EBV Rta and KSHV ORF50 proteins was analyzed by immunoblotting of cell extracts prepared in the same experiment (Fig. 8) in which the analysis of EBV BRLF1 and ORF50 mRNA by Northern blotting was studied. Cells were untreated or treated with the inducing agents, NaB or TPA. CHX was added to one aliquot of cells together with the inducing agent. Cell extracts were prepared 24 h after induction. Immunoblots were probed with monospecific polyclonal rabbit antibodies to the EBV Rta (A) or KSHV ORF50 (B) protein.
The experiments of Fig. 8 and 9 showed that while CHX differed in its ability to inhibit EBV BRLF1 and KSHV ORF50 mRNAs induced by TPA, CHX was equally effective at inhibiting EBV Rta and KSHV ORF50 protein expression induced by TPA.
Effects of CHX on EBV and KSHV lytic cycle induction by NaB in BC-1 cells.
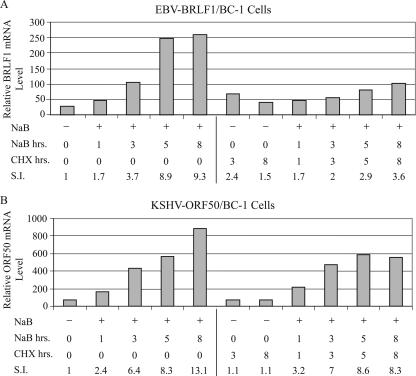
The abundance of EBV BZLF1 and BRLF1 mRNAs induced by NaB in BC-1 cells was too low to be able to use Northern blotting to quantitate their response in the presence of a protein synthesis inhibitor. Therefore, the effects of CHX on induction of EBV BRLF1 and KSHV ORF50 lytic cycle mRNAs by NaB in BC-1 cells was studied by using QRT-PCR (Fig. 10). CHX inhibited the level of EBV BRLF1 mRNA induced by NaB at 3 h and 5 h by 46% and 68%, respectively. CHX produced no inhibitory effects on the ORF50 mRNA level measured 3 h or 5 h after addition of NaB.
FIG. 10.
Quantitation of effects of CHX on EBV BRLF1 mRNA and KSHV ORF50 mRNA expression in BC-1 cells treated with NaB. BC-1 cells were treated with NaB for the indicated times. RNA was harvested at 1, 3, 5, and 8 h after treatment. One aliquot of cells received CHX simultaneously with NaB. The content of EBV BRLF1 mRNA (A) or KSHV ORF50 mRNA (B) was measured by QRT-PCR. The stimulation index (S.I.) was calculated relative to the content of RNA in untreated cells harvested at time zero.
Thus, in BC-1 cells, in which the lytic cycle of two different gammaherpesviruses was activated by two different inducing stimuli, the EBV BRLF1 mRNA was relatively sensitive to inhibition by CHX and the ORF50 mRNA level was relatively resistant. These results were validated by two different readout assays, Northern blotting and quantitative RT-PCR.
DISCUSSION
Using expression of the EBV BZLF1 and BRLF1 and KSHV ORF50 genes, which encode trans-acting factors that regulate early viral genes and DNA replication, as markers for lytic cycle activation, we found that new protein synthesis was required for lytic activation of EBV but was not essential to activate KSHV. The newly made proteins that activate EBV were present within 4 to 6 h after application of the inducing stimulus. Thereafter, CHX no longer inhibited, and could enhance, expression of the BZLF1 and BRLF1 mRNAs. Depending on the lytic cycle inducing agent, resistance to the inhibitory effects of CHX developed with different kinetics. The newly made proteins needed for activation of EBV were present at earlier times after treatment with 5Aza2′dC (Fig. 3C) or TPA (Fig. 4) than after treatment with the HDAC inhibitors (Fig. 3A and B). The results indicate that EBV BZLF1 and BRLF1 are not “immediate-early” genes upon reactivation from latency. The experiments do not specifically address the question of whether these genes would or would not behave with immediate-early kinetics upon de novo infection.
Cell background effects.
Lytic cycle activation of gammaherpesviruses in cultured cells is strongly influenced by poorly characterized interactions between the viruses and the host cells. The same inducing stimulus does not activate EBV to enter the lytic cycle in all cell backgrounds. For example, HDAC inhibitors activate EBV in HH514-16 cells but not in B95-8 cells; conversely, TPA activates EBV to enter the lytic cycle in B95-8 cells but not in HH514-16 cells (21). Some cell lines are notoriously resistant to lytic cycle activation by external stimuli. Even in cell lines that are responsive to lytic induction stimuli, a subpopulation of cells remains refractory to lytic cycle activation (6). Therefore, we needed to address the possibility that the requirement for new protein synthesis to activate EBV and not KSHV was independent of cell background effects.
The availability of PEL cell lines that are dually infected with EBV and KSHV offered the unique opportunity to control for influences of cell background. In BC-1 cells, EBV lytic cycle activation, as assessed by BRLF1 mRNA expression induced either by TPA or by NaB, was transiently inhibited by CHX, whereas ORF50 expression was resistant to CHX. One possible interpretation of this experiment is that the BC-1 cell line is heterogeneous. One cell subpopulation, which harbors EBV, may require protein synthesis, while another population, in which new protein synthesis is dispensable, harbors KSHV. This result is unlikely, since previously we showed that clones of BC-1 derived from single cells harbor both genomes (42). However, the experiments do not address the possibility that lytic activation of EBV and KSHV occur in different cells.
Inducing agent-specific effects.
The results show that new protein synthesis is required for four different inducing stimuli, two HDAC inhibitors, NaB and TSA, as well as TPA and 5Aza2′dC, to stimulate EBV to enter the lytic cycle. These agents work by at least three different mechanisms. However, the inducing agents could not all be studied with the same cell line. Therefore, our conclusion about the requirements for new protein synthesis must be tempered by the reservation that it applies to the combinations of inducing agents and cells that we studied. In Akata cells, for example, EBV is activated to enter the lytic cycle following cross-linking of surface Ig (40, 54, 55). Others have reported that activation of EBV gene expression following this stimulus does not require de novo protein synthesis (44, 56). Therefore, the mechanisms and pathways of lytic cycle activation are likely to be heterogeneous and depend on the cell, virus, and inducing agent. Nonetheless, the experiments with BC-1 cells show that for two different stimuli operating in the same cell background, the requirement for protein synthesis differs for the two viruses.
Comparing response times of EBV BZLF1, BRLF1, and KSHV ORF50.
As assessed by Northern blotting and quantitative RT PCR, the kinetics of response of the EBV and KSHV lytic activator genes differed. The rapid response of ORF50 mRNA and the relatively slow response of the BRLF1 and BZLF1 mRNAs are consistent with the hypothesis that the activation pathways of the two viruses differ. The longer time preceding activation of EBV BZLF1 and BRLF1 may reflect the requirement for new protein synthesis. The shorter time for activation of ORF50 mRNA by the same stimuli may result from the presence in the cell of preformed complexes containing transcription factors that activate the ORF50 promoter. Earlier studies have mapped the butyrate response element of the ORF50 promoter to Sp1/Sp3 sites (60). Members of the Sp1 family are constitutively expressed. HDAC inhibitors may rapidly modify transcription factors such as Sp1 and alter the chromatin structure of the ORF50 promoter, allowing preexisting transcription factors, such as Sp1, to bind or repressors to be removed (37, 60). A recent study indicates that HDAC inhibitors may also promote acetylation of the latency-associated nuclear antigen. This activity may impair the capacity of the latency-associated nuclear antigen to act as a repressor of the ORF50 promoter (36).
CHX can increase the abundance of the EBV BZLF1 and BRLF1 mRNAs and the KSHV ORF50 mRNA.
At least two theories account for the well-described enhancing effects of CHX on mRNA abundance. CHX may enhance transcription of some genes (16, 33, 51, 59). CHX may inhibit the synthesis of proteins involved in mRNA degradation (25). When translation is inhibited by CHX, mRNA may remain associated with polysomes and not be transferred to organelles, where mRNA degradation occurs (4, 50). We observed a twofold increase in the level of BRLF1 mRNA in HH514-16 cells that had been treated with 5Aza2′dC for 6 h, followed by addition of CHX for 2 h, compared with the level in cells treated with 5Aza2′dC for 8 h without CHX (Fig. 3, compare lanes 3 and 4). Similarly, the level of BRLF1 mRNA was twofold higher in B95-8 cells that had been treated with TPA for 6 h before CHX was added than in cells which received only TPA (Fig. 4, compare lanes 4 and 5). The enhancing effects of CHX were always more evident with the 3.0-kb BRLF1 mRNA than with the 1.0-kb BZLF1 mRNA. These results favor the hypothesis that once the new proteins that stimulate BRLF1 expression are present at optimal levels, CHX exerts a stabilizing effect on the mRNA or enhances transcription of the mRNA. We do not understand why CHX preferentially enhances expression of the 3.0-kb BRLF1 mRNA rather than the 1.0-kb BZLF1 mRNA.
CHX increased the ORF50 mRNA level two- to threefold when added simultaneously with NaB or TSA to HH-B2 cells as the inducing agents (Fig. 5 and 6). CHX also markedly enhanced ORF50 mRNA expression in HH-B2 cells that had not been treated with any other chemical inducing agent. This effect was ∼19-fold when measured by Northern blotting and 60- to 78-fold when measured by QRT-PCR. CHX may exert effects other than those conventionally ascribed to mRNA stabilization to mediate this marked increase in ORF50 mRNA abundance. CHX may induce ORF50 transcription, perhaps by inhibiting production of a repressor protein.
Why is new protein synthesis required to activate EBV lytic gene expression?
We have obtained preliminary data that address three hypotheses to answer this question. We explored whether the CHX-induced block in expression of the EBV BZLF1 and BRLF1 mRNAs affected global hyperacetylation of histone H3 stimulated by the HDAC inhibitors. The HDAC inhibitors induced a two- to threefold increase in H3 hyperacetylation; this was not affected by CHX treatment (not shown).
The second hypothesis was that CHX inhibited the expression of the ZEBRA protein, which might be required for optimal levels of BZLF1 and BRLF1 expression, via autostimulatory and cross-stimulatory loops (18, 30, 47). We measured the time after addition of the lytic cycle-inducing stimulus when the ZEBRA protein could first be detected. We used immunoblotting and electrophoretic mobility shift assays with the high-affinity ZIIIB ZEBRA response element to detect ZEBRA. By immunoblotting of HH514-16 and B95-8 cell extracts with high-affinity antibody to ZEBRA, we never detected the ZEBRA protein above the background level until 8 h after addition of the inducing stimulus (data not shown). In these cells, the BRLF1 and BZLF1 mRNAs were refractory to the action of CHX by 4 to 6 h. When an electrophoretic mobility shift assay was used to detect ZEBRA, it was also first observed at 8 h after addition of the HDAC inhibitors and 6 h after addition of azacytidine (data not shown). Thus, ZEBRA could be detected only 2 to 4 h after expression of the BRLF1 and BZLF1 mRNAs had become refractory to the inhibitory effects of CHX. In further experiments, CHX was added at intervals after TSA and ZEBRA expression was monitored at 12 h. ZEBRA expression became resistant to the inhibitory effect of CHX only 8 h after exposure of the cells to TSA. We also found that CHX inhibits induction of BZLF1 by TPA in Raji cells (data not shown). In Raji cells, there is no autostimulation of the BZLF1 promoter (30, 34). From these experiments we think it is unlikely that the inhibitory effects of CHX on BZLF1 mRNA are due to failure of autostimulation.
We prefer the hypothesis that one or more new cellular proteins must be made in order to activate the BRLF1 and BZLF1 mRNAs. The challenge for the future will be to determine whether one or many cellular proteins are required, whether the same cellular proteins are involved in all cell backgrounds, and whether lytic cycle inducing agents that act by different mechanisms activate the same or different cellular proteins to mediate the latency-to-lytic-cycle switch of EBV.
Acknowledgments
This study was supported by grants CA12055 and CA16038 from the NIH/NCI.
We are grateful to Ethel Cesarman for gifts of the BC-1 cell line and to Daniel DiMaio and Ayman El-Guindy for discussions and critical reading of the manuscript.
Footnotes
Published ahead of print on 27 June 2007.
REFERENCES
- 1.Amon, W., U. K. Binne, H. Bryant, P. J. Jenkins, C. E. Karstegl, and P. J. Farrell. 2004. Lytic cycle gene regulation of Epstein-Barr virus. J. Virol. 78:13460-13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartkiewicz, M., H. Gold, and S. Altman. 1989. Identification and characterization of an RNA molecule that copurifies with RNase P activity from HeLa cells. Genes Dev. 3:488-499. [DOI] [PubMed] [Google Scholar]
- 3.Batterson, W., and B. Roizman. 1983. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J. Virol. 46:371-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beelman, C. A., and R. Parker. 1994. Differential effects of translational inhibition in cis and in trans on the decay of the unstable yeast MFA2 mRNA. J. Biol. Chem. 269:9687-9692. [PubMed] [Google Scholar]
- 5.Ben-Sasson, S. A., and G. Klein. 1981. Activation of the Epstein-Barr virus genome by 5-aza-cytidine in latently infected human lymphoid lines. Int. J. Cancer 28:131-135. [DOI] [PubMed] [Google Scholar]
- 6.Bhaduri-McIntosh, S., and G. Miller. 2006. Cells lytically infected with Epstein-Barr virus are detected and separable by immunoglobulins from EBV-seropositive individuals. J. Virol. Methods 137:103-114. [DOI] [PubMed] [Google Scholar]
- 7.Biggin, M., M. Bodescot, M. Perricaudet, and P. Farrell. 1987. Epstein-Barr virus gene expression in P3HR1-superinfected Raji cells. J. Virol. 61:3120-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binne, U. K., W. Amon, and P. J. Farrell. 2002. Promoter sequences required for reactivation of Epstein-Barr virus from latency. J. Virol. 76:10282-10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell, M. E., J. W. Palfreyman, and C. M. Preston. 1984. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J. Mol. Biol. 180:1-19. [DOI] [PubMed] [Google Scholar]
- 10.Cesarman, E., P. S. Moore, P. H. Rao, G. Inghirami, D. M. Knowles, and Y. Chang. 1995. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood 86:2708-2714. [PubMed] [Google Scholar]
- 11.Cho, M. S., G. W. Bornkamm, and H. zur Hausen. 1984. Structure of defective DNA molecules in Epstein-Barr virus preparations from P3HR-1 cells. J. Virol. 51:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clements, J. B., R. J. Watson, and N. M. Wilkie. 1977. Temporal regulation of herpes simplex virus type 1 transcription: location of transcripts on the viral genome. Cell 12:275-285. [DOI] [PubMed] [Google Scholar]
- 13.Countryman, J., H. Jenson, R. Seibl, H. Wolf, and G. Miller. 1987. Polymorphic proteins encoded within BZLF1 of defective and standard Epstein-Barr viruses disrupt latency. J. Virol. 61:3672-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Countryman, J., and G. Miller. 1985. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc. Natl. Acad. Sci. USA 82:4085-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies, A. H., R. J. Grand, F. J. Evans, and A. B. Rickinson. 1991. Induction of Epstein-Barr virus lytic cycle by tumor-promoting and non-tumor-promoting phorbol esters requires active protein kinase C. J. Virol. 65:6838-6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai, D. S., and R. M. Niles. 1995. Expression and regulation of retinoid X receptors in B16 melanoma cells. J. Cell Physiol. 165:349-357. [DOI] [PubMed] [Google Scholar]
- 17.Feng, W. H., J. I. Cohen, S. Fischer, L. Li, M. Sneller, R. Goldbach-Mansky, N. Raab-Traub, H. J. Delecluse, and S. C. Kenney. 2004. Reactivation of latent Epstein-Barr virus by methotrexate: a potential contributor to methotrexate-associated lymphomas. J. Natl. Cancer Inst. 96:1691-1702. [DOI] [PubMed] [Google Scholar]
- 18.Flemington, E., and S. H. Speck. 1990. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J. Virol. 64:1227-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flemington, E. K., A. E. Goldfeld, and S. H. Speck. 1991. Efficient transcription of the Epstein-Barr virus immediate-early BZLF1 and BRLF1 genes requires protein synthesis. J. Virol. 65:7073-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gradoville, L., J. Gerlach, E. Grogan, D. Shedd, S. Nikiforow, C. Metroka, and G. Miller. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gradoville, L., D. Kwa, A. El-Guindy, and G. Miller. 2002. Protein kinase C-independent activation of the Epstein-Barr virus lytic cycle. J. Virol. 76:5612-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gruffat, H., S. Portes-Sentis, A. Sergeant, and E. Manet. 1999. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) encodes a homologue of the Epstein-Barr virus bZip protein EB1. J. Gen. Virol. 80:557-561. [DOI] [PubMed] [Google Scholar]
- 23.Hardwick, J. M., P. M. Lieberman, and S. D. Hayward. 1988. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J. Virol. 62:2274-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaiswal, A. S., and S. Narayan. 1999. Protein synthesis inhibitor-mediated stability of adenomatous polyposis coli mRNA levels in HCT-116 colon cancer cells. Int. J. Oncol. 14:1045-1048. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins, P. J., U. K. Binne, and P. J. Farrell. 2000. Histone acetylation and reactivation of Epstein-Barr virus from latency. J. Virol. 74:710-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenson, H. B., P. J. Farrell, and G. Miller. 1987. Sequences of the Epstein-Barr Virus (EBV) large internal repeat form the center of a 16-kilobase-pair palindrome of EBV (P3HR-1) heterogeneous DNA. J. Virol. 61:1495-1506. (Erratum, 61:2950). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katan, M., A. Haigh, C. P. Verrijzer, P. C. van der Vliet, and P. O'Hare. 1990. Characterization of a cellular factor which interacts functionally with Oct-1 in the assembly of a multicomponent transcription complex. Nucleic Acids Res. 18:6871-6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kedes, D. H., and D. Ganem. 1997. Sensitivity of Kaposi's sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J. Clin. Investig. 99:2082-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolman, J. L., N. Taylor, L. Gradoville, J. Countryman, and G. Miller. 1996. Comparing transcriptional activation and autostimulation by ZEBRA and ZEBRA/c-Fos chimeras. J. Virol. 70:1493-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kristie, T. M., and P. A. Sharp. 1990. Interactions of the Oct-1 POU subdomains with specific DNA sequences and with the HSV alpha-trans-activator protein. Genes Dev. 4:2383-2396. [DOI] [PubMed] [Google Scholar]
- 32.Laux, G., U. K. Freese, R. Fischer, A. Polack, E. Kofler, and G. W. Bornkamm. 1988. TPA-inducible Epstein-Barr virus genes in Raji cells and their regulation. Virology 162:503-507. [DOI] [PubMed] [Google Scholar]
- 33.Lee, C. H. 2001. Induction of P-glycoprotein mRNA transcripts by cycloheximide in animal tissues: evidence that class I Pgp is transcriptionally regulated whereas class II Pgp is post-transcriptionally regulated. Mol. Cell Biochem. 216:103-110. [DOI] [PubMed] [Google Scholar]
- 34.Le Roux, F., A. Sergeant, and L. Corbo. 1996. Epstein-Barr virus (EBV) EB1/Zta protein provided in trans and competent for the activation of productive cycle genes does not activate the BZLF1 gene in the EBV genome. J. Gen. Virol. 77:501-509. [DOI] [PubMed] [Google Scholar]
- 35.Lin, S. F., D. R. Robinson, G. Miller, and H. J. Kung. 1999. Kaposi's sarcoma-associated herpesvirus encodes a bZIP protein with homology to BZLF1 of Epstein-Barr virus. J. Virol. 73:1909-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu, F., L. Day, S. J. Gao, and P. M. Lieberman. 2006. Acetylation of the latency-associated nuclear antigen regulates repression of Kaposi's sarcoma-associated herpesvirus lytic transcription. J. Virol. 80:5273-5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu, F., J. Zhou, A. Wiedmer, K. Madden, Y. Yuan, and P. M. Lieberman. 2003. Chromatin remodeling of the Kaposi's sarcoma-associated herpesvirus ORF50 promoter correlates with reactivation from latency. J. Virol. 77:11425-11435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luka, J., B. Kallin, and G. Klein. 1979. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology 94:228-231. [DOI] [PubMed] [Google Scholar]
- 39.Lukac, D. M., R. Renne, J. R. Kirshner, and D. Ganem. 1998. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252:304-312. [DOI] [PubMed] [Google Scholar]
- 40.Mellinghoff, I., M. Daibata, R. E. Humphreys, C. Mulder, K. Takada, and T. Sairenji. 1991. Early events in Epstein-Barr virus genome expression after activation: regulation by second messengers of B cell activation. Virology 185:922-928. [DOI] [PubMed] [Google Scholar]
- 41.Milanesi, G., E. N. Brody, O. Grau, and E. P. Geiduschek. 1970. Transcriptions of the bacteriophage T4 template in vitro: separation of “delayed early” from “immediate early” transcription. Proc. Natl. Acad. Sci. USA 66:181-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller, G., L. Heston, E. Grogan, L. Gradoville, M. Rigsby, R. Sun, D. Shedd, V. M. Kushnaryov, S. Grossberg, and Y. Chang. 1997. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J. Virol. 71:314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller, G., M. O. Rigsby, L. Heston, E. Grogan, R. Sun, C. Metroka, J. A. Levy, S. J. Gao, Y. Chang, and P. Moore. 1996. Antibodies to butyrate-inducible antigens of Kaposi's sarcoma-associated herpesvirus in patients with HIV-1 infection. N. Engl. J. Med. 334:1292-1297. [DOI] [PubMed] [Google Scholar]
- 44.Packham, G., M. Brimmell, D. Cook, A. J. Sinclair, and P. J. Farrell. 1993. Strain variation in Epstein-Barr virus immediate early genes. Virology 192:541-550. [DOI] [PubMed] [Google Scholar]
- 45.Pellett, P. E., J. L. McKnight, F. J. Jenkins, and B. Roizman. 1985. Nucleotide sequence and predicted amino acid sequence of a protein encoded in a small herpes simplex virus DNA fragment capable of trans-inducing alpha genes. Proc. Natl. Acad. Sci. USA 82:5870-5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ragoczy, T., L. Heston, and G. Miller. 1998. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J. Virol. 72:7978-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ragoczy, T., and G. Miller. 2001. Autostimulation of the Epstein-Barr virus BRLF1 promoter is mediated through consensus Sp1 and Sp3 binding sites. J. Virol. 75:5240-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 49.Serio, T. R., J. L. Kolman, and G. Miller. 1997. Late gene expression from the Epstein-Barr virus BcLF1 and BFRF3 promoters does not require DNA replication in cis. J. Virol. 71:8726-8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheth, U., and R. Parker. 2003. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 300:805-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stordeur, P., L. Schandene, P. Durez, C. Gerard, M. Goldman, and T. Velu. 1995. Spontaneous and cycloheximide-induced interleukin-10 mRNA expression in human mononuclear cells. Mol. Immunol. 32:233-239. [DOI] [PubMed] [Google Scholar]
- 52.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun, R., S. F. Lin, K. Staskus, L. Gradoville, E. Grogan, A. Haase, and G. Miller. 1999. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J. Virol. 73:2232-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takada, K. 1984. Cross-linking of cell surface immunoglobulins induces Epstein-Barr virus in Burkitt lymphoma lines. Int. J. Cancer 33:27-32. [DOI] [PubMed] [Google Scholar]
- 55.Takada, K., K. Horinouchi, Y. Ono, T. Aya, T. Osato, M. Takahashi, and S. Hayasaka. 1991. An Epstein-Barr virus-producer line Akata: establishment of the cell line and analysis of viral DNA. Virus Genes 5:147-156. [DOI] [PubMed] [Google Scholar]
- 56.Takada, K., and Y. Ono. 1989. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J. Virol. 63:445-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor, N., J. Countryman, C. Rooney, D. Katz, and G. Miller. 1989. Expression of the BZLF1 latency-disrupting gene differs in standard and defective Epstein-Barr viruses. J. Virol. 63:1721-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wen, W., D. Iwakiri, K. Yamamoto, S. Maruo, T. Kanda, and K. Takada. 2007. Epstein-Barr virus BZLF1 gene, a switch from latency to lytic infection, is expressed as an immediate-early gene after primary infection of B lymphocytes. J. Virol. 81:1037-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Won, J. S., J. K. Lee, D. K. Song, S. O. Huh, J. S. Jung, Y. H. Kim, M. R. Choi, and H. W. Suh. 2000. Cycloheximide increases proenkephalin and tyrosine hydroxylase gene expression in rat adrenal medulla. Mol. Pharmacol. 57:1173-1181. [PubMed] [Google Scholar]
- 60.Ye, J., D. Shedd, and G. Miller. 2005. An Sp1 response element in the Kaposi's sarcoma-associated herpesvirus open reading frame 50 promoter mediates lytic cycle induction by butyrate. J. Virol. 79:1397-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zalani, S., E. Holley-Guthrie, and S. Kenney. 1996. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc. Natl. Acad. Sci. USA 93:9194-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu, F. X., T. Cusano, and Y. Yuan. 1999. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J. Virol. 73:5556-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.zur Hausen, H., F. O'Neil, and U. Freese. 1978. Persisting oncogenic herpesviruses induced by the tumor promoter TPA. Nature 272:373-375. [DOI] [PubMed] [Google Scholar]