Abstract
Adiponectin is secreted from adipose tissue in response to metabolic effectors in order to sensitize the liver and muscle to insulin. Reduced circulating levels of adiponectin that usually accompany obesity contribute to the associated insulin resistance. The molecular mechanisms controlling the production of adiponectin are essentially unknown. In this report, we demonstrate that the endoplasmic reticulum (ER) oxidoreductase Ero1-Lα and effectors modulating peroxisome proliferator-activated receptor γ (PPARγ) and SIRT1 activities regulate secretion of adiponectin from 3T3-L1 adipocytes. Specifically, adiponectin secretion and Ero1-Lα expression are induced during the early phase of adipogenesis but are then down-regulated during the terminal phase, coincident with an increased expression of SIRT1. Suppression of SIRT1 or activation of PPARγ enhances Ero1-Lα expression and stimulates secretion of high-molecular-weight complexes of adiponectin in mature adipocytes. Suppression of Ero1-Lα through expression of a corresponding small interfering RNA reduces adiponectin secretion during the differentiation of 3T3-L1 preadipocytes. Moreover, ectopic expression of Ero1-Lα in Ero1-Lα-deficient 3T3 fibroblasts stimulates the secretion of adiponectin following their conversion into adipocytes and prevents the suppression of adiponectin secretion in response to activation of SIRT1 by exposure to resveratrol. These findings provide a framework to understand the mechanisms by which adipocytes regulate secretion of adiponectin in response to various metabolic states.
The rapid rise in the prevalence of obesity is a major factor contributing to the dramatic increase in insulin resistance and type 2 diabetes among the young as well as the elderly in Western society (22). Understanding the link between adiposity and glucose homeostasis is the focus of many investigations. Recent studies have identified the adipocyte as an endocrine cell that functions not only to store and metabolize lipids but also to secrete a plethora of biologically active molecules (adipokines) that participate in overall energy balance (19). Notable among these adipokines is adiponectin, which has been shown to play an important role in regulating insulin control of glucose metabolism and whose secretion from the adipocyte is modulated in response to various metabolic states (17, 35). Specifically, circulating adiponectin levels are decreased in obese and insulin-resistant subjects, and many clinical investigations support the notion that metabolic suppression of adiponectin production in obese individuals contributes significantly to the metabolic syndrome, including insulin resistance, atherosclerosis, and hypertension (35).
Adiponectin is synthesized as a single polypeptide of 30 kDa and is then assembled in the endoplasmic reticulum (ER) into higher-molecular-weight (HMW) complexes prior to its secretion. Circulating adiponectin consists of an array of complexes composed of multimers of the 30-kDa polypeptide facilitated by distinct disulfide bonds generating trimers, middle-molecular-weight (MMW) hexamers, and an elaborate HMW complex that has recently been suggested to possess the most potent insulin-sensitizing activity of all the complexes (18, 25, 39). In fact, it appears that the ratio of HMW adiponectin to other complexes changes in direct response to perturbations in the metabolic state accompanying obesity and its associated disorders (25, 37). It is important, therefore, to define the molecular mechanisms controlling production of HMW adiponectin and determine how these processes respond to changes in metabolism.
Recent investigations suggest that a major mode of action of the thiazolidinedione (TZD) class of insulin-sensitizing drugs is to increase the circulating levels of HMW adiponectin (18, 25). Since TZDs are also potent ligands for the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ), these observations support a role for PPARγ in regulating adiponectin production. In fact, studies by Shimomura and colleagues (16) have provided evidence that PPARγ can regulate expression of adiponectin by promoting transcription of the corresponding mRNA through a PPARγ response element in the promoter of its gene. A recent study, however, stated that increased plasma adiponectin in response to the TZD pioglitazone does not result from increased gene expression (29). Additional mechanisms must, therefore, be operating, some of which might involve a PPARγ-dependent expression of proteins participating in the formation and secretion of HMW adiponectin. Furthermore, PPARγ might also function to mediate the response of adipocytes to metabolic perturbations associated with obesity and insulin resistance. In this regard, the NAD-dependent deacetylase SIRT1 has recently been shown to suppress PPARγ activity in response to calorie restriction in fasted animals, leading to fat mobilization in adipose depots (26). In the present study, we questioned whether changes in metabolites, such as glucose, regulate secretion of HMW adiponectin through the expression of a component of the secretory process whose production is controlled by SIRT1 modulation of PPARγ activity.
Formation of disulfide bonds occurs in the lumen of the ER by at least two pathways that oxidize cysteine pairs to form native bonds as well as isomerization of nonnative disulfide bonds (32). The major pathway is comprised principally of the flavoprotein Ero1 and members of the protein disulfide isomerase (PDI) family. PDI is a multidomain member of the thioredoxin superfamily that can catalyze thiol-disulfide oxidation, reduction, and isomerization that facilitate the formation of intra- as well as intermolecular disulfide bonds (10). Ero1 is an ER membrane-associated oxidoreductase that utilizes the oxidizing power of oxygen to generate disulfide bonds in itself, which it then transfers to PDI (12, 13, 27). Oxidized PDI is then able to transfer its disulfide bonds to appropriate substrates. The entire process consists, therefore, of transmission of oxidizing equivalents between Ero1, PDI, and secretory proteins that involves a series of direct thiol-disulfide exchange reactions between each of the proteins (8, 11, 36). Mammals express two related Ero1 proteins, Ero1-Lα and Ero1-Lβ. Ero1-Lβ is produced primarily in secretory cells, and its expression is induced by the unfolded protein response (9, 24). In contrast, Ero1-Lα is expressed in most cell types, where it is considered to be the rate-limiting step in disulfide bond formation (4, 24).
In the following studies, we demonstrate that secretion of HMW adiponectin from 3T3-L1 adipocytes is regulated through the nutrient control of SIRT1 activity. Inhibition of SIRT1 and/or activation of PPARγ leads to increased expression of Ero1-Lα and a corresponding increase in HMW adiponectin secretion. Suppression of Ero1-Lα through expression of a corresponding small interfering RNA (siRNA) reduces adiponectin secretion during the differentiation of 3T3-L1 preadipocytes. Moreover, ectopic expression of Ero1-Lα in 3T3 fibroblasts stimulates the secretion of adiponectin during their differentiation into adipocytes. These data suggest that nutrient control of adiponectin secretion is mediated through SIRT1-associated regulation of the PPARγ-responsive gene Ero1-Lα.
MATERIALS AND METHODS
Materials.
Dulbecco's modified Eagle's medium (DMEM) with and without 4.5 g/liter glucose was purchased from Mediatech, Inc. (Herndon, VA), fetal bovine serum (FBS) came from Gemini Bio-Products, calf serum and TRIzol were from Invitrogen, and d-glucose was obtained from American Bioanalytical (Natick, MA). The PPARγ agonist troglitazone was obtained from Biomol International, while the PPARγ antagonist T0070907 was purchased from Tocris. Resveratrol was from Cayman Chemical, and lactate, pyruvate, and nicotinamide were all from Sigma. All other chemicals were supplied by American Bioanalytical.
Cell culture.
Murine 3T3-L1 preadipocytes were cultured and maintained in DMEM supplemented with 10% calf serum. Differentiation was induced by exposure of postconfluent cells to DMEM containing 10% FBS, 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, and 1.67 μM insulin. After 48 h, the medium was changed to DMEM containing 10% FBS every 2 days. Human embryonic kidney HEK-293T cells were cultured in DMEM with 10% FBS. The Swiss F-PPARγ cells were differentiated as recently described (21).
Plasmid construction and retrovirus transfection/infection.
A PCR fragment corresponding to the coding region of murine Ero1-Lα mRNA was generated using 3T3-L1 adipocyte cDNA as template and the following oligonucleotides, also containing either a BamHI or a SalI restriction site, as primers: 5′-GAA GGA TCC ATG GGC CGC GCC TGG GGC TTG CTC GTT-3′ (sense) and 5′-CGC CGT CGA CGG CAC ATT CCA ACC GTC CTC CTC AGT G-3′ (antisense). The PCR product was subcloned into the multicloning site of pRevTRE retrovirus using BamHI and SalI restriction endonucleases. pSUPER-SIRT1 siRNA retroviral vector was generously provided by Jim Xiao of Boston University School of Medicine and consisted of the plasmid recently described (26). HEK-293T cells were grown to 70% confluence in 100-mm-diameter dishes, at which stage they were transfected with a DNA-FUGENE cocktail consisting of 36 μl Fugene 6, 6 μg retrovirus plasmid, 6 μg pVPack-VSV-G vector, 6 μg pVPack-GAG-POL vector, and 164 μl DMEM without FBS. Twenty-four hours later, the medium was replaced with 6 ml fresh DMEM containing 10% FBS. One day after that, the culture medium containing high-titer retrovirus was harvested and filtered through a 0.45-μm-pore-size filter. The viral filtrate was used to infect both 3T3-L1 preadipocytes and Swiss 3T3 fibroblasts.
Transient knockdown of Ero1-Lα in 3T3-L1 adipocytes.
3T3-L1 preadipocytes were cultured in 60-mm dishes and induced to differentiate as described above. On day 4, the differentiation-inducing medium was replaced with 2.5 ml fresh DMEM containing 10% FBS. Fifteen microliters of TransIT-TKO transfection reagent (Mirus Bio Corporation, Madison, WI) was diluted into 250 μl serum-free DMEM and incubated at room temperature for 20 min, after which time 100 nM Ero1-Lα RNA inhibition (RNAi) duplex or control duplex (final concentration in the dish) was added and mixed thoroughly. After incubation at room temperature for another 20 min, the resulting TransIT-TKO-RNAi mixture was gently mixed into the adipocyte cultures. The next day, the medium was changed to 2.5 ml fresh DMEM containing 10% FBS, and the transfection was repeated as on day 4. On day 6, the total cellular proteins were harvested and subjected to Western blot analysis. The modified synthetic Ero1-Lα RNAi duplexes (stealth siRNA) were from Invitrogen Life Technologies. The sequences are as follows: siRNA 1, GCUGAGUAUGUGGACUUACUCCUUA; siRNA 2, GGGCACUGCUCUGAAGAUCUUGUUU; siRNA 3, GGGCUCUCUCCAAAGUGCUUCCAUU. A green fluorescent protein-RNAi duplex was used as the negative control.
RNA analysis.
Total RNA was isolated using TRIzol following the manufacturer's instructions and was subjected to reverse transcription-PCR (RT-PCR) analysis as outlined in the Promega product manual as previously described (31). Primers used for the RT-PCR analysis were as follows: PPARγ, 5′-CCA GAG CAT GGT GCC TTC GCT G-3′ and 5′-GAG CTG ACC CAA TGG TTG CTG-3′; adiponectin, 5′-ACT CCT GGA GAG AAG GAG AA-3′ and 5′-TTG TCC TTC TTG AAG AGG CTC ACC-3′. In the case of analyzing Ero1-Lα mRNA, primers were the same as those listed above for generating the Ero1-Lα pRevTRE expression vector.
Western blot analysis of proteins.
Isolation and Western blot analysis of total cell proteins was performed as outlined previously (21). The antibodies employed in the analysis were as follows: mouse polyclonal antiadiponectin antibody (Affinity BioReagents, Golden, CO), polyclonal anti-SIRT1 antibody (Upstate), anti-PPARγ and anti-C/EBPα (Santa Cruz Biotechnology), anti-Ero1 polyclonal antibody (Abnova Co., Taiwan, China), and polyclonal anti-aP2 serum (David Benlohr, University of Minnesota). For analysis of secreted proteins, aliquots of the culture medium were centrifuged at low speed to remove debris and then subjected to standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as above or nonreducing SDS-PAGE as outlined by Kadowaki and collaborators (37). Prior to electrophoresis, the samples were mixed with 4× nonreducing protein sample buffer (200 mM Tris pH 6.8, 8% SDS, 0.4% bromophenol blue, 40% glycerol) and incubated at room temperature for 1 hour. Care was taken to ensure that all components of the SDS-PAGE system were completely free of reducing agents.
All experiments were performed at least three times, and figures presented below are representative of the data.
RESULTS
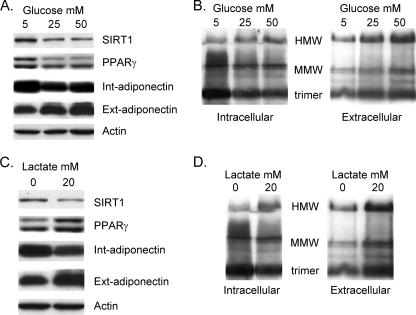
To gain insight into mechanisms by which nutrients might regulate adiponectin synthesis and secretion, we exposed 3T3-L1 adipocytes to increasing doses of either glucose or lactate for 72 h and analyzed adiponectin production in Western blot assays. Figure 1A shows that exposing 3T3-L1 adipocytes to increasing concentrations of glucose dramatically increases the amount of adiponectin secreted into the medium while decreasing the level of intracellular adiponectin. We also measured the abundance of the NAD-dependent deacetylase SIRT1, since its expression and activity have been shown to be regulated by nutrients, such as glucose and lactate, that influence the NAD+/NADH ratio (30). In fact, there is a significant decrease of SIRT1 expression in response to an increase in glucose concentration.
FIG. 1.
Nutrients selectively increase secretion of HMW complexes of adiponectin. 3T3-L1 cells were cultured normally until day 4 of differentiation, at which stage the medium was changed to either standard DMEM containing 5 mM glucose (C and D) or glucose-free DMEM (A and B), and in each case the DMEM was supplemented with 10% FBS. Cultures in panels A and B were also exposed to the indicated concentration of d-glucose, while those in panels C and D were exposed to 20 mM lactate. On day 7, the medium and cell layer were harvested and subjected to either reducing (A and C) or nonreducing (B and D) SDS-PAGE as outlined in Materials and Methods. Int, intracellular; Ext, extracellular (secreted into the medium).
The secreted adiponectin detected by Western blot analysis of SDS-PAGE performed under reducing conditions as shown in Fig. 1A corresponds to the monomer polypeptide of 30-kDa mass. Recent studies have demonstrated that adiponectin is processed in the endoplasmic reticulum prior to secretion into an elaborate set of higher-ordered structures involving disulfide bond linkage of each of the monomers into HMW complexes composed of several hexamers. In addition, investigations have also shown that the HMW forms of adiponectin possess the highest insulin-sensitizing activity. It is important, therefore, to identify the specific forms of adiponectin that are secreted from 3T3-L1 adipocytes in response to various effectors. Recent studies have shown that analysis of adiponectin on nonreducing SDS-PAGE faithfully represents the complexity of the secreted adiponectin molecules produced in adipose tissue and present in the circulation (37). Figure S1 in the supplemental material shows the separation of the various forms of adiponectin secreted into the media of cultures of 3T3-L1 preadipocytes following nonreducing SDS PAGE under conditions that preserve the disulfide bonds.
To assess the effect of glucose on the complexity of the secreted adiponectin, intracellular and extracellular protein samples were subjected to nonreducing SDS-PAGE without heat. Figure 1B demonstrates a significant increase in the appearance of the HMW species in the medium in response to glucose, with a corresponding decrease in the abundance of the trimer within the cell. Mature adipocytes rapidly metabolize glucose to pyruvate during glycolysis. Lactate is also converted to pyruvate through the action of lactate dehydrogenase, with conversion of NAD+ to NADH. It is encouraging, therefore, that lactate has a similar effect on enhancing the secretion of HMW forms of adiponectin at the expense of a decrease in intracellular trimers (Fig. 1C and D).
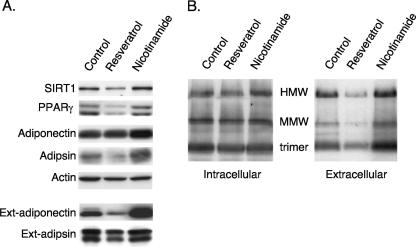
Calorie restriction (low glucose) enhances the activity of the NAD-dependent deacetylase SIRT1 due to an increase in the NAD+/NADH ratio. Consequently, we questioned whether the effects of glucose and lactate on adiponectin secretion involved SIRT1. 3T3-L1 adipocytes were, therefore, exposed to an activator (resveratrol) or inhibitor (nicotinamide) of SIRT1 activity. Figure 2A demonstrates that activation of SIRT1 reduces the secretion of adiponectin (external) without significantly affecting synthesis of the protein (intracellular) or without affecting secretion of adipsin. In contrast, inhibition of SIRT1 has the opposite effect, enhancing secretion of adiponectin many times greater than the slight increase in the production of the protein and has no effect on adipsin. Furthermore, modulation of SIRT1 activity leads to a corresponding change in the relative abundance of the different adiponectin complexes. Specifically, resveratrol decreases, whereas nicotinamide enhances, secretion of the HMW complexes (Fig. 2B).
FIG. 2.
Perturbation of SIRT1 activity affects secretion of HMW forms of adiponectin. 3T3-L1 adipocytes (4 days) were exposed to either resveratrol (50 μM) or nicotinamide (5 mM) in standard DMEM containing 10% FBS for 2 days. The cells were then cultured in fresh DMEM overnight, at which time the medium and total cell layer were harvested for Western blot analysis of intra- and extracellular proteins on reducing (A) and nonreducing (B) SDS-PAGE as outlined in Materials and Methods.
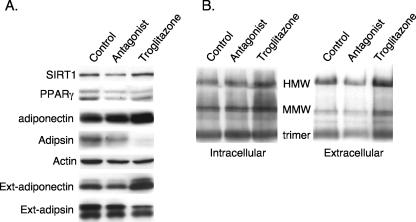
SIRT1 can regulate PPARγ activity; we questioned, therefore, whether PPARγ was also involved in controlling adiponectin secretion. Treatment of 3T3-L1 adipocytes with the PPARγ ligand troglitazone significantly increases the amount of secreted HMW adiponectin and attenuates secretion of adipsin, whereas T0070907, a PPARγ antagonist, has the opposite effect (Fig. 3A and B). Interestingly, activation of PPARγ induces expression of SIRT1 (Fig. 3A), consistent with the fact that SIRT1 expression is enhanced during adipogenesis (26).
FIG. 3.
Perturbation of PPARγ activity affects secretion of adiponectin. 3T3-L1 adipocytes were exposed to troglitazone (5 μM) or a PPARγ antagonist (10 μM T0070907) in standard DMEM containing 10% FBS for 3 days, at which time the medium and total cell layer were harvested for Western blot analysis of intra- and extracellular proteins on reducing (A) and nonreducing (B) SDS-PAGE as outlined in Materials and Methods.
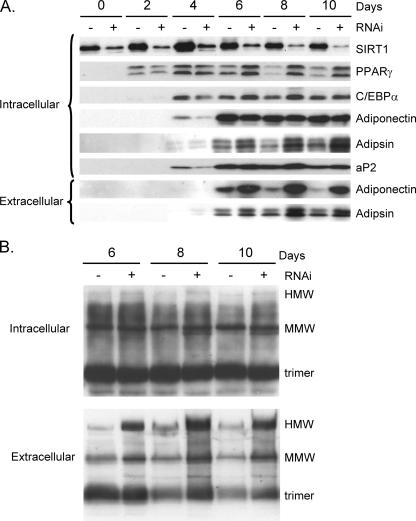
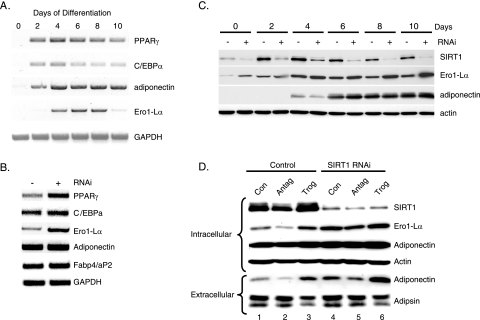
To confirm that SIRT1 regulates adiponectin secretion, we generated a 3T3-L1 cell line in which SIRT1 was knocked down using RNAi technology. Western blot analysis of proteins extracted from vector control cells and SIRT1 knockdown cells at different times during their differentiation into adipocytes demonstrated an extensive increase in SIRT1 expression at 2 to 4 days of differentiation of control cells. In contrast, the SIRT1 RNAi effectively down-regulated SIRT1 expression in the knockdown cells and in so doing prevented the decrease in PPARγ expression during terminal adipogenesis. More importantly, secretion of adiponectin (extracellular) decreased significantly during adipogenesis in control cells in a manner coincident with the increase in SIRT1 and decrease in PPARγ (Fig. 4A, minus lanes). In contrast, knockdown of SIRT1 resulted in high levels of adiponectin secretion that consisted of abundant amounts of HMW species (Fig. 4B). There was no dramatic effect of suppression of SIRT1 on secretion of adipsin or on the production of adiponectin (intracellular), but there was a slight enhancement of synthesis of the fatty acid binding protein 4 (aP2) and adipsin (Fig. 4A).
FIG. 4.
Inhibition of SIRT1 expression enhances secretion of HMW adiponectin. 3T3-L1 preadipocytes expressing a control vector or SIRT1 siRNA were differentiated for the indicated number of days, and medium (extracellular) as well as the total cell layer (intracellular) was harvested for Western blot analysis of proteins on reducing (A) or nonreducing (B) SDS-PAGE employing antibodies to the following proteins: SIRT1, PPARγ, C/EBPα, adiponectin, adipsin, and aP2/FABP4.
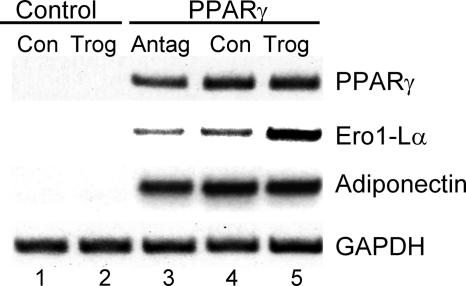
Together, these data suggest that SIRT1 and PPARγ are regulating a component of the machinery involved in the processing and secretion of adiponectin. Recent studies have identified an elaborate process in which a series of endoplasmic reticulum proteins, including the oxidoreductase Ero1-Lα and protein disulfide isomerase, regulate secretion of a variety of molecules. In fact, investigations by others (38) have shown that Ero1-Lα mRNA expression is enhanced during adipogenesis in 3T3-L1 preadipocytes; consequently, we questioned whether the Ero1-Lα gene is a direct target of PPARγ and whether Ero1-Lα regulates secretion of adiponectin. To address the first question, we ectopically expressed PPARγ in Swiss 3T3 fibroblasts to create a cell line referred to as WT-PPARγ cells, which are capable of undergoing complete conversion into adipocytes in response to inducers of adipogenesis, including dexamethasone, isobutylmethylxanthine, and insulin, with or without troglitazone. Figure 5 demonstrates that ectopic PPARγ induces expression of Ero1-Lα mRNA in these cells in response to dexamethasone, isobutylmethylxanthine, or insulin alone to levels that are significantly higher than those expressed in control cells containing the empty retroviral vector (Fig. 5, compare lane 4 with lane 1). Furthermore, the expression of Ero1-Lα mRNA is enhanced severalfold by exposure of the PPARγ cells to troglitazone with no apparent increase in adiponectin expression (Fig. 5, compare lane 5 with lane 4). Similarly, inhibition of PPARγ activity by the antagonist T0070907 attenuates Ero1-Lα expression (Fig. 5, compare lane 3 with lane 4). These data are consistent, therefore, with the notion that Ero1-Lα gene expression is directly responsive to the action of PPARγ. As stated above, other investigators have shown that expression of Ero1-Lα is regulated during adipogenesis, and the data in Fig. 6A are consistent with those studies, since there was an extensive induction of Ero1-Lα mRNA expression in differentiating 3T3-L1 preadipocytes at a time coinciding with the increase in PPARγ activity (days 2 to 4). Interestingly, its expression declines significantly during the latter stages of differentiation, similar to that observed for adiponectin secretion (Fig. 4A). To determine whether this decrease in Ero1-Lα expression is due to enhanced SIRT1 activity, Ero1-Lα mRNA was analyzed in SIRT1 knockdown cells. Figures 6B and C show that levels of Ero1-Lα mRNA and protein are much higher in the knockdown cells compared to vector controls. In addition, Fig. 6C shows the decrease in Ero1-Lα during adipogenesis in control cells that is consistent with the drop in corresponding mRNA levels (Fig. 6A).
FIG. 5.
Activation of PPARγ in Swiss 3T3 fibroblasts induces Ero1-Lα expression as well as adipogenesis. Swiss 3T3 fibroblasts expressing wild-type PPARγ or a control vector were induced to differentiate by exposure to a differentiation cocktail of dexamethasone, isobutylmethylxanthine, and insulin in 10% FBS as outlined in Materials and Methods (Con). Some cultures were also exposed to 5 μM troglitazone (Trog) or 10 μM PPARγ antagonist T0070907 (Antag) along with the differentiation cocktail. At day 6, cells were harvested for analysis of individual mRNAs as indicated using RT-PCR.
FIG. 6.
SIRT1 regulates expression of the ER oxidoreductase Ero1-Lα. (A) 3T3-L1 preadipocytes were differentiated for the indicated days, and total cell RNA was extracted for RT-PCR analysis of mRNAs corresponding to PPARγ, C/EBPα, adiponectin, Ero1-Lα, FABP4/aP2, and GAPDH. (B and C) 3T3-L1 preadipocytes expressing a control vector (-) or a SIRT1 siRNA (+) were differentiated for 8 days for RT-PCR analysis of mRNAs (B) or for the indicated days for Western blot analysis of intracellular proteins (C). (D) Control and SIRT1 siRNA-expressing 3T3-L1 preadipocytes were differentiated in the absence (Con) or presence of either a PPARγ antagonist (Antag; 10 μM T0070907) or PPARγ agonist (Trog; 5 μM troglitazone), and the medium (extracellular) and total cell layer (intracellular) were harvested for Western blot analysis of the indicated proteins.
It is very likely that SIRT1 is suppressing adiponectin secretion by suppressing expression of the PPARγ-responsive gene Ero1-Lα. The data in Fig. 6D are consistent with this notion, since the effect of knocking down SIRT1 in combination with exposure to troglitazone (lane 4) significantly enhanced Ero1-Lα expression and adiponectin secretion (extracellular) compared to the modest increase in adiponectin production (intracellular) (Fig. 6D, compare lane 6 with lane 1). It is important to mention that there is no significant effect of SIRT1 knockdown on adipsin secretion (extracellular), but treatment with troglitazone in both cell lines causes a slight decrease in production of adipsin. Additionally, inhibition of PPARγ activity by treatment with the PPARγ antagonist T0070907 inhibits Ero1-Lα expression and suppresses adiponectin secretion in both control and SIRT1 knockdown cells (Fig. 6D, compare lanes 2 and 1 as well as 5 and 4).
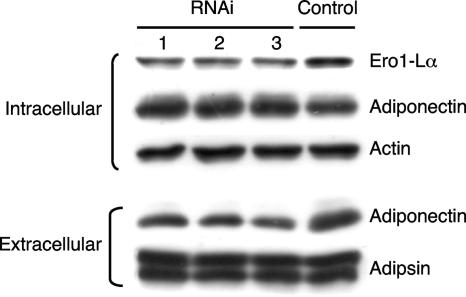
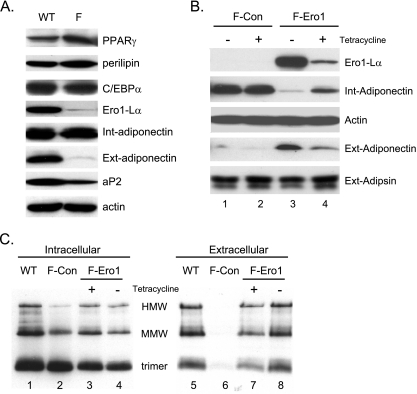
To definitively show a role for Ero1-Lα in controlling adiponectin secretion, we suppressed its expression during the terminal phase of adipogenesis in 3T3-L1 preadipocytes through the transient expression of corresponding siRNAs. This was achieved by transfecting cultures of preadipocytes individually with three separate siRNAs at day 4 of their differentiation into adipocytes. Cells were then harvested at day 6 for analysis of expression of select proteins as well as secretion of adiponectin. Figure 7 demonstrates that all three siRNAs attenuate Ero1-lα expression ∼50% during this 2-day period, with siRNA number 3 being the most effective. The reduction in Ero1-Lα expression had no significant effect on synthesis of adiponectin or actin or secretion of adipsin, but it did significantly reduce the amount of adiponectin secreted from the cells (Fig. 7, compare lanes 1 to 3 with lane 4). In addition to suppression of Ero1-Lα, we also investigated the effect of overexpressing the protein in cells in which adiponectin secretion and Ero1-Lα expression have been compromised. The data in Fig. 5 show that ectopic expression of a wild-type PPARγ in Swiss fibroblasts (Swiss-WT-PPARγ cells) induces adipogenesis, which includes expression of Ero1-Lα. In contrast, expression of a PPARγ molecule in which F372 has been modified to alanine (Swiss-PPARγF372A) (21) leads to cells that are capable of undergoing conversion into adipocytes in the presence of troglitazone, but their level of secretion of adiponectin (extracellular) is significantly reduced relative to the total amount of adiponectin synthesized (intracellular) (Fig. 8A). Additionally, Ero1-Lα expression is virtually undetectable in the Swiss-PPARγF372A adipocytes compared to the amount produced in Swiss-WT-PPARγ adipocytes (Fig. 8A). To test the function of Ero1-Lα, we stably introduced Ero1-Lα cDNA into the Swiss-PPARγF372A cells using a pREV-TET-Ero1-Lα retrovirus that generated a cell line in which we could control the level of Ero1-Lα expression by exposure of the cells to varying concentrations of tetracycline. Figure 8B shows that conversion of the Swiss-PPARγF372A fibroblasts expressing a pREV-TET vector alone (F-Con) into adipocytes induces synthesis of adiponectin (intracellular) without any apparent production of Ero1-Lα or secretion of adiponectin (extracellular) (Fig. 8B, lanes 1 and 2). In contrast, conditional ectopic expression of Ero1-Lα in Swiss-PPARγF372A cells (F-Ero1) leads to abundant production of Ero1-Lα and a corresponding increase in the secretion of adiponectin (extracellular) (Fig. 8B, lanes 3 and 4). We additionally analyzed adiponectin under nonreducing conditions to determine the effect of ectopic Ero1-Lα on formation of the higher-ordered complexes. Figure 8C, lane 1, shows the production of the three major complexes of adiponectin (trimer, MMW, and HMW) by Swiss adipocytes expressing a WT-PPARγ similar to the complexes produced by 3T3-L1 adipocytes (Fig. 1B). Furthermore, these Swiss-WT-PPARγ adipocytes are able to secrete these adiponectin complexes into the extracellular medium (Fig. 8C, lane 5). Swiss cells expressing mutant PPARγF372A, however, produce adiponectin complexes (Fig. 8C, lane 2), but they remain within the cell and are not secreted into the medium (Fig. 8C. lane 6). It is relevant that the complexes produced in the F-Con cells differ somewhat from those in the WT cells, since the relative abundance of the MMW and HMW species is very low in the F-Con cell extracts (Fig. 8C, compare lane 2 with lane 1). Interestingly, ectopic expression of Ero1-Lα in the Swiss-PPARγF372A (F-Ero1) cells led to a significant increase in the production of MMW and HMW complexes in the cells (Fig. 8C, lanes 3 and 4), but more importantly, it also led to secretion of abundant amounts of all three adiponectin complexes (trimer, MMW, and HMW) into the medium (Fig. 8C, lanes 7 and 8).
FIG. 7.
Knockdown of Ero1-Lα expression in 3T3-L1 adipocytes inhibits secretion of adiponectin. 3T3-L1 adipocytes were transiently transfected with siRNAs corresponding to Ero1-Lα (1, 2, and 3) or a control siRNA as described in Materials and Methods. On day 6, total cell extracts (intracellular) and medium (extracellular) were harvested and subjected to Western blot analysis of Ero1-Lα, adiponectin, actin, and adipsin.
FIG. 8.
Ectopic expression of Ero1-Lα enhances secretion of adiponectin in 3T3 adipocytes. (A) Swiss 3T3 fibroblasts expressing either WT-PPARγ (WT) or the mutant F372APPARγ (F) were induced to differentiate as described in Materials and Methods, and total cell extracts (Int) as well as medium (Ext) were harvested at day 5 for Western blot analysis of the indicated proteins. (B and C) Swiss-F372APPARγ cells expressing either a pREV-TET Ero1-Lα retrovirus (F-Ero) or a control vector (F-Con) were induced to differentiate in the presence (+) or absence (-) of tetracycline for 5 days as described in Materials and Methods. At this stage, medium (Ext) and total cell protein (Int) were harvested and subjected to reducing (B) or nonreducing without heat (C) SDS-PAGE followed by Western blot analysis using antibodies against the indicated proteins. In panel C, WT proteins from panel A were also analyzed as a control.
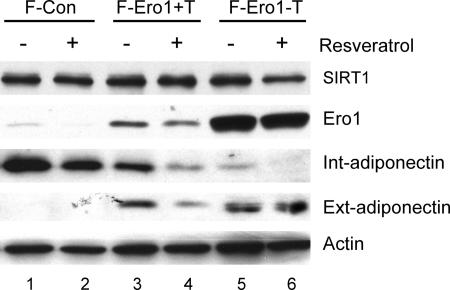
These data demonstrate a definite role for Ero1-Lα in promoting the secretion of HMW adiponectin from adipocytes and suggest that Ero1-Lα likely mediates the effects of metabolic perturbations on adiponectin secretion. To begin to test this notion, we questioned whether ectopic expression of Ero1-Lα could overcome the negative effects of enhanced SIRT1 activity on adiponectin secretion. Consequently, Swiss-F-PPARγ cells expressing either a control vector (F-Con) or ectopic Ero1-Lα (F-Ero1) were induced to differentiate into adipocytes in the presence or absence of tetracycline to facilitate control of ectopic Ero1-Lα. At day 4, cells were treated with or without resveratrol at concentrations known to activate SIRT1. Figure 9 shows that ectopic expression of Ero1-Lα significantly enhances secretion of adiponectin (compare lane 3 with lane 1). This effect is increased more when cells are cultured in the absence of tetracycline, which also increases the ectopic production of Ero1-Lα (compare lane 5 with lane 3). Interestingly, activation of SIRT1 by treatment with resveratrol inhibits secretion of adiponectin in F-Ero1 cells exposed to tetracycline, consistent with the data in Fig. 2, which show that resveratrol attenuates adiponectin secretion in 3T3-L1 adipocytes. Furthermore, enhanced production of ectopic Ero1-Lα in F-Ero1 cells by culture in the absence of tetracycline overcomes the Sirt1-associated inhibition of adiponectin secretion (Fig. 9, compare lanes 4 and 6).
FIG. 9.
Ectopic expression of Ero1-Lα attenuates the inhibitory effects of resveratrol on adiponectin secretion. Swiss-F372APPARγ cells expressing either a pREV-TET Ero1-Lα retrovirus (F-Ero) or a control vector (F-Con) were induced to differentiate by exposure to differentiation medium and 5 μM troglitazone in the presence (+T) or absence (-T) of tetracycline for 5 days as described in Materials and Methods. At day 4, cells were treated with or without 50 μM resveratrol for 2 days, and medium (Ext) and total cell protein (Int) were harvested for Western blot analysis of the indicated proteins.
DISCUSSION
Studies during the last few years have clearly shown that circulating adiponectin plays a direct role in sensitizing both liver and muscle to insulin, thus contributing directly to overall glucose homeostasis (18, 35). Moreover, additional studies have identified the HMW complexes of circulating adiponectin as principal mediators of its insulin-sensitizing activity (25, 37). Consequently, we considered it important to identify the effectors and mechanisms controlling the production and secretion of HMW adiponectin from the adipocyte. To this end, the present data show that exposing 3T3-L1 adipocytes to increasing doses of either glucose or lactate significantly enhances the secretion of HMW complexes of adiponectin without having a significant effect on synthesis of the monomeric protein. Since glucose and lactate are metabolized by the adipocyte to pyruvate, converting NAD+ into NADH, we questioned whether the NAD-dependent deacetylase SIRT1 was involved in facilitating this response. The data show that inhibition of SIRT1 activity, by exposure to nicotinamide, also enhances the secretion of HMW adiponectin, whereas treatment of cells with resveratrol, an activator of SIRT1, significantly reduces secretion of the complexes. Moreover, knockdown of SIRT1 in 3T3-L1 adipocytes through the ectopic expression of a corresponding siRNA also increases secretion of HMW adiponectin as well as preventing the decline in PPARγ activity that normally occurs during terminal adipogenesis in 3T3-L1 cells. We next questioned whether SIRT1 controls the expression or activity of a component of the machinery participating in the formation of HMW adiponectin in the endoplasmic reticulum of the adipocyte. The results demonstrate an extensive induction of the ER oxidoreductase Ero1-Lα during the differentiation of 3T3-L1 preadipocytes into adipocytes that coincides with the differentiation-associated increase in secretion of adiponectin. Moreover, the data show that expression of Ero1-Lα mRNA is enhanced severalfold following exposure of adipocytes to the PPARγ ligand troglitazone, consistent with its gene being responsive to PPARγ activity. Additionally, suppression of SIRT1 activity through expression of siRNA also leads to a dramatic increase in Ero1-Lα production, along with enhanced secretion of HMW adiponectin. A direct role for Ero1-Lα in controlling adiponectin secretion was demonstrated by suppressing its production through the transient expression of Ero1-Lα RNAi during the terminal phase of adipogenesis in 3T3-L1 preadipocytes. Finally, we demonstrated that ectopic expression of Ero1-Lα leads to an extensive increase in secretion of HMW adiponectin in 3T3 adipocytes which overcomes the negative effects of activation of SIRT1 on adiponectin secretion.
The most likely mechanism by which SIRT1 regulates adiponectin secretion is through its ability to regulate PPARγ activity and, in so doing, also regulate expression of the PPARγ-responsive gene Ero1-Lα. It is interesting that not all PPARγ-responsive genes are influenced to the same extent as Ero1-Lα by SIRT1. Specifically, knockdown of SIRT1 in 3T3-L1 adipocytes leads to a dramatic increase in Ero1-Lα production but results in no detectable change in adiponectin or C/EBPα synthesis and only a modest increase in expression of fatty acid binding protein 4 (aP2). It is important to mention that recent studies by Qiao and Shao (28) have suggested that SIRT1 positively regulates adiponectin gene expression, which would appear to contradict with the results presented here. The data in those studies, however, showed that suppression of SIRT1 by siRNA technology leads to a decrease in cellular amounts of adiponectin based on Western blot analysis of total cell proteins. Those observations are consistent with data presented here in Fig. 4, since suppression of SIRT1 enhances adiponectin secretion with a corresponding decrease in intracellular adiponectin. Since Qiao and Shao did not measure adiponectin secretion or mRNA levels, it was not possible for them to draw any strong conclusions about the precise mechanism by which SIRT1 controls adiponectin production.
Taken together, the data suggest that SIRT1 might be controlling the activity of factors, aside from PPARγ, that control Ero1-Lα gene expression but have no role to play in control of other adipogenic genes. This notion is supported by the fact that Ero1-Lα is also produced in a nonadipogenic manner, since it is expressed in preadipocytes as well as other cell types (24). The other adipogenic genes, such as adiponectin and aP2, are adipocyte specific and, consequently, their expression in the adipocyte depends primarily on PPARγ activity. It is important to point out, however, that the enhancement of Ero1-Lα expression during adipogenesis does involve PPARγ. In this regard, it is conceivable that SIRT1 regulates PPARγ on the Ero1-Lα gene promoter differently from PPARγ associated with the other adipogenic gene promoters. In fact, our data show that mutation of F372 within helix 7 of the ligand-binding domain of PPARγ to an alanine generates a nuclear receptor that can respond to troglitazone by inducing adiponectin and aP2 expression but is incapable of enhancing production of Ero1-Lα (Fig. 8A, lane F). Wild-type PPARγ, however, is capable of inducing all three genes in response to the ligand (Fig. 8A, lane WT). These observations suggest that PPARγ might be capable of regulating multiple functions of the adipocyte by regulating defined groups of genes in response to a select set of metabolic effectors. For instance, PPARγ might respond to one set of effectors to enhance target gene expression while responding to another set to reduce expression of a different target gene. In fact, studies by Lazar and coworkers (6, 14, 20) have shown that PPARγ represses the expression of the glycerol kinase and oxidized low-density lipoprotein receptor genes in mature adipocytes while activating expression of other adipogenic genes, such as aP2. We suggest that Ero1-Lα also belongs to a similar group of adipocyte genes that are actively repressed by PPARγ in adipocytes by mechanisms that involve SIRT1 (Wang et al., unpublished data).
The data in Fig. 8C support the notion that Ero1-Lα plays a critical role in controlling the secretion of HMW adiponectin from adipocytes. The most likely mechanism involves Ero1-Lα functioning as an ER membrane-associated oxidoreductase by utilizing the oxidizing power of oxygen to generate disulfide bonds in itself, which it then transfers to PDI, and the resulting oxidized PDI is then able to transfer its disulfide bonds to adiponectin. It is also possible that Ero1-Lα participates in the release of adiponectin from the adipocyte by disrupting its retention in the endoplasmic reticulum by a process referred to as thiol retention of secretory proteins (1, 3, 15, 23). Studies have shown that another ER resident enzyme, ERp44, retains unassembled immunoglobulin μ chains in the cell through direct disulfide bond linkage (1). Disruption of this linkage through treatment of cells with reducing agents causes release of the immunoglobulin chains into the medium. Additionally, investigations have also shown that ERp44 can interact with Ero1-Lα through a similar thiol retention process (23). It is conceivable, therefore, that adiponectin might also be retained in the adipocyte through disulfide bond formation with ERp44 or other related proteins and that overexpression of Ero1-Lα disrupts this interaction, thereby enhancing the release of HMW adiponectin into the medium. In fact, our preliminary data support the notion of thiol retention of adiponectin, since treatment of 3T3-L1 adipocytes with β-mercaptoethanol significantly enhances the secretion of adiponectin (data not shown).
It is also important to consider other processes that might result in reduced adiponectin secretion in addition to the apparent decrease in Ero1-Lα expression. In this regard, it is possible that mature adipocytes suffer from both oxidative as well ER stress as a direct result of the extensive secretion of disulfide-linked proteins. The secretory process can generate reactive oxygen species as a by-product of the oxidoreductases in producing disulfide bonds. Additionally, it is very likely that unfolded proteins will also accumulate in the ER due to inefficient folding being a by-product of the active secretion process. Such stress responses could possibly feed back on the secretion of adipocyte proteins to allow for degradation of any unfolded proteins via ERAD and glutathione-mediated neutralization of reactive oxygen species (5, 7, 34). It will be important in future investigations to determine whether there is a direct link between ER stress and adiponectin secretion. In this regard, it will also be interesting to determine whether Ero1-Lβ, which is involved in the unfolded protein response, plays a role in regulating the function of adipocytes.
In conclusion, these investigations have uncovered a novel mechanism by which adipocytes can regulate secretion of adiponectin in response to changes in nutrient status. Of interest is the demonstration that SIRT1 appears to play an important role in mediating the response of adipocytes to perturbations in overall metabolism. SIRT1 has also been implicated in processes contributing to diminishment of bodily functions associated with aging (2, 33); consequently, it will be important to determine whether there is any role for the SIRT1-associated regulation of adiponectin in the development of insulin resistance and type 2 diabetes in the elderly. Since it is well accepted that adiponectin acts to sensitize the organism to insulin, these observations should provide information that leads to development of therapeutics to combat insulin resistance and type 2 diabetes.
Supplementary Material
Acknowledgments
We are grateful to Kathryn Davis for critical reading of the manuscript and constructive comments. We thank L. Guarente for the pSUPER SIRT1 siRNA plasmid.
This work was supported by U.S. PHS grants DK51586 and DK58825.
Footnotes
Published ahead of print on 23 April 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Anelli, T., M. Alessio, A. Bachi, L. Bergamelli, G. Bertoli, S. Camerini, A. Mezghrani, E. Ruffato, T. Simmen, and R. Sitia. 2003. Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44. EMBO J. 22:5015-5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bordone, L., and L. Guarente. 2005. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat. Rev. Mol. Cell Biol. 6:298-305. [DOI] [PubMed] [Google Scholar]
- 3.Brewer, J. W., and R. B. Corley. 1996. Quality control in protein biogenesis: thiol-mediated retention monitors the redox state of proteins in the endoplasmic reticulum. J. Cell Sci. 109:2383-2392. [DOI] [PubMed] [Google Scholar]
- 4.Cabibbo, A., M. Pagani, M. Fabbri, M. Rocchi, M. R. Farmery, N. J. Bulleid, and R. Sitia. 2000. ERO1-L, a human protein that favors disulfide bond formation in the endoplasmic reticulum. J. Biol. Chem. 275:4827-4833. [DOI] [PubMed] [Google Scholar]
- 5.Chakravarthi, S., C. E. Jessop, and N. J. Bulleid. 2006. The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep. 7:271-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chui, P. C., H. P. Guan, M. Lehrke, and M. A. Lazar. 2005. PPARγ regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J. Clin. Investig. 115:2244-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denic, V., E. M. Quan, and J. S. Weissman. 2006. A luminal surveillance complex that selects misfolded glycoproteins for ER-associated degradation. Cell 126:349-359. [DOI] [PubMed] [Google Scholar]
- 8.Dias-Gunasekara, S., and A. M. Benham. 2005. Defining the protein-protein interactions of the mammalian endoplasmic reticulum oxidoreductases (EROs). Biochem. Soc. Trans. 33:1382-1384. [DOI] [PubMed] [Google Scholar]
- 9.Dias-Gunasekara, S., J. Gubbens, M. van Lith, C. Dunne, J. A. Williams, R. Kataky, D. Scoones, A. Lapthorn, N. J. Bulleid, and A. M. Benham. 2005. Tissue-specific expression and dimerization of the endoplasmic reticulum oxidoreductase Ero1β. J. Biol. Chem. 280:33066-33075. [DOI] [PubMed] [Google Scholar]
- 10.Ellgaard, L., and L. W. Ruddock. 2005. The human protein disulphide isomerase family: substrate interactions and functional properties. EMBO Rep. 6:28-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fassio, A., and R. Sitia. 2002. Formation, isomerisation and reduction of disulphide bonds during protein quality control in the endoplasmic reticulum. Histochem. Cell Biol. 117:151-157. [DOI] [PubMed] [Google Scholar]
- 12.Frand, A. R., and C. A. Kaiser. 1998. The ERO1 gene of yeast is required for oxidation of protein dithiols in the endoplasmic reticulum. Mol. Cell 1:161-170. [DOI] [PubMed] [Google Scholar]
- 13.Frand, A. R., and C. A. Kaiser. 1999. Ero1p oxidizes protein disulfide isomerase in a pathway for disulfide bond formation in the endoplasmic reticulum. Mol. Cell 4:469-477. [DOI] [PubMed] [Google Scholar]
- 14.Guan, H. P., Y. Li, M. V. Jensen, C. B. Newgard, C. M. Steppan, and M. A. Lazar. 2002. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat. Med. 8:1122-1128. [DOI] [PubMed] [Google Scholar]
- 15.Isidoro, C., C. Maggioni, M. Demoz, A. Pizzagalli, A. M. Fra, and R. Sitia. 1996. Exposed thiols confer localization in the endoplasmic reticulum by retention rather than retrieval. J. Biol. Chem. 271:26138-26142. [DOI] [PubMed] [Google Scholar]
- 16.Iwaki, M., M. Matsuda, N. Maeda, T. Funahashi, Y. Matsuzawa, M. Makishima, and I. Shimomura. 2003. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 52:1655-1663. [DOI] [PubMed] [Google Scholar]
- 17.Kadowaki, T., and T. Yamauchi. 2005. Adiponectin and adiponectin receptors. Endocr. Rev. 26:439-451. [DOI] [PubMed] [Google Scholar]
- 18.Kadowaki, T., T. Yamauchi, N. Kubota, K. Hara, K. Ueki, and K. Tobe. 2006. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 116:1784-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kershaw, E. E., and J. S. Flier. 2004. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 89:2548-2556. [DOI] [PubMed] [Google Scholar]
- 20.Lehrke, M., and M. A. Lazar. 2005. The many faces of PPARgamma. Cell 123:993-999. [DOI] [PubMed] [Google Scholar]
- 21.Liu, J., H. Wang, Y. Zuo, and S. R. Farmer. 2006. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol. Cell. Biol. 26:5827-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Must, A., and S. E. Anderson. 2003. Effects of obesity on morbidity in children and adolescents. Nutr. Clin. Care 6:4-12. [PubMed] [Google Scholar]
- 23.Otsu, M., G. Bertoli, C. Fagioli, E. Guerini-Rocco, S. Nerini-Molteni, E. Ruffato, and R. Sitia. 2006. Dynamic retention of Ero1α and Ero1β in the endoplasmic reticulum by interactions with PDI and ERp44. Antioxid. Redox Signal. 8:274-282. [DOI] [PubMed] [Google Scholar]
- 24.Pagani, M., M. Fabbri, C. Benedetti, A. Fassio, S. Pilati, N. J. Bulleid, A. Cabibbo, and R. Sitia. 2000. Endoplasmic reticulum oxidoreductin 1-lbeta (ERO1-Lβ), a human gene induced in the course of the unfolded protein response. J. Biol. Chem. 275:23685-23692. [DOI] [PubMed] [Google Scholar]
- 25.Pajvani, U. B., M. Hawkins, T. P. Combs, M. W. Rajala, T. Doebber, J. P. Berger, J. A. Wagner, M. Wu, A. Knopps, A. H. Xiang, K. M. Utzschneider, S. E. Kahn, J. M. Olefsky, T. A. Buchanan, and P. E. Scherer. 2004. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J. Biol. Chem. 279:12152-12162. [DOI] [PubMed] [Google Scholar]
- 26.Picard, F., M. Kurtev, N. Chung, A. Topark-Ngarm, T. Senawong, R. Machado De Oliveira, M. Leid, M. W. McBurney, and L. Guarente. 2004. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429:771-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollard, M. G., K. J. Travers, and J. S. Weissman. 1998. Ero1p: a novel and ubiquitous protein with an essential role in oxidative protein folding in the endoplasmic reticulum. Mol. Cell 1:171-182. [DOI] [PubMed] [Google Scholar]
- 28.Qiao, L., and J. Shao. 2006. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J. Biol. Chem. 281:39915-39924. [DOI] [PubMed] [Google Scholar]
- 29.Rasouli, N., A. Yao-Borengasser, L. M. Miles, S. C. Elbein, and P. A. Kern. 2006. Increased plasma adiponectin in response to pioglitazone does not result from increased gene expression. Am. J. Physiol. Endocrinol. Metab. 290:E42-E46. [DOI] [PubMed] [Google Scholar]
- 30.Rodgers, J. T., C. Lerin, W. Haas, S. P. Gygi, B. M. Spiegelman, and P. Puigserver. 2005. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434:113-118. [DOI] [PubMed] [Google Scholar]
- 31.Schadinger, S. E., N. L. Bucher, B. M. Schreiber, and S. R. Farmer. 2005. PPARγ2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am. J. Physiol. Endocrinol. Metab. 288:E1195-E1205. [DOI] [PubMed] [Google Scholar]
- 32.Sevier, C. S., and C. A. Kaiser. 2002. Formation and transfer of disulphide bonds in living cells. Nat. Rev. Mol. Cell Biol. 3:836-847. [DOI] [PubMed] [Google Scholar]
- 33.Sinclair, D. A., and L. Guarente. 2006. Unlocking the secrets of longevity genes. Sci. Am. 294:48-51. [DOI] [PubMed] [Google Scholar]
- 34.Sitia, R., and I. Braakman. 2003. Quality control in the endoplasmic reticulum protein factory. Nature 426:891-894. [DOI] [PubMed] [Google Scholar]
- 35.Trujillo, M. E., and P. E. Scherer. 2005. Adiponectin: journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J. Intern. Med. 257:167-175. [DOI] [PubMed] [Google Scholar]
- 36.Tu, B. P., and J. S. Weissman. 2004. Oxidative protein folding in eukaryotes: mechanisms and consequences. J. Cell Biol. 164:341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waki, H., T. Yamauchi, J. Kamon, Y. Ito, S. Uchida, S. Kita, K. Hara, Y. Hada, F. Vasseur, P. Froguel, S. Kimura, R. Nagai, and T. Kadowaki. 2003. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J. Biol. Chem. 278:40352-40363. [DOI] [PubMed] [Google Scholar]
- 38.Wilson-Fritch, L., A. Burkart, G. Bell, K. Mendelson, J. Leszyk, S. Nicoloro, M. Czech, and S. Corvera. 2003. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol. Cell. Biol. 23:1085-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamauchi, T., J. Kamon, H. Waki, Y. Terauchi, N. Kubota, K. Hara, Y. Mori, T. Ide, K. Murakami, N. Tsuboyama-Kasaoka, O. Ezaki, Y. Akanuma, O. Gavrilova, C. Vinson, M. L. Reitman, H. Kagechika, K. Shudo, M. Yoda, Y. Nakano, K. Tobe, R. Nagai, S. Kimura, M. Tomita, P. Froguel, and T. Kadowaki. 2001. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 7:941-946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.