Abstract
The nuclear export and cytoplasmic degradation of the cyclin-dependent kinase inhibitor p27 are required for effective progression of the cell cycle through the G0-G1 transition. The mechanism responsible for this translocation of p27 has remained unclear, however. We now show that cyclin D2 directly links growth signaling with the nuclear export of p27 at the G0-G1 transition in some cell types. The up-regulation of cyclin D2 in response to mitogenic stimulation was found to occur earlier than that of other D-type cyclins and in parallel with down-regulation of p27 at the G0-G1 transition. RNA interference-mediated depletion of cyclin D2 inhibited the nuclear export of p27 and delayed its degradation at the G0-G1 transition. In contrast, overexpression of cyclin D2 in G0 phase shifted the localization of p27 from the nucleus to the cytoplasm and reduced the stability of p27. Overexpression of the cyclin D2(T280A) mutant, whose export from the nucleus is impaired, prevented the translocation and degradation of p27. These results indicate that cyclin D2 translocates p27 from the nucleus into the cytoplasm for its KPC-dependent degradation at the G0-G1 transition.
Progression of the cell cycle in eukaryotic cells depends on the activities of a series of protein complexes composed of cyclins and cyclin-dependent kinases (CDKs). The activities of these complexes are regulated by various mechanisms, including inhibition by CDK inhibitors (CKIs) (70). The CKI p27Kip1, which belongs to the Cip/Kip family of proteins, plays a pivotal role in the control of cell proliferation. Transition from G0 phase of the cell cycle to S phase is promoted by complexes of G1 cyclins (cyclins D1, D2, D3, and E) and CDKs (CDK4 or -6 for cyclin D and CDK2 for cyclin E), and p27 inhibits the activities of these complexes through direct interaction (70). However, p27 also promotes the assembly of D-type cyclins with CDK4 or CDK6 (43). Mice homozygous for deletion of the p27 gene are larger than normal mice and exhibit multiple-organ hyperplasia as well as a predisposition to both spontaneous and radiation- or chemical-induced tumors, reflecting the function of p27 in cell cycle regulation (22, 23, 37, 58).
In normal cells, the level of p27 is high during G0 phase but decreases rapidly on reentry of the cells into G1 phase (62, 66). This rapid removal of p27 at the G0-G1 transition is required for effective progression of the cell cycle to S phase. The abundance of p27 is thought to be controlled by multiple mechanisms that operate at the level of the synthesis (transcription and translation), degradation, and localization of this protein (1, 16, 29, 31, 52, 55, 63, 67). The ubiquitin-proteasome pathway contributes to such control by mediating the degradation of p27. In S and G2 phases of the cell cycle, degradation of p27 is promoted by its phosphorylation on Thr187 by the cyclin E-CDK2 complex; this reaction is required for the binding of p27 to Skp2, an F-box protein that functions as the receptor component of an SCF-type ubiquitin ligase complex (12, 56, 59-61, 69, 74, 77). Although SCFSkp2 has been shown to be largely responsible for the ubiquitylation of p27 in the nucleus during S and G2 phases, the ubiquitylation of p27 at the G0-G1 transition is independent of Skp2 and occurs in the cytoplasm (28). We recently showed that a cytoplasmic ubiquitin ligase complex, designated KPC, is important for proteolysis of p27 at the G0-G1 transition (27, 33, 40).
The translocation of p27 from the nucleus to the cytoplasm is necessary for KPC-dependent proteolysis. However, the mechanism responsible for such translocation in response to growth signals has been unclear. We and others have obtained biochemical evidence suggesting that the phosphorylation of p27 on Ser10 is important for this process (9, 16, 31, 67). However, the observation that Ser10 of p27 is phosphorylated in G0 phase in the absence of mitogenic signals (17, 32, 39) is not consistent with the notion that phosphorylation of this residue occurs in response to mitogenic stimulation and that it triggers the nuclear export of p27 (9). Furthermore, our analysis of mice that express a form of p27 in which Ser10 is replaced by alanine (p27S10A) revealed that the phosphorylation of Ser10 is not required for p27 translocation (39). Although a similar analysis by other researchers yielded the opposite conclusion (8), both we and this other group demonstrated that phosphorylation of Ser10 is an important determinant of the stability of p27 in G0 phase (8, 39). Other studies have implicated Jab1 or other phosphorylation sites of p27, such as Thr198 in the human protein, in the cytoplasmic translocation and localization of p27 (24, 46, 71, 76, 80). Not only phosphorylation of serine and threonine but also that of tyrosine by Src family kinase or c-Abl affects the stability of p27 protein (15, 26). A unified explanation for the nuclear export and degradation of p27 has thus been lacking.
D-type cyclins (cyclins D1, D2, and D3) were discovered to be factors whose expression is increased by growth signals and are therefore considered mediators of signaling that links extracellular stimuli to the cell cycle machinery (5, 51). These proteins form complexes with CDK4 or CDK6 that phosphorylate and inactivate the product (pRb) of the retinoblastoma tumor suppressor gene and the pRb-related proteins p107 and p130 (7, 49, 50, 53). This process is thought to be indispensable for progression of the cell cycle from G0-G1 to S phase, given that cells deficient in D-type cyclins or in CDK4 and -6 show marked defects in cell cycle progression when stimulated with serum (41, 48). D-type cyclins are also thought to contribute to another process important for the G0-G1 transition. Lymphocytes that lack cyclin D2 do not proliferate in response to mitogenic stimuli such as immunoglobulin M, largely as a result of a defect in the rapid elimination of p27 (45, 73). This observation suggested that D-type cyclins, in particular, cyclin D2, play an important role in the down-regulation of p27 at the G0-G1 transition. Indeed, the abundance of cyclin D2 has been shown to increase in association with the decrease in p27 expression in various cell types (6, 11, 45, 64).
We have now investigated whether cyclin D2 might be an effector that directly links growth factor signaling with the nuclear export and subsequent cytoplasmic degradation of p27 at the G0-G1 transition in NIH 3T3 cells and some other cells. We found that the expression of cyclin D2 was up-regulated earliest among the three D-type cyclins after stimulation of cells with serum. Furthermore, cyclin D2 was indeed found to mediate the translocation of p27 from the nucleus to the cytoplasm that precedes the KPC-dependent proteolysis of this CKI. Cyclins D1 and D3 appear not to participate directly in this process, indicating that cyclin D2 has a specific role in the down-regulation of p27 at the G0-G1 transition at least in some cell types.
MATERIALS AND METHODS
Cell culture, cell synchronization, and cell cycle analysis.
NIH 3T3 and HEK293T cells as well as mouse peripheral lymphocytes were cultured as described previously (31, 58). C2C12 myoblasts were cultured under the same conditions as HEK293T cells. Mouse embryonic fibroblasts (MEFs) were isolated and cultured as described previously (39, 59). For analysis of synchronized cells, MEFs as well as C2C12 and NIH 3T3 cells were arrested in G0 phase by serum deprivation for 48 to 96 h in medium supplemented with 0.1% fetal bovine serum or 0.1% calf serum and were then cultured in medium containing 10% fetal bovine serum or 10% calf serum to induce reentry into the cell cycle. Lymphocytes were stimulated as described previously (28). Phases of the cell cycle were determined by flow cytometry as described previously (28) but with slight modifications.
Construction of expression plasmids and their introduction into cultured cells.
Complementary DNAs encoding mouse cyclin D1 or D2 were cloned by reverse transcription-PCR, and the cDNA for mouse p27 was cloned as described previously (58). Complementary DNAs for dominant negative forms of human CDK4 [CDK4(D158N)] and CDK6 [CDK6(D163N)] (79) were generated by PCR. For retroviral expression, cDNAs for cyclin D1, D2, or D2(T280A) or p27 tagged with two copies of the Myc epitope (M2) or with the hemagglutinin epitope (HA) at their NH2 termini and those for wild-type CDK4 or the dominant negative forms of CDK4 or CDK6 tagged with HA at their COOH termini were subcloned into pMX-puro (kindly provided by T. Kitamura, University of Tokyo) (57), and the resulting vectors were introduced into Plat E cells by calcium phosphate-mediated transfection. The recombinant retroviruses thereby generated were used to infect C2C12 cells or NIH 3T3 cells, which were then subjected to selection in medium containing puromycin (5 to 10 μg/ml). Cells stably expressing the recombinant proteins were pooled for experiments. For expression in HEK293T cells, cDNAs for cyclins D1 and D2 tagged with two copies of the Myc epitope at their NH2 termini, for NH2-terminally HA-tagged p27, and for COOH-terminally HA-tagged dominant negative CDK4 or CDK6 were subcloned into pcDNA3 (Invitrogen). The cDNA for cyclin D2 was also subcloned into p3×FLAG-CMV7.1 (Sigma) for expression of a protein with three copies of the FLAG epitope at its NH2 terminus. Cells were transfected with the resulting vectors by the calcium phosphate method and were harvested after 48 h for experiments.
Establishment and maintenance of ecdysone-inducible cell lines.
Ecdysone-inducible cell lines were established with the use of an ecdysone-inducible mammalian expression system (Invitrogen). The pVgRXR vector was introduced into NIH 3T3 cells by calcium phosphate-mediated transfection, and cell colonies resistant to zeocin (700 μg/ml) were isolated and grown. These cells were then transfected with a pIND vector containing the cDNA for mouse cyclin D2 tagged with two copies of the Myc epitope. The cells were exposed to the ecdysone analog ponasterone A (10 μM) for induction of exogenous cyclin D2 expression.
Immunoprecipitation, immunoblot, immunofluorescence, and pulse-chase analyses.
Immunoprecipitation, immunoblot, and immunofluorescence analyses were performed as described previously (31) but with some modifications. Immunoprecipitation was performed with antibodies to p27 (C-19 [Santa Cruz Biotechnology]), to the Myc epitope (9E10 [Sigma]), to HA (HA11 [Covance] or Y-11 [Santa Cruz Biotechnology]), to FLAG (M2 [Sigma]), or to pRb (Pharmingen). For detection of p27-CRM1 binding, cells were lysed with CRM1 binding buffer (0.1% Triton X-100, 50 mM HEPES-NaOH [pH 7.5], 50 mM potassium acetate, 5 mM magnesium acetate, 150 mM NaCl, 10% glycerol, 1 mM dithiothreitol, 10 mM sodium pyrophosphate, 10 mM NaF, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, aprotinin [10 μg/ml], leupeptin [20 μg/ml]), and lysates (300 μg of protein) were subjected to immunoprecipitation with the use of Affi-Prep protein A support (Bio-Rad). Immunoblots were probed with antibodies to HA (HA11), to FLAG (M2), to the Myc epitope (9E10), to p27 (Transduction Laboratories), to cyclin D1 (72-13G [Santa Cruz Biotechnology]), to cyclin D2 (M-20 [Santa Cruz Biotechnology]), to cyclin D3 (Transduction Laboratories), to CDK4 (DCS-31 [Sigma] or C-22 [Santa Cruz Biotechnology]), to CDK6 (C-21 [Santa Cruz Biotechnology]), to human pRb phosphorylated on Ser780 (MBL), to phosphothreonine (Cell Signaling), to HSP70 (Transduction Laboratories), to CRM1 (Transduction Laboratories), or to KPC1 or KPC2 (33). Immunofluorescence analysis was performed with antibodies to p27 (Neomarkers), to cyclin D2 (Neomarkers), to the Myc epitope (9E10), or to HA (Y-11). Pulse-chase analysis was performed as described previously (33).
RNAi.
Construction of short hairpin RNA (shRNA) vectors and RNA interference (RNAi) were performed as described previously (33). The sequences targeted to mouse p27, Skp2, and cyclin D1, D2, or D3 mRNAs were 5′-GACAATCAGGCTGGGTTAGCG-3′ (p27-KD), 5′-GCTCTTCCTCGGCTGCAGATT-3′ (Skp2-KD), 5′-GTTGTGCATCTACACTGACAA-3′ (D1-KD), 5′-GGATGATGAAGTGAACACACT-3′ (D2-KD), and 5′-GCCGCACATGCGGAAGATGCT-3′ (D3-KD), respectively.
RESULTS
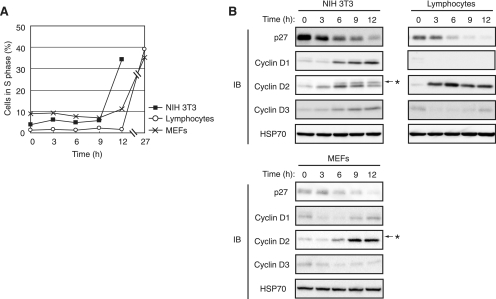
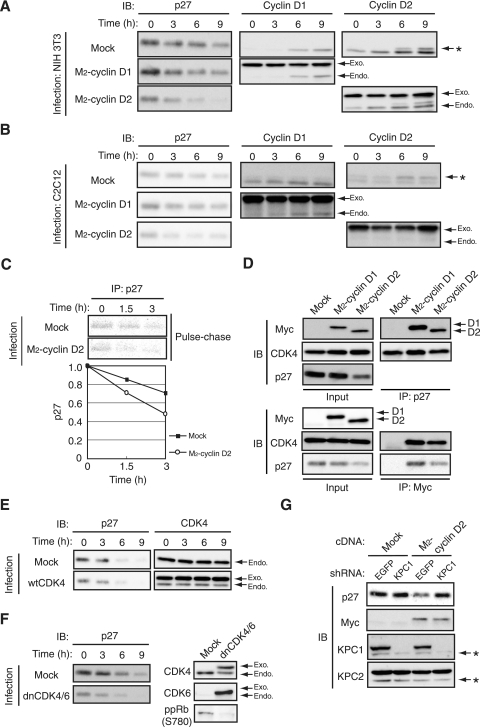
We examined changes in abundance of p27 and D-type cyclins in NIH 3T3 fibroblasts that had been arrested in G0 phase by serum deprivation for 3 days and then reexposed to serum in order to induce synchronized progression of the cell cycle to S phase (Fig. 1A). The amount of p27 was decreased as early as 3 h after serum stimulation (Fig. 1B). Among D-type cyclins, the increase in the amount of cyclin D2 was evident after serum stimulation for 3 h, unequivocally earlier than the increases in abundance of cyclins D1 and D3, which were first apparent at 6 to 9 h (Fig. 1B). Similar results were also obtained with MEFs arrested in G0 phase by serum deprivation for 4 days and then stimulated by serum. We also examined the changes in p27 and D-type cyclin abundance in mouse primary lymphocytes stimulated with the combination of a phorbol ester and Ca2+ ionophore (Fig. 1A). The increase in the amount of cyclin D2 again coincided with the decrease in that of p27, whereas the levels of cyclins D1 and D3 were very low or virtually undetectable (Fig. 1B). These findings suggested that cyclin D2 is up-regulated in parallel with the down-regulation of p27 in quiescent cells subjected to mitogenic stimulation. We next explored the possibility that cyclin D2 contributes to the down-regulation of p27 at the G0-G1 transition.
FIG. 1.
Up-regulation of cyclin D2 occurs in parallel with down-regulation of p27 at the G0-G1 transition. (A) Serum-deprived NIH 3T3 cells, MEFs, or mouse primary lymphocytes were stimulated for the indicated times by exposure to medium containing 10% calf serum, 10% fetal bovine serum, or 10 nM phorbol 12,13-dibutyrate and 300 nM ionomycin, respectively. The percentage of cells in S phase was determined by flow cytometry. (B) Lysates prepared from the cells described above were subjected to immunoblot (IB) analysis with antibodies to the indicated proteins. HSP70 was examined as an internal control. The band indicated by the asterisk is attributable to cross-reaction of the antibodies to cyclin D2 with cyclin D1.
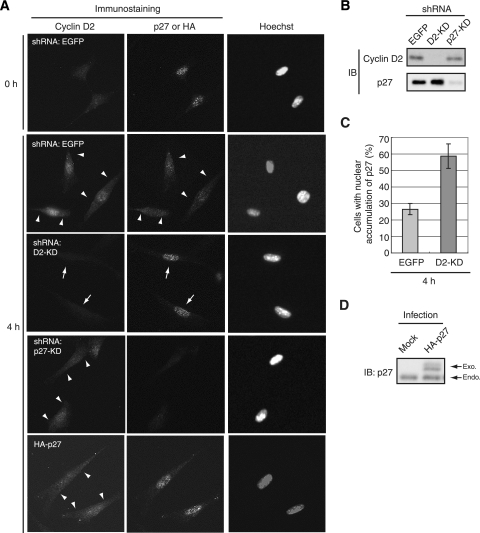
Immunofluorescence analysis revealed that endogenous cyclin D2 and p27 were each distributed in both the nucleus and the cytoplasm of NIH 3T3 cells in early G1 phase (Fig. 2A). To confirm that the observed signals were specific for cyclin D2 and p27, we performed RNAi to deplete cells of the endogenous proteins. The cells were thus infected with retroviral vectors encoding shRNAs specific for enhanced green fluorescent protein (EGFP) (control), cyclin D2, or p27 mRNAs. These shRNAs were highly efficient and specific in inducing depletion of the corresponding target protein (Fig. 2B). Immunofluorescence for cyclin D2 or p27 was not detected (or was greatly reduced) in cells subjected to RNAi for the corresponding mRNA (Fig. 2A), suggesting that the signals initially observed were not attributable to nonspecific staining with the antibodies. The amount of p27 in the nucleus was found to be increased in cells depleted of endogenous cyclin D2 by RNAi (Fig. 2A and C), whereas depletion or overexpression of p27 did not affect the distribution of cyclin D2 (Fig. 2A and D). These results suggested that depletion of cyclin D2 resulted in inhibition of the nuclear export of p27 and that cyclin D2 is thus a determinant of the localization of p27, whereas the opposite is not the case.
FIG. 2.
Depletion of cyclin D2 inhibits the cytoplasmic translocation of p27 in early G1 phase. (A) NIH 3T3 cells were infected with retroviral vectors encoding shRNAs specific for EGFP (control), cyclin D2 (D2-KD), or p27 (p27-KD) mRNAs or HA-tagged p27 and were arrested in G0 phase by serum deprivation for 72 h. The cells were then fixed immediately (0 h) or after stimulation for 4 h with 10% calf serum to induce synchronized progression of the cell cycle from G0 to early G1 phase. The cells were subjected to immunofluorescence analysis with antibodies to cyclin D2 and to p27 or HA and were stained with Hoechst 33258. Arrowheads indicate cytoplasmic signals of each protein. Arrows indicate nuclear accumulation of p27 in cells devoid of endogenous cyclin D2. Magnification, ×40. (B) The cells depleted of cyclin D2 or p27 and stimulated for 4 h as described above were lysed and subjected to immunoblot (IB) analysis with antibodies to cyclin D2 or p27. (C) Quantitative analysis of the subcellular localization of p27 in cells depleted of cyclin D2 and control cells stimulated for 4 h as described above. A total of 65 to 120 cells was scored for each sample. Data represent the percentages of cells showing a predominantly nuclear localization of p27 and are means ± standard errors of the means from three independent experiments. (D) Cells expressing HA-p27 (or infected with the corresponding empty vector [Mock]) and stimulated for 4 h as described above were lysed and subjected to IB analysis with antibodies to p27. Bands corresponding to the exogenous (Exo.) and endogenous (Endo.) proteins are indicated.
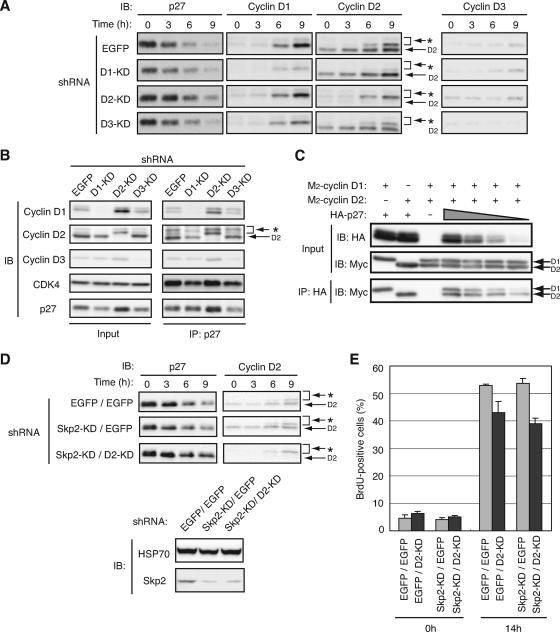
We investigated the possible contribution of endogenous D-type cyclins to the degradation of p27 at the G0-G1 transition by infection of NIH 3T3 cells with retroviral vectors encoding shRNAs specific for EGFP (control) or cyclin D1, D2, or D3 mRNAs. Depletion of cyclin D2 resulted in a delay in p27 degradation, whereas that of cyclin D1 or D3 did not (Fig. 3A). Given that depletion of cyclin D2 prevented nuclear export of p27 in early G1 phase (Fig. 2A), this delay is likely attributable to inhibition of the translocation of p27 into the cytoplasm. Similar results were obtained with another set of shRNAs that target different sequences in cyclin D1, D2, or D3 mRNAs (data not shown), suggesting that the observed effects were not nonspecific artifacts. We also found that a substantial amount of endogenous D-type cyclins and CDK4 associated with endogenous p27 even in G0 phase (Fig. 3B), consistent with previous observations (44, 81). These results thus suggested that depletion of cyclin D2 inhibited the translocation and degradation of p27 at the G0-G1 transition.
FIG. 3.
Depletion of cyclin D2 delays p27 degradation at the G0-G1 transition and inhibits progression of the cell cycle. (A) NIH 3T3 cells were infected with retroviral vectors for EGFP (control), cyclin D1 (D1-KD), cyclin D2 (D2-KD), or cyclin D3 (D3-KD) shRNAs, arrested in G0 phase by serum deprivation, and stimulated by reexposure to 10% serum for the indicated times. Cell lysates were then subjected to immunoblot (IB) analysis with antibodies to p27 or to cyclin D1, D2, or D3. The band indicated by the asterisk is attributable to cross-reaction of the antibodies to cyclin D2 with cyclin D1. (B) NIH 3T3 cells were subjected to RNAi as described for panel A, arrested in G0 phase, lysed, and subjected to immunoprecipitation (IP) with antibodies to p27. The resulting precipitates as well as the original cell lysates (Input) were subjected to IB analysis with antibodies to cyclin D1, D2, or D3, to CDK4, or to p27. (C) Lysates of HEK293T cells expressing various amounts of HA-tagged p27 and similar amounts of M2-cyclin D1 or M2-cyclin D2 were subjected to IP with antibodies to HA (HA11). The resulting precipitates as well as the original cell lysates (Input) were subjected to IB analysis with antibodies to HA or to the Myc epitope. (D) NIH 3T3 cells were subjected to RNAi for EGFP or cyclin D2 followed by RNAi for EGFP or Skp2, arrested in G0 phase by serum deprivation for 72 h, and stimulated by reexposure to 10% calf serum for the indicated times. Cell lysates were then subjected to IB analysis with antibodies to p27 or to cyclin D2. Those cells which were growing in asynchronized conditions were also harvested, and the lysates from the cells were subjected to IB analysis with antibodies to HSP70 (control) and Skp2. (E) NIH 3T3 cells were subjected to RNAi as described for panel D, arrested in G0 phase by serum deprivation for 72 h, and harvested or stimulated by reexposure to 10% serum for 14 h. Cells were exposed to 10 μM bromodeoxyuridine (BrdU) for 1 h before harvest. The cells were then subjected to fluorescence-activated cell sorter analysis with antibodies to BrdU. Data represent the percentages of cells positive for BrdU staining and are means ± standard errors of the means from two independent experiments.
To examine the functional difference between cyclins D1 and D2, we compared the binding affinities of p27 for these two cyclins. HEK293T cells that expressed approximately equal amounts of Myc epitope-tagged cyclins D1 and D2 together with various amounts of HA-tagged p27 were lysed and subjected to immunoprecipitation with antibodies to HA. The association of cyclins D1 and D2 with the precipitated HA-p27 was then detected by immunoblot analysis with antibodies to the Myc epitope (Fig. 3C). The amounts of cyclins D1 and D2 bound to p27 were similar when the expression level of p27 was high. In contrast, at lower levels of p27 expression, the amount of cyclin D2 bound to p27 was greater than that of cyclin D1. These results suggested that the functional difference between cyclins D1 and D2 in the regulation of p27 translocation and degradation depends not only on the subcellular localization of these proteins (see Fig. 4) but also on their binding affinity for p27.
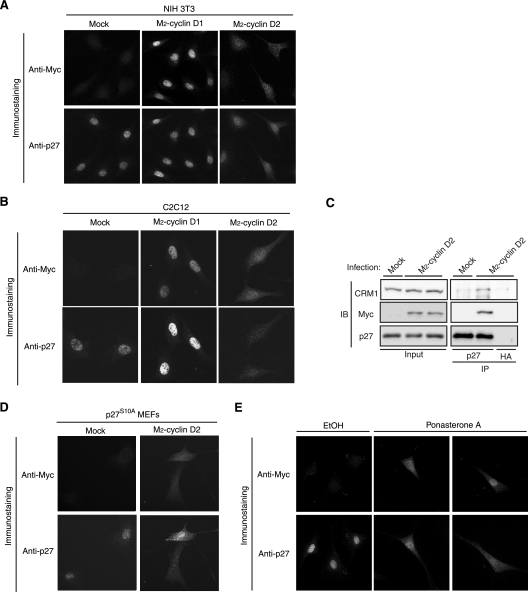
FIG. 4.
Overexpression of cyclin D2 induces translocation of p27 from the nucleus to the cytoplasm. (A) NIH 3T3 cells were infected with a retroviral vector for M2-cyclin D1 or M2-cyclin D2 or with the corresponding empty vector (Mock) and were then arrested in G0 phase by serum deprivation for 72 h. The cells were fixed and subjected to immunofluorescence analysis with antibodies to the Myc epitope and to p27. (B) C2C12 cells were infected as described above, arrested in G0 phase by serum deprivation for 48 h, and subjected to immunofluorescence analysis as described above. (C) NIH 3T3 cells infected with the vector for M2-cyclin D2 or the empty vector as described above were arrested in G0 phase, after which cell lysates were prepared and subjected to immunoprecipitation (IP) with antibodies to p27 or to HA (Y-11). The resulting precipitates as well as the original cell lysates (Input) were subjected to immunoblot (IB) analysis with antibodies to CRM1, to the Myc epitope, or to p27. The abundance of exogenous cyclin D2 was relatively low intentionally because a high level of cyclin D2 expression results in a substantial reduction in the amount of p27 (see Fig. 5A), making it difficult to compare precisely the amounts of CRM1 bound to p27. (D) MEFs derived from p27S10A knockin mice were subjected to overexpression of cyclin D2 as described above, arrested in G0 phase by serum deprivation for 72 h, fixed, and subjected to immunofluorescence analysis as described above. (E) NIH 3T3 cells of a line that expresses M2-cyclin D2 under the control of ecdysone were deprived of serum for 48 h and then incubated in the presence of ponasterone A (10 μM) or vehicle (ethanol [EtOH]) for 24 h. Cells were then fixed and subjected to immunofluorescence analysis as described above. Two independent representative images of ponasterone A-treated cells are shown. Magnification, ×40.
Given that the promotion of p27 degradation by cyclin D2 appeared to be mediated at the level of the cytoplasmic translocation of p27, we hypothesized that this effect of cyclin D2 would be dependent on the KPC-mediated pathway of p27 degradation rather than on the Skp2-mediated pathway. To test this hypothesis, we examined the effects of cyclin D2 and Skp2 depletion by RNAi on p27 degradation in NIH 3T3 cells. Depletion of Skp2 alone did not affect p27 degradation at the G0-G1 transition, consistent with our previous observation for primary lymphocytes (28). On the other hand, depletion of cyclin D2 together with Skp2 increased the abundance of p27 (Fig. 3D), as with the results for NIH 3T3 cells (Fig. 3A). Furthermore, the progression into S phase was substantially inhibited by depletion of cyclin D2 in both mock- and Skp2-depleted cells (Fig. 3E), suggesting that the promotion by cyclin D2 of both p27 degradation and cell cycle progression is largely independent of Skp2.
To examine whether cyclin D2 participates in p27 down-regulation at the G0-G1 transition, we infected NIH 3T3 or C2C12 cells with a retroviral vector for Myc epitope-tagged cyclin D2. Expression of the ectopic protein in G0 phase was used to recapitulate conditions of early G1 phase. As controls, cells were infected with the empty retroviral vector (mock) or with a vector for Myc epitope-tagged cyclin D1. The expression levels of exogenous cyclins D1 and D2 were similar to those of the corresponding endogenous proteins (see Fig. 5A). Immunofluorescence analysis revealed that p27 was localized exclusively in the nucleus of NIH 3T3 (Fig. 4A) or C2C12 (Fig. 4B) cells infected with the empty vector or the vector for cyclin D1, whereas cells overexpressing cyclin D2 manifested a reduced extent of nuclear staining for p27 and an increase in the amount of p27 in the cytoplasm. In both cell types, the localizations of exogenous cyclins D1 and D2 appeared to differ, with the former being located predominantly in the nucleus and the latter being detected in both the nucleus and the cytoplasm, a pattern similar to that for endogenous cyclin D2 in early G1 phase (Fig. 2A). The localizations of exogenous cyclins D1 (nucleus) and D2 (nucleus and cytoplasm) were similar to those of endogenous p27 in the respective cells. Furthermore, expression of exogenous cyclin D2 increased the binding of p27 to CRM1, a carrier protein for nuclear export (31) (Fig. 4C). These results thus suggested that cyclin D2 is able to change the subcellular localization of p27 in G0-arrested cells. To examine whether the translocation of p27 induced by cyclin D2 expression is dependent on the phosphorylation of p27 on Ser10, we expressed exogenous cyclin D2 in MEFs derived from p27S10A knockin mice (39). We found that p27S10A was translocated into the cytoplasm in G0 phase in response to ectopic expression of cyclin D2 (Fig. 4D), indicating that the nuclear export of p27 promoted by cyclin D2 in G0 phase is independent of phosphorylation of p27 on Ser10.
FIG. 5.
Overexpression of cyclin D2 promotes p27 degradation at the G0-G1 transition. (A) NIH 3T3 cells infected with a retroviral vector encoding M2-cyclin D1 or M2-cyclin D2 or with the corresponding empty vector were arrested in G0 phase by serum deprivation for 72 h and then reexposed to 10% calf serum for the indicated times. Cell lysates were subjected to immunoblot (IB) analysis with antibodies to p27, to cyclin D1, or to cyclin D2. The band indicated by the asterisk is attributable to cross-reaction of the antibodies to cyclin D2 with cyclin D1. The positions of bands corresponding to exogenous (Exo.) and endogenous (Endo.) proteins are shown. (B) C2C12 cells infected with retroviral vectors as described above were arrested in G0 phase by serum deprivation for 48 h and then reexposed to 10% fetal bovine serum for the indicated times. Cell lysates were subjected to IB analysis as described above. (C) NIH 3T3 cells infected with a retroviral vector for M2-cyclin D2 or with the empty vector were deprived of serum for 72 h, pulse-labeled with [35S]methionine and [35S]cysteine for 2 h, washed, and incubated for the indicated chase times in serum-deficient medium. Cell lysates were subjected to immunoprecipitation (IP) with antibodies to p27, and the resulting precipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Labeled proteins were detected by autoradiography (top), and the intensity of the 35S-p27 bands was quantified and plotted relative to the values at time zero. Data are means from three independent experiments. (D) NIH 3T3 cells infected with retroviral vectors and arrested in G0 phase as described above were lysed and subjected to IP with antibodies to p27 or to the Myc epitope. The resulting precipitates as well as the original cell lysates (Input) were subjected to IB analysis with antibodies to the Myc epitope, to CDK4, and to p27. The relative level of exogenous cyclin D1 was lower than that used in the experiment described in panel A to make it similar to that of cyclin D2. (E) NIH 3T3 cells infected with a retroviral vector encoding wild-type (wt) CDK4 or with the corresponding empty vector were arrested in G0 phase by serum deprivation for 72 h and then reexposed to 10% calf serum for the indicated times. Cell lysates were subjected to IB analysis with antibodies to p27 or to CDK4. (F) NIH 3T3 cells infected with retroviral vectors encoding dnCDK4/6 or with the corresponding empty vector were arrested in G0 phase and then reexposed to 10% calf serum for the indicated times. Cell lysates were subjected to IB analysis with antibodies to p27. Lysates of asynchronous cells were also subjected to IB analysis with antibodies to CDK4 and to CDK6. In addition, cells harvested in S phase (14 h after the onset of serum stimulation) were lysed and subjected to IP with antibodies to pRb. The resulting precipitates were subjected to IB analysis with antibodies to the phospho-Ser780 form of human pRb (ppRb) (Ser773 in mouse pRb). (G) NIH 3T3 cells expressing M2-cyclin D2 or those infected with the corresponding empty vector were infected with retroviral vectors encoding shRNAs specific for EGFP (control) or KPC1 mRNAs. The cells were arrested in G0 phase, lysed, and subjected to IB analysis with antibodies to the indicated proteins. Asterisks indicate nonspecific bands.
To eliminate the possibility that these observations were artifacts of retroviral infection, we established an NIH 3T3 cell line in which the expression of Myc epitope-tagged cyclin D2 is induced by ponasterone A, an analog of the steroid hormone ecdysone. These cells were arrested in G0 phase, and expression of exogenous cyclin D2 was induced. Consistent with the results obtained by retroviral infection, induction of cyclin D2 expression by ponasterone A resulted in the translocation of p27 from the nucleus to the cytoplasm (Fig. 4E), whereas ethanol treatment (control) did not affect the nuclear localization of p27 at G0 phase. Similar results were obtained with other independent cell lines (data not shown). These data thus indicate that cyclin D2 possesses an intrinsic ability to alter the subcellular localization of p27, and they suggest that the rapid increase in cyclin D2 expression at the G0-G1 transition is important for the concomitant nuclear export of p27.
We next investigated further the decrease in the abundance of p27 at the G0-G1 transition in NIH 3T3 cells that overexpress cyclin D1 or D2. Overexpression of cyclin D1 did not markedly affect the kinetics of p27 down-regulation (Fig. 5A). Consistent with the results of our immunofluorescence analysis (Fig. 4A), the amount of p27 in G0-arrested cells overexpressing cyclin D2 was smaller than that in mock-infected cells or in cells overexpressing cyclin D1 (Fig. 5A). Similar results were obtained with C2C12 cells (Fig. 5B). To confirm that this decrease in p27 expression resulted from an increased turnover rate of p27, we performed a pulse-chase analysis. The half-life of p27 in cyclin D2-overexpressing NIH 3T3 cells was indeed shorter than that in control cells (Fig. 5C). Overexpression of cyclin D2 also accelerated the decrease in the amount of p27 that occurs at the G0-G1 transition in both NIH 3T3 and C2C12 cells (Fig. 5A and B). A coimmunoprecipitation assay showed that endogenous p27 was associated with the recombinant cyclin D1 or D2 as well with CDK4 in the transfected NIH 3T3 cells (Fig. 5D). These results suggested that cyclin D2 has the ability to promote p27 degradation at the G0-G1 transition. The specificity of this effect of cyclin D2 may be attributable in part to the difference between the subcellular localizations of cyclins D1 and D2 (Fig. 4A).
We then examined whether the kinase activity of CDK4 or CDK6 is required for the degradation of p27 in G1 phase by expressing wild-type or dominant negative mutant (dnCDK4/6) forms of these enzymes in NIH 3T3 cells. Expression of wild-type CDK4 promoted the degradation of p27 in G1 phase (Fig. 5E). A similar accelerated decrease in the amount of p27 was apparent in cells expressing dnCDK4/6 (Fig. 5F), indicating that CDK4/6 kinase activity is not necessary for this effect. We confirmed that expression of dnCDK4/6 inhibited the phosphorylation of pRb on Ser773 (equivalent to Ser780 of human pRb) (Fig. 5F), a reaction known to be mediated by CDK4 or -6 (36). The abundance of p27 in G0 phase was reduced in cells expressing wild-type CDK4 or dnCDK4/6 (Fig. 5E and F), similar to the effect of overexpression of cyclin D2. The presence of CDK4 or -6 thus appears to promote the degradation of p27 independently of its kinase activity, suggesting that the cyclin D2-CDK complex may mediate the translocation of p27 into the cytoplasm more effectively than does cyclin D2 alone.
Given that overexpression of cyclin D2 induced the translocation of p27 from the nucleus to the cytoplasm and its down-regulation in G0-arrested cells, it was likely that cyclin D2 increased the extent of p27 degradation in a manner dependent on the cytoplasmic ubiquitin ligase KPC, which is constitutively active throughout the cell cycle (33). To test whether KPC contributes to the destabilization of p27 induced by cyclin D2, we depleted cells of the KPC1 subunit of KPC by RNAi (33). In G0 phase, p27 is normally localized to the nucleus and unavailable for degradation by KPC. Depletion of KPC1 thus had virtually no effect on the abundance of p27 in G0-arrested control NIH 3T3 cells (Fig. 5G). In cells overexpressing cyclin D2, however, in which the amount of p27 was reduced compared with that in control cells, depletion of KPC1 restored the level of p27 to that apparent in control cells. These data thus suggested that cyclin D2 facilitates the KPC-dependent degradation of p27 by escorting p27 into the cytoplasm.
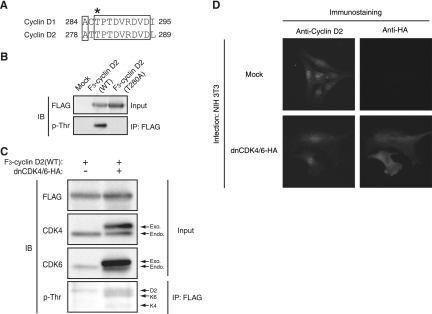
If cyclin D2 has the ability to change the subcellular localization of p27, expression of a mutant form of cyclin D2 that localizes only to the nucleus would be expected to inhibit p27 translocation to the cytoplasm. Glycogen synthase kinase-3β has been shown to induce the nuclear export and destabilization of cyclin D1 through phosphorylation of this cyclin on Thr286. The cyclin D1(T286A) mutant thus exhibited a prolonged half-life and nuclear localization as a result of its impaired export from the nucleus (18, 19). Given that the amino acid sequences of cyclins D1 and D2 are well conserved in the region containing this phosphorylation site (Fig. 6A), we generated cyclin D2(T280A) as the equivalent mutant of cyclin D1(T286A). We expressed FLAG-tagged wild-type or T280A mutant forms of cyclin D2 in HEK293T cells, subjected cell lysates to immunoprecipitation with antibodies to the FLAG epitope, and subjected the immunoprecipitates to immunoblot analysis with antibodies to phosphothreonine. Wild-type cyclin D2 was found to contain phosphothreonine, whereas the T280A mutant did not (Fig. 6B). These results thus indicated that cyclin D2 is phosphorylated predominantly on Thr280, as cyclin D1 is phosphorylated on Thr286. Expression of dnCDK4/6 did not inhibit the threonine phosphorylation of ectopic wild-type cyclin D2 (Fig. 6C) or the translocation of endogenous cyclin D2 (Fig. 6D), suggesting that the phosphorylation of cyclin D2 on Thr280 and its translocation (see Fig. 7) are independent of CDK activity.
FIG. 6.
Cyclin D2 is phosphorylated on Thr280 in a manner independent of CDK4/6 activity. (A) Alignment of amino acid sequences in the COOH-terminal region of mouse cyclins D1 and D2. Conserved amino acids are boxed. The Thr286 phosphorylation site of cyclin D1 and the corresponding residue (Thr280) of cyclin D2 are indicated by the asterisk. (B) HEK293T cells expressing 3×FLAG (F3)-tagged wild-type (WT) cyclin D2 or cyclin D2(T280A), or those transfected with the corresponding empty vector, were incubated for 4 h with 10 μM MG132 (proteasome inhibitor) and exposed for the final 30 min of the incubation to 50 nM calyculin A and 5 μM cyclosporine A (phosphatase inhibitors). Cell lysates were then subjected to immunoprecipitation (IP) with antibodies to FLAG, and the resulting precipitates were subjected to immunoblot (IB) analysis with antibodies to phosphothreonine (p-Thr). The original cell lysates (Input) were also subjected to IB analysis with antibodies to FLAG. (C) HEK293T cells expressing F3-tagged WT cyclin D2 and HA-tagged dnCDK4/6, as indicated, were incubated with MG132, calyculin A, and cyclosporine A as described for panel B. Cell lysates were then subjected to IP with antibodies to FLAG, and the resulting precipitates were subjected to IB analysis with antibodies to p-Thr. p-Thr was detected in dnCDK4/6 as well as in cyclin D2 (arrows). The original cell lysates (Input) were also subjected to IB analysis with antibodies to FLAG, to CDK4, and to CDK6. Exo., exogenous; Endo., endogenous. (D) NIH 3T3 cells infected with retroviral vectors for HA-tagged dnCDK4/6 or with the corresponding empty vector (Mock) were arrested in G0 phase, stimulated by exposure to 10% calf serum for 4 h to induce synchronized progression from G0 to early G1 phase, fixed, and subjected to immunofluorescence analysis with antibodies to cyclin D2 and to HA (Y-11). Magnification, ×40.
FIG. 7.
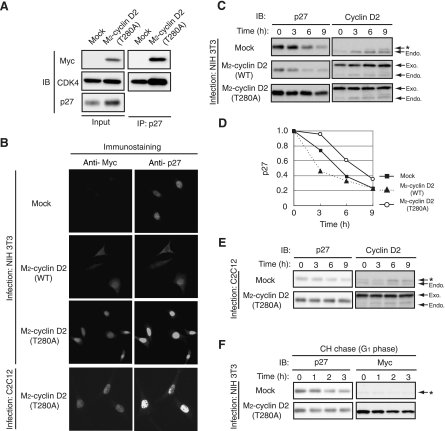
Cyclin D2(T280A) inhibits nuclear export of p27 and delays its degradation. (A) NIH 3T3 cells infected with a retroviral vector for M2-cyclin D2(T280A) or with the corresponding empty vector (Mock) were arrested in G0 phase by serum deprivation for 72 h. Cell lysates were then subjected to immunoprecipitation (IP) with antibodies to p27. The resulting precipitates as well as the original cell lysates (Input) were subjected to immunoblot (IB) analysis with antibodies to the Myc epitope, to CDK4, and to p27. (B) NIH 3T3 or C2C12 cells infected with a retroviral vector for M2-tagged wild-type (WT) cyclin D2 or cyclin D2(T280A) or with the corresponding empty vector were arrested in G0 phase by serum deprivation, fixed, and subjected to immunofluorescence analysis with antibodies to the Myc epitope and to p27. Magnification, ×40. (C) NIH 3T3 cells infected with a retroviral vector for M2-tagged WT cyclin D2 or cyclin D2(T280A) or with the corresponding empty vector were arrested in G0 phase by serum deprivation and then reexposed to 10% serum for the indicated times. Cell lysates were subjected to IB analysis with antibodies to p27 or to cyclin D2. The positions of the exogenous (Exo.) and endogenous (Endo.) cyclin D2 proteins are indicated. The band indicated by the asterisk is attributable to cross-reaction of the antibodies to cyclin D2 with cyclin D1. (D) Quantitation of p27 band intensities relative to the corresponding values for time zero in experiments similar to the experiment with results shown in panel C. Data are means from three independent experiments. (E) An experiment similar to that with results shown in panel C was performed with C2C12 cells. (F) NIH 3T3 cells infected with a retroviral vector for M2-cyclin D2(T280A) or with the corresponding empty vector were arrested in G0 phase by serum deprivation, reexposed to 10% serum for 3 h, and then incubated in the additional presence of cycloheximide (CH) (50 μg/ml) for the indicated times. Cell lysates were subjected to IB analysis with antibodies to p27 or to the Myc epitope. The asterisk indicates nonspecific bands.
Coimmunoprecipitation analysis also confirmed that cyclin D2(T280A) interacted with endogenous p27 in NIH 3T3 cells and that expression of cyclin D2(T280A) increased the abundance of p27 in NIH 3T3 cells arrested in G0 (Fig. 7A). Similarly to previous observations with cyclin D1(T286A) (19), cyclin D2(T280A) was more stable and accumulated in the nucleus to a greater extent than the wild-type protein in G0-arrested NIH 3T3 or C2C12 cells (Fig. 7B; data not shown). Furthermore, endogenous p27 accumulated in the nucleus to a greater extent in cells expressing cyclin D2(T280A) than in control cells, an effect opposite that apparent in cells overexpressing wild-type cyclin D2. These results suggested that the subcellular localization of cyclin D2 is an important determinant of p27 localization and stability. We therefore next examined whether cyclin D2(T280A) affected p27 degradation at the G0-G1 transition. Whereas overexpression of wild-type cyclin D2 markedly reduced the abundance of p27 in G0 phase and promoted p27 degradation at the G0-G1 transition, expression of cyclin D2(T280A) inhibited p27 degradation at the G0-G1 transition in NIH 3T3 cells (Fig. 7C and D). A similar effect was observed to occur in C2C12 cells (Fig. 7E). A cycloheximide chase experiment also revealed that p27 stability was increased in NIH 3T3 cells expressing cyclin D2(T280A) compared with that in control cells (Fig. 7F). These results indicate that cytoplasmic translocation of cyclin D2 is required for the cytoplasmic translocation and degradation of p27 and that this function of cyclin D2 depends on its phosphorylation on Thr280.
DISCUSSION
The rapid degradation of p27 at the G0-G1 transition is necessary for effective progression of the cell cycle to S phase. We have shown previously that the nuclear export and KPC-dependent ubiquitylation of p27, rather than the classical SCFSkp2-dependent p27 ubiquitylation that takes place in the nucleus, are largely responsible for such progression of cells to S phase (27, 28, 31, 33, 40, 61). However, the precise mechanism by which the growth factor-dependent translocation of p27 from the nucleus to the cytoplasm is achieved has been controversial. We have now shown that cyclin D2 is required for such translocation of p27 at the G0-G1 transition. Cyclin D2 is expressed in G0 phase, albeit at a relatively low level. In asynchronously growing cells, cyclin D2 appears to be localized predominantly in the nucleus (47). In G1 cells, however, we found that a subset of cyclin D2 molecules was clearly localized to the cytoplasm, consistent with previous observations (47, 68, 75). Specific depletion of cyclin D2 by RNAi greatly reduced the cytoplasmic immunofluorescence signal obtained with antibodies to cyclin D2, excluding the possibility that it was an artifact due to a nonspecific reaction of the antibodies. We thus propose that cyclin D2 is localized in the cytoplasm at least in some phases of the cell cycle in some cell types.
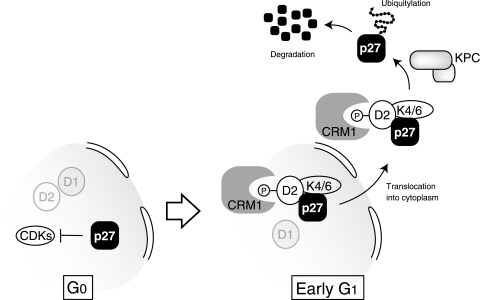
The expression of cyclin D2 was found to increase in parallel with the down-regulation of p27 at the G0-G1 transition, and this up-regulation of cyclin D2 occurred earlier than did that of cyclins D1 and D3. Forced expression of cyclin D2 with either a retroviral or an inducible expression system triggered the cytoplasmic translocation of p27 in G0-arrested cells, and this effect appeared to underlie the associated destabilization of p27 by cyclin D2. Cyclin D2(T280A), a mutant form of cyclin D2 that lacks the ability to translocate from the nucleus to the cytoplasm, inhibited the cytoplasmic translocation and degradation of p27, suggesting that the translocation of cyclin D2 is required for that of p27. Furthermore, depletion of endogenous cyclin D2 by RNAi slowed the rate of cytoplasmic translocation and degradation of p27 at the G0-G1 transition, a finding that is consistent with previous observations on the kinetics of p27 degradation in lymphocytes derived from cyclin D2-deficient mice (45). On the basis of our results, we propose that cyclin D2 mediates the translocation of p27 from the nucleus to the cytoplasm and thereby promotes the KPC-dependent degradation of p27 at the G0-G1 transition (Fig. 8). Consistent with this model, the promotion of p27 degradation by cyclin D2 was found to be dependent on KPC but largely independent of Skp2.
FIG. 8.
Model for cyclin D2-dependent cytoplasmic translocation and degradation of p27. The CKI p27 is translocated from the nucleus to the cytoplasm at the G0-G1 transition by cyclin D2 molecules that have been phosphorylated on Thr280. Our data suggest that p27, cyclin D2, and CDK4 or -6 form a complex in early G1 phase. The nuclear transporter CRM1 may recognize phosphorylated cyclin D2 and thereby mediate p27 translocation. Once in the cytoplasm, p27 is ubiquitylated by KPC and degraded by the 26S proteasome.
Phosphorylation of p27 on Ser10 has been thought to occur in response to mitogenic signaling and to be required for the cytoplasmic translocation of p27 (9, 16, 31, 67). However, our results suggest that p27 is phosphorylated on Ser10 in G0 phase in the absence of mitogenic stimulation. Furthermore, our analysis of p27S10A knockin mice revealed that p27 phosphorylation on Ser10 is not necessary for the translocation of p27 into the cytoplasm (39). Rather, Ser10 phosphorylation is important for the stability of p27 in the nucleus at G0 phase (8, 39). We have now extended these observations by showing that cyclin D2 also promotes the cytoplasmic translocation of p27 in MEFs derived from p27S10A knockin mice. Furthermore, KPC1 was found to bind to p27S10A as well as it did to wild-type p27 (T. Kamura and K. I. Nakayama, unpublished data), suggesting that KPC-mediated degradation of p27 in the cytoplasm is independent of p27 phosphorylation on Ser10. Together, these observations suggest that the mechanism responsible for the control of p27 stability by Ser10 phosphorylation in G0 phase differs from the pathway mediated by cyclin D2 and KPC.
Cyclins D1 and D3 did not show the same effects on p27 translocation and degradation as did cyclin D2. Although the three D-type cyclins share substantial amino acid sequence similarity (75 to 78% identity) in the cyclin box, a conserved domain important for binding to CDKs, the extent of sequence identity outside of this domain is only 39 to 47%. Indeed, previous studies have provided evidence of functional differences among the D-type cyclins. For example, although cyclin D2 activates CDK2, cyclin D1 does not (20, 21, 50, 53). Conversely, cyclin D1 binds to and activates the estrogen receptor, whereas cyclins D2 and D3 do not (83). Inhibitory activities toward other proteins, including pRb and v-Myb, also appear to differ among the D-type cyclins (4, 25). In addition, we have now shown that the subcellular localization and p27 binding affinity of cyclin D1 differ from those of cyclin D2. The contributions of D-type cyclins to cell cycle arrest or cell differentiation also appear to differ (34, 35, 54).
Analysis of genetically engineered mice has revealed functional redundancy and specificity for the D-type cyclins. Cyclin D2-deficient lymphocytes show a reduced responsiveness to mitogenic signals as a result of the accumulation of p27 (45, 73), with cyclin D2 appearing to be indispensable for p27 degradation in lymphocytes. In addition to lymphocytes, the ovaries, testes, and pancreas manifest phenotypes in cyclin D2-deficient mice (42, 72) that appear opposite to those in p27-deficient mice (23, 37, 58, 78). In the ovaries, cyclin D2 seems to antagonize the inhibition of granulosa cell proliferation mediated by p27 (10). Mice engineered to express cyclin D2 instead of cyclin D1 still manifest neural defects characteristic of cyclin D1 knockout mice, despite the fact that other defects of the latter animals were corrected by cyclin D2 gene knockin, suggestive of functional differences between cyclins D1 and D2 in neural development (13). Such genetic evidence suggests that cyclin D2 antagonizes p27 function, presumably through its control of p27 stability (45), and that cyclin D2 has a specific role in this regard that is not mimicked by cyclin D1 or D3. These previous and our present observations indicate that cyclin D2 plays an important role in p27 degradation under physiological conditions, at least in cell types in which cyclin D2 is dominant among the three D-type cyclins.
The mechanisms underlying the nuclear export of cyclin D1 and that of cyclin D2 appear to be similar. Cyclin D1 is exported from the nucleus in a CRM1-dependent manner, and the phosphorylation of cyclin D1 on Thr286 is required for its binding to CRM1 (2, 18). The sequence surrounding Thr286 of cyclin D1 is well conserved in cyclin D2, and our data now indicate that Thr280 of cyclin D2 (corresponding to Thr286 of cyclin D1) is indeed phosphorylated. Consistent with this notion, a recent study showed that glycogen synthase kinase-3β regulates the stability of cyclin D2 (30). We hypothesized that CRM1 interacts with cyclin D2 in a Thr280 phosphorylation-dependent manner, resulting in the translocation of p27 associated with the CRM1-cyclin D2 complex into the cytoplasm (Fig. 8). Despite these similarities, the localizations of cyclins D1 and D2 as well as their effects on p27 stability are substantially different. In contrast to our proposal, some previous studies have suggested that p27 (or p21) determines the nuclear localization of the cyclin D1-CDK4 complex (3, 43, 65). At least under the conditions of our experiments, however, forced expression or RNAi-mediated depletion of p27 did not affect the localization of cyclin D2, whereas such modulation of cyclin D2 abundance markedly affected the localization and stability of p27. Given that most of these previous studies referred only to cyclin D1 and that we have shown here that cyclins D1 and D2 differ in both subcellular localization and binding affinity for p27, the previous data for cyclin D1 cannot necessarily be extended to cyclin D2.
Differences in the spatial and temporal regulations of the D-type cyclins likely contribute to the functional differences among these proteins. The difference in subcellular localization between (endogenous or overexpressed) cyclins D1 and D2 observed in the present study is probably attributable to a difference in phosphorylation status of these molecules. Furthermore, the difference in timing of the up-regulation of the D-type cyclins at the G0-G1 transition may contribute to the individual characteristics of these proteins. It is also possible that the modification of p27 in response to growth signals affects its binding affinity for cyclin D1 or D2. Previous studies have shown that human p27 is phosphorylated on Ser10 or Thr198 (Thr197 in mouse p27) by kinases activated in response to growth signaling and that such phosphorylation may affect the interaction between p27 and cyclins/CDKs (9, 24, 82). Alternatively, degradation of cyclin D1- or cyclin D2-associated p27 might be intrinsically different regardless of p27 modification status. It is also possible that the different affinities of p27 for cyclins D1 and D2 might reflect a difference in the associations with CDK4 or CDK6. These possibilities remain to be tested in future studies.
Previous studies have suggested that cyclin D2 contributes to the stabilization and titration of p27 (38, 41). This apparent discrepancy with our present findings may be attributable to a difference in phosphorylation status of cyclin D2 on Thr280. The nonphosphorylated form of cyclin D2, which is mimicked by the cyclin D2(T280A) mutant, was found to accumulate in the nucleus and to increase the abundance of p27. However, our results suggest that a subset of cyclin D2 molecules phosphorylated on Thr280 translocates from the nucleus to the cytoplasm in association with p27, with the result that p27 is destabilized by KPC-dependent ubiquitylation. According to our model, the phosphorylation of cyclin D2 on Thr280 may function as a molecular switch to control these two functions (p27 stabilization versus destabilization) of cyclin D2.
The possible contribution of CDK4 or -6 to the cytoplasmic translocation of p27 remains to be determined. The interaction between exogenous cyclin D1 and CDK4 appears weak but is detectable in G0 phase (14). It was shown previously that p27 binds to free cyclin D1 or free CDK4 as well as to the cyclin D1-CDK4 complex in experiments with recombinant proteins (81). In quiescent NIH 3T3 cells, ectopic cyclin D1 associates efficiently with CDK4, but the resulting complex is inactive (44). It remains unclear how much p27 binds to free cyclin D2 and the cyclin D2-CDK4 complex. We found that exogenous cyclins D1 and D2 interacted with endogenous CDK4 of NIH 3T3 cells in G0 phase. We also detected interaction between endogenous p27 and endogenous cyclins D1, D2, and D3 as well as CDK4. Together, these data suggest that p27, cyclin D, and CDK4 form a complex in G0 phase. Although the kinase activity of CDK4 or -6 did not seem necessary for the degradation of p27 in G1 phase, our results indicate that these kinases nevertheless promote p27 degradation. We propose that the ability of the cyclin D2-CDK4 complex to escort p27 into the cytoplasm is greater than that of cyclin D2 alone.
The expression of D-type cyclins shows tissue specificity, and it is unlikely that p27 degradation is dependent on the cyclin D2-KPC pathway in all cell types. Our present data indicate that cyclin D2 promotes the nuclear export of p27 in at least three different cell types: NIH 3T3 fibroblasts, mouse primary lymphocytes, and C2C12 myoblasts. In addition, previous genetic evidence has shown that cyclin D2-deficient lymphocytes have a reduced responsiveness to mitogenic signals as a result of p27 accumulation (45), suggesting that cyclin D2 is indispensable for p27 degradation in these cells. However, we do not exclude the possibility that p27 translocation and stability are controlled by other D-type cyclins in cells that do not express cyclin D2. The regulation of p27 abundance in such cells remains to be elucidated.
In conclusion, we have identified a novel role for cyclin D2 in the translocation of p27 from the nucleus to the cytoplasm at the G0-G1 transition. The rapid up-regulation of cyclin D2 in response to mitogenic stimulation is thus important for the down-regulation of p27 at this transition. Our results thus indicate that cyclin D2 is one of the missing links between growth signals and the export of p27 from the nucleus.
Acknowledgments
We thank N. Ishida and T. Kamura for helpful discussions; N. Nishimura, R. Mitsuyasu, F. Matsuzaki, and other laboratory members for technical assistance; and M. Kimura and A. Ohta for help in preparation of the manuscript.
Footnotes
Published ahead of print on 23 April 2007.
REFERENCES
- 1.Agrawal, D., P. Hauser, F. McPherson, F. Dong, A. Garcia, and W. J. Pledger. 1996. Repression of p27kip1 synthesis by platelet-derived growth factor in BALB/c 3T3 cells. Mol. Cell. Biol. 16:4327-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alt, J. R., J. L. Cleveland, M. Hannink, and J. A. Diehl. 2000. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 14:3102-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alt, J. R., A. B. Gladden, and J. A. Diehl. 2002. p21Cip1 promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J. Biol. Chem. 277:8517-8523. [DOI] [PubMed] [Google Scholar]
- 4.Baker, G. L., M. W. Landis, and P. W. Hinds. 2005. Multiple functions of D-type cyclins can antagonize pRb-mediated suppression of proliferation. Cell Cycle 4:330-338. [PubMed] [Google Scholar]
- 5.Baldin, V., J. Lukas, M. J. Marcote, M. Pagano, and G. Draetta. 1993. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 7:812-821. [DOI] [PubMed] [Google Scholar]
- 6.Banerji, L., J. Glassford, N. C. Lea, N. S. Thomas, G. G. Klaus, and E. W. Lam. 2001. BCR signals target p27Kip1 and cyclin D2 via the PI3-K signalling pathway to mediate cell cycle arrest and apoptosis of WEHI 231 B cells. Oncogene 20:7352-7367. [DOI] [PubMed] [Google Scholar]
- 7.Bates, S., L. Bonetta, D. MacAllan, D. Parry, A. Holder, C. Dickson, and G. Peters. 1994. CDK6 (PLSTIRE) and CDK4 (PSK-J3) are a distinct subset of the cyclin-dependent kinases that associate with cyclin D1. Oncogene 9:71-79. [PubMed] [Google Scholar]
- 8.Besson, A., M. Gurian-West, X. Chen, K. S. Kelly-Spratt, C. J. Kemp, and J. M. Roberts. 2006. A pathway in quiescent cells that controls p27Kip1 stability, subcellular localization, and tumor suppression. Genes Dev. 20:47-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehm, M., T. Yoshimoto, M. F. Crook, S. Nallamshetty, A. True, G. J. Nabel, and E. G. Nabel. 2002. A growth factor-dependent nuclear kinase phosphorylates p27Kip1 and regulates cell cycle progression. EMBO J. 21:3390-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burns, K. H., J. E. Agno, P. Sicinski, and M. M. Matzuk. 2003. Cyclin D2 and p27 are tissue-specific regulators of tumorigenesis in inhibin alpha knockout mice. Mol. Endocrinol. 17:2053-2069. [DOI] [PubMed] [Google Scholar]
- 11.Busk, P. K., R. Hinrichsen, J. Bartkova, A. H. Hansen, T. E. Christoffersen, J. Bartek, and S. Haunso. 2005. Cyclin D2 induces proliferation of cardiac myocytes and represses hypertrophy. Exp. Cell Res. 304:149-161. [DOI] [PubMed] [Google Scholar]
- 12.Carrano, A. C., E. Eytan, A. Hershko, and M. Pagano. 1999. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1:193-199. [DOI] [PubMed] [Google Scholar]
- 13.Carthon, B. C., C. A. Neumann, M. Das, B. Pawlyk, T. Li, Y. Geng, and P. Sicinski. 2005. Genetic replacement of cyclin D1 function in mouse development by cyclin D2. Mol. Cell. Biol. 25:1081-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng, M., V. Sexl, C. J. Sherr, and M. F. Roussel. 1998. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1). Proc. Natl. Acad. Sci. USA 95:1091-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu, I., J. Sun, A. Arnaout, H. Kahn, W. Hanna, S. Narod, P. Sun, C. K. Tan, L. Hengst, and J. Slingerland. 2007. p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2. Cell 128:281-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connor, M. K., R. Kotchetkov, S. Cariou, A. Resch, R. Lupetti, R. G. Beniston, F. Melchior, L. Hengst, and J. M. Slingerland. 2003. CRM1/Ran-mediated nuclear export of p27Kip1 involves a nuclear export signal and links p27 export and proteolysis. Mol. Biol. Cell 14:201-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng, X., S. E. Mercer, S. Shah, D. Z. Ewton, and E. Friedman. 2004. The cyclin-dependent kinase inhibitor p27Kip1 is stabilized in G0 by Mirk/dyrk1B kinase. J. Biol. Chem. 279:22498-22504. [DOI] [PubMed] [Google Scholar]
- 18.Diehl, J. A., M. Cheng, M. F. Roussel, and C. J. Sherr. 1998. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 12:3499-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diehl, J. A., F. Zindy, and C. J. Sherr. 1997. Inhibition of cyclin D1 phosphorylation on threonine-286 prevents its rapid degradation via the ubiquitin-proteasome pathway. Genes Dev. 11:957-972. [DOI] [PubMed] [Google Scholar]
- 20.Dulic, V., L. F. Drullinger, E. Lees, S. I. Reed, and G. H. Stein. 1993. Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-Cdk2 and cyclin D1-Cdk2 complexes. Proc. Natl. Acad. Sci. USA 90:11034-11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ewen, M. E., H. K. Sluss, C. J. Sherr, H. Matsushime, J. Kato, and D. M. Livingston. 1993. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell 73:487-497. [DOI] [PubMed] [Google Scholar]
- 22.Fero, M. L., E. Randel, K. E. Gurley, J. M. Roberts, and C. J. Kemp. 1998. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 396:177-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fero, M. L., M. Rivkin, M. Tasch, P. Porter, C. E. Carow, E. Firpo, K. Polyak, L. H. Tsai, V. Broudy, R. M. Perlmutter, K. Kaushansky, and J. M. Roberts. 1996. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell 85:733-744. [DOI] [PubMed] [Google Scholar]
- 24.Fujita, N., S. Sato, K. Katayama, and T. Tsuruo. 2002. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J. Biol. Chem. 277:28706-28713. [DOI] [PubMed] [Google Scholar]
- 25.Ganter, B., S. Fu, and J. S. Lipsick. 1998. D-type cyclins repress transcriptional activation by the v-Myb but not the c-Myb DNA-binding domain. EMBO J. 17:255-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimmler, M., Y. Wang, T. Mund, Z. Cilensek, E. M. Keidel, M. B. Waddell, H. Jakel, M. Kullmann, R. W. Kriwacki, and L. Hengst. 2007. Cdk-inhibitory activity and stability of p27Kip1 are directly regulated by oncogenic tyrosine kinases. Cell 128:269-280. [DOI] [PubMed] [Google Scholar]
- 27.Hara, T., T. Kamura, S. Kotoshiba, H. Takahashi, K. Fujiwara, I. Onoyama, M. Shirakawa, N. Mizushima, and K. I. Nakayama. 2005. Role of the UBL-UBA protein KPC2 in degradation of p27 at G1 phase of the cell cycle. Mol. Cell. Biol. 25:9292-9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hara, T., T. Kamura, K. Nakayama, K. Oshikawa, S. Hatakeyama, and K. I. Nakayama. 2001. Degradation of p27Kip1 at the G0-G1 transition mediated by a Skp2-independent ubiquitination pathway. J. Biol. Chem. 276:48937-48943. [DOI] [PubMed] [Google Scholar]
- 29.Hengst, L., and S. I. Reed. 1996. Translational control of p27Kip1 accumulation during the cell cycle. Science 271:1861-1864. [DOI] [PubMed] [Google Scholar]
- 30.Huang, W., H. Y. Chang, T. Fei, H. Wu, and Y. G. Chen. 2007. GSK3β mediates suppression of cyclin D2 expression by tumor suppressor PTEN. Oncogene 26:2471-2482. [DOI] [PubMed] [Google Scholar]
- 31.Ishida, N., T. Hara, T. Kamura, M. Yoshida, K. Nakayama, and K. I. Nakayama. 2002. Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export. J. Biol. Chem. 277:14355-14358. [DOI] [PubMed] [Google Scholar]
- 32.Ishida, N., M. Kitagawa, S. Hatakeyama, K. Nakayama, and K. I. Nakayama. 2000. Phosphorylation at serine 10, a major phosphorylation site of p27Kip1, increases its protein stability. J. Biol. Chem. 275:25146-25154. [DOI] [PubMed] [Google Scholar]
- 33.Kamura, T., T. Hara, M. Matsumoto, N. Ishida, F. Okumura, S. Hatakeyama, M. Yoshida, K. Nakayama, and K. I. Nakayama. 2004. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27Kip1 at G1 phase. Nat. Cell Biol. 6:1229-1235. [DOI] [PubMed] [Google Scholar]
- 34.Kato, J. Y., and C. J. Sherr. 1993. Inhibition of granulocyte differentiation by G1 cyclins D2 and D3 but not D1. Proc. Natl. Acad. Sci. USA 90:11513-11517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kerkhoff, E., and E. B. Ziff. 1995. Cyclin D2 and Ha-Ras transformed rat embryo fibroblasts exhibit a novel deregulation of cell size control and early S phase arrest in low serum. EMBO J. 14:1892-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitagawa, M., H. Higashi, H. K. Jung, I. Suzuki-Takahashi, M. Ikeda, K. Tamai, J. Kato, K. Segawa, E. Yoshida, S. Nishimura, and Y. Taya. 1996. The consensus motif for phosphorylation by cyclin D1-Cdk4 is different from that for phosphorylation by cyclin A/E-Cdk2. EMBO J. 15:7060-7069. [PMC free article] [PubMed] [Google Scholar]
- 37.Kiyokawa, H., R. D. Kineman, K. O. Manova-Todorova, V. C. Soares, E. S. Hoffman, M. Ono, D. Khanam, A. C. Hayday, L. A. Frohman, and A. Koff. 1996. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell 85:721-732. [DOI] [PubMed] [Google Scholar]
- 38.Kong, G., S. S. Chua, Y. Yijun, F. Kittrell, R. C. Moraes, D. Medina, and T. K. Said. 2002. Functional analysis of cyclin D2 and p27Kip1 in cyclin D2 transgenic mouse mammary gland during development. Oncogene 21:7214-7225. [DOI] [PubMed] [Google Scholar]
- 39.Kotake, Y., K. Nakayama, N. Ishida, and K. I. Nakayama. 2005. Role of serine 10 phosphorylation in p27 stabilization revealed by analysis of p27 knock-in mice harboring a serine 10 mutation. J. Biol. Chem. 280:1095-1102. [DOI] [PubMed] [Google Scholar]
- 40.Kotoshiba, S., T. Kamura, T. Hara, N. Ishida, and K. I. Nakayama. 2005. Molecular dissection of the interaction between p27 and Kip1 ubiquitylation-promoting complex, the ubiquitin ligase that regulates proteolysis of p27 in G1 phase. J. Biol. Chem. 280:17694-17700. [DOI] [PubMed] [Google Scholar]
- 41.Kozar, K., M. A. Ciemerych, V. I. Rebel, H. Shigematsu, A. Zagozdzon, E. Sicinska, Y. Geng, Q. Yu, S. Bhattacharya, R. T. Bronson, K. Akashi, and P. Sicinski. 2004. Mouse development and cell proliferation in the absence of D-cyclins. Cell 118:477-491. [DOI] [PubMed] [Google Scholar]
- 42.Kushner, J. A., M. A. Ciemerych, E. Sicinska, L. M. Wartschow, M. Teta, S. Y. Long, P. Sicinski, and M. F. White. 2005. Cyclins D2 and D1 are essential for postnatal pancreatic β-cell growth. Mol. Cell. Biol. 25:3752-3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.LaBaer, J., M. D. Garrett, L. F. Stevenson, J. M. Slingerland, C. Sandhu, H. S. Chou, A. Fattaey, and E. Harlow. 1997. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11:847-862. [DOI] [PubMed] [Google Scholar]
- 44.Ladha, M. H., K. Y. Lee, T. M. Upton, M. F. Reed, and M. E. Ewen. 1998. Regulation of exit from quiescence by p27 and cyclin D1-CDK4. Mol. Cell. Biol. 18:6605-6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lam, E. W., J. Glassford, L. Banerji, N. S. Thomas, P. Sicinski, and G. G. Klaus. 2000. Cyclin D3 compensates for loss of cyclin D2 in mouse B-lymphocytes activated via the antigen receptor and CD40. J. Biol. Chem. 275:3479-3484. [DOI] [PubMed] [Google Scholar]
- 46.Liang, J., J. Zubovitz, T. Petrocelli, R. Kotchetkov, M. K. Connor, K. Han, J. H. Lee, S. Ciarallo, C. Catzavelos, R. Beniston, E. Franssen, and J. M. Slingerland. 2002. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat. Med. 8:1153-1160. [DOI] [PubMed] [Google Scholar]
- 47.Lukas, J., J. Bartkova, M. Welcker, O. W. Petersen, G. Peters, M. Strauss, and J. Bartek. 1995. Cyclin D2 is a moderately oscillating nucleoprotein required for G1 phase progression in specific cell types. Oncogene 10:2125-2134. [PubMed] [Google Scholar]
- 48.Malumbres, M., R. Sotillo, D. Santamaria, J. Galan, A. Cerezo, S. Ortega, P. Dubus, and M. Barbacid. 2004. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 118:493-504. [DOI] [PubMed] [Google Scholar]
- 49.Matsushime, H., M. E. Ewen, D. K. Strom, J. Y. Kato, S. K. Hanks, M. F. Roussel, and C. J. Sherr. 1992. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell 71:323-334. [DOI] [PubMed] [Google Scholar]
- 50.Matsushime, H., D. E. Quelle, S. A. Shurtleff, M. Shibuya, C. J. Sherr, and J. Y. Kato. 1994. D-type cyclin-dependent kinase activity in mammalian cells. Mol. Cell. Biol. 14:2066-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsushime, H., M. F. Roussel, R. A. Ashmun, and C. J. Sherr. 1991. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell 65:701-713. [DOI] [PubMed] [Google Scholar]
- 52.Medema, R. H., G. J. Kops, J. L. Bos, and B. M. Burgering. 2000. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404:782-787. [DOI] [PubMed] [Google Scholar]
- 53.Meyerson, M., and E. Harlow. 1994. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol. Cell. Biol. 14:2077-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyyappan, M., H. Wong, C. Hull, and K. T. Riabowol. 1998. Increased expression of cyclin D2 during multiple states of growth arrest in primary and established cells. Mol. Cell. Biol. 18:3163-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Millard, S. S., A. Vidal, M. Markus, and A. Koff. 2000. A U-rich element in the 5′ untranslated region is necessary for the translation of p27 mRNA. Mol. Cell. Biol. 20:5947-5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montagnoli, A., F. Fiore, E. Eytan, A. C. Carrano, G. F. Draetta, A. Hershko, and M. Pagano. 1999. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 13:1181-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morita, S., T. Kojima, and T. Kitamura. 2000. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7:1063-1066. [DOI] [PubMed] [Google Scholar]
- 58.Nakayama, K., N. Ishida, M. Shirane, A. Inomata, T. Inoue, N. Shishido, I. Horii, D. Y. Loh, and K. I. Nakayama. 1996. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85:707-720. [DOI] [PubMed] [Google Scholar]
- 59.Nakayama, K., H. Nagahama, Y. A. Minamishima, M. Matsumoto, I. Nakamichi, K. Kitagawa, M. Shirane, R. Tsunematsu, T. Tsukiyama, N. Ishida, M. Kitagawa, K. I. Nakayama, and S. Hatakeyama. 2000. Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploidy and centrosome overduplication. EMBO J. 19:2069-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakayama, K., H. Nagahama, Y. A. Minamishima, S. Miyake, N. Ishida, S. Hatakeyama, M. Kitagawa, S. Iemura, T. Natsume, and K. I. Nakayama. 2004. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev. Cell 6:661-672. [DOI] [PubMed] [Google Scholar]
- 61.Nakayama, K. I., and K. Nakayama. 2006. Ubiquitin ligases: cell-cycle control and cancer. Nat. Rev. Cancer 6:369-381. [DOI] [PubMed] [Google Scholar]
- 62.Nourse, J., E. Firpo, W. M. Flanagan, S. Coats, K. Polyak, M. H. Lee, J. Massague, G. R. Crabtree, and J. M. Roberts. 1994. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature 372:570-573. [DOI] [PubMed] [Google Scholar]
- 63.Pagano, M., S. W. Tam, A. M. Theodoras, P. Beer-Romero, G. Del Sal, V. Chau, P. R. Yew, G. F. Draetta, and M. Rolfe. 1995. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269:682-685. [DOI] [PubMed] [Google Scholar]
- 64.Parada, Y., L. Banerji, J. Glassford, N. C. Lea, M. Collado, C. Rivas, J. L. Lewis, M. Y. Gordon, N. S. Thomas, and E. W. Lam. 2001. BCR-ABL and interleukin 3 promote haematopoietic cell proliferation and survival through modulation of cyclin D2 and p27Kip1 expression. J. Biol. Chem. 276:23572-23580. [DOI] [PubMed] [Google Scholar]
- 65.Reynisdottir, I., and J. Massague. 1997. The subcellular locations of p15Ink4b and p27Kip1 coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 11:492-503. [DOI] [PubMed] [Google Scholar]
- 66.Reynisdottir, I., K. Polyak, A. Iavarone, and J. Massague. 1995. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-β. Genes Dev. 9:1831-1845. [DOI] [PubMed] [Google Scholar]
- 67.Rodier, G., A. Montagnoli, L. Di Marcotullio, P. Coulombe, G. F. Draetta, M. Pagano, and S. Meloche. 2001. p27 cytoplasmic localization is regulated by phosphorylation on Ser10 and is not a prerequisite for its proteolysis. EMBO J. 20:6672-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmidt, B. A., A. Rose, C. Steinhoff, T. Strohmeyer, M. Hartmann, and R. Ackermann. 2001. Up-regulation of cyclin-dependent kinase 4/cyclin D2 expression but down-regulation of cyclin-dependent kinase 2/cyclin E in testicular germ cell tumors. Cancer Res. 61:4214-4221. [PubMed] [Google Scholar]
- 69.Sheaff, R. J., M. Groudine, M. Gordon, J. M. Roberts, and B. E. Clurman. 1997. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 11:1464-1478. [DOI] [PubMed] [Google Scholar]
- 70.Sherr, C. J., and J. M. Roberts. 2004. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 18:2699-2711. [DOI] [PubMed] [Google Scholar]
- 71.Shin, I., F. M. Yakes, F. Rojo, N. Y. Shin, A. V. Bakin, J. Baselga, and C. L. Arteaga. 2002. PKB/Akt mediates cell-cycle progression by phosphorylation of p27Kip1 at threonine 157 and modulation of its cellular localization. Nat. Med. 8:1145-1152. [DOI] [PubMed] [Google Scholar]
- 72.Sicinski, P., J. L. Donaher, Y. Geng, S. B. Parker, H. Gardner, M. Y. Park, R. L. Robker, J. S. Richards, L. K. McGinnis, J. D. Biggers, J. J. Eppig, R. T. Bronson, S. J. Elledge, and R. A. Weinberg. 1996. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 384:470-474. [DOI] [PubMed] [Google Scholar]
- 73.Solvason, N., W. W. Wu, D. Parry, D. Mahony, E. W. Lam, J. Glassford, G. G. Klaus, P. Sicinski, R. Weinberg, Y. J. Liu, M. Howard, and E. Lees. 2000. Cyclin D2 is essential for BCR-mediated proliferation and CD5 B cell development. Int. Immunol. 12:631-638. [DOI] [PubMed] [Google Scholar]
- 74.Sutterluty, H., E. Chatelain, A. Marti, C. Wirbelauer, M. Senften, U. Muller, and W. Krek. 1999. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat. Cell Biol. 1:207-214. [DOI] [PubMed] [Google Scholar]
- 75.Takano, Y., Y. Kato, P. J. van Diest, M. Masuda, H. Mitomi, and I. Okayasu. 2000. Cyclin D2 overexpression and lack of p27 correlate positively and cyclin E inversely with a poor prognosis in gastric cancer cases. Am. J. Pathol. 156:585-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tomoda, K., Y. Kubota, and J. Kato. 1999. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature 398:160-165. [DOI] [PubMed] [Google Scholar]
- 77.Tsvetkov, L. M., K. H. Yeh, S. J. Lee, H. Sun, and H. Zhang. 1999. p27Kip1 ubiquitination and degradation is regulated by the SCFSkp2 complex through phosphorylated Thr187 in p27. Curr. Biol. 9:661-664. [DOI] [PubMed] [Google Scholar]
- 78.Uchida, T., T. Nakamura, N. Hashimoto, T. Matsuda, K. Kotani, H. Sakaue, Y. Kido, Y. Hayashi, K. I. Nakayama, M. F. White, and M. Kasuga. 2005. Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat. Med. 11:175-182. [DOI] [PubMed] [Google Scholar]
- 79.van den Heuvel, S., and E. Harlow. 1993. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262:2050-2054. [DOI] [PubMed] [Google Scholar]
- 80.Viglietto, G., M. L. Motti, P. Bruni, R. M. Melillo, A. D'Alessio, D. Califano, F. Vinci, G. Chiappetta, P. Tsichlis, A. Bellacosa, A. Fusco, and M. Santoro. 2002. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27Kip1 by PKB/Akt-mediated phosphorylation in breast cancer. Nat. Med. 8:1136-1144. [DOI] [PubMed] [Google Scholar]
- 81.Vlach, J., S. Hennecke, and B. Amati. 1997. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 16:5334-5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang, W., D. Bergamaschi, B. Jin, and X. Lu. 2005. Posttranslational modifications of p27kip1 determine its binding specificity to different cyclins and cyclin-dependent kinases in vivo. Blood 105:3691-3698. [DOI] [PubMed] [Google Scholar]
- 83.Zwijsen, R. M., E. Wientjens, R. Klompmaker, J. van der Sman, R. Bernards, and R. J. Michalides. 1997. CDK-independent activation of estrogen receptor by cyclin D1. Cell 88:405-415. [DOI] [PubMed] [Google Scholar]