Abstract
The mRNAs encoding postsynaptic components at the neuromuscular junction are concentrated in the synaptic region of muscle fibers. Accumulation of these RNAs in the synaptic region is mediated, at least in part, by selective transcription of the corresponding genes in synaptic myofiber nuclei. The transcriptional mechanisms that are responsible for synapse-specific gene expression are largely unknown, but an Ets site in the promoter regions of acetylcholine receptor (AChR) subunit genes and other “synaptic” genes is required for synapse-specific transcription. The Ets domain transcription factor GA-binding protein (GABP) has been implicated to mediate synapse-specific gene expression. Inactivation of GABPα, the DNA-binding subunit of GABP, leads to early embryonic lethality, preventing analysis of synapse formation in gabpα mutant mice. To study the role of GABP at neuromuscular synapses, we conditionally inactivated gabpα in skeletal muscle and studied synaptic differentiation and muscle gene expression. Although expression of rb, a target of GABP, is elevated in muscle tissue deficient in GABPα, clustering of synaptic AChRs at synapses and synapse-specific gene expression are normal in these mice. These data indicate that GABP is dispensable for synapse-specific transcription and maintenance of normal AChR expression at synapses.
During neuromuscular synapse formation, postsynaptic proteins, including acetylcholine receptors (AChRs) and the muscle-specific receptor tyrosine kinase, MuSK, become localized to the synaptic membrane of skeletal muscle fibers. Similarly, the mRNAs encoding many postsynaptic components become concentrated in the synaptic region of myofibers, because their corresponding genes are transcribed selectively in subsynaptic muscle nuclei (4, 28). The transcription factor GA-binding protein (GABP) has been implicated to mediate synapse-specific gene expression (32).
GABP is a dimer of GABPα, which contains an Ets domain that binds DNA, and GABPβ, which contributes a nuclear localization sequence and the transcriptional activation domain to GABP (18, 31). Dimerization of GABPα and GABPβ is mediated by interactions between four amino-terminal ankyrin repeats in GABPβ and the Ets domain, plus a short α-helix adjacent to the Ets domain, in GABPα (1, 39). While there is one known gabpα gene, two gabpβ genes, gabpβ1 and gabpβ2, have been found in mammals, and gabpβ1 gives rise to at least four distinct splice isoforms (7, 10, 18, 41). Certain GABPβ isoforms mediate formation of a heterotetramer, composed of two GABPα/β dimers, which binds to paired Ets sites, whereas other GABPβ isoforms are recruited to a single Ets site as part of a GABPα/β dimer (7).
Synapse-specific transcription of the AChR δ gene is dependent upon a single Ets site in its promoter region (16). GABP is the major Ets protein in myotube nuclear extracts that binds this Ets site (9, 33). The AChR ɛ promoter also contains an Ets site that binds GABP, and mutations in this Ets site are responsible for certain congenital myasthenic syndromes in humans (22, 23, 35). While the importance of Ets sites in synapse-specific gene expression is widely accepted, the role of GABP in synapse formation is less clear. Transfection of a dominant-negative form of GABPβ, lacking sequences required for transcriptional activation, inhibits AChR cluster formation and induction of an AChR ɛ reporter construct by ectopic agrin expression in adult skeletal muscle (2). Transfection of a dominant-negative form of GABPβ also attenuates the activation of a MuSK reporter construct by agrin in vitro (17). Furthermore, transfection of a mutant form of GABPα that cannot be phosphorylated at threonine 280 interferes with the induction of AChR ɛ gene expression by neuregulin 1 (Nrg-1) in cultured muscle cells (38). Forced expression of the DNA-binding domain of Ets2, another Ets domain-containing transcription factor, in skeletal muscle leads to defects in the organization and size of primary gutters and secondary folds at neuromuscular synapses and reduces the expression levels of “synaptic” genes (6). These results indicate that interfering with the function of multiple members of the Ets family of transcription factors by transfection of dominant-negative constructs affects the expression of genes that are preferentially transcribed in subsynaptic nuclei at the neuromuscular synapse. These studies, however, do not address whether GABP is required for neuromuscular synapse formation or synapse-specific gene expression.
Deletion of GABPα in mice results in embryonic lethality prior to embryonic day 7.5 (E7.5) of development (26), preventing an analysis of a potential role for GABP in synapse formation, which begins at E13. Here we conditionally inactivated gabpα specifically in skeletal muscle and analyzed synaptic development. We find that GABPα is dispensable for synapse-specific gene expression and clustering of synaptic AChRs during synapse formation. Furthermore, postnatal synaptic maturation is normal in conditional gabpα mutant mice. These findings suggest that additional proteins bind the Ets site in AChR genes and stimulate their expression in synaptic nuclei.
MATERIALS AND METHODS
Generation of mice carrying a loxP-flanked allele of gabpα.
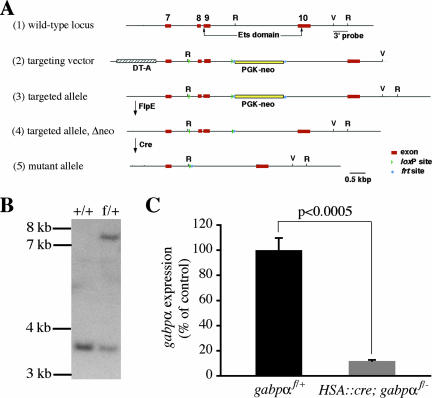
To generate the loxP-flanked gabpα allele (gabpαf), we introduced loxP sites into introns 7 and 9 of a gabpα genomic DNA fragment encompassing exons 7 through 10; furthermore, we introduced a frt-flanked neomycin resistance cassette into intron 9 and a diphtheria toxin A cassette at the 5′ end of the targeting vector (Fig. 1A). Because exons 8 and 9 encode the majority of the Ets domain and sequences immediately amino-terminal to the Ets domain, which are required for GABP function, deletion of these exons is likely to result in a null allele (gabpα−). Indeed, gabpα−/− mice died during embryogenesis, whereas gabpα+/− mice survived as adults (see below). 129S6/SvEvTac-derived W4 embryonic stem (ES) cells were electroporated with the targeting vector and selected with neomycin, and surviving clones were screened for homologous recombination by Southern blotting using a 3′ EcoRV/EcoRI fragment as a probe (Fig. 1A and B). One gabpαf/+ ES cell clone was chosen for blastocyst injections, and resulting chimeras were crossed to C57BL/6 mice. Phenotypic analyses were carried out in a mixed background.
FIG. 1.
Generation of null and loxP-flanked gabpα alleles and deletion of gabpα in skeletal muscle. (A) The cartoon shows the targeting strategy to generate the gabpαf and gabpα− alleles. The following are depicted: (1) the normal gabpα locus (exons are numbered, and the sequence encoding the Ets domain is indicated by arrows); (2) the targeting vector, which includes a frt-flanked neomycin resistance cassette (PGK-neo) and a diphtheria toxin A (DT-A) cassette; (3) the loxP-flanked, targeted allele following homologous recombination; (4) the targeted allele after removal of PGK-neo by FlpE; and (5) the mutant allele after Cre recombinase-mediated removal of exons 8 and 9, which encode the bulk of the Ets domain. EcoRI (R) and EcoRV (V) restriction sites and the position of the probe used for Southern blot hybridization are indicated. (B) Southern blot analysis of EcoRI-digested DNA from ES cells carrying two wild-type alleles (+/+) or one loxP-flanked allele and one wild-type allele (f/+) of gabpα. (C) RNA isolated from gastrocnemius muscles from P21 gabpαf/+ (n = 3) or HSA::cre; gabpαf/− mice (n = 4) was reverse transcribed into cDNA, and expression of the wild-type gabpα transcript was measured by quantitative PCR using primers in exons that are deleted after Cre-mediated recombination of loxP sites (see Materials and Methods). gabpα expression in muscle tissue is reduced by 88% for gabpα mutant mice compared to levels for control littermates (P < 0.0005). Error bars in panel C indicate standard errors of the means.
Gabpαf/+ mice were crossed to FlpE-expressing mice (8) to remove the neomycin resistance cassette from intron 9 (Fig. 1A). We generated gabpα+/− mice by crossing gabpαf/f mice with CMV::cre mice, which express Cre recombinase in the germ line (42).
Mouse strains and genotyping.
HSA::cre mice have been described previously (20) and were genotyped as reported previously (13). loxP-flanked and wild-type gabpα alleles were detected by PCR using primers that hybridize to sequences in gabpα (CTTACAATTTTGAGGTGCATAGACC and CCAAAGGAATTAGGGGAATCTTTCC). The null allele was detected using a separate pair of primers (GGCCAGCCAAGAGCAACA and TCCACCCTTGGACAGATCCTGCATGGC).
Immunohistochemistry.
Motor axons and nerve terminals were visualized by staining with antibodies against neurofilament and synaptophysin, respectively; muscle fibers and AChR clusters were stained with Alexa660-phalloidin and Alexa594-α-bungaratoxin (Alexa594-α-Bgt), as described previously (13).
To determine the branch point number of postsynaptic AChR clusters in P21 diaphragm muscles, we analyzed 10 synapses per animal for 4 animals per genotype. We determined the means for each genotype and compared them in a two-tailed Student t test.
Quantitation of synaptic AChRs.
The number and density of synaptic AChRs were determined by measuring Alexa594-α-Bgt binding, as described previously (13). We analyzed at least 62 synapses in each P0 diaphragm muscle, at least 27 synapses in each P21 gastrocnemius muscle, and a minimum of 23 synapses in each P21 diaphragm muscle. The mean AChR level and density from multiple mice (numbers of mice are indicated in the figure legends) with the same genotype were determined. Because the density and level of synaptic AChRs were not significantly different in P21 gabpαf/− and gabpαf/+ mice (data not shown), the data from these two genotypes were grouped.
In situ hybridization.
Intercostal and diaphragm muscles were processed for in situ hybridization and hybridized with digoxigenin-labeled riboprobes that recognize the AChR α-subunit (5), AChR δ-subunit (36), AChR ɛ-subunit (13), or MuSK mRNA (11) as described elsewhere (13). Labeling with sense probes resulted in weak, uniform staining for each gene (data not shown).
The width of the in situ hybridization signal was measured relative to the distance between two adjacent ribs using ImageJ (NIH). Means were determined from four tissue samples (from two mice) of the same genotype, and genotypes were compared in a two-tailed Student t test.
Quantitative RT-PCR.
RNA isolation from gastrocnemius muscle, reverse transcription (RT), and quantitative PCR were carried out essentially as described previously (13). Relative expression levels of gabpα and the AChR ɛ-subunit were normalized to muscle creatine kinase (mck) expression. retinoblastoma (rb) and cytochrome c oxidase subunit IV (coxIV) RNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (gapdh) expression. The primers used for PCR amplification were TGCATCCTGCACCACCAACT and ATGCCTGCTTCACCACCTTC for gapdh, CGTGTCACCTCTGCTGCT and CCTTCATATTGCCTCCCTTCT for mck, AATGGGGACAACGTAAGAACA and GTACACAAATCTCTTGCCTTGAAC for wild-type gabpα, TGCTAGCCCAGACTGTCTTCTT and GTCGTTGGCGTCCTCAAAG for AChR ɛ, CTTGGCTAACTTGGGAGAAAG and GCTCAGTAAAAGTGAATGGCAT for rb, and GGGAGTGTTGTGAAGAGTGAAG and CCTTCTCCTTCTCCTTCAGC for coxIV.
RESULTS
Generation of gabpα mutant alleles and conditional inactivation of GABPα in skeletal muscle.
GABP has been implicated in the regulation of a wide variety of genes, including but not limited to nuclear genes encoding mitochondrial proteins, rb, and AChR subunit genes (14, 27, 32). To determine the function of GABP in vivo, we generated mice carrying a loxP-flanked allele of gabpα (gabpαf; Fig. 1A and B). In these mice, exons 8 and 9, which encode the majority of the GABPα Ets domain, are flanked by loxP sites, allowing Cre-mediated deletion of these sequences (Fig. 1A). We also generated mice with a gabpα allele lacking exons 8 and 9 (gabpα−) by crossing gabpαf/+ mice to CMV::cre mice, which express Cre in all cell types, including the germ line (Fig. 1A) (see Materials and Methods). We intercrossed gabpα+/− mice and failed to obtain gabpα−/− newborn mice (data not shown). At E8.5, the earliest stage we examined, we failed to recover homozygous mutant embryos; however, genotyping of extraembryonic membrane tissue from empty deciduas showed that this tissue contained gabpα−/− embryos, which had apparently resorbed prior to E8.5 (data not shown). These results indicate that GABPα is required for survival during early embryonic development, as reported previously (26, 43), preventing analysis of its function at later stages of development.
To determine the role of GABP in skeletal muscle, we inactivated GABPα specifically in skeletal muscle. To this end, we generated mice carrying a human skeletal actin (HSA)::cre transgene and null and loxP-flanked alleles of gabpα. HSA::cre; gabpαf/− mice were born at the expected Mendelian frequency. Because mice die at birth if the diaphragm and intercostal muscles fail to form or function, these findings suggest that GABP is not essential for the formation of skeletal muscles. To measure the extent of gabpα inactivation in skeletal muscle tissue, we isolated RNA from P21 HSA::cre; gabpαf/− mice and gabpαf/+ littermates and used a quantitative, real-time PCR assay to measure the level of gabpα expression (Materials and Methods). We found that the expression of RNA encoding wild-type GABPα is reduced nearly 10-fold in muscle tissue from HSA::cre; gabpαf/− mice (11.8% ± 0.9% of the level found in muscle tissue from gabpαf/+ mice; P < 0.0005) (Fig. 1C). Because GABPα is expressed in most if not all cell types (19, 24, 26) and because muscle tissue contains fibroblasts, smooth muscle cells, Schwann cells, and endothelial cells in addition to muscle fibers, these data establish a minimal reduction of GABPα expression within skeletal myofibers. Residual expression of GABPα in vascular, interstitial, and neural cell types is likely to contribute to most if not all of the remaining expression of GABPα in skeletal muscle tissue. Thus, our data indicate that GABPα expression is substantially reduced in skeletal myofibers of HSA::cre; gabpαf/− mice.
rb gene expression is regulated by GABP.
GABP has been implicated in the regulation of nuclear genes encoding mitochondrial proteins, including cytochrome c oxidase subunits and the rb gene (14, 27). GABP binds the promoter regions of these target genes, and GABP overexpression induces their transcription; conversely, their transcription is attenuated by expression of dominant-negative forms of GABP (25, 30, 37, 40).
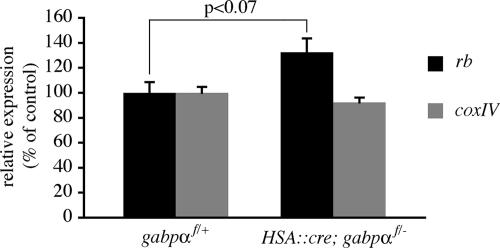
To determine whether GABP regulates the expression of rb and coxIV, we measured their expression in gastrocnemius muscles from P21 HSA::cre; gabpαf/− mice and gabpαf/+ littermates by quantitative RT-PCR. We found that coxIV mRNA levels are normal in muscle from HSA::cre; gabpαf/− mice (92.2% ± 3.9% of control; P > 0.2) (Fig. 2). rb expression, on the other hand, was elevated in muscle from HSA::cre; gabpαf/− mice (132.5% ± 11.1% of control; P < 0.07) (Fig. 2). These findings indicate that coxIV expression in muscle is not dependent upon GABP and suggest that rb is negatively regulated by GABP.
FIG. 2.
rb gene expression is elevated in skeletal muscle of HSA::cre; gabpαf/− mice. RNA from gastrocnemius muscle of P21 control mice (n = 3) or gabpα mutant littermates (n = 5) was reverse transcribed, and gene expression was measured by quantitative PCR. rb mRNA levels are increased by 32% (P < 0.07), whereas coxIV mRNA levels are unchanged in muscle from gabpα mutant mice (P > 0.2). Error bars indicate standard errors of the means.
Skeletal muscle GABPα is not required for synapse formation and synaptic gene expression during embryonic development.
GABP has been implicated in synapse-specific gene expression and neuromuscular synapse formation (32). To study presynaptic and postsynaptic differentiation in HSA::cre; gabpαf/− mice, we stained whole mounts of diaphragm muscle from P0 HSA::cre; gabpαf/− mice and control littermates with probes that allowed us to visualize motor axons, nerve terminals, muscle fibers, and AChRs (Materials and Methods). Muscle fibers in HSA::cre; gabpαf/− mice are of normal arrangement and size, and the positions of the main intramuscular nerve and synaptic sites appear normal (Fig. 3A and B) (data not shown). Moreover, in HSA::cre; gabpαf/− mice, as in wild-type mice, AChRs are clustered at synaptic sites, and the size and shape of presynaptic nerve terminals and postsynaptic AChR clusters appear normal (Fig. 3A and B). Similar results were obtained for gastrocnemius muscle (data not shown). These results indicate that expression of GABPα in skeletal muscle is not essential for the formation of muscle fibers, growth of motor axons to muscle, or the formation of neuromuscular synapses.
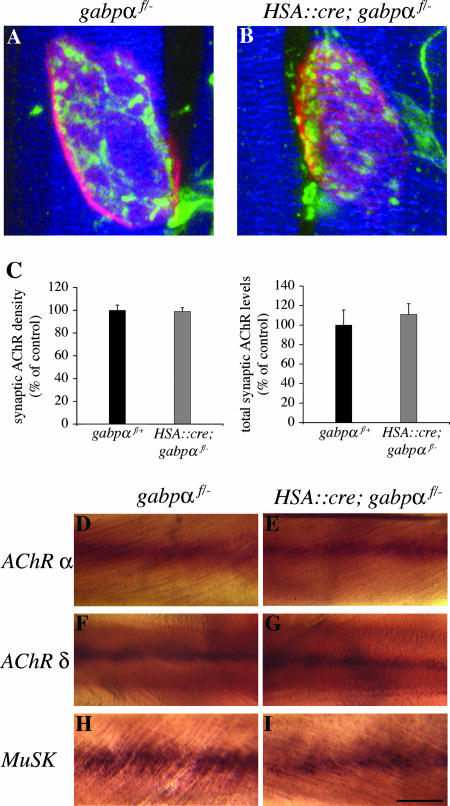
FIG. 3.
GABPα is dispensable for AChR clustering and synapse-specific gene expression. (A and B) Whole mounts of diaphragm muscles from P0 HSA::cre; gabpαf/− mice (B) or control littermates (A) were stained with Alexa594-α-Bgt to visualize postsynaptic AChRs (red), antibodies against neurofilament and synaptophysin to visualize motor axons and nerve terminals (green), respectively, and Alexa660-phalloidin to visualize muscle fibers (blue). AChR clusters are of similar sizes and shapes in control mice and gabpα mutant mice. (C) Synaptic AChR density and total synaptic AChR levels are indistinguishable in conditional gabpα mutant mice (n = 2) and control littermates (n = 4). (D to I) Whole mounts of intercostal muscles from newborn control mice (D, F, and H) or HSA::cre; gabpαf/− mice (E, G, and I) were processed for in situ hybridization. AChR α-subunit (D and E), AChR δ-subunit (F and G), and MuSK (H and I) mRNAs are concentrated in the central region of muscle from HSA::cre; gabpαf/− mice, as with control mice. Scale bar = 5 μm for panels A and B or 120 μm for panels D to I. Error bars in panel C indicate standard errors of the means.
To determine whether the number and density of synaptic AChRs are normal in the absence of skeletal muscle GABPα, we measured synaptic AChR protein expression by quantitating the amount of Alexa594-α-Bgt bound to synaptic AChRs in diaphragm muscles from P0 HSA::cre; gabpαf/− mice and control mice (Fig. 3C) (Materials and Methods). We found no significant difference in the density or number of synaptic AChRs in gabpα mutant mice and control littermates (density, 99% ± 3.3% of control, P > 0.2; total number, 111% ± 10.9% of control, P > 0.2). These findings indicate that the number and density of AChRs at developing synapses do not depend upon GABPα expression in muscle.
To analyze the role of GABP in synaptic transcription, we examined the pattern of AChR gene expression in intercostal muscles from P0 mice by in situ hybridization. We found that AChR α-subunit and AChR δ-subunit mRNAs are concentrated in the central region of muscle from HSA::cre; gabpαf/− mice, as with control mice (Fig. 3D to G). We measured the width of the AChR α and AChR δ expression domains relative to the distance between individual ribs and found no significant difference (P > 0.2) between control mice (AChR α, 16% ± 1.1%, n = 4; AChR δ, 16% ± 2.2%, n = 4) and HSA::cre; gabpαf/− littermates (AChR α, 17% ± 2.2%, n = 4; AChR δ, 17% ± 1.9%, n = 4). We also analyzed the pattern of MuSK gene expression and found that MuSK mRNA is patterned normally in HSA::cre; gabpαf/− mice (Fig. 3H and I) and that there is no significant difference (P > 0.2) in the width of the MuSK expression domain between control mice (21% ± 2.5%; n = 4) and conditional gabpα mutant mice (17% ± 3.7%; n = 4). These findings indicate that GABP function in skeletal muscle is not required to establish the pattern of synapse-specific gene expression during development.
GABPα expression in muscle is dispensable for postnatal maturation of neuromuscular synapses.
Postnatally, neuromuscular synapses undergo extensive remodeling and maturation. Hence, we analyzed whether GABP is required for the maturation or maintenance of neuromuscular synapses after birth. Postnatal HSA::cre; gabpαf/− mice appear healthy and do not display any overt phenotype (data not shown). We visualized motor axons, nerve terminals, muscle fibers, and AChRs in whole mounts of diaphragm muscle from P21 mice. We found that AChRs are concentrated at synaptic sites in gabpα mutant mice; these AChR clusters are apposed by nerve terminals that have the same size and shape as nerve terminals in wild-type mice (Fig. 4A and B). Similar results were obtained for gastrocnemius muscle (data not shown). To quantitatively compare the morphology of neuromuscular synapses in diaphragms of HSA::cre; gabpαf/− mice and gabpαf/+ mice, we determined the number of branch points in postsynaptic AChR clusters (see Materials and Methods). We found no significant difference (P > 0.2) in branch point number between control mice (5.5 ± 0.58) and conditional gabpα mutant mice (5.1 ± 0.30). Thus, GABPα is not essential to maintaining the normal arrangement of AChRs and nerve terminals at neuromuscular synapses in postnatal mice.
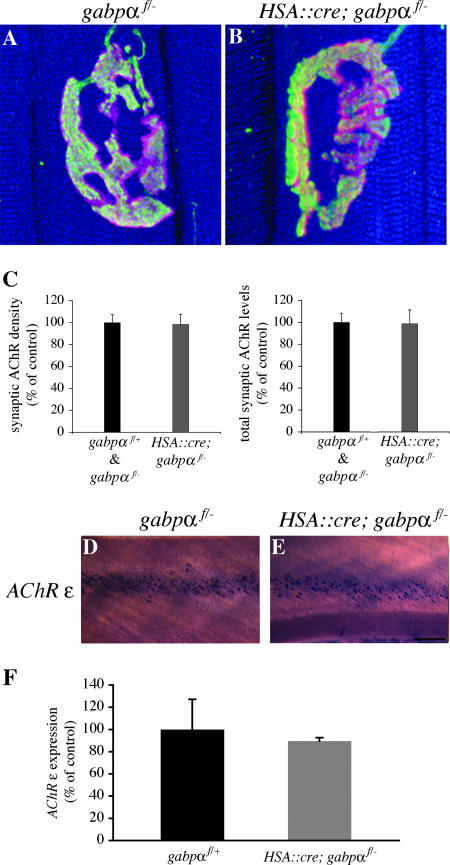
FIG. 4.
Neuromuscular synapses mature normally in HSA::cre; gabpαf/− mice. (A and B) Whole mounts of diaphragm muscles from P21 control mice and HSA::cre; gabpαf/− mice were stained as described in the legend to Fig. 3. Neuromuscular synapses exhibit a branched, pretzel-like structure in control mice (A) or gabpα mutant mice (B). Furthermore, the sizes of myofibers are similar in gabpα mutant mice and control mice (A and B). (C) The density and total number of synaptic AChRs are similar in muscle from control mice (n = 4) and HSA::cre; gabpαf/− mice (n = 6). (D and E) Whole mounts of intercostal muscles from P21 control mice (D) and HSA::cre; gabpαf/− mice (E) were processed for in situ hybridization. AChR ɛ mRNA is concentrated in the central region of muscle from gabpα mutant P21 mice, similar to the case with control mice. (F) RNA from gastrocnemius muscles of P21 control mice (n = 3) or gabpα mutant littermates (n = 5) was reverse transcribed, and AChR ɛ gene expression was measured by quantitative PCR. Expression of AChR ɛ is similar in HSA::cre; gabpαf/− mice and control mice (P > 0.2). Scale bar = 10 μm for panels A and B or 200 μm for panels D and E. Error bars in C and F indicate standard errors of the means.
To determine whether GABPα is required to maintain AChR expression postnatally, we measured the densities and numbers of synaptic AChRs in diaphragm muscles from P21 HSA::cre; gabpαf/− mice and control mice (Fig. 4C). We found no significant difference in the density or number of synaptic AChRs in gabpα mutant mice and control littermates (density, 98% ± 9.3% of control, P > 0.2; total number, 99% ± 12.7% of control, P > 0.2). Similar results were obtained for gastrocnemius muscles (density, 109% ± 3.1% of control, P > 0.2; total number, 93% ± 4.2% of control, P > 0.2; n = 3 for control mice; n = 5 for mutant mice). These findings indicate that the number and density of AChRs at mature synapses do not depend upon GABPα expression in muscle.
During the first week of postnatal life, expression of the AChR γ-subunit is down-regulated and expression of the AChR ɛ-subunit is induced; this postnatal switch in AChR subunit expression is critically responsible for a change in the kinetics and conductance of synaptic AChRs (21). Because GABPα has been proposed to play a key role in inducing AChR ɛ-subunit expression (2, 22, 23, 35), we analyzed AChR ɛ expression in HSA::cre; gabpαf/− mice. We examined the expression pattern of the AChR ɛ-subunit gene in intercostal muscles of P21 gabpα mutant mice by in situ hybridization and found that AChR ɛ-subunit mRNA is concentrated in the central region of muscle from HSA::cre; gabpαf/− mice, as with control littermates (Fig. 4D and E; width of AChR ɛ expression domain, 13% ± 1.5% for controls, n = 4; 14% ± 1.1% for mutants, n = 4; P > 0.2). Similar results were obtained with diaphragm muscle (data not shown). These results indicate that skeletal muscle GABPα is not required for activating AChR ɛ-subunit transcription in subsynaptic nuclei and patterning synapse-specific gene expression postnatally.
To determine whether GABP regulates the level of AChR ɛ-subunit gene expression, we measured AChR ɛ mRNA in gastrocnemius muscle from P21 HSA::cre; gabpαf/− mice and control mice by quantitative RT-PCR. We found that AChR ɛ mRNA levels are normal in HSA::cre; gabpαf/− mice (89.6% ± 2.9% of control; P > 0.2) (Fig. 4F). Thus, the AChR ɛ expression level is not dependent upon GABP.
DISCUSSION
The genes encoding several postsynaptic proteins, including AChRs, AChE, and MuSK, are transcribed specifically in myofiber nuclei positioned near the synaptic site. GABP has been implicated as a key transcription factor regulating synapse-specific gene expression, largely because it can bind the cis-regulatory element required for synaptic transcription of AChR and AChE genes. We conditionally inactivated the DNA-binding subunit of GABP, GABPα, in skeletal muscle and found that the pattern of synapse-specific gene expression is normal in these mutant mice. Synapses develop and mature normally in these mice, forming an elaborate, branched shape that contains normal numbers of postsynaptic AChRs. Moreover, AChR ɛ-subunit gene expression is induced postnatally and patterned normally in gabpα conditionally mutant mice. These results provide strong evidence against an essential role for GABP in neuromuscular synapse formation and synapse-specific transcription. O'leary et al. have recently described their analysis of mice that are deficient in skeletal muscle GABPα (24a). Although they report that the arborization of nerve terminals is simplified at a subset of synapses and that AChR ɛ gene expression is reduced in the diaphragm muscle, similar to our findings, they report that skeletal muscle GABPα is not essential for viability, growth, muscle development, or neuromuscular synapse formation.
GABP has been implicated in the induction of the rb gene, since the rb promoter region contains a binding site for GABP, and overexpression of GABP stimulates expression of a reporter gene controlled by the rb promoter in cultured cells (30, 37). Surprisingly, we find that the expression of rb is elevated (1.3-fold) in muscle of HSA::cre; gabpαf/− mice, suggesting that GABP suppresses rb gene expression, possibly directly, in skeletal muscle. These data demonstrate that deletion of gabpα in skeletal muscle of HSA::cre; gabpαf/− mice causes misregulation of at least one proposed GABP target gene, while neuromuscular synapse formation and synapse-specific transcription are unaffected. We cannot exclude the possibility that these mutant mice still express a low level of GABPα, insufficient to repress rb expression but fully capable of stimulating synapse-specific gene expression.
We used HSA::cre mice to conditionally inactivate gabpα in skeletal muscle. Previously we demonstrated that the HSA::cre transgene mediates efficient (>95%) deletion of loxP-flanked target sequences in muscle fibers (13). Here we show that the levels of wild-type gabpα transcript are reduced approximately 10-fold in muscle tissue of HSA::cre; gabpαf/− mice compared to those for gabpαf/+ mice. Because gabpα is likely to be expressed in nonmuscle cells within muscle tissue (see Results) and because fibroblasts, smooth muscle cells, Schwann cells, and endothelial cells constitute approximately 50% of the nuclei within muscle tissue (34), the reduction of gabpα within muscle fibers of HSA::cre; gabpαf/− mice is likely greater than 10-fold.
The gabpα− allele described here is likely to be a null allele, because it lacks the sequences in gabpα that encode the DNA-binding domain. Consistent with this idea, gabpα−/− mice die before E8.5 (data not shown), as do mice that are homozygous for a gabpα allele lacking the first protein-coding exon (26). Because the gabpα gene encodes the DNA-binding portion of GABP and GABPβ does not bind directly to DNA (3), deletion of gabpα in HSA::cre; gabpαf/− mice abolishes the ability of GABP to stimulate transcription in skeletal muscle.
Expression of a dominant-negative form of GABPβ, which can interact with GABPα but lacks the transcriptional activation domain, inhibits ectopic induction of AChR ɛ gene expression by agrin in adult skeletal muscle, suggesting that GABP can regulate synaptic AChR gene expression (2). These findings and our results would be reconciled if a complex of GABPα and transcriptionally defective GABPβ remained bound to the Ets site in the AChR ɛ gene, preventing other, “compensating” Ets proteins from binding to the Ets site and substituting for GABPα. In contrast, removal of GABPα in HSA::cre; gabpαf/− mice may allow for other Ets proteins to occupy the Ets site in AChR genes and compensate for the absence of GABP. Consistent with this idea, the DNA-binding specificities of different members of the family of Ets domain-containing transcription factors are very similar (3), and multiple Ets proteins are expressed in muscle (12, 29). Thus, although it is possible that Ets domain-containing transcription factors other than GABP normally confer synapse-specific gene expression, other Ets domain proteins may only compensate for the loss of GABPα. Notably, one Ets family member, Erm, is a particularly attractive candidate for regulating synapse-specific transcription, since erm RNA is highly concentrated at synaptic sites in skeletal muscle (12, 15). Further analysis of erm mutant mice should reveal whether Erm alone or Erm together with GABP has a role in synapse-specific gene expression.
Acknowledgments
This work was supported by grants from the NIH (NS27963 and NS31963) to S.J.B.
We thank Judith Melki and Kevin Campbell for HSA::cre mice and Xiang-Qing Li and Jihua Fan for expert technical assistance.
Footnotes
Published ahead of print on 7 May 2007.
REFERENCES
- 1.Batchelor, A. H., D. E. Piper, F. C. de la Brousse, S. L. McKnight, and C. Wolberger. 1998. The structure of GABPα/β: an ETS domain-ankyrin repeat heterodimer bound to DNA. Science 279:1037-1041. [DOI] [PubMed] [Google Scholar]
- 2.Briguet, A., and M. A. Ruegg. 2000. The Ets transcription factor GABP is required for postsynaptic differentiation in vivo. J. Neurosci. 20:5989-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, T. A., and S. L. McKnight. 1992. Specificities of protein-protein and protein-DNA interaction of GABP alpha and two newly defined ets-related proteins. Genes Dev. 6:2502-2512. [DOI] [PubMed] [Google Scholar]
- 4.Burden, S. J. 2002. Building the vertebrate neuromuscular synapse. J. Neurobiol. 53:501-511. [DOI] [PubMed] [Google Scholar]
- 5.DeChiara, T. M., D. C. Bowen, D. M. Valenzuela, M. V. Simmons, W. T. Poueymirou, S. Thomas, E. Kinetz, D. L. Compton, E. Rojas, J. S. Park, C. Smith, P. S. DiStefano, D. J. Glass, S. J. Burden, and G. D. Yancopoulos. 1996. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 85:501-512. [DOI] [PubMed] [Google Scholar]
- 6.de Kerchove D'Exaerde, A., J. Cartaud, A. Ravel-Chapuis, T. Seroz, F. Pasteau, L. M. Angus, B. J. Jasmin, J. P. Changeux, and L. Schaeffer. 2002. Expression of mutant Ets protein at the neuromuscular synapse causes alterations in morphology and gene expression. EMBO Rep. 3:1075-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de la Brousse, F. C., E. H. Birkenmeier, D. S. King, L. B. Rowe, and S. L. McKnight. 1994. Molecular and genetic characterization of GABP beta. Genes Dev. 8:1853-1865. [DOI] [PubMed] [Google Scholar]
- 8.Farley, F. W., P. Soriano, L. S. Steffen, and S. M. Dymecki. 2000. Widespread recombinase expression using FLPeR (flipper) mice. Genesis 28:106-110. [PubMed] [Google Scholar]
- 9.Fromm, L., and S. J. Burden. 1998. Synapse-specific and neuregulin-induced transcription require an ets site that binds GABPalpha/GABPbeta. Genes Dev. 12:3074-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gugneja, S., J. V. Virbasius, and R. C. Scarpulla. 1995. Four structurally distinct, non-DNA-binding subunits of human nuclear respiratory factor 2 share a conserved transcriptional activation domain. Mol. Cell. Biol. 15:102-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbst, R., E. Avetisova, and S. J. Burden. 2002. Restoration of synapse formation in Musk mutant mice expressing a Musk/Trk chimeric receptor. Development 129:5449-5460. [DOI] [PubMed] [Google Scholar]
- 12.Hippenmeyer, S., N. A. Shneider, C. Birchmeier, S. J. Burden, T. M. Jessell, and S. Arber. 2002. A role for neuregulin1 signaling in muscle spindle differentiation. Neuron 36:1035-1049. [DOI] [PubMed] [Google Scholar]
- 13.Jaworski, A., and S. J. Burden. 2006. Neuromuscular synapse formation in mice lacking motor neuron- and skeletal muscle-derived Neuregulin-1. J. Neurosci. 26:655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly, D. P., and R. C. Scarpulla. 2004. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 18:357-368. [DOI] [PubMed] [Google Scholar]
- 15.Kishi, M., T. T. Kummer, S. J. Eglen, and J. R. Sanes. 2005. LL5beta: a regulator of postsynaptic differentiation identified in a screen for synaptically enriched transcripts at the neuromuscular junction. J. Cell Biol. 169:355-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koike, S., L. Schaeffer, and J. P. Changeux. 1995. Identification of a DNA element determining synaptic expression of the mouse acetylcholine receptor delta-subunit gene. Proc. Natl. Acad. Sci. USA 92:10624-10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacazette, E., S. Le Calvez, N. Gajendran, and H. R. Brenner. 2003. A novel pathway for MuSK to induce key genes in neuromuscular synapse formation. J. Cell Biol. 161:727-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaMarco, K., C. C. Thompson, B. P. Byers, E. M. Walton, and S. L. McKnight. 1991. Identification of Ets- and notch-related subunits in GA binding protein. Science 253:789-792. [DOI] [PubMed] [Google Scholar]
- 19.Martin, M. E., Y. Chinenov, M. Yu, T. K. Schmidt, and X. Y. Yang. 1996. Redox regulation of GA-binding protein-alpha DNA binding activity. J. Biol. Chem. 271:25617-25623. [DOI] [PubMed] [Google Scholar]
- 20.Miniou, P., D. Tiziano, T. Frugier, N. Roblot, M. Le Meur, and J. Melki. 1999. Gene targeting restricted to mouse striated muscle lineage. Nucleic Acids Res. 27:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishina, M., T. Takai, K. Imoto, M. Noda, T. Takahashi, S. Numa, C. Methfessel, and B. Sakmann. 1986. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature 321:406-411. [DOI] [PubMed] [Google Scholar]
- 22.Nichols, P., R. Croxen, A. Vincent, R. Rutter, M. Hutchinson, J. Newsom-Davis, and D. Beeson. 1999. Mutation of the acetylcholine receptor epsilon-subunit promoter in congenital myasthenic syndrome. Ann. Neurol. 45:439-443. [PubMed] [Google Scholar]
- 23.Ohno, K., B. Anlar, and A. G. Engel. 1999. Congenital myasthenic syndrome caused by a mutation in the Ets-binding site of the promoter region of the acetylcholine receptor epsilon subunit gene. Neuromuscul. Disord. 9:131-135. [DOI] [PubMed] [Google Scholar]
- 24.O'Leary, D. A., D. Koleski, I. Kola, P. J. Hertzog, and S. Ristevski. 2005. Identification and expression analysis of alternative transcripts of the mouse GA-binding protein (Gabp) subunits alpha and beta1. Gene 344:79-92. [DOI] [PubMed] [Google Scholar]
- 24a.O'Leary, D. A., P. G. Noakes, N. A. Lavidis, I. kola, P. J. Hertzog, and S. Ristevski. 2007. Targeting of the ETS factor Gabp disrupts Neuromuscular junction synapstic function. Mol. Cell. Biol. 27:3470-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ongwijitwat, S., and M. T. Wong-Riley. 2005. Is nuclear respiratory factor 2 a master transcriptional coordinator for all ten nuclear-encoded cytochrome c oxidase subunits in neurons? Gene 360:65-77. [DOI] [PubMed] [Google Scholar]
- 26.Ristevski, S., D. A. O'Leary, A. P. Thornell, M. J. Owen, I. Kola, and P. J. Hertzog. 2004. The ETS transcription factor GABPα is essential for early embryogenesis. Mol. Cell. Biol. 24:5844-5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosmarin, A. G., K. K. Resendes, Z. Yang, J. N. McMillan, and S. L. Fleming. 2004. GA-binding protein transcription factor: a review of GABP as an integrator of intracellular signaling and protein-protein interactions. Blood Cells Mol. Dis. 32:143-154. [DOI] [PubMed] [Google Scholar]
- 28.Sanes, J. R., and J. W. Lichtman. 2001. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2:791-805. [DOI] [PubMed] [Google Scholar]
- 29.Sapru, M. K. 2001. Neuregulin-1 regulates expression of the Ets-2 transcription factor. Life Sci. 69:2663-2674. [DOI] [PubMed] [Google Scholar]
- 30.Savoysky, E., T. Mizuno, Y. Sowa, H. Watanabe, J. Sawada, H. Nomura, Y. Ohsugi, H. Handa, and T. Sakai. 1994. The retinoblastoma binding factor 1 (RBF-1) site in RB gene promoter binds preferentially E4TF1, a member of the Ets transcription factors family. Oncogene 9:1839-1846. [PubMed] [Google Scholar]
- 31.Sawa, C., M. Goto, F. Suzuki, H. Watanabe, J. Sawada, and H. Handa. 1996. Functional domains of transcription factor hGABP beta1/E4TF1-53 required for nuclear localization and transcription activation. Nucleic Acids Res. 24:4954-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaeffer, L., A. de Kerchove d'Exaerde, and J. P. Changeux. 2001. Targeting transcription to the neuromuscular synapse. Neuron 31:15-22. [DOI] [PubMed] [Google Scholar]
- 33.Schaeffer, L., N. Duclert, M. Huchet-Dymanus, and J. P. Changeux. 1998. Implication of a multisubunit Ets-related transcription factor in synaptic expression of the nicotinic acetylcholine receptor. EMBO J. 17:3078-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmalbruch, H., and U. Hellhammer. 1977. The number of nuclei in adult rat muscles with special reference to satellite cells. Anat. Rec. 189:169-175. [DOI] [PubMed] [Google Scholar]
- 35.Si, J., D. S. Miller, and L. Mei. 1997. Identification of an element required for acetylcholine receptor-inducing activity (ARIA)-induced expression of the acetylcholine receptor epsilon subunit gene. J. Biol. Chem. 272:10367-10371. [DOI] [PubMed] [Google Scholar]
- 36.Simon, A. M., P. Hoppe, and S. J. Burden. 1992. Spatial restriction of AChR gene expression to subsynaptic nuclei. Development 114:545-553. [DOI] [PubMed] [Google Scholar]
- 37.Sowa, Y., Y. Shiio, T. Fujita, T. Matsumoto, Y. Okuyama, D. Kato, J. Inoue, J. Sawada, M. Goto, H. Watanabe, H. Handa, and T. Sakai. 1997. Retinoblastoma binding factor 1 site in the core promoter region of the human RB gene is activated by hGABP/E4TF1. Cancer Res. 57:3145-3148. [PubMed] [Google Scholar]
- 38.Sunesen, M., M. Huchet-Dymanus, M. O. Christensen, and J. P. Changeux. 2003. Phosphorylation-elicited quaternary changes of GA binding protein in transcriptional activation. Mol. Cell. Biol. 23:8008-8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson, C. C., T. A. Brown, and S. L. McKnight. 1991. Convergence of Ets- and notch-related structural motifs in a heteromeric DNA binding complex. Science 253:762-768. [DOI] [PubMed] [Google Scholar]
- 40.Virbasius, J. V., C. A. Virbasius, and R. C. Scarpulla. 1993. Identity of GABP with NRF-2, a multisubunit activator of cytochrome oxidase expression, reveals a cellular role for an ETS domain activator of viral promoters. Genes Dev. 7:380-392. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe, H., J. Sawada, K. Yano, K. Yamaguchi, M. Goto, and H. Handa. 1993. cDNA cloning of transcription factor E4TF1 subunits with Ets and notch motifs. Mol. Cell. Biol. 13:1385-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White, J. K., W. Auerbach, M. P. Duyao, J. P. Vonsattel, J. F. Gusella, A. L. Joyner, and M. E. MacDonald. 1997. Huntingtin is required for neurogenesis and is not impaired by the Huntington's disease CAG expansion. Nat. Genet. 17:404-410. [DOI] [PubMed] [Google Scholar]
- 43.Xue, H. H., J. Bollenbacher, V. Rovella, R. Tripuraneni, Y. B. Du, C. Y. Liu, A. Williams, J. P. McCoy, and W. J. Leonard. 2004. GA binding protein regulates interleukin 7 receptor alpha-chain gene expression in T cells. Nat. Immunol. 5:1036-1044. [DOI] [PubMed] [Google Scholar]