Abstract
BACKGROUND
The degree of variability in prostate-specific antigen (PSA) measurements is important for interpreting test results in screening programs, and particularly for interpreting the significance of changes between repeated tests. This study aimed to determine the long-term intra-individual variation for PSA in healthy men.
METHODS
A randomly selected cohort of men in a biennial prostate cancer screening program (ERSPC) conducted in Sweden from 1995–1996 to 2001–2002. We studied men who had total PSA (tPSA) levels <2.0 ng/ml in 2001–2002. This included 791 men with tPSA ≤ 0.61 ng/ml (group A), 1,542 men with tPSA ≤ 0.99 ng/ml (group B), and 1,029 men with tPSA 1.00–1.99 ng/ml (group C). The intra-individual variability of free PSA (fPSA) and tPSA was assessed by calculating coefficients of variation (CV) for each individual’s PSA measurements from the first and second round of screening (1995–1996 and 1997–1998).
RESULTS
Intra-individual CV (geometric means) for tPSA were 13.7%, 12.7%, and 11.5% in groups A, B, and C, respectively. Corresponding CVs for fPSA were significantly lower, ranging from 12.1% to 10.4%. The estimated biological variation of tPSA and fPSA in groups A to C were 12.5%, 11.4%, 10.0% and 9.7%, 7.8%, 7.5%, respectively.
CONCLUSIONS
In healthy men with PSA levels less than 2 ng/ml, the natural long-term variability for tPSA was less than 14%, and with 95% probability, a change in tPSA greater than 30% indicates a change beyond normal random variation.
Keywords: PSA, biological variation, critical difference, screening
INTRODUCTION
A prostate-specific antigen (PSA) value of 4 ng/ml or higher has been considered predictive of cancer of the prostate (CaP). Several recent studies have also found PSA levels of less than 4 ng/ml to be associated with a substantial number of cases of CaP [1,2], and several studies have suggested that a PSA level of 2.5 ng/ml or greater has a predictive value similar to that of a level of 4 ng/ml or greater [3,4]. The interpretation of PSA levels of less than 4.0 ng/ml is a challenge in decision-making for clinicians, as there are no widely accepted and carefully validated upper limits of normal PSA level. The generally accepted practice of performing prostate biopsy when PSA levels are 4.0 ng/ml or higher is based on a prospective study of 6,630 men aimed at optimizing cancer detection rates relative to test specificity [5]. Another proposed predictor of CaP is the rate of increase in PSA levels over time (PSA velocity). Carter et al. [6], based on the study of increase in PSA in men who later diagnosed with CaP, suggested that an annual increase in PSA of greater than 0.75 ng/ml per year is indicative of cancer. Contradictory data have been reported regarding the clinical usefulness of PSA velocity in men with PSA levels less than 4.0 ng/ml [7–9].
The variability of PSA is an important factor to be considered in screening and in monitoring of individuals over time. Total variation in PSA includes analytical and biological variation. Analytical variation depends on assay performance, sample handling, and laboratory processing [10]. Biological variation relates to individual factors such as PSA metabolism, renal elimination, and physical and sexual activity [11,12].
A large normal variability requires larger changes between two consecutive measurements to distinguish pathological changes from changes resulting from analytical and biological variations. Several studies have described intra-individual variation of PSA over short time periods, at most 5-months [13]. Nixon et al. [14] calculated the coefficient of variation (CV) over 2 weeks and demonstrated that a change between two PSA measurements of approximately 25% indicated a significant change. However, the only long-term study has been performed in patients with CaP [15]. In that study, Bunting et al. reported a critical difference, defined as the minimum percent change between two consecutive measurements that suggests a significant change beyond the normal variation, close to 60% over a time period of 1 year. As CaP is a slowly progressive disease in most cases, PSA screening is often performed annually or biannually. To our knowledge, there have been no reports describing long-term variability of PSA in healthy men. We evaluated the long-term intra-individual variability for the different forms of PSA in seemingly healthy men, defined as having tPSA levels of less than 2 ng/ml after 8 years of observation.
MATERIALS AND METHODS
Subjects
We used data from men who participated in a biennial CaP screening program (part of the European Randomized Screening for Prostate Cancer (ERSPC) study) conducted in Göteborg, Sweden from 1995 to 2002 [16]. The participants, 10,000 men aged 50–66 years, were randomly selected and offered PSA testing between 1995 and 1996. Informed consent was obtained from all participants. They were then invited to receive testing every second year thereafter for 8 years (for a total of four PSA tests). In the first and second rounds of screening, men with a total PSA (tPSA) of ≥ 3 ng/ml were offered digital rectal examination, transrectal ultrasound, and sextant biopsy, whereas in the third and fourth rounds of screening, men with a tPSA of ≥ 2.5 ng/ml underwent prostate biopsy. Men with PSA <1 ng/ml in the second round of PSA screening were not invited to participate in the third screening round. Because this altered screening protocol could have biased our data considerably, data from the third round were not included in this study. The observed cumulative incidence of prostate carcinoma was 7.3% after 8 years [16].
From the fourth round of screening, we selected the 3,568 men with tPSA <2.0 ng/ml. PSA data from the first and second rounds of screening were available for 2,571 of these men. The subjects were split into three groups, according to tPSA level measured during the fourth round of screening: 791 with tPSA ≤ 0.61 ng/ml (group A), 1,542 with tPSA ≤ 0.99 ng/ml (group B), and 1,029 with tPSA 1.00–1.99 ng/ml (group C). The median age at screening in 1995–1996 was 55 years in group A, 56 years in group B, and 55 years in group C.
Laboratory Methods and Sample Handling
Serum was separated from blood cells by centrifugation, frozen within 3 hr of venipuncture, and stored at −20°C. tPSA and free PSA (fPSA) were analyzed within 2 weeks of sample collection and within 3 hr of thawing. tPSA and fPSA were measured with the ProStatus™ assay from Perkin Elmer Life Sciences (Turku, Finland), from which we calculated the percentage fPSA (%fPSA) and the level of complexed PSA (cPSA = tPSA − fPSA). Samples were analyzed on a DELFIA™ 1234 Plate fluorometer (Perkin Elmer Life Sciences). This commercial assay is based on a dual-label detection technique, which uses three carefully characterized, distinctly unique binding monoclonal antibodies to PSA. The assay for tPSA measures fPSA and PSA in complex with alpha-1-antichymotrypsin in an equimolar fashion [17]. The lower limit of detection is 0.04 ng/ml for fPSA and 0.1 ng/ml for tPSA. Analytical coefficients of variation (CVa) were calculated from the data from control samples used during the study period. The mean CVa for tPSA at a level of 2.4 ng/ml was 5.6%, and for fPSA at a level of 0.25 ng/ml was 7.2%. The mean CVa for cPSA at a level of 2.2 ng/ml was 5.5% and for %fPSA at a level of 10% was 7.7%.
Statistical Methods
Statistical analysis was performed with the SPSS program version 10.0 (SPSS, Inc., Chicago).
Bland Altman plots, with and without logarithmic transformation, were performed to determine whether the changes varied in a systematic way over the range of measurements in the whole study population [18].
Our estimations of variation and trend over time for PSA were calculated from levels measured in samples collected from the first (1995–1996) and second (1997–1998) rounds of screening.
The coefficient of total intra-individual variation (CVt) was calculated from the ratio between standard deviation (SD) and the mean of the two measurements of each patient: 1995–1996 screening and 1997–1998 screening (CVt = SD/mean 100). The CVt represents a combination of the biological (CVb) and analytical (CVa) components of variation. CVb was calculated from the following equation: CVb2 = CVt2−CVa2. As the CVs obtained for fPSA, tPSA, cPSA, and %fPSA showed a log-normal distribution, the CVs are presented as geometric means. Patients with SDs resulting in zero were excluded from log transformation. Ninety-five percent confidence intervals (CIs) of the means of the logarithmically transformed CVs were used to evaluate statistical significance of differences between the CVs. Slightly overlapping CIs were also tested by t-test or paired t-test. To calculate the critical difference at the 95% probability level CVt was multiplied by 2.32 (1.64 √2) (1-tailed estimation) [19]. Trend over time of tPSA, fPSA, cPSA, and %fPSA was calculated by using linear regression analysis with logarithmically transformed values. Exact time between the two measurements was calculated for all patients (as an independent factor in the analysis). P-values less than 0.05 were considered to be statistically significant. Model assumptions were checked by residual analysis.
RESULTS
The distributions of levels of fPSA, tPSA, cPSA, and %fPSA in serum, measured during each of the three screening occasions, are shown in Table I.
TABLE I.
Distribution of Serum fPSA, tPSA, cPSA, and Percent fPSA Values at the Three Rounds of Screening
| Median (25th–75th centiles)
|
|||||
|---|---|---|---|---|---|
| Group | Year of screening | fPSA (ng/ml) | tPSA (ng/ml) | cPSA (ng/ml) | %fPSA |
| A | 1995–1996 | 0.15 (0.12–0.20) | 0.45 (0.35–0.59) | 0.28 (0.20–0.40) | 34.5 (26.2–43,4) |
| 1997–1998 | 0.15 (0.11–0.19) | 0.48 (0.35–0.62) | 0.31 (0.22–0.43) | 31.2 (24.0–39.7) | |
| 2001–2002 | 0.15 (0.11–0.19) | 0.43 (0.34–0.53) | 0.27 (0.20–0.35) | 35.8 (27.8–43.0) | |
| B | 1995–1996 | 0.19 (0.14–0.25) | 0.58 (0.42–0.79) | 0.38 (0.25–0.55) | 32.9 (25.0–42.0) |
| 1997–1998 | 0.18 (0.13–0.25) | 0.62 (0.45–0.79) | 0.41 (0.28–0.56) | 31.3 (24.3–38.7) | |
| 2001–2002 | 0.19 (0.14–0.16) | 0.61 (0.43–0.78) | 0.39 (0.27–0.52) | 33.3 (26.1–41.1) | |
| C | 1995–1996 | 0.29 (0.23–0.37) | 1.05 (0.84–1.35) | 0.75 (0.56–1.02) | 27.5 (21.5–34.6) |
| 1997–1998 | 0.30 (0.23–0.40) | 1.15 (0.93–1.46) | 0.84 (0.63–1.10) | 26.2 (20.6–33.1) | |
| 2001–2002 | 0.36 (0.28–0.46) | 1.36 (1.15–1.63) | 0.98 (0.83–1.21) | 26.5 (20.7–32.7) | |
Group A represents men with tPSA ≤ 0.61 ng/ml, group B men with tPSA ≤ 0.99 ng/ml, and group C men with tPSA 1.00–1.99 ng/ml, in 2001–2002.
Calculated parameters are shown in italics.
For each of the three groups with different final PSA levels, total intra-individual variation (expressed as CVt, a measure of individual variation as a percentage of the individual mean values) was assessed for fPSA, tPSA, cPSA, and %fPSA (Table II). Total CV for tPSA was 13.7% for group A (final PSA ≤ 0.61 ng/ml), 12.7% for group B (final PSA ≤ 0.99 ng/ml), and 11.5% for group C (final PSA 1.00–1.99 ng/ml). Men in group C showed significantly lower total variation of tPSA than the men in groups A and B. In all three groups, CVt for tPSA was significantly higher than that for fPSA and %fPSA. The critical differences (i.e., the sizes of increase between two consecutive measurements that are statistically significant at the 95% probability level) for the different PSA forms and %fPSA are also given in Table II. The critical differences for tPSA ranged from 31.8% to 26.7% in groups A to C.
TABLE II.
Total Coefficient of Variation (CVt) and Critical Difference of the Different Forms of PSA Based on Values From the Screenings of 1995–1996 and 1997–1998
| Group | fPSA | tPSA | CPSA | %fPSA |
|---|---|---|---|---|
| Geometric mean CVt, % (95% CI) | ||||
| A | 12.1 (11.2–13.0)b | 13.7 (12.7–14.6)b | 13.8 (12.3–15.6) | 9.6 (8.8–10.5) |
| B | 10.6 (10.0–11.2) | 12.7 (12.1–13.4)c | 13.4 (12.4–14.4) | 9.0 (8.5–9.6) |
| C | 10.4 (9.7–11.0)a | 11.5 (10.7–12.2)a,c | 12.5 (11.4–13.6) | 7.9 (7.4–8.5) |
| Critical difference, % | ||||
| A | 28.1 | 31.8 | 32.0 | 22.3 |
| B | 24.6 | 29.5 | 31.1 | 20.9 |
| C | 24.1 | 26.7 | 29.0 | 18.3 |
P <0.01 by paired t-test.
P <0.01 by paired t-test.
P <0.05 by t-test.
Group A represents men with tPSA ≤ 0.61ng/ml, group B men with tPSA ≤ 0.99 ng/ml and group C men with tPSA 1.00–1.99 ng/ml, in 2001–2002.
Calculated parameters are shown in italics.
Critical differences are estimated with one-tailed 95% probability.
The estimated biological variations of tPSA in groups A, B, and C was 12.5%, 11.4%, and 10.0%, respectively (Table III).
TABLE III.
Coefficient of Analytical Variation (CVa) and Calculated Coefficient of Biological Variation (CVb) Between Screenings of 1995–1996 and 1997–1998
| Group | fPSA | tPSA | cPSA | %fPSA |
|---|---|---|---|---|
| CVa, % | ||||
| Controls | 7.2 | 5.6 | 5.5 | 7.7 |
| CVb, % | ||||
| A | 9.7 | 12.5 | 12.7 | 5.7 |
| B | 7.8 | 11.4 | 12.2 | 4.7 |
| C | 7.5 | 10.0 | 11.2 | 1.8 |
Group A represents men with tPSA ≤ 0.61 ng/ml, group B men with tPSA ≤ 0.99 ng/ml and group C men with tPSA 1.00–1.99 ng/ml, in 2001–2002.
CVa is expressed as mean and CVb as geometric mean.
Calculated parameters are shown in italics.
Bland Altman analysis showed that differences in tPSA levels between the first and the second screening round were proportional to the mean (results not shown). However, logarithmic transformation of the values minimized this relationship between the differences and means (Fig. 1). For men in group C, estimation of trend over time on logarithmically transformed values (Table IV) showed a significant increase in levels of tPSA (4.1% per year; P <0.001) and fPSA (1.9% per year; P <0.05). In contrast, men in group A and B showed no significant increase over time.
Fig. 1.
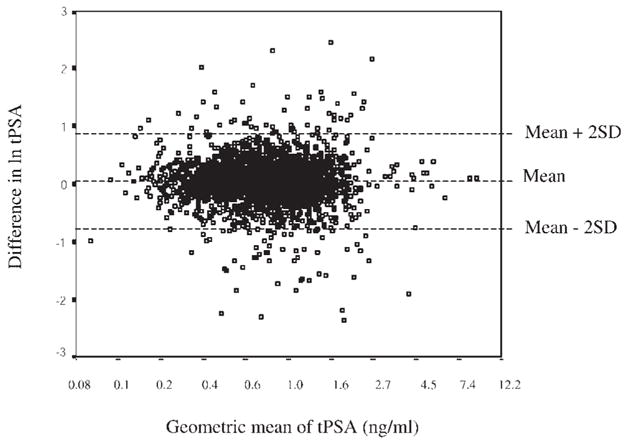
Bland Altman plot showing the correlation of the means and differences of logarithmically transformed tPSA values measured at screening of the whole study population in 1995–1996 and 1997–1998. Values on the x-axis have been transformed anti-logarithmically.
TABLE IV.
Mean Trend Over Time Between the Screening Rounds of 1995–1996 and 1997–1998
| Mean percent change per year (95% CI)
|
||||
|---|---|---|---|---|
| Group | FPSA | tPSA | cPSA | %fPSA |
| A | −2.5 (−0.4 to −4.5)a | 0.4 (−1.7 to 2.5) | 2.7 (0.1 to 5.3)a | −32.9 (−4.5 to −1.3)b |
| B | −1.0 (−2.5 to 0.6) | 1.1 (−0.5 to 2.6) | 2.7 (0.8 to 4.7)b | −2.0 (−3.2 to −0.9)b |
| C | 1.9 (0.3 to 3.6)a | 4.1 (2.5 to 5.8)c | 5.1 (3.1 to 7.0)c | −2.2 (−3.5 to −0.8)b |
P <0.05.
P <0.01.
P <0.001.
Group A represents men with tPSA ≤ 0.61 ng/ml, group B men with tPSA ≤ 0.99 ng/ml and group C men with tPSA 1.00–1.99 ng/ml, in 2001–2002.
DISCUSSION
Repeated measurements of PSA are used in clinical routines, often on a yearly or biannual basis. To assess the relevance of changes between two time points, we have studied the long-term variability of the different forms of PSA at several different PSA levels in a randomly selected population of asymptomatic and apparently healthy men. To minimize the risk of our data being confounded by incident CaP among the study participants, we focused on men whose PSA levels were low (tPSA <2.0 ng/ml) at the end of the 8-year observation period. Data from the final round of screening were used to select participants and divide them into three groups by tPSA level, while data from the first and second rounds of screening were used to assess the intra-individual variation.
At all three tPSA levels, the current study demonstrated that the total intra-individual variation of tPSA was significantly higher than the intra-individual variation for either fPSA or %fPSA. This suggests that fPSA concentration in blood may vary less than cPSA concentration, which is the major contributor to tPSA. One explanation is that fPSA and cPSA may have different elimination pathways, and hence different elimination rates [20–23]. The finding of higher total variation in the group of men with the lowest PSA levels in this study cohort is more likely to be a reflection of analytical imprecision at the lower ranges.
This study also aimed at evaluating the contribution of biological to the total intra-individual variability. The biological variation was calculated from the total intra-individual and the analytical variability. The analytical variation was assessed from control samples analyzed during the study period instead of being obtained from manufacturer-generated data, which has made our calculations more reliable. We found that men with low PSA levels and low risk of CaP have a rather low biological variability in both fPSA and tPSA (less than 14%). In contrast to this, Bunting et al. [15] have presented a long-term study of patients with CaP demonstrating a variation of approximately 20% for both fPSA and tPSA. These data would therefore suggest that the variability of PSA is considerably less in seemingly healthy men than in men with CaP.
Our study did not aim to evaluate the long-term trend of PSA in healthy men, as this would have required a different study setup. However, we were interested in estimating time-dependent changes, since these contribute to the total intra-individual variation. In our study population of seemingly healthy men, the annual change in tPSA contributed to our reported variability only to a small extent.
We found no significant elevation of tPSA over time in groups A and B, which consisted of men with final tPSA levels <0.99 ng/ml. However, in group C, consisting of men with final tPSA 1.00–1.99 ng/ml, tPSA increased 4% per year between screening rounds 1 and 2. This increase is consistent with the previously reported 3.2% increase in tPSA per year in a population study of men in Olmsted County, Minnesota [24].
Recently, Eastham et al. [25] evaluated the year-to-year fluctuations in PSA levels over a period of 4 years in a cohort of men selected from a polyp-prevention trial study group. Several cut-off points for PSA were studied; 30% and 26% of the men with a PSA level >4 ng/ml and >2.5 ng/ml, respectively, had a PSA value below these cut-offs at the next PSA-testing. The variation for PSA was not calculated and the results are therefore not directly comparable with our study, but the results of Eastham et al. certainly indicate the importance of considering the variability of PSA in a screening protocol for CaP.
Total intra-individual variability of PSA is the parameter that determines the critical difference, which is defined as the minimum percent change between two consecutive PSA measurements that suggest a significant change beyond the normal biological and analytical variation. In our study cohort (with a very low risk of confounding CaP), an increase in tPSA of approximately 30% indicates a true increase at the 95% confidence level and may thus reflect a clinically significant change. In the Bunting et al. [15] study of patients with CaP, a critical difference close to 60% was found.
Complexed PSA and %fPSA are calculated from fPSA and tPSA. Therefore, estimations of the variability of cPSA and %fPSA are dependent on the variations of each of the two PSA forms. Due to this fact, we have chosen not to draw any extensive conclusions on the variability of cPSA and %fPSA.
To improve our understanding of the natural long-term variability in PSA, we have focused on men with low PSA levels (less than 2 ng/ml) after an observation period of 8 years. We found that the intra-individual variability for tPSA, in seemingly healthy men, was less than 14% and that an increase in tPSA of approximately 30% indicates a true change in these low PSA ranges. We do not aim to recommend a particular PSA change as the criterion for biopsy. However, our findings may be helpful in the evaluation of small fluctuations in PSA levels and in deciding the time interval for PSA tests in screening programs.
Acknowledgments
We thank Jan-Åke Nilsson and Per Fernlund for help with statistics and Gun-Britt Eriksson and Kerstin Håkansson for laboratory work.
Grant sponsor: Swedish Research Council; Grant number: 7903; Grant sponsor: Swedish Cancer Society; Grant numbers: 3555, 4715; Grant sponsor: Faculty of Medicine, Lund University; Grant sponsor: Research Fund and the Cancer Research Fund of University Hospital, Malmö; Grant sponsor: Regional Research Foundation, Skane, Sweden; Grant sponsor: Fundation Federico S.A.; Grant sponsor: European Union contract (P-Mark); Grant number: LSHC-CT-2004-50301; Grant sponsor: National Cancer Institute (SPORE Pilot Project 7, USA); Grant number: P50-CA92629.
References
- 1.Kobayashi T, Nishizawa K, Ogura K, Mitsumori K, Ide Y. Detection of prostate cancer in men with prostate-specific antigen levels of 2.0 to 4.0 ng/mL equivalent to that in men with 4.1 to 10.0 ng/mL in a Japanese population. Urology. 2004;63:727–731. doi: 10.1016/j.urology.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 2.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, Minasian LM, Ford LG, Lippman SM, Crawford ED, Crowley JJ, Coltman CA., Jr Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 3.Lodding P, Aus G, Bergdahl S, Frosing R, Lilja H, Pihl CG, Hugosson J. Characteristics of screening detected prostate cancer in men 50 to 66 years old with 3 to 4 ng./ml. Prostate specific antigen. J Urol. 1998;159:899–903. [PubMed] [Google Scholar]
- 4.Krumholtz JS, Carvalhal GF, Ramos CG, Smith DS, Thorson P, Yan Y, Humphrey PA, Roehl KA, Catalona WJ. Prostate-specific antigen cutoff of 2.6 ng/mL for prostate cancer screening is associated with favorable pathologic tumor features. Urology. 2002;60:469–473. doi: 10.1016/s0090-4295(02)01875-7. discussion 473–464. [DOI] [PubMed] [Google Scholar]
- 5.Catalona WJ, Hudson MA, Scardino PT, Richie JP, Ahmann FR, Flanigan RC, deKernion JB, Ratliff TL, Kavoussi LR, Dalkin BL, Waters WB, MacFarlane MT, Southwick PC. Selection of optimal prostate specific antigen cutoffs for early detection of prostate cancer: Receiver operating characteristic curves. J Urol. 1994;152:2037–2042. doi: 10.1016/s0022-5347(17)32300-5. [DOI] [PubMed] [Google Scholar]
- 6.Carter HB, Pearson JD, Metter EJ, Brant LJ, Chan DW, Andres R, Fozard JL, Walsh PC. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. Jama. 1992;267:2215–2220. [PMC free article] [PubMed] [Google Scholar]
- 7.Fang J, Metter EJ, Landis P, Carter HB. PSA velocity for assessing prostate cancer risk in men with PSA levels between 2.0 and 4.0 ng/ml. Urology. 2002;59:889–893. doi: 10.1016/s0090-4295(02)01646-1. discussion 893–884. [DOI] [PubMed] [Google Scholar]
- 8.Riffenburgh RH, Amling CL. Use of early PSA velocity to predict eventual abnormal PSA values in men at risk for prostate cancer. Prostate Cancer Prostatic Dis. 2003;6:39–44. doi: 10.1038/sj.pcan.4500614. [DOI] [PubMed] [Google Scholar]
- 9.Roobol MJ, Kranse R, de Koning HJ, Schroder FH. Prostate-specific antigen velocity at low prostate-specific antigen levels as screening tool for prostate cancer: Results of second screening round of ERSPC (ROTTERDAM) Urology. 2004;63:309–313. doi: 10.1016/j.urology.2003.09.083. discussion 313–305. [DOI] [PubMed] [Google Scholar]
- 10.Piironen T, Pettersson K, Suonpaa M, Stenman UH, Oesterling JE, Lovgren T, Lilja H. In vitro stability of free prostate-specific antigen (PSA) and prostate-specific antigen (PSA) complexed to alpha 1-antichymotrypsin in blood samples. Urology. 1996;48:81–87. doi: 10.1016/s0090-4295(96)00616-4. [DOI] [PubMed] [Google Scholar]
- 11.Bruun L, Bjork T, Lilja H, Becker C, Gustafsson O, Christensson A. Percent-free prostate specific antigen is elevated in men on haemodialysis or peritoneal dialysis treatment. Nephrol Dial Transplant. 2003;18:598–603. doi: 10.1093/ndt/18.3.598. [DOI] [PubMed] [Google Scholar]
- 12.Tchetgen MB, Oesterling JE. The effect of prostatitis, urinary retention, ejaculation, and ambulation on the serum prostate-specific antigen concentration. Urol Clin North Am. 1997;24:283–291. doi: 10.1016/s0094-0143(05)70374-8. [DOI] [PubMed] [Google Scholar]
- 13.Price CP, Allard J, Davies G, Dawnay A, Duffy MJ, France M, Mandarino G, Ward AM, Patel B, Sibley P, Sturgeon C. Pre- and post-analytical factors that may influence use of serum prostate specific antigen and its isoforms in a screening programme for prostate cancer. Ann Clin Biochem. 2001;38:188–216. doi: 10.1258/0004563011900632. [DOI] [PubMed] [Google Scholar]
- 14.Nixon RG, Wener MH, Smith KM, Parson RE, Blase AB, Brawer MK. Day to day changes in free and total PSA: Significance of biological variation. Prostate Cancer Prostatic Dis. 1997;1:90–96. doi: 10.1038/sj.pcan.4500212. [DOI] [PubMed] [Google Scholar]
- 15.Bunting PS, DeBoer G, Choo R, Danjoux C, Klotz L, Fleshner N. Intraindividual variation of PSA, free PSA and complexed PSA in a cohort of patients with prostate cancer managed with watchful observation. Clin Biochem. 2002;35:471–475. doi: 10.1016/s0009-9120(02)00345-4. [DOI] [PubMed] [Google Scholar]
- 16.Hugosson J, Aus G, Lilja H, Lodding P, Pihl CG. Results of a randomized, population-based study of biennial screening using serum prostate-specific antigen measurement to detect prostate carcinoma. Cancer. 2004;100:1397–1405. doi: 10.1002/cncr.20126. [DOI] [PubMed] [Google Scholar]
- 17.Mitrunen K, Pettersson K, Piironen T, Bjork T, Lilja H, Lovgren T. Dual-label one-step immunoassay for simultaneous measurement of free and total prostate-specific antigen concentrations and ratios in serum. Clin Chem. 1995;41:1115–1120. [PubMed] [Google Scholar]
- 18.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 19.Fraser CG, Petersen PH. The importance of imprecision. Ann Clin Biochem. 1991;28(Pt 3):207–211. doi: 10.1177/000456329102800301. [DOI] [PubMed] [Google Scholar]
- 20.Bjork T, Ljungberg B, Piironen T, Abrahamsson PA, Pettersson K, Cockett AT, Lilja H. Rapid exponential elimination of free prostate-specific antigen contrasts the slow, capacity-limited elimination of PSA complexed to alpha 1-antichymotrypsin from serum. Urology. 1998;51:57–62. doi: 10.1016/s0090-4295(97)00572-4. [DOI] [PubMed] [Google Scholar]
- 21.Lilja H, Haese A, Bjork T, Friedrich MG, Piironen T, Pettersson K, Huland E, Huland H. Significance and metabolism of complexed and noncomplexed prostate specific antigen forms, and human glandular kallikrein 2 in clinically localized prostate cancer before and after radical prostatectomy. J Urol. 1999;162:2029–2034. doi: 10.1016/S0022-5347(05)68093-7. discussion 2034–2025. [DOI] [PubMed] [Google Scholar]
- 22.Birkenmeier G, Struck F, Gebhardt R. Clearance mechanism of prostate specific antigen and its complexes with alpha2-macroglobulin and alpha1-antichymotrypsin. J Urol. 1999;162:897–901. doi: 10.1097/00005392-199909010-00086. [DOI] [PubMed] [Google Scholar]
- 23.Bruun L, Ekberg H, Bjork T, Lilja H, Hoglund P, Christensson A. Rapid elimination by glomerular filtration of free prostate specific antigen and human kallikrein 2 after renal transplantation. J Urol. 2004;171:1432–1435. doi: 10.1097/01.ju.0000118580.19344.68. [DOI] [PubMed] [Google Scholar]
- 24.Oesterling JE, Jacobsen SJ, Chute CG, Guess HA, Girman CJ, Panser LA, Lieber MM. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. Jama. 1993;270:860–864. [PubMed] [Google Scholar]
- 25.Eastham JA, Riedel E, Scardino PT, Shike M, Fleisher M, Schatzkin A, Lanza E, Latkany L, Begg CB. Variation of serum prostate-specific antigen levels: An evaluation of year-to-year fluctuations. Jama. 2003;289:2695–2700. doi: 10.1001/jama.289.20.2695. [DOI] [PubMed] [Google Scholar]


