Abstract
A comparative study of chimpanzee (Pan troglodytes) and capuchin monkey (Cebus apella) cerebellar asymmetry and its relationship to handedness was conducted. Magnetic resonance images of the brain and behavioral data on a coordinated bimanual task were obtained from 16 chimpanzees and 11 capuchins. Chimpanzees displayed a greater rightward bias of the posterior cerebellum and capuchins displayed a greater leftward bias of the anterior cerebellum. Cerebellar asymmetries were significantly associated with handedness in capuchins but not chimpanzees, and this effect was most pronounced in right-handed capuchins.
Keywords: Handedness, asymmetry, cerebellum, chimpanzees, capuchins
1. Introduction
Most studies on the neurobiology of handedness have focused on areas of the neocortex, including the planum temporale (Beaton, 1997; Moffat, Hampson, & Lee, 1998; Shapleske, Rossell, Woodruff, & David, 1999), precentral gyrus (Amunts et al., 1996; Foundas, Leonard, & Heilman, 1995; Hammond, 2002), and inferior frontal gyrus (Foundas, Hong, Leonard, & Heilman, 1996; Taglialeta, Cantalupo, & Hopkins, 2006). The role of structures in the metencephalon, particularly the cerebellum, has received considerably less attention. Snyder, Bilder, Wu, Bogerts, and Lieberman (1995) reported dextral humans had greater cerebellar torque (a right anterior, left posterior bias of the cerebellum) than did nondextrals (left-handed and ambidextrous) and the pattern of cerebellar torque matched the pattern of developmental torque seen in the neocortex. Functional magnetic resonance imaging studies have demonstrated cerebellar activation during unimanual and bimanual actions of the hand (Nair, Purcott, Fuchs, Steinberg, & Kelso, 2003; Nitschke, Kleinschmidt, Wessel, & Frahm, 1996). Given the role of the cerebellum in coordinated motor tasks, it is likely that asymmetries associated with hand preference are present in nonhuman primates, but this has yet to be investigated.
Despite a plethora of studies on handedness in nonhuman primates (see Hook-Costigan & Rogers, 1996; Hopkins, 2006; MacNeilage et al., 1987; McGrew & Marchant, 1997; Ward & Hopkins, 1993 for reviews), our understanding of the neurobiological substrates of handedness in nonhuman primates remains poorly understood. A few studies have reported neural correlates associated with hand preference in nonhuman primates. For example, evidence from chimpanzees indicates that hand preferences for non-communicative actions are correlated with asymmetries of the KNOB, a region of the precentral gyrus, but not language area homologues (Dadda, Cantalupo, & Hopkins, 2006; Hopkins & Cantalupo, 2004). Similarly, asymmetries of the dorsal portion of the precentral gyrus are associated with hand preference in capuchin monkeys (Phillips & Sherwood, 2005). In squirrel monkeys, single cell recording studies have shown greater activity in the dorsal portion of the motor cortex in the hemisphere contralateral to the preferred hand (Nudo et al., 1992).
Here we examine whether cerebellar asymmetries are associated with handedness in two nonhuman primate species, chimpanzees and capuchin monkeys. While there is increasing evidence that captive and wild chimpanzees are similar to humans in expressing a tendency towards population-level right-handedness (Hopkins, Stoinski, Lucas, Ross & Wesley, 2003; Hopkins, Wesley, Izard, Hook & Shapiro, 2004; Lonsdorf & Hopkins, 2005) albeit to a lesser degree, the data for capuchins are less clear, with some research groups reporting population-level preferences (e.g., Spinozzi, Castorina, & Truppa, 1998) and others not (e.g.,Fragaszy, Fedigan, & Visalberghi, 2004; Westergaard & Suomi, 1996). Individual capuchins do, however, express strong and consistent hand preferences during tasks that require complex bimanual coordination (Fragaszy & Mitchell, 1990; Limongelli, Sonetti, & Visalberghi, 1994; Westergaard & Suomi, 1993a, 1993b, 1996). Chimpanzees and capuchins share similar characteristics regarding hand morphology and use (Spinozzi et al., 2004), making them ideal species for comparison in brain-behavior relationships. Therefore, these two species provide an interesting opportunity to investigate the relationship between variation in handedness and cerebellar asymmetry.
The cerebellum was selected as a region of interest because it plays an important role in both the coordination of motor actions and the processing of cognitive information via indirect projections to cortical motor areas and prefrontal cortex (Ramnani, 2006). Cerebellar loops with motor and prefrontal areas are distinct and separate, providing differential functional circuitry for motor and cognitive operations (Kelly & Strick, 2003). Moreover, recent comparative studies have shown that apes have a larger cerebellum than would be predicted for an anthropoid primate of the same brain size (Rilling & Insel, 1998), suggesting evolutionary selection for increasing motor planning and coordination. Thus, it might be argued that changes in cerebellar size or connectivity to cortical areas may have been associated with the evolution of population-level handedness. If this hypothesis is correct, then associations between handedness and cerebellar asymmetry would be present in apes but not capuchin monkeys, based on the extant behavioral data. In contrast, if neural correlates of handedness are highly conserved in primates, as suggested by the previous findings of an association between handedness and asymmetries in the dorsal portion of the precentral gyrus, then significant associations should be evident in both capuchin monkeys and chimpanzees. Lastly, it is possible that the evolution of cerebellar asymmetries are unrelated to handedness in primates, in which case, no associations should be found in either species.
2. Method
2.1 Subjects
Magnetic resonance images and behavioral data were collected from sixteen chimpanzees (Pan troglodytes) and eleven capuchin monkeys (Cebus apella). All of the chimpanzees were members of a captive colony at the Yerkes National Primate Research Center (YNPRC) in Atlanta, Georgia. Nine capuchin monkeys were housed at the Laboratory for Neurobehavioral Investigations in Hiram, Ohio and two were housed at the Northeastern Ohio Universities College of Medicine (NEOUCOM) in Rootstown, Ohio. All subjects were mother-reared and socially housed. Chimpanzees ranged in age from 6 – 44 years (M = 21.13, SD = 12.53); capuchins ranged in age from 1 – 18 years (M = 7.00, SD = 6.03).
2.2 Behavioral Measures
Studies across several primate taxa provide support for the notion that more complex tasks elicit a greater consistency and strength of hand preference than do more simple tasks such as reaching (Cebus: Anderson, Degiorgio, Lamarque, & Fagot, 1996; Spinozzi et al., 1998; Cercocebus: Blois-Heulin, Guitton, Nedellec, Bienvenue, Ropars, & Vallet, 2006; Pan: Hopkins & Rabinowitz, 1997; Papio: Vauclair et al., 2005). The TUBE task (Hopkins, 1995) is one measure of complex bimanual coordination that has been tested in several primate species, including chimpanzees and capuchins. We chose to use this task as our measure of handedness for several reasons. First, the TUBE task shows good test-retest correlation, even after as many as 6 years separating tests, in different species (Hopkins & Cantalupo, 2003; Vauclair et al., 2003). Second, the tube task elicits consistent and significant hand preferences in the majority of subjects tested with a given species (see Vauclair et al,.2005). Lastly, recent studies in capuchin monkeys and chimpanzees have shown that handedness for this task correlates with neuroanatomical structures associated with cortical motor areas representing hand (chimpanzees: Hopkins & Cantalupo, 2004; capuchins: Phillips & Sherwood, 2005). Therefore, in the present study, hand preference for chimpanzees and capuchins was determined for each subject using this coordinated bimanual task. Subjects were individually presented with a piece of poly-vinyl-chloride (PVC) tube with peanut butter smeared inside (chimpanzee TUBE dimensions: 15 cm in length and 2.5 cm in diameter; capuchin TUBE dimensions: 6 cm in length and 1.5 cm in diameter). To remove the food, subjects had to hold the tube in one hand and use the other hand to retrieve the peanut butter. The holding, stabilizing hand is regarded as subordinate while the active, reaching hand is considered dominant and was the hand we considered to be the preferred hand for this task. Thus, the hand used to retrieve the food from inside the tube was recorded as left or right for each individual response. Chimpanzees were tested two times with the TUBE task, or more if fewer than 30 responses were produced. Capuchins received four sessions with the task.
2.3 MRI Procedure and Image Quantification Method
Chimpanzees
Magnetic resonance images (MRI) were obtained in vivo using either a 1.5 or 3T scanner. For all scans, subjects were first immobilized by ketamine injection (10 mg/kg) and subsequently anaesthetized with propofol (40–60 mg/(kg/h)) following standard procedures at the YNPRC. Subjects were then transported to the MRI facility. The subjects remained anaesthetized for the duration of the scans as well as the time needed to transport them between their home cage and the imaging facility (total time ~ 2 h). Subjects were placed in the scanner chamber in a supine position with their head fitted inside the human-head coil. Scan duration ranged between 40 and 80 min as a function of brain size. The majority of the subjects (n = 13) were scanned using a 1.5 Tesla scanner (Phillips, Model 51). The remaining chimpanzees (n = 3) were scanned using a 3.0 T scanner (Siemens Trio, Siemens Medical Solutions USA, Inc., Malvern, Pennsylvania, USA) at the YNPRC. Although images acquired on the 3T machine did have a higher resolution, for the measurements of the cerebellum this difference was slight and did not impact measures of the anterior and posterior subregions.
For all chimpanzees scanned in vivo using the 1.5 T machine, T1-weighted images were collected in the transverse plane using a gradient echo protocol (pulse repetition = 19.0 ms, echo time = 8.5 ms, number of signals averaged 8, and a 256 × 256 matrix). For the chimpanzees scanned using a 3.0 T scanner (Siemens Trio), T1-weighted images were collected using a three-dimensional gradient echo sequence (pulse repetition = 2300 ms, echo time = .4.4 ms, number of signals averaged = 3, matrix size = 320 × 320). After completing MRI procedures, the subjects were returned to the YNPRC and temporarily housed in a single cage for 6–12 h to allow the effects of the anesthesia to wear off, after which they were returned to their home cage.
Capuchins
Capuchins were transported to the Brain Imaging Research Center in Pittsburgh, Pennsylvania for the MR procedure. Once at the facility, subjects were immobilized with ketamine (25 mg/kg) and acetylpromazine (1 mg/kg) injection intramuscularly; atropine (0.05 mg/kg) was given subcutaneously. A constant intravenous drip of 160–330 micrograms/kg/minutes of propofol was administered via intravenous catheter in the saphenous vein to maintain anesthesia. Subjects were placed into the scanner chamber and their heads were fitted inside a 16 cm head coil. Subjects remained anesthetized throughout the MR procedure (approximately 50 minutes). While under sedation, respiration rate, heart rate, and oxygen consumption were continually monitored. For the capuchins, T1-weighted images were acquired on a 3.0 T scanner (Siemens Allegra). Images were collected in saggital plane using a gradient echo protocol (pulse repetition = 1500 ms, echo time = 3.04 ms, number of signals averaged 25, and a 256 × 256 matrix). Subjects were allowed to completely recover from the effects of the anesthesia before transport back to Hiram College or NEOUCOM.
2.4 Data Analysis
Handedness index (HI) scores were determined for each subject by using the formula (#R − #L)/(#R + #L), where #R is the number of instances in which the right hand was used and #L equals the number of instances in which the left hand was used. The HI produces scores ranging from − 1.0 to +1.0, with negative scores indicating a preference for the left hand and positive scores indicating a preference for the right hand. More extreme absolute scores reflect a stronger preference for a preferred hand. A mean handedness index (MHI) was calculated for each individual by taking the average HI across all trials for the TUBE task. To determine if the hand preference of an individual was significantly different from chance, z-scores were calculated based on the total frequency in left and right hand use. Subjects with z-scores greater than 1.96 were classified as right-handed. Subjects with z-scores less than 1.96 were classified as non-right-handed.
To measure cerebellar asymmetry, raw images were reformatted into the ANALYZE 3D volume file format to facilitate re-slicing into orthogonal planes prior to morphometric analysis. Volumetric measurements were performed using either ANALYZE 6.0 for chimpanzees (Mayo Clinic, Mayo Foundation, Rochester, Minnesota, USA)) or MRIcro 1.39 for capuchins (Rorden & Brett, 2000). For each subject, measures were taken from coronal view from two cerebellar subregions, anterior and posterior, and an asymmetry quotient calculated (AQ = R − L/[(R + L) × 0.5]) for each subregion. Positive asymmetry quotients represent a right-side bias, whereas negative asymmetry quotients represent a left-side bias. Anterior and posterior portions of the cerebellum were determined by the uvula-tonsils landmark (see Figure 1) (Snyder et al., 1995). Although this approach of bisecting the cerebellum into anterior and posterior subregions does not necessarily reflect functional distinctions, we took this approach so that our results would be comparable to data of cerebellar asymmetries and handedness in humans (Snyder et al., 1995).
Figure 1.
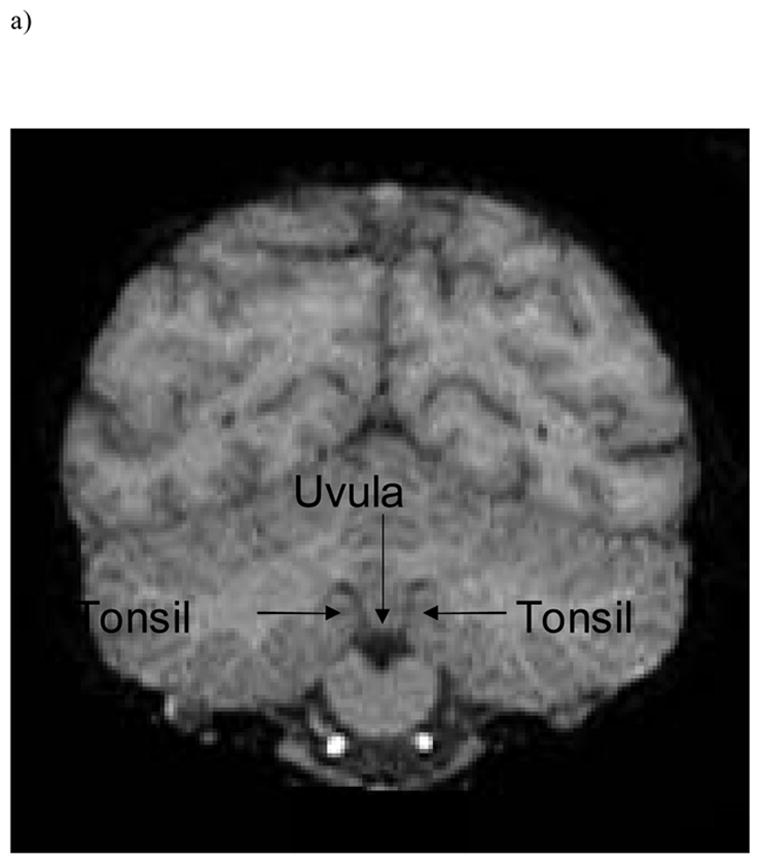
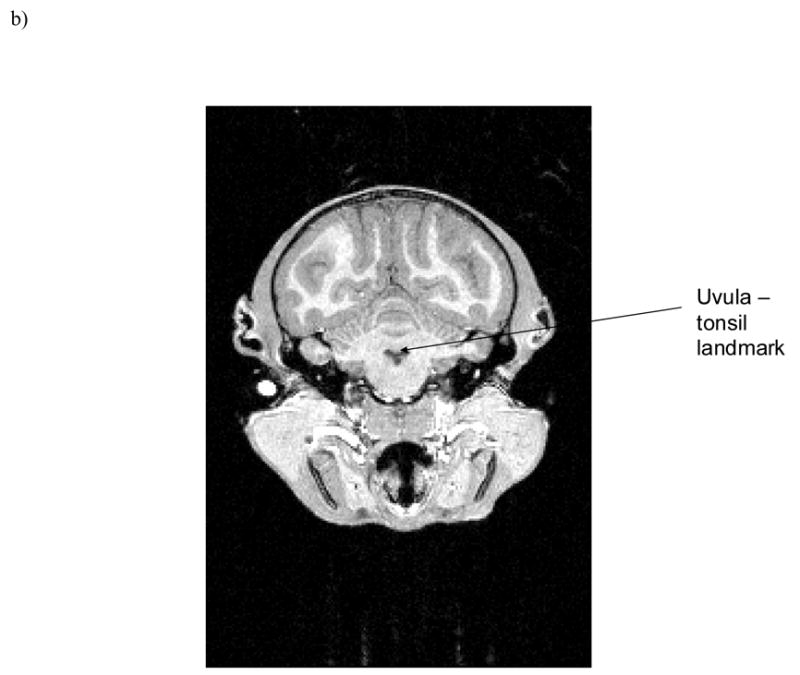
Uvula-tonsils landmark in coronal view from a) chimpanzee and b) capuchin.
3. Results
Descriptive Information
Individual MHI and absolute MHI (ABS-MHI) values for the TUBE task, classification into dextral group, cerebellar volume, and anterior and posterior AQ values are presented in Table 1. Data were initially analyzed by sex for each species. No differences in cerebellar asymmetry for males or females were found in chimpanzees or capuchins; subsequent analyses thus pooled male and female data for each species. We compared the MHI and ABS-MHI scores on the TUBE task between species. No significant differences in MHI scores were found but capuchin monkeys (ABS-M = .75, SD = .27) were significantly more lateralized on the TUBE task than chimpanzees (ABS-M = .35, SD = .29), t(25)=3.59, p < .001.
Table 1.
Mean handedness indices (MHI), classification into dextral group, cerebellar volume, and anterior and posterior cerebellar asymmetry quotients (AQ) for chimpanzees and capuchins.
| MHI | Dextral Group | Whole Cerebellar Volume, cc | Anterior Cerebellar AQ | Posterior Cerebellum AQ | |
|---|---|---|---|---|---|
|
Chimpanzees
| |||||
| Agatha | .52 | R | 46.23 | −.14 | .14 |
| Beleka | −.05 | NR | 48.64 | −.24 | .18 |
| Bo | .47 | R | 49.08 | −.13 | .09 |
| Brandy | −.19 | NR | 56.60 | .08 | −.01 |
| Cheeta | .85 | R | 44.47 | .01 | .06 |
| Christa | −.13 | NR | 61.40 | −.07 | .03 |
| Dara | −.20 | NR | 50.17 | 0 | 0 |
| Elvira | −.09 | NR | 45.53 | −.08 | .12 |
| Jacqueline | −.26 | NR | 40.12 | −.04 | −.03 |
| Jolson | .17 | NR | 46.82 | −.06 | .02 |
| Lulu | −.91 | NR | 27.37 | .03 | 0 |
| Melissa | .67 | R | 41.56 | .21 | −.03 |
| Rogger | −.10 | NR | 57.57 | −.15 | .14 |
| Rowena | .69 | R | 46.60 | −.04 | .09 |
| Suwanee | .32 | R | 48.05 | .07 | −.05 |
| Sylvia | .05 | NR | 46.53 | −.01 | .02 |
|
Capuchins
| |||||
| Alou | .81 | R | 7.77 | −.18 | −.11 |
| Carlos | −.95 | NR | 8.89 | .08 | −.02 |
| DC | .96 | R | 7.28 | −.26 | .04 |
| DiMaggio | .39 | R | 7.16 | −.30 | .13 |
| Georgia | −.75 | NR | 6.80 | −.17 | 0 |
| LC | .85 | R | 6.50 | −.16 | −.29 |
| Miro | 1.00 | R | 7.12 | −.38 | −.05 |
| Noel | −.82 | NR | 6.25 | −.12 | .02 |
| Shoeless | −.14 | NR | 8.68 | .02 | .11 |
| Sosa | −.62 | NR | 7.67 | −.01 | .07 |
| Vincent | −1.00 | NR | 8.70 | .06 | 0.1 |
In terms of the cerebellum asymmetries, the mean AQ values for the anterior and posterior regions are shown in Figure 2. Cerebellar AQ values were examined for population-level asymmetry by using a single sample t-test (tested against a population mean of zero). Chimpanzees displayed no population-level asymmetry for the anterior but showed a significant rightward bias for the posterior cerebellum (anterior cerebellum: t(15) = 1.31, p > .05; posterior cerebellum: t(15) = 2.73, p < .05). In contrast to chimpanzees, capuchin anterior cerebellum AQ values showed population-level leftward asymmetry (t(10) = −2.82, p < .05), but the posterior cerebellum did not (t(10) = 0.00, p >.05). Chimpanzees and capuchins differed in the magnitude of cerebellar asymmetry for the anterior sub-region t(25) = −2.10, p < .05) but not the posterior sub-region of the cerebellum: (t(25) = −.84, p > .05).
Figure 2.
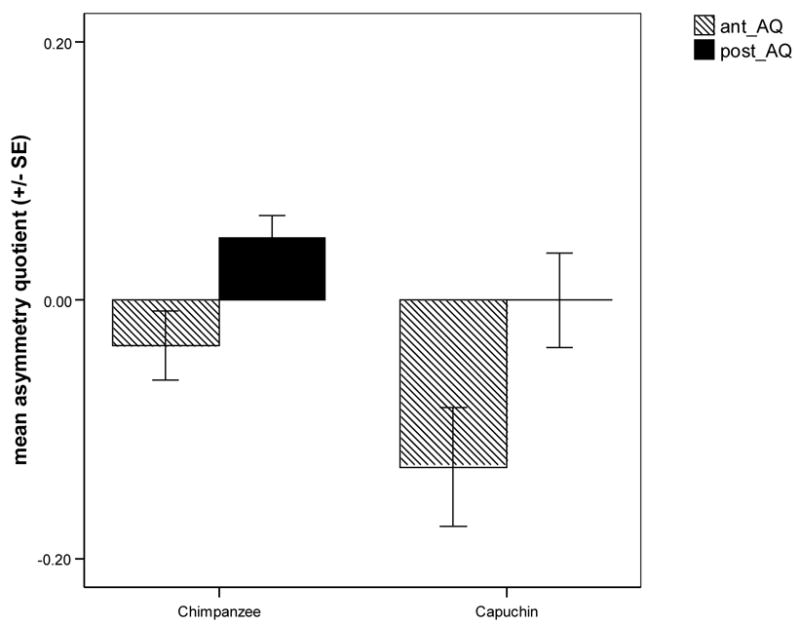
Mean (± SE) anterior and posterior cerebellar asymmetry quotients (AQ) for chimpanzees and capuchins. Chimpanzees showed a significant rightward bias for the posterior cerebellum; capuchins displayed a significant leftward bias for the anterior cerebellum.
Cerebellar torque
To determine whether the cerebellum of chimpanzees or capuchins displayed torque, a Pearson correlation coefficient was conducted between the anterior and posterior AQ values. In chimpanzees, leftward bias of the anterior cerebellum was associated with rightward bias of the posterior cerebellum, (r = −.83, p < .001), indicating significant torquing. In contrast, capuchins showed no significant association between anterior and posterior cerebellar AQ values (r = .22, p > .05).
Hand and Species Effects
To evaluate the relationships between species, handedness and cerebellar asymmetry, an analysis of variance was conducted with species and handedness on the TUBE task as between-subjects factors and anterior and posterior cerebellar AQs as the within-subject factor. A significant interaction was found between species and handedness (F(1, 23) = 27.84, p < .001) Post-hoc analysis showed that right-handed capuchins showed the most pronounced leftward cerebellar asymmetry compared to non-right-handed capuchins (F(2, 35) = 17.54, p < .01; see Figure 3). Right- and non-right-handed chimpanzees did not differ significantly in cerebellar asymmetry.
Figure 3.
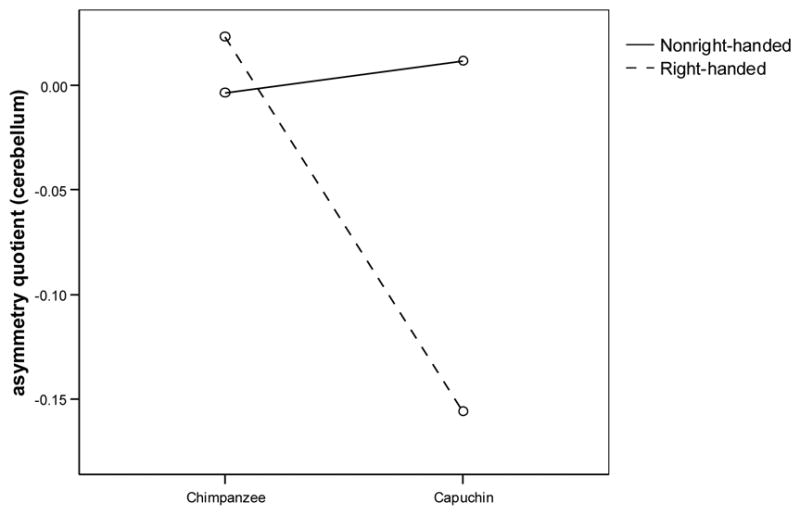
Interaction of hand preference (right, nonright-handed) on the TUBE task and species (Pan, Cebus) with mean cerebellar asymmetry quotient. Right-handed capuchins displayed a leftward cerebellar bias, whereas right-handed chimpanzees displayed a rightward cerebellar bias.
A Pearson correlation coefficient was calculated relating AQ of both cerebellar subregions to the MHI on the TUBE task for each species. Chimpanzees showed no correlation between MHI and anterior cerebellar AQ (r = .10, p > .05) or posterior cerebellar AQ (r = .17, p > .05). In capuchins, anterior cerebellar AQ was significantly correlated with MHI (r = −.76, p < .01; see Figure 3) but posterior cerebellar AQ and MHI were not correlated (r = −.45, p > .05).
4. Discussion
Although incongruent with our hypotheses, our results do indicate that cerebellar asymmetry is significantly associated with handedness in capuchins and not in chimpanzees. This effect was most pronounced in capuchins that displayed a right-hand preference on the TUBE task. Chimpanzees and capuchins differed with respect to patterns of cerebellar asymmetry, with chimpanzees having a greater rightward bias of the posterior cerebellum and capuchins having a greater leftward bias of the anterior cerebellum. Additionally, cerebellar torque was present in chimpanzees and not in capuchins. In humans, right-frontal and left-occipital petalias are more prominent in right-handed individuals and cerebellar asymmetry follows a similar pattern, displaying a right anterior, left posterior bias (Snyder et al. 1995). Chimpanzees show a similar pattern of cortical petalias (Hopkins & Marino, 2000; Pilcher, Hammock, & Hopkins, 2001), but cerebellar torque obtained in the present study showed an opposite pattern. Capuchins display a leftward anterior frontal petalia and no occipital petalia (Phillips & Sherwood, unpub. data). The functional significance of this pattern of cerebellar torque (or lack thereof, in the case of capuchins) and its relationship to cerebellar asymmetry is unclear.
Given that the left cerebellar cortex projects to motor areas of the right cerebral hemisphere and the right cerebral hemisphere controls the left hand, the pattern of association between cerebellar asymmetry and handedness we found in capuchins was unexpected. Ipislateral cerebellar activity is typically associated with hand movements. However, contralateral cerebellar activity is higher in sequential finger movements than simple finger movements (Solodkin, Hlustik, Noll & Small, 2001). Thus, certain motor areas, including ipsilateral and contralateral cerebellum, are more sensitive to task complexity than others. Our results may reflect the differences in structural and functional organization of motor areas as a function of species, hand preference, and motor task complexity.
While these results are seemingly unrelated to the evolution of population-level handedness, as chimpanzees display population-level handedness on the TUBE task (Hopkins, 2006) and capuchins do not (but see Spinozzi et al., 1998), they may shed light on how the cerebellum connects with cortical motor and prefrontal areas for each species, and how these projections are involved in hand movement for specific tasks. The fact that capuchins displayed a greater absolute strength of handedness on the TUBE task compared to chimpanzees suggests the degree of complexity of the TUBE task differs across species. In macaques, cortico-pontine projections from the primary motor cortex and premotor cortex are denser than the projections from prefrontal cortex (Ramnani, 2006). While similar data in great apes are not available, recent evidence indicates a major connection from the prefrontal cortex to the cortico-ponto-cerebellar system in humans, a pattern not seen in monkeys (Ramnani et al., 2006). The “mosaic hypothesis” of brain evolution proposes that distinct brain areas did not evolve in isolation, but rather functional circuitry systems evolved together (Barton & Harvey, 2000). Cerebellum size correlates with neocortex size in primates (Barton & Harvey, 2000) and as the neocortical loops with the cerebellum become more developed control of handedness may be delegated outside of the cerebellum. Functional MRI or lesion data are necessary to evaluate this hypothesis that complexity of the TUBE task (and resulting handedness) is related to the amount of prefrontal input to the pons.
The TUBE task was the only behavior we considered in evaluating handedness in the two species and additional studies using additional and arguably more complex measures, such as tool use, might be if interest. This seems particularly relevant in light of the finding that the TUBE task elicited a more pronounced manifestation of hand use in the capuchins compared to the chimpanzees. Capuchins and chimpanzees are well know for their tool using abilities in captivity and the wild (Fragaszy et al., 2004; Goodall, 1986) and, at least in chimpanzees, hand use for tool use correlates with asymmetries in the inferior frontal gyrus and planum temporale (Hopkins, Russell, & Cantalupo, in press). Thus, whether asymmetries in handedness for tool use correlate with lateralization in the cerebellum would be of great interest.
In conclusion, the results of the study provide evidence of a relationship between cerebellar asymmetry and handedness in capuchins but not chimpanzees. Together with recent studies on the relationship between handedness and areas of the primary motor cortex in chimpanzees (Dadda, Cantalupo, & Hopkins, 2006; Hopkins & Cantalupo, 2004) and capuchins (Phillips & Sherwood, 2005), these results contribute to the growing body of literature on the neurobiology of handedness. However, much remains to be addressed concerning the evolution of cerebellar specialization and its relationship to handedness. Further research, collecting combined behavioral and brain data on additional chimpanzees and capuchins, as well as broader array of primate species, is necessary to elucidate cerebellar contributions to handedness.
Figure 4.
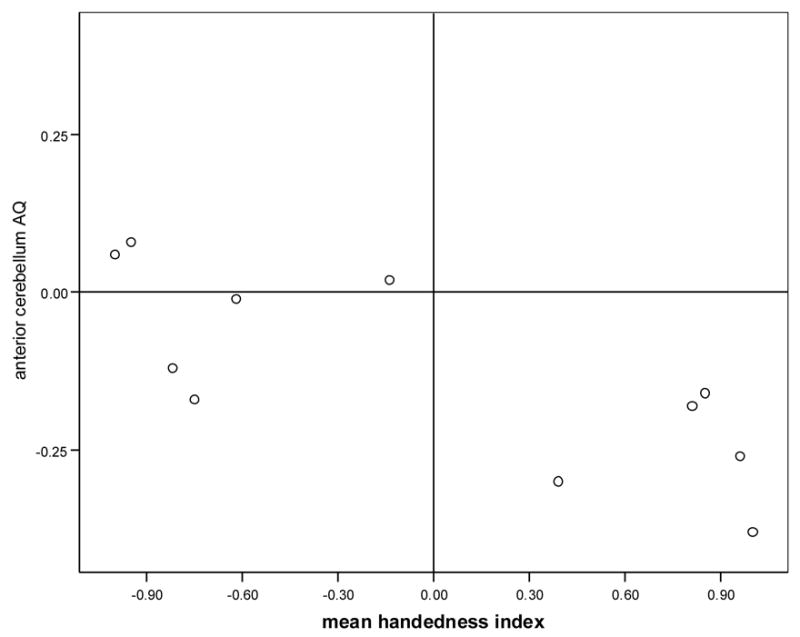
Capuchin anterior cerebellum asymmetry quotient (AQ) correlated with mean handedness index on the TUBE task. Right-handed individuals displayed a greater leftward bias of the anterior cerebellum.
Acknowledgments
This research was supported by NIH grants NS-36605, NS-42867, HD-38105; HHMI #52005125 and the Wenner-Gren Foundation (to CCS). The Yerkes Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care. APA Guidelines for the ethical treatment of animals were adhered to during all aspects of this study. We thank Leslie Dunham and Dr. Claudio Cantalupo for assistance in tracing the chimpanzee brain images and Holly Koslosky for assistance in tracing the capuchin brain images, Dr. Kwan-Jin Jung of BIRC for his assistance in optimizing the imaging protocol, and Dr. Chet Sherwood for enlightening discussion and for providing valuable comments on a previous draft of this manuscript. Special thanks to our respective veterinary staffs for the care of the animals during scanning. Correspondence should be addressed to: Dr. Kimberley Phillips, Department of Psychology, Hiram College, 11715 Garfield Road, Hiram, Ohio 44234. Email: phillipsk@hiram.edu.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amunts K, Schlaug G, Schleicher A, Steinmetz H, Dabringhaus A, Roland PE, Zilles K. Asymmetry in the human motor cortex and handedness. Neuroimage. 1996;4:216–222. doi: 10.1006/nimg.1996.0073. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Degiorgio C, Lamarque C, Fagot J. A multi-task assessment of hand lateralization in capuchin monkeys (Cebus apella) Primates. 1996;37:97–103. [Google Scholar]
- Barton RA, Harvey PH. Mosaic evolution of brain structure in mammals. Nature. 2000;405:1055–1058. doi: 10.1038/35016580. [DOI] [PubMed] [Google Scholar]
- Beaton AA. The relation of planum temporale asymmetry and morphology of the corpus callosum to handedness, gender and dyslexia: A review of the evidence. Brain and Language. 1997;60:255–322. doi: 10.1006/brln.1997.1825. [DOI] [PubMed] [Google Scholar]
- Blois-Heulin C, Guitton JS, Nedellec-Bienvenue L, Ropars L, Vallet E. Hand preference in unimanual and bimanual tasks and postural effect on manual laterality in captive red-capped mangabeys (Cercocebus torquatus torquatus) American Journal of Primatology. 2006;68:429–444. doi: 10.1002/ajp.20239. [DOI] [PubMed] [Google Scholar]
- Dadda M, Cantalupo C, Hopkins WD. Further evidence of an association between handedness and neuroanatomical asymmetries in the primary motor cortex of chimpanzees (Pan troglodytes) Neuropsychologia. 2006 doi: 10.1016/j.neuropsychologia.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas AL, Hong K, Leonard CM, Heilman KM. Hand preference and magnetic resonance imaging asymmetries of the central sulcus. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1998;11(2):65–71. [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Heilman KM. Morphological cerebral asymmetries and handedness. The pars triangularis and planum temporale. Archives of Neurology. 1995;52:501–508. doi: 10.1001/archneur.1995.00540290091023. [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Mitchell SR. Hand preference and performance on unimanual and bimanual tasks in capuchin monkeys (Cebus apella) Journal of Comparative Psychology. 1990;104:272–282. doi: 10.1037/0735-7036.104.3.275. [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Visalberghi E, Fedigan LM. The Complete Capuchin. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- Goodall J. The chimpanzees of Gombe: Patterns of behavior. Cambridge: Harvard University Press; 1986. [Google Scholar]
- Hammond G. Correlates of human handedness in primary motor cortex: a review and hypothesis. Neuroscience & Biobehavioral Reviews. 2002;26:285–292. doi: 10.1016/s0149-7634(02)00003-9. [DOI] [PubMed] [Google Scholar]
- Hook-Costigan MA, Rogers LJ. Hand preferences in New World primates. International Journal of Comparative Psychology. 1996;9:173–207. [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees: Cross-sectional analysis. Journal of Comparative Psychology. 1995;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Comparative and familial analysis of handedness in great apes. Psychological Bulletin. 2006;132:538–559. doi: 10.1037/0033-2909.132.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. Handedness in chimpanzees (Pan troglodytes) is associated with asymmetries of the primary motor cortex but not with homologous language areas. Behavioral Neuroscience. 2004;118:1176–1183. doi: 10.1037/0735-7044.118.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Marino L. Asymmetries in cerebral width in nonhuman primate brains as revealed by magnetic resonance imaging (MRI) Neuropsychologia. 2000;38:493–499. doi: 10.1016/s0028-3932(99)00090-1. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Rabinowitz D. Manual specializations and tool use in captive chimpanzees (Pan troglodytes): The effect of unimanual and bimanual strategies on hand preference. Laterality. 1997;2:267–277. doi: 10.1080/713754273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Cantalupo C. Does variation in sample size explain individual differences in hand preferences of chimpanzees (Pan troglodytes)? An empirical study and reply to Palmer (2002) American Journal of Physical Anthropology. 2003;121:878–881. doi: 10.1002/ajpa.10170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Russell J, Cantalupo C. Handedness for tool use correlates with neuroanatomical asymmetries in the language homologs of chimpanzees (Pan troglodytes) Psychological Science in press. [Google Scholar]
- Hopkins WD, Stoinski T, Lucas K, Ross S, Wesley MJ. Comparative assessment of handedness for a coordinated bimanual task in chimpanzees (Pan), gorillas (Gorilla), and orangutans (Pongo) Journal of Comparative Psychology. 2003;117:302–308. doi: 10.1037/0735-7036.117.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins WD, Wesley MJ, Izard MK, Hook M, Schapiro SJ. Chimpanzees (Pan troglodytes) are predominantly right-handed: replication in three populations of apes. Behavioral Neuroscience. 2004;118:659–663. doi: 10.1037/0735-7044.118.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993;259:819–821. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. The organization of cerebello- and pallido-thalamic projections to primary motor cortex: An investigation employing retrograde transneuronal transport of herpres simplex virus type I. Journal of Neuroscience. 1999;19:1446–1463. doi: 10.1523/JNEUROSCI.19-04-01446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. The Journal of Neuroscience. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limongelli L, Sonetti MG, Visalberghi E. Hand preference of tufted capuchins (Cebus apella) in tool-using tasks. In: Anderson JR, Roeder JJ, Thierry B, Hammerschmidt N, editors. Current Primatology. Strasbourg: University Louis Pasteur Press; 1994. pp. 9–15. [Google Scholar]
- Lonsdorf EV, Hopkins WD. Wild chimpanzees show population-level handedness for tool use. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12634–12638. doi: 10.1073/pnas.0505806102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behavioral and Brain Sciences. 1987;10:247–303. [Google Scholar]
- Moffat SD, Hampson E, Lee DH. Morphology of the planum temporale and corpus callosum in left handers with evidence of left and right hemisphere speech representation. Brain. 1998;121:2369–2379. doi: 10.1093/brain/121.12.2369. [DOI] [PubMed] [Google Scholar]
- McGrew WC, Marchant LF. On the other hand: Current issues in and metaanalysis of the behavioral laterality of hand function in nonhuman primates. Yearbook of Physical Anthropology. 1997;40:201–232. [Google Scholar]
- Nair DG, Purcott KL, Fuchs A, Steinberg F, Kelso JAS. Cortical and cerebellar activity of the human brain during imagined and executed unimanual and bimanual action sequences: A functional MRI study. Cognitive Brain Research. 2003;15:250–260. doi: 10.1016/s0926-6410(02)00197-0. [DOI] [PubMed] [Google Scholar]
- Nitschke MF, Kleinschmidt A, Wessel K, Frahm J. Somatotopic motor representation in the human anterior cerebellum: A high-resolution functional MRI study. Brain. 1996;119:1023–1029. doi: 10.1093/brain/119.3.1023. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Jenkins WM, Merzenich MM, Prejean T, Grenda R. Neuropsychological correlates of hand preference in primary motor cortex of adult squirrel monkeys. Journal of Neuroscience. 1992;12(8):2918–2947. doi: 10.1523/JNEUROSCI.12-08-02918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Sherwood CC. Primary motor cortex asymmetry is correlated with handedness in capuchin monkeys (Cebus apella) Behavioral Neuroscience. 2005;119:1701–1704. doi: 10.1037/0735-7044.119.6.1701. [DOI] [PubMed] [Google Scholar]
- Pilcher D, Hammock L, Hopkins WD. Cerebral volume asymmetries in non-human primates as revealed by magnetic resonance imaging. Laterality. 2001;6:165–180. doi: 10.1080/13576500042000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: Anatomy and function. Nature Reviews Neuroscience. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Behrens TEJ, Johansen-Berg H, Richter MC, Pinsk MA, Anserddon JLR, et al. The evolution of prefrontal inputs to the cortico-pontine system: Diffusion imaging evidence from macaque monkeys and humans. Cerebral Cortex. 2006;16:811–818. doi: 10.1093/cercor/bhj024. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. Evolution of the cerebellum in primates: Differences in relative volume among monkeys, apes and humans. Brain, Behavior and Evolution. 1998;52:308–314. doi: 10.1159/000006575. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behavioral Neurology. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PWR, David AS. The planum temporale: A systematic, quantitative review of its structural, functional and clinical significance. Brain Research Reviews. 1999;2:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Bilder RM, Wu H, Bogerts B, Lieberman JA. Cerebellar volume asymmetries are related to handedness: A quantitative MRI study. Neuropsychologia. 1995;33:407–419. doi: 10.1016/0028-3932(94)00125-9. [DOI] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, Noll DC, Small SL. Lateralization of motor circuits and handedness during finger movements. European Journal of Neurology. 2001;8:425–434. doi: 10.1046/j.1468-1331.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- Spinozzi G, Castorina M, Truppa V. Hand preferences in unimanual and coordinated bimanual tasks by tufted capuchin monkeys (Cebus apella) Journal of Comparative Psychology. 1998;112:183–191. [Google Scholar]
- Spinozzi G, Truppa V, Lagana T. Grasping behavior in tufted capuchin monkeys (Cebus apella): Grip types and manual laterality for picking up a small food item. American Journal of Physical Anthropology. 2004;125:30–41. doi: 10.1002/ajpa.10362. [DOI] [PubMed] [Google Scholar]
- Taglialatela JP, Cantalupo C, Hopkins WD. Gesture handedness predicts asymmetry in the chimpanzee inferior frontal gyrus. NeuroReport. 2006;17:923–927. doi: 10.1097/01.wnr.0000221835.26093.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauclair J, Meguerditchian A, Hopkins WD. Hand preferences for unimanual and coordinated bimanual tasks in baboons (Papio anubis) Cognitive Brain Research. 2005;25:210–216. doi: 10.1016/j.cogbrainres.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JP, Hopkins WD. Primate laterality: Current behavioral evidence of primate asymmetries. New York: Springer-Verlag; 1993. [Google Scholar]
- Westergaard GC, Suomi SJ. Hand preference in the use of nut-cracking tools by tufted capuchin monkeys (Cebus apella) Folia Primatologica. 1993a;61:38–42. doi: 10.1159/000156726. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Suomi SJ. Hand preference in capuchin monkeys varies with age. Primates. 1993b;34:295–299. [Google Scholar]
- Westergaard GC, Suomi SJ. Hand preference for a bimanual task in tufted capuchins (Cebus apella) and rhesus macaques (Macaca mulatta) Journal of Comparative Psychology. 1996;110:406–411. doi: 10.1037/0735-7036.110.4.406. [DOI] [PubMed] [Google Scholar]


