Abstract
Purpose
We investigated whether repeat prostate biopsies are associated with more favorable prognoses, less extensive disease or higher rates of IC in patients who are ultimately diagnosed with prostate cancer and treated with RRP.
Materials and Methods
We examined standard clinical and pathological data on 1,357 patients treated with RRP from 1983 to 2001. In addition, we noted the rate of IC in a subgroup of 847 patients in whom tumor volume was measured.
Results
Cancer was found in 1,042 patients (77%) at the first biopsy, in 227 (17%) at the second biopsy, in 59 (4%) at the third biopsy and in 29 (2%) at the fourth or later biopsy. Patients with 2 or greater biopsies had a higher rate of clinical T1c stage cancer and larger prostates than patients with only 1 biopsy (each p <0.0001). After RRP patients with 1 biopsy had a lower rate of organ confined tumors (61% vs 75%, p <0.0001), and a higher rate of extracapsular extension, seminal vesicle invasion, lymph node metastases and Gleason sum 7 or greater than other patients. IC was found in 10% of patients with 1 biopsy and 18% of those with 2 or greater biopsies (p = 0.018). Despite these more favorable pathological outcomes there was no difference in biochemical recurrence rate.
Conclusions
Although we found that a greater number of biopsies was related to a better pathological outcome after RRP, the number of biopsies did not predict disease recurrence. The increasing number of biopsies currently being performed, especially in patients with larger prostates, likely results in higher rates of IC.
Keywords: prostate, prostatic neoplasms, prostatectomy, biopsy
Increased PSA and/or abnormal DRE is the most frequent finding prompting prostate biopsy. At initial biopsy 20% to 30% of patients are diagnosed with prostate cancer and the rest are followed clinically and possibly undergo repeat biopsies.1,2 The decision to repeat the biopsy is generally supported by increasing PSA, PSA velocity greater than 0.75 ng/ml yearly, low free PSA, abnormal DRE or a pathological finding related to an increased risk of cancer, such as high grade prostatic intraepithelial neoplasia or atypical small acinar proliferation.3–9
The clinical significance of cancer detected on repeat biopsy has been studied previously. Epstein et al reported that cancers were found more frequently in the transitional zone or laterally in the peripheral zone in patients with a previously negative biopsy.10 However, cancers detected at the initial biopsy were more often found in the posterior or posterolateral zone of the prostate. Patients diagnosed with cancer at the first biopsy and patients with cancer diagnosed at repeat biopsy did not have different pathological stages or cancer volumes. However, Epstein et al found that these patients had higher volume prostate glands and a higher rate of IC. We determined if repeat prostate biopsies before the diagnosis and treatment of prostate cancer with RRP are associated with more favorable prognosis, less extensive disease or a higher rate of IC.
MATERIALS AND METHODS
Patient characteristics
The subjects of our study were 1,357 patients diagnosed with prostate cancer (clinical stages T1 to T3a) and treated with RRP, as performed by 1 surgeon (PTS) from 1983 to 2001. Clinical and pathological data were collected prospectively. The 1992 TNM staging classification was used for clinical staging.11 No patients received prior radiotherapy, cryotherapy or neoadjuvant hormonal therapy. Biopsies were performed with an 18 gauge needle biopsy gun under transrectal ultrasound guidance.
Clinical and pathological analysis
Clinical variables were patient age, serum PSA (natural logarithm), clinical stage, biopsy Gleason grade, prostate volume on transrectal ultrasound, the number of prostate biopsies performed and year of surgery. PSA was measured with the Hybritech Tandem-R® assay.
RRP specimens were reviewed according to a previously described protocol.12,13 A primary and secondary Gleason grade was assigned to the entire cancer in the specimen. Pathological stage was classified as organ confined, extra-capsular extension, seminal vesicle invasion or positive lymph nodes. Cancer at the inked margin was considered a positive surgical margin. In a subset of 847 patients tumor volume was measured using a planimetric method. In these patients the rate of IC was determined and defined as an organ confined tumor less than 0.5 cm3 in volume with Gleason grade 3 or less.14
Followup
After RRP patients were scheduled for followup every 3 months during year 1, every 6 months until the fifth year and then annually. PSA was assessed at each visit and DRE was performed annually. Disease recurrence was defined as local recurrence, evidence of distant metastasis on bone scan or other tests, or a persistently increased PSA of 0.4 ng/ml or greater.
Statistical analysis
The variables used were final Gleason sum, total tumor volume, IC, pathological stage and surgical margin status. The chi-square test was used for comparisons between categorical variables and to analyze trends. The Spearman rank correlation test compared tumor volume and prostate gland size with the year of surgery, number of biopsies and patient age. For comparing continuous variables, we used the Wilcoxon rank sum test. Statistical significance was considered at p <0.05. Multivariate logistic regression assessed the relationship between clinical variables and the presence of nonorgan confined and IC. Progression-free probabilities were obtained using the Kaplan-Meier method and we calculated the association between progression-free probabilities and the number of biopsies using the log rank test. We analyzed preoperative clinical findings and number of biopsies with regard to progression-free probabilities using Cox proportional regression. The analyses were performed using SPSS 9.0 (SPSS, Chicago, Illinois).
RESULTS
Table 1 lists clinical stage, PSA and biopsy Gleason sum. Mean patient age was 62.1 year (range 37 to 81) and mean PSA was 9.36 ng/ml (range 0.5 to 100). Of these patients 75% had PSA less than 10 ng/ml.
Table 1.
Clinical characteristics in 1,357 patients treated with RRP
| Clinical Findings | No. Pts (%) |
|---|---|
| Clinical stage: | |
| T1c | 543 (40) |
| T2a | 282 (21) |
| T2b | 304 (22) |
| T2c | 150 (11) |
| T3 | 78 (6) |
| PSA (ng/ml): | |
| Less than 4 | 222 (16) |
| 4–10 | 764 (56) |
| Greater than 10 | 321 (24) |
| Unavailable | 50 (4) |
| Biopsy Gleason sum: | |
| 2–4 | 83 (6) |
| 5–6 | 889 (66) |
| 3 + 4 | 237 (17) |
| 4 + 3 | 74 (5) |
| 8–10 | 65 (5) |
| Undetermined | 9 (1) |
The majority of the patients (94%) had clinical stages T1–T2 disease and 40% had nonpalpable T1c cancer. Prostate cancer was diagnosed at the first biopsy in 1,042 patients (77%), at the second biopsy in 227 (17%), after a third biopsy in 59 (4%) and at the fourth or later biopsy in 29 (2%). The number of biopsies needed to diagnose cancer increased historically (p <0.0001, fig. 1). For example, only 7 of 355 patients (2%) treated before 1993 underwent more than 1 biopsy compared to 154 of 385 (40%) treated after 1998. For biopsies in which the number of cores taken was available a median of 6 cores was obtained at the first biopsy, 8 were obtained at the second, 7.5 were obtained at the third and 8 were obtained in patients with 4 or greater biopsies. A median of 8.5 months elapsed between the first and second biopsies, 26.5 months elapsed between the first and third biopsies, and 41.1 months elapsed between the first and fourth or final biopsies.
Fig. 1.
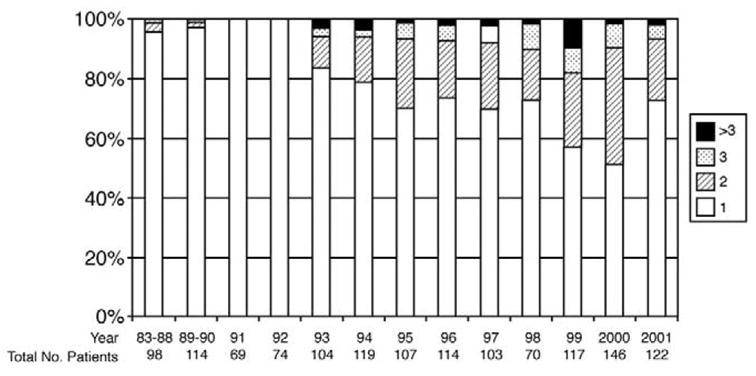
Number of prostate biopsies performed before radical prostatectomy with time in 1,375 patients (test for trend p = 0001).
Table 2 shows the association between the number of biopsies and clinical features. Compared to patients diagnosed after 2 or greater biopsies those diagnosed at the initial biopsy were more likely to have a smaller prostate gland (p = 0.0001) and abnormal DRE (clinical stage T2 or T3, p <0.0001). There was no difference in the number of patients diagnosed with a biopsy Gleason sum of 7 or greater whether they were diagnosed with cancer at the initial biopsy or after repeat biopsies (p = 0.14).
Table 2.
Clinical features of prostate cancer by number of biopsies
| No. Biopsies
|
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 or Greater | p Value | Totals | |
| No. pts | 1,042 | 227 | 59 | 29 | 1,357 | |
| Median ng/ml PSA (range)* | 6.6 (0.5–100) | 6.3 (0.8–41.8) | 7.0 (1.2–22.2) | 7.41 (0.5–78.4) | 0.86 | 6.6 (0.5–100) |
| No. Gleason sum 7 or greater (%) | 313 (30) | 53 (23) | 11 (19) | 8 (28) | 0.14 | 385 (28) |
| No. clinical stage (%): | <0.001 | |||||
| T1c | 374 (36) | 123 (54) | 33 (56) | 13 (45) | 543 (40) | |
| T2a | 208 (20) | 53 (23) | 13 (22) | 8 (28) | 262 (21) | |
| T2b | 259 (25) | 31 (14) | 9 (15) | 5 (17) | 304 (22) | |
| T2c | 132 (13) | 11 (5) | 4 (7) | 3 (10) | 150 (11) | |
| T3a | 69 (7) | 9 (4) | 0 | 0 | 78 (6) | |
| Mean prostate vol (cm3)† | 38.2 | 48.5 | 59.6 | 40.1 | <0.0001 | 39.9 |
Spearman correlation coefficient 0.005.
Spearman correlation coefficient 0.169.
Table 3 shows the relationship between the number of biopsies and the pathological features of cancer in the RRP specimen. Of the 1,042 patients diagnosed on the initial biopsy 61% had organ confined cancer compared to 75% of 315 diagnosed after 2 or greater biopsies (p <0.0001). Similarly the frequency of extracapsular extension, seminal vesicle invasion and positive lymph nodes was higher in patients with 1 biopsy than in those with 2 or greater biopsies. The frequency of poorly differentiated cancer (Gleason sum 7 or greater) in the RRP specimen was higher in patients diagnosed with cancer at the first biopsy than in those diagnosed after 2 or greater biopsies (p = 0.038). The rate of positive surgical margins was the same in all groups (13% overall).
Table 3.
Pathological features of prostate cancer by number of biopsies
| No. Biopsies
|
||||||
|---|---|---|---|---|---|---|
| 1 (%) | 2 (%) | 3 (%) | 4 or Greater (%) | p Value | Totals | |
| Overall | 1,042 | 227 | 59 | 29 | 1,357 | |
| Gleason sum 7 or greater | 566 (54) | 105 (46) | 24 (41) | 14 (48) | 0.038 | 709 (52) |
| Pathological stage:* | 0.002 | |||||
| T2 | 637 (61) | 172 (76) | 45 (76) | 21 (72) | 875 (64) | |
| T3a + b | 251 (24) | 40 (18) | 11 (19) | 6 (20) | 308 (23) | |
| T3c | 85 (8) | 8 (4) | 1 (2) | 1 (3) | 95 (7) | |
| N1 | 69 (7) | 7 (3) | 2 (3) | 1 (3) | 5 (17) | |
| Pos surgical margins | 146 (14) | 25 (11) | 7 (12) | 5 (17) | 0.589 | 183 (13) |
T2—organ confined, T3a + b—extracapsular extension, T3c—seminal vesicle invasion and N1—positive lymph nodes.
In a subgroup of 847 patients in whom total tumor volume was measured, including 717 with 1, 96 with 2, 21 with 3 and 13 with 4 biopsies, we found a significant decrease in tumor volume associated with an increase in the number of biopsies. Patients with 1 biopsy had a median cancer volume of 2.02 cm3 compared to 1.13 cm3 in those with 4 or greater biopsies (p = 0.001, table 4). Reflecting this result, IC was more common in patients with 2 or greater biopsies than in those with only 1 biopsy (18% vs 10%, p = 0.018). In this subgroup we also found that patient age, the number of biopsy sessions and the IC rate increased significantly as prostate gland size increased (each p <0.0001). Overall 16.6% of patients with a prostate of more than 50 cm3 had indolent tumors compared to 9% of those with a prostate of less than 50 cm3.
Table 4.
Tumor volume and IC by number of biopsies in 847 patients after RRP
| No. Biopsies | Median Tumor Vol (cm3) (range)* | No. IC (%)† |
|---|---|---|
| 1 | 2.02 (0.01–26) | 75 (10) |
| 2 | 1.62 (0.01–16) | 18 (19) |
| 3 | 1.18 (0.01–6) | 3 (14) |
| 4 or Greater | 1.13 (0.01–5.7) | 2 (15)
|
| Totals | 1.89 (.01–26) | 98 (12) |
Available in 847 patients (Spearman correlation coefficient −0.117, p = 0.001).
p = 0.018.
The number of previous biopsies predicted extracapsular extension (p = 0.029), as did PSA (p <0.001), clinical stage (p <0.001) and biopsy Gleason sum (p <0.001, table 5). Similarly in the 847 patient subgroup the number of biopsies significantly predicted IC when other clinical features were controlled (p = 0.027, table 5).
Table 5.
Multivariate logistic regression of preoperative clinical features to predict extracapsular extension and IC in 1,357 radical prostatectomy specimens
| Clinical Variable | Coefficient | OR | 95% CI | p Value |
|---|---|---|---|---|
| Extracapsular extension | ||||
| PSA | 0.154 | 0.85 | 0.80–0.91 | <0.001 |
| Clinical stage:* | <0.001 | |||
| T2a | 0.418 | 1.58 | 1.05–2.19 | <0.001 |
| T2b | 1.402 | 4.06 | 2.89–5.70 | 0.025 |
| T2c | 1.466 | 4.33 | 2.85–6.58 | <0.001 |
| T3 | 1.822 | 6.18 | 3.50–10.94 | <0.001 |
| Gleason sum | 0.378 | 1.45 | 1.26–1.69 | <0.001 |
| Previous neg biopsies | −0.360 | 0.69 | 0.50–0.96 | 0.029 |
| IC | ||||
| PSA: | −0.154 | 0.85 | 0.80–0.91 | <0.001 |
| Clinical stage:* | 0.020 | |||
| T2a | −0.403 | 0.66 | 0.35–1.23 | 0.200 |
| T2b | −0.634 | 0.53 | 0.29–0.96 | 0.038 |
| T2c | −1.006 | 0.36 | 0.15–0.84 | 0.019 |
| T3 | −2.355 | 0.09 | 0.01–0.77 | 0.028 |
| Gleason sum | −1.214 | 0.29 | 0.22–0.38 | <0.001 |
| Previous neg biopsies | 0.636 | 1.88 | 1.07–3.32 | 0.027 |
Referent cT1c.
Mean followup in the entire group was 32 months (range 1 to 169). The progression-free probability at 5 years was 78.7%. Patients with 1 biopsy had progression-free probabilities similar to those in patients with 2 or greater biopsies (77.4 and 80%, respectively, p = 0.64, fig. 2). PSA (p <0.0005), biopsy Gleason score (p <0.0005) and clinical stage (p = 0.002) were significant predictors of progression, although the number of biopsies was not a significant predictor (p = 0.76).
Fig. 2.
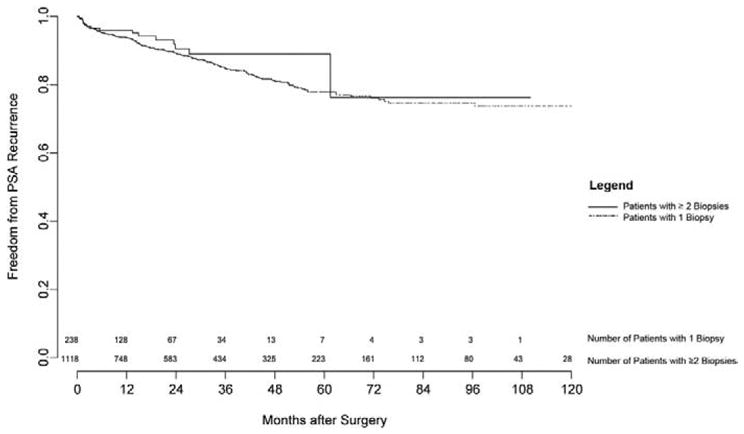
PSA recurrence-free survival after RRP in 1,357 patients by number of biopsies.
DISCUSSION
There is significant controversy about repeat biopsies and their relationship to prostate cancer outcomes.9,10 In the European Prostate Cancer Detection study Djavan et al studied 1,051 patients with PSA 4 to 10 ng/ml who underwent sextant prostate biopsies.9 In 820 patients with negative initial biopsies they performed subsequent biopsies at 6 to 8-week intervals and found that cancer detected at the second biopsy was similar in pathological stage and volume to cancer detected at the first biopsy. However, cancers detected at the third and fourth biopsies were smaller, more often unifocal and had a lower pathological stage than those found at the initial biopsy. They concluded that second biopsies are justified in patients with negative first biopsies but 3 or greater biopsies should be obtained only in select patients.9 In contrast to their study, we found significant differences between the pathological features of cancer found at the initial biopsy and cancer found at 2 or greater biopsies. Of the patients in our series who underwent 2 biopsies and in whom tumor volume was measured 76% had organ confined disease and a tumor volume of 1.62 cm3, in contrast to the 60.9% and 4.9 cm3, respectively, in the 83 patients in the study of Djavan et al. Furthermore, in our 768 patients with PSA between 4 and 10 ng/ml we found an increased rate of organ confined tumors (70% vs 81%, p = 0.006) and IC (4% vs 9.5%, p = 0.018) between those diagnosed at the initial biopsy and those diagnosed after 2 or greater biopsies. However, the patient population in the screening trial of Djavan et al differed from ours. The study of Djavan et al was prospective with patients undergoing early repeat biopsy (within 6 to 8 weeks). Patients were mainly referred from elsewhere and different criteria were used for repeat biopsy.
Epstein et al analyzed the use of repeat sextant biopsies to evaluate the extent of prostate cancer in patients with a previous positive biopsy.15 Patients in whom cancer was not discovered during repeat biopsy (31%) had a higher probability of confined (89%) and indolent (66%) tumors than patients in whom cancer was discovered during initial plus repeat biopsies. Interestingly the false-negative rate was even higher (50%) in their patients with prostate volume more than 70 cm3. This study highlights the overall false-negative rate of 31% for sextant biopsies and shows that these particular patients, who are similar to our repeat biopsy group, have a better pathological stage and a higher rate of indolent tumors after surgery.
Our multivariate analysis demonstrated that the number of previous biopsies as well as the standard preoperative variables (PSA, clinical stage and Gleason score) is related to a higher probability of organ confined tumors and IC. These findings are likely explained by the smaller tumor burden found in this group of patients. These patients were also found to have higher total prostate volumes, lowering the proportion of cancer in the prostate gland.
Patients with repeat biopsies tend to have larger prostate glands. This could explain why these patients have higher than normal PSA even if cancer is minimal. Epstein el al reported that patients with repeat biopsies had high total prostate volume.10 Patients with prostates more than 75 cm3 had twice the chance (32.5%) of having had a previously negative biopsy compared to those with smaller prostates (15.2%). Patients with repeat biopsies had better progression-free probability on univariate analysis than patients with only 1 biopsy (77% vs 90%). However, according to Cox proportional hazards regression the number of biopsies was not a significant predictor of progression-free probability.
In a subgroup of 847 patients those with repeat biopsies had smaller cancer volumes and higher rates of IC. Epstein et al compared patients with and without previous negative biopsies, and found a higher incidence of indolent tumors in the former group (28.4% vs 14.6%).15 This rate of IC is higher than what we found even after acknowledging that they used a cutoff volume of 0.2 cm3 to consider a tumor as “very limited.”15 Conversely they did not find a difference in pathological stage between the groups.
Prostate size may help explain why the rate of indolent tumors increases with the number of biopsies.16,17 Patients in whom cancer is discovered after only 1 biopsy frequently have prostate volumes less than 40 cm3. Increasing the number of cores taken in the initial biopsy will increase tumor detection without increasing the detection of IC, a fact that has been previously suggested.17 However, patients with repeat biopsies have larger prostates and increasing the number of cores taken will increase the detection of IC in these patients. After analyzing tumor maps from 180 prostatectomy specimens Chen et al found that prostates more than 50 cm3 had a 33% rate of small cancers (less than 0.5 cm3) compared to 14% in those less than 50 cm3.18 They concluded that the higher detection rate of smaller cancers in large glands is the result of a higher proportion of low volume cancers in these glands. They suggested that larger prostates tend to be biopsied due to benign increases in PSA rather than to significant cancer and they suggested against increasing the number of cores to compensate for the increase in size, which would risk increasing the detection of low volume tumors.
Despite the more favorable pathological findings we found no difference in biochemical outcomes. This may reflect the relatively short followup in our study (mean 32 months). An alternative hypothesis is that, while the pathological features are more worrisome in men diagnosed with prostate cancer after a single biopsy than after multiple biopsies, radical prostatectomy remains an effective cure even in the face of microscopic extraprostatic disease.
There are several limitations to our retrospective study. There was a lack of standardization of biopsy technique during the study course. This is more reflective of what happens in clinical practice, and it certainly has changed with time and will continue to do so. The long time frame of our study may also have confounded our results since there was substantial stage migration during this period. Lastly, there was a relatively small number of patients who underwent multiple biopsies before the diagnosis of prostate cancer. Despite these limitations our results suggest the more favorable pathological nature of cancers diagnosed after more than 1 biopsy session.
CONCLUSIONS
Patients with previous negative biopsies before the diagnosis of cancer were more likely to have favorable pathological results after RRP compared to patients diagnosed at the initial biopsy. The rate of clinically IC increased with time, which may be explained by the increased number of biopsies obtained before identifying cancer and by increasing prostate gland volume in patients with repeat biopsy sessions. The number of biopsies did not improve predictions of treatment outcomes based on standard clinical information, including PSA, clinical T stage and biopsy Gleason sum. However, we believe that this variable may be useful in the future in a mathematical model predicting extracapsular extension and clinically indolent tumors.
Acknowledgments
Supported by a gift from the Leon Lowenstein Foundation.
Abbreviations and Acronyms
- DRE
digital rectal examination
- IC
indolent cancer
- PSA
prostate specific antigen
- RRP
radical retropubic prostatectomy
References
- 1.Djavan B, Zlotta A, Remzi M, Ghawidel K, Basharkhah A, Schulman CC, et al. Optimal predictors of prostate cancer on repeat prostate biopsy: a prospective study of 1,051 men. J Urol. 2000;163:1144. [PubMed] [Google Scholar]
- 2.Fink KG, Hutarew G, Lumper W, Jungwirth A, Dietze O, Schmeller NT. Prostate cancer detection with two sets of ten-core compared with two sets of sextant biopsies. Urology. 2001;58:735. doi: 10.1016/s0090-4295(01)01352-8. [DOI] [PubMed] [Google Scholar]
- 3.Potter SR, Horniger W, Tinzl M, Bartsch G, Partin AW. Age, prostate-specific antigen, and digital rectal examination as determinants of the probability of having prostate cancer. Urology. 2001;57:1100. doi: 10.1016/s0090-4295(01)00980-3. [DOI] [PubMed] [Google Scholar]
- 4.Carter HB, Pearson JD, Metter EJ, Brant LJ, Chan DW, Andres R, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA. 1992;267:2215. [PMC free article] [PubMed] [Google Scholar]
- 5.Catalona WJ, Partin AW, Slawin KM, Brawer MK, Flanigan RC, Patel A, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter clinical trial. JAMA. 1998;279:1542. doi: 10.1001/jama.279.19.1542. [DOI] [PubMed] [Google Scholar]
- 6.O’dowd GJ, Miller MC, Orozco R, Veltri RW. Analysis of repeated biopsy results within 1 year after a noncancer diagnosis. Urology. 2000;55:553. doi: 10.1016/s0090-4295(00)00447-7. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang RC, Kenwright DN, Nacey JN, Delahunt B. The presence of atypical small acinar proliferation in prostate needle biopsy is predictive of carcinoma on subsequent biopsy. BJU Int. 2001;87:70. doi: 10.1046/j.1464-410x.2001.00989.x. [DOI] [PubMed] [Google Scholar]
- 8.Park S, Shinohara K, Grossfeld GD, Carroll PR. Prostate cancer detection in men with prior high grade prostatic intraepithelial neoplasia or atypical prostate biopsy. J Urol. 2001;165:1409. [PubMed] [Google Scholar]
- 9.Djavan B, Ravery V, Zlotta A, Dobronski P, Dobrovits M, Fakhari M, et al. Prospective evaluation of prostate cancer detected on biopsies 1, 2, 3 and 4: when should we stop? J Urol. 2001;166:1679. [PubMed] [Google Scholar]
- 10.Epstein JI, Walsh PC, Akingba G, Carter HB. The significance of prior benign needle biopsies in men subsequently diagnosed with prostate cancer. J Urol. 1999;162:1649. [PubMed] [Google Scholar]
- 11.Ohori M, Wheeler TM, Scardino PT. The New American Joint Committee on Cancer and International Union Against Cancer TNM classification of prostate cancer. Clinicopathologic correlations. Cancer. 1994;74:104. doi: 10.1002/1097-0142(19940701)74:1<104::aid-cncr2820740119>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler TM. Anatomy and pathology of prostate cancer. In: Vogelzang NJ, Scardino PT, Shipley WU, Coffey DS, editors. Comprehensive Textbook of Genitourinary Oncology. 2. chapt. 36. Philadelphia: Lippincott Williams & Wilkins; 2000. p. 587. [Google Scholar]
- 13.Rabbani F, Bastar A, Fair WR. Site specific predictors of positive margins at radical prostatectomy: an argument for risk based modification of technique. J Urol. 1998;160:1727. [PubMed] [Google Scholar]
- 14.Ohori M, Wheeler TM, Dunn JK, Stamey TA, Scardino PT. The pathological features and prognosis of prostate cancer detectable with current diagnostic tests. J Urol. 1994;152:1714. doi: 10.1016/s0022-5347(17)32369-8. [DOI] [PubMed] [Google Scholar]
- 15.Epstein JI, Walsh PC, Sauvageot J, Carter HB. Use of repeat sextant and transition zone biopsies for assessing extent of prostate cancer. J Urol. 1997;158:1886. doi: 10.1016/s0022-5347(01)64159-4. [DOI] [PubMed] [Google Scholar]
- 16.Goto Y, Ohori M, Arakawa A, Kattan MW, Wheeler TM, Scardino PT. Distinguishing clinically important from unimportant prostate cancers before treatment: value of systematic biopsies. J Urol. 1996;156:1059. [PubMed] [Google Scholar]
- 17.Grossklaus DJ, Coffey CS, Shappell SB, Jack GS, Cookson MS. Prediction of tumour volume and pathological stage in radical prostatectomy specimens is not improved by taking more prostate needle-biopsy cores. BJU Int. 2001;88:722. doi: 10.1046/j.1464-4096.2001.02413.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen ME, Troncoso P, Johnston D, Tang K, Babaian RJ. Prostate cancer detection: relationship to prostate size. Urology. 1999;53:764. doi: 10.1016/s0090-4295(98)00574-3. [DOI] [PubMed] [Google Scholar]


