Abstract
Valproic acid (VPA) is used to treat epilepsy and bipolar disorder and to prevent migraine. It is also undergoing trials for cancer therapy. However, the biochemical and molecular biological actions of VPA are poorly understood. Using the social amoeba Dictyostelium discoideum, we show that an acute effect of VPA is the inhibition of chemotactic cell movement, a process partially dependent upon phospholipid signaling. Analysis of this process shows that VPA attenuates the signal-induced translocation of PHCrac-green fluorescent protein from cytosol to membrane, suggesting the inhibition of phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) production. Direct labeling of lipids in vivo also shows a reduction in PIP and PIP2 phosphorylation following VPA treatment. We further show that VPA acutely reduces endocytosis and exocytosis—processes previously shown to be dependent upon PIP3 production. These results suggest that in Dictyostelium, VPA rapidly attenuates phospholipid signaling to reduce endocytic trafficking. To examine this effect in a mammalian model, we also tested depolarization-dependent neurotransmitter release in rat nerve terminals, and we show that this process is also suppressed upon application of VPA and an inhibitor of phosphatidylinositol 3-kinase. Although a more comprehensive analysis of the effect of VPA on lipid signaling will be necessary in mammalian systems, these results suggest that VPA may function to reduce phospholipid signaling processes and thus may provide a novel therapeutic effect for this drug.
Valproic acid (VPA), or 2-propyl pentanoic acid, was accidentally found to be an effective antiepileptic treatment in the 1960s. The drug is now used to treat around one-third of patients newly diagnosed with epilepsy (24), although its mechanism of action remains unclear (26). VPA is also licensed by the U.S. Food and Drug Administration for the treatment of bipolar disorder and is used for migraine prophylaxis. In addition, the drug has a neuroprotective role, which may contribute to several therapeutic actions. Finally, it is being evaluated in the treatment of certain cancers, including leukemia and solid tumors such as colon and breast cancer, since it induces cell differentiation and inhibits cell proliferation and metastasis (reviewed in reference 4).
The proposed cellular targets of VPA are numerous. It directly inhibits class I and II histone deacetylase (HDAC) enzymes (15), leading to the elevated expression of approximately 2% of the protein-coding genes (60). This effect may be important in its role in cancer treatment and as a neuroprotective agent. With regard to epilepsy, VPA directly interrupts γ-amino butyric acid (GABA) signaling by inhibiting GABA breakdown and thereby elevating GABA levels (26). It has also been shown to modulate sodium channel activity (61), by an unknown mechanism. In the treatment of bipolar disorder, it has been proposed to act through both direct and indirect inhibition of glycogen synthase kinase 3 (8, 43), although this mechanism has been disputed (12, 43, 47). Finally, some of the neuroprotective effects of VPA may result from its effects on mitogen-activated protein kinase/extracellular signal-regulated kinase signaling (5, 18), which modulates processes such as neuronal differentiation, neuronal survival, and long-term neuroplasticity. In addition to these effects, we have previously shown that prolonged VPA treatment attenuates inositol-(1,4,5)-trisphosphate (InsP3) signaling (12, 50, 62) using the eukaryotic model system Dictyostelium discoideum, and this effect also occurs in primary rat dorsal root ganglia neurons (12, 62). These data support a role for VPA in the inositol depletion theory of bipolar disorder drug action as proposed by Berridge et al. (3).
We now extend these studies with the identification of a rapid effect of VPA in blocking Dictyostelium chemotactic cell movement. Analysis of the signaling components involved in this effect shows that VPA acutely reduces signal-induced translocation of PHCrac-green fluorescent protein (GFP) from the cytosol to the membrane and that this is likely to be caused by a reduction in production of the PHCrac-GFP binding substrate, PIP3. We show that in the presence of VPA, in vivo labeling of phospholipids in Dictyostelium is reduced, in agreement with a VPA-dependent reduction in the production of phosphatidylinositol-(3,4,5)-trisphosphate (PIP3). Since previous studies in Dictyostelium have shown that endocytosis and exocytosis are dependent upon PIP3 production (7, 46, 66), we analyzed the effect of VPA on these functions, and we show that VPA also reduces these processes. Translation of these studies to a mammalian synapse model shows that both VPA and a phosphatidylinositol 3-kinase (PI3K) inhibitor attenuate glutamate release. These data propose a novel mechanism of action of VPA by the modulation of phospholipid signaling.
MATERIALS AND METHODS
Reagents.
All compounds used were supplied by Sigma-Aldrich Co., Ltd., apart from latrunculin B, which was from Molecular Probes, and the enhanced chemiluminescence reagent from Amersham. Complete protease inhibitor cocktail was from Roche Ltd. Phosphate buffer (PB) contained 16.5 mM potassium dihydrogen orthophosphate, 3.8 mM dipotassium hydrogen orthophosphate, pH 6.2.
Chemotaxis experiments.
Chemotaxis assays, live cell imaging, and imaging analysis were performed as described previously (63). Dictyostelium strain AX2 cells, developed for 6 h, were plated on a four-well chamber for uniformly applied stimulation and a one-well chamber for the microinjector-delivered cyclic AMP (cAMP) stimulation (Nalgene Nunc International, Naperville, IL) and allowed to adhere to the cover glass for 10 min in PB. Live cells were imaged using a Zeiss laser scanning microscope, LSM 510 META, with a 40× objective and numerical aperture of 1.3 or a 63×, 1.4 numerical aperture oil differential interference contrast Plan-Neofluar objective. To monitor PHCrac-GFP, the specimens were excited with a laser line at 488 nm for GFP, and fluorescent emission was recorded between 505 and 530 nm. Live cells were imaged during stimulations with a uniform concentration (100 nM) or a gradient of cAMP as previously described (63) in the presence or absence of 1 mM VPA. To quantify cell movement, images were analyzed using the DIAS software package (55).
Phospholipid labeling.
A saponin-based cell permeabilization protocol for Dictyostelium was adapted from one for mammalian cells (17). Dictyostelium strain AX2 cells were developed for 5 h as previously described (5), transferred to still dishes (2.5 cm), allowed to settle to give a confluent monolayer in PB, and pretreated with drug (0.5 mM VPA or 50 μM LY294002) for 5 min. At regular time intervals, buffer was replaced with labeling solution {139 mM sodium glutamate, 5 mM glucose, 5 mM EDTA, 20 mM piperazine-N,N′-bis(2-ethanesulfonic acid) pH 6.6, 1 mM MgSO4·2H2O, 300 μg/ml saponin, 1× phosphatase inhibitor cocktails 1 and 2 (Roche Ltd.), and 1 μCi/ml [γ-32P]ATP} supplemented with drugs at defined concentrations. Following a 5-min incubation, labeling solution was removed, cells were lysed in acidified methanol, and phospholipids were separated as previously detailed (25). Phospholipid labeling was quantified using a Typhoon PhosphorImager and normalized using quantification of nonpolar, labeled lipids.
Endocytic trafficking.
The uptake of particles and fluid, as well as exocytosis, was measured in AX2 cells as described previously (16, 33, 45).
Synaptosome preparation and glutamate release.
Isolated nerve terminals (synaptosomes) were prepared from the cerebral cortices of 2-month-old male Sprague-Dawley rats by homogenization, differential centrifugation, and Percoll gradient purification as described elsewhere (52). Glutamate release from synaptosomes (0.1 to 0.2 mg of total protein) resuspended in 1 ml of HEPES-buffered incubation medium (140 mM NaCl, 5 mM KCl, 5 mM NaHCO3, 1 mM MgCl2, 1.2 mM Na2HPO4, 10 mM glucose, and 20 mM HEPES, pH 7.4) was assayed by online fluorimetry (21, 40, 53). CaCl2 (1 mM) and drugs were added as indicated at the start of incubation at 37°C in a spectrofluorometer chamber with constant stirring. Release was stimulated as indicated with the K+ channel inhibitor 4-aminopyridine (1 mM) (21) and monitored at 2-s intervals.
RESULTS
VPA inhibits chemotactic cell movement.
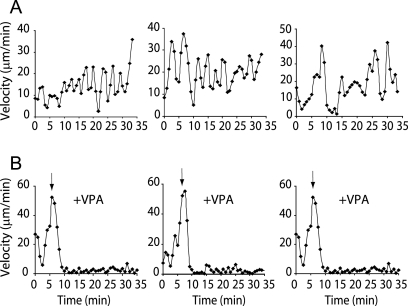
The social amoeba Dictyostelium has been used to elucidate signaling pathways involved in cell movement (11, 14, 63) and to examine the cellular targets of bipolar disorder treatments (12, 62). The latter work demonstrated that VPA blocks the aggregation of cells into fruiting bodies. To examine this process in more detail, we analyzed the movement of aggregation-competent cells in a chemoattractant (cAMP) gradient (see the movie in Fig. S1 in the supplemental material). Cell movement towards the cAMP source is pulsatile, with periods of rapid migration followed by a transient slowing (Fig. 1A). Exposure to VPA at 1 mM, a concentration close to clinical plasma levels in treated patients of 0.3 to 0.6 mM (23), blocked chemotaxis within 5 min (Fig. 1B). This result was of particular interest since the analysis of this rapid effect may help in understanding the acute mechanism by which intravenous VPA can stop seizures (within 20 min of administration) (64).
FIG. 1.
VPA blocks chemotactic movement in wild-type Dictyostelium cells. (A) Quantification of speed for chemotaxing cells moving towards a source of cAMP in a pulsatile way, alternately increasing and decreasing speed. (B) Addition of VPA (1 mM) to cells (arrow) acutely blocks cell movement within 5 min. Three typical experiments are shown in panels A and B. See also the movie in Fig. S1 in the supplemental material.
VPA inhibits signal-induced translocation of PHCrac-GFP.
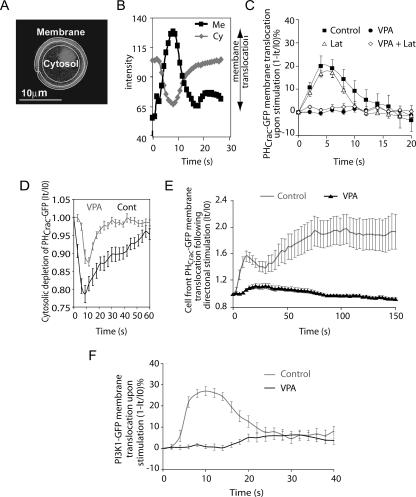
The receptor-mediated regulation of phosphatidylinositol signaling plays an important role in the chemotactic response (49), and this pathway is highly conserved in eukaryotic cells. In Dictyostelium, cAMP binds to the G-protein-coupled receptor cAR1 and induces the dissociation of heterotrimeric G-proteins into Gα and Gβγ subunits (19, 20), leading to the activation of PI3K, which converts phosphatidylinositol-(4,5)-bisphosphate (PIP2) to PIP3 on the inner plasma membrane (14, 48). Function of this pathway is necessary for chemotaxis under defined conditions (29). PIP3 mediates cellular responses by recruiting proteins with pleckstrin homology (PH) domains, such as CRAC (cytosolic regulator of adenylyl cyclase) and Akt/PKB (protein kinase B), to the plasma membrane (36, 41). It has been shown that cAMP-induced PI3K activation results in an increase in PIP3 levels that can be monitored by the membrane translocation of PHCrac-GFP (29, 42, 63), and in Dictyostelium, translocation can be used to measure PIP3 generation (29). To examine the effect of VPA on the cAMP-induced signal transduction pathway leading to PIP3 generation, we visualized the membrane translocation of PHCrac-GFP (Fig. 2A and B) in living cells upon cAMP stimulation. Following uniform cAMP stimulation, a transient increase in PIP3 generation caused a transient PHCrac-GFP membrane translocation, which peaked at about 5 s (Fig. 2C), corresponding to the temporal signal-induced production of PIP3 by PI3K. Treatment of cells with VPA (1 mM, 10 min) blocked this membrane translocation, suggesting that VPA reduces PIP3 production (Fig. 2C). Since VPA is used therapeutically at plasma concentrations of 0.3 to 0.6 mM, we repeated these experiments by treating the cells with 0.5 mM VPA for 10 min and monitored PHCrac-GFP cytosolic depletion (Fig. 2D). This treatment reduced the rate and magnitude of the PHCrac-GFP translocation without totally abolishing it. The block in PHCrac-GFP translocation was not caused by an inhibitory effect on protein translocation, since movement of an unrelated fusion protein, yellow fluorescent protein (YFP)-actin-binding domain (YFP-ABD) (6), to the F-actin-rich cortex was not altered following VPA treatment (see the movie in Fig. S2 in the supplemental material). We previously showed that treatment with VPA (1 mM, 10 min) affected neither cAMP-induced cAR1 phosphorylation nor cAMP-triggered dissociation of Gα2Gβγ (5), thus suggesting that VPA reduces PIP3 production downstream of cAR1/G-protein activation. Treatment of cells with LY294002 (a PI3K inhibitor) phenocopied the effect of VPA on PHCrac-GFP membrane translocation, consistent with the idea that VPA reduces PIP3 production (29).
FIG. 2.
Effect of VPA on Dictyostelium PIP3 production as measured by PHCrac-GFP translocation. (A to C) Cytosolic (Cy) and membrane (Me) PHCrac-GFP (fluorescence) levels were used to monitor PIP3 production on the membrane in cells expressing PHCrac-GFP after uniform cAMP stimulation (C) with or without VPA treatment (1 mM, 10 min) or in the presence or absence of latrunculin B (Lat). (D and E) Reduction of VPA concentration (0.5 mM, 10 min) reduced and slowed the depletion of cytosolic PHCrac-GFP as a measure of PIP3 production (D), and PHCrac-GFP localization was measured at the front of chemotaxing cells in a cAMP gradient with or without VPA treatment (1 mM, 10 min) (E). (F) Cells expressing a PI3K1-GFP construct were exposed to a uniform pulse of cAMP with or without VPA (1 mM, 10 min), and PI3K-GFP membrane translocation (fluorescence) was measured at given time intervals IO and It. Data are shown as means ± standard errors (n = 8).
Since we have shown that VPA blocks chemotaxis by inhibiting cell movement in a chemotactic gradient, it is possible that VPA blocks motility but not cAMP gradient sensing. Analysis of this effect has employed latrunculin-treated cells, exposed to a cAMP gradient, giving rise to PHCrac-GFP translocation to the membrane in a polarized manner (41, 48). To examine whether VPA treatment inhibits the gradient-sensing ability of the cells, we visualized dynamics of PHCrac-GFP translocation in VPA- and latrunculin-treated cells directionally exposed to cAMP. Without VPA treatment, PHCrac-GFP translocated to the front of the cell in a biphasic pattern (Fig. 2E), as has been previously shown (63). In contrast, treatment of cells with 1 mM VPA for 10 min blocked the formation of both peaks of PHCrac-GFP translocation (Fig. 2E). These results suggest that VPA blocks the gradient sensing ability of cells by inhibiting both the first peak of PIP3 production and the amplification of this signal shown in the second peak.
Activation of cAR1/G-proteins leads to a recruitment of PI3K from the cytosol to the membrane, increasing the amount of membrane-bound active PI3K and therefore PIP3 production and thus playing a role in amplifying a cAMP gradient in highly polarized chemotaxing cells (19, 48). To examine whether VPA affects PI3K membrane translocation, we pulsed cells containing a PI3K-GFP construct with cAMP and measured PI3K-GFP translocation in the presence of VPA. Cells without VPA treatment showed transient membrane localization of PI3K-GFP, peaking at around 10 s after cAMP stimulation (Fig. 2F). Treatment of cells with 1 mM VPA for 10 min prior to stimulation blocked this translocation (Fig. 2F). However, as previously mentioned, VPA does not reduce the translocation of a marker for filamentous actin, YFP-ABD, suggesting that VPA treatment does not generally affect protein redistribution (see the movies in Fig. S2 in the supplemental material). Thus, the positive feedback loop that amplifies the cellular PIP3 gradient in chemotaxing cells is abrogated by VPA through the inhibition of both the initial production of PIP3 and the subsequent PI3K translocation (56).
VPA treatment and inhibition of PI3K activity attenuate in vivo phospholipid phosphorylation.
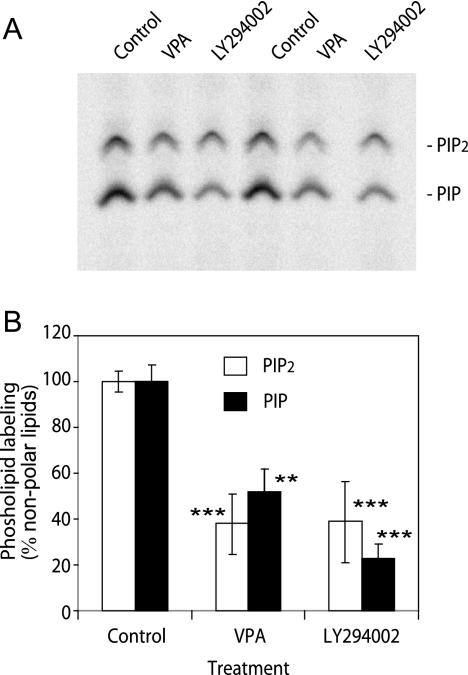
To investigate a potential change in phospholipid turnover caused by VPA in Dictyostelium, we developed cells for 5 h by pulsing with cAMP (5), pretreated cells for 5 min with defined drugs, permeabilized cells, and labeled lipids with [γ-32P]ATP for 5 min. Extraction of lipids and separation by thin-layer chromatography allowed the quantification of phospholipid turnover. Strong labeling of PIP and PIP2 was observed under control conditions (Fig. 3A). PIP3 labeling was not detected due to the comparatively small amount of this compound in the cell, and the addition of exogenous cAMP (10 μM) showed no quantifiable change in phospholipid labeling (data not shown). Under these conditions, VPA (1 mM) produced a significant (P > 0.01) reduction in both PIP and PIP2 turnover (Fig. 3B). Treatment of cells with LY294002 (50 μM) also caused a significant reduction in lipid turnover (P > 0.005) (Fig. 3B). These results show that acute VPA treatment causes a reduction in lipid phosphorylation. Although this may be due to reduced kinase or increased phosphatase levels, these results are consistent with a reduction in PIP3 production, as suggested by PHCrac-GFP translocation studies.
FIG. 3.
Effects of VPA and PI3K inhibition on in vivo Dictyostelium phospholipid turnover. Aggregation-competent wild-type (AX2) Dictyostelium cells were permeabilized, and lipids were labeled using [γ-32P]ATP in the presence of VPA (0.5 mM) or LY294002 (50 μM). (A) Thin-layer chromatography separation of phospholipids for two independent samples is shown. (B) Quantification of phospholipids (±standard deviation) (n = 4). **, P < 0.01; ***, P < 0.001.
VPA affects endocytosis and exocytosis.
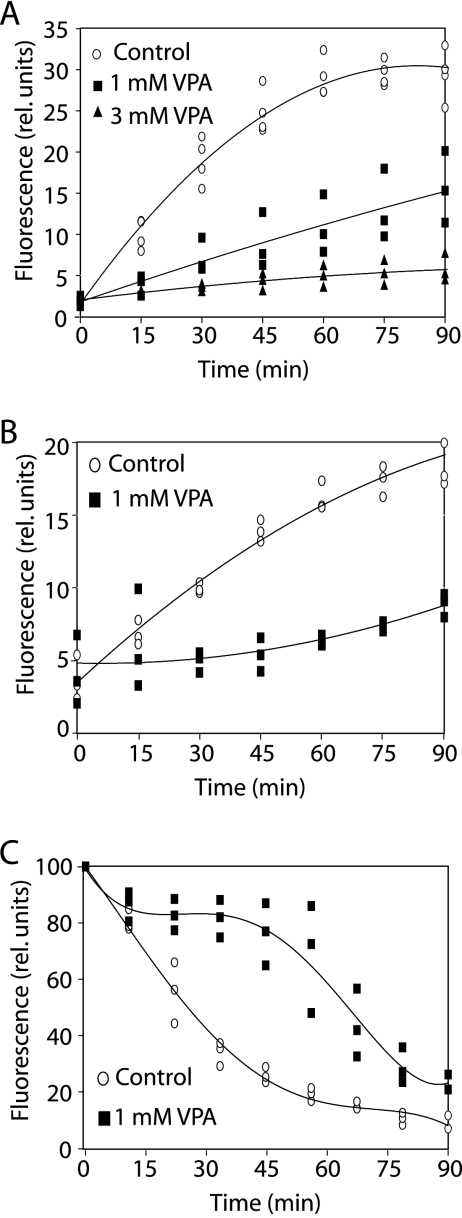
The inhibition of cAMP-induced PHCrac-GFP translocation and the reduction in phospholipid phosphorylation by VPA in Dictyostelium suggests it causes a reduction in phospholipid signaling, and this signaling pathway has been shown to affect vesicle trafficking in this model (7, 46, 65). In order to identify whether VPA may affect this process, we examined the acute effect of VPA on phagocytosis, pinocytosis, and exocytosis. To measure phagocytosis, we added VPA (1 or 3 mM) to growing cells and immediately recorded the uptake of labeled yeast particles over time (Fig. 4A). Treatment of cells with 1 mM VPA strongly reduced phagocytosis, while 3 mM VPA completely abolished particle uptake. Fluid-phase uptake was measured by quantifying the uptake of a soluble fluorescent marker (Fig. 4B). VPA (1 mM) caused an immediate, substantial reduction in fluid uptake, suggesting that this process is more sensitive to VPA than is phagocytosis. This concurs with previous work, as mutants lacking PI3K 1 and 2, or wild-type cells treated with a PI3K inhibitor, showed strongly blocked pinocytosis (46, 65) but only partially reduced phagocytosis (65). Since cellular components involved in endocytosis are likely to be necessary also for exocytosis, we characterized the effect of VPA on exocytosis. In these experiments, cells were allowed to take up fluorescent fluid-phase marker until saturation in the absence of VPA. Subsequent challenge with 1 mM VPA substantially delayed the release of fluorescence, compared with untreated cells (Fig. 4C). Similarly, the release of marker from cells loaded with endocytic tracer is also delayed in PI3K1/2 double mutants (7), as well as in PI3K inhibitor-treated cells (46). These data suggest that VPA acts to reduce endocytic trafficking through the attenuation of the phospholipid signaling.
FIG. 4.
Effects of VPA on Dictyostelium endocytosis and exocytosis. Growing wild-type cells were assayed for vesicle trafficking by adding the indicated amounts of VPA starting at 0 min. (A) Quantitative measurement of uptake of fluorescently labeled yeast particles. (B) Uptake of fluorescently labeled dextran as a fluid-phase marker. (C) Release of fluorescent dextran from cells loaded for 3 h.
VPA treatment and inhibition of PI3K activity reduces neurotransmitter release.
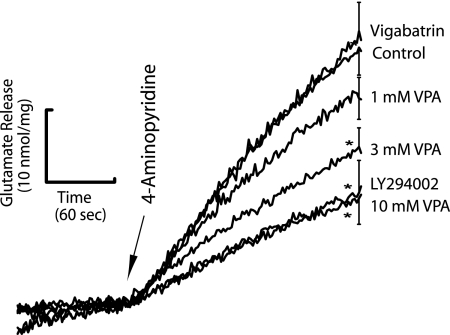
Since our data suggest that, in Dictyostelium, VPA reduces the phospholipid-dependent signaling and vesicle release, we examined an effect of VPA and PI3K inhibition in the modulation of synaptic signaling. We thus assessed the effect of VPA and a PI3K inhibitor on depolarization-dependent neurotransmitter glutamate release from isolated rat cerebrocortical nerve terminals (synaptosomes), an established model for assessing presynaptic function (54). In this model, VPA dose dependently inhibited 4-aminopyridine-evoked glutamate release from synaptosomes (Fig. 5), as measured by online fluorimetry (40). VPA at 1 mM showed a slight decrease in glutamate release (19%) and, at 3 mM and 10 mM, VPA produced clear decreases of glutamate release of 39% and 59%, respectively. High VPA concentrations were necessary in this model to overcome high membrane content of the synaptic preparation, which tends to sequester lipophilic agents like VPA. This effect was reproduced using the PI3K inhibitor LY294002, giving a 57% suppression of glutamate release at a concentration shown to affect glutamate release in the same manner as wortmannin (100 μM) (9). This effect was not through modulation of GABA levels, as the established GABA-transaminase inhibitor vigabatrin caused no significant reduction (Fig. 5).
FIG. 5.
Effects of VPA and PI3K inhibition on rat synaptosomal glutamate release. VPA (1, 3, and 10 mM), PI3K inhibitor (LY294002; 100 μM), and a GABA transaminase inhibitor (vigabatrin; 100 μM) were incubated with synaptosomes for 10 min before depolarization with 4-aminopyridine to evoke calcium-dependent glutamate release. Release traces shown are means ± standard errors (n = 3 to 5) from independent synaptosomal preparations. Only the error bar at 4 min after initiation of release is shown. Significant changes were determined by one-way analysis of variance followed by a post-hoc Fisher's least-significant-difference procedure to compare release in the presence of drug with control release. *, significant difference versus control release (P < 0.05). Each n indicates the number of experiments carried out using individual synaptosome preparations independently derived from five to seven animals.
DISCUSSION
In this work we have identified a novel acute effect of VPA in blocking movement in chemotaxing Dictyostelium cells (Fig. 1). We showed this effect occurs with a block in the production of PIP3, as measured by PHCrac-GFP translocation (Fig. 2), whereby high cellular concentrations of VPA strongly reduced signal-induced PIP3 production while lower concentrations slowed and attenuated this process. We also showed that VPA exposure acutely reduces phospholipid phosphorylation (Fig. 3), consistent with a reduction in the production of PIP3 caused by the drug. We further showed that VPA modulates Dictyostelium endo- and exocytosis, processes previously associated with PIP3 production (7, 46, 65). VPA concentrations used in these experiments are around therapeutic limits, since plasma concentrations of VPA are 0.3 to 0.6 mM (31) and chronic treatment of animal models can give rise to brain concentrations of 1.0 to 1.5 mM (2). We further showed that in a mammalian synaptic model for vesicle release—a key process in seizure occurrence—both VPA and a block in phospholipid signaling caused by PI3K inhibition significantly reduced glutamate release.
Results presented in this paper suggest that VPA causes an acute reduction in PIP3 production; however, it remains unclear how VPA reduces the level of this critical signaling lipid. This effect could be caused by a variety of methods, including reduced supply of the PIP3 precursor PIP2, direct or indirect inhibition of phosphatidylinositol kinase enzymes (e.g., PI3K), or direct or indirect activation of phosphatidylinositol phosphatase enzymes (e.g., PTEN). The coordinate decrease in phosphorylated PIP and PIP2 formation suggests that VPA's effect on PIP3 production may be caused by an effect on an enzyme responsible for phosphorylation or dephosphorylation of multiple phospholipid targets (as we have shown for PI3K inhibition). Further work will examine the role of phosphatidylinositol kinases (3′ and 5′) and phosphatases as targets for VPA. It is also of interest that sequence variations have been found in 5′ kinase (intron 9 PIP5K2A) that have been linked with bipolar disorder and schizophrenia (57). These results contrast with work in cerebellar granule cells, whereby VPA functions in a neuroprotective role by the activation of the PI3K/PKB signaling pathway (38).
It remains a formal possibility that the reduction in PHCrac-GFP translocation is caused by reduced movement of the reporter construct, not the production of PIP3. This seems unlikely, though, as another fluorescently tagged construct was not grossly affected in cellular localization by VPA treatment. Another possibility, as shown by Luo et al. (30), is that InsP7/8 competes with PIP3 for binding PH domain proteins, such as PHCrac, in the physiological regulation of high-order phosphoinositides during chemotaxis (30). However, the coordinate reduction in PIP and PIP2 production caused by VPA, and the common effect of VPA and the PI3K inhibitor, strongly suggest the observed results occur via a VPA-catalyzed reduction in phospholipid phosphorylation (and PIP3 production).
Investigating a role for a VPA-catalyzed reduction in phospholipid turnover that modulates synaptic function showed that both VPA and PI3K inhibition reduced glutamate release in purified mammalian synaptic preparations. The acute nature of this effect is of importance, as the intravenous administration of VPA inhibits epileptic seizures within 20 min (64), and few studies have identified short-term effects of the drug (26). The modulation of phospholipid signaling also provides an attractive and sensible mechanism for the function of branched short chain fatty acids, such as VPA. In GT1 to -7 neurons, the drug has been shown to be incorporated into lipids (51), although the resulting valproyl-phospholipid has not been identified, nor is its function understood. Incorporation of VPA into the lipid bilayer, either free or as a phospholipid, may modify the normal function of membrane-bound signaling components.
The modulation of phospholipid signaling, and specifically the function of PIP3-dependent processes, has been widely linked to potential epilepsy-related targets. For example, epileptic seizures have traditionally been thought to result from changes in ion channel activity, and PI3K (59) and phospholipid signaling (32) have both been implicated in the regulation of ion channels. Conversely, the deletion of the enzyme PTEN, the PI3K antagonist, causes seizures in mice (1), and Storey et al. (58) suggested that altered PI3K signaling might contribute to the pathophysiology of epilepsy.
The acute nature of the block in PIP3 production suggests that the observed effects of VPA are not caused by InsP3 depletion, which requires >5-h treatment in Dictyostelium (unpublished data) or mammalian neurons (12, 62). However, phospholipase C functions to regulate stimulation-induced production of InsP3 from PIP2, and its activity can be directly regulated by PIP3 (44). Thus, a potential inhibition of both PIP2 and PIP3 production by VPA treatment may suppress an overactive InsP3 signaling cascade, hence proposing a mechanism by which VPA functions, through phospholipid modulation, in the inositol depletion hypothesis.
Although VPA's role as an anticancer agent may be through HDAC inhibition (4), our data also suggest two additional potential mechanisms. Firstly, VPA may act by directly attenuating PIP3 production, which is elevated in many cancer types (22). In support of this, Denlinger et al. (10) recently found that the combined inhibition of PI3K and HDAC induced lung cancer cell apoptosis in vitro and in vivo. Secondly, it may suppress tumor metastasis (4) by inhibiting chemotaxis, and VPA has also been shown to inhibit chemotaxis in basic fibroblast growth factor-induced human umbilical vein endothelial cell migration (37) and to reduce individual neural crest cell migration (13). It is also interesting that the role of PIP2 is increasingly being recognized in cell migration (28).
The attenuation of synaptosomal glutamate release by VPA through the modulation of phospholipid signaling may also play a therapeutic role for this drug. Alterations in the release of glutamate and other neurotransmitters have been widely associated with seizures and as targets for antiepileptic treatments. Elevated glutamate release has been shown in the hippocampus of rats following pentylenetetrazol kindling (27) as a seizure model, and VPA reduced glutamate release in this system by an unknown mechanism. VPA also suppresses status epilepticus induced by 4-aminopyridine in the hippocampus (34) and 4-aminopyridine-induced excitatory synaptic activity (35). Our results, therefore, suggest a potential mechanism for these VPA effects—through phospholipid modulation to alter vesicle release. This latter process is also targeted by the new epilepsy treatment levetiracetam (31).
To conclude, our data identify a mechanism of action of valproic acid via the attenuation of phospholipid signaling and downstream phospholipid-dependent endocytic trafficking in the biomedical model system Dictyostelium. We further show that both VPA and a PI3K inhibitor attenuate glutamate release in a mammalian synaptic model. Although further work will be necessary in mammalian systems, these results propose a novel role for valproic acid in the attenuation of phospholipid-dependent signaling which may provide a new insight into the therapeutic function of the drug.
Supplementary Material
Acknowledgments
R.S.B.W. was funded by a Wellcome Trust RCD Fellowship.
Footnotes
Published ahead of print on 13 April 2007.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Backman, S. A., V. Stambolic, A. Suzuki, J. Haight, A. Elia, J. Pretorius, M. S. Tsao, P. Shannon, B. Bolon, G. O. Ivy, and T. W. Mak. 2001. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat. Genet. 29:396-403. [DOI] [PubMed] [Google Scholar]
- 2.Bazinet, R. P., M. T. Weis, S. I. Rapoport, and T. A. Rosenberger. 2006. Valproic acid selectively inhibits conversion of arachidonic acid to arachidonoyl-CoA by brain microsomal long-chain fatty acyl-CoA synthetases: relevance to bipolar disorder. Psychopharmacology (Berlin) 184:122-129. [DOI] [PubMed] [Google Scholar]
- 3.Berridge, M. J., C. P. Downes, and M. R. Hanley. 1989. Neural and developmental actions of lithium: a unifying hypothesis. Cell 59:411-419. [DOI] [PubMed] [Google Scholar]
- 4.Blaheta, R. A., M. Michaelis, P. H. Driever, and J. Cinatl, Jr. 2005. Evolving anticancer drug valproic acid: insights into the mechanism and clinical studies. Med. Res. Rev. 25:383-397. [DOI] [PubMed] [Google Scholar]
- 5.Boeckeler, K., K. Adley, X. Xu, A. Jenkins, T. Jin, and R. S. Williams. 2006. The neuroprotective agent, valproic acid, regulates the mitogen-activated protein kinase pathway through modulation of protein kinase A signalling in Dictyostelium discoideum. Eur. J. Cell Biol. 85:1047-1057. [DOI] [PubMed] [Google Scholar]
- 6.Bretschneider, T., S. Diez, K. Anderson, J. Heuser, M. Clarke, A. Müller-Taubenberger, J. Kohler, and G. Gerisch. 2004. Dynamic actin patterns and Arp2/3 assembly at the substrate-attached surface of motile cells. Curr. Biol. 14:1-10. [DOI] [PubMed] [Google Scholar]
- 7.Buczynski, G., B. Grove, A. Nomura, M. Kleve, J. Bush, R. A. Firtel, and J. Cardelli. 1997. Inactivation of two Dictyostelium discoideum genes, DdPIK1 and DdPIK2, encoding proteins related to mammalian phosphatidylinositide 3-kinases, results in defects in endocytosis, lysosome to postlysosome transport, and actin cytoskeleton organization. J. Cell Biol. 136:1271-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, G., L. D. Huang, Y. M. Jiang, and H. K. Manji. 1999. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J. Neurochem. 72:1327-1330. [DOI] [PubMed] [Google Scholar]
- 9.Cousin, M. A., C. S. Malladi, T. C. Tan, C. R. Raymond, K. J. Smillie, and P. J. Robinson. 2003. Synapsin I-associated phosphatidylinositol 3-kinase mediates synaptic vesicle delivery to the readily releasable pool. J. Biol. Chem. 278:29065-29071. [DOI] [PubMed] [Google Scholar]
- 10.Denlinger, C. E., B. K. Rundall, and D. R. Jones. 2005. Inhibition of phosphatidylinositol 3-kinase/Akt and histone deacetylase activity induces apoptosis in non-small cell lung cancer in vitro and in vivo. J. Thorac. Cardiovasc. Surg. 130:1422-1429. [DOI] [PubMed] [Google Scholar]
- 11.Dormann, D., G. Weijer, C. A. Parent, P. N. Devreotes, and C. J. Weijer. 2002. Visualizing PI3 kinase-mediated cell-cell signaling during Dictyostelium development. Curr. Biol. 12:1178-1188. [DOI] [PubMed] [Google Scholar]
- 12.Eickholt, B. J., G. Towers, W. J. Ryves, D. Eikel, K. Adley, L. Ylinen, N. Chadborn, A. Harwood, H. Nau, and R. S. Williams. 2005. Effects of valproic acid derivatives on inositol trisphosphate depletion, teratogenicity, GSK-3β inhibition and viral replication. A screening approach for new bipolar disorder drugs based on the valproic acid core structure. Mol. Pharmacol. 67:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller, L. C., S. K. Cornelius, C. W. Murphy, and D. J. Wiens. 2002. Neural crest cell motility in valproic acid. Reprod. Toxicol. 16:825-839. [DOI] [PubMed] [Google Scholar]
- 14.Funamoto, S., K. Milan, R. Meili, and R. A. Firtel. 2001. Role of phosphatidylinositol 3′ kinase and a downstream pleckstrin homology domain-containing protein in controlling chemotaxis in Dictyostelium. J. Cell Biol. 153:795-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurvich, N., O. M. Tsygankova, J. L. Meinkoth, and P. S. Klein. 2004. Histone deacetylase is a target of valproic acid-mediated cellular differentiation. Cancer Res. 64:1079-1086. [DOI] [PubMed] [Google Scholar]
- 16.Hacker, U., R. Albrecht, and M. Maniak. 1997. Fluid-phase uptake by macropinocytosis in Dictyostelium. J. Cell Sci. 110:105-112. [DOI] [PubMed] [Google Scholar]
- 17.Hamodeh, S. A., M. Rehn, G. Haschke, and M. Diener. 2004. Mechanism of butyrate-induced hyperpolarization of cultured rat myenteric neurones. Neurogastroenterol. Motil. 16:597-604. [DOI] [PubMed] [Google Scholar]
- 18.Hao, Y., T. Creson, L. Zhang, P. Li, F. Du, P. Yuan, T. D. Gould, H. K. Manji, and G. Chen. 2004. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J. Neurosci. 24:6590-6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janetopoulos, C., T. Jin, and P. Devreotes. 2001. Receptor-mediated activation of heterotrimeric G-proteins in living cells. Science 291:2408-2411. [DOI] [PubMed] [Google Scholar]
- 20.Jin, T., N. Zhang, Y. Long, C. A. Parent, and P. N. Devreotes. 2000. Localization of the G protein βγ complex in living cells during chemotaxis. Science 287:1034-1036. [DOI] [PubMed] [Google Scholar]
- 21.Jovanovic, J. N., A. J. Czernik, A. A. Fienberg, P. Greengard, and T. S. Sihra. 2000. Synapsins as mediators of BDNF-enhanced neurotransmitter release. Nat. Neurosci. 3:323-329. [DOI] [PubMed] [Google Scholar]
- 22.Kang, S., A. G. Bader, and P. K. Vogt. 2005. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc. Natl. Acad. Sci. USA 102:802-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerwin, R. 1999. The Bethlem and Maudsley NHS Trust prescribing guidelines. Martin Duntiz, London, England.
- 24.Knoester, P. D., S. V. Belitser, C. L. Deckers, A. Keyser, W. O. Renier, A. C. Egberts, and Y. A. Hekster. 2004. Diffusion of the new antiepileptic drug lamotrigine in Dutch clinical practice. Eur. J. Clin. Pharmacol. 60:751-758. [DOI] [PubMed] [Google Scholar]
- 25.Kular, G., M. Loubtchenkov, P. Swigart, J. Whatmore, A. Ball, S. Cockcroft, and R. Wetzker. 1997. Co-operation of phosphatidylinositol transfer protein with phosphoinositide 3-kinase gamma in the formylmethionyl-leucylphenylalanine-dependent production of phosphatidylinositol 3,4,5-trisphosphate in human neutrophils. Biochem. J. 325:299-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lagace, D. C., W. T. O'Brien, N. Gurvich, M. W. Nachtigal, and P. S. Klein. 2005. Valproic acid: how it works. Or not. Clin. Neurosci. Res. 4:215-225. [Google Scholar]
- 27.Li, Z. P., X. Y. Zhang, X. Lu, M. K. Zhong, and Y. H. Ji. 2004. Dynamic release of amino acid transmitters induced by valproate in PTZ-kindled epileptic rat hippocampus. Neurochem. Int. 44:263-270. [DOI] [PubMed] [Google Scholar]
- 28.Ling, K., N. J. Schill, M. P. Wagoner, Y. Sun, and R. A. Anderson. 2006. Movin' on up: the role of PtdIns(4,5)P(2) in cell migration. Trends Cell Biol. 16:276-284. [DOI] [PubMed] [Google Scholar]
- 29.Loovers, H. M., M. Postma, I. Keizer-Gunnink, Y. E. Huang, P. N. Devreotes, and P. J. Van Haastert. 2006. Distinct roles of PI(3,4,5)P3 during chemoattractant signaling in Dictyostelium: a quantitative in vivo analysis by inhibition of PI3-kinase. Mol. Biol. Cell 17:1503-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo, H. R., Y. E. Huang, J. C. Chen, A. Saiardi, M. Iijima, K. Ye, Y. Huang, E. Nagata, P. Devreotes, and S. H. Snyder. 2003. Inositol pyrophosphates mediate chemotaxis in Dictyostelium via pleckstrin homology domain-PtdIns(3,4,5)P3 interactions. Cell 114:559-572. [DOI] [PubMed] [Google Scholar]
- 31.Lynch, B. A., N. Lambeng, K. Nocka, P. Kensel-Hammes, S. M. Bajjalieh, A. Matagne, and B. Fuks. 2004. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc. Natl. Acad. Sci. USA 101:9861-9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma, H. P., S. Saxena, and D. G. Warnock. 2002. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC). J. Biol. Chem. 277:7641-7644. [DOI] [PubMed] [Google Scholar]
- 33.Maniak, M., R. Rauchenberger, R. Albrecht, J. Murphy, and G. Gerisch. 1995. Coronin involved in phagocytosis: dynamics of particle-induced relocalization visualized by a green fluorescent protein Tag. Cell 83:915-924. [DOI] [PubMed] [Google Scholar]
- 34.Martin, E. D., and M. A. Pozo. 2003. Valproate suppresses status epilepticus induced by 4-aminopyridine in CA1 hippocampus region. Epilepsia 44:1375-1379. [DOI] [PubMed] [Google Scholar]
- 35.Martin, E. D., and M. A. Pozo. 2004. Valproate reduced synaptic activity increase induced by 4-aminopyridine at the hippocampal CA3-CA1 synapse. Epilepsia 45:436-440. [DOI] [PubMed] [Google Scholar]
- 36.Meili, R., C. Ellsworth, S. Lee, T. B. Reddy, H. Ma, and R. A. Firtel. 1999. Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 18:2092-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michaelis, M., U. R. Michaelis, I. Fleming, T. Suhan, J. Cinatl, R. A. Blaheta, K. Hoffmann, R. Kotchetkov, R. Busse, H. Nau, and J. Cinatl, Jr. 2004. Valproic acid inhibits angiogenesis in vitro and in vivo. Mol. Pharmacol. 65:520-527. [DOI] [PubMed] [Google Scholar]
- 38.Mora, A., G. Sabio, J. C. Alonso, G. Soler, and F. Centeno. 2002. Different dependence of lithium and valproate on PI3K/PKB pathway. Bipolar Disord. 4:195-200. [DOI] [PubMed] [Google Scholar]
- 39.Reference deleted.
- 40.Nicholls, D. G., and T. S. Sihra. 1986. Synaptosomes possess an exocytotic pool of glutamate. Nature 321:772-773. [DOI] [PubMed] [Google Scholar]
- 41.Parent, C. A., B. J. Blacklock, W. M. Froehlich, D. B. Murphy, and P. N. Devreotes. 1998. G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95:81-91. [DOI] [PubMed] [Google Scholar]
- 42.Parent, C. A., and P. N. Devreotes. 1999. A cell's sense of direction. Science 284:765-770. [DOI] [PubMed] [Google Scholar]
- 43.Phiel, C. J., F. Zhang, E. Y. Huang, M. G. Guenther, M. A. Lazar, and P. S. Klein. 2001. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 276:36734-36741. [DOI] [PubMed] [Google Scholar]
- 44.Piechulek, T., T. Rehlen, C. Walliser, P. Vatter, B. Moepps, and P. Gierschik. 2005. Isozyme-specific stimulation of phospholipase C-gamma 2 by Rac GTPases. J. Biol. Chem. 280:38923-38931. [DOI] [PubMed] [Google Scholar]
- 45.Rauchenberger, R., U. Hacker, J. Murphy, J. Niewohner, and M. Maniak. 1997. Coronin and vacuolin identify consecutive stages of a late, actin-coated endocytic compartment in Dictyostelium. Curr. Biol. 7:215-218. [DOI] [PubMed] [Google Scholar]
- 46.Rupper, A. C., J. M. Rodriguez-Paris, B. D. Grove, and J. A. Cardelli. 2001. p110-related PI 3-kinases regulate phagosome-phagosome fusion and phagosomal pH through a PKB/Akt dependent pathway in Dictyostelium. J. Cell Sci. 114:1283-1295. [DOI] [PubMed] [Google Scholar]
- 47.Ryves, W. J., E. Dalton, A. Harwood, and R. S. B. Williams. 2005. GSK-3 activity in neocortical cells is inhibited by lithium but not carbamazepine or valproic acid. Bipolar Disord. 7:260-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasaki, T., J. Irie-Sasaki, R. G. Jones, A. J. Oliveira-dos-Santos, W. L. Stanford, B. Bolon, A. Wakeham, A. Itie, D. Bouchard, I. Kozieradzki, N. Joza, T. W. Mak, P. S. Ohashi, A. Suzuki, and J. M. Penninger. 2000. Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science 287:1040-1046. [DOI] [PubMed] [Google Scholar]
- 49.Servant, G., O. D. Weiner, P. Herzmark, T. Balla, J. W. Sedat, and H. R. Bourne. 2000. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 287:1037-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimshoni, J. A., E. C. Dalton, A. Jenkins, S. Eyal, K. Kwan, R. S. Williams, N. Pessah, B. Yagen, A. J. Harwood, and M. Bialer. 2007. The effects of CNS-active valproic acid constitutional isomers, cyclopropyl analogues and amide derivatives on neuronal growth cone behaviour. Mol. Pharmacol. 71:884-892. [DOI] [PubMed] [Google Scholar]
- 51.Siafaka-Kapadai, A., M. Patiris, C. Bowden, and M. Javors. 1998. Incorporation of [3H]valproic acid into lipids in GT1-7 neurons. Biochem. Pharmacol. 56:207-212. [DOI] [PubMed] [Google Scholar]
- 52.Sihra, T. S. 1997. Protein phosphorylation and dephosphorylation in isolated nerve terminals (synaptosomes), p. 67-119. In H. C. Hemmings, Jr. (ed.), Regulatory protein modifications: techniques and protocols. Humana Press, Totowa, NJ.
- 53.Sihra, T. S., E. Bogonez, and D. G. Nicholls. 1992. Localized Ca2+ entry preferentially effects protein dephosphorylation, phosphorylation, and glutamate release. J. Biol. Chem. 267:1983-1989. [PubMed] [Google Scholar]
- 54.Sihra, T. S., and R. A. Nichols. 1993. Mechanisms in the regulation of neurotransmitter release from brain nerve terminals: current hypotheses. Neurochem. Res. 18:47-58. [DOI] [PubMed] [Google Scholar]
- 55.Soll, D. R. 1999. Computer-assisted three-dimensional reconstruction and motion analysis of living, crawling cells. Comput. Med. Imag. Graph. 23:3-14. [DOI] [PubMed] [Google Scholar]
- 56.Srinivasan, S., F. Wang, S. Glavas, A. Ott, F. Hofmann, K. Aktories, D. Kalman, and H. R. Bourne. 2003. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J. Cell Biol. 160:375-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stopkova, P., T. Saito, C. S. Fann, D. F. Papolos, J. Vevera, I. Paclt, I. Zukov, R. Stryjer, R. D. Strous, and H. M. Lachman. 2003. Polymorphism screening of PIP5K2A: a candidate gene for chromosome 10p-linked psychiatric disorders. Am. J. Med. Genet. B 123:50-58. [DOI] [PubMed] [Google Scholar]
- 58.Storey, N. M., J. P. O'Bryan, and D. L. Armstrong. 2002. Rac and Rho mediate opposing hormonal regulation of the ether-a-go-go-related potassium channel. Curr. Biol. 12:27-33. [DOI] [PubMed] [Google Scholar]
- 59.Tong, Q., N. Gamper, J. L. Medina, M. S. Shapiro, and J. D. Stockand. 2004. Direct activation of the epithelial Na+ channel by phosphatidylinositol 3,4,5-trisphosphate and phosphatidylinositol 3,4-bisphosphate produced by phosphoinositide 3-OH kinase. J. Biol. Chem. 279:22654-22663. [DOI] [PubMed] [Google Scholar]
- 60.Van Lint, C., S. Emiliani, and E. Verdin. 1996. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr. 5:245-253. [PMC free article] [PubMed] [Google Scholar]
- 61.Vreugdenhil, M., and W. J. Wadman. 1999. Modulation of sodium currents in rat CA1 neurons by carbamazepine and valproate after kindling epileptogenesis. Epilepsia 40:1512-1522. [DOI] [PubMed] [Google Scholar]
- 62.Williams, R. S. B., L. Cheng, A. W. Mudge, and A. J. Harwood. 2002. A common mechanism of action for three mood-stabilizing drugs. Nature 417:292-295. [DOI] [PubMed] [Google Scholar]
- 63.Xu, X., M. Meier-Schellersheim, X. Jiao, L. E. Nelson, and T. Jin. 2005. Quantitative imaging of single live cells reveals spatiotemporal dynamics of multistep signaling events of chemoattractant gradient sensing in Dictyostelium. Mol. Biol. Cell 16:676-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu, K. T., S. Mills, N. Thompson, and C. Cunanan. 2003. Safety and efficacy of intravenous valproate in pediatric status epilepticus and acute repetitive seizures. Epilepsia 44:724-726. [DOI] [PubMed] [Google Scholar]
- 65.Zhou, K., S. Pandol, G. Bokoch, and A. E. Traynor-Kaplan. 1998. Disruption of Dictyostelium PI3K genes reduces [32P]phosphatidylinositol 3,4 bisphosphate and [32P]phosphatidylinositol trisphosphate levels, alters F-actin distribution and impairs pinocytosis. J. Cell Sci. 111:283-294. [DOI] [PubMed] [Google Scholar]
- 66.Zhou, K., K. Takegawa, S. D. Emr, and R. A. Firtel. 1995. A phosphatidylinositol (PI) kinase gene family in Dictyostelium discoideum: biological roles of putative mammalian p110 and yeast Vps34p PI 3-kinase homologs during growth and development. Mol. Cell. Biol. 15:5645-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.