Abstract
Dd-STATa, a Dictyostelium discoideum homologue of metazoan STAT transcription factors, is necessary for culmination. We created a mutant strain with partial Dd-STATa activity and used it to screen for unlinked suppressor genes. We screened approximately 450,000 clones from a slug-stage cDNA library for their ability to rescue the culmination defect when overexpressed. There were 12 multicopy suppressors of Dd-STATa, of which 4 encoded segments of a known noncoding RNA, dutA. Expression of dutA is specific to the pstA zone, the region where Dd-STATa is activated. In suppressed strains the expression patterns of several putative Dd-STATa target genes become similar to the wild-type strain. In addition, the amount of the tyrosine-phosphorylated form of Dd-STATa is significantly increased in the suppressed strain. These results indicate that partial copies of dutA may act upstream of Dd-STATa to regulate tyrosine phosphorylation by an unknown mechanism.
The JAK/STAT signal transduction pathway is known to regulate the immune response, cell fate, proliferation, cell migration, and programmed cell death in multicellular organisms (33). STAT (signal transducers and activators of transcription) proteins are transcription factors located at the end of these signaling pathways, and Dd-STATa is a functional Dictyostelium discoideum homologue of metazoan STAT proteins (19, 32). The existence of a STAT in a facultative multicellular organism such as Dictyostelium suggests that SH2 domain signaling played an important role in the evolutionary transition to multicellularity (6, 20). Dd-STATa is activated by extracellular cyclic AMP (cAMP) through the serpentine-type receptor cAR1 (2), after which Dd-STATa binds to regulatory promoter elements of ecmB and cudA genes that are necessary for normal development (12, 19). As disruption of the Dd-STATa gene causes the failure of culmination, Dd-STATa has functions essential for the final stages of Dictyostelium development (32). In general, however, which molecules regulate Dd-STATa activity and which genes are regulated by Dd-STATa remain poorly understood.
To search for molecules linked to the STAT signaling pathway, we previously identified 13 potential Dd-STATa target genes by use of in situ hybridization (42-44). Another powerful approach is to isolate genetic suppressors of STAT mutants (39). In Dictyostelium, REMI (restriction enzyme-mediated integration) mutagenesis is widely used to isolate loss-of-function mutants (24, 41). However, isolating the gene of interest from the mutant strain can be challenging, and REMI mutants often have complicated genomic rearrangements, making it difficult to prove which gene is affected. Library complementation using a cDNA expression library circumvents these issues by simplifying and accelerating gene identification and validation. Further, various types of genetic interactions are possible (overexpression, gain-of-function, dominant-negative, and attenuated functions), increasing the richness of the approach (14, 35, 40). Here, we describe the isolation of multicopy suppressor clones of Dd-STATa using this approach.
Recently, a large number of noncoding RNAs (ncRNA) have been reported with a range of developmental functions in different organisms (10, 48). In Dictyostelium, an ncRNA is also described as an antisense regulator of the EB4 gene locus (17); dutA, development-specific but untranslatable RNA, has also been reported as an ncRNA, although its function is unknown (52). Since then, other ncRNAs have been identified (3, 18, 22, 37). Here, we show that one of the suppressor genes of Dd-STATa isolated in an overexpression genetic screen is dutA.
MATERIALS AND METHODS
Plasmids.
pREP plasmid harbors a replicase open reading frame (ORF) required for replication of Ddp2 origins in D. discoideum (5, 46). pLD1A15SN is a plasmid used for cDNA library construction and has a Ddp2 origin (40). pLD1-HygT (a kind gift of Masashi Fukuzawa, Hirosaki University, Japan) harbors a Ddp2 replication origin and hygromycin B-resistant selectable marker cassette (Hygr). For detection of ecmF(SLF308) promoter activity, an ecmF(SLF308)::lacZ vector was used (a kind gift of Mineko Maeda, Osaka University, Japan).
Creation of D. discoideum strains used for genetic screening.
For a genetic screening of the Dd-STATa suppressor, the pREP ORF was stably integrated into the genome of a Dd-STATa-null strain to make a Dd-STATa−::ORF+ strain as described previously (40). For a selection of the pREP ORF-integrated transformants, pLD1-HygT was cotransformed. Hygromycin B-resistant colonies were selected in HL5 medium, and integration of the pREP ORF was confirmed by PCR. The ORF-integrated strain that showed the best efficiency (∼1,500 colonies/μg cDNA plasmid) was chosen. The strain was further cultured in the medium without hygromycin B to cure the pLD1-HygT plasmid. The resultant hygromycin B-sensitive strain was named Dd-STATa−::ORF+. Using the same procedure, pREP ORF was integrated into the genome of the Ax2 strain to make the Ax2::ORF+ strain.
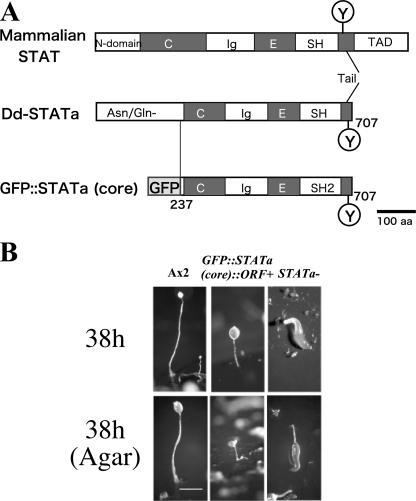
Green fluorescent protein (GFP) was fused with a truncated Dd-STATa gene which encodes a STAT core region, corresponding to amino acids 237 to 707 of Dd-STATa (47). The fusion gene was integrated into the genome of Dd-STATa−::ORF+ to make the strain Dd-STATa(core)::ORF+. The Dd-STATa(core)::ORF+ strain was easily identified, as it eventually forms fruiting bodies.
Cell culture and growth conditions.
Cells of various strains were cultured axenically in HL5 medium at 22°C. Dd-STATa-null cells were grown in HL5 supplemented with 10 μg/ml blasticidine S (Kaken Pharmaceutical, Tokyo, Japan). Transformants having Neor cassettes were selected in HL5 supplemented with 10 μg/ml G418 (Geneticin; ICN Biochemicals Inc., Ohio); the concentration of G418 was increased up to 20 μg/ml subsequently. The dutA-null mutant cells (a kind gift of Koji Okamoto, Kyoto University) were grown in HL5 supplemented with 20 μg/ml of G418.
Construction of the cDNA library.
Ax2 cells (1 × 108) were allowed to develop on water agar plates (22- by 22-cm Nunc dishes) until they reached the slug stage (15, 16, 17, and 18 h). Cells from all four time points were combined, and poly(A)+ RNA was purified using the poly(A) Pure mRNA purification kit (Ambion Inc., Texas). The poly(A)+ RNA was further purified with oligo(dT) latex beads (Oligotex-dT30 Super mRNA purification kit; TaKaRa, Kyoto, Japan). Poly(A)+ RNA was converted into cDNA ligated to pLD1A15SN as described previously (40).
Ligated plasmids were transformed into Escherichia coli DH5α Electro-Cells (TaKaRa, Kyoto, Japan) by electroporation. A Mini-prep analysis revealed that a minimum of 90% of the plasmid clones contained inserts. Inserts averaged 0.8 to 0.9 kb in size and ranged from 0.4 to >4 kb. Library plasmid was prepared from 1 × 107 independent primary E. coli colonies using a Mega-prep plasmid preparation kit (QIAGEN, Germany).
Screening the multicopy suppressor of Dd-STATa.
The cDNA library plasmid was transformed into the GFP::STATa(core)::ORF+ strain by electroporation using two square wave pulses of 900 V for 1.0 ms with a 5-s pulse interval (Gene Pulser Xcell; Bio-Rad). The transformed cells were selected in HL5 medium supplemented with 10 μg/ml G418 and plated at approximately 1,000 cells per plate with Klebsiella aerogenes on SM agar. Candidate suppressor clones that made fruiting bodies more efficiently than the parental strain were visually selected. cDNA plasmids were recovered from possible suppressed clones and retransformed into the parental strain to confirm suppression. The cDNA fragment in each plasmid was digested with NotI and SalI and subcloned into pSPORT1 (Invitrogen, CA) for sequencing.
Suppression assay.
Cells of Ax2, Dd-STATa-null, and GFP::STATa(core)::ORF+ cells transformed with various pLD1-dutA(cDNA) constructs were axenically grown in shaking culture, and 1 × 106 cells were allowed to develop at 22°C on water agar poured onto glass plates. After 38 h of development, the numbers of slugs and fruiting bodies (including culminants) were counted, and the percent fruiting body formation ratio was calculated as follows: fruiting body formation ratio (%) = [(total number of fruiting bodies)/(total number of slugs and fruiting bodies)] × 100.
Analysis of gene expression by RT-PCR.
Total RNA was extracted from each strain at desired developmental stages of Ax2, GFP::STATa(core)::ORF, dutA-null strains and various pLD1-dutA(cDNA) transformants with an RNeasy extraction kit (QIAGEN). cDNA synthesis and reverse transcription-PCR (RT-PCR) were performed with an RNA LA PCR kit (avian myeloblastosis virus), version 1.1 (TaKaRa, Kyoto, Japan) using primers described previously (42, 44) and listed in Table 1.
TABLE 1.
Sequences of oligonucleotides used for RT-PCR to detect the dutA transcript
| Primer name | Sequence | Reference |
|---|---|---|
| dutAd2-Sal | 5′-ACG CGT CGA CAC TCT CTC AAA TGT ATA AGC AGTC-3′ | This work |
| dutA2 | 5′-CAA ACT GGA CCT AAC AGC ATA CAC-3′ | This work |
| dutA-RT1 | 5′-TCC TTC TAC TTT TTG GTG CTT CTG-3′ | This work |
| dutAd2 | 5′-CCC AAG CTT AAA GAC TGC TTA TAC ATT TGA GAG-3′ | This work |
| dutA1 | 5′-AAT GAT TGG CTA TGG CCC AAA TGG-3′ | This work |
| LD1SM | 5′-AAA AGT CGA CCC ACG CGT CC-3′ | 40 |
In situ hybridization.
Whole-mount in situ hybridization was performed as described previously (11, 29, 30, 43).
Construction of extrachromosomal lacZ constructs.
Exp4(+) plasmid (8) was digested with NotI to remove the Neor cassette and the ends converted to a SalI site by linker ligation to make pExp4(+)Sal. Then, the 2H3 terminator region was excised from this vector by digestion with XhoI and SalI and ligated into the XhoI site of pLD1A15SN to make pLD1::2H3term. The orientation of the 2H3 terminator was confirmed by digestion with EcoRI, SalI, and XhoI. As pLD1::2H3term originally contained the actin15 promoter and actin15 terminator, the vector was digested with XbaI and XhoI to remove these regions, and the remaining vector was gel purified. The XbaI/XhoI-digested and gel-purified ecmF(SLF308)::lacZ DNA fragment or cudA(pst)::lacZ was ligated into the modified vector to make pLD1-ecmF(SLF308)::lacZ or pLD1-cudA(pst)::lacZ. For the suppression construct, the XbaI cassette of suppressor clone 0114 was ligated into the XbaI site of the above lacZ vector to make pLD1-0114::ecmF(SLF308)::lacZ or pLD1-0114::cudA(pst)::lacZ.
β-Galactosidase staining.
For the detection of promoter activity of specific genes, cells transformed with plasmids containing promoter fragments fused to the lacZ coding sequence were grown and developed on nitrocellulose filters as above. Fixation and staining were performed as described elsewhere (7).
Western analysis.
Western analysis was performed as described previously (1). Mouse anti-Dd-STATa monoclonal antibody D4 (1:50 dilution), rabbit anti-phospho-Dd-STATa polyclonal antibody SC7 (1:333 dilution; a kind gift of Tsuyoshi Araki and Jeffrey Williams, University of Dundee, United Kingdom) (2), or anti-GFP monoclonal antibody (1:2,000 dilution; Roche, Germany) was used as a primary antibody, and alkaline phosphatase-conjugated anti-mouse immunoglobulin G (IgG; 1:20,000 dilution; Promega) or anti-rabbit IgG (HL) antibody (1:2,500 dilution; Promega) was used as a secondary antibody.
RESULTS
Generation of a strain expressing partially active Dd-STATa protein.
Inactivation of the Dd-STATa gene blocks culmination and results in aberrant terminally differentiated structures, primarily slug-like structures, even after prolonged development (32). When a histidine-tagged core fragment of Dd-STATa (residues 237 to 707) is expressed in Dd-STATa null cells, it partially rescues development and allows fruiting body formation (47; M. Fukuzawa, personal communication).
We generated a strain which we have called GFP::STATa(core)::ORF+ that has integrated a low copy number of the GFP-fused Dd-STATa core fragment, GFP::STATa(core) (Fig. 1A), into a Dd-STATa-null strain harboring an integrated replicase gene. Development of the GFP::STATa(core)::ORF+ strain resembled that of the histidine-tagged Dd-STATa core strain. However, the former formed fruiting bodies with shorter stalks than wild type on buffered filters (Fig. 1B, upper panel). More strikingly, the GFP::STATa(core)::ORF+ strain hardly formed fruiting bodies on water agar plates even after 38 h of development (Fig. 1B, see the legend for details; see also Fig. 3B and C, below). Once the GFP::STATa(core)::ORF+ strain had formed fruiting bodies, normal spores were formed and their viability was indistinguishable from that of Ax2 (data not shown). Expression of several potential Dd-STATa target genes examined by microarrays indicated that the GFP::STATa(core)::ORF+ strain had an intermediate behavior between Ax2 (wild type) and Dd-STATa-null strains; thus, it behaves like a partially active Dd-STATa strain (N. Shimada, G. Bloomfield, A. Sakurai, J. Skelton, M. Fukuzawa, Z. Katagiri, R. R. Kay, A. Ivens, and T. Kawata, unpublished data) (see also Fig. 5, below).
FIG. 1.
Domain organization of the mammalian STAT, Dictyostelium Dd-STATa, and GFP::STATa(core). (A) GFP::STATa(core) contains an N-terminal fusion of GFP and a core fragment of Dd-STATa (residues 237 to 707) which lacks the Asn/Gln-rich domain but includes the natural C terminus. N domain, N-terminal conserved domain; C, coiled-coil domain; Ig, immunoglobulin-like domain; E, a connector or linker domain with EF-hand hold; SH2, Src homology 2 domain; Y, phosphorylated tyrosine residue; TAD, transcriptional activator domain (47). (B) Developmental morphology of a strain expressing GFP::STATa(core) protein, GFP::STATa(core)::ORF+ strain. Ax2 (left column), GFP::STATa(core)::ORF+ (center column), and STATa-null (right column) cells were allowed to develop on either black filters (upper panel) or water agar plates (lower panel). The GFP::STATa(core)::ORF+ cells eventually form fruiting bodies. Note that the GFP::STATa(core)::ORF+ cells form fruiting bodies as effectively as Ax2 cells do on black filters, but only ∼5% of GFP::STATa(core)::ORF+ slugs can form a fruiting body even after 38 h of development on water agar. A rare fruiting body of the GFP::STATa(core)::ORF+ strain on water agar is shown.
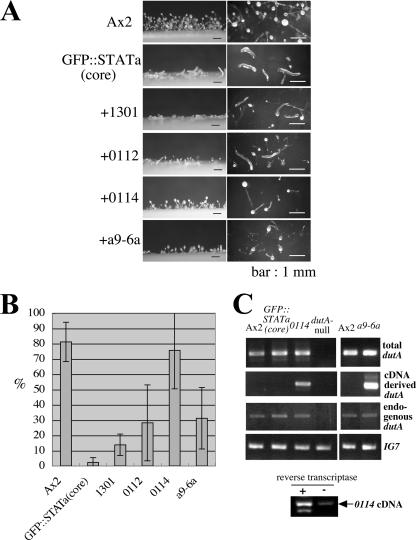
FIG. 3.
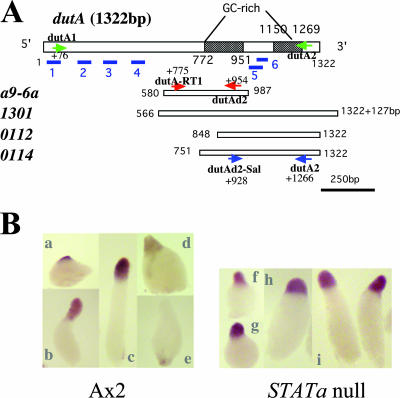
dutA cDNA clones as Dd-STATa multicopy suppressors. (A) Morphology of each clone on thin water agar plates after 38 h of development. The name of each strain is shown at the left of the photo. The left column shows the side view, and the right column shows the overhead view. Bars, 1 mm. Expression of clone 0114 most effectively suppressed the phenotype of the parental strain, GFP::STATa(core)::ORF+. (B) Efficiency of fruiting body formation. Four independent clones (1301, a9-6a, 0112, and 0114) were isolated as overexpression suppressors of Dd-STATa. The name of each strain is shown at the bottom of the bar. The height of the bar denotes the efficiency of fruiting body formation (see Materials and Methods for further detail). For each calculation, at least four experiments were performed. Error bars indicate standard deviations. (C) Overexpression of dutA RNA from the pLD1-dutA vector in the suppressed strain. The level of dutA expression was monitored by semiquantitative RT-PCR. For each assay, 0.2 μg of total RNA was used. Sequences of specific dutA primers to detect total dutA RNA for Ax2, GFP::STATa(core)::ORF+, 0114, and dutA-null strains were dutAd2-Sal and dutA2. For a comparison between Ax2 and a9-6a strains, primers dutA-RT1 and dutAd2 were used. For detection of dutA RNA from the pLD1-dutA vector, the 5′ primer was LD1SM, which does not detect the endogenous dutA RNA (40). The 3′ primer was dutA2 for Ax2, GFP::STATa(core)::ORF+, 0114, and dutA-null strains; primer dutAd2 was used for comparison with strains Ax2 and a9-6a. Transcripts of the dutA RNA from the pLD1-dutA vector have short actin15 and adaptor sequences where there is a priming site for LD1SM. To detect endogenous dutA RNA, primers dutA1 and dutA2 were used. The sequence of each oligonucleotides is shown in Table 1. The bottom panel shows the PCR product from the 0114 RNA sample with (+) or without (−) reverse transcriptase in the reaction mixture. The positions of each primer are indicated in Fig. 4A. IG7 was detected in the same reaction as a normalization control.
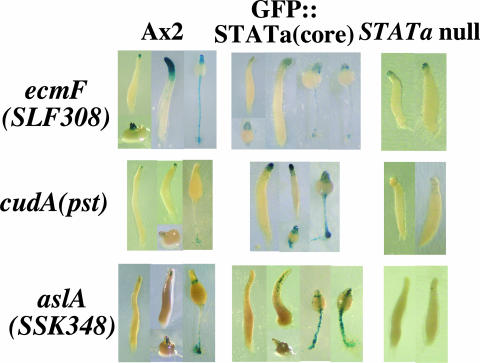
FIG. 5.
Expression of potential Dd-STATa target genes in the parental strain. Spatial expression patterns of ecmF(SLF308) (upper panel) and aslA(SSK348) (lower panel) promoter activity were detected by lacZ reporter constructs. Ax2 (left column), GFP::STATa(core)::ORF+ (center column), and Dd-STATa-null (right column) cells were transformed with the ecmF(SLF308), cudA(pst), or aslA(SSK348) promoter region fused to the β-galactosidase (lacZ) gene and developed on filters to a range of developmental stages. Promoter activity was detected by staining as described in Materials and Methods.
Genetic search for multicopy suppressors of Dd-STATa.
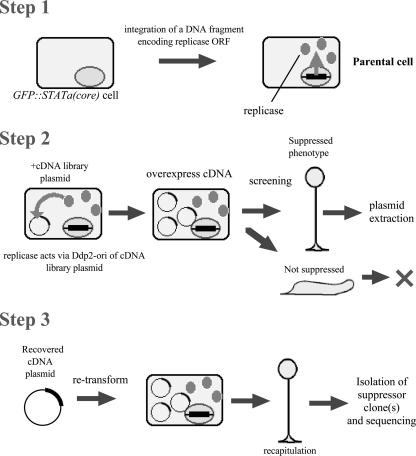
In order to isolate multicopy suppressor clones of Dd-STATa, we modified a library complementation method developed by Robinson and Spudich (40). The screening strategy is shown in Fig. 2. First, a replicase gene was integrated into the genome of the Dd-STATa-null strain to make a Dd-STATa-null::ORF+ strain. Next the linearized GFP::STATa(core) fusion gene was integrated to make a GFP::STATa(core)::ORF+ strain, the parental strain for our screen. For the second step, the cDNA library made from slug-stage mRNA was transformed into the parental strain. Transformants were plated with K. aerogenes on SM agar to search for clones that made fruiting bodies more efficiently than the parental strain. Finally, cDNA plasmids were recovered from presumptive suppressed clones and retransformed into the parental strain to confirm suppression, and each suppressor cDNA was sequenced. In this way, we screened approximately 450,000 clones for their ability to partially rescue the culmination defect when overexpressed. So far, we have identified 26 putative suppressors. Twelve clones were recapitulated and confirmed to be multicopy suppressors of Dd-STATa (details will be published elsewhere).
FIG. 2.
Screening strategy to identify multicopy suppressor clones of Dd-STATa. The screening method developed by Robinson and Spudich (40) was modified as detailed in the text and in Materials and Methods.
The noncoding RNA gene dutA is a multicopy suppressor of Dd-STATa.
Four independent clones (a9-6a, 1301, 0112, and 0114) of 12 confirmed suppressors encode different regions of dutA RNA, a Dictyostelium development-specific but untranslatable RNA (see Fig. 4A, below) (52). When these clones were overexpressed in the parental strain, GFP::STATa(core)::ORF+, the efficiency of fruiting body formation on water agar plates increased, although it varied among the four clones (Fig. 3A and B). Most of the suppression was not perfect complementation, as the stalk formed was shorter than that of wild type (Ax2) (Fig. 3A). However, the parental strain hardly formed fruiting bodies under the same conditions. Therefore, these clones are overexpression suppressors of Dd-STATa. Importantly, suppression was not limited to a particular parental strain: using two more independently isolated Dd-STATa-null::ORF+ strains, the 0114 cDNA clone again suppressed the culmination defect, although the efficiency was only half of that seen in the first isolated GFP::STATa(core)::ORF+ strain (data not shown). Overexpression of control cDNA never suppressed the phenotype of the parent strain (data not shown). Furthermore, overexpression of 0114 cDNA never suppressed the STATa-null::ORF+ strain, either (data not shown).
FIG. 4.
Schematic representation of the dutA gene and its expression. (A) The dutA transcribed region contains two GC-rich regions and six putative hairpin-loop structures identified by blue underlining (52). Four independent clones (a9-6a, 1301, 0112, and 0114) isolated as overexpression suppressors of Dd-STATa encode different regions of dutA RNA. The efficiency of fruiting body formation varied among the four clones (Fig. 3B). The positions of primers used for RT-PCR to detect dutA expression are indicated as arrows (Fig. 3C). (B) Spatial expression patterns of dutA RNA detected by in situ hybridization in Ax2 (left panel; a to e) and in the Dd-STATa-null strain (right panel; f to i). The dutA RNA probe was synthesized from EST clone SSJ314.
Overexpression of the 0114 cDNA (we simply call this strain 0114 hereafter) most effectively suppressed the Dd-STATa phenotype, forming fruiting bodies as effectively as Ax2 (Fig. 3A and B). Expression of other dutA cDNA clones a9-6a and 0112 moderately suppressed the parental phenotype, while clone 1301 suppressed it very weakly (Fig. 3A and B). In situ hybridization analysis showed the dutA gene was expressed in pstA cells, where Dd-STATa is activated at the slug stage (Fig. 4B, patterns a to c). Interestingly, the expression of the dutA gene gradually decreased as culmination proceeded (Fig. 4B, patterns d and e). In contrast to the potential Dd-STATa target genes (43), dutA was also expressed in pstA cells in the Dd-STATa null mutant (Fig. 4B, patterns f to h).
Overexpression of truncated dutA RNA in all four suppressed clones was confirmed by RT-PCR using a primer that does not detect endogenous dutA RNA (Fig. 3C; the results of the 0112 and a1301 clones are not shown, as they were principally the same as the result with the 0114 clone). Indeed, the PCR product derived from RNA transcribed from the pLD1-dutA vector was detected only in the suppressed strains (Fig. 3C). To eliminate the possibility that the PCR product was mainly derived from contamination of pLD1 vector DNA in the total RNA sample, PCR was performed with or without reverse transcriptase. Although there is trace contamination by vector DNA, the PCR product was mainly dependent on the presence of reverse transcriptase (Fig. 3C, bottom panel). Interestingly, the level of endogenous dutA expression was almost equal in all strains tested (Fig. 3C).
Partially reverted expression of potential Dd-STATa target genes in the suppressed strain.
To confirm that morphological suppression is the effect of Dd-STATa function, we compared the expression patterns of the potential and proven Dd-STATa target genes ecmF(SLF308) and cudA(pst) (12, 42) by β-galactosidase staining in Ax2, the parental strain, and 0114.
We first introduced the normal authentic ecmF(SLF308)::lacZ, cudA(pst)::lacZ, and aslA(SSK348)::lacZ reporter constructs into Ax2, GFP::STATa(core)::ORF+, and Dd-STATa-null strains and compared expression patterns when these cells were allowed to develop on buffered filters (Fig. 5). As reported previously (42), the ecmF(SLF308) gene was expressed strongly in pstA cells at late stages. Its expression was hardly detectable in the Dd-STATa-null strain, although a very weak tip staining was visible. In the parental GFP::STATa(core)::ORF+ strain, expression of the ecmF(SLF308) gene was intermediate between the Ax2 and the Dd-STATa-null strains both in intensity and spatial distribution. aslA(SSK348) is another potential Dd-STATa target gene and is also expressed in cells near the tip, the “pstAB core” cells (see Fig. 9, below) (44). Surprisingly, the expression of aslA(SSK348) increased in the GFP::STATa(core)::ORF+ strain, and ectopic expression in stalk cells was observed (Fig. 5). Increased expression in prestalk cells in the GFP::STATa(core)::ORF+ strain was similarly observed for cudA(pst)::lacZ, which is a confirmed Dd-STATa target marker also expressed in tip cells (Fig. 5) (12).
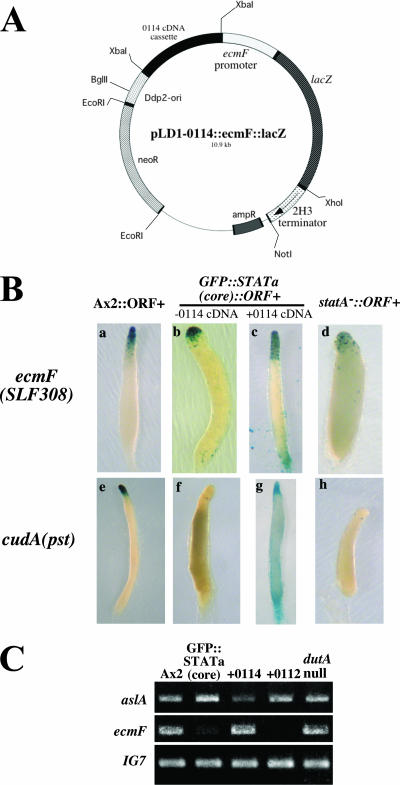
As the suppressed clones were already resistant to G418, hygromycin B, and blasticidine S, in order to introduce the lacZ vectors into these strains we created new reporter constructs, pLD1-0114::ecmF(SLF308)::lacZ and pLD1-0114::cudA(pst)::lacZ (Fig. 6A). They harbor both a lacZ reporter cassette and the 0114 cDNA fragment in the same G418 resistance-conferring extrachromosomal vector, pLD1. For the nonsuppressed control, the lacZ reporter cassette alone was integrated into pLD1 to make pLD1-ecmF(SLF308)::lacZ and pLD1-cudA(pst)::lacZ. Transformation of pLD1-0114::ecmF(SLF308)::lacZ or pLD1-0114::cudA(pst)::lacZ plasmid suppressed the parental phenotypes, although the suppression was weaker than when the original 0114 cDNA was transformed (∼40% fruiting body formation ratio) (data not shown). However, as the transformants formed fruiting bodies more efficiently than the parental strain, the 0114 cDNA fragment in the new lacZ plasmid was also functional.
FIG. 6.
Expression of potential Dd-STATa target genes in the suppressed strain. (A) Map of the extrachromosomal lacZ vector, pLD1-0114::ecmF::lacZ, which contains both ecmF::lacZ and the 0114 cDNA fragment. The map of pLD1-0114::cudA(pst)::lacZ is the same except for the promoter region. (B) Spatial expression patterns of ecmF(SLF308) and cudA(pst) promoter activity driving an extrachromosomal lacZ reporter construct. Cells of different types were allowed to develop on filters to slug, Mexican hat, and culminant stages; although only late slugs of each transformant are shown here, staining patterns of ecmF at other stages were principally the same as observed in Fig. 5. The cell types are Ax2::ORF+ (a), GFP::STATa(core)::ORF+ (b), and Dd-STATa−::ORF+ cells (d) transformed with pLD1-ecmF(SLF308)::lacZ; GFP::STATa(core)::ORF+ cells transformed with pLD1-0114::ecmF(SLF308)::lacZ (c), Ax2::ORF+ (e), GFP::STATa(core)::ORF+ (g), and Dd-STATa−::ORF+ (h) transformed with pLD1-cudA(pst)::lacZ; and GFP::STATa(core)::ORF+ cells transformed with pLD1-0114::cudA(pst)::lacZ (g). Cells were stained as a pooled population; the duration of staining at 37°C for the transformants was 3 h (a), 16 h (b and d), 5 h (c), 2 h (e), 48 h (f and h), and 1 h (g). (C) Comparison of aslA(SSK348) and ecmF(SLF308) gene expression levels in Ax2, GFP::STATa(core)::ORF+, 0114, 0112, and dutA-null cells as detected by semiquantitative RT-PCR. Total RNA was extracted from each population at the late slug stage and used to amplify the specific aslA(SSK348) and ecmF(SLF308) DNA fragment as described in Materials and Methods. IG7 was detected in the same reaction as a normalization control.
The Ax2::ORF+, GFP::STATa(core)::ORF+, and Dd-STATa−::ORF+ strains transformed with pLD1-ecmF(SLF308)::lacZ showed an ecmF gene expression pattern similar to the normal ecmF(SLF308)::lacZ construct (Fig. 6B, patterns a, b, and d). When the GFP::STATa(core)::ORF+ strain was transformed with pLD1-0114::ecmF(SLF308)::lacZ, the ecmF promoter was active in all prestalk cells (pstAO cells) at late slug stages, though the level of expression was slightly weaker than that observed in the Ax2::ORF+ strain (note that the staining length was different for each strain). Instead, the rear guard cells were ectopically stained (Fig. 6B, pattern c). Reversion of marker gene expression in the suppressed strain was also observed for cudA(pst)::lacZ (Fig. 6B, pattern g), although the extrachromosomal construct showed a different staining pattern from that of the normal, integrated marker construct (Fig. 5 and 6B, patterns e and f).
The above results were further tested by RT-PCR (Fig. 6C). As shown, aslA(SSK348) was up regulated slightly in the GFP::STATa(core)::ORF+ strain. This is presumably due to an increased expression in prestalk cells (Fig. 5). The expression increase in the parental strain reverted in strain 0114, in which the aslA transcript level was almost equal to that seen in Ax2 (Fig. 6C, upper panel). In strain 0112, reversion of the aslA transcript level was partial. The aslA transcript level in the dutA-null strain was also almost equal to that seen in Ax2 (Fig. 6C, upper panel). The ecmF(SLF308) transcripts were faintly detectable in the GFP::STATa(core)::ORF+ strain. In the 0114 strain, the ecmF transcript level almost equaled that seen in Ax2 (Fig. 6C, middle panel). Unexpectedly, the transcript was almost undetectable in the 0112 strain for an unknown reason. Again, the ecmF transcript level in the dutA-null strain was also almost equal to that seen in Ax2 (Fig. 6C, middle panel). Although there are some exceptions, the overall results of both β-galactosidase staining and RT-PCR show that expression of potential Dd-STATa target genes was partially reverted to wild type when the parental strain was suppressed morphologically.
Overexpression of dutA segments increased the level of tyrosine phosphorylation of the GFP::Dd-STATa(core) protein.
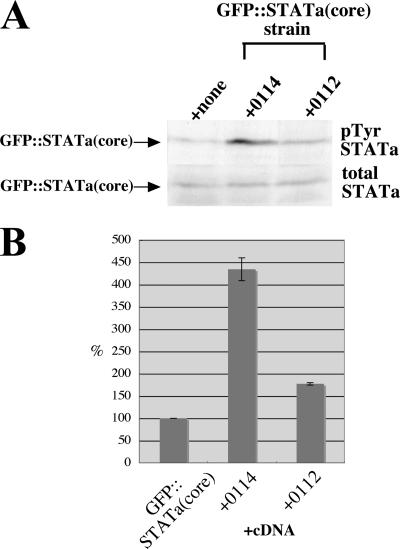
To understand how overexpression of dutA RNA segments suppressed the phenotype of the parental strain, we investigated the phosphorylation level of tyrosine residues located near the C terminus of the GFP::STATa(core) protein. The phosphorylated form of GFP::STATa(core) was detected by Western blot analysis with anti-phospho-Dd-STATa polyclonal antibody SC7 (2). Total GFP::STATa(core) protein was detected with either anti-Dd-STATa monoclonal antibody D4 or anti-GFP antibody. The level of the phosphorylated form was normalized to the total GFP::STATa(core) protein amount. In the most effective suppressor, clone 0114, the relative level of activated, tyrosine-phosphorylated GFP::STATa(core) protein increased by approximately 4.5-fold compared to that of the parental strain (Fig. 7). In the weak 0112 suppressor, the level of tyrosine phosphorylation increased by approximately twofold compared to that of the parental strain (Fig. 7).
FIG. 7.
Enhanced phosphorylation of STATa(core) protein. (A) Western blot analysis of total slug-stage proteins of various strains expressing the GFP::STATa(core) protein. Ten micrograms of protein was loaded in each gel lane and blotted onto filters. The activated form of STATa (upper panel, pTyr STATa) was detected by anti-phospho-Dd-STATa polyclonal antibody SC7; total GFP::STATa(core) protein (lower panel, total STATa) was detected by anti-GFP monoclonal antibody as described in Materials and Methods. The parental strain used for the screening, GFP::STATa(core)::ORF+, is indicated as +none. The GFP::STATa(core)::ORF+ strains expressing clones 0114 and 0112 are indicated as +0114 and +0112. Note that there is no endogenous Dd-STATa protein in the GFP::STATa(core)::ORF+ strain, as it is made from the Dd-STATa-null strain. Because the GFP::STATa(core) protein lacks the N-terminal GSK3 phosphorylation sites (13), there is no hypershifted doublet Dd-STATa band as seen for endogenous Dd-STATa protein in the Ax2 strain. (B) Quantification of phosphorylation levels. The intensity of each band from Western blotting was measured by use of the software NIH Image 1.63. Phosphorylated GFP::STATa(core) protein was normalized against total GFP::STATa(core) protein. The ratio in the parental GFP::STATa(core)::ORF+ strain was taken as 100%, and the ratio in each clone is shown graphically. STATa is phosphorylated at a tyrosine residue near the C-terminal end that is necessary for reciprocal interaction with the SH2 domain of the partner STATa protein (19).
DISCUSSION
A strain expressing N-terminal-truncated GFP::STATa(core) protein.
Expression of an N-terminal-truncated Dd-STATa fusion protein, the GFP::STATa(core) protein, in STATa-null mutant cells partially rescues the phenotype of the mutant so that it enters culmination to form fruiting bodies (Fig. 1). Unlike the Dd-STATa-null strain, cells expressing GFP::STATa(core) formed fruiting bodies when developed on buffered filters (Fig. 1B). However, the same strain barely formed fruiting bodies even after 38 h of development on non-nutrient agar plates (Fig. 3B). Such a phenotype is slightly different from that of a previously reported STATa(core) strain that formed fruiting bodies more efficiently on non-nutrient agar (47). The phenotypic difference may be attributed to the different tags used, His versus GFP, and especially to the difference in copy number of the truncated Dd-STATa gene. The GFP::STATa(core)::ORF+ strain used in this study harbors a single copy of the integrated GFP::STATa(core) gene, and the cognate protein was not overexpressed when compared to endogenous Dd-STATa protein in strain Ax2 as detected by Western blotting (data not shown).
The increased expression of aslA(SSK348) and cudA genes in the GFP::STATa(core)::ORF+ strain is likely due to increased accumulation in all prestalk and/or stalk cells (Fig. 5). Both promoters consist of complicated subfragments, one of which is reported to be a prestalk repressor element (12, 44). As a possibility, the reduction of Dd-STATa activity or truncation of the N-terminal 236 amino acids of Dd-STATa may diminish the binding of putative repressor protein(s) to the DNA, activating transcription in all prestalk or stalk cells.
Isolation of genetic suppressors for genes specific to the multicellular stages of development.
A developmentally blocked strain with integrated GFP::STATa(core) and replicase genes, GFP::STATa(core)::ORF+, was used as the parental strain for a genetic screen (Fig. 2). We have to date identified 26 putative Dd-STATa suppressor clones out of approximately 450,000 clones examined. Genetic screening as used here is a powerful method for isolating suppressors and overexpression mutants for genes specific to the multicellular stages of development. Because the complexity of the cDNA library is very large (1 × 107 independent primary E. coli colonies), further screening will identify more novel suppressor genes.
Most of the suppressor genes so far identified, including dutA, are expressed in pstA cells where STATa is specifically activated (Fig. 4B and data to be published elsewhere). This also indicates the signaling relationships between the STATa and suppressor clones (genes).
Noncoding RNA as a Dd-STATa multicopy suppressor.
It is surprising that dutA was isolated as a multicopy suppressor of Dd-STATa, because the dutA product is known as a noncoding RNA (52). The independent dutA suppressor clones identified in this study all encoded 3′ portions of the transcript. This may indicate the greater significance of the 3′ portion of the dutA RNA than the 5′ region (Fig. 4A). Clone 0114 most efficiently suppressed the parental phenotype and formed fruiting bodies as efficiently as Ax2 (Fig. 3). Yoshida and coworkers reported that the phenotype of the dutA-null mutant is morphologically normal (52). Although we need further experiments to verify a conclusion, it is likely that the high-copy suppressor fragment of dutA is acting in a dominant-negative fashion. This is based on several observations: (i) that expression of truncated dutA suppresses the phenotype of a partial-loss-of-function Dd-STATa mutant, GFP::STATa(core)::ORF+ (Fig. 3A); (ii) that truncated dutA expression does not rescue the Dd-STATa-null mutant (data not shown); (iii) that the dutA-null mutant shows normal expression of genes affected in the GFP::STATa(core)::ORF+ strain, aslA and ecmF (Fig. 6C). Unfortunately, we have been unable to make a construct encoding the full-length dutA RNA in a Ddp2-ori-based extrachromosomal vector. This failure is likely due to the extreme instability in E. coli of AT-rich sequences at the 5′ end of the dutA gene. This may be the simplest reason why all our suppressor clones contained only 3′ portions of the dutA cDNA.
The reason why the extrachromosomal cudA(pst) construct showed a different staining pattern from that of the normal, integrated marker construct in Ax2 and GFP::STATa(core)::ORF+ strains (Fig. 6B, patterns e and f) is unknown. In Ax2, staining was seen in pstA cells rather than tip cells (Fig. 6B, pattern e), while there were only a few scattered cells staining, rather than strong prestalk staining, in the GFP::STATa(core)::ORF+ strain. There was also a high background in the prespore zone (Fig. 6B, pattern f). Perhaps a particular chromatin structure is necessary for an accurate expression pattern of the cudA gene.
As shown in Fig. 3 and 4A, the difference between clones 0114 and 0112 is a 98-nucleotide region, and 0114 suppressed the deficiency in fruiting approximately threefold better than clone 0112 did. Both 0114 and full-length dutA RNAs show similar predicted secondary structures for the region in common, but 0112 RNA has a slightly different structure (data not shown). Therefore, the distinguishing 98-nucleotide region, which includes the 5′ GC-rich sequence of the dutA RNA (52), could be important for secondary structure and perhaps exhibits dutA function. Although 1301 RNA encompasses the entire region of 0114 RNA, its suppression efficiency was very low (Fig. 3 and 4). The presence of extra RNA 3′ sequence in 1301 causes its predicted secondary structure to differ from that of 0114 (data not shown), and this may be the reason for the low suppression efficiency.
Can dutA RNA act as a precursor of miRNA or small interfering RNA?
The fact that the dutA RNA is a known ncRNA raised the possibility that it may act as a precursor of micro RNA (miRNA) or small interfering RNA. A relationship between ncRNA and STAT signaling has been reported in Arabidopsis thaliana, where Scarecrow-like family mRNAs encoding putative STAT homologues are cleaved by miRNA 39 (miR171) (27). In Dictyostelium, two Dicer homologue proteins, DrnA and DrnB (31, 38), are present, and RNA interference techniques have been reported (22, 31, 38). There are also many RNA helicase genes, of which at least two, helC and helF, are important for normal development (28, 38). Although these observations support the possibility of dutA being a precursor RNA as described above, it is supposed to be difficult to detect small RNAs derived from dutA RNA because all possible loop structures are extremely AU rich. Therefore, we await genetic dissection of the Dictyostelium RNA interference pathway in considering dutA function.
Similarities of dutA RNA to other ncRNA.
The dutA transcript is 1,322 nucleotides long, which is larger than most miRNA precursors. Human SRA RNA has a relatively similar 875-nucleotide length, is known to interact with the progestin steroid receptor, and serves as a coactivator of transcription (25). Human 7SK RNA, 331 nucleotides long, binds and inhibits the transcriptional elongation factor P-TEFb (34, 51). Because a relatively large region including the GC-rich sequence region is important for suppression, the dutA RNA could have a similar function to regulate the expression of a wide range of genes at the transcriptional level. It has also been reported that an RNA molecule, TSU, binds to a STAT1 dimer and suppresses STAT1-induced major histocompatibility complex genes by preventing STAT1 nuclear localization (36). Several transcription factors, including STAT1, are reported to bind both DNA and RNA, raising the possibility of regulation of transcription factors by RNA binding (4). There is also the possibility that the dutA RNA is a natural antisense transcript and regulates expression of other genes in trans (26).
Mechanism of suppression by dutA overexpression.
The mechanism of suppression by dutA RNA largely remains to be elucidated. Many genes involving cAMP signaling at multicellular stages are expressed in pstA cells at the slug stage (49), corresponding to the increased tyrosine phosphorylation level of the GFP::STATa(core) protein in the suppressed strains (Fig. 7). Thus, suppression may act through the increase of cAMP signaling activity at the slug stage. There could be changes in the activities of adenylyl cyclase (ACA, ACR, and ACG), phosphodiesterase (including PdeA and RegA), and RdeA. However, at least for three genes which are known to regulate Dd-STATa signaling, regA, pdeA, and acaA, expression levels in suppressed and parental strains, as well as in Ax2, were constant as measured by RT-PCR (data not shown).
An increase of cAMP is unlikely, as the level of total cAMP at the slug stage was constant among the suppressed clones and parental and wild-type strains (data not shown). This may indicate that some other pathway is regulating the level of GFP::STATa(core) phosphorylation. Such a pathway may explain why the suppressed 0114 clone showed enhanced nuclear localization of the GFP::STATa(core) protein (data not shown) and increased expression of putative Dd-STATa target genes in rear guard cells, where the endogenous dutA gene is inactive. dutA RNA might regulate bypath signaling independent of cAMP and/or activate a yet-unidentified tyrosine kinase which would then activate Dd-STATa (Fig. 8). In support of this hypothesis, Okamoto and coworkers reported that the expression of dutA is independent of cAMP pulses but requires cAMP-dependent protein kinase A (PKA) (23); PKA is also required for the repression of ecmB in pstA cells by Dd-STATa, an essential feature of normal culmination (15, 16, 19, 32). However, we cannot rule out the possibility that the constant cAMP level in major cell types masks an increased level of cAMP in the tip cells, where Dd-STATa protein and endogenous dutA gene expression are activated but the cell number is very restricted.
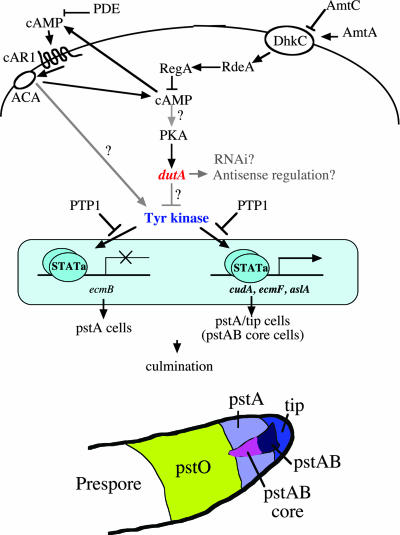
FIG. 8.
Model of Dd-STATa suppression by dutA overexpression. Overexpression of dutA RNA reverts the expression of various potential Dd-STATa target genes, including cudA (12), ecmF (42), and aslA (44), in the parental strain, GFP::STATa(core)::ORF+. In Ax2, ecmF and cudA are expressed in the pstA/tip cells, whereas the aslA gene is expressed in the pstAB core cells. The expression of the ecmB gene is repressed in the pstA and tip cells by Dd-STATa (32). Because patterns of cudA(pst) expression are complicated, we have not shown the data; nevertheless, cudA(pst)::lacZ expression is clearly influenced by dutA overexpression. We have not yet proved that the expression of the ecmB gene is influenced by dutA overexpression. Adenylyl cyclase (ACA), phosphodiesterase (PDE), and RegA regulate the level of cAMP. Although PKA is activated by cAMP and dutA by PKA, dutA expression is independent of cAMP pulses (23). Ammonium transporter C (AmtC) regulates culmination by repressing histidine kinase (DhkC), which otherwise activates RegA via RdeA (21). Inversely, AmtA may promote phosphorelay in response to high ammonia levels (45). Dd-STATa is activated by elevation of extracellular cAMP and the signal relayed through the cAMP receptor cAR1 (2, 50). The protein tyrosine phosphatase PTP1 is thought to negatively regulate Dd-STATa (9). The tyrosine kinase which activates Dd-STATa has yet to be identified. Most likely, overexpression of the dutA RNA regulates the activity of the tyrosine kinase but could work as a regulatory RNA at other points in the pathway. The effect of overexpression of truncated dutA may be dominant negative, and endogenous dutA could repress Dd-STATa activity. Subtypes of prestalk cells are illustrated at the bottom.
In summary, this study shows that ncRNAs are likely to have significant roles during Dictyostelium development and in particular in regulating STATa. It also demonstrates the utility of using multicopy suppression to identify genes important in multicellular development in Dictyostelium.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science (JSPS) to T. Kawata (no. 17570190) and by a Grant-in-Aid for JSPS Fellowships for Young Scientists to N. Shimada (no. 17.2751). N.S. is a JSTS Research Fellow. This work was also partly supported by The Yamada Foundation to T.K.
We are indebted to Douglas N. Robinson of The Johns Hopkins University for his helpful advice concerning the genetic screening method and for providing plasmids, Hidekazu Kuwayama of the University of Tsukuba for quantification of cAMP, and Kakeru Minemura for help with the screening. We also thank Masashi Fukuzawa of Hirosaki University and Mineko Maeda of Osaka University for plasmids, Koji Okamoto of Kyoto University for the dutA-null strain, and Tsuyoshi Araki and Jeffrey G. Williams, University of Dundee, United Kingdom, for providing anti-Dd-STATa antibodies. We are grateful to Jeffrey G. Williams, Douglas N. Robinson, Kei Inouye of Kyoto University, and Hidekazu Kuwayama for their valuable comments on the manuscript and to David Ratner, Amherst College, for proofreading the manuscript.
Footnotes
Published ahead of print on 13 April 2007.
REFERENCES
- 1.Aoshima, R., R. Hiraoka, N. Shimada, and T. Kawata. 2006. Analysis of a homologue of the adducin head gene which is a potential target for the Dictyostelium STAT protein Dd-STATa. Int. J. Dev. Biol. 50:523-532. [DOI] [PubMed] [Google Scholar]
- 2.Araki, T., M. Gamper, A. Early, M. Fukuzawa, T. Abe, T. Kawata, E. Kim, R. A. Firtel, and J. G. Williams. 1998. Developmentally and spatially regulated activation of a Dictyostelium STAT protein by a serpentine receptor. EMBO J. 17:4018-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspegren, A., A. Hinas, P. Larsson, A. Larsson, and F. Soderbom. 2004. Novel non-coding RNAs in Dictyostelium discoideum and their expression during development. Nucleic Acids Res. 32:4646-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassiday, L. A., and L. J. Maher III. 2002. Having it both: transcription factors that bind DNA and RNA. Nucleic Acids Res. 30:4118-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, A. C. M., M. B. Slade, and K. L. Williams. 1990. Identification of the origin of replication of the eukaryote Dictyostelium discoideum nuclear plasmid Ddp2. Plasmid 24:208-217. [DOI] [PubMed] [Google Scholar]
- 6.Darnell, J. E., Jr. 1997. STATs and gene regulation. Science 277:1630-1635. [DOI] [PubMed] [Google Scholar]
- 7.Dingermann, T., N. Reindl, H. Werner, M. Hildebrandt, W. Nellen, A. Harwood, J. Williams, and K. Nerke. 1989. Optimization and in situ detection of Escherichia coli β-galactosidase gene expression in Dictyostelium discoideum. Gene 85:353-362. [DOI] [PubMed] [Google Scholar]
- 8.Dynes, J. L., A. M. Clark, G. Shaulsky, A. Kuspa, W. F. Loomis, and R. A. Firtel. 1994. LagC is required for cell-cell interactions that are essential for cell-type differentiation in Dictyostelium. Genes Dev. 8:948-958. [DOI] [PubMed] [Google Scholar]
- 9.Early, A., M. Gamper, J. Moniakis, E. Kim, T. Hunter, J. G. Williams, and R. A. Firtel. 2001. Protein tyrosine phosphatase PTP1 negatively regulates Dictyostelium STATa and is required for proper cell-type proportioning. Dev. Biol. 232:233-245. [DOI] [PubMed] [Google Scholar]
- 10.Eddy, S. R. 2001. Non-coding RNA genes and the modern RNA world. Nat. Rev. Genet. 2:919-929. [DOI] [PubMed] [Google Scholar]
- 11.Escalante, R., and W. F. Loomis. 1995. Whole-mount in situ hybridization of cell-type-specific mRNAs in Dictyostelium. Dev. Biol. 43:262-266. [DOI] [PubMed] [Google Scholar]
- 12.Fukuzawa, M., and J. G. Williams. 2000. Analysis of the promoter of the cudA gene reveals novel mechanisms of Dictyostelium cell type differentiation. Development 127:2705-2713. [DOI] [PubMed] [Google Scholar]
- 13.Ginger, R. S., E. C. Dalton, W. J. Ryves, M. Fukuzawa, J. G. Williams, and A. J. Harwood. 2000. Glycogen synthase kinase-3 enhances nuclear export of a Dictyostelium STAT protein. EMBO J. 19:5483-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girard, K. D., C. Chaney, M. Delannoy, S. C. Kuo, and D. N. Robinson. 2004. Dynacortin contributes to cortical viscoelasticity and helps define the shape changes of cytokinesis. EMBO J. 23:1536-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harwood, A. J., N. A. Hopper, M. N. Simon, D. M. Driscoll, M. Veron, and J. G. Williams. 1992. Culmination in Dictyostelium is regulated by the cAMP-dependent protein kinase. Cell 69:615-624. [DOI] [PubMed] [Google Scholar]
- 16.Harwood, A. J., A. Early, and J. G. Williams. 1993. A repressor controls the timing and spatial localisation of stalk cell-specific gene expression in Dictyostelium. Development 118:1041-1048. [DOI] [PubMed] [Google Scholar]
- 17.Hildebrandt, M., and W. Nellen. 1992. Differential antisense transcription from the Dictyostelium EB4 gene locus: implications on antisense-mediated regulation of mRNA stability. Cell 69:197-204. [DOI] [PubMed] [Google Scholar]
- 18.Hinas, A., P. Larsson, L. Avesson, L. A. Kirsebom, A. Virtanen, and F. Soderbom. 2006. Identification of the major spliceosomal RNAs in Dictyostelium discoideum reveals developmentally regulated U2 variants and polyadenylated snRNAs. Eukaryot. Cell 5:924-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawata, T., A. Shevchenko, M. Fukuzawa, K. A. Jermyn, N. F. Totty, N. V. Zhukovskaya, A. E. Sterling, M. Mann, and J. G. Williams. 1997. SH2 signaling in a lower eukaryote: a STAT protein that regulates stalk cell differentiation in Dictyostelium. Cell 89:909-916. [DOI] [PubMed] [Google Scholar]
- 20.Kay, R. R. 1997. Dictyostelium development: lower STATs. Curr. Biol. 7:R723-R725. [DOI] [PubMed] [Google Scholar]
- 21.Kirsten, J. H., Y. Xiong, A. J. Dunbar, M. Rai, and C. K. Singleton. 2005. Ammonium transporter C of Dictyostelium discoideum is required for correct prestalk gene expression and for regulating the choice between slug migration and culmination. Dev. Biol. 287:146-156. [DOI] [PubMed] [Google Scholar]
- 22.Kuhlmann, M., B. E. Borisova, M. Kaller, P. Larsson, D. Stach, J. Na, L. Eichinger, F. Lyko, V. Ambros, F. Söderbom, C. Hammann, and W. Nellen. 2005. Silencing of retrotransposons in Dictyostelium by DNA methylation and RNAi. Nucleic Acids Res. 33:6405-6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumimoto, H., H. Yoshida, and K. Okamoto. 1996. Expression of Dictyostelium early gene, dutA, is independent of cAMP pulses but dependent on protein kinase A. FEMS Microbiol. Lett. 140:121-124. [DOI] [PubMed] [Google Scholar]
- 24.Kuspa, A., and W. F. Loomis. 1992. Tagging developmental genes in Dictyostelium by restriction enzyme-mediated integration of plasmid DNA. Proc. Natl. Acad. Sci. USA 89:8803-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanz, R. B., N. J. McKenna, S. A. Onate, U. Albrecht, J. Wong, S. Y. Tsai, M.-J. Tsai, and B. W. O'Malley. 1999. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell 97:17-27. [DOI] [PubMed] [Google Scholar]
- 26.Lavorgna, G., D. Dahary, B. Lehner, R. Sorek, C. M. Sanderson, and G. Casari. 2004. In search of antisense. Trends Biochem. Sci. 29:88-94. [DOI] [PubMed] [Google Scholar]
- 27.Llave, C., Z. Xie, K. D. Kasschau, and J. C. Carrington. 2002. Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 297:2053-2056. [DOI] [PubMed] [Google Scholar]
- 28.Machesky, L. M., R. H. Insall, and R. R. Kay. 1998. The helC gene encodes a putative DEAD-box RNA helicase required for development in Dictyostelium discoideum. Curr. Biol. 8:607-610. [DOI] [PubMed] [Google Scholar]
- 29.Maeda, M., H. Kuwayama, M. Yokoyama, K. Nishio, T. Morio, H. Urushihara, M. Katoh, Y. Tanaka, T. Saito, H. Ochiai, K. Takemoto, H. Yasukawa, and I. Takeuchi. 2000. Developmental changes in the spatial expression of genes involved in myosin function in Dictyostelium. Dev. Biol. 43:114-119. [DOI] [PubMed] [Google Scholar]
- 30.Maeda, M., H. Sakamoto, T. Maruo, S. Ogihara, N. Iranfar, D. Fuller, T. Morio, H. Urushihara, Y. Tanaka, and W. F. Loomis. 2003. Changing patterns of gene expression in prestalk cell subtypes of Dictyostelium recognised by in situ hybridization with genes from microarray analyses. Eukaryot. Cell 2:627-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martens, H., J. Novotny, J. Oberstrass, T. L. Steck, P. Postlethwait, and W. Nellen. 2002. RNAi in Dictyostelium: the role of RNA-directed RNA polymerases and double-stranded RNase. Mol. Biol. Cell 13:445-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohanty, S., K. A. Jermyn, A. Early, T. Kawata, L. Aubry, A. Ceccarelli, P. Schaap, J. G. Williams, and R. A. Firtel. 1999. Evidence that the Dictyostelium Dd-STATa protein is a repressor that regulates commitment to stalk cell differentiation and is also required for efficient chemotaxis. Development 126:3391-3405. [DOI] [PubMed] [Google Scholar]
- 33.Mui, A. L.-F. 1999. The role of STATs in proliferation, differentiation, and apoptosis. Cell. Mol. Life Sci. 55:1547-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen, V. T., T. Kiss, A. A. Michels, and O. Bensaude. 2001. 7SK small nuclear RNA binds to and inhibits the activity of CDK9/cyclin T complexes. Nature 414:322-325. [DOI] [PubMed] [Google Scholar]
- 35.Octtaviani, E., J. C. Effler, and D. N. Robinson. 2006. Enlazin, a natural fusion of two classes of canonical cytoskeletal proteins, contributes to cytokinesis dynamics. Mol. Biol. Cell 17:5275-5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peyman, J. A. 1999. Repression of major histocompatibility complex genes by a human trophoblast ribonucleic acid. Biol. Reprod. 60:23-31. [DOI] [PubMed] [Google Scholar]
- 37.Pi, M., T. Morio, H. Urushihara, and Y. Tanaka. 1998. Characterization of a novel small RNA encoded by Dictyostelium discoideum mitochondrial DNA. Mol. Gen. Genet. 257:124-131. [DOI] [PubMed] [Google Scholar]
- 38.Popova, B., M. Kuhlmann, A. Hinas, F. Soderbom, and W. Nellen. 2006. HelF, a putative RNA helicase acts as a nuclear suppressor of RNAi but not antisense mediated gene silencing. Nucleic Acids Res. 34:773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prelich, G. 1999. Suppression mechanisms: themes from variations. Trends Genet. 15:261-266. [DOI] [PubMed] [Google Scholar]
- 40.Robinson, D. N., and J. A. Spudich. 2000. Dynacortin, a genetic link between equatorial contractility and global shape control discovered by library complementation of a Dictyostelium discoideum cytokinesis mutant. J. Cell Biol. 150:823-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaulsky, G., R. Escalante, and W. F. Loomis. 1996. Developmental signal transduction pathways uncovered by genetic suppressors. Proc. Natl. Acad. Sci. USA 92:5660-5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimada, N., K. Nishio, M. Maeda, H. Urushihara, and T. Kawata. 2004. Extracellular matrix family proteins that are potential targets of Dd-STATa in Dictyostelium discoideum. J. Plant Res. 117:345-353. [DOI] [PubMed] [Google Scholar]
- 43.Shimada, N., M. Maeda, H. Urushihara, and T. Kawata. 2004. Identification of new modes of Dd-STATa regulation of gene expression in Dictyostelium by in situ hybridisation. Int. J. Dev. Biol. 48:679-682. [DOI] [PubMed] [Google Scholar]
- 44.Shimada, N., T. Maruo, M. Maeda, H. Urushihara, and T. Kawata. 2005. Evidence that the Dictyostelium STAT protein Dd-STATa plays a role in the differentiation of inner basal disc cells and identification of a promoter element essential for expression in these cells. Differentiation 73:50-60. [DOI] [PubMed] [Google Scholar]
- 45.Singleton, C. K., J. H. Kirsten, and C. Dinsmore. 2006. Function of ammonium transporter A in the initiation of culmination of development in Dictyostelium discoideum. Eukaryot. Cell 5:991-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slade, M. B., A. C. M. Chang, and K. L. Williams. 1990. The sequence and organization of Ddp2, a high-copy-number nuclear plasmid of Dictyostelium discoideum. Plasmid 24:197-207. [DOI] [PubMed] [Google Scholar]
- 47.Soler-Lopez, M., C. Petosa, M. Fukuzawa, R. Ravelli, J. G. Williams, and C. W. Muller. 2004. Structure of an activated Dictyostelium STAT in its DNA-unbound form. Mol. Cell 13:791-804. [DOI] [PubMed] [Google Scholar]
- 48.Storz, G. 2002. An expanding universe of noncoding RNAs. Science 296:1260-1263. [DOI] [PubMed] [Google Scholar]
- 49.Tsujioka, M., M. Yokoyama, K. Nishio, H. Kuwayama, T. Morio, M. Katoh, H. Urushihara, T. Saito, H. Ochiai, Y. Tanaka, I. Takeuchi, and M. Maeda. 2001. Spatial expression patterns of genes involved in cyclic AMP responses in Dictyostelium discoideum development. Dev. Growth Differ. 43:275-283. [DOI] [PubMed] [Google Scholar]
- 50.Williams, J. G. 2006. Transcriptional regulation of Dictyostelium pattern formation. EMBO Rep. 7:694-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang, Z., Q. Zhu, K. Luo, and Q. Zhou. 2001. The 7SK small nuclear RNA inhibits the CDK9/cyclin T1 kinase to control transcription. Nature 414:317-322. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida, H., H. Kumimoto, and K. Okamoto. 1994. dutA RNA functions as an untranslatable RNA in the development of Dictyostelium discoideum. Nucleic Acids Res. 22:41-46. [DOI] [PMC free article] [PubMed] [Google Scholar]