Abstract
The nucleoporin Nup124p is a host protein required for the nuclear import of both, retrotransposon Tf1-Gag as well as the retroviral HIV-1 Vpr in fission yeast. The human nucleoporin Nup153 and the Saccharomyces cerevisiae Nup1p were identified as orthologs of Nup124p. In this study, we show that all three nucleoporins share a large FG/FXFG-repeat domain and a C-terminal peptide sequence, GRKIxxxxxRRKx, that are absolutely essential for Tf1 retrotransposition. Though the FXFG domain was essential, the FXFG repeats themselves could be eliminated without loss of retrotransposon activity, suggesting the existence of a common element unrelated to FG/FXFG motifs. The Nup124p C-terminal peptide, GRKIAVPRSRRKR, was extremely sensitive to certain single amino acid changes within stretches of the basic residues. On the basis of our comparative study of Nup124p, Nup1p, and Nup153 domains, we have developed peptides that specifically knockdown retrotransposon activity by disengaging the Tf1-Gag from its host nuclear transport machinery without any harmful consequence to the host itself. Our results imply that those domains challenged a specific pathway affecting Tf1 transposition. Although full-length Nup1p or Nup153 does not complement Nup124p, the functionality of their conserved domains with reference to Tf1 activity suggests that these three proteins evolved from a common ancestor.
INTRODUCTION
The propagation of Tf1, a long terminal repeat (LTR)-containing retrotransposon in the fission yeast Schizosaccharomyces pombe (Levin et al., 1990; Levin and Boeke, 1992) is known to require many of the same processes used by retroviruses to complete its life cycle within the host cell. Yeast retrotransposons can therefore serve as very useful models for the replication of retroviruses (Levin et al., 1993; Irwin et al., 2005; Kelly and Levin, 2005). The full-length transcript of the Tf1 retroelement encodes gag, protease (PR), reverse transcriptase (RT), and integrase (IN) proteins that assemble along with copies of RNA to form 50-nm virus-like particles (VLPs; Levin et al., 1993; Haag et al., 2000; Teysset et al., 2003). To integrate its genome into the target cell, it is assumed that Tf1-VLPs move to the nucleus and are transported through the central channel of the nuclear pore complex (NPC). In fact, Tf1 particles were observed within the nucleus associated with heterochromatin (Teysset et al., 2003). Though details of the nuclear import mechanism remain unclear, there is substantial evidence that both Tf1-Gag and the NPC nucleoporin Nup124p are key determinants of this process (Atwood et al., 1996; Balasundaram et al., 1999; Dang and Levin, 2000; Teysset et al., 2003; Kim et al., 2005; Varadarajan et al., 2005). Similarly, NPC elements (including nucleoporins) governing the nuclear entry of the retrotransposon Ty3 cDNA in Saccharomyces cerevisiae had either a positive or negative impact on its replication (transposition), implying the crucial role of the nuclear pore in regulating retroviral activity (Irwin et al., 2005). Although an NLS required for nuclear entry of Ty1 and Ty3 was identified within the C-terminal domain of their respective integrases (Kenna et al., 1998; Lin et al., 2001), a possible interaction of Gag3 with the NPC was suggested with Ty3 (Irwin et al., 2005).
The NPC is a large structure embedded in the double membrane of the nuclear envelope, with a molecular weight of approximately 125 MDa in vertebrates and 66 MDa in yeast (Fahrenkrog and Aebi, 2003; Antonin and Mattaj, 2005). Despite differences in both size and composition, yeast and vertebrate NPCs have a number of common features including their general overall structure, protein composition, and mechanism of nuclear transport (Fabre and Hurt, 1997; Yang et al., 1998; Stoffler et al., 1999; Rout et al., 2000; Ryan and Wente, 2000; Wente, 2000; Cronshaw et al., 2002; Suntharalingam and Wente, 2003; Kiseleva et al., 2004; Devos et al., 2006; Tran and Wente, 2006). Fission yeast has NPC features and transport factors comparable to S. cerevisiae and vertebrate systems and the appropriate tools to study these features are now available (Ding et al., 2000; Chen et al., 2004; Kiseleva et al., 2004; Yoshida and Sazer, 2004). In general, the bulk of macromolecular traffic through the NPC is mediated by a system of mobile transporter proteins, multiple importins α and β and the nucleoporins (Nups), the latter, a unique and conserved group of structural proteins of the pore complex. The most highly conserved feature between yeast and mammalian nucleoporins is a dipeptide repeat motif, in the form phenylalanine-glycine (FG), glycine-leucine-phenylalanine-glycine (GLFG), or phenylalanine-X (any amino acid)-phenylalanine-glycine (FXFG), present in approximately one-third of all core components of the pore (Rout and Aitchison, 2000; Rout et al., 2000; Cronshaw et al., 2002). Many NPC-binding sites for importins/exportins and NTF2 carriers are supplied by these FG-repeat–containing Nups (Ryan and Wente, 2000). Although the presence of FG repeats is a feature shared by many Nups, the intervening sequences between FG repeats differ drastically among them. What contribution, if any, the surrounding amino acids make to the FG-binding sites is unclear (Cushman et al., 2006). One possibility is that FG-Nups may form a meshwork within the central channel of the pore where they are involved in translocation (Ribbeck and Gorlich, 2001). However, the essential functionality of the FG/FXFG/GLFG repeats in Nups is in question because most asymmetrically localized Nups (either to the cytoplasmic or nucleoplasmic faces of the NPC) may be eliminated without loss of viability (Strawn et al., 2004). FG/GLFG/FXFG-repeat–containing Nups are known to bind HEAT repeats on importin β (Bayliss et al., 2002b). Kap95, an importin β, is an essential protein (Chen et al., 2004), and there are two importin α type 1, Imp1p and Cut15p, both of which have partially overlapping and distinct functions in S. pombe (Umeda et al., 2005). Both Cut15p and Imp1p were able to import classical NLS-containing substrates in vivo and in vitro into the nucleus, implying that each is an authentic importin α with possibly common binding partners (Umeda et al., 2005). There is compelling in vivo evidence that interaction between importin α and FG Nups may be the basis for the latter to either concentrate carrier-cargo complexes at the NPC entrance and/or participate in a sequence of docking and undocking interactions as the carrier-cargo complexes transit through NPCs (Stewart et al., 2001).
We had previously demonstrated that the fission yeast Nup124p, a nonessential FG repeat–containing nucleoporin was a host factor required for Tf1 transposition (Balasundaram et al., 1999; Varadarajan et al., 2005). We also predicted that such a function was a unique one, because no housekeeping functions were disrupted in the absence of Nup124p (Balasundaram et al., 1999). Indeed, nuclear import of Tf1-Gag was impeded, and Tf1 transposition was markedly reduced in the nup124 null mutant (Balasundaram et al., 1999; Varadarajan et al., 2005), despite the presence of at least eight other FG-nucleoporins (Denning and Rexach, 2007). Furthermore, the N-terminus contains a putative Tf1-Gag–binding domain and the C-terminus of Nup124p contains the FXFG domain essential for retrotransposition but not for localization of either Nup124p or Tf1-Gag to the NPC, reiterating the fact that Nup124p possessed special features required for the nuclear import/translocation of the retrotransposon Tf1 (Tf1-Gag; Balasundaram et al., 1999; Dang and Levin, 2000; Varadarajan et al., 2005). In this report, we have investigated the mechanism of Nup124p-mediated Tf1 import into the nucleus. We have studied the regions at the C-terminus of Nup124p required for Tf1 transposition by mapping two key domains, the FXFG-repeat domain and a well-conserved C-terminal peptide, and identified key residues within a well-conserved C-terminal peptide. We present evidence to show that though the FXFG repeats themselves are redundant, the FXFG domain and a conserved C-terminal motif GRKIxxxxxRRKx of Nup124p and those of its orthologs, Nup1p and Nup153, play a critical and unique role in Tf1 activity. We also show that Nup124p interacts with key components of the fission yeast nuclear transport system, Imp1p and Cut15p (importin α) and Kap95 (importin β), in addition to Tf1-Gag. Finally, we demonstrate that overexpressing the conserved domains of Nup124p selectively knocks down Tf1 activity by preventing Tf1-Gag from being imported into the nucleus without affecting the growth of the host cell, making elements of the nuclear pore complex an attractive option for the development of antiviral strategies.
MATERIALS AND METHODS
Yeast Strains and Media
All strains are listed in Table 1. The S. pombe minimal liquid and plate media were composed of EMM (Balasundaram et al., 1999). Strain YNB19, h-ura4-294 leu 1-32 his3Δ nup124::HIS3 ade6-M216 and YNB16, h-leu1-32 ura4-294 were the principal yeast strains used in this study and are referred to in the text as null mutant (Δnup124) and wild type (WT), respectively. YNB19 and YNB16 were transformed with various plasmids described in Table 2(see also Supplementary Table S1). Transformants were routinely propagated in EMM −ura − leu + 15 μM thiamine. When expression of the desired sequence was required, strains were grown in EMM −ura −leu without thiamine to induce the nmt1 (strong) or nmt81 (weak) promoter (nmt, no messsage in thiamine).
Table 1.
Yeast strains
| Strain (YNB) | Parent strain/source | Plasmid |
|||
|---|---|---|---|---|---|
| Ura+ |
Leu+ |
||||
| No. | Character | No. | Character | ||
| 10 | YHL 4990 | pHL490 | PROTfs (frameshift mutation in Tf1-protease) | pSP1 | Empty vector |
| 11 | YHL 4992 | pHL476 | Infs (frameshift mutation in Tf1-integrasen) | pSP1 | Empty vector |
| 16 | YHL 912 | — | — | ||
| 19 | YHL 7143 | — | — | ||
| 58 | YNB 19 | pHL449-1 | Tf1 reporter: neoAI-marked version of nmt1:Tf1 in an ura4 selectable marker | pBNB 12 | 3HA:Nup124 |
| 59 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 31 | 3HA:nup124ΔAA571-615 |
| 65 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 21 | 3HA:nup124ΔAA1089-1111 |
| 235 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 103 | 3HA:nup124ΔAA828-967 |
| 236 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 105 | 3HA:nup124ΔAA998-1026 |
| 350 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 136 | 3HA:nup124 R1154A |
| 352 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 139 | 3HA:nup124 R1156A |
| 354 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 142 | 3HA:nup124 R1157A |
| 356 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 148 | 3HA:nup124 K1158A |
| 357 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 149 | 3HA:nup124 R1159A |
| 549 | YNB 19 | BNB 193 | Tf1-Gag:YFP | pBNB 12 | 3HA:nup124 |
| 550 | YNB 19 | BNB 193 | Tf1-Gag:YFP | pBNB 155 | 3HA:nup124AA1154RSRRKR1159-ASAAAA |
| 552 | YNB 19 | BNB 193 | Tf1-Gag:YFP | pBNB 93 | 3HA:nup124-1 |
| 553 | YNB 19 | BNB 193 | Tf1-Gag:YFP | pBNB 117 | 3HA:nup124ΔAA571-1111 |
| 554 | YNB 19 | BNB 193 | Tf1-Gag:YFP | pBNB 119 | 3HA:nup124Δ111-331 |
| 893 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB418 | nmt81:empty vector |
| 967 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 492 | nmt81:nup153 |
| 1017 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 572 | nmt81:nup1 |
| 1018 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 524 | 3HA:nup124 G1147S |
| 1020 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 526 | 3HA:nup124 R1148A |
| 1028 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 552 | 3HA:nup124ΔAA1151-1155:nup153AA1468-1472 |
| 1030 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 554 | 3HA:nup124ΔAA1156-1159:nup153AA1473-75 |
| 1032 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 556 | 3HA:nup124ΔAA1151-1155 |
| 1034 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 558 | 3HA:nup124ΔAA1156-57 |
| 1040 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 531 | 3HA:nup124 I1150A |
| 1042 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 532 | 3HA:nup124Δ1147-1150 |
| 1044 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 541 | 3HA:nup124 K1158A R1159A |
| 1046 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 542 | 3HA:nup124 AA1157-59A |
| 1048 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 544 | 3HA:nup124 AA1156-59A |
| 1050 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 533 | 3HA:nup124 ΔAA 1147-1159 |
| 1052 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 535 | 3HA:nup124ΔAA 571-1146 |
| 1054 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 539 | 3HA:nup124 ΔAA1147-1159:nup153 AA 1464-1475 |
| 1058 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 550 | 3HA:nup124 ΔAA1147-1159:nup1AA1065-1076 |
| 1062 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 601 | 3HA:nup124 ΔAA1147-1159:nup1AA1065-1076 +R |
| 1064 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 573 | nmt81:nup124 |
| 1066 | YNB 16 | pHL449-1 | Tf1-reporter (see above) | pBNB 579 | nmt1:GFP:GRKIAVPRSRRKR |
| 1070 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 610 | 3HA:nup124 AA1156-57AA |
| 1086 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 623 | nmt81:nup124Δ AA828-967 |
| 1096 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 652 | nmt81: nup124 ΔAA570-1146:nup153 AA 903-1463 |
| 1098 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 664 | nmt81:nup1AA1-1076+R |
| 1117 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 672 | nmt81:nup124 ΔAA571-1146:nup1 AA 406-1065 |
| 1121 | YNB 16 | pHL449-1 | Tf1-reporter (see above) | pBNB 647 | nmt1:nup124 AA 571-1111 |
| 1125 | YNB 16 | pHL449-1 | Tf1-reporter (see above) | pBNB 649 | nmt1:nup153 AA 903-1418 |
| 1140 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 640 | Nup124ΔAA828-967+ AA571-574,612-615, 998-1001, 1023-026,1089-1092, 1108-1111-AAAA |
| 1141 | YNB 16 | pHL449-1 | Tf1-reporter (see above) | pBNB 696 | nmt1:nup1 AA 406-988 |
| 1189 | YNB 16 | BNB 271 | pREP82 | pBNB 736 | nmt1:RFP:YAHSDATMVCMFS |
| 1191 | YNB 16 | BNB 193 | Tf1-Gag:YFP | pBNB 736 | nmt1:RFP:YAHSDATMVCMFS |
| 1197 | YNB 16 | BNB 271 | pREP82 | pBNB 671 | nmt1:RFP:GRKIAVPRSRRKR |
| 1198 | YNB 16 | BNB 193 | Tf1-Gag:YFP | pBNB 671 | nmt1:RFP:GRKIAVPRSRRKR |
| 1201 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 725 | nmt81: nup124ΔAA571-1146:nup120 AA517-1137 |
| 1205 | YNB 16 | pHL449-1 | Tf1-reporter (see above) | pBNB 699 | nmt1:nup120AA 517-1137 |
| 1209 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 527 | 3HA:nup124 K1149A |
| 1211 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 546 | 3HA:nup124 AA1155-59A |
| 1213 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 548 | 3HA:nup124 AA1154-59A |
| 1215 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 577 | 3HA:nup124 ΔAA1147-1159:nup153 AA 1464-1475 + R |
| 1217 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 564 | nmt81:nup153 Δ AA1468-1475:nup124 AA 1147-1159 |
| 1219 | YNB 16 | pHL449-1 | Tf1-reporter (see above) | pBNB 736 | nmt1:RFP:YAHSDATMVCMFS |
| 1221 | YNB 16 | BNB 193 | Tf1-Gag:YFP | pBNB 776 | nmt1:RFP:nup120AA517-1137 |
| 1223 | YNB 16 | BNB 193 | Tf1-Gag:YFP | pBNB 778 | nmt1:RFP:nup124 AA571-1111 |
| 1227 | YNB 16 | — | pBNB 773 | nmt1:GRKIAVPRSRRKR:GFP-LacZ | |
| 1205 | YNB 16 | pHL449-1 | Tf1-reporter (see above) | pBNB 699 | nmt1:nup120AA 517-1137 |
| 1209 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 527 | 3HA:nup124 K1149A |
| 1211 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 546 | 3HA:nup124 AA1155-59A |
| 1213 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 548 | 3HA:nup124 AA1154-59A |
| 1215 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 577 | 3HA:nup124 ΔAA1147-1159:nup153 AA 1464-1475 + R |
| 1217 | YNB 19 | pHL449-1 | Tf1-reporter (see above) | pBNB 564 | nmt81:nup153 Δ AA1468-1475:nup124 AA 1147-1159 |
| 1219 | YNB 16 | pHL449-1 | Tf1-reporter (see above) | pBNB 736 | nmt1:RFP:YAHSDATMVCMFS |
| 1221 | YNB 16 | BNB 193 | Tf1-Gag:YFP | pBNB 776 | nmt1:RFP:nup120AA517-1137 |
| 1223 | YNB 16 | BNB 193 | Tf1-Gag:YFP | pBNB 778 | nmt1:RFP:nup124 AA571-1111 |
| 1227 | YNB 16 | — | pBNB 773 | nmt1:GRKIAVPRSRRKR:GFP-LacZ | |
| 1230 | YNB 16 | BNB 271 | pREP82 | pBNB 776 | nmt1:RFP:nup120AA517-1137 |
| 1232 | YNB 16 | BNB 271 | pREP82 | pBNB 778 | nmt1:RFP:nup124 AA571-1111 |
| 1234 | YNB 16 | BNB 193 | Tf1-Gag:YFP | pBNB 418 | nmt81:empty vector |
| 1253 | YNB 16 | pHL449-1 | Tf1-reporter (see above) | pBNB 8 | nmt1:GFP |
Table 2.
Construct details
| Construct | Details |
|---|---|
| 3HA:nup124ΔFXFG1-2 | Nup124ΔAA571-615 |
| 3HA:nup124ΔFXFG3-7 | Nup124ΔAA828-967 |
| 3HA:nup124ΔFXFG8-9 | Nup124ΔAA998-1026 |
| 3HA:nup124ΔFXFG10-11 | Nup124ΔAA1089-1111 |
| 3HA:nup124ΔFXFG3-7* | Nup124ΔAA828-967 |
| 3HA:nup124ΔFXFG3-7*,FXFG1-2,8-11→ AAAA | Nup124ΔAA828-967+ AA571-574,612-615, 998-1001,1023-026,1089-1092, 1108-1111-AAAA |
| 3HA:nup124ΔFXFG | Nup124ΔAA571-1146 |
| Nup124FXFG | Nup124AA571-1146 |
| Nup153FXFG | Nup153AA903-1418 |
| Nup1FXFG | Nup1pAA406-988 |
| Nup120p noFXFG | Nup120pAA517-1137 |
| Nup124pΔFXFG:Nup153FXFG | Nup124pΔAA571-1111-Nup153AA903-1418 |
| Nup124pΔFXFG:Nup1pFXFG | Nup124pΔAA571-1111-Nup1pAA406-888 |
| Nup124pΔFXFG:Nup120p noFXFG | Nup124pΔAA571-1111-Nup120pAA517-1137 |
| 3HA:Nup124pR-A | 3HA:nup124pR1148A |
| 3HA:Nup124pRR-AA | 3HA:nup124pRR1156-57AA |
| 3HA:Nup124pΔRR | 3HA:nup124pΔ1156-57 |
| 3HA:Nup124pΔCT | 3HA:nup124pΔ1147-59 |
| 3HA:Nup124pΔCT* | 3HA:nup124p Δ1151-55 |
| Nup124pΔCT:Nup1pCT | Nup124pΔAA1147-59:Nup1pAA1065-76 |
| Nup124pΔCT:Nup153CT | Nup124pΔAA1147-59:Nup153pAA1464-1475 |
| Nup124pΔCT:Nup1pCT+ | Nup124pΔAA1147-59:Nup1pAA1065-1076+R |
| Nup124pΔCT:Nup153CT@ | Nup124pΔAA1147-59:Nup153pAA1464-1475+R |
| Nup124pCT | Nup124pAA1147-1159 |
| Nup1p+R | Nup1pAA1-1076+R |
| Nup153ΔCT:Nup124CT | Nup153pΔAA1464-1475:Nup124pAA1147-59 |
| Nup124pCT* | Nup124pAA1153RSRRKR1159-ASAAAA |
| RFP:Nup120p seq | RFP:Nup120pAA517-1137 |
| RFP:Nup124pFXFG | RFP:Nup124pAA571-1111 |
| Nup124ΔCT*:Nup153CT# | Nup124pΔAA1151-55:Nup153AA1468-72 |
| Nup124pm | Nup124AA1153RSRRKR1159-ASAAAA |
| Nup124p∧ | 3HA:nup124ΔAA571-1111::AA1151-1159 |
Plasmid Constructions
DNA fragments used to create plasmids for this study (Supplementary Table S1) were generated by PCR. All constructs were confirmed by DNA sequencing. Because many of the constructs were of a complex nature, a simplified naming system was adopted and used throughout this work (Table 2), unless otherwise specified.
Tf1 Transposition and Tf1 cDNA Recombination Assays
Tf1 transposition and Tf1 cDNA recombination were assayed as previously described (Balasundaram et al., 1999; Varadarajan et al., 2005). Briefly, Tf1 transposition was monitored by placing a neo-marked Tf1 element under the control of the inducible nmt1 promoter. The bacterial neo gene allowed cells to grow in the presence of 500 μg of G418/ml, and thus Tf1 transposition activity could be correlated with the ability of cells to grow on G418-containing medium. S. pombe strains that contained a Tf1-neo plasmid (pHL449-1) were grown as patches on EMM −ura dropout agar plates in the presence of thiamine (Tf1 Off) or in the absence of thiamine (Tf1 On). The transcription of the nmt1-Tf1-neo gene is induced in the absence of thiamine (Tf1 On). After 4 d of incubation at 32°C, these plates were replica printed to medium containing 5-fluoroorotic acid (5-FOA; 100 mg/ml) to eliminate the URA3-Tf1-neo plasmid. Finally, 5-FOAr patches were printed to medium containing both 5-FOA and G418 and incubated at 32°C for 38 h to detect strains that became resistant to G418 as the result of insertions of the neo-marked Tf1 element into the genome. The presence of Tf1 cDNA in the nucleus was examined by cDNA recombination assays. This protocol is similar to that of the transposition assay in that strains with the neoAI-marked Tf1 plasmid were induced for the expression of Tf1 on EMM −Ura plates without thiamine for 4 d at 32°C. The plates were then replica printed to YES medium that contained 500 μg of G418/ml. Recombination between cDNA and cellular transposon sequences was scored on the G418 plates after 38 h of growth at 32°C. Because the main goal of this study was to examine the role(s) of nuclear pore/transport components and their ability to import Tf1 components into the nucleus, only cDNA recombination assays are depicted and referred to throughout the text and in the figures as “Tf1 Activity.”
Fluorescence Microscopy
Cells totaling ∼1.0 OD600 (optical density at 600 nm) were harvested and fixed in 3.7% formaldehyde in PBS for 5 min at room temperature. Fixed cells were washed twice in 1× phosphate-buffered saline with 0.1% Triton-X and mounted onto glass slides with Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA). Laser-scanning confocal microscopy was performed using an Olympus upright Confocal microscope (Fluoview; Melville, NY), with red fluorescent protein (RFP) visualized using the Cy3 laser (543 nm) and yellow fluorescent protein (YFP) using the enhanced green fluorescent protein (GFP) laser (488 nm). DAPI was visualized using the DAPI laser at 405 nm. For indirect immunofluorescence, cells were harvested at 0.4–0.8 OD600 and treated as previously described (Balasundaram et al., 1999; Varadarajan et al., 2005). Hemagglutinin epitope (HA)-tagged proteins were visualized with anti-hemagglutinin antibody (clone 12CA5, Roche Biochemicals, Basel, Switzerland) at a dilution of 1:1000. Either Alexa Fluor 488 or Alexa Fluor 594 (Molecular Probes, Eugene, OR) was used as the secondary antibody at a 1:1000 dilution and mounted with Vectashield mounting medium containing DAPI (Vector Laboratories). Epifluorescence microscopy was performed at 1000× magnification using a Leica DMLB microscope (Deerfield, IL) equipped with an Optronics DEI-750T coded CCD camera (Optronics, Goleta, CA). Leica Qwin proprietary software was used to capture images. All images were processed for figure presentation using Adobe Photoshop 7.0 software.
Coimmunoprecipitation and In Vitro Binding Assay
All incubations were carried out at 4°C unless otherwise noted. Nup124p, Tf1-Gag, Kap95, Cut15p, Imp1p, Grn1p, and HIV-1 Gag proteins were synthesized using a TNT-coupled reticulocyte lysate system (Promega, Madison, WI), according to the manufacturer's instructions. Equal amounts of in vitro–translated proteins were mixed and incubated for 60 min at 4°C in binding buffer (25 mM HEPES, pH 7.9, 150 mM KCl, 0.1% Nonidet P-40, 5% glycerol, 0.5 mM dithiothreitol, and 0.4 mM phenyl methyl sulfonyl fluoride). Respective antibodies were added to each tube and incubated for 90 min at 4°C. Protein A/G-agarose (Santa Cruz Biotechnology, Santa Cruz, CA; 5 mg per tube) was added to all the tubes, which were then incubated overnight on a nutator. The beads were washed three times with the binding buffer. The immunoprecipitated protein complexes were eluted from the Agarose beads and subjected to SDS/PAGE in 4-15% gels. The gels were processed for fluorography.
RESULTS
The Nup124p FXFG-Repeats Are Not Required for Tf1 Transposition
We had previously reported that the 550-amino acid long 11-FXFG-repeat domain of Nup124p was absolutely required for Tf1 transposition and that the absence of retrotransposition activity in mutants lacking the FXFG domain was associated with the inability of Tf1-Gag to be localized in the nucleus (Balasundaram et al., 1999; Varadarajan et al., 2005). In an attempt to identify whether all of the 11 or certain crucial FXFG repeats were required for function, various deletions of the FXFG domain were created as indicated in Figure 1(see Table 2 for details) and expressed in S. pombe along with a Tf1 reporter plasmid, pHL449-1 to determine the levels of Tf1 cDNA recombination and transposition (Tf1 activity) by in vivo complementation of a null mutant using published genetic assays (Balasundaram et al., 1999; Varadarajan et al., 2005) as described in Materials and Methods. As seen in Figure 1 (see Table 2 for details), although the entire FXFG-repeat–containing region (patches 17-18) was required for Tf1 activity, all the FXFG-domain deletion mutants tested (patches 5-14) yielded wild-type Tf1 activity. Such a result implied that the subdomains of FXFG domain were redundant and that one subdomain could compensate for another. We therefore used a nup124 mutant tagged with three copies of the HA epitope at its N-terminus, 3HA:nup124 ΔFXFG 3-7 as the parent, to systematically and sequentially mutate the remaining FXFG repeats to stretches of alanine quadruples (AAAA). Each of the mutants generated was tested for its ability to support Tf1 transposition (only the mutant Nup124ΔFXFG 3-7, FXFG 1-2, 8-11 → AAAA (see Table 2 for details) is shown in patches 15 and 16). As can be seen from Figure 1, patches 15 and 16, mutation of the FXFG repeats did not have any effect on transposition activity. Thus, we conclude that the FXFG repeats themselves are not required for Tf1 activity.
Figure 1.
FXFG repeats of Nup124p are not required for Tf1 activity. Multicopy plasmids containing full-length and mutant versions of Nup124 whose transcription was under the control of its native promoter or the low strength, nmt81 (no messsage in thiamine) promoter were transformed into a Δnup124 mutant strain YNB19, along with the Tf1 reporter plasmid pHL499-1. Genetic assays measured the ability of these mutant strains to reinstate levels of Tf1 cDNA recombination and transposition to that of epigenetically expressed WT levels. PROTfs (YNB10) containing a Tf1-reporter plasmid with a protease frame shift that blocks the expression of (Tf1) protease, reverse transcriptase, and integrase, serves as a negative control for the assay. Because protease is involved in the processing of the Tf1 genome, all the activities of the retrotransposon are blocked as a result of the frame shift mutation. INfs (YNB11) contains a Tf1-reporter plasmid with a frame shift in the integrase so Tf1 transposition is decreased but not Tf1 cDNA recombination and serves as a negative control for the assay. S. pombe strains containing a Tf1-neo plasmid (pHL449-1) were grown as patches as shown, on EMM −ura dropout agar plates in the presence of thiamine (Tf1 Off) or in the absence of thiamine (Tf1 On). The transcription of the nmt1-Tf1-neo gene is induced in the absence of thiamine (Tf1 On). After 4 d of incubation at 32°C, these plates were replica printed to medium containing 5-FOA (100 mg/ml) to eliminate the URA3-Tf1-neo plasmid. Finally, 5-FOAr patches were printed to medium containing both 5-FOA + G418 and incubated at 32°C for 38 h to detect strains that became resistant to G418 as the result of insertions of the neo-marked Tf1 element into the genome. For cDNA recombination assays, strains with the neoAI-marked Tf1 plasmid were induced for the expression of Tf1 on EMM −Ura plates without thiamine for 4 d at 32°C. The plates were then replica printed to YES medium that contained 500 μg of G418/ml. Recombination between cDNA and cellular transposon sequences was scored on the G418 plates after 38 h of growth at 32°C. The numbering of the patches in the figure corresponds to strains diagrammatically represented in the bottom panel. Each oval shape in the diagrammatic representation indicates one FXFG repeat. (1) Epigenetically expressed WT (3HA:nup124), YNB58; (2) Null mutant containing an empty vector (nmt81:empty vector), YNB893; (3) PROTfs, YNB10; (4) INfs, YNB11; (5 and 6) Nup124ΔFXFG1-2, YNB59; (7 and 8) Nup124ΔFXFG3-7, YNB235; (9 and 10) Nup124ΔFXFG8-9, YNB236; (11 and 12) Nup124ΔFXFG10-11, YNB65; (13 and 14) Nup124ΔFXFG3-7*, YNB 1086; (15 and 16) Nup124ΔFXFG3-7*, FXFG1-2,8-11 AAAA, YNB1140; and (17 and 18) Nup124ΔFXFG, YNB1052 (see Table 2 for details).
FXFG-Repeat Domains of Nup1p and Nup153 Are Functional in Nup124p
In a previous report, we had identified the S. cerevisiae and vertebrate (human) nucleoporins Nup1p and Nup153, respectively, as orthologs of Nup124p (Varadarajan et al., 2005). When Nup1p and Nup153 C-terminal regions are aligned independently with Nup124p C-terminal region as shown in Figure 2, there appears to be a low to moderate similarity/identity in regions besides the FXFG/FG repeats themselves. Because the entire FXFG-repeat–containing domain of Nup124p was required for Tf1 activity (Figure 1, patches 17 and 18; Balasundaram et al., 1999; Varadarajan et al., 2005), we wanted to know if such a requirement was inherent within the FXFG repeat–containing domain but not with the repeats themselves. One interesting possibility we pursued was a set of key observations by Rexach and coworkers that the large FG-repeat regions of Nups behaved in situ as natively unfolded structures thus giving them special features that may be critical for NPC function (Denning et al., 2002, 2003; Denning and Rexach, 2007). In Figure 3A, we show that the percentage of “disorder amino acids” in the C-terminal half of Nup124p is comparable to its orthologs Nup1p and Nup153 but significantly higher than Nup120p, a nucleoporin that does not contain any FXFG/FG repeats. We therefore asked if the FXFG domains from Nup1p and Nup153 would functionally replace the FXFG region of Nup124p. As a control, the region from Nup120p lacking either FG or FXFG repeats replaced the FXFG region of Nup124p. All the above chimeric proteins were expressed in S. pombe along with the Tf1 reporter plasmid, pHL449-1, to determine the levels of Tf1 activity by in vivo complementation of the defect in a null mutant as previously described (Balasundaram et al., 1999; Varadarajan et al., 2005). As seen in Figure 3B, the chimeras Nup124pΔFXFG-Nup153FXFG and Nup124pΔFXFG-Nup1pFXFG (see Table 2 for details) were able to rescue Tf1 activity comparable to wild-type levels. On the other hand, the Nup124pΔFXFG-Nup120p no FXFG chimera did not rescue Tf1 transposition. From Figures 1 and 3, we infer that although the Nup124p FXFG repeats themselves are not required, the FXFG domains of Nup124p (S. pombe), Nup1p (S. cerevisiae), and Nup153 (mammals) contain a common element(s) required for Tf1 activity.
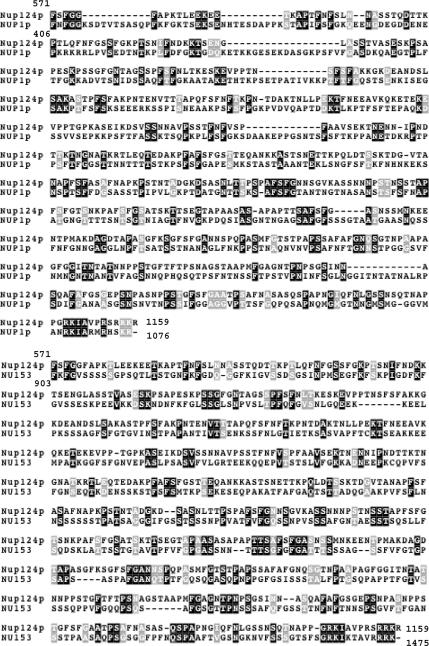
Figure 2.
Sequence alignment of Nup124p, Nup1p, and Nup153 FXFG-repeat domains. The alignment of the C-terminal region of SpNup124p with the C-terminal domain of ScNup1p and HuNup153. Identical residues are boxed in black and similar/conserved residues in gray. Amino acid ranges are indicated at the start and finish of each sequence.
Figure 3.
FXFG/FG-domains of Nup1p and Nup153 are functional in Nup124p in the context of Tf1 activity. (A) Disorder amino acids (% by frequency) in the indicated FXFG/FG region of SpNup124p, ScNup1p, HuNup153, and SpNup120p (a random non-repeat region corresponding to the C-terminal half of the protein) (B) Yeast strains expressing full-length Nup124p (YNB 1064), Nup124p with FXFG deletion (YNB 1052), chimeras of Nup124p with the indicated FXFG repeat (YNB 1117, Nup124pΔFXFG:Nup1pFXFG, or YNB 1096, Nup124pΔFXFG:Nup153FXFG), or a non-repeat–bearing chimeric fusion protein (YNB 1201, Nup124pΔFXFG:Nup120p no-FXFG) were assayed for Tf1 activity as described earlier. +++, Tf1 activity equivalent to the epigenetically expressed WT nup124 (YNB 1064); −, lack of Tf1 activity comparable to the null mutant (see Table 2 for details).
Analysis of a Highly Conserved C-terminal Polypeptide GRKIAVPRSRRKR of Nup124p That Is Absolutely Essential for Tf1 Transposition
In our initial experiments, deletion of the last 48 amino acids of Nup124p resulted in the abolition of Tf1 activity (unpublished results). Closer inspection of the three orthologs Nup1p, Nup124p, and Nup153 revealed a highly conserved sequence motif at the C-terminal end (Figure 4A). We therefore asked if this sequence, AA1147-GRKIAVPRSRRKR-AA1159 was critical for Nup124p function and if so, which residues contributed to its activity. Amino acids were either deleted or replaced with alanine as indicated in Figure 4. B–D. Constructs were expressed in the null mutant along with a Tf1 reporter plasmid, pHL449-1 (Table 2 and Supplementary Table S1) to determine the levels of Tf1 activity by genetic assays described earlier (Figure 1) As depicted in Figure 4B, the most dramatic results were associated with the 3HA:nup124pR-A (YNB1020), 3HA:nup124pRR-AA (YNB1070), 3HA:nup124pΔRR (YNB1034), and 3HA:nup124pΔCT (YNB1050) mutants, where complete elimination of Tf1 activity was noted. All other single amino acid changes yielded WT Tf1 activity and 3HA:nup124p ΔCT* (YNB1032) resulted in a significant lowering, but not elimination of Tf1 activity (Figure 4C), suggesting that the arginine residues at positions 1148 and 1156-1157 may constitute a bipartite nuclear localization signal (NLS) separated by the spacer “AVPRS.”
Figure 4.
Identification of a highly conserved peptide sequence xRKIxxxxSRRKx at the C-terminus of SpNup124p, ScNup1p, and Nup153. (A) Sequence alignment of the C-terminal 13-amino acid region of Nup124p (AA 1147-1159), Nup1p, and Nup153 from various species. Representatives were chosen from a broad selection of eukaryotes: S. pombe (Sp), S. cerevisiae (Sc), Human (Hu), Mouse, Mus musculus (Mus), Rattus norvegicus (Rat), Xenopus laevis (Xe), and Gallus gallus (Gg). (B) Tf1 activity of wild-type and mutant Nup124p at the C-terminal polypeptide, GRKIAVPRSRRKR (Nup124pCT) was measured as described in Materials and Methods. (C) The amino acids 1151-1155 (AVPRS) of the C-terminal polypeptide were mutated or deleted. Their effect on Tf1 activity was measured. (D) Tf1 activity was measured upon swapping the C-terminal peptide of Nup1p and Nup153 with the C-terminal peptide of Nup124p (YNB1058 and YNB1054, respectively), and swapping followed by addition of an arginine residue at the C-terminus (YNB1062 and YNB1215). Tf1 activity was also assayed in the presence of Nup1p (YNB 1017), upon addition of an arginine to Nup1p (YNB1098), Nup153 (YNB967) and upon swapping the C-terminal 12 amino acids of Nup153 with the C-terminal 13 amino acids of Nup124 (YNB1217; see Table 2 for details).
Because both Nup1p and Nup153 C-termini contain the equivalents of the Nup124p-R1148 and RR1156-57 residues described above, we wanted to know if the 12 amino acid sequences NRKIARMRHSKR (from Nup1p) or GRKIKTAVRRRK (from Nup153) could functionally replace the 13 amino acids, GRKIAVPRSRRKR in Nup124p. Therefore, Nup124pΔCT:Nup1pCT (YNB1058) and Nup124pΔCT:Nup153CT (YNB1054) chimeras were generated and tested for support of Tf1 activity. Figure 4D shows that both chimeras were unable to promote Tf1 activity in a nup124 null background. We reasoned that the inability to rescue Tf1 activity was because of the disparity in the required numbers of amino acids in this domain. Another reason could be that because they do not have an amino acid corresponding to R1159 of Nup124p, they lacked a consensus “R” quorum in a contextual sense. We therefore added an arginine to the above chimeras to form Nup124pΔCT:Nup1pCT+ (YNB1062) and Nup124pΔCT:Nup153CT@ (YNB1215) and tested transformants for their ability to rescue Tf1 activity (Figure 4D). Interestingly, both chimeras restored Tf1 activity to wild-type levels. Our results are indicative of a specialized requirement fulfilled only by the Nup124p sequence GRKIAVPRSRRKR (Nup124pCT). We also noted that Nup1p+R (YNB1098) or Nup153ΔCT:Nup124CT (YNB1217) did not rescue Tf1 activity in their “native” Nup1p and Nup153 contexts, respectively, indicating that there may be other factors required for Tf1 activity besides the C-terminal peptide that are unique to Nup124p (Varadarajan et al., 2005).
The Putative Gag-binding Domain, the FXFG-Repeat Domain and the C-terminal Peptide of Nup124p Are Independently Required for Nuclear Import of Tf1-Gag and Retrotransposon Activity
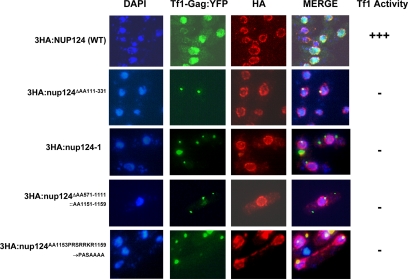
In earlier studies, we had demonstrated that Nup124p-mediated nuclear import of Tf1-Gag was critical for Tf1 activity and that deleting the putative Gag-binding or the FXFG-repeat domains caused a loss of Tf1 transposition, although the mutant proteins were localized at the NPC (Balasundaram et al., 1999; Varadarajan et al., 2005). We wanted to know if abrogation of Tf1 activity in the absence of the above domains correlated with the inability of these mutants to import Tf1-Gag into the nucleus. Null mutant strains episomally expressing Tf1-Gag:YFP cotransformed with plasmids containing 3HA-epitope–tagged WT (3HA:nup124) or mutant nup124 sequences were tested for Tf1 activity. Nup124p and Tf1-Gag:YFP were identified in situ by epifluorescence microscopy. As depicted in Figure 5, in the presence of wild-type Nup124p, Tf1-Gag was found colocalizing with the DAPI stain, indicating that Gag was imported into the nucleus. On the other hand, in the presence of Nup124p mutations that delete N-terminus (Nup124pΔ111-331; Varadarajan et al., 2005), the entire C-terminus (nup124-1; Balasundaram et al., 1999), the FXFG-repeat–containing domain (nup124ΔFXFG) or mutations in the C-terminal peptide, GRKIAVPRSRRKR (nup124pm), Tf1-Gag was found localized as cytoplasmic or perinuclear fluorescent aggregates. Similar cytoplasmic and perinuclear aggregation of Tf1-Gag has been observed (Balasundaram et al., 1999; Varadarajan et al., 2005) and may be a feature of VLPs (Teysset et al., 2003; Kim et al., 2005). Tf1-gag localizes to the NPC, even in the absence of Nup124p (Varadarajan et al., 2005). It is possible, therefore, that in the presence of Nup124p mutants, Tf1-Gag would localize at or close to the NPC, although it might not interact with it. Correspondingly, those mutants do not support Tf1 activity (Figure 5). We noted that none of the mutants exhibited any altered nuclear targeting or localization patterns when compared with the WT. Our results therefore suggest that all the above tested domains are critically important for importing Tf1-Gag into the nucleus as also to reconfirm our earlier conclusions that the Nup124-mediated translocation of Tf1-Gag from its perinuclear position into the nucleus was an absolute requirement for Tf1 retrotransposon activity.
Figure 5.
Mutations in Nup124p prevent Tf1-Gag from being imported into the nucleus resulting in loss of Tf1-transposon activity. YNB19 was transformed with Tf1-Gag:YFP and 3HA-tagged Nup124p (YNB549). Mutant Nup124p strains are shown, and their details provided in Table 2. Cells were grown in −Ura-Leu medium with or without 15 μM thiamine. Cells were processed and visualized as indicated in Materials and Methods. Only cells grown in the absence of thiamine are shown. Strains shown were also tested for their ability to support Tf1 activity. +++, Tf1 activity equivalent to the epigenetically expressed WT (YNB549); −, lack of activity.
Epigenetic Overexpression of Nup124p, Nup1p, and Nup153 FXFG/FG Domains or the Nup124p GRKIAVPRSRRKR Peptide in a Wild-type Strain Knockdown Tf1 Activity
A second approach to identifying the specificity of a domain required for activity was to observe if overexpression of FXFG-repeat–containing regions of Nup124p, Nup153, Nup1p, and the no-FXFG/FG-repeat region of Nup120p would cause an inhibition of Tf1 transposition in a WT background without inhibiting growth of the host. These experiments were performed based on the expectation that peptides whose overexpression interferes with Tf1-Gag function but do not influence cell growth will act by disengaging Tf1-Gag from Nup124p. Such a dominant negative phenotype would be consistent with titration of an FXFG-domain–interacting protein by an excess of the Nup124p, Nup1p, or Nup153-FXFG-domain fragment. Also, it was anticipated that excess of Nup120p region with no FXFG/FG-repeats would not lead to a dominant negative inhibition of Tf1 activity because it did not support wild-type levels of Tf1 transposition as demonstrated in Figure 3B. Such a result would also imply a Tf1-related function rather than a global effect. Nucleotides encoding the FXFG regions of Nup124p, Nup1p, Nup153, and the no-FXFG region of Nup120 were cloned into a vector in which their transcription was under the control of the thiamine repressible nmt1 promoter (highest strength; Table 2). These constructs were expressed in the WT along with a Tf1 reporter plasmid, pHL449-1 (Table 1) to determine levels of Tf1 activity. As depicted in Figure 6A, overexpression of FXFG/FG domains of Nup124p, Nup1p, and Nup153 (patched 5-6, 7-8, and 9-10, respectively) caused an equal and dramatic loss of Tf1 activity. Furthermore, no difference was observed in growth between Tf1-induced (No B1) or uninduced (+B1) cultures. In complete contrast, however, overexpression of the no-FXFG/FG region of Nup120p resulted in no loss of Tf1 activity (patches 11 and 12). Our results are consistent with observations that the FXFG domains of Nup1p and Nup153 act in a manner similar to the Nup124p FXFG domain, with respect to dominant negative inhibition of Tf1 activity. Our observation that a non-FXFG/FG- (Nup120p) nucleoporin chimera is unable to support wild-type levels of Tf1 transposition in a nup124 null mutant or knockdown transposition in a wild type when overexpressed, suggests a specific requirement of the FXFG domain.
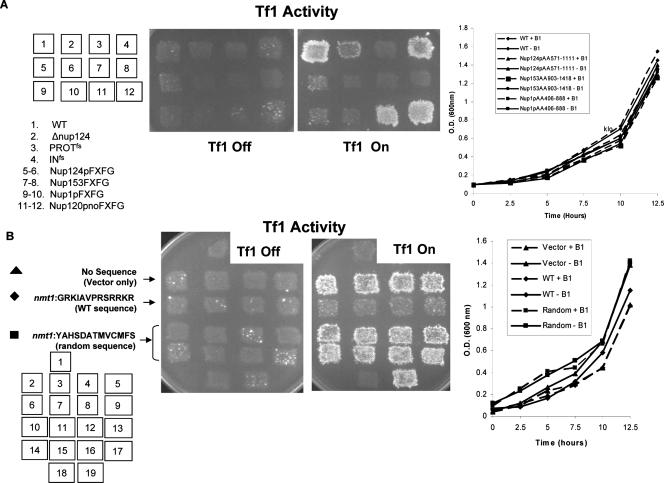
Figure 6.
Overexpression of the FXFG domain (Nup124pFXFG) or GRKIAVPRSRRKR (Nup124pCT) peptide sequence of Nup124p inhibits Tf1 activity without significantly affecting growth. (A) Genetic assays were conducted exactly as described in Figure 1 so as to measure the ability of epigenetically expressed mutants (patches 5-12) to reinstate levels of Tf1 activity in YNB16 (wild type). The indicated strains were grown in EMM −Ura-Leu+B1 (Tf1-off) and EMM −Ura-Leu-B1 (Tf1-on), and growth was measured as a function of optical density (OD 600 nm). Details of the indicated constructs provided in Table 2. (B) Multicopy plasmids containing fragments encoding the indicated amino acids, GRKIAVPRSRRKR from Nup124p (Nup124pCT) and a random sequence, YAHSDATMVCMFS, whose transcription was under the control of the high strength, nmt1 promoter were tested in a WT strain (YNB16) for their effect on Tf1 activity. (1) Null mutant containing an empty vector served as a negative control, (YNB893); (2-5) nmt1:GFP, YNB1253; (6-9) nmt1:GFP-GRKIAVPRSRRKR, YNB1066; (10-17) nmt1:GFP-YAHSDATMVCMFS, YNB1219; (18) PROTfs(YNB10) described earlier (19) INfs (YNB11) described earlier. The indicated strains were grown in EMM −Ura-Leu+B1 (Tf1-off) and EMM −Ura-Leu-B1 (Tf1-on), and growth was measured as a function of optical density (OD 600 nm; see Table 2 for details).
We used the test described above to observe if overexpression of the Nup124p C-terminal peptide, GRKIAVPRSRRKR (Nup124pCT) would cause a similar dominant negative inhibition of Tf1 transposition in a WT background. Nucleotides encoding the WT peptide sequence, GRKIAVPRSRRKR and a random sequence, YAHSDATMVCMFS were cloned into a vector in which their transcription was under the control of the thiamine repressible nmt1 promoter (highest strength). The nmt1:RFP-GRKIAVPRSRRKR and nmt1:RFP-YAHSDATMVCMFS constructs were expressed in the WT along with a Tf1 reporter plasmid, pHL449-1 (Table 1) to determine the levels of Tf1 activity by genetic assays described earlier. As depicted in Figure 6B, overexpression of nmt1:RFP-GRKIAVPRSRRKR caused a comprehensive knock down of Tf1 activity when compared with expression of the random sequence nmt1:RFP-YAHSDATMVCMFS. No difference was observed in growth between Tf1-induced (No B1) or noninduced (+B1) cultures. Taken together, our data demonstrate the Nup124pCT, GRKIAVPRSRRKR sequence is specific and critical for Nup124p function in Tf1 retrotransposon activity.
Overexpression of the Nup124p FXFG/FG Domain or the Nup124p GRKIAVPRSRRKR in WT Blocks the Nuclear Import of Tf1-Gag
We wanted to investigate the biological basis for the knockdown of Tf1 activity described above. Because overexpression of these domains did not affect the general housekeeping attributes of the NPC to the extent at which growth would be adversely affected (Figure 6), it was safe to presume that the Nup124p FXFG/FG-repeat domain (Nup124p FXFG) and the GRKIAVPRSRRKR-peptide (Nup124pCT) were involved in a Tf1-specific function. Furthermore, because both domains were required for nuclear import of Tf1-Gag (Figure 5), it was possible that their overexpression would have a similar result because the domains would compete for the same sites as the native (wild-type) protein and as a consequence, Tf1-Gag would fail to be imported into the nucleus, resulting in loss of Tf1 activity. We therefore tested Tf1-Gag localization in wild-type strains overexpressing the Nup124p FXFG/FG-repeat domain (RFP:Nup124pFXFG) or the Nup120 sequence (RFP:Nup120pseq) in the presence of nmt1Tf1-Gag:YFP. Similarly, the Nup124CT (GRKIAVPRSRRKR) or a random sequence, YAHSDATMVCMFS, were expressed as RFP-tagged peptides alongside YFP-tagged Tf1-Gag. The Nup120p sequence and the random sequence served as appropriate negative controls because their overexpression did not result in loss of Tf1 activity (see Figure 6). Strains harboring the above plasmids were grown in growth medium lacking thiamine to induce expression of Tf1 and processed for confocal fluorescence microscopy. Localization of Tf1-Gag:YFP is depicted in Figures 7 and 8. Overexpression of RFP:Nup120p seq does not affect the nuclear import of Tf1-Gag:YFP (Figure 7, row B). In fact, both RFP:Nup120p seq and Tf1-Gag:YFP colocalize in the nucleus (Nup120p seq has no canonical or characterized NLS, and we noted that RFP:Nup120p seq remained outside the nucleus in 30% of fluorescing cells). In stark contrast, however, overexpression of RFP:Nup124pFXFG causes Tf1-Gag:YFP to be mislocalized outside the nucleus (Figure 7, row D). Furthermore, in all cells exhibiting both YFP and RFP fluorescence we observed a colocalization of the RFP:Nup124pFXFG with the Tf1-Gag:YFP outside the nucleus suggesting a close association of the Nup124p FXFG domain and Tf1-Gag. The colocalization of Nup124p FXFG domain with Tf1-Gag is consistent with our observation that the C-terminus of Nup124p does indeed bind Tf1-Gag (see below).
Figure 7.
Tf1-Gag is mislocalized upon over expression of the Nup124p, Nup1p, and Nup153-FXFG/FG domains in the WT strain. YNB16 was transformed with RFP:Nup124pFXFG (YNB1223) and RFP:Nup120p seq (YNB1221) along with nmt1:Tf1-Gag:YFP. YNB 16 was also transformed with RFP:Nup124pFXFG (YNB1232) and RFP:Nup120p seq (YNB1230) alone (see Table 2 for details). Cells were grown in EMM −Ura-Leu medium with or without 15 mM thiamine. Only cells grown in the absence of thiamine are shown.
Figure 8.
Tf1-Gag is mislocalized upon over expression of the C-terminal GRKIAVPRSRRKR (Nup124pCT) polypeptide in a WT strain. YNB16 was transformed with nmt1:RFP-GRKIAVPRSRRKR (YNB1198) or nmt1:RFP-YAHSDATMVCMFS (YNB1191) along with nmt1:Tf1-Gag:YFP. YNB16 was transformed with nmt1:RFP-GRKIAVPRSRRKR (YNB1197), nmt1:RFP-YAHSDATMVCMFS (YNB1189) and nmt1:Tf1-Gag:YFP (YNB1234) alone. Cells were grown in EMM −Ura-Leu medium with or without 15 mM thiamine. Only cells that were induced are shown (see Table 2 for details).
As described in this and earlier reports (Balasundaram et al., 1999; Varadarajan et al., 2005), Tf1-Gag:YFP localizes to the nucleus as shown in Figure 8, column A. The small peptides including the random sequence YAHSDATMVCMFS (column B) or the Nup124 C-terminal peptide GRKIAVPRSRRKR (Nup124pCT; column C) shows a diffused localization pattern throughout the cell in the absence of Tf1-Gag:YFP, which is consistent with the diffusion of a small peptide fused to RFP. When RFP:YAHSDATMVCMFS and Tf1-Gag:YFP were expressed together, the latter was found in the nucleus (column D), consistent with the observation that overexpression of YAHSDATMVCMFS does not impede the nuclear import of Tf1-Gag and as a consequence does not diminish Tf1 activity as shown in Figure 6. On the other hand, overexpression of RFP:GRKIAVPRSRRKR together with Tf1-Gag:YFP (column E) results in blocking the nuclear import of Tf1-Gag:YFP, and results in its localization outside the nucleus. Absence of Tf1-Gag from the nucleus leads to loss of Tf1 activity (Figure 6). Taking our results from Figures 6–8 together, we demonstrate that epigenetic overexpression of Nup124p domains in a wild-type strain inhibit retrotransposon activity by effectively uncoupling Tf1-Gag from its nuclear import process.
Nup124p Interacts In Vitro with Tf1-Gag, the Importin β Kap95 and the Importin α's Imp1p and Cut15
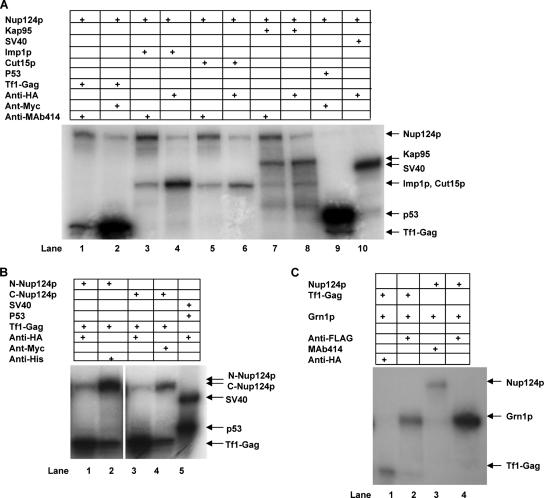
FG-Nups interact directly with a broad range of transport factors and there is compelling in vivo evidence that interaction between the carriers and FG-Nups is important for at least one stage of translocation through NPCs (reviewed in Ryan and Wente, 2000; Allen et al., 2001; Stewart et al., 2001). Studies have demonstrated interactions between FXFG or GLFG cores of nucleoporins and transport factors (Bayliss et al., 2000, 2002a,b) and also binding of transport factors to non-FG regions of nucleoporins (Pyhtila and Rexach, 2003). Importin α, Kap60 (Srp1p) and importin β, Kap95 bind to the VXXRKIXXXXRR-C-terminal motif of Nup1p (Floer et al., 1997; Gilchrist and Rexach, 2003; Pyhtila and Rexach, 2003; Liu and Stewart, 2005), and the closely similar Nup153 C-terminal motif binds to importin α (Moroianu et al., 1997). We were interested in understanding the possible involvement of transport factors in Nup124p mediated transport of Tf1-Gag across the nuclear membrane. Like its S. cerevisiae homolog, the S. pombe Kap95 (SPAC1B1.03c) is an essential gene that encodes a protein localizing to the nuclear rim (Chen et al., 2004). In contrast to the budding yeast's single importin α, fission yeast has two importin αs, Cut15p and Imp1p, which have partially overlapping and distinct functions (Matsusaka et al., 1998; Umeda et al., 2005). We wanted to know if Nup124p's role in nuclear import of Tf1 (Gag), vis-à-vis Tf1 transposition, was contingent on its interaction with Kap95 and/or one or both importin α. We performed in vitro binding assays and coimmunoprecipitation with Nup124p, Tf1-Gag, Kap95, Cut15p, Imp1p, Grn1p, and HIV-1 Gag proteins synthesized using a TNT-coupled reticulocyte lysate system. In Figure 9A, we show that full-length Tf1-Gag (lanes 1 and 2), Imp1p (lanes 3 and 4), Cut15p (lanes 5 and 6), and Kap95 (lanes 7 and 8) bind independently to full-length Nup124p. To establish that the above interactions were bonafide and specific, we showed that Nup124p did not coimmunoprecipitate with either p53 or SV40 (lanes 9 and 10). Similarly, a nucleolar protein Grn1p (Du et al., 2006) does not interact with either Tf1-Gag or Nup124p (Figure 9C, lanes 1-2 and 3-4, respectively). In Figure 9B we show that Tf1-Gag binds both, the N- and C-terminal halves of Nup124p. Although the N-terminus binding to Tf1-Gag reconfirms our earlier conclusions (Balasundaram et al., 1999; Varadarajan et al., 2005) and those of Kim et al. (2005), the C-terminal half of Nup124p (containing the entire FXFG/FG repeat domain) binding to Tf1-Gag reverses our earlier conclusion that no binding was observed for Nup124pAA 522-1152 to Tf1-Gag (Balasundaram et al., 1999). It is possible that the binding of the C-terminal (AA522-1152) of Nup124p to Tf1-Gag could be indirect, mediated via importins, an integral part of reticulocyte lysates. The known interaction between SV40 and p53 was performed routinely as a positive control to monitor the efficacy and robustness of the in vitro interaction assay (Figure 9B, lane 5).
Figure 9.
Coimmunoprecipitation of Nup124p with Tf1-Gag, Kap95, Imp1p, and Cut15p from rabbit reticulocyte lysates. (A) The following sequences: Nup124, Tf1-Gag, Kap95, Imp1, Cut15, and Grn1:GFP from BNB401, BNB706, BNB659, BNB704, BNB702, and BNB373, respectively (Supplementary Table S1), were transcribed and translated in rabbit reticulocyte lysates in the presence of [35S]methionine. Translated products were mixed in various combinations as indicated. Coimmunoprecipitation assays were performed as described in Materials and Methods with the appropriate antibodies, as indicated. (B) Nup124AA1-570 (BNB566) and Nup124 AA571-1159 (BNB761) were transcribed and translated using the rabbit reticulocyte lysate in the presence of [35S]methionine. The products were incubated with Tf1-Gag (BNB706) and immunoprecipitated with antibodies as indicated. (C) Grn1p (BNB338), a nucleolar protein of S. pombe was incubated with Nup124 (BNB401) and Tf1-Gag (BNB706) and immunoprecipitated with antibodies as indicated in Materials and Methods. The immunoprecipitates were resolved on 4-15% SDS-PAGE and detected by fluorography. The relative mobilities of full-length/+tag proteins are indicated by arrows.
DISCUSSION
The C-terminal Halves of Nup124p (S. pombe), Nup1p (S. cerevisiae), and Nup153 (Human) Contain Common Elements That Are Essential for Tf1 Activity
In general, sequence comparisons between nucleoporins from mammalian and yeast (S. cerevisiae) yield very poor alignments, and in many cases direct sequence homology is not apparent or even feasible (Varadarajan et al., 2005). Poor sequence conservation between FG Nups throughout eukarya, is suggestive of rapid evolution (Denning and Rexach, 2007). Nup124p shares certain sequence and functional features with Nup1p and Nup153 (Cronshaw et al., 2002; Hase and Cordes, 2003; Ball and Ullman, 2005; Varadarajan et al., 2005). One of those features, the FXFG-repeat domain spans a major portion of the C-terminus (see Figure 6A in Varadarajan et al., 2005). Although full-length Nup153 does not complement Nup124p, a region of Nup153 (aa448-634) was found capable of substituting for a domain within Nup124p (Varadarajan et al., 2005). Nucleoporins containing the FXFG variant of the FG motif have more disorder amino acids than Nups containing mostly GLFG or xxFG motifs (Denning et al., 2003). FXFG-repeat–containing nucleoporins, such as Nup1p, Nup2p, and Nup60p are located in the nuclear basket structure (Rout et al., 2000) and mostly bind Kap95p-Kap60p heterodimers (Allen et al., 2001). In vertebrates, it has been shown that either the FXFG nucleoporins or proteins bound to them are required for transport (Finlay and Forbes, 1990).
Our initial premise for assuming a Tf1-specific role for the FXFG-repeat domain was threefold. First, Nup124p is one of several FXFG-containing nucleoporins in fission yeast and yet its specific requirement was crucial in supporting Tf1 activity. Second, the FXFG domain was required for Tf1 activity (Varadarajan et al., 2005) but not for recruitment of Tf1-Gag to the nucleus (Balasundaram et al., 1999; Varadarajan et al., 2005). Finally, in the absence of the FXFG domain, Tf1-Gag failed to be translocated into the nucleus (Figure 5). Deleting sections of the FXFG-repeat domain that included FXFGs 1-2, 3-7, 8-9, or 10-11 did not affect Tf1 activity. We assumed that the repeats were redundant functionally and thus compensated each other. We therefore used the largest deletion construct 3HA:nup124ΔFXFG3-7* to substitute the remaining FXFGs 1, 2, 8, 9, 10, and 11 with alanine tetrapeptides. This strain which has no FXFG repeats, had no effect on Tf1 activity either, implying that the FXFG repeats themselves had an insignificant role in Nup124p-mediated nuclear import of Tf1. If not the FXFG repeats themselves, it could be some other motif inherent in stretches between the FXFG repeats or a signature constituting the entire domain that is required for function.
In vivo studies implicate non-FG-Nups in control of permeability across the nuclear membrane (Shulga and Goldfarb, 2003). Furthermore, Kap95-binding sites in the non-FG domains of Nup60 (Denning et al., 2001) and Nup1p (Pyhtila and Rexach, 2003) have been documented. The asymmetric FG repeats of Nup1p and Nup159 are not required for Kap95-mediated import, XpoI-mediated export, or mRNA export (Zeitler and Weis, 2004). Similarly, export of heat-shock RNAs are mediated by the unique carboxy-terminus of Rip1p, with no substantial contribution from the FG-repeat region (Stutz et al., 1997). These findings raise the interesting possibility that Tf1-Gag import is mediated through transport molecules binding to high-affinity non-FG–binding sites found within the C-terminus of Nup124p.
Various models have been proposed in order to explain translocation across the nuclear membrane. An interacting meshwork of FG repeats forms a hydrophobic sieve blocking translocation of molecules not bound by transport factors (Ribbeck and Gorlich, 2001, 2002). Transport factors (specifically, importin β) bind sequentially to different FG domains with progressively increasing affinity from one NPC side to the other, with highest affinity sites at respective exit points (Ben-Efraim and Gerace, 2001; Pyhtila and Rexach, 2003). An elaborate study in S. cerevisiae demonstrated redundancy in the FG domains, that a large proportion of the FG domain mass could be eliminated without affecting NPC permeability. Indeed, in that same study, it was shown that the entire FG content of Nup1p (localizing to the nuclear side of NPC) could be eliminated without loss of nuclear import function or viability (Strawn et al., 2004). Of the eight FXFG/FG-repeat Nups in S. pombe (Denning and Rexach, 2007), three including Nup124p are not essential for cell viability (Balasundaram et al., 1999; Chen et al., 2004). Of all the nonessential nucleoporins tested, only Nup124p was required for Tf1 transposition (unpublished data). Therefore, the requirement for the FXFG/FG domain of Nup124p by Tf1 and its functional replacement only by the Nup1p or Nup153 domains (and not by the FG/FXFG-less Nup120p), is intriguing and indicative of conservation of function. Although these results might suggest a specific nature of Nup124p's FXFG-domain requirement, it is possible that Nup124p interacts in a similar manner with proteins, such as transport factors, other nucleoporins, none of which are essential for viability and therefore unaffected by overexpression of the FXFG domain. The growth rate is not affected upon overexpressing these domains, indicating that essential functions were not compromised, even if overexpression were to dislodge cargoes from other FXFG-containing nucleoporins. Special features on nucleoporins (like Nup124p) may be needed to import large macromolecular cargos like the virus-like particles (VLP) of Tf1 (Kim et al., 2005). Such features may be closely related to the observation(s) that Nup1p and Nup153 contain a significantly higher number of “disorder-promoting” amino acid residues, are natively unfolded, display an extended conformation, are flexible, practically lack a significant secondary structure, or are devoid of any possible steric hindrances (Dunker et al., 2001; Fahrenkrog et al., 2002; Denning et al., 2003; Paulillo et al., 2005; Denning and Rexach, 2007; Lim et al., 2006). Such qualities are likely to confer the nucleoporin with multiple binding capacities that allow them to act as large surface–area scaffolds for assemblages of nuclear pore complexes (Paulillo et al., 2005; Denning and Rexach, 2007) or hydrogel-like elastic properties that would enhance fluid movement within the NPC (Frey et al., 2006). Like its orthologs Nup153 and Nup1p, the fission yeast Nup124p has a significantly higher frequency (%) of disorder-promoting residues at its C-terminus (FXFG-domain end; Figure 3A; see also Supplementary Table S3 of Denning and Rexach, 2007) when compared with Nup120p, which possibly explains why the respective domains from Nup153 and Nup1p may functionally replace the FXFG-repeat region of Nup124p, whereas the Nup120p sequence does not. In addition to earlier reports that the N-terminal region of Nup124p is a critical determinant of its association with Tf1-Gag (Balasundaram et al., 1999; Kim et al., 2005), in vitro–binding assays reported in this study suggest that there may be more than one point-of-contact between the two proteins because both the N- and C-termini of Nup124p bind Tf1-Gag (Figure 9). It is possible that while the interaction between Tf1-Gag and the N-terminus of Nup124p is direct, the interaction with the C-terminus is indirect, mediated via other factors, such as importin α-β recognition of Gag NLSs.
The extended FXFG-rich C-terminal half of Nup153 is highly mobile (Fahrenkrog et al., 2002) and might increase the efficiency of cargo translocation thorough the NPC (Stoffler et al., 2006). Given the ability of the 50-nm Tf1 particles to traverse the pore (Teysset et al., 2003), one could envisage the critical importance of an extended FXFG-repeat domain of Nup124p in engaging Tf1-Gag at the NPC in translocating the same into the nucleus. Because Nup124p binds to Tf1-Gag (Figure 9; Balasundaram et al., 1999; Varadarajan et al., 2005), it is possible that the Nup124p FXFG-repeat domain may be crucial in conjunction with its N-terminus in serving as a large surface-area receptor for the 50-nm Tf1 particle.
The Conserved Sequence Motif GRKIAVPRSRRKR at the C Terminus of Nup124p (Nup124pAA1147-1159) Is a Critical Determinant of Tf1 Activity
The Nup124p C-terminal sequence motif GRKIAVPRSRRKR (Nup124pCT) closely resembles the C-terminus of Nup1p (VMXXRKIA), Nup153 (VXXRKIXXXXRRK; Figure 4A), the KaRF (karyopherin release factors) motifs present at the N termini of Nup2p (VMXXRKIAXXKRR), and human Nup50 (VXXXRAIKXXRR; Gilchrist and Rexach, 2003). Mutation studies (Figure 4) clearly establish the singular importance of the arginine residue in the RKIA tetrapeptide because the 3HA:nup124pR-A mutant lacked the ability to support Tf1 activity. Similarly, the RR basic residues in the tetrapeptide RRKR (AA1156-1159) were critically important. All four basic residues were dispensable when deleted one at a time, suggesting functional redundancy. The sequence motif in general resembles a bipartite NLS with two basic peptide motifs separated by an interchangeable spacer sequence. Indeed, the motif does possess the ability to transport a GFP-LacZ fusion protein into the nucleus (unpublished data). When we replaced the intervening (spacer) sequence with the KTAVR from the Nup153 intervening sequence (Nup124ΔCT*:Nup153CT#), no loss of activity was observed, but deleting the same resulted in a significant drop in Tf1 activity. Replacing the Nup124p motif GRKIAVPRSRRKR (Nup124pCT) with either Nup1p or Nup153 C-terminal sequence motifs did not generate WT levels of Tf1 activity. Addition of an arginine residue to the termini of the above Nup124p-chimeras restored WT levels of Tf1 activity that may represent a gain-of-function mutation in these chimeras, although ironically, this arginine residue itself was not essential in the WT Nup124p context. Indeed, previous studies (Booth et al., 1999; Gilchrist and Rexach, 2003) demonstrated that a very similar sequence in S. cerevisiae Nup2 RRKIAMPKRRMAFK can actively displace NLSs from importin α, whereas the very similar, endogenous Nup1 sequence NRKIARMRHSKR cannot and instead, provides a docking site for importin α-β-cargo complexes. Because S. pombe does not appear to have a Nup2 (Chen et al., 2004; Denning and Rexach, 2007), an intriguing possibility is that the C-terminus of Nup124 retains this critical Nup2 activity. Human Nup153 and S. cerevisiae Nup1 may have lost that activity during evolution, given their content of active Nup50 and Nup2p, respectively. The full-length Nup1p and Nup153 with an arginine added at the C-terminus did not rescue Tf1 activity in the Nup124 null mutant possibly because Nup1p lacks the putative Gag-binding domain and Nup153 has other components that could adversely affect Tf1 transposition (Varadarajan et al., 2005).
The C termini of Nup1p (1065NRKIARMRHSKR1076) and Nup153 (1464GRKIKTAVRRKK1475) are known to directly bind Kap60 and importin α2, respectively, and in the case of Nup1p, is a part of the high-affinity binding site for the importin β homolog, Kap95 (Moroianu et al., 1997; Gilchrist and Rexach, 2003; Pyhtila and Rexach, 2003; Liu and Stewart, 2005). Our observation that adding back an arginine to the existing Nup1p-1065NRKIARMRHSKR1076 or Nup153 1464GRKIKTAVRRKK1475 restores Tf1 activity suggests a conservation of function. Additionally, our in vitro–binding studies suggest that like its orthologs, Nup124p interacts with the fission yeast transport factors importin β, Kap95 and the two importin αs, Cut15p and Imp1p. However, because Kap95 and Cut15 are essential genes in S. pombe (Matsusaka et al., 1998; Chen et al., 2004; Umeda et al., 2005), we could not test their role(s) in Tf1 transposition. On the other hand, deleting the nonessential Imp1p did not result in reduced Tf1 function (unpublished data). This is possibly because Cut15p could replace Imp1p functionally, because both importin αs are known to have overlapping functions (Umeda et al., 2005).
Knockdown of Retrotransposon Activity by Disengaging Tf1-Gag from Its Host Nuclear Transport Machinery
Overexpression of either the FXFG-repeat domain or the C-terminal peptide of Nup124p comprehensively knocked down Tf1 activity in the presence of a WT copy of the gene. This dominant negative inhibition was not observed when the Nup120p or a random peptide sequence was overexpressed, suggesting the involvement of a highly specific mechanism-based inhibition. On the basis of our observation(s) that these domains of Nup124p may be required for the nuclear import of Tf1-Gag and that the nuclear uptake of the latter was a prerequisite for Tf1 activity, we assumed that overexpressing them would likely affect Tf1-Gag localization. Although sequences from Nup120p, a nucleoporin with no FXFG/FG-repeats or a random sequence, YAHSDATMVCMFS did not prevent Tf1-Gag from entering the nucleus, cells overexpressing either FXFG-repeat region of Nup124p, RFP:Nup124pFXFG, or the Nup124pCT, RFP:GRKIAVPRSRRKR, showed that Tf1-Gag:YFP was not imported into the nucleus or at least not accumulated within the nucleus at concentrations that would enable Tf1 transposition to occur. Interestingly, in a majority (>90%) of these cells, Tf1-Gag:YFP is typically disengaged from the medially placed interphase nucleus and appears closer to either or both polar ends. An intriguing possibility is that the FXFG-repeat domain does in fact, bind to some cytoplasmic component(s) because it localizes in large aggregates at distal locations from the nucleus even in the absence of Tf1-Gag (Figure 7C). We noted that such localization is not without some specificity, because overexpression of the Nup120-domain does not localize to those same areas. Moreover, under certain conditions, the cytoplasmic accumulation of nuclear proteins could alter functions in viral translation, RNA synthesis, packaging, or even assembly (Gustin and Sarnow, 2002). Because TF1-Gag is recruited to the nuclear rim/perinuclear area in the absence of Nup124p (Varadarajan et al., 2005) or in the presence of nup124 mutants (Figure 5), the large cytoplasmic aggregates that we observe when either one of the two domains is over expressed may prevent Tf1-Gag from “hitching” its natural ride to the nucleus. Clearly, an understanding of this process will shed light on some of the major upstream events from the formation of the virus-like particle in the cytoplasm to its recruitment at the nucleus. Though Nup124p is at the NPC/nuclear membrane throughout the cell cycle and nuclear division (unpublished data), our current over expression data uncover a link between upstream elements far removed from the nucleus that may have a bearing on Tf1-Gag being imported into the nucleus. Lastly, as in the case of Nup124p, Nup1p, and Nup153 conserved domains, there are likely to be other similarly conserved host nuclear transport factors that may be manipulated to serve as biological (host-mediated) attenuators affecting either specific or large groups (families) of viruses that have similar modes of intracellular transport.
Supplementary Material
ACKNOWLEDGMENTS
We thank Padmapriya Varadarajan and Ng Su Ying for technical assistance. This study was funded by the Agency for Science, Technology, and Research (A*STAR), Republic of Singapore.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-12-1062) on July 5, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Allen N. P., Huang L., Burlingame A., Rexach M. Proteomic analysis of nucleoporin interacting proteins. J. Biol. Chem. 2001;276:29268–29274. doi: 10.1074/jbc.M102629200. [DOI] [PubMed] [Google Scholar]
- Antonin W., Mattaj I. W. Nuclear pore complexes: round the bend? Nat. Cell Biol. 2005;7:10–12. doi: 10.1038/ncb0105-10. [DOI] [PubMed] [Google Scholar]
- Atwood A., Lin J. H., Levin H. L. The retrotransposon Tf1 assembles virus-like particles that contain excess Gag relative to integrase because of a regulated degradation process. Mol. Cell. Biol. 1996;16:338–346. doi: 10.1128/mcb.16.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasundaram D., Benedik M. J., Morphew M., Dang V. D., Levin H. L. Nup124p is a nuclear pore factor of Schizosaccharomyces pombe that is important for nuclear import and activity of retrotransposon Tf1. Mol. Cell. Biol. 1999;19:5768–5784. doi: 10.1128/mcb.19.8.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball J. R., Ullman K. S. Versatility at the nuclear pore complex: lessons learned from the nucleoporin Nup153. Chromosoma. 2005;114:319–330. doi: 10.1007/s00412-005-0019-3. [DOI] [PubMed] [Google Scholar]
- Bayliss R., Corbett A. H., Stewart M. The molecular mechanism of transport of macromolecules through nuclear pore complexes. Traffic. 2000;1:448–456. doi: 10.1034/j.1600-0854.2000.010602.x. [DOI] [PubMed] [Google Scholar]
- Bayliss R., Leung S. W., Baker R. P., Quimby B. B., Corbett A. H., Stewart M. Structural basis for the interaction between NTF2 and nucleoporin FxFG repeats. EMBO J. 2002a;21:2843–2853. doi: 10.1093/emboj/cdf305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss R., Littlewood T., Strawn L. A., Wente S. R., Stewart M. GLFG and FxFG nucleoporins bind to overlapping sites on importin-beta. J. Biol. Chem. 2002b;277:50597–50606. doi: 10.1074/jbc.M209037200. [DOI] [PubMed] [Google Scholar]
- Ben-Efraim I., Gerace L. Gradient of increasing affinity of importin beta for nucleoporins along the pathway of nuclear import. J. Cell Biol. 2001;152:411–417. doi: 10.1083/jcb.152.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J. W., Belanger K. D., Sannella M. I., Davis L. I. The yeast nucleoporin Nup2p is involved in nuclear export of importin alpha/Srp1p. J. Biol. Chem. 1999;274:32360–32367. doi: 10.1074/jbc.274.45.32360. [DOI] [PubMed] [Google Scholar]
- Chen X. Q., Du X., Liu J., Balasubramanian M. K., Balasundaram D. Identification of genes encoding putative nucleoporins and transport factors in the fission yeast Schizosaccharomyces pombe: a deletion analysis Yeast. 2004;21:495–509. doi: 10.1002/yea.1115. [DOI] [PubMed] [Google Scholar]
- Cronshaw J. M., Krutchinsky A. N., Zhang W., Chait B. T., Matunis M. J. Proteomic analysis of the mammalian nuclear pore complex. J. Cell Biol. 2002;158:915–927. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman I., Palzkill T., Moore M. S. Using peptide arrays to define nuclear carrier binding sites on nucleoporins. Methods. 2006;39:329–341. doi: 10.1016/j.ymeth.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Dang V. D., Levin H. L. Nuclear import of the retrotransposon Tf1 is governed by a nuclear localization signal that possesses a unique requirement for the FXFG nuclear pore factor Nup124p. Mol. Cell Biol. 2000;20:7798–7812. doi: 10.1128/mcb.20.20.7798-7812.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning D., Mykytka B., Allen N. P., Huang L., Al B., Rexach M. The nucleoporin Nup60p functions as a Gsp1p-GTP-sensitive tether for Nup2p at the nuclear pore complex. J. Cell Biol. 2001;154:937–950. doi: 10.1083/jcb.200101007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning D. P., Patel S. S., Uversky V., Fink A. L., Rexach M. Disorder in the nuclear pore complex: the FG repeat regions of nucleoporins are natively unfolded. Proc. Natl. Acad. Sci. USA. 2003;100:2450–2455. doi: 10.1073/pnas.0437902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning D. P., Rexach M. F. Rapid evolution exposes the boundaries of domain structure and function in natively unfolded FG nucleoporins. Mol. Cell Proteomics. 2007;6:272–282. doi: 10.1074/mcp.M600309-MCP200. [DOI] [PubMed] [Google Scholar]
- Denning D. P., Uversky V., Patel S. S., Fink A. L., Rexach M. The Saccharomyces cerevisiae nucleoporin Nup2p is a natively unfolded protein. J. Biol. Chem. 2002;277:33447–33455. doi: 10.1074/jbc.M203499200. [DOI] [PubMed] [Google Scholar]
- Devos D., Dokudovskaya S., Williams R., Alber F., Eswar N., Chait B. T., Rout M. P., Sali A. Simple fold composition and modular architecture of the nuclear pore complex. Proc. Natl. Acad. Sci. USA. 2006;103:2172–2177. doi: 10.1073/pnas.0506345103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D. Q., Tomita Y., Yamamoto A., Chikashige Y., Haraguchi T., Hiraoka Y. Large-scale screening of intracellular protein localization in living fission yeast cells by the use of a GFP-fusion genomic DNA library. Genes Cells. 2000;5:169–190. doi: 10.1046/j.1365-2443.2000.00317.x. [DOI] [PubMed] [Google Scholar]
- Du X., Rao M. R., Chen X. Q., Wu W., Mahalingam S., Balasundaram D. The homologous putative GTPases Grn1p from fission yeast and the human GNL3L are required for growth and play a role in processing of nucleolar pre-rRNA. Mol. Biol. Cell. 2006;17:460–474. doi: 10.1091/mbc.E05-09-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., et al. Intrinsically disordered protein. J. Mol. Graph. Model. 2001;19:26–59. doi: 10.1016/s1093-3263(00)00138-8. [DOI] [PubMed] [Google Scholar]
- Fabre E., Hurt E. Yeast genetics to dissect the nuclear pore complex and nucleocytoplasmic trafficking Annu. Rev. Genet. 1997;31:277–313. doi: 10.1146/annurev.genet.31.1.277. [DOI] [PubMed] [Google Scholar]
- Fahrenkrog B., Aebi U. The nuclear pore complex: nucleocytoplasmic transport and beyond. Nat. Rev. Mol. Cell Biol. 2003;4:757–766. doi: 10.1038/nrm1230. [DOI] [PubMed] [Google Scholar]
- Fahrenkrog B., Maco B., Fager A. M., Koser J., Sauder U., Ullman K. S., Aebi U. Domain-specific antibodies reveal multiple-site topology of Nup153 within the nuclear pore complex. J. Struct. Biol. 2002;140:254–267. doi: 10.1016/s1047-8477(02)00524-5. [DOI] [PubMed] [Google Scholar]
- Finlay D. R., Forbes D. J. Reconstitution of biochemically altered nuclear pores: transport can be eliminated and restored. Cell. 1990;60:17–29. doi: 10.1016/0092-8674(90)90712-n. [DOI] [PubMed] [Google Scholar]
- Floer M., Blobel G., Rexach M. Disassembly of RanGTP-karyopherin beta complex, an intermediate in nuclear protein import. J. Biol. Chem. 1997;272:19538–19546. doi: 10.1074/jbc.272.31.19538. [DOI] [PubMed] [Google Scholar]
- Frey S., Richter R. P., Gorlich D. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science. 2006;314:815–817. doi: 10.1126/science.1132516. [DOI] [PubMed] [Google Scholar]
- Gilchrist D., Rexach M. Molecular basis for the rapid dissociation of nuclear localization signals from karyopherin alpha in the nucleoplasm. J. Biol. Chem. 2003;278:51937–51949. doi: 10.1074/jbc.M307371200. [DOI] [PubMed] [Google Scholar]
- Gustin K. E., Sarnow P. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J. Virol. 2002;76:8787–8796. doi: 10.1128/JVI.76.17.8787-8796.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag A. L., Lin J. H., Levin H. L. Evidence for the packaging of multiple copies of Tf1 mRNA into particles and the trans priming of reverse transcription. J. Virol. 2000;74:7164–7170. doi: 10.1128/jvi.74.15.7164-7170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase M. E., Cordes V. C. Direct interaction with nup153 mediates binding of Tpr to the periphery of the nuclear pore complex. Mol. Biol. Cell. 2003;14:1923–1940. doi: 10.1091/mbc.E02-09-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin B., Aye M., Baldi P., Beliakova-Bethell N., Cheng H., Dou Y., Liou W., Sandmeyer S. Retroviruses and yeast retrotransposons use overlapping sets of host genes. Genome Res. 2005;15:641–654. doi: 10.1101/gr.3739005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly F. D., Levin H. L. The evolution of transposons in Schizosaccharomyces pombe. Cytogenet. Genome Res. 2005;110:566–574. doi: 10.1159/000084990. [DOI] [PubMed] [Google Scholar]
- Kenna M. A., Brachmann C. B., Devine S. E., Boeke J. D. Invading the yeast nucleus: a nuclear localization signal at the C terminus of Ty1 integrase is required for transposition in vivo. Mol. Cell. Biol. 1998;18:1115–1124. doi: 10.1128/mcb.18.2.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. K., Claiborn K. C., Levin H. L. The long terminal repeat-containing retrotransposon Tf1 possesses amino acids in gag that regulate nuclear localization and particle formation. J. Virol. 2005;79:9540–9555. doi: 10.1128/JVI.79.15.9540-9555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiseleva E., Allen T. D., Rutherford S., Bucci M., Wente S. R., Goldberg M. W. Yeast nuclear pore complexes have a cytoplasmic ring and internal filaments. J. Struct. Biol. 2004;145:272–288. doi: 10.1016/j.jsb.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Levin H. L., Boeke J. D. Demonstration of retrotransposition of the Tf1 element in fission yeast. EMBO J. 1992;11:1145–1153. doi: 10.1002/j.1460-2075.1992.tb05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin H. L., Weaver D. C., Boeke J. D. Two related families of retrotransposons from Schizosaccharomyces pombe. Mol. Cell. Biol. 1990;10:6791–6798. doi: 10.1128/mcb.10.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin H. L., Weaver D. C., Boeke J. D. Novel gene expression mechanism in a fission yeast retroelement: Tf1 proteins are derived from a single primary translation product. EMBO J. 1993;12:4885–4895. doi: 10.1002/j.1460-2075.1993.tb06178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R. Y., Aebi U., Stoffler D. From the trap to the basket: getting to the bottom of the nuclear pore complex. Chromosoma. 2006;115:15–26. doi: 10.1007/s00412-005-0037-1. [DOI] [PubMed] [Google Scholar]
- Lin S. S., Nymark-McMahon M. H., Yieh L., Sandmeyer S. B. Integrase mediates nuclear localization of Ty3. Mol. Cell. Biol. 2001;21:7826–7838. doi: 10.1128/MCB.21.22.7826-7838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. M., Stewart M. Structural basis for the high-affinity binding of nucleoporin Nup1p to the Saccharomyces cerevisiae importin-beta homologue, Kap95p. J. Mol. Biol. 2005;349:515–525. doi: 10.1016/j.jmb.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Matsusaka T., Imamoto N., Yoneda Y., Yanagida M. Mutations in fission yeast Cut15, an importin alpha homolog, lead to mitotic progression without chromosome condensation. Curr. Biol. 1998;8:1031–1034. doi: 10.1016/s0960-9822(07)00425-3. [DOI] [PubMed] [Google Scholar]
- Moroianu J., Blobel G., Radu A. RanGTP-mediated nuclear export of karyopherin alpha involves its interaction with the nucleoporin Nup153. Proc. Natl. Acad. Sci. USA. 1997;94:9699–9704. doi: 10.1073/pnas.94.18.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulillo S. M., Phillips E. M., Koser J., Sauder U., Ullman K. S., Powers M. A., Fahrenkrog B. Nucleoporin domain topology is linked to the transport status of the nuclear pore complex. J. Mol. Biol. 2005;351:784–798. doi: 10.1016/j.jmb.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Pyhtila B., Rexach M. A gradient of affinity for the karyopherin Kap95p along the yeast nuclear pore complex. J. Biol. Chem. 2003;278:42699–42709. doi: 10.1074/jbc.M307135200. [DOI] [PubMed] [Google Scholar]
- Ribbeck K., Gorlich D. Kinetic analysis of translocation through nuclear pore complexes. EMBO J. 2001;20:1320–1330. doi: 10.1093/emboj/20.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbeck K., Gorlich D. The permeability barrier of nuclear pore complexes appears to operate via hydrophobic exclusion. EMBO J. 2002;21:2664–2671. doi: 10.1093/emboj/21.11.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout M. P., Aitchison J. D. Pore relations: nuclear pore complexes and nucleocytoplasmic exchange. Essays Biochem. 2000;36:75–88. doi: 10.1042/bse0360075. [DOI] [PubMed] [Google Scholar]
- Rout M. P., Aitchison J. D., Suprapto A., Hjertaas K., Zhao Y., Chait B. T. The yeast nuclear pore complex: composition, architecture, and transport mechanism. J. Cell Biol. 2000;148:635–651. doi: 10.1083/jcb.148.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K. J., Wente S. R. The nuclear pore complex: a protein machine bridging the nucleus and cytoplasm. Curr. Opin. Cell Biol. 2000;12:361–371. doi: 10.1016/s0955-0674(00)00101-0. [DOI] [PubMed] [Google Scholar]
- Shulga N., Goldfarb D. S. Binding dynamics of structural nucleoporins govern nuclear pore complex permeability and may mediate channel gating. Mol. Cell. Biol. 2003;23:534–542. doi: 10.1128/MCB.23.2.534-542.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M., Baker R. P., Bayliss R., Clayton L., Grant R. P., Littlewood T., Matsuura Y. Molecular mechanism of translocation through nuclear pore complexes during nuclear protein import. FEBS Lett. 2001;498:145–149. doi: 10.1016/s0014-5793(01)02489-9. [DOI] [PubMed] [Google Scholar]
- Stoffler D., Fahrenkrog B., Aebi U. The nuclear pore complex: from molecular architecture to functional dynamics. Curr. Opin. Cell Biol. 1999;11:391–401. doi: 10.1016/S0955-0674(99)80055-6. [DOI] [PubMed] [Google Scholar]
- Stoffler D., Schwarz-Herion K., Aebi U., Fahrenkrog B. Getting across the nuclear pore complex: new insights into nucleocytoplasmic transport. Can. J. Physiol. Pharmacol. 2006;84:499–507. doi: 10.1139/y06-001. [DOI] [PubMed] [Google Scholar]
- Strawn L. A., Shen T., Shulga N., Goldfarb D. S., Wente S. R. Minimal nuclear pore complexes define FG repeat domains essential for transport. Nat. Cell Biol. 2004;6:197–206. doi: 10.1038/ncb1097. [DOI] [PubMed] [Google Scholar]
- Stutz F., Kantor J., Zhang D., McCarthy T., Neville M., Rosbash M. The yeast nucleoporin rip1p contributes to multiple export pathways with no essential role for its FG-repeat region. Genes Dev. 1997;11:2857–2868. doi: 10.1101/gad.11.21.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntharalingam M., Wente S. R. Peering through the Pore. Nuclear pore complex structure, assembly, and function. Dev. Cell. 2003;4:775–789. doi: 10.1016/s1534-5807(03)00162-x. [DOI] [PubMed] [Google Scholar]
- Teysset L., Dang V. D., Kim M. K., Levin H. L. A long terminal repeat-containing retrotransposon of Schizosaccharomyces pombe expresses a Gag-like protein that assembles into virus-like particles which mediate reverse transcription. J. Virol. 2003;77:5451–5463. doi: 10.1128/JVI.77.9.5451-5463.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran E. J., Wente S. R. Dynamic nuclear pore complexes: life on the edge. Cell. 2006;125:1041–1053. doi: 10.1016/j.cell.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Umeda M., Izaddoost S., Cushman I., Moore M. S., Sazer S. The fission yeast Schizosaccharomyces pombe has two importin-alpha proteins, Imp1p and Cut15p, which have common and unique functions in nucleocytoplasmic transport and cell cycle progression. Genetics. 2005;171:7–21. doi: 10.1534/genetics.105.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadarajan P., Mahalingam S., Liu P., Ng S. B., Gandotra S., Dorairajoo D. S., Balasundaram D. The functionally conserved nucleoporins Nup124p from fission yeast and the human Nup153 mediate nuclear import and activity of the Tf1 retrotransposon and HIV-1 Vpr. Mol. Biol. Cell. 2005;16:1823–1838. doi: 10.1091/mbc.E04-07-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente S. R. Gatekeepers of the nucleus. Science. 2000;288:1374–1377. doi: 10.1126/science.288.5470.1374. [DOI] [PubMed] [Google Scholar]
- Yang Q., Rout M. P., Akey C. W. Three-dimensional architecture of the isolated yeast nuclear pore complex: functional and evolutionary implications. Mol. Cell. 1998;1:223–234. doi: 10.1016/s1097-2765(00)80023-4. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Sazer S. Nucleocytoplasmic transport and nuclear envelope integrity in the fission yeast Schizosaccharomyces pombe. Methods. 2004;33:226–238. doi: 10.1016/j.ymeth.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Zeitler B., Weis K. The FG-repeat asymmetry of the nuclear pore complex is dispensable for bulk nucleocytoplasmic transport in vivo. J. Cell Biol. 2004;167:583–590. doi: 10.1083/jcb.200407156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.