Abstract
Cystic fibrosis results from mutations in the cystic fibrosis conductance regulator protein (CFTR), a cAMP/protein kinase A (PKA) and ATP-regulated Cl− channel. CFTR is increasingly recognized as a component of multiprotein complexes and although several inhibitory proteins to CFTR have been identified, protein complexes that stimulate CFTR function remain less well characterized. We report that annexin 2 (anx 2)–S100A10 forms a functional cAMP/PKA/calcineurin (CaN)-dependent complex with CFTR. Cell stimulation with forskolin/3-isobutyl-1-methylxanthine significantly increases the amount of anx 2–S100A10 that reciprocally coimmunoprecipitates with cell surface CFTR and calyculin A. Preinhibition with PKA or CaN inhibitors attenuates the interaction. Furthermore, we find that the acetylated peptide (STVHEILCKLSLEG, Ac1-14), but not the nonacetylated equivalent N1-14, corresponding to the S100A10 binding site on anx 2, disrupts the anx 2–S100A10/CFTR complex. Analysis of 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) and CFTRinh172-sensitive currents, taken as indication of the outwardly rectifying Cl− channels (ORCC) and CFTR-mediated currents, respectively, showed that Ac1-14, but not N1-14, inhibits both the cAMP/PKA-dependent ORCC and CFTR activities. CaN inhibitors (cypermethrin, cyclosporin A) discriminated between ORCC/CFTR by inhibiting the CFTRinh172-, but not the DIDS-sensitive currents, by >70%. Furthermore, peptide Ac1-14 inhibited acetylcholine-induced short-circuit current measured across a sheet of intact intestinal biopsy. Our data suggests that the anx 2–S100A10/CFTR complex is important for CFTR function across epithelia.
INTRODUCTION
Cystic fibrosis (CF) is caused by a mutated Cl− channel, the cystic fibrosis transmembrane conductance regulator (CFTR), and manifests as a series of disorders that affect the respiratory, digestive, and reproductive systems. Mature wild-type CFTR resides in the apical membrane where it controls ion and fluid transport. A fraction of CFTR is detectable in association with membranes of the secretory pathway (Bradbury, 1999). The majority of CF patients carry the F508del-CFTR mutation, which causes improper folding of the CFTR protein affecting its traffic through the secretory pathway (Kerem et al., 1989; Bertrand and Frizzell, 2003).
Although there is no significant difference between wild-type and CF cells with regard to forskolin (FSK)-stimulated adenylyl cyclase activity (Mak et al., 2002), CF airway and gut epithelia are nevertheless characterized by a failure to generate Cl− flux after stimulation with cAMP agonists. In addition, Bradbury et al. (1992) found that mutant CF epithelia exhibited no cAMP-dependent regulation of endocytosis or exocytosis until they were transfected with cDNA encoding wild-type CFTR. Furthermore, Bebok et al. (2005) failed to restore cAMP-dependent Cl− flux in F508del-CFTR CFBE41o− cells grown at 27°C to allow “rescue” of F508del-CFTR by promoting its maturation to the apical membrane. These data suggest the existence of a signaling defect downstream of cAMP generation in CF cells. However, signal transduction pathways and resulting protein complexes that regulate CFTR function at the apical membrane are unknown. For example, although phosphorylation by cAMP-dependent protein kinase A (PKA) is the major intracellular signaling mechanism for activation of CFTR, the precise means by which PKA phosphorylation of CFTR induces Cl− flux is unknown (Naren et al., 1999; Dahan et al., 2001). Cyclic AMP also triggers CFTR processing and trafficking to the plasma membrane in various cell types, and at the same time it activates other cellular processes that may or may not be related to CFTR function (Nakamura and Gold, 1987; DiFrancesco and Tortora, 1991; Bos et al., 2003). In some polarized epithelial cells, CFTR is internalized rapidly from the plasma membrane solely through the clathrin-mediated pathway (Bradbury et al., 1999) indicating that endocytosis may influence the residence time of CFTR at the cell surface. It has also been suggested that cAMP-stimulated CFTR may regulate plasma membrane recycling. It is clear that CFTR mutation disrupts intracellular trafficking of CFTR (Bradbury, 1999; Weixel and Bradbury, 2000), and although controversial, increasing evidence indicates that in some cell types, F508del-CFTR is potentially functional and present at the plasma membrane (Kalin et al., 1999; Penque et al., 2000). Furthermore, recent work showed that CFTR is efficiently processed to post-Golgi compartments (Varga et al., 2004) and defective intracellular trafficking, endocytosis and exocytosis observed in CF strongly suggest that CFTR may interact with and regulate proteins of the secretory pathway.
Annexins bind negatively charged phospholipids and cellular membranes in a calcium-dependent manner (Moss, 1992; Rety et al., 1999; Gerke and Moss, 2002; Santamaria-Kisiel et al., 2006), share significant sequence homology with CFTR around the region of the most common CF mutation (Chap et al., 1991), and are also implicated in the regulation of vesicular traffic (Creutz, 1992). They are frequently associated with membrane compartments engaged in endocytosis and exocytosis (Turpin et al., 1998; Gerke and Moss, 2002; Zobiack et al., 2003). In addition, annexins interact with cytoskeletal proteins, to modulate formation of membrane vesicles and membrane fusion.
Annexin 2 (anx 2) forms a heterotetrameric complex with S100A10 (Santamaria-Kisiel et al., 2006). S100A10 (previously known as p11, calpactin I light chain, and annexin II ligand) is a dimer composed of two 11-kDa subunits, which belongs to the S100 calcium binding protein superfamily, and it functions as one of the mediators of calcium-dependent signaling pathway. Anx 2–S100A10 complex, located at the inner surface of the plasma membrane, is found associated with clathrin-coated vesicles and early endosomes (Emans et al., 1993; Turpin et al., 1998), and it regulates vesicle routing from the trans-Golgi network to the apical membrane (Jacob et al., 2004). Thus, anx 2 is involved in membrane fusion, trafficking, and regulating the interaction among ion channels, S100A10, and the cytoskeleton (Ali et al., 1989; Gerke and Moss, 2002). In this regard, both anx 2 and S100A10 interact with and regulate the translocation and function of Na+, K+, and Ca2+ channels (Girard et al., 2002; Okuse et al., 2002; van de Graaf et al., 2003).
Association between several annexins and specific S100 proteins is calcium dependent (Santamaria-Kisiel et al., 2006). However, the anx 2–S100A10 interaction is uniquely calcium independent (Rety et al., 1999; Gerke and Moss, 2002; Santamaria-Kisiel et al., 2006). We recently found that cAMP/PKA regulates the anx 2–S100A10 complex in epithelia (Muimo, 2006). Given that anx 2–S100A10 complex is cAMP/PKA dependent and that CFTR is also regulated by cAMP/PKA (Naren et al., 1999; Dahan et al., 2001), we speculated that anx 2–S100A10 complex may be important for CFTR function.
In this study, we report the formation of a cAMP/PKA-dependent complex between anx 2, S100A10, and CFTR in epithelia, which is important for CFTR function. We report that cAMP/PKA, in a pathway involving calcineurin (CaN, protein phosphatase [PP]2B), induces formation of the anx 2–S100A10/CFTR complex, leading to an increase in CFTR-mediated currents. Accordingly, CFTR function is inhibited when the anx 2–S100A10 complex is disrupted by pretreatment of the cells with CaN inhibitors or a specific peptide corresponding to the anx 2 binding site on S100A10 before FSK stimulation. This study identifies and provides insight into a dynamic cAMP/PKA-dependent CFTR-associated macromolecular complex that may play an important role in regulating CFTR activity in epithelia.
MATERIALS AND METHODS
Cell Culture
Cells, human bronchial epithelial cell line (16HBE14o−) (Gruenert et al., 2004), were cultured in medium 199 plus fetal calf serum as described previously (Cozens et al., 1994) until confluent. Membrane and cytosolic fractions were prepared as described previously (Muimo et al., 2000).
Human Nasal Epithelium (HNE)
HNE were obtained as described previously from healthy young adults undergoing surgery for reasons unrelated to nasal mucosal disease (Mwimbi et al., 2003). Local ethical committee approval and written informed consent were obtained. Nasal brushings were suspended in complete medium 199 until use or storage in liquid nitrogen.
Gut Biopsy
With local ethical committee approval and written informed consent, a sheet of stripped intestine was obtained endoscopically from the distal ileum and the potential difference (PD), short-circuit current (SCC), and tissue resistance were measured using a modified Ussing chamber technique as described previously (Hardcastle et al., 2001). Briefly, the sample was mounted in an Ussing chamber with an aperture of 0.03 cm2 and incubated at 37°C in Krebs bicarbonate saline gassed with 95% O2, 5% CO2. The serosal fluid contained 10 mM glucose and the mucosal fluid 10 mM mannitol. Tissue resistance was determined from the PD change induced by a 50-μA current pulse. SCC was calculated from PD and resistance measurements using Ohm's law. After 10-min stabilization, readings of electrical activity were taken at 1-min intervals. Acetylcholine (Ach; 10−3 M) was added to the serosal solution after 5 min of basal readings, and measurements were taken for a further 5 min before washout of Ach. Glucose (10 mM) was then added mucosally to confirm tissue viability. After removal of glucose, the tissue was allowed to recover for 10 min, and then N1-14 was added to mucosal and serosal solutions, and tissue incubated for 30 min. Readings were repeated for Ach and glucose as described above. After washout of N1-14 and glucose (10 min), the procedure was repeated for Ac1-14.
Immunoprecipitation, Immunoblotting, and Overlay or Far Western Assays
Immunoprecipitation and immunoblotting procedures were conducted essentially as described previously (Muimo et al., 2000). For overlay analysis, proteins were extracted from postnuclear membranes of airway epithelia with Triton X-100. Extracted proteins, CFTR, or anx 2 immunoprecipitate, separated by SDS-polyacrylamide gel electrophoresis (PAGE) were blotted onto polyvinylidene difluoride (PVDF). Blot was blocked with 1× Tris-buffered saline containing 5% nonfat dry milk ± extract (500 μg of protein) and incubated at room temperature for 60 min. Blot was washed (4 times) and then probed with anti-anx 2 (1:2000) and anti-S100A10 (1:1000).
Laser Confocal Microscopy
HNE suspended in complete medium 199 were treated with either FSK/3-isobutyl-1-methylxanthine (IBMX) for 30 min or protein kinase inhibitor (PKI) for 5 min before the addition of FSK/IBMX for a further 30 min. Control cells were incubated in complete medium 199 alone. HNE were fixed in 4% paraformaldehyde for 30 min at RT, quenched with 100 mM glycine, permeabilized (1% Triton X-100, 1X phosphate-buffered saline [PBS]) for 30 min at room temperature (RT), washed (3 times), and blocked with 1% bovine serum albumin (BSA) for 60 min at RT. Cells were incubated overnight at 4°C with anti-anx 2 goat (1:100), anti-S100A10 mouse (1:100) in PBS for 60 min, washed (3 times), and then incubated with with anti-mouse fluorescein isothiocyanate and anti-goat rhodamine (1:100) for 60 min RT. Cells were washed five times with 1X PBS and resuspended in 70% glycerol. Slides were examined by laser confocal microscopy (LSM-510; Carl Zeiss, Jena, Germany). Images were acquired and analyzed using Zeiss software.
Biotinylation of Surface Membrane Proteins
Surface biotinylation of cell surface CFTR was performed as described by Ramjeesingh et al. (2003) with some modifications. Briefly, confluent cells were treated with FSK/IBMX ± PKI for 30 min, washed with ice-cold 1X PBS, and then biotinylated using 1 mg/ml EZ-Link sulfosuccinimidyl 2-(biotinamido)-ethyl-1,3-dithiopropionate for 30 min at 4°C. Free biotin was washed three times with ice-cold 1X PBS containing 0.1% BSA and then with ice-cold 1X PBS. Cells were then scraped in ice-cold homogenization buffer (Muimo et al., 2000) and sonicated. Cell lysate was centrifuged at 300 × g for 2 min, and the pellet was discarded. Prewashed avidin agarose beads in PBS were added to the supernatant and incubated for 30 min at RT. Avidin-bound complexes were pelleted (350 × g) for 2 min and washed five times. Biotinylated proteins were eluted in Laemmli buffer, resolved by SDS-PAGE, electrotransferred, and immunoblotted with the CFTR, anx 2, and S100A10 antibody.
Whole Cell Recordings
16HBE14o− grown on plastic coverslips were placed in a perspex bath on the stage of an inverted microscope (Olympus IX70; Olympus, Tokyo, Japan). Standard patch-clamp experiments were used to investigate whole-cell currents (Hamill et al., 1981). Voltage protocols were controlled by an IBM-compatible computer, equipped with a Digidata interface (Axon Instruments, Foster City, CA) and pClamp software, Clampex 8.0 (Axon Instruments). A List EPC-7 amplifier was used to make recordings.
Whole cell recordings were obtained at room temperature with Na+ Ringer in the bath, containing 140 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 40 mM mannitol, and 10 mM HEPES (titrated to pH 7.4 with NaOH). The pipette contained 135 mM CsCl, 2 mM EGTA, 2 mM MgCl2, 2 mM Na2ATP, and 10 mM HEPES (titrated to pH 7.4 with CsOH). Whole cell currents were saved onto the hard disk of the computer after low-pass filtering (5 kHz). Cell potential was clamped to −40 mV, and then it was stepped to between+100 and −100 mV, in −20 mV steps. Average currents were derived using Excel 2000 (Microsoft, Redmond, WA). Cell area was calculated from capacity transients seen in response to a 20-mV potential step, with membrane capacitance assumed to be 1 μF/cm2. The mean capacitance of cells was 23.2 ± 1.30 pF (n = 57). Previous studies have indicated that 16HBE14o− contain both CFTR and 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS)-sensitive Cl− conductances (Schwiebert et al., 1994). Therefore, 500 μM DIDS was added to the bath to provide the magnitude of the DIDS-sensitive conductance. The magnitude of the CFTR currents was determined by the further addition of 10 μM CFTRinh172 to the bath (in the continued presence of DIDS) (Ma et al., 2002).
To activate cAMP/PKA, cells were incubated with FSK/IBMX for 30 min. To examine the effect of CaN inhibitors on CFTR function, cells were incubated for 5 min in the presence of either 5 nM cypermethrin or 1 μM cyclosporin A, before incubation for 30 min in the presence of the inhibitor plus FSK/IBMX. When the effect of Ac1-14 and N1-14 was tested, cells were incubated in the presence of the peptides (0.16 mg/ml for each) for 30 min before an additional 30 min in the presence of the peptides plus FSK/IBMX. For all experiments, a separate control data set was obtained in the absence of inhibitor or peptide on the same day.
Solutions, Chemicals, Reagents, and Antibodies Used in This Study
Osmolality of the experimental solutions was checked using a Roebling osmometer and adjusted to 300 ± 1 mOsm·kg−1 H2O by using mannitol or water as appropriate. All chemicals unless otherwise indicated were purchased from Sigma-Aldrich (Poole, Dorset, United Kingdom). PVDF membranes were from Millipore (Watford, United Kingdom), and acrylamide and other electrophoretic reagents were from Bio-Rad (Hemel Hempsted, United Kingdom). Calyculin A, okadaic acid, cypermethrin, N-[2-(p-bromocinnamylamino) ethyl]-5-isoquinolinesulfonamide (H-89), and myristoylated protein kinase A inhibitor amide 14-22 were from Calbiochem (Nottingham, United Kingdom). Peptides (>95% purity) were from Sigma Genosys (Haverhill, Suffolk, United Kingdom). Fetal calf serum was from Invitrogen (Paisley, UK). Anti-phosphoserine and anti-phosphothreonine monoclonal antibodies (Q5 and Q7, dilution 1:500; QIAGEN, Dorking, Surrey, United Kingdom), anti-phosphotyrosine (PY99, 1:5000; Autogen Bioclear, Wiltshire, United Kingdom), anti-CaN (1:1000; Sigma-Aldrich), anti-CFTR monoclonal (Lab Vision Products, Cheshire, United Kingdom), and polyclonal (1:1000, R&D Systems Europe, Abingdon, Oxfordshire, United Kingdom), anti-S100A10 (H21; 1:4000), anti-anx 2 monoclonal (HH7, 1:7000), polyclonal (goat 1:2000; all Autogen Bioclear) have been described previously (Thiel et al., 1992).
Data Analysis
Results are presented as mean ± SEM. Effects of experimental interventions were assessed by Student's t test (or analysis of variance [ANOVA]) and significance was assumed at the 5% level. Unless otherwise indicated all immunoblots are representative of at least three independent experiments.
RESULTS
Anx 2–S100A10 Forms a cAMP/PKA-dependent Complex with CFTR
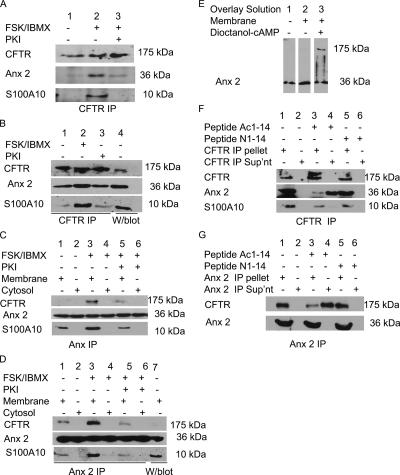
Because the anx 2–S100A10 complex regulates a number of ion channels (Girard et al., 2002; Okuse et al., 2002; van de Graaf et al., 2003) and cAMP/PKA regulates CFTR Cl− flux (Naren et al., 1999; Dahan et al., 2001), we predicted that anx 2–S100A10 may form a cAMP/PKA-dependent complex with CFTR. Immunoblot analysis of CFTR immunoprecipitates for anx 2 and S100A10 showed that both proteins coprecipitated with CFTR (Figure 1A). However, when the immortalized human bronchial epithelial cells (16HBE14o−) (Figure 1A) and HNE cells (Figure 1B) were stimulated with 10 μM FSK/100 μM IBMX, increased amounts of anx 2 and S100A10 coimmunoprecipitated with CFTR. Pretreatment of the cells with 100 mM PKI (myristoylated PKA inhibitor amide 14-22; Cheng et al., 1986) before FSK/IBMX stimulation reduced the level of anx 2 and S100A10 that coimmunoprecipitated with CFTR (Figure 1, A and B). Additionally, the reverse experiment also showed that FSK/IBMX stimulation increased the level of CFTR precipitating with anx 2 (Figure 1, C and D), and inhibition of PKA using PKI attenuated the amount of CFTR that coimmunoprecipitated with anx 2–S100A10. Figure 1E shows that when a blot containing membrane proteins (100 μg) from 16HBE14o− is overlaid with solubilized membrane proteins (0.5 mg/ml), anx 2 staining is additionally observed at 175 kDa (expected size for CFTR) in the presence of 100 μM dioctanoyl-cAMP. Thus, cAMP/PKA may regulate the anx 2–S100A10/CFTR interaction in bronchial and airway epithelia.
Figure 1.
cAMP/PKA-dependent association of anx 2–S100A10 and CFTR in 16HBE14o− and HNE. (A) PKA regulates coimmunoprecipitation of anx 2 and S100A10 with CFTR in 16HBE14o−. Immunoblots of CFTR immunoprecipitates from 16HBE14o− lysates ± FSK/IBMX or PKI/FSK/IBMX probed for CFTR, S100A10, and anx 2. (B) PKA regulates coimmunoprecipitation of anx 2 and S100A10 with CFTR in HNE. Immunoblot of CFTR immunoprecipitates from HNE, untreated (lane 1), FSK/IBMX treated for 30 min (lane 2), or treated with PKI for 5 min before FSK/IBMX treatment (lane 3) probed for CFTR, S100A10, and anx 2. Control loaded with membrane proteins (lane 4). (C) PKA regulates coimmunoprecipitation of CFTR and S100A10 with anx 2 in 16HBE14o−. Immunoblot of anx 2 immunoprecipitate from 16HBE14o− membrane and cytosol ± FSK/IBMX or PKI (5 min) before FSK/IBMX, probed for CFTR, S100A10, and anx 2. Anx 2 immunoprecipitate from untreated cells (lanes 1 and 2), FSK/IBMX-treated cells (lanes 3 and 4), or cells treated with PKI (5 min) before FSK/IBMX for 30 min (lanes 5 and 6). (D) PKA controls coimmunoprecipitation of CFTR and S100A10 with anx 2 in HNE. Immunoblot of anx 2 immunoprecipitate from membrane (lanes 1, 3, and 5) and cytosol (lanes 2, 4, and 6) of HNE ± FSK or PKI/FSK/IBMX probed for CFTR, S100A10. Anx 2 immunoprecipitates from cells untreated (lanes 1 and 2), FSK/IBMX-treated cells (lanes 3 and 4), or cells treated with PKI for 5 min before FSK/IBMX for 30 min (lanes 5 and 6). Control loaded with membrane proteins (lane 7). (E) cAMP-dependent anx 2 binding to a 175-kDa protein in overlay assays. Identical immunoblots of 16HBE14o− membrane proteins (100 μg) probed for anx 2. Lanes: control (1), blot overlaid with 0.5 mg/ml solubilized membrane proteins (2), and blot overlaid with solution 2 containing dioctanoyl cAMP (3). Results are representative of four separate experiments. (F) Ac1-14 dissociates anx 2, but not S100A10, from CFTR immunoprecipitate. Immunoblots of CFTR immunoprecipitates from 16HBE14o− lysates treated with FSK/IBMX and probed for S100A10 and anx 2 show Ac1-14 (lanes 3 and 4), but not N1-14 (lanes 5 and 6), released anx 2 from CFTR immunoprecipitate. (G) Ac1-14 dissociates CFTR from anx 2 immunoprecipitate. Immunoblots of anx 2 immunoprecipitate from 16HBE14o− cells treated with FSK/IBMX and probed for CFTR and anx 2. Ac1-14 (lanes 3 and 4), but not N1-14 (lanes 5 and 6), released CFTR from anx 2 immunoprecipitate. To confirm equal loading, blots were stripped and reprobed with anti-CFTR (R&D Systems Europe) (A, B, and F) or anti-anx 2 (HH7) (C, D, and G).
The acetylated synthetic peptide comprising the N-terminal 14 amino acids of anx 2 [Acetyl-STVHEILCKLSLEG (Ac1-14)] specifically disrupts anx 2 binding to S100A10 (Becker et al., 1990; Kube et al., 1992; Nilius et al., 1996; Konig et al., 1998). In contrast, the nonmodified peptide STVHEILCKLSLEG (N1-14) (Nilius et al., 1996; Konig et al., 1998), fails to disrupt the anx 2–S100A10 complex. To analyze the ability of Ac1-14 to disrupt the anx 2–S100A10/CFTR complex, immunoprecipitates were incubated with 100 μM peptide for 30 min at 30°C and then centrifuged. Ac1-14 (Figure 1F, lanes 3 and 4), but not N1-14 (Figure 1F, lanes 5 and 6), released anx 2 into supernatant, whereas S100A10 remained bound to the CFTR immunoprecipitate. In the reverse experiment, Ac1-14 (Figure 1G, lanes 3 and 4), but not N1-14 (Figure 1G, lanes 5 and 6) released CFTR from the anx 2 immunoprecipitate. This suggested that Ac1-14 can disrupt preformed cAMP/PKA-dependent anx 2–S100A10/CFTR complex and that association of anx 2 with CFTR requires the presence of S100A10. In the reverse experiment, release of CFTR (alongside S100A10) from the anx 2 immunoprecipitate provided further supportive evidence for the potential role of S100A10 as a bridging molecule between CFTR and anx 2.
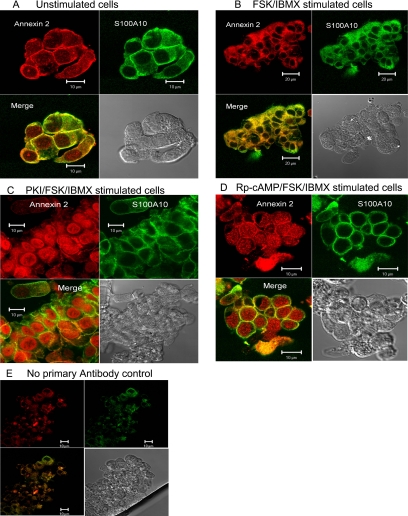
Immunolocalization of anx 2 and S100A10 in HNE treated with or without FSK and/or PKA inhibitors was used to examine the impact of cAMP and PKA on cellular distribution of these proteins. In unstimulated HNE, S100A10 staining was observed predominantly at or near the plasma membrane/cell junction, whereas anx 2, although predominately located at the plasma membrane, was also distributed throughout the cell, including the nucleus, Figure 2A. Colocalization of S100A10 and anx 2 was limited to the plasma membrane/cell junctions. After cell stimulation with FSK/IBMX, a reduction in anx 2 nuclear staining was observed combined with an enhanced staining of the protein both intracellularly and at the plasma membrane/cell junction (Figure 2B). FSK/IBMX stimulation resulted in a more even distribution of S100A10 throughout the cell except the nuclei. Therefore, enhanced colocalization of anx 2 and S100A10 was observed throughout the cell, including the plasma membrane, but not the nuclei of stimulated cells. Additionally, inhibition of PKA activity using PKI or the inhibitory Rp-cAMP analogue (100 μM) before FSK/IBMX stimulation resulted in enhanced anx 2 nuclear staining and a reduced colocalization between anx 2 and S100A10 within the cytoplasm and plasma membrane (Figure 2, C and D). PKI alone also generated enhanced nuclear localization of anx 2, confirmed by Western blot analysis of fractionated cells (not shown). These data suggested that cAMP/PKA might regulate the interaction, distribution, and function of anx 2 and S100A10 in airway epithelia.
Figure 2.
PKA regulates anx 2 and S100A10 cellular distribution in HNE cells. Modulation of PKA activity alters localization and distribution of anx 2–S100A10 in HNE. Immunocytochemical staining of HNE for anx 2 and S100A10 in cells that were untreated (A), treated with FSK/IBMX for 30 min (B), treated with PKI (5 min) before FSK/IBMX for 30 min (C), and treated with Rp-cAMP for 5 min before FSK/IBMX for 30 min (D). (E) No primary antibody control. The result is representative of three independent experiments.
Anx 2–S100A10 Complex Binds Cell Surface CFTR
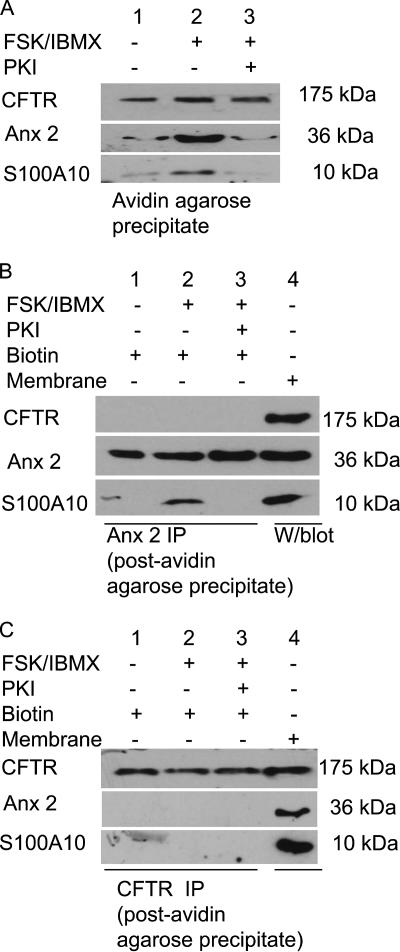
We speculated that the cAMP/PKA-induced anx 2–S100A10 complex might associate with cell surface CFTR and regulate CFTR function. After cell surface biotinylation, the membrane fraction was depleted of biotin-labeled (cell surface/integral membrane) proteins by avidin agarose precipitation. Figure 3A shows the presence of CFTR in all avidin precipitates, with no detectable change in the amount of cell surface CFTR in FSK stimulated cells. In contrast, anx 2 and S100A10 staining increased in the avidin precipitate after FSK stimulation (Figure 3A, lane 2) and preinhibition of PKA attenuated the increase (Figure 3A, lane 3). To assess whether anx 2–S100A10 also associates with noncell surface CFTR, CFTR immunoprecipitates from cell extracts (±FSK/IBMX stimulation) depleted of cell surface/integral membrane proteins, by avidin precipitation, were probed for anx 2 and S100A10. Figure 3, B and C, show that, despite the presence of all three proteins, anx 2 and S100A10 did not coimmunoprecipitate with CFTR from these fractions. Similarly, in the reverse experiment, CFTR staining is undetectable in anx 2 immunoprecipitates from fractions depleted of biotin-labeled proteins. Conversely, it is worth noting that the FSK-dependent interaction between anx 2 and S100A10 occurred in fractions depleted of biotin-labeled proteins (Figure 3C). Thus, after cAMP/PKA stimulation, there was increased coprecipitation of anx 2 and S100A10 with cell surface CFTR, but not noncell surface CFTR, suggesting that the cAMP/PKA-induced anx 2–S100A10 complex tethers to cell surface CFTR.
Figure 3.
cAMP/PKA-dependent anx 2–S100A10 complex binds cell surface CFTR. (A) Immunoblots of avidin agarose precipitates from 16HBE14o− lysates ± FSK or PKI/FSK/IBMX, biotinylated for 30 min at 4°C and probed for CFTR, anx 2, and S100A10. Cell surface CFTR associates with anx 2 and S100A10. (B) Immunoblots of anx 2 immunoprecipitates from 16HBE14o− lysates ± FSK or PKI/FSK/IBMX (postavidin precipitation in A) probed for CFTR, S100A10, and anx 2. Anx 2 does not associate with noncell surface CFTR. (C) Immunoblots of CFTR immunoprecipitates from 16HBE14o− lysates ± FSK or PKI/FSK/IBMX (postavidin precipitation in A) probed for CFTR, S100A10, and anx 2. Noncell surface CFTR does not associate with S100A10 and anx 2. To confirm equal loading of the immunoprecipitates, blots were stripped and reprobed with anti-anx 2 (HH7; B) or anti-CFTR (R&D Systems Europe; C).
CaN Mediates the cAMP/PKA-dependent Anx 2–S100A10/CFTR Interaction
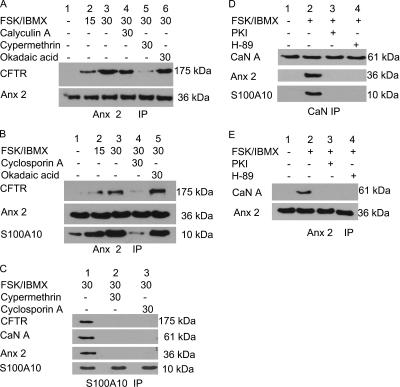
Because CaN is important for the cAMP/PKA-dependent anx 2–S100A10 complex formation (Muimo, 2006), we hypothesized that CaN may also be relevant to the cAMP/PKA-induced anx 2–S100A10 interaction with CFTR. We analyzed the composition of the complex in membranes of 16HBE14o− pretreated with phosphatase inhibitors (100 nM calyculin A, 5 nM cypermethrin, or 1 μm okadaic acid) before FSK/IBMX treatment. We found that, alongside anx 2–S100A10 complex formation, the FSK-induced anx 2–S100A10/CFTR complex was time dependent (Figure 4, A and B, lanes 1–3). Importantly, cypermethrin, a CaN phosphatase selective inhibitor that does not inhibit PP1, PP2A, or other PPs (Liu et al., 1991; Sistiaga and Sanchez-Prieto, 2000), disrupted complex formation and reduced CFTR coimmunoprecipitation with anx 2 (Figure 4A, lane 5). Conversely, okadaic acid or calyculin A, at concentrations well above the reported IC50 values for PP1 and PP2A (MacKintosh and MacKintosh, 1994; Herzig and Neumann, 2000), showed little or no inhibitory effect on the cAMP/PKA-dependent complex (Figure 4, A and B). Additionally, preincubation of the cells with 1 μM cyclosporin A, an established potent and selective inhibitor for CaN (Liu et al., 1991; Yakel, 1997) inhibited the FSK-induced complex (Figure 4B, lane 4). Analysis of S100A10 immunoprecipitates from membranes of 16HBE14o− pretreated with cyclosporin A and cypermethrin before FSK stimulation showed that both CaN inhibitors inhibit interaction between S100A10, annexin 2, and CFTR (Figure 4C).
Figure 4.
CaN mediates FSK-dependent interaction between anx 2–S100A10 and CFTR. (A) Cypermethrin inhibits time-dependent coimmunoprecipitation of anx 2 and CFTR. Immunoblot of anx 2 immunoprecipitates from 16HBE14o− membranes probed for CFTR and anx 2. The immunoprecipitates were from cells treated with FSK/IBMX for 0, 15, and 30 min (lanes 1, 2, and 3, respectively) or calyculin A (lane 4), cypermethrin (lane 5), or okadaic acid (lane 6) for 30 min before FSK/IBMX for 30 min. (B) Cyclosporin A inhibits CFTR coimmunoprecipitation with anx 2. Immunoblot of anx 2 immunoprecipitate from 16HBE14o− membranes probed for CFTR, anx 2, and S100A10. Anx 2 immunoprecipitates were from cells treated with FSK/IBMX for 0, 15, and 30 min (lanes 1, 2, and 3, respectively) or cyclosporin A (lane 4) or okadaic acid (lane 5) for 30 min before FSK/IBMX for 30 min. (C) Immunoblots of S100A10 immunoprecipitates from membranes of 16HBE14o− probed for CFTR, CaN A, and anx 2. Lanes 1, treated with FSK/IBMX for 30 min; lane 2, treated with cypermethrin; or lane 3, cyclosporin A for 30 min before FSK/IBMX for 30 min. (D) Immunoblots of CaN A immunoprecipitate from 16HBE14o− membranes probed for anx 2 and S100A10. CaN immunoprecipitate from cells: lane 1, untreated; lane 2, treated with FSK/IBMX for 30 min, and lane 3) treated with PKI or lane 4) H-89 for 5 min before FSK/IBMX for 30 min. CaN A association with anx 2–S100A10 is cAMP/PKA dependent. (E) CaN A coimmunoprecipitates with anx 2. Immunoblots of anx 2 immunoprecipitate from 16HBE14o− membranes probed for CaN A. Anx 2 immunoprecipitate from cells that were untreated (lane 1), treated with FSK/IBMX for 30 min (lane 2), and treated with PKI (lane 3) or H-89 for 5 min before FSK/IBMX for 30 min (land 4). To confirm equal loading, the blots were stripped and reprobed for anx 2 (A, B, and D), S100A10 (C), or CaN (E).
Disruption of the cAMP/PKA-induced complex by CaN inhibitors suggested that CaN might be part of the cAMP/PKA-dependent anx 2/S100A10/CFTR macromolecular complex (Figure 4C). Figure 4, D and E, showed that anx 2 coimmunoprecipitates with CaN only from cells stimulated with FSK/IBMX. CaN could not be detected in anx 2 immunoprecipitates from cells pretreated with PKA inhibitors, PKI, or 1 μM H-89, before FSK/IBMX stimulation (Figure 4D). Similar results were obtained in the reverse experiment, when CaN immunoprecipitates from similar cell preparations were probed for anx 2 (Figure 4E). Thus, CaN may form part of the cAMP/PKA-dependent anx 2–S100A10/CFTR complex.
Anx 2–S100A10 Complex Regulates CFTR Function
The data mentioned above suggested that anx 2–S100A10 might regulate CFTR function. The functional significance of the complex to CFTR was determined by analyzing both CFTR-mediated short-circuit current (SSC) in intact primary tissue and whole cell currents in 16HBE14o−.
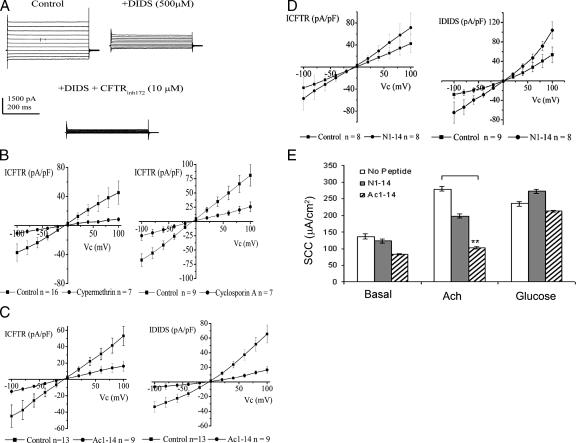
Whole cell currents were recorded from 16HBE14o− grown on plastic coverslips. Cells demonstrated large anion-selective currents that were slightly outwardly rectifying after incubation with FSK and IBMX; Figure 5A. The current at +100 mV was 118.7 ± 35.59 pA/pF, whereas the current at −100 mV was −84.6 ± 24.2 pA/pF (n = 16). DIDS at 500 μM decreased whole cell currents (see Supplemental Material) (n = 16). This DIDS sensitive current was outwardly rectifying. The remaining current was sensitive to 10 μM CFTRinh172 (see Supplemental Material) (n = 16). This CFTRihn172 sensitive current was ohmic. Currents sensitive to DIDS and CFTRinh172 were taken as the DIDS-sensitive current (IDIDS) and CFTR mediated current (ICFTR), respectively.
Figure 5.
Impact of complex disruption on whole cell outwardly rectifying and CFTR mediated Cl− currents in 16HBE14o− stimulated with FSK/IBMX. (A) Outwardly rectifying and CFTR-mediated Cl− currents in 16HBE14o− cells stimulated with FSK/IBMX for 30 min. Whole cell currents recorded from a typical cell under the control circumstance and in the presence of 500 μM DIDS and 500 μM DIDS plus 10 μM CFTRihn172. Clamp potential was stepped from −40 mV to between +100 and −100 mV, in −20 mV steps. (B) Effect of CaN inhibitors cypermethrin (5 nM) and cyclosporin A (1 μM) on ICFTR. Cells were incubated with CaN inhibitors for 5 min before exposure to FSK/BMX plus inhibitor for 30 min. Control currents for each data set were day matched. CaN inhibitors inhibited the cAMP/PKA-dependent CFTR, but not ORCC, activity. (C and D) Effect of Ac1-14 and N1-14 on IDIDS and ICFTR. Cells were incubated for 30 min in the presence of peptide, followed by incubation with the peptide plus FSK/IBMX for 30 min. Control currents for each data set were day matched, with control cells incubated for 30 min in control solution, followed by 30 min in the presence of FSK/IBMX. Ac1-14, but not N1-14, inhibited both the cAMP/PKA-dependent ORCC and CFTR activities. (E) Effect of Ac1-14 and N1-14 on SCC in gut epithelia. SCC measurements were obtained, in the presence or absence of Ac1-14 or N1-14 (100 μM), in response to Ach and glucose stimulation of mounted gut epithelia biopsies (n = 3). **p < 0.05, ANOVA.
To establish the relevance of the PKA/CaN-mediated dephosphorylation of anx 2 to CFTR function, we analyzed the effect of the CaN inhibitors cypermethrin and cyclosporin A on CFTR-mediated currents. These inhibitors both reduced the magnitude of ICFTR (Figure 5B), but they had no effect on IDIDS (see Supplemental Material).
To distinguish between the effect of PKA-mediated CFTR phosphorylation and PKA/CaN-mediated dephosphorylation of anx 2 on CFTR function, we analyzed the impact of Ac1-14, which specifically disrupts anx 2–S100A10 complex, on ICFTR. Ac1-14 inhibited both IDIDS and ICFTR (Figure 5C) (n = 13 and n = 9 in the absence and presence of Ac1-14, respectively). However, N1-14 had no effect (Figure 5D). This suggests that PKA/CaN-mediated dephosphorylation of anx 2, is important for CFTR function.
For the SSC measurements, Cl− secretion in response to the intestinal secretagogue Ach (10−3 M) ± Ac1-14 or N1-14, was measured in gut biopsies mounted in modified Ussing chambers (Hardcastle et al., 2001) (n = 3). Ach induced a transient increase in SCC in the control measurements (ΔSCC; +136 ± 7.63 μA/cm2). N1-14 was without effect on the Ach-induced increase in SCC (ΔSCC; +129 ± 7.03 μA/cm2). However, in the presence of Ac1-14, Ach induced an attenuated increase in SCC (ΔSCC; +20.0 ± 3.01 μA/cm2). Washing out Ac1-14 did not restore the Ach response, indicating possible diffusion of peptide into the tissue. Tissue viability pre/posttreatment was confirmed using sodium linked glucose (10 mM) absorption (peak response +235 ± 6.9 μA/cm2 and +213 ± 9.9 μA/cm2, respectively) (Figure 5E).
DISCUSSION
This study describes a multiprotein complex concomitantly associated with the PKA-dependent activation of CFTR. We provide evidence that a macromolecular complex associated with CFTR function assembles at the plasma membrane of epithelia upon cell stimulation with FSK or Ach. We also show that a protein phosphatase regulates the PKA-dependent activation of CFTR function. We propose that the anx 2–S100A10 cAMP/PKA-dependent complex associates with CFTR through a PKA signaling pathway involving CaN. Disruption of the cAMP/PKA-dependent complex, by pharmacological inhibitors of CaN or by Ac1-14, attenuates CFTR function.
Stimulation of normal epithelia with FSK generates Cl− flux through CFTR phosphorylation by PKA (Gadsby and Nairn, 1999). This pathway is the major recognized intracellular signaling mechanism for activation of CFTR-dependent Cl− flux. Our data demonstrate that CFTR activation by PKA is dependent on CaN and anx 2–S100A10 complex formation; preincubation of the cells with either CaN inhibitors or Ac1-14, which disrupts anx 2–S100A10 complex, before FSK stimulation inhibits both anx 2–S100A10/CFTR complex formation and CFTR Cl− conductance. Importantly, our data have a wider relevance to epithelial function because inhibition of the Ach-dependent SCC by Ac1-14 in human gut biopsies suggests that the macromolecular complex is likely to be important in vivo in epithelia affected by cystic fibrosis. Thus, these observations highlight the fact that regulation of CFTR by cAMP/PKA in vivo is a complex process and may have implications for conclusions drawn from studies conducted in vitro and in heterologous systems.
CaN is a serine/threonine protein phosphatase regulated by [Ca2+]i and calmodulin (Crabtree, 1999). The disruption or stimulation of anx 2/CaN coimmunoprecipitation by PKA inhibition or activation, respectively, indicates that cAMP/PKA regulates physical association of CaN with the anx 2/S100A10/CFTR complex. The mechanism involved is unclear, but it is likely to involve protein phosphorylation, because catalytic inhibitors of PKA disrupt coimmunoprecipitation. CFTR phosphorylation by PKA may induce a structural modification of CFTR and facilitate anx 2–S100A10 binding; meanwhile, our unpublished data show that PKA also concurrently induces CaN-dependent loss of phosphate from anx 2, which is important for complex formation with S100A10 (Muimo, 2006). Because CaN does not dephosphorylate CFTR (Zhu et al., 1999; Thelin et al., 2005), our application of Ac1-14 provides a novel means to distinguish between effect of inhibitors on PKA-mediated CFTR phosphorylation and PKA/CaN-mediated dephosphorylation of anx 2 on CFTR function. Inhibition of CFTR function by CaN inhibitors, despite PKA activation, suggests CaN plays an important role in the regulation of CFTR function. Interestingly, CaN A regulates PKA by dephosphorylating the PKA II regulatory subunit (Blumenthal et al., 1986; Klee et al., 1998). Previous analyses of protein phosphatase and CFTR function have focused on CFTR dephosphorylation and inactivation by phosphatases (Zhu et al., 1999; Thelin et al., 2005). Both PP2A and PP2C dephosphorylate and inactivate CFTR (Berger et al., 1993; Travis et al., 1997). In contrast, CaN and PP1 failed to inactivate and dephosphorylate CFTR after PKA phosphorylation in vitro when CaN was added directly to CFTR in excised patches. Additionally, 1 μM FK506, a cell-permeant CaN inhibitor, did not alter the amount of current activated by cAMP agonists in T84 and human airway epithelia (Berger et al., 1993; Travis et al., 1997). These studies were designed to analyze the effect of CaN on dephosphorylation and inactivation of CFTR, and they are therefore not in contradiction with our findings.
CFTR regulates other Cl− channels (Fulmer et al., 1995; Jovov et al., 1995). The inhibition of both types of anion selective currents by Ac1-14 is in agreement with a previous study that demonstrated that disruption of the anx 2–S100A10 complex using Ac1-14 results in a gradual decrease of volume-activated Cl− currents in vascular endothelial cells (Nilius et al., 1996). Because CFTR regulates DIDS-sensitive currents (Fulmer et al., 1995; Jovov et al., 1995), Ac1-14 inhibition of DIDS-sensitive currents may result from CFTR inhibition. Alternatively, anx 2–S100A10 may target or regulate the DIDS-sensitive channels independently. However, these channels remain to be cloned. In contrast to Ac1-14, inhibition of CaN attenuated the CFTR-mediated currents, but it was without effect on the DIDS-sensitive ORCC. The reason for this difference is unclear, but it may reflect a differential regulatory role for CaN on the ORCCs. One possibility is that CaN stimulates the DIDS-sensitive current through formation of the annexin-2/S100A10 complex while simultaneously inhibiting these Cl− channels through a direct mechanism involving dephosphorylation. Overall, this would lead to inhibition of the DIDS channels, both by a loss of complex formation and by CaN-mediated inhibition. Further work will be needed to determine whether inhibition of CaN can block complex formation with concomitant loss of the inhibitory action of CaN on the DIDS-sensitive currents. The net effect is predicted to alter the balance between inhibition (mediated via complex loss), versus activation (via loss of the direct action of CaN). In the current study, there was no difference in the DIDS-sensitive current with CaN inhibitors, suggesting that inhibition of CaN was sufficient to overcome the loss of complex formation. Taken, these findings suggest that the cAMP/PKA-dependent anx 2–S100A10 complex may play a significant role in the regulation of ion homeostasis in epithelia.
Anx 2–S100A10 regulates the translocation and function of various ion channels, including K+, Na+, and Ca2+ channels (Girard et al., 2002; Okuse et al., 2002; van de Graaf et al., 2003). It is worth noting that, in many cell types, full activation of CFTR depends on vesicular transport and subsequent fusion of vesicles containing mature CFTR with the plasma membrane (Bradbury et al., 1994; Bradbury, 1999). This Ca2+-dependent vesicle-mediated process is triggered by cAMP/PKA and requires the C terminus of CFTR (Weber et al., 1999). Anx 2–S100A10 regulate exocytic apical transport in polarized epithelia (Jacob et al., 2004). Because only cell surface associated CFTR exists in complex with anx 2–S100A10, the cAMP/PKA-dependent anx 2–S100A10/CFTR complex may tether to the plasma membrane and further work will be need to determine how this affects CFTR open probability and/or channel number in the membrane.
Several CFTR-associated proteins have been identified and some, including syntaxin and AMP-activated protein kinase (AMPK), are inhibitory to CFTR (Naren et al., 1997; Hallows et al., 2000). Syntaxin 1A exists at the apical pole of airway epithelia and binds the N terminus of CFTR and inhibits CFTR. Reagents that disrupt the syntaxin 1A/CFTR interaction potentiate CFTR activity (Naren et al., 2000). AMPK also binds and inhibits CFTR activity. We speculate that the binding of anx 2–S100A10 to CFTR may provide a cellular mechanism to overcome or reverse the inhibition of CFTR function induced by constitutively bound inhibitory proteins such as syntaxin 1A and AMPK.
In conclusion, our work reveals a functional interaction of annexin-2–S100A10 with CFTR that is dependent on the activity of cAMP/PKA/CaN and that complements the significant body of data showing that activation of CFTR occurs in a cAMP/PKA-dependent process. The identified interaction forms an important regulatory mechanism for CFTR function across epithelia.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported, in part, by grants from the Cystic Fibrosis Trust and the Deutsche Forschungsgemeinschaft. The 16HBE14o− were provided by Dr. Gruenert (California Pacific Medical Center, CA).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-02-0126) on June 20, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Ali S. M., Geisow M. J., Burgoyne R. D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature. 1989;340:313–315. doi: 10.1038/340313a0. [DOI] [PubMed] [Google Scholar]
- Bebok Z., Collawn J., Wakefield J., Parker W., Varga K., Li Y., Sorscher E. J., Clancy J. P. Failure of cAMP agonists to activate rescued {Delta}F508 CFTR in CFBE41o− airway epithelial monolayers. J. Physiol. 2005;569:601–615. doi: 10.1113/jphysiol.2005.096669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T., Weber K., Johnsson N. Protein-protein recognition via short amphiphilic helices; a mutational analysis of the binding site of annexin II for p11. EMBO J. 1990;9:4207–4213. doi: 10.1002/j.1460-2075.1990.tb07868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. A., Travis S. M., Welsh M. J. Regulation of the cystic fibrosis transmembrane conductance regulator Cl− channel by specific protein kinases and protein phosphatases. J. Biol. Chem. 1993;268:2037–2047. [PubMed] [Google Scholar]
- Bertrand C. A., Frizzell R. A. The role of regulated CFTR trafficking in epithelial secretion. Am. J. Physiol. 2003;285:C1–C18. doi: 10.1152/ajpcell.00554.2002. [DOI] [PubMed] [Google Scholar]
- Blumenthal D., Takio K., Hansen R., Krebs E. Dephosphorylation of cAMP-dependent protein kinase regulatory subunit (type II) by calmodulin-dependent protein phosphatase. Determinants of substrate specificity. J. Biol. Chem. 1986;261:8140–8145. [PubMed] [Google Scholar]
- Bos J. L., de Bruyn K., Enserink J., Kuiperij B., Rangarajan S., Rehmann H., Riedl J., de Rooij J., van Mansfeld F., Zwartkruis F. The role of Rap1 in integrin-mediated cell adhesion. Biochem. Soc. Trans. 2003;31:83–86. doi: 10.1042/bst0310083. [DOI] [PubMed] [Google Scholar]
- Bradbury N. A. Intracellular CFTR: localization and function. Physiol. Rev. 1999;79:S175–S191. doi: 10.1152/physrev.1999.79.1.S175. [DOI] [PubMed] [Google Scholar]
- Bradbury N. A., Clark J. A., Watkins S. C., Widnell C. C., Smith H. S., Bridges R. J. Characterization of the internalization pathways for the cystic fibrosis transmembrane conductance regulator. Am. J. Physiol. 1999;276:L659–L668. doi: 10.1152/ajplung.1999.276.4.L659. [DOI] [PubMed] [Google Scholar]
- Bradbury N. A., Cohn J. A., Venglarik C. J., Bridges R. J. Biochemical and biophysical identification of cystic fibrosis transmembrane conductance regulator chloride channels as components of endocytic clathrin-coated vesicles. J. Biol. Chem. 1994;269:8296–8302. [PubMed] [Google Scholar]
- Bradbury N. A., Jilling T., Berta G., Sorscher E. J., Bridges R. J., Kirk K. L. Regulation of plasma membrane recycling by CFTR. Science. 1992;256:530–532. doi: 10.1126/science.1373908. [DOI] [PubMed] [Google Scholar]
- Chap H., Fauvel J., Gassama-Diaggne A., Ragab-Thomas J., Simon M.-F. Une homologie frappante entre le CFTR et les annexines. Med. Sci. 1991;7:8–9. [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J. Biol. Chem. 1986;261:989–992. [PubMed] [Google Scholar]
- Cozens A. L., Yezzi M. J., Kunzelmann K., Ohrui T., Chin L., Eng K., Finkbeiner W. E., Widdicombe J. H., Gruenert D. C. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am. J. Respir. Cell. Mol. Biol. 1994;10:38–47. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R. Pi-by-no signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- Creutz C. E. The annexins and exocytosis. Science. 1992;258:924–931. doi: 10.1126/science.1439804. [DOI] [PubMed] [Google Scholar]
- Dahan D., Evagelidis A., Hanrahan J. W., Hinkson D. A., Jia Y., Luo J., Zhu T. Regulation of the CFTR channel by phosphorylation. Pflugers Arch. 2001;443(Suppl 1):S92–S96. doi: 10.1007/s004240100652. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- Emans N., Gorvel J. P., Walter C., Gerke V., Kellner R., Griffiths G., Gruenberg J. Annexin II is a major component of fusogenic endosomal vesicles. J. Cell Biol. 1993;120:1357–1369. doi: 10.1083/jcb.120.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulmer S. B., Schwiebert E. M., Morales M. M., Guggino W. B., Cutting G. R. Two cystic fibrosis transmembrane conductance regulator mutations have different effects on both pulmonary phenotype and regulation of outwardly rectified chloride currents. Proc. Natl. Acad. Sci. USA. 1995;92:6832–6836. doi: 10.1073/pnas.92.15.6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D. C., Nairn A. C. Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol. Rev. 1999;79:S77–S107. doi: 10.1152/physrev.1999.79.1.S77. [DOI] [PubMed] [Google Scholar]
- Gerke V., Moss S. E. Annexins: from structure to function. Physiol. Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- Girard C., Tinel N., Terrenoire C., Romey G., Lazdunski M., Borsotto M. p11, an annexin II subunit, an auxiliary protein associated with the background K+ channel, TASK-1. EMBO J. 2002;21:4439–4448. doi: 10.1093/emboj/cdf469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenert D. C., Willems M., Cassiman J. J., Frizzell R. A. Established cell lines used in cystic fibrosis research. J. Cyst Fibros. 2004;3(suppl 2):191–196. doi: 10.1016/j.jcf.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Hallows K. R., Raghuram V., Kemp B. E., Witters L. A., Foskett J. K. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J. Clin. Invest. 2000;105:1711–1721. doi: 10.1172/JCI9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hardcastle J., Hardcastle P. T., Chapman J., Taylor C. J. Ursodeoxycholic acid action on the transport function of the small intestine in normal and cystic fibrosis mice. J. Pharm. Pharmacol. 2001;53:1457–1467. doi: 10.1211/0022357011777990. [DOI] [PubMed] [Google Scholar]
- Herzig S., Neumann J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol. Rev. 2000;80:173–210. doi: 10.1152/physrev.2000.80.1.173. [DOI] [PubMed] [Google Scholar]
- Jacob R., Heine M., Eikemeyer J., Frerker N., Zimmer K. P., Rescher U., Gerke V., Naim H. Y. Annexin II is required for apical transport in polarized epithelial cells. J. Biol. Chem. 2004;279:3680–3684. doi: 10.1074/jbc.C300503200. [DOI] [PubMed] [Google Scholar]
- Jovov B., Ismailov I. I., Benos D. J. Cystic fibrosis transmembrane conductance regulator is required for protein kinase A activation of an outwardly rectified anion channel purified from bovine tracheal epithelia. J. Biol. Chem. 1995;270:1521–1528. doi: 10.1074/jbc.270.4.1521. [DOI] [PubMed] [Google Scholar]
- Kalin N., Claass A., Sommer M., Puchelle E., Tummler B. DeltaF508 CFTR protein expression in tissues from patients with cystic fibrosis. J. Clin. Invest. 1999;103:1379–1389. doi: 10.1172/JCI5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Ren H., Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J. Biol. Chem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- Konig J., Prenen J., Nilius B., Gerke V. The annexin II-p11 complex is involved in regulated exocytosis in bovine pulmonary artery endothelial cells. J. Biol. Chem. 1998;273:19679–19684. doi: 10.1074/jbc.273.31.19679. [DOI] [PubMed] [Google Scholar]
- Kube E., Becker T., Weber K., Gerke V. Protein-protein interaction studied by site-directed mutagenesis. Characterization of the annexin II-binding site on p11, a member of the S100 protein family. J. Biol. Chem. 1992;267:14175–14182. [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Ma T., Thiagarajah J. R., Yang H., Sonawane N. D., Folli C., Galietta L. J., Verkman A. S. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J. Clin. Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh C., MacKintosh R. W. Inhibitors of protein kinases and phosphatases. Trends Biochem. Sci. 1994;19:444–448. doi: 10.1016/0968-0004(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Mak J. C., Chuang T. T., Harris C. A., Barnes P. J. Increased expression of G protein-coupled receptor kinases in cystic fibrosis lung. Eur. J. Pharmacol. 2002;436:165–172. doi: 10.1016/s0014-2999(01)01625-9. [DOI] [PubMed] [Google Scholar]
- Moss S. E., editor. London, United Kingdom: Portland Press; 1992. The Annexins. [Google Scholar]
- Muimo R., Hornickova Z., Riemen C. E., Gerke V., Matthews H., Mehta A. Histidine phosphorylation of annexin I in airway epithelia. J. Biol. Chem. 2000;275:36632–36636. doi: 10.1074/jbc.M000829200. [DOI] [PubMed] [Google Scholar]
- Muimo R., Borthwick L. A. The annexin 2-S100A10 complex mediates cross-talk between cAMP and Ca2+ signalling in airway epithelia. Pediatr. Pulmonol. 2006;S29:113. [Google Scholar]
- Mwimbi X. K., Muimo R., Green M. W., Mehta A. Making human nasal cilia beat in the cold: a real time assay for cell signalling. Cell Signal. 2003;15:395–402. doi: 10.1016/s0898-6568(02)00143-2. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Gold G. H. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature. 1987;325:442–444. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- Naren A. P., Cormet-Boyaka E., Fu J., Villain M., Blalock J. E., Quick M. W., Kirk K. L. CFTR chloride channel regulation by an interdomain interaction. Science. 1999;286:544–548. doi: 10.1126/science.286.5439.544. [DOI] [PubMed] [Google Scholar]
- Naren A. P., et al. Syntaxin 1A is expressed in airway epithelial cells, where it modulates CFTR Cl(−) currents. J. Clin. Invest. 2000;105:377–386. doi: 10.1172/JCI8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naren A. P., Nelson D. J., Xie W., Jovov B., Pevsner J., Bennett M. K., Benos D. J., Quick M. W., Kirk K. L. Regulation of CFTR chloride channels by syntaxin and Munc18 isoforms. Nature. 1997;390:302–305. doi: 10.1038/36882. [DOI] [PubMed] [Google Scholar]
- Nilius B., Gerke V., Prenen J., Szucs G., Heinke S., Weber K., Droogmans G. Annexin II modulates volume-activated chloride currents in vascular endothelial cells. J. Biol. Chem. 1996;271:30631–30636. doi: 10.1074/jbc.271.48.30631. [DOI] [PubMed] [Google Scholar]
- Okuse K., Malik-Hall M., Baker M. D., Poon W. Y., Kong H., Chao M. V., Wood J. N. Annexin II light chain regulates sensory neuron-specific sodium channel expression. Nature. 2002;417:653–656. doi: 10.1038/nature00781. [DOI] [PubMed] [Google Scholar]
- Penque D., Mendes F., Beck S., Farinha C., Pacheco P., Nogueira P., Lavinha J., Malho R., Amaral M. D. Cystic fibrosis F508del patients have apically localized CFTR in a reduced number of airway cells. Lab. Invest. 2000;80:857–868. doi: 10.1038/labinvest.3780090. [DOI] [PubMed] [Google Scholar]
- Ramjeesingh M., Kidd J. F., Huan L. J., Wang Y., Bear C. E. Dimeric cystic fibrosis transmembrane conductance regulator exists in the plasma membrane. Biochem. J. 2003;374:793–797. doi: 10.1042/BJ20030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rety S., Sopkova J., Renouard M., Osterloh D., Gerke V., Tabaries S., Russo-Marie F., Lewit-Bentley A. The crystal structure of a complex of p11 with the annexin II N-terminal peptide. Nat. Struct. Biol. 1999;6:89–95. doi: 10.1038/4965. [DOI] [PubMed] [Google Scholar]
- Santamaria-Kisiel L., Rintala-Dempsey A. C., Shaw G. S. Calcium-dependent and -independent interactions of the S100 protein family. Biochem. J. 2006;396:201–214. doi: 10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiebert E. M., Flotte T., Cutting G. R., Guggino W. B. Both CFTR and outwardly rectifying chloride channels contribute to cAMP-stimulated whole cell chloride currents. Am. J. Physiol. 1994;266:C1464–C1477. doi: 10.1152/ajpcell.1994.266.5.C1464. [DOI] [PubMed] [Google Scholar]
- Sistiaga A., Sanchez-Prieto J. Protein phosphatase 1 and 2A inhibitors prolong the switch in the control of glutamate release by group I metabotropic glutamate receptors: characterization of the inhibitory pathway. J. Neurochem. 2000;75:1566–1574. doi: 10.1046/j.1471-4159.2000.0751566.x. [DOI] [PubMed] [Google Scholar]
- Thelin W. R., Kesimer M., Tarran R., Kreda S. M., Grubb B. R., Sheehan J. K., Stutts M. J., Milgram S. L. The cystic fibrosis transmembrane conductance regulator is regulated by a direct interaction with the protein phosphatase 2A. J. Biol. Chem. 2005;280:41512–41520. doi: 10.1074/jbc.M507308200. [DOI] [PubMed] [Google Scholar]
- Thiel C., Osborn M., Gerke V. The tight association of the tyrosine kinase substrate annexin II with the submembranous cytoskeleton depends on intact p11-and Ca(2+)-binding sites. J. Cell Sci. 1992;103:733–742. doi: 10.1242/jcs.103.3.733. [DOI] [PubMed] [Google Scholar]
- Travis S. M., Berger H. A., Welsh M. J. Protein phosphatase 2C dephosphorylates and inactivates cystic fibrosis transmembrane conductance regulator. Proc. Natl. Acad. Sci. USA. 1997;94:11055–11060. doi: 10.1073/pnas.94.20.11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin E., Russo-Marie F., Dubois T., de Paillerets C., Alfsen A., Bomsel M. In adrenocortical tissue, annexins II and VI are attached to clathrin coated vesicles in a calcium-independent manner. Biochim. Biophys. Acta. 1998;1402:115–130. doi: 10.1016/s0167-4889(97)00151-1. [DOI] [PubMed] [Google Scholar]
- van de Graaf S. F., Hoenderop J. G., Gkika D., Lamers D., Prenen J., Rescher U., Gerke V., Staub O., Nilius B., Bindels R. J. Functional expression of the epithelial Ca(2+) channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex. EMBO J. 2003;22:1478–1487. doi: 10.1093/emboj/cdg162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K., Jurkuvenaite A., Wakefield J., Hong J. S., Guimbellot J. S., Venglarik C. J., Niraj A., Mazur M., Sorscher E. J., Collawn J. F., Bebok Z. Efficient Intracellular processing of the endogenous cystic fibrosis transmembrane conductance regulator in epithelial cell lines. J. Biol. Chem. 2004;279:22578–22584. doi: 10.1074/jbc.M401522200. [DOI] [PubMed] [Google Scholar]
- Weber W. M., Cuppens H., Cassiman J. J., Clauss W., Van Driessche W. Capacitance measurements reveal different pathways for the activation of CFTR. Pflugers Arch. 1999;438:561–569. doi: 10.1007/s004249900086. [DOI] [PubMed] [Google Scholar]
- Weixel K. M., Bradbury N. A. The carboxyl terminus of the cystic fibrosis transmembrane conductance regulator binds to AP-2 clathrin adaptors. J. Biol. Chem. 2000;275:3655–3660. doi: 10.1074/jbc.275.5.3655. [DOI] [PubMed] [Google Scholar]
- Yakel J. L. Calcineurin regulation of synaptic function: from ion channels to transmitter release and gene transcription. Trends Pharmacol. Sci. 1997;18:124–134. doi: 10.1016/s0165-6147(97)01046-8. [DOI] [PubMed] [Google Scholar]
- Zhu T., Dahan D., Evagelidis A., Zheng S., Luo J., Hanrahan J. W. Association of cystic fibrosis transmembrane conductance regulator and protein phosphatase 2C. J. Biol. Chem. 1999;274:29102–29107. doi: 10.1074/jbc.274.41.29102. [DOI] [PubMed] [Google Scholar]
- Zobiack N., Rescher U., Ludwig C., Zeuschner D., Gerke V. The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol. Biol. Cell. 2003;14:4896–4908. doi: 10.1091/mbc.E03-06-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.