Abstract
Both farnesyl diphosphate synthase (FPS) and geranylgeranyl diphosphate synthase (GGPS) are key enzymes in the synthesis of various isoprenoid-containing compounds and proteins. Here, we describe two novel Schizosaccharomyces pombe genes, fps1+ and spo9+, whose products are similar to FPS in primary structure, but whose functions differ from one another. Fps1 is essential for vegetative growth, whereas, a spo9 null mutant exhibits temperature-sensitive growth. Expression of fps1+, but not spo9+, suppresses the lethality of a Saccharomyces cerevisiae FPS-deficient mutant and also restores ubiquinone synthesis in an Escherichia coli ispA mutant, which lacks FPS activity, indicating that S. pombe Fps1 in fact functions as an FPS. In contrast to a typical FPS gene, no apparent GGPS homologues have been found in the S. pombe genome. Interestingly, although neither fps1+ nor spo9+ expression alone in E. coli confers clear GGPS activity, coexpression of both genes induces such activity. Moreover, the GGPS activity is significantly reduced in the spo9 mutant. In addition, the spo9 mutation perturbs the membrane association of a geranylgeranylated protein, but not that of a farnesylated protein. Yeast two-hybrid and coimmunoprecipitation analyses indicate that Fps1 and Spo9 physically interact. Thus, neither Fps1 nor Spo9 alone functions as a GGPS, but the two proteins together form a complex with GGPS activity. Because spo9 was originally identified as a sporulation-deficient mutant, we show here that expansion of the forespore membrane is severely inhibited in spo9Δ cells. Electron microscopy revealed significant accumulation membrane vesicles in spo9Δ cells. We suggest that lack of GGPS activity in a spo9 mutant results in impaired protein prenylation in certain proteins responsible for secretory function, thereby inhibiting forespore membrane formation.
INTRODUCTION
Prenyltransferases are key enzymes in isoprenoid biosynthesis, and a number of prenyltransferases have been described in a variety of organisms. Among them, farnesyl diphosphate synthase (FPS) and geranylgeranyl diphosphate synthase (GGPS) have been well characterized (for review, see Koyama, 1999; Szkopinska and Plochocka, 2005). FPS catalyzes the sequential condensation of dimethylallyl diphosphate (DMAPP) and geranyl diphosphate (GPP) with isopentenyl diphosphate (IPP). The product, farnesyl diphosphate (FPP), is the precursor for several important metabolites, including sterols, dolichols, ubiquinones, and heme a (for review, see Kawamukai, 2002). GGPS catalyzes a similar condensation reaction as FPS. It uses DMAPP, GPP, and FPP as allylic substrates to generate an amphipathic molecule containing four isoprene units, GGPP, which is a precursor of carotenoids and chlorophylls. FPP and GGPP are also substrates for protein prenylation mediated by farnesyl transferase and geranylgeranyl transferase. Protein prenylation is a posttranslational modification by which isoprenoid compounds are covalently attached to cysteine residues at or near the C terminus of proteins (Casey 1992, 1994; Schafer and Rine, 1992; Omer and Gibbs, 1994). Prenylated proteins, which include small GTP-binding proteins such as Ras and Rab, account for ∼2% of total cellular proteins (Epstein et al., 1991). Prenylation has generally been found to be essential for proper localization of membrane proteins.
Sporulation in the fission yeast Schizosaccharomyces pombe is equivalent to gametogenesis in higher eukaryotes. This unique process includes two overlapping processes, meiosis and spore formation. Four haploid nuclei produced by meiosis are packaged into individual spores. During meiosis II, membrane vesicles accumulate in the vicinity of the spindle pole body, a structure equivalent to the centrosome in animal cells, and they fuse to generate a double unit membrane, termed the forespore membrane. The forespore membrane expands by fusing with membrane vesicles, and eventually it encapsulates each of the four nuclei produced by meiosis. Spore wall material is deposited in the lumenal space of the forespore membrane, and its inner membrane becomes the plasma membrane of the spores. Assembly of forespore membranes provides a model system for studying the de novo biogenesis of membrane compartments within the cytoplasm (Yoo et al., 1973; Tanaka and Hirata, 1982; Hirata and Shimoda, 1992, 1994). Many sporulation-deficient S. pombe mutants have been isolated (Bresch et al., 1968; Kishida and Shimoda, 1986). Thus far, we have analyzed spo3+, spo4+, spo6+, spo14+, spo15+, spo19+, and spo20+ (Ikemoto et al., 2000; Nakamura et al., 2000, 2001, 2002; Nakase et al., 2001; Nakamura-Kubo et al., 2003). spo3+, spo14+, and spo20+ have been shown to be involved in expansion of the forespore membrane. spo3+ encodes a membrane protein. Spo3 is expressed exclusively during meiosis and localizes to the forespore membrane (Nakamura et al., 2001). Both spo14+ and spo20+ encode components of a vesicle-trafficking pathway (Nakase et al., 2001; Nakamura-Kubo et al., 2003). In addition to these spo+ genes, several other genes are necessary for expansion of the forespore membrane. psy1+ and sec9+, both of which encode components of the plasma membrane target membrane-associated soluble N-ethylmaleimide-sensitive factor-attachment protein receptor complex, are required for both vegetative growth and sporulation (Nakamura et al., 2001, 2005). vps genes, which are involved in vacuolar protein sorting, are also responsible for expansion of the forespore membrane (Iwaki et al., 2003; Onishi et al., 2003; Koga et al., 2004; Kashiwazaki et al., 2005).
Among many sporulation-deficient mutants, spo9 has not been well characterized. In the present study, we isolated and analyzed the spo9+ gene, which encodes a protein with significant homology to a typical FPS. We also identified another gene, fps1+, which encodes FPS. Although fps1+ was found to be essential for growth, spo9+ was required for proper assembly of the forespore membrane. Despite its high sequence homology with FPS, Spo9 was largely responsible for GGPS activity. We report the novel finding that S. pombe GGPS consists of a heteromer made up of a typical FPS protein, Fps1, and an FPS-like protein, Spo9.
MATERIALS AND METHODS
Strains, Media, and Plasmids
Yeast strains used in this study are listed in Table 1. For S. pombe, complete medium (YE) was used for vegetative growth. Malt extract medium MEA and synthetic sporulation media SSA, SSL-N, and MM-N were used for mating and sporulation. Saccharomyces cerevisiae strains were grown in either YPD, YP-galactose, or selective minimal medium (SC) supplemented with appropriate amino acids, unless otherwise indicated. Plasmids used in this study are listed in Table 2.
Table 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| S. pombe | ||
| MKW5 (FY7456)a | h90 | Nakamura-Kubo et al. (2003) |
| TN8 (FY7132)a | h90 leu1-32 | Nakamura et al. (2001) |
| TN75 | h90/h90 ade6-M210/ade6-M216 leu1-32/leu1-32 ura4-D18/ura4-D18 | Nakamura et al. (2001) |
| B261 (FY12194)a | h90 spo9-B261 ade6-M210 | Bresch et al. (1968) |
| YF2 (FY13113)a | h90 spo9-B261 leu1-32 | This study |
| YF5 | h90spo9-B261 leu1<< GFP-psy1 leu1-32 | This study |
| YF9 | h90/h90 fps1Δ::ura4+/fps1+ade6-M210/ade6-M216 leu1-32/leu1-32 ura4-D18/ura4-D18 | This study |
| YF16 | h90 spo9Δ::ura4+leu1-32 ura4-D18 | This study |
| YF51 | h90leu1<< GFP-ypt7 leu1-32 | This study |
| YF54 | h90 spo9Δ::ura4+leu1<<GFP-ypt7 leu1-32 ura4-D18 | This study |
| YF69 | h90 leu1-32 ura4-D18 pAL(fps1-GFP) pDS473(spo9) | This study |
| YF75 | h90 leu1-32 ura4-D18 pTN54 pDS473(spo9) | This study |
| YF76 | h90 leu1-32 ura4-D18 | This study |
| YF77 | h90 fps1Δ::ura4+ade6 leu1-32 ura4-D18 pREP81(fps1) | This study |
| YF78 | h90 fps1Δ::ura4+ade6 leu1-32 ura4-D18 pREP81(ERG20) | This study |
| YF81 | h90 spo9Δ::ura4+leu1-32 ura4-D18 pAL(spo9) | This study |
| YN68 | h90leu1<< GFP-psy1 leu1-32 | Nakase et al. (2004) |
| S. cerevisiae | ||
| W303 (BY4502)a | MATa/α ade2-1/ade2-1 his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 trp1-1/trp1-1 ura3-1/ura3-1 | R. Rothstein (Columbia Univ.) |
| SFNY368 | MATα bts1Δ::URA3 ura3-52 leu2-3,112 | Jiang et al. (1995) |
| YF73 | MATa/α ERG20/erg20Δ::URA3 ade2-1/ade2-1 his3-11,15/his3-11,15 leu2-3,112/leu2-3,112 trp1-1/trp1-1 ura3-1/ura3-1 | This study |
| YF79 | MATaerg20Δ::URA3 ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 pYEL2(ERG20) | This study |
| YF80 | MATaerg20Δ::URA3 ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 pYEL2(fps1) | This study |
| AH109 | MATaura3-52 his3-200 ade2-101 trp1-901 leu2-3,112 gal4Δ met−gal80Δ LYS2::GAL1UAS-GAL1TATA-HIS3 GAL2UAS-GAL2TATA-ADE2 ura3::MEL1 UAS-MEL1TATA-lacZ | Takara-Bio |
| E. coli | ||
| SF7 | ΔispA::neo Δ(srl-recA)306::Tn10 | Fujisaki et al. (2005) |
| DH5α | supE44 ΔlacU169(=80lacZ ΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Sambrook et al. (1989) |
| DH10B | Δ(mrr-hsd RMS-mcrBC) mcrA recA1 | Sambrook et al. (1989) |
a The strain was obtained from the Yeast Genetic Resource Center of Japan supported by the National BioResource Project (YGRC/NBRP) (http:/yeast.lab.nig.ac.jp/nig/). The S. pombe strains constructed in this study are deposited at the YGRC/NBRP.
Table 2.
Plasmids used in this study
| Plasmid | Characteristics | Source |
|---|---|---|
| S. pombe | ||
| pAL-KS | ars1, LEU2-based vector | Tanaka et al. (2000) |
| pREP1 | ars1, LEU2-based vector carrying a thiamine-repressible nmt1 promoter | Maundrell (1993) |
| pREP81 | ars1, LEU2-based vector carrying a thiamine-repressible nmt81 promoter | Maundrell (1993) |
| pREP1(spo9) | spo9+ in pREP1 | This study |
| pREP1(fps1) | fps1+ in pREP1 | This study |
| pREP1(ERG20) | ERG20 in pREP1 | This study |
| pREP1(BTS1) | BTS1 in pREP1 | This study |
| pREP81(fps1) | fps1+ in pREP81 | This study |
| pREP81(ERG20) | ERG20 in pREP81 | This study |
| pBS(fps1) | fps1+ in pBluescript II-KS(+) | This study |
| pTN143 | GFP and nmt1 terminator in pAL-KS | Ikemoto et al. (2000) |
| pTN54 | ars1, LEU2-based vector carrying an N-terminal GFP tag and nmt41 promoter | Nakamura et al. (2001) |
| pAL(fps1-GFP) | fps1+ in pTN143 | This study |
| pAL(spo9) | spo9+ in pAL-KS | This study |
| pDS473a | ars1, ura4+-based vector carrying an N-terminal GST tag and nmt42 promoter | Forsburg and Sherman (1997) |
| pDS473(spo9) | spo9+ in pDS473a | This study |
| pTN381(GFP-ypt7) | leu1+-based vector carrying an N-terminal GFP tag and ypt7+ native promoter | Kashiwazaki et al. (2005) |
| pAL(GFP-psy1) | LEU2-based vector carrying an N-terminal GFP tag and psy1+ native promoter | Nakamura (unpublished) |
| S. cerevisiae | ||
| pYES2 | 2μ origin, URA3-based vector carrying a galactose-inducible GAL1 promoter | Invitrogen |
| pYEL2 | LEU2 in pYES2 | This study |
| pYEL2(spo9) | spo9+ in pYEL2 | This study |
| pYEL2(fps1) | fps1+ in pYEL2 | This study |
| pYEL2(ERG20) | ERG20 in pYEL2 | This study |
| pYEL2(BTS1) | BTS1 in pYEL2 | This study |
| pGBKT7 | 2μ origin, TRP1-based vector carrying a DNA-binding domain of Gal4 and ADH1 promoter | Takara-Bio |
| pGBK(spo9) | spo9+ in pGBKT7 | This study |
| pGBK(fps1) | fps1+ in pGBKT7 | This study |
| pGADT7 | 2μ origin, LEU2-based vector carrying a activation domain of Gal4 and ADH1 promoter | Takara-Bio |
| pGAD(spo9) | spo9+ in pGADT7 | This study |
| pGAD(fps1) | fps1+ in pGADT7 | This study |
| E. coli | ||
| pGEX-KG | amp based vector carrying an N-terminal GST tag | Guan and Dixon (1991) |
| pGEX(spo9) | spo9+ in pGEX-KG | This study |
| pGEX(fps1) | fps1+ in pGEX-KG | This study |
| pMAL-c | amp based vector carrying an N-terminal maltose-binding protein (MBP) tag | New England Biolabs (Ipswich, MA) |
| pMAL(spo9) | spo9+ in pMAL-c | This study |
| pMAL(fps1) | fps1+ in pMAL-c | This study |
| pMAL(BTS1) | BTS1 in pMAL-c | This study |
| pMAL(spo9+fps1) | spo9+ and fps1+ in pMAL-c | This study |
| pMAL(spo9+ fps1-R104Q) | spo9+ and fps1-R104Q in pMAL-c | This study |
| pMAL(spo9-R109Q+ fps1) | spo9-R109Q and fps1+ in pMAL-c | This study |
| pMAL(spo9-R109Q+ fps1-R104Q) | spo9-R109Q and fps1-R104Q in pMAL-c | This study |
| pBluescript II-KS(−) | amp-based vector | Stratagene |
| pBH | Human GGPS1 in pBluescript II-KS(−) | Kainou et al. (1999) |
Cloning of spo9+ and fps1+
The spo9-B261 mutant YF2 was transformed with the S. pombe genomic library, pTN-L1 (Nakamura et al., 2001). Leu+ transformants on SSA (∼7500 colonies) were then exposed to iodine vapor (Gutz et al., 1974). The few colonies that turned brown were taken as candidates for sporulation-proficient transformants. Two plasmids were isolated from these candidates and their nucleotide sequences were determined. One of these plasmids, pYF1, was further analyzed (Table 2).
Cloning of spo9+ and fps1+
The spo9-B261 mutant YF2 was transformed with the S. pombe genomic library, pTN-L1 (Nakamura et al., 2001). Leu+ transformants on SSA (∼7500 colonies) were then exposed to iodine vapor (Gutz et al., 1974). The few colonies that turned brown were taken as candidates for sporulation-proficient transformants. Two plasmids were isolated from these candidates and their nucleotide sequences were determined. One of these plasmids, pYF1, was further analyzed (Table 2).
The fps1+ gene was cloned as follows. Polymerase chain reaction (PCR) primers 5′-ATATTTTTCTCCTCAACCTCTAGTAGCTTT-3′ and 5′-GCGCGGTCGAC(SalI)ATTATTAATGGATTTGCTG-3′ were used to amplify a 3.5-kb fragment encompassing the entire fps1+ gene, and the resulting BamHI–SalI fragment was subcloned into pBluescript II-KS+ (Stratagene, La Jolla, CA) to generate pBS(fps1).
Disruption of spo9+ and fps1+
Disruptions of the spo9+ and fps1+ genes by using ura4+ were constructed as follows. PCR primers 5′-AGAGAGCGGCCGC(NotI)AAACGGATCCAGATGGTTA-3′ and 5′-AGAGACTCGAG(XhoI)TTCGGAGCGCTTTCGCGTAGAATT-3′ were used to amplify a 2.7-kb fragment encompassing the entire spo9+ gene. The resulting NotI–XhoI fragment was then subcloned into pGEM-T easy vector (Promega, Madison, WI) to generate pGEM(spo9). After digestion with HindIII and XbaI, pGEM(spo9) was filled in and ligated to a HindIII linker. The resulting plasmid was digested with HindIII, and a 1.7-kb ura4+ fragment was inserted at the HindIII site. The resulting plasmid was digested with BglII and BamHI and used to transform strain TN75.
To disrupt fps1+, pBS(fps1) was digested with PstI and EcoRI to remove a 1.1-kb fragment of the open reading frame (ORF). A HindIII linker was then inserted into at these restriction sites. A 1.7-kb ura4+ fragment was inserted at the HindIII site. The resulting plasmid was digested with ClaI and BamHI and used to transform strain TN75. Disruptions were confirmed by Southern hybridization of genomic DNA (data not shown).
Southern and Northern Blot Analyses
Genomic DNA was digested, separated in a 1% agarose gel, and then transferred onto nylon membranes for Southern blotting. Total RNA was isolated from cells harvested at specific times. The isolated RNA was separated on a 1% agarose gel, transferred to a nylon membrane, and subjected to Northern blot analysis (Thomas, 1980).
Nucleotide Sequence Analysis of the spo9-B261 Mutant Allele
The entire spo9+ ORF and promoter region were amplified by PCR with primers 5′-CTCCACTCGAG(XhoI)CATATAGCCATGGGTTCTC-3′ and 5′-AGAGACTCGAG(XhoI)TTCGGAGCGCTTTCGCGTAGAATT-3′, by using genomic DNA from the spo9-B261 mutant as template. The amplified DNA fragment was cloned into pGEM-T easy vector and sequenced.
Ubiquinone Extraction and Measurement
Ubiquinone was extracted as described previously (Kainou et al., 1999). The extracted crude ubiquinone was analyzed by normal phase thin layer chromatography (TLC) with an authentic standard UQ6. Normal phase TLC was carried out on a Kiesel gel 60 F254 plate (Merck, Frankfurt, Germany) by using benzene:acetone (97:3, vol/vol). The UV-visualized band containing ubiquinone was collected from the TLC plate and extracted with chloroform:methanol (1:1, vol/vol). Samples were dried, and the pellets were redissolved in ethanol. The purified ubiquinone was further analyzed by high-performance liquid chromatography using ethanol as the solvent.
Prenyl Diphosphate Synthase Assay
Prenyl diphosphate synthase activity was measured by detection of [1-14C]IPP incorporated into reaction products as described previously (Kainou et al., 2001; Saiki et al., 2003). E. coli DH5α harboring a plasmid containing the human GGPS1 gene was incubated to late log phase in LB medium containing ampicillin at 37°C. Cells were harvested by centrifugation, suspended in buffer A (100 mM potassium phosphate, pH 7.4, 5 mM EDTA, and 1 mM 2-mercaptoethanol), and ruptured by sonication (six 30-s pulses with 30-s intervals in an ice bath between sonications). After centrifugation of the homogenate, the supernatant was used as a crude enzyme extract. Similarly, S. pombe cells were grown to mid- to late log phase in minimum medium. Cells were harvested by centrifugation and suspended in buffer A. The washed cells were ruptured by vigorous mixing with glass beads 14 times for 30 s with 60-s intervals in an ice bath between mixings. After centrifugation of the homogenate, the supernatant was used as a crude enzyme extract. The assay reaction mixture contained 1.0 mM MgCl2, 0.1% (wt/vol) Triton X-100, 50 mM potassium phosphate buffer, pH 7.5, 10 μM [1-14C]IPP (specific activity 0.92 TBq/mol), 5 μM FPP, and 200 μg of crude extract containing the enzyme in a final volume of 0.4 ml. Sample mixtures were incubated for 60 min at 30°C. Reaction products such as prenyl diphosphates were extracted with 1-butanol–saturated water and hydrolyzed with acid phosphatase. Hydrolysis products were extracted with hexane and analyzed by reversed phase TLC by using acetone:water (19:1, vol/vol). Radioactivity on the TLC plate was detected with an imaging analyzer BAS1500-Mac (Fuji Film, Tokyo, Japan). The plate was exposed to iodine vapor to detect marker prenols.
Yeast Two-Hybrid Analysis
For yeast two-hybrid analysis, PCR primers 5′-GCGCGCCATGG(NcoI)AAATGGTCAACGATTTTAA-3′ and 5′-GCGCGCTCGAG(XhoI)CTACCGTGACCTTTTGTAA-3′ were used to amplify the spo9+ gene. The amplified fragment was digested with NcoI and XhoI and inserted into the corresponding sites of pGBKT7 (GAL4 DNA binding domain; Takara-Bio, Otsu, Japan) and pGADT7 (GAL4 activation domain; Takara-Bio), yielding pGBK(spo9) and pGAD(spo9), respectively. Similarly, PCR primers 5′-GCGCGCCATGG(NcoI)AGATGTCTGCAGTTGATAA-3′ and 5′-GCGCGCTCGAG(XhoI)TTACTTATTTCTTTTGTAA-3′ were used to amplify the fps1+ gene. The amplified fragment was digested with NcoI and XhoI, and inserted into the corresponding sites of pGBKT7 and pGADT7 yielding pGBK(fps1) and pGAD(fps1), respectively. These plasmids were introduced into S. cerevisiae AH109 (Takara-Bio), and transformants were replicated onto selective minimal medium supplemented with appropriate amino acids, except for His to assay for protein interactions in vivo.
Coimmunoprecipitation Assays
The wild-type strain (YF76) was transformed with pDS473(spo9) plus pAL(fps1-GFP), or with pDS473(spo9) plus pTN54 to yield YF69 and YF75 strains, respectively. YF69 and YF75 were grown overnight at 28°C in MM. The cells were harvested in log phase, resuspended in TES (20 mM Tris-HCl, pH 7.5, 1 mM EDTA, and 150 mM NaCl) and then ruptured with glass beads. The lysate was centrifuged at 800 × g for 5 min to remove cell debris. The supernatant was then centrifuged at 13,000 × g for 20 min to prepare a soluble fraction. The soluble fraction was incubated with mouse anti-green fluorescent protein (GFP) antibody (Roche Diagnostics, Mannheim, Germany) at 4°C for 1 h, and then mixed with protein G-Sepharose (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). After further incubation at 4°C for 1 h, the solution was centrifuged at 800 × g for 5 min. The pellet was washed with TES three times and resuspended in sample buffer. Samples were electrophoresed in SDS-polyacrylamide gels, and the target polypeptides were detected by Western blot analysis using rat anti-GFP (a generous gift from S. Fujita) or mouse anti-glutathione S-transferase (GST) (Sigma-Aldrich, St. Louis, MO) antibody.
Subcellular Fractionation
pTN381(GFP-ypt7) was digested with SnaBI and the linear plasmid was integrated into the leu1 locus of wild type (TN8) and the spo9Δ mutant (YF16) to yield YF51 and YF54, respectively. The YF51 and YF54 strains were grown overnight at 28°C in MM. The cells were harvested in log phase, ruptured with glass beads as described above, and centrifuged at 800 × g for 5 min. The supernatant was further centrifuged at 100,000 × g for 1 h to separate the soluble fraction, the pellet being resuspended in an equal volume of resuspension buffer. The samples from each fraction were subjected to Western blot analysis by using either mouse anti-GFP (Roche Diagnostics), rabbit anti-Rhb1 (Nakase et al., 2006), or rabbit anti-Spo14 (Nakamura-Kubo et al., 2003) antibodies.
Fluorescence Microscopy
Cells were fixed as described previously (Hagan and Hyams, 1988) by use of glutaraldehyde and paraformaldehyde. The Psy1 protein was visualized by fusing it to GFP. The nuclear chromatin region was stained with 4,6-diamidino-2-phenylindole (DAPI). Stained cells were observed under a fluorescence microscope (model BX51; Olympus, Tokyo, Japan), and images were obtained using a CoolSNAP charge-coupled device camera (Roper Scientific, San Diego, CA).
Electron Microscopy
Cells were mounted on copper grids to form a thin layer, and then they were submerged in liquid propane cooled with liquid N2. Frozen cells were transferred to 2% OsO4 in anhydrous acetone, held at −80°C for 48 h in a solid CO2-acetone bath, and then transferred to −35°C for 2 h, 4°C for 2 h, and room temperature for 2 h. After washing with anhydrous acetone three times, samples were infiltrated with increasing concentrations of Spurr's resin in anhydrous acetone, and finally with 100% Spurr's resin. Samples were then polymerized at 50°C for 5 h and 60°C for 50 h. Thin sections were cut on a Reichert Ultracut S ultramicrotome, and then stained with uranyl acetate and lead citrate. Sections were viewed on an electron microscope H-7600 (Hitachi, Tokyo, Japan) at 100 kV.
RESULTS
spo9+ Encodes a Farnesyl Diphosphate Synthase-like Protein
To further investigate phenotypes of spo9-B261 mutant, we monitored vegetative growth and sporulation. As reported previously, the spo9-B261 mutant exhibits defects in ascospore formation (Bresch et al., 1968) (Figure 1A). In addition, the spo9-B261 mutant also exhibited temperature-sensitive vegetative growth (Figure 1B). These results indicate that the spo9+ gene is required not only for sporulation but also for vegetative growth. To identify the spo9+ gene product and its biological function, we isolated the spo9+ gene by functional complementation (see Materials and Methods). Two plasmids were isolated that were able to rescue both the temperature sensitivity and sporulation deficiency of spo9-B261. Subcloning and partial sequencing revealed that the spo9-complementing ability was due to one ORF composed of 1.2 kb (SPBC36.06c) (Figure 2A). The spo9+ gene encodes a 40.0-kDa protein consisting of 351 amino acid residues (Figure 2B). The predicted Spo9 protein shares 42 and 38% identity and 64 and 61% similarity with the S. cerevisiae FPS, Erg20, and human FPS, respectively (Figure 2B). FPS catalyzes chain elongation of the C5 substrate DMAPP to the C15 product FPP by addition of two molecules of IPP. Spo9 contains two aspartate-rich domains, first aspartate-rich motif: consensus DDXX(XX)D (FARM), and second aspartate-rich motif: consensus DDXXD (SARM), conserved among many prenyltransferases, including FPSs and GGPSs (Koyama et al., 1993; Chen et al., 1994; Song and Poulter, 1994; Szkopinska and Plochocka, 2005) (Figure 3A).
Figure 1.
spo9 mutant exhibits a sporulation deficiency and temperature-sensitive growth. (A) Wild type (TN8), spo9-B261 (YF2), and spo9Δ (YF16) were incubated at 28°C on sporulation medium (MEA) for 2 d. Bar, 10 μm. (B) The same strain as shown in A was streaked on complete medium (YEA) and incubated at 25 or 36°C for 3 d.
Figure 2.
Structure of the spo9+ gene. (A) Restriction map, subcloning, and construction of null mutants. The arrow indicates the region and direction of the spo9+ ORF, which encodes a protein composed of 351 amino acid residues. All the subclones were derived from pYF1. Complementation by each subclone: +, complemented; −, did not complement. Restriction enzyme sites: A, AvrII; B, BamHI; Bg, BglII; H, HindIII; S, StuI; and X, XbaI. (B) Comparison of the amino acid sequences two of S. pombe FPS-like proteins, Spo9 (CAA19054), and Fps1 (CAB11097) and other members of the FPS family. SpSpo9, S. pombe Spo9; SpFps1, S. pombe Fps1; ScErg20, S. cerevisiae FPS (CAA89462); AtFPS, Arabidopsis thaliana FPS2 (AAB07247); and HsFPS, Homo sapiens FPS (P14324). Identical amino acid residues are shown in white against black; similar residues are shaded. (C) Determination of spo9-B261 mutation. Duplicated nucleotide region is shown in white against black.
Figure 3.
(A) Comparison of the amino acid sequences of conserved regions of Fps1 and Spo9 and other members of FPSs and GGPSs. ScBts1, S. cerevisiae GGPS, Bts1 (Q12051); AtGGPS, Arabidopsis thaliana GGPS6 (BAA23157); and HsGGPS, Homo sapiens GGPS1 (AAH05252). FARM and SARM are boxed. The GQ motif is shown in bold. The arrow indicates the fifth amino acid before the FARM motif, which is important for determination of the final product. (B) A phylogenetic tree of GGPSs and FPSs from various organisms calculated by the neighbor joining method. Fps1 and Spo9 are marked by asterisks. Abbreviations: Ag, Abies grandis; At, A. thaliana; Bm, Bombyx mori; Bt, Bos taurus; Cp, Claviceps purpurea; Dj, Dendroctonus jeffreyi; Dm, Drosophila melanogaster; Ec, E. coli; Gf, Gibberella fujikuroi; Gg, Gallus gallus; Hb, Hevea brasiliensis; Hs, Homo sapiens; Kg, Kitasatospora griseola; Kl, Kluyveromyces lactis; La, Lupinus albus; Lc, Lactarius chrysorrheus; Ld, Leishmania donovani; Mm, Mus musculus; Mp, Myzus persicae; Mt, Methanothermobacter thermautotrophicus; Nc, Neurospora crassa; Pa, Parthenium argentatum; Rn, Rattus norvegicus; Sa, Sulfolobus acidocaldarius; Sar, Streptomyces argenteolus; Sc, S. cerevisiae; Sm, Sphaceloma manihoticola; Sp, S. pombe; Tb, Trypanosoma brucei; Tg, Toxoplasma gondii; Tt, Thermus thermophilus; and Zm, Zea mays.
To investigate the biological function of spo9+, a null mutant was created by conventional gene disruption by using ura4+ as a marker (Figure 2A). The spo9 deletion mutant (spo9Δ) was viable but displayed temperature sensitivity and sporulation deficiency like the original spo9-B261 mutant (Figure 1, A and B). Because most of the meiosis-defective mutants isolated to date are unable to sporulate (Bresch et al., 1968; Kishida and Shimoda, 1986), one cannot rule out the possibility that the spo9Δ mutant has a defect in meiosis. Therefore, we analyzed the meiotic nuclear divisions in spo9Δ. The first and second meiotic divisions were found to proceed with kinetics similar to that observed in an isogenic wild-type strain, with the final yield of tetranucleate cells reaching ∼90% (data not shown). These results suggest that the spo9Δ mutant is able to complete meiosis but that it is defective in ascospore formation.
Sequencing of the spo9-B261 allele revealed a direct duplication of 20-base pairs (from the 926th to 945th nucleotide of wild-type spo9+) (Figure 2C). This duplication caused a frameshift, resulting in a truncated protein of 272 amino acid residues. This truncated protein lacked the conserved hydrophilic C terminus, which is important for FPS activity (Song and Poulter, 1994). The spo9-B261 mutant protein seems to have lost all activity, like the spo9 disruptant.
Although the S. pombe genes responsible for mating, meiosis, and sporulation are generally transcribed under conditions of nutritional starvation (Yamamoto et al., 1997; Watanabe et al., 2001; Shimoda, 2004), Northern analysis revealed that spo9+ transcription occurred during vegetative growth and that it was not enhanced, but rather diminished, after the shift to a nitrogen-free medium (Figure 4).
Figure 4.
Transcription of spo9+ and fps1+ genes. Wild type (MKW5) precultured in growth medium (MM+N) was incubated in liquid sporulation medium (MM−N) at 28°C. After the shift, samples were harvested at the indicated times and subjected to Northern blotting. Top, spo9+ mRNA; middle, fps1+ mRNA; bottom, rRNA stained with ethidium bromide.
S. pombe Has a Second FPS-like Gene
A homology search performed against the fission yeast genome database (The Wellcome Trust Sanger Institute, Hinxton, Cambridge, United Kingdom; http://www.sanger.ac.uk/) detected another gene encoding an FPS-like protein (SPAC6F12.13C). We designated this gene fps1+. Fps1 shares 44, 58, and 48% identity and 64, 73, and 66% similarity with fission yeast Spo9, S. cerevisiae Erg20, and human FPS, respectively. Like Spo9, Fps1 also contains two aspartate-rich domains, which are conserved among FPSs and GGPSs as described above (Figure 3A). The fps1+ mRNA was also detectable during vegetative growth, but transcription did not increase after nitrogen starvation (Figure 4). It was also noted that the level of transcription of fps1+ was apparently higher than that of spo9+. Next, we disrupted the fps1+ gene. Tetrad analysis of the heterozygous diploid (YF9: fps1+/fps1Δ) indicated that only two spores per ascus were viable and produced Ura− colonies. Microscopic observation of the inviable spores showed that most germinated, but they seemed to have undergone only one or two divisions before growth arrest. Therefore, we conclude that fps1+ is essential for cell viability, but not for germination.
Genetic Relationship among S. pombe and S. cerevisiae FPS- and GGPS-related Genes
To test the possibility of functional redundancy between Fps1 and Spo9, we examined whether overexpression of fps1+ could rescue the phenotypes of spo9Δ. fps1+ was placed under control of the thiamine-repressible nmt1 promoter. The resultant expression plasmid, pREP1(fps1), rescued both the temperature-sensitive growth and sporulation deficiency, when introduced into spo9Δ cells (Figure 5, C and D). In contrast, pREP1(spo9) could not rescue the lethality of the fps1 null mutant (data not shown).
Figure 5.
(A) S. cerevisiae strains YF79 [erg20Δ harboring pYEL2(ERG20)] or YF80 [erg20Δ harboring pYEL2(fps1)] was incubated on YP-galactose (induced) or YPD medium (repressed) at 30°C for 2 d. (B) S. pombe strains YF77 [fps1Δ harboring pREP81(fps1)] and YF78 [fps1Δ harboring pREP81(ERG20)] were incubated on MM medium with (repressed) or without thiamine (induced) at 25°C for 3 d. (C) Overexpression of various genes in spo9Δ. S. pombe strain YF16 (spo9Δ) was transformed with either empty pREP1, pREP1(fps1), pREP1(spo9), pREP1(ERG20), or pREP1(BTS1). The Leu+ transformants were incubated on sporulation medium (MEA) at 28°C for 2 d. Bar, 10 μm. (D) The same transformants as shown in C were streaked on YEA medium and incubated at 25 or 36°C for 3 d. (E) Overexpression of various genes in S. cerevisiae bts1Δ. S. cerevisiae strain SFNY368 (bts1Δ) was transformed with empty pYEL2, pYEL2(BTS1), pYEL2(spo9), pYEL2(fps1), or pYEL2(ERG20). The Leu+ transformants were incubated on YP-galactose medium at 30°C for 3 d and at 15°C for 9 d.
In S. cerevisiae, FPS is encoded by the ERG20 gene. ERG20 was originally identified by genetic analysis of an inviable ergosterol-deficient mutant (Chambon et al., 1990; Blanchard and Karst, 1993). To assess the level of functional relatedness between S. pombe and S. cerevisiae FPSs, spo9+ and fps1+ were expressed under control of the GAL1 promoter in an erg20Δ mutant of S. cerevisiae. Ectopic expression of fps1+ but not spo9+ rescued the lethality of the erg20 null mutation in S. cerevisiae (Figure 5A). In a reciprocal manner, S. cerevisiae ERG20 was placed under the control of the thiamine-repressible nmt1 promoter and introduced into S. pombe fps1Δ and spo9Δ mutants. ERG20 rescued both the lethality of the fps1Δ mutant (Figure 5B) and the temperature sensitivity and sporulation deficiency of a spo9Δ mutant (Figure 5, C and D). These data establish that fission yeast Fps1 is able to fulfill all essential Erg20 functions.
GGPS and FPS belong to a family of prenyltransferases that catalyze consecutive condensations of IPP with allylic primer substrates. A number of genes of GGPS (Misawa et al., 1990; Ohnuma et al., 1994; Jiang et al., 1995; Zhu et al., 1997; Kainou et al., 1999; Kuzuguchi et al., 1999; Okada et al., 2000; Engprasert et al., 2004) and FPS (Chambon et al., 1990; Fujisaki et al., 1990; Koyama et al., 1993; Delourme et al., 1994; Cunillera et al., 1996; Koyama, 1999; Szkopinska and Plochocka, 2005) have been isolated from various organisms. Sequence alignment of the encoded enzymes reveals limited partial homology (Figure 3A). Apparently, the enzymes have been grouped based on their primary structures (Chen et al., 1994) (Figure 3B). However both enzymes share two aspartate-rich motifs as described above. A BLAST search of the nonredundant proteins in the database at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST/) was carried out using human GGPS (accession no. AAH05252) as a template to identify its S. pombe orthologue. We selected proteins whose e-value is <1.0. Two S. pombe prenyl diphosphate syntheses, Dps1 (e-value 4e-06) and Fps1 (e-value 2e-02) were hit. Dps1 has been known to function as a component of decaprenyl diphosphate synthase (Saiki et al., 2003), but Fps1 remained to be elucidated. These facts suggest that S. pombe has no apparent orthologue of GGPS as deduced from the primary structure, and they also imply the possibility that Fps1 and/or Spo9 function(s) as a GGPS. To test this possibility, we examined the relationship between S. pombe FPS-like proteins and S. cerevisiae GGPS. Both fps1+ and spo9+ complemented the cold sensitivity of an S. cerevisiae bts1Δ mutant (Figure 5E) and reciprocally, BTS1 complemented the temperature sensitivity and sporulation deficiency of an S. pombe spo9Δ mutant (Figure 5, C and D), but not the lethality of the fps1Δ mutant (data not shown). Erg20 also complemented the cold sensitivity of the S. cerevisiae bts1Δ mutant (Figure 5E). However, BTS1 could not rescue the lethality of the S. cerevisiae erg20Δ mutation (data not shown).
S. pombe fps1+ Complements an E. coli ispA Mutant
We next examined whether Fps1 and Spo9 possessed FPS activity by expressing the genes in an E. coli ispA mutant that lacks FPS (Fujisaki et al., 1990). The ubiquinone level in the ispA mutant is significantly lower than that in wild type, because ubiquinone is one of the end products of FPP (Fujisaki et al., 2005, Figure 6, A and B). If fps1+ and/or spo9+ encoded an FPS, we presumed that the ubiquinone level of the ispA strain expressing these genes would be restored. Ubiquinone was extracted from ispA strains either harboring the empty vector, fps1+-, or spo9+-carrying plasmids, and it was assayed by HPLC. As shown in Figure 6, the ubiquinone level in fps1+-expressing cells (Figure 6C) was significantly higher than in cells harboring the control vector (Figure 6B). In contrast, the level in the spo9+-expressing strain was almost identical to that in the control strain (Figure 6D). These data suggest that Fps1 but not Spo9 has FPS activity.
Figure 6.
fps1+, but not spo9+, complements an E. coli ispA deletion mutant. Ubiquinone was extracted from E. coli wild-type strain DH5α and SF7 (ΔispA) harboring pGEX-KG, pGEX(fps1) or pGEX(spo9). UQ6 was included as an internal standard. The amount of UQ-8 plus UQ-7 was estimated to be 64, 9.2, 24, and 8.6 ng per A600 for growth in DH5α (wild type), SF7 harboring pGEX-KG, pGEX(fps1), and pGEX(spo9), respectively.
Fps1 and Spo9 Are Sufficient for GGPS Activity
Phenotypes of mutants of S. pombe and S. cerevisiae harboring disruptions in FPS- and GGPS-related genes are summarized in Table 3 and indicate that the behavior of spo9+ is similar to that of BTS1. Although S. pombe has no apparent GGPS-like genes, these data suggest the possibility that Spo9 functions as a GGPS in S. pombe. GGPP is also known as a precursor of carotenoids. Several bacterial groups, including Erwinia, possess a gene cluster, crt, required for carotenoid biosynthesis that contains crtE (geranylgeranyl diphosphate synthase), crtX (zeaxanthin β-glucosidase), crtY (lycopene cyclase), crtI (phytoene desaturase), crtB (phytoene synthase), and crtZ (β-carotene hydroxylase) genes (Misawa et al., 1990). Because E. coli does not possess GGPS, E. coli cells transformed with plasmid pACCAR25ΔcrtE containing all the crt genes except for crtE from E. uredovora, are only able to accumulate zeaxanthin, which has a yellow color, if GGPP is produced (Zhu et al., 1997; Kainou et al., 1999; Engprasert et al., 2004). Therefore, we tested whether spo9+ has GGPS activity by use of this carotenoid formation test. fps1+, spo9+, and S. cerevisiae BTS1 were cloned into a bacterial expression plasmid, and introduced into DH10B harboring pACCAR25ΔcrtE. BTS1 transformants (positive control) produced the expected yellow pigment (Figure 7). However, neither fps1+ nor spo9+ transformants formed yellow colonies. Because it is known that FPS and GGPS from many organisms form a dimer and that dimer formation is essential for enzyme activity (Tarshis et al., 1994; Szkopinska and Plochocka, 2005), we speculated that if coexpression of Fps1 and Spo9 is needed for GGPS activity, cotransformation of these genes in E. coli might result in formation of a functional GGPP-producing enzyme. As shown in Figure 7, transformants expressing fps1+ and spo9+ simultaneously produced a notable yellow pigment. These data suggest that Fps1 and Spo9 together form a heteromeric GGPS.
Table 3.
Genetic relationship among S. cerevisiae and S. pombe FPS- and GGPS- like proteins
| Phenotype | Complementation by |
||||
|---|---|---|---|---|---|
| ERG20 | BTS1 | fps1+ | spo9+ | ||
| S. cerevisiae | |||||
| erg20Δ | Lethal | + | − | + | − |
| bts1Δ | Cold sensitive | + | + | + | + |
| S. pombe | |||||
| fps1Δ | Lethal | + | − | + | − |
| spo9Δ | Temperature sensitive | + | + | + | + |
| Sporulation deficient | + | + | + | + | |
+, complemented; −, did not complement.
Figure 7.
Coexpression of Spo9 and Fps1 is required for GGPS activity. E. coli DH10B carrying pACCAR25ΔcrtE was transformed with pMAL-c, pMAL(BTS1), pMAL(spo9), pMAL(fps1), pMAL (spo9+fps1), pMAL(spo9-R109Q+fps1), pMAL(spo9+fps1-R104Q), or pMAL(spo9-R109Q+fps1-R104Q); plated on LB medium supplemented with ampicillin and chloramphenicol; and incubated at 28°C for 3 d.
We next examined whether Fps1 and Spo9 physically interact each other, by use of the yeast two-hybrid assay. The assay indicated a positive interaction between Fps1 and Spo9 (Figure 8A). An interaction was also observed between Fps1 and Fps1 and between Spo9 and Spo9, but the level was considerably lower than between Fps1 and Spo9 (Figure 8A). To assess the association of Fps1 and Spo9 in S. pombe cells, a chimeric protein consisting of Fps1 fused to GFP was coexpressed with GST-tagged Spo9 in S. pombe. Fps1-GFP was immunoprecipitated with anti-GFP antibody, and copurification of GST-Spo9 was verified by Western blotting by using anti-GST antibody. As shown in Figure 8B, GST-Spo9 copurified with Fps1-GFP but not with unfused GFP. The interaction between Spo9 and Fps1 was also observed in an E. coli lysate (data not shown). Together, these data indicate that Fps1 and Spo9 interact directly with each other.
Figure 8.
Spo9 interacts with Fps1. (A) Yeast two-hybrid analysis. Plasmids expressing the respective Gal4 activation domain (AD) and Gal4 DNA-binding domain (BD) fusions were tested for two-hybrid interaction. (B) Coimmunoprecipitation of Spo9 and Fps1. Cell extracts were prepared from vegetative cells expressing tagged proteins, GFP and GST-Spo9 or Fps1-GFP and GST-Spo9, and subjected to immunoprecipitation with anti-GFP antibody. Precipitates were analyzed by Western blotting by using anti-GFP, or anti-GST antibody.
To understand the role of Fps1 and Spo9 in GGPS activity, we constructed two mutants, Fps1R104Q and Spo9R109Q, in which the conserved arginine just after the FARM motif was replaced with glutamine. These arginines seem to be critical for function of Fps1 and Spo9 because fps1-R104Q and spo9-R109Q were unable to complement the phenotype of fps1Δ and spo9Δ, respectively. An E. coli strain that harbored plasmid pACCAR25ΔcrtE coexpressing either Fps1 plus Spo9 R109Q or Fps1R104Q plus Spo9R109Q failed to produce the yellow pigment. Surprisingly, the same E. coli strain expressing Fps1R104Q plus Spo9 formed yellow colonies (Figure 7). These data suggest that GGPS is dependent on the enzymatic activity of Spo9 but not on that of Fps1.
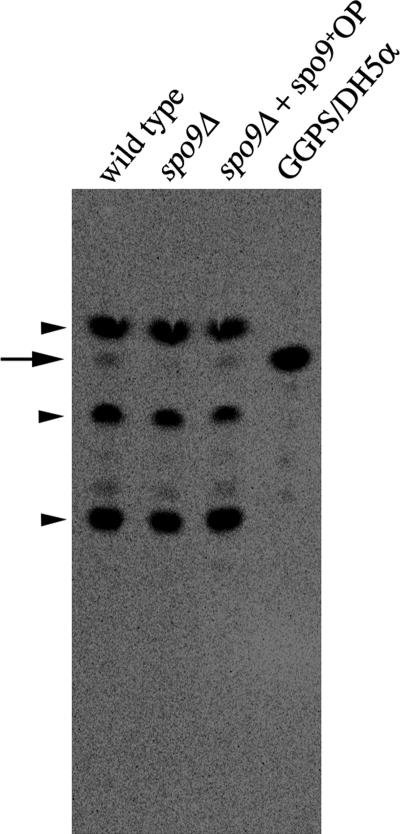
If spo9+ encodes a component of GGPS, GGPS activity should be detected in S. pombe wild type, but not in the spo9Δ mutant. In vitro GGPS activity was then measured in cell-free extracts from these strains. Cells were homogenized, and [14C]IPP and FPP were used as substrates to detect prenyl diphosphate synthase activity. The extracts were subsequently hydrolyzed with acid phosphatase, and the products were separated by reversed phase TLC. Although wild type was able to produce geranylgeraniol (GGOH), the spo9Δ strain barely showed any GGPS activity (Figure 9). The GGOH spot in the spo9Δ strain was intensified by the overexpression of the spo9+ gene. These data indicate that Spo9 indeed has GGPS activity.
Figure 9.
GGPS activity is significantly reduced in spo9 mutant. GGPS was assayed using [1-14C]IPP and FPP as substrates with crude extracts from wild-type (TN8), spo9Δ (YF16), and the spo9Δ harboring spo9+ (YF81) cells and E. coli DH5α harboring pBH expressing human GGPS1. The reaction products were hydrolyzed by acid phosphatase, and the resulting alcohols were analyzed by reversed phase TLC. An arrow indicates the product of GGOH. Other than GGOH, there are three intense spots; the uppermost and the lowest spots are farnesol and decaprenol, respectively, and a middle one is presumably ergosterol. These spots are indicated by arrowheads.
Protein prenylation is a posttranslational modification by which isoprenoid compounds are covalently attached to cysteine residues at or near the C terminus of proteins (Casey 1992, 1994; Schafer and Rine, 1992; Omer et al., 1994). A number of proteins are known to be prenylated, and in the most cases, the prenylation is essential for their membrane localization. Indeed, in the S. cerevisiae bts1Δ mutant, the membrane association of the small GTPases, Ypt1 and Sec4, is defective due to lack of geranylgeranylation (Jiang et al., 1995). Ypt7, a fission yeast orthologue of Rab7 GTPase, mediates fusion of endosomes to vacuoles and homotypic vacuole fusion (Iwaki et al., 2004). Ypt7 has a Cys-X-Cys at its C terminus, which is recognized by geranylgeranyl transferase. If spo9+ encodes a sole GGPS, disruption of the gene should result in depletion of GGPP and loss of geranylgeranylation of Ypt7. To test this hypothesis, we examined the membrane association of Ypt7 in the spo9Δ mutant. Cell extracts were fractionated into a membrane fraction and a cytosolic fraction, and the distribution of Ypt7 was examined in each by Western blot analysis. Spo14, a membrane-bound protein was used as a probe for the membrane fraction (Nakamura-Kubo et al., 2003). Most Ypt7 was detected in the membrane fraction prepared from wild-type cells. However, in spo9Δ cells, Ypt7 was detected in both cytosolic and membrane fractions (Figure 10). Thus, the membrane association of Ypt7 is partially defective in spo9Δ cells. In the unfractionated extract from the spo9Δ cells, Ypt7 was detected as a doublet. In contrast, in both cytosolic and membrane fractions from spo9Δ cells, Ypt7 was detected as a single band, although migration of Ypt7 detected in the membrane fraction was slightly faster than that in the cytosolic fraction. Generally, the prenylated protein migrates faster than its unmodified form (Figure 10). These data support the notion that the extent of geranylgeranylation was lower in spo9Δ cells. Rhb1, a fission yeast orthologue of the human small GTPase Rheb, is known to be farnesylated and the farnesylation is important for membrane association (Yang et al., 2001; Nakase et al., 2006). If Spo9 mainly functions as a GGPS and not an FPS, the distribution of Rhb1 should not be affected by the spo9 mutation. As shown in Figure 10, the distribution of Rhb1 in the spo9Δ cells in cytosolic and membrane fractions was almost identical to that in wild-type cells. Together, these data are consistent with the possibility that Spo9 is an essential component of GGPS in fission yeast.
Figure 10.
spo9 mutation causes mislocalization of Ypt7 but not Rhb1. Wild-type cells (YF51) carrying GFP-Ypt7 and spo9Δ cells (YF54) were ruptured, and then they were subjected to differential centrifugation to fractionate into a P100 membrane fraction and an S100 supernatant. Each fraction was resolved by SDS-polyacrylamide gel electrophoresis and subjected to Western blotting using either anti-GFP, anti-Rhb1, or anti-Spo14 (control) antibody.
The spo9 Mutant Is Defective in Forespore Membrane Formation
Phenotypic analyses of many sporulation-deficient mutants have revealed that most are defective in forespore membrane formation (Nakamura et al., 2001, 2005; Nakase et al., 2001; Nakamura-Kubo et al., 2003; Onishi et al., 2003; Koga et al., 2004; Kashiwazaki et al., 2005). To examine in detail how the spo9 mutation impairs sporulation, the assembly of forespore membrane in the spo9-B261 mutant was observed using GFP-tagged Psy1, a syntaxin-like protein (Nakamura et al., 2001). Progression of meiosis was monitored by observing the formation and elongation of spindle microtubules. In wild-type cells, most haploid nuclei produced by meiotic second divisions were encapsulated by the forespore membrane. In the spo9-B261 mutant, forespore membrane formation initiated normally during meiosis II (Figure 11A), but subsequent development was blocked (Figure 11B). Only 40% of the zygotes contained four complete sets of nucleated prespores (membrane-bounded spore precursors), although they were remarkably small (type I in Figure 11B). Anucleated prespores were observed in 50% of the zygotes (type II in Figure 11B). In type III, assembly of the forespore membrane seemed to be arrested halfway (Figure 11B). These results indicate that forespore membrane formation initiates normally, but that subsequent development and integrity of the forespore membrane are impaired in spo9. Essentially identical data were obtained when spo9Δ cells were used.
Figure 11.
Aberrant assembly of the forespore membrane in spo9 mutant. (A) Assembly of the forespore membrane during metaphase II. Wild type (YN68) and spo9-B261 (YF5) were cultured in SSL-N to induce meiosis at 28°C for 8 h. Fixed cells were doubly stained with anti-α-tubulin and DAPI. (B) Classification of terminal phenotypes of the forespore membrane in spo9 zygotes. Strain, culture conditions and staining procedures are the same as described in Figure 11A. Type I, forespore membranes engulfed each nucleus but prespores were remarkably small. Type II, four aggregates of GFP-Psy1 were formed close to nuclei. Type III, forespore membrane formation arrested. Bars, 10 μm.
To investigate the structure of the forespore membrane in more detail, we observed the fine structures of spo9Δ cells by thin-section electron microscopy. As shown in Figure 12, membrane vesicle accumulation was notable in the cytoplasm of immature spo9Δ asci, but such vesicle accumulation was not observed in wild-type asci. The vesicles in the spo9Δ mutant had an average diameter of 79 ± 42 nm, consistent with previous measurements of post-Golgi secretory vesicles in S. cerevisiae. Furthermore, Golgi-like structures were often observed in the spo9Δ mutant (Figure 12B). In addition, apparently abnormal forespore membranes were also observed. Anucleated spore-like bodies were often observed as well (Figure 12, B–D). These data are essentially identical to those obtained by fluorescence microscopy described above. Thus, spo9+ is important for the assembly of the forespore membrane, especially the expansion of the membrane.
Figure 12.
Fine structures of spo9Δ asci. Mature spores in wild type (A) and anucleated spore-like bodies in spo9Δ mutant (B–D). N, SPB, and FSM denote the nucleus, spindle pole body, and forespore membrane, respectively. Note that many membrane vesicles (indicated by arrows) and Golgi-like structures (indicated by arrowheads) are present in the cytoplasm of spo9Δ. Bar, 0.5 μm.
DISCUSSION
Isolation and Characterization of Two Novel Genes Encoding a Farnesyl Diphosphate Synthase-like Protein in S. pombe
Both FPS and GGPS are highly conserved enzymes from bacteria to humans. Here, we isolated two novel genes, fps1+ and spo9+. Both Fps1 and Spo9 share significant identity (40–50%) with typical FPSs, but only modest identity (∼20%) with typical GGPSs. Thus, both Fps1 and Spo9 more closely resemble FPS rather than GGPS in amino acid sequence. Based on database search and phylogenetic analysis, S. pombe has no other GGPS-encoding gene. In S. cerevisiae, Erg20 and Bts1 are specifically recognized as an FPS and a GGPS, respectively. Overexpression of fps1+ in an E. coli ispA mutant that lacks FPS restored the production of ubiquinone, which is one of the end products of FPP, suggesting that Fps1 functions as a typical FPS in S. pombe. Because GGPP can be a substrate for octaprenyl diphosphate synthase, a key enzyme of ubiquinone synthesis, it is also possible that the same complementation of the ispA mutation is due to the GGPS activity of Fps1. However, this possibility seems unlikely by the following reasons. First, the overexpression of Fps1 but not S. cerevisiae GGPS Bts1 could complement the lethality of an S. cerevisiae erg20 mutant that lacks FPS. Second, ERG20 but not BTS1 could complement the lethality of an S. pombe fps1Δ mutant. Third, overexpression of fps1+ alone in E. coli cells cannot produce the substantial amount of GGPP by judging from the result of carotenoid producing assay (Figure 7). In similar experiments, no evidence was obtained to support the possibility that Spo9 functions as an FPS, although it resembled other FPSs in primary structure. The failure of spo9+ to complement the phenotype of either fps1Δ or erg20Δ makes it unlikely that Spo9 functions as an FPS.
S. pombe Geranylgeranyl Diphosphate Synthase Is Composed of a Typical FPS, Fps1, and an FPS-like Protein, Spo9
Although S. pombe has two FPS-like proteins, a protein that shares significant similarity with GGPS in the S. pombe genome database was not found. Several lines of evidence obtained in this study suggest that both Spo9 and Fps1 form a heteromer and that this complex functions as GGPS. First, spo9+ was able to complement the phenotype of an S. cerevisiae bts1Δ mutant, which lacks GGPS, and BTS1 was able to rescue spo9Δ. Second, GGPS activity was significantly lower in S. pombe spo9Δ cells than in wild type. Third, the spo9 mutation perturbed the membrane association of a geranylgeranylated protein but not that of a farnesylated protein. Fourth, Spo9 directly interacts with Fps1. Fifth, E. coli cells that express a set of genes required for carotenoid biosynthesis but without a GGPS gene, only produced carotenoid when spo9+ and fps1+ were expressed simultaneously.
Most short-chain prenyltransferases are known to function as homodimers (Koyama, 1999). However, in other cases, as in spearmint (Burke et al., 1999), geranyl diphosphate synthase forms a heterodimer and decaprenyl diphosphate synthase in S. pombe and humans forms a heterotetramer (Saiki et al., 2003, 2005). Each of these heteromers includes a subunit that has significant homology with typical prenyltransferases that contain two aspartate-rich motifs. For example, Dps1, a component of decaprenyl diphosphate synthase from S. pombe has a high similarity (∼40%) with typical long-chain prenyl diphosphate synthases, whereas the other component, Dlp1, has only weak similarity (∼20%) with prenyl diphosphate synthases (Suzuki et al., 1997; Saiki et al., 2003, 2005). In marked contrast to these proteins, both subunits of S. pombe GGPS share high similarity with FPSs, but only weak similarity with typical GGPSs. We attempted to determine whether the S. pombe GGPS is composed of a heterodimer or a heterotetramer by a gel filtration analysis. However, neither protein could be detected in any fractions corresponding to heterodimers or heterooligomers (data not shown). This fact suggests that the interaction between Fps1 and Spo9 is so weak that the complex readily dissociates during gel filtration. In the experiment showing the coimmunoprecipitation of Spo9 and Fps1 in S. pombe cells, a density of Spo9 relative to Fps1 in lane 2 (IP) is lower than that in lane 2 (lysates) (Figure 8B). This result might be attributed to their dissociation during the immunoprecipitation. Alternatively, the result could be interpreted by the formation of homodimers of Fps1, as revealed in Figure 8A.
We obtained clear evidence that S. pombe GGPS is composed of Fps1 and Spo9, but at the same time, we cannot rule out the possibility that Fps1 might have weak GGPS activity for the following reasons. First, although GGPS activity was significantly reduced in spo9Δ cells, it was not absent (Figure 9). In addition, geranylgeranylation-dependent membrane association of Ypt7 was observed to some extent in spo9Δ cells (Figure 10). These results suggest that GGPP is still synthesized in spo9Δ cells. Second, overexpression of fps1+ was able to suppress the temperature sensitivity and sporulation deficiency of spo9Δ, and GGPS activity was detected in those cells (data not shown). Several FPSs have been known to retain GGPS activity as well (Chen and Poulter, 1993; Fujiwara et al., 2004). It is reasonable that GGPS activity was detected under nonphysiological conditions, i.e., overexpression of the Fps1 protein. At present, we cannot examine the GGPS activity and membrane association of Ypt7 and Rhb1 in the fps1 disruptant, because fps1+ is essential for growth. Analysis of conditional fps1 mutants will allow us to elucidate the role of Fps1 as a GGPS.
Previous studies have revealed that the amino acid residue located on the fifth position before the FARM of FPS is important for the determination of chain length of a final product. In all of known FPSs, including S. pombe Fps1, the corresponding amino acid is either phenylalanine or tyrosine, but the corresponding amino acid is cysteine in Spo9 (Figure 3A). Ohnuma et al. (1996) constructed 20 FPS mutant proteins from Bacillus stearothermophilus, each of which has a different amino acid at position 81 located on the fifth position before the FARM, and revealed that the average chain length of products is inversely proportional to the accessible surface area of substituted amino acids. Interestingly, B. stearothermophilus FPS Y81C mutant, in which tyrosine 81 was replaced with cysteine, effectively produced GGPP (Ohnuma et al., 1996). These data suggest the possibility that cysteine 95 is important for the Spo9 function as GGPS. To test this possibility, we constructed the mutant protein Spo9C95F, in which cysteine 95 was replaced with phenylalanine, and introduced it into fps1Δ. However, the mutant protein could not complement the lethality of fps1Δ, and it did complement the phenotypes of spo9Δ. Conversely, the fps1-F90C mutant gene could complement the lethality of fps1Δ. Thus, it is unlikely that cysteine 95 is the only important determinant for the function of S. pombe Spo9 as a GGPS.
Role of Spo9 in Sporulation
Our study also demonstrates that GGPS plays a crucial role in sporulation. Fluorescence microscopy by using the forespore membrane marker protein GFP-Psy1 revealed that the forespore membrane formation initiates normally, but that the subsequent process, expansion of the membrane was severely impaired in spo9Δ. GGPP is the precursor for several important metabolites, including dolichols, ubiquinones, and heme a in S. pombe. One possibility is that the decrease in these metabolites leads to the defect in sporulation. If so, various steps in sporulation should be affected. However, the spo9 mutation perturbed only expansion of forespore membrane. The other events of sporulation, the meiotic nuclear division and the initiation of forespore membrane formation, seemed to proceed normally in spo9Δ. Therefore, it seems unlikely that the decrease of end products such as heme a and/or ubiquinone directly affects the sporulation-defective phenotype of spo9Δ. At least there is no defect in sporulation in ubiquinone-deficient strains (unpublished observations). Alternatively, it is possible that the spo9 mutation perturbs the membrane association of a geranylgeranylated protein necessary for sporulation. Indeed, the membrane association of Ypt7, a putative geranylgeranylated protein, is significantly diminished in spo9Δ. Our previous study revealed that Ypt7 is essential for proper spore formation (Kashiwazaki et al., 2005). Thus, the defective membrane association of Ypt7 could plausibly result in the spo9Δ mutant phenotype. Nonetheless, the phenotype of spo9Δ is much severe than that of ypt7Δ, suggesting that other geranylgeranylated proteins are involved in sporulation as well (Giannakouros et al., 1992). In an S. cerevisiae bts1 mutant, the failure to geranylgeranylate Ypt1 and Sec4 leads to a defect in the membrane association of these proteins. The bts1 mutation may impair intracellular membrane trafficking, because these proteins play essential roles in this process (Rossi et al., 1991; Jiang et al., 1995). A number of genes that play roles in the general secretion machinery have been reported to be necessary for sporulation in both S. cerevisiae and S. pombe (Neiman, 1998; Nakase et al., 2001; Nakamura et al., 2001, 2005; Jantti et al., 2002; Nakamura-Kubo et al., 2003; Nakanishi et al., 2006). Among them, sec4 mutant also exhibits a sporulation deficiency in S. cerevisiae (Neiman, 1998). S. pombe possesses a Sec4 homologue, Ypt2, which also has a consensus sequence for geranylgeranylation (Haubruck et al., 1990). Defective geranylgeranylation of Ypt2 in the spo9 mutant might cause a sporulation deficiency. This possibility is further supported by our observation that many membrane vesicles accumulate in the cytoplasm during sporulation in spo9Δ cells.
In summary, we report here that S. pombe FPS is composed of Fps1 and that GGPS is composed of a typical FPS, Fps1, and an FPS-like protein, Spo9. To our knowledge, a heteromer of two FPS-like proteins is a novel type of GGPS that has not been previously reported. We speculate that S. pombe developed this GGPS by duplicating an FPS gene followed by limited mutational changes in the duplicated copy resulting in the present heteromeric structure.
ACKNOWLEDGMENTS
We thank S. Forsburg (University of Southern California) for plasmids; Ferro-Novick (University of Utah), S. Fujisaki (Toho University), and the Yeast Genetic Resource Center Japan supported by the National BioResource Project (YGRC/NBRP; http://yeast.lab.nig.ac.jp/nig/) for strains; K. Gull (University of Manchester), S. Fujita (Mitsubishi Chemical), and T. Matsumoto (Kyoto University) for antibodies. This study was supported by grant-in-aid for scientific research on priority areas “Genome Biology” to C.S., for Scientific Research (B) to M.K., and on “Cell Cycle Control” and “Life of Proteins” to T.N. from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and The Sumitomo Foundation to T.N.
Abbreviations used:
- DMAPP
dimethylallyl diphosphate
- FARM
first aspartate-rich motif
- FPP
farnesyl diphosphate
- FPS
farnesyl diphosphate synthase
- GGPP
geranylgeranyl diphosphate
- GGPS
geranylgeranyl diphosphate synthase
- GPP
geranyl diphosphate
- IPP
isopentenyl diphosphate
- SARM
second aspartate-rich motif
- TLC
thin-layer chromatography.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-02-0112) on June 27, 2007.
REFERENCES
- Blanchard L., Karst F. Characterization of a lysine-to-glutamic acid mutation in a conservative sequence of farnesyl diphosphate synthase from Saccharomyces cerevisiae. Gene. 1993;125:185–189. doi: 10.1016/0378-1119(93)90326-x. [DOI] [PubMed] [Google Scholar]
- Bresch C., Muller G., Egel R. Genes involved in meiosis and sporulation of a yeast. Mol. Gen. Genet. 1968;102:301–306. doi: 10.1007/BF00433721. [DOI] [PubMed] [Google Scholar]
- Burke C. C., Wildung M. R., Croteau R. Geranyl diphosphate synthase: cloning, expression, and characterization of this prenyltransferase as a heterodimer. Proc. Natl. Acad. Sci. USA. 1999;96:13062–13067. doi: 10.1073/pnas.96.23.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. J. Biochemistry of protein prenylation. J. Lipid Res. 1992;33:1731–1740. [PubMed] [Google Scholar]
- Casey P. J. Lipid modifications of G proteins. Curr. Opin. Cell Biol. 1994;6:219–225. doi: 10.1016/0955-0674(94)90139-2. [DOI] [PubMed] [Google Scholar]
- Chambon C., Ladeveze V., Oulmouden A., Servouse M., Karst F. Isolation and properties of yeast mutants affected in farnesyl diphosphate synthetase. Curr. Genet. 1990;18:41–46. doi: 10.1007/BF00321113. [DOI] [PubMed] [Google Scholar]
- Chen A., Kroon P. A., Poulter C. D. Isoprenyl diphosphate synthases: protein sequence comparisons, a phylogenetic tree, and predictions of secondary structure. Protein Sci. 1994;3:600–607. doi: 10.1002/pro.5560030408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A., Poulter C. D. Purification and characterization of farnesyl diphosphate/geranylgeranyl diphosphate synthase. A thermostable bifunctional enzyme from Methanobacterium thermoautotrophicum. J. Biol. Chem. 1993;268:11002–11007. [PubMed] [Google Scholar]
- Cunillera N., Arro M., Delourme D., Karst F., Boronat A., Ferrer A. Arabidopsis thaliana contains two differentially expressed farnesyl-diphosphate synthase genes. J. Biol. Chem. 1996;271:7774–7780. doi: 10.1074/jbc.271.13.7774. [DOI] [PubMed] [Google Scholar]
- Delourme D., Lacroute F., Karst F. Cloning of an Arabidopsis thaliana cDNA coding for farnesyl diphosphate synthase by functional complementation in yeast. Plant Mol. Biol. 1994;26:1867–1873. doi: 10.1007/BF00019499. [DOI] [PubMed] [Google Scholar]
- Engprasert S., Taura F., Kawamukai M., Shoyama Y. Molecular cloning and functional expression of geranylgeranyl pyrophosphate synthase from Coleus forskohlii Briq. BMC. Plant Biol. 2004;4:18. doi: 10.1186/1471-2229-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W. W., Lever D., Leining L. M., Bruenger E., Rilling H. C. Quantitation of prenylcysteines by a selective cleavage reaction. Proc. Natl. Acad. Sci. USA. 1991;88:9668–9670. doi: 10.1073/pnas.88.21.9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsburg S. L., Sherman D. A. General purpose tagging vectors for fission yeast. Gene. 1997;191:191–195. doi: 10.1016/s0378-1119(97)00058-9. [DOI] [PubMed] [Google Scholar]
- Fujisaki S., Hara H., Nishimura Y., Horiuchi K., Nishino T. Cloning and nucleotide sequence of the ispA gene responsible for farnesyl diphosphate synthase activity in Escherichia coli. J. Biochem. 1990;108:995–1000. doi: 10.1093/oxfordjournals.jbchem.a123327. [DOI] [PubMed] [Google Scholar]
- Fujisaki S., Takahashi I., Hara H., Horiuchi K., Nishino T., Nishimura Y. Disruption of the structural gene for farnesyl diphosphate synthase in Escherichia coli. J. Biochem. 2005;137:395–400. doi: 10.1093/jb/mvi049. [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Yamanaka A., Hirooka K., Kobayashi A., Imanaka T., Fukusaki E. Temperature-dependent modulation of farnesyl diphosphate/geranylgeranyl diphosphate synthase from hyperthermophilic archaea. Biochem. Biophys. Res. Commun. 2004;325:1066–1074. doi: 10.1016/j.bbrc.2004.10.129. [DOI] [PubMed] [Google Scholar]
- Giannakouros T., Armstrong J., Magee A. I. Protein prenylation in Schizosaccharomyces pombe. FEBS Lett. 1992;297:103–106. doi: 10.1016/0014-5793(92)80337-g. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Gutz H., Heslot H., Leupold U., Loprieno N. Schizosaccharomyces pombe. Handb. Genet. 1974;1:395–446. [Google Scholar]
- Hagan I. M., Hyams J. S. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 1988;89:343–357. doi: 10.1242/jcs.89.3.343. [DOI] [PubMed] [Google Scholar]
- Haubruck H., Engelke U., Mertins P., Gallwitz D. Structural and functional analysis of ypt2, an essential ras-related gene in the fission yeast Schizosaccharomyces pombe encoding a Sec4 protein homologue. EMBO J. 1990;9:1957–1962. doi: 10.1002/j.1460-2075.1990.tb08323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A., Shimoda C. Electron microscopic examination of sporulation-deficient mutants of the fission yeast Schizosaccharomyces pombe. Arch. Microbiol. 1992;158:249–255. doi: 10.1007/BF00245240. [DOI] [PubMed] [Google Scholar]
- Hirata A., Shimoda C. Structural modification of spindle pole bodies during meiosis II is essential for the normal formation of ascospores in Schizosaccharomyces pombe: ultrastructural analysis of spo mutants. Yeast. 1994;10:173–183. doi: 10.1002/yea.320100205. [DOI] [PubMed] [Google Scholar]
- Ikemoto S., Nakamura T., Kubo M., Shimoda C. S. pombe sporulation-specific coiled-coil protein Spo15p is localized to the spindle pole body and essential for its modification. J. Cell Sci. 2000;113:545–554. doi: 10.1242/jcs.113.3.545. [DOI] [PubMed] [Google Scholar]
- Iwaki T., Osawa F., Onishi M., Koga T., Fujita Y., Hosomi A., Tanaka N., Fukui Y., Takegawa K. Characterization of vps33+, a gene required for vacuolar biogenesis and protein sorting in Schizosaccharomyces pombe. Yeast. 2003;20:845–855. doi: 10.1002/yea.1011. [DOI] [PubMed] [Google Scholar]
- Iwaki T., Tanaka N., Takagi H., Giga-Hama Y., Takegawa K. Characterization of end4+, a gene required for endocytosis in Schizosaccharomyces pombe. Yeast. 2004;21:867–881. doi: 10.1002/yea.1134. [DOI] [PubMed] [Google Scholar]
- Jantti J., Aalto M. K., Oyen M., Sundqvist L., Keranen S., Ronne H. Characterization of temperature-sensitive mutations in the yeast syntaxin 1 homologues Sso1p and Sso2p, and evidence of a distinct function for Sso1p in sporulation. J. Cell Sci. 2002;115:409–420. doi: 10.1242/jcs.115.2.409. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Proteau P., Poulter D., Ferro-Novick S. BTS1 encodes a geranylgeranyl diphosphate synthase in Saccharomyces cerevisiae. J. Biol. Chem. 1995;270:21793–21799. doi: 10.1074/jbc.270.37.21793. [DOI] [PubMed] [Google Scholar]
- Kainou T., Kawamura K., Tanaka K., Matsuda H., Kawamukai M. Identification of the GGPS1 genes encoding geranylgeranyl diphosphate synthases from mouse and human. Biochim. Biophys. Acta. 1999;1437:333–340. doi: 10.1016/s1388-1981(99)00028-1. [DOI] [PubMed] [Google Scholar]
- Kainou T., Okada K., Suzuki K., Nakagawa T., Matsuda H., Kawamukai M. Dimer formation of octaprenyl-diphosphate synthase (IspB) is essential for chain length determination of ubiquinone. J. Biol. Chem. 2001;276:7876–7883. doi: 10.1074/jbc.M007472200. [DOI] [PubMed] [Google Scholar]
- Kashiwazaki J., Nakamura T., Iwaki T., Takegawa K., Shimoda C. A role for fission yeast Rab GTPase Ypt7p in sporulation. Cell Struct. Funct. 2005;30:43–49. doi: 10.1247/csf.30.43. [DOI] [PubMed] [Google Scholar]
- Kawamukai M. Biosynthesis, bioproduction and novel roles of ubiquinone. J. Biosci. Bioeng. 2002;94:511–517. doi: 10.1016/s1389-1723(02)80188-8. [DOI] [PubMed] [Google Scholar]
- Kishida M., Shimoda C. Genetic mapping of eleven spo genes essential for ascospore formation in the fission yeast Schizosaccharomyces pombe. Curr. Genet. 1986;10:443–447. doi: 10.1007/BF00419871. [DOI] [PubMed] [Google Scholar]
- Koga T., Onishi M., Nakamura Y., Hirata A., Nakamura T., Shimoda C., Iwaki T., Takegawa K., Fukui Y. Sorting nexin homologues are targets of phosphatidylinositol 3-phosphate in sporulation of Schizosaccharomyces pombe. Genes Cells. 2004;9:561–574. doi: 10.1111/j.1356-9597.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- Koyama T., Obata S., Osabe M., Takeshita A., Yokoyama K., Uchida M., Nishino T., Ogura K. Thermostable farnesyl diphosphate synthase of Bacillus stearothermophilus: molecular cloning, sequence determination, overproduction, and purification. J. Biochem. 1993;113:355–363. doi: 10.1093/oxfordjournals.jbchem.a124051. [DOI] [PubMed] [Google Scholar]
- Koyama T. Molecular analysis of prenyl chain elongating enzymes. Biosci. Biotechnol. Biochem. 1999;63:1671–1676. doi: 10.1271/bbb.63.1671. [DOI] [PubMed] [Google Scholar]
- Kuzuguchi T., Morita Y., Sagami I., Sagami H., Ogura K. Human geranylgeranyl diphosphate synthase. cDNA cloning and expression. J. Biol. Chem. 1999;274:5888–5894. doi: 10.1074/jbc.274.9.5888. [DOI] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- Misawa N., Nakagawa M., Kobayashi K., Yamano S., Izawa Y., Nakamura K., Harashima K. Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J. Bacteriol. 1990;172:6704–6712. doi: 10.1128/jb.172.12.6704-6712.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Kashiwazaki J., Shimoda C. A fission yeast SNAP-25 homologue, SpSec9, is essential for cytokinesis and sporulation. Cell Struct. Funct. 2005;30:15–24. doi: 10.1247/csf.30.15. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Kishida M., Shimoda C. The Schizosaccharomyces pombe spo6+ gene encoding a nuclear protein with sequence similarity to budding yeast Dbf4 is required for meiotic second division and sporulation. Genes Cells. 2000;5:463–479. doi: 10.1046/j.1365-2443.2000.00343.x. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nakamura-Kubo M., Hirata A., Shimoda C. The Schizosaccharomyces pombe spo3+ gene is required for assembly of the forespore membrane and genetically interacts with psy1+-encoding syntaxin-like protein. Mol. Biol. Cell. 2001;12:3955–3972. doi: 10.1091/mbc.12.12.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Nakamura-Kubo M., Nakamura T., Shimoda C. Novel fission yeast Cdc7-Dbf4-like kinase complex required for the initiation and progression of meiotic second division. Mol. Cell Biol. 2002;22:309–320. doi: 10.1128/MCB.22.1.309-320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Kubo M., Nakamura T., Hirata A., Shimoda C. The fission yeast spo14+ gene encoding a functional homologue of budding yeast Sec12 is required for the development of forespore membranes. Mol. Biol. Cell. 2003;14:1109–1124. doi: 10.1091/mbc.E02-08-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H., Morishita M., Schwartz C. L., Coluccio A., Engebrecht J., Neiman A. M. Phospholipase D and the SNARE Sso1p are necessary for vesicle fusion during sporulation in yeast. J. Cell Sci. 2006;119:1406–1415. doi: 10.1242/jcs.02841. [DOI] [PubMed] [Google Scholar]
- Nakase Y., Fukuda K., Chikashige Y., Tsutsumi C., Morita D., Kawamoto S., Ohnuki M., Hiraoka Y., Matsumoto T. A defect in protein farnesylation suppresses a loss of Schizosaccharomyces pombe tsc2+, a homolog of the human gene predisposing to tuberous sclerosis complex. Genetics. 2006;173:569–578. doi: 10.1534/genetics.106.056895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase Y., Nakamura T., Hirata A., Routt S. M., Skinner H. B., Bankaitis V. A., Shimoda C. The Schizosaccharomyces pombe spo20+ gene encoding a homologue of Saccharomyces cerevisiae Sec14 plays an important role in forespore membrane formation. Mol. Biol. Cell. 2001;12:901–917. doi: 10.1091/mbc.12.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase Y., Nakamura T., Okazaki K., Hirata A., Shimoda C. The Sec14 family glycerophospholipid-transfer protein is required for structural integrity of the spindle pole body during meiosis in fission yeast. Genes Cells. 2004;9:1275–1286. doi: 10.1111/j.1365-2443.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- Neiman A. M. Prospore membrane formation defines a developmentally regulated branch of the secretory pathway in yeast. J. Cell Biol. 1998;140:29–37. doi: 10.1083/jcb.140.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma S., Narita K., Nakazawa T., Ishida C., Takeuchi Y., Ohto C., Nishino T. A role of the amino acid residue located on the fifth position before the first aspartate-rich motif of farnesyl diphosphate synthase on determination of the final product. J. Biol. Chem. 1996;271:30748–30754. doi: 10.1074/jbc.271.48.30748. [DOI] [PubMed] [Google Scholar]
- Ohnuma S., Suzuki M., Nishino T. Archaebacterial ether-linked lipid biosynthetic gene. Expression cloning, sequencing, and characterization of geranylgeranyl-diphosphate synthase. J. Biol. Chem. 1994;269:14792–14797. [PubMed] [Google Scholar]
- Okada K., Saito T., Nakagawa T., Kawamukai M., Kamiya Y. Five geranylgeranyl diphosphate synthases expressed in different organs are localized into three subcellular compartments in Arabidopsis. Plant Physiol. 2000;122:1045–1056. doi: 10.1104/pp.122.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer C. A., Gibbs J. B. Protein prenylation in eukaryotic microorganisms: genetics, biology and biochemistry. Mol. Microbiol. 1994;11:219–225. doi: 10.1111/j.1365-2958.1994.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Onishi M., Koga T., Morita R., Nakamura Y., Nakamura T., Shimoda C., Takegawa K., Hirata A., Fukui Y. Role of phosphatidylinositol 3-phosphate in formation of forespore membrane in Schizosaccharomyces pombe. Yeast. 2003;20:193–206. doi: 10.1002/yea.953. [DOI] [PubMed] [Google Scholar]
- Rossi G., Yu J. A., Newman A. P., Ferro-Novick S. Dependence of Ypt1 and Sec4 membrane attachment on Bet2. Nature. 1991;351:158–161. doi: 10.1038/351158a0. [DOI] [PubMed] [Google Scholar]
- Saiki R., Nagata A., Kainou T., Matsuda H., Kawamukai M. Characterization of solanesyl and decaprenyl diphosphate synthases in mice and humans. FEBS J. 2005;272:5606–5622. doi: 10.1111/j.1742-4658.2005.04956.x. [DOI] [PubMed] [Google Scholar]
- Saiki R., Nagata A., Uchida N., Kainou T., Matsuda H., Kawamukai M. Fission yeast decaprenyl diphosphate synthase consists of Dps1 and the newly characterized Dlp1 protein in a novel heterotetrameric structure. Eur. J. Biochem. 2003;270:4113–4121. doi: 10.1046/j.1432-1033.2003.03804.x. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E. F., Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schafer W. R., Rine J. Protein prenylation: genes, enzymes, targets, and functions. Annu. Rev. Genet. 1992;26:209–237. doi: 10.1146/annurev.ge.26.120192.001233. [DOI] [PubMed] [Google Scholar]
- Shimoda C. Forespore membrane assembly in yeast: coordinating SPBs and membrane trafficking. J. Cell Sci. 2004;117:389–396. doi: 10.1242/jcs.00980. [DOI] [PubMed] [Google Scholar]
- Song L., Poulter C. D. Yeast farnesyl-diphosphate synthase: site-directed mutagenesis of residues in highly conserved prenyltransferase domains I and II. Proc. Natl. Acad. Sci. USA. 1994;91:3044–3048. doi: 10.1073/pnas.91.8.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Okada K., Kamiya Y., Zhu X. F., Nakagawa T., Kawamukai M., Matsuda H. Analysis of the decaprenyl diphosphate synthase (dps) gene in fission yeast suggests a role of ubiquinone as an antioxidant. J. Biochem. 1997;121:496–505. doi: 10.1093/oxfordjournals.jbchem.a021614. [DOI] [PubMed] [Google Scholar]
- Szkopinska A., Plochocka D. Farnesyl diphosphate synthase; regulation of product specificity. Acta Biochim. Pol. 2005;52:45–55. [PubMed] [Google Scholar]
- Tanaka K., Hirata A. Ascospore development in the fission yeasts Schizosaccharomyces pombe and S. japonicus. J. Cell Sci. 1982;56:263–279. doi: 10.1242/jcs.56.1.263. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Yonekawa T., Kawasaki Y., Kai M., Furuya K., Iwasaki M., Murakami H., Yanagida M., Okayama H. Fission yeast Eso1p is required for establishing sister chromatid cohesion during S phase. Mol. Cell Biol. 2000;20:3459–3469. doi: 10.1128/mcb.20.10.3459-3469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarshis L. C., Yan M., Poulter C. D., Sacchettini J. C. Crystal structure of recombinant farnesyl diphosphate synthase at 2.6-A resolution. Biochemistry. 1994;33:10871–10877. doi: 10.1021/bi00202a004. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc. Natl. Acad. Sci. USA. 1980;77:5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Miyashita K., Saito T. T., Yoneki. T, Kakihara. Y, Nabeshima K., Kishi Y. A., Shimoda C., Nojima H. Comprehensive isolation of meiosis-specific genes identifies novel proteins and unusual non-coding transcripts in Schizosaccharomyces pombe. Nucleic Acids Res. 2001;29:2327–2337. doi: 10.1093/nar/29.11.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Imai Y., Watanabe Y. Mating and sporulation in Schizosaccharomyces pombe. In: Pringle J. R., Broach J. B., Jones E. W., editors. Molecular and Cellular Biology of the Yeast Saccharomyces. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 1037–1106. [Google Scholar]
- Yang W., Tabancay A. P., Jr, Urano J., Tamanoi F. Failure to farnesylate Rheb protein contributes to the enrichment of G0/G1 phase cells in the Schizosaccharomyces pombe farnesyltransferase mutant. Mol. Microbiol. 2001;41:1339–1347. doi: 10.1046/j.1365-2958.2001.02599.x. [DOI] [PubMed] [Google Scholar]
- Yoo B. Y., Calleja G. B., Johnson B. F. Ultrastructural changes of the fission yeast (Schizosaccharomyces pombe) during ascospore formation. Arch. Mikrobiol. 1973;91:1–10. doi: 10.1007/BF00409533. [DOI] [PubMed] [Google Scholar]
- Zhu X. F., Suzuki K., Saito T., Okada K., Tanaka K., Nakagawa T., Matsuda H., Kawamukai M. Geranyl geranyl pyrophosphate synthase encoded by the newly isolated gene GGPS6 from Arabidopsis thaliana is localized in mitochondria. Plant Mol. Biol. 1997;35:331–341. doi: 10.1023/a:1005898805326. [DOI] [PubMed] [Google Scholar]