Abstract
Gene silencing using small interfering RNA (siRNA) is a valuable laboratory tool and a promising approach to therapeutics for a variety of human diseases. Recently, RNA interference (RNAi) has been linked to cytoplasmic GW bodies (GWB). However, the correlation between RNAi and the formation of GWB, also known as mammalian processing bodies, remains unclear. In this report, we show that transfection of functional siRNA induced larger and greater numbers of GWB. This siRNA-induced increase of GWB depended on the endogenous expression of the target mRNA. Knockdown of GW182 or Ago2 demonstrated that the siRNA-induced increase of GWB required these two proteins and correlated with RNAi. Furthermore, knockdown of rck/p54 or LSm1 did not prevent the reassembly of GWB that were induced by and correlated with siRNA-mediated RNA silencing. We propose that RNAi is a key regulatory mechanism for the assembly of GWB, and in some cases, GWB may serve as markers for RNAi in mammalian cells.
INTRODUCTION
GW bodies (GWB), also known as mammalian processing bodies (P bodies), are cytoplasmic foci that contain multiple decay factors and that are involved in the 5′→3′ mRNA degradation pathway. GWB are named from the marker protein GW182, which contains multiple glycine (G) and tryptophan (W) repeats and a classic RNA binding domain at the carboxyl terminus (Eystathioy et al., 2002a). The mRNA decay factors/complexes found in GWB include the deadenylase Ccr4, the decapping complex Dcp1a/1b/Dcp2, the LSm1-7 complex, Ge-1 (also known as Hedls), rck/p54, and exonuclease Xrn1 (Bashkirov et al., 1997; van Dijk et al., 2002; Ingelfinger et al., 2002; Lykke-Andersen, 2002; Eystathioy et al., 2003; Cougot et al., 2004; Andrei et al., 2005; Yu et al., 2005; Fenger-Gron et al., 2005). GWB are physically juxtaposed to and transiently interact with stress granules (SG). SG process cytoplasmic aggregates of stalled translational preinitiation complexes that accumulate during stress responses and that share certain components with GWB (Kedersha et al., 2005).
In addition to mRNA decay, a crucial role of GWB and their components in RNA interference (RNAi) was recently uncovered (Anderson and Kedersha, 2006; Eulalio et al., 2007a; Jakymiw et al., 2007). RNAi is a posttranscriptional gene silencing mechanism that uses specific double-stranded RNA to silence genes in a sequence-specific manner (Meister and Tuschl, 2004; Mello and Conte, 2004). In brief, the double-stranded RNA is processed by Dicer into small interfering RNA (siRNA) or microRNA (miRNA). The 21- to 26-nucleotide siRNA and miRNA are then incorporated in the effector complex, RNA-induced silencing complex (RISC), which either cleaves or inhibits translation of the target mRNA. In 2005, two key components of RISC, Argonaute2 (Ago2) and siRNA/miRNA, were found to be enriched in GWB (Sen and Blau, 2005; Pillai et al., 2005; Jakymiw et al., 2005; Liu et al., 2005b; Pauley et al., 2006). miRNA-targeted mRNA also localizes to GWB in a miRNA-dependent manner (Liu et al., 2005b). These observations provide the first evidence that RNAi is linked to GWB, and they have opened a new era in our understanding of intracellular RNAi processing. In addition, Ago2 interacts with GW182 in human cells (Jakymiw et al., 2005; Liu et al., 2005a), and this interaction is conserved in Caenorhabditis elegans and Drosophila (Ding et al., 2005; Behm-Ansmant et al., 2006). Disruption of GWB either by a dominant-negative effect or by GW182-knockdown impairs siRNA and miRNA activities (Jakymiw et al., 2005; Liu et al., 2005a), indicating that GW182 and/or GWB are important for RNAi function. Furthermore, miRNA-mediated mRNA degradation requires GWB components such as GW182, the decapping complex Dcp1/Dcp2, and the deadenylase Ccr4:Not (Rehwinkel et al., 2005; Behm-Ansmant et al., 2006), whereas miRNA-mediated translational repression requires rck/p54 (Chu and Rana, 2006).
GWB are highly dynamic structures. First, GWB change in size and number in response to cell proliferation, nutrient conditions and the cell cycle. GWB are larger and more numerous in proliferating cells, whereas they are apparently fewer in resting and nutrient-starved cells (Yang et al., 2004). During cell cycle, smaller GWB are seen in early S phase and larger GWB are seen in late S and G2 phases. The majority of GWB disassembled before mitosis and small GWB reassembled in early G1 (Yang et al., 2004). Second, as sites for 5′→3′ mRNA decay, the size and number of GWB are affected by blocking deadenylation, decapping, and 5′→3′ mRNA degradation or translation (Sheth and Parker, 2003; Cougot et al., 2004; Andrei et al., 2005; Teixeira et al., 2005). GWB require RNA for assembly, and the amount of mRNA or mRNA decay intermediates accumulated in GWB affects the size and number of these foci (Sen and Blau, 2005; Brengues et al., 2005; Teixeira et al., 2005). Third, and more interestingly, our recent studies show that blocking the genesis of miRNA disassembles GWB and introducing siRNA into these cells reassembles these foci, implicating that either miRNA or the miRNA activities are crucial for the formation of GWB (Pauley et al., 2006). Because siRNA is very similar to miRNA structurally, we are interested to determine whether siRNA or siRNA-mediated activities also have an effect on the assembly of GWB. The answer to this question will help us understand the correlation between RNAi activity and the formation of GWB.
MATERIALS AND METHODS
Antibodies
The human prototype anti-GWB (anti-GW182 and anti-Ago2) sera were obtained from serum banks at the Advanced Diagnostics Laboratory, University of Calgary (Calgary, AB, Canada). The selection of sera was based on specific reactivity to either GW182 or Ago2 (Jakymiw et al., 2006). Rabbit anti-Ago2 was a gift from Dr. Tom Hobman (University of Alberta, Edmonton, AB, Canada), and rabbit anti-Dcp1a was obtained from Dr. Jens Lykke-Andersen (University of Colorado). Rabbit polyclonal anti-LSm4 was produced as described previously (Eystathioy et al., 2002b). Mouse monoclonal anti-lamin A/C 636, anti-TIAR, and anti-tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), BD Biosciences (San Jose, CA), and Sigma-Aldrich (St. Louis, MO), respectively. Rabbit polyclonal anti-green fluorescent protein (GFP) and anti-rck/p54 were purchased from Invitrogen (Carlsbad, CA) and MBL International (Boston, MA), respectively. Chicken polyclonal anti-LSm1 was purchased from GenWay Biotech (San Diego, CA).
siRNA
The siRNAs used in the current study were all purchased from Dharmacon RNA Technologies (Lafayette, CO). The siRNAs were dissolved in 1X Universal buffer (provided by Dharmacon RNA Technologies), and the resulting 20 μM stock was stored in aliquots at −20°C before use. The predesigned siRNAs include siCONTROL siRNA for human/mouse/rat lamin A/C (catalog no. D-001050-01-05), siCONTROL RISC-Free siRNA (catalog no. D-001220-01), and siGENOME SMARTpool siRNA for human receptor for advanced glycation end-product (RAGE) (catalog no. M-003625-01). The sense and antisense strand, respectively, of the rest of siRNAs with known sequences are as follows: individual siGENOME ON-TARGET Human TNRC6 (GW182) siRNA duplex: 5′-GAA AUG CUC UGG UCC GCU AUU-3′ and 5′-P UAG CGG ACC AGA GCA UUU CUU-3′ (catalog D-014107-01-0020); hAgo2: 5′-GCA CGG AAG UCC AUC UGA A dTdT-3′ and 5′-UUC AGA UGG ACU UCC GUG C dTdT-3′ (Chu and Rana, 2006); hrck/p54: 5′-GCA GAA ACC CUA UGA GAU UUU-3′ and 5′-AAU CUC AUA GGG UUU CUG CUU-3′ (Chu and Rana, 2006); hLSm1: 5′-GUG ACA UCC UGG CCA CCU CAC UU-3′ and 5′-GUG AGG UGG CCA GGA UGU CAC UU-3′ (Chu and Rana, 2006); hLamin A/C: 5′P-CUG GAC UUC CAG AAG AAC A dTdT-3′ and 5′-Cy3-UGU UCU UCU GGA AGU CCA G dTdT-3′; Luciferase GL2 duplex: 5′-CGU ACG CGG AAU ACU UCG A dTdT-3′ and 5′-U CGA AGU AUU CCG CGU ACG dTdT-3′ (catalog D-001100-01-20); and EGFP: 5′-P GGC UAC GUC CAG GAG CGC ACC-3′ and 5′-P U GCG CUC CUG GAC GUA GCC UU-3′.
Construction of Inducible GFP3T3 Fibroblast (TRE-GFP3T3) Cells
To establish a reliable 3T3 fibroblast cell line expressing tTA, both constructs (pCAG 20-1 and pUHD10-3 Puro) (Era and Witte, 2000) were transfected into 3T3 cells by FuGENE 6 (Roche Diagnostics, Indianapolis, IN) and selected with 1 μg/ml puromycin in doxycycline-free medium. Clones, which proliferated in doxycycline-free medium but died in the presence of 1 μg/ml doxycycline (Sigma-Aldrich) and 1 μg/ml puromycin were selected as primary parental doxycycline-regulatory 3T3 cells. The open reading frame of enhanced GFP (EGFP) was amplified by polymerase chain reaction (PCR) by using LA-Taq polymerase (Takara Bio, Otsu, Japan) from pCX-GFP vector (Ikawa et al., 1995). Primers used were 5′-TGCCGACGCGTGCCACC ATGGTGAGCAAGG and 5′-ATAAGAATGCGGCCGCTGAGGAGTGAATTCTTACTT. The PCR fragment was ligated into the MluI–NotI restriction site of the pTRE2hyg expression vector (Clontech, Palo Alto, CA), which contained a tetracycline-responsive element. This vector was introduced into the doxycycline-regulated 3T3 cells by FuGENE 6 and selected with 200 μg/ml hygromycin B (Invitrogen). Doxcycline-dependent expression of EGFP was confirmed by the GFP expression with or without 1 μg/ml hygromycin B.
Cell Culture and Transfection
HeLa, HSG, NIH 3T3, and GFP3T3 cells were cultured in DMEM containing 10% fetal bovine serum in a 37°C incubator with 5% CO2. siRNA was transiently transfected into cells grown on glass coverslips in a six-well plate by using Oligofectamine (Invitrogen). Briefly, the cultured cells were grown to 30–40% confluence. Then, 100 nM, or in cotransfection of two different siRNAs, 100 nM of each siRNA, was transfected into cells. Usually, cells were fixed 2 d after the transfection. In the 4-day time point experiment, cells were fixed at day 1, day 2, day 3, and day 4 after transfection. In the sequential transfection experiment, the second siRNA transfection was performed 24 h after the initial transfection, and the cells were fixed at 2 or 3 d after the second transfection. In the plasmid and siRNA cotransfecting experiment, HeLa cells were grown to 50–70%. Then, the GFP vector was cotransfected with siRNA either for Ago2, or for rck/p54, or for LSm1 at 1:3 ratio (wt/wt) by using Lipofectamine 2000 (Invitrogen). To test the efficiency of Ago2-knockdown by siRNA, GFP-Ago2 was cotransfected with siRNA for Ago2 at 1:1 ratio (wt/wt), and then the cells were lysed 2 d later. The transfected cells from all the above-mentioned experiments were either processed for indirect immunofluorescence (IIF) or lysed for Western blot analysis.
Western Blot Analysis
Cells were harvested in radioimmunoprecipitation assay buffer (150 mM NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, and 50 mM Tris-HCl, pH 7.5) containing complete protease cocktail inhibitors (Roche Diagnostics). When whole cells lysate was used to detect the expression level of GWB components upon siRNA transfection, cells were lysed in Laemmli sample buffer directly. Afterward, equal amounts of protein extract were separated on 7.5 or 10% polyacrylamide gel and transferred to nitrocellulose. The nitrocellulose membrane was blocked in 5% nonfat dried milk in phosphate-buffered saline-Tween for 1 h at room temperature, and then it was probed with primary antibodies to the following proteins for 1 h: Ago2 (1:200), Dcp1a (1:1000), rck/p54 (1:500), LSm1 (1:2000), LSm4 (1:200), tubulin (1:3000), and GFP (1:200). The membrane was then incubated with horseradish peroxidase-conjugated goat antibodies for 1 h, and immunoreactive bands were detected by the Supersignal Chemiluminescent system (Pierce Chemical, Rockford, IL).
Fluorescence Microscopy
Cells were fixed and permeabilized as described previously (Jakymiw et al., 2005). For colocalization studies, cells were incubated at room temperature with primary antibodies to the following proteins for 1 h: GW182 (human serum; 1:6000), lamin A/C (1:100), Dcp1a (1:500), rck/p54 (1:500), and TIAR (1:100). Afterward, cells were incubated with the corresponding secondary fluorochrome-conjugated goat antibodies at room temperature for 1 h. Alexa Fluor 488 (1:400), Alexa Fluor 568 (1:400), Alexa Fluor 350 (1:100) (Invitrogen), and Cy5 (1:100) (Jackson ImmunoResearch Laboratories, West Grove, PA) were the primary fluorochromes used. Last, glass coverslips were mounted onto the glass slides by using either VECTASHIELD mounting medium with or without 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories). Fluorescent images were captured with a Zeiss Axiovert 200M microscope fitted with a Zeiss AxioCam MRm camera (Carl Zeiss, Jena, Germany) by using 10× 0.75 numerical aperture (NA), 20× 0.75 NA, 40× 0.75 NA, or 63× 1.4 NA objectives. All the exposure times and gain settings within one set of experiment are equivalent. Color images were processed using Adobe Photoshop, version 7 (Adobe Systems, San Jose, CA).
Statistical Analysis
GWB/P bodies in each cell were monitored based on light intensity by using the Axio Vs40 software, version 4.5.0.0; Carl Zeiss). Images from a complete experiment were taken using the same exposure time, and about two or three different areas (100–300 cells) were randomly selected for the measurement of the number of GWB by using CellProfiler object counting software program (Carpenter et al., 2006). The threshold was set to a value so that the background signal was erased and the quantitated foci were confirmed by being overlaid with the original image. The number of foci in each cell was counted by correlating the position of each focus with the area around each nucleus, which was defined as the coverage of a cell. Statistical analysis was performed using Prism 4.0c for Macintosh (GraphPad Software, San Diego, CA). Data between groups were compared using Kruskal–Wallis with Dunn's multiple comparison tests or Fisher's exact test with Bonferroni's correction. For the measurement of RNAi activity, ∼70–110 cells from each data group were randomly selected for the measurement of lamin A/C intensity by using the AxioVs40 software. The area from each cell nuclei was selected based on DAPI staining and then switched to lamin A/C staining for measurement. The median values of lamin A/C signal in the mock-transfected (or luciferase siRNA-transfected) HeLa cells and in the luciferase siRNA–lamin siRNA sequentially transfected (or lamin siRNA singly transfected) HeLa cells were defined as 0 and 100% siRNA function, respectively. In the sequential transfection experiment, the lamin A/C silencing efficiency of the sample group was calculated based on (median fluorescent intensity in mock group − median fluorescent intensity in sample group)/(median fluorescent intensity in mock group − median fluorescent intensity in luciferase siRNA and lamin A/C siRNA sequentially transfected group) × 100%. In the cotransfection experiment, the lamin A/C silencing efficiency was calculated based on a similar formula: (median fluorescent intensity in luciferase siRNA group − median fluorescent intensity in sample group)/(median fluorescent intensity in luciferase siRNA group − median fluorescent intensity in lamin A/C siRNA group) × 100%.
RESULTS
The Size and Number of GWB Increased in Cells Transfected with siRNA Eliciting RNA Silencing of Its Endogenous Target
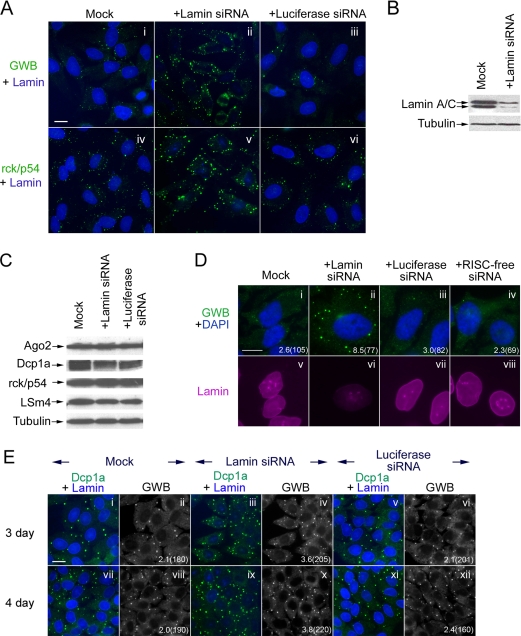
To address the question of how siRNA affects the formation of GWB, we transfected HeLa cells with lamin A/C siRNA, which targets an endogenous mRNA, or luciferase siRNA, which does not target an endogenous mRNA. Interestingly, we detected larger and greater numbers of GWB in cells with efficient lamin A/C-knockdown by siRNA than in the mock-transfected cells or in the cells transfected with luciferase siRNA (Figure 1, A and B). The accumulation of rck/p54 in GWB also increased in lamin A/C siRNA-transfected cells (Figure 1A, iv–vi). In comparison, cells transfected with luciferase siRNA had comparable GWB to those in the mock-transfected cells (Figure 1A). In addition, another siRNA for a different endogenous target, RAGE (Bierhaus et al., 2005), also induced larger and greater numbers of GWB (Supplemental Figure S1). Notably, the “RISC-free” siRNA, a siRNA chemically modified by Dharmacon RNA Technologies to lose its silencing ability, is similar to luciferase siRNA in that it did not affect the size or number of GWB either (Figure 1D). Together, these data suggested that siRNA, which elicited RNA silencing of its endogenous target, was able to increase the size and number of GWB. Interestingly, the protein expression level of GWB components, including Ago2, Dcp1a, rck/p54, and LSm4, did not increase upon transfection of siRNA for lamin A/C (Figure 1C). This supports a hypothesis that these components were recruited to GWB from preexisting or nascent pools of protein upon the transfection of siRNA for lamin A/C.
Figure 1.
Transfection of siRNA for lamin A/C increased the size and number of GWB. (A) Lamin A/C siRNA induced an increase in the size and number of GWB (ii compared with i, v compared with iv) in HeLa cells, whereas luciferase siRNA did not (iii compared with i, vi compared with iv). HeLa cells were mock transfected or transfected with siRNA for either lamin A/C or luciferase. Cells were fixed 3 d after transfection and stained with index human anti-GWB serum (green, i–iii) or rabbit anti-rck/p54 (green, iv–vi) for visualizing GWB and mouse anti-lamin A/C to monitor the knockdown of lamin A/C (blue, i–vi). Bar, 10 μm. (B) Western blot analysis demonstrated that siRNA for lamin A/C achieved efficient gene silencing. The level of tubulin reactivity served as a loading control. (C) Expression of components of GWB was not apparently affected upon transfection of siRNA either for lamin A/C or luciferase. HeLa cells were transfected with 100 nM siRNA either for lamin A/C or luciferase and lysed 3 d later. The whole cell lysates were analyzed by Western blot for the expression of GWB components, including Ago2, Dcp1a, rck/p54, and LSm4. The level of tubulin reactivity served as a loading control. (D) Transfection of RISC-free siRNA had no detectable effect on GWB (green, iv compared with i) as luciferase siRNA (green, iii compared with i). HeLa cells were mock transfected (i and v) or transfected with 100 nM lamin A/C siRNA (ii and vi), or luciferase siRNA (iii and vii), or RISC-free siRNA (iv and viii). Cells were fixed on day 3 after transfection and stained with human anti-GWB serum (green), mouse anti-lamin A/C (magenta), and DAPI (blue). The average number of GWB per cell is shown with the total number of cells counted indicated in parentheses. Bar, 10 μm. (E) Transfection of lamin A/C siRNA induced more GWB (iii–iv compared with i–ii, ix–x compared with vii–viii) in HSG cell line on both day 3 and 4 after transfection, whereas luciferase siRNA did not (v and vi compared with i and ii, xi and xii compared with vii and viii). The HSG cells were mock transfected or transfected with 100 nM siRNA either for lamin A/C or luciferase. The transfected cells were fixed 3 and 4 d after transfection, and then they were stained with human anti-GWB serum and rabbit anti-Dcp1a for GWB, mouse anti-lamin A/C for detecting the knockdown of lamin A/C. The average number of GWB per cell is shown with the total number of cells counted indicated in parentheses. Bar, 10 μm.
In addition to HeLa cells, we transfected siRNA for lamin A/C into a different cell line, human salivary gland (HSG), and a similar increase of GWB was detected (Figure 1E). This demonstrated that the observed effect of siRNA on GWB was not restricted to HeLa cells. As is shown in subsequent experiments, this siRNA-induced increase of GWB is also observed in mouse cells (Figure 3).
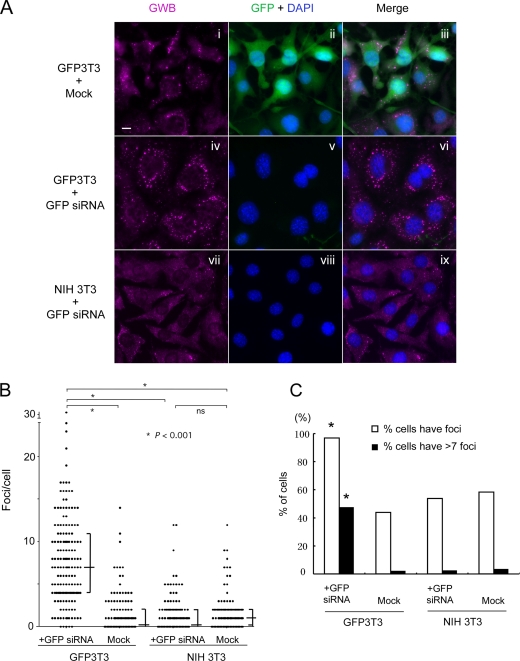
Figure 3.
The siRNA-induced increase of GWB is target dependent. (A) GFP siRNA induced an increase in the size and number of GWB in GFP3T3 cells (iv compared with i), but it did not in NIH 3T3 cells (vii compared with i). Expression of GFP is efficiently inhibited in GFP siRNA-transfected GFP3T3 cells (green, v compared with ii). The cells were fixed on day 3 after mock transfection or transfection of GFP siRNA, and they were costained with human anti-GWB serum (magenta, i, iv, and vii) for GWB and DAPI for nuclei (blue). Merged image of each row is shown in the right column. Bar, 10 μm. (B and C) Quantitative analyses indicated that GFP siRNA induced an increase both in the number of GWB per cell and in the percentage of cells with GWB in GFP3T3 cells. The number of GWB per cell was counted in 100–200 cells for each group. Each dot represented a single cell, and it was plotted on a graph with the number of GWB per cell as y-axis (B). The median with the interquartile range is indicated for each data group. *, significant difference between groups indicated by bracket (Dunn's multiple comparison test, p < 0.001); ns, no significant difference between groups indicated by bracket (Dunn's multiple comparison test, p > 0.05). Based on the data from B, the percentage of cells with GWB (open bars) or increased GWB (filled bars) was calculated, and the result is shown for each individual group (C). Seven foci per cell, the median value of GFP siRNA-transfected GFP3T3 group, are set as the standard value to define cells with increased GWB. The bars indicated by * are significantly higher than the others (Fisher's exact test, p < 0.001).
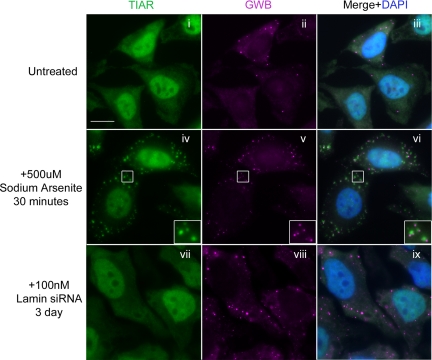
To exclude the possibility that the transfected siRNA might act like certain stressors, such as sodium arsenite, which is reported to induce the formation of both GWB and SG (Kedersha et al., 2005), we examined the effect of lamin A/C siRNA on the formation of SG (Figure 2). As a result, only numerous large GWB but no anti-TIAR (a marker protein for SG)-labeled SG were detected in lamin A/C siRNA-transfected cells (Figure 2, viii and vii). In comparison, many GWB (Figure 2v) and SG (Figure 2iv) were observed in the arsenite-treated cells, a positive control for the stress response, where GWB and SG are often juxtaposed (Figure 2vi, inset). These data indicated that the siRNA-induced increase of GWB was independent of stress response.
Figure 2.
Lamin A/C siRNA did not induce stress granules. Lamin A/C siRNA induced numerous large GWB (viii compared with ii), but it did not induce SG (vii compared with i and iv). In contrast, sodium arsenite induced SG (iv compared with i) and an increase of GWB (v compared with ii). An enlarged cell section is shown in the bottom right corner for arsenite-treated cells (iv–vi), illustrating that SG were often adjacent to GWB (vi, inset). The cells were counterstained with human anti-GWB (magenta), mouse anti-TIAR for SG (green), and DAPI for nuclei (blue). Merged image of each row are shown in the right column. Bar, 10 μm.
siRNA Required Endogenous Expression of Its Target for Inducing an Increase in Size and Number of GWB
To further verify that the siRNA-induced increase of GWB is dependent on the presence of the siRNA target, we transfected siRNA for GFP into a mouse fibroblast cell line (NIH 3T3) engineered to express GFP (GFP3T3) integrally (Figure 3A, iv–vi). Mock-transfected GFP3T3 cells (Figure 3A, i–iii) or NIH 3T3 cells transfected with GFP siRNA (Figure 3A, vii–ix) served as controls that either missed the siRNA or the target of siRNA, respectively. Our data showed that the siRNA for GFP efficiently silenced the expression of its target (Supplemental Figures S2 and 3A) and induced a prominent increase of GWB only in the GFP3T3 cells (Figure 3A). By using the CellProfiler object counting software (Carpenter et al., 2006) to quantitate GWB in each cell, we showed that the number of GWB per cell in GFP siRNA-transfected GFP3T3 cells was significantly higher than that either in the mock GFP3T3 cells or in the GFP siRNA-transfected NIH 3T3 cells (Figure 3B). In addition, the percentage of cells with GWB or with increased GWB in the GFP siRNA-transfected GFP3T3 cells was also remarkably higher than that in the mock GFP3T3 cells or in the GFP siRNA-transfected NIH 3T3 cells (Figure 3C). In comparison, there was no significant difference between the mock-transfected NIH 3T3 cells and GFP siRNA-transfected NIH 3T3 in either the number of GWB per cell (Figure 3B) or the percentage of cells with GWB (or with increased GWB) (Figure 3C). These data demonstrated that GFP siRNA induced an increase in both the number of GWB and the percentage of cells with GWB only in the 3T3 cells expressing GFP, a finding supporting the conclusion that siRNA-induced increase of GWB is target-dependent and may correlate with siRNA-elicited silencing activities.
The siRNA-Induced Increase of GWB Started on Day 1 after Transfection and Lasted for at Least 4 d
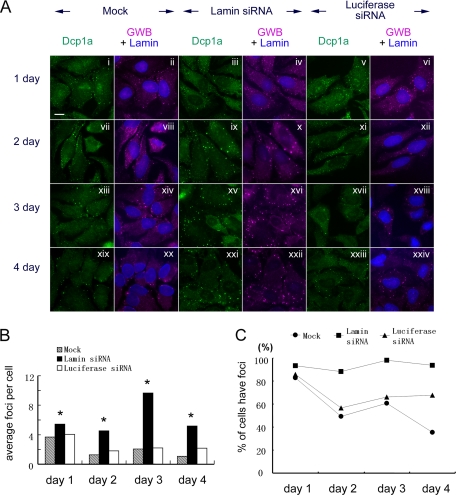
To determine the temporal increase of GWB induced by siRNA, we performed a 4-d time point experiment to monitor the changes of GWB as well as the accumulation of Dcp1a in GWB at each time point. Interestingly, after transfection of siRNA for lamin A/C, GWB were much larger on day 3 and 4 than on day 1 (Figure 4, A and B, and Supplemental Figure S3, A and B). Quantitative analysis showed that the average number of GWB in lamin A/C siRNA-transfected cells was higher than that of the mock cells or luciferase siRNA-transfected cells through day 1–4. The maximal increase of GWB was approximately five-fold of mock cells on day 3 (Figure 4B and Supplemental Figure S3B). In addition, the percentage of cells with GWB in lamin A/C siRNA-transfected cells (88–98%) was higher than that of the other two groups (35–67%) through day 2–4 (Figure 4C). In comparison, the number of GWB through day 1–4 was similar between luciferase siRNA-transfected cells and the mock-transfected cells (Figure 4B). The percentage of cells with GWB was also similar between these two groups (Figure 4C). Notably, the variation of GWB (in size and number) during cell cycle was more easily detected in these two groups than in the lamin A/C siRNA-transfected cells (Supplemental Figure S3A; data not shown). A similar temporal increase in the accumulation of rck/p54 in GWB was observed (data not shown). Together, these data demonstrated that siRNA that elicits RNA silencing could induce an increase both in the number of GWB and in the percentage of cells with GWB. This increase in number and size of GWB occurred on day 1 after transfection and lasted for at least 4 d.
Figure 4.
The increases of GWB started on day 1, and they were most prominent on day 3 after transfection of siRNA for lamin A/C. (A) HeLa cells were mock transfected, or they were transfected with 100 nM siRNA for lamin A/C or for luciferase, and then fixed on day 1 (i–vi), day 2 (vii–xii), day 3 (xiii–xviii), and day 4 (xix–xxiv) after transfection. Cells were counterstained with human anti-GWB serum (magenta) and rabbit anti-Dcp1a (green) to monitor the changes of GWB. The level of lamin A/C was evaluated by using mouse anti-lamin A/C (blue). Bar, 10 μm. (B) Quantitative analysis indicated that cells transfected with lamin A/C siRNA had a significant increase in the average number of GWB per cell through day 1–4. The number of GWB per cell was quantitated as described in Figure 3B and Materials and Methods. The groups indicated by * are significantly higher than the other groups on the same day (Dunn's multiple comparison test, p < 0.001). (C) The percentage of cells with GWB in lamin A/C siRNA-transfected cells (filled square) is significantly higher than mock cells (filled circle) and luciferase siRNA-transfected cells (filled triangle) through day 2–4 (Fisher exact test, p < 0.001). In comparison, the latter two groups were very similar to each other (Fisher exact test, no significant difference through day 1–3, p > 0.05).
GW182 Was Required for the siRNA-induced Increase of GWB
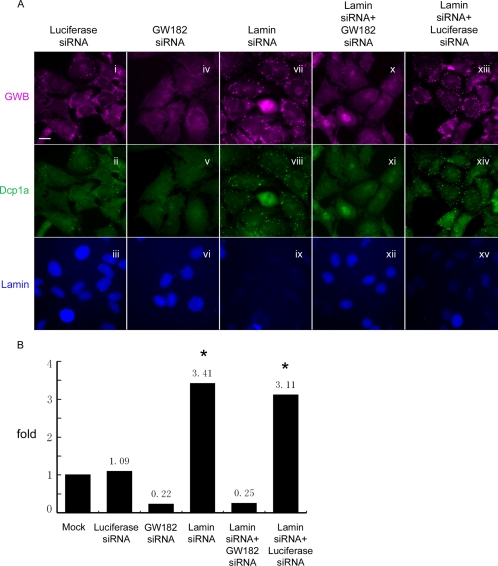
GW182 is important for both GWB formation (Yang et al., 2004) and miRNA/siRNA activity (Jakymiw et al., 2005; Liu et al., 2005a; Chu and Rana, 2006). We were interested to examine how the knockdown of GW182 affects the siRNA-induced increase of GWB. We used the same GW182 siRNA tested previously to be efficient in silencing the target (Jakymiw et al., 2005). As shown, the transfected GW182 siRNA disassembled GWB and it abolished the accumulation of Dcp1a in foci without affecting the level of lamin A/C expression (Figure 5A, iv–vi). Quantitative analysis confirmed that GW182 siRNA-transfected cells only had 0.22-fold of the GWB in mock cells (Figure 5B). More interestingly, numerous large GWB induced by lamin A/C siRNA (Figure 5A, vii–ix, xiii–xv) were absent in cells where siRNA for GW182 and lamin A/C were cotransfected and where RNA silencing was impaired (Figure 5A, x–xii). This observation was supported by the quantitative data that indicated the number of siRNA-induced GWB dropped from 3.11- to 0.25-fold upon GW182-knockdown (Figure 5B). Luciferase siRNA served as a control siRNA that did not affect the assembly of GWB. In summary, these data indicated that the siRNA-induced increase of GWB required GW182, suggesting that the integrity of GWB and/or RNAi activity are very important for the siRNA-induced increase of GWB.
Figure 5.
GW182 was required for the siRNA-induced increase of GWB. (A) GW182-knockdown inhibited both the increase of GWB (x and xi compared with xiii and xiv) and the lamin A/C-knockdown (xii compared with xv) induced by lamin A/C siRNA. HeLa cells were transfected with 100 nM siRNA for GW182 (iv–vi), 100 nM siRNA for lamin A/C (vii–ix), or both (x–xii) for 3 d. siRNA for luciferase in both the single siRNA transfection (i–iii) and cotransfection (xiii–xv) served as controls. Cells were stained with human anti-GWB serum (top) and rabbit anti-Dcp1a (middle) for GWB, mouse anti-lamin A/C for monitoring lamin A/C-knockdown (bottom). Bar, 10 μm. (B) Quantitative analysis showed that knockdown of GW182 abolished the siRNA-induced increase of GWB. The number of GWB in ∼300 cells collected from three randomly selected fields was counted for each group. The average number of GWB per cell in each group was divided by that of mock cells to calculate the fold difference. The bars indicated by * are significantly higher than others (Dunn's multiple comparison test, p < 0.001).
The siRNA-induced Increase of GWB Required Ago2 and Correlated with RNA Silencing Activities
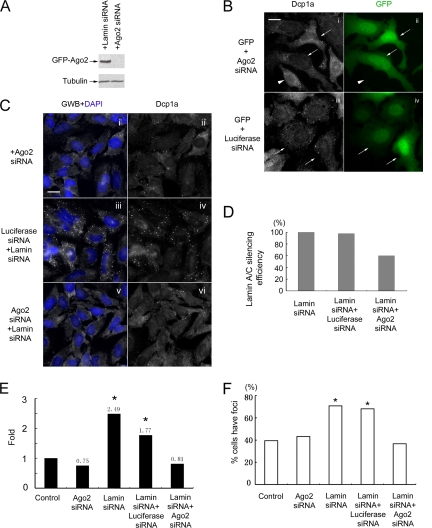
To further determine the correlation between RNAi activity and the siRNA-induced increase of GWB, we were interested in the knockdown of Ago2, another GWB component that is a key component of RNA silencing (Liu et al., 2004). The siRNA used in this study was shown to efficiently silence the expression of Ago2 by both another group (Chu and Rana, 2006) and by us (Figure 6A). Interestingly, when cotransfecting the Ago2 siRNA and the GFP vector at a 3:1 ratio (wt/wt), we detected discrete foci in the GFP-positive cells, which likely contained the siRNA for Ago2 (Figure 6B, i and ii). Consistently, the number of GWB (labeled by anti-Dcp1a) in Ago2-knockdown cells was only slightly less than that of control cells (0.75-fold; Figure 6E), indicating Ago2 is not essential for the formation of GWB. To examine how Ago2-knockdown affects the siRNA-induced increase of GWB, we cotransfected Ago2 siRNA and lamin A/C siRNA into HeLa cells. As a control, luciferase siRNA was cotransfected with lamin A/C siRNA. Notably, Ago2-knockdown impaired the silencing function of lamin A/C siRNA (60% remained; Figure 6D), and it abolished the increased size and number of GWB (labeled by anti-Dcp1a) induced by lamin A/C siRNA (Figure 6C, v–vi), resulting in the number of foci decreasing from 1.77- to 0.81-fold (Figure 6E). Ago2-knockdown also decreased the percentage of cells with foci induced by lamin A/C siRNA, which dropped to a percentage similar to that of control cells (Figure 6F). In comparison, cells transfected with siRNA for lamin A/C and luciferase had high efficiency of RNA silencing (97.8%; Figure 6D) and large numbers of prominent foci (Figure 6C, iii and iv). Together, these data indicated that Ago2 was not essential for GWB formation, but it was required for the siRNA-induced increase of GWB. The observation strongly suggested that impairment of RNAi function affects GWB dynamics.
Figure 6.
The siRNA-induced increase of GWB required Ago2 and correlated with RNA silencing activities. (A) Western blot analysis showed that siRNA for Ago2 efficiently knocked down Ago2. GFP–Ago2 was cotransfected with siRNA either for Ago2 or for lamin A/C at 1:1 ratio (wt/wt) for 2 d. Tubulin reactivity served as a loading control. (B) Ago2-knockdown barely affected the formation of GWB. siRNA for Ago2 (i and ii) or luciferase (iii and iv) was cotransfected with GFP vector at 3:1 ratio (wt/wt) into HeLa cells for 2 d. The cells were counterstained with rabbit anti-Dcp1a. In cells transfected with Ago2 siRNA, discrete GWB were detected in GFP-positive cells (i and ii, arrows), which were comparable to the GWB in GFP-negative cells in the same panel (i and ii, arrowhead) or to the GWB in GFP-positive cells transfected with luciferase siRNA (iii and iv, arrows). Bar, 10 μm. (C–F) siRNA-induced increase of GWB was abolished when Ago2 was knocked down and RNA silencing efficiency was impaired. HeLa cells were transfected with 100 nM siRNA for Ago2 (i and ii), 100 nM siRNA for lamin A/C, or both (v and vi) for 2 d. siRNA for luciferase in both the single siRNA transfection and cotransfection (iii and iv) served as controls. Cells were stained with human anti-GWB serum (left), and rabbit anti-Dcp1a (right) for GWB, DAPI (blue) and mouse anti-lamin A/C (lamin A/C staining is not shown). (C) Ago2 siRNA diminished the increase of GWB induced by lamin A/C siRNA. Fewer GWB were observed in cells cotransfected with siRNA for Ago2 and lamin A/C (v and vi) than cells with siRNA for both luciferase and lamin A/C (iii and iv). Bar, 10 μm. (D) Quantitative analysis of lamin A/C silencing efficiency indicated that Ago2-knockdown impaired RNAi activity. AxioV40 software was used to measure the fluorescent intensity of nuclear lamin A/C staining in each cell for each group (70 cells/group). The median value of lamin A/C fluorescent intensity in each group was used to calculate its corresponding RNA silencing efficiency based on the formula described under Materials and Methods. y-axis, lamin A/C silencing efficiency. (E) Quantitative analysis showed that Ago2-knockdown significant decreased the average number of GWB induced by siRNA. The number of GWB in each cell was counted as described in Figure 3B and under Materials and Methods. Then, the resulted average number of GWB per cell in each group was divided by that of control cells to calculate the fold difference. The bars indicated by * are significantly higher than others (Dunn's multiple comparison test, p < 0.001). (F) Ago2-knockdown decreased the percentage of cells with GWB induced by siRNA. Based on the data from (E), the percentage of cells with GWB was calculated and shown for each individual group. The bars indicated by * are significantly higher than other groups (Fisher's exact test, p < 0.001).
Knockdown of LSm1 or rck/p54 Did Not Inhibit the Assembly of GWB Induced by siRNA
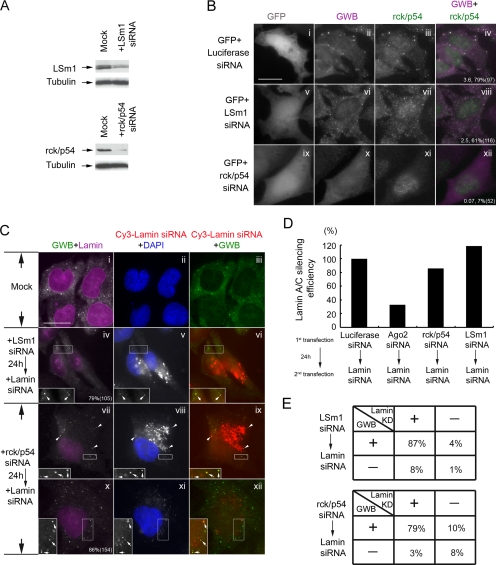
To further dissect the correlation of RNAi activity with the siRNA-induced increase of GWB, we performed knockdown experiments for LSm1 and rck/p54, both reported to be important for the formation of GWB but that have no effect on siRNA-mediated silencing (Chu and Rana, 2006). The siRNA for LSm1 and rck/p54 are shown by us (Figure 7A) and by other investigators (Chu and Rana, 2006) to inhibit the expression of its target. To confirm the roles of the LSm1 and rck/p54 in the formation of GWB, we cotransfected a GFP vector with siRNA either for LSm1 or for rck/p54 at a ratio of 1:3 (wt/wt) into HeLa cells for 2 d (Figure 7B). GFP vector was cotransfected with luciferase siRNA as a control. GWB were barely detected in rck/p54-knockdown cells (Figure 7B, ix–xii). In comparison, a few small GWB were observed in LSm1-knockdown cells (Figure 7B, v–viii), implying that LSm1 may be required for the formation of a subset of GWB. In general, both LSm1-knockdown and rck/p54-knockdown prevented the formation of large prominent GWB induced by lamin A/C siRNA (Figure 7C; data not shown). Nevertheless, sequentially transfecting lamin A/C siRNA reassembled many small GWB in rck/p54-knockdown cells, resulting in the percentage of cells with GWB increasing drastically from 7% (0.07 GWB/cell) (Figure 7B, x–xii) to 86% (∼20 GWB/cell) (Figure 7C, vii and x; data not shown). Apparently, the residual rck/p54 was recruited to the newly assembled GWB in rck/p54-knockdown cells, despite the highly efficient silencing of rck/p54 in these cells (Figure 7A and Supplemental Figure S4). In LSm1-knockdown cells, sequentially transfecting lamin A/C siRNA reassembled fewer small GWB (Figure 7B, vi–viii, compared with C, iv). Furthermore, Cy3-labeled lamin A/C siRNA localized to these reassembled GWB, and it efficiently silenced the expression of its target both in LSm1-knockdown cells (Figure 7C, iv–vi) and in rck/p54-knockdown cells (Figure 7C, vii–xii). Notably, the localization of Cy3-lamin A/C siRNA to GWB was not only found in Cy3-siRNA strongly transfected cells (Figure 7C, iv–ix) but also found in Cy3-siRNA weakly transfected cells (Figure 7C, x–xii), which was more easily detected in rck/p54-knockdown cells. Consistent with the above-mentioned data and data from others (Chu and Rana, 2006), knockdown of LSm1 or rck/p54 did not affect siRNA-mediated silencing (Figure 7D). Most importantly, the assembly of these siRNA-induced GWB correlated with the silencing of lamin A/C both in LSm1-knockdown cells (87%) and in rck/p54-knockdown cells (79%) (Figure 7E). In summary, LSm1 and rck/p54 contributed to the formation of GWB to different degrees. However, knockdown of either did not prevent the siRNA-induced assembly of GWB or localization of siRNA to GWB. The siRNA-induced assembly of GWB in LSm1-knockdown cells and in rck/p54-knockdown cells correlated with RNA silencing activities.
Figure 7.
Knockdown of LSm1 or rck/p54 disassembled GWB, but it did not inhibit the assembly of GWB induced by siRNA. (A) Knockdown of LSm1 or rck/p54 in HeLa cells by siRNA. HeLa cells were transfected with siRNA for LSm1 or rck/p54, harvested 2 d later and analyzed by Western blot with antibodies to LSm1 or rck/p54. Tubulin reactivity served as a loading control. (B) Rck/p54 is required for the assembly of majority of GWB, whereas LSm1 is important only for a portion of GWB. GFP vector were cotransfected with siRNA for LSm1, rck/p54, or luciferase at 1:3 ratio (wt/wt) into HeLa cells for 2 d. The transfected cells were counterstained with human anti-GWB serum and rabbit anti-rck/p54. Merge images of anti-GWB and anti-rck/p54 are shown in the right column. The average number of GWB per cell and the percentage of cells with GWB are shown with the total number of cells counted indicated in parentheses. Bar, 10 μm. (C) Lamin A/C siRNA induced the assembly of GWB and localized to these GWB in spite of LSm1-knockdown or rck/p54-knockdown. siRNA (100 nM) either for LSm1 (iv–vi) or rck/p54 (vii–xii) were transfected into HeLa cells, and 24 h later, 100 nM Cy3-labeled lamin A/C siRNA (red) was sequentially transfected. The transfected cells were fixed 3 d after the second transfection and stained with human anti-GWB serum, mouse anti-lamin A/C (magenta), and DAPI (blue). Merged images of Cy3-siRNA (red) and anti-GWB (green) are shown in the right column. The percentages of cells exhibiting a similar or identical GWB staining to the cells presented are shown in iv and x with the total number of cells counted indicated in parentheses. iv-ix show representative localization of siRNA to GWB in Cy3-siRNA strongly transfected cells, whereas x-xii show the localization in weakly transfected cells. Insets are enlarged by 1.5- to 2-fold, and the Cy3 signal is enhanced to show the localization of siRNA to GWB. Arrows indicate the colocation for the weak Cy3 signals in enlarged insets. Arrowheads indicate the colocalization for the strong Cy3 signals in GWB. Bar, 10 μm. (D) Knockdown of LSm1 or rck/p54 did not affect RNA silencing activities. AxioV40 software was used to measure the fluorescent intensity of nuclear lamin A/C staining in each cell for each group (∼100 cells/group). The median value of lamin A/C fluorescent intensity in each group was used to calculate its corresponding RNA silencing efficiency based on the formula described under Materials and Methods. y-axis, lamin A/C silencing efficiency. (E) The siRNA-induced assembly of GWB correlated with RNA silencing activities. One hundred to 150 cells from each group were randomly selected and subjected to quantitation according to the assembly of GWB and the knockdown of lamin A/C. GWB +, cells exhibiting similar or identical GWB staining to the cells presented in C panel iv for LSm1-knockdown or C panel x for rck/p54-knockdown; GWB −, cells without microscopic detectable GWB; Lamin KD +, the fluorescent intensity of nuclear lamin A/C staining is <50% of the median value of lamin A/C fluorescent intensity of the mock cells; Lamin KD −, the lamin A/C intensity is >50% of the median value of lamin A/C intensity of mock cells.
DISCUSSION
The major and highly reproducible observation reported in the current study is that siRNA:mRNA induces the appearance of numerous large GWB in the majority of cells where the normal variation of GWB in size and number during the cell cycle was greatly obscured, whereas in untransfected cells large GWB were only detected in a small fraction of cells at late S and G2 stage of the cell cycle. Further study indicated that this siRNA-induced increase in size and number of GWB was regulated by RNAi activity. Our results provide novel insight into the correlations between siRNA function and the assembly of GWB, suggesting that GWB could serve as markers for siRNA-mediated activity in mammalian cells.
siRNA:mRNA Initiates Assembly of Microscopic Detectable GWB by Recruiting GWB Components
siRNA:mRNA nucleated the assembly of GWB, possibly by recruiting GWB components. We hypothesize that GWB components are actively exchanged between the cytoplasmic pool and GWB based on the actively ongoing siRNA/miRNA function and the mediated mRNA decay/translational repression. One model is that siRNA:mRNA is targeted to or recruits components to pre-existing submicroscopic GWB, which then develop into larger cytoplasmic structures detectable by conventional microscopy. Alternatively, siRNA:mRNA itself forms de novo GWB by recruiting necessary components/complexes for silencing. The greatest numbers of large GWB were induced on day 3 after siRNA transfection, suggesting that the large GWB may be more related to siRNA-mediated mRNA decay processes. We postulate that the smaller GWB (or the submicroscopic GWB), which increased on day 1 or even earlier, may be related to the early stage of RNA silencing. The formation of large GWB could be attributed to the siRNA-mediated degradation of large amounts of mRNAs, which may have exceeded the maximal capacity of the mRNA decay machinery. As proposed recently, this could then lead to accumulation of these mRNAs or mRNA decay intermediates in GWB (Franks and Lykke-Andersen, 2007). Similarly, increased accumulation of mRNA decay intermediates in GWB due to possible interference by Cy3 dye in the degradation of target mRNA is a reasonable interpretation for why Cy3-lamin A/C siRNA induces more numerous large GWB than does unlabeled lamin A/C siRNA with exactly the same sequence (data not shown).
The Role of GWB Components for the Assembly of GWB
Based on the requirement of different GWB components examined in this study for the assembly of GWB, we can deduce some scenarios for GWB assembly. Because GW182 and rck/p54 are important for miRNA-mediated decay/translational repression, their requirement in GWB formation may be attributed, at least in part, to the amount of miRNA-mediated repressed mRNPs maintained in GWB. Notably, GW182-knockdown greatly inhibited the reassembly of GWB induced by siRNA:mRNA, resulting in very few detectable GWB. This may suggest that GW182 is required at the early stage of GWB assembly. In contrast, rck/p54-knockdown prevented the formation of large GWB but not small GWB induced by siRNA:mRNA, suggesting that rck/p54 may function at a later stage after the initial trigger of GWB formation. Moreover, rck/p54-knockdown may limit the amount of mRNPs shuttled to one GWB and the excess RNPs have to be shuttled to other “unsaturated” GWB, thereby forming more numerous but smaller detectable GWB. Furthermore, Cy3-lamin A/C siRNA localized to these reassembled small GWB and mediated efficient silencing, indicating that small GWB are capable of carrying out RNA silencing. It is possible that the mRNA decay in small GWB is less efficient than that in large GWB; however, this speculation will need to be addressed in future experiments. Interestingly, even in cells with efficient rck/p54-knockdown, the residual rck/p54 was detected and concentrated in the reassembled GWB (Supplemental Figure S4), suggesting that the recruitment of rck/p54 to GWB is very efficient. This recruitment may be via siRNA:mRNA-associated Ago2, because rck/p54 directly interacts with Ago2 (Chu and Rana, 2006). It is possible that rck/p54 contributes to the assembly of these newly formed GWB or that it is recruited there for downstream functions. Nevertheless, we cannot differentiate whether the assembly of these siRNA-induced GWB is required for siRNA-mediated silencing or whether it is the consequence of siRNA-mediated silencing. Because almost complete knockdown of rck/p54 barely affected RNA silencing efficiency, we postulate that, in general, rck/p54 is not important for siRNA function, unless it efficiently fulfills functions with only residual amounts of the protein. In contrast to GW182 and rck/p54, LSm1 has a less profound effect on the assembly of GWB. The incomplete disassembly of GWB by LSm1-knockdown was reported previously (Andrei et al., 2005), whereas complete disappearance of GWB by LSm1-knockdown was reported in another study (Chu and Rana, 2006). The reasons for this discrepancy can be attributed to different ways of defining “foci”, different ways of determining cells with knockdown, or different efficiency/specificity of the antibody used to detect foci. Nevertheless, our data are in agreement with the conclusion that LSm1 contributes to the formation of GWB. The reassembly of GWB induced by siRNA:mRNA in LSm1-knockdown cells implies that LSm1, like rck/p54, is not required to initiate the assembly of GWB, and it is possibly involved in the formation of larger foci. Similar to LSm1, Ago2 has less profound effect on the assembly of GWB compared with GW182 and rck/p54. Knockdown of Ago2 had a minor effect on the accumulation of Dcp1a indicating that Ago2 is not required to stabilize mRNA decay factors in GWB and that the mRNA processing stage could be independent of the siRNA/miRNA-mediated silencing stage. Furthermore, the function of Ago2 in miRNA-mediated translational repression could possibly be compensated by other Argonaute proteins in mammalian cells. This explanation was supported by previous studies where Ago2-knockdown did not affect miRNA function profoundly (Chu and Rana, 2006) and where Agos1-4 had equal capability in binding miRNAs (Meister et al., 2004).
Regulation of GWB Assembly
An understanding of the regulation of GWB assembly in mammalian cells has been greatly advanced by the current study. Our data suggest that, in mammalian cells, the majority of mRNAs degraded via the 5′→3′ pathway or translationally repressed in GWB are mediated by siRNA or miRNA. We speculate that under certain circumstances, GWB may serve as markers for siRNA/miRNA activity; therefore, the variation in number and size of GWB may correlate with the activities of miRNA during different stages of the cell cycle and proliferation (Yang et al., 2004; Hatfield et al., 2005; He et al., 2005; O'Donnell et al., 2005; Lian et al., 2006).
Interestingly, a recent publication reported that Drosophila siRNA:mRNA or miRNA:mRNA also nucleated the formation of GWB (Eulalio et al., 2007b), an observation that strongly supports our finding that siRNA/miRNA-mediated function is a key regulatory mechanism of GWB assembly. Nonetheless, their data also implicated differences in the regulation of GWB formation in Drosophila from that in human. For example, Ago2-knockdown disassembled GWB and long double-stranded RNA did not restore GWB in LSm1-knockdown or rck/p54-knockdown cells in Drosophila (Eulalio et al., 2007b). These apparent discrepancies with our data may be attributed to the potential difference in the function of GWB components and in the RNAi pathway between Drosophila and human cells (Lee et al., 2004; Okamura et al., 2004).
Depending on the presence of the RNAi machinery, the regulation of GWB assembly might vary between species. For example, the RNAi machinery as well as related cofactors, such as Argonaute proteins and GW182, are absent in Saccharomyces cerevisiae. The absence of RNAi in S. cerevisiae may explain the observed differences between yeast P bodies and mammalian P bodies (GWB) in responding to stresses (Sheth and Parker, 2003; Yang et al., 2004; Brengues et al., 2005; Teixeira et al., 2005). Yeast P bodies are considered sites for processing global messages, whereas GWB are more like specific cellular structures regulating and organizing siRNA/miRNA-mediated function. This is consistent with the concept that most mRNAs are degraded via the 5′→3′ pathway in yeast, whereas in mammalian cells only a portion of mRNAs are degraded via the 5′→3′ pathway (Wilusz et al., 2001; Tourriere et al., 2002; Coller and Parker, 2004; Parker and Song, 2004). The correlations between GWB and RNAi are proven to be strong.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Jens Lykke-Andersen for providing the rabbit anti-Dcp1 antibody and Dr. Tom Hobman for providing the hAgo2 rabbit antibody. We also thank Drs. Anne E. Carpenter and Thouis R. Jones (Whitehead Institute for Biomedical Research) for providing the CellProfiler software and technical support. This work was supported in part by the Canadian Institutes for Health Research grant MOP-38034, Canadian Breast Cancer Research Foundation grant 16992, and National Institutes of Health (NIH) grants AI-47859 and AR-42455. S.L. is supported by NIH training grant DE-007200. M.J.F. holds the Arthritis Society Chair.
Abbreviations used:
- GWB
GW bodies
- IIF
indirect immunofluorescence
- miRNA
microRNA
- P bodies
processing bodies
- RISC
RNA-induced silencing complex
- SG
stress granules.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-01-0070) on June 27, 2007.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Anderson P., Kedersha N. RNA granules. J. Cell Biol. 2006;172:803–808. doi: 10.1083/jcb.200512082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrei M. A., Ingelfinger D., Heintzmann R., Achsel T., Rivera-Pomar R., Luhrmann R. A role for eIF4E and eIF4E-transporter in targeting mRNPs to mammalian processing bodies. RNA. 2005;11:717–727. doi: 10.1261/rna.2340405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashkirov V. I., Scherthan H., Solinger J. A., Buerstedde J. M., Heyer W. D. A mouse cytoplasmic exoribonuclease (mXRN1p) with preference for G4 tetraplex substrates. J. Cell Biol. 1997;136:761–773. doi: 10.1083/jcb.136.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm-Ansmant I., Rehwinkel J., Doerks T., Stark A., Bork P., Izaurralde E. mRNA degradation by miRNAs and GW182 requires both CCR 4, NOT deadenylase and DCP 1, DCP2 decapping complexes. Genes Dev. 2006;20:1885–1898. doi: 10.1101/gad.1424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A., Humpert P. M., Morcos M., Wendt T., Chavakis T., Arnold B., Stern D. M., Nawroth P. P. Understanding RAGE, the receptor for advanced glycation end products. J. Mol. Med. 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- Brengues M., Teixeira D., Parker R. Movement of eukaryotic mRNAs between polysomes and cytoplasmic processing bodies. Science. 2005;310:486–489. doi: 10.1126/science.1115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter A. E. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C. Y., Rana T. M. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol. 2006;4:e210. doi: 10.1371/journal.pbio.0040210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller J., Parker R. Eukaryotic mRNA decapping. Annu. Rev. Biochem. 2004;73:861–890. doi: 10.1146/annurev.biochem.73.011303.074032. [DOI] [PubMed] [Google Scholar]
- Cougot N., Babajko S., Seraphin B. Cytoplasmic foci are sites of mRNA decay in human cells. J. Cell Biol. 2004;165:31–40. doi: 10.1083/jcb.200309008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Spencer A., Morita K., Han M. The developmental timing regulator AIN-1 interacts with miRISCs and may target the argonaute protein ALG-1 to cytoplasmic P bodies in C. elegans. Mol. Cell. 2005;19:437–447. doi: 10.1016/j.molcel.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Era T., Witte O. N. Regulated expression of P210 Bcr-Abl during embryonic stem cell differentiation stimulates multipotential progenitor expansion and myeloid cell fate. Proc. Natl. Acad. Sci. USA. 2000;97:1737–1742. doi: 10.1073/pnas.97.4.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 2007a;8:9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Schweizer D., Izaurralde E. P-body formation is a consequence, not the cause of RNA-mediated gene silencing. Mol. Cell Biol. 2007b;27:3970–3981. doi: 10.1128/MCB.00128-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eystathioy T., Chan E.K.L., Tenenbaum S. A., Keene J. D., Griffith K., Fritzler M. J. A phosphorylated cytoplasmic autoantigen, GW182, associates with a unique population of human mRNAs within novel cytoplasmic speckles. Mol. Biol. Cell. 2002a;13:1338–1351. doi: 10.1091/mbc.01-11-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eystathioy T., Jakymiw A., Chan E.K.L., Seraphin B., Cougot N., Fritzler M. J. The GW182 protein colocalizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies. RNA. 2003;9:1171–1173. doi: 10.1261/rna.5810203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eystathioy T., Peebles C. L., Hamel J. C., Vaughn J. H., Chan E.K.L. Autoantibody to hLSm4 and the heptameric LSm complex in anti-Sm sera. Arthritis Rheum. 2002b;46:726–734. doi: 10.1002/art.10220. [DOI] [PubMed] [Google Scholar]
- Fenger-Gron M., Fillman C., Norrild B., Lykke-Andersen J. Multiple processing body factors and the ARE binding protein TTP activate mRNA decapping. Mol. Cell. 2005;20:905–915. doi: 10.1016/j.molcel.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Franks T. M., Lykke-Andersen J. TTP and BRF proteins nucleate processing body formation to silence mRNAs with AU-rich elements. Genes Dev. 2007;21:719–735. doi: 10.1101/gad.1494707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield S. D., Shcherbata H. R., Fischer K. A., Nakahara K., Carthew R. W., Ruohola-Baker H. Stem cell division is regulated by the microRNA pathway. Nature. 2005;435:974–978. doi: 10.1038/nature03816. [DOI] [PubMed] [Google Scholar]
- He L. A microRNA polycistron as a potential human oncogene. Nature. 2005;435:828–833. doi: 10.1038/nature03552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa M., Kominami K., Yoshimura Y., Tanaka K., Nishimune Y., Okabe M. A rapid and non-invasive selection of transgenic embryos before implantation using green fluorescent protein (GFP) FEBS Lett. 1995;375:125–128. doi: 10.1016/0014-5793(95)01162-8. [DOI] [PubMed] [Google Scholar]
- Ingelfinger D., rndt-Jovin D. J., Luhrmann R., Achsel T. The human LSm1-7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci. RNA. 2002;8:1489–1501. [PMC free article] [PubMed] [Google Scholar]
- Jakymiw A., Ikeda K., Fritzler M. J., Reeves W. H., Satoh M., Chan E.K.L. Autoimmune targeting of key components of RNA interference. Arthritis Res. Ther. 2006;8:R87. doi: 10.1186/ar1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakymiw A., Lian S., Eystathioy T., Li S., Satoh M., Hamel J. C., Fritzler M. J., Chan E.K.L. Disruption of GW bodies impairs mammalian RNA interference. Nat. Cell Biol. 2005;7:1267–1274. doi: 10.1038/ncb1334. [DOI] [PubMed] [Google Scholar]
- Jakymiw A., Pauley K. M., Li S., Ikeda K., Lian S., Eystathioy T., Satoh M., Fritzler M. J., Chan E. K. The role of GW/P-bodies in RNA processing and silencing. J Cell Sci. 2007;120:1317–1323. doi: 10.1242/jcs.03429. [DOI] [PubMed] [Google Scholar]
- Kedersha N., Stoecklin G., Ayodele M., Yacono P., Lykke-Andersen J., Fitzler M. J., Scheuner D., Kaufman R. J., Golan D. E., Anderson P. Stress granules and processing bodies are dynamically linked sites of mRNP remodeling. J. Cell Biol. 2005;169:871–884. doi: 10.1083/jcb.200502088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. S., Nakahara K., Pham J. W., Kim K., He Z., Sontheimer E. J., Carthew R. W. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- Lian S., Jakymiw A., Eystathioy T., Hamel J. C., Fritzler M. J., Chan E.K.L. GW bodies, microRNAs and the cell cycle. Cell Cycle. 2006;5:242–245. doi: 10.4161/cc.5.3.2410. [DOI] [PubMed] [Google Scholar]
- Liu J., Carmell M. A., Rivas F. V., Marsden C. G., Thomson J. M., Song J. J., Hammond S. M., Joshua-Tor L., Hannon G. J. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- Liu J., Rivas F. V., Wohlschlegel J., Yates J. R., Parker R., Hannon G. J. A role for the P-body component GW182 in microRNA function. Nat. Cell Biol. 2005a;7:1261–1266. doi: 10.1038/ncb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Valencia-Sanchez M. A., Hannon G. J., Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat. Cell. Biol. 2005b;7:719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke-Andersen J. Identification of a human decapping complex associated with hUpf proteins in nonsense-mediated decay. Mol. Cell Biol. 2002;22:8114–8121. doi: 10.1128/MCB.22.23.8114-8121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G., Landthaler M., Patkaniowska A., Dorsett Y., Teng G., Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Meister G., Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- Mello C. C., Conte D., Jr Revealing the world of RNA interference. Nature. 2004;431:338–342. doi: 10.1038/nature02872. [DOI] [PubMed] [Google Scholar]
- O'Donnell K. A., Wentzel E. A., Zeller K. I., Dang C. V., Mendell J. T. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–843. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- Okamura K., Ishizuka A., Siomi H., Siomi M. C. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004;18:1655–1666. doi: 10.1101/gad.1210204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R., Song H. The enzymes and control of eukaryotic mRNA turnover. Nat. Struct. Mol. Biol. 2004;11:121–127. doi: 10.1038/nsmb724. [DOI] [PubMed] [Google Scholar]
- Pauley K. M., Eystathioy T., Jakymiw A., Hamel J. C., Fritzler M. J., Chan E.K.L. Formation of GW bodies is a consequence of microRNA genesis. EMBO Rep. 2006;7:904–910. doi: 10.1038/sj.embor.7400783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai R. S., Bhattacharyya S. N., Artus C. G., Zoller T., Cougot N., Basyuk E., Bertrand E., Filipowicz W. Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science. 2005;309:1573–1576. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- Rehwinkel J., Behm-Ansmant I., Gatfield D., Izaurralde E. A crucial role for GW182 and the DCP 1, DCP2 decapping complex in miRNA-mediated gene silencing. RNA. 2005;11:1640–1647. doi: 10.1261/rna.2191905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G. L., Blau H. M. Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat. Cell Biol. 2005;7:633–636. doi: 10.1038/ncb1265. [DOI] [PubMed] [Google Scholar]
- Sheth U., Parker R. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science. 2003;300:805–808. doi: 10.1126/science.1082320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira D., Sheth U., Valencia-Sanchez M. A., Brengues M., Parker R. Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA. 2005;11:371–382. doi: 10.1261/rna.7258505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourriere H., Chebli K., Tazi J. mRNA degradation machines in eukaryotic cells. Biochimie. 2002;84:821–837. doi: 10.1016/s0300-9084(02)01445-1. [DOI] [PubMed] [Google Scholar]
- van Dijk E., Cougot N., Meyer S., Babajko S., Wahle E., Seraphin B. Human Dcp 2, a catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. EMBO J. 2002;21:6915–6924. doi: 10.1093/emboj/cdf678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz C. J., Wormington M., Peltz S. W. The cap-to-tail guide to mRNA turnover. Nat. Rev. Mol. Cell Biol. 2001;2:237–246. doi: 10.1038/35067025. [DOI] [PubMed] [Google Scholar]
- Yang Z., Jakymiw A., Wood M. R., Eystathioy T., Rubin R. L., Fritzler M. J., Chan E.K.L. GW182 is critical for the stability of GW bodies expressed during the cell cycle and cell proliferation. J. Cell Sci. 2004;117:5567–5578. doi: 10.1242/jcs.01477. [DOI] [PubMed] [Google Scholar]
- Yu J. H., Yang W. H., Gulick T., Bloch K. D., Bloch D. B. Ge-1 is a central component of the mammalian cytoplasmic mRNA processing body. RNA. 2005;11:1795–1802. doi: 10.1261/rna.2142405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.