Abstract
In Saccharomyces cerevisiae, the initial reaction of the tricarboxylic acid cycle is catalyzed by the mitochondrial citrate synthase Cit1. The function of Cit1 has previously been studied mainly in terms of acetate utilization and metabolon construction. Here, we report the relationship between the function of Cit1 and apoptosis. Yeast cells with cit1 deletion showed a temperature-sensitive growth phenotype, and they displayed a rapid loss in viability associated with typical apoptotic hallmarks, i.e., reactive oxygen species (ROS) accumulation and nuclear fragmentation, DNA breakage, and phosphatidylserine translocation, when exposed to heat stress. On long-term cultivation, cit1 null strains showed increased potentials for both aging-induced apoptosis and adaptive regrowth. Activation of the metacaspase Yca1 was detected during heat- or aging-induced apoptosis in cit1 null strains, and accordingly, deletion of YCA1 suppressed the apoptotic phenotype caused by cit1 null mutation. Cells with cit1 deletion showed higher tendency toward glutathione (GSH) depletion and subsequent ROS accumulation than the wild type, which was rescued by exogenous GSH, glutamate, or glutathione disulfide (GSSG). These results led us to conclude that GSH deficiency in cit1 null cells is caused by an insufficient supply of glutamate necessary for biosynthesis of GSH rather than the depletion of reducing power required for reduction of GSSG to GSH.
INTRODUCTION
In multicellular organisms, apoptosis is a highly regulated process of cell death that allows a cell to self-degrade for the body to eliminate potentially threatening or undesired cells; thus, it is a crucial event for common defense mechanisms and in development (Lam et al., 1999). The process of cellular suicide is also present in unicellular organisms such as the yeast Saccharomyces cerevisiae (Madeo et al., 1997). When unicellular organisms are exposed to harsh conditions, apoptosis may serve as a defense mechanism for the preservation of cell populations through the sacrifice of some members of a population to promote the survival of others (Frohlich and Madeo, 2000). Apoptosis in S. cerevisiae shows some typical features of mammalian apoptosis such as flipping of phosphatidylserine, membrane blebbing, chromatin condensation and margination, and DNA cleavage (Ligr et al., 1998).
In S. cerevisiae, apoptotic cell death was first described for a temperature-sensitive (ts) cdc48 mutant that exhibited typical apoptotic markers, i.e., exposition of phosphatidylserine, DNA fragmentation, and chromatin condensation at nonpermissive temperatures (Madeo et al., 1997). A correlation between aging and apoptosis in yeast has been evidenced by the presence of apoptotic markers in old cells (Frohlich and Madeo, 2000; Laun et al., 2001). Although the genome of S. cerevisiae does not encode obvious homologues to any of the core apoptotic machinery proteins, e.g., the Bcl-2/Bax family of proteins or the caspase family, a metacaspase called yeast caspase-1 (Yca1), which is required for the H2O2- or aging-induced (Madeo et al., 2002) and telomere-initiated apoptotic pathway (Qi et al., 2003), was identified in this organism. Evidence has indicated that exposure of yeast cells to high salinity induces apoptosis, which is caused by intracellular ion disequilibria rather than by an osmotic imbalance (Huh et al., 2002). Yeast cells deleted for the SRO7/SOP1 encoding a tumor suppressor homologue show increased sensitivity to NaCl stress, and on exposure to growth-inhibiting NaCl concentrations, Δsro7 mutants display a rapid loss in viability caused by Yca1-dependent apoptosis (Wadskog et al., 2004). Hyperosmotic stress caused by a high glucose or sorbitol concentration in culture medium also triggers S. cerevisiae to undergo Yca1- and mitochondria-dependent apoptosis (Silva et al., 2005). Pheromone-induced cell death, which might have evolved to remove yeast cells that fail to mate by using α-factor, has also been reported to show several traits typical for the mitochondria-dependent apoptosis of animal cells (Severin and Hyman, 2002). In addition, yeast N-glycosylation mutants with defects in the hetero-oligomeric membrane complex oligosaccharyltransferase, such as ost1 and wbp1-1, have recently been shown to undergo apoptosis that seemingly occurs independently of YCA1 (Hauptmann et al., 2006). Mitochondria can contribute to cell death in both mammalian and yeast cells when Bax is activated to permeabilize the mitochondrial outer membrane, resulting in the release of cytochrome c and other factors that trigger caspase activation and enhance cell death (Pastorino et al., 1998). The members of the mitochondrial fission machinery, Dnm1, Mdv1/Net2, and Fis1, have been shown to be related to programmed cell death involving mitochondria (Fannjiang et al., 2004).
The first reaction of the tricarboxylic acid (TCA) cycle is condensation of acetyl-CoA and oxaloacetate to form citrate, and it is catalyzed by the major mitochondrial citrate synthase (CS) Cit1 in S. cerevisiae. Thus, Cit1 functions as a rate-limiting enzyme of the TCA cycle (Suissa et al., 1984), whereas the peroxisomal CS Cit2 is involved in the glyoxylate cycle (Kim et al., 1986; Lewin et al., 1990). Deletion of CIT1 results in cells that are unable to grow on acetate (Kispal et al., 1988), despite the fact that the inner mitochondrial membrane is provided with a citrate transporter (Grigorenko et al., 1990), which could allow the extramitochondrial isoform (Cit2) to act as a shunt for missing Cit1. A cytosolically localized form of Cit1 is also incompetent for restoration of growth of the Δcit1 strain on acetate, which suggests that mitochondrial localization of Cit1 is essential for its function in the TCA cycle. When mislocalized in mitochondria, Cit2 is able to restore the wild-type phenotype in a strain lacking Cit1 (Velot et al., 1999; Lee et al., 2000). These phenomena support the view that the metabolic function of the TCA cycle may require structural as well as catalytic roles for CS. Accordingly, a portion of the TCA cycle enzymes, such as CS (Cit1), succinate dehydrogenase (Sdh1), malate dehydrogenase (Mdh1), and fumarate hydratase (Fum1), have been shown to be included in a supramolecular complex, in which there is channeling of intermediates between enzyme active sites (Velot et al., 1997; Velot and Srere, 2000). Double deletion of both CIT1 and CIT2 causes the cells to require glutamate when grown on a minimal medium. However, deletion of either CIT1 or CIT2 does not lead to glutamate auxotrophy (Kim et al., 1986). In addition, it has been shown in that the presence of any active CS in mitochondria, peroxisomes, or cytosol suffices for abolition of glutamate auxotrophy of the Δcit1Δcit2 mutant through the provision of citrate, which is expected to be converted into glutamate via α-ketoglutarate (Lee et al., 2006).
In previous reports, the function of yeast mitochondrial CS has been described mainly in terms of acetate utilization and metabolon construction. In the present study, our efforts were focused on investigating the relationship between the function of Cit1 and apoptosis. Here, we present evidence that deletion of CIT1 causes hypersusceptibility to heat- or aging-induced Yca1-dependent apoptosis. Our results also suggest that the apoptosis occurring under the stress conditions is mediated by depletion of glutamate that is required for biosynthesis of the tripeptide glutathione (l-γ-glutamyl-l-cysteinylglycine, GSH) in cit1 null mutants.
MATERIALS AND METHODS
Yeast Strains, Media, and Transformation
The strains of S. cerevisiae used in this study are listed in Table 1. Rich medium consisted of 1% yeast extract, 2% peptone, 75 μM adenine sulfate, and 2% glucose (YPD). Synthetic complete medium consisted of 0.7% yeast nitrogen base (Difco, Detroit, MI), 2 mM uracil, and 1 mM supplementary amino acids, such as glutamate, histidine, leucine, and methionine, and 2% glucose (SCD). Plates contained 2% agar (Difco). Transformation of yeast strains was performed by the lithium acetate method (Ito et al., 1983).
Table 1.
S. cerevisiae strains and plasmids used in this study
| Strain and plasmid | Relevant genotype | Reference or source |
|---|---|---|
| BY4741 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | American Type Culture Collection (Manassas, VA) |
| BY4741-YNR001C | Δcit1::KanMX4 MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | American Type Culture Collection |
| BY4741-YCR005C | Δcit2::KanMX4 MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | American Type Culture Collection |
| BY4741-YOR197W | Δyca1::KanMX4 MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | American Type Culture Collection |
| MCBY001 | Δcit1::URA3 Δcit2::KanMX4 MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | This study |
| MCBY002 | Δcit1::URA3 Δyca1::KanMX4 MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | This study |
| MCBY003 | Δcit1::URA3 Δcit2::KanMX4 Δyca1::LEU2 MATa his3Δ1 leu2Δ0 met15zΔ0 ura3Δ0 | This study |
| YEpCit1 | CIT1 URA3 | Lee et al. (2006) |
| YEpCit2 | CIT2 URA3 | Lee et al. (2006) |
| YCpCit1 | CIT1 LEU2 | This study |
| YCpCit2 | CIT2 LEU2 | This study |
Plasmids
The plasmids used in this study are listed in Table 1. For construction of YCpCit1, a 2.0-kb SacI–BamHI fragment containing the CIT1 gene was excised from YEpCit1 (Lee et al., 2006), and it was then inserted into SacI–BamHI-digested YCp111. Similarly, a 1.7-kb HindIII–SacI fragment containing the CIT2 gene was excised from YEpCit2 (Lee et al., 2006), and it was then ligated with SacI–BamHI-digested YCp111 plasmid to yield YCpCit2.
Gene Disruption
For construction of the Δcit1Δcit2 double mutant, a 1.8-kb polymerase chain reaction (PCR) product containing cit1::URA3 fusion construct was amplified from the yeast genomic DNA with the primers 5′-TAAAAAGAAAATAAGGCAAAACATATAGCAATATAATACTATTTACGAAGatgcagctcagattctttgtt tgaaaaattagcgctctcgcgttgc and 5′-AAATACGTGTTTGAATAGTCGCATACC CTGAATCAAAAATCAAATTTTCCtaattaaattgaagctctaatttgtgagtttagtatacatg cattt, where the uppercase nucleotides correspond to CIT1 and the lowercase nucleotides correspond to URA3. The Δcit2 mutant (BY4741-YCR005C) was then transformed with the fusion construct to yield the Δcit1Δcit2 (MCBY001) strain. Similarly, the Δyca1 mutant (BY4741-YOR197W) was transformed with the 1.8-kb cit1::URA3 fusion to yield the Δcit1Δyca1 (MCBY002) strain. To construct Δcit1Δcit2Δyca1 mutant, a 2.2-kb PCR product containing yca1::LEU2 fusion construct was amplified by double-joint PCR with the following primers: for the 5′ flanking region of YCA1, 5′-GAATTCTAGCTTCCTCTGTATTTTGC and 5′-agttaagaaaatccttgcttAAATATATGAATGTGT ACGT; for the 3′ flanking region of YCA1, 5′-gctgtgatttcttgaccaacGATTGTAAATCTAGTCGGTC and 5′-GAATTCACACTGAAAATGAGCAACCT; for LEU2 gene, 5′-aagcaaggattttcttaact and 5′-gttggtcaagaaatcacagc; and for final PCR round, 5′-TAGCTTCCTCTGTATTTTGC and 5′-ACACTGAAAATGAGCAACCT, where the uppercase nucleotides correspond to the 5′ or 3′ flanking sequence of YCA1 open reading frame, the lowercase nucleotides correspond to LEU2, and the underlined nucleotides correspond to additional EcoRI restriction sites. The Δcit1Δcit2 mutant (MCBY001) was then transformed with the yca1::LEU2 fusion construct to yield the Δcit1Δcit2Δyca1 (MCBY003) strain.
Growth and Survival Tests
For analysis of growth by plate assays, yeast cells grown in SCD for 1 d at 30°C were washed and resuspended in phosphate-buffered saline (PBS) to a concentration of 1.0 × 108 cells ml−1. After the cell suspensions were serially diluted in 10-fold steps, a 5-μl aliquot of each dilution was spotted onto SCD plates, and the plates were then incubated at the desired temperatures. For cell survival experiments, yeast cells grown in SCD broth at 30°C for 1 d, unless otherwise indicated, were suspended in PBS to a concentration of 1.0 × 108 cells ml−1. Two-milliliter samples were taken and subjected to one or more of the following physical and chemical treatments: heat, H2O2, acetic acid, antimycin, rotenone, and Tiron. One hundred microliter aliquots of each sample were taken at intervals and serially diluted in 10-fold steps. To determine viability by plate assay, a 5-μl aliquot of each dilution was spotted onto SCD plates and incubated at 30°C for 3 d. For the colony-forming unit (CFU) assay, a 100-μl aliquot of each dilution was spread onto an SCD plate, and colonies were scored after incubation at 30°C for 3 d.
Test for Apoptotic Markers
The terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay was performed as described by Madeo et al. (1999). In brief, cells were fixed with 3.7% formaldehyde, digested with lyticase (Sigma-Aldrich, St. Louis, MO), applied to a polylysine-coated slide, treated with 0.3% H2O2 to block endogenous peroxidases, permeabilized with 0.1% Triton X-100, incubated with 10 μl of TUNEL reaction mixture (Roche Diagnostics, Mannheim, Germany) for 60 min at 37°C, incubated with 10 μl of Converter-POD (Roche Diagnostics) for 30 min at 37°C, and then stained with DAB-substrate solution (Roche Diagnostics) for 10 min. A coverslip was mounted with a drop of Kaiser's glycerol gelatin (Merck, Darmstadt, Germany), and then bright-field images of cells were acquired using the BX51 universal research microscope (Olympus, Tokyo, Japan) equipped with a digital camera (DP11; Olympus).
To monitor the levels of intracellular reactive oxygen species (ROS), cells were stained with 10 μg ml−1 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) for 2 h with shaking at 30°C (Madeo et al., 1999). For analysis of nuclear fragmentation, cells were fixed in 3.7% formaldehyde, and then they were stained with 0.5 μg ml−1 DAPI (Sigma-Aldrich) (Madeo et al., 1997). Externalization of phosphatidylserine was assayed using fluorescein isothiocyanate (FITC)-coupled Annexin V (BD Biosciences, San Diego, CA) as described by Madeo et al. (1997). Cells were suspended in digestion buffer, and cell walls were digested as described above. The spheroplasts were stained with both FITC-Annexin V and 500 μg ml−1 propidium iodide (PI; BD Biosciences, San Jose, CA).
Fluorescence microscopy was performed under a BX51 universal research microscope (Olympus) equipped with a digital camera (DP11; Olympus) by using an appropriate filter set: an FITC filter for DCFH-DA and FITC-Annexin V, a 4,6-diamidino-2-phenylindole (DAPI) filter for DAPI, or a rhodamine filter for PI staining. To determine the frequencies of morphological phenotypes (TUNEL, DCFH-DA, DAPI, FITC-Annexin V, and PI), at least 300 cells from three independent experiments were evaluated.
Detection of Metacaspase Activity
Active metacaspase was detected using FITC-VAD-fmk (CaspACE; Promega) and PI (Sigma-Aldrich) (Wysocki and Kron, 2004). Yeast cells (5 × 106 cells) were harvested, washed in 1 ml of PBS, and resuspended in 200 μl of staining solution containing 50 μM FITC-VAD-fmk. After incubation for 20 min at 30°C, cells were washed and resuspended in 1 ml of PBS. To detect loss of membrane integrity, 5 μl of 1 mg ml−1 PI was added to cell suspensions. For flow cytometric analysis, the stained cells were counted using FACSCalibur (BD Biosciences) and CellQuest analysis software (BD Biosciences) with FL-1 for FITC green fluorescence and FL-2 for PI red fluorescence.
GSH and Glutamate Assay
Intracellular levels of GSH were determined by measuring the rate of 2-nitro-5-thiobenzoic acid formation from 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB; Sigma-Aldrich) in the GSH recycling system (Anderson, 1985). Five microliters of each cell lysate was added to 1 ml of 100 mM phosphate buffer, pH 7.5, containing 0.6 mM DTNB, 5 mM EDTA, 0.2 mM NADPH, and 1 U/ml GSH reductase (GR) (Sigma-Aldrich), and the rate of increase in A412 was monitored. Intracellular levels of glutamate were determined by using a colorimetric glutamate analysis kit (R-Biopharm, Darmstadt, Germany) according to the instructions of the manufacturer.
RESULTS
Deletion of CIT1 Causes Increased ts Phenotype
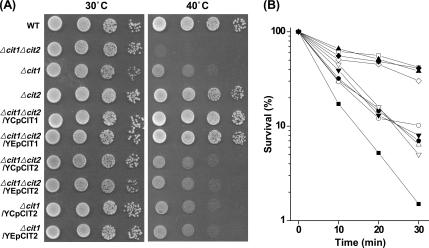
Temperature sensitivity of the CS mutants, i.e., Δcit1, Δcit2, and Δcit1Δcit2, and their derivatives carrying ectopic CIT1 or CIT2 gene was assessed by both growth assay and survival test at elevated temperatures in comparison with those of the wild-type strain (BY4741). All of the yeast strains developed normal-sized colonies after 3-d cultivation at 30°C on SCD plates (Figure 1A, left), which indicates that none of the two CS genes is essential to the growth on the complete medium. After 3-d cultivation at 40°C, not only Δcit1 and Δcit1Δcit2 cells but also Δcit1/YCpCit2, Δcit1/YEpCit2, Δcit1Δcit2/YCpCit2, and Δcit1Δcit2/YEpCit2 cells failed to develop colonies on SCD plates, whereas Δcit2, Δcit1Δcit2/YCpCit1, and Δcit1Δcit2/YEpCit1 cells grew normally, as did the wild-type cells (Figure 1A, right).
Figure 1.
Deletion of CIT1 causes increased sensitivity to heat stress. (A) Analysis of growth of yeast strains at elevated temperatures. Yeast strains (wild type, Δcit1, Δcit2, Δ cit1Δcit2, Δcit1Δcit2/YCpCit1, Δcit1Δcit2/YEp Cit1, Δcit1Δcit2/YCpCit2, Δcit1Δcit2/YEpCit2, Δcit1/YCpCit2, and Δcit1/YEpCit2) grown in SCD were serially diluted, spotted on SCD agar plates, and incubated at 30 or 40°C for 3 d. (B) Analysis of survival rates of yeast strains at lethal temperatures. Cells of the yeast strains listed above were shifted to 50°C, and aliquots of the samples were taken at intervals. Surviving cells were evaluated by CFU assays carried out in triplicate. □, wild type; ▵, Δcit1; ▴, Δcit2; ■, Δcit1Δcit2; ◇, Δcit1Δcit2/YCpCit1; ♦, Δcit1 Δcit2/YEpCit1; ○, Δcit1Δcit2/YCpCit2; ·, Δcit1 Δcit2/YEpCit2; ▿, Δcit1/YCpCit2; ▾, Δcit1/YEpCit2.
Survival tests of the yeast strains demonstrated that Δcit1, Δcit1Δcit2, Δcit1/YCpCit2, Δcit1/YEpCit2, Δcit1Δcit2/YCpCit2, and Δcit1Δcit2/YEpCit2 cells are highly sensitive to heat stress (Figure 1B). Although the survival rates of wild-type, Δcit2, Δcit1Δcit2/YCpCit1, and Δcit1Δcit2/YEpCit1 cells were 35–50% after 30 min of exposure to heat stress at 50°C, only 5–10% of the initial population of Δcit1, Δcit1/YCpCit2, Δcit1/YEpCit2, Δcit1Δcit2/YCpCit2, and Δcit1Δcit2/YEpCit2 cells survived after the same treatment. In addition, 99% of the Δcit1Δcit2 mutant cells were killed within the first 30 min of exposure to heat stress. This result indicates that Δcit1 cells have the ts phenotype, whereas Δcit2 cells do not. It was also found that the ts phenotype caused by deletion of CIT1 could not be suppressed by CIT2, although the heat sensitivity of Δcit1Δcit2 cells was even more severe than that of Δcit1 and the transformants lacking Cit1.
Heat-induced Death of the Cells with cit1 Deletion Is Mediated by ROS
To determine whether the increased death rates of Δcit1 and Δcit1Δcit2 cells under heat stress conditions are caused by a higher tendency of the mutant populations toward stress-induced apoptosis, we looked for cytological and biochemical features of apoptosis in the mutant cells. First, we analyzed the cells for the presence of ROS, which has been shown to be both necessary and sufficient for inducing apoptosis in yeast (Madeo et al., 1999).
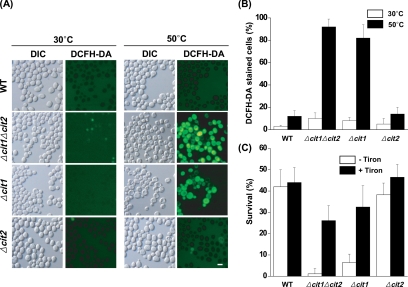
DCFH-DA, which is oxidized to the fluorescent chromophore, 2′,7′-dichlorofluorescein (DCF), through the action of peroxide (H2O2), was used as a probe for the detection of ROS. As indicated by fluorescence microscopy (Figure 2, A and B), the majority of Δcit1 (82%) and Δcit1Δcit2 cells (92%) precultured for 2 d in SCD broth at 30°C were fluorescent after 10 min of exposure to heat stress at 50°C. In contrast, wild-type and Δcit2 cells under these conditions did not show any detectable levels of DCF fluorescence. These results indicate that deletion of CIT1 causes heat stress-dependent accumulation of ROS.
Figure 2.
Heat-induced death of cit1 null cells is mediated by ROS. (A) Observation of ROS accumulation in the cells exposed to heat stress. Yeast strains (wild type, Δcit1, Δcit2, and Δcit1Δcit2) grown in SCD were incubated at 30 or 50°C for 10 min. The cells were then stained with DCFH-DA and examined by fluorescence microscopy. Bar, 10 μm. (B) Percentage of ROS-accumulating cells after exposure to heat stress. DCFH-DA–positive cells were counted in fluorescence images and total cells in corresponding differential interference contrast (DIC) images. (C) Effect of Tiron on the temperature-dependent survival rate of yeast cells. Cells of the strains listed above were incubated at 50°C for 30 min with or without the addition of 10 mM Tiron, and aliquots of the sample were then taken. Surviving cells were evaluated by CFU assays carried out in triplicate.
To confirm the presence of a direct correlation between the ts phenotype and the heat stress-dependent ROS accumulation observed in the cells with a cit1 deletion, we analyzed the temperature-dependent survival rate of the yeast strains in the presence of Tiron, a cell-permeable ROS scavenger. As presented in Figure 2C, after 30-min heat treatment at 50°C in the presence of 10 mM Tiron, the survival rates of Δcit1 and Δcit1Δcit2 cells were estimated to be ∼33 and 26%, respectively. Considering that only ∼7 and 1% of the initial population of Δcit1 and Δcit1Δcit2 cells, respectively, remained alive after the same heat treatment in the absence of Tiron, it seems that Tiron rescues the mutant cells from the heat-induced death. Thus, this result indicates that the heat-induced death of cit1 deletion mutants is mediated by the accumulation of ROS.
Cells with the cit1 Deletion Display Markers of Apoptosis under Heat Stress Conditions
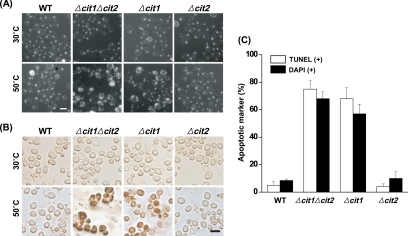
Having established increased occurrence of ROS in heat-stressed Δcit1 and Δcit1Δcit2 mutants, we attempted to examine the mutant cells subjected to heat stress for nuclear fragmentation, a well-established cytological hallmark of apoptosis. A normal, single round-shaped nucleus was detected by DAPI staining in each of the wild-type and mutant cells under normal conditions (30°C; 10 min) (Figure 3, A and C). Conversely, ∼57 and 68% of Δcit1 and Δcit1Δcit2 cells, respectively, displayed irregularly shaped and fragmented nuclei 10 min after exposure to 50°C. In contrast, only ∼10% of the similarly treated wild-type and Δcit2 strain showed abnormal nuclei.
Figure 3.
Cells with the cit1 deletion display fragmentation of nuclei and DNA under heat stress conditions. (A) Observation of nuclear fragmentation in the cells exposed to heat stress. Yeast strains (wild type, Δcit1, Δcit2, and Δcit1Δcit2) grown in SCD were incubated at 30 or 50°C for 10 min. The cells were then stained with DAPI and examined by fluorescence microscopy. Bar, 10 μm. (B) Observation of DNA fragmentation in the cells exposed to heat stress. Yeast strains prepared as described above were stained with TUNEL reagent, and then they were examined by bright-field microscopy. Bar, 10 μm. (C) Percentage of cells displaying fragmentation of nuclei or DNA after exposure to heat stress (50°C for 10 min). Numbers of the cells with fragmented nuclei (DAPI+) and DNA (TUNEL+) were determined from DAPI fluorescence and bright-field images, respectively.
We also examined the fragmentation of DNA in the nucleus, another familiar feature of apoptosis, by using a TUNEL analysis, in which fluorescent nucleotides are added to the 3′-OH ends of the DNA fragments, making the phenomenon visible by fluorescent microscopy. None of the wild-type or mutant cells showed TUNEL-positive nuclei under normal conditions (30°C; 10 min) (Figure 3, B and C). After 10 min of exposure to 50°C, ∼68 and 75% of Δcit1 and Δcit1Δcit2 populations, respectively, showed intensely stained nuclei, indicating DNA strand breakage. However, the nuclei of both the wild-type and Δcit2 cells remained unstained or only slightly stained after the same treatment.
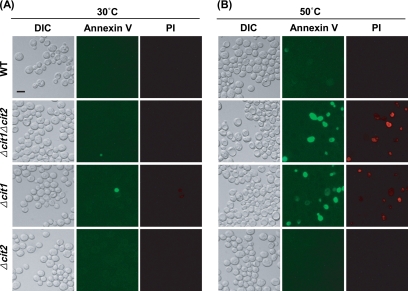
In yeast cells as well as mammalian cells, translocation of phosphatidylserine, which is predominantly located on the inner leaflet of the plasma membrane under normal conditions, to the outer leaflet serves as a sensitive marker for early stages of apoptosis (Martin et al., 1995). For detection of phosphatidylserine in the outer phase of the cytoplasmic membrane, spheroplasts formed from the heat-stressed cells of wild-type and mutant strains were stained with both FITC-Annexin V and PI, and they were analyzed by fluorescence microscopy.
When exposed to heat stress (50°C; 10 min), a portion of Δcit1 and Δcit1Δcit2 cells were exclusively stained with FITC-Annexin V, indicating that they were undergoing early stage of apoptosis (Figure 4). In addition, some of the heat-stressed cells were dually stained with both FITC-Annexin V and PI, suggesting the presence of cells of late apoptotic phases or necrosis. On the contrary, only a negligible portion of similarly treated wild-type and Δcit2 cells were solely stained with FITC-Annexin V, and thus, they were considered to be apoptotic.
Figure 4.
Cells with the cit1 deletion display phosphatidylserine translocation under heat stress conditions. Yeast cells (wild type, Δcit1, Δcit2, and Δcit1Δcit2) grown in SCD were incubated at 30 or 50°C for 10 min. The cells were stained with both FITC-Annexin V and PI, and then they were examined by fluorescence microscopy. Bar, 10 μm.
In summary, these results lead us to conclude that compared with the isogenic wild-type strains, mutant strains with the cit1 deletion are much more susceptible to heat stress-induced cell death that bears the structural attributes of apoptosis with respect to ROS accumulation, nuclear fragmentation, externalization of phosphatidylserine, and DNA strand breakage.
Cells with cit1 Deletion Exhibit Accelerated Aging Mediated by Apoptosis and Adaptive Regrowth
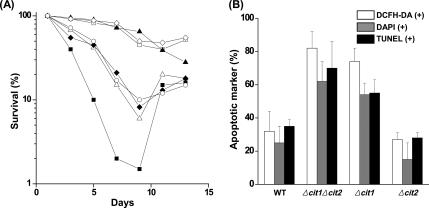
It has been demonstrated that chronologically aged yeast cultures die exhibiting typical markers of apoptosis, accumulate oxygen radicals, and show caspase activation (Laun et al., 2001; Herker et al., 2004). To determine whether the deletion of the CIT1 gene affects the survival of cells in chronologically aged yeast cultures, we cultured wild-type and mutant strains in SCD continuously for 13 d, and we monitored the portion of living cells by CFU assay. Although about one-half of the maximum populations of the wild-type, Δcit2, and Δcit1Δcit2/YEpCit1 cells stayed alive after a 9-d culture, only 1–10% of Δcit1, Δcit1Δcit2, Δcit1/YEpCit2, and Δcit1Δcit2/YEpCit2 cells survived after the 9-d culture period (Figure 5A). This result indicates that cells with the cit1 deletion are subject to a much faster and more severe aging process than their isogenic wild-type population.
Figure 5.
Deletion of CIT1 causes accelerated aging associated with typical hallmarks of apoptosis and subsequent adaptive regrowth. (A) Analysis of the rate of aging of yeast strains. Yeast strains (wild type, Δcit1, Δcit2, Δcit1Δcit2, Δcit1Δcit2/YEpCit1, Δcit1Δcit2/YEpCit2, and Δcit1/YEpCit2) were grown in SCD for 13 d. Aliquots of the cultures were taken at intervals, and the portion of living cells was then monitored by CFU assays carried out in triplicate. □, wild type; ▵, Δcit1; ▴, Δcit2; ■, Δcit1Δcit2; ◇, Δcit1Δcit2/YEpCit1; ○, Δcit1Δcit2/YEpCit2; ♦, Δcit1/YEpCit2. (B) Percentage of cells exhibiting the typical hallmarks of apoptosis in aged cultures. The yeast strains listed above were grown in SCD for 7 d. To estimate the proportion of ROS-accumulating cells, yeast cells were stained with DCFH-DA, and then they were examined by fluorescence microscopy. DCFH-DA–positive cells were counted in fluorescence images and total cells in corresponding DIC images. For observation of nuclear fragmentation, cells were stained with DAPI, and the proportions of the cells with abnormal nuclei were estimated by fluorescence microscopy. For observation of DNA fragmentation, cells were stained with TUNEL reagent. The proportions of cells exhibiting DNA fragmentation were estimated by bright-field microscopy.
It has been reported in the previous research that during the chronological aging process of yeast, the premature apoptotic death promotes the regrowth of a subpopulation of better-adapted mutants (Fabrizio et al., 2004). After 9-d culture of Δcit1, Δcit1Δcit2, Δcit1/YEpCit2, and Δcit1Δcit2/YEpCit2 cells, when 90–99% of the populations died, the number of viable cells in the culture started to increase and then reached up to 15–20% of the initial population after 2 d (Figure 5A). Thus, this result indicates that mutants with the cit1 deletion have higher potentials for adaptive regrowth after the accelerated aging-dependent cell death upon long-term cultivation than wild-type strains.
To determine whether the accelerated aging-dependent death of Δcit1 and Δcit1Δcit2 populations is programmed, we looked for cytological and biochemical features of apoptosis. When stained with DCFH-DA, a majority of the 7-d-old populations of Δcit1 (74%) and Δcit1Δcit2 cells (82%) were fluorescent, whereas only a minority of wild-type (32%) and Δcit2 cells (27%) of similar age showed detectable levels of DCF fluorescence (Figure 5B). This result indicates that the accelerated chronological aging of cit1 null cells is accompanied by the accumulation of ROS. Chromatin staining of Δcit1 and Δcit1Δcit2 cells with DAPI showed abnormal nuclear morphology in >55% of the populations during the major mortality phase (Figure 5B). In contrast, ∼80% of the 7-d-old populations of the wild-type and Δcit2 mutant maintained normal nuclear morphology similar to that of young cells. In TUNEL analysis, more than one-half of both the 7-d-old populations of Δcit1 and Δcit1Δcit2 strains showed intensely stained nuclei, which indicated that most of the cells contained DNA strand breaks (Figure 5B). In contrast, the majority of the nuclei of both wild-type (65%) and Δcit2 cells (73%) remained unstained or only slightly stained after 7-d cultivation. Together, these results suggest that mutants with the cit1 deletion are also much more susceptible than wild-type cells to chronological aging-induced cell death accompanied by the typical hallmarks of apoptosis, including ROS accumulation, nuclear fragmentation, and DNA strand breakage.
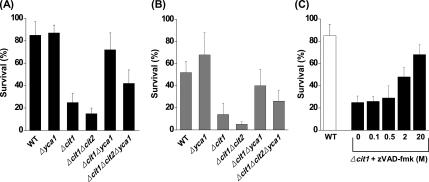
Yca1 Is Involved in the Increased Susceptibility of the Cells with the cit1 Deletion to the Apoptotic Process Induced by Heat Stress and Chronological Aging
Yeast YCA1 gene codes for a metacaspase that regulates apoptosis through a protein-cutting activity reminiscent of that exhibited by mammalian caspases (Madeo et al., 2002). To address the role of the metacaspase Yca1 in the increased susceptibility of the cells with the cit1 deletion to heat-induced cell death, we constructed Δcit1Δyca1 and Δcit1Δcit2Δyca1 mutants, and then we examined their phenotypes in comparison with Δcit1 and Δcit1Δcit2 mutants. Although only ∼25% of the initial population of Δcit1 cells survived after 10 min of heat treatment at 50°C, 72% of Δcit1Δyca1 cells exposed to a similar heat stress were alive (Figure 6A). In addition, the survival rates of Δcit1Δcit2 and Δcit1Δcit2Δyca1 cells subjected to similar heat treatment were 15 and 42%, respectively. We also attempted to address the involvement of YCA1-mediated apoptosis in the accelerated aging phenotype of the yeast cells with the cit1 deletion. The survival rates of Δcit1 and Δcit1Δcit2 cells were only ∼14 and 5% after 7-d culture in SCD broth, respectively. In contrast, ∼40 and 26% of Δcit1Δyca1 and Δcit1Δcit2Δyca1 cells, respectively, remained alive under similar conditions (Figure 6B). These results indicate that deletion of YCA1 significantly suppresses both the ts and accelerated aging phenotypes of the cit1 null cells.
Figure 6.
Deletion of YCA1 or inhibition of Yca1 activity suppresses the phenotype of cells with the cit1 deletion. (A) Effect of YCA1 deletion on the ts phenotype caused by deletion of CIT1. Yeast strains (wild type, Δyca1, Δcit1, Δcit1Δyca1, and Δcit1Δcit2Δyca1) grown in SCD were incubated at 50°C for 10 min, and aliquots of the samples were taken. Surviving cells were evaluated by CFU assays carried out in triplicate in all cases. (B) Effect of YCA1 deletion on the accelerated chronological aging caused by the deletion of CIT1. The yeast strains listed above were grown in SCD for 7 d, and surviving cells were then evaluated by CFU assay. (C) Effect of Yca1 inhibition on the ts phenotype caused by the deletion of CIT1. Cells of the Δcit1 strain grown in SCD were incubated at 50°C for 10 min in the presence of 0–20 μM zVAD-fmk, a metacaspase inhibitor, and surviving cells were then evaluated by CFU assay.
The effect of Yca1 inhibition on the ts phenotype of Δcit1 cells was examined using a metacaspase inhibitor, zVAD-fmk. The survival rate of Δcit1 cells exposed to heat stress (50°C; 10 min) increased from ∼25 to 69% in accordance with increasing concentration of zVAD-fmk, from 0 to 20 μM (Figure 6C). This result suggests that inhibition of Yca1 activity suppresses the ts phenotype of Δcit1 cells.
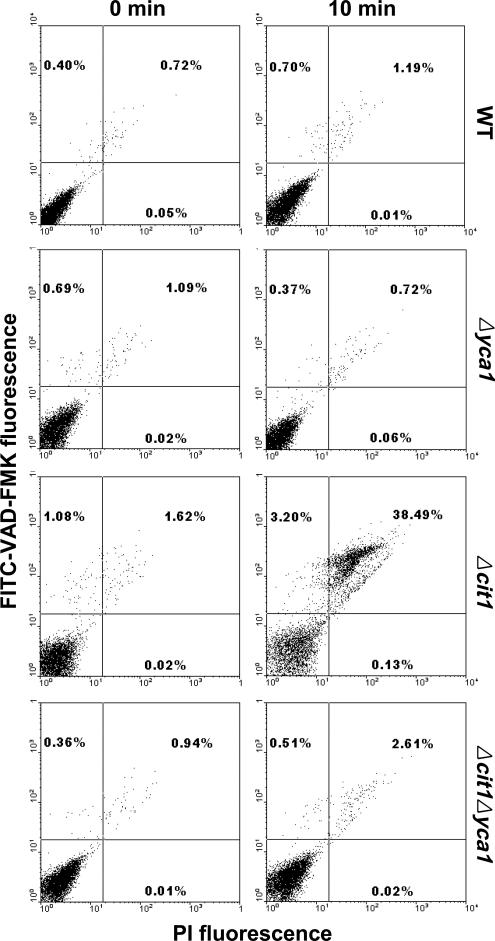
To assess the involvement of Yca1 activation in the increased susceptibility of cit1 null cells to heat-induced cell death, we performed dual staining of yeast cells exposed to heat stress with the FITC-labeled pan-caspase inhibitor VAD-fmk that binds to activated caspases, and PI, which is a reporter of cell membrane integrity. Using bivariate flow cytometry analysis, cells detected by forward scatter were plotted by red fluorescence of PI on the x-axis and by green fluorescence of FITC on the y-axis (Wysocki and Kron, 2004). Only negligible portions of CIT1 wild-type, Δyca1, and Δcit1Δyca1 cells became either FITC-positive/PI-negative (activated caspase/early apoptotic) or FITC-positive/PI-positive (postapoptotic cells) after 10-min heat treatment at 50°C] (Figure 7). On the contrary, in Δcit1 population significantly increased proportions of both FITC-positive/PI-negative (3.20%) and FITC-positive/PI-positive cells (38.49%) were detected after 10 min of similar treatment. It thus seems that although little metacaspase activation occurs in CIT1 wild-type, Δyca1, and Δcit1Δyca1 cells exposed to heat stress, considerable level of metacaspase activation is induced in heat-stressed Δcit1 cells, which eventually leads to apoptotic cell death. Together, these results indicate that the ts and accelerated aging phenotype of the mutants carrying the cit1 deletion are caused by apoptosis that is mediated by the action of the metacaspase Yca1.
Figure 7.
The yeast metacaspase Yca1 is activated during heat-induced death of cells with the cit1 deletion. Yeast strains (wild type, Δyca1, Δcit1, and Δcit1Δyca1) grown in SCD were exposed to heat stress (50°C; 10 min). Metacaspase activation was analyzed by dual staining with FITC-VAD-fmk and PI, followed by bivariate flow cytometry analysis. Cells detected by forward scatter were plotted by PI fluorescence on the x-axis and by FITC fluorescence on the y-axis (Wysocki and Kron, 2004).
Cells with the cit1 Deletion Exhibit Increased Sensitivity to the Stresses Causing Accumulation of ROS
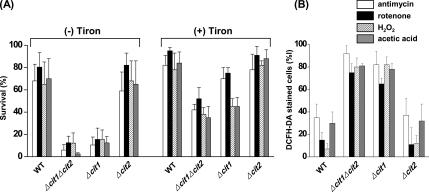
As described above, the yeast cells with the cit1 deletion showed increased susceptibility to the apoptotic cell death mediated by ROS accumulation under the conditions of heat stress or chronological aging. It could be expected that any of the ROS-generating chemicals can affect the survival of cit1 null cells by inducing apoptosis. Thus, we performed survival tests of the yeast strains in the presence of ROS-generating chemicals such as antimycin (100 μM), rotenone (100 μM), H2O2 (10 mM), and acetic acid (20 mM). Although the survival rates of wild-type and Δcit2 cells ranged from 60 to 80% after 1-h exposure to the chemicals, <15% of the initial population of Δcit1 and Δcit1Δcit2 cells survived after the same treatment (Figure 8A). The lethal effect of the ROS-generating chemicals on the cit1 deletion mutants was significantly attenuated by the ROS scavenger Tiron, so that the survival rates of the Δcit1 and Δcit1Δcit2 cells exposed to the chemicals increased up to 45–75 and 35–52%, respectively, in the presence of 10 mM Tiron. Accordingly, 65–90% of the initial population of Δcit1 and Δcit1Δcit2 cells showed ROS accumulation, as determined by DCFH-DA staining and subsequent fluorescence microscopy after 1-h exposure to the ROS-generating chemicals (Figure 8B). However, only 8–37% of the initial population of wild-type and Δcit2 cells exposed to the chemicals for 1 h exhibited signs of ROS accumulation. These results indicate that mutants with the cit1 deletion have much higher sensitivity to the ROS-generating chemicals, and they further suggest that the increased rate of stress-induced apoptosis of the cells with the cit1 deletion is correlated with their higher tendency to accumulate ROS compared with that of wild-type cells.
Figure 8.
Cells with the cit1 deletion exhibit increased sensitivity to ROS-generating chemicals. (A) Effect of CIT1-deletion on the survival of yeast cells in the presence of ROS-generating chemicals. Yeast cells (wild type, Δcit1, Δcit2, and Δcit1Δcit2) grown in SCD were exposed to 100 μM antimycin, 100 μM rotenone, 10 mM H2O2, or 20 mM acetic acid for 1 h in the presence or absence of 10 mM Tiron. Surviving cells were evaluated by CFU assays carried out in triplicate. (B) Effect of CIT1-deletion on the intracellular levels of ROS in the presence of ROS-generating chemicals. The yeast strains were prepared and treated with the ROS-generating chemicals as described above. The cells were then stained with DCFH-DA and examined by fluorescence microscopy. The numbers of DCFH-DA–positive cells were estimated in fluorescence images and total cells in corresponding DIC images.
Cells with the cit1 Deletion Exhibit Depletion of GSH, Which Leads to Increased Susceptibility to Heat-induced and ROS-mediated Apoptosis
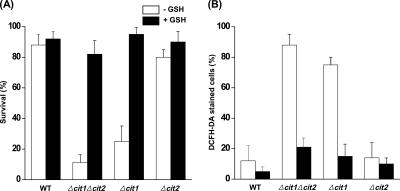
To determine whether the accumulation of ROS in the cells with the cit1 deletion exposed to stress conditions is facilitated by the depletion of GSH, which is used by many peroxidases to reduce H2O2 and a multitude of organic hydroperoxides (Grant, 2001), we analyzed the levels of GSH in the mutant and wild-type cells. When grown in SCD for 1 d, the levels of GSH in Δcit1 and Δcit1Δcit2 cells were estimated to be ∼48 and 27% of that in wild-type cells, respectively (Table 2). In contrast, when the cells were grown in SDC supplemented with 10 mM GSH, Δcit1 and Δcit1Δcit2 mutants exhibited similar levels of GSH as wild-type cells, i.e., ∼78 and 88%, respectively. We also examined the effect of exogenous GSH on the temperature-dependent survival of the yeast strains. In accordance with the restoration of the intracellular GSH levels, Δcit1 and Δcit1Δcit2 cells grown in GSH-supplemented SCD medium showed almost the same temperature-dependent survival rates as wild-type and Δcit2 cells (Figure 9A). In addition, when stained with DCFH-DA, Δcit1 and Δcit1Δcit2 cells did not show any detectable levels of DCF fluorescence under heat stress conditions, indicating the absence of heat-induced ROS accumulation (Figure 9B). These results altogether suggest that deletion of CIT1 causes the depletion of intracellular GSH, which, in turn, induces the accumulation of ROS and accompanying apoptotic cell death under stress conditions.
Table 2.
Intracellular levels of GSH and glutamate in S. cerevisiae strains grown in different media
| Strain | GSH (nmol/mg protein)a Additions to SCD |
Glutamate (nmol/mg protein)b Additions to SCD |
||||
|---|---|---|---|---|---|---|
| Glutamate (1 mM) | Glutamate (10 mM) | GSH (10 mM) | Glutamate (1 mM) | Glutamate (10 mM) | GSH (10 mM) | |
| WT | 117.8 + 10.5 | 114.8 + 12.7 | 127.7 + 20.2 | 12.2 + 2.6 | 20.1 + 2.2 | 14.9 + 1.5 |
| Δcit1Δcit2 | 32.1 + 5.7 | 88.5 + 15.2 | 99.2 + 10.9 | 1.2 + 0.5 | 15.1 + 5.2 | 9.6 + 2.9 |
| Δcit1 | 56.4 + 9.8 | 99.9 + 5.7 | 112.4 + 11.1 | 3.5 + 1.8 | 14.2 + 3.3 | 10.0 + 1.8 |
| Δcit2 | 105.4 + 17.4 | 112.2 + 10.3 | 124.5 + 15.0 | 11.4 + 2.5 | 22.2 + 4.7 | 15.4 + 2.6 |
a Intracellular levels of GSH were analyzed by the DTNB method carried out in triplicate.
b Intracellular levels of glutamate were analyzed using a colorimetric glutamate analysis kit carried out in triplicate.
Figure 9.
Cells with the cit1 deletion exhibit depletion of GSH, which leads to increased susceptibility to heat-induced and ROS-mediated apoptosis. (A) Effect of exogenous GSH on the ts phenotype caused by the deletion of CIT1. Yeast strains (wild type, Δcit1, Δcit2, and Δcit1Δcit2) were grown in SCD broth with or without 10 mM GSH, and then they were incubated at 50°C for 10 min. Aliquots of the samples were taken, and surviving cells were evaluated by CFU assays carried out in triplicate. (B) Effect of exogenous GSH on ROS accumulation in CIT1-deleted cells exposed to heat stress. The yeast strains listed above were grown in SCD with or without 10 mM GSH, and then they were incubated at 50°C for 10 min. The cells were then stained with DCFH-DA and examined by fluorescence microscopy. The numbers of DCFH-DA–positive cells were estimated in fluorescence images and total cells in corresponding DIC images.
Depletion of GSH in Cells with the cit1 Deletion Is Caused by Shortage of Glutamate
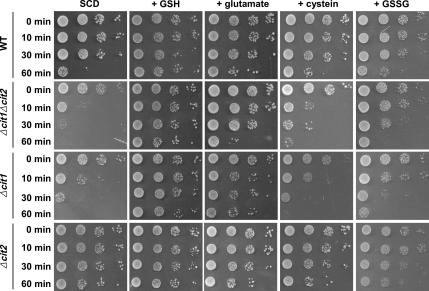
Intracellular GSH levels are maintained by a complex series of reactions that balance its rate of biosynthesis and reduction (Ohtake and Yabuuchi, 1991; Grant et al., 1996a; Inoue et al., 1998). To determine whether the biosynthetic process of GSH is defective in cit1 deletion mutants, we examined the effect of several amino acids used as precursors for GSH synthesis, such as glutamate, glycine, and cysteine, on the survival of the yeast strains under heat stress conditions. Mutant Δcit1 and Δcit1Δcit2 cells grown in SCD supplemented with 10 mM glutamate showed similar survival rates as wild-type and Δcit2 cells after exposure to the heat stress at 50°C (Figure 10). In contrast, the addition of either 10 mM cysteine or 10 mM glycine (data not shown) did not rescue the ts phenotype of either Δcit1 or Δcit1Δcit2 mutant. These results suggest that the depletion of GSH, which occurs in cells with the cit1 deletion that are exposed to heat stress, is caused by a shortage of glutamate but not by cysteine or glycine deficiencies.
Figure 10.
Depletion of GSH leading to heat-induced apoptosis in cells with the cit1 deletion is caused by a shortage of glutamate. Yeast cells (wild type, Δcit1, Δcit2, and Δcit1Δcit2) grown in SCD or in SCD with 10 mM GSH, 10 mM glutamate, 10 mM cysteine, or 10 mM GSSG were incubated at 50°C. To evaluate the surviving cells, aliquots of the sample were taken at intervals, serially diluted, spotted on SCD agar plates, and incubated at 30°C for 3 d.
When grown for 1 d in SCD that contained 1 mM glutamate, the levels of glutamate in Δcit1 and Δcit1Δcit2 cells were estimated to be only ∼29 and 10% of that in wild-type cells, and the levels of GSH in the mutant cells were 48 and 27%, respectively (Table 2). The low level of glutamate and GSH in Δcit1Δcit2 cells is consistent with the previous observation that the double mutant showed glutamate auxotrophy under normal growth conditions (Kim et al., 1986; Lee et al., 2000), as well as with the present result that it showed much higher susceptibility to heat and aging stress. It is also apparent that although the level of glutamate detected in Δcit1 cells can support their growth on minimal medium lacking glutamate, it cannot reach the minimum level required for maintaining the normal level of resistance to heat and aging stress. As expected, when grown in SCD supplemented with 10 mM glutamate for 1 d, the levels of glutamate and GSH in both the Δcit1 and Δcit1Δcit2 cells reached roughly 70–87% of the levels found in the wild-type and Δcit2 mutant cells (Table 2), which shows good agreement with the result that 10 mM glutamate added to the medium can rescue the ts phenotype caused by the deletion of CIT1 (Figure 10).
To determine whether the shortage of GSH in mutants with the cit1 deletion resulted from any defect in the process of reduction of glutathione disulfide (GSSG) to GSH, we analyzed the effect of GSSG used as a supplement in medium on the survival of yeast cells under heat stress. As presented in Figure 10, the ts phenotype of Δcit1 and Δcit1Δcit2 cells was noticeably rescued by 10 mM GSSG contained in the medium. This result demonstrates that the reduction of GSSG to GSH catalyzed by GR by using reducing power donated by NADPH is functional in mutants with the cit1 deletion.
DISCUSSION
The yeast mitochondrial CS Cit1 plays a pivotal role in the TCA cycle, not only with its catalytic function producing the first TCA cycle intermediate citrate (Suissa et al., 1984) but also with its key structural role in construction of the TCA cycle metabolon (Velot et al., 1997; Velot and Srere, 2000). Disruption of the CIT1 gene results in cells that are unable to grow on acetate (Kispal et al., 1988). Cit1 is also involved in glutamate synthesis together with Cit2 by supplying citrate, which is expected to be converted into glutamate via α-ketoglutarate. Thus, the absence of both Cit1 and Cit2 causes glutamate auxotrophy, whereas the presence of either Cit1 or Cit2 does not (Kim et al., 1986; Lee et al., 2006). In the present study, we attempted to determine the effect of the deletion of CIT1 on the susceptibility of yeast cells to apoptosis induced by heat or aging stress. Thus far, the relationship between the function of CS and apoptosis has not yet been reported in either multicellular or unicellular organisms. In the present report, we describe the hypersusceptibility to heat- or aging-induced apoptosis in mutants lacking the mitochondrial CS Cit1.
The prime clue to the involvement of Cit1 in heat- or aging-induced apoptosis was first derived from the observation that cells with the cit1 deletion, but not the cit2 deletion, showed the ts phenotype, which was not suppressed by the introduction of a single-copy or multicopy plasmid carrying CIT2 (Figure 1). We were curious to know which of the two different modes of cell death, apoptosis, and necrosis is responsible for the ts phenotype of the cit1 deletion mutants. We observed several typical morphological and cytological hallmarks of apoptosis, such as accumulation of ROS (Figure 2), nuclear and DNA fragmentation (Figure 3), and phosphatidylserine translocation (Figure 4), in cells with the cit1 deletion that were exposed to heat stress. These results led us to conclude that deletion of CIT1 causes apoptotic cell death under nonpermissive conditions.
We also attempted to determine whether other types of stress experienced by the cells carrying the deletion of CIT1 can also lead to apoptosis. Compared with wild-type strains, the cit1 null strains showed much higher susceptibility to chronological aging-induced cell death accompanied by the typical hallmarks of apoptosis, such as ROS accumulation, nuclear fragmentation, and DNA breakage (Figure 5). Furthermore, the aging-induced apoptosis occurring in the cells with the cit1 deletion was followed by the regrowth of a subpopulation that was expected to consist of better-adapted cells to prolonged culture conditions (Figure 5). The phenomenon of adaptive regrowth and the preceding ROS-mediated altruistic apoptotic program observed during the chronological aging process of yeast have been suggested to have evolved to promote adaptation to changing environments (Fabrizio et al., 2004). In accordance with this result, yeast cells harboring a mutation causing increased levels of cytosolic superoxide such as Δsod1 (deletion of superoxide dismutase gene) or Δctt1 (deletion of catalase gene) caused increased potential for adaptive regrowth (Fabrizio et al., 2004). Accordingly, in the present study, we found that cit1 deletion mutants showed much higher sensitivity to the ROS-generating chemicals such as antimycin, rotenone, H2O2, and acetic acid (Figure 8), which suggests that the hypersusceptibility to stress-induced apoptosis of cit1 deletion mutants is correlated with their higher tendencies to accumulate ROS compared with that of CIT1 wild-type cells.
We could see considerable increase in the survival rates of Δcit1Δyca1 and Δcit1Δcit2Δyca1 cells subjected to either heat stress or prolonged culture compared with those of Δcit1 and Δcit1Δcit2 cells similarly treated, respectively (Figure 6). This supports that deletion of YCA1 suppresses both the ts and accelerated aging phenotypes of the cit1 null cells. We also found that inhibition of Yca1 activity with zVAD-fmk suppresses the ts phenotype of Δcit1 cells in a concentration-dependent manner (Figure 6). In contrast to the CIT1 wild-type, Δyca1, and Δcit1Δyca1 cells, which showed negligible heat-induced metacaspase activation, Δcit1 cells exhibited considerable level of metacaspase activation triggered by heat stress, which consequently caused apoptosis (Figure 7). These results lead us to conclude that the ts and accelerated aging phenotype of cit1 deletion mutants is caused by Yca1-mediated apoptosis.
Similar observations have been reported for the involvement of yeast Yca1 in apoptotic cell death. Bettiga et al. (2004) reported that disruption of the UBP10 gene encoding a deubiquitinating enzyme of S. cerevisiae results in a complex phenotype characterized by a subpopulation of cells that exhibits the typical cellular markers of apoptosis and that the phenotype of ubp10 disruptant is suppressed by an additional deletion of YCA1. Consistent with these findings, it was shown that deletion of YCA1 leads to a large H2O2-dependent accumulation of oxidized proteins and up-regulation of 20S proteasome activity and that apoptosis is abrogated in the Δyca1 strain, whereas the YCA1 wild-type undergoes apoptosis as measured by externalization of phosphatidylserine and the display of TUNEL-positive nuclei (Khan et al., 2005). It was also demonstrated that in the presence of 25 mM valproic acid, which inhibits the growth of yeast in a dose-dependent manner with complete inhibition attained at 100 mM, YCA1-deleted cells do not show any signs of apoptosis, whereas YCA1 wild-type cells die showing apoptotic markers (Mitsui et al., 2005).
To eliminate the harmful effect of ROS, cells contain effective defense mechanisms, including enzymes such as catalase, superoxide dismutase, and GSH peroxidase, and antioxidants such as GSH and vitamins C and E (Yu, 1994). The superoxide radical is dismutated to H2O2 by Cu/Zn-dependent (Bermingham-McDonogh et al., 1988) or Mn-dependent variant superoxide dismutase (Chang and Kosman, 1989). Detoxification of H2O2, which gives rise to the highly reactive hydroxyl radical in the presence of transition metals, can be achieved by catalase (Grant et al., 1998) and by various types of peroxidase, which generate water and molecular oxygen (Inoue et al., 1999; Kowaltowski et al., 2000). Many peroxidases use GSH to reduce H2O2 and a multitude of organic hydroperoxides (Galiazzo et al., 1987; Inoue et al., 1999); thus, GSH is required for protection against oxidative stress (Grant et al., 1996b; Stephen and Jamieson, 1996) and heat stress (Sugiyama et al., 2000). To address whether accumulation of ROS in cit1 deletion mutants under stress conditions results from the depletion of GSH, we analyzed the intracellular levels of GSH in the mutant and wild-type cells. Cells carrying the deletion of CIT1 were subject to depletion of GSH when grown in SCD medium. However, these cells exhibited similar levels of GSH as wild-type cells in SGH-supplemented SDC (Table 2). Consistently, cit1 deletion mutants grown in the presence of GSH did not show the ts phenotype or heat stress-induced ROS accumulation (Figure 9). These results have led us to propose that the deletion of CIT1 causes depletion of intracellular GSH, which results in the accumulation of ROS and accompanying apoptosis under stress conditions.
GSH is one of the most prevalent reducing thiol compounds in nearly every aerobic organism, and it performs diverse functions in many cellular processes, including amino-acid transport, synthesis of protein and DNA, modulation of enzyme activity, detoxification of damaging molecules, and maintenance of intracellular redox state (Douglas, 1987). One of the most important functions of GSH is its activity as an antioxidant. GSH serves as an electron donor for antioxidant enzymes such as glutaredoxin and GSH peroxidase (Grant, 2001). The intracellular levels of GSH are maintained by a complex series of reactions that balance its rate of synthesis and reduction. GSH is synthesized via two consecutive ATP-dependent reactions, i.e., formation of the dipeptide γ-glutamylcysteine (l-γ-Glu-Cys) from glutamate and cysteine catalyzed by γ-glutamylcysteine synthetase (Gsh1) and ligation of glycine with l-γ-Glu-Cys to yield GSH catalyzed by GSH synthetase (Gsh2) (Ohtake and Yabuuchi, 1991; Inoue et al., 1998). Reduction of GSSG to GSH is efficiently mediated by GR, by using NADPH specifically as a reducing equivalent. In S. cerevisiae, GSH is critically required for aerobic survival, and GR is required to protect cells against oxidative stress (Grant et al., 1996a). To determine whether the biosynthetic process of GSH is defective in the mutants in which the CIT1 gene was deleted, we examined the effects of several amino acids used as precursors for GSH synthesis, such as glutamate, glycine, and cysteine, on the survival of the yeast strains under heat stress conditions. The cells of cit1 deletion mutants grown in SCD supplemented with 10 mM glutamate showed similar survival rates as wild-type cells after exposure to the heat stress at 50°C (Figure 10). In contrast, addition of either 10 mM cysteine or glycine did not rescue the ts phenotype of the cit1 deletion mutants. These results suggest that depletion of GSH, which occurs in cells with the cit1 deletion that are exposed to heat stress, is caused by a shortage of glutamate but not by cysteine or glycine deficiencies.
In normal aerobic conditions, yeast cells in exponential phase that have been grown on rich media have a high redox ratio (GSH/GSSG) of ∼11–16:1, which supports the view that the majority of total intracellular glutathione is maintained in a reduced form, GSH (Grant et al., 1996a; Grant, 2001). Exposure of yeast cells to oxidative stress, such as H2O2, causes a reduction in GSH levels, as well as a shift in the redox balance toward the more oxidized form, GSSG (Grant et al., 1998). This increase in GSSG is consistent with the role of GSH as both a free radical scavenger and a cofactor for various antioxidant enzymes, including GSH peroxidase, GSH S-transferases, and glutaredoxins (Grant, 2001). GSSG is reduced to GSH via a multistep reaction catalyzed by GR, which is essential for the GSH redox cycle that maintains adequate levels of reduced cellular GSH. For reduction of GSSG, GR should be initially reduced by NADPH, and the reduced enzyme, in turn, reacts with a molecule of GSSG, resulting in two consecutive disulfide interchanges to yield two molecules of GSH and restoring of the enzyme to the oxidized form (Massey and Williams, 1965). The ts phenotype of the cit1 deletion mutants was noticeably rescued by 10 mM of exogenous GSSG (Figure 10), which supports the view that the reduction of GSSG to GSH catalyzed by NADPH-dependent GR is functional in the cells carrying the cit1 deletion. Consequently, it can be concluded that the deficiency of GSH observed in the mutant cells with the cit1 deletion does not result from the depletion of reducing power required for the GR reaction, but instead it is due to an insufficient supply of glutamate, which is necessary for biosynthesis of GSH.
ACKNOWLEDGMENTS
This work was supported by the KOSEF R01-2003-000-10162-0, the New University for Regional Innovation, and the 2nd stage of Brain Korea 21 projects.
Abbreviations used:
- CFU
colony-forming unit
- CS
citrate synthase
- DCFH-DA
2′,7′-dichlorodihydrofluorescein diacetate
- DCF
2′,7′-dichlorofluorescein diacetate
- DTNB
5,5′-dithiobis-(2-nitrobenzoic acid)
- GR
glutathione reductase
- GSH
glutathione
- GSSG
glutathione disulfide
- ROS
reactive oxygen species
- SCD
synthetic complete glucose
- TCA
tricarboxylic acid
- ts
temperature-sensitive
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick-end labeling.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-02-0118) on July 5, 2007.
REFERENCES
- Anderson M. E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O., Gralla E. B., Valentine J. S. The copper, zinc-superoxide dismutase gene of Saccharomyces cerevisiae: cloning, sequencing, and biological activity. Proc. Natl. Acad. Sci. USA. 1988;85:4789–4793. doi: 10.1073/pnas.85.13.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettiga M., Calzari L., Orlandi I., Alberghina L., Vai M. Involvement of the yeast metacaspase Yca1 in ubp10Δ-programmed cell death. FEMS Yeast Res. 2004;5:141–147. doi: 10.1016/j.femsyr.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Chang E. C., Kosman D. J. Intracellular Mn (II)-associated superoxide scavenging activity protects Cu, Zn superoxide dismutase-deficient Saccharomyces cerevisiae against dioxygen stress. J. Biol. Chem. 1989;264:12172–12178. [PubMed] [Google Scholar]
- Douglas K. T. Mechanism of action of glutathione-dependent enzymes. Adv. Enzymol. Relat. Areas Mol. Biol. 1987;59:103–167. doi: 10.1002/9780470123058.ch3. [DOI] [PubMed] [Google Scholar]
- Fabrizio P., Battistella L., Vardavas R., Gattazzo C., Liou L. L., Diaspro A., Dossen J. W., Gralla E. B., Longo V. D. Superoxide is a mediator of an altruistic aging program in Saccharomyces cerevisiae. J. Cell Biol. 2004;166:1055–1067. doi: 10.1083/jcb.200404002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannjiang Y., Cheng W. C., Lee S. J., Qi B., Pevsner J., McCaffery J. M., Hill R. B., Basanez G., Hardwick J. M. Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev. 2004;18:2785–2797. doi: 10.1101/gad.1247904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich K. U., Madeo F. Apoptosis in yeast-a monocellular organism exhibits altruistic behaviour. FEBS Lett. 2000;473:6–9. doi: 10.1016/s0014-5793(00)01474-5. [DOI] [PubMed] [Google Scholar]
- Galiazzo F., Schiesser A., Rotilio G. Glutathione peroxidase in yeast. Presence of the enzyme and induction by oxidative conditions. Biochem. Biophys. Res. Commun. 1987;147:1200–1205. doi: 10.1016/s0006-291x(87)80197-3. [DOI] [PubMed] [Google Scholar]
- Grant C. M. Role of the glutathione/glutaredoxin and thioredoxin systems in yeast growth and response to stress conditions. Mol. Microbiol. 2001;39:533–541. doi: 10.1046/j.1365-2958.2001.02283.x. [DOI] [PubMed] [Google Scholar]
- Grant C. M., Collinson L. P., Roe J. H., Dawes I. W. Yeast glutathione reductase is required for protection against oxidative stress and is a target gene for yAP-1 transcriptional regulation. Mol. Microbiol. 1996a;21:171–179. doi: 10.1046/j.1365-2958.1996.6351340.x. [DOI] [PubMed] [Google Scholar]
- Grant C. M., MacIver F. H., Dawes I. W. Glutathione is an essential metabolite required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. Curr. Genet. 1996b;29:511–515. doi: 10.1007/BF02426954. [DOI] [PubMed] [Google Scholar]
- Grant C. M., Perrone G., Dawes I. W. Glutathione and catalase provide overlapping defenses for protection against hydrogen peroxide in the yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 1998;253:893–898. doi: 10.1006/bbrc.1998.9864. [DOI] [PubMed] [Google Scholar]
- Grigorenko E. V., Small W. C., Persson L. O., Srere P. A. Citrate synthase 1 interacts with the citrate transporter of yeast mitochondria. J. Mol. Recognit. 1990;3:215–219. doi: 10.1002/jmr.300030508. [DOI] [PubMed] [Google Scholar]
- Hauptmann P., Riel C., Kunz-Schughart L. A., Frohlich K. U., Madeo F., Lehle L. Defects in N-glycosylation induce apoptosis in yeast. Mol. Microbiol. 2006;59:765–778. doi: 10.1111/j.1365-2958.2005.04981.x. [DOI] [PubMed] [Google Scholar]
- Herker E., Jungwirth H., Lehmann K. A., Maldener C., Frohlich K. U., Wissing S., Buttner S., Fehr M., Sigrist S., Madeo F. Chronological aging leads to apoptosis in yeast. J. Cell Biol. 2004;164:501–507. doi: 10.1083/jcb.200310014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh G. H., Damsz B., Matsumoto T. K., Reddy M. P., Rus A. M., Ibeas J. I., Narasimhan M. L., Bressan R. A., Hasegawa P. M. Salt causes ion disequilibrium-induced programmed cell death in yeast and plants. Plant J. 2002;29:649–659. doi: 10.1046/j.0960-7412.2001.01247.x. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Matsuda T., Sugiyama K., Izawa S., Kimura A. Genetic analysis of glutathione peroxidase in oxidative stress response of Saccharomyces cerevisiae. J. Biol. Chem. 1999;274:27002–27009. doi: 10.1074/jbc.274.38.27002. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Sugiyama K., Izawa S., Kimura A. Molecular identification of glutathione synthetase (GSH2) gene from Saccharomyces cerevisiae. Biochim. Biophys. Acta. 1998;1395:315–320. doi: 10.1016/s0167-4781(97)00199-1. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. A., Chock P. B., Stadtman E. R. Knockout of caspase-like gene, YCA1, abrogates apoptosis and elevates oxidized proteins in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 2005;102:17326–17331. doi: 10.1073/pnas.0508120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Rosenkrantz M. S., Guarente L. Saccharomyces cerevisiae contains two functional citrate synthase genes. Mol. Cell. Biol. 1986;6:1936–1942. doi: 10.1128/mcb.6.6.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispal G., Rosenkrantz M., Guarente L., Srere P. A. Metabolic changes in Saccharomyces cerevisiae strains lacking citrate synthases. J. Biol. Chem. 1988;263:11145–11149. [PubMed] [Google Scholar]
- Kowaltowski A. J., Vercesi A. E., Rhee S. G., Netto L. E. Catalases and thioredoxin peroxidase protect Saccharomyces cerevisiae against Ca2+-induced mitochondrial membrane permeabilization and cell death. FEBS Lett. 2000;473:177–182. doi: 10.1016/s0014-5793(00)01526-x. [DOI] [PubMed] [Google Scholar]
- Lam E., Pontier D., del Pozo O. Die and let live–programmed cell death in plants. Curr. Opin. Plant Biol. 1999;2:502–507. doi: 10.1016/s1369-5266(99)00026-6. [DOI] [PubMed] [Google Scholar]
- Laun P., Pichova A., Madeo F., Fuchs J., Ellinger A., Kohlwein S., Dawes I., Frohlich K. U., Breitenbach M. Aged mother cells of Saccharomyces cerevisiae show markers of oxidative stress and apoptosis. Mol. Microbiol. 2001;39:1166–1173. [PubMed] [Google Scholar]
- Lee J. G., Cho S. P., Lee H. S., Lee C. H., Bae K. S., Maeng P. J. Identification of a cryptic N-terminal signal in Saccharomyces cerevisiae peroxisomal citrate synthase that functions in both peroxisomal and mitochondrial targeting. J. Biochem. 2000;128:1059–1072. doi: 10.1093/oxfordjournals.jbchem.a022834. [DOI] [PubMed] [Google Scholar]
- Lee J. G., Lee Y. J., Lee C. H., Maeng P. J. Mutational and functional analysis of the cryptic N-terminal targeting signal for both mitochondria and peroxisomes in yeast peroxisomal citrate synthase Cit2p. J. Biochem. 2006;140:121–133. doi: 10.1093/jb/mvj136. [DOI] [PubMed] [Google Scholar]
- Lewin A. S., Hines V., Small G. M. Citrate synthase encoded by the CIT2 gene of Saccharomyces cerevisiae is peroxisomal. Mol. Cell. Biol. 1990;10:1399–1405. doi: 10.1128/mcb.10.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligr M., Madeo F., Frohlich E., Hilt W., Frohlich K. U., Wolf D. H. Mammalian Bax triggers apoptotic changes in yeast. FEBS Lett. 1998;438:61–65. doi: 10.1016/s0014-5793(98)01227-7. [DOI] [PubMed] [Google Scholar]
- Madeo F., Frohlich E., Frohlich K. U. A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell Biol. 1997;139:729–734. doi: 10.1083/jcb.139.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F., Frohlich E., Ligr M., Grey M., Sigrist S. J., Wolf D. H., Frohlich K. U. Oxygen stress: a regulator of apoptosis in yeast. J. Cell Biol. 1999;145:757–767. doi: 10.1083/jcb.145.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F., et al. A caspase-related protease regulates apoptosis in yeast. Mol. Cell. 2002;9:911–917. doi: 10.1016/s1097-2765(02)00501-4. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Newmeyer D. D., Mathias S., Farschon D. M., Wang H. G., Reed J. C., Kolesnick R. N., Green D. R. Cell-free reconstitution of Fas-, UV radiation- and ceramide-induced apoptosis. EMBO J. 1995;14:5191–5200. doi: 10.1002/j.1460-2075.1995.tb00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V., Williams C. H., Jr On the reaction mechanism of yeast glutathione reductase. J. Biol. Chem. 1965;240:4470–4480. [PubMed] [Google Scholar]
- Mitsui K., Nakagawa D., Nakamura M., Okamoto T., Tsurugi K. Valproic acid induces apoptosis dependent of Yca1p at concentrations that mildly affect the proliferation of yeast. FEBS Lett. 2005;579:723–727. doi: 10.1016/j.febslet.2004.12.051. [DOI] [PubMed] [Google Scholar]
- Ohtake Y., Yabuuchi S. Molecular cloning of the gamma-glutamylcysteine synthetase gene of Saccharomyces cerevisiae. Yeast. 1991;7:953–961. doi: 10.1002/yea.320070907. [DOI] [PubMed] [Google Scholar]
- Pastorino J. G., Chen S. T., Tafani M., Snyder J. W., Farber J. L. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J. Biol. Chem. 1998;273:7770–7775. doi: 10.1074/jbc.273.13.7770. [DOI] [PubMed] [Google Scholar]
- Qi H., Li T. K., Kuo D., Nur E.K.A., Liu L. F. Inactivation of Cdc13p triggers MEC1-dependent apoptotic signals in yeast. J. Biol. Chem. 2003;278:15136–15141. doi: 10.1074/jbc.M212808200. [DOI] [PubMed] [Google Scholar]
- Severin F. F., Hyman A. A. Pheromone induces programmed cell death in S. cerevisiae. Curr. Biol. 2002;12:R233–R235. doi: 10.1016/s0960-9822(02)00776-5. [DOI] [PubMed] [Google Scholar]
- Silva R. D., Sotoca R., Johansson B., Ludovico P., Sansonetty F., Silva M. T., Peinado J. M., Corte-Real M. Hyperosmotic stress induces metacaspase- and mitochondria-dependent apoptosis in Saccharomyces cerevisiae. Mol. Microbiol. 2005;58:824–834. doi: 10.1111/j.1365-2958.2005.04868.x. [DOI] [PubMed] [Google Scholar]
- Stephen D. W., Jamieson D. J. Glutathione is an important antioxidant molecule in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1996;141:207–212. doi: 10.1111/j.1574-6968.1996.tb08386.x. [DOI] [PubMed] [Google Scholar]
- Sugiyama K., Kawamura A., Izawa S., Inoue Y. Role of glutathione in heat-shock-induced cell death of Saccharomyces cerevisiae. Biochem. J. 2000;352:71–78. [PMC free article] [PubMed] [Google Scholar]
- Suissa M., Suda K., Schatz G. Isolation of the nuclear yeast genes for citrate synthase and fifteen other mitochondrial proteins by a new screening method. EMBO J. 1984;3:1773–1781. doi: 10.1002/j.1460-2075.1984.tb02045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velot C., Lebreton S., Morgunov I., Usher K. C., Srere P. A. Metabolic effects of mislocalized mitochondrial and peroxisomal citrate synthases in yeast Saccharomyces cerevisiae. Biochemistry. 1999;38:16195–16204. doi: 10.1021/bi991695n. [DOI] [PubMed] [Google Scholar]
- Velot C., Mixon M. B., Teige M., Srere P. A. Model of a quinary structure between Krebs TCA cycle enzymes: a model for the metabolon. Biochemistry. 1997;36:14271–14276. doi: 10.1021/bi972011j. [DOI] [PubMed] [Google Scholar]
- Velot C., Srere P. A. Reversible transdominant inhibition of a metabolic pathway. In vivo evidence of interaction between two sequential tricarboxylic acid cycle enzymes in yeast. J. Biol. Chem. 2000;275:12926–12933. doi: 10.1074/jbc.275.17.12926. [DOI] [PubMed] [Google Scholar]
- Wadskog I., Maldener C., Proksch A., Madeo F., Adler L. Yeast lacking the SRO7/SOP1-encoded tumor suppressor homologue show increased susceptibility to apoptosis-like cell death on exposure to NaCl stress. Mol. Biol. Cell. 2004;15:1436–1444. doi: 10.1091/mbc.E03-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki R., Kron S. J. Yeast cell death during DNA damage arrest is independent of caspase or reactive oxygen species. J. Cell Biol. 2004;166:311–316. doi: 10.1083/jcb.200405016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B. P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994;74:139–162. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]