Abstract
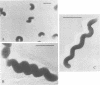
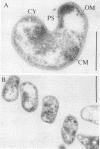
The importance of Leptospirillum ferrooxidans for leach processes has been evaluated by studying the lithotrophic flora of three mine biotopes and a heap leaching operation, by percolation experiments with inoculated, sterilized ore, and by morphological, physiological, and genetic investigations of pure and mixed cultures of L. ferrooxidans, Thiobacillus ferrooxidans, and Thiobacillus thiooxidans. In biotopes of 20°C or above, Leptospirillum-like bacteria are as abundant as T. ferrooxidans. Leptospirilli represent at least one-half of the ferrous-iron-oxidizing population. Percolation experiments confirmed this result. Leptospirilli were as numerous as T. ferrooxidans. At reduced temperatures, the generation times of leptospirilli increase more so than those of T. ferrooxidans. At 14°C, Leptospirillum grows slowly and T. ferrooxidans dominates the population. Physiological investigations indicate that L. ferrooxidans is a strict chemolithoautotroph, metabolizing only ferrous iron and pyrite. Even an addition of 0.05% (wt/vol) yeast extract inhibited its growth. The maximum ferrous-iron-oxidizing activity of L. ferrooxidans amounts to about 40% of the activity of T. ferrooxidans. After growth on sulfidic ore, both species exhibit reduced iron-oxidizing activities, L. ferrooxidans exhibiting one-third and T. ferrooxidans exhibiting one-seventh of their maximum activities. Surprisingly, the absolute values are similar. For indirect leaching, L. ferrooxidans is as important as T. ferrooxidans. This was confirmed by the results of percolation experiments. L. ferrooxidans together with T. thiooxidans mobilized metals at least as well as T. ferrooxidans did. The best results were obtained with a mixed culture of all three species.
Full text
PDF

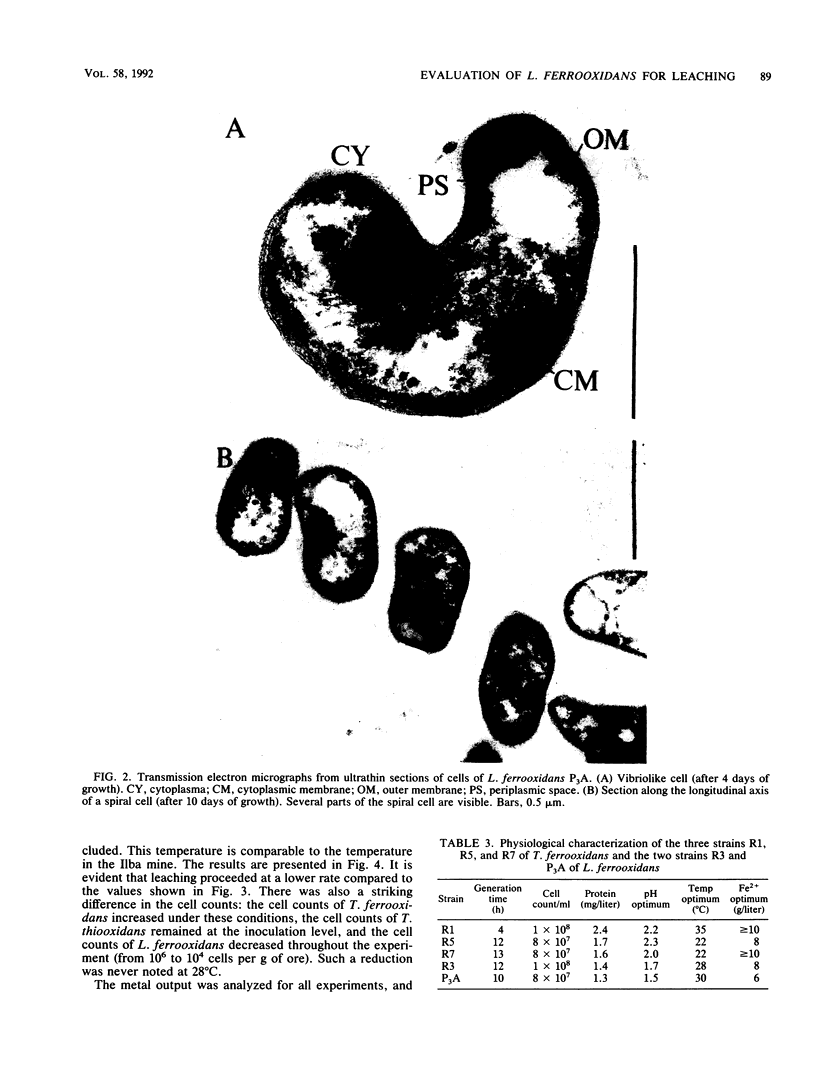
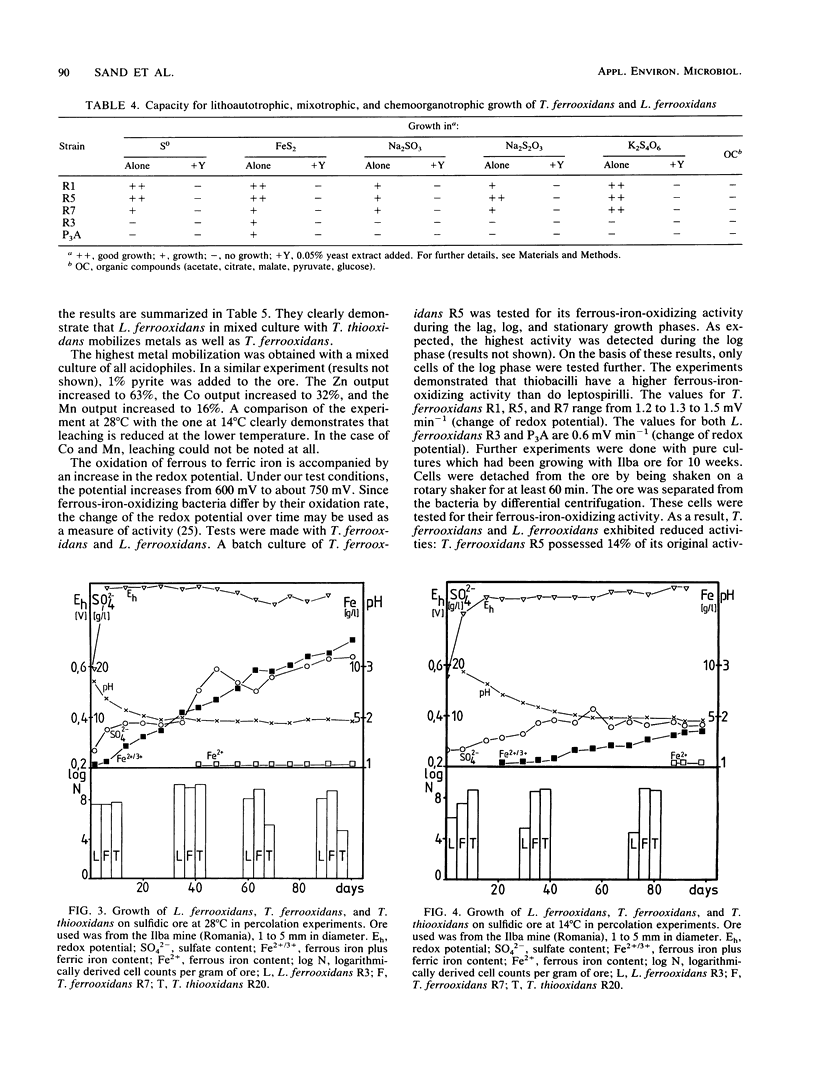


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahonen L., Tuovinen O. H. Microbiological oxidation of ferrous iron at low temperatures. Appl Environ Microbiol. 1989 Feb;55(2):312–316. doi: 10.1128/aem.55.2.312-316.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- EHRLICH H. L. MICROORGANISMS IN ACID DRAINAGE FROM A COPPER MINE. J Bacteriol. 1963 Aug;86:350–352. doi: 10.1128/jb.86.2.350-352.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A. P., Jr Microbial succession and mineral leaching in an artificial coal spoil. Appl Environ Microbiol. 1978 Dec;36(6):861–869. doi: 10.1128/aem.36.6.861-869.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins S. R., Davidson M. S., Brierley J. A., Brierley C. L. Microorganisms in reclamation of metals. Annu Rev Microbiol. 1986;40:311–336. doi: 10.1146/annurev.mi.40.100186.001523. [DOI] [PubMed] [Google Scholar]
- Hutchinson M., Johnstone K. I., White D. The taxonomy of certain thiobacilli. J Gen Microbiol. 1965 Dec;41(3):357–366. doi: 10.1099/00221287-41-3-357. [DOI] [PubMed] [Google Scholar]
- Koops H. P., Harms H. Deoxyribonucleic acid homologies among 96 strains of ammonia-oxidizing bacteria. Arch Microbiol. 1985 Apr;141(3):214–218. doi: 10.1007/BF00408061. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Muyzer G., de Bruyn A. C., Schmedding D. J., Bos P., Westbroek P., Kuenen G. J. A Combined Immunofluorescence-DNA-Fluorescence Staining Technique for Enumeration of Thiobacillus ferrooxidans in a Population of Acidophilic Bacteria. Appl Environ Microbiol. 1987 Apr;53(4):660–664. doi: 10.1128/aem.53.4.660-664.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand W. Importance of Hydrogen Sulfide, Thiosulfate, and Methylmercaptan for Growth of Thiobacilli during Simulation of Concrete Corrosion. Appl Environ Microbiol. 1987 Jul;53(7):1645–1648. doi: 10.1128/aem.53.7.1645-1648.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. M., Bock E., Westphal K., Cannon G. C. Icosahedral inclusions (carboxysomes) of Nitrobacter agilis. J Bacteriol. 1977 Nov;132(2):673–675. doi: 10.1128/jb.132.2.673-675.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]