Abstract
We have previously shown that Ca2+ directly activates ATP-sensitive microtubule binding by a Chlamydomonas outer arm dynein subparticle containing the β and γ heavy chains (HCs). The γ HC–associated LC4 light chain is a member of the calmodulin family and binds 1-2 Ca2+ with KCa = 3 × 10−5 M in vitro, suggesting it may act as a Ca2+ sensor for outer arm dynein. Here we investigate interactions between the LC4 light chain and γ HC. Two IQ consensus motifs for binding calmodulin-like proteins are located within the stem domain of the γ heavy chain. In vitro experiments indicate that LC4 undergoes a Ca2+-dependent interaction with the IQ motif domain while remaining tethered to the HC. LC4 also moves into close proximity of the intermediate chain IC1 in the presence of Ca2+. The sedimentation profile of the γ HC subunit changed subtly upon Ca2+ addition, suggesting that the entire complex had become more compact, and electron microscopy of the isolated γ subunit revealed a distinct alteration in conformation of the N-terminal stem in response to Ca2+ addition. We propose that Ca2+-dependent conformational change of LC4 has a direct effect on the stem domain of the γ HC, which eventually leads to alterations in mechanochemical interactions between microtubules and the motor domain(s) of the outer dynein arm.
INTRODUCTION
Eukaryotic cilia and flagella are highly conserved organelles involved in cellular motility, fluid transport, and development. Motile cilia/flagella are powered by dynein motor proteins that form the inner and outer rows of arms attached to the outer doublet microtubules. These enzymes contain one or more heavy chain (HC) motor units associated with a variety of other components that serve to attach the motor at the appropriate location within the flagellum and to regulate activity so as to result in coordinated movement. Dynein HCs are members of the AAA+ family of ATPases and consist of an N-terminal stem region necessary for assembly, and a motor unit that contains six AAA+ domains and a C-terminal globular segment arranged in a heptameric ring (Samso et al., 1998; King, 2000; Samso and Koonce, 2004). The ATP-sensitive microtubule-binding site is located at the tip of a coiled-coil stalk protruding from between the AAA4 and AAA5 subdomains. In general, the region N-terminal of AAA1 is poorly conserved between the various axonemal and cytoplasmic dyneins and is involved in HC-HC interactions as well as association with various intermediate (ICs), light intermediate chains, and light chains (LCs) that may confer chain-specific regulatory and cargo attachment functions (for review see King, 2002).
To generate coordinated flagellar beating, dynein motor activity must be tightly controlled. Ca2+-regulated waveform alterations have been observed in the flagella of various cells including Paramecium (Naitoh and Kaneko, 1972), and sea urchin (Brokaw et al., 1974) and mammalian (Lindemann and Goltz, 1988) sperm. In demembranated and reactivated Chlamydomonas cell models, the cis- and trans- flagellar axonemes respond differentially to variations in Ca2+ concentration in the range pCa 8 to pCa 61 (Kamiya and Witman, 1984). Modulation of intraflagellar Ca2+ in the submicromolar range allows the cell to undergo phototaxis (directed movement toward or away from a light source), and mutant studies indicate that this control system requires the inner, but not outer, row of dynein arms (Kamiya and Okamoto, 1985; Mitchell and Rosenbaum, 1985). In addition, reactivated Chlamydomonas axonemes display an asymmetric beat pattern at Ca2+ concentrations below pCa 6, become quiescent at pCa 5, and then resume beating, but with a symmetric waveform at pCa 4 (Bessen et al., 1980). This waveform conversion provides the physiological basis for the photophobic (avoidance) response and either does not occur or is aberrant in strains lacking outer arms (Kamiya and Okamoto, 1985; Mitchell and Rosenbaum, 1985), suggesting that this motor is essential for flagellar reversal.
The Chlamydomonas outer arm contains three HCs (α, β, and γ) that have distinct assembly and enzymatic properties (Pfister et al., 1982; Pfister and Witman, 1984; Sakakibara et al., 1991, 1993). These motor units are associated with two WD-repeat intermediate chains (IC1 and IC2), at least 10 light chains (LCs), and a trimeric docking complex (DC) necessary for attachment of the arm to the appropriate axonemal location (Takada and Kamiya, 1994). We demonstrated previously that ATP-sensitive microtubule binding by an outer arm dynein subparticle containing only the β and γ HCs can be maximally activated above pCa 6 (Sakato and King, 2003). This observation suggested that outer arm dynein function is regulated by Ca2+ binding directly to a component of the motor complex in vitro.
The purified Chlamydomonas outer arm contains two potential candidates for this putative Ca2+ regulatory subunit. The docking complex protein DC3 has two consensus EF hands and binds one Ca2+ with Kd = 1 × 10−5 M in a redox-sensitive manner; it also binds Mg2+ but with a much lower affinity (Casey et al., 2003a,b). However, a DC3-null mutant (oda14) rescued with a defective form of DC3 that cannot bind Ca2+ displays apparently normal photobehavior, suggesting that Ca2+-binding by this protein is not required for these responses (Casey et al., 2003a). The LC4 light chain, which directly associates with the γ HC, is also related to calmodulin and contains four helix-loop-helix motifs, two of which conform to the EF hand consensus for Ca2+-binding loops (Pfister et al., 1982; King and Patel-King, 1995). This LC binds 1-2 Ca2+ with KCa = 3 × 10−5 M in vitro; it does not bind Mg2+ (King and Patel-King, 1995). The phenotypic consequences due to a lack of LC4 are unknown, because no mutants defective for this protein currently exist. However, the direct association of LC4 with a HC makes it a promising candidate for an outer arm Ca2+ sensor. Here we focus on the detailed interactions between LC4 and the γ HC and explore the molecular mechanism by which conformational alterations involving LC4 might regulate dynein motor activity in response to Ca2+ binding.
MATERIALS AND METHODS
Strains and Culture Conditions
The Chlamydomonas reinhardtii strains used in this study are detailed in Table 1. These strains (except the oda11 oda4-s7 and oda11 sup1-2 double mutants constructed in this laboratory) are available from the Chlamydomonas Genetics Center (Duke University, Durham, NC). Cells were cultured in R medium (M medium plus 0.0075 M sodium acetate) with a light/dark cycle of 15 h/9 h and constant aeration (Witman, 1986).
Table 1.
Strains used in this study
| Strain | Description | References |
|---|---|---|
| cc124 | Wild type | |
| ida1 | Defective for 1α HC. Lacks inner arm I1/f | Kamiya et al. (1991) |
| Porter et al. (1992) | ||
| ida4 | Defective for p28. Lacks a subset of inner arms | Kamiya et al. (1991) |
| LeDizet and Piperno (1995) | ||
| oda2 | Defective for γ HC. Lacks outer arms | Kamiya (1988) |
| Wilkerson et al. (1994) | ||
| oda3 | Defective for DC1. Lacks outer arms and the outer arm docking complex | Kamiya (1988) |
| Koutoulis et al. (1997) | ||
| oda4-s7 | Expresses truncated β HC lacking motor domain | Sakakibara et al. (1993) |
| oda11 oda4-s7 | Double mutant defective for α and β HCs. Contains only intact γ HC | Sakakibara et al. (1991, 1993) |
| oda11 | Defective for α HC. Lacks α HC and LC5 | Sakakibara et al. (1991) |
| oda11 sup1-2 | Double mutant that lacks the α HC and contains a suppressor mutation within the microtubule binding stalk of the β HC | Sakakibara et al. (1991) |
| sup2 (sup-pf2) | Suppressor mutation within the γ HC that restores motility to strains paralyzed due to lack of radial spokes and central pair complex | Huang et al. (1982) |
| Rupp et al. (1996) | ||
| pf14 | Defective for RSP3. Lacks radial spokes. Paralyzed flagella | Huang et al. (1981) |
| pf18 | Lacks central pair microtubule complex. Paralyzed flagella | Adams et al. (1981) |
Preparation of Flagellar Axonemes and Dynein
Flagellar axonemes were prepared by standard methods (Witman, 1986). Intact outer arm dynein (αβγ HCs) and an α-γ HC subparticle that lacks the β HC motor unit were extracted from ida1 and oda4-s7 mutant strains, respectively. Dyneins were purified by sucrose density gradient centrifugation in the presence of Mg2+ and at low hydrostatic pressure as previously described (Takada et al., 1992; Sakato and King, 2003). To obtain αβ and γ HC subparticles, sucrose was removed from purified intact outer arm dynein by ultrafiltration, and the sample was subjected to a second sucrose gradient centrifugation in the absence of Mg2+. Peak fractions containing each subparticle were pooled and kept at −75°C until use.
Coimmunoprecipitation of Preassembled Dynein Subunits from Cytoplasm
Cytoplasmic extract preparation and coimmunoprecipitation were performed by following the method of Fowkes and Mitchell (1998) with minor modifications including autolysin treatment (Qin et al., 2004). Chlamydomonas cells were grown to a density of ∼1.0 × 106 cells/ml in 500 ml liquid medium, harvested, treated with autolysin, and resuspended in immunoprecipitation (IP) buffer (30 mM HEPES, pH 7.4, 5 mM MgSO4, 0.5 mM EDTA, 25 mM KCl, 1 mM dithiothreitol [DTT]) plus a 1/100× volume of protease inhibitor cocktail (P8849, Sigma, St. Louis, MO) to a total volume of ∼0.5 ml. The cell suspension was homogenized with an equal volume of acid-washed glass beads (diameter ∼1 mm) by vortexing for 1 min. The homogenate was clarified in a TLA100.2 rotor (Beckman, Fullerton, CA) at 33,000 rpm for 2 h at 4°C. The clarified cytoplasmic extract was supplemented with 75 mM NaCl and 0.05% Triton X-100 and incubated with CT240 antibody (generated in this study) or preimmune serum for 1 h at 4°C and for 1 more hour after the addition of 10 μl settled volume of ImmunoPure Immobilized protein G Plus beads (Pierce Biotechnology, Rockford, IL). The beads were washed three times with IP buffer containing 75 mM NaCl and 0.05% Triton X-100 and once with IP buffer only. The immunoprecipitates were eluted by adding 2× gel loading buffer (0.1 M Tris-Cl, pH 6.8, 0.2 M DTT, 4% SDS, 0.2% bromophenol blue, and 20% glycerol) and boiling. Twenty micrograms of cytoplasmic extracts and equal volumes of immunoprecipitates were analyzed by electrophoresis and immunoblotting.
Ca2+ Effects on γ HC Subparticle Sedimentation
The purified γ HC subparticle was fractionated in a 5–20% sucrose gradient in HME buffer (30 mM HEPES, pH 7.4, 5 mM MgSO4, 1 mM EGTA) containing 1 mM DTT and 1 mM phenylmethylsulfonyl fluoride either in the absence of Ca2+, or at pCa 5 or pCa 3, in a SW55 rotor (Beckman) at 30,000 rpm for 12 h at 4°C. Appropriate amounts of a CaCl2 stock solution were added to yield the desired Ca2+ concentration. Fifteen fractions of 350 μl were collected from each gradient. Recombinant LC4 protein (see below) was sedimented in parallel with the γ HC subparticle to confirm that this LC does not migrate at 12S in the absence of the HC. As additional controls, bovine brain tubulin (Cytoskeleton, Denver, CO), and the outer arm αβ subunits were also sedimented in additional gradients. Equal volumes of each fraction were electrophoresed in 10% tricine SDS gels, 8% SDS gels, and 4% acrylamide 4 M urea gels and transferred to nitrocellulose for immunoblotting. Densitometric analysis was performed using ImageJ.
Preparation of Recombinant Proteins and Antibodies
Using the full-length γ HC cDNA2 (constructed by Dr. C. G. Wilkerson, Michigan State University.) as template, the following fragments of the γ HC stem domain were amplified with Pfu DNA polymerase (Stratagene, La Jolla, CA) and cloned into the pMAL-c2 vector (New England Biolabs, Ipswich, MA); residues 1-442, 1-754, 1-1089, 1-1486, 1432-1848, 338-754, 691-1089, 691-1486, 875-893, 875-1167, 875-1182, 890-1167, 890-1182, 1014-1486, and 1164-1182. This resulted in fusion of these regions to the C-terminus of maltose-binding protein (MBP) via a hydrophilic linker containing a Factor Xa cleavage site. Fragments 338-754, 691-1089, 1014-1486, and 691-1486 were either expressed very poorly or showed very limited solubility and could not be used further. The control MBP-LacZ protein derived from the original pMAL-c2 vector; the MBP-LC4 construct was described previously (King and Patel-King, 1995). To generate an N-terminal 10× His-tagged LC4 construct, full-length LC4 was amplified with Pfu DNA polymerase using the original LC4 cDNA (King and Patel-King, 1995) as template and cloned into the pET16b vector (Novagen, Madison, WI).
Recombinant proteins were overexpressed in Escherichia coli strains XL1-Blue (Stratagene) or BL21(DE3)pLysS (Novagen). MBP fusion proteins were purified by amylose affinity chromatography (New England Biolabs). His-tagged LC4 was purified using His-Bind Resin (Novagen). Recombinant LC4 was obtained by digesting MBP-LC4 with Factor Xa and separating the products by anion exchange chromatography using a HiTrap ANX FF column (Amersham Biosciences, Piscataway, NJ) on a Biologic chromatography workstation (Bio-Rad Laboratories, Hercules, CA).
The MBP-LC4 and MBP-γ HC stem domain N1 (residues 1-442) proteins were used as the immunogens to obtain rabbit polyclonal antibodies CT61 and CT240, respectively. Sera were blot-purified against the appropriate recombinant proteins lacking the MBP moiety before use; for some preparations of CT61 His-tagged LC4 was used. Other antibodies used include rabbit polyclonals against LC1 (R5932; Benashski et al., 1999), LC3 (R4930; Patel-King et al., 1996; Harrison et al., 2002), LC5 (R4929; Patel-King et al., 1996), and DC1 (Wakabayashi et al., 2001) and murine monoclonals DM1A (Sigma), 1878A, 18αA, 18βC, and 12γB versus α-tubulin, IC1, and the α, β, and γ HCs, respectively (King et al., 1985, 1986; King and Witman, 1988a; Wilkerson et al., 1994). Immunoblotting was performed as described previously (Harrison et al., 1998).
In Vitro Expression of 35S-labeled Proteins
To generate a LC4 construct for the binding assay, a PstI-XhoI fragment of the original LC4 cDNA was subcloned into pBluescript II KS+ (Stratagene) downstream of the T7 promoter and the SmaI and blunted BstBI sites were ligated to remove a part of the 5′-untranslated region (UTR) that contained additional out-of-frame ATG codons. For the chemical crosslinking experiment, a Kozak sequence was incorporated into the full-length and N-terminal–truncated versions of the LC4 construct to enhance translation initiation at the first AUG.
Radiolabeled LC4 proteins were synthesized using the TnT T7-coupled reticulocyte lysate system (Promega, Madison, WI). Each 50 μl reaction contained the amino acid mixture minus methionine and 20 μCi of EasyTag l-[35S]methionine (PerkinElmer Life Sciences, Boston, MA). Reactions were incubated for 1.5 h at 30°C, chilled on ice, and clarified by centrifugation. Supernatants were pooled and subsequently used for binding assays or chemical crosslinking experiments. In addition to full-length LC4, the translation products from the Δ5′UTR LC4 construct included a series of N-terminal–truncated forms that derived from translation initiation at internal downstream Met residues.
In Vitro LC4-γ HC Binding Assay
MBP fusion proteins containing segments of the γ HC IQ motif region or LacZ were bound to a 100-μl settled volume of amylose beads in 100 μl of HMET (HME buffer plus 0.1% Tween 20) or HMECT (HMEC [HME buffer containing 1 mM Ca2+] and 0.1% Tween 20). The latter buffer was used to ensure that LC4 was fully saturated with Ca2+. Additional control samples containing no MBP fusion protein were processed in parallel. Beads were mixed with 2 μl of the Δ5′UTR LC4 in vitro translation reaction and incubated for 4 h at 4°C. Samples were washed five times with HMET or HMECT buffer, once with HME or HMEC buffer, and resuspended in 20 μl of 4× gel loading buffer. Samples were denatured for 30 min at 56°C, separated in 10–20% acrylamide tricine SDS gels, and stained with Coomassie blue. The 35S-labeled proteins were detected by autoradiography using Fuji super RX film (Tokyo, Japan).
LC4-γ HC Chemical Crosslinking
Five micrograms of MBP protein fused to the γ HC stem fragments, the LacZ control, or no protein, in 40 μl of HME or HMEC buffer containing 1 mM DTT and 10 mM maltose were preincubated with 5 μl of the full-length or N-terminal–truncated form of 35S-LC4 protein for 1 h on ice. Maltose was necessary for the solubility of the MBP-γ HC stem fragment fusion proteins. Crosslinking was initiated by the addition of 2 μl of 200 mM DMP in methanol (final concentration 10 mM), and samples were incubated for 1 h at room temperature; methanol without DMP was used as a control. After incubation, 20 μl of each sample was mixed with 5 μl of 5× gel loading buffer and denatured for 30 min at 56°C. Equal amounts of denatured samples were separated in 8% acrylamide SDS gels and stained with Coomassie blue, and 35S-labeled protein was detected by autoradiography.
Crosslinking of native dynein samples with 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), 1,5-difluoro-2,4-dinitrobenzene (DFDNB), dimethylpimelimidate (DMP), and disuccinimidyl suberate (DSS) and vanadate-mediated photolysis of native dynein HCs was performed as described previously (Benashski and King, 2000). All chemical crosslinking reagents used in this study were obtained from Pierce Biotechnology.
Immunoprecipitation of Crosslinked Products and Mass Spectrometry
Crosslinking of purified outer arm dynein was performed by the addition of 10 mM DMP or solvent only in the presence of 1 mM Ca2+ as described above. The reaction was quenched by 0.1 M Tris-Cl, pH7.4, denatured in 2% SDS, and diluted with more than 20× volume of Tris-buffered saline (TBS; 50 mM Tris-Cl, pH 7.4, 150 mM NaCl) containing 1% Triton X-100 (to reduce the SDS level to below 0.1%). Twenty microliters of settled volume of CT61 antibody-bound protein G Plus beads was added to the diluted sample, incubated with gentle agitation overnight at 4°C, and washed five times with TBS. The immunoprecipitates were eluted by the addition of 40 μl of 2× gel loading buffer and boiling and analyzed by electrophoresis and immunoblotting. Twenty-five microliters of the eluate from the crosslinked sample was separately electrophoresed in an 8% acrylamide SDS gel and stained with Coomassie blue. The crosslinked product band was excised for mass spectrometry. This method is modified from King et al. (1991).
After trypsin digestion, peptides were identified by mass spectrometry at the University of Massachusetts Medical School Proteomic Mass Spectrometry Facility (Worcester, MA).
Negative Stain Electron Microscopy and Single Particle Analysis
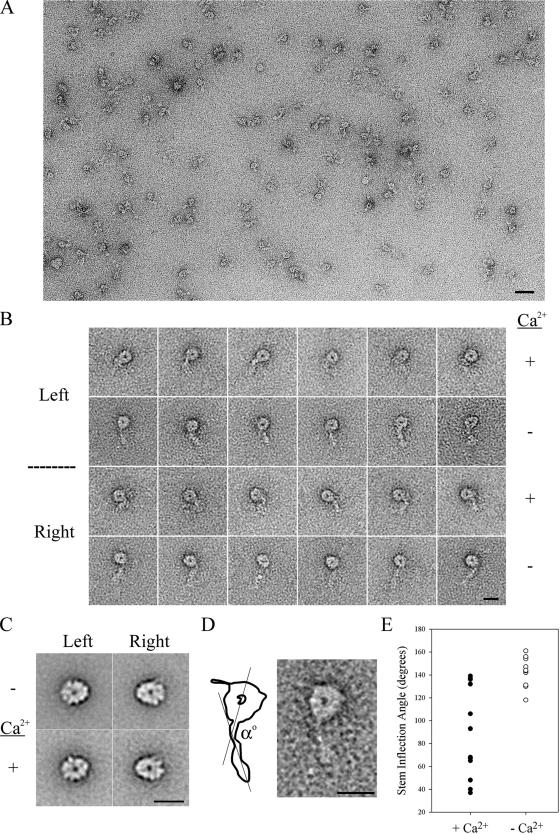
For negative-stain electron microscopy, the γ HC subparticle was isolated by anion-exchange chromatography (Sakakibara and Nakayama, 1998) to avoid the use of sucrose, which adversely affects staining. Before electron microscopic observation, samples were examined by SDS-PAGE and staining with a fluorescent dye (SYPRO Ruby stain, Bio-Rad) to ensure that LC4 had not dissociated from the γ HC during purification. Purified γ HC subparticles were diluted to 20 nM with MMEK buffer (30 mM MOPS-K, 5 mM MgCl2, 1 mM EGTA, 100 mM KCl, pH7.4) with or without 1.1 mM CaCl2 (final concentration), fixed briefly with 2% glutaraldehyde, and applied to carbon-coated copper grids that had been treated with ozone to make the carbon film hydrophilic. Samples on grids were stained with 1.0% (wt/vol) uranyl acetate. Micrographs were taken with a JEM2000EX electron microscope (JEOL, Tokyo, Japan) operated at an accelerating voltage of 80 kV with a nominal magnification of 50,000×. Electron micrographs were digitized on an EPSON GTX-700 scanner (Seiko Epson, Nagano, Japan) at 1000 dpi, corresponding to a pixel size of 0.54 nm on the grid. Digital micrographs were imported into the SPIDER suite of programs (Frank et al., 1996) for all subsequent image processing (Burgess et al., 2003, 2004a,b). For the analysis, we used 703 particles imaged in plus Ca2+ conditions and 582 particles prepared in the absence of Ca2+. The particles were aligned using a reference-free alignment procedure and classified using K-means clustering (Burgess et al., 2004a). We often observed that there is a kink or inflection in the tail of the γ HC subparticle, and we set the kink point to the center of the alignment. Image classification was performed based on images of the N-terminal tail region.
Other Computational Methods
The γ HC IQ motifs were identified using the calmodulin target database (http://calcium.uhnres.utoronto.ca/; Yap et al., 2000).
RESULTS
LC4 Associates with the γ HC
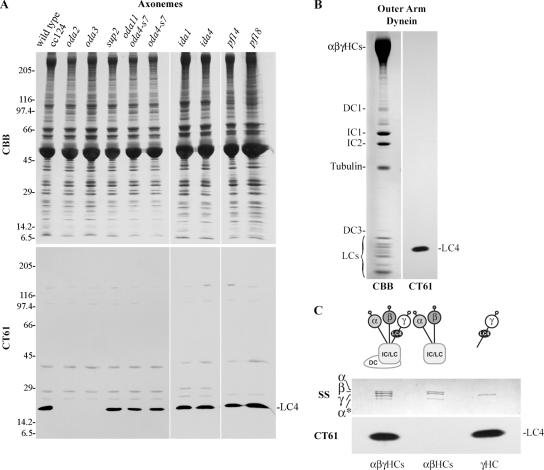
We raised rabbit polyclonal antiserum CT61 against an MBP/LC4 fusion protein and blot-purified the resulting serum using full-length recombinant LC4. Immunoblot analysis of wild-type flagellar axonemes (Figure 1A) and purified outer arm dynein (Figure 1B) revealed that this antibody detected a single major band corresponding to LC4. In axonemal, but not dynein, samples, at least four additional very minor bands were also observed. LC4 was missing in mutant axonemes (oda2 and oda3) lacking outer dynein arms, but was present in strains defective for other axonemal substructures (Figure 1A). In addition, LC4 was present in strains lacking the outer arm α HC (oda11), the β HC motor domain (oda4-s7), and an uncharacterized alteration in the γ HC that results in suppression of paralysis due to lack of the radial spokes or central pair microtubule complex (sup2 previously termed sup-pf2). Fractionation of outer arm dynein into two subparticles revealed that all LC4 is directly associated with the γ HC (Figure 1C) as suggested previously (Pfister et al., 1982), unlike the LC3 thioredoxin that binds to both the β and γ HCs (Harrison et al., 2002).
Figure 1.
Characterization of the CT61 antibody against LC4. (A) Axonemes prepared from wild-type and mutant strains (see Table 1) were electrophoresed in a 5–15% acrylamide gradient gel and stained with Coomassie blue (CBB, top). Similar samples were transferred to nitrocellulose and probed with blot-purified CT61 antibody (bottom). A single major band corresponding to LC4 was observed. This protein was missing only in mutants that lack the outer dynein arm (oda2 and oda3). Several minor bands were also detected in whole axonemal samples. Mr standards (×103) are indicated at left. (B) Left, electrophoretic analysis of purified intact outer arm dynein (including the docking complex) in a 5–15% acrylamide gradient gel; Coomassie blue stain. Right, an immunoblot of an identical sample probed with CT61. Only a single band that comigrates with native LC4 was detected. (C) Top, intact outer arm dynein and the αβ and γ HC subunits electrophoresed in a 3–5% acrylamide 3–8 M urea gel to separate the individual HCs; silver stain. The band marked α* is a proteolytic fragment previously referred to as “band 11” (Pfister et al., 1982) that is derived by cleavage at a site located ∼90 kDa from the C-terminus of the intact HC (King and Witman, 1988a). Similar samples were also electrophoresed in a 5–15% acrylamide gradient gel and probed with CT61 (bottom). The LC4 protein is present only in the intact dynein particle and the isolated γ HC subunit.
Cytoplasmic Preassembly of the LC4/γ HC Complex
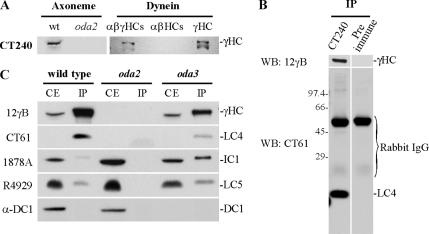
Previous studies have revealed that HCs and ICs are preassembled within the cytoplasm before transport into the flagellum (Fowkes and Mitchell, 1998). To determine whether LC4 is also present in this cytoplasmic complex, we immunoprecipitated the γ HC from cytoplasmic extracts. Because our anti-γ HC mAb (12γ B; King et al., 1985) does not work well for this assay, we prepared a polyclonal antibody (CT240) against the most N-terminal region of this HC (residues 1-442) expressed as a fusion with MBP. This antibody reacts solely with the γ HC and specifically does not recognize other axonemal HCs (Figure 2A). Both the γ HC and LC4 were present in immunoprecipitates from wild-type cytoplasmic extracts obtained using CT240, but were absent from the preimmune control (Figure 2, B and C). These precipitates contained only small amounts of the outer arm proteins IC1 and the α HC-associated LC5. Furthermore, the outer arm docking complex protein (DC1) was not detectable in these samples (Figure 2C), suggesting either that the docking complex is transported to the flagellum as part of a separate unit (Wakabayashi et al., 2001) or that antibody binding induces dissociation as we observed previously for the interaction between the IC/LC complex and the α and β HCs after binding of the mAb 1869A (King and Witman, 1990). As a further control, similar experiments were performed with oda2 (lacks the γ HC) and oda3 (lacks DC1) extracts (Figure 2C). No dynein proteins were obtained from oda2 extracts using the γ HC antibody as expected, whereas all dynein components tested, including considerably enhanced quantities of IC1, were associated in oda3 extracts. These data indicate that LC4 and the γ HC are preassembled within the cytoplasm and suggest that binding of IC1 to the cytoplasmic γ HC-containing complex may be enhanced in the absence of the outer arm docking complex protein DC1.
Figure 2.
Cytoplasmic preassembly of the LC4/γ HC complex. (A) Axonemes from wild type and the oda2 mutant strain, purified intact outer arm dynein, and the αβ and γ HC subparticles were probed with the CT240 antibody raised against the γ HC N-terminal domain. This antibody specifically reacts with the γ HC and did not recognize other HCs within either oda2 axonemes or the αβ HC subparticle from the outer dynein arm. (B) The CT240 antibody was used to immunoprecipitate the γ HC from a wild-type cytoplasmic extract. Immunoblot analysis (WB) revealed that the pellet contained the γ HC (detected using the 12γB mAb) and LC4; neither protein was present in the preimmune control sample. (C) To further address the preassembly state of these components, cytoplasmic extracts (CE) prepared from wild-type, oda2, and oda3 cells were incubated with CT240 antibody, and the resulting immunoprecipitates (IP) were analyzed for the presence of the γ HC, LC4, IC1, LC5, and DC1 using antibodies 12γB, CT61, 1878A, R4929, and anti-DC1, respectively. Note that LC4 was not detected in the original extracts at a loading of 20 μg, but could be observed when protein amount was increased and exposure times were lengthened (not shown).
LC4 Interacts with the N-terminal Domain of the γ HC
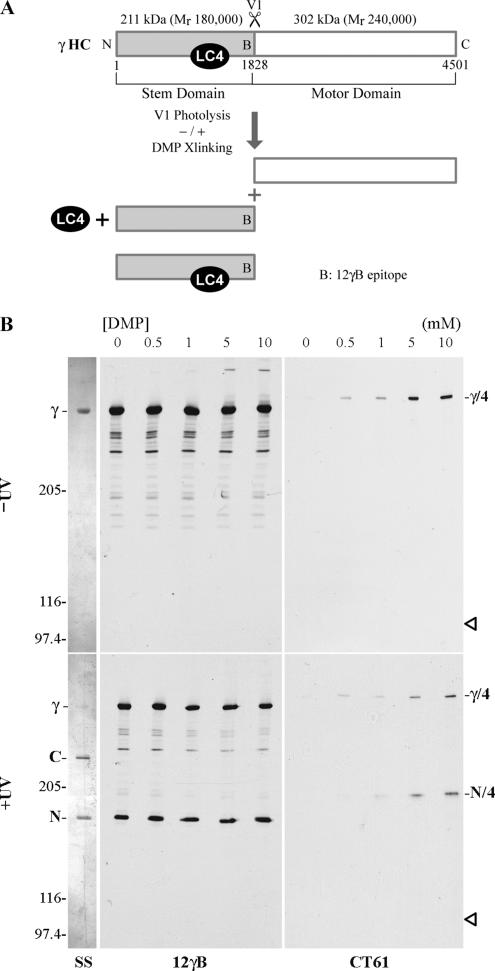
To initially define the γ HC region with which LC4 interacts, we combined vanadate-mediated photolysis and chemical crosslinking. UV irradiation of dynein HCs in the presence of vanadate and ATP results in cleavage at the V1 site within the P-loop of the first AAA+ domain (Lee-Eiford et al., 1986; King and Witman, 1987). For the γ HC, this generates two fragments: an N-terminal stem domain of 211 kDa (Mr180,000) and a C-terminal motor domain of 302 kDa (Mr240,000;King and Witman, 1988b; Figure 3, A and B). The epitope recognized by the 12γB mAb is located near the site of V1 cleavage within the N-terminal fragment (Wilkerson et al., 1994). DMP treatment covalently links primary amines via a linker of 9.2 Å. Treatment of the native γ HC particle with DMP resulted in crosslinking of LC4 to the smaller N-terminal V1 photocleavage fragment (Figure 3, A and B). This crosslinked product was detected by the CT61 antibody, but was not readily observed with CT240 or with the 12γB mAb, presumably due to epitope modification; the 12γB epitope (Wilkerson et al., 1994) and the γ HC region used as immunogen for CT240 contain 6 and 38 Lys residues, respectively.
Figure 3.
Association of LC4 with the γ HC N-terminal domain. (A) Map of the γ HC indicating the origin of the products generated by photocleavage at the V1 site and the location at which LC4 binds; this interaction may be stabilized by crosslinking. (B) The purified γ HC subunit was treated with 0–10 mM DMP in the presence of 1 mM ATP and 100 μM vanadate, electrophoresed in 4% acrylamide 4 M urea gels, and either stained with silver (SS) or blotted and probed with 12γB and CT61 to reveal the γ HC and LC4, respectively (top). Similar samples were photocleaved at the V1 site within the HC before crosslinking (bottom). The N- and C-terminal γ HC photocleavage products are indicated at left. LC4 is crosslinked to the smaller, N-terminal fragment.
Stable Association of LC4 and the γ HC within the Native Complex
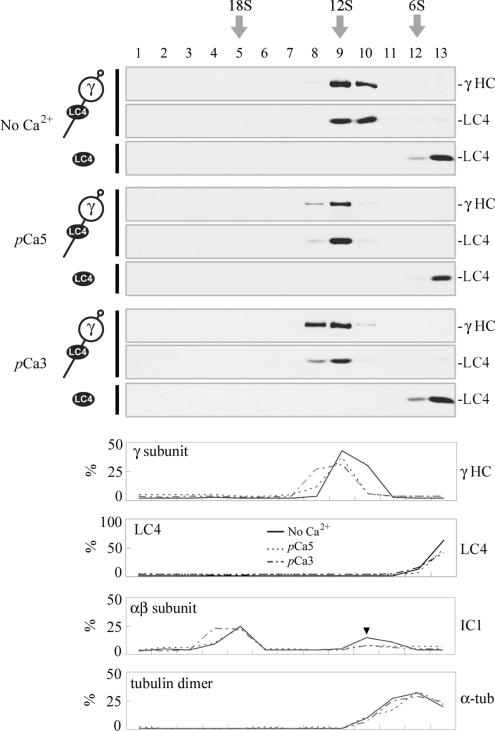
In many calmodulin-regulated systems (e.g., myosin V), calmodulin dissociates from the enzyme upon Ca2+ binding (for review see Bahler and Rhoads, 2002). To test whether Ca2+ addition affected LC4-γ HC association, we fractionated the native γ HC complex (containing the γ HC, LC1, and LC4) by sucrose density gradient centrifugation either in the absence of Ca2+ or at pCa 5 or pCa 3 (Figure 4); LC4 precisely cosedimented with the γ HC at ∼12S under all Ca2+ conditions. Recombinant LC4 was found at the top of the gradient, indicating that this protein does not migrate at 12 S in the absence of the γ HC. Thus, LC4 remains attached to the γ HC under conditions in which the LC is saturated with Ca2+ (King and Patel-King, 1995), and ATP-sensitive microtubule binding by the -βγ HC subparticle is maximally activated (Sakato and King, 2003). Intriguingly, we did observe a small and reproducible shift in the sedimentation profile for the ∼12S γ HC complex as a result of Ca2+ addition; the LC4/γ HC peak was in fractions 9–10 in the absence of Ca2+, fraction 9 at pCa 5, and fractions 8–9 at pCa 3. This shift suggests that the complex may undergo a Ca2+-dependent change to a more compact form in the presence of ligand. In contrast, neither LC4 nor tubulin dimer peaks shifted upon Ca2+ addition; the outer arm αβ subunit peak did not shift at pCa5 but became spread out across one additional fraction at pCa3.
Figure 4.
The native LC4/γ HC complex is stable in both high and low Ca2+. The purified γ HC complex was sedimented in a 5–20% sucrose density gradient in the absence of Ca2+ (top) or at pCa 5 and pCa 3 (middle and bottom); the bottom of the gradient is at left. Equal volumes of each fraction were electrophoresed and the γ HC and LC4 detected by immunoblotting with 12γB and CT61 antibodies, respectively. Recombinant LC4 was processed in parallel to ensure that it did not sediment at ∼12S in the absence of the γ HC. LC4 does not dissociate from the γ HC in a Ca2+-dependent manner. However, there is a small shift in the sedimentation coefficient of the complex toward a more rapidly migrating species at high Ca2+ levels. To further quantify this shift, the % of the total γ HC, recombinant LC4, the outer arm αβ subunit, and tubulin dimer present in each fraction was determined in the absence of Ca2+ and at pCa 5 and 3; the latter two samples were sedimented in additional gradients (blots not shown). Unlike the γ subunit, neither LC4 nor tubulin shift upon Ca2+ addition; the αβ subunit peak did not shift at pCa 5, but spread out by one additional fraction only at pCa 3. The arrowhead in the αβ subunit plot indicates a small peak of dissociated IC/LC complex detected by the IC1 antibody (1878A).
Ca2+-dependent Alteration of the γ HC N-terminal Region
To test whether the apparent Ca2+-dependent shift of the γ HC complex in sucrose gradients derived from an alteration in conformation, we examined the isolated γ HC subunit by negative-stain electron microscopy in the presence and absence of Ca2+, followed by single-particle image processing (Burgess et al., 2003, 2004a,b). A survey micrograph of a representative preparation is shown in Figure 5A. We then identified dynein particles that had adsorbed to the carbon film in one of two orientations (termed “left” and “right”) such that the face of the AAA ring with central cavity is observed. A montage of class-averaged particles in both orientations prepared in either the presence or absence of Ca2+ is shown in Figure 5B. Image averaging of the γ HC head domain revealed no major differences in conformation in either orientation after Ca2+ addition (Figure 5C). However, the N-terminal stem domain was affected by Ca2+. In many averaged images, this region showed an inflection point at about two-thirds of the distance from the N-terminal tip to the motor unit. To quantify this inflection, we measured the angle (α°) between a line drawn along the length of the N-terminal region starting at the tip and a second line that passed through the center of the AAA ring and the point where the N-terminal domain joined the ring (Figure 5D). For dynein particles prepared in the absence of Ca2+, a relatively constant angle α of 120–160° (144 ± 12°; mean ± SD) was obtained (Figure 5E). However, in the presence of Ca2+ a much larger angular dispersion about the inflection point, ranging from ∼35 to 140° (89 ± 40°; mean ± SD), was observed (Figure 5E). These observations suggest that the γ HC N-terminal region is relatively flexible about this inflection point in the presence of Ca2+ but that it becomes locked in a more extended conformation in the absence of ligand.
Figure 5.
Electron microscopic analysis of the γ heavy chain subunit. (A) Survey micrograph of γ HC subunit particles prepared in the presence of Ca2+ and negatively stained with uranyl acetate. Scale bar, 40 nm. (B) Montage of class-averaged images of isolated γ HC subunit particles prepared for negative stain electron microscopy in either the presence or absence of Ca2+; both left and right views are shown. Scale bar, 15 nm. (C) Image averages of left and right views of the γ HC motor domain in the presence and absence of Ca2+ are shown. Scale bar, 15 nm. (D) Enlargement of the left view of one γ HC subunit particle class average imaged in the absence of Ca2+ and interpretative diagram illustrating the method used to measure the stem inflection angle α. Scale bar, 15 nm. (E) The stem inflection angle α was measured for particles prepared in the presence and absence of Ca2+. In the absence of ligand, a low angular dispersion (between ∼120 and 160°) about the inflection point was observed, whereas considerably greater variability in α (between ∼35 and 140°) was found for particles prepared in the presence of Ca2+.
Ca2+-independent Attachment of Full-Length and Truncated LC4 to the γ HC N-terminal Domain
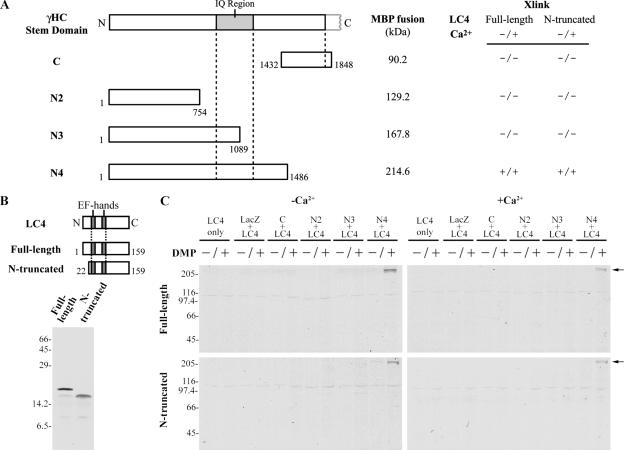
Because we observed a Ca2+-independent interaction between LC4 and the γ HC within the native complex, we examined segments from the γ HC N-terminal region to assess where the interaction site might be located. Various portions of this γ HC region (see Figure 6A) were fused to MBP. Full-length and N-terminal–truncated (residues 22-159; see below) forms of 35S-labeled LC4 were synthesized in a reticulocyte lysate (Figure 6B) and incubated with the MBP-γ HC proteins. Because 10 mM maltose was necessary for the solubility of these constructs, we used DMP crosslinking to stabilize any interactions before electrophoresis. Only the MBP-N4 construct (γ HC residues 1-1486) was crosslinked to the LC4 proteins (Figure 6C); no products were obtained with any other γ HC region. The interaction between MBP-N4 and LC4 was not Ca2+-dependent. Thus, it appears that the γ HC region bounded by residues 1089–1486 is essential for the Ca2+-independent association of this LC and that the N-terminal region of LC4 is not involved in the interaction.
Figure 6.
A Ca2+-independent LC4-binding region on the γ HC. (A) Map of the γ HC N-terminal region indicating the location and mass of the various segments used to assess LC4 binding; the interaction properties of each fragment in the presence and absence of Ca2+ are indicated (see also C). (B) Map of the LC4 constructs used and autoradiograph of the 35S-labeled in vitro–translated full-length and N-terminal–truncated proteins. (C) After incubation of LC4 with the γ HC stem domain fusion proteins, interactions were stabilized by DMP crosslinking, and the samples were electrophoresed. Only the γ HC N4 segment was competent to bind LC4 (both full-length and truncated forms) in a Ca2+-independent manner (arrows). No interaction was observed between LC4 and other parts of the γ HC N-terminal domain.
Ca2+-dependent Interaction of Truncated LC4 with the IQ Motif Region of the γ HC
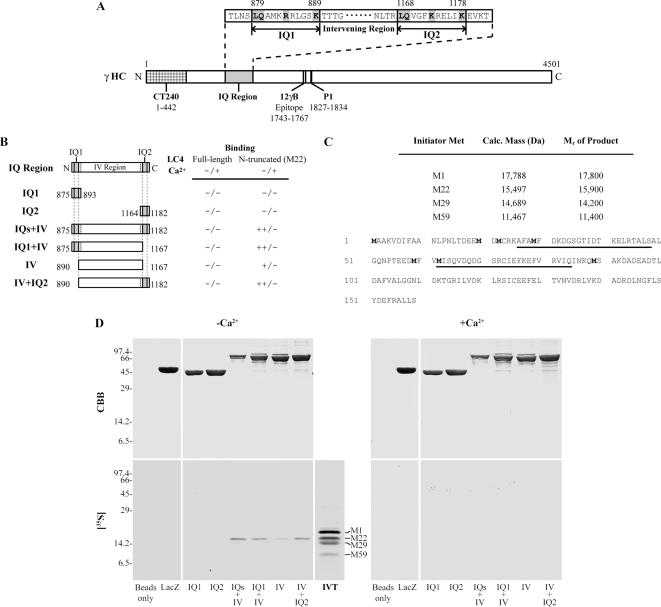
The IQ motif {(I/V/L)Qxxx(R/K)xxxx(R/K)} is a consensus sequence for binding calmodulin-family proteins that is widely distributed in eukaryotes (Rhoads and Friedberg, 1997). Sequence analysis revealed two IQ motifs within the N-terminal domain (residues 875-1182) of the γ HC (Figure 7A); this motif is not present in either the α or β outer arm HCs. To test whether one or both IQ motifs might be involved in binding LC4, we used an in vitro binding assay using MBP fusion proteins containing various parts of this γ HC region (Figure 7B) and 35S-labeled LC4 synthesized in a reticulocyte lysate (Figure 7, C and D). Full-length LC4 (indicated as M1) did not bind to any of the fusion proteins in either the presence or absence of Ca2+ (Figure 7D). However, a truncated LC4 product containing both N-terminal EF-hands and derived from translation initiation at either M20 or M22, bound to this γ HC region in a Ca2+-dependent manner. Other truncated products that lack intact N-terminal EF-hands (derived from initiation at M29 and M59) did not bind. Thus, the 22-159 segment of LC4, allows for Ca2+-dependent interaction with the IQ region of the γ HC. This association requires that the first EF hand within LC4 (residues 25-48) be intact, and, at least in this minimal in vitro system, is abolished by a short sequence at the N-terminus of LC4. Furthermore, this observation implies that the Ca2+-independent association of truncated LC4 (see Figure 6) involves a region of the γ HC that is C-terminal of residue 1182.
Figure 7.
Ca2+-dependent interaction of LC4(22-159) with the γ HC IQ region. (A) Map of the γ HC, indicating the location and sequence of the two IQ motifs. (B) Map indicating the various γ HC MBP fusion proteins used and whether they bound either full-length or the N-terminal–truncated form of LC4 in the presence or absence of Ca2+. Constructs containing the intervening (IV) region plus one or both IQ motifs bound LC4(22-159) well only in the absence of Ca2+ (++); the intervening region between the IQ motifs exhibited detectable binding (+). (C) Sequence of LC4 indicating the location of Met residues (bold) at which translation initiation occurred and the calculated mass and measured Mr of the products. The two EF hands are underlined. (D) MBP fusion proteins containing various segments of the IQ motif region and MBP-LacZ and bead-only controls were incubated with in vitro–translated LC4 (IVT) in the presence and absence of 1 mM Ca2+. Full-length LC4 did not bind to any of the constructs. In contrast, N-terminal truncated LC4 obtained by translation initiation at M22 bound to this γ HC region in a Ca2+-dependent manner.
LC3 Also Interacts with the N-terminal Domain of the γ HC
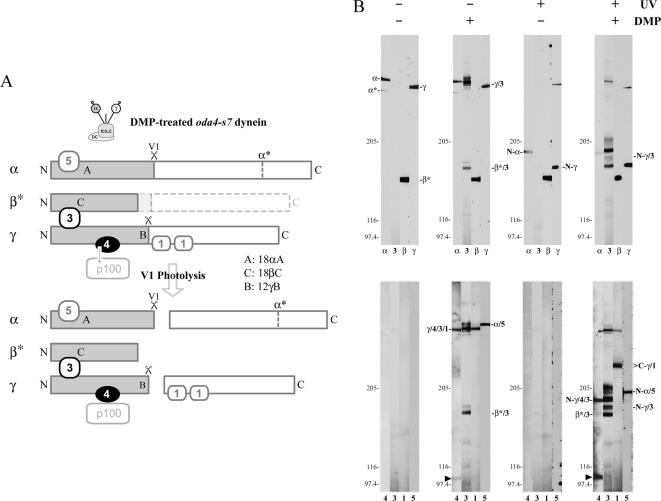
To assess whether LC4 and the γ HC N-terminal domain interact with other components within the isolated dynein particle, we used a combination of chemical crosslinking and vanadate-mediated photocleavage to probe intradynein associations (Figure 8, A and B). To help avoid confusion between the various HCs, this experimental series was performed using intact outer arm dynein purified from oda4-s7 mutant axonemes that contain a truncated ∼160 kDa form of the β HC (indicated as β* in Figure 8) that completely lacks the motor domain (Sakakibara et al., 1993). In the absence of DMP and UV irradiation, only intact α and γ HCs and the truncated β* were detected on immunoblots (Figure 8B, left panels). DMP treatment resulted in multiple crosslinked products containing various combinations of HCs and LCs. A single high molecular weight product containing the α HC and LC5 thioredoxin (α/5) was obtained; photocleavage revealed that this interaction involved the α HC N-terminal domain (N-α/5), confirming our previous observation (Harrison et al., 2002). In contrast, multiple products were generated containing the LC3 thioredoxin that is known to interact with both the β and γ HCs (Pfister and Witman, 1984; Harrison et al., 2002). The β* HC region became crosslinked to LC3 (β*/3), and this complex was insensitive to UV irradiation as expected. In DMP-treated samples, we also observed several high molecular weight species containing LC3, including one consisting of the γ HC and LC3 (γ/3) and the quaternary complex of LC1, LC3, LC4, and the γ HC (γ/4/3/1). Photocleavage of this latter product at the V1 site yielded one product containing LC3, LC4, and the N-terminal domain of the γ HC (N-γ/4/3) and a pair of bands consisting of the γ HC C-terminal region and either one or two copies of LC1 (C-γ/1). In addition two other crosslinked products with Mr greater than that of N-γ/4/3 were generated; however, their origin remains uncertain. In the crosslinked quaternary complex, LC3 could associate either directly with the γ HC or via interaction with LC4. However, we did not observe a LC3/LC4 DMP crosslinked product (predicted molecular weight of ∼34 kDa) as would be expected in the latter case (not shown). Therefore, LC3 is most likely crosslinked to, and presumably interacts directly with, the N-terminal region of the γ HC.
Figure 8.
Chemical crosslinking defines intradynein interactions. (A) Diagram illustrating the crosslinked products generated by DMP treatment and subsequent V1 photocleavage of oda4-s7 dynein, which contains a truncated form of the β HC (β*). The relative size of the HC fragments corresponds to their apparent size after electrophoresis; it does not directly relate to their actual mass based on sequence. (B) Outer arm dynein containing a ∼160-kDa truncated form of the β HC motor domain from oda4-s7 was incubated with 1 mM ATP plus 100 μM vanadate, and half the samples were irradiated with UV light to cleave the α and γ HCs at their V1 sites. After the photolysis reaction, proteins were then subject to crosslinking with 10 mM DMP or were treated with solvent alone. Samples were electrophoresed and probed with 18αA, 18βC, and 12γB to detect the N-terminal regions of the three HCs, and with R5932, R4930, CT61, and R4924, which recognize the HC-associated LC1, LC3, LC4, and LC5 proteins, respectively. The top series of blots indicate the location of HC bands and LC3, whereas the other LCs were analyzed with respect to LC3 in the bottom series. In the presence of DMP, LC3 is crosslinked to both the β and γ HCs (labeled β*/3 and γ/3); after photolysis the γ HC/LC3 product (N-γ/3) lacks the C-terminal motor unit and consequently migrates more rapidly. Further analysis identified a crosslinked band containing the γ HC and LC1, LC3, and LC4 (γ/4/3/1). After photolysis, this complex yields two products: the γ HC N-terminal region crosslinked to LC3 and LC4 (N-γ/4/3) and a γ HC C-terminal domain linked to LC1 (C-γ/1). The arrowhead marks an additional product containing LC4 that is obtained in enhanced yield after UV irradiation to cleave the HCs at the V1 site; this product is further analyzed in Figure 9.
LC4 Comes into Proximity of IC1 in a Ca2+-dependent Manner
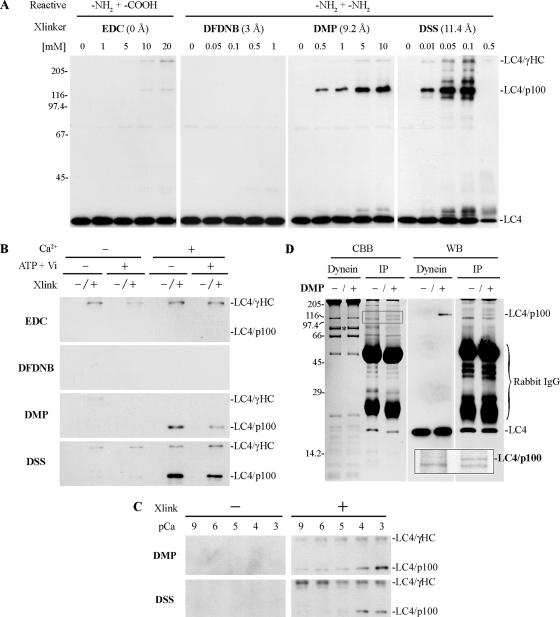
A band of Mr ∼110,000 in urea gels (Mr >120,000 in SDS gels) was detected by the LC4 antibody (Figure 8B, black arrowheads) after DMP treatment of intact oda4-s7 dynein in the presence of ATP/vanadate; the yield of this product was significantly enhanced upon photolysis, which mimics the “no ATP-bound” state. This suggests that LC4 also interacts with a protein of ∼90–100 kDa; a similar crosslinked product was obtained with oda11 sup1-2 dynein, which contains a β HC with abrogated microtubule binding activity (not shown). Analysis of wild-type dynein using reagents with varying linker lengths of 0–11.4 Å revealed that DMP and DSS gave the highest yields of LC4-p100 in the presence of Ca2+ (Figure 9A); use of the zero-length reagent EDC generated a small amount of product, indicating that the two proteins indeed interact directly. The short-length amine-reactive linker DFDNB generated a barely detectable amount of LC4-crosslinked product at concentrations of 0.05–0.5 mM, suggesting that the amines crosslinked by DMP and DSS are >3 Å apart. We also noticed that the presence of Ca2+ was necessary to obtain this LC4-p100 product in high yield as very little was generated in the presence of EGTA (Figure 9B). Indeed, with both DSS and DMP significant amounts of LC4-p100 were obtained only with Ca2+ levels at or above pCa 4 (Figure 9C). Furthermore, addition of ATP/vanadate reduced the amount of LC4-p100 even in the presence of the metal ligand, suggesting that a further change in conformation occurs upon nucleotide binding (Figure 9B).
Figure 9.
Ca2+-dependent crosslinking of LC4 to IC1. (A) Purified outer arm dynein was treated with the carbodiimide EDC or with the amine-selective reagents DFDNB, DMP, and DSS in the presence of 1 mM Ca2+. After electrophoresis in 8% acrylamide SDS gels, samples were probed for the presence of LC4. Crosslinked products containing LC4 and either the γ HC or p100 are evident in the EDC, DMP, and DSS samples; upon prolonged exposure a very small amount of product was detectable in the DFDNB-treated sample (not shown). (B) Purified outer arm dynein in the presence or absence of 1 mM ATP/100 μM vanadate, and 1 mM Ca2+ was treated with the indicated crosslinking reagents (20 mM EDC, 1 mM DFDNB, 10 mM DMP, and 0.01 mM DSS). Formation of the LC4-γ HC product was not dependent on either nucleotide or Ca2+. In contrast, LC4-p100 crosslinking was dramatically increased in the presence of Ca2+, and the product yield was also nucleotide-dependent. (C) Purified dynein was incubated at pCa 9–pCa 3 in the presence or absence of 10 mM DMP or 0.01 mM DSS. Significant levels of the LC4-p100 product were only obtained at and above pCa 4. (D) Immunoprecipitation of crosslinked LC4-p100 using the CT61 antibody. Purified outer arm dynein was crosslinked with 10 mM DMP in the presence of Ca2+ (Dynein), denatured, refolded, and immunoprecipitated (IP). Samples were electrophoresed in 10% tricine SDS gels and either stained with Coomassie blue (CBB) or transferred and probed with the CT61 antibody (WB). A LC4-p100 crosslinked product was immunoprecipitated in addition to LC4 as indicated at right. An inset at the bottom right shows an enlarged image of the boxed region of the Coomassie blue–stained gel. Mass spectrometry identified p100 as IC1. The asterisk indicates non-crosslinked IC1 that migrates with Mr78,000.
As the LC4-p100 product was not observed after crosslinking of the purified γ HC subparticle (Figure 3, open arrowheads), it presumably derived from interaction of LC4 with a component of the αβ HC subparticle or the outer arm docking complex. Accordingly, we isolated the crosslinked product by immunoprecipitation with CT61 (Figure 9D), excised the band after electrophoresis, and analyzed its composition by mass spectrometry. Five peptides derived from IC1 of the outer dynein arm were obtained, unambiguously identifying p100 as IC1.
DISCUSSION
In Chlamydomonas, isolated flagellar axonemes exhibit waveform conversion from an asymmetric to a symmetric pattern in response to an increase in Ca2+ from pCa 6 to pCa 4 (Bessen et al., 1980). Mutational studies suggest that the outer row of axonemal dynein arms may be the ultimate target of this Ca2+-mediated signaling pathway (Kamiya and Okamoto, 1985; Mitchell and Rosenbaum, 1985). Previously we found that Ca2+ concentrations above pCa 6 maximally activate ATP-sensitive microtubule binding by purified -βγ HC subparticles (Sakato and King, 2003). This indicates that outer arm dynein contains an integral Ca2+ sensor. Two Ca2+-binding proteins of the EF-hand superfamily have been identified within this structure: the docking complex protein DC3 (Casey et al., 2003a) and the γ HC–associated LC4 light chain (King and Patel-King, 1995). DC3 seems not to be involved in Ca2+ sensing because a null mutant (oda14) rescued with a DC3 variant unable to bind Ca2+ exhibits normal photo-induced behavioral responses, including waveform conversion (Casey et al., 2003a). Here we have examined the detailed interaction of the other candidate Ca2+ sensor (LC4) with the γ HC and describe structural alterations in this complex that occur in response to Ca2+ binding.
Interaction of LC4 with the γ HC
Four LCs (LCs 1, 3, 4, and 5) within the Chlamydomonas outer arm interact directly with their target HCs. LC3 and LC5 are thioredoxins and we have previously observed that at least one of these (LC3) interacts with two HCs (β and γ; Harrison et al., 2002). The crosslinking experiments reported here further confirm this observation. In contrast, within the outer arm, the motor domain–associated protein LC1 interacts only with the γ HC (Benashski et al., 1999). Subfractionation of purified dynein and immunoblotting revealed that LC4 is similarly associated exclusively with the γ HC, as suggested previously from fractionation studies (Pfister et al., 1982). Furthermore, we observed that this LC4-γHC interaction occurs within the cytoplasm, implying the preassembly of this HC/LC complex before its transport and incorporation into the flagellum.
Preassembled Outer Arm Dynein Complexes in the Cytoplasm
Previous studies of outer arm complexes within Chlamydomonas cytoplasm have revealed that all three HCs and both ICs can be immunoprecipitated by an anti-β HC antibody (Fowkes and Mitchell, 1998) and that the dynein motor unit and docking complex are independently preassembled in the cytoplasm (Wakabayashi et al., 2001). Using an antibody directed against the γ HC N-terminal domain, we observed that LC4 was immunoprecipitated from wild-type extracts, indicating that HC-LC assembly also occurs in the cytoplasm. Intriguingly, in the same samples we obtained only very small amounts of IC1 and no DC1. However, the amount of immunoprecipitated IC1 increased very significantly in the DC1 mutant oda3, suggesting that the presence of the docking complex influences retention of IC1 and potentially the cytoplasmic assembly state of the outer arm dynein particle. Alternatively, because the N-terminal region of the γ HC must be located close to where these proteins interact, one possible reason for the low level of IC1 is that, in the presence of the docking complex, the CT240 antibody disrupts an interaction between the γ HC (or an associated protein) and components of the αβ subunit; for example, we previously found that binding of an antibody against IC2 can disrupt association of the IC/LC complex with the α and β HCs (King and Witman, 1990).
Why Does the γ HC Contain Two IQ Motifs?
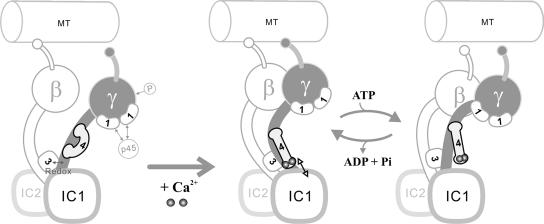
The IQ motif is an established binding consensus for calmodulin family proteins (Rhoads and Friedberg, 1997), although several distinct molecular mechanisms have been described for these interactions (Hoeflich and Ikura, 2002). The γ HC N-terminal domain contains two IQ motifs that promote interaction with LC4 and are thus functional. However, stoichiometry measurements based on both Coomassie blue dye binding and 35S-labeling have indicated that there is a single copy of LC4 per dynein particle (King and Witman, 1989). The question therefore arises as to why there are two IQ motifs but only one bound copy of LC4. First, two copies of LC4 might interact with the HC in vivo, only one of which is bound sufficiently tightly to copurify with dynein after high salt extraction. Second, although both motifs can interact with LC4 in vitro, one might be used in vivo to bind a related protein, e.g., calmodulin, centrin, or the docking complex protein DC3. Although there is no direct evidence in support of this possibility, it cannot be ruled out at the present time. Finally, recent studies of calmodulin binding to the neck domain of myosin V (Martin and Bayley, 2004) and the small conductance Ca2+-activated K+ channel (Schumacher et al., 2001) have revealed that the N- and C-terminal domains of a single calmodulin molecule can independently bind to two different IQ motifs aligned in parallel, thereby forming a bridge between them; in the case of the K+ channel, this involves both Ca2+-dependent and -independent interactions and leads to Ca2+-dependent dimerization. If LC4 bound the γ HC N-terminal domain in a similar manner, it would readily explain the interaction with both IQ motifs as well as the apparently critical role played by the 278-residue intervening region. Furthermore, as only the two N-terminal helix-loop-helix motifs of LC4 conform to the consensus for Ca2+ binding (King and Patel-King, 1995), this model could accommodate a Ca2+-dependent interaction with one IQ domain and a Ca2+-independent association with the other. The differential interactions of these LC4 regions with the γ HC might alter the conformation of the complex and provide the physical basis for the shift in sedimentation coefficient, enhanced flexibility of the N-terminal region and interaction with IC1 observed upon Ca2+ addition. A model depicting these interactions is shown in Figure 10.
Figure 10.
A model for LC4 interactions within outer arm dynein. In the absence of Ca2+, LC4 is tightly bound to the γ HC (left); this complex cannot efficiently form a rigor bond with MTs (Sakato and King, 2003). After Ca2+ addition, LC4 changes conformation and its Ca2+-bound EF hand motifs detach from the IQ region and come into proximity of or attach to IC1 (center). This is predicted to lead to an alteration in the N-terminal stem domain of the γ HC and the activation of motor activity. After ATP addition, a further change in γ HC conformation occurs, causing LC4 to move somewhat away from IC1 (right). Other outer arm dynein components including the α HC are not incorporated into this diagram because their functional interactions with either the γ HC, LC1, or LC4 have not been defined.
The coiled-coil docking complex protein DC2 also has two IQ motifs (Takada et al., 2002). However, we did not observe any interaction between DC2 and LC4, and these motifs more likely bind other calmodulin-family proteins, such as DC3.
The γ HC Is Regulated by Multiple Signaling Pathways
Previously, we found that motor activity of the β HC is required for Ca2+-dependent activation of ATP-sensitive MT binding by the -βγ HC subparticle (Sakato and King, 2003). Furthermore, we observed that crosslinking of -βγ HC dynein from oda11 sup1-2 that contains a β HC with a defective coiled coil stalk domain still generated the LC4-IC1 product. This indicates that LC4 can undergo a Ca2+-dependent conformational change in this mutant dynein, even though it does not result in the activation of ATP-dependent microtubule binding. This suggests that the configuration of these two HCs is important to exert full motor activity at high Ca2+ concentrations. Because LC4 comes into close proximity of IC1 at high Ca2+, Ca2+-dependent conformational change of LC4 likely has a direct effect on the γ HC stem rather than its motor domain. This would be consistent with our observation that the stem domain adopts a relatively constant orientation in the absence of Ca2+ but is much more variable in its presence. Furthermore, recent studies revealed that the stem domain of the dynein HC undergoes a dynamic structural change during the ATP hydrolytic cycle and that this motion contributes to formation of the power stroke (Burgess et al., 2003; Kon et al., 2005). In addition, we observed that the Ca2+ sensitivity of the -βγ HC subparticle was altered by binding tubulin to the basal ATP-insensitive site (our unpublished observations), providing further evidence that dynein motor activity can be modulated through the stem domain.
Chlamydomonas flagellar motility is also controlled by redox poise (Wakabayashi and King, 2006). The LC3 thioredoxin interacts with the γ HC and ATPase activity of this motor unit, but not the α and β HCs or the inner arms, is activated by sulfhydryl modification (Harrison et al., 2002). These observations suggest that the redox control mechanism may involve or be mediated through the γ HC. Furthermore, the γ HC has two potential regulatory inputs directly to the motor domain. Two copies of the leucine-rich repeat protein LC1 are associated with the nucleotide binding sites of the γ HC motor domain and also interact with an additional axonemal component (Benashski et al., 1999; Wu et al., 2000; DiBella et al., 2005) We have proposed previously that Arg residues within the terminal helix of LC1 control ATPase activity in a manner similar to the “arginine fingers” that promote GTPase activity of Ras and Rho (Wu et al., 2000; King, 2002); recent work has shown that the RNA interference–mediated knock down of an LC1 orthologue in trypanosomes leads to defective motility (Baron et al., 2007). Furthermore, analysis of the Chlamydomonas phosphoproteome (Wagner et al., 2006) has revealed that the γ HC is phosphorylated on Ser2467, which is located within the third AAA+ domain. Mutational studies of cytoplasmic dynein indicate that this domain plays an important role in dynein motor function (Silvanovich et al., 2003), and single molecule analysis (Mallik et al., 2004) suggests that nucleotide binding at domains other than AAA1 regulates the power stroke mechanism.
In conclusion, we have defined specific structural alterations within the outer dynein arm that involve the γ HC, LC4, and IC1 and occur in response to an increase in Ca2+. These results provide further evidence for the regulation of outer dynein arm structure and activity by Ca2+ and impart insight into a potential molecular mechanism.
ACKNOWLEDGMENTS
We thank Sharon Benashski and Oksana Gorbatyuk for antibody preparation, Ramila Patel-King for the sequence of the γ heavy chain genomic region, Drs. Curtis Wilkerson (Michigan State University) and George Witman (University of Massachusetts Medical School) for the full-length γ heavy chain cDNA construct, Drs. Ken-ichi Wakabayashi and Ritsu Kamiya (University of Tokyo) for anti-DC1 antibody, Dr. John Leszyk (University of Massachusetts Medical School) for mass spectrometry, Dr. Stanley Burgess (University of Leeds) for kindly providing us with the batch programs for single-particle image-processing using SPIDER, and Yukako Sakai (National Institute of Information and Communications Technology) for assistance with electron microscopy. M.S. thanks Dr. Karina Nikulina (University of California, San Francisco) for technical advice. This study was supported by Grant GM51293 from the National Institutes of Health. S.M.K. is an investigator of the Patrick and Catherine Weldon Donaghue Medical Research Foundation.
Abbreviations used:
- DC
docking complex
- DFDNB
1,5-difluoro-2,4-dinitrobenzene
- DMP
dimethylpimelimidate
- DSS
disuccinimidyl suberate
- EDC
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
- HC
heavy chain
- IC
intermediate chain
- LC
light chain
- UTR
untranslated region.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-10-0917) on July 18, 2007.
1 pCa refers to the negative log of the Ca2+ concentration.
2 We have reanalyzed both cDNA and genomic γ HC clones and identified a number of differences compared to the original cDNA sequence. The amino acid numbering scheme for the γ HC used in this report derives from this revised sequence.
REFERENCES
- Adams G., Huang B., Piperno G., Luck D. Central pair microtubule complex of Chlamydomonas flagella: polypeptide composition as revealed by analysis of mutants. J. Cell Biol. 1981;91:69–76. doi: 10.1083/jcb.91.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler M., Rhoads A. Calmodulin signaling via the IQ motif. FEBS Lett. 2002;513:107–113. doi: 10.1016/s0014-5793(01)03239-2. [DOI] [PubMed] [Google Scholar]
- Baron D. M., Kabututu Z. P., Hill K. L. Stuck in reverse: loss of LC1 in Trypanosoma brucei disrupts outer dynein arms and leads to reverse flagellar beat and backward movement. J. Cell Sci. 2007;120:1513–1520. doi: 10.1242/jcs.004846. [DOI] [PubMed] [Google Scholar]
- Benashski S. E., King S. M. Investigation of protein-protein interactions within flagellar dynein using homobifunctional and zero-length crosslinking reagents. Methods. 2000;22:365–371. doi: 10.1006/meth.2000.1088. [DOI] [PubMed] [Google Scholar]
- Benashski S. E., Patel-King R. S., King S. M. Light chain 1 from the Chlamydomonas outer dynein arm is a leucine-rich repeat protein associated with the motor domain of the γ heavy chain. Biochemistry. 1999;38:7253–7264. doi: 10.1021/bi990466y. [DOI] [PubMed] [Google Scholar]
- Bessen M., Fay R. B., Witman G. B. Calcium control of waveform in isolated flagellar axonemes of Chlamydomonas. J. Cell Biol. 1980;86:446–455. doi: 10.1083/jcb.86.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokaw C. J., Josslin R., Bobrow L. Calcium ion regulation of flagellar beat symmetry in reactivated sea urchin spermatozoa. Biochem. Biophys. Res. Commun. 1974;58:795–800. doi: 10.1016/s0006-291x(74)80487-0. [DOI] [PubMed] [Google Scholar]
- Burgess S., Walker M., Thirumurugan K., Trinick J., Knight P. Use of negative stain and single-particle image processing to explore dynamic properties of flexible macromolecules. J. Struct. Biol. 2004a;147:247–258. doi: 10.1016/j.jsb.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Burgess S. A., Walker M., Sakakibara H., Oiwa K., Knight P. The structure of dynein-c by negative stain electron microscopy. J. Struct. Biol. 2004b;146:205–216. doi: 10.1016/j.jsb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Burgess S. A., Walker M. L., Sakakibara H., Knight P. J., Oiwa K. Dynein structure and power stroke. Nature. 2003;421:715–718. doi: 10.1038/nature01377. [DOI] [PubMed] [Google Scholar]
- Casey D., Inaba K., Pazour G., Takada S., Wakabayashi K., Wilkerson C., Kamiya R., Witman G. DC3, the 21-kD subunit of the outer dynein arm-docking complex (ODA-DC), is a novel EF-hand protein important for assembly of both the outer arm and the ODA-DC. Mol. Biol. Cell. 2003a;14:3650–3663. doi: 10.1091/mbc.E03-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey D., Yagi T., Kamiya R., Witman G. DC3, the smallest subunit of the Chlamydomonas flagellar outer dynein arm-docking complex, is a redox-sensitive calcium-binding protein. J. Biol. Chem. 2003b;278:42652–42659. doi: 10.1074/jbc.M303064200. [DOI] [PubMed] [Google Scholar]
- DiBella L. M., Gorbatyuk O., Sakato M., Wakabayashi K., Patel-King R. S., Pazour G. J., Witman G. B., King S. M. Differential light chain assembly influences outer arm dynein motor function. Mol. Biol. Cell. 2005;16:5661–5674. doi: 10.1091/mbc.E05-08-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowkes M. E., Mitchell D. R. The role of preassembled cytoplasmic complexes in assembly of flagellar dynein subunits. Mol. Biol. Cell. 1998;9:2337–2347. doi: 10.1091/mbc.9.9.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J., Radermacher M., Penczek P., Zhu J., Li Y., Ladjadj M., Leith A. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J. Struct. Biol. 1996;116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- Harrison A., Olds-Clarke P., King S. M. Identification of the t complex-encoded cytoplasmic dynein light chain Tctex1 in inner arm I1 supports the involvement of flagellar dyneins in meiotic drive. J. Cell Biol. 1998;140:1137–1147. doi: 10.1083/jcb.140.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison A., Sakato M., Tedford H. W., Benashski S. E., Patel-King R. S., King S. M. Redox-based control of the γ heavy chain ATPase from Chlamydomonas outer arm dynein. Cell Motil. Cytoskelet. 2002;52:131–143. doi: 10.1002/cm.10044. [DOI] [PubMed] [Google Scholar]
- Hoeflich K. P., Ikura M. Calmodulin in action: diversity in target recognition and activation mechanisms. Cell. 2002;108:739–742. doi: 10.1016/s0092-8674(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Huang B., Piperno G., Ramanis Z., Luck D. Radial spokes of Chlamydomonas flagella: genetic analysis of assembly and function. J. Cell Biol. 1981;88:80–88. doi: 10.1083/jcb.88.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Ramanis Z., Luck D. J. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for flagellar function. Cell. 1982;28:115–124. doi: 10.1016/0092-8674(82)90381-6. [DOI] [PubMed] [Google Scholar]
- Kamiya R. Mutations at twelve independent loci result in absence of outer dynein arms in Chlamydomonas reinhardtii. J. Cell Biol. 1988;107:2253–2258. doi: 10.1083/jcb.107.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya R., Kurimoto E., Muto E. Two types of Chlamydomonas flagellar mutants missing different components of inner-arm dynein. J. Cell Biol. 1991;112:441–447. doi: 10.1083/jcb.112.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya R., Okamoto M. A mutant of Chlamydomonas reinhardtii that lacks the flagellar outer dynein arm but can swim. J. Cell Sci. 1985;74:181–191. doi: 10.1242/jcs.74.1.181. [DOI] [PubMed] [Google Scholar]
- Kamiya R., Witman G. B. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J. Cell Biol. 1984;98:97–107. doi: 10.1083/jcb.98.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. M. AAA domains and organization of the dynein motor unit. J. Cell Sci. 2000;113:2521–2526. doi: 10.1242/jcs.113.14.2521. [DOI] [PubMed] [Google Scholar]
- King S. M. Dynein motors: structure, mechanochemistry and regulation. In: Schliwa M., editor. Molecular Motors. Weinheim: Wiley-VCH Verlag GmbH; 2002. pp. 45–78. [Google Scholar]
- King S. M., Otter T., Witman G. B. Characterization of monoclonal antibodies against Chlamydomonas flagellar dyneins by high-resolution protein blotting. Proc Natl Acad Sci USA. 1985;82:4717–4721. doi: 10.1073/pnas.82.14.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. M., Otter T., Witman G. B. Purification and characterization of Chlamydomonas flagellar dyneins. Methods Enzymol. 1986;134:291–306. doi: 10.1016/0076-6879(86)34097-7. [DOI] [PubMed] [Google Scholar]
- King S. M., Patel-King R. S. Identification of a Ca(2+)-binding light chain within Chlamydomonas outer arm dynein. J. Cell Sci. 1995;108:3757–3764. doi: 10.1242/jcs.108.12.3757. [DOI] [PubMed] [Google Scholar]
- King S. M., Wilkerson C. G., Witman G. B. The Mr 78,000 intermediate chain of Chlamydomonas outer arm dynein interacts with α-tubulin in situ. J. Biol. Chem. 1991;266:8401–8407. [PubMed] [Google Scholar]
- King S. M., Witman G. B. Structure of the α and β heavy chains of the outer arm dynein from Chlamydomonas flagella. Masses of chains and sites of ultraviolet-induced vanadate-dependent cleavage. J. Biol. Chem. 1987;262:17596–17604. [PubMed] [Google Scholar]
- King S. M., Witman G. B. Structure of the α and β heavy chains of the outer arm dynein from Chlamydomonas flagella. Location of epitopes and protease- sensitive sites. J. Biol. Chem. 1988a;263:9244–9255. [PubMed] [Google Scholar]
- King S. M., Witman G. B. Structure of the γ heavy chain of the outer arm dynein from Chlamydomonas flagella. J. Cell Biol. 1988b;107:1799–1808. doi: 10.1083/jcb.107.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. M., Witman G. B. Molecular structure of Chlamydomonas outer arm dynein. In: Warner F. D., Satir P., Gibbons I. R., editors. Cell Movement. The Dynein ATPases. Vol. 1. New York: Alan R. Liss; 1989. pp. 61–75. [Google Scholar]
- King S. M., Witman G. B. Localization of an intermediate chain of outer arm dynein by immunoelectron microscopy. J. Biol. Chem. 1990;265:19807–19811. [PubMed] [Google Scholar]
- Kon T., Mogami T., Ohkura R., Nishiura M., Sutoh K. ATP hydrolysis cycle-dependent tail motions in cytoplasmic dynein. Nat. Struct. Mol. Biol. 2005;12:513–519. doi: 10.1038/nsmb930. [DOI] [PubMed] [Google Scholar]
- Koutoulis A., Pazour G. J., Wilkerson C. G., Inaba K., Sheng H., Takada S., Witman G. B. The Chlamydomonas reinhardtii ODA3 gene encodes a protein of the outer dynein arm docking complex. J. Cell Biol. 1997;137:1069–1080. doi: 10.1083/jcb.137.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDizet M., Piperno G. ida4-1, ida4-2, and ida4-3 are intron splicing mutations affecting the locus encoding p28, a light chain of Chlamydomonas axonemal inner dynein arms. Mol. Biol. Cell. 1995;6:713–723. doi: 10.1091/mbc.6.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Eiford A., Ow R. A., Gibbons I. R. Specific cleavage of dynein heavy chains by ultraviolet irradiation in the presence of ATP and vanadate. J. Biol. Chem. 1986;261:2337–2342. [PubMed] [Google Scholar]
- Lindemann C. B., Goltz J. S. Calcium regulation of flagellar curvature and swimming pattern in triton X-100-extracted rat sperm. Cell Motil. Cytoskelet. 1988;10:420–431. doi: 10.1002/cm.970100309. [DOI] [PubMed] [Google Scholar]
- Mallik R., Carter B. C., Lex S. A., King S. J., Gross S. P. Cytoplasmic dynein functions as a gear in response to load. Nature. 2004;427:649–652. doi: 10.1038/nature02293. [DOI] [PubMed] [Google Scholar]
- Martin S. R., Bayley P. M. Calmodulin bridging of IQ motifs in myosin-V. FEBS Lett. 2004;567:166–170. doi: 10.1016/j.febslet.2004.04.053. [DOI] [PubMed] [Google Scholar]
- Mitchell D. R., Rosenbaum J. L. A motile Chlamydomonas flagellar mutant that lacks outer dynein arms. J. Cell Biol. 1985;100:1228–1234. doi: 10.1083/jcb.100.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naitoh K., Kaneko H. Reactivated Triton-extracted, models of Paramecium: modification of ciliary movement by calcium ions. Science. 1972;176:523–524. doi: 10.1126/science.176.4034.523. [DOI] [PubMed] [Google Scholar]
- Patel-King R. S., Benashki S. E., Harrison A., King S. M. Two functional thioredoxins containing redox-sensitive vicinal dithiols from the Chlamydomonas outer dynein arm. J. Biol. Chem. 1996;271:6283–6291. doi: 10.1074/jbc.271.11.6283. [DOI] [PubMed] [Google Scholar]
- Pfister K. K., Fay R. B., Witman G. B. Purification and polypeptide composition of dynein ATPases from Chlamydomonas flagella. Cell Motil. 1982;2:525–547. doi: 10.1002/cm.970020604. [DOI] [PubMed] [Google Scholar]
- Pfister K. K., Witman G. B. Subfractionation of Chlamydomonas 18 S dynein into two unique subunits containing ATPase activity. J. Biol. Chem. 1984;259:12072–12080. [PubMed] [Google Scholar]
- Porter M. E., Power J., Dutcher S. K. Extragenic suppressors of paralyzed flagellar mutations in Chlamydomonas reinhardtii identify loci that alter the inner dynein arms. J. Cell Biol. 1992;118:1163–1176. doi: 10.1083/jcb.118.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H., Diener D. R., Geimer S., Cole D. G., Rosenbaum J. L. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J. Cell Biol. 2004;164:255–266. doi: 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads A., Friedberg F. Sequence motifs for calmodulin recognition. FASEB J. 1997;11:331–340. doi: 10.1096/fasebj.11.5.9141499. [DOI] [PubMed] [Google Scholar]
- Rupp G., O'Toole E., Gardner L. C., Mitchell B. F., Porter M. E. The sup-pf-2 mutations of Chlamydomonas alter the activity of the outer dynein arms by modification of the γ-dynein heavy chain. J. Cell Biol. 1996;135:1853–1865. doi: 10.1083/jcb.135.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H., Mitchell D. R., Kamiya R. A Chlamydomonas outer arm dynein mutant missing the α heavy chain. J. Cell Biol. 1991;113:615–622. doi: 10.1083/jcb.113.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H., Nakayama H. Translocation of microtubules caused by the αβ, β and γ outer arm dynein subparticles of Chlamydomonas. J. Cell Sci. 1998;111:1155–1164. doi: 10.1242/jcs.111.9.1155. [DOI] [PubMed] [Google Scholar]
- Sakakibara H., Takada S., King S. M., Witman G. B., Kamiya R. A Chlamydomonas outer arm dynein mutant with a truncated β heavy chain. J. Cell Biol. 1993;122:653–661. doi: 10.1083/jcb.122.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakato M., King S. Calcium regulates ATP-sensitive microtubule binding by Chlamydomonas outer arm dynein. J. Biol. Chem. 2003;278:43571–43579. doi: 10.1074/jbc.M305894200. [DOI] [PubMed] [Google Scholar]
- Samso M., Koonce M. 25 Å-resolution structure of a cytoplasmic dynein motor reveals a seven-member planar ring. J. Mol. Biol. 2004;340:1059–1072. doi: 10.1016/j.jmb.2004.05.063. [DOI] [PubMed] [Google Scholar]
- Samso M., Radermacher M., Frank J., Koonce M. P. Structural characterization of a dynein motor domain. J. Mol. Biol. 1998;276:927–937. doi: 10.1006/jmbi.1997.1584. [DOI] [PubMed] [Google Scholar]
- Schumacher M. A., Rivard A. F., Bachinger H. P., Adelman J. P. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410:1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- Silvanovich A., Li M.-g., Serr M., Mische S., Hays T. The third P-loop domain in cytoplasmic dynein heavy chain is essential for dynein motor function and ATP-sensitive microtubule binding. Mol. Biol. Cell. 2003;14:1355–1365. doi: 10.1091/mbc.E02-10-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S., Kamiya R. Functional reconstitution of Chlamydomonas outer dynein arms from α- β and γ subunits: requirement of a third factor. J. Cell Biol. 1994;126:737–745. doi: 10.1083/jcb.126.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S., Sakakibara H., Kamiya R. Three-headed outer arm dynein from Chlamydomonas that can functionally combine with outer-arm-missing axonemes. J. Biochem. (Tokyo) 1992;111:758–762. doi: 10.1093/oxfordjournals.jbchem.a123832. [DOI] [PubMed] [Google Scholar]
- Takada S., Wilkerson C. G., Wakabayashi K., Kamiya R., Witman G. B. The outer dynein arm-docking complex: composition and characterization of a subunit (Oda1) necessary for outer arm assembly. Mol. Biol. Cell. 2002;13:1015–1029. doi: 10.1091/mbc.01-04-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner V., Gessner G., Heiland I., Kaminski M., Hawat S., Scheffler K., Mittag M. Analysis of the phosphoproteome of Chlamydomonas reinhardtii provides new insights into various cellular pathways. Eukaryot. Cell. 2006;5:457–468. doi: 10.1128/EC.5.3.457-468.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., King S. M. Modulation of Chlamydomonas reinhardtii flagellar motility by redox poise. J. Cell Biol. 2006;173:743–754. doi: 10.1083/jcb.200603019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., Takada S., Witman G. B., Kamiya R. Transport and arrangement of the outer-dynein-arm docking complex in the flagella of Chlamydomonas mutants that lack outer dynein arms. Cell Motil. Cytoskelet. 2001;48:277–286. doi: 10.1002/cm.1015. [DOI] [PubMed] [Google Scholar]
- Wilkerson C. G., King S. M., Witman G. B. Molecular analysis of the γ heavy chain of Chlamydomonas flagellar outer-arm dynein. J. Cell Sci. 1994;107:497–506. doi: 10.1242/jcs.107.3.497. [DOI] [PubMed] [Google Scholar]
- Witman G. B. Isolation of Chlamydomonas flagella and flagellar axonemes. Methods Enzymol. 1986;134:280–290. doi: 10.1016/0076-6879(86)34096-5. [DOI] [PubMed] [Google Scholar]
- Wu H., Maciejewski M. W., Marintchev A., Benashski S. E., Mullen G. P., King S. M. Solution structure of a dynein motor domain associated light chain. Nat. Struct. Biol. 2000;7:575–579. doi: 10.1038/76804. [DOI] [PubMed] [Google Scholar]
- Yap K., Kim J., Truong K., Sherman M., Yuan T., Ikura M. Calmodulin target database. J. Struct. Funct. Genom. 2000;1:8–14. doi: 10.1023/a:1011320027914. [DOI] [PubMed] [Google Scholar]