Abstract
Acetyl phosphate, the intermediate of the AckA-Pta pathway, acts as a global signal in Escherichia coli. Although acetyl phosphate clearly signals through two-component response regulators, it remains unclear whether acetyl phosphate acts as a direct phospho donor or functions through an indirect mechanism. We used two-dimensional thin-layer chromatography to measure the relative concentrations of acetyl phosphate, acetyl coenzyme A, ATP, and GTP over the course of the entire growth curve. We estimated that the intracellular concentration of acetyl phosphate in wild-type cells reaches at least 3 mM, a concentration sufficient to activate two-component response regulators via direct phosphoryl transfer.
Bacterial cells must respond properly to diverse external and internal cues, a process that requires global signaling. The ideal global signal is metabolically inexpensive, short-lived, indicative only of significant changes, and capable of effecting the coordinated regulation of diverse cellular processes. Several lines of evidence suggest that the small molecule acetyl phosphate (acetyl-P) can serve as such a global signal.
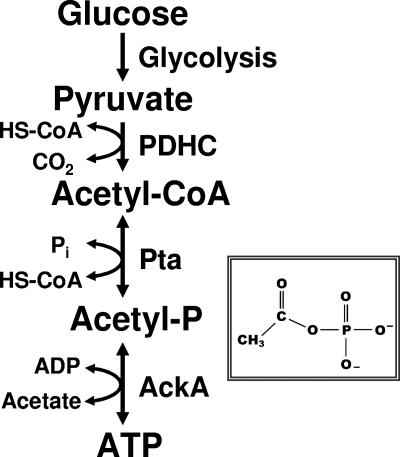
Acetyl-P is the high-energy, acid/base-labile intermediate of the reversible Pta-AckA pathway (Fig. 1). This pathway interconverts coenzyme A (HS-CoA), ATP, and acetate with acetyl coenzyme A (acetyl-CoA), ADP, and orthophosphate (Pi) (9, 43). The reversibility of this pathway permits both acetyl-CoA synthesis (acetate activation) and acetate evolution (acetogenesis). During acetogenesis, Pta synthesizes acetyl-P and HS-CoA from acetyl-CoA and Pi, while AckA generates ATP from acetyl-P and ADP. Simultaneously, AckA produces acetate, which cells excrete into the environment. Thus, the steady-state concentration of acetyl-P depends upon the rate of its formation catalyzed by Pta and the rate of its degradation catalyzed by AckA (for reviews, see references 45 and 59).
FIG. 1.
Schematic diagram of the acetate kinase (AckA)-phosphotransacetylase (Pta) pathway that interconverts acetyl-CoA and acetate. PDHC, pyruvate dehydrogenase complex. (Inset) Molecular formula of acetyl-P.
Acetyl-P has been proposed to act as a global regulator by direct phosphorylation of response regulators (RRs) of the family of two-component signal transduction (2CST) pathways (34, 55). The simplest of these 2CST pathways consists of a histidine kinase (HK) and an RR. Using ATP as its phosphoryl donor, the HK autophosphorylates a conserved histidine residue. In turn, the RR autophosphorylates a conserved aspartate residue, using its phosphorylated cognate HK as its phosphoryl donor. Experiments performed in vitro have clearly demonstrated that acetyl-P can donate its phosphoryl group to purified RRs but not to HKs (for reviews, see references 52 and 57). This ability arises from its capacity to store energy. Acetyl-P possesses a larger ΔG° of hydrolysis (−43.3 kJ/mol) than ATP possesses (−30.5 kJ/mol in complex with Mg2+). This difference forms the basis for the role of acetyl-P in generating ATP (for a review, see reference 59).
Although acetyl-P can function as a phosphoryl donor in vitro, its ability to function in vivo as a global signal has remained essentially unproven, despite a wealth of seemingly supportive data (for a review, see reference 59). There are two prime reasons for this lack of proof. First, direct observation in vivo of phosphoryl transfer from a donor to an RR is extremely challenging from a technical standpoint (14), and even this difficult procedure cannot distinguish the phosphoryl donor. Second, the genetic experiments performed in lieu of such a direct demonstration have been less than definitive (for a review, see reference 59). Recently, however, some of us provided conclusive genetic evidence that acetyl-P can indeed function as a global signal in Escherichia coli. Using epistasis analysis, we showed that acetyl-P could regulate the biogenesis of flagella and the biosynthesis of capsule via the 2CST RR RcsB (16). Since RcsB is known to regulate some 5% of the E. coli genome (for reviews, see references 28 and 39), acetyl-P must now be considered a global signal.
Controversy still simmers, however, around the question of mechanism. In vitro, acetyl-P clearly donates its phosphoryl group to many RRs (13, 27, 59). In vivo in wild-type (WT) cells, however, the biochemical relevance of this mechanism remains unclear. The problem hinges on the discordance between estimates of the acetyl-P concentration in vivo and the concentration required for efficient phosphorylation in vitro. The reported concentrations necessary to efficiently autophosphorylate two purified RRs (CheY and PhoB) tend to be in the low to middle millimolar range (12, 31, 32). However, most measurements of the intracellular concentration of acetyl-P have yielded values in the 40 to 300 μM range, with one study reporting a value of 1.3 mM (33, 40), Thus, the intracellular concentration of acetyl-P appears to be about 10% of that necessary for acetyl-P to function as a direct phosphoryl donor in vivo.
A variety of biochemical and enzymatic assays and growth conditions have been employed to investigate the intracellular concentration of acetyl-P, and the resulting values have varied widely (19-21, 33, 40). Moreover, the previous reports have (with limited exceptions) relied upon data gathered from only one or two time points, which may not represent the peak internal concentrations of acetyl-P. Furthermore, the lability of acetyl-P makes determination of its concentration a significant challenge. Clearly, fluctuations in the acetyl-P concentration or the lability of the molecule could contribute to a significant underestimate of the actual intracellular acetyl-P concentration. If so, then a difference between the required concentration in vitro and the availability in vivo would not exist. Such a result would influence our thinking regarding potential mechanisms of acetyl-P function as a global regulator.
To address this gap in our knowledge, we optimized a simple two-dimensional thin-layer chromatographic (2D-TLC) method pioneered by Bochner and Ames (4, 5) and adapted by McCleary and Stock (33) to measure the relative concentrations of acetyl-P, acetyl-CoA, ATP, and GTP over the course of the entire growth curve under defined growth conditions. Using this assay, we estimated the intracellular concentration of acetyl-P in WT cells to be approximately 3 mM, a concentration high enough for acetyl-P to potentially function as a direct phospho donor.
MATERIALS AND METHODS
Bacterial strains.
All bacterial strains used in this study are listed in Table 1. All strains evaluated were derivatives of AJW678, which is WT for acetate metabolism (23). Derivatives were constructed by generalized transduction with P1kc, as described previously (48).
TABLE 1.
Strains used in this study
| Strain | Relevant genotype | Reference or source |
|---|---|---|
| AJW678 | thi-1 thr-1(Am) leuB6 metF159(Am) rpsL136 lacX74 | 23 |
| AJW1729 | AJW678 poxB::Km | 23 |
| AJW1781 | AJW678 Δ(acs::Km-1) | 24 |
| AJW1939 | AJW678 ackA::Km | 23 |
| AJW2013 | AJW678 (ackA pta hisJ hisP dhu) zej223::Tn10 | 60 |
| AJW2399 | AJW678 (ackA pta hisJ hisP dhu) zej223::Tn10 poxB::Km | (P1)AJW1729 × AJW2013 (Kmr) |
| AJW3000 | MC4100 (ackA pta hisJ hisP dhu) zej223::Tn10 tdcD | (P1)AJW2013 × RM227 (Tcr/AceHI−) |
| CP904 | thi-1 thr-1(Am) leuB6 metF159(Am) rpsL136 Δ(pta-1::Tn10-lacZ) | 40 |
| MC4100 | F−araD139 (argF-lac)U169 ptsF25 deoC1 relA1 flbB530 rpsL150 | 10 |
| RM227 | MC4100 tdcD | 18 |
Media and growth conditions.
Cells were grown on LB plates containing 1% (wt/vol) tryptone, 0.5% (wt/vol) yeast extract, 0.5% (wt/vol) NaCl, and 1.5% (wt/vol) Bacto agar. When required, kanamycin or tetracycline was added to a final concentration of 40 μg/ml or 15 μg/ml, respectively. 10× MOPS (morpholinepropanesulfonic acid) salts was prepared as described previously (36), with the exception of the K2HPO4 concentration. To facilitate the uptake of 32Pi, the K2HPO4 concentration was reduced 10-fold so that the final concentration in 1× modified MOPS medium (mMOPS) was 0.2 mM (4). 1× mMOPS was supplemented with 0.8% sodium pyruvate, 100 μg/ml l-threonine, l-histidine, l-leucine, l-methionine, and 10 μg/ml thiamine.
Labeling of cells.
All cultures were shaken at 250 rpm and incubated at 37°C. To achieve microaerophilic conditions, overnight cultures were diluted to an optical density at 600 nm of approximately 0.03 in enough medium to fill approximately 70 to 80% of a 12-mm test tube. This ensured that enough culture would be available for sampling without reducing the culture volume to less than 30% of the tube volume. For each experiment, two parallel cultures were prepared. One culture was labeled with 30 μCi/ml 32Pi (carrier free in dilute HCl [pH 2 to 3]; GE Healthcare, Piscataway, NJ). The other was used to determine optical density at 600 nm. All cultures were incubated for approximately 1.5 h prior to the first sampling.
Preparation of labeled extracts.
Aliquots (100 μl) were collected from 32Pi-labeled cultures at 35- to 90-min intervals, placed into 1.5-ml microcentrifuge tubes with 10 μl ice-cold 11 N formic acid (Fisher, Hanover Park, IL), and incubated in an ice bath for 30 min (4).
Following incubation, unincorporated Pi was precipitated with 16.5 μl precipitate solution (200 mM sodium tungstate, 200 mM tetraethylammonium-HCl, 50 mM procaine) as described previously (5). Samples were either processed immediately or shock frozen in an ethanol-dry ice bath until further use. The precipitate was pelleted in a microcentrifuge at 4°C and 15,800 × g for 15 min. One hundred microliters of the supernatant was immediately neutralized with 8 μl of 2-picoline (4), and samples were stored at −20°C until 2D-TLC separation.
2D-TLC.
Separation of acetyl-P and acetyl-CoA was performed on EMD polyethyleneimine cellulose-F plates (EMD Chemicals, La Jolla, CA). A 5-μl sample was spotted on the lower left corner of a 10- by 10-cm plate, allowed to dry under a fume hood for 40 min, and then washed in methanol (each plate was taken by the margin corner, immersed in methanol, agitated back and forth eight or nine times, removed, and shaken gently to eliminate excess methanol). This washing process was repeated two more times, and the plate was dried for 40 min under the hood and then subjected to 2D-TLC as described by McCleary and Stock (33). The first dimension consisted of development in 0.52 M LiCl (Mallinckrodt, Phillipsburg, NJ) and 1% (vol/vol) glacial acetic acid (Fisher, Hanover Park, IL); the second dimension consisted of development in 1.0 M ammonium acetate and 0.35 M ammonium chloride (both from Sigma, St. Louis, MO), with the pH adjusted to 3.5 with glacial acetic acid. Plates were developed for 45 min in the first dimension, dried thoroughly for 40 min, soaked in methanol for 15 min, dried again, and then developed in the second dimension for 90 min.
Determining the position of acetyl-P on a 2D-TLC Plate.
The migration of acetyl-P in E. coli extracts was determined by comparison to a labeled standard. Synthetic [32P]acetyl-P was prepared as previously described (33) and diluted 1:1,000. Five-microliter aliquots were processed by 2D-TLC (as described above), yielding signals corresponding to the positions of acetyl-P and Pi.
Instability of acetyl-P during TLC analysis.
Analysis of labeled extracts resulted in detection of significant quantities of Pi in ackA mutant cells (strain AJW1939 [Table 1]) and very little Pi in ackA pta mutant cells (strain AJW2013 [Table 1]). Since the precipitation procedure removes more than 99.9% of the Pi present in the formic acid extract (5), this suggested that the appearance of Pi might result from instability of acetyl-P during 2D-TLC. To test this hypothesis, we prepared synthetic [32P]acetyl-P, precipitated the unincorporated Pi, subjected the acetyl-P to 2D-TLC, and found that the separation procedure released Pi (data not shown). Furthermore, regression analysis of separated extracts from both mutants and their WT parent (strain AJW678 [Table 1]) suggested a precursor-product relationship between acetyl-P and the Pi (data not shown) and that 45% of the acetyl-P signal may be present in the form of hydrolyzed Pi. Because of uncertainty regarding its source, however, the Pi was not used to determine internal concentrations of acetyl-P. To minimize the effect of breakdown and to ensure the comparability of samples taken at each time point, all samples representing a single time point harvested from multiple cultures and, wherever possible, all samples representing sets of two or more time points were processed simultaneously.
Quantification of incorporated 32P.
Processed plates were thoroughly dried and exposed to a phosphor screen for 18 to 24 h. Detection of radioactivity was performed with a Typhoon 8600 variable-mode imager (Molecular Dynamics Inc., Sunnyvale, CA), and the results were analyzed using the bundled ImageQuant software without background correction. The signal produced by either acetyl-P or acetyl-CoA was calculated as a percentage of the total signal of the TLC plate.
Detection of ATP and GTP.
ATP and GTP also were separated on EMD polyethyleneimine cellulose-F plates, using the Tb and Sb buffers as described by Bochner and Ames (4) with the exception that the development times were shortened for the first and second dimensions to 30 and 45 min, respectively. The incorporation of radiolabeled phosphate into ATP and GTP was quantified in the manner described above for acetyl-P and acetyl-CoA.
Acetate assay.
Acetate measurements for the culture supernatant were obtained using an acetic acid kit according the manufacturer's instructions (r-biopharm, Darmstadt, Germany).
RESULTS
Growth effects of Pi concentration.
In an effort to distinguish among potential models of acetyl-P action upon 2CST pathways, we sought to monitor the acetyl-P pool in WT cells and in isogenic mutants defective in acetate metabolism. Although several indirect techniques for the measurement of acetyl-P have been reported, we chose to use a modified version of the method of Bochner and Ames (4, 5, 33), which involves labeling of cells with 32Pi and separation of phosphorylated molecules by 2D-TLC. This method has two primary advantages. First, it allows rapid direct analysis of acetyl-P, which is critical because of the extreme lability of the molecule. Second, it also allows simultaneous measurement of acetyl-P, acetyl-CoA, ATP, and other small, phosphorylated compounds.
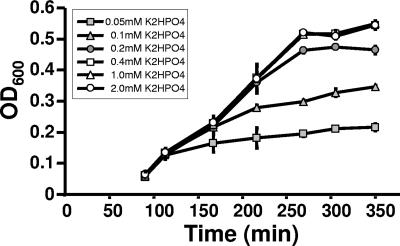
To facilitate the uptake of 32Pi, we reduced the concentration of K2HPO4 (1.32 mM) in MOPS minimal medium (complete MOPS) (36). To determine the minimal phosphate concentration necessary to maintain the growth rate and endpoint optical density at levels comparable to those of cells cultured in complete MOPS, we monitored the growth of WT cells (strain AJW678 [Table 1]) exposed to a range of K2HPO4 concentrations from 0.05 to 2.0 mM (Fig. 2). When the medium was supplemented with 0.2 mM K2HPO4, WT cells grew at the same rate and to almost the same optical density as cells grown in the presence of higher concentrations, as reported previously by Bochner and Ames (4). Although cells cultured at concentrations below 0.2 mM also began to grow at the same growth rate, the cultures inevitably had reduced endpoint optical densities. When these stationary-phase cultures were provided with additional K2HPO4 (up to 2.0 mM), growth resumed and the cultures reached an endpoint optical density similar to that obtained with 2.0 mM (data not shown). Thus, we concluded that entry into stationary phase occurred because the phosphate became depleted. Since isogenic ackA and ackA pta mutants assayed similarly yielded similar results (data not shown), we routinely used MOPS medium supplemented with 0.2 mM K2HPO4 (referred to as mMOPS) for all further studies.
FIG. 2.
Effect of orthophosphate on growth of WT cells (strain AJW678) aerated at 37°C in MOPS medium supplemented with 0.8% pyruvate and containing K2HPO4 at the following concentrations: 2.0, 1.0, 0.4, 0.2, 0.1, and 0.05 mM. The plotted values are means of two independent experiments ± standard deviation. OD600, optical density at 600 nm.
Identification of signals.
To identify the signals that correspond to acetyl-P, Pi, and acetyl-CoA, we used three different approaches.
(i) Identification by spiking.
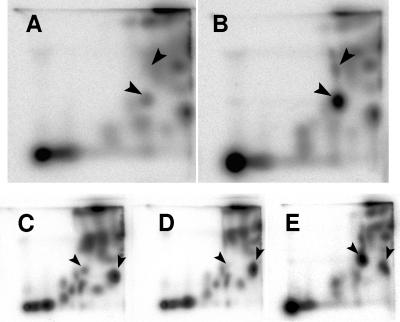
Synthetic [32P]acetyl-P was used to spike formic acid extracts of 32Pi-labeled WT cells (AJW678) and thereby determine the position corresponding to acetyl-P (Fig. 3A and B). To determine the positions of Pi and acetyl-CoA, WT cell extracts were similarly spiked with 32Pi and [1-14C]acetyl-CoA (data not shown).
FIG. 3.
Identification of the acetyl-P signal following separation of small molecules by 2D-TLC. (A and B) Extracts of (A) WT cells (strain AJW678) and (B) WT cells spiked with synthetic [32P]acetyl-P. The arrowheads indicate orthophosphate (upper arrowhead) and acetyl-P (lower arrowhead). (C to E) Extracts of (C) WT (strain AJW678), (D) ackA pta mutant (strain AJW2013), and (E) ackA mutant (strain AJW1939). The arrowheads indicate acetyl-P (left arrowhead) and acetyl-CoA (right arrowhead).
(ii) Verification using isogenic Pta-AckA pathway mutants.
Pta and AckA are the only enzymes known to produce acetyl-P in E. coli (for a review, see reference 59); therefore, an isogenic ackA pta mutant (strain AJW2013 [Table 1]) could be expected to produce no signal corresponding to acetyl-P. Conversely, an isogenic ackA mutant (strain AJW1939 [Table 1]) should be unable to convert acetyl-P to acetate and could, therefore, be expected to exhibit an increased signal corresponding to the position of acetyl-P. Indeed, relative to their WT parent, the ackA pta mutant and the ackA mutant exhibited a significant decrease and a significant increase, respectively, in the signal at the location that corresponds to acetyl-P (Fig. 3C to E).
(iii) Further verification by acetyl-P production.
To verify the assignment, a pta mutant (strain AJW612 [Table 1]), which can utilize exogenous acetate to form acetyl-P via AckA but cannot convert that acetyl-P to acetyl-CoA, was grown on pyruvate or on pyruvate supplemented with acetate. When grown on pyruvate, this pta mutant (and all other cells that lacked Pta, including cells with two different ackA pta deletions in three different genetic backgrounds) produced a signal that comigrated with acetyl-P (referred to as spot X [see below]). However, when grown on pyruvate supplemented with acetate, this strain produced a significant spot at a position corresponding to the position of acetyl-P (data not shown). These data confirm the position of the signal corresponding to acetyl-P. Interestingly, cells cultured with pyruvate supplemented with propionate (reported to be a secondary substrate for AckA [6, 53]) produced a species that comigrated with acetyl-P.
Characterization of spot X.
To determine the lower limit of detection for acetyl-P, we next investigated the nature of spot X, the faint signal that comigrates with acetyl-P in pta ackA and pta mutants. Several attempts to separate spot X from acetyl-P using different buffer pairs failed (data not shown). Furthermore, spot X exhibited lability during extraction similar to that of acetyl-P (data not shown). Thus, we hypothesized that the comigrating signal might result from a compound X with properties quite similar to those of acetyl-P. Because we had observed that acetyl-P and propionyl phosphate (propionyl-P) comigrate, we hypothesized that spot X might represent propionyl-P. Alternatively, E. coli could possess an AckA-Pta-independent means of producing acetyl-P.
Three distinct E. coli enzymes are known to synthesize propionyl-P: Pta (from acetyl-CoA), AckA (from acetate), and TdcD (a propionyl kinase involved in threonine degradation; also from acetate) (18, 59). Since an ackA pta tdcD mutant produced spot X (data not shown), we concluded that spot X is unlikely to be propionyl-P or that E. coli possesses a novel means of producing propionyl-P.
Having eliminated Pta, AckA, and TdcD as possible sources of spot X, we considered the possibility that E. coli harbors an additional pathway for synthesis of acetyl-P. One possible source of acetyl-P is the enzyme EutD, a Pta paralog proposed to convert acetyl-CoA to acetyl-P during ethanolamine catabolism (50). However, the eut operon is not induced unless both ethanolamine and adenosyl-B12 are present in the environment (42, 47). Therefore, EutD would not be expressed under the conditions tested. A second source might be the enzyme PoxB, which is reported to oxidize pyruvate to acetate (2, 3, 17). The instability of acetyl-P combined with the fact that at least one other “acetate-forming” enzyme (Xsc) was recently shown to produce acetyl-P (44) suggested to us that the major product of PoxB activity might be acetyl-P that was destroyed in the assay process. Therefore, the WT and mutants isogenic for poxB (strain AJW1729 [Table 1]), pta (strain AJW612), and ackA pta poxB (strain AJW2399 [Table 1]) were assayed. No difference in the spot X signals produced by the pta and ackA pta poxB mutants was observed (data not shown). Thus, PoxB is not responsible for the synthesis of compound X.
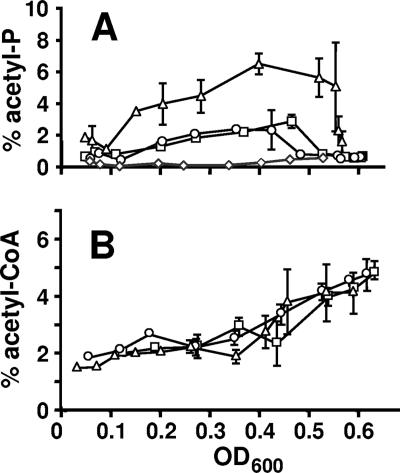
Although we were unable to eliminate the synthesis of spot X or to determine its origin, it appeared to be a relatively minor species during much of the growth curve. During early exponential growth (Fig. 4A), the signal corresponding to acetyl-P in WT cells accounted for approximately 0.6% of the total phosphorylated compounds, whereas the signal corresponding to spot X accounted for approximately 0.2%. Similarly, after entry into stationary phase, the acetyl-P signal in WT cells and spot X in ackA pta mutant cells both accounted for approximately 0.7%. Thus, assuming that the status of the Pta-AckA pathway does not influence the synthesis or degradation of compound X in WT cells, the background contributed by spot X constituted a substantial amount of the signal ascribed to acetyl-P during early exponential growth and the vast majority during stationary phase. Thus, while the steady-state concentration of acetyl-P throughout exponential growth greatly exceeded that of compound X, the concentrations at the earliest and latest time points may represent the lower limit of detection rather than the actual acetyl-P concentration. Because of uncertainty as to the nature of spot X, we chose not to subtract this background from our measurements.
FIG. 4.
Comparison of acetyl-P and acetyl-CoA pools from cells aerated at 37°C in mMOPS supplemented with 0.8% pyruvate. Samples were harvested at regular intervals, extracts were prepared, and small molecules were separated by 2D-TLC. (A) Signal ascribed to acetyl-P plotted as a percentage of the total radioactivity applied to the plate. □, WT (strain AJW678); ○, acs mutant (strain AJW1781); ▵, ackA mutant (strain AJW1939); ⋄, ackA pta mutant (strain AJW2013). (B) Signal ascribed to acetyl-CoA plotted as a percentage of the total radioactivity applied to the plate. □, WT (strain AJW678); ○, acs mutant (strain AJW1781); ▵, ackA mutant (strain AJW1939). The plotted values are the means of two independent experiments ± standard deviation. OD600, optical density at 600 nm.
Characterization of acetyl-P.
To characterize the acetyl-P pool, we simultaneously analyzed the WT (strain AJW678) and isogenic mutants defective for ackA (strain AJW1939) or ackA pta (strain AJW2013). We also analyzed a mutant defective for acetyl-CoA synthetase (Acs), a high-affinity enzyme responsible for the assimilation of millimolar amounts (<5 mM) of environmental acetate (strain AJW1781 [24]). For each of these four strains, we monitored growth, the extracellular acetate concentration, and the intracellular concentrations of both acetyl-CoA and acetyl-P.
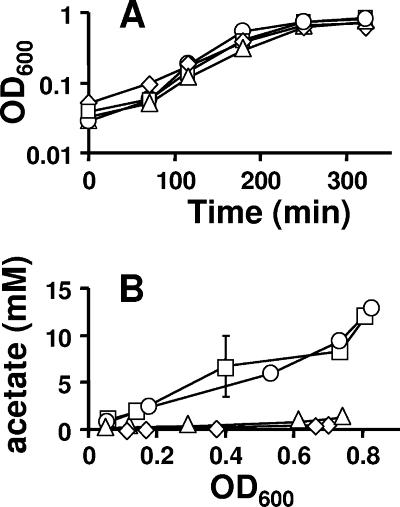
All four strains grew at approximately the same rate and to nearly the same final density (Fig. 5A). WT cells and mutants lacking Acs excreted equally large amounts of acetate (upwards of 15 mM); in contrast, cells lacking AckA or both AckA and Pta excreted very little acetate (<1 mM) (Fig. 5B), as reported previously (11, 40, 50). The percentages of phosphorylated molecules corresponding to acetyl-CoA were similar in all four strains, holding steady between 1.5 and 3% of the total phosphorylated molecules until just prior to entry into stationary phase, whereupon they increased rapidly to about 5% (Fig. 4B and data not shown). In contrast, the percentage of phosphorylated molecules corresponding to acetyl-P (and/or spot X) varied from strain to strain. In WT cells and in cells that lacked Acs, the signal reached about 2 to 3%; in cells that lacked AckA, it reached about 7%, and in cells that lacked both AckA and Pta, it remained considerably below 1% for much of the growth curve (Fig. 4A).
FIG. 5.
Comparison of growth (A) and extracellular acetate concentration (B) of cells aerated at 37°C in mMOPS supplemented with 0.8% pyruvate. □, WT (strain AJW678); ○, acs mutant (strain AJW1781); ▵, ackA mutant (strain AJW1939); ⋄, ackA pta mutant (strain AJW2013). The plotted values are the means of two independent experiments ± standard deviation. OD600, optical density at 600 nm.
Relative concentration of acetyl-P.
Because ATP is well studied, well separated by 2D-TLC, and relevant to the current study, we chose it as a standard against which we could evaluate the intracellular acetyl-P concentration. Samples collected from WT cells (Fig. 6) and from cells of the acs mutant (data not shown) were separated by 2D-TLC using a solvent system known to isolate ATP and GTP (4).
FIG. 6.
Comparison of ATP (▵), acetyl-P (□), acetyl-CoA (○), and GTP (⋄) from WT cells (strain AJW678) aerated at 37°C in mMOPS supplemented with 0.8% pyruvate. Samples were harvested at regular intervals, extracts were prepared, and small molecules were separated by 2D-TLC. Signals ascribed to small molecules were plotted as percentages of the total radioactivity applied to the plate. The plotted values are the means of two independent experiments ± standard deviation. OD600, optical density at 600 nm; phos'd cmpd, phosphorylated compound.
At the height of WT acetyl-P accumulation, approximately four generations after labeling with 32Pi, incorporation into ATP accounted for nearly 9.5% of the signal produced by the 32P label, whereas acetyl-P accounted for nearly 3.0%. In contrast, GTP and acetyl-CoA each accounted for about 2% of the total signal.
DISCUSSION
Evidence that acetyl-P can function as a global signal.
Acetyl-P was first advanced as a global signal (34, 55) on the basis of two observations: (i) select RRs became phosphorylated in vitro in the presence of acetyl-P alone (13, 27) and (ii) known targets of these RRs became activated in vivo in an HK-independent manner (13, 56).
It is now known that many purified RRs can autophosphorylate in vitro using acetyl-P as their phospho donor and that numerous RR targets can be regulated in vivo in an AckA-Pta pathway-dependent manner (for a review, see reference 59). Moreover, DNA array analysis has implicated acetyl-P in the regulation of almost 100 genes, most of which are involved in the assembly of surface structures required for proper biofilm development (60). Recently, the RR RcsB, which controls as much as 5% of the E. coli genome (for reviews, see references 28 and 39), was shown to mediate much of the demonstrated AckA-Pta pathway-dependent regulation (16). These observations lend strong support to the hypothesis that acetyl-P can function as a global signal by directly affecting the activation state of at least one global RR, but they do not directly address the issue of mechanism.
Here, we showed that the intensity of the signal ascribed to acetyl-P was approximately one-third the signal intensity ascribed to ATP. Since each molecule of ATP represents three equivalents of 32P label and each molecule of acetyl-P represents one such equivalent, our measurements indicate that the concentrations of ATP and acetyl-P are approximately equivalent under the conditions tested. Since the intracellular ATP concentration of enteric bacteria has been reported to be about 3 mM (4, 26, 29, 58; for a review, see reference 37), we concluded that the concentration of acetyl-P is also about 3 mM. Implicit in this calculation is the assumption that all three of the phosphates within ATP are turned over at approximately the same rate. However, we are unaware of any report where this has been tested. It is also clear that the concentration of acetyl-P reported here is a peak value. The acetyl-P concentration clearly varies with the type of carbon source. For example, glucose-grown cells show a 50% reduction in acetyl-P compared to cells cultured in the presence of pyruvate. Future studies will examine the relationship between carbon source and acetyl-P in greater detail.
Furthermore, the results of this study suggest that this value may underrepresent the actual concentration of acetyl-P. We consistently observed a species that comigrated with Pi during analysis of acetyl-P in extracts. We believe that this Pi is derived at least in part from the breakdown of acetyl-P during analysis. First, the sodium tungstate precipitation method is expected to remove more than 99.9% of the unincorporated Pi. Second, increases in Pi correlate with increases in acetyl-P. Third, the first-dimension buffer is relatively acidic (pH 2), suggesting that a substantial amount of the acid-labile acetyl-P could be hydrolyzed during the TLC process. If we assume that the Pi is derived from hydrolyzed acetyl-P, then the concentration of acetyl-P in WT cells may actually reach 4.5 mM. If so, then the concentration of acetyl-P in ackA mutants may reach 15 mM.
Elevated ionic strength inhibits the autophosphorylation of CheY by small-molecule phospho donors, including acetyl-P (31), and if the ionic strength is kept constant, the autophosphorylation rate increases linearly with substrate concentration and does not saturate (12). Thus, CheY autophosphorylation does not follow Michaelis-Menten kinetics and hence the Km cannot be calculated. Rather, it appears that autophosphorylation occurs as rapidly as the substrate-enzyme complex can form. If so, then as the intracellular acetyl-P concentration increases, so too should autophosphorylation.
To the best of our knowledge, a corresponding experiment has not been performed with any other RR. However, there is no reason to believe that other RRs would behave differently than CheY. Indeed, it is anticipated that different RRs would possess distinct intrinsic rate constants for autophosphorylation and thus be differentially sensitive to the status of the acetyl-P pool. Even then, the status of the acetyl-P pool would significantly influence the activation state of an RR only when the rate of phosphoryl transfer from a phosphorylated HK was not substantially greater than the rate of transfer from acetyl-P. This would explain why CheY activation responds to the status of the Pta-AckA pathway in the absence of its cognate HK CheA but not in its presence; i.e., the rate of transfer from phospho-CheA is several orders of magnitude greater than that from acetyl-P (12, 31). However, some RRs do not possess a cognate HK or are present in large excess over their cognate HKs. At least one example of each class of RR (RssB and OmpR, respectively) appears to be sensitive to the intracellular acetyl-P pool (1, 8, 30). Also, some RRs have cognate HKs that possess phospho-aspartyl phosphatase activity in addition to their kinase activity. Under conditions that favor the phosphatase activity of its cognate HK, an intrinsically sensitive RR could respond to the status of the acetyl-P pool. This appears to be the case with NRI (NtrC) (38) and RcsB (16). Thus, we would expect that the concentration of acetyl-P in a WT cell would be capable of altering the phosphorylation state of a subset of intrinsically sensitive RRs. Furthermore, the larger pool found in ackA mutants would permit activation of a larger subset of RRs (for a review, see reference 59).
Acetyl-P-acetyl-CoA relationship.
The steady-state concentration of acetyl-CoA remained constant throughout most of exponential growth before increasing about twofold just prior to entry into stationary phase (Fig. 4B). Interestingly, the intracellular concentration of acetyl-CoA was unaffected by the status of the AckA-Pta pathway. Given that disruption of this pathway causes increased expression of genes that encode ATPase and enzymes of the tricarboxylic acid cycle but not HS-CoA biosynthesis (60), it appears that the mutants compensate for the inability to use the Pta-AckA pathway to recycle HS-CoA by increasing carbon flux into the tricarboxylic acid cycle and respiration.
In WT cells, the increase in the acetyl-CoA pool observed during entry into stationary phase coincided with the decline in the acetyl-P pool. Since both acs and ackA mutants also exhibited an increase in acetyl-CoA concentration, the increase in acetyl-CoA cannot be explained by reactivation of previously excreted acetate. It more likely results from a reduction in the conversion of acetyl-CoA to acetyl-P. We can envision two potential mechanisms for the increase in acetyl-CoA. First, the reduction may be due to a shift in the equilibrium of Pta activity towards the synthesis of acetyl-CoA. This could result from a lack of sufficient Pi to drive the acetyl-P-forming reaction (although the concentration would have to be higher than that required for phosphate starvation because entry into stationary phase occurred about one-half a generation later). Second, the increase in acetyl-CoA could result from a reduction in Pta activity altogether.
Coincident with the increase in acetyl-CoA, we observed a decrease in acetyl-P during entry into stationary phase. Furthermore, the acetyl-P pool declined rapidly even in the absence of AckA. Acetyl-P is stable at neutral pH and 30°C for 10 to 15 h (54); thus, it is unlikely that the pool degraded by noncatalytic hydrolysis. Instead, acetyl-P depletion likely resulted from transfer of the phosphoryl group to RRs and from the activity of other enzymes that can hydrolyze acetyl-P, albeit with lower efficiency than AckA. These enzymes include the putative acylphosphatase YccX (51) and a large number of HD hydrolases (25).
Concluding remarks.
We have shown that acetyl-P reaches a concentration of no less than 3 mM and that the concentration varies by at least fivefold. In the context of the current literature, these observations strongly suggest that acetyl-P can act as a direct phosphoryl donor in vivo and that no novel mechanism has to be envisioned to explain the effect of acetyl-P on RR-dependent transcription and/or behavior.
It should be noted, however, that these observations do not argue against any alternative mechanism. It has been reported that acetyl-P can substitute for phosphoenolpyruvate as the phospho donor for purified enzyme I of the phosphoenolpyruvate:carbohydrate phosphotransferase system (15) and can act as an allosteric effector for HPr kinase/phosphatase purified from the gram-positive organism Bacillus subtilis (41), for certain eukaryotic ATPases (7, 22, 49), and for poly-β-hydroxybutyrate synthase from cyanobacteria (35, 46). To the best of our knowledge, however, there have been no reports demonstrating that any of these interactions can occur in vivo.
Acknowledgments
We thank David Thach for help in optimizing the sodium tungstate precipitation, Gary Sawers (Max-Planck Institut für Terrestrische Mikrobiologie, Marburg, Germany) for providing strains, and Robert Bourret (University of North Carolina, Chapel Hill) for critical discussions.
We thank the National Institute of General Medical Sciences (grant GM066130) for providing funding to A.J.W. and the United States Department of Agriculture (award 2005-35319-15304) for providing funding to D.H.K.
Footnotes
Published ahead of print on 1 June 2007.
REFERENCES
- 1.Bang, I. S., J. P. Audia, Y. K. Park, and J. W. Foster. 2002. Autoinduction of the ompR response regulator by acid shock and control of the Salmonella enterica acid tolerance response. Mol. Microbiol. 44:1235-1250. [DOI] [PubMed] [Google Scholar]
- 2.Bertagnolli, B. L., and L. P. Hager. 1991. Activation of Escherichia coli pyruvate oxidase enhances the oxidation of hydroxyethylthiamin pyrophosphate. J. Biol. Chem. 266:10168-10173. [PubMed] [Google Scholar]
- 3.Bertagnolli, B. L., and L. P. Hager. 1993. Role of flavin in acetoin production by two bacterial pyruvate oxidases. Arch. Biochem. Biophys. 300:364-371. [DOI] [PubMed] [Google Scholar]
- 4.Bochner, B. R., and B. N. Ames. 1982. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J. Biol. Chem. 257:9759-9769. [PubMed] [Google Scholar]
- 5.Bochner, B. R., and B. N. Ames. 1982. Selective precipitation orthophosphate from mixtures containing labile phosphorylated metabolites. Anal. Biochem. 122:100-107. [DOI] [PubMed] [Google Scholar]
- 6.Bock, A.-K., J. Glasemacher, R. Schmidt, and P. Schonheit. 1999. Purification and characterization of two extremely thermostable enzymes, phosphate acetyltransferase and acetate kinase, from the hyperthermophilic eubacterium Thermotoga maritima. J. Bacteriol. 181:1861-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodley, A. L., and W. P. Jencks. 1987. Acetyl phosphate as a substrate for the calcium ATPase of sarcoplasmic reticulum. J. Biol. Chem. 262:13997-14004. [PubMed] [Google Scholar]
- 8.Bouche, S., E. Klauck, D. Fischer, M. Lucassen, K. Jung, and R. Hengge-Aronis. 1998. Regulation of RssB-dependent proteolysis in Escherichia coli: a role for acetyl phosphate in a response regulator-controlled process. Mol. Microbiol. 27:787-795. [DOI] [PubMed] [Google Scholar]
- 9.Brown, T. D. K., M. C. Jones-Mortimer, and H. L. Kornberg. 1977. The enzymic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J. Gen. Microbiol. 102:327-336. [DOI] [PubMed] [Google Scholar]
- 10.Casadaban, M. J., and S. N. Cohen. 1979. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc. Natl. Acad. Sci. USA 76:4530-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, D.-E., S. Shin, J.-S. Rhee, and J.-G. Pan. 1999. Acetate metabolism in a pta mutant of Escherichia coli W3110: importance of maintaining acetyl-CoA flux for the growth and survival. J. Bacteriol. 181:6656-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Da Re, S. S., D. Deville-Bonne, T. Tolstykh, M. Veron, and J. B. Stock. 1999. Kinetics of CheY phosphorylation by small molecule phosphodonors. FEBS Lett. 457:323-326. [DOI] [PubMed] [Google Scholar]
- 13.Feng, J., M. R. Atkinson, W. McCleary, J. B. Stock, B. L. Wanner, and A. J. Ninfa. 1992. Role of phosphorylated metabolic intermediates in the regulation of glutamine synthetase synthesis in Escherichia coli. J. Bacteriol. 174:6061-6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forst, S., J. Gelgado, A. Rampersaud, and M. Inouye. 1990. In vivo phosphorylation of OmpR, the transcription activator of the ompF and ompC genes in Escherichia coli. J. Bacteriol. 172:3473-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox, D. K., N. D. Meadow, and S. Roseman. 1986. Phosphate transfer between acetate kinase and enzyme I of the bacterial phosphotransferase system. J. Biol. Chem. 261:13498-13503. [PubMed] [Google Scholar]
- 16.Fredericks, C. E., S. Shibata, S.-I. Aizawa, S. A. Reimann, and A. J. Wolfe. 2006. Acetyl phosphate-sensitive regulation of flagellar biogenesis and capsular biosynthesis depends on the Rcs phosphorelay. Mol. Microbiol. 61:734-747. [DOI] [PubMed] [Google Scholar]
- 17.Gennis, R. B., and L. P. Hager. 1976. Pyrvuate oxidase, p. 493-504. In A. Martonosi (ed.), The enzymes and biological membranes, vol. 2. Plenum, New York, NY. [Google Scholar]
- 18.Hesslinger, C., S. A. Fairhurst, and G. Sawers. 1998. Novel keto acid formate-lyase and propionate kinase enzymes are components of an anaerobic pathway in Escherichia coli that degrades l-threonine to propionate. Mol. Microbiol. 27:477-492. [DOI] [PubMed] [Google Scholar]
- 19.Hong, J.-S., and A. G. Hunt. 1980. The role of acetylphosphate in active transport. J. Supramol. Struct. 4:77. [Google Scholar]
- 20.Hong, J. S., A. G. Hunt, P. S. Masters, and M. A. Lieberman. 1979. Requirements of acetyl phosphate for the binding protein-dependent transport systems in Escherichia coli. Proc. Natl. Acad. Sci. USA 76:1213-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunt, A. G. 1986. Micromethod for the measurement of acetyl phosphate and acetyl coenzyme A. Methods Enzymol. 122:43-50. [DOI] [PubMed] [Google Scholar]
- 22.Kaya, S., T. Yokoyama, Y. Hayashi, K. Taniguchi, and T. Tsuda. 1998. ATP and acetyl phosphate induces molecular events near the ATP binding site and the membrane domain of Na+,K+-ATPase. The tetrameric nature of the enzyme. J. Biol. Chem. 273:24334-24338. [DOI] [PubMed] [Google Scholar]
- 23.Kumari, S., C. M. Beatty, D. F. Browning, S. J. Busby, E. J. Simel, G. Hovel-Miner, and A. J. Wolfe. 2000. Regulation of acetyl coenzyme A synthetase in Escherichia coli. J. Bacteriol. 182:4173-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumari, S., R. Tishel, M. Eisenbach, and A. J. Wolfe. 1995. Cloning, characterization, and functional expression of acs, the gene which encodes acetyl coenzyme A synthetase in Escherichia coli. J. Bacteriol. 177:2878-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuznetsova, E., M. Proudfoot, C. F. Gonzalez, G. Brown, M. V. Omelchenko, I. Borozan, L. Carmel, Y. I. Wolf, H. Mori, A. V. Savchenko, C. H. Arrowsmith, E. V. Koonin, A. M. Edwards, and A. F. Yakunin. 2006. Genome-wide analysis of substrate specificities of the Escherichia coli haloacid dehalogenase-like phosphatase family. J. Biol. Chem. 281:36149-36161. [DOI] [PubMed] [Google Scholar]
- 26.Lasko, D. R., and D. I. C. Wang. 1996. On-line monitoring of intracellular ATP concentration in Escherichia coli fermentations. Biotechnol. Bioeng. 52:364-372. [DOI] [PubMed] [Google Scholar]
- 27.Lukat, G. S., W. R. McCleary, A. M. Stock, and J. B. Stock. 1992. Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. Proc. Natl. Acad. Sci. USA 89:718-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majdalani, N., and S. Gottesman. 2005. The Rcs phosphorelay: a complex signal transduction system. Annu. Rev. Microbiol. 59:379-405. [DOI] [PubMed] [Google Scholar]
- 29.Maloney, P. C., E. R. Kashket, and T. H. Wilson. 1974. A protonmotive force drives ATP synthesis in bacteria. Proc. Natl. Acad. Sci. USA 71:3896-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsubara, M., and T. Mizuno. 1999. EnvZ-independent phosphotransfer signaling pathway of the OmpR-mediated osmoregulatory expression of OmpC and OmpF in Escherichia coli. Biosci. Biotechnol. Biochem. 63:408-414. [DOI] [PubMed] [Google Scholar]
- 31.Mayover, T. L., C. J. Halkides, and R. C. Stewart. 1999. Kinetic characterization of CheY phosphorylation reactions: comparison of P-CheA and small-molecule phosphodonors. Biochemistry. 38:2259-2271. [DOI] [PubMed] [Google Scholar]
- 32.McCleary, W. R. 1996. The activation of PhoB by acetylphosphate. Mol. Microbiol. 20:1155-1163. [DOI] [PubMed] [Google Scholar]
- 33.McCleary, W. R., and J. B. Stock. 1994. Acetyl phosphate and the activation of two-component response regulators. J. Biol. Chem. 269:31567-31572. [PubMed] [Google Scholar]
- 34.McCleary, W. R., J. B. Stock, and A. J. Ninfa. 1993. Is acetyl phosphate a global signal in Escherichia coli? J. Bacteriol. 175:2793-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyake, M., K. Kataoka, M. Shirai, and Y. Asada. 1997. Control of poly-beta-hydroxybutyrate synthase mediated by acetyl phosphate in cyanobacteria. J. Bacteriol. 179:5009-5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neuhard, J., and P. Nygaard. 1987. Purines and pyrimidines, p. 445-473. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, DC.
- 38.Ninfa, A. J., P. Jiang, M. R. Atkinson, and J. A. Peliska. 2000. Integration of antagonistic signals in the regulation of nitrogen assimilation in Escherichia coli. Curr. Top. Cell. Regul. 36:31-75. [DOI] [PubMed] [Google Scholar]
- 39.Pruss, B. M., C. Besemann, A. Denton, and A. J. Wolfe. 2006. A complex transcription network controls the early stages of biofilm development by Escherichia coli. J. Bacteriol. 188:3731-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pruss, B. M., and A. J. Wolfe. 1994. Regulation of acetyl phosphate synthesis and degradation, and the control of flagellar expression in Escherichia coli. Mol. Microbiol. 12:973-984. [DOI] [PubMed] [Google Scholar]
- 41.Ramstrom, H., S. Sanglier, E. Leize-Wagner, C. Philippe, A. Van Dorsselaer, and J. Haiech. 2003. Properties and regulation of the bifunctional enzyme HPr kinase/phosphatase in Bacillus subtilis. J. Biol. Chem. 278:1174-1185. [DOI] [PubMed] [Google Scholar]
- 42.Roof, D. M., and J. R. Roth. 1992. Autogenous regulation of ethanolamine utilization by a transcriptional activator of the eut operon in Salmonella typhimurium. J. Bacteriol. 174:6634-6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rose, I. A., M. Grunsberg-Manago, S. R. Korey, and S. Ochoa. 1954. Enzymatic phosphorylation of acetate. J. Biol. Chem. 211:737-756. [PubMed] [Google Scholar]
- 44.Ruff, J., K. Denger, and A. M. Cook. 2003. Sulphoacetaldehyde acetyltransferase yields acetyl phosphate: purification from Alcaligenes defragrans and gene clusters in taurine degradation. Biochem. J. 369:275-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sawers, R. G., and D. P. Clark. 27 July 2004, posting date. Chapter 3.5.3, Fermentative pyruvate and acetyl-coenzyme A metabolism. In R. Curtiss III et al. (ed.), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC. http://www.ecosal.org. [DOI] [PubMed]
- 46.Sharma, L., B. Panda, A. K. Singh, and N. Mallick. 2006. Studies on poly-beta-hydroxybutyrate synthase activity of Nostoc muscorum. J. Gen. Appl. Microbiol. 52:209-214. [DOI] [PubMed] [Google Scholar]
- 47.Sheppard, D. E., and J. R. Roth. 1994. A rationale for autoinduction of a transcriptional activator: ethanolamine ammonia-lyase (EutBC) and the operon activator (EutR) compete for adenosyl-cobalamin in Salmonella typhimurium. J. Bacteriol. 176:1287-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silhavy, T. J., M. L. Berman, and L. W. Enquist. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 49.Soler, F., M.-I. Fortea, A. Lax, and F. Fernandez-Belda. 2002. Dissecting the hydrolytic activities of sarcoplasmic reticulum ATPase in the presence of acetyl phosphate. J. Biol. Chem. 277:38127-38132. [DOI] [PubMed] [Google Scholar]
- 50.Starai, V. J., J. Garrity, and J. C. Escalante-Semerena. 2005. Acetate excretion during growth of Salmonella enterica on ethanolamine requires phosphotransacetylase (EutD) activity, and acetate recapture requires acetyl-CoA synthetase (Acs) and phosphotransacetylase (Pta) activities. Microbiol. 151:3793-3801. [DOI] [PubMed] [Google Scholar]
- 51.Stefani, M., N. Taddei, and G. Ramponi. 1997. Insights into acylphosphatase structure and catalytic mechanism. Cell. Mol. Life Sci. 53:141-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stock, A. M., V. L. Robinson, and P. N. Goudreau. 2000. Two-component signal transduction. Annu. Rev. Biochem. 69:183-215. [DOI] [PubMed] [Google Scholar]
- 53.Van Dyk, T. K., and R. A. LaRossa. 1987. Involvement of ack-pta operon products in alpha-ketobutyrate metabolism by Salmonella typhimurium. Mol. Gen. Genet. 207:435-440. [DOI] [PubMed] [Google Scholar]
- 54.Walsh, C. T. 1979. Enzymatic reaction mechanisms. Freeman, New York, NY.
- 55.Wanner, B. L. 1993. Gene regulation by phosphate in enteric bacteria. J. Cell. Biochem. 51:47-54. [DOI] [PubMed] [Google Scholar]
- 56.Wanner, B. L., and M. R. Wilmes-Riesenberg. 1992. Involvement of phosphotransacetylase, acetate kinase, and acetyl phosphate synthesis in control of the phosphate regulon in Escherichia coli. J. Bacteriol. 174:2124-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.West, A. H., and A. M. Stock. 2001. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 26:369-376. [DOI] [PubMed] [Google Scholar]
- 58.Wilson, D. M., J. F. Alderette, P. C. Maloney, and T. H. Wilson. 1976. Protonmotive force as the source of energy for adenosine 5′-triphosphate synthesis in Escherichia coli. J. Bacteriol. 126:327-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolfe, A. J. 2005. The acetate switch. Microbiol. Mol. Biol. Rev. 69:12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolfe, A. J., D.-E. Chang, J. D. Walker, J. E. Seitz-Partridge, M. D. Vidaurri, C. F. Lange, B. M. Pruess, M. C. Henk, J. C. Larkin, and T. Conway. 2003. Evidence that acetyl phosphate functions as a global signal during biofilm development. Mol. Microbiol. 48:977-988. [DOI] [PubMed] [Google Scholar]