Abstract
Oxidative damage of DNA is a source of mutation in living cells. Although all organisms have evolved mechanisms of defense against oxidative damage, little is known about these mechanisms in nonenteric bacteria, including pseudomonads. Here we have studied the involvement of oxidized guanine (GO) repair enzymes and DNA-protecting enzyme Dps in the avoidance of mutations in starving Pseudomonas putida. Additionally, we examined possible connections between the oxidative damage of DNA and involvement of the error-prone DNA polymerase (Pol)V homologue RulAB in stationary-phase mutagenesis in P. putida. Our results demonstrated that the GO repair enzymes MutY, MutM, and MutT are involved in the prevention of base substitution mutations in carbon-starved P. putida. Interestingly, the antimutator effect of MutT was dependent on the growth phase of bacteria. Although the lack of MutT caused a strong mutator phenotype under carbon starvation conditions for bacteria, only a twofold increased effect on the frequency of mutations was observed for growing bacteria. This indicates that MutT has a backup system which efficiently complements the absence of this enzyme in actively growing cells. The knockout of MutM affected only the spectrum of mutations but did not change mutation frequency. Dps is known to protect DNA from oxidative damage. We found that dps-defective P. putida cells were more sensitive to sudden exposure to hydrogen peroxide than wild-type cells. At the same time, the absence of Dps did not affect the accumulation of mutations in populations of starved bacteria. Thus, it is possible that the protective role of Dps becomes essential for genome integrity only when bacteria are exposed to exogenous agents that lead to oxidative DNA damage but not under physiological conditions. Introduction of the Y family DNA polymerase PolV homologue rulAB into P. putida increased the proportion of A-to-C and A-to-G base substitutions among mutations, which occurred under starvation conditions. Since PolV is known to perform translesion synthesis past damaged bases in DNA (e.g., some oxidized forms of adenine), our results may imply that adenine oxidation products are also an important source of mutation in starving bacteria.
Bacteria spend most of the time under starvation conditions, when their growth is inhibited due to a shortage of energy. Still, populations of stationary-phase bacteria undergo rapid evolution because of mutations. Mutagenesis occurring in resting cells is called adaptive mutation or stationary-phase mutation (22, 23). Bacteria have several stress responses (including induction of the error-prone DNA polymerases carrying out translesion synthesis) that provide ways in which mutation rates can be increased in stationary-phase cells (for reviews, see references 24, 47, and 64). Oxidative DNA damage is also an important source of mutagenesis. It is known that the formation of 7,8-dihydro-8-oxo-2′-deoxyguanine (8-oxoG or GO) can give rise to stationary-phase mutations in Escherichia coli (13, 14). Also, products of the oxidative damage of adenine have been shown to be mutagenic (36). However, so far, these oxidation products have received less attention.
Bacteria have evolved different ways to protect their DNA from oxidative damage. For example, stationary-phase-specific protein Dps protects E. coli against multiple stresses, including the avoidance of oxidative damage of DNA by exogenous agents (2, 46, 56). The protection is promoted by the formation of a crystalline structures composed of DNA and Dps (26, 78). Dps homologues are present in many distantly related bacteria, indicating that DNA protection by Dps may be crucial and widespread in prokaryotes. Martinez and Kolter (46) have demonstrated that in the presence of exogenously added hydrogen peroxide, the lack of Dps resulted in a 10-fold increase in the frequency of mutations in E. coli. However, whether the presence of Dps could influence the frequency of mutations under physiological conditions in bacteria is still unexplored.
In order to mitigate the mutagenic effect of 8-oxoG, bacteria have developed an oxidized guanine (GO) repair system (52, 54). Oxidatively damaged guanine is removed by MutM glycosylase. If MutM fails to remove 8-oxoG adducts before DNA replication, translesion synthesis can be inaccurate, leading to the misincorporation of A opposite the 8-oxoG adduct (53, 69). Thus, another glycosylase, MutY, removes adenine from A·(8-oxoG) and from A·G mispairings (54). Defective mutM and mutY alleles both result in enhanced production of G·C-to-T·A transversions (17, 59). The spontaneous mutation frequency is elevated more than 10-fold in MutM-defective E. coli (17, 51) and 20- to 100-fold in MutY-defective bacteria (59). MutT pyrophosphohydrolase hydrolyzes 8-oxodGTP to 8-oxodGMP and pyrophosphate to prevent its use as a substrate by replicative DNA polymerase III (PolIII) (45) or by Y family DNA polymerases (70, 80). Inactivation of MutT in E. coli leads to a specific increase in A·T-to-C·G transversions, resulting from a misincorporation of 8-oxodGTP opposite template A (45, 81).
It has been demonstrated that alterations in the genes of DNA mismatch repair (MMR) and in the mutY gene were responsible for increased mutation frequency in mutator Pseudomonas aeruginosa strains from cystic fibrosis patients, whereas no mutator strains were identified with the inactivation of the mutM or mutT gene (60, 61). Additionally, a mutY-defective derivative of Pseudomonas fluorescens showed a strongly enhanced competitive root-tip-colonizing phenotype through accelerated evolution (20). The fact that the absence of MutY increases the mutation frequency of Pseudomonas species has also been demonstrated in our work with P. putida (67). P. aeruginosa mutY, mutM, and mutT genes were shown to complement those of E. coli GO repair-deficient strains, but no knockouts of these genes were constructed in P. aeruginosa (61). Thus, it is still unclear whether, besides MutY, the absence of MutM or MutT activity could also cause a mutator phenotype in Pseudomonas species.
8-oxoG is not generally considered to be a replication-blocking lesion (10). On the other hand, in vitro studies have revealed that 8-oxoG is highly susceptible to further oxidation and yields a variety of additional products, some of which block DNA replication (reviewed in reference 58). Translesion synthesis DNA polymerases PolII, PolIV, and PolV allow replication to progress in the presence of DNA lesions that are strongly inhibitory to the replicative DNA polymerase PolIII (28). Translesion synthesis by PolIV and PolV is error prone (28, 73) and has been shown to participate in stationary-phase mutagenesis (8, 16, 49, 72, 75, 82). Recently published studies by Neeley et al. (57) suggest a major role for PolV and minor roles for PolII and PolIV in the mechanism of guanine oxidation mutagenesis in E. coli strains containing engineered M13 viral genomes with guanine oxidation products.
P. putida, like many other bacterial species, does not carry PolV genes in its chromosome. Instead, DNA polymerase PolV homologues are frequently encoded by naturally occurring conjugative plasmids in these bacteria (e.g., see reference 74 and references therein). Our previous studies have revealed that the PolV homologue RulAB from TOL plasmid pWW0 increases the evolutionary fitness of starving P. putida bacteria (74). This raises the question of whether this polymerase could be involved in stationary-phase mutagenesis via a mechanism which is dependent on oxidative damage of DNA.
Here we have focused our studies on mechanisms of mutagenesis caused by oxidative damage with P. putida. We have investigated the role of MutY, MutM, and MutT homologues in DNA repair in this organism and the roles of Dps in the protection of DNA and in avoidance of mutations. We have also examined the possible relationship between RulAB-dependent mutagenesis and oxidative damage of DNA in starving P. putida organisms. Our results demonstrated that MutT is involved in the avoidance of mutations in starving populations of P. putida, whereas the absence of this enzyme has only a slight effect on the mutation frequency in growing bacteria. The phenotypic effect of the absence of MutM appeared in changes in the spectrum of stationary-phase mutations but not in the mutation frequency. Additionally, our results implied that although Dps protected P. putida cells against sudden exposure to the oxidizing agent hydrogen peroxide, this protein had no role in the avoidance of stationary-phase mutations under physiological conditions of bacteria. Introduction of the translesion synthesis DNA polymerase PolV genes rulAB from TOL plasmid pWW0 into the P. putida chromosome, which originally lacks PolV genes, resulted in an increased frequency of A-to-G and A-to-C base substitutions. These data hinted that in addition to the 8-oxoG lesion, damage of adenine may also be an important endogenously produced mutagen in starved bacteria.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this study are described in Table 1. Complete media used were Luria-Bertani (LB) medium (55) and minimal medium M9 (1). Solid medium contained 1.5% Difco agar. Casamino Acids (CAA) and glucose were added to the minimal medium at final concentrations of 0.4% and 0.2%, respectively. Phenol-minimal plates contained 2.5 mM phenol (Phe) as the sole carbon and energy source. Antibiotics were added at the following concentrations: ampicillin, 100 μg/ml; kanamycin (Km), 50 μg/ml; tetracycline, 80 μg/ml; rifampin (Rif), 100 μg/ml; and carbenicillin, 1,500 to 3,000 μg/ml. E. coli was incubated at 37°C and P. putida at 30°C. E. coli and P. putida were electrotransformed as described by Sharma and Schimke (68). E. coli strains DH5α (Invitrogen) and CC118 λpir (32) were used for the DNA cloning procedures, and HB101 (12) was used as a host for helper plasmid pRK2013 (21), necessary for mobilization of nonconjugative plasmids.
TABLE 1.
Bacterial strains and plasmids used for this study
| Strain or plasmid | Genotype or construction | Source or reference |
|---|---|---|
| E. coli | ||
| TG1 | supE hsd Δ5 thi Δ(lac-proAB) F′ (traD36 proAB+lacIqlacZΔM15) | 18 |
| DH5α | supE44 ΔlacU169 (Φ80 lacZΔM15) recA1 endA1 hsdR17 thi-1 gyrA96 relA1 | Invitrogen |
| HB101 | subE44 subF58 hsdS3 (rB− mB−) recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 | 12 |
| CC118 λpir | Δ(ara-leu) araD ΔlacX74 galE galK phoA20 thi-1 rpsE rpoB argE (Am) recA1 λpir phage lysogen | 32 |
| P. putida | ||
| PaW85 | Wild type | 6 |
| PaWRulAB | PaW85, carrying the rulAB genes from pWW0 in chromosome in attTn7 site | This study |
| PaWMutY | PaW85, mutY::tet | This study |
| PaWMutM | PaW85, ΔmutM::km | This study |
| PaWMutT | PaW85, ΔmutT::km | This study |
| PaWDps | PaW85, Δdps::km | This study |
| PaWMutYRulAB | PaWRulAB, mutY::tet | This study |
| PaWMutMRulAB | PaWRulAB, ΔmutM::km | This study |
| PaWMutTRulAB | PaWRulAB, ΔmutT::km | This study |
| Plasmids | ||
| pBluescript KS(+) | Cloning vector (Apr) | Stratagene |
| pUTmini-Tn5 Km2 | Delivery plasmid for mini-Tn5 Km2 (Apr Kmr) | 19 |
| pGP704 L | Delivery plasmid for homologous recombination (Apr) | 62 |
| pRK2013 | Helper plasmid for conjugal transfer of pGP704 L (Kmr) | 21 |
| pBK-miniTn7-ΩSm1 | pUC19-based delivery plasmid for miniTn7-ΩSm1 (Smr, Apr) | 42 |
| pUX-BF13 | R6K replicon-based helper plasmid, providing the Tn7 transposase proteins (Apr, mob+) | 4 |
| pGP704mutY::tet | mutY::tet-sequence-containing SacI-KpnI fragment from pB1scrMutY-Tet cloned into pGP704L | 67 |
| pKSmutM | pBluescript KS(+) containing the PCR-amplified mutM gene region inserted in EcoRV-opened vector plasmid | This study |
| pKSΔmutM::km | mutM in pKSmutM is interrupted with the Kmr gene from pUTmini-Tn5 Km2 by replacing PaeI- and Van9II-generated fragment from mutM by Kmr gene | This study |
| pGP704ΔmutM::km | pGP704 L with Acc65I-XbaI fragment of ΔmutM::km from pKSΔmutM::km in vector plasmid opened with the same restrictases | This study |
| pKSmutT | pBluescript KS(+) containing the PCR-amplified mutT gene region inserted into EcoRV-opened vector plasmid | This study |
| pKSΔmutT::km | mutT in pKSmutT is interrupted with Kmr gene from pUTmini-Tn5 Km2 by replacing Bpu1102I-Van91I-generated fragment in mutT with Kmr gene | This study |
| pGP704ΔmutT::km | pGP704 L with Acc65I-XbaI fragment of ΔmutT::km from pKSΔmutT::km in vector plasmid opened with the same restrictases | This study |
| pKSdps | pBluescript KS(+) containing the PCR-amplified dps gene region inserted into EcoRV-opened vector plasmid | This study |
| pKSΔdps::km | dps in pKSdps is interrupted with Kmr gene from pUTmini-Tn5 Km2 by replacing Eco130I-EcoRV-generated fragment in dps with Kmr gene | This study |
| pGP704Δdps::km | pGP704 L with Acc65I-XbaI fragment of Δdps::km from pKSΔdps::km in vector plasmid opened with the same restrictases | This study |
| pUCNotrulAB | pUC18Not containing rulAB genes | 74 |
| pBK-miniTn7-ΩSm1rulAB | rulAB genes from pUC18NotrulAB in NotI site of pBK-miniTn7-ΩSmI | This study |
| pKTpheA56+A | Test system for detection of Phe+ revertants occurring due to 1-bp deletions | 76 |
| pKTpheA22TAG | Test system for detection of Phe+ revertants occurring due to base substitutions | 75 |
| pKT240 | Medium-copy broad-host-range cloning vector (Apr Kmr) | 3 |
Construction of P. putida GO repair-deficient strains.
The mutM (PP5125) and mutT (PP1348) sequences of P. putida KT2440 were obtained from the Institute for Genomic Research website (http://www.tigr.org). These genes were amplified by PCR from genomic DNA of P. putida strain PaW85 (6) (this strain is isogenic to KT2440). Thereafter, the internal sequences of the amplified genes were replaced with antibiotic resistance marker genes and DNA fragments carrying an antibiotic resistance determinant, and sequences from 5′ and 3′ ends of particular mut genes were inserted into plasmid pGP704 L (62), not able to replicate in hosts other than E. coli CC118 λpir. The wild-type sequences of the mutM and mutT genes present in the chromosome of P. putida strain PaW85 were replaced with the interrupted ones by homologous recombination. Derivatives of the plasmid pGP704 L carrying replacement cassettes were conjugatively transferred into P. putida PaW85 by using helper plasmid pRK2013 (21). The integration of whole-delivery plasmid into a target site was excluded by testing transconjugants for their resistance to carbenicillin (only those unable to grow in the presence of 3,000 μg/ml carbenicillin were considered to be true recombinants, generated as a result of double recombination events). PaW85 derivatives with desired gene knockouts were verified by PCR analysis.
The 810-bp mutM gene was amplified by PCR from the genomic DNA of P. putida strain PaW85. The primers mutMylem (5′-CATTGGATTGTCGGAATACGA-3′), complementary to the nucleotide sequence −70 to −50 relative to the ATG initiator codon of the mutM gene, and mutMalum (5′-CGGATCACTGGCAAGGGCT-3′), complementary to the nucleotide sequence +111 to +93 downstream of the TGA stop codon of the mutM gene, were used for the amplification of the mutM gene. The amplified 995-bp DNA fragment containing the mutM gene was inserted into the EcoRV-cleaved vector plasmid pBluescript KS(+) to obtain plasmid pKSmutM. The Kmr gene was amplified by PCR from plasmid pUTmini-Tn5 Km2 by using the primer KmSac, which binds at positions 105 to 126 upstream of the ATG start codon of the Kmr gene and at positions 61 to 62 downstream of the TAA stop codon of the Kmr gene (33). The Ecl136II-cleaved DNA fragment containing the Kmr gene was used to replace the PaeI- and Van91I-generated 525-bp fragment in the mutM gene in plasmid pKSmutM. The PaeI ends were blunt ended before the ligation. The Acc65I- and XbaI-generated 1,531-bp ΔmutM::Km sequence from plasmid pKSΔmutM::Km was inserted into vector plasmid pGP704 L, opened with the same restriction enzymes. The plasmid pGP704ΔmutM::Km was selected in E. coli strain CC118 λpir. The interrupted mutM gene containing the internal deletion was inserted into the chromosome of P. putida PaW85 by homologous recombination. Plasmid pGP704ΔmutM::km, not able to replicate in hosts other than E. coli CC118 λpir, was conjugatively transferred into P. putida PaW85 by using helper plasmid pRK2013. The PaW85 ΔmutM::km knockout strain PaWMutM was verified by PCR analysis using primers mutMlookus (5′-ATCTGCGCGTCCTACCGGG-3′), complementary to nucleotide sequence −150 to −132 upstream of the ATG initiator codon of the mutM gene, and KmOc (5′-TCGAGCAAGACGTTTCCC-3′), complementary to nucleotide sequence 34 to 16 downstream of the ATG initiator codon of the Kmr gene.
The primers mutTylem (5′-AGTAGCGGCCGTCATTCGCG-3′), complementary to nucleotide sequence +18 to +37 downstream of the ATG initiator codon of the mutT gene, and mutTalum (5′-TTGCAGAAGTGTTGTGGCAGC-3′), complementary to nucleotide sequence +79 to +59 downstream of the TGA stop codon of the mutT gene, were used for the amplification of the 942-bp mutT gene. The amplified 1,005-bp DNA fragment containing the mutT gene was inserted into the EcoRV-cleaved vector plasmid pBluescript KS(+) to obtain plasmid pKSmutT. The Kmr gene was amplified by PCR from plasmid pUTmini-Tn5 Km2 by using the primer KmSac. The Ecl136II-cleaved DNA fragment containing the Kmr gene was used to replace the Bpu1102I- and Van91I-generated 450-bp fragment in the mutT gene in plasmid pKSmutT. The Bpu1102I ends were blunt ended before the ligation. The XbaI- and Acc65I-generated DNA fragment containing the ΔmutT::Km sequence from plasmid pKSΔmutT::Km was inserted into plasmid pGP704 L, cleaved with the Acc65I and XbaI enzymes. The resulting plasmid, pGP704ΔmutT::Km, was selected in E. coli strain CC118 λpir. The interrupted mutT gene containing the internal deletion was inserted into the chromosome of P. putida PaW85 by homologous recombination. The PaW85 ΔmutT::km knockout mutant PaWMutT was verified by PCR analysis using primers mutTlookus1 (5′-CCTACATCGAGACCATTTATCAG-3′), complementary to the nucleotide sequence from −73 to −51 upstream of the ATG initiator codon of the mutT gene, and KmOc (5′-TCGAGCAAGACGTTTCCC-3′), complementary to the nucleotide sequence 34 to 16 downstream of the ATG initiator codon of the Kmr gene.
In order to obtain independently isolated clones of P. putida PaW85 mutY knockouts, the previously constructed plasmid, pGP704mutY::tet (67), was used to carry out homologous recombination between the wild-type mutY allele and the interrupted one. The strain PaWMutY containing the disrupted mutY gene (mutY::tet) was verified by PCR analysis. In addition, the mutator phenotype of the clones containing the interrupted mutY sequence but lacking the original sequence was examined by measuring the spontaneous frequency of mutation to rifampin resistance.
Construction of P. putida strains carrying the DNA polymerase PolV homologue-encoding rulAB genes in chromosome.
The plasmid pUCNotrulAB (74) carrying the rulAB operon, originated from the catabolic TOL plasmid pWW0 (29, 77), was used to subclone these genes as a NotI-cleaved DNA fragment into the NotI-cleaved mini-Tn7 transposon delivery plasmid pBK-miniTn7-ΩSm1 (42). The plasmid pBK-miniTn7-ΩSm1rulAB, which is not able to replicate in Pseudomonas spp., was selected in E. coli strain DH5α and was further used in mobilization by four-parent conjugation into various P. putida strains. Plasmid pBK-miniTn7-ΩSm1rulAB carrying the rulAB genes was introduced into P. putida PaW85 by four-parent mating. More precisely, the following strains were used in four-parental mating: E. coli strain DH5α containing the delivery plasmid pBK-miniTn7-ΩSm1rulAB; E. coli CC118 λpir carrying the helper plasmid pUX-BF13 (4) for the transposition event to occur, as it contains the Tn7 transposase genes; E. coli HB101 carrying the plasmid pRK2013 (the helper plasmid for other plasmids' mobilization); and P. putida strain PaW85. Consequently, integration of the rulAB genes in the composition of mini-Tn7 into the specific attTn7 site located downstream of the glmS gene in P. putida chromosome was verified by PCR analysis with primers Tn7GlmS and Tn7R (43). The P. putida strain carrying the rulAB genes in its chromosome was designated as PaWRulAB. The expression of the rulAB genes in the P. putida chromosome was verified by using a UV irradiation survival assay described previously (74). In order to obtain P. putida GO repair-deficient strains carrying the rulAB genes in their chromosomes (strains PaWMutYRulAB, PaWMutMRulAB, and PaWMutTRulAB), PaWRulAB was used as a recipient for the replacement of original mut sequences with the interrupted ones by homologous recombination, as described above.
Construction of the P. putida PaW85 dps knockout strain.
The dps (PP1210) gene sequence of P. putida KT2440 was obtained from the Institute for Genomic Research website (http://www.tigr.org). The 471-bp dps gene was amplified by PCR from the genomic DNA of P. putida strain PaW85. The primers dpsylem (5′-TTGAGTGCCGCGTGCCTT-3′), complementary to the nucleotide sequence −641 to −623 upstream of the ATG initiator codon of the dps gene, and dpsalum (5′-AGGTGGCCTATGAAGCGCTGA-3′), complementary to the nucleotide sequence +183 to +164 downstream of the TAA stop codon of the dps gene, were used for amplification of the dps gene. The amplified 1,350-bp DNA fragment containing the dps gene was inserted into the EcoRV-cleaved vector plasmid pBluescript KS(+) to obtain plasmid pKSdps. The Kmr gene was amplified by PCR from plasmid pUTmini-Tn5 Km2 by using the primer KmSac. The Ecl136II-cleaved DNA fragment containing the Kmr gene was used to replace the Eco130I- and EcoRV-generated 200-bp fragment in the dps gene in plasmid pKSdps. The ends of the Eco130I were blunt ended before the ligation reaction. The XbaI- and Acc65I-generated DNA fragment containing the Δdps::Km sequence from plasmid pKSΔdps::Km was inserted into plasmid pGP704 L, cleaved with the same enzymes. The resulting plasmid, pGP704Δdps::Km, was selected in E. coli strain CC118 λpir. The interrupted dps gene containing the internal deletion was inserted into the chromosome of P. putida PaW85 by homologous recombination. Plasmid pGP704Δdps::km, not able to replicate in hosts other than E. coli CC118 λpir, was conjugatively transferred into P. putida PaW85 by using helper plasmid pRK2013. The PaW85 Δdps::km knockout mutant PaWDps was verified by PCR analysis using primers dpslookus (5′-CGGTTGCCGGCATCTTGTG-3′), complementary to the nucleotide sequence −667 to −649 upstream of the ATG initiator codon of the dps gene, and KmOc (5′-TCGAGCAAGACGTTTCCC-3′), complementary to the nucleotide sequence 34 to 16 downstream of the ATG initiator codon of Kmr gene.
Isolation and analysis of Phe+ revertants and Rifr mutants.
Assay systems used to measure different types of point mutations in starving P. putida were based on the activation of phenol monooxygenase gene pheA, enabling bacteria to utilize phenol as the growth substrate and to form colonies on selective plates. The reporter gene pheA was altered in RSF1010-derived tester plasmids either by +1 frameshift mutation or by introducing a TAG translational stop codon into the pheA gene (75). Conditions for the isolation of phenol-degrading Phe+ revertants were the same as those described in our previous study (66). Carbenicillin at a concentration of 1,500 μg/ml was used in selective plates to maintain selectin for plasmid. Several independently isolated clones of the same gene knockouts were used in mutagenesis studies to control reproducibility of the results. We have previously shown (40, 75) that Phe+ colonies appearing on phenol-minimal plates on day 2 contained mutations that occurred before the plating in a growing culture, whereas colonies that emerged on selective plates on day 3 and later contained mutations that occurred after the cells were plated. The latter were called stationary-phase mutations. About 5 × 108 cells of the P. putida wild-type strain and its derivatives were plated onto phenol-minimal plates from independent cultures that were gown overnight in liquid M9 medium containing glucose and CAA. When larger amounts of cells of the tester strains were plated onto selective plates, they were plated with approximately 5 × 108 scavenger cells (P. putida PaW85 carrying pKT240 [3]). In order to test whether bacteria would die during starvation on the selective medium, we measured the number of CFU for at least five independent starving cultures of the particular strain studied. The viability of bacteria was determined on the same plates that were used for the isolation of Phe+ revertants. No differences were found between the viability of wild-type strain PaW85 and that of its derivatives during incubation of bacteria under starvation conditions.
The frequency of spontaneous mutations in growing cells was determined in at least four separate experiments, in each of 20 independent cultures. Independent cultures of P. putida tester strains were generated by growing cells to late logarithmic growth phase in M9 medium containing glucose and CAA, diluting this culture by 105 into fresh glucose and CAA-containing M9 medium, and dispensing 2-ml aliquots into test tubes and allowing cells to reach saturation by growing cells for 18 to 20 h. Cells sampled from the same cultures were used for determination of the frequency of Phe+ revertants and Rifr mutants. Phe+ revertants appearing on phenol-minimal plates were counted on day 2 after plating. To determine the frequency of Rifr mutants, colonies were counted on 100-μg/ml-rifampin-supplemented plates incubated for 24 h.
The frequency of spontaneous mutations in independently growing cultures was calculated per 1 × 109 plated cells by using the Lea-Coulson method of the median (44, 63). Data were analyzed by a software program for statistical analyses (Statgraphics Centurion XV; Statpoint Inc.). P values of the medians for Rifr or Phe+ mutations were calculated by using the Mann-Whitney (Wilcoxon) W test (71). Differences between average accumulation rates of Phe+ mutants were analyzed using the Student t test. The chi-square test of independence was used for comparing DNA sequencing results.
For Phe+ revertants, an approximately 350-bp DNA region covering the area of the pheA gene containing potential reversion mutations was analyzed by DNA sequencing, as described previously (75). The DNA sequencing reactions were analyzed with an ABI Prism 377 DNA sequencer (Perkin-Elmer).
Oxidative-stress assay.
The oxidative stress assay was performed as described in reference 56, with some modifications. The overnight stationary-phase cultures of wild-type P. putida strain PaW85 and its dps-defective derivative were incubated in 30 ml of LB broth at 30°C with aeration in the presence or absence of hydrogen peroxide (Sigma) at a concentration of 200 mM. After the addition of hydrogen peroxide, viable cell counts were determined as CFU, by serial dilution of cells eliminated periodically from cultures and plated to the LB agar plates. All assays were done at least four times.
RESULTS AND DISCUSSION
Mutation frequency in growing cells of P. putida strains lacking different GO repair enzymes.
The GO system has been studied mainly in E. coli, and it is known to involve at least three enzymes, MutY and MutM glycosylases and MutT pyrophosphohydrolase (52). The absence of MutY or MutT causes a strong mutator phenotype in E. coli, whereas the lack of MutM has a milder effect on mutation frequency (17, 45, 59, 81). Mutators isolated from natural populations of Pseudomonas species have been found to be defective in either enzymes of DNA mismatch repair or MutY, but no mutators were identified with inactivation of the mutT or mutM gene (20, 60, 61). This raised the question of how essential MutT and MutM are in the avoidance of mutations in pseudomonads. We have previously shown that the lack of MutY leads to the mutator phenotype in P. putida (67). To further elucidate the role of the GO repair system in the avoidance of mutations in this organism, we constructed P. putida strains PaWMutM and PaWMutT, which were defective in MutM or MutT, respectively. MutT, first characterized in E. coli (9, 45), belongs to the Nudix family of hydrolases. Enzymes containing the Nudix box invariably catalyze the hydrolysis of nucleoside diphosphates linked to some other moiety, X (7, 50). According to annotation of the P. putida genome at the Institute for Genomic Research website (http://www.tigr.org), this organism contains several open reading frames encoding putative Nudix family (known also as MutT family) proteins. Based on DNA sequence data, PP1062 and PP1348 are the closest homologues to E. coli mutT (they share 41% identity at the deduced amino acid level with E. coli mutT). Among the 10 different genes encoding putative Nudix hydrolases, PP1348 is the closest homologue to the P. aeruginosa mutT gene PA4400 (they share 72% identity at the deduced amino acid sequence level), which has been demonstrated to complement E. coli mutT deficiency (61). Hence, PP1348 was chosen as the target to knock out MutT activity in P. putida. Construction of GO repair-deficient strains is described in detail in Materials and Methods.
We examined the frequency of spontaneous mutations of growing cells in the P. putida wild-type strain and its GO repair-defective derivatives, using two different assay systems. The chromosomal Rifr system enabled the detection of Rifr colonies occurring due to mutations in the rpoB gene. The other test system measured base substitutions by eliminating the TAG stop codon in the phenol monooxygenase (pheA) gene present in the RSF1010-derived plasmid (75). Point mutations that restored the functional pheA sequence enabled bacteria to utilize phenol as a growth substrate (we counted Phe+ colonies appearing on day 2 after plating onto phenol-minimal plates). The results presented in Table 2 show that the median values of frequency of spontaneous Rifr mutations and Phe+ mutations were increased ∼90-fold and ∼80-fold, respectively, in PaWMutY compared to that of the wild-type strain. At the same time, the absence of MutM or MutT elevated the frequency of Rifr mutations by only two- to threefold. No significant effect of the lack of MutM or MutT on the frequency of mutations was detected with the test system which measured the appearance of Phe+ revertants. Thus, our results clearly demonstrated that the lack of MutY activity and not the knockout of MutM or MutT caused the mutator phenotype in growing cells of P. putida.
TABLE 2.
Effect of inactivation of GO repair enzymes on frequency of mutations in growing cells of P. putidaa
| Strain genotype | Median frequency for Rifr mutation | Phenotypic effectb | Median frequency for Phe+ mutation | Phenotypic effectb |
|---|---|---|---|---|
| WT | 3.8 | 1 | 1.5 | 1 |
| mutY | 345 | 91 | 120 | 80 |
| ΔmutM | 8.4 | 2.2 | 2 | 1.3 |
| ΔmutT | 11 | 2.9 | 2 | 1.3 |
The median value for frequency of mutation per 1 × 109 cells is shown. This was calculated using the Lea-Coulson method of the median (44, 63). The frequency of mutation was determined for at least 60 independent cultures. Differences between frequencies of Rifr mutations of PaWMutY, PaWMutM, and PaWMutT relative to that of the wild-type strain and Phe+ mutations of PaWMutY relative to that of the wild-type strain were statistically significant (P value less than 0.05) at the 95% confidence level, based on the Mann-Whitney test (71).
The phenotypic effect is given as a ratio calculated by dividing the mutation frequency of a particular GO repair-deficient strain by the mutation frequency of wild-type strain PaW85.
Roles of MutT and MutM in stationary-phase mutagenesis of P. putida.
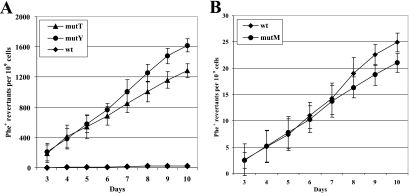
We have previously shown that the absence of MutY had a strongly increasing effect on the frequency of mutations in starved populations of P. putida (67). To study the effect of various GO repair enzymes on the avoidance of stationary-phase mutations in P. putida, we used different assay systems that enabled us to monitor base substitutions and frameshift mutations separately (75). Phe+ revertants accumulating on phenol-minimal plates on day 3 and later were counted on each day during 2 weeks of starvation. We found that the frequency of accumulation of 1-bp deletion mutants was not affected by the absence of GO repair enzymes (data not shown). At the same time, the deficiency of either MutY or MutT greatly elevated the occurrence of base substitution mutations, resulting in about 100-fold and 75-fold higher frequencies of accumulation of Phe+ revertants, respectively, than that of wild-type bacteria (Fig. 1A). However, bacteria with the mutM-defective allele did not reveal statistically significant changes in frequency of stationary-phase mutations compared to that of the wild-type strain (Fig. 1B). These data demonstrated that both MutY and MutT are important in decreasing the rate of mutation in starving P. putida.
FIG. 1.
Accumulation of Phe+ revertants on phenol-minimal plates in P. putida wild-type strain PaW85 (wt) and in its GO repair-defective derivatives PaWMutY (mutY), PaWMutT (mutT), and PaWMutM (mutM). (A) Accumulation of stationary-phase mutations in strains PaW85, PaWMutY, and PaWMutT. (B) Accumulation of stationary-phase mutations in strains PaW85 and PaWMutM. About 2 × 107 cells of tester strain PaWMutY or PaWMutT carrying the plasmid pKTpheA22TAG with 5 × 108 scavenger cells (PaW85 cells carrying pKT240) or 5 × 108 cells of tester strain PaW85 or PaWMutM carrying the plasmid pKTpheA22TAG were plated from independent liquid M9 medium cultures grown overnight onto phenol-minimal plates. Data for at least five parallel experiments are presented. In all cases, means ± standard deviations (error bars) for at least 10 plates calculated per 1 × 109 cells are shown.
Analysis of the Phe+ revertants which appeared due to base substitutions revealed that the spectrum of mutations identified in the wild-type strain was distinct from the spectrum of changes characterized with different GO repair-defective strains (Table 3). Whereas the T-to-C transition was the most prominent change in wild-type bacteria (77% among all changes identified), the revertants which were collected from populations of MutY-defective bacteria contained predominantly G-to-T transversions (85%). Revertants isolated from MutT-defective bacteria contained mostly two types of transversions, A-to-C (70%) or T-to-G (20%). These data are consistent with known functions of MutY and MutT in E. coli (54).
TABLE 3.
Reversion of nonsense mutation (TAG) in Phe+ mutants accumulating in P. putida wild-type strain and its GO repair-defective derivatives
| Targeta | DNA change | Occurrences (%)
|
Occurrences with rulAB genes inserted into chromosome (%)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild type | mutM | mutT | mutY | Wild type | mutM | mutT | mutY | ||
| TAG | T → C | 164 (77) | 103 (66) | 14 (8.3) | 24 (12) | 115 (60)c | 59 (63) | 7 (5) | 23 (12) |
| T → G | 19 (9) | 11 (7) | 34 (20) | 5 (2) | 11 (6) | 4 (4.3) | 36 (25) | 5 (2.5) | |
| T → A | 1 (0.5) | 0 | 0 | 0 | 0 | 1 (1.1) | 0 | 0 | |
| G → T | 12 (5.6) | 24 (15.4)b | 1 (0.6) | 176 (85) | 6 (3) | 16 (17.2) | 0 | 168 (84) | |
| A → C | 0 | 3 (2) | 118 (70) | 0 | 4 (2)c | 7 (8)c | 102 (70) | 1 (0.5) | |
| A → G | 13 (6) | 10 (6.4) | 2 (1.1) | 3 (1) | 51 (27)c | 3 (3.2) | 0 | 1 (0.5) | |
| A → T | 4 (1.9) | 5 (3.2) | 0 | 0 | 4 (2) | 3 (3.2) | 0 | 1 (0.5) | |
Phe+ mutant colonies used for identification of stationary-phase mutations were picked up on days 3 to 15. Approximately 15 mutants were analyzed per each day. We did not notice remarkable changes in the spectrum of mutations in revertants derived from the earlier or the later period of starvation.
Bold type denotes statistically significant differences (P < 0.05) between the mutM and wild-type strains (marked also in bold).
Bold type denotes statistically significant differences (P < 0.05) in the presence or absence of rulAB genes (marked also in bold).
MutT hydrolyzes 8-oxodGTP to prevent its incorporation into DNA (45). Our results demonstrated that P. putida carrying the mutT (PP1348) null allele expressed a strong mutator phenotype in carbon-starved bacteria but affected only slightly the mutation frequency in growing bacteria. Annotation of the P. putida genome (www.tigr.org) indicates the presence of 10 putative Nudix family proteins in this organism. Some Nudix family members, such as MutT, are known to have the ability to degrade potentially mutagenic, oxidized nucleotides, while others control the levels of metabolic intermediates and signaling molecules (50). The presence of multiple genes for Nudix family proteins is common in bacteria. For example, E. coli has 13 Nudix hydrolases (including MutT), 11 of which are characterized by their enzymatic activities (50, 79). Inactivation of the mutT gene in E. coli leads to an approximately 1,000-fold increase in spontaneous mutation frequency due to increased A·T-to-C·G transversions (81). Studies over the past 10 years have revealed that at least three other proteins besides MutT could eliminate 8-oxodGTP from the nucleotide pool in growing E. coli cells (34, 37, 41). However, compared to MutT, the antimutator effect of these proteins is considerably weaker (about two- to threefold effects). Orf135 (NudG) is a Nudix family protein which hydrolyzes 2-hydroxy-dATP and 8-oxoG (39). The lack of Orf135 enzyme resulted in a mild mutator phenotype, and overexpression of Orf135 protein reduced mutation frequency in E. coli (37). Overexpression of Orf17 (NtpA, NudB) protein, another Nudix family enzyme, decreased mutation frequency in MutT-defective E. coli (34). Additionally, GTP cyclohydrolase II, which is not related to Nudix family proteins, may also serve as a backup enzyme for the MutT protein, since it is able to hydrolyze 8-oxoG and its absence increases mutation frequency in MutT-defective E. coli (41). The possible antimutator function of these proteins in starving bacteria has not been studied. The biological function and substrate specificity of P. putida Nudix family proteins are entirely unexplored. Based on results of the current study, we hypothesize that the role of some of the other nine putative P. putida Nudix family proteins is to back up the PP1348-encoded MutT function in a growth phase-dependent manner. It is possible that any of these proteins not expressed in starving bacteria but active in growing bacteria may suppress the lack of PP1348-encoded activity in growing P. putida.
A search of the www.tigr.org website revealed that the P. aeruginosa PAO1 genome encodes nine putative Nudix family proteins. Drawing parallels with the results of our study demonstrating that the absence of the PP1348-encoded MutT homologue had only a mild effect on mutation frequency in growing bacteria, we propose that similarly to P. putida, some other Nudix protein(s) could serve as a backup for MutT in P. aeruginosa. This might be a reason why no mutT-defective variants were identified among P. aeruginosa clinical isolates expressing a strong mutator phenotype (see, e.g., references 60 and 61).
Although the absence of MutM did not elevate the mutation frequency in starving P. putida, the proportion of G-to-T transversions increased significantly in this strain (15.4% were identified in PaWMutM versus 5.6% detected in the wild-type strain [P = 0.0018]) (Table 3). This hints that MutM actually is functional in starving P. putida. Compared to the other GO repair system enzymes MutY and MutT, a deficiency in MutM also had a much smaller effect on stationary-phase mutations in other bacterial species including the best-studied microorganism, E. coli (15). E. coli has additional enzymes to remove 8-oxoG from mispairs. Hazra et al. (31) found that the Nei protein (endonuclease VIII) could excise 8-oxoG from 8-oxoG/A and 8-oxoG/G mispair-containing oligonucleotides. Triple mutants lacking MutY, MutM, and Nei showed an increase in G·C-to-T·A transversions, which indicated that nei serves as a backup to remove 8-oxoG (11). Additionally, the E. coli Nth protein (endonuclease III) has an 8-oxoG DNA glycosylase/AP lyase activity which removes 8-oxoG preferentially from 8-oxoG/G mispairs (48). Mutants defective in Nei exhibited no mutator phenotype, and mutants defective in Nth showed a weak mutator phenotype, while double mutants lacking both Nei and Nth exhibited a strong mutator phenotype (35, 65). Annotation of the P. putida genome did not indicate the presence of putative endonuclease VIII (Nei) homologues in this organism but did reveal that P. putida has an Nth homologue. The deduced amino acid sequence of PP1092 showed 67.8% identity with the sequence of E. coli endonuclease III (Nth protein). Thus, one may hypothesize that the P. putida Nth homologue may suppress a mutator phenotype of P. putida lacking the functional mutM gene.
The DNA polymerase PolV homologue RulAB influences the spectrum of stationary-phase mutations.
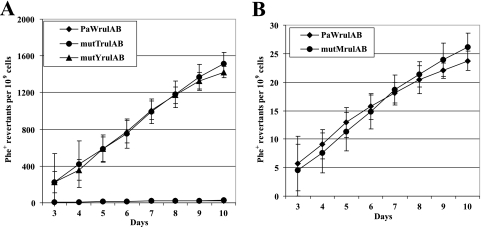
It was reported that stationary-phase populations of MutY-defective E. coli had, in addition to G·C-to-T·A transversions, an enhanced rate of G·C-to-C·G transversions, and evidence was presented that MutY protein possesses a DNA glycosylase activity to remove unmodified G from an 8-oxoG·G mispair (83). The study by Timms et al. (76) revealed that the pathway which generates G·C-to-C·G transversions in starved E. coli requires the umuDC genes encoding DNA polymerase PolV. PolV is involved in the appearance of mutants able to utilize novel growth substrates and mutants with enhanced abilities to scavenge amino acids released from dead cells in starving populations of E. coli (8, 16, 49, 82). Similar results have been obtained with P. putida. We have recently shown that the PolV homologue encoded by TOL plasmid pWW0 significantly enhances the fitness of bacteria under conditions of long-term starvation (74). These results indicated that PolV genes rulAB from the TOL plasmid increase the probability of accumulation of beneficial mutations in P. putida cells, allowing genetic adaptation of bacterial populations under conditions of environmental stress. Here, in order to study the possible relationship between RulAB-dependent mutagenesis and oxidative damage of DNA in starving P. putida, we looked at the effect of the rulAB genes on the frequency of mutations and spectra of base substitution mutations in a P. putida wild-type strain and in its GO repair-defective derivatives. The frequency of accumulation of Phe+ revertants in the P. putida wild-type strain and its MutY-, MutM- or MutT-defective derivative in the presence of the rulAB genes is shown in Fig. 2. The presence of the rulAB genes did not significantly affect the accumulation rate of Phe+ revertants compared to that measured in strains lacking these genes, except for MutY-defective bacteria, where the accumulation rate of the Phe+ revertants was slightly reduced in the rulAB-proficient strain PaWMutYRulAB compared to that in strain PaWMutY. However, a comparison of results of the DNA sequence analysis of Phe+ mutants accumulated in the presence or absence of the rulAB genes in the bacterial chromosome revealed differences between the spectra of base substitutions identified in the wild-type strain and those in the strain lacking MutM (Table 3). These data demonstrated that rulAB-encoded PolV contributes to the occurrence of base substitution mutations in stationary-phase populations of P. putida. At the same time, and differing from our expectations, this DNA polymerase did not increase the proportion of G-to-C substitutions. Instead, the presence of the rulAB genes in the bacterial chromosome enhanced the replacement of nucleotide A with nucleotide G or C. In GO repair-proficient bacteria, the proportion of A-to-G transitions was increased from 6% in the PaW85 strain lacking rulAB genes to 27% in the PaWRulAB strain carrying rulAB genes (P < 0.001). There was also an increase in A-to-C transversions in the following strains: the proportion of A-to-C changes was 2% in strain PaWRulAB, whereas A-to-C substitutions were not detected among 213 mutants picked up from starved populations of strain PaW85 (P = 0.03). In the case of a MutM-defective background, the presence of the rulAB genes increased the proportion of A-to-C transversions from 2%, identified in bacteria in the absence of RulAB (strain PaWMutM), to 8% in RulAB-proficient cells (strain PaWMutMRulAB) (P = 0.029). Notably, the frequency of A-to-C substitutions was not affected by the presence of rulAB genes in MutT-defective bacteria (Table 3). This indicates that this type of transversion was not caused by misincorporation of 8-oxoG opposite template A.
FIG. 2.
Study of the effect of rulAB genes on the accumulation of Phe+ revertants on phenol-minimal plates in P. putida wild-type strain PaW85 (PaWrulAB) and in its GO repair-defective derivatives PaWMutY (mutYrulAB), PaWMutT (mutTrulAB), and PaWMutM (mutMrulAB). (A) Accumulation of stationary-phase mutations in strains PaWRulAB, PaWMutYRulAB, and PaWMutTRulAB. (B) Accumulation of stationary-phase mutations in strains PaW85RulAB and PaWMutMRulAB. Data for at least five parallel experiments are presented. In all cases, means ± standard deviations (error bars) for at least 10 plates calculated per 1 × 109 cells are shown. Differences between average accumulation rates of Phe+ mutations per day per 1 × 109 cells measured in the presence of the rulAB genes during a 3- to 10-day starvation period were not statistically significant (except for strain PaWMutYRulAB) compared to those measured in counterpart strains without the rulAB genes (P = 0.17 for PaW85; P = 0.27 for PaWMutM; P = 0.31 for PaWMutT; and P < 0.001 for PaWMutY) at the 95% confidence level, based on the Student t test.
So far, in contrast to 8-oxoG, 2-hydroxyadenine (2-OH-A), an oxidized form of adenine, has received less attention because its steady-state levels in cellular DNA are lower than those of 8-oxoG (25, 38). However, 2-OH-A is as mutagenic as 8-oxoG is (36). Most recently, it was demonstrated that replication fork block is the likely outcome of a replicative DNA polymerase encountering a template 2-OH-A, and its bypass by translesion synthesis polymerases is mutagenic (5). Bypass of 2-OH-A by two Y family polymerases, archaeal polymerase Dpo4 and human polymerase η, was associated with A-to-G, A-to-C, and A-to-T base substitutions (5). Since PolV also belongs to a Y family of DNA polymerases and plays a crucial role in carrying out translesion synthesis past damaged bases in DNA, one may speculate that the effects of RulAB observed in our studies are due to the presence of oxidatively damaged adenine in DNA. Interestingly, the proportion of A-to-G transitions also increased in starved cells of P. putida lacking DNA MMR (66). Notably, Barone et al. (5) have suggested that MMR would prevent the accumulation of 2-OH-A in DNA and thereby the accumulation of A-to-G and A-to-T base substitutions. Whether this is also true in our case needs further studies.
Paradoxically, the proportion of A-to-C transversions also increased in MutM-defective bacteria. According to the known substrate specificity of MutM, this enzyme, in addition to 8-oxoG, repairs a number of alternative substrates (e.g., see references 27 and 30). However, to our knowledge, no 2-OH-A glycosylase activity has been reported for MutM. Thus, the mechanism of enhancement of A-to-C transversions in MutM-defective bacteria in the presence of RulAB remains obscure.
Dps does not affect stationary-phase mutations in P. putida.
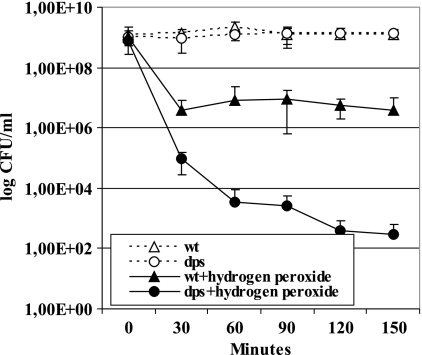
Dps, a stationary-phase-specific DNA-binding protein, is present in a wide variety of organisms. It has been demonstrated that Dps protects cells against multiple stresses during the stationary phase (56). Among these various functions, Dps protects DNA against oxidative damage by serving as an alternative target for reactive oxygen species (26, 46, 56, 78). So far, the effect of Dps on mutagenic processes has been investigated only in the presence of exogenously added hydrogen peroxide and by overexpressing this enzyme in GO repair-deficient E. coli (46). In that particular study, the lack of Dps resulted in an approximately 10-fold increase in G·C-to-T·A transversions (46). Whether Dps prevents mutations under physiological conditions was not investigated in that report. Hence, we decided to examine whether Dps could have an effect on the frequency of stationary-phase mutations in P. putida. For that purpose, we constructed a Dps-defective mutant of P. putida. First, in order to determine whether Dps is involved in the protection of P. putida against oxidative damage, we performed a hydrogen peroxide sensitivity test of the P. putida wild-type strain and its Dps-defective derivative strain PaWDps. We found that strain PaWDps was more sensitive to hydrogen peroxide stress than the wild type (Fig. 3). The viability of PaWDps treated with 200 mM H2O2 was reduced about 104-fold after 30 min of treatment compared to about 300-fold reduction in viability of wild-type cells. Later, wild-type bacteria adapted to this stress as they maintained viability at 4 × 106 CFU/ml for 150 min. Adaptation to hydrogen peroxide stress also took place in cultures of bacteria lacking Dps: viable cell titers of Dps-defective bacteria were reduced by more than 106-fold for 150 min (we detected 3 × 102 CFU/ml), but this decline was much slower than the rapid death that occurred during the first 30 min after the addition of hydrogen peroxide to the growth medium of bacteria. These data indicate that Dps is involved in the protection of P. putida cells against sudden exposure to H2O2.
FIG. 3.
Effect of the absence of Dps on survival of bacteria in the presence of 200 mM H2O2. Overnight cultures of wild-type (wt) or Dps-defective (dps) cells were treated with H2O2. After the addition of hydrogen peroxide, viable cell counts were determined as CFU by serial dilution of cells eliminated periodically from cultures and plated to the LB agar plates. Open symbols designate nontreated cells. All assays were done at least four times.
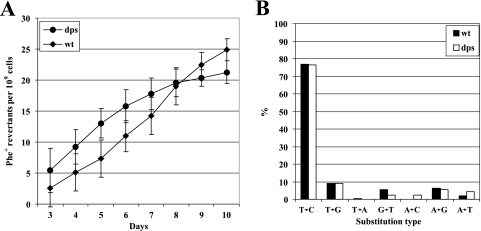
Next, we compared the frequencies of accumulation of Phe+ revertants from starving populations of the P. putida wild-type strain with those of its Dps-defective derivative strain PaWDps (Fig. 4A). Although the curves of accumulation of the mutants seem slightly different, these differences appeared not to be statistically significant. Additionally, the spectrum of base substitutions occurring in starving populations of Dps-defective bacteria was similar to that of the wild-type bacteria (Fig. 4B). Here we can state that the introduction of the dps null allele into mutY-defective bacteria also did not elevate the rate of accumulation of Phe+ revertants (data not shown). Thus, our results imply that Dps has no role in the avoidance of stationary-phase mutations, at least in P. putida. It is also possible that the protective role of Dps in genome integrity becomes essential only when bacteria are exposed to exogenous DNA-damaging agents.
FIG. 4.
Study of the effect of Dps on the occurrence of stationary-phase mutations in P. putida. (A) Accumulation of Phe+ revertants on phenol-minimal plates of P. putida wild-type strain (wt) and its Dps-defective derivative PaWDps (dps). Data for at least five parallel experiments are presented. In both cases, means ± standard deviations (error bars) for at least 10 plates calculated per 1 × 109 cells are shown. Differences between average accumulation rates of Phe+ mutations per day per 1 × 109 cells measured in these strains during 3- to 10-day starvation periods are not statistically significant (P value of 0.77) at the 95% confidence level, based on the Student t test. (B) The spectrum of Phe+ mutations occurring in P. putida wild-type strain and its Dps-defective derivative PaWDps (dps). Minor differences in the spectra of base substitutions seen in this figure are not statistically significant (P > 0.05).
To summarize our results, we conclude that the oxidative damage of DNA is an important source of stationary-phase mutagenesis in P. putida. The lack of activity of the GO repair system significantly increases the frequency of base substitutions in starved populations of P. putida. However, although Dps protects P. putida against oxidative stress, Dps did not counteract mutagenesis under starvation conditions of bacteria. Additionally, the presence of the Y family DNA polymerase PolV homologue elevated the frequency of A-to-C and A-to-G base substitutions, which might be a consequence of the presence of oxidized adenine on DNA. Hence, it would be interesting to study whether an enzyme(s) which may serve as a backup for MutM or any of numerous Nudix family proteins present in P. putida could be involved in avoidance of mutations caused by oxidative damage.
Acknowledgments
We thank laboratory members for their comments on the manuscript.
This work was supported by grant 5757 from the Estonian Science Foundation and by grant 55005614 from the Howard Hughes Medical Institute International Research Scholars Program.
Footnotes
Published ahead of print on 1 June 2007.
REFERENCES
- 1.Adams, M. H. 1959. Bacteriophages. Interscience Publishers Inc., New York, NY.
- 2.Almiron, M., A. J. Link, D. Furlong, and R. Kolter. 1992. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 6:2646-2654. [DOI] [PubMed] [Google Scholar]
- 3.Bagdasarian, M. M., E. Amann, R. Lurz, B. Ruckert, and M. Bagdasarian. 1983. Activity of the hybrid trp-lac (tac) promoter of Escherichia coli in Pseudomonas putida. Construction of broad-host-range, controlled-expression vectors. Gene 26:273-282. [DOI] [PubMed] [Google Scholar]
- 4.Bao, Y., D. P. Lies, H. Fu, and G. P. Roberts. 1991. An improved Tn7-based system for the single-copy insertion of cloned genes into chromosomes of gram-negative bacteria. Gene 109:167-168. [DOI] [PubMed] [Google Scholar]
- 5.Barone, F., S. D. McCulloch, P. Macpherson, G. Maga, M. Yamada, T. Nohmi, A. Minoprio, F. Mazzei, T. A. Kunkel, P. Karran, and M. Bignami. 2007. Replication of 2-hydroxyadenine-containing DNA and recognition by human MutSalpha. DNA Repair 6:355-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayley, S. A., C. J. Duggleby, M. J. Worsey, P. A. Williams, K. G. Hardy, and P. Broda. 1977. Two modes of loss of the Tol function from Pseudomonas putida mt-2. Mol. Gen. Genet. 154:203-204. [DOI] [PubMed] [Google Scholar]
- 7.Bessman, M. J., D. N. Frick, and S. F. O'Handley. 1996. The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J. Biol. Chem. 271:25059-25062. [DOI] [PubMed] [Google Scholar]
- 8.Bhamre, S., B. B. Gadea, C. A. Koyama, S. J. White, and R. G. Fowler. 2001. An aerobic recA-, umuC-dependent pathway of spontaneous base-pair substitution mutagenesis in Escherichia coli. Mutat. Res. 473:229-247. [DOI] [PubMed] [Google Scholar]
- 9.Bhatnagar, S. K., and M. J. Bessman. 1988. Studies on the mutator gene, mutT of Escherichia coli. Molecular cloning of the gene, purification of the gene product, and identification of a novel nucleoside triphosphatase. J. Biol. Chem. 263:8953-8957. [PubMed] [Google Scholar]
- 10.Bjelland, S., and E. Seeberg. 2003. Mutagenicity, toxicity and repair of DNA base damage induced by oxidation. Mutat. Res. 531:37-80. [DOI] [PubMed] [Google Scholar]
- 11.Blaisdell, J. O., Z. Hatahet, and S. S. Wallace. 1999. A novel role for Escherichia coli endonuclease VIII in prevention of spontaneous G→T transversions. J. Bacteriol. 181:6396-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyer, H. W., and D. Roulland-Dussoix. 1969. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J. Mol. Biol. 41:459-472. [DOI] [PubMed] [Google Scholar]
- 13.Bridges, B. A. 1996. Mutation in resting cells: the role of endogenous DNA damage. Cancer Surv. 28:155-167. [PubMed] [Google Scholar]
- 14.Bridges, B. A. 1993. Spontaneous mutation in stationary-phase Escherichia coli WP2 carrying various DNA repair alleles. Mutat. Res. 302:173-176. [DOI] [PubMed] [Google Scholar]
- 15.Bridges, B. A., M. Sekiguchi, and T. Tajiri. 1996. Effect of mutY and mutM/fpg-1 mutations on starvation-associated mutation in Escherichia coli: implications for the role of 7,8-dihydro-8-oxoguanine. Mol. Gen. Genet. 251:352-357. [DOI] [PubMed] [Google Scholar]
- 16.Bull, H. J., M. J. Lombardo, and S. M. Rosenberg. 2001. Stationary-phase mutation in the bacterial chromosome: recombination protein and DNA polymerase IV dependence. Proc. Natl. Acad. Sci. USA 98:8334-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabrera, M., Y. Nghiem, and J. H. Miller. 1988. mutM, a second mutator locus in Escherichia coli that generates G·C → T·A transversions. J. Bacteriol. 170:5405-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carter, P., H. Bedouelle, and G. Winter. 1985. Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res. 13:4431-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Lorenzo, V., M. Herrero, U. Jakubzik, and K. N. Timmis. 1990. Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol. 172:6568-6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Weert, S., L. C. Dekkers, I. Kuiper, G. V. Bloemberg, and B. J. Lugtenberg. 2004. Generation of enhanced competitive root-tip-colonizing Pseudomonas bacteria through accelerated evolution. J. Bacteriol. 186:3153-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster, P. L. 1993. Adaptive mutation: the uses of adversity. Annu. Rev. Microbiol. 47:467-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foster, P. L. 1999. Mechanisms of stationary phase mutation: a decade of adaptive mutation. Annu. Rev. Genet. 33:57-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster, P. L. 2005. Stress responses and genetic variation in bacteria. Mutat. Res. 569:3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frelon, S., T. Douki, and J. Cadet. 2002. Radical oxidation of the adenine moiety of nucleoside and DNA: 2-hydroxy-2′-deoxyadenosine is a minor decomposition product. Free Radic. Res. 36:499-508. [DOI] [PubMed] [Google Scholar]
- 26.Frenkiel-Krispin, D., S. Levin-Zaidman, E. Shimoni, S. G. Wolf, E. J. Wachtel, T. Arad, S. E. Finkel, R. Kolter, and A. Minsky. 2001. Regulated phase transitions of bacterial chromatin: a non-enzymatic pathway for generic DNA protection. EMBO J. 20:1184-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fromme, J. C., and G. L. Verdine. 2003. DNA lesion recognition by the bacterial repair enzyme MutM. J. Biol. Chem. 278:51543-51548. [DOI] [PubMed] [Google Scholar]
- 28.Goodman, M. F. 2002. Error-prone repair DNA polymerases in prokaryotes and eukaryotes. Annu. Rev. Biochem. 71:17-50. [DOI] [PubMed] [Google Scholar]
- 29.Greated, A., L. Lambertsen, P. A. Williams, and C. M. Thomas. 2002. Complete sequence of the IncP-9 TOL plasmid pWW0 from Pseudomonas putida. Environ. Microbiol. 4:856-871. [DOI] [PubMed] [Google Scholar]
- 30.Hatahet, Z., Y. W. Kow, A. A. Purmal, R. P. Cunningham, and S. S. Wallace. 1994. New substrates for old enzymes. 5-Hydroxy-2′-deoxycytidine and 5-hydroxy-2′-deoxyuridine are substrates for Escherichia coli endonuclease III and formamidopyrimidine DNA N-glycosylase, while 5-hydroxy-2′-deoxyuridine is a substrate for uracil DNA N-glycosylase. J. Biol. Chem. 269:18814-18820. [PubMed] [Google Scholar]
- 31.Hazra, T. K., T. Izumi, R. Venkataraman, Y. W. Kow, M. Dizdaroglu, and S. Mitra. 2000. Characterization of a novel 8-oxoguanine-DNA glycosylase activity in Escherichia coli and identification of the enzyme as endonuclease VIII. J. Biol. Chem. 275:27762-27767. [DOI] [PubMed] [Google Scholar]
- 32.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hõrak, R., H. Ilves, P. Pruunsild, M. Kuljus, and M. Kivisaar. 2004. The ColR-ColS two-component signal transduction system is involved in regulation of Tn4652 transposition in Pseudomonas putida under starvation conditions. Mol. Microbiol. 54:795-807. [DOI] [PubMed] [Google Scholar]
- 34.Hori, M., T. Asanuma, O. Inanami, M. Kuwabara, H. Harashima, and H. Kamiya. 2006. Effects of overexpression and antisense RNA expression of Orf17, a MutT-type enzyme. Biol. Pharm. Bull. 29:1087-1091. [DOI] [PubMed] [Google Scholar]
- 35.Jiang, D., Z. Hatahet, J. O. Blaisdell, R. J. Melamede, and S. S. Wallace. 1997. Escherichia coli endonuclease VIII: cloning, sequencing, and overexpression of the nei structural gene and characterization of nei and nei nth mutants. J. Bacteriol. 179:3773-3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamiya, H. 2003. Mutagenic potentials of damaged nucleic acids produced by reactive oxygen/nitrogen species: approaches using synthetic oligonucleotides and nucleotides: survey and summary. Nucleic Acids Res. 31:517-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamiya, H., E. Iida, N. Murata-Kamiya, Y. Yamamoto, T. Miki, and H. Harashima. 2003. Suppression of spontaneous and hydrogen peroxide-induced mutations by a MutT-type nucleotide pool sanitization enzyme, the Escherichia coli Orf135 protein. Genes Cells 8:941-950. [DOI] [PubMed] [Google Scholar]
- 38.Kamiya, H., and H. Kasai. 1995. Formation of 2-hydroxydeoxyadenosine triphosphate, an oxidatively damaged nucleotide, and its incorporation by DNA polymerases. Steady-state kinetics of the incorporation. J. Biol. Chem. 270:19446-19450. [DOI] [PubMed] [Google Scholar]
- 39.Kamiya, H., N. Murata-Kamiya, E. Iida, and H. Harashima. 2001. Hydrolysis of oxidized nucleotides by the Escherichia coli Orf135 protein. Biochem. Biophys. Res. Commun. 288:499-502. [DOI] [PubMed] [Google Scholar]
- 40.Kasak, L., R. Hõrak, and M. Kivisaar. 1997. Promoter-creating mutations in Pseudomonas putida: a model system for the study of mutation in starving bacteria. Proc. Natl. Acad. Sci. USA 94:3134-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobayashi, M., Y. Ohara-Nemoto, M. Kaneko, H. Hayakawa, M. Sekiguchi, and K. Yamamoto. 1998. Potential of Escherichia coli GTP cyclohydrolase II for hydrolyzing 8-oxo-dGTP, a mutagenic substrate for DNA synthesis. J. Biol. Chem. 273:26394-26399. [DOI] [PubMed] [Google Scholar]
- 42.Koch, B., L. E. Jensen, and O. Nybroe. 2001. A panel of Tn7-based vectors for insertion of the gfp marker gene or for delivery of cloned DNA into Gram-negative bacteria at a neutral chromosomal site. J. Microbiol. Methods 45:187-195. [DOI] [PubMed] [Google Scholar]
- 43.Lambertsen, L., C. Sternberg, and S. Molin. 2004. Mini-Tn7 transposons for site-specific tagging of bacteria with fluorescent proteins. Environ. Microbiol. 6:726-732. [DOI] [PubMed] [Google Scholar]
- 44.Lea, D. E., and C. A. Coulson. 1949. The distribution of the numbers of mutants in bacterial populations. J. Genet. 49:264-265. [DOI] [PubMed] [Google Scholar]
- 45.Maki, H., and M. Sekiguchi. 1992. MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature 355:273-275. [DOI] [PubMed] [Google Scholar]
- 46.Martinez, A., and R. Kolter. 1997. Protection of DNA during oxidative stress by the nonspecific DNA-binding protein Dps. J. Bacteriol. 179:5188-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matic, I., F. Taddei, and M. Radman. 2004. Survival versus maintenance of genetic stability: a conflict of priorities during stress. Res. Microbiol. 155:337-341. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto, Y., Q. M. Zhang, M. Takao, A. Yasui, and S. Yonei. 2001. Escherichia coli Nth and human hNTH1 DNA glycosylases are involved in removal of 8-oxoguanine from 8-oxoguanine/guanine mispairs in DNA. Nucleic Acids Res. 29:1975-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKenzie, G. J., P. L. Lee, M. J. Lombardo, P. J. Hastings, and S. M. Rosenberg. 2001. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Mol. Cell 7:571-579. [DOI] [PubMed] [Google Scholar]
- 50.McLennan, A. G. 2006. The Nudix hydrolase superfamily. Cell Mol. Life Sci. 63:123-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michaels, M. L., C. Cruz, A. P. Grollman, and J. H. Miller. 1992. Evidence that MutY and MutM combine to prevent mutations by an oxidatively damaged form of guanine in DNA. Proc. Natl. Acad. Sci. USA 89:7022-7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Michaels, M. L., and J. H. Miller. 1992. The GO system protects organisms from the mutagenic effect of the spontaneous lesion 8-hydroxyguanine (7,8-dihydro-8-oxoguanine). J. Bacteriol. 174:6321-6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michaels, M. L., L. Pham, C. Cruz, and J. H. Miller. 1991. MutM, a protein that prevents G·C—-T·A transversions, is formamidopyrimidine-DNA glycosylase. Nucleic Acids Res. 19:3629-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Michaels, M. L., J. Tchou, A. P. Grollman, and J. H. Miller. 1992. A repair system for 8-oxo-7,8-dihydrodeoxyguanine. Biochemistry 31:10964-10968. [DOI] [PubMed] [Google Scholar]
- 55.Miller, J. H. 1992. A short course in bacterial genetics: a laboratory manual and handbook for Echerichia coli and related bacteria. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 56.Nair, S., and S. E. Finkel. 2004. Dps protects cells against multiple stresses during stationary phase. J. Bacteriol. 186:4192-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neeley, W. L., S. Delaney, Y. O. Alekseyev, D. F. Jarosz, J. C. Delaney, G. C. Walker, and J. M. Essigmann. 2007. DNA polymerase V allows bypass of toxic guanine oxidation products in vivo. J. Biol. Chem. 282:12741-12748. [DOI] [PubMed] [Google Scholar]
- 58.Neeley, W. L., and J. M. Essigmann. 2006. Mechanisms of formation, genotoxicity, and mutation of guanine oxidation products. Chem. Res. Toxicol. 19:491-505. [DOI] [PubMed] [Google Scholar]
- 59.Nghiem, Y., M. Cabrera, C. G. Cupples, and J. H. Miller. 1988. The mutY gene: a mutator locus in Escherichia coli that generates G·C—-T·A transversions. Proc. Natl. Acad. Sci. USA 85:2709-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oliver, A., R. Canton, P. Campo, F. Baquero, and J. Blazquez. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288:1251-1254. [DOI] [PubMed] [Google Scholar]
- 61.Oliver, A., J. M. Sanchez, and J. Blazquez. 2002. Characterization of the GO system of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 217:31-35. [DOI] [PubMed] [Google Scholar]
- 62.Pavel, H., M. Forsman, and V. Shingler. 1994. An aromatic effector specificity mutant of the transcriptional regulator DmpR overcomes the growth constraints of Pseudomonas sp. strain CF600 on para-substituted methylphenols. J. Bacteriol. 176:7550-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosche, W. A., and P. L. Foster. 2000. Determining mutation rates in bacterial populations. Methods 20:4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenberg, S. M. 2001. Evolving responsively: adaptive mutation. Nat. Rev. Genet. 2:504-515. [DOI] [PubMed] [Google Scholar]
- 65.Saito, Y., F. Uraki, S. Nakajima, A. Asaeda, K. Ono, K. Kubo, and K. Yamamoto. 1997. Characterization of endonuclease III (nth) and endonuclease VIII (nei) mutants of Escherichia coli K-12. J. Bacteriol. 179:3783-3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saumaa, S., K. Tarassova, M. Tark, A. Tover, R. Tegova, and M. Kivisaar. 2006. Involvement of DNA mismatch repair in stationary-phase mutagenesis during prolonged starvation of Pseudomonas putida. DNA Repair 5:505-514. [DOI] [PubMed] [Google Scholar]
- 67.Saumaa, S., A. Tover, L. Kasak, and M. Kivisaar. 2002. Different spectra of stationary-phase mutations in early-arising versus late-arising mutants of Pseudomonas putida: involvement of the DNA repair enzyme MutY and the stationary-phase sigma factor RpoS. J. Bacteriol. 184:6957-6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma, R. C., and R. T. Schimke. 1996. Preparation of electrocompetent E. coli using salt-free growth medium. BioTechniques 20:42-44. [DOI] [PubMed] [Google Scholar]
- 69.Shibutani, S., M. Takeshita, and A. P. Grollman. 1991. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature 349:431-434. [DOI] [PubMed] [Google Scholar]
- 70.Shimizu, M., P. Gruz, H. Kamiya, S. R. Kim, F. M. Pisani, C. Masutani, Y. Kanke, H. Harashima, F. Hanaoka, and T. Nohmi. 2003. Erroneous incorporation of oxidized DNA precursors by Y-family DNA polymerases. EMBO Rep. 4:269-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sokal, R. R., and F. J. Rohlf. 1981. Biometry. W. H. Freeman, San Francisco, CA.
- 72.Sung, H. M., G. Yeamans, C. A. Ross, and R. E. Yasbin. 2003. Roles of YqjH and YqjW, homologs of the Escherichia coli UmuC/DinB or Y superfamily of DNA polymerases, in stationary-phase mutagenesis and UV-induced mutagenesis of Bacillus subtilis. J. Bacteriol. 185:2153-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sutton, M. D., and G. C. Walker. 2001. Managing DNA polymerases: coordinating DNA replication, DNA repair, and DNA recombination. Proc. Natl. Acad. Sci. USA 98:8342-8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tark, M., A. Tover, K. Tarassova, R. Tegova, G. Kivi, R. Hõrak, and M. Kivisaar. 2005. A DNA polymerase V homologue encoded by TOL plasmid pWW0 confers evolutionary fitness on Pseudomonas putida under conditions of environmental stress. J. Bacteriol. 187:5203-5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tegova, R., A. Tover, K. Tarassova, M. Tark, and M. Kivisaar. 2004. Involvement of error-prone DNA polymerase IV in stationary-phase mutagenesis in Pseudomonas putida. J. Bacteriol. 186:2735-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Timms, A. R., W. Muriel, and B. A. Bridges. 1999. A UmuD,C-dependent pathway for spontaneous G:C to C:G transversions in stationary phase Escherichia coli mut Y. Mutat. Res. 435:77-80. [DOI] [PubMed] [Google Scholar]
- 77.Williams, P. A., and K. Murray. 1974. Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J. Bacteriol. 120:416-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolf, S. G., D. Frenkiel, T. Arad, S. E. Finkel, R. Kolter, and A. Minsky. 1999. DNA protection by stress-induced biocrystallization. Nature 400:83-85. [DOI] [PubMed] [Google Scholar]
- 79.Xu, W., C. A. Dunn, F. O'Handley, S. D. L. Smith, and M. J. Bessman. 2006. Three new Nudix hydrolases from Escherichia coli. J. Biol. Chem. 281:22794-22798. [DOI] [PubMed] [Google Scholar]
- 80.Yamada, M., T. Nunoshiba, M. Shimizu, P. Gruz, H. Kamiya, H. Harashima, and T. Nohmi. 2006. Involvement of Y-family DNA polymerases in mutagenesis caused by oxidized nucleotides in Escherichia coli. J. Bacteriol. 188:4992-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yanofsky, C., E. C. Cox, and V. Horn. 1966. The unusual mutagenic specificity of an E. coli mutator gene. Proc. Natl. Acad. Sci. USA 55:274-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yeiser, B., E. D. Pepper, M. F. Goodman, and S. E. Finkel. 2002. SOS-induced DNA polymerases enhance long-term survival and evolutionary fitness. Proc. Natl. Acad. Sci. USA 99:8737-8741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang, Q. M., N. Ishikawa, T. Nakahara, and S. Yonei. 1998. Escherichia coli MutY protein has a guanine-DNA glycosylase that acts on 7,8-dihydro-8-oxoguanine:guanine mispair to prevent spontaneous G:C→C:G transversions. Nucleic Acids Res. 26:4669-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]