Abstract
Type 1 fimbriae and flagella have been previously shown to contribute to the virulence of uropathogenic Escherichia coli (UPEC) within the urinary tract. In this study, the relationship between motility and type 1 fimbrial expression was tested for UPEC strain CFT073 by examining the phenotypic effect of fimbrial expression on motility and the effect that induction of motility has on type 1 fimbrial expression. While constitutive expression of type 1 fimbriae resulted in a significant decrease in motility and flagellin expression (P < 0.0001), a loss of type 1 fimbrial expression did not result in increased motility. Additionally, hypermotility and flagellar gene over- and underexpression were not observed to affect the expression of type 1 fimbriae. Hence, it appeared that the relationship between type 1 fimbrial expression and motility is unidirectional, where the overexpression of type 1 fimbriae dramatically affects motility and flagellum expression but not vice versa. Moreover, the constitutive expression of type 1 fimbriae in UPEC cystitis isolate F11 and the laboratory strain E. coli K-12 MG1655 also resulted in decreased motility, suggesting that this phenomenon is not specific to CFT073 or UPEC in general. Lastly, by analyzing the repression of motility caused by constitutive type 1 fimbrial expression, it was concluded that the synthesis and presence of type 1 fimbriae at the bacterial surface is only partially responsible for the repression of motility, as evidenced by the partial restoration of motility in the CFT073 fim L-ON ΔfimAICDFGH mutant. Altogether, these data provide further insight into the complex interplay between type 1 fimbrial expression and flagellum-mediated motility.
Uropathogenic Escherichia coli (UPEC) causes the majority of urinary tract infections in otherwise healthy individuals. Studies suggest that up to 95% of all urinary tract infections develop in an ascending manner (3), beginning with periurethral colonization, followed by bladder infection (cystitis) and in some cases, if left untreated, ascension of the ureters to the kidney (pyelonephritis). Sequencing of the genome of pyelonephritis isolate E. coli CFT073 revealed 12 putative fimbrial gene clusters, including a type 1 fimbrial operon (60). UPEC utilizes type 1 fimbriae for adherence to N-linked oligomannose glycoproteins or uroplakin receptors found on transitional epithelia of the bladder (28, 46, 62). Our laboratory has established that type 1 fimbriae are highly expressed during murine urinary tract infection (54) and are important in infection, particularly in the first 24 h of experimental bladder colonization (22). Type 1 fimbriae have also been shown to satisfy molecular Koch's postulates as a virulence factor (15).
The promoter upstream of the type 1 fimbrial operon resides within an invertible element (IE) of DNA that upon inversion changes the promoter orientation, which in turn affects the transcription of the fimAICDFGH genes (1). The switching of promoter orientation ultimately leads to the expression (phase on) or loss of expression (phase off) of type 1 fimbriae and is mediated by the extensively studied FimB and FimE recombinases (8, 25, 41), in addition to the recently identified FimB- and FimE-like recombinases IpuA and IpbA (12). While FimB promotes both “on-to-off” and “off-to-on” phase variation, FimE has been shown to promote primarily “on-to-off” switching (19, 25).
While fimbria-mediated adherence is important during the initial stages of host colonization, bacterial motility and chemotaxis provide a means for UPEC to disseminate to new sites of the urinary tract to obtain nutrients when they become limited as well as to escape host immune responses. Recently, our laboratory and others have demonstrated that flagellum-mediated motility and chemotaxis also contribute to the fitness of UPEC during urinary tract colonization (32, 61). Genes for flagellum synthesis form an ordered and highly regulated cascade of three classes (26, 27, 38). The class I master regulon flhDC encodes a transcription factor necessary for class 2 gene transcription (38). Class 2 genes encode the basal body and hook of the flagellum in addition to FliA (σ28) and FlgM (anti-σ28 factor) (38). FliA is the sigma factor required for the transcription of the class 3 flagellar genes (29, 48). The class 3 genes encode hook-associated proteins and the filament of the flagellum (FliC) as well as proteins necessary for motility and chemotaxis (such as CheY) (38). CheY is a response regulator (of a two-component regulatory system) that, upon phosphorylation, interacts with the flagellar motor switch complex and in turn influences the direction of flagellar rotation and speed of swimming (11, 42, 51, 53). Due to the fact that the flagellar filament of E. coli is highly immunogenic (59, 64) and that flagellum-mediated motility is very energy expensive (57), it is logical that flagellum synthesis be tightly regulated. This regulation is focused primarily at the level of the flhDC master regulon.
Reciprocal regulation between flagellum synthesis and fimbria production may allow bacteria to coordinate two counterproductive properties, motility and adherence. Indeed, it would not be advantageous for a bacterium tethered to an epithelial surface by fimbriae to attempt swimming (or swarming) at a high rate of speed using its flagellar motor. Thus, it is intuitive that an adherent bacterium should not be highly motile and that a highly motile bacterium should be less adherent. Several genera of pathogenic and nonpathogenic bacteria have been shown to reciprocally regulate motility and adherence through different mechanisms. For example, the two-component BvgAS system of Bordetella pertussis acts as a switch between two states: the Bvg+ phase activates the expression of adhesin and toxin genes, whereas the Bvg− phase promotes motility (17). In a different mode of regulation, mutations that alter motility in Vibrio cholerae directly feed back to the ToxR regulatory system to alter the expression of the toxin-coregulated pilus (20). Interestingly, genes within fimbrial operons have also been implicated in the repression of flagellum synthesis. For example, increased expression of FimZ of the type 1 fimbrial operon of Salmonella enterica serovar Typhimurium has been shown to cause hyperfimbriation and a loss of motility in soft agar (14). A gene, mrpJ, of the MR/P fimbrial operon in Proteus mirabilis was shown by our laboratory to encode a protein that, when overexpressed, repressed flagellum-mediated motility (35). Li et al. (35) also demonstrated that PapX, an MrpJ homolog encoded by the pheV-associated but not the pheU-associated pap allele of CFT073, also caused reduced motility when overexpressed in P. mirabilis. Moreover, the overexpression of PapX was recently shown in our laboratory to result in a similar reduction of flagellum-mediated motility in the parent UPEC strain CFT073 (A. N. Simms and H. L. T. Mobley, unpublished data).
To explore the potential relationship between motility and adherence of UPEC, we meticulously examined the connection between type 1 fimbrial expression, motility, and flagellum expression. Overall, we demonstrate that the interplay between type 1 fimbrial expression and motility is unidirectional. By examining motility and chemotaxis in 0.25% agar under phase-contrast microscopy and using a capillary chemotaxis assay, we present evidence that two UPEC strains constitutively producing type 1 fimbriae are significantly less motile and chemotactic to l-aspartate than the wild type. Moreover, we demonstrate that this phenomenon is specific not only to UPEC but also to the laboratory E. coli K-12 strain MG1655. Using transmission electron microscopy, Western blot analysis, and quantitative real-time reverse transcription-PCR (qPCR), we determined that CFT073 fim L-ON expresses significantly fewer flagella than wild-type CFT073. By deleting the fimAICDFGH genes in CFT073 fim L-ON (where the IE remains in the on position but the structural genes for type 1 fimbriae have been deleted), we observed a partial restoration of wild-type motility and flagellum expression, thus suggesting that the expression of type 1 fimbriae at the bacterial surface is only partially responsible for the decrease in motility and flagellum expression observed for the CFT073 fim L-ON strain. Therefore, it is concluded that other unknown regulatory mechanisms, such as those that are upregulated when the fim switch is in the on orientation (irrespective of fim expression), that are also important for the reduction of motility and flagellum expression in the fim L-ON variant must exist. Ultimately, data generated from these studies provide insight into the complex interplay between flagellum-mediated motility and a known adherence factor of E. coli. Furthermore, these findings aid in the understanding of the balance between motility and type 1 fimbria-mediated adherence during urinary tract pathogenesis.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
E. coli strain CFT073 was isolated from the blood and urine of a patient diagnosed with acute pyelonephritis (43). All E. coli strains and plasmids used in these studies are shown in Table 1. For initial propagation, all strains were grown on LB agar plates with appropriate antibiotics (50 μg/ml nalidixic acid, 25 μg/ml kanamycin, or 100 μg/ml ampicillin) and incubated at 37°C for 18 h. Liquid cultures grown overnight were obtained by inoculating a single colony into LB broth containing appropriate antibiotics and incubation at 37°C for 18 h with aeration (200 rpm).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Reference or source |
|---|---|---|
| E. coli strains | ||
| CFT073 | Wild-type pyelonephritis isolate (O6:K2:H1) | 44 |
| CFT073 fim L-ON | CFT073 fim IE phase locked; constitutive type 1 fimbrial expression | 22 |
| CFT073 fim L-OFF | CFT073 fim IE phase locked; no type 1 fimbrial expression | 22 |
| fim L-ON ΔfimAICDFGH | CFT073 fim L-ON with deletion of fimAICDFGH | This study |
| UPEC76 | CFT073 Nalr derivative; both pap operons disrupted | 45 |
| Δfim pap | UPEC76 fimAICDFGH::aphA (Kanr) | 55 |
| ΔflhDC | CFT073 flhDC::aphA (Kanr) | This study |
| ΔflhC | CFT073 flhC::aphA (Kanr) | This study |
| ΔfliA | CFT073 fliA::aphA (Kanr) | 32 |
| ΔflgM | CFT073 flgM::aphA (Kanr) | 32 |
| ΔfliC | CFT073 fliC::aphA (Kanr) | 32 |
| ΔcheY | CFT073 cheY::aphA (Kanr) | 32 |
| F11 | Wild-type cystitis isolate (O6:H31) | 58 |
| F11 fim L-ON | F11 fim IE phase locked; constitutive type 1 fimbrial expression | 56 |
| F11 fim L-OFF | F11 fim IE phase locked; no type 1 fimbrial expression | 56 |
| K-12 | Wild-type K-12 strain MG1655 | Gift from R. Welch (6) |
| K-12 fim L-ON | K-12 strain MG1655 fim IE phase locked; constitutive type 1 fimbrial expression (AAEC554) | Gift from I. Blomfield (40) |
| K-12 fim L-OFF | K-12 strain MG1655 fim IE phase locked; no type 1 fimbrial expression (AAEC358) | Gift from I. Blomfield (40) |
| Plasmids | ||
| pBAD/Myc-HisA | Arabinose-inducible expression vector (Ampr) | Invitrogen |
| pBAD-flhDC | pBAD/Myc-HisA digested with NcoI and PmeI replacing Myc-His site with flhDC (Ampr) | This study |
To culture wild-type and mutant strains of CFT073 in conditions that favor optimized wild-type motility, bacteria were cultivated from outer motility rings in 0.25% tryptone motility agar (see below) and grown in tryptone broth (10 g tryptone and 5 g NaCl per liter) at 30°C with aeration (200 rpm) for 16 h. Upon enriching for motile bacterial populations, the bacterial cultures were then stored for up to 1 week at 4°C. For the capillary chemotaxis assay, most Western blot analyses, transmission electron microscopy, and qPCR experiments, 400 μl of stored culture was resuspended into 12 ml of tryptone broth (10 g tryptone and 5 g NaCl per liter) in a 125-ml flask. Cultures were incubated at 30°C with aeration (200 rpm) until the optical density at 600 nm (OD600) reached ≅0.3, corresponding to optimal wild-type motility. Motility was verified by viewing wet mounts of cultures by phase-contrast microscopy as described below. At this time, the cultures were immediately prepared for the capillary chemotaxis assay, sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), transmission electron microscopy, or RNA extraction. For RNA preparation, 1 ml of RNAprotect bacterial reagent (QIAGEN) was added immediately to a 500-μl sample of bacterial culture to stabilize RNA according to the manufacturer's specifications. After incubation at 23°C for 10 min, bacteria were harvested by centrifugation (10 min, 10,000 × g, 23°C), and the bacterial pellet was stored at −80°C for up to 4 weeks until RNA extraction.
Construction and characterization of CFT073 deletion mutants.
Deletion mutants of flhDC, flhC, and fimAICDFGH were generated using the lambda red recombinase designed by Datsenko and Wanner (16) and as described previously for the fliC, fliA, flhM, and cheY deletion mutants (32). The flhDC and flhC deletion mutants were constructed in the wild-type CFT073 strain, while the fimAICDFGH deletion mutant was made in a type 1 fimbrial phase-locked derivative of CFT073 that constitutively expresses type 1 fimbriae (CFT073 fim L-ON). Primers homologous to sequences within the 5′ and 3′ regions of flhDC, flhC, fimA (5′, fimAICDFGH), or fimH (3′, fimAICDFGH) were designed and used to replace these genes with a nonpolar kanamycin resistance cassette derived from the template plasmid pKD4 (Table 2). Less than 10% of the flhDC, flhC, and fimAICDFGH gene sequence remained after recombination. Kanamycin (25 μg/ml) was used for the selection of all mutants. The disruption and deletion of flhDC, flhC, and fimAICDFGH were confirmed by using a PCR/digest assay as described previously (32). Both of the flagellar mutants were characterized as nonmotile in 0.25% tryptone broth agar and by phase-contrast microscopy (data not shown). Additionally, the flhDC and flhC deletion mutants did not produce any detectable levels of flagellin as determined by Western blot analysis using H1 flagellin antiserum (data not shown). The loss of type 1 fimbrial expression of the fimAICDFGH deletion mutant CFT073 fim L-ON ΔfimAICDFGH was monitored by the loss of the ability to agglutinate guinea pig erythrocytes (data not shown) and by Western blotting with FimH antiserum (see Fig. 2).
TABLE 2.
Oligonucleotides used in this study
| Primer | Sequence (5′-3′) | Description |
|---|---|---|
| flhDCP1 (forward) | TAATGCATACCTCCGAGTTGCTGAAACACATTTATGACATATGGGAATTAGCCATGGTCC | Construction of isogenic flhDC mutant |
| flhDCP2 (reverse) | AAGGTATAAACGGTAGGCTTTGATCACCGCATCGACGCCAGTGTAGGCTGGAGCTGCTTC | Construction of isogenic flhDC mutant |
| flhCP1 (forward) | AAAGCATTGTTCAGGAAGCGCGGGATATTCAGCTGGCAATGTGTAGGCTGGAGCTGCTTC | Construction of isogenic flhC mutant |
| flhCP2 (reverse) | CTGTACTCTCTGTTCATCCAGCAGTTGTGGGATAATATCGATGGGAATTAGCCATGGTCC | Construction of isogenic flhC mutant |
| fimA-HP1 (forward) | GGAAAACTGTGCAGTGTTGGCAGTCAAACTCGTTGACAAAGTGTAGGCTGGAGCTGCTTC | Construction of isogenic fim L-ON fimAICDFGH mutant |
| fimA-HP2 (reverse) | GTGCAGGTTTTAGCTTCAGGTAATATTGCGTACCTGCATTATGGGAATTAGCCATGGTCC | Construction of isogenic fim L-ON fimAICDFGH mutant |
| flhDCBAD (forward) | CTGATTCCATGGGAATAATGCATACCTCCGAGTTG | Construction of pBAD-flhDC |
| flhDCBAD (reverse) | ACTGGTGTTTAAACTTAAACAGCCTGTACTCTCTGTTC | Construction of pBAD-flhDC |
| gapA (forward) | CGTTAAAGGCGCTAACTTCG | qPCR |
| gapA (reverse) | ACGGTGGTCATCAGACCTTC | qPCR |
| fliC (forward) | ACAGCCTCTCGCTGATCACTCAAA | qPCR |
| fliC (reverse) | GCGCTGTTAATACGCAAGCCAGAA | qPCR |
| fimA (forward) | GTTGGCTTTGGATGGTGCGTCATT | qPCR |
| fimA (reverse) | GGTTGCGGCACCAATGGCATAATA | qPCR |
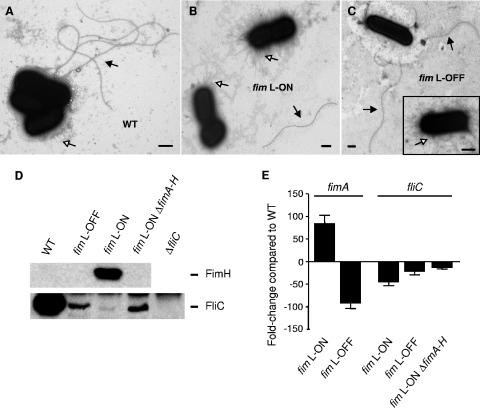
FIG. 2.
Effect of type 1 fimbrial expression on flagellum expression in UPEC strain CFT073. (A to C) Transmission electron microscopy of cultures prepared for the chemotaxis assay. (A) Wild-type CFT073 (WT). (B) CFT073 fim L-ON. (C) CFT073 fim L-OFF. Bacteria were visualized at magnifications between ×7,900 and ×25,000. Black arrowheads, flagella; white arrowheads, fimbriae. Bar, 0.5 μm. (D) Strains were cultured from motility agar and grown to early exponential phase (OD600 of ∼0.30) in tryptone broth at 30°C with shaking (200 rpm). Whole-cell lysates of these cultures were denatured, electrophoresed onto SDS-PAGE gels, and subjected to Western blot analyses using rabbit antiserum against H1 flagellin or mouse antiserum against FimH. CFT073 ΔfliC and CFT073 fim L-OFF were used as the negative controls for FliC and FimH expression, respectively. The molecular masses of FliC and FimH of UPEC strain CFT073 are predicted to be 60.9 kDa and 29.3 kDa, respectively. (E) qPCR analysis of fimA and fliC transcription. Black bars represent the average changes (n-fold) (n = 3) in gene expression of fimA and fliC between fim L-ON, fim L-OFF, and fim L-ON ΔfimAICDFGH (fimA-H). Changes (n-fold) were calculated using wild-type CFT073 as the relative measure of comparison. Error bars represent the SEM.
Motility assays.
Motility was initially determined by using soft agar plates. Specifically, 50 μl of a culture grown overnight was reinoculated into 5 ml of sterile LB broth and incubated at 37°C with aeration (200 rpm) to an OD600 of 0.9 to 1.0. At that point, cultures were standardized to an OD600 of ∼0.9 and stabbed into 0.25% tryptone agar plates using a sterile inoculating needle. The plates were incubated for 16 h at 30°C (or, for one experiment, at 37°C) before the diameter of swimming was assessed for each strain. Since the diameter of swimming in soft agar can be attributable to motility and chemotaxis, all motility agar results were confirmed by phase-contrast microscopy. To do this, wet mounts of bacterial cultures, grown to an OD600 of ∼0.3, corresponding to optimal wild-type motility, were viewed at ×400 magnification using a Zeiss Axioplan microscope. For both motility assays, wild-type CFT073 served as the positive control, and the nonmotile mutant CFT073 ΔfliC served as the negative control.
Capillary chemotaxis assay.
A modified version of a standard capillary chemotaxis assay was performed as previously described (2, 31, 50). Bacteria were cultured for optimized wild-type motility as described above for the bacterial culture conditions. Cultures were harvested by centrifugation (2,000 × g, 10 min, 23°C), spent medium was aspirated, and bacteria were resuspended in chemotaxis buffer (10 mM potassium phosphate buffer [pH 7.0], 0.10 mM potassium EDTA [pH 8.0]) and incubated for 1 h at 30°C. Starved bacterial suspensions (500 μl) were added to the wells of the chambers, followed by the addition of microcapillaries (1 μl) filled with either 10 mM l-aspartate (Sigma) or chemotaxis buffer as a control to measure random movement into the capillary. Chemotaxis chambers were incubated for 90 min at 30°C before capillaries were removed for serial dilution. Dilutions were spiral plated with an Autoplate 4000 apparatus (Spiral Biotech) and incubated at 37°C overnight. Colonies were enumerated using a Q-Count apparatus with automated colony-counting software (Spiral Biotech) to determine CFU/ml. l-Aspartate was diluted in chemotaxis buffer, adjusted to pH ≅8.0 to match that of chemotaxis buffer alone, 0.2-μm-filter sterilized (Millipore), and stored at room temperature.
Cloning and overexpression of flhDC.
To induce flagellar gene expression and motility, a 963-bp fragment containing the full open reading frame encoding FlhDC was cloned into pBAD/Myc-HisA (Invitrogen) under the control of the arabinose-inducible promoter. Briefly, primers containing NcoI and PmeI restriction sites were designed to amplify and directionally clone flhDC directly downstream of the araBAD promoter, replacing the Myc-His region (Table 2). The resulting construct, pBAD-flhDC, and the empty pBAD vector were transformed into wild-type CFT073, CFT073 ΔflhDC, and CFT073 fim L-ON. Restriction digestion of isolated plasmid DNA was performed to confirm the presence and direction of the flhDC insert. The induction and repression of motility were determined for wild-type CFT073, CFT073 ΔflhDC, and CFT073 fim L-ON containing pBAD-flhDC or vector alone by subculturing into LB broth, culturing the strains to an OD600 of ∼0.8, and stabbing the standardized cultures into soft agar plates (1% tryptone, 0.5% NaCl, 0.25% agar) containing either 0.2% arabinose (for induction) or 0.4% glucose (for repression). Plates were incubated for 16 h at 23°C before the degree of motility was assessed for each strain.
Induction of flhDC expression in the CFT073 fim L-ON strain containing pBAD-flhDC (and the empty pBAD vector as a control) was achieved by culturing bacteria in LB broth containing 100 μg/ml of ampicillin with shaking (200 rpm) at 37°C to an OD600 of ∼0.5 and then adding either 0.2% arabinose (for induction) or 0.4% glucose (for repression) to the cultures for induction or repression, respectively, of the araBAD promoter. At this point, the cultures were further incubated with shaking (200 rpm) at 37°C for an additional 2 h for complete induction or repression. Induction of flhDC expression in wild-type CFT073 was conducted differently, because the wild type does not constitutively express type 1 fimbriae, as does the fim L-ON strain, and does not optimally produce type 1 fimbriae under the growth conditions described above. Therefore, for optimal type 1 expression, wild-type CFT073 containing pBAD-flhDC (and the empty pBAD vector as a control) was cultured statically in LB broth containing 100 μg/ml of ampicillin for 48 h at 37°C and then subcultured (1:100) into fresh LB broth containing 100 μg/ml of ampicillin and cultured statically for an additional 48 h at 37°C. For the induction or repression of flhDC expression, 0.2% arabinose or 0.4% glucose, respectively, was added at the start of the initial culture and after every 24 h of culturing. After incubation, both wild-type CFT073 and the CFT073 fim L-ON mutant were prepared immediately for Western blot analysis or RNA extraction. For RNA preparation, 500 μl of culture was added to 1 ml of RNAprotect bacterial reagent (QIAGEN) and prepared as described above.
Transmission electron microscopy.
Samples of wild-type CFT073 and the fim-locked mutant cultures were obtained from the capillary chemotaxis assay (after they had been starved in phosphate-buffered saline for 1 h at 30°C). Carbon-only copper grids were coated with poly-l-lysine (Sigma) to enhance the attachment of bacteria to the grid. A droplet (8 μl) of culture was placed onto the grid for 5 min. Bacteria were then fixed with 2.5% glutaraldehyde and stained with 1% sodium phosphotungstic acid (pH 5.8) for 30 s. The grids were examined with a Philips CM-100 transmission electron microscope at an operating voltage of 60 kV. Bacteria were examined between the magnifications of ×7,900 and ×25,000. Digital images of bacteria were captured with an automated compustage and a Kodak 1.6 Megaplus high-resolution digital camera. All materials and solutions, unless otherwise noted, were obtained from Electron Microscopy Sciences (Fort Washington, PA).
Detection of flagellum and fimbria expression.
For the wild type, the fim-locked mutants, and the flagellar mutants, bacteria were cultured for optimized wild-type motility as described above in bacterial culture conditions. Whole-cell lysates were prepared for electrophoresis by adding either an equal volume of 2× SDS sample buffer (flagellum samples) or 2× SDS sample buffer acidified with 1 N HCl (fimbria samples), followed by boiling for 10 min. After boiling, the fimbrial samples were neutralized with 1 N NaOH. Samples were electrophoresed using discontinuous one-dimensional SDS-PAGE as described previously by Laemmli (30). After electrophoresis, gels were transferred onto a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corp.). Western blots for the detection of flagella were incubated with a 1:40,000 dilution of rabbit polyclonal antiserum to H1 flagellin (Statens Serum Institute, Copenhagen, Denmark), followed by a 1:20,000 dilution of peroxidase-conjugated goat anti-rabbit immunoglobulin G (Sigma). Western blots for the detection of type 1 fimbriae were incubated with a 1:5,000 dilution of murine antiserum against FimH (courtesy of S. Langermann), followed by a 1:100,000 dilution of peroxidase-conjugated goat anti-mouse immunoglobulin G. Both were developed using a chemiluminescent detection system (ECL; Amersham Pharmacia Biotech).
For the flhDC induction studies, wild-type CFT073 and CFT073 fim L-ON containing pBAD-flhDC or pBAD alone were cultured as described above. Whole-cell lysates were prepared for electrophoresis by adding either 6× SDS sample buffer (flagellum samples) and boiling for 10 min or 6× SDS sample buffer acidified with 1 N HCl (fimbria samples) followed by boiling for 10 min. After boiling, fimbrial samples were neutralized with 1 N NaOH. Samples were electrophoresed by SDS-PAGE and subjected to Western blot analysis and detection as described above.
RNA isolation and cDNA synthesis.
Total RNA from bacterial samples was extracted using the RNeasy Mini kit (QIAGEN) according to the RNeasy Mini handbook. Following treatment of RNA with TURBO DNase (Ambion), cDNA was synthesized using the SuperScript First-Strand Synthesis system for reverse transcriptase PCR according to the manufacturer's protocols (Invitrogen). In parallel, RNA samples were subjected to agarose gel electrophoresis to verify quality and yield. Subsequently, RNA present in the cDNA samples was digested with RNase H (Invitrogen), and cDNA was purified using the QIAquick PCR purification kit (QIAGEN) according to the QIAquick Spin handbook. PCR with primers specific to the gapA gene (glyceraldehyde-3-phosphate dehydrogenase) (Table 2) of CFT073 was performed on cDNA samples prepared with and without reverse transcriptase to confirm no genomic DNA contamination of the RNA preparations following DNase treatment.
Comparative qPCR.
Primers designed to amplify fliC and fimA (Table 2) were targeted to regions of unique sequence within each gene. For each sample, 30 ng of cDNA and 300 nM of each primer set were mixed with 12.5 μl 2× SYBR green PCR master mix (Stratagene) per well. Assays were performed in triplicate with the Stratagene Mx3000P instrument. All data were normalized to the endogenous reference gene gapA. Also, melting curve analysis demonstrated that the accumulation of SYBR green-bound DNA was gene specific and not due to primer dimers. Data were analyzed by the 2−ΔΔCT method (37). The choice of a baseline calibrator varied depending upon what strains or conditions were examined. These data were transformed to log2 to obtain a change difference (n-fold) between strains.
Statistical analysis.
For motility and capillary chemotaxis assays, an unpaired Student's t test with Welch correction was used to determine significant differences in chemotaxis and motility between strains (InStat; GraphPad Software). For qPCR experiments, significant differences in the average change (n-fold) of three separate replicates were determined using a paired two-tailed Student's t test (Prism; GraphPad Software).
RESULTS
Constitutive expression of type 1 fimbriae results in decreased motility and chemotaxis to l-aspartate in UPEC strain CFT073.
Two surface organelles, flagella and fimbriae, are important in microbial pathogenesis but mediate opposite and likely antagonistic actions. We hypothesized that UPEC reciprocally regulates motility and adherence. Previously, our laboratory constructed type 1 fimbrial phase-locked derivatives of CFT073 that bear a mutation in the left inverted repeat of the type 1 fimbrial IE that blocks DNA inversion by FimB and FimE, thus resulting in either a locked-on (fim L-ON) or locked-off (fim L-OFF) promoter orientation (22). To determine the effect that type 1 fimbrial expression has on motility, wild-type strain CFT073 and the fim L-ON phase-locked derivative of CFT073 that constitutively expresses type 1 fimbriae were evaluated for their motility in 0.25% tryptone soft agar. The average diameter of swimming of CFT073 fim L-ON was dramatically less (about fourfold; P < 0.0001) than that of wild-type CFT073 (Fig. 1A, C, and G). A flagellum-negative mutant, CFT073 ΔfliC, used as a negative control, was nonmotile in 0.25% soft agar (Fig. 1B). Since the diameter of swimming in soft agar can be attributable to both motility and chemotaxis, the repression of motility in CFT073 fim L-ON was confirmed by phase-contrast microscopy. Indeed, we observed that CFT073 fim L-ON was much less motile than wild-type CFT073 and CFT073 fim L-OFF (a phase-locked derivative of CFT073 unable to produce type 1 fimbriae).
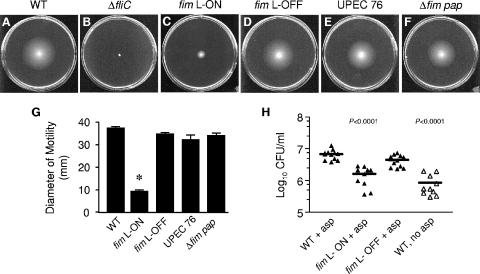
FIG. 1.
Effect of type 1 and P fimbrial expression on the motility and chemotaxis of UPEC strain CFT073. Motilities of the wild type (WT) (A), CFT073 ΔfliC (flagellum-negative mutant) (B), CFT073 fim L-ON (C), CFT073 fim L-OFF (D), UPEC76 (CFT073 pap double mutant) (E), and Δfim pap (fim mutant of UPEC76) (F) in soft agar are shown. Standardized cultures of each strain were stabbed into 0.25% soft agar plates and incubated for 16 h at 30°C. (G) Black bars represent the average diameters of swimming (in mm) of triplicate motility plates. Error bars represent the standard errors of the means (SEM). (H) Chemotaxis of the wild type and the fim-locked mutants to 10 mM l-aspartate (asp). Each data point represents the log10 CFU/ml per 1 μl capillary. Bars indicate the mean log10 CFU/ml. Filled symbols indicate capillaries filled with attractant, while open symbols indicate capillaries filled with chemotaxis buffer only. Significant differences in motility and chemotaxis between the wild type and each strain (or control) were determined using an unpaired Student's t test with Welch correction (Instat; GraphPad). *, P < 0.0001.
Although the expression of type 1 fimbriae has been shown to be poorly expressed, if at all, by E. coli at temperatures lower than 37°C owing to the differential temperature modulation by H-NS of the fimB and fimE genes (49), we demonstrate by transmission electron microscopy and Western blot analysis that CFT073 fim L-ON (phase-locked mutant that constitutively expresses type 1 fimbriae and cannot undergo FimB and FimE recombination) is heavily fimbriated and abundantly expresses FimH at 30°C throughout the motility assays (Fig. 2B and D, respectively). Therefore, it is possible that the presence and abundance of type 1 fimbriae at the bacterial surface may be contributing to the reduction in motility observed in the fim L-ON variant. To determine whether these results would be consistent at higher temperatures that favor type 1 fimbrial expression, we also compared the motilities of wild-type CFT073 and CFT073 fim L-ON in 0.25% tryptone soft agar after incubation for 16 h at 37°C. Once more, we observed that the average motility diameter of the wild type (44 ± 4 mm) was significantly greater than the average motility diameter observed for CFT073 fim L-ON (8 ± 2 mm) (P < 0.0001) (data not shown).
In view of the fact that the CFT073 fim L-ON mutant was shown to have reduced motility, it was not surprising that it was also observed to be significantly less chemotactic to l-aspartate than wild-type CFT073 by using a capillary chemotaxis assay (Fig. 1H) (P < 0.0001). To account for the random movement of bacteria into the capillaries during the chemotaxis assay, wild-type CFT073 chemotaxis to buffer alone (with no aspartate) was used as a negative control (Fig. 1H). As expected, there were significantly more wild-type CFT073 bacteria present in the capillaries filled with aspartate than with buffer alone (Fig. 1H) (P < 0.0001).
Constitutive expression of type 1 fimbriae results in decreased flagellin expression and transcription of fliC in UPEC strain CFT073.
To determine whether the reduced motility of CFT073 fim L-ON was due to a decrease in flagellum expression, wild-type CFT073 and the CFT073 fim L-ON mutant were cultured in tryptone broth with aeration to a density corresponding to optimal motility (for the wild type, an OD600 of ∼0.3). The production of flagella was initially assessed by Western blot analysis using H1 flagellin antiserum. To verify that CFT073 fim L-ON was still producing type 1 fimbriae under these conditions, which were not optimal for type 1 fimbria expression, the same lysates were subjected to Western blot analysis using FimH antiserum. As expected, wild-type CFT073 did not produce detectable levels of type 1 fimbriae, whereas strain CFT073 fim L-ON was shown to express abundant FimH (Fig. 2D). The increased expression of type 1 fimbriae correlated with a reduction in flagellin expression in CFT073 fim L-ON compared to wild-type CFT073 (Fig. 2D). Levels of fimbriation and flagellation were also assessed by transmission electron microscopy. While wild-type CFT073 was frequently observed to be flagellated, CFT073 fim L-ON was rarely observed to produce flagella (Fig. 2A and B, respectively, black arrowheads). Conversely, wild-type CFT073 was rarely observed to express fimbriae, whereas CFT073 fim L-ON was observed to express an abundant amount of fimbriae (presumably type 1 fimbriae) (Fig. 2A and B, respectively, white arrowheads). As a negative control, the CFT073 ΔfliC mutant was never observed to produce flagella (data not shown).
To determine whether the decrease in flagellin expression is regulated at the level of transcription, the amounts of fliC transcripts between wild-type CFT073 and the CFT073 fim L-ON mutant were compared using qPCR. Consistent with the Western results, the level of fliC transcripts in the CFT073 fim L-ON strain was decreased 46-fold (n = 3) relative to wild-type CFT073 (Fig. 2E). Additionally, the levels of fimA transcripts were observed to be 85-fold (n = 3) greater in the CFT073 fim L-ON mutant than in wild-type CFT073, thus confirming that this mutant constitutively expresses type 1 fimbriae (Fig. 2E). Overall, these data were consistent with an inverse relationship between type 1 fimbria and flagellum expression.
Loss of type 1 and P fimbrial expression does not have an inverse effect on motility in UPEC strain CFT073.
To further examine the effect of fimbrial expression on motility, we analyzed the ability of various fimbrial mutants of CFT073 to swim in 0.25% soft agar. Based on the reciprocal nature of flagellum and fimbria expression of the CFT073 fim L-ON strain, we hypothesized that the loss of fimbrial production would result in increased flagellation and motility. Surprisingly, we noticed a slight reduction (not increase) of CFT073 fim L-OFF motility and chemotaxis compared to wild-type CFT073 (Fig. 1A, D, G, and H). Likewise, a double pap mutant (UPEC76) and triple fim pap mutant of CFT073 were also shown to be slightly less motile than the wild type in soft agar (Fig. 1A, E, F, and G). The decrease in motility of the CFT073 fim L-OFF variant also correlated with a decrease in FliC expression as assessed by Western blot analysis using H1 flagellin antiserum (Fig. 2D). Moreover, the level of fliC transcription in the CFT073 fim L-OFF mutant was observed to be 23-fold less than that in wild-type CFT073 (Fig. 2E). Although the CFT073 fim L-OFF mutant displayed only a slight decrease in motility compared to the wild type, it is interesting that a dramatic decrease in flagellin expression was observed. It is possible that the expression of FliC in a motile liquid culture is not fully representative of FliC expression in motility agar. However, a general trend that decreases in motility always correlated with decreases in FliC expression and vice versa was always observed.
To further investigate the slight reduction of motility exhibited by the CFT073 fim L-OFF mutant, we used transmission electron microscopy to visualize the surface expression of flagella and fimbriae. As observed with the Western analyses and qPCR, CFT073 fim L-OFF appeared to be less flagellated than wild-type CFT073 (Fig. 2A and C, black arrowheads). Interestingly, however, the CFT073 fim L-OFF mutant was observed to produce more fimbriae than wild-type CFT073 (Fig. 2A and C, white arrowheads). We demonstrate in Fig. 2D that the fimbriae produced by CFT073 fim L-OFF are not type 1 fimbriae. This is not too surprising, since previous studies from our laboratory have demonstrated that a loss of one fimbrial type leads to the expression of another in UPEC (33, 55). Therefore, it is possible that the increased fimbria production of the fim L-OFF mutant may be responsible for its decreased motility. Also, the slight decreases in motility observed for CFT073 fim L-OFF, UPEC76, and CFT073 Δfim pap may not be as biologically significant as the dramatic decrease in motility observed for the CFT073 fim L-ON mutant (a strain that is forced to constitutively produce type 1 fimbriae). Thus, flagellum expression and motility appear to be mostly affected when there is an abundance of fimbria expression and not a loss of fimbria expression.
Induction of flhDC expression does not affect expression of type 1 fimbriae in UPEC strain CFT073.
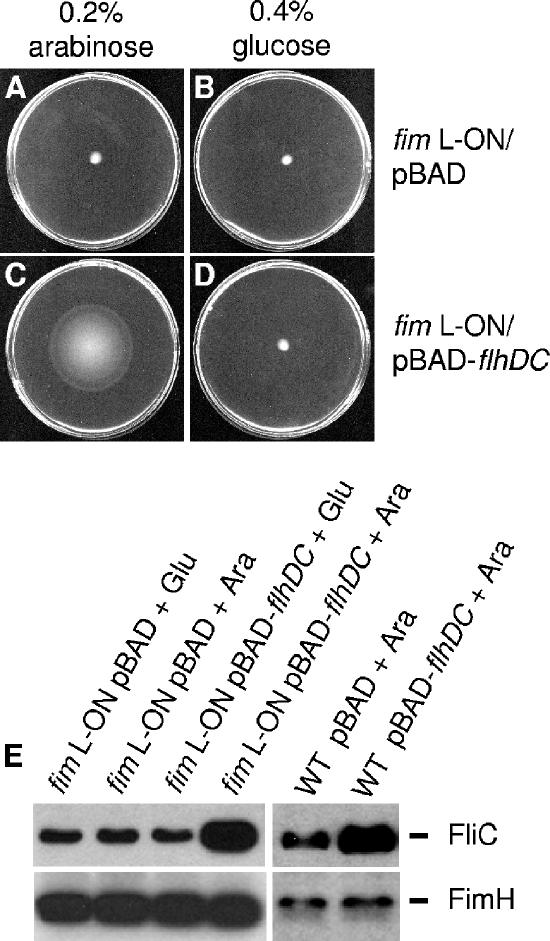
To examine the effect that a motility phenotype has upon type 1 fimbria expression, we introduced the flagellar class I master regulon flhDC under the inducible/repressible arabinose promoter contained on a plasmid (pBAD-flhDC). When introduced into an flhDC mutant of CFT073, the induction of pBAD-flhDC with 0.2% arabinose restored motility in soft agar and FliC expression (data not shown). Interestingly, the induction of pBAD-flhDC with 0.2% arabinose also resulted in increased motility of the CFT073 fim L-ON mutant in soft agar, which was repressed upon the addition of 0.4% glucose (Fig. 3C and D). The increased motility was not observed in the CFT073 fim L-ON mutant containing the empty pBAD vector (Fig. 3A and B). Therefore, these data demonstrated that the induced expression of flhDC on a plasmid could reliably create a hypermotile state by activating the hierarchical flagellar machinery within the bacterial cell.
FIG. 3.
Effect of flhDC overexpression on motility and expression of FliC and FimH by wild-type CFT073 and CFT073 fim L-ON. (A to D) Motility of CFT073 fim L-ON containing pBAD-flhDC or pBAD alone in 0.25% agar containing 0.2% arabinose or 0.2% glucose for induction or repression of flhDC expression, respectively. (E) Western blot analysis using rabbit antiserum against H1 flagellin or mouse antiserum against FimH. Cultures of fim L-ON or the wild type (WT) containing either the pBAD-flhDC or empty pBAD vector, grown either aerated or statically in Luria broth with 0.2% arabinose (Ara) or 0.4% glucose (Glu) at 37°C, respectively, were adjusted to the same optical density. Equal volumes of each sample were electrophoresed onto SDS-PAGE gels and subjected to Western blot analysis.
Since we observed a decrease in motility of the CFT073 fim L-ON mutant, we initially monitored the amounts of FliC and FimH expressed on the surface of CFT073 fim L-ON containing either the empty pBAD vector or pBAD-flhDC (in the presence of 0.2% arabinose or 0.4% glucose) by Western blot analysis. As the expression of FliC increased in the CFT073 fim L-ON mutant containing pBAD-flhDC (induced with 0.2% arabinose), the levels of FimH expression were comparable to that of CFT073 fim L-ON containing either pBAD-flhDC (repressed with 0.4% glucose) or the empty pBAD vector (Fig. 3E). Since it is possible that the CFT073 fim L-ON mutant may have stably produced type 1 fimbriae prior to the induction of flhDC expression, we also determined the level of FimH expression of wild-type CFT073 during the induction of pBAD-flhDC. For these experiments, we induced flhDC expression during serial passage of static culture in LB medium for optimal type 1 fimbrial expression. The induction of pBAD-flhDC with 0.2% arabinose in wild-type CFT073 consistently resulted in increased FliC expression as assessed by Western blot analysis using H1 flagellin antiserum (Fig. 3E). On the other hand, we observed that the induction of flhDC in wild-type CFT073 did not result in a decrease of FimH expression compared to wild-type CFT073 containing the empty pBAD vector (Fig. 3E). While the constitutive expression of type 1 fimbriae results in decreased motility and FliC expression, the inverse does not appear to be true; thus, induced expression of FliC and motility does not result in decreased type 1 fimbria expression.
Class I, II, and III flagellar mutants do not upregulate type 1 fimbria expression in UPEC strain CFT073.
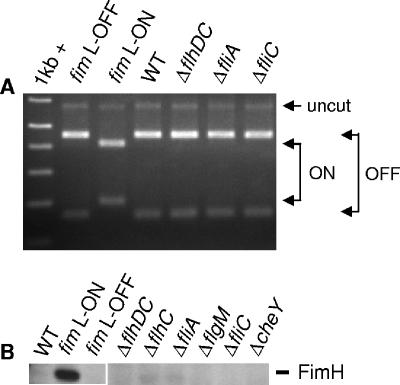
Finally, to fully characterize the relationship between type 1 fimbria expression and motility, we investigated the possible effect of flagellar mutation on type 1 fimbrial expression. In particular, we hypothesized that flagellar mutants of CFT073 may upregulate type 1 fimbrial expression. For this study, isogenic flagellar mutants of CFT073 carrying disruptions in various flagellar genes within the three different hierarchical classes were cultured in tryptone broth with aeration to the early exponential phase for optimal motility (although mutants remained nonmotile) and then examined by a type 1 fimbrial IE assay and Western blot analysis for the expression of type 1 fimbriae. The IE PCR assay was conducted as described previously (36). For the IE assay and Western analysis, the CFT073 fim L-ON and fim L-OFF mutants were used as positive and negative controls, respectively, for type 1 fimbrial expression. Wild-type CFT073 and each flagellar mutant appeared to have their type 1 fimbrial IEs in the off orientation and also appeared not to express FimH (Fig. 4A and B). Therefore, it seems that overall, motility and flagellar gene over- and underexpression do not affect the expression of type 1 fimbriae.
FIG. 4.
Effect of class I, II, and III flagellar gene mutations on the orientation of the type 1 fimbrial IE and expression of FimH in CFT073. Strains were grown to early exponential phase (OD600 of ∼0.30) in tryptone broth at 30°C with shaking (200 rpm). A type 1 fimbrial IE assay (A) and Western blot analysis using mouse antiserum against FimH (B) are depicted. WT, wild type.
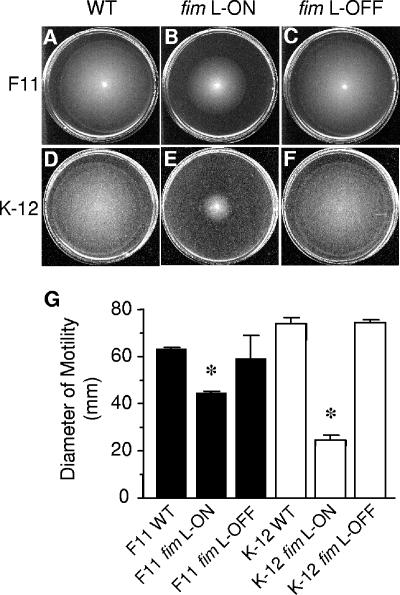
Constitutive expression of type 1 fimbriae results in decreased motility in other E. coli strains.
The majority of E. coli strains express type 1 fimbriae and are capable of flagellum-mediated motility (24, 38). Therefore, to examine whether the repression of motility during constitutive expression of type 1 fimbriae is a phenomenon specific to UPEC strain CFT073, we examined the motility of phase-locked type 1 fimbrial variants of other E. coli strains: the cystitis isolate F11 (56) and the laboratory K-12 strain MG1655 (40). As observed with CFT073 fim L-ON, the motility of F11 fim L-ON was significantly lower than that of wild-type F11 (Fig. 5A, B, and G) (P < 0.0001), indicating that at least one other UPEC isolate exhibits the same repression-of-motility phenotype during constitutive expression of type 1 fimbriae. Also, this phenotype does not appear to be specific to UPEC, in that the motility of the fim L-ON variant of the laboratory K-12 strain MG1655 was also significantly decreased compared to that of the K-12 fim L-OFF strain (Fig. 5D, E, and G). Interestingly, the F11 fim L-OFF mutant was observed to be slightly less motile than wild-type F11 (Fig. 5A, C, and G), which was similar to that seen with the CFT073 fim L-OFF mutant (Fig. 1A, D, and G). On the other hand, the K-12 fim L-OFF mutant appeared to be as motile as wild-type K-12. This result suggests that other factors that inhibit motility in CFT073 and F11 may not be present or expressed in K-12. However, as mentioned above, the differences in motility of the fim L-OFF mutants are not as biologically significant as those seen with the fim L-ON mutants.
FIG. 5.
Effect of constitutive type 1 fimbrial expression on the motility of UPEC strain F11 and the laboratory E. coli K-12 strain MG1655. Motility of wild-type (WT) F11 (A), F11 fim L-ON (B), F11 fim L-OFF (C), wild-type K-12 (D), K-12 fim L-ON (AAEC554) (E), and K-12 fim L-OFF (AAEC358) (F) in soft agar. Standardized cultures of each strain were stabbed into 0.25% soft agar plates and incubated for 16 h at 30°C. (G) Black and white bars represent the average diameters of swimming (in mm) of triplicate motility plates for the F11 strains and the K-12 strains, respectively. Error bars represent the SEM. Significant differences in motility between the wild type and the respective phase-locked mutants were determined using an unpaired Student's t test with Welch correction (Instat; GraphPad). *, P < 0.0001.
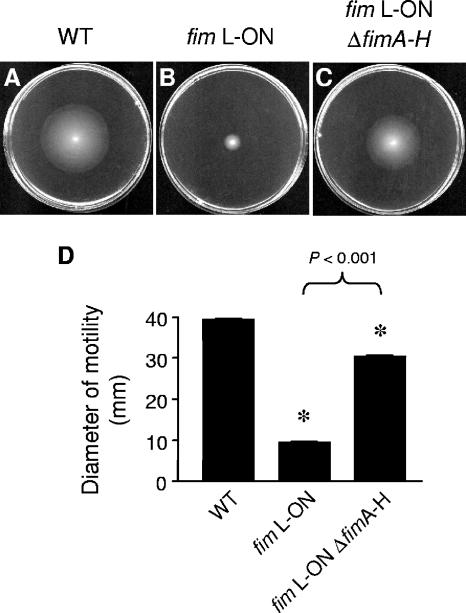
Deletion of the type 1 fimbrial operon of fim L-ON partially restores motility and flagellum expression in UPEC strain CFT073.
The observation that the CFT073 fim L-ON mutant was significantly less motile than wild-type CFT073 led us to investigate whether the expression of type 1 fimbriae on the surface of the bacterium is responsible for the reduced motility seen in CFT073 fim L-ON. Conversely, it is possible that the orientation of the fim promoter, rather than the presence of the fimbriae, leads to the observed repression of motility. To test these alternatives, the major structural and assembly genes for type 1 fimbriae were deleted to create a fim null mutant of CFT073 fim L-ON, CFT073 fim L-ON ΔfimAICDFGH (as described in Materials and Methods). The loss of expression of type 1 fimbriae in this mutant was confirmed via Western blot analysis using FimH antiserum (Fig. 2D) and mannose-sensitive hemagglutination (data not shown). The motility of CFT073 fim L-ON ΔfimAICDFGH was significantly higher than that of its CFT073 fim L-ON parent but not of the same magnitude as that of wild-type CFT073 (Fig. 6). Moreover, fliC transcription and expression, as assessed by qPCR and Western analysis, respectively, were also partially restored in the CFT073 fim L-ON ΔfimAICDFGH mutant (Fig. 2A and B). This partial restoration of motility and FliC expression observed with CFT073 fim L-ON ΔfimAICDFGH led us to conclude that the constitutive expression of type 1 fimbriae partially represses motility and fliC expression in CFT073 fim L-ON, but the appearance of the fimbriae on the surface of the bacteria is not solely responsible for this phenotype.
FIG. 6.
Deletion of the type 1 fimbrial operon of CFT073 fim L-ON and its effect on motility. Motilities of wild-type (WT) CFT073 (A), CFT073 fim L-ON (B), and CFT073 fim L-ON ΔfimAICDFGH (fimA-H) (C) in soft agar are shown. Standardized cultures of each strain were stabbed into 0.25% soft agar plates and incubated for 16 h at 30°C. (D) Black bars represent the average diameters of swimming (in mm) of triplicate motility plates. Error bars represent the SEM. Significant differences in motility (comparing the wild type to fim L-ON and fim L-ON ΔfimAICDFGH) were determined using an unpaired Student's t test with Welch correction (Instat; GraphPad). *, P < 0.0001.
DISCUSSION
The objective of these studies was to examine a possible connection between the adherence and motility of UPEC pyelonephritis strain CFT073 in order to gain further insight into the interplay between these two important pathogenic traits. Initially, we hypothesized that motility and adherence were reciprocally regulated, since it would clearly be disadvantageous for a bacterium to stick and swim at the same time. Since flagella are organelles that are generally used for motility, and fimbriae are utilized for adherence, we presumed that these two surface structures would be reciprocally expressed by an individual bacterium. Recently, it has been demonstrated that unlinked FimB- and FimE-like recombinase mutants in the fim switch-on orientation are nonmotile in soft agar, while recombinase mutants in the fim switch-off orientation are motile (12). In parallel, our laboratory showed that the constitutive expression of type 1 fimbriae results in decreased motility, chemotaxis to l-aspartate, and decreased flagellum expression; however, flagellar mutants deficient in motility or chemotaxis did not upregulate the expression of type 1 fimbriae (33a). Here, we examined the relationship between type 1 fimbria, motility, and flagellum expression in detail.
As type 1 fimbriae are thought to promote the autoaggregation of E. coli (52), we thought it possible that type 1 fimbriae may contribute to the autoaggregation of CFT073 fim L-ON, thus physically limiting its ability to swim in soft agar. However, upon further investigation, we noted that autoaggregation could not be solely responsible for the reduced motility of CFT073 fim L-ON in that CFT073 fim L-ON produced significantly fewer fliC transcripts than wild-type CFT073 (Fig. 2A). Therefore, it is most likely that the down-regulation of fliC transcription is the cause of the decrease in motility. Nevertheless, it is possible that autoaggregation of strain CFT073 fim L-ON may result in some feedback regulatory mechanism that results in decreased fliC transcription. If this were the case, further studies would be needed to characterize the potential role of autoaggregation in the reduction of motility.
While the constitutive expression of type 1 fimbriae resulted in a significant decrease in motility and flagellin expression, the loss of type 1 and P fimbrial expression did not result in increased motility. In fact, both of the UPEC fim L-OFF mutants, as well as UPEC76 and the CFT073 Δfim pap mutant, displayed slight decreases in motility compared to wild-type CFT073 and F11. Upon further investigation, we observed that the CFT073 fim L-OFF mutant produces more fimbriae than wild-type CFT073 by transmission electron microscopy. This is consistent with previous studies from our laboratory and others that have shown that the loss of one fimbrial type generally leads to the expression of another fimbrial type (33). In particular, Snyder et al. previously demonstrated that the loss of both type 1 and P fimbriae in UPEC pyelonephritis strain CFT073 leads to the expression of F1C fimbriae (55). A similar study conducted previously by Wright et al. demonstrated that the loss of type 1 fimbriae in UPEC cystitis strain UTI89 led to the increased expression of S fimbriae (61a). Therefore, it is possible that the loss of type 1 or P fimbrial expression leads to the production of another fimbrial type, which in the end may be the cause of the slight decrease (and not increase) in motility in the fim L-OFF (and other fimbrial deletion mutant) strains. Since these strains are still able to shut off and regulate fimbrial expression, we speculate that this is the reason why their motility is not as significantly or drastically reduced as that of the fim L-ON strains, which are forced to constitutively express type 1 fimbriae. Ultimately, we conclude that flagellum expression and motility appear to be mostly affected when there is an abundance of fimbria expression and not a loss of fimbria expression.
As there are several studies that demonstrate a reciprocal balance between motility and adherence (14, 17, 20, 35), there are also a few studies that provide evidence that is inconsistent with our original hypothesis of reciprocal regulation. For instance, while flagella are more thoroughly documented for their involvement in motility and fimbriae for their adhesiveness, there is also evidence for type IV pilus-mediated twitching motility and flagellum-mediated adherence (21, 39). Additionally, flagella and type 1 fimbriae have been shown to be integral components of adherent-invasive E. coli strain LF82 in the adherence to and invasion of intestine-407 cells (4, 10). In particular, Barnich et al. previously revealed that flagella and type 1 fimbriae of LF82 are regulated in a coordinate manner, as evidenced by the reduction of type 1 fimbria expression in a fliC mutant, and went on to speculate that flagella, type 1 fimbriae, and other unknown factors coordinately contribute to the invasive ability of adherent-invasive E. coli strain LF82 (4). It has also been shown that both fimbria- and flagellum-mediated motility are important for the association and invasion of cultured epithelial cells by Salmonella enterica serovar Enteritidis (18). Therefore, it is possible that both adherence and motility act in unison during different stages of colonization, including invasion of and attachment to host cells.
Finally, in this report, we demonstrate a unidirectional relationship between flagellum-mediated motility and type 1 fimbrial expression (and presumably adherence) that appears to be common among motile strains of both UPEC and other E. coli strains. In particular, we observed that type 1 fimbrial phase-locked derivatives of UPEC strains CFT073 and F11 as well as E. coli K-12 strain MG1655 that constitutively express type 1 fimbriae (fim L-ON) are much less motile and express fewer flagella than their respective wild-type or fim L-OFF strains. Moreover, we demonstrated that the decrease in motility of the fim L-ON strains occurs at the level of fliC transcription. By examining a fim null mutant of CFT073 fim L-ON, we observed a significant increase in motility compared to the fim L-ON parent; however, the level of motility was still significantly lower than that of wild-type CFT073. This observation led us to conclude that the presence of type 1 fimbriae on the surface of the bacterium is not solely responsible for the decreased motility of CFT073 fim L-ON. Indeed, it is possible that factors that influence the orientation or induction of the fim promoter, rather than the physical presence of fimbriae at the bacterial surface, also contribute to the repression of motility. This is intriguing because there are a number of transcriptional regulators, including LrhA (9, 34), Lrp (7, 13, 23, 47), H-NS (5, 49), and IHF (8, 63), that have been shown to modulate the expression of both flagella and fimbriae. Currently, our laboratory is in the process of determining the mechanism(s) of regulation that results in the decreased motility observed in the fim L-ON strain.
Acknowledgments
This work was supported by Public Health Service grants AI43363 and AI059722 from the National Institutes of Health.
Footnotes
Published ahead of print on 18 May 2007.
REFERENCES
- 1.Abraham, J. M., C. S. Freitag, J. R. Clements, and B. I. Eisenstein. 1985. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc. Natl. Acad. Sci. USA 82:5724-5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler, J., and M. M. Dahl. 1967. A method for measuring the motility of bacteria and for comparing random and non-random motility. J Gen. Microbiol. 46:161-173. [DOI] [PubMed] [Google Scholar]
- 3.Bacheller, C. D., and J. M. Bernstein. 1997. Urinary tract infections. Med. Clin. N. Am. 81:719-730. [DOI] [PubMed] [Google Scholar]
- 4.Barnich, N., J. Boudeau, L. Claret, and A. Darfeuille-Michaud. 2003. Regulatory and functional co-operation of flagella and type 1 pili in adhesive and invasive abilities of AIEC strain LF82 isolated from a patient with Crohn's disease. Mol. Microbiol. 48:781-794. [DOI] [PubMed] [Google Scholar]
- 5.Bertin, P., E. Terao, E. H. Lee, P. Lejeune, C. Colson, A. Danchin, and E. Collatz. 1994. The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. J. Bacteriol. 176:5537-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 7.Blomfield, I. C., P. J. Calie, K. J. Eberhardt, M. S. McClain, and B. I. Eisenstein. 1993. Lrp stimulates phase variation of type 1 fimbriation in Escherichia coli K-12. J. Bacteriol. 175:27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blomfield, I. C., D. H. Kulasekara, and B. I. Eisenstein. 1997. Integration host factor stimulates both FimB- and FimE-mediated site-specific DNA inversion that controls phase variation of type 1 fimbriae expression in Escherichia coli. Mol. Microbiol. 23:705-717. [DOI] [PubMed] [Google Scholar]
- 9.Blumer, C., A. Kleefeld, D. Lehnen, M. Heintz, U. Dobrindt, G. Nagy, K. Michaelis, L. Emödy, T. Polen, R. Rachel, V. F. Wendisch, and G. Unden. 2005. Regulation of type 1 fimbriae synthesis and biofilm formation by the transcriptional regulator LrhA of Escherichia coli. Microbiology 151:3287-3298. [DOI] [PubMed] [Google Scholar]
- 10.Boudeau, J., N. Barnich, and A. Darfeuille-Michaud. 2001. Type 1 pili-mediated adherence of Escherichia coli strain LF82 isolated from Crohn's disease is involved in bacterial invasion of intestinal epithelial cells. Mol. Microbiol. 39:1272-1284. [DOI] [PubMed] [Google Scholar]
- 11.Bren, A., and M. Eisenbach. 1998. The N terminus of the flagellar switch protein, FliM, is the binding domain for the chemotactic response regulator, CheY. J. Mol. Biol. 278:507-514. [DOI] [PubMed] [Google Scholar]
- 12.Bryan, A., P. Roesch, L. Davis, R. Moritz, S. Pellett, and R. A. Welch. 2006. Regulation of type 1 fimbriae by unlinked FimB- and FimE-like recombinases in uropathogenic Escherichia coli strain CFT073. Infect. Immun. 74:1072-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvo, J. M., and R. G. Matthews. 1994. The leucine-responsive regulatory protein, a global regulator of metabolism in Escherichia coli. Microbiol. Rev. 58:466-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clegg, S., and K. T. Hughes. 2002. FimZ is a molecular link between sticking and swimming in Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:1209-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connell, I., W. Agace, P. Klemm, M. Schembri, S. Marild, and C. Svanborg. 1996. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA 93:9827-9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deora, R., H. J. Bootsma, J. F. Miller, and P. A. Cotter. 2001. Diversity in the Bordetella virulence regulon: transcriptional control of a Bvg-intermediate phase gene. Mol. Microbiol. 40:669-683. [DOI] [PubMed] [Google Scholar]
- 18.Dibb-Fuller, M. P., E. Allen-Vercoe, C. J. Thorns, and M. J. Woodward. 1999. Fimbriae- and flagella-mediated association with and invasion of cultured epithelial cells by Salmonella enteritidis. Microbiology 145:1023-1031. [DOI] [PubMed] [Google Scholar]
- 19.Gally, D. L., J. Leathart, and I. C. Blomfield. 1996. Interaction of FimB and FimE with the fim switch that controls the phase variation of type 1 fimbriae in Escherichia coli K-12. Mol. Microbiol. 21:725-738. [DOI] [PubMed] [Google Scholar]
- 20.Gardel, C. L., and J. J. Mekalanos. 1996. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect. Immun. 64:2246-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girón, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44:361-379. [DOI] [PubMed] [Google Scholar]
- 22.Gunther, N. W., IV, J. A. Snyder, V. Lockatell, I. Blomfield, D. E. Johnson, and H. L. Mobley. 2002. Assessment of virulence of uropathogenic Escherichia coli type 1 fimbrial mutants in which the invertible element is phase-locked on or off. Infect. Immun. 70:3344-3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hay, N. A., D. J. Tipper, D. Gygi, and C. Hughes. 1997. A nonswarming mutant of Proteus mirabilis lacks the Lrp global transcriptional regulator. J. Bacteriol. 179:4741-4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson, J. R. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 4:80-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klemm, P. 1986. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J. 5:1389-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komeda, Y. 1982. Fusions of flagellar operons to lactose genes on a mu lac bacteriophage. J. Bacteriol. 150:16-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komeda, Y. 1986. Transcriptional control of flagellar genes in Escherichia coli K-12. J. Bacteriol. 168:1315-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korhonen, T. K., R. Virkola, and H. Holthofer. 1986. Localization of binding sites for purified Escherichia coli P fimbriae in the human kidney. Infect. Immun. 54:328-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kutsukake, K., S. Iyoda, K. Ohnishi, and T. Iino. 1994. Genetic and molecular analyses of the interaction between the flagellum-specific sigma and anti-sigma factors in Salmonella typhimurium. EMBO J. 13:4568-4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 31.Lane, M. C., A. L. Lloyd, T. A. Markyvech, E. C. Hagan, and H. L. Mobley. 2006. Uropathogenic Escherichia coli strains generally lack functional Trg and Tap chemoreceptors found in the majority of E. coli strains strictly residing in the gut. J. Bacteriol. 188:5618-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lane, M. C., V. Lockatell, G. Monterosso, D. Lamphier, J. Weinert, J. R. Hebel, D. E. Johnson, and H. L. Mobley. 2005. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect. Immun. 73:7644-7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lane, M. C., and H. L. Mobley. Role of P-fimbrial-mediated adherence in pyelonephritis and persistence of uropathogenic Escherichia coli (UPEC) in the mammalian kidney. Kidney Int., in press. [DOI] [PubMed]
- 33a.Lane, M. C., C. V. Lockatell, D. Lamphier, J. Weinert, J. R. Hebel, D. E. Johnson, and H. L. T. Mobley. 2005. Abstr. 105th Gen. Meet. Am. Soc. Microbiol., abstr. B-269, p. 79.
- 34.Lehnen, D., C. Blumer, T. Polen, B. Wackwitz, V. F. Wendisch, and G. Unden. 2002. LrhA as a new transcriptional key regulator of flagella, motility and chemotaxis genes in Escherichia coli. Mol. Microbiol. 45:521-532. [DOI] [PubMed] [Google Scholar]
- 35.Li, X., D. A. Rasko, C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2001. Repression of bacterial motility by a novel fimbrial gene product. EMBO J. 20:4854-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lim, J. K., N. W. Gunther IV, H. Zhao, D. E. Johnson, S. K. Keay, and H. L. Mobley. 1998. In vivo phase variation of Escherichia coli type 1 fimbrial genes in women with urinary tract infection. Infect. Immun. 66:3303-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 38.Macnab, R. M. 1996. Flagella and motility, p. 123-145. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 39.Mattick, J. S. 2002. Type IV pili and twitching motility. Annu. Rev. Microbiol. 56:289-314. [DOI] [PubMed] [Google Scholar]
- 40.McClain, M. S., I. C. Blomfield, K. J. Eberhardt, and B. I. Eisenstein. 1993. Inversion-independent phase variation of type 1 fimbriae in Escherichia coli. J. Bacteriol. 175:4335-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McClain, M. S., I. C. Blomfield, and B. I. Eisenstein. 1991. Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli. J. Bacteriol. 173:5308-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McEvoy, M. M., A. Bren, M. Eisenbach, and F. W. Dahlquist. 1999. Identification of the binding interfaces on CheY for two of its targets, the phosphatase CheZ and the flagellar switch protein FliM. J. Mol. Biol. 289:1423-1433. [DOI] [PubMed] [Google Scholar]
- 43.Mobley, H. L., and G. R. Chippendale. 1990. Hemagglutinin, urease, and hemolysin production by Proteus mirabilis from clinical sources. J. Infect. Dis. 161:525-530. [DOI] [PubMed] [Google Scholar]
- 44.Mobley, H. L., D. M. Green, A. L. Trifillis, D. E. Johnson, G. R. Chippendale, C. V. Lockatell, B. D. Jones, and J. W. Warren. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58:1281-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mobley, H. L., K. G. Jarvis, J. P. Elwood, D. I. Whittle, C. V. Lockatell, R. G. Russell, D. E. Johnson, M. S. Donnenberg, and J. W. Warren. 1993. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of αGal(1-4)βGal binding in virulence of a wild-type strain. Mol. Microbiol. 10:143-155. [DOI] [PubMed] [Google Scholar]
- 46.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494-1497. [DOI] [PubMed] [Google Scholar]
- 47.Newman, E. B., and R. Lin. 1995. Leucine-responsive regulatory protein: a global regulator of gene expression in E. coli. Annu. Rev. Microbiol. 49:747-775. [DOI] [PubMed] [Google Scholar]
- 48.Ohnishi, K., K. Kutsukake, H. Suzuki, and T. Iino. 1990. Gene fliA encodes an alternative sigma factor specific for flagellar operons in Salmonella typhimurium. Mol. Gen. Genet. 221:139-147. [DOI] [PubMed] [Google Scholar]
- 49.Olsen, P. B., M. A. Schembri, D. L. Gally, and P. Klemm. 1998. Differential temperature modulation by H-NS of the fimB and fimE recombinase genes which control the orientation of the type 1 fimbrial phase switch. FEMS Microbiol. Lett. 162:17-23. [DOI] [PubMed] [Google Scholar]
- 50.Palleroni, N. J. 1976. Chamber for bacterial chemotaxis experiments. Appl. Environ. Microbiol. 32:729-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sagi, Y., S. Khan, and M. Eisenbach. 2003. Binding of the chemotaxis response regulator CheY to the isolated, intact switch complex of the bacterial flagellar motor: lack of cooperativity. J. Biol. Chem. 278:25867-25871. [DOI] [PubMed] [Google Scholar]
- 52.Schembri, M. A., G. Christiansen, and P. Klemm. 2001. FimH-mediated autoaggregation of Escherichia coli. Mol. Microbiol. 41:1419-1430. [DOI] [PubMed] [Google Scholar]
- 53.Schuster, M., R. Zhao, R. B. Bourret, and E. J. Collins. 2000. Correlated switch binding and signaling in bacterial chemotaxis. J. Biol. Chem. 275:19752-19758. [DOI] [PubMed] [Google Scholar]
- 54.Snyder, J. A., B. J. Haugen, E. L. Buckles, C. V. Lockatell, D. E. Johnson, M. S. Donnenberg, R. A. Welch, and H. L. Mobley. 2004. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 72:6373-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Snyder, J. A., B. J. Haugen, C. V. Lockatell, N. Maroncle, E. C. Hagan, D. E. Johnson, R. A. Welch, and H. L. Mobley. 2005. Coordinate expression of fimbriae in uropathogenic Escherichia coli. Infect. Immun. 73:7588-7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snyder, J. A., A. L. Lloyd, C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2006. Role of phase variation of type 1 fimbriae in a uropathogenic Escherichia coli cystitis isolate during urinary tract infection. Infect. Immun. 74:1387-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soutourina, O. A., and P. N. Bertin. 2003. Regulation cascade of flagellar expression in gram-negative bacteria. FEMS Microbiol. Rev. 27:505-523. [DOI] [PubMed] [Google Scholar]
- 58.Stapleton, A., S. Moseley, and W. E. Stamm. 1991. Urovirulence determinants in Escherichia coli isolates causing first-episode and recurrent cystitis in women. J. Infect. Dis. 163:773-779. [DOI] [PubMed] [Google Scholar]
- 59.Steiner, T. S., J. P. Nataro, C. E. Poteet-Smith, J. A. Smith, and R. L. Guerrant. 2000. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J. Clin. Investig. 105:1769-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Welch, R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 99:17020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wright, K. J., P. C. Seed, and S. J. Hultgren. 2005. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect. Immun. 73:7657-7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61a.Wright, K. J., P. C. Seed, and S. J. Hultgren. 2005. Abstr. 105th Gen. Meet. Am. Soc. Microbiol., abstr. B-176, p. 116.
- 62.Wu, X. R., T. T. Sun, and J. J. Medina. 1996. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc. Natl. Acad. Sci. USA 93:9630-9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yona-Nadler, C., T. Umanski, S. Aizawa, D. Friedberg, and I. Rosenshine. 2003. Integration host factor (IHF) mediates repression of flagella in enteropathogenic and enterohaemorrhagic Escherichia coli. Microbiology 149:877-884. [DOI] [PubMed] [Google Scholar]
- 64.Zhou, X., J. A. Girón, A. G. Torres, J. A. Crawford, E. Negrete, S. N. Vogel, and J. B. Kaper. 2003. Flagellin of enteropathogenic Escherichia coli stimulates interleukin-8 production in T84 cells. Infect. Immun. 71:2120-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]