Abstract
Myxococcus xanthus is a predatory bacterium that exhibits complex social behavior. The most pronounced behavior is the aggregation of cells into raised fruiting body structures in which cells differentiate into stress-resistant spores. In the laboratory, monocultures of M. xanthus at a very high density will reproducibly induce hundreds of randomly localized fruiting bodies when exposed to low nutrient availability and a solid surface. In this report, we analyze how M. xanthus fruiting body development proceeds in a coculture with suitable prey. Our analysis indicates that when prey bacteria are provided as a nutrient source, fruiting body aggregation is more organized, such that fruiting bodies form specifically after a step-down or loss of prey availability, whereas a step-up in prey availability inhibits fruiting body formation. This localization of aggregates occurs independently of the basal nutrient levels tested, indicating that starvation is not required for this process. Analysis of early developmental signaling relA and asgD mutants indicates that they are capable of forming fruiting body aggregates in the presence of prey, demonstrating that the stringent response and A-signal production are surprisingly not required for the initiation of fruiting behavior. However, these strains are still defective in differentiating to spores. We conclude that fruiting body formation does not occur exclusively in response to starvation and propose an alternative model in which multicellular development is driven by the interactions between M. xanthus cells and their cognate prey.
Fruiting bodies formed by myxobacterium species such as Myxococcus xanthus were one of the first examples of social, multicellular behavior in bacteria (16, 27). Fruiting bodies are macroscopic structures that can be as large as a few millimeters in size and can consist of millions of cells. M. xanthus cells are predators, growing by lysing prey bacteria and metabolizing the macromolecules released (1, 4, 20). As such, they thrive in soil niches such as herbivorous mammalian dung pellets, and fruiting bodies can be readily observed forming on dung. Fruiting body morphogenesis is thought to require the sensing of intracellular nutritional status as well as self-generated extracellular cell-to-cell communication signals (22). In this study, we examine the hypothesis that the direct perception of prey cells could also play an important role in the cell-cell communication process of fruiting body formation.
Vegetative cells of M. xanthus are motile on solid surfaces through the use of gliding motility. Gliding motility utilizes both type IV pili and fixed adhesion sites to propel the cells forward in the direction of the long axis of the cell (14, 26). The direction of cell movement periodically reverses, and modulation of cellular reversals through the chemotaxis-like Frz pathway allows cells to direct their movements (13). Directed cell movement is used during surface colonization, predatory rippling, and fruiting body aggregation. In the laboratory, fruiting bodies can be induced by incubating vegetative cells on a solid surface with low nutrient availability and high cell density. Under these conditions, most cells direct their movement into the formation of hundreds of raised aggregates containing 105 to 106 cells each (15). In addition to aggregating, sporulation is induced, with cells inside the fruiting aggregates much more likely to differentiate into spores than cells that occupy the spaces in between.
Several investigations into the early stages of fruiting body development have been performed. The synthesis of (p)ppGpp as an intracellular signal is a common event during the stringent response in bacteria (2). In M. xanthus, the production of (p)ppGpp during amino acid starvation has been proposed to be sufficient and necessary for the induction of fruiting body development (7, 24). The RelA protein is essential for converting GTP to (p)ppGpp during ribosomal stalling that occurs during the stringent response. Ectopic expression of the Escherichia coli relA gene in M. xanthus causes premature aggregation (24). The M. xanthus relA gene was previously shown to be required for aggregation, sporulation, and expression of a variety of developmental gene reporters, making it the earliest known requirement for the fruiting program (7, 8). The relA gene product is also required for the production of another early developmental marker, intercellular A-signal activity (7).
A-signal mutants (asg) are considered to be deficient in producing one of the earliest-acting signals in fruiting body development (the A signal). The asg mutants have a general defect in protein secretion that includes an inability to secrete at least two proteases that are thought to be required for generating the amino acid and small peptide pool, which suffices for complementing developmental reporter gene expression levels in asg mutants (17). The A signal has often been proposed to fulfill a quorum-sensing function, providing the cell density information necessary for cells to decide if there is a population that is sufficiently large enough to be able to construct a fruiting body. However, the A signal has only a rough correlation to cell density and lacks the specificity of other quorum-sensing systems, since the peptide pool produced by starving M. xanthus cells can vary significantly from culture to culture (8, 10, 11, 17, 29).
Most experiments studying fruiting body formation examine the process in pure M. xanthus cultures incubated with little or no nutrients. However there is evidence that the presence of prey, or prey components, can greatly affect the developmental program (23). Rippling behavior, which has often been interpreted as a discrete stage in fruiting body development, is also a predatory behavior that is observed during growth of M. xanthus on prey or large, nondiffusing substrates such as peptidoglycan (1, 19). Starvation-induced rippling has recently been shown to occur in a strain that has a high level of autolysis but not in a strain with a lower autolytic rate, indicating that the rippling stage of starvation-induced development may be due solely to the predation of surviving cells on the lysed cell debris of their sister cells (1). In this report, we examine further how the presence of prey alters the developmental program by examining changes in the process of fruiting body formation.
MATERIALS AND METHODS
Strains and growth conditions.
M. xanthus strain DZ2 was analyzed in this study. Escherichia coli strain β2155 was supplied as prey since it is a Kanr diaminopimelic acid (DAP) auxotroph, and prey cell densities can be controlled on any medium lacking DAP. For routine culturing, M. xanthus was grown in CYE broth and E. coli was grown in LB broth (1, 21). Kanamycin was supplied at 100 μg/ml when appropriate, and DAP was supplied at 100 μg/ml. A modified version of CF agar (1) with reduced nutrient levels, termed CFL, was utilized for the analysis of predation under very-low-nutrient conditions. CFL contains 1/10 the normal levels of pyruvate and citrate, and the recipe is 10 mM MOPS (morpholinepropanesulfonic acid) (pH 7.6), 1 mM KH2PO4, 8 mM MgSO4, 0.02% (NH4)2SO4, 0.02% citrate, 0.02% pyruvate, and 0.1 g/liter Casitone. The Casitone level was varied in several experiments to yield 0, 0.1, 1.0, and 10.0 g/liter Casitone.
Preliminary experiments on signaling were performed with strains MS1000 (DK101/ΔrelA) and DK4208 (DK1622/asgB mutant) (both strains were gifts from M. Singer). Strains JK1701 (DZ2/relA mutant) and JK1702 (DZ2/asgD mutant) were constructed by PCR amplifying a ∼500-bp internal gene fragment with engineered restriction sites and cloning each fragment into the Kanr plasmid pBJ113. The new plasmid constructs were electroporated into M. xanthus strain DZ2, and recombinants were selected on CYE plates with 40 mg/ml kanamycin. Individual colonies were isolated, and clones with the correct disruption were confirmed by using colony PCR.
Predation assays.
Cultures of M. xanthus and E. coli cells were harvested at mid-log phase and washed three times in 10 mM MOPS (pH 7.6) buffer. E. coli cells were concentrated to a final cell density of 1 × 1011 cells/ml, and M. xanthus cells were concentrated to a final cell density of 1 × 109 cells/ml. In the initial colony predation assays, 20 μl of E. coli cells was pipetted onto the plates and allowed to dry. M. xanthus cells were mixed 2:1 with India ink, and 1 μl was added to the center of the E. coli prey colony. In the basal nutrient level assays, 10 μl of E. coli cells was pipetted onto the plates and allowed to dry. M. xanthus cells were mixed with India ink as described above, and 1 μl was added to the edge of the E. coli prey colony. For the analysis of step changes in prey, E. coli prey cells were pipetted in linear strips consisting of 5 aliquots of 2 μl each that were pipetted close enough that the drops could adhere to each other before drying. Cells were allowed to dry before another strip of cells was added at a different cell density. Last, M. xanthus cells mixed with India ink were added to the broad edge of the E. coli strip. Plates were incubated at 32°C for the times indicated in each predation experiment. Assays were performed a minimum of three times, with representative images selected in each case. Fruiting behavior was observed by using a Nikon SMZ1000 dissecting microscope, and wet mounts were analyzed with a Nikon Eclipse E400 phase-contrast microscope. Images were captured using a Q Imaging camera and software. Fruiting body localization was analyzed by creating 100-pixel horizontal slices of images in Adobe Photoshop.
Sporulation counts.
To determine the level of spores formed in monoculture compared to cocultures with prey, M. xanthus and E. coli cells were added to the CFL-based plates listed to yield aliquots of ∼107 M. xanthus cells only, ∼108 M. xanthus cells only, and ∼107 M. xanthus cells added to a dried colony of ∼109 E. coli cells. After 4 days of incubation at 32°C, cells were harvested in MOPS buffer and sonicated at minimum power for 1 s to disperse large aggregates without causing a major change in cell viability. Serial dilutions were added to melted top agar and plated onto CYE plates to obtain a total cell count. Dispersed cell suspensions were also incubated at 50°C for 2 h to kill vegetative cells. The heat-resistant population was determined through serial dilution and plating of cells onto CYE plates as described above. CYE plates were incubated at 32°C for 4 days, and the CFU were determined for the total cell count and the number of heat-resistant spores. CFU were assessed in triplicate on three independent cultures for each condition.
RESULTS
Predation-induced fruiting body formation.
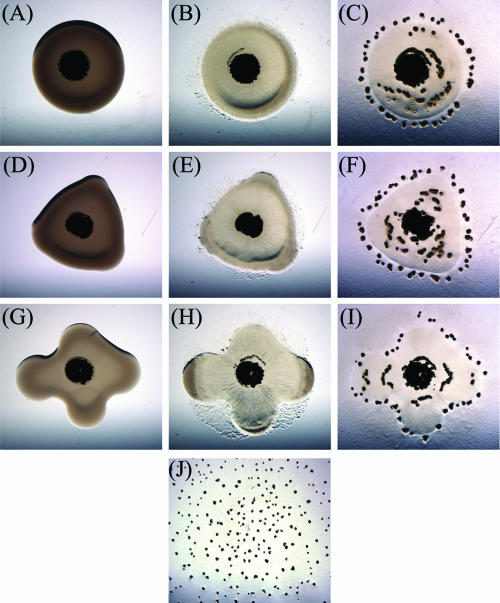
M. xanthus cell development is routinely analyzed in monocultures. It has been proposed that several cell-cell signaling events are required for the development of multicellular fruiting bodies (8). Because M. xanthus is a microbial predator, we hypothesized that the presence of prey could alter the dynamics of fruiting body construction by providing additional interspecies signals that are lacking in monocultures. To examine the impact of prey on fruiting body formation, E. coli strain β2155 prey cells were pipetted in 20-μl aliquots containing ∼2 × 109 total cells onto a low-nutrient medium designated CFL, which contains 0.1 g/liter Casitone as the primary carbon and energy source. The β2155 strain is a DAP auxotroph and is useful in predation assays since in any medium lacking DAP, the E. coli cell density remains relatively constant (1). The prey cell aliquots were pipetted in a variety of patterns and allowed to dry (Fig. 1). Approximately 1 × 107 cells of wild-type M. xanthus strain DZ2 mixed with India ink were pipetted onto the center of the E. coli prey colonies, and the cocultures were incubated at 32°C. In control samples lacking E. coli, M. xanthus cells migrate away from the initial inoculum and form fruiting bodies in a well-spaced but random pattern (Fig. 1J).
FIG. 1.
Prey-directed fruiting body formation. Twenty microliters of E. coli β2155 cells was pipetted and allowed to dry on CFL agar medium as prey for 1 μl of M. xanthus DZ2 cells mixed with India ink, which were pipetted onto the E. coli prey. As the M. xanthus swarm expands, the E. coli cells are lysed, and fruiting body formation is induced along the perimeter of the original prey colony. Photographs were captured at ×8 magnification at 0 h (A, D, and G), 48 h (B, E, and H), and 72 h (C, F, and I). J shows a control experiment with no E. coli prey at 120 h.
In the presence of E. coli prey, the M. xanthus swarm expands from the initial inoculum, lysing the E. coli cells as they migrate. After 72 h, many large fruiting bodies were observed to be forming along the perimeter of the predation zone marked by the original E. coli colony, resulting in a fruiting body pattern that mimics the initial E. coli inoculum (Fig. 1C, F, and I). The association of fruiting bodies with the predation zone edge occurs regardless of the distance of the edge from the initial M. xanthus inoculum, indicating that it is not a timed event. The rate of swarm expansion is nearly identical in every direction, regardless of whether E. coli prey cells are present or not (data not shown); this indicates that cell movement is not significantly impeded by the presence of prey.
The marked formation of fruiting bodies around the perimeter of prey colonies could be occurring for several reasons. Due to surface tension, cell suspensions do not dry uniformly, resulting in slightly more prey cells near the colony edge than in the center. This generates an increasing gradient of prey cells directed outward from the center of the inoculum. The presence of more prey cells may lead to localized increases in M. xanthus cell density after consuming prey-derived nutrients. If cell density is the major factor in the initiation of fruiting body aggregation, there should be a corresponding gradual increase in the number of M. xanthus fruiting bodies reflecting the gradual increase of prey cells available from the center to the edge. However, this is not the pattern that emerges. In fact, a superimposition of the images captured at time zero and at 72 h indicates that the fruiting body aggregates form adjacent to but immediately outside of the predation zone delineated by the original prey colony (Fig. 1). Additionally, native E. coli colonies with a typical convex shape provided as prey will also stimulate fruiting body aggregation along the outside edge of the prey colony perimeter (data not shown).
The presence of prey stimulates fruiting body aggregation at nutrient levels that normally inhibit development.
The stimulation of fruiting bodies around the perimeter of prey colonies may also be due to M. xanthus cells sensing a sharp decline in nutrient levels as they leave the prey locale. This could cause a localized starvation response, which rapidly triggers the induction of a developmental program. If this is the case, then raising the basal nutrient level in the medium should result in a more gradual nutrient decline and a spatial dissociation of fruiting bodies from the perimeter of the predation zone, as cells should be able to travel farther before sensing starvation. If this is not the case, it would indicate that some component of prey cells directs fruiting body formation in a way that is distinct from the sensing of the overall nutrient status.
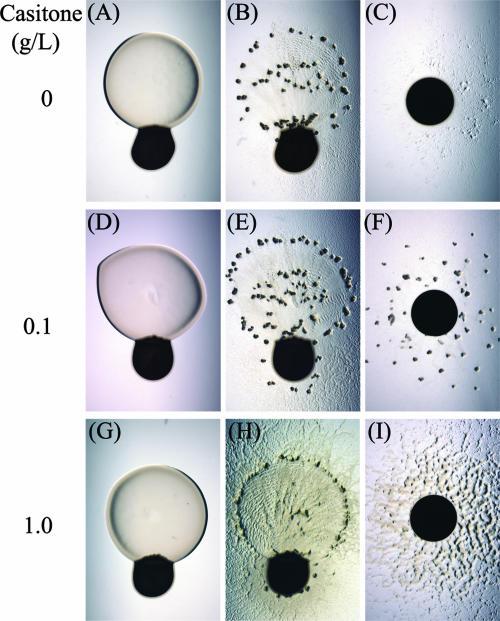
M. xanthus cells were added to a series of CFL plates containing various levels of Casitone as a carbon and energy source. As shown in Fig. 2, the Casitone level in the medium has a profound effect on the formation of fruiting bodies under monoculture conditions. At 0.1 g/liter Casitone, fruiting bodies form readily within 72 h, but both lower (0 g/liter) and higher (1.0 g/liter) nutrient levels inhibit rapid fruiting body development. In the presence of E. coli prey cells, fruiting bodies forming in a ring around the predation zone at 0, 0.1, and 1.0 g/liter Casitone are observed. The increased number of fruiting bodies observed at 0 g/liter Casitone in the presence of prey may be due either directly to the additional nutrients supplied by prey, providing the energy necessary to develop properly, or indirectly through growth and a consequent increase in the overall cell density of M. xanthus. However, neither of these possibilities can be used to rationalize why rapid fruiting body aggregation would occur at higher nutrient levels (1.0 g/liter Casitone) without any observed spatial or temporal shift. The induction of fruiting bodies in the presence of prey at nutrient levels that are typically prohibitive of fruiting body formation indicates that prey-induced aggregation is not occurring due to a sharp decrease in nutrient availability. As observed previously, there remains a close association of fruiting body formation adjacent to the edge of the initial E. coli colony perimeter.
FIG. 2.
Effect of basal nutrient level on prey-directed fruiting body formation. M. xanthus DZ2 cells were pipetted adjacent to prey colonies (first two columns) and alone (third column) on CFL plates containing (A to C) 0.0 g/liter, (D to F) 0.1 g/liter, and (G to I) 1.0 g/liter Casitone. In the presence of prey, fruiting aggregates were observed at 72 h under conditions ranging from 0.0 to 1.0 g/liter Casitone. In the absence of prey, fruiting aggregates were observed only at 0.1 g/liter Casitone.
Analysis of cellular differentiation in predation-induced aggregation.
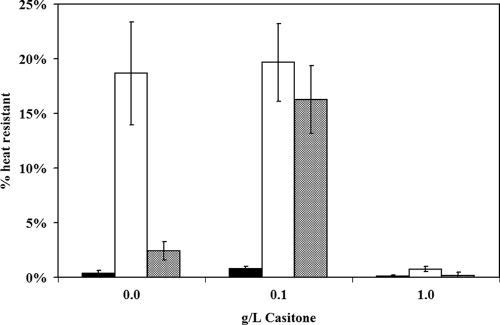
The surprising result that the presence of prey is able to induce fruiting bodies at nutrient levels that should be prohibitive led us to examine the effect of prey on sporulation levels as well. M. xanthus cells were harvested after 4 days of incubation on CFL-based medium, and the total number of viable CFU was determined and compared to the number of CFU after exposure to 2 h of heat stress at 50°C (Fig. 3). On CFL plates containing 0 g/liter Casitone, spores comprise 19% of the population in the presence of prey but <1% of the population in the absence of prey. The presence of prey in this assay causes an increase in the total number of M. xanthus cells harvested, typically about two- to threefold higher. To determine if the increase in the sporulation percentage is due solely to the increased cell density achieved by consuming prey-derived nutrients, we repeated the assay with a 10-fold-higher inoculum of M. xanthus cells. The increased inoculum resulted in more total cells than the samples incubated with prey but consisted of only 2% heat-resistant spores. At 0.1 g/liter Casitone, the sporulation percentage was again higher in the presence of prey, but in this case, increasing the initial M. xanthus cell density 10-fold increased the percentage of spore cells in the population to nearly that of the sample incubated with prey. This indicates that a combination of increased cell density and increased nutrient level, through either Casitone or prey cells, is sufficient to stimulate higher sporulation levels.
FIG. 3.
Prey effect on sporulation levels. M. xanthus DZ2 cells were incubated in either the presence or the absence of prey for 4 days on CFL plates with various levels of Casitone. Cells were harvested, and the viable M. xanthus cell count was determined through plating serial dilutions before and after a 2-h 50°C heat stress. Closed bars, 107 M. xanthus cells; open bars, 107 M. xanthus cells with 109 E. coli cells; hatched bars, 108 M. xanthus cells. The presence of prey increases the sporulation percentage of the population at 0 g/liter Casitone but not at 1.0 g/liter Casitone.
At 1.0 g/liter Casitone, conditions in which fruiting body aggregates are observed only in the presence of prey, a measurement of the heat-resistant population indicates that sporulation levels are <1% under all conditions. This indicates that while the edge of the predation zone serves to stimulate the early stages of fruiting body aggregation, a rapid morphological change in the spore cell type is not concurrently induced. Microscopic examination of wet mounts prepared from the fruiting aggregates formed in the presence of prey after 1 and 2 weeks of incubation indicates that the number of spores increases after prolonged incubation such that they are eventually indistinguishable from fruiting bodies formed under more stringent conditions (data not shown). These results support the hypothesis that predation-induced aggregation does not require starvation, since sporulation is delayed at 1.0 g/liter Casitone and yet aggregation is not.
Analysis of predation-induced aggregation in developmental signaling mutants.
Two early events in the monoculture starvation-induced development of M. xanthus cells are the production of (p)ppGpp during ribosomal stalling, which is produced through the activity of the RelA protein, and the generation of the cell density-related A signal, which requires the asg genes for proper expression. If the presence of prey is sufficient to stimulate the early stages of fruiting body formation, then an examination of the predation phenotypes of relA and asg mutants should provide insight into whether or not (p)ppGpp and A-signal production are required for prey-directed fruiting body formation. Preliminary experiments performed with strains MS1000 (ΔrelA) and DK4208 (asgB mutant) indicated that aggregation would still occur at the predation zone edge but with a severe delay with respect to strain DZ2 (data not shown). However, both of these mutant strains are known to have motility defects. MS1000 is derived from the DK101 background, which harbors a defect in the PilQ type IV pilus secretin protein (28). DK4208 is a tan-phase-locked mutant and displays the reduced motility phenotype common to this phase variant (9, 12).
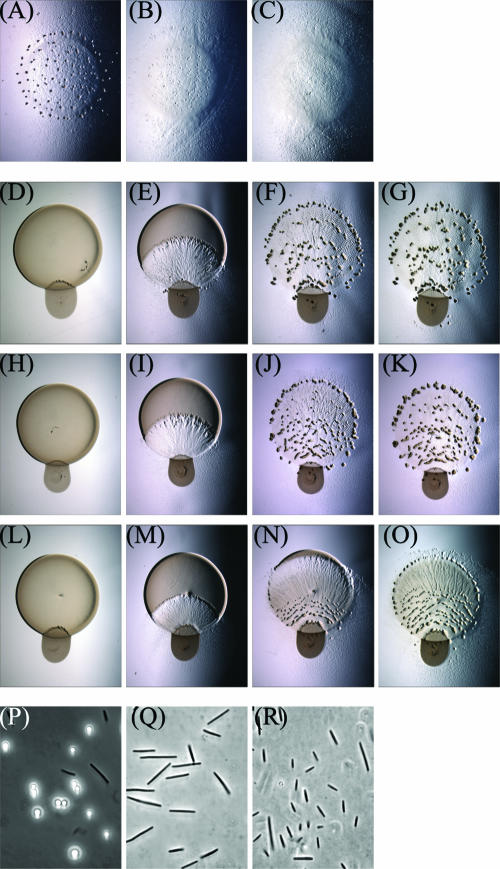
To eliminate potential motility artifacts, insertion mutants were constructed in the relA and asgD genes in the DZ2 parental strain. Under monoculture starvation conditions, both the relA and asgD mutants are defective in fruiting body aggregation compared to DZ2 (Fig. 4A to C). In the presence of prey, all three of these strains are able to penetrate the prey colony and lyse the E. coli cells with similar timing (Fig. 4D to O). Fruiting aggregates forming within the predation zone in all strains were observed as well. Both the DZ2 and asgD mutant strains form the characteristic ring of fruiting bodies around the predation zone edge by 64 h into the assay. The relA mutant has reduced swarm expansion and thus exhibits an aggregation delay with respect to the parent. However, the induction of a ring of fruiting aggregates was still observed in the relA mutant. This induction can be rapid, since areas that contain macroscopic amounts of prey can be converted to sites of fruiting aggregates in as little as 10 h (Fig. 4N and O). The final localization of the aggregates is also similar to that observed for DZ2. Microscopic examination of wet mounts prepared by harvesting cells from the fruiting aggregates indicates that neither mutant produces any spherical, phase-bright spore cells (Fig. 4P to R). This indicates that while the macroscopic developmental process of fruiting aggregation is rescued by the presence of prey, the conversion of individual cells to spores is still defective in both the relA and asgD mutant backgrounds. This also demonstrates that the starvation and cell density signals produced by RelA and AsgD are not required for multicellular behavior but are required only for cellular differentiation.
FIG. 4.
Analysis of early signaling mutants. In a monoculture starvation assay, (A) DZ2 forms fruiting bodies in 48 h, whereas (B) asgD mutant and (C) relA mutant strains are defective at aggregation. In a coculture predation assay, (D to G) DZ2, (H to K) asgD mutant, and (L to O) relA mutant strains are all proficient at inducing fruiting aggregation, particularly at the edges of the predation zone. Pictures were captured at 0, 40, 64, and 74 h (left to right) during predation. Analysis of wet mounts indicated that phase-bright spores are present in the predation-induced aggregates of (P) DZ2 but not (Q) asgD mutant or (R) relA mutant cells.
A step-down in prey cell density will induce fruiting body formation.
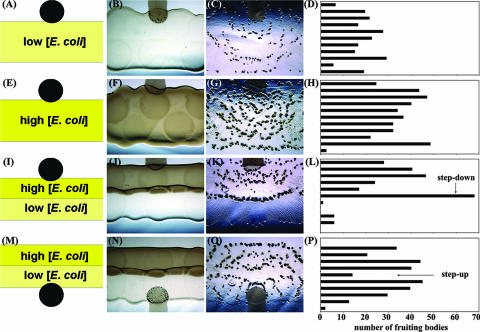
Because prey-directed fruiting bodies are not dependent on early intracellular or intercellular signals and also not dependent on the overall level of nutrient availability, we hypothesized that the impetus for forming a fruiting body aggregate at the predation zone edge could be due to the direct sensing of a loss of prey by the M. xanthus swarm. If aggregation occurs due to the direct monitoring of prey in the environment, rather than internal metabolic status, then we predict that a step-down in prey availability could suffice to trigger multicellular aggregation similar to that with a total loss of prey. To test this hypothesis, E. coli cells were added to CFL plates in long, thin adjacent strips containing either of two cell densities, ∼4 × 107 cells/mm2 (10 μl of a suspension of 1 × 1011 cells/ml) and ∼1 × 107 cells/mm2 (10 μl of a suspension of 2.5 × 1010 cells/ml). M. xanthus cells were pipetted onto the edge of these strips such that as the M. xanthus swarm expands out from the initial inoculum, cells that move in the direction of the E. coli prey will encounter sharp changes in prey availability (Fig. 5).
FIG. 5.
Response to stepped changes in prey cell density. E. coli β2155 cell suspensions were pipetted in a series of small drops and allowed to dry to generate relatively straight lines of prey at 4 × 107 cells/mm2 and 1 × 107 cells/mm2. M. xanthus cells were mixed with India ink and added to the edge of the dried prey colonies in a 1-μl aliquot. Prey cells were provided at (A to D) a constant low cell density, (E to H) a constant high cell density, (I to L) a step change down from a high cell density to a low cell density, or (M to P) a step change up from a low cell density to a high cell density. The left column portrays a conceptual diagram of the assay followed by images captured at 0 h and 72 h showing the pattern of fruiting bodies formed. For each assay, the field of view was sliced into 11 equivalent horizontal sections, and quantification of the fruiting body localization pattern is provided in the right column. The arrows highlight the location at which a step-up or a step-down in prey availability occurs.
Analysis of control samples in which there is a constant density of E. coli cells indicates that fruiting bodies form within the predation zone in vague arcs emanating from the initial inoculum (Fig. 5A to H). At a constant high cell density of prey, there are more fruiting bodies formed, and the average size of fruiting bodies observed is larger than that at the constant low cell density of prey. Under each of these conditions, fruiting bodies are distributed evenly such that when the predation zone is sliced into horizontal sections, no section in either control sample contains more than 15% of the total number of fruiting bodies. When cells are exposed to a sharp step-down in prey availability, however, a corresponding line of fruiting bodies was observed to form along the site of the step-down in prey (Fig. 5I to L). The horizontal section encompassing the site of the step-down contains 28% of the total fruiting bodies in the sample, while the two sections immediately following have a combined 0.4% of the total fruiting bodies, indicating that the step-down in prey availability provides a strong aggregation signal such that adjacent areas are devoid of aggregates.
M. xanthus cell movement is dependent on the elasticity of the surface, and stressed agar will bias the movement of cells through a process called elasticotaxis (5). Thus, the aggregation of cells at the site of a loss or step-down in prey availability could be due to physical forces such as a change in the overall topology or elasticity of the medium caused by the change in prey. To exclude this possibility, M. xanthus cells were also challenged with a step-up in prey availability (Fig. 5 M to P). At the site of a step-up in prey, there is actually a decrease in the probability of forming a fruiting body aggregate, with 5% of the total fruiting bodies found in this section. The neighboring sections contain 16% and 14% of the total fruiting body counts. This indicates that fruiting bodies formed at the edges of the predation zone or at a step-down in prey availability are not due simply to changes in topology or elasticity. Nutrient depletion should occur everywhere in the sample eventually and should occur earlier in the sections closest to the initial inoculum of M. xanthus, yet the pattern of fruiting body localization does not change significantly after 72 h. The site of a step-up in prey availability remains nearly devoid of fruiting bodies, even as fruiting bodies are induced in the succeeding sections. The ability of M. xanthus cells to react to essentially the same stimulus in two different ways depending on their prior environment indicates that they are responding directly to changes in the availability of prey using a system of cellular memory reminiscent of bacterial chemotaxis.
DISCUSSION
In 1963, Dworkin demonstrated that it is possible to manipulate the life cycle of M. xanthus solely by altering the amino acid content of a defined growth medium (4). This was important evidence that the developmental program of M. xanthus is a response to environmental conditions rather than an inexorable sequence of a predetermined growth cycle. Further analysis of this laboratory phenomenon has led to a model in which the developmental program of M. xanthus is initiated by starvation and then coordinated through a series of self-generated extracellular signals (6, 22). However, there has always been some difficulty in rationalizing why M. xanthus would initiate a complex multicellular program requiring significant time and energy after its nutrient supply has been exhausted. One model for starvation-induced development is that M. xanthus cells cannibalize each other, taking a step backward in growth in order to have enough energy to convert a fraction of the population into hardy spores (8). An alternative model, which stems from our analysis of predation-induced development, is that fruiting bodies are induced by a perceived reduction in prey availability. In this model, M. xanthus cells initiate multicellular development before nutrients are exhausted and without a requirement for self-sacrifice.
In this study, we examine how fruiting body development occurs in cocultures with suitable prey. We find that the presence of prey has a dramatic impact on the localization of fruiting bodies. In particular, the boundaries of a prey colony seem to provide a specific signal for fruiting body aggregation. Although this may at first glance seem to be a consequence of starvation, a deeper analysis indicates that this is not the case. By changing the basal nutrient level in medium, we observed that the presence of prey stimulates fruiting body aggregation across a wider range of nutrient levels than was observed under monoculture conditions. We also demonstrate that fruiting body aggregation will occur without an immediate commitment to sporulation in the presence of prey and higher nutrient availability. There have been several indications from genetic evidence that the processes of sporulation and aggregation may not be inextricably linked in M. xanthus. A DZ2/espB mutant forms fruiting aggregates, but sporulation occurs both outside and inside the aggregates (3). A similar result was observed in the DK1622/rodK mutant (18). It is now possible to provide a concrete biological rationalization for these genetic observations of how multicellular behavior can be uncoupled from cellular differentiation.
The earliest stages of fruiting body formation are currently thought to be controlled by a combination of sensing starvation and a sufficiently high cell density to initiate a developmental program (8). However, the ability of prey to also stimulate multicellular behavior allows new ways to test the interpretations of monoculture experiments to determine if they apply to a broader set of environmental circumstances. Two of the early signals for fruiting body aggregation have been proposed to be (p)ppGpp and the self-generated mixture of amino acids and small peptides that comprise the A signal. The relA and asgD gene products are required for (p)ppGpp production and A-signal production, respectively, and cause severe defects in fruiting body formation during monoculture development. However, under predatory conditions, neither of these genetic loci are required for the formation of fruiting body aggregates, yet they are still defective in the differentiation of vegetative cells to spores. This indicates that they are not necessary for the early signaling events of fruiting body aggregation but raises the question of why fruiting aggregates are not observed in monocultures of these strains. A close examination of these mutants in monoculture indicates that small, poorly defined aggregates are present in both strains. Perhaps the ability to differentiate into spores, which includes the loss of motility at some undetermined checkpoint, is necessary to stabilize the random aggregates formed in monoculture. Recent work by Sliusarenko et al. supports this idea, where an examination of cell movement during the early stages of fruiting body formation showed that reduced cell movement was more important than the control of cell reversals for the stability of early aggregates (25). Thus, mutants such as relA and asgD, which do not form stable aggregates in monoculture and are also not able to transition to nonmotile spores, may be able to utilize a prey-derived stimulus to halt in order to aggregate under predatory conditions without differentiation.
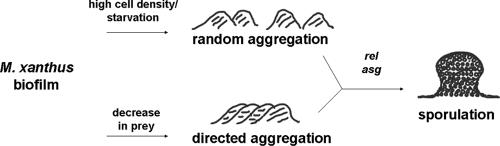
We propose a model in which cells are able to monitor the availability of prey or prey-derived macromolecules and use this information to initiate multicellular development (Fig. 6). A previous study demonstrated that the presence of prey or macromolecular growth substrates will induce multicellular rippling behavior in M. xanthus (1). In addition to rippling within a predation zone, our results indicate that M. xanthus cells respond to a loss of prey by forming fruiting bodies outside the edge of a predation zone. Additionally, this does not appear to be an on/off switch triggered only by a complete loss of prey, as M. xanthus cells are also capable of inducing fruiting bodies after a step-down in prey availability and inhibiting fruiting bodies after a step-up in prey availability. The typical laboratory method for inducing fruiting bodies in monoculture would thus fit into this model as an extreme circumstance in which M. xanthus cells suddenly find themselves in the peculiar situation of having a very high density of mid-log-phase vegetative cells and absolutely no prey or suitable macromolecular growth substrates. In nature, starvation is not likely to occur so suddenly to such a large population of M. xanthus cells; thus, it is important to closely examine the relationship that M. xanthus cells have with their prey in order to fully understand the multicellular behaviors displayed by this organism. Determining the specific components of prey that M. xanthus detects to make these decisions will be the challenge for understanding how cell-cell communication functions within this predator-prey relationship. An examination of the correlation between rippling behavior in the presence of macromolecular growth substrates and fruiting body aggregation at the edges of prey should also be a focus of study in the very near future.
FIG. 6.
Model for the initiation of multicellular development. Although a combination of high cell density and starvation will induce fruiting body formation in monoculture, it is important to consider that M. xanthus is a predatory bacterium and that prey availability alters the timing and localization of fruiting body aggregation as well. The early signals for this process are therefore dependent on interspecies signals, and self-generated signals from the relA and asg loci are not required for cellular differentiation until later in development.
Acknowledgments
We thank Mitch Singer for generously providing strains MS1000 (ΔrelA) and DK4208 (asgB mutant), which were extremely valuable to our initial analysis.
Funding was provided by The University of Iowa and NIH grant AI059682 to J.R.K.
Footnotes
Published ahead of print on 18 May 2007.
REFERENCES
- 1.Berleman, J. E., T. Chumley, P. Cheung, and J. R. Kirby. 2006. Rippling is a predatory behavior in Myxococcus xanthus. J. Bacteriol. 188:5888-5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braeken, K., M. Moris, R. Daniels, J. Vanderleyden, and J. Michiels. 2006. New horizons for (p)ppGpp in bacterial and plant physiology. Trends Microbiol. 14:45-54. [DOI] [PubMed] [Google Scholar]
- 3.Cho, K., and D. R. Zusman. 1999. Sporulation timing in Myxococcus xanthus is controlled by the espAB locus. Mol. Microbiol. 34:714-725. [DOI] [PubMed] [Google Scholar]
- 4.Dworkin, M. 1963. Nutritional regulation of morphogenesis in Myxococcus xanthus. J. Bacteriol. 86:67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontes, M., and D. Kaiser. 1999. Myxococcus cells respond to elastic forces in their substrate. Proc. Natl. Acad. Sci. USA 96:8052-8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagen, D. C., A. P. Bretscher, and D. Kaiser. 1978. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev. Biol 64:284-296. [DOI] [PubMed] [Google Scholar]
- 7.Harris, B. Z., D. Kaiser, and M. Singer. 1998. The guanosine nucleotide (p)ppGpp initiates development and A-factor production in Myxococcus xanthus. Genes Dev. 12:1022-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaiser, D. 2004. Signaling in myxobacteria. Annu. Rev. Microbiol. 58:75-98. [DOI] [PubMed] [Google Scholar]
- 9.Kuspa, A., and D. Kaiser. 1989. Genes required for developmental signalling in Myxococcus xanthus: three asg loci. J. Bacteriol. 171:2762-2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuspa, A., L. Plamann, and D. Kaiser. 1992. Identification of heat-stable A-factor from Myxococcus xanthus. J. Bacteriol. 174:3319-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuspa, A., L. Plamann, and D. Kaiser. 1992. A-signalling and the cell density requirement for Myxococcus xanthus development. J. Bacteriol. 174:7360-7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laue, B. E., and R. E. Gill. 1994. Use of a phase variation-specific promoter of Myxococcus xanthus in a strategy for isolating a phase-locked mutant. J. Bacteriol. 176:5341-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McBride, M. J., R. A. Weinberg, and D. R. Zusman. 1989. “Frizzy” aggregation genes of the gliding bacterium Myxococcus xanthus show sequence similarities to the chemotaxis genes of enteric bacteria. Proc. Natl. Acad. Sci. USA 86:424-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mignot, T., J. W. Shaevitz, P. L. Hartzell, and D. R. Zusman. 2007. Evidence that focal adhesion complexes power bacterial gliding motility. Science 315:853-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Connor, K. A., and D. R. Zusman. 1991. Development in Myxococcus xanthus involves differentiation into two cell types, peripheral rods and spores. J. Bacteriol. 173:3318-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oetker, H. 1953. Studies on the nutrition of various myxobacteria. Arch. Mikrobiol. 19:206-246. [PubMed] [Google Scholar]
- 17.Plamann, L., A. Kuspa, and D. Kaiser. 1992. Proteins that rescue A-signal-defective mutants of Myxococcus xanthus. J. Bacteriol. 174:3311-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen, A. A., S. L. Porter, J. P. Armitage, and L. Sogaard-Andersen. 2005. Coupling of multicellular morphogenesis and cellular differentiation by an unusual hybrid histidine protein kinase in Myxococcus xanthus. Mol. Microbiol. 56:1358-1372. [DOI] [PubMed] [Google Scholar]
- 19.Reichenbach, H. 1966. Myxococcus spp. (Myxobacteriales) Schwarmentwicklung und bildung von protocysten, p. 557-578. In G. Wolf (ed.), Encyclop. Cinematogr. Film E778/1965.
- 20.Rosenberg, E., and M. Varon. 1984. Antibiotics and lytic enzymes, p. 109-125. In E. Rosenberg (ed.), Myxobacteria: development and cell interactions. Springer, New York, NY.
- 21.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 22.Shimkets, L. J. 1999. Intercellular signaling during fruiting-body development of Myxococcus xanthus. Annu. Rev. Microbiol. 53:525-549. [DOI] [PubMed] [Google Scholar]
- 23.Shimkets, L. J., and D. Kaiser. 1982. Induction of coordinated movement of Myxococcus xanthus cells. J. Bacteriol. 152:451-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer, M., and D. Kaiser. 1995. Ectopic production of guanosine penta- and tetraphosphate can initiate early developmental gene expression in Myxococcus xanthus. Genes Dev. 9:1633-1644. [DOI] [PubMed] [Google Scholar]
- 25.Sliusarenko, O., D. R. Zusman, and G. Oster. 2007. Aggregation during fruiting body formation in Myxococcus xanthus is driven by reducing cell movement. J. Bacteriol. 189:611-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun, H., D. R. Zusman, and W. Shi. 2000. Type IV pilus of Myxococcus xanthus is a motility apparatus controlled by the frz chemosensory system. Curr. Biol. 10:1143-1146. [DOI] [PubMed] [Google Scholar]
- 27.Thaxter, R. 1892. On the Myxobacteriaceae, a new order of Schizomycetes. Bot. Gaz. 17:389-406. [Google Scholar]
- 28.Wall, D., P. E. Kolenbrander, and D. Kaiser. 1999. The Myxococcus xanthus pilQ (sglA) gene encodes a secretin homolog required for type IV pilus biogenesis, social motility, and development. J. Bacteriol. 181:24-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waters, C. M., and B. L. Bassler. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21:319-346. [DOI] [PubMed] [Google Scholar]