Abstract
F1Fo ATP synthases function by a rotary mechanism. The enzyme's peripheral stalk serves as the stator that holds the F1 sector and its catalytic sites against the movement of the rotor. In Escherichia coli, the peripheral stalk is a homodimer of identical b subunits, but photosynthetic bacteria have open reading frames for two different b-like subunits thought to form heterodimeric b/b′ peripheral stalks. Chimeric b subunit genes have been constructed by substituting sequence from the Thermosynechococcus elongatus b and b′ genes in the E. coli uncF gene, encoding the b subunit. The recombinant genes were expressed alone and in combination in the E. coli deletion strain KM2 (Δb). Although not all of the chimeric subunits were incorporated into F1Fo ATP synthase complexes, plasmids expressing either chimeric bE39-I86 or b′E39-I86 were capable of functionally complementing strain KM2 (Δb). Strains expressing these subunits grew better than cells with smaller chimeric segments, such as those expressing the b′E39-D53 or bL54-I86 subunit, indicating intragenic suppression. In general, the chimeric subunits modeled on the T. elongatus b subunit proved to be more stable than the b′ subunit in vitro. Coexpression of the bE39-I86 and b′E39-I86 subunits in strain KM2 (Δb) yielded F1Fo complexes containing heterodimeric peripheral stalks composed of both subunits.
F1Fo ATP synthases generate the majority of ATP consumed by living organisms and are found in the plasma membrane of bacteria, the inner membrane of mitochondria, and the thylakoid membrane of chloroplasts. All F1Fo ATP synthases function by a conserved mechanism in which the electrochemical energy of a proton or sodium gradient is harnessed to synthesize ATP from ADP and inorganic phosphate (for reviews, see references 3, 22, and 30). In Escherichia coli the membrane-embedded Fo sector housing the proton channel is composed of three different subunits in the stoichiometry ab2c10. The F1 sector consists of five subunits in the stoichiometry α3β3γδɛ. F1Fo ATP synthase functions as a rotary motor. The translocation of protons through the Fo channel results in movement of the rotor stalk consisting of the c10γɛ subunits relative to the remainder of the complex. The rotation drives ATP synthesis via conformation shifts in the catalytic sites located at the interfaces between the α3β3 subunits. The α3β3 subunits are held in place against the rotation of the rotor stalk by the peripheral stalk composed of the b2δ subunits.
In E. coli, the peripheral stalk is an extended homodimer of identical b subunits (2, 9, 35). Each b subunit has an N-terminal transmembrane domain and stretches the length of the F1Fo complex, making direct contact with the δ subunit perched atop and to one side of the F1 sector (8, 19). Three distinct functional domains have been defined within the hydrophilic portion of the b subunits (26). First, the tether domain roughly corresponds to the segment between the surface of the membrane and a position beneath the F1 sector. Both large deletions and insertions can be readily accommodated in this domain (32, 34), and functional F1Fo complexes with peripheral stalks containing two b subunits with tether domains differing in length have been engineered (15). The dimerization domain extends up into one of the noncatalytic αβ interfaces in F1 (18). This section is essential and sufficient for the formation of a dimer of the soluble form of the b subunit (26). A high-resolution structure of a polypeptide modeling the dimerization domain from amino acids 62 to 122 revealed a linear α-helix (6). Recent analysis of disulfide cross-link formation within the b subunit dimerization domains suggested a right-handed coiled-coil arrangement (5). The C-terminal F1 binding domain is required for interaction of the peripheral stalk and F1 (1, 19). The structure of the soluble portions of the peripheral stalk from bovine mitochondrial F1Fo ATP synthase was recently solved by crystallography and showed a stalk with three different subunits that formed an extended curving subcomplex (7).
In contrast, the peripheral stalks found in chloroplast F1Fo ATP synthase consist of two different b-like polypeptides, named subunits I and II, that form a heterodimer (27). Some eubacteria, encompassing photosynthetic bacteria but including some other species, also have genes encoding two b-like subunits designated b and b′. Both polypeptides have been shown to be present in purified ATP synthase from Aquifex aeolicus (25). The general expectation that b and b′ form a heterodimer has been supported by studies of expressed hydrophilic domains of the subunits from the cyanobacterium Synechocystis sp. strain PCC6803 (10). Sedimentation analyses showed that the predominant species present in equimolar mixtures of the two polypeptides had a molecular weight expected for the heterodimer, whereas the individual polypeptides gave molecular weights corresponding to monomers. Heterodimer formation was also supported by chemical cross-linking. To our knowledge, however, it has never been demonstrated that only heterodimeric stalks are formed within the enzyme, or, alternatively, whether b2 and b′2 homodimeric stalks might also exist and support function.
Thermosynechococcus elongatus BP-1 is a thermophilic cyanobacterium whose entire genome has been sequenced (21). Although little is known about the F1Fo ATP synthase of T. elongatus other than the sequence information, it has been used as a model organism for the study of photosynthesis (23) and circadian rhythms (24). T. elongatus BP-1 has genes encoding both b and b′ subunits, and these genes share a relatively high degree of sequence identity with the Synechocystis genes. There are approximately 50% identity and 70% similarity in the deduced amino acid sequences of both b and b′ genes between the two organisms. Therefore, it seems reasonable to assume that the b and b′ subunits of T. elongatus will preferentially form heterodimeric peripheral stalks.
In this work we have generated chimeric b subunits by replacing segments of the E. coli uncF gene, encoding the b subunit, with either b or b′ sequence based on the T. elongatus genes (Fig. 1). The recombinant subunit genes were expressed alone and in combination in an E. coli uncF deletion strain. Although some of the chimeric subunits failed to assemble into F1Fo ATP synthase complexes, others were incorporated and were capable of functional complementation of the deletion strain. Expression of chimeric subunits to form only homodimeric stalks was in some cases sufficient for activity. When expressed together, the b and b′ chimeric subunits readily formed heterodimeric peripheral stalks in F1Fo ATP synthase complexes.
FIG. 1.
Alignment of the E. coli b subunit sequence with the sequences of the T. elongatus b and b′ subunits over the E39-to-A107 region. Identical amino acids are shaded, and the positions mentioned in the text are labeled.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli strain KM2 (Δb), which carries a chromosomal deletion of the uncF (b subunit) gene, and strain 1100 ΔBC (Δunc), lacking the entire unc operon, were used as the host strains (17). Cells were grown on minimal A medium supplemented with succinate (0.2%, wt/vol) to determine enzyme viability. Cells harvested for membrane preparations were grown in Luria broth supplemented with glucose (0.2%, wt/vol) and isopropyl-1-thio-β-d-galactopyranoside (IPTG; 40 μg/ml). IPTG, ampicillin (100 μg/ml), and chloramphenicol (25 μg/ml) were added as needed. All cells were grown at 37°C unless otherwise noted.
Expression plasmid construction.
Ten plasmids were constructed for expression of chimeric E. coli/T. elongatus b or b′ subunits (Table 1). Five of these plasmids expressed a chimeric b subunit with a histidine tag (his6) on the amino terminus, and five expressed a chimeric b′ subunit with a V5 tag (GKPIPNPLLGLDST) on the carboxyl terminus. The plasmids expressing chimeric b and b′ subunits were constructed using expression plasmids pTAM37 (bhis; Cmr) and pTAM46 (bV5; Apr), respectively (15). Sections of nucleotide sequences of the uncF (b subunit) gene from E. coli codons E39 to A107 were replaced with the homologous T. elongatus b and b′ sequences. This was accomplished by cassette site-directed mutagenesis essentially as described previously (16). The E. coli sequence was replaced in three steps using four restriction sites located within the uncF gene: SnaBI, which cuts at nucleotide 72; PpuMI, cutting at nucleotide 156; XbaI, at nucleotide 258; and SapI, at nucleotide 347 (Table 2). The mutated sequences were E39 to D53 for the SnaBI and PpuMI sites, L54 to I86 for the PpuMI and XbaI sites, and L87 to A107 for the XbaI and SapI sites. The native cysteine in the b subunit was mutated to a serine (C25S) in all constructs, and the chimeric b subunit genes were confirmed by direct nucleotide sequencing.
TABLE 1.
Plasmids used in this study
| Plasmid | Gene producta | Antibiotic resistanceb | Source or reference |
|---|---|---|---|
| pBR322 | Δb | Ap | New England Biolabs |
| pTAM37 | bhis | Cm | 15 |
| pTAM46 | bV5 | Ap | 15 |
| pSBC56 | bE39-D53, his | Cm | This study |
| pSBC57 | b′E39-D53, V5 | Ap | This study |
| pSBC58 | bL54-I86, his | Cm | This study |
| pSBC76 | b′L54-I86, V5 | Ap | This study |
| pSBC97 | bE39-I86, his | Cm | This study |
| pSBC98 | b′E39-I86, V5 | Ap | This study |
| pSBC94 | bL54-A107, his | Cm | This study |
| pSBC79 | b′L54-A107, V5 | Ap | This study |
| pSBC95 | bE39-A107, his | Cm | This study |
| pSBC96 | b′E39-A107, V5 | Ap | This study |
his, six-histidine epitope tag at the amino terminus; V5, epitope tag with the sequence GKPIPNPLLGLDS appended to the carboxyl terminus. The replaced regions are designated by the first and last E. coli amino acids that were replaced with T. elongatus sequence.
Ap, ampicillin; Cm, chloramphenicol.
TABLE 2.
Synthetic oligonucleotides used in this study
| Oligonucleotide pair (gene) | Annealed oligonucleotide | Sequencea |
|---|---|---|
| TG1/2b (bE39-D53) | TG1 | 5′-GTATGGCCGCCACTGATGGCAGCCATCGAAAAACGTCAAAAACAGATCGCTACTGCTATCGCTGAAGCTGAAGAACGTCAGAAG-3′ |
| TG2 | 3′-CATACCGGCGGTGACTACCGTCGGTAGCTTTTTGCAGTTTTTGTCTAGCGATGACGATAGCGACTTCGACTTCTTGCAGTCTTCCTG-5′ | |
| TG3/4 (b′E39-D53) | TG3 | 5′-GTATGGCCGCCACTGATGGCAGCCATCGAAAAACGTCAAAAATACATCCGTACTAACCTGCAGCAGGCTAAAGAACGTCTGCAGCAG-3′ |
| TG4 | 3′-CATACCGGCGGTGACTACCGTCGGTAGCTTTTTGCAGTTTTTATGTAGGCATGATTGGACGTCGTCCGATTTCTTGCAGACGTCGTCCTG-5′ | |
| TG13/10c (bL54-I86) | TG13 | 5′-GACGTTGCTGCTGCTCGTCTGGCTGAAGCTCAGCAGAAACTGACTCAGGCTAAACAGGAGGCTCAGCGTATCCGTGAAGATGCTCTGACTCGTGCTAAAGCTGTT-3′ |
| TG10 | 3′-CAACGACGACGAGCAGACCGACTTCGAGTCGTCTTTGACTGAGTCCGATTTGTCCTCCGAGTCGCATAGGCACTTCTACGAGACTGAGCACGATTTCGACAAGATC-5′ | |
| TG14/12 (b′L54-I86) | TG14 | 5′-GACGCTACTGAACTGGCTCAGCAGTACGAACAGGAGCTGGCTTCCACTCGTCGTCAGGCTCAGGCTCTGATCGAAGAAGCTCGTGTTGAAGCTCAGAAAATT-3′ |
| TG12 | 3′-CGATGACTTGACCGAGTCGTCATGCTTGTCCTCGACCGAAGGTGAGCAGCAGTCCGAGTCCGAGACTAGCTTCTTCGAGCACAACTTCGAGTCTTTTAAGATC-5′ | |
| SC39/40d (bL87-A107) | SC39 | 5′-CTAGAAGAAATTATTGCTCAGGCTAAACGTGAAATTGAACGTCTGCAGGAGACCGCTAGCCAGGAAATTGAAGCCGAGCGTAAACGTGC-3′ |
| SC40 | 3′-TTCTTTAATAACGAGTCCGATTTGCACTTTAACTTGCAGACGTCCTCTGGCGATCGGTCCTTTAACTTCGGCTCGCATTTGCACGGGC-5′ | |
| SC43/44 (b′L87-A107) | SC43 | 5′-CTAGATGCGGAAATTGCGGAAGCGCAGCAGGCGGTCCAGGCGGAGCTCTTGAAAATTCAGGCGGAAATTGAAGCCGAGCGTAAACGTGC-3′ |
| SC44 | 3′-TACGCCTTTAACGCCTTCGCGTCGTCCGCCAGGTCCGCCTCGAGAACTTTTAAGTCCGCCTTTAACTTCGGCTCGCATTTGCACGGGC-5′ | |
| SC45/46 (bC25S + AflIII) | SC45 | 5′-CTGTTCGTTCTGTTCTCCATGAAGTACGTGTGGCCGCC-3′ |
| SC46 | 3′-GACAAGCAAGACAAGAGGTACTTCATGCACACCGGCGG-5′ | |
| SC59/60e (b−D + SacII) | SC59 | 5′-ACTGCTATCGCTGAAGCTGAAGAACGTCAGAAGGTTGCCGCGGCTCGTCTGGCTGAAGCTCAGCAGAAACTG-3′ |
| SC60 | 3′-TGACGATAGCGACTTCGACTTCTTGCAGTCTTCCAACGGCGCCGAGCAGACCGACTTCGAGTCGTCTTTGAC-5′ | |
| SC61/62 (b′−D + NheI) | SC61 | 5′-ACTAACCTGCAGCAGGCTAAAGAACGTCTGCAGCAGGCTACTGAGCTAGCTCAGCAGTACGAACAGGAGCTGGCT-3′ |
| SC62 | 3′-TGATTGGACGTCGTCCGATTTCTTGCAGACGTCGTCCGATGACTCGATCGAGTCGTCATGCTTGTCCTCGACCGA-5′ | |
| SC53/54 (b + SpeI) | SC53 | 5′-GCTGCTAACAGCGACATCGTGGATAAACTAGTCGCTGAACTGTAAGGAGGGAGG-3′ |
| SC54 | 3′-CGACGATTGTCGCTGTAGCACCTATTTGATCAGCGACTTGACATTCCTCCCTCC-5′ | |
| SC57/58 (b + SnaBI) | SC57 | 5′-CTGTTCGTTCTGTTCTCCATGAAGTACGTATGGCCGCC-3′ |
| SC58 | 3′-GACAAGCAAGACAAGAGGTACTTCATGCATACCGGCGG-5′ | |
| TB38/39 (bV5 + SacI) | TB38 | 5′-CGTGGATAAACTTGTCGCTGAGCTCGGTAAACCGATCCCGAACCCGCTGCTGGGTCTGGACTCTACCTAAGGAGGGAGGGGCTGATG-3′ |
| TB39 | 3′-GCACCTATTTGAACAGCGACTCGAGCCATTTGGCTAGGGCTTGGGCGACGACCCAGACCTGAGATGGATTCCTCCCTCCCCGACTAC-5′ |
Added restriction sites are underlined, and the V5 epitope tag is boldfaced.
Oligonucleotides TG1/2 (b) and TG3/4 (b′) were used to replace DNA between restriction sites SnaBI and PpuMI.
Oligonucleotides TG13/10 (b) and TG14/12 (b′) were used to replace DNA between restriction sites PpuMI and XbaI.
Oligonucleotides SC39/40 (b) and SC43/44 (b′) were used to replace DNA between restriction sites XbaI and SapI.
Oligonucleotides SC59/60 (b) and SC61/62 (b′) were used to remove an unwanted aspartic acid between the E39-to-D53 and L54-to-I86 regions.
Preparation of membranes.
Membranes were prepared using the method of Caviston et al. (4). Bacterial cells were harvested upon reaching an optical density at 600 nm of approximately 1.0. The cells were collected by centrifugation in a Du Pont Sorvall RC-5B Superspeed centrifuge with a GSA rotor at 10,000 × g for 10 min and were resuspended in TM buffer (50 mM Tris-HCl [pH 7.5], 10 mM MgSO4). DNase I was added to a final concentration of 10 μg/ml, and the cells were broken by passing them twice through a French pressure cell at 14,000 lb/in2. Two sequential centrifugation steps in the same Sorvall centrifuge with an SS-34 rotor at 10,000 × g for 10 min were carried out to remove unbroken cells and debris. Membrane vesicles were recovered by centrifugation in a Beckman-Coulter Optima LE-80K ultracentrifuge with a 70.1 Ti rotor at 150,000 × g for 1.5 h. Membranes were washed by resuspending them in TM buffer using a glass homogenizer and a Teflon pestle and then centrifuging them again in the Beckman centrifuge for 1 h. The washed membranes were suspended again with the homogenizer in a final volume of 1 ml TM buffer, and the protein concentration was determined using the bicinchoninic acid method (31).
Activity assays.
The ATP hydrolysis activity of membrane samples was determined using the acid molybdate method to monitor the release of inorganic phosphate as previously described (32). Reactions were carried out with 60 μg of protein suspended in 4 ml of reaction buffer (50 mM Tris-HCl [pH 7.5], 1 mM MgCl2) at 37°C unless otherwise specified. Lauryl dimethylamine oxide (LDAO) stimulation of ATP hydrolysis activity was determined by adding LDAO to the ATP hydrolysis reaction mixture at a final concentration of 0.5% as previously described (13). ATP- and NADH-driven proton pumping assays were conducted by adding 500 μg of membrane protein to 3.5 ml reaction buffer (50 mM morpholinepropanesulfonic acid [MOPS; pH 7.3], 10 mM MgCl2) and monitoring the fluorescence quenching of 9-amino-6-chloro-2-methoxyacridine (ACMA) as previously described (33). Fluorescence quenching was detected using two spectrofluorometers, either a Perkin-Elmer LS-3B or a Photon Technologies International QuantaMaster 4.
Trypsin digestion.
The incorporation of chimeric b subunits into intact F1Fo ATP synthase complexes was detected by the digestion of membrane samples with trypsin to degrade unincorporated b subunit as previously described (34). The b subunits were digested by bringing a total of 0.1 mg of membrane protein to a volume of 90 μl using TM buffer and adding 10 μl of 2-mg/ml trypsin to start the reaction. Aliquots containing 10 μg of digested protein were removed at 1, 2, 3, 4, 6, 8, and 16 h, and the reaction was stopped by the addition of 2 μl of 10-mg/ml trypsin inhibitor from Glycine max (soybean; Sigma-Aldrich). For long digestions, additional trypsin was added at 3, 6, and 8 h to compensate for the estimated activity loss of 25%/3 h. The samples were studied by Western blot analysis with 1 μg of each sample loaded per lane. The presence of heterodimeric peripheral stalks in intact F1Fo complexes was examined by the digestion of 0.5 mg of membrane protein in a reaction volume of 100 μl. The entire reaction was stopped by the addition of 30 μl of 10-mg/ml trypsin inhibitor, and the product was purified over a nickel resin and analyzed by Western blot analysis (see below).
Nickel resin purification.
Membrane samples were solubilized and purified over a high-capacity nickel chelate affinity matrix (Ni-CAM), purchased from Sigma, to selectively retain F1Fo ATP synthase complexes containing at least one histidine tag as previously described (15). A total of 0.5 mg of membrane protein was brought up to a volume of 150 μl with a final concentration of 1 mM imidazole, 0.2 M NaCl, and 0.2% Tegamine oxide WS-35. The solubilized membrane protein was purified over 75 μl of Ni-CAM resin essentially as described by the manufacturer. The resin was incubated with the membrane protein for 10 min and then washed five times with wash buffer (50 mM Na2PO4 [pH 8.0], 0.2 M NaCl, 1 mM imidazole, 0.2% Tegamine oxide WS-35). Any bound protein was eluted in elution buffer (50 mM Na2PO4 [pH 8.0], 0.3 M NaCl, 250 mM imidazole) by incubating the resin twice for 10 min with 125 μl of elution buffer. The eluted protein was concentrated using a Microcon YM-10 centrifugal filter device, and the volume of the eluant was estimated by weight.
Western blotting.
Proteins were separated on a 15% sodium dodecyl sulfate-polyacrylamide gel, and Western blotting was performed as described previously (14). The primary and secondary antibodies used here were the anti-b subunit antibody (a gift from G. Deckers-Hebestreit) and the anti-V5 antibody (Invitrogen). The presence of bound secondary antibody was detected by chemiluminescence using Amersham Biosciences' ECL Plus Western blotting detection system.
RESULTS
Complementation analysis.
For complementation studies, plasmids pTAM37 (bhis; Cmr) and pTAM46 (bV5; Apr) were transformed into deletion strain KM2 (Δb) to serve as positive controls (15). The b subunits expressed by these plasmids were equipped with different epitope tags to facilitate the detection of heterodimeric peripheral stalks. The epitope tags had very little effect on enzyme activity. Ten additional plasmids have been constructed to express chimeric subunits in which parts of the tether and dimerization domains of the E. coli b subunit have been replaced with the homologous b or b′ segments from T. elongatus (Fig. 1). This organism was chosen in an attempt to increase the stability of the chimeric constructs. The amino and carboxyl boundaries of the substituted region were selected to avoid disturbing the environment of the critical R36 residue and to prevent disruption of essential interactions between the peripheral stalk and F1. The abilities of these plasmids to complement deletion strain KM2 (Δb) were studied by growth on minimal A medium supplemented with succinate (Table 3). Plasmids that contained T. elongatus b or b′ sequence between either D54 and A107 or E39 and A107 were unable to complement strain KM2. Similarly, KM2/pSBC57 (b′E39-D53, V5) failed to grow on succinate medium, but a weakly positive result was obtained with strain KM2/pSBC56 (bE39-D53, his). Much more impressive complementation was obtained with strain KM2/pSBC76 (b′L54-I86, V5), but KM2/pSBC58 (bL54-I86, his) did not grow. Interestingly, colony formation was in evidence when either chimeric bE39-I86, his or b′E39-I86, V5 subunits were expressed.
TABLE 3.
Growth properties and ATPase activities in cells expressing chimeric subunits
| Strain/plasmid(s) | Gene product(s) | Growth on succinatea | ATP hydrolysis activityb
|
|
|---|---|---|---|---|
| Without LDAO | With 0.5% LDAOc | |||
| KM2/pTAM37/pTAM46 | bhis/bV5 | +++ | 0.62 ± 0.04 | 1.21 ± 0.01 |
| KM2/pBR322 | Δb | − | 0.13 ± 0.02 | 0.22 ± 0.01 |
| KM2/pSBC56 | bE39-D53, his | + | 0.58 ± 0.03 | 1.27 ± 0.07 |
| KM2/pSBC57 | b′E39-D53, V5 | − | 0.20 ± 0.01 | 0.18 ± 0.04 |
| KM2/pSBC56/pSBC57 | bE39-D53, his/b′E39-D53, V5 | + | 0.61 ± 0.02 | 1.26 ± 0.06 |
| KM2/pSBC58 | bL54-I86, his | − | 0.42 ± 0.01 | 0.71 ± 0.02 |
| KM2/pSBC76 | b′L54-I86, V5 | +++ | 0.22 ± 0.01 | 0.31 ± 0.02 |
| KM2/pSBC58/pSBC76 | bL54-I86, his/b′L54-I86, V5 | +++ | 0.47 ± 0.03 | 0.76 ± 0.06 |
| KM2/pSBC97 | bE39-I86, his | + | 0.50 ± 0.04 | 0.87 ± 0.08 |
| KM2/pSBC98 | b′E39-I86, V5 | ++ | 0.19 ± 0.01 | 0.26 ± 0.01 |
| KM2/pSBC97/pSBC98 | bE39-I86, his/b′E39-I86, V5 | ++ | 0.49 ± 0.04 | 0.88 ± 0.02 |
| KM2/pSBC94 | bL54-A107, his | − | 0.22 ± 0.01 | 0.30 ± 0.01 |
| KM2/pSBC79 | b′L54-A107, V5 | − | 0.15 ± 0.01 | 0.44 ± 0.03 |
| KM2/pSBC94/pSBC79 | bL54-A107, his/b′L54-A107, V5 | − | 0.23 ± 0.03 | 0.29 ± 0.03 |
| KM2/pSBC95 | bE39-A107, his | − | 0.12 ± 0.03 | 0.34 ± 0.01 |
| KM2/pSBC96 | b′E39-A107, V5 | − | 0.12 ± 0.02 | 0.16 ± 0.03 |
| KM2/pSBC95/pSBC96 | bE39-A107, his/b′E39-A107, V5 | − | 0.14 ± 0.03 | 0.37 ± 0.02 |
Symbols: +++, wild-type growth; ++, colonies smaller than wild type; +, small-colony formation; −, no growth.
Reported in μmol of Pi/mg of membrane protein/min.
Used to release F1 from the inhibitory effect of Fo.
Stability of F1Fo complexes.
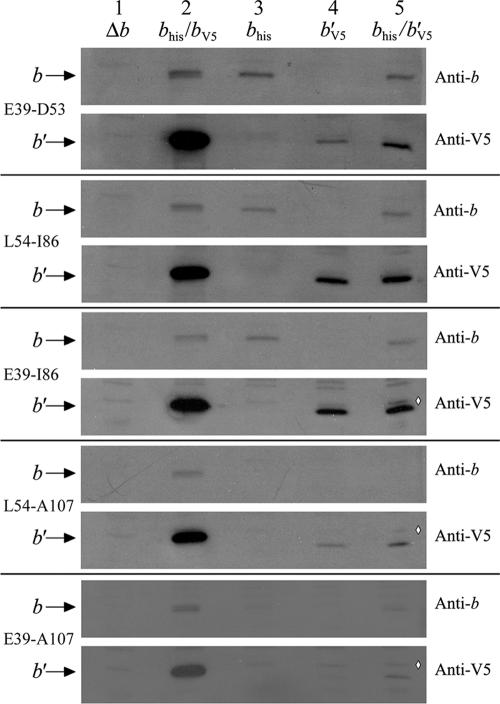
In order to detect the expression of the recombinant b subunits and their incorporation into membranes, the presence of b subunit proteins was studied by Western blot analysis. The experiments were performed on membrane samples prepared from cells expressing chimeric b or b′ subunits either alone or together (Fig. 2). The Western blots were probed with a polyclonal antibody against the b subunit or a monoclonal antibody against the V5 epitope tag. The E. coli anti-b subunit antibody recognized the chimeric bhis subunits in membranes prepared from KM2 cells expressing the bE39-D53, his, bL54-I86, his, or bE39-I86, his subunit. Although b′V5 subunits appeared to be present at lower levels than the controls, these subunits were detected by the anti-V5 antibody in membranes prepared from cells expressing b′E39-D53, V5, b′L54-I86, V5, or b′E39-I86, V5 subunits. The chimeric b′ subunits were not detectable with the antibody against the E. coli b subunit. The first indication of a likely interaction between chimeric b and b′ subunits within an F1Fo complex was the observation that the b′E39-D53, V5 subunit seemed to be stabilized in the membrane in the presence of the bE39-D53, his subunit (Fig. 2, E39-D53, lanes 4 and 5). The absence of signals for the chimeric b or b′ subunit with T. elongatus sequence from D54 to A107 or from E39 to A107 with either antibody suggested that the chimeric segment could not be extended further toward the C terminus. These proteins apparently failed to be stably incorporated into the membranes and were degraded.
FIG. 2.
Immunoblot of membranes prepared from E. coli strain KM2 (Δb) expressing chimeric b subunits. Aliquots of membrane proteins (1 μg) were separated on 15% sodium dodecyl sulfate-polyacrylamide gels and transferred to nitrocellulose membranes as previously described (14). The presence of the b subunit was detected using both a polyclonal antibody raised against the b subunit and an antibody against the V5 epitope tag appended to the carboxyl terminus of the chimeric b′ subunits. The position of the b subunit band and the region that was replaced with T. elongatus sequence are indicated on the left and the primary antibody on the right. Membrane samples were loaded as follows: lane 1, negative control KM2/pBR322 (Δb); lane 2, positive control KM2/pTAM37/pTAM46 (bhis/bV5); lane 3, chimeric bhis subunits; lane 4, chimeric b′V5 subunits; lane 5, coexpression of chimeric bhis and b′V5 subunits. The commercial anti-V5 antibody gave a much stronger signal than the polyclonal anti-b subunit antibody and also detected an extra band running near the level of the b subunit, indicated in lane 5 by a white diamond (⋄).
Membrane-associated ATP hydrolysis was determined for KM2 cells expressing chimeric b and b′ subunits (Table 3). Since the b subunit is required for association of the F1 complex with the Fo complex, membrane-associated ATPase activity can in some cases be used as an indirect indication of the relative amounts of intact F1Fo complexes stably incorporated into the membrane. As expected, only very low levels of ATP hydrolysis were observed in membranes prepared from cells expressing chimeric b or b′ subunits with T. elongatus sequence replacing L54 to A107 or E39 to A107, a result that correlated well with failed complementation tests and negative Western blotting results. In contrast, substantial ATP hydrolysis activity was seen with all chimeric b subunits with replacements between E39 and D53, L54 and I86, or E39 and I86. Strangely, this was not true of the chimeric b′ subunits. Membranes prepared from KM2/pSBC57 (b′E39-D53, V5), KM2/pSBC76 (b′L54-I86, V5), and KM2/pSBC98 (b′E39-I86,V5) all had very little membrane-associated ATP hydrolysis activity. Given that two of the strains had solidly positive complementation test results, it was necessary to look into this issue further. We successfully reproduced the complementation analysis and then sequenced plasmid DNA prepared from the colonies grown on succinate minimal medium. The uncF (b subunit) genes in these plasmids retained the designed chimeric b subunit gene and carried no other mutations. A recent publication suggested that a cold-stabilized form of the complex had a significant lag in ATP hydrolysis activity (12). Therefore, ATP hydrolysis was carefully reexamined under conditions that would account for a potential lag, and results essentially identical to those shown in Table 3 were obtained.
Another possibility was that the chimeric b′ subunits inhibited F1Fo ATP hydrolysis. LDAO releases F1 from the influence of Fo-associated mutations (13) and promotes release of the inhibitory ɛ subunit (11), so determination of ATP hydrolysis activity in the presence of LDAO yields a value reflecting the total F1 present in a membrane sample. The amount of LDAO stimulation observed indicated that the KM2/pSBC76 (b′L54-I86, V5) and KM2/pSBC98 (b′E39-I86,V5) samples had only minimal F1 bound. Therefore, it seems that although the two strains grew well, indicating abundant F1Fo ATP synthase function in vivo, the F1Fo complexes were not stable and were lost during membrane preparation. The reduced levels of b′V5 subunits seen during the Western blot analysis (Fig. 2) lent further support to this interpretation.
Coupled F1Fo activity.
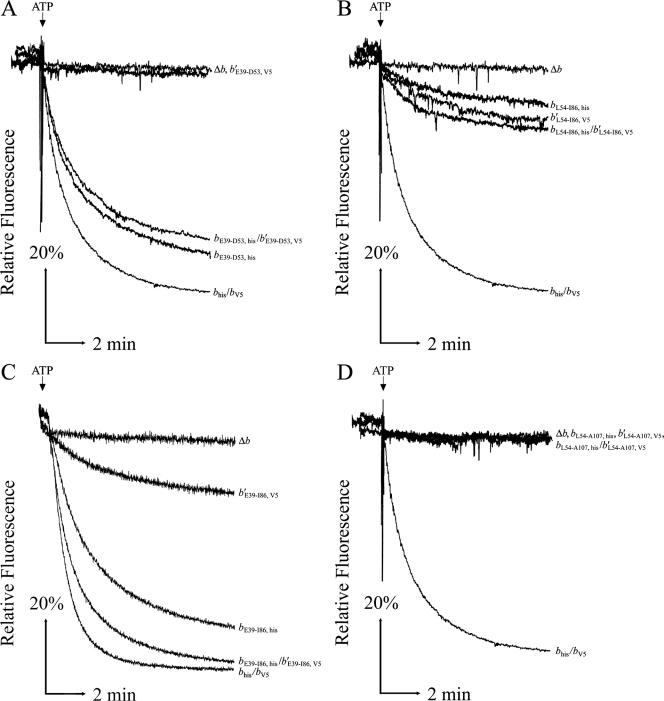
ATP-driven proton pumping in membrane vesicles was used to detect coupled activity in F1Fo complexes with chimeric peripheral stalks (Fig. 3). Acidification of inverted membrane vesicles was monitored by fluorescence quenching of ACMA. All membrane samples had strong ACMA quenching upon addition of NADH, confirming that the membrane vesicles were intact and closed (data not shown). KM2/pSBC57 (b′E39-D53, V5) membranes showed no proton pumping activity, but strong fluorescence quenching was seen in KM2/pSBC56 (bE39-D53, his) membranes (Fig. 3A). KM2/pSBC58 (bL54-I86, his) and KM2/pSBC76 (b′L54-I86, V5) membranes showed low but detectable levels of ATP-driven proton pumping (Fig. 3B). No activity was observed in membranes prepared from either KM2/pSBC94 (bL54-A107, his) or KM2/pSBC79 (b′L54-A107, V5) (Fig. 3D). Membranes from KM2/pSBC95 (bE39-A107, his) showed limited fluorescence quenching under our most sensitive assay conditions (data not shown). The most interesting results were obtained by looking at the E39-to-I86 chimeric subunits (Fig. 3C). Coexpression of the chimeric bE39-I85, his and b′E39-I86, V5 subunits reproducibly yielded higher proton pumping activity than expression of either of the individual subunits alone. Moreover, the initial rate of fluorescence quenching was substantially higher in the KM2/pSBC97/pSBC98 (bE39-I86, his/b′E39-I86, V5) membranes (Table 4). These results might have reflected either the additive activity from the (bE39-I86, his)2 and (b′E39-I86, V5)2 homodimeric complexes or, more likely, the presence of F1Fo complexes containing bE39-I86, his/b′E39-I86, V5 heterodimeric complexes.
FIG. 3.
ATP-driven energization of membrane vesicles prepared from KM2 (Δb) cells expressing chimeric b subunits. Aliquots of membrane proteins (500 μg) were suspended in 3.5 ml assay buffer (50 mM MOPS [pH 7.3], 10 mM MgCl2), and fluorescence quenching of ACMA was used to detect energization of membrane vesicles after the addition of ATP as previously described (33). Traces are plotted as relative fluorescence over time. The point where ATP was added is indicated above each graph, and chimeric subunits are given on the right. Each panel shows traces of membranes from the negative control KM2/pBR322 (Δb), the positive control KM2/pTAM37/pTAM46 (bhis/bV5), and the b and b′ chimeric subunits expressed individually and together. The chimeric subunits in the panels are as follows: E39 to D53 (A), L54 to I86 (B), E39 to I86 (C), and L54 to A107 (D). Traces were obtained using a Perkin-Elmer (A, B, and D) or Photon Technologies International (C) spectrofluorometer.
TABLE 4.
Proton pumping rates of membranes prepared from cells expressing chimeric E39-to-I86 subunits
| Strain/plasmid(s) | Gene product(s) | Final fluorescence (% of initial fluorescence) | Initial rate of decrease (%/min) |
|---|---|---|---|
| KM2/pTAM37/pTAM46 | bhis/bV5 | 22.2 ± 0.1 | 66.9 ± 0.6 |
| KM2/pBR322 | Δb | 91.7 ± 0.5 | 0.5 ± 0.2 |
| KM2/pSBC97 | bE39-I86, his | 34.5 ± 0.8 | 27 ± 2 |
| KM2/pSBC98 | b′E39-I86, V5 | 76.0 ± 0.4 | 6.1 ± 1.1 |
| KM2/pSBC97/pSBC98 | bE39-I86, his/ b′E39-I86, V5 | 28.0 ± 0.5 | 48 ± 3 |
Detection of heterodimers.
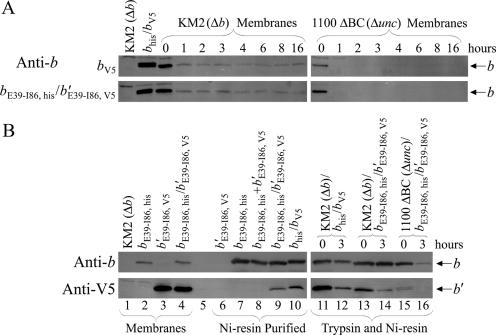
In order to detect F1Fo complexes with heterodimeric peripheral stalks, a nickel resin purification procedure developed previously in our lab (15) was used to examine membranes prepared from KM2/pSBC97/pSBC98 (bE39-I86, his/b′E39-I86, V5) (Fig. 4B, lanes 1 to 10). Four important controls were included in the experiment. To confirm that the resin was retaining only F1Fo complexes containing the chimeric bE39-I86, his subunit, membranes prepared from KM2/pSBC98 (b′E39-I86, V5) were processed, and no bands were observed using either the anti-b or the anti-V5 antibody (Fig. 4B, lane 6). F1Fo complexes from KM2/pSBC97 (bE39-I86, his) were purified as a positive control to demonstrate that the resin retained histidine-tagged complexes and the anti-V5 antibody detected nothing in samples lacking a V5 epitope tag (lane 7). To address possible aggregation of F1Fo complexes during sample preparation, membranes from strains KM2/pSBC97 (bE39-I86, his) and KM2/pSBC98 (b′E39-I86, V5) were mixed prior to solubilization. As expected, no band was observed using the anti-V5 antibody (lane 8). The positive control was nickel resin-purified F1Fo complexes from KM2/pTAM37/pTAM46 (bhis/bV5). The presence of complexes containing heterodimeric bhis/bV5 peripheral stalks was demonstrated by signals from both the anti-b and anti-V5 antibodies (lane 10). An essentially identical result was obtained using membranes from KM2/pSBC97/pSBC98 (bE39-I86, his/b′E39-I86, V5) (lane 9). The b′E39-I86, V5 subunit could have been retained in the nickel resin-purified material only if it were part of an F1Fo complex containing a bE39-I86, his subunit.
FIG. 4.
Incorporation of heterodimeric peripheral stalks into F1Fo ATP synthase complexes. (A) Trypsin digestion of either pTAM46 (bV5) or pSBC97/pSBC98 (bE39-I86, his/b′E39-I86, V5) expressed in either KM2 (Δb) or 1100 ΔBC (Δunc) cells. The strains and expressed subunits are given on the figure. Proteolytic digestion and collection of aliquots were performed as described in Materials and Methods. Western blotting was performed, and blots were probed with an antibody against the b subunit as for Fig. 2. (B) Aliquots of membrane samples were solubilized and purified over Ni-CAM as previously described (15). Ten percent of the total eluant from the nickel resin purification was loaded in each lane of purified protein. Western blotting was performed with antibodies against both the b subunit and the V5 epitope tag. Membranes were loaded into lanes as follows: lanes 1 to 4, 1 μg of nonpurified membranes from KM2/pBR322 (Δb), KM2/pSBC97 (bE39-I86, his), KM2/pSBC98 (b′E39-I86, V5), and KM2/pSBC97/pSBC98 (bE39-I86, his/b′E39-I86, V5), respectively; lane 5, intentionally left empty; lane 6, nickel resin negative control KM2/pSBC98 (b′E39-I86, V5); lane 7, nickel resin positive control KM2/pSBC97 (bE39-I86, his); lane 8, nickel resin aggregation control KM2/pSBC97 (bE39-I86, his) plus KM2/pSBC98 (b′E39-I86, V5); lane 9, purification of KM2/pSBC97/pSBC98 (bE39-I86, his/b′E39-I86, V5); lane 10, purification of heterodimer positive control KM2/pTAM37/pTAM46 (bhis/bV5); lanes 11 and 12, purification of trypsin-digested KM2/pTAM37/pTAM46 (bhis/bV5) at 0 and 3 h, respectively; lanes 13 and 14, purification of trypsin-digested KM2/pSBC97/pSBC98 (bE39-I86, his/b′E39-I86, V5) at 0 and 3 h, respectively; lanes 15 and 16, purification of trypsin-digested 1100 ΔBC/pSBC97/pSBC98 (Δunc/bE39-I86, his/b′E39-I86, V5) at 0 and 3 h, respectively.
Trypsin digestion of membranes samples was used to confirm that the heterodimeric peripheral stalks were incorporated into intact F1Fo complexes. A portion of the wild-type bV5 subunit remained resistant to degradation during an extended overdigestion with trypsin when expressed in the presence of the other F1Fo ATP synthase genes (Fig. 4A, KM2 digestion). The same subunit was degraded when expressed alone in strain 1100 ΔBC (Δunc), demonstrating that the incorporation of the b subunit into an intact F1Fo complex protects the peripheral stalk from trypsin digestion. This result is consistent with what has been observed previously (34). Likewise, chimeric pSBC97/pSBC98 (bE39-I86, his/b′E39-I86, V5) subunits were partially protected by incorporation into F1Fo complexes. A very small amount of bV5 subunit was detected in membranes exposed to trypsin in the absence of the rest of the complex using the higher-sensitivity antibody against the V5 epitope tag (data not shown). Nickel resin purification of samples digested with trypsin demonstrated that the heterodimeric peripheral stalks were incorporated into intact F1Fo complexes (Fig. 4B, lanes 11 to 16). A band was seen using the anti-V5 antibody after 3 h of trypsin digestion for both the positive control KM2/pTAM37/pTAM46 (bhis/bV5) and the chimeric KM2/pSBC97/pSBC98 (bE39-I86, his/b′E39-I86, V5) sample but not for the 1100 ΔBC/pSBC97/pSBC98 (bE39-I86, his/b′E39-I86, V5) sample. The b′E39-I86, V5 subunit survived trypsin digestion and nickel resin purification as a component of a heterodimeric F1Fo complex containing the bE39-I86, his subunit.
DISCUSSION
A series of plasmids have been constructed that were designed to express chimeric subunits such that portions of the tether and dimerization domains of the E. coli F1Fo ATP synthase b subunit were replaced with homologous sequences from the b and b′ subunits of the thermophilic cyanobacterium T. elongatus. The chimeric subunits with the largest successful T. elongatus segments had replacements spanning positions E39 to I86. The chimeric bE39-I86 and b′E39-I86 subunits each contained a total of 48 replaced amino acids, or almost one-third of the entire b subunit. Plasmids expressing either recombinant chimeric subunit were individually capable of genetic complementation of an uncF (b subunit) deletion strain. In addition to the homodimeric stalks formed by the expression of each subunit alone, coexpression of the bE39-I86 and b′E39-I86 subunits yielded readily detectable F1Fo complexes with heterodimeric peripheral stalks. Attempts to extend the T. elongatus segment further toward the carboxyl terminus resulted in failure. The most logical interpretation was that critical interactions between the peripheral stalk and F1 known to occur in this area of the b subunit (18, 20) were interrupted, leading to a general defect in assembly of the F1Fo complex.
The secondary structure of the b subunit throughout the area under study here is thought to be an extended, linear α-helix (35). This has been confirmed by X-ray crystallography for the section covering amino acids 66 to 122 (6). In view of the properties of tether domain insertion and deletion mutants (15, 32, 34), one might have expected that any α-helical sequence substituted in the tether domain would be sufficient if the protein-protein contacts within Fo and F1 were maintained. However, the b′E39-D53, V5 chimeric subunit was fully defective. Extension of the chimeric region in the b′E39-I86, V5 subunit resulted in formation of an active F1Fo ATP synthase. Therefore, the structural defect induced by the substitutions in the b′E39-D53 region was dramatically suppressed within the b′E39-I86 subunit. The phenotype of the defective bL54-I86, his subunit was suppressed to a lesser degree in the bE39-I86, his subunit. The evidence suggests that there must be determinants throughout the E39-to-I86 section of the b subunit that act together to specify the structure of the peripheral stalk. The obvious interpretation is that multiple protein-protein interactions between the two b subunits likely provide these structural determinants. There is ample evidence for direct contacts within the dimerization domain (28, 29). To date, there is no evidence of intimate interactions between the two b subunits within the tether domain between positions K23 and D53.
F1Fo complexes containing chimeric b′ subunits were much less stable in vitro than complexes with chimeric b subunits. Within the E39-to-I86 segment there are 14 amino acids in the E. coli and T. elongatus b subunits that are different in subunit b′ (Fig. 1). While it is possible that these specific amino acids have an important influence on F1Fo complex stability, it seems more likely that the overall structure of the b′ subunit does not necessarily favor maintenance of the intersubunit contacts needed for a stable enzyme in aqueous buffers. This view is supported by the observation that the levels of b′E39-D53, b′L54-I86, and b′E39-I86 were all increased by coexpression with the cognate b subunit (Fig. 2). Therefore, the formation of chimeric b/b′ heterodimeric F1Fo complexes seems to stabilize the chimeric b′ subunits in vitro.
Acknowledgments
This work was supported by Public Health Service grant GM70978.
We thank Mac O'Neil Plancher for participation in the study.
Footnotes
Published ahead of print on 25 May 2007.
REFERENCES
- 1.Bhatt, D., S. P. Cole, T. B. Grabar, S. B. Claggett, and B. D. Cain. 2005. Manipulating the length of the b subunit F1 binding domain in F1Fo ATP synthase from Escherichia coli. J. Bioenerg. Biomembr. 37:67-74. [DOI] [PubMed] [Google Scholar]
- 2.Cain, B. 2000. Mutagenic analysis of the Fo stator subunits. J. Bioenerg. Biomembr. 32:365-371. [DOI] [PubMed] [Google Scholar]
- 3.Capaldi, R. A., and R. Aggeler. 2002. Mechanism of the F1Fo-type ATP synthase, a biological rotary motor. Trends Biochem. Sci. 27:154-160. [DOI] [PubMed] [Google Scholar]
- 4.Caviston, T. L., C. J. Ketchum, P. L. Sorgen, R. K. Nakamoto, and B. D. Cain. 1998. Identification of an uncoupling mutation affecting the b subunit of F1Fo ATP synthase in Escherichia coli. FEBS Lett. 429:201-206. [DOI] [PubMed] [Google Scholar]
- 5.Del Rizzo, P. A., Y. Bi, and S. D. Dunn. 2006. ATP synthase b subunit dimerization domain: a right-handed coiled coil with offset helices. J. Mol. Biol. 364:735-746. [DOI] [PubMed] [Google Scholar]
- 6.Del Rizzo, P. A., Y. Bi, S. D. Dunn, and B. H. Shilton. 2002. The “second stalk” of Escherichia coli ATP synthase: structure of the isolated dimerization domain. Biochemistry 41:6875-6884. [DOI] [PubMed] [Google Scholar]
- 7.Dickson, V. K., J. A. Silvester, I. M. Fearnley, A. G. W. Leslie, and J. E. Walker. 2006. On the structure of the stator of the mitochondrial ATP synthase. EMBO J. 25:2911-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn, S. D., and J. Chandler. 1998. Characterization of the b2δ complex from Escherichia coli ATP synthase. J. Biol. Chem. 273:8646-8651. [DOI] [PubMed] [Google Scholar]
- 9.Dunn, S. D., D. T. McLachlin, and M. Revington. 2000. The second stalk of Escherichia coli ATP synthase. Biochim. Biophys. Acta 1458:356-363. [DOI] [PubMed] [Google Scholar]
- 10.Dunn, S. D., E. Kellner, and H. Lill. 2001. Specific heterodimer formation by the cytoplasmic domains of the b and b′ subunits of cyanobacterial ATP synthase. Biochemistry 40:187-192. [DOI] [PubMed] [Google Scholar]
- 11.Dunn, S. D., R. G. Tozer, and V. D. Zadorozny. 1990. Activation of Escherichia coli F1-ATPase by lauryldimethylamine oxide and ethylene glycol: relationship of ATPase activity to the interaction of the ɛ and β subunit. Biochemistry 29:4335-4340. [DOI] [PubMed] [Google Scholar]
- 12.Galkin, M. A., R. R. Ishmukhametov, and S. B. Vik. 2006. A functionally inactive, cold-stabilized form of the Escherichia coli F1Fo ATP synthase. Biochim. Biophys. Acta 1757:206-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner, J. L., and B. D. Cain. 1999. Amino acid substitutions in the a subunit affect the ɛ subunit of F1Fo ATP synthase from Escherichia coli. Arch. Biochem. Biophys. 361:302-308. [DOI] [PubMed] [Google Scholar]
- 14.Grabar, T. B., and B. D. Cain. 2004. Genetic complementation between mutant b subunits in F1Fo ATP synthase. J. Biol. Chem. 279:31205-31211. [DOI] [PubMed] [Google Scholar]
- 15.Grabar, T. B., and B. D. Cain. 2003. Integration of b subunits of unequal length into F1Fo-ATP synthase. J. Biol. Chem. 278:34751-34756. [DOI] [PubMed] [Google Scholar]
- 16.Hardy, A. W., T. B. Grabar, D. Bhatt, and B. D. Cain. 2003. Mutagenesis studies of the F1Fo ATP synthase b subunit membrane domain. J. Bioenerg. Biomembr. 35:389-397. [DOI] [PubMed] [Google Scholar]
- 17.McCormick, K. A., and B. D. Cain. 1991. Targeted mutagenesis of the b subunit of F1Fo ATP synthase in Escherichia coli: Glu-77 through Gln-85. J. Bacteriol. 173:7240-7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLachlin, D. T., A. M. Coveny, S. M. Clark, and S. D. Dunn. 2000. Site-directed cross-linking of the b to the α, β, and a subunits of the Escherichia coli ATP synthase. J. Biol. Chem. 275:17571-17577. [DOI] [PubMed] [Google Scholar]
- 19.McLachlin, D. T., J. A. Bestard, and S. D. Dunn. 1998. The b and δ subunit of the Escherichia coli ATP synthase interact via residues in their C-terminal regions. J. Biol. Chem. 273:15162-15168. [DOI] [PubMed] [Google Scholar]
- 20.Motz, C., T. Hornung, M. Kersten, D. T. McLachlin, S. D. Dunn, J. G. Wise, and P. D. Vogel. 2004. The subunit b dimer of the FoF1-ATP synthase: interaction with F1-ATPase as deduced by site-specific spin-labeling. J. Biol. Chem. 279:49074-49081. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura, Y., T. Kaneko, S. Sato, M. Ikeuchi, H. Katoh, S. Sasamoto, A. Watanabe, M. Iriguchi, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2002. Complete genome structure of the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1. DNA Res. 9:123-130. [DOI] [PubMed] [Google Scholar]
- 22.Noji, H., and M. Yoshida. 2001. The rotary machine in the cell, ATP synthase. J. Biol. Chem. 276:1665-1668. [DOI] [PubMed] [Google Scholar]
- 23.Okajima, K., S. Yoshihara, Y. Fukushima, X. Geng, M. Katayama, S. Higashi, M. Watanabe, S. Sato, S. Tabata, Y. Shibata, S. Itoh, and M. Ikeuchi. 2005. Biochemical and functional characterization of BLUF-type flavin-binding proteins of two species of cyanobacteria. J. Biochem. 137:741-750. [DOI] [PubMed] [Google Scholar]
- 24.Pattanayek, R., D. R. Williams, S. Pattanayek, Y. Xu, T. Mori, C. H. Johnson, P. L. Stewart, and M. Egli. 2006. Analysis of KaiA-KaiC protein interactions in the cyanobacterial circadian clock using hybrid structural methods. EMBO J. 25:2017-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng, G., M. Bostina, M. Radermacher, I. Rais, M. Karas, and H. Michel. 2006. Biochemical and electron microscopic characterization of the F1Fo ATP synthase from the hyperthermophilic eubacterium Aquifex aeolicus. FEBS Lett. 580:5934-5940. [DOI] [PubMed] [Google Scholar]
- 26.Revington, M., D. T. McLachlin, G. S. Shaw, and S. D. Dunn. 1999. The dimerization domain of the b subunit of the Escherichia coli F1Fo-ATPase. J. Biol. Chem. 274:31094-31101. [DOI] [PubMed] [Google Scholar]
- 27.Richter, M. L., R. Hein, and B. Huchzermeyer. 2000. Important subunit interactions in the chloroplast ATP synthase. Biochim. Biophys. Acta 1458:326-342. [DOI] [PubMed] [Google Scholar]
- 28.Rodgers, A. J. W., and R. A. Capaldi. 1998. The second stalk composed of the b- and δ-subunits connects Fo to F1 via an α-subunit in the Escherichia coli ATP synthase. J. Biol. Chem. 273:29406-29410. [DOI] [PubMed] [Google Scholar]
- 29.Rodgers, A. J. W., S. Wilkens, R. Aggeler, M. B. Morris, S. M. Howitt, and R. A. Capaldi. 1997. The subunit δ-subunit b domain of Escherichia coli F1Fo ATPase. J. Biol. Chem. 272:31058-31064. [DOI] [PubMed] [Google Scholar]
- 30.Senior, A. E., S. Nadanaciva, and J. Weber. 2002. The molecular mechanism of ATP synthesis by F1Fo-ATP synthase. Biochim. Biophys. Acta 1553:188-211. [DOI] [PubMed] [Google Scholar]
- 31.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 32.Sorgen, P. L., M. R. Bubb, and B. D. Cain. 1999. Lengthening the second stalk of F1Fo ATP synthase in Escherichia coli. J. Biol. Chem. 274:36261-36266. [DOI] [PubMed] [Google Scholar]
- 33.Sorgen, P. L., M. R. Bubb, K. A. McCormick, A. S. Edison, and B. D. Cain. 1998. Formation of the b subunit dimer is necessary for interaction with F1-ATPase. Biochemistry 37:923-932. [DOI] [PubMed] [Google Scholar]
- 34.Sorgen, P. L., T. L. Caviston, R. C. Perry, and B. D. Cain. 1998. Deletions in the second stalk of F1Fo-ATP synthase in Escherichia coli. J. Biol. Chem. 273:27873-27878. [DOI] [PubMed] [Google Scholar]
- 35.Weber, J. 2006. ATP synthase: subunit-subunit interactions in the stator stalk. Biochim. Biophys. Acta 1757:1162-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]