Abstract
Lactococcus lactis, a facultative anaerobic lactic acid bacterium, is known to have an increased growth yield when grown aerobically in the presence of heme. We have now established the presence of a functional, proton motive force-generating electron transfer chain (ETC) in L. lactis under these conditions. Proton motive force generation in whole cells was measured using a fluorescent probe (3′,3′-dipropylthiadicarbocyanine), which is sensitive to changes in membrane potential (Δψ). Wild-type cells, grown aerobically in the presence of heme, generated a Δψ even in the presence of the F1-Fo ATPase inhibitor N,N′-dicyclohexylcarbodiimide, while a cytochrome bd-negative mutant strain (CydAΔ) did not. We also observed high oxygen consumption rates by membrane vesicles prepared from heme-grown cells, compared to CydAΔ cells, upon the addition of NADH. This demonstrates that NADH is an electron donor for the L. lactis ETC and demonstrates the presence of a membrane-bound NADH-dehydrogenase. Furthermore, we show that the functional respiratory chain is present throughout the exponential and late phases of growth.
Lactococcus lactis has a long history of use in the production of fermented dairy products, such as cheese and buttermilk, under mainly anaerobic conditions. Studies on the aerobic growth of L. lactis have therefore been focused mainly on the effect of oxygen on fermentation patterns (25) or cell damage due to the formation of reactive oxygen species (3, 8, 32).
These damaging effects of oxygen on L. lactis cells are not observed when cells are grown in the presence of both oxygen and a heme source (9, 30, 45). Aerated, heme-grown L. lactis cells display new characteristics such as increased growth yield, resistance to oxidative and acid stress, and improved long-term survival when stored at low temperatures (40). These traits are important for industrial applications, and the use of heme to increase the efficiency of biomass production of starter cultures has been described previously (10, 13, 37). The increased growth efficiency of aerated heme-grown L. lactis cells is due to a shift from homolactic to mixed-acid fermentation, more complete glucose utilization in non-pH-controlled batch cultures, and possibly energy generation by NADH oxidation via the electron transfer chain (ETC) (9). The ability to generate metabolic energy via NADH oxidation by the ETC will be the subject of this work. Increased growth efficiency will make L. lactis more useful as a cell factory for the production of biomass-related compounds such as proteins and vitamins.
Heme is an essential cofactor of cytochrome complexes in the electron transport chains of respiring cells (14, 52). Furthermore, the genomes of several L. lactis strains contain genes which, when expressed, could form a simple ETC if supplied with heme (13). Genes encoding menaquinone biosynthesis enzymes and a bd-type cytochrome (mena)quinoloxidase have, for example, been identified in the genomes of strains IL-1403 and SK11 (http://genome.ornl.gov/microbial/lcre/) (6). The (mena)quinoloxidase is a membrane-bound enzyme consisting of two subunits, which are encoded by cydA and cydB. The cydC and cydD genes encode an ABC transporter, which is required for the assembly of the oxidase (7). This type of cytochrome-containing enzyme is found in a variety of (facultative) aerobic bacteria (16), where it functions as a (alternative) terminal electron acceptor capable of working under low-oxygen conditions (17, 43).
The higher growth yield in the presence of heme and the presence of ETC-related genes in the genome suggest active respiration in aerated heme-grown cells of L. lactis (5). To prove that actual respiration occurs, the formation of a proton motive force (PMF) as a result of ETC activity still needs to be demonstrated. In this paper, we present genetic and physiological evidence for cytochrome bd-associated PMF formation and thus the presence of a functional ETC in L. lactis.
MATERIALS AND METHODS
Cultures and growth conditions.
The strains used in these studies were Lactococcus lactis MG1363 (11) or derivatives of this strain: a cytochrome-negative mutant (CydAΔ Cmr) and a cytochrome negative mutant complemented with plasmid pIL253CydABCD. Plasmid pIL253CydABCD is a pIL253 derivative (46) carrying the cydABCD genes (Cmr Eryr). Cells were grown on M17 medium (Difco, Detroit, MI) supplemented with glucose (GM17) to a final concentration of 1% (wt/vol). When indicated, cells were grown in GM17 medium supplemented with heme (hemin) (stock solution, 0.5 mg/ml in 0.05 M NaOH; Sigma) to a final concentration of 2 μg/ml or with the equivalent volume of 0.05 M NaOH as a control. When indicated, chloramphenicol and/or erythromycin was added to a final concentration of 10 μg/ml. Cultures were grown aerobically in 100-ml flasks with shaking at 250 rpm or anaerobically in tubes/glass bottles at 30°C.
Annotation of L. lactis MG1363 genes.
Sequence data for L. lactis MG1363 were obtained from the L. lactis MG1363 sequencing consortium. DNA sequences of open reading frames (ORFs) were translated into amino acid sequences and annotated by homology using the BLAST algorithm as described previously (1). Prediction of membrane-spanning helices was performed as described previously (53).
Mutant construction.
Molecular cloning techniques were carried out in accordance with standard laboratory procedures (44). For the construction of the knockout plasmid, primers were designed on the basis of MG1363 genome sequence data. A 1-kb fragment upstream of cydA (forward primer p39 [GATCGTCAACCATCAACCAT] and reverse primer p40 [GGTTAGCATTGTTTATCTCC]) and a 1-kb fragment downstream of cydA (forward primer p41 [GTGGATGAATAATGACTGGA] and reverse primer p42 [CCAGCGATAGCAATAAACTG]) were amplified using PCR. The flanking fragments were cloned blunt ended into vector pNZ5317 (23) digested with SwaI (upstream fragment) and Ecl36II (downstream fragment) to produce the knockout vector pRB6671_CydA_KO. The knockout plasmid was transformed into L. lactis MG1363, and a chloramphenicol replacement of the cydA gene was obtained by a double-crossover event by homologous recombination as described previously (22), which resulted in mutant strain CydAΔ. For complementation studies of the cytochrome-negative mutant (CydAΔ), a vector carrying the cyd operon (cydABCD) was constructed. The operon was amplified using PCR techniques (forward primer P43 [TGACGCATGCGAGGCCTCAAGAAAGCACTT] and reverse primer P44 [TGACGAGCTCCGTAGACGAGTAACGCATCT]) using the genome of MG1363 as a template. The primer tails (underlined) carried recognition sequences for restriction enzymes SphI and SacI for easy cloning. The PCR product and vector pIL253 were digested with SphI and SacI, purified from gel, and cloned sticky blunt to construct pIL253_CydABCD. Finally, to complement the cytochrome-negative mutant, this plasmid was transformed into the CydAΔ strain.
Isolation of membrane vesicles.
Cells from a 2-liter culture were grown aerobically to late exponential phase (optical density [OD] of about 2.5 to 3.0), washed twice in 100 mM potassium phosphate (pH 7.0), and resuspended in 20 ml of the same buffer. The cell suspension was incubated with 10 mg/ml egg lysozyme (Merck, Darmstadt, Germany) for 30 min at 30°C. Cell lysis was achieved by passage two times through a French pressure cell (American Instrument Corp., Silver Spring, MD) at an operating pressure of 20,000 lb/in2. The orientation of bacterial membrane vesicles prepared by French press is predominantly inside-out (2, 27). The suspension was supplemented with 10 mM MgSO4 and 100 μg/ml DNase and incubated for 15 min at 30°C, followed by the addition of 15 mM K-EDTA. A low spin at 12,000 rpm was performed to remove cell debris and whole cells. The vesicle-containing supernatant was centrifuged at 150,000 × g to harvest the membranes, which were resuspended in 50 mM potassium phosphate (pH 7.0) containing 10% glycerol to a final concentration of 10 to 20 μg/ml, divided into 500-μl aliquots, and stored at −80°C.
Extrusion of membrane vesicles.
To obtain single unilamellar vesicles suitable for comparing enzyme activities, 500 μl of membrane suspension was thawed and diluted with 500 μl of 50 mM potassium phosphate (pH 5.5). The 1-ml mixture was extruded using a Miniextruder (Avanti Polar Lipids Inc., Alabaster, United Kingdom) with a 0.4-μm-size nucleopore polycarbonate track etch membrane (Whatman International Ltd., Kent, United Kingdom) to generate inside-out, single laminar vesicles with an average size of 0.4 μm (50).
Measurements of membrane potential.
The fluorescent probe 3′,3′-dipropylthiadicarbocyanine [DiSC3(5)] was used to monitor the membrane potential (ΔΨ) in intact cells (51). The distribution of the probe over the cytoplasmic membrane and the soluble phase is sensitive to changes in the ΔΨ. More probe molecules from the soluble phase will dissolve in the membrane with increasing ΔΨ, causing the quenching of the fluorescence signal by aggregation (20, 48). Nigericin (K+/H+ exchange) was added to convert ΔpH into ΔΨ, making it possible to estimate the contribution of the pH gradient to the PMF. Valinomycin (K+ ionophore) was added, in combination with nigericin, to cause a total dissipation of the PMF. The fluorescence was measured with a Cary Eclipse fluorescence spectrophotometer combined with a Cary Single Cell Peltier accessory (Varian, Palo Alto, CA) or an SPF-500C spectrofluorometer (SLM Aminco). The fluorescence was measured at an emission wavelength of 666 nm with an excitation wavelength of 643 nm (both with a 5-nm band pass).
Wild-type and CydAΔ cells were supplemented with heme and grown aerobically. During growth, samples of cells were harvested at early exponential phase (OD at 600 nm [OD600] of 0.4 to 0.48), early/mid-exponential phase (OD600 of 1.04 to 1.08) and mid-exponential phase (OD600 of 1.49 to 1.53) and after overnight growth (OD600 of 4.49 for the wild type and 2.6 for CydAΔ). Cell samples were washed twice in 50 mM KPi (pH 5.0) and resuspended in the same buffer to an OD600 of 5.0. Subsequently, samples were diluted to an OD600 of 0.3, and N,N′-dicyclohexylcarbodiimide (DCCD; Sigma-Aldrich) (stock, 1 M in ethanol) was added to a final concentration of 1 mM when indicated, or, as a control, the equivalent volume of ethanol was added. DCCD is a well-known inhibitor of the F1-Fo ATPase and prevents PMF formation by the hydrolysis of ATP (15, 47). The samples were incubated for 45 min on ice in the presence of DCCD or ethanol. After incubation, 2 ml of fresh buffer was added to the 1-ml samples to optimize the cell density for measuring fluorescence, after which the samples were transferred into 3-ml cuvettes. Finally, DiSC3(5) was added to a final concentration of 133 nM. Cuvettes containing this mixture were warmed at room temperature for 3 min prior to measuring; 15 mM glucose, 0.1 μM nigericin, or 2 μM valinomycin was added when indicated.
Oxygen uptake measurements.
A biological oxygen monitor (model 5300; YSI Scientific, OH) with a Clark-type polarographic oxygen probe and a 15-ml sample chamber was used to measure dissolved oxygen. To measure oxygen consumption, cells were washed twice in 50 mM potassium phosphate (pH 5.0), resuspended in the same buffer to an OD600 of 5.0, and placed on ice. Prior to each measurement, the buffer was heated to 30°C, and the electrode was allowed to equilibrate for 10 min. At time zero, cells were added to a final concentration of an OD600 of 0.2. After 5 min, 13 mM glucose was added. The dissolved oxygen of air-saturated buffer was calibrated using air-saturated water. To measure oxygen consumption by membrane vesicles, 1 ml of membrane vesicle mixture was added to 10 ml 50 mM potassium phosphate (pH 5.0). After 5 min, either 5 mM NADH or NAD+ was added (both obtained from Sigma-Aldrich).
Other analytical procedures.
Protein concentrations of membrane preparations were determined using the bicinchoninic acid protein assay reagent (Omnilabo Int., Breda, The Netherlands) (49).
RESULTS
In silico evidence for an ETC in L. lactis MG1363.
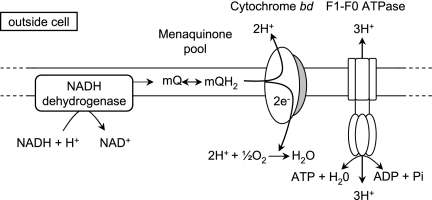
An in silico analysis was performed on the genome of L. lactis MG1363 to identify possible components of an electron transfer chain. Two type II NADH dehydrogenases, encoded by noxA and noxB (Tables 1 and 2), were predicted on the basis of the L. lactis MG1363 genome sequence (13). Both genes are characterized as having flavin adenine dinucleotide binding motifs but differ in the numbers of predicted membrane-spanning segments (four in NoxB and one in NoxA). Although the operon-like structure of noxA and noxB on the genome suggests coregulation (28), these type II NADH dehydrogenases are always observed to function as separate, individual polypeptides. The genes for the biosynthesis of riboflavin, the precursor of flavin adenine dinucleotide, can also be found, and the biosynthesis of this vitamin by L. lactis has been experimentally verified in L. lactis MG1363 (6). Menaquinone production has been observed in many L. lactis strains (33), and menaquinone biosynthesis genes (menFDXBEC and preA-menA) were identified. Genes encoding a (mena)quinoloxidase have also been identified in the L. lactis MG1363 genome (cydABCD), which show a high degree of similarity to the well-characterized Bacillus subtilis strain 168 cyd genes (55, 57, 58). This type of cytochrome (bd type) is not considered to be an actual proton pump, as scalar chemistry alone could account for the stoichiometry of 1H+/e−, during the reduction of oxygen to water (16, 31, 39). Thus, all the genetic elements needed to form a functional ETC are present in the genome of MG1363, with the exception of a complete heme biosynthesis pathway (Fig. 1).
TABLE 1.
Identification by homology searches of NADH-dehydrogenase, (mena)quinoloxidase, and menaquinone biosynthesis genes in the genome of L. lactis MG1363 compared to B. subtilis 168
| L. lactis MG1363 ORF | Homology to B. subtilis 168 | % DNA identity/ % aa identitya | Identity |
|---|---|---|---|
| llmg_1864 | cydA | 48/68 | Cytochrome d ubiquinol oxidase subunit I (EC 1.10.3.-) |
| llmg_1883 | cydB | 43/62 | Cytochrome d ubiquinol oxidase subunit II (EC 1.10.3.-) |
| llmg_1862 | cydC | 47/65 | ABC transporter component CydC |
| llmg_1861 | cydD | 44/64 | ABC transporter component CydD |
Predicted identity on the level of amino acid (aa) composition.
TABLE 2.
Identification by homology searches of NADH-dehydrogenase, (mena)quinoloxidase, and menaquinone biosynthesis genes in the genome of L. lactis MG1363 compared to L. lactis IL-1403
| L. lactis MG1363 ORF | Homology to L. lactis IL-1403 | % DNA identity/ % aa identity (%)a | Identity |
|---|---|---|---|
| llmg_0196 | preA | 95/98 | Farnesyl pyrophosphate synthetase (EC 2.5.1.1) |
| llmg_0197 | ybiG/menA | 88/95 | 1,4-Dihydroxy-2-naphthoate polyprenyltransferase (EC 2.5.1.-) |
| llmg_1828 | menF | 80/89 | Isochorismate synthase (EC 5.4.4.2) |
| llmg_1829 | menD | 85/91 | 2-Succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase (EC 2.5.1.64) |
| llmg_1830 | menX | 77/85 | YtxM-like protein/menaquinone biosynthesis-related protein |
| llmg_1831 | menB | 98/100 | Naphthoate synthase (EC 4.1.3.36) |
| llmg_1832 | menE | 85/93 | O-Succinylbenzoic acid coenzyme A ligase (EC 6.2.1.26) |
| llmg_1833 | yhdB/menC | 91/97 | O-Succinylbenzoate synthase (EC 4.2.1.-) |
| llmg_1735 | noxA | 99/99 | NADH dehydrogenase (EC 1.6.99.3) |
| llmg_1734 | noxB | 99/99 | NADH dehydrogenase (EC 1.6.99.3) |
Predicted identity on the level of amino acid (aa) composition.
FIG. 1.
Predicted aerobic ETC of heme-grown L. lactis MG1363. The three principal components of the ETC are the type II NADH dehydrogenase complex, the menaquinol/menaquinone couple, and the cytochrome bd complex. A PMF can be also formed by the hydrolysis of ATP via F1-Fo ATPase.
Growth improvement by heme supplementation.
Wild-type L. lactis cells, when grown aerobically and supplemented with heme, showed increased biomass and less acidification of their medium (Table 3), suggesting the presence of a functional ETC. We constructed an isogenic mutant of L. lactis MG1363 that lacks these characteristics by replacing the cydA gene with a chloramphenicol marker gene (CydAΔ). As anticipated, the increased biomass formation and higher pH observed with aerated heme-grown wild-type cells were absent in the CydAΔ strain, and the growth characteristics resembled those of non-heme-grown wild-type cells. Subsequent complementation of the CydAΔ mutant with a vector carrying the (mena)quinoloxidase coding operon (pIL253CydABCD) restored the wild-type-like phenotype when grown aerobically with heme.
TABLE 3.
Aerobic growth (OD600) and acidification of L. lactis with and without hemea
| Membrane preparation | OD600 | pH |
|---|---|---|
| Wild type + heme | 5.25 | 5.04 |
| Wild type | 2.60 | 4.34 |
| CydAΔ + heme | 2.57 | 4.33 |
| CydAΔ | 2.66 | 4.27 |
| CydAΔ + pIL253CydABCD + heme | 5.07 | 5.06 |
| CydAΔ + pIL253CydABCD | 2.62 | 4.30 |
L. lactis wild-type, CydAΔ, and CydAΔ cells, complemented with plasmid pIL253CydABCD (carrying the cydABCD operon), were grown aerobically overnight in M17G medium at 30°C in the presence or absence of heme.
Measurement of ΔΨ in whole cells of L. lactis.
The formation of a membrane potential by L. lactis, as a result of electron transport, was determined with the fluorescent probe DiSC3(5). The intensity of fluorescence of the probe is sensitive to changes in the ΔΨ, and it decreases with increasing ΔΨ and visa versa. The PMF is composed of ΔpH and ΔΨ. In order to estimate the contribution of a pH gradient to the PMF, we added nigericin (a K+/H+ exchanger) to convert the ΔpH into a ΔΨ. Furthermore, the addition of valinomycin (K+ ionophore) plus nigericin collapsed the ΔΨ completely. The main proton pump in L. lactis responsible for PMF generation is the F1-Fo ATPase, by pumping protons at the expense of metabolic ATP (26). To discriminate between PMF generation by the ETC and that by the F1-Fo ATPase, the F1-Fo ATPase-specific inhibitor DCCD was used (47). To further validate PMF formation via the ETC, we used a cytochrome bd-negative mutant (CydAΔ) as a control.
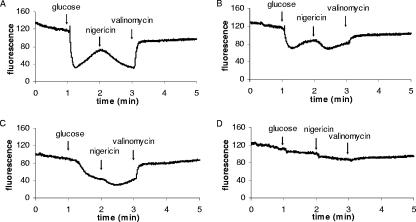
The changes in membrane potential, DiSC3(5) fluorescence, were recorded as a function of time (Fig. 2). In wild-type cells and CydAΔ cells with no DCCD treatment, the addition of glucose led to an increase in ΔΨ. However, for CydAΔ cells incubated with DCCD, this increase in ΔΨ was negligible. The increase in ΔΨ after the addition of glucose was transient, since the membrane potential is subsequently converted into a pH gradient (see also Discussion). Accordingly, the addition of nigericin resulted in an increase in ΔΨ. The gradual decrease of the ΔΨ, that is, upon the addition of nigericin to wild-type cells inhibited with DCCD and of CydAΔ cells (Fig. 2B and C), was caused by the excess of nigericin and could be prevented by using lower nigericin concentrations (data not shown). The subsequent addition of valinomycin dissipated the ΔΨ completely. The fluorescence measurements clearly show that the cytochrome-negative CydAΔ strain is unable to generate a ΔΨ when the F1-Fo ATPase is inhibited by DCCD. In contrast, wild-type heme-grown cells are able to build up ΔΨ even in the presence of DCCD. Taken together, these findings indicate the presence of a cytochrome bd-dependent mechanism of PMF generation in wild-type L. lactis cells.
FIG. 2.
Fluorescence traces of DiSC3(5) in whole cells of L. lactis showing the generation of a membrane potential. Cells were prepared as described in Materials and Methods. A decrease in fluorescence signifies an increase in membrane potential. At 1 min, glucose (15 mM) was added; at 2 min, nigericin (0.1 μM) was added; and at 3 min, valinomycin (2 μM) was added. (A) Wild-type cells. (B) Wild-type cell treated with DCCD. (C) CydAΔ cells. (D) CydAΔ cells treated with DCCD.
It has been suggested that respiration is induced in the late exponential/early stationary phases of growth when glucose becomes limiting or the pH drops below a certain threshold (9, 12, 54). To investigate this hypothesis, ETC activity measurements were performed by using cells harvested at different phases of growth, corresponding to early, mid-, and late exponential phase (OD600 of ∼0.5, ∼1.0, and ∼1.5, respectively) and the late stationary phase (cultures grown overnight). A clear buildup of ΔΨ was observed upon the addition of glucose to DCCD-treated and untreated wild-type cells and untreated CydAΔ cells but never in DCCD-treated CydAΔ cells, irrespective of the growth phase (Table 4). These results show that a functional ETC in wild-type cells is formed in all stages and not restricted to late exponential and stationary growth phases.
TABLE 4.
Relative drop in fluorescence of early-exponential-phase, mid-exponential-phase, and overnight (or late-stationary-phase) cultures of DCCD-treated and untreated wild-type and CydAΔ cells after glucose additiona
| Strain | OD600 (phase) | % Fluorescence drop
|
|
|---|---|---|---|
| −DCCD | +DCCD | ||
| Wild type | 0.48 (early) | 60.7 | 26.7 |
| CydAΔ | 0.4 (early) | 40.0 | 5.1 |
| Wild type | 1.08 (early/mid)b | 71.8 | 37.2 |
| CydAΔ | 1.04 (early/mid)b | 58.6 | 4.9 |
| Wild type | 1.53 (mid) | 38.0 | 22.1 |
| CydAΔ | 1.49 (mid) | 46.9 | 7.0 |
| Wild type | 4.49 (O/N)c | 46.9 | 63.1 |
| CydAΔ | 2.6 (O/N)c | 45.7 | 5.7 |
The percent fluorescence drop compares two fluorescence values in one trace at one time point with a maximal difference in value: the low trace value is the measured level of fluorescence after the addition of glucose; the high trace value was extrapolated from the fluorescence recording before the addition of glucose. Experimental conditions are described in Materials and Methods.
These fluorescence traces are described in detail in the legend of Fig. 2.
Cultures grown overnight (O/N) were grown for more than 20 h and considered (late) stationary-phase cells.
Rate of oxygen uptake by whole cells.
The terminal electron acceptor of aerobic ETC in L. lactis is oxygen through the activity of the oxygen-requiring cytochrome bd complex. This complex most likely oxidizes menaquinol and reduces oxygen to water. ETC activity should thus lead to increased oxygen consumption. For the measurements of oxygen uptake, aerobically grown, early-exponential-phase cells (OD600 of 0.5 to 0.58) were used, rather than stationary-phase cells, to eliminate possible differences in cell viability. Upon the addition of glucose, heme-supplemented wild-type cells showed a higher oxygen consumption rate (25.72 ± 2.76 nmol O2 depletion/min/mg [dry weight] at an OD of 0.50 to 0.58) than heme-supplemented CydAΔ cells (13.51 ± 0.02 nmol O2 depletion/min/mg [dry weight] at an OD of 0.50 to 0.58) or wild-type cells grown in the absence of heme (13.02 ± <0.01 nmol O2 depletion/min/mg [dry weight] at an OD of 0.50 to 0.58) (oxygen consumption rates for buffer with heme were not detected). These results confirm that respiration in L. lactis is not a growth-phase-dependent event but is present throughout aerated heme-supplemented growth.
NADH-dependent oxygen consumption by membrane vesicles.
The results with whole cells of L. lactis led to a model of a simple ETC, which uses NADH as an electron donor and oxygen as an electron acceptor (Fig. 1). To prove that NADH can indeed serve as an electron donor, oxygen consumption by membrane vesicles of aerobically grown wild-type cells (with and without heme) and CydAΔ cells (with heme) was measured (Table 5). Membrane vesicles prepared from heme-grown wild-type cells showed a greater-than-6.5-fold increase in oxygen consumption upon the addition of NADH compared to membrane vesicles from wild-type cells grown without heme or to membrane vesicles from heme-grown CydAΔ cells. The oxygen consumptions by the latter two were comparable. No oxygen consumption was observed when membrane vesicles of heme-grown wild-type cells were supplied with NAD+, demonstrating the need for the reduced form of NAD. Furthermore, NADH added to buffer without membrane vesicles led to only a limited amount of oxygen consumption (data not shown).
TABLE 5.
Oxygen consumption by membrane vesicles at 25°C after the addition of NADH or NADa
| Membrane preparation | Addition | mean μmol O2 consumption/min/mg protein ± SD |
|---|---|---|
| Wild type + heme | NADH | 42.18 ± 0.12 |
| Wild type | NADH | 6.62 ± 0.35 |
| CydAΔ + heme | NADH | 8.41 ± 1.32 |
| Wild type + heme | NAD | ND |
Membrane vesicles were prepared from the different cells as described in Materials and Methods. ND, no consumption of oxygen detected after the addition of NAD.
DISCUSSION
In this report, we have shown, for the first time, that aerobic electron transport in L. lactis MG1363 actually leads to the generation of a PMF. A cytochrome bd-negative mutant was constructed (CydAΔ) as a negative control lacking the respiratory phenotype. We used DCCD to inhibit the F1-Fo ATPase, the primary PMF-generating system in fermentative lactic acid bacteria (LAB) using ATP as the energy source (19). DCCD-treated heme-supplemented wild-type cells were still capable of generating a PMF, while in contrast, DCCD-treated heme-supplemented CydAΔ cells were not. These two observations demonstrate that heme-supplemented wild-type cells have an additional PMF-generating system besides the F1-Fo ATPase. This PMF-generating system requires a functional cytochrome bd complex and implies the presence of a functional ETC.
The slight decrease in fluorescence upon the addition of glucose to DCCD-treated CydAΔ cells is explained by the incomplete inhibition of the F1-Fo ATPase by DCCD (23). In addition to the F1-Fo ATPase and the ETC, the contribution of alternative mechanisms of PMF generation, if present at all, to the overall energy conservation in L. lactis seems minimal (24, 29, 34, 35). What is clearly seen in the fluorescence recordings of the heme-supplemented wild-type cells is that the addition of glucose leads to an initial rapid increase in ΔΨ and a subsequent conversion into a ΔpH. This conversion in ΔpH is deduced from the increase in ΔΨ upon the addition of nigericin. Although the membrane of a cell acts as a capacitor, its capacitance is low. Consequently, the extrusion of a few protons already leads to a large ΔΨ. To generate a ΔpH of a similar size, the cell needs to pump out far more protons, and this would lead to a very high ΔΨ. Therefore, mechanisms are present to increase the ΔpH at the expense of ΔΨ, i.e., through the electrogenic uptake of K+ ions, which allows more protons to be pumped out (4, 21, 36).
Respiring cells (aerated and heme supplemented) from an exponentially growing culture showed an increased oxygen consumption rate compared to similarly grown cells containing a disruption in the cytochrome genes or compared to non-heme-grown wild-type cells. These results confirm that the L. lactis ETC leads to the reduction of oxygen. An indication of the fact that respiration is not growth phase dependent is the observation that in heme-supplemented early-exponential-phase wild-type cells, respiration is already maximal. Additionally, we have observed that heme-grown wild-type cells incubated with DCCD can still form a clear PMF, irrespective of the growth phase from which they were harvested. Therefore, we can conclude that a fully functional ETC is present in heme-grown wild-type cells throughout growth and is not limited to the late exponential or stationary phase.
In this work and that of others (13), it has been proposed that NADH is an important electron donor for the ETC, which explains the observation of mixed acid fermentation under respiratory conditions. Membrane vesicles prepared from wild-type cells grown with heme showed a greater-than-6.5-fold increase in oxygen consumption compared to wild-type cells grown without heme and CydAΔ cells grown with heme. Furthermore, this oxygen consumption was dependent on the reduced form of NAD (NADH). We have thus clearly demonstrated that NADH is a likely electron donor for the ETC in L. lactis and that a membrane-bound NADH dehydrogenase is present.
When NADH is added to membrane vesicles of heme-grown CydAΔ or non-heme-grown wild-type cells, there is still some oxygen consumed. Roughly 10% of this oxygen consumption can be attributed to a direct chemical reaction of NADH with oxygen. The rest of the observed NADH-dependent oxygen consumption in the control experiments can be attributed to the NADH oxidases that are known to be present in L. lactis. Although these NADH oxidases do not contain any membrane-spanning helices, they can be (loosely) associated with the membrane fraction (http://genome.ornl.gov/microbial/lcre/). This could explain the NADH-dependent, membrane-associated oxygen consumption seen in the membrane fractions from non-heme-grown wild-type cells or heme-grown CydAΔ cells.
Since in the dairy environment, little or no heme or oxygen is present, respiration is not expected to contribute significantly to growth and metabolic conversion. Heme-dependent respiration is therefore most likely a trait that confers a significant selective advantage in the original habitat of L. lactis: the plant surface or phyllosphere. An intriguing question, then, is the origin of the heme source in the phyllosphere. This and other questions concerning the respiratory capacities of LAB remain and promise increased scientific insight and novel industrial applications.
The definition of L. Lactis as a facultative anaerobe seems not to be true in all situations (e.g., when heme and oxygen are present). In light of this study, a better definition of L. lactis would be a facultative aerobe. It is still largely unknown how many other LAB with a similar facultative aerobic metabolism exist. An extensive screening among the different species (pediococci, lactococci, and lactobacilli) is required to better define and characterize LAB as a group. Some information on the respiratory capabilities of a limited number of LAB, mostly streptococci and enterococci, can already be found in the literature (41, 42, 45, 56, 59, 60). Interestingly, analysis of the large 3.3-Mb genome of Lactobacillus plantarum WCFS revealed the presence of genes coding for a fumarate reductase and heme-dependent nitrate reductase complex, creating a branched ETC capable of oxygen and nitrate respiration (18, 38). This would point to more possibilities for electron transfer and energy conservation in Lactobacillus plantarum than in L. lactis. The future exploitation of the respiratory capacities of LAB could result in improved industrially important traits (higher biomass/gram carbon source, increased resistance to acid stress and oxygen stress, and increased survival rate when stored at low temperatures), making the organisms even more attractive as cell factories.
Acknowledgments
The gene sequences of L. lactis MG1363 were a kind gift of the L. lactis MG1363 sequencing consortium; we thank Oscar Kuipers for providing the genome sequence data. Furthermore, we acknowledge Jolanda Lambert and Michiel Kleerebezem for providing us with plasmid pNZ5317.
Footnotes
Published ahead of print on 11 May 2007.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ames, G. F., K. Nikaido, J. Groarke, and J. Petithory. 1989. Reconstitution of periplasmic transport in inside-out membrane vesicles. Energization by ATP. J. Biol. Chem. 264:3998-4002. [PubMed] [Google Scholar]
- 3.Anders, R. F., D. M. Hogg, and G. R. Jago. 1970. Formation of hydrogen peroxide by group N streptococci and its effect on their growth and metabolism. Appl. Microbiol. 19:608-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakker, E. P., and W. E. Mangerich. 1981. Interconversion of components of the bacterial proton motive force by electrogenic potassium transport. J. Bacteriol. 147:820-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blank, L. M., B. J. Koebmann, O. Michelsen, L. K. Nielsen, and P. R. Jensen. 2001. Hemin reconstitutes proton extrusion in an H+-ATPase-negative mutant of Lactococcus lactis. J. Bacteriol. 183:6707-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz-Ramos, H., G. M. Cook, G. Wu, M. W. Cleeter, and R. K. Poole. 2004. Membrane topology and mutational analysis of Escherichia coli CydDC, an ABC-type cysteine exporter required for cytochrome assembly. Microbiology 150:3415-3427. [DOI] [PubMed] [Google Scholar]
- 8.Duwat, P., S. D. Ehrlich, and A. Gruss. 1995. The recA gene of Lactococcus lactis: characterization and involvement in oxidative and thermal stress. Mol. Microbiol. 17:1121-1131. [DOI] [PubMed] [Google Scholar]
- 9.Duwat, P., S. Sourice, B. Cesselin, G. Lamberet, K. Vido, P. Gaudu, Y. Le Loir, F. Violet, P. Loubiere, and A. Gruss. 2001. Respiration capacity of the fermenting bacterium Lactococcus lactis and its positive effects on growth and survival. J. Bacteriol. 183:4509-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrigues, C., E. Johansen, M. B. Pedersen, H. Mollgaard, K. I. Sorensen, P. Gaudu, A. Gruss, and G. Lamberet. 2006. Getting high (OD) on heme. Nat. Rev. Microbiol. 4:c2-c3. [DOI] [PubMed] [Google Scholar]
- 11.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaudu, P., G. Lamberet, S. Poncet, and A. Gruss. 2003. CcpA regulation of aerobic and respiration growth in Lactococcus lactis. Mol. Microbiol. 50:183-192. [DOI] [PubMed] [Google Scholar]
- 13.Gaudu, P., K. Vido, B. Cesselin, S. Kulakauskas, J. Tremblay, L. Rezaiki, G. Lamberret, S. Sourice, P. Duwat, and A. Gruss. 2002. Respiration capacity and consequences in Lactococcus lactis. Antonie Leeuwenhoek 82:263-269. [PubMed] [Google Scholar]
- 14.Gennis, R. B. 1987. The cytochromes of Escherichia coli. FEMS Microbiol. 46:387-399. [Google Scholar]
- 15.Harold, F. M., J. R. Baarda, C. Baron, and A. Abrams. 1969. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N′-dicyclohexylcarbodiimide. J. Biol. Chem. 244:2261-2268. [PubMed] [Google Scholar]
- 16.Jünemann, S. 1997. Cytochrome bd terminal oxidase. Biochim. Biophys. Acta 1321:107-127. [DOI] [PubMed] [Google Scholar]
- 17.Kita, K., K. Konishi, and Y. Anraku. 1986. Purification and properties of two terminal oxidase complexes of Escherichia coli aerobic respiratory chain. Methods Enzymol. 126:94-113. [DOI] [PubMed] [Google Scholar]
- 18.Kleerebezem, M., J. Boekhorst, R. van Kranenburg, D. Molenaar, O. P. Kuipers, R. Leer, R. Tarchini, S. A. Peters, H. M. Sandbrink, M. W. Fiers, W. Stiekema, R. M. Lankhorst, P. A. Bron, S. M. Hoffer, M. N. Groot, R. Kerkhoven, M. de Vries, B. Ursing, W. M. de Vos, and R. J. Siezen. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 100:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konings, W. N. 2002. The cell membrane and the struggle for life of lactic acid bacteria. Antonie Leeuwenhoek 82:3-27. [PubMed] [Google Scholar]
- 20.Krasne, S. 1980. Interactions of voltage-sensing dyes with membranes. II. Spectrophotometric and electrical correlates of cyanine-dye adsorption to membranes. Biophys. J. 30:441-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krulwich, T. A. 1983. Na+/H+ antiporters. Biochim. Biophys. Acta 726:245-264. [DOI] [PubMed] [Google Scholar]
- 22.Lambert, J. M., R. S. Bongers, and M. Kleerebezem. 2006. Cre-lox-based system for multiple gene deletions and selectable-marker removal in Lactobacillus plantarum. Appl. Environ Microbiol. 73:1126-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leimgruber, R. M., C. Jensen, and A. Abrams. 1981. Purification and characterization of the membrane adenosine triphosphatase complex from the wild-type and N,N′-dicyclohexylcarbodiimide-resistant strains of Streptococcus faecalis. J. Bacteriol. 147:363-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lolkema, J. S., B. Poolman, and W. N. Konings. 1995. Role of scalar protons in metabolic energy generation in lactic acid bacteria. J. Bioenerg. Biomembr. 27:467-473. [DOI] [PubMed] [Google Scholar]
- 25.Lopez de Felipe, F., M. Kleerebezem, W. M. de Vos, and J. Hugenholtz. 1998. Cofactor engineering: a novel approach to metabolic engineering in Lactococcus lactis by controlled expression of NADH oxidase. J. Bacteriol. 180:3804-3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maloney, P. C. 1982. Energy coupling to ATP synthesis by the proton-translocating ATPase. J. Membr. Biol. 67:1-12. [DOI] [PubMed] [Google Scholar]
- 27.Matsuura, K., and M. Nishimura. 1977. Sidedness of membrane structures in Rhodopseudomonas sphaeroides. Electrochemical titration of the spectrum changes of carotenoid in spheroplasts, spheroplast membrane vesicles and chromatophores. Biochim. Biophys. Acta 459:483-491. [DOI] [PubMed] [Google Scholar]
- 28.Melo, A. M., T. M. Bandeiras, and M. Teixeira. 2004. New insights into type II NAD(P)H:quinone oxidoreductases. Microbiol. Mol. Biol. Rev. 68:603-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michels, P. A. M., J. P. J. Michels, J. Boonstra, and W. N. Konings. 1979. Generation of a electrochemical proton gradient in bacteria by the excretion of metabolic end products. FEMS Microbiol. Lett. 53:357-364. [Google Scholar]
- 30.Mickelson, M. N. 1972. Glucose degradation, molar growth yields, and evidence for oxidative phosphorylation in Streptococcus agalactiae. J. Bacteriol. 109:96-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller, M. J., and R. B. Gennis. 1985. The cytochrome d complex is a coupling site in the aerobic respiratory chain of Escherichia coli. J. Biol. Chem. 260:14003-14008. [PubMed] [Google Scholar]
- 32.Miyoshi, A., T. Rochat, J. J. Gratadoux, Y. Le Loir, S. C. Oliveira, P. Langella, and V. Azevedo. 2003. Oxidative stress in Lactococcus lactis. Genet. Mol. Res. 2:348-359. [PubMed] [Google Scholar]
- 33.Morishita, T., N. Tamura, T. Makino, and S. Kudo. 1999. Production of menaquinones by lactic acid bacteria. J. Dairy Sci. 82:1897-1903. [DOI] [PubMed] [Google Scholar]
- 34.Otto, R., J. Hugenholtz, W. N. Konings, and H. Veldkamp. 1980. Increase of molar growth yield of Streptococcus cremoris for lactose as a consequence of lactate consumption by Pseudomonas stutzeri in mixed culture. FEMS Microbiol. Lett. 9:85-88. [Google Scholar]
- 35.Otto, R., A. S. M. Sonnenberg, H. Veldkamp, and W. N. Konings. 1980. Generation of an electrochemical proton gradient in Streptococcus cremoris by lactate efflux. Proc. Natl. Acad. Sci. USA 77:5502-5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padan, E., and S. Schuldiner. 1987. Intracellular pH and membrane potential as regulators in the prokaryotic cell. J. Membr. Biol. 95:189-198. [DOI] [PubMed] [Google Scholar]
- 37.Pedersen, M. B., S. L. Iversen, K. I. Sorensen, and E. Johansen. 2005. The long and winding road from the research laboratory to industrial applications of lactic acid bacteria. FEMS Microbiol. Rev. 29:611-624. [DOI] [PubMed] [Google Scholar]
- 38.Philippot, L., and O. Hojberg. 1999. Dissimilatory nitrate reductases in bacteria. Biochim. Biophys. Acta 1446:1-23. [DOI] [PubMed] [Google Scholar]
- 39.Puustinen, A., M. Finel, T. Haltia, R. B. Gennis, and M. Wikstrom. 1991. Properties of the two terminal oxidases of Escherichia coli. Biochemistry 30:3936-3942. [DOI] [PubMed] [Google Scholar]
- 40.Rezaiki, L., B. Cesselin, Y. Yamamoto, K. Vido, E. van West, P. Gaudu, and A. Gruss. 2004. Respiration metabolism reduces oxidative and acid stress to improve long-term survival of Lactococcus lactis. Mol. Microbiol. 53:1331-1342. [DOI] [PubMed] [Google Scholar]
- 41.Ritchey, T. W., and H. W. Seeley. 1974. Cytochromes in Streptococcus faecalis var. zymogenes grown in a haematin-containing medium. J. Gen. Microbiol. 85:220-228. [DOI] [PubMed] [Google Scholar]
- 42.Ritchey, T. W., and H. W. Seely, Jr. 1976. Distribution of cytochrome-like respiration in streptococci. J. Gen. Microbiol. 93:195-203. [DOI] [PubMed] [Google Scholar]
- 43.Sakamoto, J., E. Koga, T. Mizuta, C. Sato, S. Noguchi, and N. Sone. 1999. Gene structure and quinol oxidase activity of a cytochrome bd-type oxidase from Bacillus stearothermophilus. Biochim. Biophys. Acta 1411:147-158. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 45.Sijpesteijn, A. K. 1970. Induction of cytochrome formation and stimulation of oxidative dissimilation by hemin in Streptococcus lactis and Leuconostoc mesenteroides. Antonie Leeuwenhoek 36:335-348. [DOI] [PubMed] [Google Scholar]
- 46.Simon, D., and A. Chopin. 1988. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie 70:559-566. [DOI] [PubMed] [Google Scholar]
- 47.Simoni, R. D., and P. W. Postma. 1975. The energetics of bacterial active transport. Annu. Rev. Biochem. 44:523-554. [DOI] [PubMed] [Google Scholar]
- 48.Sims, P. J., A. S. Waggoner, C. H. Wang, and J. F. Hoffman. 1974. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry 13:3315-3330. [DOI] [PubMed] [Google Scholar]
- 49.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 50.Subbarao, N. K., R. I. MacDonald, K. Takeshita, and R. C. MacDonald. 1991. Characteristics of spectrin-induced leakage of extruded, phosphatidylserine vesicles. Biochim. Biophys. Acta 1063:147-154. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki, H., Z. Y. Wang, M. Yamakoshi, M. Kobayashi, and T. Nozawa. 2003. Probing the transmembrane potential of bacterial cells by voltage-sensitive dyes. Anal. Sci. 19:1239-1242. [DOI] [PubMed] [Google Scholar]
- 52.Thony-Meyer, L. 1997. Biogenesis of respiratory cytochromes in bacteria. Microbiol. Mol. Biol. Rev. 61:337-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tusnady, G. E., and I. Simon. 1998. Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J. Mol. Biol. 283:489-506. [DOI] [PubMed] [Google Scholar]
- 54.Vido, K., D. Le Bars, M. Y. Mistou, P. Anglade, A. Gruss, and P. Gaudu. 2004. Proteome analyses of heme-dependent respiration in Lactococcus lactis: involvement of the proteolytic system. J. Bacteriol. 186:1648-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Wachenfeldt, C., and L. Hederstedt. 1992. Molecular biology of Bacillus subtilis cytochromes. FEMS Microbiol. Lett. 79:91-100. [DOI] [PubMed] [Google Scholar]
- 56.Winstedt, L., L. Frankenberg, L. Hederstedt, and C. von Wachenfeldt. 2000. Enterococcus faecalis V583 contains a cytochrome bd-type respiratory oxidase. J. Bacteriol. 182:3863-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winstedt, L., and C. von Wachenfeldt. 2000. Terminal oxidases of Bacillus subtilis strain 168: one quinol oxidase, cytochrome aa3 or cytochrome bd, is required for aerobic growth. J. Bacteriol. 182:6557-6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winstedt, L., K. Yoshida, Y. Fujita, and C. von Wachenfeldt. 1998. Cytochrome bd biosynthesis in Bacillus subtilis: characterization of the cydABCD operon. J. Bacteriol. 180:6571-6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto, Y., C. Poyart, P. Trieu-Cuot, G. Lamberet, A. Gruss, and P. Gaudu. 2005. Respiration metabolism of group B Streptococcus is activated by environmental haem and quinone and contributes to virulence. Mol. Microbiol. 56:525-534. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto, Y., C. Poyart, P. Trieu-Cuot, G. Lamberet, A. Gruss, and P. Gaudu. 2006. Roles of environmental heme, and menaquinone, in Streptococcus agalactiae. Biometals 19:205-210. [DOI] [PubMed] [Google Scholar]