Abstract
The two-component signal transduction system PhoRS of Corynebacterium glutamicum is involved in the phosphate (Pi) starvation response. To analyze the binding of unphosphorylated and phosphorylated PhoR to the promoters of phosphate starvation-inducible (psi) genes, this response regulator and the kinase domain of its cognate sensor, PhoS (MBP-PhoSΔ1-246), were overproduced and purified. MBP-PhoSΔ1-246 showed constitutive autophosphorylation activity, and a rapid phosphoryl group transfer from phosphorylated MBP-PhoSΔ1-246 to PhoR was observed. Gel mobility shift assays revealed that phosphorylation increases the DNA-binding affinity of PhoR. The affinity of PhoR∼P to different promoters varied and decreased in the order pstSCAB > phoRS > phoC > ushA > porB > ugpA > pitA > nucH and phoH1 > glpQ1. The binding sites in front of pstSCAB and phoRS were localized at positions −194 to −176 and −61 to −43 upstream of the transcriptional start sites, respectively. Alignment of these two 19-bp binding sites revealed a high identity in the 5′-terminal part, but not in the 3′-terminal part. As many OmpR-type response regulators bind to direct repeats, the 19-bp sequence might be interpreted as a loosely conserved 8-bp direct repeat separated by 3 bp. This idea was supported by the fact that the highest binding affinity was observed with a perfect 8-bp direct repeat of the sequence CCTGTGAAaatCCTGTGAA. Inspection of the other target promoters revealed sequences with some similarity to this binding motif, which might represent PhoR binding sites. The in vivo relevance of the PhoR-binding site within the phoRS promoter was supported by reporter gene studies.
Phosphorus is an essential nutrient for all cells and is required, e.g., for the biosynthesis of nucleotides, DNA, and RNA, but also for the regulation of protein activity by phosphorylation of histidine, aspartate, serine, threonine, or tyrosine residues. A common phosphorus source is inorganic phosphate (Pi), and cells have developed mechanisms for the acquisition, assimilation, and storage of phosphate. When Pi becomes limiting, many bacteria induce the synthesis of proteins that enable them to capture the residual Pi resources more efficiently and to make alternative phosphorus sources accessible. The corresponding genes are collectively named Pi starvation-inducible genes, or psi genes. The phosphate starvation response, particularly its regulation, has been most carefully studied in Escherichia coli (28) and Bacillus subtilis (12).
We recently started to characterize the phosphate starvation response in Corynebacterium glutamicum (13), a gram-positive soil bacterium used industrially for the production of more than 2 million tons of amino acids per year, mainly l-glutamate and l-lysine (11). An overview of the biology, genetics, physiology, and application of C. glutamicum can be found in a recent monograph (5). Phosphorus constitutes 1.5% to 2.1% of the cell dry weight of C. glutamicum (18), part of which is present as polyphosphate (15, 17, 21). The phosphate starvation stimulon of C. glutamicum was determined using whole-genome DNA microarrays (13). Comparison of the mRNA profiles before and at different times after a shift from Pi excess to Pi starvation led to the identification of a group of genes that are presumably required to cope with a limited Pi supply. This group includes the pstSCAB operon, encoding an ABC transporter for high-affinity Pi uptake; the ugpAEBC operon, encoding an ABC transporter for uptake of glycerol 3-phosphate; glpQ1, encoding a glycerophosphoryl diester phosphodiesterase; ushA, encoding a secreted enzyme with UDP sugar hydrolase and 5′-nucleotidase activity (22); nucH, encoding a putative secreted nuclease that possibly plays a role in liberating Pi from extracellular nucleic acids; phoC (NCgl2959/cg3393), which may encode a cell wall-associated phosphatase (29); phoH1, encoding an ATPase of unknown function; and the pctABCD operon, encoding an ABC transport system that might be involved in the uptake of a yet-unknown phosphorus-containing compound (13).
In most bacteria analyzed in this respect, the phosphate starvation response is controlled by two-component signal transduction systems, e.g., the PhoBR system in E. coli (28) or the PhoPR system in B. subtilis (12). When we screened a set of 12 C. glutamicum mutants, each lacking the genes for one two-component system, one of the strains showed a growth defect under Pi starvation compared to the wild type (16). The genes lacking in this strain were named phoS and phoR and encode a histidine kinase and a response regulator, respectively. The role of the PhoRS system in the adaptation to Pi starvation was supported by DNA microarray studies that showed that the psi genes, except those of the pstSCAB operon, were not induced in the ΔphoRS mutant within 1 hour after a shift from Pi excess to Pi limitation, in contrast to the wild type (16). In the study presented here, we analyzed whether the C. glutamicum response regulator PhoR is able to interact with the promoters of the psi genes, determined the influence of phosphorylation on the DNA-binding affinity, and characterized the binding sites in front of pstSCAB and phoRS in detail.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Bacterial strains and plasmids used or constructed in this work are listed in Table 1. E. coli DH5α (Invitrogen) was used as a host during the construction of recombinant plasmids and was routinely grown aerobically at 37°C on a rotary shaker (120 rpm) in Luria-Bertani (LB) medium (23). E. coli BL21(DE3) (26) was used for overproduction of the proteins MBP-PhoSΔ1-246 and PhoR and was grown aerobically at 30°C on a rotary shaker (120 rpm) in LB medium. When appropriate, ampicillin was added at a concentration of 100 μg/ml. The C. glutamicum strain ATCC 13032 (1) was used as the wild type. For chloramphenicol-acetyltransferase (CAT) assays, 5 ml of brain heart infusion (BHI) medium (Difco) was inoculated with colonies from a fresh BHI agar plate and incubated for about 8 h at 30°C and 170 rpm. The cells of this first preculture were used to inoculate a 500-ml shake flask containing 50 ml of CGXII minimal medium with 4% (wt/vol) glucose (14) and 30 mg/liter 3,4-dihydroxybenzoic acid as an iron chelator. The second preculture was incubated overnight at 30°C and 120 rpm and then used to inoculate the main culture (300 ml of the CGXII medium described above in a 1-liter shake flask) to an optical density at 600 nm (OD600) of 1. This culture was incubated at 30°C and 120 rpm until an OD600 of 4 to 5 was reached. At that time, cells from 30 ml culture were harvested by centrifugation on ice and stored at −20°C until they were used. Two hundred milliliters of the remaining culture was harvested, washed twice in CGXII medium lacking phosphate and glucose, resuspended in 200 ml CGXII-glucose medium without phosphate, and incubated as described above. Ten, 30, 60, 90, and 120 min after the transfer to Pi-free medium, cells from 30 ml culture were harvested and stored at −20°C until they were used. For growth of C. glutamicum harboring pET2-phoR plasmids, the medium was supplemented with 25 μg/ml kanamycin.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strains | Relevant characteristics | Source/reference |
|---|---|---|
| E. coli DH5α | F−thi-1 endA1 hsdR17(r− m+) supE44 ΔlacU169 (φ80lacZΔM15) recA1 gyrA96 relA1; host for cloning procedures | Invitrogen |
| E. coli BL21(DE3) | F−ompT hsdSB(rB− mB−) gal dcm (λcIts857 ind1 Sam7 nin5 lacUV5-T7 gene 1); host for overproduction of plasmid-encoded recombinant proteins | Novagen |
| C. glutamicum ATCC 13032 | Biotin-auxotrophic wild-type strain | 1 |
| Plasmids | ||
| pET16b | Ampr; PT7lacI oriV from pBR322; E. coli expression vector for overproduction of proteins with an N-terminal decahistidine tag that can be cleaved off by factor Xa | Novagen |
| pET16b-PhoR | Ampr; pET16b derivative for overproduction of PhoR with an N-terminal decahistidine tag (PhoRN-His10) | This work |
| pMal-c | Ampr; PtaclacIq ColE1 oriV; E. coli expression vector for construction and overproduction of fusion proteins containing the MBP of E. coli (MalE) without its signal peptide | New England Biolabs |
| pMBP-PhoSΔ1-246 | Ampr; pMal-c derivative for overproduction of the kinase domain of C. glutamicum PhoS (amino acid residues 247 to 485) fused to the C-terminus of the E. coli MBP without signal peptide (MBP-PhoSΔ1-246) | This work |
| pET2 | Kanr; promoter-probe vector | 27 |
| pET2-phoR1 | Kanr; pET2 with a 233-bp fragment of the phoR promoter | This work |
| pET2-phoR2 | Kanr; pET2 with a 200-bp fragment of the phoR promoter | This work |
| pET2-phoR3 | Kanr; pET2 with a 180-bp fragment of the phoR promoter | This work |
General DNA techniques and sequence analyses.
The enzymes for recombinant DNA work were obtained from Roche Diagnostics (Mannheim, Germany) or New England Biolabs (Frankfurt, Germany). The oligonucleotides used in this study were obtained from Operon (Cologne, Germany) and are listed in Table S1 in the supplemental material. Routine methods, like PCR, DNA restriction, or DNA ligation, were carried out according to standard protocols (23). Chromosomal DNA from C. glutamicum was prepared as described previously (6). Plasmids from E. coli were isolated with the QIAprep spin miniprep kit (QIAGEN). E. coli was transformed by the RbCl method (9). DNA sequencing was performed with a Genetic Analyzer 3100-Avant (Applied Biosystems). Sequencing reactions were carried out with the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems).
Construction of expression plasmids pMBP-PhoSΔ1-246 and pET16b-PhoR.
For overproduction and purification of the kinase domain of the C. glutamicum sensor kinase PhoS, the expression plasmid pMBP-PhoSΔ1-246 was constructed. The DNA region encoding the kinase domain (amino acids 247 to 485) was amplified by PCR using primers that introduced an EcoRI restriction site before codon 247 (primer phoSΔ1-246-EcoRI-fw) and a PstI restriction site after the stop codon (primer phoSΔ1-246-PstI-rv). The resulting PCR product was cloned into pMal-c (New England Biolabs, Frankfurt, Germany) cut with EcoRI and PstI, and the relevant portion of the resulting plasmid, pMBP-PhoSΔ1-246, was checked for the absence of spurious mutations by DNA sequencing. The cloning strategy resulted in a protein (MBP-PhoSΔ1-246; 624 amino acids; 67.7 kDa) in which the kinase domain of PhoS was fused to the carboxy terminus of the E. coli maltose binding protein (MBP) lacking its signal peptide.
For overproduction and purification of the response regulator PhoR with an N-terminal cleavable decahistidine tag, the expression plasmid pET16b-PhoR was constructed. The phoR coding region was amplified using primers that introduced an NdeI restriction site overlapping the start codon (primer phoR-NdeI-fw) and an XhoI restriction site after the stop codon (primer phoR-XhoI-rv). After digestion with NdeI and XhoI, the 767-bp PCR product was cloned into the expression vector pET16b (Novagen). The PCR-derived part of the resulting pET16b-PhoR plasmid and the ligation sites were sequenced in order to exclude unwanted mutations. The PhoRN-His10 protein encoded by this plasmid contained 21 additional amino acids (MGHHHHHHHHHHSSGHIEGRH) at the amino terminus, including a factor Xa cleavage site (SGHIEGR). After cleavage of PhoRN-His10 with factor Xa, the resulting PhoR protein contained only one additional amino acid (H) at the amino terminus.
Overproduction and purification of PhoR and MBP-PhoSΔ1-246.
For overproduction of PhoR and MBP-PhoSΔ1-246, E. coli BL21(DE3) transformed with one of the expression plasmids was cultivated in LB medium up to an OD600 of 0.3. Then, expression of the target gene was induced by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and the culture was incubated for another 3 h at room temperature. Cells were harvested, washed once in TNGI5 buffer (20 mM Tris-HCl, pH 7.9, 300 mM NaCl, 5% [vol/vol] glycerol, and 5 mM imidazole; the number indicates the millimolar imidazole concentration), and resuspended in the same buffer (∼1.5 g [wet weight] in 6 ml buffer) supplemented with 1 mM phenylmethylsulfonyl fluoride and 1 mM diisopropylfluorophosphate. After disruption using a French pressure cell, intact cells and cell debris were removed by centrifugation (20 min at 5,000 × g; 4°C), and the supernatant was subjected to ultracentrifugation (1 h at 150,000 × g; 4°C). The resulting supernatant was passed through a 0.2-μm filter and used for protein purification.
MBP-PhoSΔ1-246 was purified by affinity chromatography on amylose resin (New England Biolabs) equilibrated with TNGI5 buffer. After being washed with 30 ml TNGI5 buffer, MBP-PhoSΔ1-246 was eluted with 10 1-ml portions TNGI5 buffer containing 10 mM maltose. Fractions containing MBP-PhoSΔ1-246 were pooled, the buffer was exchanged for GMS buffer (50 mM Tris-HCl, pH 7.5, 50 mM KCl, 10 mM MgCl2, 0.5 mM EDTA, and 10% [vol/vol] glycerol) using PD-10 columns (GE Healthcare), and the purified protein was stored at −20°C.
PhoRN-His10 was purified by Ni2+ chelate affinity chromatography using His-bind resin (Novagen) equilibrated with TNGI5 buffer. After being washed using buffers with increasing imidazole concentrations (20 ml TNGI5, 15 ml TNGI20, 15 ml TNGI50, and 10 ml TNGI70), PhoRN-His10 was eluted with eight 1-ml portions TNGI200 buffer. Fractions containing PhoRN-His10 were pooled, and the buffer was exchanged for GMS buffer using PD-10 columns (GE Healthcare). The purified protein was stored at −20°C. As initial gel mobility shift assays revealed that the His tag decreased the DNA-binding affinity, the tag was routinely cleaved off using factor Xa (Novagen). Briefly, purified PhoRN-His10 was digested for about 14 h at 20°C with factor Xa (20 U/mg PhoRN-His10), and subsequently, factor Xa was removed with Xarrest agarose. The purified PhoR was stored at −20°C. Overproduction and purification of MBP-PhoSΔ1-246 and PhoR, as well as the proteolytic His tag cleavage, were monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and staining of the proteins with Coomassie brilliant blue.
In vitro phosphorylation assays.
To determine its autophosphorylation activity, 12 μM MBP-PhoSΔ1-246 was incubated with 0.17 μM [γ-32P]ATP (10 mCi/ml; GE Healthcare) and 80 μM nonradioactive ATP. The assay mixture was incubated at room temperature, and at different time points, aliquots were removed, mixed with an equal volume of 2× SDS loading buffer (124 mM Tris-HCl, pH 6.8, 20% glycerol, 4.6% SDS, 1.4 M β-mercaptoethanol, 0.01% bromophenol blue), and placed one ice. Subsequently, without prior heating, the samples were subjected to SDS-PAGE using a 12% separating gel. After being dried, the gel was analyzed with a BAS-1800 phosphorimager (Fujifilm). For analyzing phosphorylation of PhoR by MBP-PhoSΔ1-246, a twofold molar excess of purified PhoR was added to the MBP-PhoSΔ1-246-containing assay mixture described above (after it had been incubated for 5 min), and the sample was incubated for another 61 min. At different time points, aliquots were removed (taking into consideration the changed MBP-PhoSΔ1-246 concentration) and handled as described above.
Gel mobility shift assays.
For gel mobility shift assays, a variety of different DNA fragments (89 to 704 bp) generated by PCR and purified using the QIAquick PCR Purification Kit (QIAGEN, Hilden, Germany) were mixed with PhoRN-His10 or with PhoR, either in the unphosphorylated state or after phosphorylation with MBP-PhoSΔ1-246. The reaction mixture included ∼100 ng target DNA (10 to 55 nM), a 0- to 300-fold molar excess of protein, and GMS buffer in a total volume of 20 μl. For phosphorylation of PhoR, it was incubated for 60 min with MBP-PhoSΔ1-246 (half the concentration of PhoR) and 5 mM ATP before the DNA fragments were added. In general, the protein-DNA mixtures were incubated for 30 min at room temperature and then separated on a 15% native polyacrylamide gel. Electrophoresis and staining were performed as described previously (30) but using precooled electrophoresis buffer.
For testing the effect of mutations in PhoR-binding sites, the desired mutated DNA fragments were constructed by overlap extension PCR. For this purpose, a 22-bp region harboring the presumed binding site was placed between two fragments of the pstS promoter that do not bind PhoR. These two flanking regions extend from positions −72 to +162 (flanking region 1) and from positions −369 to −283 (flanking region 2) relative to the transcriptional start site (+1) determined previously (16). For amplification of flanking region 1, primer PpstS rev, covering nucleotides +162 to +140 of the pstS promoter, was used in combination with a 49-mer oligonucleotide. The 5′-terminal 22 nucleotides of this 49-mer covered the PhoR-binding site, including the desired mutations, and the 3′-terminal 27 nucleotides corresponded to positions −72 to −45 of the pstS promoter. For amplification of flanking region 2, primer PpstS fw, covering nucleotides −369 to −347 of the pstS promoter, was used in combination with a second 49-mer oligonucleotide. Its 5′-terminal 22 nucleotides covered the complementary PhoR-binding site, including the desired mutations, and its 3′-terminal 27 nucleotides corresponded to nucleotides −283 to −310 of the pstS promoter. The PCR fragments obtained with these two primer pairs were purified using the QIAquick PCR Purification Kit (QIAGEN, Hilden, Germany), and 50 ng of each fragment was used as a template for overlap extension PCR with the primers PpstS fw and PpstS rev. The resulting PCR product was purified as described above and used in the gel mobility shift assays.
Construction of transcriptional fusions and CAT assays.
For CAT assays, the full-length phoR promoter (fragment phoR1) and two phoR promoter subfragments (fragments phoR2 and phoR3) were amplified and cloned into the corynebacterial promoter-probe vector pET2 (27), resulting in plasmids pET2-phoR1, pET2-phoR2, and pET2-phoR3. The cloned fragments were controlled by DNA sequence analysis. The plasmids were introduced into wild-type C. glutamicum, and the transformed strains were cultivated as outlined above. The CAT assays were performed as described previously (7).
RESULTS
Autophosphorylation of MBP-PhoSΔ1-246 and phosphoryl group transfer to the response regulator PhoR.
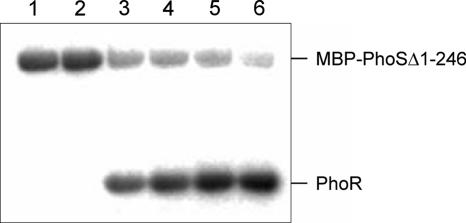
To demonstrate the phosphorylation activities that are characteristic of two-component signal transduction systems, the kinase domain of PhoS (MBP-PhoSΔ1-246) and the entire PhoR protein were overproduced in E. coli and purified (Fig. 1). To determine whether MBP-PhoSΔ1-246 possesses autophosphorylation activity, the protein was incubated with [γ-32P]ATP, and samples taken at different times were analyzed by SDS-PAGE and autoradiography. As shown in Fig. 2, MBP-PhoSΔ1-246 catalyzed its own phosphorylation, reaching a maximal phosphorylation level after about 5 min of incubation (data not shown). When purified PhoR was added at this time, an efficient transfer of the phosphoryl group from MBP-PhoSΔ1-246 to PhoR was observed within 1 minute (Fig. 2). Maximal PhoR phosphorylation levels were reached after 30 to 60 min. No phosphorylation of PhoR was observed when the protein was incubated with [γ-32P]ATP for 60 min in the absence of MBP-PhoSΔ1-246 (data not shown).
FIG. 1.
Coomassie-stained SDS-polyacrylamide gels showing overproduction and purification of PhoRN-His10 and MBP-PhoSΔ1-246. Additionally, the successful cleavage of the N-terminal His tag of PhoRN-His10 by factor Xa is shown. Lanes 1, 4, 7, and 10, protein standards (Precision Plus Protein Prestained Standard; Bio-Rad); lanes 2 and 3, whole-cell lysates of E. coli BL21(DE3)/pET16b-PhoR before and 3 h after IPTG induction, respectively; lane 5, 1.3 μg of purified PhoRN-His10; lane 6, 1.3 μg of PhoR after factor Xa digestion of PhoRN-His10; lanes 8 and 9, whole-cell lysates of E. coli BL21(DE3)/pMBP-PhoSΔ1-246 before and 3 h after IPTG induction, respectively; lane 11, 3 μg of purified MBP-PhoSΔ1-246.
FIG. 2.
Autophosphorylation of MBP-PhoSΔ1-246 and phosphoryl group transfer from MBP-PhoSΔ1-246∼P to PhoR. MBP-PhoSΔ1-246 (12 μM) was incubated in TKMD buffer (50 mM Tris-HCl [pH 7.5], 200 mM KCl, 5 mM MgCl2, and 5 mM dithiothreitol) at room temperature with 0.17 μM [γ-32P]ATP (∼3,000 Ci/mmol; 10 mCi/ml) and 80 μM nonradioactive ATP in a total volume of 60 μl; 1 min (lane 1) and 5 min (lane 2) after ATP addition, 7-μl samples were removed. After 5 min, 30 μl of a 25.8 μM PhoR solution was added to 30 μl of the remaining MBP-PhoSΔ1-246 solution, and 14-μl samples were taken after 1 min (lane 3), 6 min (lane 4), 21 min (lane 5), and 61 min (lane 6). All samples were mixed immediately with 2× SDS loading buffer and stored on ice. Subsequently, without prior heating, the samples were subjected to SDS-PAGE using a 12% separating gel. After being dried, the gel was analyzed with a BAS-1800 phosphorimager (Fujifilm).
Binding of PhoR to putative target promoters and influence of phosphorylation on the binding affinity.
According to our previous data, rapid induction of the psi genes after a shift from Pi excess to Pi starvation is dependent on the PhoRS two-component system (16). We therefore analyzed whether the response regulator PhoR can bind to the promoter regions of the psi genes. In a first experiment, the promoter regions of the pstSCAB operon and of nucH, together with those of acn (aconitase) and gpmA (cg0482; phosphoglycerate mutase), which served as negative controls, were incubated with PhoR up to a 300-fold molar excess of protein. As shown in the upper part of Fig. 3, unphosphorylated PhoR was able to bind to the pstS promoter. About 50% of the pst promoter fragment was shifted at a PhoR concentration of 2.1 μM. In contrast, PhoR did not bind to the nucH promoter or to the promoter regions used as controls. In a parallel experiment, PhoR was preincubated for 60 min with MBP-PhoSΔ1-246 and 5 mM ATP before addition of the DNA fragments. As shown in the lower part of Fig. 3, the phosphorylation of PhoR had a significant influence on its binding properties. In the case of the pstS promoter, the affinity was increased about fivefold, as about 50% of the pstS promoter fragment was shifted at a PhoR∼P concentration of ∼0.45 μM. In the case of the nucH promoter, about 50% of the fragment was shifted at the maximal PhoR∼P concentration used in this experiment (3.15 μM), whereas no shift was observed again with the two control fragments. This experiment showed that, as in the cases of E. coli PhoB (28) and B. subtilis PhoP (19), phosphorylation increases the DNA-binding affinity of C. glutamicum PhoR. At the same time, the experiment indicates that PhoR∼P has different binding affinities to different target promoters.
FIG. 3.
Comparison of the DNA-binding affinities of unphosphorylated and phosphorylated PhoR to the promoter regions of pstS and nucH. Fragments covering the promoter regions of nucH (10 nM), pstS (10 nM), and the control genes gpmA (15 nM) and acn (27 nM) were incubated for 30 min at room temperature with increasing concentrations PhoR (A) or PhoR∼P (B). Then, the 20-μl reaction mixtures were separated by electrophoresis on 15% native polyacrylamide gels to separate free DNA and DNA-protein complexes, and the gels were stained with SybrGreen I. For phosphorylation of PhoR, the protein was preincubated for 60 min at room temperature with a twofold-lower concentration of MBP-PhoSΔ1-246 and 5 mM ATP.
After it had been shown successfully that PhoR interacts with two of the predicted target promoters, binding of PhoR to the promoter regions of pstSCAB, phoRS, phoC, ushA, ugpAEBC, nucH, phoH1, glpQ1, and pctABCD was tested individually. In all these binding assays, the promoter region of the acn gene served as a negative control. As shown in Fig. 4, PhoP phosphorylated by MBP-PhoSΔ1-246 was able to bind to each of the promoter fragments tested; however, the binding affinities differed. They decreased in the order pstS (∼35×) > phoR (∼45×) > phoC (∼50×) > ushA (∼60×) > ugpA (∼70×) > nucH and phoH1 (∼120×) > glpQ1 (>120×). The values in parentheses roughly indicate the molar excess of PhoR∼P required to shift 50% of the DNA fragment. In the case of the promoter region of the pctABCD operon, very weak binding was observed, requiring a 150- to 200-fold molar excess of PhoR∼P to shift half of the DNA fragments (data not shown). The acn control promoter was not shifted in any of the experiments.
FIG. 4.
Binding of PhoR∼P and PhoR to individual promoter regions. (A) DNA fragments (14 to 38 nM) covering the promoter regions of pstS, phoR, phoC, ushA, porB, ugpA, pitA, nucH, phoH1, glpQ1, and acn (negative control) were incubated for 30 min at room temperature with a 0- to 120-fold molar excess of PhoR∼P. Subsequently, the reaction mixtures (20 μl) were separated by electrophoresis on a native 15% polyacrylamide gel, and the gels were stained with SybrGreen. (B) The same DNA fragments used in panel A were incubated with a 0- to 300-fold molar excess of unphosphorylated PhoR and then treated as described above.
Another gene whose promoter region was tested for binding of PhoR was porB, which was described as an anion-specific channel or porin in the cell wall of C. glutamicum (3). The mRNA level of the porB gene was reproducibly reduced in the ΔphoRS mutant, under both Pi excess and Pi limitation (data not shown). As shown in Fig. 4, the porB promoter region was shifted by PhoR, requiring an ∼70-fold molar excess of PhoR∼P for binding half of the DNA fragments. This suggests that PhoR might be an activator of porB expression, independent of Pi starvation conditions. As porins were recently reported to be required for Pi uptake in Mycobacterium smegmatis (31), PorB might have such a function in C. glutamicum.
Reinspection of our previous microarray data (13) revealed that the mRNA level of the pitA gene encoding a low-affinity phosphate uptake system (29) was reproducibly reduced in the wild type after a shift from Pi excess to Pi starvation, although the value did not match the arbitrarily chosen criteria (fourfold-changed mRNA level, on average) used to summarize the genes of the Pi starvation stimulon (see Table 2 in reference 13). Therefore, we also analyzed the binding of PhoR to the promoter region of pitA. As shown in Fig. 4, the pitA promoter fragment was indeed shifted, requiring a 90-fold molar excess of PhoR∼P to bind half of the DNA fragments. PhoR might thus function as a repressor of the pitA gene.
In parallel to binding assays with phosphorylated PhoR, unphosphorylated PhoR was also tested. For all promoters analyzed, the binding affinity of unphosphorylated PhoR was much lower than that of the phosphorylated form or binding was even undetectable (Fig. 4B).
Delimitation of the PhoR-binding sites in the pstS and phoR promoter regions.
Attempts to find a conserved sequence motif in the promoter regions of the putative PhoR target genes were not successful. Therefore, gel mobility shift assays with subfragments of the promoter regions of pstS and phoR were performed in order to confine the PhoR-binding sites in these promoters (Fig. 5). The promoters of pstS and phoR were chosen because they showed the highest affinity to PhoR in the preceding experiments. In the case of the pstS promoter, the binding assays suggested the presence of two binding regions, one located between −175 and −201 and another one between −99 and −136 relative to the transcriptional start site (Fig. 5). In the case of the phoR promoter, the most likely binding site was located between −40 and −65 relative to the transcriptional start site (Fig. 5). Comparison of the corresponding sequences still did not allow the identification of a common sequence motif.
FIG. 5.
Search for PhoR-binding sites within the promoter regions of pstS and phoR. Gel mobility shift assays were performed with subfragments (14 to 55 nM) of these promoter regions and PhoR∼P (for details, see the legend to Fig. 3). The numbers indicate the ends of the fragments relative to the transcription start site (+1). Based on the observed shifts, the fragments were divided into five categories: +++, 50% of the fragment shifted with a <30-fold molar excess of PhoR∼P; ++, 50% of the fragment shifted with an ∼30-fold molar excess of PhoR∼P; +, 50% of the fragment shifted with a >30- to 60-fold molar excess of PhoR∼P; (+), 20 to 50% of the fragment shifted at a >60- to 90-fold molar excess of PhoR∼P; and −, 0 to 20% of the fragment shifted at a 120-fold molar excess of PhoR∼P. Based on these results, two putative PhoR-binding sites were located in the pstS promoter (−99 to −136 and −175 to −201) and one in the phoR promoter region (−40 to −65).
Mutational analysis of the promoter-distal PhoR-binding site within the pstS promoter.
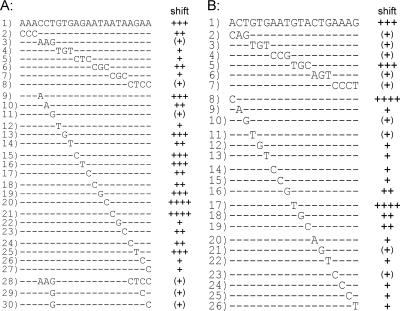
Further gel mobility shift assays with DNA fragments containing the region from −175 to −201 of the pstS promoter confirmed the presence of a PhoR-binding site between −176 and −194 relative to the transcription start site, as the insertion of this 22-bp sequence between two fragments of 87 bp and 234 bp that are not shifted resulted in a 343-bp fragment that was shifted by PhoR with high affinity. To test which of the 22 bp are critical for PhoR binding, a mutational analysis was performed, and the results are summarized in Fig. 6A. In a first series of experiments, 3 or 4 bp were exchanged simultaneously (fragments 2 to 8). The mutations in fragments 3 and 8 strongly inhibited PhoR binding, and those in fragments 2, 4, 5, 6, and 7 led to a weaker decrease in the binding affinity. In a second series of experiments, each of bp 4 to 22 was exchanged separately (fragments 9 to 27). The exchanges in fragments 9, 13, 15, 16, 19, and 25 had no obvious effect on PhoR binding; those in fragments 10, 14, 17, 23, and 24 decreased binding weakly; those in fragments 18, 22, 26, and 27 decreased binding more strongly; and those in fragments 11 and 12 decreased binding very strongly. Remarkably, the mutations in fragments 20 and 21 had a positive influence on the binding affinity. In a third series of experiments, the base pairs whose exchange had the strongest negative influence on PhoR binding were combined (fragments 28 to 30). As expected, an additive effect was observed: exchange of only 2 of the 22 bp was sufficient to prevent PhoR binding almost completely (fragments 29 and 30). Thus, specific nucleotides that are of prime importance for PhoR binding could be determined.
FIG. 6.
Mutational analysis of the PhoR-binding sites within the pstS promoter (A) and the phoR promoter (B). Previous experiments had indicated that fragments 1 have a high affinity to PhoR∼P. To determine the relevance of individual base pairs for binding, fragments with different mutations were tested in gel mobility shift assays for the ability to bind PhoR∼P (for details, see the legend to Fig. 3). According to the results of the shift (see Fig. S1 in the supplemental material), the fragments were divided into five categories: ++++, 50% of the fragment shifted with a <30-fold molar excess of PhoR∼P, +++, 50% of the fragment shifted with an ∼30-fold molar excess of PhoR∼P; ++, 50% of the fragment shifted with a >30- to 45-fold molar excess of PhoR∼P and 100% of the fragment shifted at a 60-fold molar excess of PhoR∼P; +, <50% of the fragment shifted with a >30- to 45-fold molar excess of PhoR∼P and 100% shifted at a 90-fold molar excess of PhoR∼P; and (+), 50% of the fragment shifted at a >45- to 90-fold molar excess of PhoR∼P.
Mutational analysis of the PhoR-binding site within the phoR promoter.
Further analysis of the phoR promoter region led to the identification of a putative PhoR-binding site located between −43 and −61 with respect to the transcriptional start site. This 19-bp region was subjected to the same kind of mutational analysis described above, and the results are depicted in Fig. 6B. When 3 or 4 bp were exchanged simultaneously, five of the resulting fragments (fragments 2, 3, 4, 6, and 7) showed a strongly reduced binding affinity and one (fragment 5) behaved like the parent fragment. These effects were reflected by the results obtained with fragments containing single exchanges (fragments 8 to 26). All mutations except two reduced the PhoR-binding affinity. The exchanges in fragments 8 and 17 led to an increased PhoR binding affinity.
Deduction and verification of a putative consensus binding site for PhoR.
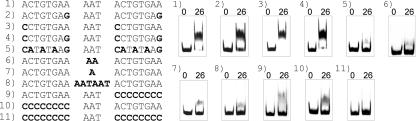
An alignment of the PhoR-binding sites within the pstS promoter and the phoRS promoter (Fig. 7, no. 1 and 2) showed that 11 of the 19 bp were identical in the two sequences. Most prominent was the sequence CTGTGA in the 5′ half of the 19-bp motif. One may interpret the binding sites as two very loosely conserved 8-bp direct repeats separated by a 3-bp spacer. To follow up this idea, binding of PhoR∼P to a fragment containing the 8-bp sequence ACTGTGAA twice separated by the 3-bp spacer AAT and mutated derivatives of this sequence were tested in gel mobility shift assays. As shown in Fig. 8, the fragment containing this artificial sequence showed the highest affinity for PhoR of all fragments tested hitherto, and this affinity could still be improved by changing the 5′-terminal A residues of the 8-bp repeats to C residues (fragment 3). In this case, a 26-fold molar excess of PhoR was sufficient for a complete shift. The other mutations within the 8-bp repeat decreased the binding affinity, in the case of fragment 5 almost completely. When the 3-bp spacer region between the two 8-bp repeats was shortened to either 2 or 1 bp (fragments 6 and 7), binding was also almost completely prevented. The same was true for an increase of the spacer region to 6 bp (fragment 8). Similarly, there was essentially no binding of PhoR if either the 5′-terminal or the 3′-terminal 8-bp repeat was completely exchanged for C residues. These data support the idea that the PhoR-binding site represents a degenerated 8-bp repeat separated by 3 bp and reveal CCTGTGAANNNCCTGTGAA as currently the best binding site.
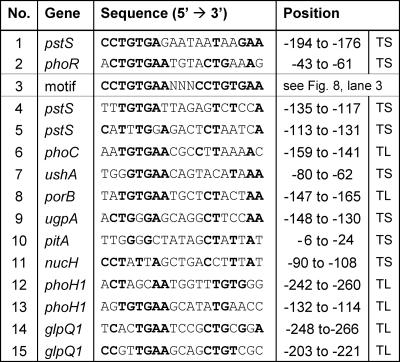
FIG. 7.
Alignment of verified and putative PhoR binding sites. Binding sites 1 and 2 were identified in this work, and binding site 3 is an artificial binding site with the currently highest affinity for PhoR∼P. It represents an 8-bp direct repeat separated by 3 bp. Sequences 4 to 15 were identified by visual inspection of the promoter regions to which PhoR binds in vitro. Positions are relative either to the transcriptional start site (TS) or, if this is not known, to the proposed translational start site (TL).
FIG. 8.
Experimental analysis of the putative PhoR consensus binding motif. Based on sequences 1 and 2 in Fig. 7, an artificial 19-bp consensus sequence consisting of two direct 8-bp repeats of the sequence ACTGTGAA and the spacer AAT was inserted between two fragments that were not bound by PhoR∼P. The resulting fragment and mutated derivatives were tested in gel mobility shift assays for the ability to bind PhoR∼P (for details, see the legends to Fig. 3 and 4).
In vivo relevance of the PhoR-binding site in front of phoRS.
In order to determine whether the PhoR-binding site within the phoRS promoter region is necessary for transcriptional activation of phoRS in vivo, the expression of three transcriptional fusions with a reporter gene encoding CAT in C. glutamicum was measured. The phoR promoter fragments that were cloned in front of the promoterless cat gene in plasmid pET2 (27) are shown in Fig. 9A. Plasmid pET2-phoR1 contained the entire phoR promoter region, including the PhoR-binding site; plasmid pET2-phoR2 lacked the PhoR-binding site but still contained the −10 and −35 regions of the promoter; and plasmid pET2-phoR3 also lacked these regions. The strains carrying these plasmids were cultivated in regular CGXII minimal medium with 4% (wt/vol) glucose until mid-exponential phase (OD600, ∼5). Then, the cells were harvested, washed two times with CGXII without phosphate and glucose, and resuspended in CGXII glucose medium without phosphate. Immediately before harvest and 10, 30, 60, 90, and 120 min after the transfer to Pi-free medium, the CAT activities of cell extracts were determined. As shown in Fig. 9B, the strain with plasmid pET2-phoR1 grown under Pi excess showed a specific activity of about 300 mU/mg. Under Pi limitation, the specific activity increased to about 500 mU/mg within 60 min. The strain carrying plasmid pET2-phoR2 showed a threefold-lower CAT activity under Pi excess (100 mU/mg), and this activity remained constant under Pi limitation (Fig. 9C). The strain with plasmid pET2-phoR3 showed negligible CAT activity (<2 mU/mg) (Fig. 9D). It is not yet clear whether the threefold-lower CAT activity of the strain carrying pET2-phoR2 was due to an incomplete −35 region or to the fact that PhoR also activates phoRS expression to some extent under Pi excess. Nevertheless, the fact that there was no increase of phoRS expression under Pi limitation in the absence of the PhoR-binding site supports the relevance of this site for induction of the phoRS operon.
FIG. 9.
Test for the in vivo relevance of the proposed PhoR-binding site within the phoR promoter with plasmid-borne phoR-cat transcriptional fusions using C. glutamicum ATCC 13032 as a host. (A) Overview of the three phoR promoter fragments that were cloned in front of the promoterless cat gene in plasmid pET2. Fragment phoR1 (−75 to +158 relative to the transcriptional start site) covers the entire phoR promoter region, including the PhoR-binding site (BS; −61 to −43) and the −10 and −35 regions; fragment phoR2 (−42 to +158) lacks the PhoR-binding site but contains the −10 and −35 regions; and fragment phoR3 (+22 to +158) also lacks the −10 and −35 regions. (B to D) Expression of the transcriptional fusions in C. glutamicum ATCC 13032 was determined by measuring the CAT activities of cells after cultivation in CGXII glucose minimal medium without phosphate for 0 to 120 min. The values for each fusion represent averages from measurements of three independent cultures. The error bars represent standard deviations.
DISCUSSION
In our preceding study, we presented evidence that the PhoS-PhoR two-component signal transduction system of C. glutamicum is involved in the response to phosphate starvation. A ΔphoRS mutant showed impaired growth under Pi limitation, presumably because many of the genes required to cope with Pi starvation were not induced as rapidly and strongly as in the wild type (16). In the current study, we provide in vitro evidence for a direct regulation of the psi genes by PhoS-PhoR, as purified PhoR was able to bind to nine of the presumed target promoters. In many cases, response regulators that function as transcriptional activators and/or repressors show an increased DNA-binding affinity after phosphorylation, as reported, e.g., for CitB of Klebsiella pneumoniae (20) or NarL of E. coli (32). The isolated kinase domain of PhoS, which showed constitutive autophosphorylation activity, was used as a tool for the rapid in vitro phosphorylation of PhoR (Fig. 2). Phosphorylation led to an approximately fivefold increase of PhoR's DNA-binding affinity under our experimental conditions (Fig. 3 and 4). The factor of five is presumably a minimal value, as we do not know the percentage of PhoR that was actually phosphorylated in our binding assays, and is comparable to that observed for other response regulators, e.g., PhoP of B. subtilis, whose DNA-binding affinity is increased about 10-fold by phosphorylation (19).
PhoR was shown to bind to eight promoters of psi genes/operons, i.e., pstSCAB (∼35×) > phoRS (∼45×) > phoC (∼50×) > ushA (∼60×) > ugpAEBC (∼70×) > nucH and phoH1 (∼120×) > glpQ1 (>120×), but with significantly different affinities, as indicated by the values in parentheses, which denote the molar excess of PhoR∼P required to shift ∼50% of the DNA fragment. There is a correlation, although not strict, between the binding affinity and the induction kinetics of the psi genes after a shift from Pi excess to Pi starvation (13), with genes having a high affinity for PhoR being more rapidly induced than genes with a low affinity. Thus, the induction kinetics of the individual psi genes may in part be determined by the binding affinities of their promoters for PhoR∼P. The temporal pattern of induction of the different psi genes might allow the cells to adapt as efficiently and economically as possible to Pi limitation. The first response is the induction of the pstSCAB and phoRS genes, whose expression is increased up to eightfold within 10 min after a Pi downshift (13) and whose promoters show the highest affinity to PhoR (Fig. 4). The high-affinity ABC-type Pi uptake system encoded by pstSCAB allows the cell to scavenge those low Pi concentrations in the environment that cannot be taken up by low-affinity Pi transporters, e.g., PitA. The psi genes that are induced later than pstSCAB and phoRS and whose promoters have a lower affinity for PhoR encode transporters for the uptake of organophosphates, such as glycerol-3-phosphate, or extracytoplasmic enzymes, like PhoC, UshA (22), or NucH, that cleave phosphate from organophosphates and thus make it accessible for import by the PstSCAB system.
The promoter with the second-best affinity to PhoR was that of the phoRS operon itself (Fig. 4), supporting previous data that suggested a positive autoregulation of phoRS (16). Such a type of regulation is a common feature of many two-component systems and can have different functions (2). In the case of the PhoS-PhoR system, elevated PhoR levels formed through autoinduction could be necessary to control all of the putative target promoters (≥10, according to our results) and at the same time could be responsible for the apparently hierarchical organization of the regulon. Whereas uninduced levels of PhoR∼P are sufficient to induce the high-affinity pstSCAB and phoRS promoters, elevated levels of PhoR∼P might be required for induction of the lower-affinity target promoters. Thus, the PhoS-PhoR system may function as a rheostat rather than a simple switch (2). This kind of behavior was previously also described for the BvgA-BvgS system of Bordetella pertussis (4, 24).
Using mutational analysis, it could be shown that PhoR binds to 19-bp regions within the pstS and the phoR promoter (Fig. 6). An alignment of these two sequences (Fig. 7, no. 1 and 2) revealed that in particular the 5′-terminal half was very similar. OmpR-type regulators with a winged helix-turn-helix DNA-binding motif, to which PhoR belongs, commonly bind to direct-repeat sequences, although there is high variability in the nucleotide sequences, the spacing between repeat units, and the number of repeat units. Based on this trait, one might interpret the PhoR-binding sites as two very loosely conserved 8-bp direct repeats separated by a 3-bp spacer. A similar binding motif was recently described for the response regulator MprA of Mycobacterium tuberculosis (10). Support for the assumption that PhoR binds to a direct repeat was obtained by the finding that an artificial 19-bp binding site composed of two 8-bp direct repeats of the 5′-terminal half of the pstSCAB and phoRS binding sites showed a higher affinity for PhoR∼P than the natural binding motif with the highest affinity, i.e., that of the pst promoter. Moreover, reduction of the spacer between the two repeats from 3 to 2 bp almost completely prevented PhoR∼P binding. As the distance of 11 bp between the centers of the two repeats corresponds approximately to one full turn of the DNA helix, this spacing would allow the binding of two PhoR monomers in a side-by-side manner on the same face of the DNA helix. Gel filtration studies with unphosphorylated PhoR indicated an apparent molecular mass between 33 and 37 kDa (data not shown). Since PhoR (calculated mass, 26.5 kDa) is composed of two domains that are connected by a flexible linker (the receiver domain and the DNA-binding domain), its migration in gel filtration might be faster than that of the globular proteins used for calibration. Therefore, an apparent mass of ∼35 kDa is compatible with a monomeric form of PhoR. Gel filtration studies with PhoR that had been phosphorylated by preincubation with MBP-PhoSΔ1-246 and ATP led to the same results. However, as we do not know the percentage of PhoR that becomes phosphorylated in this way, definite conclusions as to the influence of phosphorylation on the oligomerization status of PhoR are not yet possible. If phosphorylated PhoR is also monomeric, binding of one monomer to an 8-bp repeat unit might favor the binding of a second monomer to the neighboring 8-bp repeat (cooperative binding). In the case of PhoP from M. tuberculosis, the response regulator with the highest sequence identity (65%) to C. glutamicum PhoR in this Mycobacterium species, binding to three direct 9-bp repeats of the consensus sequence A-C-T/G-T/G-T/G-T/C-A-A/G-C was reported, with spacer regions of 5 bp and 35 bp (8). In contrast to the phoRS operon of C. glutamicum, the phoPR operon of M. tuberculosis is negatively autoregulated, and binding of PhoP to the phoP promoter appears to be independent of phosphorylation (8).
With respect to the transcriptional start site, the PhoR-binding site is centered at position −185 in the pstSCAB promoter and at position −52 in the phoRS promoter. Whereas the latter position allows a direct interaction of PhoR with the α subunit of RNA polymerase or with the sigma factor, DNA bending or an interaction with additional regulatory proteins would be required for such an interaction in the pstSCAB promoter. As mentioned above, evidence for another PhoR-binding site in the pstSCAB promoter located between −99 and −136 was obtained (Fig. 5). A search in this region for the occurrence of the optimal binding motif (Fig. 7, no. 3) revealed the sequence TTTGTGATTAGAGTCTCCA (positions −135 to −117) as the best hit (Fig. 7, no. 4), but the match was weak. Transcriptional fusion studies showed that the DNA region between −194 and −176 upstream of the transcriptional start site is essential for PhoR-dependent induction of the pstSCAB operon in vivo, and therefore, the region from −135 to −117 alone is not sufficient (25). The in vivo relevance of the PhoR-binding site in the phoRS promoter was also supported by transcriptional fusion studies, as shown in Fig. 9.
A search for the occurrence of the currently best 19-bp PhoR-binding site in the upstream regions of the other proposed PhoR target genes phoC, ushA, porB, ugpAEBC, pitA, nucH, phoH, and glpQ1 revealed that all contained one or two sequences with similarity to the query motif (Fig. 7, no. 4 to 15). Whether they indeed represent PhoR-binding sites has yet to be confirmed. Besides a definition of the PhoR regulon, an understanding of the physiological function of the PhoRS two-component system requires knowledge of the stimulus sensed by the histidine kinase PhoS, a topic to be addressed in future studies.
Supplementary Material
Acknowledgments
This work was supported by the German ministry of Education and Research (BMBF) within the framework of genome research on prokaryotic organisms (GenoMik) through grant 031U113D/031U213D.
We thank Volker Wendisch for critically reading the manuscript.
Footnotes
Published ahead of print on 11 May 2007.
Supplemental material for this article may be found at http://jb.asm.org.
REFERENCES
- 1.Abe, S., K. Takayama, and S. Kinoshita. 1967. Taxonomical studies on glutamic acid producing bacteria. J. Gen. Appl. Microbiol. 13:279-301. [Google Scholar]
- 2.Bijlsma, J. J. E., and E. A. Groisman. 2003. Making informed decisions: regulatory interactions between two-component systems. Trends Microbiol. 11:359-366. [DOI] [PubMed] [Google Scholar]
- 3.Costa-Riu, N., E. Maier, A. Burkovski, R. Kramer, F. Lottspeich, and R. Benz. 2003. Identification of an anion-specific channel in the cell wall of the Gram-positive bacterium Corynebacterium glutamicum. Mol. Microbiol. 50:1295-1308. [DOI] [PubMed] [Google Scholar]
- 4.Deora, R., H. J. Bootsma, J. F. Miller, and P. A. Cotter. 2001. Diversity in the Bordetella virulence regulon: transcriptional control of a Bvg-intermediate phase gene. Mol. Microbiol. 40:669-683. [DOI] [PubMed] [Google Scholar]
- 5.Eggeling, L., and M. Bott (ed.). 2005. Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton, FL.
- 6.Eikmanns, B. J., N. Thum-Schmitz, L. Eggeling, K. U. Luedtke, and H. Sahm. 1994. Nucleotide sequence, expression and transcriptional analysis of the Corynebacterium glutamicum gltA gene encoding citrate synthase. Microbiology 140:1817-1828. [DOI] [PubMed] [Google Scholar]
- 7.Engels, V., and V. F. Wendisch. 2007. The DeoR-type regulator SugR represses expression of ptsG in Corynebacterium glutamicum. J. Bacteriol. 189:2955-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta, S., A. Sinha, and D. Sarkar. 2006. Transcriptional autoregulation by Mycobacterium tuberculosis PhoP involves recognition of novel direct repeat sequences in the regulatory region of the promoter. FEBS Lett. 580:5328-5338. [DOI] [PubMed] [Google Scholar]
- 9.Hanahan, D. 1985. Techniques for transformation of E. coli, p. 109-135. In D. M. Glover (ed.), DNA cloning, vol. 1. IRL Press, Oxford, United Kingdom. [Google Scholar]
- 10.He, H. J., and T. C. Zahrt. 2005. Identification and characterization of a regulatory sequence recognized by Mycobacterium tuberculosis persistence regulator MprA. J. Bacteriol. 187:202-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermann, T. 2003. Industrial production of amino acids by coryneform bacteria. J. Biotechnol. 104:155-172. [DOI] [PubMed] [Google Scholar]
- 12.Hulett, F. M. 2002. The Pho regulon, p. 193-201. In J. A. Sonenshein and R. M. Losick (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC.
- 13.Ishige, T., M. Krause, M. Bott, V. F. Wendisch, and H. Sahm. 2003. The phosphate starvation stimulon of Corynebacterium glutamicum determined by DNA microarray analyses. J. Bacteriol. 185:4519-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keilhauer, C., L. Eggeling, and H. Sahm. 1993. Isoleucine synthesis in Corynebacterium glutamicum: molecular analysis of the ilvB-ilvN-ilvC operon. J. Bacteriol. 175:5595-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klauth, P., S. R. Pallerla, D. Vidaurre, C. Ralfs, V. F. Wendisch, and S. M. Schoberth. 2006. Determination of soluble and granular inorganic polyphosphate in Corynebacterium glutamicum. Appl. Microbiol. Biotechnol. 72:1099-1106. [DOI] [PubMed] [Google Scholar]
- 16.Kocan, M., S. Schaffer, T. Ishige, U. Sorger-Herrmann, V. F. Wendisch, and M. Bott. 2006. Two-component systems of Corynebacterium glutamicum: deletion analysis and involvement of the PhoS-PhoR system in the phosphate starvation response. J. Bacteriol. 188:724-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lambert, C., D. Weuster-Botz, R. Weichenhain, E. W. Kreutz, A. A. De Graaf, and S. M. Schoberth. 2002. Monitoring of inorganic polyphosphate dynamics in Corynebacterium glutamicum using a novel oxygen sparger for real time P-31 in vivo NMR. Acta Biotechnol. 22:245-260. [Google Scholar]
- 18.Liebl, W. 2005. Corynebacterium taxonomy, p. 9-34. In L. Eggeling and M. Bott (ed.), Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton, FL.
- 19.Liu, W., Y. Qi, and F. M. Hulett. 1998. Sites internal to the coding regions of phoA and pstS bind PhoP and are required for full promoter activity. Mol. Microbiol. 28:119-130. [DOI] [PubMed] [Google Scholar]
- 20.Meyer, M., P. Dimroth, and M. Bott. 1997. In vitro binding of the response regulator CitB and of its carboxy-terminal domain to A+T-rich DNA target sequences in the control region of the divergent citC and citS operons of Klebsiella pneumoniae. J. Mol. Biol. 269:719-731. [DOI] [PubMed] [Google Scholar]
- 21.Pallerla, S. R., S. Knebel, T. Polen, P. Klauth, J. Hollender, V. F. Wendisch, and S. M. Schoberth. 2005. Formation of volutin granules in Corynebacterium glutamicum. FEMS Microbiol. Lett. 243:133-140. [DOI] [PubMed] [Google Scholar]
- 22.Rittmann, D., U. Sorger-Hermann, and V. F. Wendisch. 2005. Phosphate starvation inducible gene ushA encodes a 5′ nucleotidase required for growth of Corynebacterium glutamicum on media with nucleotides as the phosphorus source. Appl. Environ. Microbiol. 71:4339-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 24.Scarlato, V., A. Prugnola, B. Arico, and R. Rappuoli. 1990. Positive transcriptional feedback at the bvg locus controls expression of virulence factors in Bordetella pertussis. Proc. Natl. Acad. Sci. USA 87:6753-6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorger-Hermann, U. 2006. Analyse des Mechanismus der Phosphatregulation in Corynebacterium glutamicum. PhD thesis. University of Düsseldorf, Düsseldorf, Germany.
- 26.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-130. [DOI] [PubMed] [Google Scholar]
- 27.Vasicova, P., Z. Abrhamova, J. Nesvera, M. Patek, H. Sahm, and B. Eikmanns. 1998. Integrative and autonomously replicating vectors for analysis of promoters in Corynebacterium glutamicum. Biotechnol. Techniques 12:743-746. [Google Scholar]
- 28.Wanner, B. L. 1996. Phosphorus assimilation and control of the phosphate regulon, p. 1357-1381. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 29.Wendisch, V. F., and M. Bott. 2005. Phosphorus metabolism, p. 377-396. In L. Eggeling and M. Bott (ed.), Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton, FL.
- 30.Wennerhold, J., A. Krug, and M. Bott. 2005. The AraC-type regulator RipA represses aconitase and other iron proteins from Corynebacterium under iron limitation and is itself repressed by DtxR. J. Biol. Chem. 280:40500-40508. [DOI] [PubMed] [Google Scholar]
- 31.Wolschendorf, F., M. Mahfoud, and M. Niederweis. 2007. Porins are required for uptake of phosphates by Mycobacterium smegmatis. J. Bacteriol. 189:2435-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, J. H., G. P. Xiao, R. P. Gunsalus, and W. L. Hubbell. 2003. Phosphorylation triggers domain separation in the DNA binding response regulator NarL. Biochemistry 42:2552-2559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.