Abstract
Nontypeable Haemophilus influenzae (NTHi) organisms are obligate parasites of the human upper respiratory tract that can exist as commensals or pathogens. Toxin-antitoxin (TA) loci are highly conserved gene pairs that encode both a toxin and antitoxin moiety. Seven TA gene families have been identified to date, and NTHi carries two alleles of the vapBC family. Here, we have characterized the function of one of the NTHi alleles, vapBC-1. The gene pair is transcribed as an operon in two NTHi clinical isolates, and promoter fusions display an inverse relationship to culture density. The antitoxin VapB-1 forms homomultimers both in vitro and in vivo. The expression of the toxin VapC-1 conferred growth inhibition to an Escherichia coli expression strain and was successfully purified only when cloned in tandem with its cognate antitoxin. Using total RNA isolated from both E. coli and NTHi, we show for the first time that VapC-1 is an RNase that is active on free RNA but does not degrade DNA in vitro. Preincubation of the purified toxin and antitoxin together results in the formation of a protein complex that abrogates the activity of the toxin. We conclude that the NTHi vapBC-1 gene pair functions as a classical TA locus and that the induction of VapC-1 RNase activity leads to growth inhibition via the mechanism of mRNA cleavage.
Nontypeable Haemophilus influenzae (NTHi) organisms are small facultative gram-negative pleomorphic coccobacilli that lack the genes to produce and assemble a polysaccharide capsule. NTHi is fastidious and requires exogenous heme or protoporphyrin IX for aerobic growth as well as NAD when cultured in the absence of human cells. As these organisms are obligate parasites on the mucous membranes of humans, there are no natural animal or environmental reservoirs.
H. influenzae is acquired in the nasopharynx shortly after birth, and the organisms can exist as commensals or pathogens. Mucosal infections associated with NTHi include otitis media, conjunctivitis, sinusitis, epiglottitis, and pneumonia (24). NTHi is the leading cause of chronic bronchitis in adults and is implicated in exacerbations of chronic obstructive pulmonary disease (25). Otitis media is the second-most prevalent infection of young children worldwide. Hearing loss is the most common sequelae of otitis media, with behavioral, educational, and language development delays being additional consequences of early-onset otitis media with effusion. The estimated annual direct medical costs from otitis media range from $1.96 billion to $4.1 billion (33).
Invasive NTHi infections include bacteremia and meningitis; traversal of epithelial or endothelial cells by the bacterium is required for these infections to occur. Prior to 1985, most invasive H. influenzae infections were due to encapsulated type b strains. During that year, a polysaccharide conjugate vaccine against the type b polyribosylribitol phosphate capsule was licensed and distributed in the United States. Disease caused by type b has been drastically curtailed since. However, the vaccine is not protective against NTHi strains, since these strains lack the capsular antigen. Efforts to identify NTHi vaccine candidates are ongoing and include strategies using immunogenic surface-exposed loops of the P2 and P4 outer membrane proteins (15, 27), the C-terminal fragment of the Hap autotransporter (22), conjugate vaccines with outer membrane protein P6 as a carrier (37), and oral vaccines composed of killed bacterial extracts (2).
First identified in pathogenic strains of the gram-negative, strict anaerobe Dichelobacter nodosus, virulence-associated protein (vap) genes were found on a novel area of the chromosome that hybridized to nearly all virulent strains tested but to only 23% of the avirulent strains studied (16). The chromosomes of the NTHi strains Rd KW20 (11), R2866 (28), and 86-028NP (14) contain homologues of vapA, vapB, vapC, and vapD, with one gene pair, vapBC, in duplicate.
The genetic organization of the NTHi vap genes is similar to toxin-antitoxin (TA) loci. Characteristic features of TA loci are that the TA gene pair is an operon, consisting of an upstream antitoxin and a downstream toxin gene. The antitoxins prevent the effects of the toxins by forming tight complexes with them. The antitoxins are more labile than the toxins and under conditions of stress are quickly degraded by cellular proteases. This allows their cognate toxins to become active (13).
Seven TA gene families have been identified to date: relBE, parDE, higBA, vapBC, mazEF, phd-doc, and ccdAB (30). All except the vapBC and mazEF loci were originally discovered on plasmids. Although TA loci were first thought to be merely plasmid addiction modules, the discovery that these gene pairs were conserved in the chromosomes of numerous diverse prokaryotes (gram-negative and gram-positive as well as the Archaea) did not support that hypothesis and led to the notion that TA loci are important in the regulation of certain cellular functions. In a database-mining effort that included 126 completely sequenced prokaryotic genomes, 671 TA loci were identified (30). Of these, 285 (42%) were vapBC homologues.
The vapBC alleles in NTHi have been shown to be expressed by proteomic studies (12, 17, 19). As NTHi carries two chromosomal vapBC operons (HI0321-HI0322 and HI0948-HI0947), for clarity we shall refer to these as vapBC-1 and vapBC-2. Here, we describe the functional characteristics of NTHi vapBC-1 (HI0321-HI0322).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains and plasmids used in these studies are listed in Table 1. Escherichia coli strains were grown in LB broth or agar with or without 30 μg/ml kanamycin or 100 μg/ml ampicillin, as required. Induction of protein expression was with the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 2 h. For the protein-protein interaction studies, strain SU101 carrying pDD687 was grown overnight in LB broth containing 1 mM IPTG and then diluted and grown to log phase in LB containing 1 mM IPTG prior to β-galactosidase activity assays. H. influenzae strains were grown in brain heart infusion (BHI) broth or agar supplemented with 10 μg/ml heme-histidine and β-NAD (supplemented BHI). All were cultured at 37°C in room air.
TABLE 1.
Bacteria and plasmids used in this study
| Strain or plasmid | Description | Source |
|---|---|---|
| Strains | ||
| H. influenzae | ||
| 86-028NP | Otitis media isolate from a pediatric patient | R. S. Munson, Jr. |
| R2866 | Blood isolate from an immunocompetent child with meningitis immunized with the Hib vaccinea | A. L. Smith |
| E. coli | ||
| BL21(DE3) | F−ompT hsdSB(rB− mB−) gal dcm (DE3) | EMD Bioscience |
| DH5α | λ− φ80ΔlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | Laboratory collection |
| K-12 | Feces isolate from a diphtheria convalescent | ATCC |
| SU101 | lexA71::Tn5(Def) sulA211 Δ(IacIPOZYA)169/F lacIqlacZΔM15::Tn9 | M. Granger-Schnarr |
| Plasmids | ||
| pACYC184 | Source of cat gene | Laboratory collection |
| pDD676 | pTrcHisA with vapB-1 | This work |
| pDD677 | pTrcHisA with vapC-1 | This work |
| pDD686 | pET24b with vapBC-1 | This work |
| pDD687 | pSR658 with vapB-1 fused to the LexA DBD | This work |
| pDD689 | pET24b with cat | This work |
| pDD690 | pET24b with vapB-1 | This work |
| pDD693 | pMC1403 with R2866 vapBC-1 promoter fusion | This work |
| pDD694 | pMC1403 with 86-028NP vapBC-1 promoter fusion | This work |
| pET24b | C-terminal His tag expression vector | EMD Bioscience |
| pMC1403 | Promoter::lacZ fusion vector | 5 |
| pSR658 | LexA DBD fusion vector | Laboratory collection |
| pTrcHisA | Expression vector with N-terminal polyhistidine tag | Invitrogen |
Hib, H. influenzae type b.
Cloning of vapB-1, vapC-1, and cat.
Each vap gene was cloned into the expression vectors pTrcHisA and pET24b as a single copy. In addition, both were cloned in a tandem fashion in pET24b. The cat gene was cloned into pET24b. All primers used in this study were synthesized by Integrated DNA Technologies (Coralville, IA). For pTrcHisA cloning, vapB-1 was amplified by PCR using the primers 321 forward (5′-GAGAGAATTCATATGCTTACTAAAGTG-3′) and 321 reverse (5′-AACAAAGCTTTCATAAATTTTCTCGC-3′). The primers included the engineered restriction sites EcoRI and HindIII (underlined), respectively, and resulted in pDD676. Similarly, vapC-1 was amplified using the primers 322 forward (5′-GCGAGAATTCTTATGATTTATATGTTAG-3′) and 322 reverse (5′-TCAGAAGCTTCTATTTTGTCCAATCTTGCC-3′) with the same engineered sites, resulting in pDD677. Both constructs were ligated into EcoRI/HindIII-cut pTrcHisA and expressed in E. coli strain DH5α. For pET24b cloning, vapB-1 was amplified using the primers 2866B Sac forward (5′-GGAGGAGCTCTATGCTTACTAAAGTG-3′) and 2866 B1 Xho reverse (5′-ATATCTCGAGTAAATTTTCTCGCTCC-3′), with engineered SacI and XhoI sites (underlined), respectively. Ligation of the amplicon into SacI/XhoI-cut pET24b resulted in the fusion of VapB-1 to the C-terminal polyhistidine tag in pDD690. Both vapBC genes were cloned in tandem into pET24b using the primers 2866B Sac forward (5′-GGAGGAGCTCTATGCTTACTAAAGTG-3′) and VapC Xho reverse (5′-GAATCTCGAGTTTTGTCCAATCTTGCC-3′), and the amplicon was ligated into SacI/XhoI-cut pET24b, resulting in pDD686. This strategy resulted in the fusion of only VapC-1 to the C-terminal polyhistidine tag. The cat gene from pACYC184 was amplified using the primers CAT Sac forward (5′-AGGAGAGCTCTATGGAGAAAAAAATCACTGG-3′) and CAT Xho reverse (5′-AAAACTCGAGCGCCCCGCCCTGCCACTC-3′) and ligated to SacI/XhoI-cut pET24b, resulting in Cat with a polyhistidine-tag in pDD689. Each pET24b-based construct was expressed in E. coli BL21(DE3).
Homodimerization assays.
For these assays, vapB-1 was fused to the LexA DNA-binding domain (DBD) in the vector pSR658 (7), resulting in pDD687, and was expressed in the reporter strain SU101. This strain has a chromosomal construct that consists of a lacZ reporter gene controlled by the strong sulA promoter, which contains an LexA operator sequence. When there is no fusion to the LexA DBD, the strain constitutively expresses a high level of β-galactosidase. However, if a protein fused to the LexA DBD in pSR658 can homodimerize, this results in a competent LexA dimer that can bind to the LexA operator and repress transcription of lacZ. Expression of the LexA fusion in pSR658 is induced by IPTG, and since β-galactosidase is a very long-lived enzyme, the reporter strain is routinely grown overnight in the inducer, so that any enzyme that was transcribed prior to induction of the LexA chimera has the opportunity to degrade. This results in a more reliable and specific measurement of homodimerization.
Following overnight incubation in LB broth with 1 mM IPTG, the reporter strain carrying pDD687 was diluted and grown to log phase in LB broth with 1 mM IPTG. The amount of homodimerization was quantitated by β-galactosidase activity assays and compared to the reporter strain carrying pSR658 (no fusion).
Protein purification and antibodies.
Proteins were purified from induced cultures using a MagneHis protein purification system (catalog no. V8500; Promega) according to the manufacturer's instructions. Briefly, E. coli strains DH5α or BL21(DE3) carrying the various fusions were grown to log phase in LB broth with appropriate antibiotics and induced for 2 h with 1 mM IPTG. The cells were pelleted, frozen at −80°C, and subjected to three freeze-thaw cycles prior to being processed using the MagneHis kit protocol for native purification. Protein concentration was quantitated using the micro bicinchoninic acid protein assay (catalog no. 23235; Pierce). Aliquots of purified protein were frozen at −80°C and thawed when needed, after which the thawed aliquot was held at 4°C until used in assays.
Antibodies used to probe immunoblots were monoclonal anti-Xpress-horseradish peroxidase (HRP) (R911-25; Invitrogen) for pTrcHis fusions and monoclonal anti-His (C-terminal)-HRP (R931-25; Invitrogen) for pET24b fusions. Immunoblots were developed using the SuperSignal West Pico substrate (34080; Pierce). Sodium dodecyl sulfate (SDS)-polyacrylamide gels were stained with Coomassie blue and destained with 10% acetic acid.
Total RNA isolation.
Cultures of E. coli K-12 (ATCC 10798) and H. influenzae strains R2866 and 86-028NP were grown to log phase and processed using an SV total RNA isolation kit (Z3101; Promega) according to the manufacturer's instructions. The purified total RNA was then subjected to spectrophotometry at 260 and 280 nm to determine its purity and concentration. Aliquots were frozen at −80°C, and each aliquot was thawed only once before use in reverse transcriptase PCR or RNase activity assays.
Reverse transcriptase PCR.
First-strand cDNA synthesis was performed using avian myeloblastosis virus (AMV) reverse transcriptase according to the manufacturer's instructions (M5101; Promega). Briefly, aliquots of purified total RNA corresponding to 250 nanograms from NTHi strain R2866 or 86-028NP were incubated at 70°C for 5 min with the primer RT BC1 reverse (5′-CAATGCGTGACAAGCGATCC-3′), which anneals to vapC-1. The mixtures were chilled on ice, centrifuged to collect, and then incubated at 37°C for 1 h with 1 μl (10 U) of AMV reverse transcriptase, 4 μl of 5× AMV buffer, 1 μl of 10 mM deoxynucleotide triphosphate mixture, and nuclease-free water to a final volume of 20 μl. Control reaction mixtures were identical but included no AMV reverse transcriptase. Following this incubation, a 2.5-μl aliquot of each cDNA synthesis reaction mixture was used as the template for conventional PCR with the primers RT BC1 forward (5′-CAAAAGTGGTAACAGCCAAGC-3′), which anneals to vapB-1, and RT BC1 reverse (5′-CAATGCGTGACAAGCGATCC-3′), which anneals to vapC-1.
Promoter-reporter gene fusion construction.
A 340-bp sequence located upstream of the vapBC-1 allele in strains R2866 and 86-028NP that included the first seven amino acids of vapB-1 was amplified by PCR using the primers BC 1403 forward (5′-ACTAGAATTCATCATTTACTCATTGACTTGC-3′) and BC 1403 reverse (5′-GTTAGGATCCTGAAACACTTTAGTAAGC-3′), which included engineered EcoRI and BamHI sites (underlined), respectively. These fragments were then ligated in frame with a promoterless lacZ reporter gene in the vector pMC1403 (5), creating pDD693 (R2866 vapBC-1 promoter fusion) and pDD694 (86-028NP vapBC-1 promoter fusion). β-Galactosidase activity assays were performed in triplicate during lag phase and early, mid-, and late logarithmic phases.
RNase activity assays.
Aliquots of purified VapC-1, VapB-1, or Cat proteins were incubated at 37°C for 15 min with approximately 400 nanograms of purified total RNA from E. coli or H. influenzae in a buffer consisting of 10 mM HEPES (pH 7.4)-15 mM NaCl in a final volume of 10 μl. Negative controls consisted of the MagneHis protein elution buffer alone. For some assays, VapB-1 and VapC-1 were preincubated together at room temperature for 30 min prior to the addition of total RNA substrate, after which the reactions were held at 37°C for 15 min. Each reaction was stopped by the addition of 2 μl of 6× loading buffer and separated on 1% Tris-borate-EDTA agarose gels with 0.8 μg/ml ethidium bromide. All solutions used were nuclease free or treated with diethyl pyrocarbonate. Spot density was measured using the FluorChem IS-8900 program (Alpha Innotech, San Leandro, CA) with automatic background determination.
RESULTS
Both NTHi strains R2866 and 86-028NP express vapBC-1.
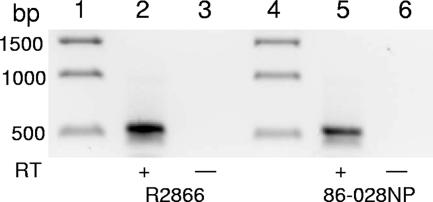
The genomes of the NTHi invasive blood isolate R2866 (28) and pediatric otitis media isolate 86-028NP (14) have been completely sequenced, and the vapBC-1 allele is identical at the DNA level in both strains. To determine if vapBC-1 was expressed, total RNA from each strain grown to log phase in supplemented BHI broth was subjected to reverse transcriptase PCR using a forward primer that annealed to vapB-1 and a reverse primer located in vapC-1. The resulting 535-bp product in the reactions that included AMV reverse transcriptase, but not in the control reactions without reverse transcriptase, showed that both R2866 and 86-028NP express vapBC-1 and transcribe the gene pair as an operon (Fig. 1, lanes 2 and 5, respectively).
FIG. 1.
NTHi strains R2866 and 86-028NP express vapBC-1 as an operon. Reverse transcriptase PCRs with a forward primer in vapB-1 and a reverse primer in vapC-1 electrophoresed on a 0.8% agarose gel. Lanes 1 and 4, 1-kb DNA ladder; lanes 2 and 5, R2866 and 86-028NP with reverse transcriptase (+RT); lanes 3 and 6, R2866 and 86-028NP without reverse transcriptase (−RT).
Expression of vapBC-1 is inversely related to culture density.
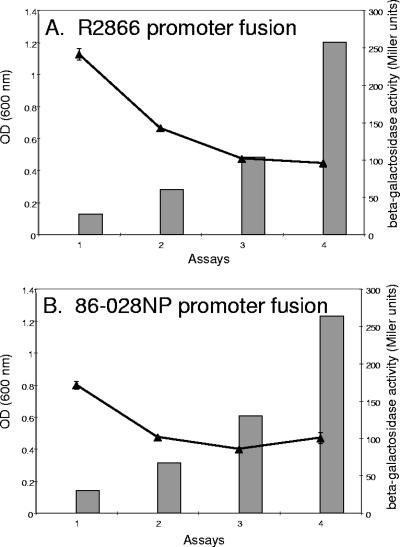
Fragments that included the predicted promoter and transcription start sites of vapBC-1 in R2866 and 86-028NP identified by a neural network program (available at http://searchlauncher.bcm.tmc.edu/seq-search/gene-search.html), which also included the first seven amino acids of vapB-1, were fused in frame with a promoterless lacZ reporter gene in pMC1403 and transferred to E. coli strain DH5α. Since there was a single-nucleotide polymorphism between strains R2866 and 86-028NP at a site located 10 bases into the predicted transcript, with five additional polymorphisms scattered upstream, we fused both promoter regions to the reporter gene and analyzed the activity of each at culture densities corresponding to lag phase and early, mid-, and late logarithmic phases (Fig. 2). We found that there was relatively greater β-galactosidase activity in the strain carrying the R2866 promoter fusion during lag phase than in the strain carrying the 86-028NP promoter fusion, with an average, respectively, of 241 ± 15 versus 171 ± 10 Miller units (Fig. 2A and B, respectively). In both cases, the activity decreased with increasing culture density and approached identical levels during late growth phase (95 ± 2 versus 100 ± 15 Miller units, respectively). The negative control, pMC1403 with no promoter fusion, did not deliver detectable β-galactosidase activity at any point during the growth cycle (data not shown).
FIG. 2.
Expression of vapBC-1 over the cell cycle. (A) The β-galactosidase activity of the R2866 vapBC-1 promoter fusion (curve) is inversely related to culture density (bars). (B) The 86-028NP vapBC-1 promoter fusion (curve) displays lower initial β-galactosidase activity than the R2866 fusion but mirrors the trend toward decreasing expression with increasing culture density (bars). OD, optical density.
Purified NTHi VapB-1 forms multimers in vitro.
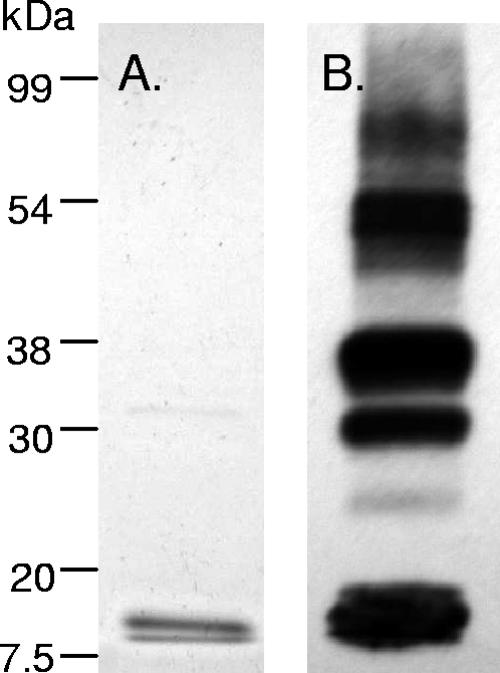
VapB-1 is the putative antitoxin protein of the vapBC-1 operon. We used the pTrcHisA vector (Invitrogen, Carlsbad, CA) to clone, overexpress, and natively purify VapB-1. This vector includes an N-terminal polyhistidine fusion that allows affinity purification using paramagnetic nickel beads, as well as an epitope tag (Xpress) for which a monoclonal HRP-linked antibody is commercially available. Figure 3A shows a Coomassie-stained 12% SDS-PAGE separation of a typical pTrc::VapB-1 purification from E. coli strain DH5α. Figure 3B is an identical immunoblot probed with the monoclonal anti-Xpress HRP-linked antibody. In this fusion, the calculated molecular mass of pTrc::VapB-1 is 13.2 kDa. Note that there is an enormous difference in sensitivity between Coomassie staining and chemiluminescent detection methods. Even after pTrc::VapB-1 is heated in SDS-polyacrylamide gel electrophoresis (PAGE) loading buffer with a reducing agent and separated on a gel, it is apparent that pTrc::VapB-1 is forming multimers.
FIG. 3.
VapB-1 forms multimers in vitro. (A) Coomassie-stained 12% SDS-PAGE separation of a typical purified pTrc::VapB-1 construct (2 μg) from DH5α. (B) An identical immunoblot probed with anti-Xpress-HRP monoclonal antibody. Note the multiple bands resulting from apparent homo-interactions.
The VapB-1 protein homodimerizes in vivo.
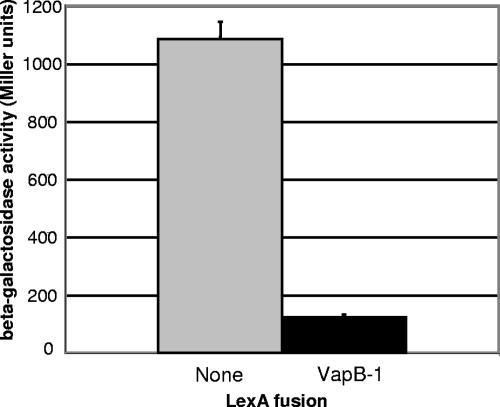
Since we observed multimerization of the pTrc::VapB-1 purified protein on an immunoblot (Fig. 3B) and because many TA antitoxins display homo-interactions (38), VapB-1 was fused to the LexA DBD in a bacterial-based protein-protein interaction reporter system to quantitate its homodimerization (7). The LexA protein consists of two domains, one that recognizes and binds to a specific operator site on DNA and one that functions as a dimerization domain. The LexA dimerization domain can be removed and replaced with another protein or fragment. Since the repressor is only active as a dimer, the homodimerization of the fused protein allows the chimeric LexA to bind to its operator site and repress transcription of a lacZ reporter gene. This interaction was quantitated by β-galactosidase activity assays (Fig. 4), and we found that the NTHi antitoxin VapB-1 interacted strongly with itself in vivo. The reporter strain SU101 expressing the control unfused LexA DBD had a mean activity of 1,086 ± 59 Miller units, and the same strain expressing the LexA-VapB-1 fusion had a mean activity of 123 ± 10 Miller units (n = 3; P < 0.0001, two-tailed t test). This represents an 88% repression of the reporter gene in this system, evidence of strong VapB-1 homo-interaction.
FIG. 4.
VapB-1 homodimerizes in vivo. With no protein fused to the LexA DBD, the repressor cannot form a dimer, and transcription of the lacZ reporter gene is constitutive (gray bar). However, when the LexA DBD is fused to VapB-1, competent dimers are formed, and the chimeras can bind to the LexA operator sequence, repressing transcription of the reporter gene (black bar). This level of repression indicates strong VapB-1 homo-interaction.
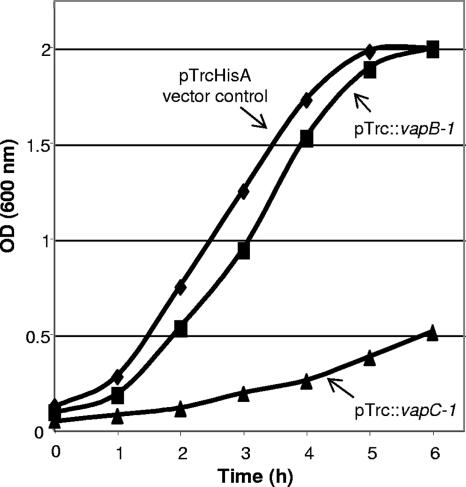
NTHi VapC-1 causes growth inhibition in vivo.
The toxin portion of the R2866 vapBC-1 allele, vapC-1, was also cloned into the pTrcHisA vector as an N-terminal polyhistidine fusion. However, even in the absence of the gratuitous inducer IPTG, carrying the plasmid containing the toxin conferred growth inhibition to the E. coli expression strain DH5α, making this construct unsuitable for protein purification. Figure 5 shows a representative growth curve of DH5α carrying either the pTrcHisA vector, the pTrc::vapB-1 fusion, or the pTrc::vapC-1 fusion in LB broth. We hypothesized that the observed attenuation of growth caused by pTrc::vapC-1 was due to the type of promoter used in the vector, which is leaky enough to allow some transcription of a cloned gene even in the absence of the inducer. The growth inhibition resulting from unintended protein expression indicated to us not only that the R2866 vapC-1 gene encoded a functional protein but also that it was toxic to E. coli cells in very small quantities.
FIG. 5.
VapC-1 causes growth inhibition in vivo. When grown in LB broth without IPTG, the pTrcHisA vector promoter allowed a small amount of transcription of pTrc::VapC-1, which conferred growth inhibition to the DH5α expression strain and made this construct unsuitable for protein isolation. No significant growth effects were observed with the pTrc::VapB-1 fusion or the vector alone. OD, optical density.
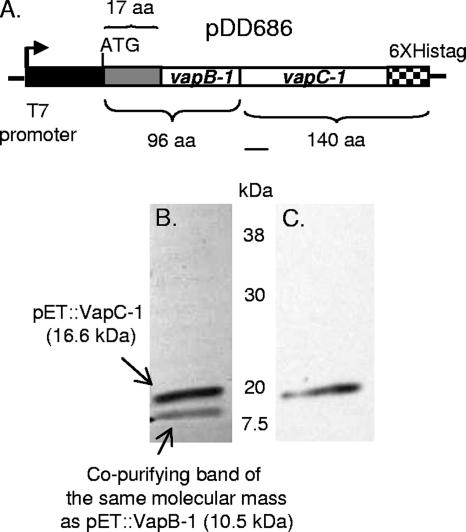
The VapC-1 toxin was natively purified by tandem cloning with the VapB-1 antitoxin.
As in most cases, nature provided the most elegant and simple solution to the biological challenge of purifying a toxin protein, and we took advantage of this by the tandem cloning of the R2866 vapBC-1 TA operon into the expression vector pET24b (69750-3; EMD Biosciences), such that the T7 promoter drove the operon's transcription, but the C-terminal polyhistidine tag was fused to VapC-1 only, creating pDD686 (Fig. 6A). The vector pET24b is highly regulated, since the T7 phage promoter that controls transcription requires prior induction of its cognate RNA polymerase. Thus, when the T7 RNA polymerase is induced by IPTG in E. coli strain BL21(DE3), both vapB-1 and vapC-1 are transcribed, but only vapC-1 has a polyhistidine tag. This strategy ensured that each full-length VapC-1 toxin was paired with at least one full-length VapB-1 antitoxin and resulted in the restoration of normal growth in the expression strain (data not shown). In this system, the apparent molecular mass of pET::VapB-1 is 10.5 kDa and that of pET::VapC-1 is 16.6 kDa. Figure 6B shows a Coomassie-stained SDS-PAGE gel of a typical VapC-1 paramagnetic bead purification. Because we were purifying VapC-1 from a tandem cloning and TA loci proteins are known to form tight heterocomplexes, we hypothesized that we might observe at least some VapB-1 copurifying with VapC-1. Note that a band of the approximate size of pET::VapB-1 appears with His-tagged pET::VapC-1 purified from BL21(DE3) on the Coomassie-stained gel (Fig. 6B). In contrast to the results observed with pTrc::VapB-1, an immunoblot of pET::VapC-1 probed with an anti-C-terminal polyhistidine tag HRP-linked monoclonal antibody showed only a single band at approximately 17 kDa (Fig. 6C). The fact that we were able to successfully purify a native His-tagged VapC-1 toxin from this tandem cloning is evidence that the presence of VapB-1 is necessary to prevent the VapC-1-induced growth inhibition of the expression strain.
FIG. 6.
VapC-1 was successfully purified via tandem cloning into pET24b and expression in BL21(DE3). (A) The vapBC-1 operon was fused to pET24b such that vapB-1 was in frame with the vector's ATG start codon at the N terminus, and vapC-1 was in frame with the C-terminal polyhistidine tag, creating pDD686. Induction of the construct with IPTG resulted in no significant growth inhibition of the expression strain. (B) Coomassie-stained 12% SDS-PAGE separation of a typical purified pET::VapC-1 construct (3.5 μg). Two bands are visible: one at the calculated molecular mass of pET::VapC-1 (16.6 kDa) and one at the size of pET::VapB-1 (10.5 kDa). (C) Identical immunoblot probed with anti-His C-terminal HRP-linked monoclonal antibody. Note that only a single band is apparent.
The VapC-1 toxin is an RNase that acts on free RNA in vitro.
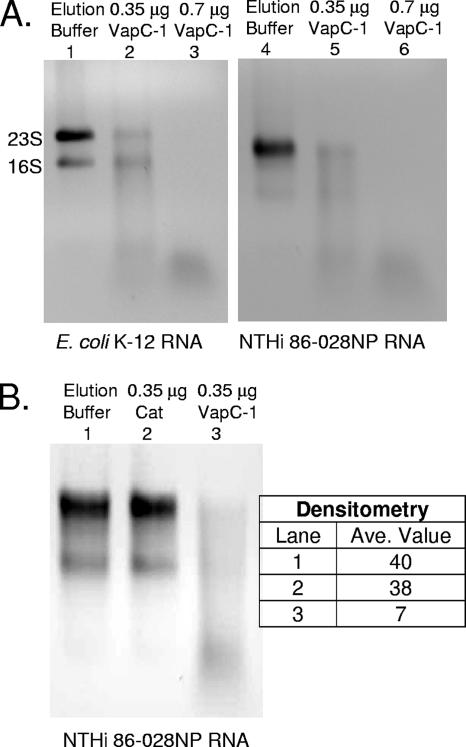
The VapC-1 toxin contains a motif known as a PilT N terminus (PIN) domain that consists of about 100 amino acids with two nearly invariant aspartate residues. These residues have been shown to be essential for metal ion coordination in other PIN domain-containing proteins that are ribonucleases (10). The PIN domain protein family displays similarity to the nuclease domains of Taq polymerase, T4 RNase H, and the 5′-3′ flap endonucleases (3). Accordingly, we performed RNase activity assays with purified VapC-1 to determine whether it was enzymatically active on purified total RNA in vitro. As a control to ensure that any activity observed with VapC-1 was specific and not due to an RNase that may have copurified with the protein, the chloramphenicol acetyltransferase (cat) gene from pACYC184 was fused to the pET24b vector, overexpressed in the E. coli strain BL21(DE3), and natively purified in an identical fashion to VapC-1. The Cat protein is a known homotrimer that displays no RNase activity.
Figure 7A shows that the VapC-1 toxin can efficiently degrade both E. coli K-12 and H. influenzae strain 86-028NP total RNA in a concentration-dependent manner in vitro. Note the natural 23S rRNA cleavage pattern of strain 86-028NP. That Haemophilus species display 23S fragmentation heterogeneity is a previously reported phenomenon (34). Figure 7B shows that the VapC-1 RNase activity is specific, as incubating strain 86-028NP total RNA substrate with the same amount of the Cat protein—which had been cloned, overexpressed, and purified in an identical fashion as VapC-1—results in no significant degradation as quantitated by densitometry.
FIG. 7.
VapC-1 is an RNase toxin. (A) Total RNA from E. coli K-12 or H. influenzae strain 86-028NP was used as the substrate in RNase activity assays with increasing amounts of the purified VapC-1 toxin. Lanes 1 and 4, MagneHis protein elution buffer control; lanes 2 and 5, 0.35 μg of VapC-1; lanes 3 and 6, 0.7 μg of VapC-1. (B) The Cat protein was cloned into pET24b and purified in the same manner as VapC-1 as a control for any copurifying RNase activity. Lane 1, MagneHis protein elution buffer control; lane 2, 0.35 μg of Cat protein; lane 3, 0.35 μg of VapC-1. Densitometry indicated that the observed RNase activity was specific to VapC-1. Ave, average.
The VapC-1 toxin cannot degrade double- or single-stranded DNA.
To determine whether VapC-1 had general nuclease activity, a 760-bp PCR product of the cat gene was used as the substrate in nuclease assays. These assays were identical to the RNase activity assays, except that the substrate was linear, double-stranded DNA. No significant difference was observed between assays containing either the elution buffer control or VapC-1, indicating that it displayed no DNase activity under these conditions (data not shown). A purified plasmid was tested as a circular double-stranded DNA substrate but was not degraded by VapC-1. Finally, an aliquot of M13mp18 single-stranded DNA (N4040S; New England Biolabs) was used as a substrate, with identical results.
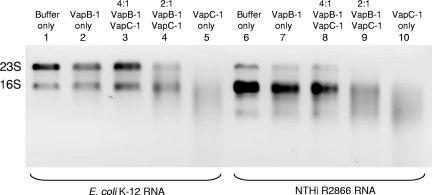
The VapB-1 antitoxin inhibits VapC-1 RNase activity.
Following translation, canonical toxin and antitoxin proteins interact to form nontoxic complexes, and we observed the rescue of growth inhibition when VapB-1 and VapC-1 were expressed together in E. coli. To investigate whether these complexes could be reconstituted in vitro with purified proteins, we preincubated various ratios of the purified VapB-1 antitoxin and the VapC-1 toxin prior to adding the total RNA substrate. For these assays, we cloned VapB-1 into the pET24b vector as a single gene and purified it in an identical manner as the VapC-1 protein, so that both proteins would be expressed from the same vector in the same expression strain. Figure 8 shows that VapB-1 has no intrinsic RNase activity by itself and that preincubation with a 4:1 ratio of VapB-1 to VapC-1 abrogates the RNase activity of VapC-1. A 2:1 ratio of VapB-1 to VapC-1 partially inhibits the RNase activity of VapC-1. For these assays, we used both E. coli K-12 total RNA and NTHi strain R2866 total RNA as substrates. Note that the natural 23S rRNA fragmentation pattern of strain R2866 differs from that of strain 86-028NP.
FIG. 8.
VapB-1 forms nontoxic complexes with VapC-1 in vitro. The antitoxin VapB-1 was cloned into pET24b as a single gene and purified using the MagneHis native protein purification protocol. Various amounts of purified VapB-1 were incubated with a constant amount of VapC-1 for 30 min prior to the addition of the total RNA substrate in RNase activity assays. A 4:1 ratio of VapB-1 to VapC-1 abrogates the RNase activity of VapC-1. Lanes 1 and 6, MagneHis protein elution buffer control; lanes 2 and 7, 0.2 μg of VapB-1; lanes 3 and 8, 0.4 μg of VapB-1 plus 0.1 μg of VapC-1 (4:1 ratio); lanes 4 and 9, 0.2 μg of VapB-1 plus 0.1 μg of VapC-1 (2:1 ratio); lanes 5 and 10, 0.1 μg of VapC-1 alone. For these assays, H. influenzae strain R2866 total RNA was used. Note the natural 23S fragmentation pattern, which differs from that of strain 86-028NP.
DISCUSSION
E. coli expresses the chromosomal TA loci relBE and mazEF.
The RelE toxin has been identified as a global inhibitor of translation and is induced under starvation conditions (6). RelE is a sequence-specific RNase, cleaving mRNA in the A site of the ribosome, but it is unable to degrade free RNA (32). The MazE antitoxin is degraded by the cellular protease ClpPA (1), and this results in the activation of the toxin MazF, which cleaves single-stranded RNA independently of ribosomes. This makes MazF functionally distinct from the RelE toxin (39). In this study, we show that NTHi VapC-1 displays MazF-like activity; that is, the toxin is active on free RNA in vitro. Interestingly, H. influenzae does not contain an mazEF homologue, whereas E. coli, on the other hand, does not contain any vapBC homologues. Further support for this apparent trend toward mutual exclusivity comes from a group that surveyed 23 separate genomes and found that 20 contained either an mazEF locus or a vapBC locus but not both (40).
Although most organisms carry various TA loci in their genomes, the reason why these genes are so highly conserved as well as what their exact purpose might be remains controversial. Some investigators posit that free-living organisms carry more TA loci in their genomes than host-adapted organisms because the former encounter harsh environmental conditions, whereas adaptation to a host organism may require only a few TA operons to serve a similar function (30). One caveat to this view is that the prevalence of TA modules in a genome may not always correlate with their expression or activity. In E. coli, it was originally thought that the toxins RelE and MazF killed the cells, since investigators observed a dramatic loss of colony formation when these toxins were released from their antitoxins (13). It was later shown, however, that these toxins did not actually kill the cells but, instead, contributed to a reversible bacteriostatic state in which the cells were unable to form a colony; they were viable but nonreplicative. Expression of the cognate antitoxins rescued these cells and allowed colony formation (6, 31). It has been suggested that induction of a reversible viable but nonreplicative state is important for cells to survive in a competitive or harsh environment in nature or inside a host (24), and there is evidence that H. influenzae enters a bacteriostatic state in the middle ear during the course of otitis media with effusion (26). In addition to enhancing survival under suboptimal conditions, a strategy of reversible growth arrest could provide a mechanism for nonspecific antibiotic resistance (nongrowing cells are not susceptible to the cidal action of most antibiotics) as well as decrease each cell's metabolic burden and energy requirements.
A recent competing hypothesis put forward is that the mazEF TA locus can induce a programmed cell death (PCD) cycle that mimics the PCD observed in multicellular organisms (8). This line of reasoning is supported by the observation that, while the MazE antitoxin can rescue MazF toxin overexpression in the short term, there seems to be a “point of no return” after which no amount of MazE antitoxin will allow the culture to recover. However, there are three points to consider in this debate. First, the results of these in vitro PCD experiments seem to show that, even after 24 h, there is a very small (1 to 5%) portion of the culture that does survive (18). This could be enough to maintain a “founder” population that could resume replication after conditions improved, particularly in vivo (20). Second, a confounding element of life on human mucosal surfaces is that many organisms, including H. influenzae, form biofilms in this environment, and growth in biofilms has been shown to modulate the metabolic characteristics of a number of organisms compared to those grown in planktonic culture (21, 36). Further, even within an in vitro planktonic culture, there is an observable difference between the amount of bacterial cells that persist following antibiotic treatment, which depends upon whether the culture is in logarithmic or stationary phase at the time the antibiotic is added (35). Third, the aforementioned experiments were performed with one type of TA locus (mazEF), and the results observed regarding this particular module might not be generally applicable to the TA superfamily as a whole. Indeed, a recent study of one of the two higBA TA alleles carried in the superintegron of chromosome II of Vibrio cholerae suggested that the expression of the HigB toxin at endogenous levels for several hours was not bactericidal, but the function of the toxin was not elucidated (4). Therefore, at this point, it may be premature to accept either view as being entirely correct.
Our results indicate that the vapBC-1 allele in two clinical isolates of NTHi is expressed as a functional TA locus with VapB-1 as the antitoxin and VapC-1 as the RNase toxin. The gene pair is transcribed as an operon in both NTHi strains, and each promoter appears to be more active during lag phase, with expression displaying an inverse relation to culture density. VapC-1 is active on free RNA purified from two different genera in vitro but does not display general nuclease activity, as it did not degrade double- or single-stranded DNA under the same conditions. The homodimerization observed with VapB-1, coupled with the ratio of antitoxin to toxin protein required to abrogate VapC-1 activity, suggests that the nontoxic VapBC-1 complex consists of VapB-1 multimers interacting with VapC-1 moieties in vivo. This is reminiscent of the MazEF complex, which has been shown to form a heterohexamer (38). The degradation of the labile VapB-1 antitoxin under stress would allow the stable VapC-1 toxin to actively inhibit protein synthesis by cleaving mRNA. The entry of NTHi into a bacteriostatic state induced by suboptimal conditions could be involved in mucosal infections that recur following antibiotic treatment, such as otitis media and bronchitis. On the other hand, even if most of the infecting population were killed by toxin induction, a small surviving segment could be responsible for the reseeding of tissues observed in chronic disease.
Interestingly, TA loci are conserved (often in multiple copies) in the genomes of many organisms that can cause persistent infections and/or persist in the environment: Mycobacterium tuberculosis, Helicobacter pylori, Coxiella burnetii, Leptospira interrogans, V. cholerae, and Salmonella enterica serovars Typhi and Typhimurium, as well as H. influenzae (9, 23, 29, 30). It is intriguing that a human-adapted organism such as H. influenzae would maintain two vapBC alleles on its very small (∼2.0 Mb) chromosome and suggests that both may be important for its lifestyle. Further studies are planned to investigate the in vivo function and synergy of the NTHi vap alleles.
Acknowledgments
We thank Arnold L. Smith and Robert S. Munson, Jr., for their kindness in supplying the NTHi strains R2866 and 86-028NP, respectively, and Huguette Albrecht for helpful discussions.
This research was supported by NIH grants R01 HL061507 and R01 HL070752 (to S.Y.Y.) and R01 HL073324 (to M.H.W.).
Footnotes
Published ahead of print on 11 May 2007.
REFERENCES
- 1.Aizenman, E., H. Engelberg-Kulka, and G. Glaser. 1996. An Escherichia coli chromosomal “addiction module” regulated by guanosine [corrected] 3′,5′-bispyrophosphate: a model for programmed bacterial cell death. Proc. Natl. Acad. Sci. USA 93:6059-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arandjus, C., P. N. Black, P. J. Poole, R. W. Baker, and C. Steurer-Stey. 2006. Oral bacterial vaccines for the prevention of acute exacerbations in chronic obstructive pulmonary disease and chronic bronchitis. Respir. Med. 100:1671-1681. [DOI] [PubMed] [Google Scholar]
- 3.Arcus, V. L., P. B. Rainey, and S. J. Turner. 2005. The PIN-domain toxin-antitoxin array in mycobacteria. TRENDS Microbiol. 13:360-365. [DOI] [PubMed] [Google Scholar]
- 4.Budde, P. P., B. M. Davis, J. Yuan, and M. K. Waldor. 2007. Characterization of a higBA toxin-antitoxin locus in Vibrio cholerae. J. Bacteriol. 189:491-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadaban, M. J., J. Chou, and S. N. Cohen. 1980. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J. Bacteriol. 143:971-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen, S. K., M. Mikkelsen, K. Pedersen, and K. Gerdes. 2001. RelE, a global inhibitor of translation, is activated during nutritional stress. Proc. Natl. Acad. Sci. USA 98:14328-14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daines, D. A., M. Granger-Schnarr, M. Dimitrova, and R. P. Silver. 2002. Use of a LexA-based system to identify protein-protein interactions in vivo. Methods Enzymol. 358:153-161. [DOI] [PubMed] [Google Scholar]
- 8.Engelberg-Kulka, H., S. Amitai, I. Kolodkin-Gal, and R. Hazan. 2006. Bacterial programmed cell death and multicellular behavior in bacteria. PLOS Genet. 2:1518-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faruque, S. M., K. Biswas, S. M. Nashir Udden, Q. S. Ahmad, D. S. Sack, G. B. Nair, and J. J. Mekalanos. 2006. Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc. Natl. Acad. Sci. USA 103:6350-6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fatica, A., D. Tollervey, and M. Dlakic. 2004. PIN domain of Nob1p is required for D-site cleavage in 20S pre-rRNA. RNA 10:1698-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleischmann, R., M. Adams, O. White, R. Clayton, E. Kirkness, A. Kerlavage, C. Bult, J. Tomb, B. Dougherty, and J. Merrick. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. [DOI] [PubMed] [Google Scholar]
- 12.Fountoulakis, M., J. F. Juranville, D. Roder, S. Evers, P. Berndt, and H. Langen. 1998. Reference map of the low molecular mass proteins of Haemophilus influenzae. Electrophoresis 19:1819-1827. [DOI] [PubMed] [Google Scholar]
- 13.Gotfredsen, M., and K. Gerdes. 1988. The Escherichia coli relBE genes belong to a new toxin-antitoxin gene family. Mol. Microbiol. 29:1065-1076. [DOI] [PubMed] [Google Scholar]
- 14.Harrison, A., D. W. Dyer, A. Gillaspy, W. C. Ray, R. Mungur, M. B. Carson, H. Zhong, J. Gipson, M. Gipson, L. S. Johnson, L. Lewis, L. O. Bakaletz, and R. S. Munson, Jr. 2005. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J. Bacteriol. 187:4627-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotomi, M., Y. Ikeda, M. Suzumoto, K. Yamauchi, B. A. Green, G. Zlotnick, D. S. Billal, J. Shimada, K. Fujihara, and N. Yamanaka. 2005. A recombinant P4 protein of Haemophilus influenzae induces specific immune responses biologically active against nasopharyngeal colonization in mice after intranasal immunization. Vaccine 23:1294-1300. [DOI] [PubMed] [Google Scholar]
- 16.Katz, M. E., R. A. Strugnell, and J. I. Rood. 1992. Molecular characterization of a genomic region associated with virulence in Dichelobacter nodosus. Infect. Immun. 60:4586-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolker, E., S. Purvine, M. Y. Galperin, S. Stolyar, D. R. Goodlett, A. I. Nesvizhskii, A. Keller, T. Xie, A. F. Picone, T. Cherny, J. K. Eng, E. Yi, L. Hood, A. F. Siegel, T. J. Reilly, K. S. Makarova, B. O. Palsson, and A. L. Smith. 2003. Initial proteome analysis of the model organism Haemophilus influenzae Rd KW20. J. Bacteriol. 185:4593-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolodkin-Gal, I., and H. Engelberg-Kulka. 2006. Induction of Escherichia coli chromosomal mazEF by stressful conditions causes an irreversible loss of viability. J. Bacteriol. 188:3420-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langen, H., B. Takacs, S. Evers, P. Berndt, H.-W. Lahm, B. Wipf, C. Gray, and M. Fountoulakis. 2000. Two-dimensional map of the proteome of Haemophilus influenzae. Electrophoresis 21:411-429. [DOI] [PubMed] [Google Scholar]
- 20.Lewis, K. 2005. Persister cells and the riddle of biofilm survival. Biochemistry 70:267-274. [DOI] [PubMed] [Google Scholar]
- 21.Lewis, K. 2007. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 5:48-56. [DOI] [PubMed] [Google Scholar]
- 22.Liu, D. F., K. M. Mason, M. Mastri, M. Pazirandeh, D. Cutter, D. L. Fink, J. W. St. Geme III, D. Zhu, and B. A. Green. 2004. The C-terminal fragment of the internal 110-kilodalton passenger domain of the Hap protein of nontypeable Haemophilus influenzae is a potential vaccine candidate. Infect. Immun. 72:6961-6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marmion, B. P., P. A. Storm, J. G. Ayres, L. Semendric, L. Mathews, W. Winslow, M. Turra, and R. J. Harris. 2005. Long-term persistence of Coxiella burnetii after acute primary Q fever. QJM 98:7-20. [DOI] [PubMed] [Google Scholar]
- 24.Murphy, T. F., and M. A. Apicella. 1987. Nontypeable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune response to infection. Rev. Infect. Dis. 9:1-15. [DOI] [PubMed] [Google Scholar]
- 25.Murphy, T. F., A. L. Brauer, A. T. Schiffmacher, and S. Sethi. 2004. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 170:266-272. [DOI] [PubMed] [Google Scholar]
- 26.Murphy, T. F., and C. Kirkham. 2002. Biofilm formation by nontypeable Haemophilus influenzae: strain variability, outer membrane antigen expression and role of pili. BMC Microbiol. 2:7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neary, J. M., and T. F. Murphy. 2006. Antibodies directed at a conserved motif in loop 6 of outer membrane protein P2 of nontypeable Haemophilus influenzae recognize multiple strains in immunoassays. FEMS Immunol. Med. Microbiol. 46:251-261. [DOI] [PubMed] [Google Scholar]
- 28.Nizet, V., K. F. Colina, J. R. Almquist, C. E. Reubens, and A. L. Smith. 1996. A virulent nonencapsulated Haemophilus influenzae. J. Infect. Dis. 173:180-186. [DOI] [PubMed] [Google Scholar]
- 29.Odontsetseg, N., Y. Sakoda, and H. Kida. 2005. Serological evidence of the persistence of infection with Leptospira interrogans serovar Hardjo in cattle in Mongolia. Microbiol. Immunol. 49:865-869. [DOI] [PubMed] [Google Scholar]
- 30.Pandey, D. P., and K. Gerdes. 2005. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 33:966-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pedersen, K., S. K. Christensen, and K. Gerdes. 2002. Rapid induction and reversal of a bacteriostatic condition by controlled expression of toxins and antitoxins. Mol. Microbiol. 45:501-510. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen, K., A. V. Zavialov, M. Y. Pavlov, J. Elf, K. Gerdes, and M. Ehrenberg. 2003. The bacterial toxin RelE displays codon-specific cleavage of mRNAs in the ribosomal A site. Cell 112:131-140. [DOI] [PubMed] [Google Scholar]
- 33.Pichichero, M. E., and D. I. Brixner. 2006. A review of recommended antibiotic therapies with impact on outcomes in acute otitis media and acute bacterial sinusitis. Am. J. Manag. Care 12:S292-S302. [PubMed] [Google Scholar]
- 34.Song, X.-M., A. Forsgren, and H. Janson. 1999. Fragmentation heterogeneity of 23S ribosomal RNA in Haemophilus species. Gene 230:287-293. [DOI] [PubMed] [Google Scholar]
- 35.Spoering, A. L., and K. Lewis. 2001. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 183:6746-6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webster, P., S. Wu, G. Gomez, M. A. Apicella, A. G. Plaut, and J. W. S. St. Geme III. 2006. Distribution of bacterial proteins in biofilms formed by non-typeable Haemophilus influenzae. J. Histochem. Cytochem. 54:829-842. [DOI] [PubMed] [Google Scholar]
- 37.Wu, T., J. Chen, T. F. Murphy, B. A. Green, and X.-X. Gu. 2005. Investigation of nontypeable Haemophilus influenzae outer membrane protein P6 as a new carrier for lipooligosaccharide conjugate vaccines. Vaccine 23:5177-5185. [DOI] [PubMed] [Google Scholar]
- 38.Zhang, J., Y. Zhang, and M. Inouye. 2003. Characterization of the interactions within the mazEF addiction module of Escherichia coli. J. Biol. Chem. 278:32300-32306. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, J., Y. Zhang, L. Zhu, M. Suzuki, and M. Inouye. 2004. Interference of mRNA function by sequence-specific endoribonuclease PemK. J. Biol. Chem. 279:20678-20684. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, Y. X., X. K. Guo, C. Wu, B. Bi, S. X. Ren, C. F. Wu, and G. P. Zhao. 2004. Characterization of a novel toxin-antitoxin module, VapBC, encoded by Leptospira interrogans chromosome. Cell Res. 14:208-216. [DOI] [PubMed] [Google Scholar]