Abstract
A spontaneous mutant of Streptococcus pneumoniae strain D39 exhibiting elevated β-galactosidase activity was identified. We determined that the β-galactosidase activity was due to BgaA, a surface protein in S. pneumoniae, and that the expression of bgaA was regulated. Transcription analyses demonstrated expression of bgaA in the constitutive β-galactosidase (BgaAC) mutant, but not in the parent. β-Galactosidase expression was induced in the parent under specific growth conditions; however, the levels did not reach those of the BgaAC mutant. We localized the mutation resulting in the BgaAC phenotype to a region upstream of bgaA and in the promoter of a phosphoenolpyruvate-dependent phosphotransferase system (PTS) operon. The mutation was in a catabolite-responsive element (cre) and affected the binding of CcpA (catabolite control protein A), a key regulator of many carbon metabolism genes. The pts operon and bgaA were cotranscribed, and their transcription was regulated by CcpA. Deletion of ccpA altered β-galactosidase activity, leading to a sevenfold increase in the parent but a fivefold decrease in the BgaAC mutant. The resulting β-galactosidase activities were the same in the two strains, suggesting the presence of a second repressor. The presence of glucose in the growth medium resulted in pts-bgaA repression by both CcpA and the second repressor, with the latter being important in responding to the glucose concentration. Expression of β-galactosidase is important for S. pneumoniae adherence during colonization of the nasopharynx, a site normally devoid of glucose. CcpA and environmental glucose concentrations thus appear to play important roles in the regulation of a niche-specific virulence factor.
Streptococcus pneumoniae is a low-GC gram-positive pathogen. It is a major cause of death worldwide, primarily due to invasive diseases, such as pneumonia and bacteremia (1). It is also a frequent cause of many other infections, including meningitis, otitis media, and sinusitis. Sixty percent of the adult population may be asymptomatically colonized in the nasopharynx by S. pneumoniae (4). Adaptation to different environmental niches in the host and changes in the expression of many virulence factors lead to the progression from colonization to invasive disease. Environmental sensing leading to changes in virulence gene expression has been shown for many important human pathogens (42, 43), although the exact mechanisms involved in S. pneumoniae are not well characterized.
The present studies were initiated when a spontaneous mutant of S. pneumoniae that exhibited constitutive β-galactosidase activity was identified. S. pneumoniae has a 6,704-bp gene, bgaA, which encodes the 2,235-amino-acid β-galactosidase (46). The protein is surface exposed and anchored to the cell wall via sortase-mediated cleavage at the LPXTG motif. The N terminus contains a putative signal sequence, consistent with the protein being exported. Typical β-galactosidases comprise approximately 1,000 amino acids and are cytoplasmic proteins. In BgaA, 365 residues located in the N-terminal half of the protein have homology to the Escherichia coli and Streptococcus thermophilus β-galactosidases. The remainder of the protein has no homology to other described proteins.
The function of BgaA has not been well characterized, but it has been reported not to be involved in lactose metabolism (46). It has been suggested to be involved in digestion of host cell polysaccharides, which may play a role in adherence or host cell interaction (46). King et al. showed that BgaA is important in adherence to upper-airway epithelial cells in the absence of capsule and that it could aid in the deglycosylation of human secretory component (24). The latter activity was increased due to increased transcription of bgaA in transparent strains, which exhibit less capsule production and more teichoic acid and colonize the host better than opaque strains (21-23). BgaA activity is specific for β,1-4 galactose linkages (25), which can be found in many glycosylated host cell proteins, as well as some S. pneumoniae capsule structures (6, 17, 35). BgaA is expressed in the host, as demonstrated using convalescent-phase serum (48), and disruption of bgaA attenuates the virulence of S. pneumoniae in a mouse pneumonia model (14). These data suggest that BgaA is important for S. pneumoniae adaptation and survival in the host.
One way in which many low-GC gram-positive bacteria, including S. pneumoniae, adapt to changing growth conditions is through catabolite repression mediated by catabolite control protein A (CcpA). The fundamental aspects of this system have been described primarily from studies with Bacillus sp. (reviewed in references 37 and 39). CcpA, a member of the LacI-GalR family, binds to catabolite-responsive elements (cre) located within or near promoters (40). cre are identified by the sequence TGWAANCGNTNWCA (the underlined nucleotides are involved in binding to CcpA) (18, 19, 31, 41). If the cre is located within the promoter region or open reading frame, binding of CcpA inhibits RNA polymerase interaction with the promoter or its progression through the DNA, thereby repressing transcription (20). Binding of CcpA to a cre located upstream of the promoter is proposed to enhance transcription by allowing CcpA to interact with RNA polymerase (38). CcpA binding to cre is enhanced by the binding of Ser46-phosphorylated HPr to CcpA. HPr is a component of the phosphoenolpyruvate-dependent phosphotransferase system (PTS), which is also regulated by CcpA (37, 39). The PTS is the main uptake system for sugars in many bacteria. It involves a series of enzymatic reactions that are responsible for the coupling of carbohydrate uptake and phosphorylation. HPr is phosphorylated on His-15 by transfer of the phosphoryl group from phosphoenolpyruvate via enzyme I (EI), which is an event that is not sugar specific. The sugar-specific components of the pathway are designated enzyme II (EII), which can be composed of multiple proteins or a single protein with specific EII domains. The phosphate is transferred from HPr (His-15) to the sugar-specific enzymes IIA (EIIA) and then EIIB, which phosphorylates the sugar upon translocation into the cell. EIIC makes up the channel involved in translocating the sugar across the membrane. When the concentrations of cellular glycolytic intermediates, such as fructose-1,6-bisphosphate, are increased, HPr kinase phosphorylates HPr on the Ser-46 residue. Upon Ser-46 phosphorylation, HPr is not a good substrate for EI in the PTS pathway but can interact with CcpA to enhance its binding to cre. When a preferred PTS sugar, such as glucose, is present, CcpA represses the transcription of other PTS transporters. bgaA is located downstream of a PTS operon, and putative cre are located upstream of both bgaA and the PTS operon.
Catabolite repression in bacilli, as well as streptococci, may involve many additional levels of regulation. For multiple streptococcal species (9, 11, 16, 33, 47) and Lactobacillus casei (12), CcpA (also referred to as RegM [11, 33]) is not responsible for all catabolite repression. For example, in Streptococcus mutans, disruption of ccpA results in increased glucose repression of enzymes involved in carbohydrate utilization (33). In Streptococcus gordonii, the arginine deaminase system (ADS) involves multiple levels of regulation (9, 47). Here, regulation involves both CcpA and ArcR, which is an activator of the ADS. The two regulators share overlapping binding sequences in the promoter regions, which results in CcpA inhibition of RNA polymerase binding, as well as ArcR binding (47). The results of previous studies of S. pneumoniae suggested that CcpA regulates both β-galactosidase and α-glucosidase but is not involved in glucose repression of either of these enzymes (11). Further studies by Iyer et al. using a different strain of S. pneumoniae suggested that glucose repression of β-galactosidase was partially mediated via CcpA; however, a secondary regulator was also involved (16). In contrast, glucose repression of α-galactosidase, α-glucosidase, and β-glucosidase was not mediated by CcpA. In both studies, CcpA was shown to be important in virulence (11, 16). The results from the streptococcal systems suggest a complex regulatory network involving CcpA, as well as secondary regulators, that allows rapid adaptation to changing environments in the host.
The two previous reports regarding S. pneumoniae noted a role for CcpA in the repression of β-galactosidase activity, but the mechanism for repression was not known (11, 16). In this study, we identified a mutant exhibiting constitutive β-galactosidase activity. Characterization of this mutant allowed us to determine that CcpA controls β-galactosidase activity through regulation of the PTS-encoding operon located upstream of bgaA. We also show that glucose regulates bgaA expression through both CcpA-dependent and -independent mechanisms.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used in this study are shown in Table 1. For growth in liquid medium, cells were inoculated into either THY (Todd-Hewitt broth supplemented with 0.5% yeast extract; Difco) or D medium (tryptone, 10 g/liter; neopeptone, 5 g/liter; Tris, 1.25 g/liter; sodium chloride, 5 g/liter; yeast extract, 1.25 g/liter; and 0.1% of the indicated sugar source) (2). For growth on solid medium, blood agar plates (blood agar base no. 2 [Difco] supplemented with 3% defibrinated sheep blood [Colorado Serum Company]) or THY or D medium with 1.5% Bacto agar (Difco) was used. Where indicated, catalase and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) were added to final concentrations of 5,000 U (Worthington Biochemicals) and 0.25 mg/ml, respectively. Where applicable, media were supplemented with erythromycin (Em) or kanamycin (Km) at concentrations of 0.3 μg/ml and 250 μg/ml, respectively. E. coli strains were maintained in L broth or on L agar supplemented as needed with ampicillin (Ap), Km, or Em at concentrations of 50, 50, and 300 μg/ml, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant properties | Reference or source |
|---|---|---|
| S. pneumoniae | ||
| D39 | Type 2 parent; BgaA+ | 4a |
| GK155 | pGK528 × GK1000; Emr downstream of bgaA | This study |
| GK157 | pGK530 × D39; Emr downstream of bgaA | This study |
| GK159 | pGK538 xGK1000; ΔbgaA in GK1000 | This study |
| GK165 | pGK538 × D39; ΔbgaA in D39 | This study |
| GK220 | pGK579 × D39; ΔccpA in D39; Kmr | This study |
| GK304, GK305 | pGK638 × D39; Emr between pts and bgaA | This study |
| GK308, GK309 | pGK638 × GK1000; Emr between pts and bgaA | This study |
| GK311 | pGK641 × GK1000; Emr upstream of pts | This study |
| GK313 | pGK646 × D39; Kmr between pts and bgaA | This study |
| GK315, GK316 | Independent isogenic BgaAC derivatives of D39 with constitutive β-Gal mutation; −56G→C mutation in pts promoter | This study |
| GK317, GK318 | pGK646 × GK1000; Kmr between pts and bgaA | This study |
| GK320 | pGK579 × GK315; ΔccpA in GK315; Kmr | This study |
| GK322 | pGK538 × GK315; ΔbgaA in GK315 | This study |
| GK338 | pGK663 × D39; pts insertion in D39; Emr | This study |
| GK339 | pGK663 × GK1000; pts insertion in GK1000; Emr | This study |
| GK344, GK345 | pGK663 × GK220; pts insertion and ΔccpA in D39; Kmr Emr | This study |
| GK346 | pGK663 × GK320; pts insertion and ΔccpA in GK1000; Kmr Emr | This study |
| GK1000 | Spontaneous BgaAC derivative of D39; −56G→C mutation in pts promoter | This study |
| GK1001 | Spontaneous BgaAC derivative of D39; −57C→T mutation in pts promoter | This study |
| E. coli | ||
| BL21(AI) | F−ompT hsdSB(rB− mB−) gal dcm lon araB::T7RNAP-tetA | Invitrogen |
| DH5αF′ | F′ φ80dlacZΔM15Δ (lacZYA-argF)U169 deoR recA1 endA1 hsdR17(rK− mK+) phoA supE44λ−thi-1 gyrA96 relA1 | Life Technologies |
| GK639 | BL21(AI); pGK639; CcpA-His; Apr | This study |
| Plasmids | ||
| pCR2.1 | PCR Cloning vector; Apr Kmr | Invitrogen |
| pET20b | T7 RNAP expression vector; Apr | Promega |
| pJY4164 | Lacks origin of replication for S. pneumoniae; Emr | 45 |
| pGK528 | pJY4164 derivative containing PCR fragments from the amplification of GK1000 chromosomal DNA by the primer pair DBgaA3/DBgaA4; for linkage of Em upstream of bgaA | This study |
| pGK530 | pJY4164 derivative containing PCR fragments from the amplification of D39 chromosomal DNA by the primer pair DBgaA3/DBgaA4; for linkage of Em downstream of bgaA | This study |
| pGK538 | pJY4164 derivative containing PCR fragments from the amplification of GK1000 DNA by the primer pairs F-UpBgaA/R-UpBgaA and F-DownBgaA/R-DownBgaA; for deletion of bgaA | This study |
| pGK579 | pJY4164 derivative containing PCR fragments from the amplification of GK1000 DNA by the primer pairs F-UpCcpA1/R-UpCcpA2 and F-downCcpA3/R-DownCcpA4 with Km resistance gene aphA-3 between two fragments; for deletion of ccpA | This study |
| pGK638 | pJY4164 derivative containing PCR fragments from the amplification of D39 chromosomal DNA by the primer pair F-UpBgaA/R-UpBgaA; for polar insertion between pts and bgaA | This study |
| pGK639 | pET-20b::XhoI-NdeI from PCR product amplified from D39 using primer pair F-CcpAORF/ R-CcpAORF; C-terminal His-tagged CcpA | This study |
| pGK641 | pJY4164 derivative containing PCR fragments from the amplification of GK1000 chromosomal DNA by the primer F-spr0562/R-spr0562; for linkage of Em downstream of bgaA | This study |
| pGK646 | pJY4164 derivative containing PCR fragments from the amplification of D39 chromosomal DNA by the primer pair F-UpBgaA/R-UpBgaA and F-BgaApro/BgaA2 with Km resistance gene aphA-3 between two fragments: used to insert Km resistance between pts and bgaA | This study |
| pGK663 | pJY4164 derivative containing PCR fragments from the amplification of D39 chromosomal DNA by the primer pair F-PTS3/R-PTS2; for polar insertion in pts operon | This study |
Deletion of bgaA and ccpA.
Allelic exchange was used to generate deletions in bgaA and ccpA. To construct plasmids for generating bgaA deletions, PCR fragments flanking bgaA were generated using S. pneumoniae constitutive β-galactosidase (BgaAC) mutant chromosomal DNAs as templates. Chromosomal DNA was extracted using QIAGEN genomic tips, as described by the manufacturer (QIAGEN). The upstream and downstream regions obtained using primer pairs F-UpBgaA/R-UpBgaA and F-DownBgaA/R-DownBgaA, respectively (Table 2), were initially cloned separately in pCR2.1 (Invitrogen). The cloned fragments were excised from pCR2.1 by digestion with EcoRI, which is contained in the multiple cloning site of the vector, and KpnI. The latter restriction site was generated by addition of the KpnI recognition sequence to the PCR primers R-UpBgaA and F-DownBgaA, which were used in the initial amplification. The excised fragments were cloned together in the S. pneumoniae suicide vector pJY4164. The deletion plasmid (pGK538) was maintained in DH5αF′, and the appropriate construction was confirmed by sequencing. pGK538 was transformed into competent S. pneumoniae, and the transformation mixture was plated on THY agar plates in the absence of antibiotic selection for the plasmid. For deletion of bgaA in strains constitutive for β-galactosidase activity, colonies were screened on plates supplemented with X-Gal and catalase to identify white isolates, indicative of loss of β-galactosidase expression. Deletion of bgaA by allelic exchange in these isolates was confirmed by PCR. For deletion of bgaA in strains that exhibited low β-galactosidase activity, the transformation mixture was plated in the absence of selection on D agar supplemented with X-Gal and catalase, where the cells exhibit more β-galactosidase activity than on THY agar. Colonies that appeared reduced in β-galactosidase activity were patched onto D agar plates supplemented with X-Gal and catalase. They were screened for deletion of bgaA by colony PCR, in which 10 patches were suspended in 300 μl of water and then boiled for 10 min. Three microliters of the mixture was used in a PCR with the primer pair F-UpBgaA/R-DownBgaA, and the resulting products were screened for reduction in size.
TABLE 2.
Oligonucleotide primers used in this study
| ORF and primera | Sequenceb | Positionc |
|---|---|---|
| spr0562-0565 | ||
| F-spr0562 | dTTGATTGAAAGGGTTAGTATTGAC | 11-34 |
| F-PTSpro2 | CTAGCTTCCTAGTTTACTCTTTG | 266-288 |
| R-PTSpro3 | CAAAGAGTAAACTAGGAAGCTAG | 288-266 |
| R-spr0562 | dCTATATGAAACCGTTGTCAATTAC | 429-406 |
| R-PTSpro | dCTAATACCATAAGTTTTCCCTTC | 483-461 |
| F-PTS3 | dGGAATTTCTAGGAAAGGACTTGC | 647-669 |
| R-PTS2 | dCGATAACTGGGATACCTGGTTC | 1201-1180 |
| F-UpBgaA | GCTACAGCAGCTATCGTTCTTG | 1818-1839 |
| R-UpBgaA | eTATTTTGCTTTTGCTGCGTACTC | 2815-2793 |
| F-bgaApro | eTGCGCTCCTATAAAATATAAACTC | 2818-2840 |
| BgaA2 | CTACGATACCAAAGTAAGAGCT | 5329-5308 |
| F-DownBgaA | eGTGCAGGATTAGTAGTTACTAAAG | 9767-9790 |
| DBgaA3 | GTCCTAAATAGAAGATAAAGAG | 9854-9875 |
| spr0566-0579 | ||
| DBgaA4 | GAACGAACGCTATCAAAACTTGAAAGC | 183-157 |
| R-DownBgaA | CAGTTCCTTCTTACCACAAGACC | 762-741 |
| spr1806-1817 | ||
| F-UpCcpA1 | dACATATGCTGGTCCTCTACCAG | 5336-5357 |
| R-UpCcpA2 | fGAAAAAATCAGGGAATCGAGAAG | 6370-6348 |
| F-CcpAORF | gTTTACGTTTTCGTGTTGAG | 6374-6392 |
| R-CcpAORF | hATGTGTGAGATAGAAAGG | 7411-7394 |
| F-DownCcpA3 | fTCTTTTACAAGTAGAGGTACTGATTG | 7412-7437 |
| R-DownCcpA4 | dCATCCAACGGAAGTGCAAGTTC | 8451-8433 |
Primers are from the R6 genome. Open reading frame (ORF) designations are given for the regions containing the groups of primers. Forward and reverse primers are represented by F and R, respectively.
Sequences are from the complete R6 genome (15).
Nucleotide positions for primers are listed in the forward or reverse orientation, as necessary.
Primer also contains an EcoRI restriction site and additional nucleotides at the 5′ end (GGAATTCC; restriction site underlined).
Primer also contains a KpnI restriction site (GGTACC) at the 5′ end.
Primer also contains a BglII restriction site and additional nucleotides at the 5′ end (GAAGATCT; restriction site underlined).
Primer also contains an XhoI restriction site and additional nucleotides at the 5′ end (CCGCTCGAG; restriction site underlined).
Primer also contains an NdeI restriction site and additional nucleotides at the 5′ end (GGAATTCCATATG; restriction site underlined).
The plasmid construct for deletion of ccpA was generated in a manner similar to that for the bgaA deletion construct. The primer pairs used for amplification of the flanking regions were F-UpCcpA1/R-UpCCpa2 and F-DownCcpA3/R-DownCcpA4 (Table 2). To allow selection of ccpA deletions, a Km resistance marker (aphA-3) was inserted between the upstream and downstream flanking regions using the restriction enzyme BglII, as described previously (44).
Linkage analysis.
For linkage analyses, insertion-duplication was used to place an Em resistance marker either 3 kb upstream, 0.5 kb downstream, or 15 kb downstream of bgaA. Target DNA fragments were cloned into pJY4164 to generate plasmids pGK641, pGK530, and pGK547 (Table 1). Recipients transformed with the plasmids were selected by plating them on blood agar plates containing Em. Competence was induced as described previously (13), except that cultures were incubated only 2 to 2.5 h prior to being plated. The location of the insertion-duplication in the expected sites was confirmed by PCR. For linkage analyses, either intact or restriction-digested chromosomal DNA from the marked strains was transformed into recipient strains. Em-resistant isolates were selected on THY agar plates supplemented with Em and catalase. Em-resistant transformants were screened for the β-galactosidase phenotype by plating them on THY-catalase-Em plates supplemented with X-Gal.
β-Galactosidase assays.
β-Galactosidase activity was determined as described by Miller (28). Briefly, cultures were grown to mid-exponential phase (cell density, 3 × 108 CFU/ml). A 0.2-ml aliquot of the culture was added directly to 0.8 ml Z buffer (28), and the suspension was incubated at 30°C with 0.2 ml of 4 mg/ml o-nitrophenol-β-d-galactopyranoside. Reactions were stopped by the addition of 0.5 ml of 1 M Na2CO3. Activity was calculated in Miller units, as described previously (28).
Transcription analysis.
Total RNA was isolated from a 50-ml culture grown in THY using a hot-acid phenol extraction, as described previously (10). RNA concentrations were determined using UV spectrophotometry. Transcript levels were determined using slot blotting (3). Briefly, samples were diluted to 3 and 0.5 μg per 30 μl for each probe and denatured for 15 min at 65°C in 90 μl of denaturing solution {500 μl formamide, 162 μl 12.3 M [37%] formaldehyde, and 100 μl MOPS [3-(N-morpholino)-propanesulfonic acid] buffer [0.2 M MOPS, pH 7.0, 0.5 M sodium acetate, and 0.01 M EDTA]}. Then, 240 μl of cold 20× SSC (1× SSC = 0.15 M NaCl, 0.015 M sodium citrate, pH 7.0) was added to each sample. Denatured samples were spotted on nylon membranes, which were UV cross-linked and then prehybridized for 3 h at 42°C in high-sodium dodecyl sulfate (SDS) hybridization buffer (7% SDS, 50% formamide, 5× SSC, 2% blocking reagent [Roche], 50 mM sodium phosphate, and 0.1% N-laurylsarcosine). The membranes were incubated overnight with denatured digoxigenin (DIG)-labeled PCR probes made with primers BgaA1/BgaA2 and F-PTS3/R-PTS2 (Table 2), which were added directly to the membranes in the high-SDS hybridization buffer. To remove nonspecifically bound probe, the membranes were washed twice with 2× SSC containing 0.1% SDS for 15 min at room temperature and then twice with 0.5× SSC containing 0.1% SDS for 15 min at 65°C. The blots were developed using the Anti-DIG-AP Fab fragments (Roche) and the Phototope-Star Detection Kit for Nucleic Acids (New England BioLabs). The relative levels of transcript were determined by densitometry using ImageJ software (http://rsb.info.nih.gov/ij). A pspA probe was used as an internal control to ensure equal loading (13).
Electrophoretic mobility shift assays.
To clone ccpA, the open reading frame was PCR amplified with primers that contained restriction enzyme sites for XhoI and NdeI on the 5′ ends of the forward and reverse primers, respectively (F-CcpAORF/R-CcpAORF) (Table 2). After NdeI and XhoI digestion, the PCR product was ligated into pET20b to generate a C-terminal His tag, and the ligation mixture was transformed into BL21-AI (Invitrogen). To induce ccpA expression, arabinose (2% final concentration) was added to a 50-ml culture at late exponential growth phase. CcpA was purified under nondenaturing conditions in wash/extraction buffer using a cobalt resin (Clontech) to affinity purify the His-tagged protein, as described by the manufacturer. Protein eluted from the cobalt column was dialyzed overnight at 4°C in 1× phosphate-buffered saline (342.5 mM NaCl, 6.75 mM KCl, 13.5 mM Na2HPO4, and 4.5 mM KH2PO4) containing 10% glycerol. The purified protein was stored at −80°C. For cellular-lysate preparation, D39 was grown to mid-exponential phase in THY and concentrated 10-fold. The cells were lysed by incubation at 37°C in 0.01 M Tris (pH 8) containing 0.5% sodium deoxycholate with protease and nuclease inhibitors. The promoter regions of the pts operons (positions −206 to +10) were PCR amplified from the indicated strains using primers F-PTSpro2/R-PTSpro (Table 2). The purified PCR products were DIG labeled as described by the manufacturer (Roche; DIG gel shift kit). Labeled probes (0.8 ng) were incubated with increasing concentrations of purified CcpA in binding buffer (10 mM Tris-HCl, pH 7.4, 1 mM dithiothreitol, 1 mM EDTA, 50 mM KCl, 5% glycerol, 50 μg/ml bovine serum albumin, 0.05% Nonidet P-40) for 20 min at 37°C (19). After incubation, samples in bromophenol blue loading buffer were separated by electrophoresis using 5% acrylamide gels in 1× Tris-borate-EDTA buffer, pH 8.0 (10.8 g Tris-HCl, 5.55 g boric acid, 0.74 g EDTA) that had been prerun in 1× Tris-borate-EDTA. The samples were electroblotted from the gels onto Zeta-Probe positively charged nylon membranes (Bio-Rad). The membranes were UV cross-linked (Stratagene) and developed using chemiluminescence, as described for the DIG gel shift kit (Roche). Chemiluminescent images were viewed using an EpiChemi3 DarkRoom (UVP).
RESULTS
Isolation of β-galactosidase constitutive mutants.
Previous studies of the S. pneumoniae capsule serotype 2 strain D39 demonstrated a low level of bgaA-encoded β-galactosidase activity (∼5 Miller units) (24). Analysis of a D39 stock culture in our laboratory that was originally obtained more than 20 years ago (26) revealed the presence of both β-galactosidase-positive and apparent β-galactosidase-negative isolates, as determined by plating them in the presence of the chromogenic substrate X-Gal to identify blue and white colonies, respectively. Following growth in THY liquid medium to a density of ∼3 × 108 CFU/ml, the β-galactosidase activity of the white isolates was 2 to 5 Miller units, whereas that of the blue isolates was 60 to 75 Miller units. Deletion of bgaA in the white and blue isolates demonstrated that BgaA was responsible for the activities observed in both. As described below, the elevated β-galactosidase activity was found to be due to constitutive expression of bgaA; therefore, these isolates are referred to as BgaAC. The isolates producing low levels of β-galactosidase are considered to be the wild type and are therefore referred to as D39.
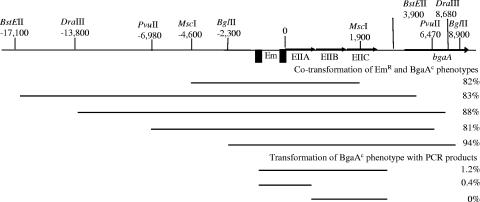
We initially chose a single BgaAC isolate (GK1000) for further characterization. D39 and the constitutive strain exhibited similar growth rates in THY. Restriction digests of chromosomal DNAs with HindIII yielded identical patterns for the two strains (data not shown). The size and monoclonal antibody reactivities of the surface protein PspA, which is size and antigenically variable among strains (7), were also identical (data not shown). These results indicated that the BgaAC strain was a D39 derivative. RNA analyses demonstrated elevated transcription of bgaA in the BgaAC mutant (see below), but no mutations were evident in the bgaA promoter region (data not shown). We therefore undertook linkage analyses to localize the mutation(s) responsible for the altered β-galactosidase levels. To determine if the mutation was located in bgaA, we introduced the D39 bgaA chromosomal region into the BgaAC derivative GK159, in which bgaA had been deleted. The ability to isolate BgaAC transformants with this cross would indicate that the mutation leading to this phenotype was not located in bgaA. For linkage analyses, an Em marker was inserted 475 bp downstream of bgaA in D39, and chromosomal DNA from this strain (GK157) was used to transform GK159. Among the Em-resistant transformants, 11% exhibited the BgaAC phenotype, as determined by blue-white colony screening on X-Gal plates. We next introduced the same Em insertion downstream of bgaA in the original BgaAC mutant (GK1000) and transformed DNA from this strain (GK155) into D39. Here, 25% of the Em-resistant transformants exhibited the BgaAC phenotype, indicating that the mutation was closely linked to bgaA. Further linkage analyses utilized derivatives of the BgaAC strain that contained Em markers located at various sites around bgaA. Following transformation of D39 with DNA from the BgaAC derivative GK311, which contained the Em marker 3 kb upstream of bgaA, 89% of the Em-resistant transformants exhibited the BgaAC phenotype. Using restriction enzyme-digested DNA from GK311 in further transformations of D39, the mutation was localized to a 4-kb region (Fig. 1). Transformation of D39 with PCR products derived from this region showed that the mutation was in a region located immediately upstream of bgaA and containing genes encoding PTS enzymes. These enzymes have homology to putative fructose and galactitol transporters in Clostridium acetobutylicum and Streptococcus agalactiae (∼38% identity; ∼65% positives) and to a putative galactose transporter from Streptococcus pyogenes (41% identity; 63% positives). Sequence analyses identified a single point mutation located between the −35 and −10 sequences of the putative promoter for the pts operon. This region contains a putative cre (Fig. 2A). Sequence analyses of the pts promoter regions from 10 additional BgaAC isolates purified from the primordial stock demonstrated that 6 of the isolates contained the same G-to-C transversion mutation found in GK1000, while the remaining 4 isolates contained a C-to-T transition mutation (GK1001) of the nucleotide immediately upstream of this position (Fig. 2A). Thus, two independent mutants appear to have arisen during the passage of the culture leading to the stock. The mutations appear to be rare, however, as we have not subsequently observed white-to-blue phenotype transition or vice versa.
FIG. 1.
Mapping of mutation leading to the BgaAC phenotype. Restriction fragments from the BgaAC strain GK311 were transformed to D39. Emr transformants were screened for the blue colony BgaAC phenotype by plating them in the presence of X-Gal. Cotransformation of the BgaAC phenotype with Emr represents close linkage to the antibiotic marker. In transformations of D39 with PCR products, the transformation mixtures were plated in the presence of X-Gal and the percentage of blue colonies was determined. Transformation of the BgaAC phenotype with the PCR fragments indicates that the mutation is located in the specific fragment. EIIA, EIIB, and EIIC encode PTS enzymes for sugar transport.
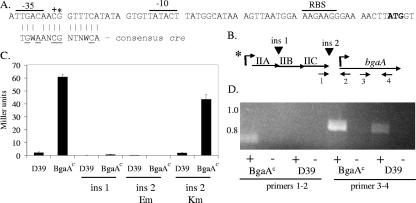
FIG. 2.
Transcription of the pts-bgaA region. (A) pts promoter region. *, point mutation (G→C) in the original BgaAC mutant GK1000 and derivatives; +, point mutation (C→T) in a second mutant, GK1001, and derivatives. The cre consensus sequence is aligned below the pts promoter. Underlined amino acids are important in CcpA binding (31, 41). N, any base, W, adenine or thymine. The lines above the sequence indicate the −35, −10, and ribosome binding site (RBS). The translation start codon is in boldface. (B) Insertions and primers used to characterize bgaA expression. Arrows and numbers beneath the figure indicate primers used in RT-PCR analyses in panel D. The asterisk indicates the location of the pts promoter point mutation in the BgaAC strain. Insertions (ins) are indicated by arrowheads above the pts region. (C) β-Galactosidase activities of insertion mutants. The locations of insertions 1 and 2 are indicated in panel B. Insertion 2, Em, is polar on downstream genes. Insertion 2, Km, is nonpolar. The results are the means (plus standard error) of three replicates. (D) RT-PCR analyses of D39 and the BgaAC strain, GK1000. + and − indicate the presence or absence, respectively, of reverse transcriptase in the reaction. The numbers below the figure represent the primer pairs used in the reaction, as shown in panel B. Molecular size (kb) is indicated on the left.
To confirm that the G-to-C mutation was responsible for the BgaAC phenotype in GK1000, an isogenic derivative (GK315) was obtained by transforming D39 with a 1-kb PCR product amplified from GK1000 and encompassing the mutation. Approximately 0.4% of the colonies on X-Gal plates were blue, indicating introduction of the BgaAC phenotype. Sequence analysis of the 1-kb region in the recipients confirmed that the only mutation occurred in the pts promoter. Subsequent studies were performed with the isogenic derivative GK315, except where stated.
Cotranscription of pts and bgaA.
To determine whether the constitutive transcription of bgaA in the BgaAC mutant GK315 was due to a cis effect of the mutation in the pts promoter, we analyzed the effect of insertion-duplication mutations in and around the pts operon. Polar insertion mutations either in the pts operon (Fig. 2B, insertion 1) or between the pts operon and bgaA (Fig. 2B, insertion 2, Em) eliminated β-galactosidase activity (Fig. 2C). However, when a nonpolar Km resistance marker lacking transcription termination sequences was inserted between the pts operon and bgaA, β-galactosidase activities were unchanged from the D39 and BgaAC parents (Fig. 2B and C, insertion 2, Km). RT-PCR analysis confirmed that bgaA and the upstream pts operon were located on the same transcript (Fig. 2D), and RNA slot blotting demonstrated that the insertion in the pts operon eliminated the bgaA transcript (data not shown). The region between the pts operon and bgaA does not contain any apparent transcription termination sequences, but a near-consensus promoter is located 14 nucleotides upstream of the putative BgaA start codon and a Box element is located between pts and bgaA. Box elements in S. pneumoniae have been predicted to contain secondary structure, but their function is unknown (27). Deletion of the Box element in D39 had no effect on β-galactosidase activity (data not shown). Thus, under the conditions examined, the bgaA promoter was not utilized, and the BgaAC phenotype resulted from cotranscription of bgaA with the pts genes from the pts promoter.
CcpA binding to the pts promoter.
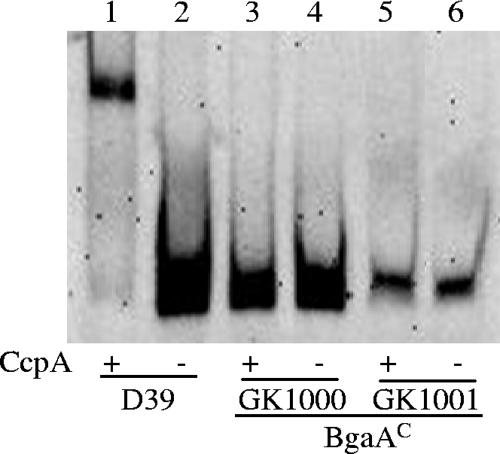
Because the point mutations affecting bgaA expression were located in a putative cre overlapping the −35 region of the pts promoter, we used gel shift assays to examine the interaction of CcpA with this region amplified from D39 and the BgaAC mutants, GK1000 and GK1001, containing the two different point mutations. Efficient binding of CcpA to the D39 pts promoter was observed, but very little binding occurred with the BgaAC mutants (Fig. 3). With the D39 pts promoter, binding was shown with CcpA protein levels as low as 3.75 ng, whereas levels as high as 50 ng were not sufficient to bind the BgaAC pts promoters (data not shown). This result suggested that CcpA binding normally represses pts and bgaA expression and that the mutations led to a decreased affinity of CcpA for the cre. We did not detect binding of CcpA to the bgaA promoter region, although a potential cre was located in this region (data not shown).
FIG. 3.
CcpA binding to the pts promoter. Electrophoretic mobility shift assays were used to determine if the point mutation identified in BgaAC resulted in altered binding of CcpA. Purified CcpA (15 ng) incubated with DIG-labeled D39 or BgaAC pts promoter DNAs was electrophoresed on a 5% nondenaturing acrylamide gel. The labeled DNA was detected by chemiluminescence.
Alteration of pts-bgaA expression and β-galactosidase activity by deletion of ccpA.
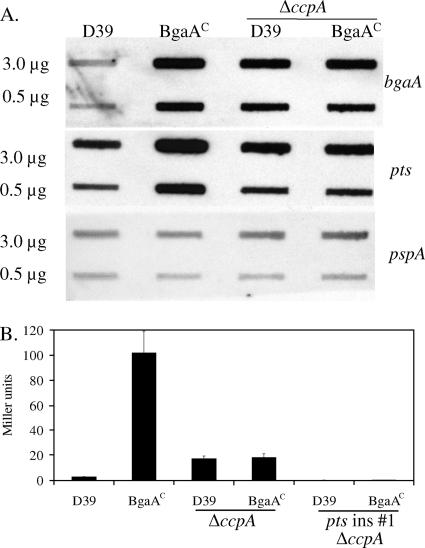
Since the transcriptional regulator, CcpA, binds to the pts promoter and coregulates bgaA, we examined the effect of the deletion of ccpA on bgaA and pts transcription. In D39, the deletion resulted in an increase in both transcript levels, whereas in the BgaAC mutant, decreases occurred for both (Fig. 4A). As a result, the respective transcript levels in the two strains were the same. We also examined levels of β-galactosidase activity in the ccpA deletion strains. The result was similar to the transcriptional analysis, so that the levels of β-galactosidase activity were the same for the D39 and BgaAC ccpA mutants (Fig. 4B, ΔccpA). To confirm that the β-galactosidase activity and bgaA expression in the ccpA deletions were not due to expression from the promoter immediately upstream of bgaA, pts was disrupted in the deletion strains. As shown in Fig. 4B, β-galactosidase activity was lost in these strains (pts insertion 1, ΔccpA). The results of these experiments suggested that there was a second repressor of pts and bgaA expression.
FIG. 4.
(A) Effects of ccpA deletion on transcription and β-galactosidase activity. Slot blots containing the amounts of RNA indicated on the left were probed with internal sequences from the genes indicated on the right. BgaAC is GK1000. pspA was used as a loading control. (B) β-Galactosidase activity. Cultures were grown to mid-exponential phase in THY. The results are the means (plus standard errors) of three replicates.
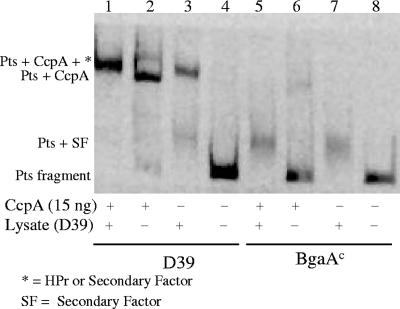
Binding of the second repressor to the pts promoter.
Total cellular lysates from D39 were used in gel shift assays to detect binding of the second repressor to the pts promoter region. Incubation of the D39 pts promoter in the presence of either lysate or lysate plus purified CcpA yielded a band slightly higher than that observed with the purified CcpA alone (Fig. 5, lanes 1 to 3). The increased size of the protein-DNA complex may have been due to the presence of HPr in the cellular lysate, which would interact with CcpA in binding to the pts promoter, or to the binding of both CcpA and the second repressor. In the absence of purified CcpA, a smaller and less intense band was also observed following incubation of the D39 pts promoter with cellular lysate alone (Fig. 5, lane 3, PTS plus SF). A more intense band of this same size was present in reactions with the BgaAC pts promoter using lysate or lysate plus purified CcpA (Fig. 5, lanes 5 and 7). In these experiments, weak binding of purified CcpA to the BgaAC pts promoter was observed in the absence of lysate (Fig. 5, lane 6). These results suggested that the D39 pts promoter had a higher affinity for CcpA than for the second repressor but the BgaAC mutant pts promoter had a higher affinity for the second repressor.
FIG. 5.
Binding of a second repressor to the pts promoter. Electorphoretic mobility shift assays were used to determine if the second repressor of pts-bgaA was a DNA binding protein. Purified CcpA and total cellular lysates from D39 incubated with DIG-labeled D39 and BgaAC (GK1000) pts promoter DNA were processed as in Fig. 3.
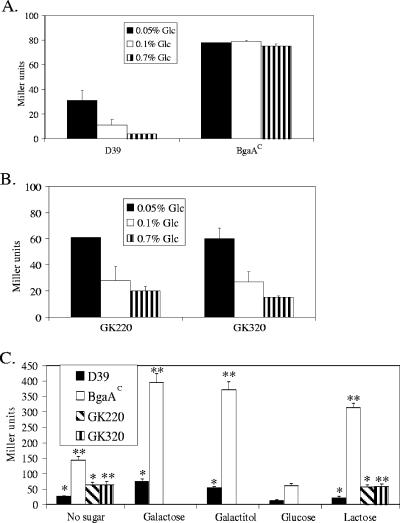
Regulation of β-galactosidase activity by carbon source.
The regulation of pts and bgaA by CcpA led us to examine conditions that might affect expression of these genes. Since both were cotranscribed, we utilized β-galactosidase activity from BgaA as a measure of expression. The standard growth medium (THY) used in the prior studies contained 0.2% glucose. By using a semidefined growth medium and altering the sugar source, we found that glucose mediated repression of pts-bgaA expression. For D39, but not the BgaAC mutant, repression was observed with increasing glucose concentrations (Fig. 6A). When ccpA was deleted, similar levels of repression were observed with both strains, and they were elevated over that observed for the CcpA+ D39 (Fig. 6B). These results suggested that glucose repression of the pts-bgaA operon involved both CcpA and the second repressor and that repression with increasing glucose concentration was due primarily to the second repressor and not CcpA. The fact that repression occurred in the BgaAC mutant when ccpA was deleted again suggested that weak binding of CcpA to the mutant cre affected binding of the second repressor to its recognition sequence in the promoter region.
FIG. 6.
β-Galactosidase activities during growth in different sugars. (A) β-Galactosidase activities of D39 and BgaAC (GK315) in increasing glucose. (B) β-Galactosidase activities of ccpA deletion mutants of D39 (GK220) and BgaAC (GK320). For both panels A and B, strains were grown to mid-exponential growth phase in D medium supplemented with 0.1% glucose and then diluted 1/12 in D medium supplemented with the indicated glucose concentration. β-Galactosidase assays were performed with mid-exponential phase cultures. The results are the means (plus standard errors) of three replicates. (C) β-Galactosidase activities in various PTS sugars. Strains were grown to mid-exponential growth phase in D medium with the indicated sugar (0.1%) and then diluted 1/12 in D medium containing the same sugar. For the no-sugar culture, strains were first grown in D medium containing 0.1% glucose and then diluted 1/12 into D medium lacking a sugar. When either no sugar or galactitol was added to the growth medium, very little growth was observed for either D39 or the BgaAC mutant. For these cultures, β-galactosidase assays were performed after growth ceased (after glucose was exhausted for the no-sugar culture). For all others, β-galactosidase was determined at mid-exponential phase. The results are the means (plus standard errors) of three replicates. *, P < 0.05 compared to D39 grown in glucose. **, P < 0.05 compared to BgaAC grown in glucose.
Because the PTS operon upstream of bgaA has homology to putative galactose, galactitol, and fructose transporters, we next examined β-galactosidase activities during growth in these sugars and in lactose, as their uptake is mediated by phosphoenolpyruvate-dependent PTSs. As shown in Fig. 6C for both D39 and the BgaAC mutant, high levels of β-galactosidase activity were seen when no sugar was added, and activity increased during growth with galactose, galactitol, and, for BgaAC, lactose. β-Galactosidase activity for D39 never reached the level of the BgaAC mutant. As shown for glucose in Fig. 6B and lactose in Fig. 6C, deletion of ccpA resulted in identical β-galactosidase levels for D39 and the BgaAC mutant due to increased activity in the former and decreased activity in the latter. For the ccpA deletions, β-galactosidase activities were the same during culture with lactose or no sugar, indicating that the observed activity in these mutants was the result of a lack of repression by glucose and not induction by lactose (Fig. 6C).
During growth of D39 in glucose or lactose, we observed increased β-galactosidase activity in the early stationary phase growth (approximate 10- and 17-fold increases, respectively, over mid-exponential phase), as previously reported for growth in glucose (24). β-Galactosidase activity in the BgaAC mutant remained essentially constant throughout growth in glucose but increased approximately twofold in lactose upon entering stationary phase.
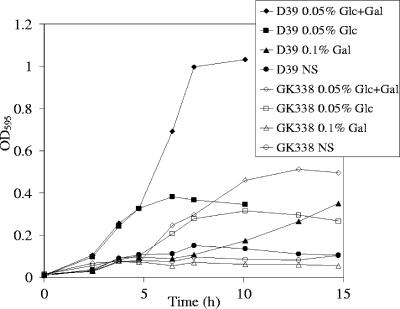
Effects of pts mutation on growth.
We examined the effect of an insertion mutation in the D39 pts on growth in glucose and galactose, as the PTS transporter had homology with a putative galactose transporter in S. pyogenes and was cotranscribed with β-galactosidase, an enzyme that cleaves terminal galactose residues. The mutation affected growth in both sugars, and the mutant appeared unable to initiate growth in the presence of galactose only (Fig. 7). Growth was also slowed in rich medium (THY; data not shown). Similar results were obtained for the BgaAC constitutive mutant GK315 and its pts insertion mutant (GK339) in glucose, galactose, and THY (data not shown). In contrast to this PTS, several of the other PTSs in D39 do not contain all of the components for sugar transport (15, 36). Therefore, this PTS may be important for the uptake of many sugars, possibly explaining the growth defects of the pts insertion mutants.
FIG. 7.
Growth of D39 and its pts insertion mutant (GK338). Strains were grown in D medium with the indicated sugar source. Gal, 0.1% galactose; NS, no sugar; OD595, optical density at 595 nm. The data presented are representative of multiple experiments.
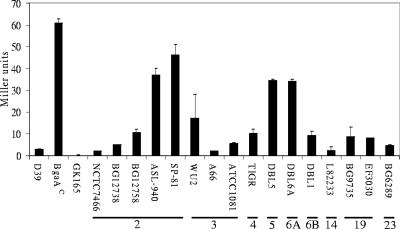
β-Galactosidase activities in other strains.
A wide variation in β-galactosidase activities was seen among other S. pneumoniae strains (Fig. 8). NCTC7466 is another D39 isolate that is separated by many years from our D39 (26). It exhibited the same level of β-galactosidase activity and the same promoter region sequence as our strain (by comparison with the available genome sequence) (15). From the genome sequence of TIGR4 (36) and sequence analyses of the capsule type 2 strains BG12738, BG12758, ASL-940, and SP-81, we found that all had pts promoter and cre regions identical to that of D39 (shown in Fig. 2A). The high levels of β-galactosidase activity in some strains, such as ASL-940 and SP-81, may therefore reflect the influence of other factors, possibly including the second repressor involved in β-galactosidase regulation, that vary between strains.
FIG. 8.
β-Galactosidase activities of other S. pneumoniae strains. The strains were grown to mid-exponential phase in THY. The results are the means (plus standard errors) of three replicates. The capsular serotype is shown below the strain name.
DISCUSSION
We have shown that a point mutation in the promoter region of the pts operon upstream of bgaA leads to constitutive β-galactosidase activity. The point mutation is located between the −35 and −10 sequences of the pts promoter in a cre. The G-to-C transversion mutation in the BgaAC mutants, GK1000 and GK315, altered the ability of CcpA to bind the cre and repress expression of the pts operon and bgaA. This residue, as well as the C immediately upstream and corresponding to the point mutation in GK1001, are universally conserved in cre (29, 41). Both residues are critical for interaction with CcpA, as the conserved Leu55 in CcpA intercalates between them to effect minor groove expansion (31). Binding of CcpA to the cre can inhibit transcription by inhibiting the binding of RNA polymerase (20). We found that the point mutations in the BgaAC mutants decreased the affinity of CcpA for the cre. These alterations apparently resulted in a reduced ability to inhibit RNA polymerase binding, thereby leading to constitutive transcription of the pts operon and bgaA.
The pts operon and bgaA are contained on the same transcript, as demonstrated by RT-PCR and the elimination of β-galactosidase activity by the insertion of a terminator between the pts operon and bgaA. CcpA thus represses β-galactosidase expression by binding to the promoter region of the upstream pts operon. A second repressor was revealed when ccpA was deleted in the D39 parent and the BgaAC mutant strains. In the BgaAC mutant, weak binding of CcpA appeared to reduce binding of the second repressor, leading to high levels of pts-bgaA expression and β- galactosidase activity. When ccpA was deleted, the second repressor was able to bind, thereby reducing pts-bgaA expression. Repression in D39 may occur by simultaneous binding of both CcpA and the second repressor or the two repressors may bind independently. As with the mutant strain, deletion of ccpA in D39 allowed binding of only the second repressor, which did not repress pts-bgaA expression to the level observed when CcpA was present. Since the second repressor could bind to both the parent and mutated pts promoter regions, it must not recognize the same sequence as CcpA. It does, however, bind in the same region as CcpA, as shown by the fact that deletion of ccpA allowed repression of bgaA expression in the BgaAC mutant, which was identical to that observed with the parent. The possibility of a second repressor was suggested in studies with the TIGR4 S. pneumoniae strain (16), although we do not know whether the two repressors are the same. In the S. gordonii ADS, the binding sequences for CcpA and the second regulator, ArcR, overlap (47), possibly similar to what we observed. The ArcR binding sequence is not, however, present in the region containing the binding site for the S. pneumoniae second repressor (unpublished observations).
Our results demonstrate that glucose-mediated repression of bgaA occurs through both CcpA and the second repressor. CcpA-mediated repression of β-galactosidase activity in the presence of glucose was also noted using the S. pneumoniae TIGR4 strain (16). In contrast, it was concluded that glucose was not involved in CcpA-mediated repression of β-galactosidase activity in S. pneumoniae D39 (11), the same strain we used in the present studies. The discrepancy appears to relate to the effect of the second repressor, which may have obscured the effect of glucose in the earlier studies (11). When ccpA is deleted, binding of the second repressor still allows some glucose-mediated repression to be observed. The effect of the second repressor was also seen during culture with other sugars. During growth in lactose, the β-galactosidase activity of the D39 ccpA deletion mutant was elevated over that of D39 and was similar to that observed for the ccpA deletion mutants in the absence of added sugar. However, the highest β-galactosidase activity was observed with the BgaAC mutant grown in lactose, galactose, or galactitol. This activity appears to result from both ineffective CcpA repression (due to the mutation in the cre) and ineffective repression by the second repressor (due to reduced accessibility to its binding site as a result of weakly bound CcpA). The difference in β-galactosidase levels between the BgaAC mutant and the ΔccpA mutants grown in lactose, therefore, appears to reflect the activity of the second repressor. Regulation of β-galactosidase expression may therefore involve multiple levels of repression, including that relating to the presence of glucose, and the activities of CcpA and the second repressor. Full alleviation of repression may occur when neither CcpA nor the second repressor is active, such as when glucose is absent and another PTS sugar is present. To date, we have not identified the second repressor but have shown that it is not the S. pneumoniae homologue of the global transcriptional regulator CodY that is found in many gram-positive bacteria and which, in Bacillus subtilis, binds near CcpA and has an additive effect (32, 34) (unpublished data).
CcpA-mediated catabolite repression allows gram-positive bacteria to utilize the most efficient carbon source available for growth through a hierarchy in which expression of PTS transporters involved in the utilization of less effective sources is repressed (8). Glucose is usually at the top of this hierarchy, and its ability to repress pts-bgaA expression fits well with the niches occupied by S. pneumoniae and the functions proposed for BgaA. S. pneumoniae is a normal and frequent colonizer of the nasopharyngeal cavity (4), a site where there is little to no glucose (30). Glucose levels are similarly low in healthy lungs (5), but higher levels occur during infection (30) and in the bloodstream. BgaA has previously been shown to cleave human glycoproteins and to be important in adherence by S. pneumoniae (24). In glucose-deficient sites, repression of pts-bgaA by CcpA and the second repressor would be relieved, resulting in high-level expression of β-galactosidase and the PTS. Cleavage of galactose from host glycoproteins by BgaA and its transport into the cell by the PTS would allow S. pneumoniae not only to adhere, but also to persist. Elevated glucose concentrations in the lung or other sites of dissemination would lead to repression of pts-bgaA expression by CcpA and the second repressor. CcpA, and possibly the second repressor, may therefore play central roles in regulating the transition from colonization to systemic infection.
Acknowledgments
We thank Karita Ambrose for her initial observations that led to this study.
This study was supported by Public Health Service grants AI28457 and T32 HL07553 from the National Institutes of Health.
Footnotes
Published ahead of print on 11 May 2007.
REFERENCES
- 1.Advisory Committee on Immunization Practice. 1997. Prevention of pneumococcal disease. Morb. Mortal. Wkly. Rep. 46:1-24. [PubMed] [Google Scholar]
- 2.Alloing, G., C. Granadel, D. A. Morrison, and J. P. Claverys. 1996. Competence pheromone, oligopeptide permease, and induction of competence in Streptococcus pneumoniae. Mol. Microbiol. 21:471-478. [DOI] [PubMed] [Google Scholar]
- 3.Alwine, J. C., D. J. Kemp, and G. R. Stark. 1977. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc. Natl. Acad. Sci. USA 74:5350-5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austrian, R. 1986. Some aspects of the pneumococcal carrier state. J. Antimicrob. Chemother. 18(Suppl. A):35-45. [DOI] [PubMed] [Google Scholar]
- 4a.Avery, O. T., C. M. MacLeod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a deoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker, E. H., N. Clark, A. L. Brennan, D. A. Fisher, K. M. Gyi, M. E. Hodson, B. J. Philips, D. L. Baines, and D. M. Wood. 2007. Hyperglycaemia and cystic fibrosis alter respiratory fluid glucose concentrations estimated by breath condensate analysis. J. Appl. Physiol. 102:1969-1975. [DOI] [PubMed] [Google Scholar]
- 6.Bentley, S. D., D. M. Aanensen, A. Mavroidi, D. Saunders, E. Rabbinowitsch, M. Collins, K. Donohoe, D. Harris, L. Murphy, M. A. Quail, G. Samuel, I. C. Skovsted, M. S. Kaltoft, B. Barrell, P. R. Reeves, J. Parkhill, and B. G. Spratt. 2006. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2:262-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crain, M. J., W. D. Waltman II, J. S. Turner, J. Yother, D. F. Talkington, L. S. McDaniel, B. M. Gray, and D. E. Briles. 1990. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect. Immun. 58:3293-3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deutscher, J., C. Francke, and P. W. Postma. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol Rev. 70:939-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong, Y., Y. Y. Chen, and R. A. Burne. 2004. Control of expression of the arginine deiminase operon of Streptococcus gordonii by CcpA and Flp. J. Bacteriol. 186:2511-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgellis, D., S. Arvidson, and A. von Gabain. 1992. Decay of ompA mRNA and processing of 9S RNA are immediately affected by shifts in growth rate, but in opposite manners. J. Bacteriol. 174:5382-5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giammarinaro, P., and J. C. Paton. 2002. Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect. Immun. 70:5454-5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosalbes, M. J., V. Monedero, C. A. Alpert, and G. Perez-Martinez. 1997. Establishing a model to study the regulation of the lactose operon in Lactobacillus casei. FEMS Microbiol. Lett. 148:83-89. [DOI] [PubMed] [Google Scholar]
- 13.Hardy, G. G., M. J. Caimano, and J. Yother. 2000. Capsule biosynthesis and basic metabolism in Streptococcus pneumoniae are linked through the cellular phosphoglucomutase. J. Bacteriol. 182:1854-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hava, D. L., and A. Camilli. 2002. Large-scale identification of serotype 4 Streptococcus pneumoniae virulence factors. Mol. Microbiol. 45:1389-1406. [PMC free article] [PubMed] [Google Scholar]
- 15.Hoskins, J., W. E. Alborn, Jr., J. Arnold, L. C. Blaszczak, S. Burgett, B. S. DeHoff, S. T. Estrem, L. Fritz, D. J. Fu, W. Fuller, C. Geringer, R. Gilmour, J. S. Glass, H. Khoja, A. R. Kraft, R. E. Lagace, D. J. LeBlanc, L. N. Lee, E. J. Lefkowitz, J. Lu, P. Matsushima, S. M. McAhren, M. McHenney, K. McLeaster, C. W. Mundy, T. I. Nicas, F. H. Norris, M. O'Gara, R. B. Peery, G. T. Robertson, P. Rockey, P. M. Sun, M. E. Winkler, Y. Yang, M. Young-Bellido, G. Zhao, C. A. Zook, R. H. Baltz, S. R. Jaskunas, P. R. Rosteck, Jr., P. L. Skatrud, and J. I. Glass. 2001. Genome of the bacterium Streptococcus pneumoniae strain R6. J. Bacteriol. 183:5709-5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer, R., N. S. Baliga, and A. Camilli. 2005. Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae. J. Bacteriol. 187:8340-8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamerling, J. P. 2000. Pneumococcal polysaccharides: a chemical view, p. 81-114. In A. Tomasz (ed.), Streptococcus pneumoniae: molecular biology and mechanisms of disease. Mary Ann. Liebert, Inc., Larchmont, NY.
- 18.Kim, J. H., and G. H. Chambliss. 1997. Contacts between Bacillus subtilis catabolite regulatory protein CcpA and amyO target site. Nucleic Acids Res. 25:3490-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, J. H., Z. T. Guvener, J. Y. Cho, K. C. Chung, and G. H. Chambliss. 1995. Specificity of DNA binding activity of the Bacillus subtilis catabolite control protein CcpA. J. Bacteriol. 177:5129-5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, J. H., Y. K. Yang, and G. H. Chambliss. 2005. Evidence that Bacillus catabolite control protein CcpA interacts with RNA polymerase to inhibit transcription. Mol. Microbiol. 56:155-162. [DOI] [PubMed] [Google Scholar]
- 21.Kim, J. O., S. Romero-Steiner, U. B. Sorensen, J. Blom, M. Carvalho, S. Barnard, G. Carlone, and J. N. Weiser. 1999. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect. Immun. 67:2327-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, J. O., and J. N. Weiser. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177:368-377. [DOI] [PubMed] [Google Scholar]
- 23.King, S. J., K. R. Hippe, J. M. Gould, D. Bae, S. Peterson, R. T. Cline, C. Fasching, E. N. Janoff, and J. N. Weiser. 2004. Phase variable desialylation of host proteins that bind to Streptococcus pneumoniae in vivo and protect the airway. Mol. Microbiol. 54:159-171. [DOI] [PubMed] [Google Scholar]
- 24.King, S. J., K. R. Hippe, and J. N. Weiser. 2006. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol. Microbiol. 59:961-974. [DOI] [PubMed] [Google Scholar]
- 25.Kojima, K., M. Iwamori, S. Takasaki, K. Kubushiro, S. Nozawa, R. Iizuka, and Y. Nagai. 1987. Diplococcal beta-galactosidase with a specificity reacting to beta 1-4 linkage but not to beta 1-3 linkage as a useful exoglycosidase for the structural elucidation of glycolipids. Anal. Biochem. 165:465-469. [DOI] [PubMed] [Google Scholar]
- 26.Lanie, J. A., W. L. Ng, K. M. Kazmierczak, T. M. Andrzejewski, T. M. Davidsen, K. J. Wayne, H. Tettelin, J. I. Glass, and M. E. Winkler. 2007. Genome sequence of Avery's virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J. Bacteriol. 189:38-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin, B., O. Humbert, M. Camara, E. Guenzi, J. Walker, T. Mitchell, P. Andrew, M. Prudhomme, G. Alloing, R. Hakenbeck, et al. 1992. A highly conserved repeated DNA element located in the chromosome of Streptococcus pneumoniae. Nucleic Acids Res. 20:3479-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 29.Miwa, Y., A. Nakata, A. Ogiwara, M. Yamamoto, and Y. Fujita. 2000. Evaluation and characterization of catabolite-responsive elements (cre) of Bacillus subtilis. Nucleic Acids Res. 28:1206-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Philips, B. J., J. X. Meguer, J. Redman, and E. H. Baker. 2003. Factors determining the appearance of glucose in upper and lower respiratory tract secretions. Intensive Care Med. 29:2204-2210. [DOI] [PubMed] [Google Scholar]
- 31.Schumacher, M. A., G. S. Allen, M. Diel, G. Seidel, W. Hillen, and R. G. Brennan. 2004. Structural basis for allosteric control of the transcription regulator CcpA by the phosphoprotein HPr-Ser46-P. Cell 118:731-741. [DOI] [PubMed] [Google Scholar]
- 32.Shivers, R. P., S. S. Dineen, and A. L. Sonenshein. 2006. Positive regulation of Bacillus subtilis ackA by CodY and CcpA: establishing a potential hierarchy in carbon flow. Mol. Microbiol. 62:811-822. [DOI] [PubMed] [Google Scholar]
- 33.Simpson, C. L., and R. R. Russell. 1998. Identification of a homolog of CcpA catabolite repressor protein in Streptococcus mutans. Infect. Immun. 66:2085-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonenshein, A. L. 2005. CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr. Opin. Microbiol. 8:203-207. [DOI] [PubMed] [Google Scholar]
- 35.Spik, G., G. Strecker, B. Fournet, S. Bouquelet, J. Montreuil, L. Dorland, H. van Halbeek, and J. F. Vliegenthart. 1982. Primary structure of the glycans from human lactotransferrin. Eur. J. Biochem. 121:413-419. [DOI] [PubMed] [Google Scholar]
- 36.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 37.Titgemeyer, F., and W. Hillen. 2002. Global control of sugar metabolism: a gram-positive solution. Antonie Leeuwenhoek 82:59-71. [PubMed] [Google Scholar]
- 38.Turinsky, A. J., F. J. Grundy, J. H. Kim, G. H. Chambliss, and T. M. Henkin. 1998. Transcriptional activation of the Bacillus subtilis ackA gene requires sequences upstream of the promoter. J. Bacteriol. 180:5961-5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warner, J. B., and J. S. Lolkema. 2003. CcpA-dependent carbon catabolite repression in bacteria. Microbiol. Mol. Biol. Rev. 67:475-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weickert, M. J., and S. Adhya. 1992. A family of bacterial regulators homologous to Gal and Lac repressors. J. Biol. Chem. 267:15869-15874. [PubMed] [Google Scholar]
- 41.Weickert, M. J., and G. H. Chambliss. 1990. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 87:6238-6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiser, J. N., D. Bae, H. Epino, S. B. Gordon, M. Kapoor, L. A. Zenewicz, and M. Shchepetov. 2001. Changes in availability of oxygen accentuate differences in capsular polysaccharide expression by phenotypic variants and clinical isolates of Streptococcus pneumoniae. Infect. Immun. 69:5430-5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.West, A. H., and A. M. Stock. 2001. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem. Sci. 26:369-376. [DOI] [PubMed] [Google Scholar]
- 44.Xayarath, B., and J. Yother. 2007. Mutations blocking side chain assembly, polymerization, or transport of a Wzy-dependent Streptococcus pneumoniae capsule are lethal in the absence of suppressor mutations and can affect polymer transfer to the cell wall. J. Bacteriol. 189:3369-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yother, J., G. L. Handsome, and D. E. Briles. 1992. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J. Bacteriol. 174:610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zahner, D., and R. Hakenbeck. 2000. The Streptococcus pneumoniae beta-galactosidase is a surface protein. J. Bacteriol. 182:5919-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng, L., Y. Dong, and R. A. Burne. 2006. Characterization of cis-acting sites controlling arginine deiminase gene expression in Streptococcus gordonii. J. Bacteriol. 188:941-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zysk, G., R. J. Bongaerts, E. ten Thoren, G. Bethe, R. Hakenbeck, and H. P. Heinz. 2000. Detection of 23 immunogenic pneumococcal proteins using convalescent-phase serum. Infect. Immun. 68:3740-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]